Abstract
Background
Antibiotic therapy for suspected acute bacterial meningitis (ABM) needs to be started immediately, even before the results of cerebrospinal fluid (CSF) culture and antibiotic sensitivity are available. Immediate commencement of effective treatment using the intravenous route may reduce death and disability.
Objectives
The objective is to compare the effectiveness and safety of third generation cephalosporins (ceftriaxone or cefotaxime) with conventional treatment using penicillin or ampicillin‐chloramphenicol in patients with community‐acquired ABM.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 1), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (January 1966 to March week 4, 2011) and EMBASE (January 1974 to April 2011). We also searched the reference list of review articles and book chapters, and contacted experts for any unpublished trials.
Selection criteria
Randomised controlled trials (RCTs) comparing third generation cephalosporins (ceftriaxone or cefotaxime) with conventional antibiotics (ampicillin‐chloramphenicol combination, or chloramphenicol alone) as empirical therapy for ABM in adults and children.
Data collection and analysis
Two review authors independently applied the study selection criteria, assessed methodological quality and extracted data.
Main results
Nineteen trials that involved 1496 patients were included in the analysis. There was no heterogeneity of results among the studies in any outcome except diarrhoea. There was no statistically significant difference between the groups in the risk of death (risk difference (RD) 0%; 95% confidence interval (CI) ‐3% to 2%), risk of deafness (RD ‐4%; 95% CI ‐9% to 1%) or risk of treatment failure (RD ‐1%; 95% CI ‐4% to 2%). However, there were significantly decreased risks of culture positivity of CSF after 10 to 48 hours (RD ‐6%; 95% CI ‐11% to 0%) and statistically significant increases in the risk of diarrhoea between the groups (RD 8%; 95% CI 3% to 13%) with the third generation cephalosporins. The risk of neutropaenia and skin rash were not significantly different between the two groups. However, due to increased antibiotic resistance since the 1980s, the finding of this review should be read with caution.
Authors' conclusions
The review shows no clinically important difference between third generation cephalosporins (ceftriaxone or cefotaxime) and conventional antibiotics (ampicillin‐chloramphenicol combination, or chloramphenicol alone). Therefore the choice of antibiotic will depend on cost and availability. The antimicrobial resistance pattern against various antibiotics needs to be closely monitored in low‐ to middle‐income countries as well as high‐income countries.
Keywords: Humans; Acute Disease; Ampicillin; Ampicillin/therapeutic use; Anti‐Bacterial Agents; Anti‐Bacterial Agents/therapeutic use; Cephalosporins; Cephalosporins/adverse effects; Cephalosporins/therapeutic use; Chloramphenicol; Chloramphenicol/therapeutic use; Community‐Acquired Infections; Community‐Acquired Infections/drug therapy; Meningitis, Bacterial; Meningitis, Bacterial/drug therapy; Penicillins; Penicillins/therapeutic use; Randomized Controlled Trials as Topic
Plain language summary
Newer, third generation cephalosporins versus conventional antibiotics for treating acute bacterial meningitis
Acute bacterial meningitis is a life‐threatening illness. Currently the evidence suggests that old and new antibiotics offer the same level of treatment. Bacteria which cause meningitis are often thought to be resistant to conventional (older) antibiotics, and so doctors often prescribe newer antibiotics (called third generation cephalosporins). Commencing treatment early is vitally important and the choice of antibiotic is often made without any knowledge of possible drug resistance. This review examined 19 studies with 1496 participants to see whether there is a difference in effectiveness between conventional and newer antibiotics. This review found no differences. Adverse effects in both approaches were similar, except for diarrhoea, which was more common in the cephalosporin group. Only three studies dealt with adults; the remaining studies recruited participants aged 15 years and younger. Therefore, we believe that the results probably pertain more to children. Conventional and newer antibiotics seem reasonable options for initial, immediate treatment. The choice may depend on availability, affordability and local policies.
Summary of findings
Summary of findings for the main comparison. Third generation cephalosporins versus conventional therapy for acute bacterial meningitis.
| Third generation cephalosporins versus conventional therapy for acute bacterial meningitis | ||||||
| Patient or population: patients treated for acute bacterial meningitis Settings: acute bacterial meningitis Intervention: third generation cephalosporins versus conventional therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Third generation cephalosporins versus conventional therapy | |||||
| Death | Study population | See comment | 1496 (19) | Risks were calculated from pooled risk differences | ||
| 67 per 1000 | 65 per 1000 (37 to 87) | |||||
| Medium risk population | ||||||
| 47 per 1000 | 46 per 1000 (26 to 61) | |||||
| Deafness | Study population | See comment | 501 (10) | Risks were calculated from pooled risk differences | ||
| 118 per 1000 | 83 per 1000 (28 to 127) | |||||
| Medium risk population | ||||||
| 72 per 1000 | 50 per 1000 (17 to 78) | |||||
| Culture positivity 10 to 48 hours start of treatment | Study population | See comment | 442 (12) | Risks were calculated from pooled risk differences | ||
| 117 per 1000 | 58 per 1000 (7 to 117) | |||||
| Medium risk population | ||||||
| 25 per 1000 | 12 per 1000 (1 to 25) | |||||
| Diarrhoea | Study population | See comment | 750 (12) | Risks were calculated from pooled risk differences | ||
| 125 per 1000 | 202 per 1000 (155 to 255) | |||||
| Medium risk population | ||||||
| 77 per 1000 | 125 per 1000 (95 to 157) | |||||
| Neutropenia | Study population | See comment | 472 (10) | Risks were calculated from pooled risk differences | ||
| 54 per 1000 | 29 per 1000 (‐16 to 74) | |||||
| Medium risk population | ||||||
| 13 per 1000 | 7 per 1000 (‐4 to 18) | |||||
| Skin rash | Study population | See comment | 533 (8) | Risks were calculated from pooled risk differences | ||
| 16 per 1000 | 4 per 1000 (‐24 to 26) | |||||
| Medium risk population | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Treatment failure (either death or deafness) | Study population | See comment | 1496 (19) | Risks were calculated from pooled risk differences | ||
| 87 per 1000 | 79 per 1000 (47 to 107) | |||||
| Medium risk population | ||||||
| 67 per 1000 | 61 per 1000 (36 to 82) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | ||||||
Summary of findings 2. Cephalosporins versus conventional therapy for H. influenzae meningitis.
| Cephalosporins versus conventional therapy for H. influenzae meningitis | ||||||
| Patient or population: patients treated for H. influenzae meningitis Settings: acute bacterial meningitis Intervention: cephalosporins versus conventional therapy for H. influenzae meningitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Cephalosporins versus conventional therapy for H. influenzae meningitis | |||||
| Death | Study population | See comment | 318 (10) | Risks were calculated from pooled risk differences | ||
| 62 per 1000 | 69 per 1000 (12 to 122) | |||||
| Medium risk population | ||||||
| 26 per 1000 | 29 per 1000 (5 to 51) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | ||||||
Summary of findings 3. Third generation cephalosporins versus conventional antibiotics for S. pneumoniae meningitis.
| Third generation cephalosporins versus conventional antibiotics for S. pneumoniae meningitis | ||||||
| Patient or population: patients treated for S. pneumoniae meningitis Settings: acute bacterial meningitis Intervention: third generation cephalosporins versus conventional antibiotics for S. pneumoniae meningitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Third generation cephalosporins versus conventional antibiotics for S. pneumoniae meningitis | |||||
| Death | Study population | See comment | 129 (11) | Risks were calculated from pooled risk differences | ||
| 255 per 1000 | 232 per 1000 (74 to 395) | |||||
| Medium risk population | ||||||
| 250 per 1000 | 228 per 1000 (72 to 387) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | ||||||
Summary of findings 4. Third generation cephalosporins versus conventional therapy for meningococcal meningitis.
| Third generation cephalosporins versus conventional therapy for meningococcal meningitis | ||||||
| Patient or population: patients treated for meningococcal meningitis Settings: acute bacterial meningitis Intervention: third generation cephalosporins versus conventional therapy for meningococcal meningitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Third generation cephalosporins versus conventional therapy for meningococcal meningitis | |||||
| Death | Study population | See comment | 477 (13) | Risks were calculated from pooled risk differences | ||
| 39 per 1000 | 35 per 1000 (‐11 to 80) | |||||
| Medium risk population | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | ||||||
Summary of findings 5. Third generation cephalosporins versus conventional antibiotics (high and low/middle‐income countries) for acute bacterial meningitis.
| Third generation cephalosporins versus conventional antibiotics (high and low/middle‐income countries) for acute bacterial meningitis | ||||||
| Patient or population: patients treated for acute bacterial meningitis Settings: acute bacterial meningitis Intervention: third generation cephalosporins versus conventional antibiotics (high and low/middle‐income countries) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Third generation cephalosporins versus conventional antibiotics (high and low/middle‐income countries) | |||||
| Death | Study population | See comment | 1301 (19) | Risks were calculated from pooled risk differences | ||
| 67 per 1000 | 62 per 1000 (37 to 87) | |||||
| Medium risk population | ||||||
| 44 per 1000 | 40 per 1000 (24 to 57) | |||||
| Death: high‐income countries | Study population | See comment | 510 (9) | Risks were calculated from pooled risk differences | ||
| 27 per 1000 | 28 per 1000 (‐3 to 66) | |||||
| Medium risk population | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Death: Low/middle‐income countries | Study population | See comment | 791 (10) | Risks were calculated from pooled risk differences | ||
| 94 per 1000 | 85 per 1000 (44 to 124) | |||||
| Medium risk population | ||||||
| 80 per 1000 | 72 per 1000 (38 to 106) | |||||
| Deafness | Study population | See comment | 501 (10) | Risks were calculated from pooled risk differences | ||
| 114 per 1000 | 76 per 1000 (24 to 124) | |||||
| Medium risk population | ||||||
| 54 per 1000 | 36 per 1000 (11 to 59) | |||||
| Deafness: high‐income countries | Study population | See comment | 380 (6) | Risks were calculated from pooled risk differences | ||
| 138 per 1000 | 90 per 1000 (28 to 148) | |||||
| Medium risk population | ||||||
| 72 per 1000 | 47 per 1000 (14 to 77) | |||||
| Deafness: low‐income countries | Study population | See comment | 121 (4) | Risks were calculated from pooled risk differences | ||
| 45 per 1000 | 39 per 1000 (‐44 to 124) | |||||
| Medium risk population | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | ||||||
Background
Description of the condition
Acute bacterial meningitis (ABM) is a major cause of death and disability, especially in low‐ to middle‐income countries. Despite advances in understanding the pathophysiology of meningitis and new brain imaging techniques, the case fatality rate of ABM is still around 10% to 30% and an additional 20% to 50% of cases have only partial recovery with long‐term disability.
Description of the intervention
One of the major concerns in the treatment of ABM is the emergence of resistant bacterial strains to conventional antibiotics. An increasing number of B‐lactamase‐producing strains of Haemophilus influenzae (H. influenzae) type b are resistant to ampicillin, and a smaller number of chloramphenicol acetyltransferase‐producing strains are resistant to chloramphenicol (Kaplan 1988b). There have been reports of penicillin resistant meningococci (Sutcliffe1988) and strains of pneumococci resistant to penicillin have also been reported (Kaplan 1988b).
How the intervention might work
Antibiotic therapy for ABM needs to be commenced before the results of cerebrospinal fluid (CSF) culture and sensitivity to antibiotics are available. Empirical therapy should be based on the most common bacterial species that cause the disease, according to the patient's age group or clinical setting and local antibiotic susceptibility patterns of the predominant pathogens. Third generation cephalosporins, including cefotaxime and ceftriaxone, are known to possess broad‐spectrum antibacterial activity against the three most common causative agents of ABM: 1. Streptococcus pneumoniae (S. pneumoniae), 2. Neisseria meningitidis (N. meningitidis) and 3. H. influenzae type b. Several authors have recommended using third generation cephalosporins as the first‐line drugs of choice. However, non‐availability of antibiotics is a major issue in low‐ to middle‐income countries. In parts of Africa where meningitis epidemics are common, oily chloramphenicol injections face uncertain production (Nathan 2005). In many low‐ to middle‐income countries, including India, large rural areas have no access to third generation cephalosporins. The lack of access is due to the expense and the fact that the drugs are not readily available. The choice, therefore, lies in using conventional antibiotics or third generation cephalosporins ‐ whichever is obtainable.
Why it is important to do this review
It is not clear whether current evidence supports the equivalence of third generation cephalosporins and conventional antibiotics. This review aimed to determine whether there is any difference in the effectiveness and safety of the third generation cephalosporins cefotaxime and ceftriaxone and conventional antibiotics in the empirical therapy of community‐acquired ABM.
Objectives
The objective of the review was to compare the effectiveness and safety of third generation cephalosporins with penicillin and ampicillin‐chloramphenicol or other conventional antibiotic therapies in patients with community‐acquired ABM.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), irrespective of publication status, in which a third generation cephalosporin was compared to a conventional antibiotic in patients with ABM.
Types of participants
We included patients of any age or sex with community‐acquired ABM. The criteria we used for diagnosis of ABM included various combinations of clinical features and CSF composition. Clinical features consisted of fever, headache, vomiting and neck stiffness, with or without altered sensorium. CSF analysis consisted of polymorphonuclear pleocytosis, increased CSF protein and diminished CSF glucose.
We excluded studies containing:
meningitis following lumbar puncture (done for unrelated reasons such as epidural anaesthesia);
meningitis associated with head trauma, CSF leak, neurosurgery, known para‐meningeal focus of infection (defined as infection at sites in close proximity to the meninges, namely otitis media, cranial osteomyelitis or brain abscess); or
known immunosuppression (defined as a condition associated with suppressed immune response). For example, intake of immunosuppressive drugs or presence of diseases like malignancy, HIV positivity or systemic lupus erythematosus etc.).
We excluded studies if their title or abstract explicitly mentioned that the focus of the studies was on meningitis associated with one of these conditions listed above.
Types of interventions
The interventions were any third generation cephalosporin compared with conventional antibiotic treatment. The third generation cephalosporins we considered were:
cefotaxime; and
ceftriaxone.
The conventional treatment may include:
penicillin alone;
ampicillin‐chloramphenicol combination;
penicillin‐chloramphenicol combination; and
chloramphenicol alone, including single oily injections of chloramphenicol.
Considering the seriousness of the disease, the preferred route of administration of the interventions was intravenous. Our protocol specified this as a preferred route in the studies to be included. However, we also considered for inclusion, trials with other or multiple routes (for example, intravenous followed by oral).
Types of outcome measures
Primary outcomes
Deaths from any cause, in hospital or during a follow‐up period.
Severe deafness, defined as interfering (or likely to interfere) with usual activity.
Other disabling sequelae, defined as any sequelae at the end of the follow‐up period which caused dependence in any activity of daily living (for example, walking, toileting, bathing, dressing and eating) or caused inability to carry out previous work.
Treatment failure, defined as presence of one or more of the primary outcomes (for example, death, disabling sequelae, or severe deafness (defined above)) at the end of follow‐up.
Secondary outcomes
Side effects of drugs, for example, diarrhoea, agranulocytosis or skin rash.
Culture positivity of CSF for the causative bacteria, after 10 to 48 hours.
Search methods for identification of studies
Electronic searches
For this review update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 1), part of www.thecochranelibrary.com (accessed 7 April 2011), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register; MEDLINE (March 2007 to March week 4, 2011); and EMBASE (March 2007 to April 2011) (see Appendix 1). We also searched the reference list of review articles and book chapters, and contacted experts for any unpublished trials. We imposed no language or publication restrictions.
We searched MEDLINE and CENTRAL using the keywords and MeSH terms in Appendix 1. We combined the MEDLINE terms with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We searched EMBASE using an amended version of this strategy (see Appendix 2). See Appendix 3 for details of previous search.
Searching other resources
We contacted content experts for any unpublished trials. To date, pharmaceutical companies manufacturing third generation cephalosporins have not responded to requests for unpublished trials.
Data collection and analysis
Selection of studies
Three review authors (AK, TS, PKG) independently selected trials for inclusion in the review. We resolved disagreements by discussion.
Data extraction and management
Two review authors (AK, KP) independently extracted and cross‐checked the outcome data. We resolved disagreements by discussion.
Assessment of risk of bias in included studies
Two review authors (AK, KP) independently assessed the risk of bias of the included trials according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a) based on the following six domains with the rating of low risk of bias, high risk of bias and uncertain risk of bias.
Random sequence generation.
Allocation concealment.
Blinding.
Incomplete outcome data.
Selective reporting.
Other bias.
We did not consider blinding to be a major issue as the included outcomes can be measured objectively. Other criteria included the prognostic balance between the two treatment arms and the completeness and length of follow‐up.
Measures of treatment effect
We intended to use risk ratio (RR) and risk difference (RD) for calculating proportional and absolute risk reductions, respectively. However, in many studies there were no deaths (or other outcomes) in any group and the only estimate which we could use to include all such studies in the analysis, was RD. Therefore, we used RD to summarise the results. Use of RR or odds ratio (OR) would have excluded many studies from the analysis.
Unit of analysis issues
The unit of analysis was the individual patients included in the study. We did not find any cross‐over or cluster‐randomised trials on this topic.
Dealing with missing data
We recorded the number of randomised and analysed studies to determine if authors conducted an intention‐to‐treat (ITT) analysis.
Assessment of heterogeneity
We assessed heterogeneity in all analyses with an I2 statistic value of ≥ 50% taken to indicate statistical heterogeneity.
Assessment of reporting biases
We conducted a visual inspection of the funnel plot of the studies for any obvious asymmetry that could indicate publication bias.
Data synthesis
We analysed the data using Review Manager 5.1 (RevMan 2011). We used the Dersimonian and Laird method to calculate the summary estimate (DerSimonian 1986). We calculated the summary estimates using the fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analyses based on the causative organism for H. influenzae, S. pneumoniae andN. meningitidis, as specified in the protocol. We also examined the outcomes of death, deafness and treatment failure for high income and low‐ to middle‐income countries separately. The definition of income categories was according to gross national income (GNI) per capita (based on World Bank criteria) as follows: low‐income countries in which the GNI per capita was $745 or less; in lower middle‐income countries, $746 to $2,975; in upper middle‐income countries, $2976 to $9205; and in high‐income countries, $9206 or more. For our analysis, we collapsed the low‐ and both middle‐income categories into one, termed as low‐ to middle‐income countries (GNI $9205 or less). An income‐based classification of countries where studies were done is represented in Table 6.
1. Income‐based classification of countries where studies were conducted.
| High‐income | Upper middle‐income | Lower middle‐income | Low‐income |
| Arnoff 1984 | Haffejee 1988 | Bryan 1985 | Nathan 2005 |
| Barson 1985 | Odio 1986 | Filali 1993 | Sharma 1996 |
| Congeni 1984 | Tuncer 1988 | Girgis 1987 | |
| Del Rio 1983 | Rodriguz 1985 | ||
| Jacobs 1985 | Girgis 1988 | ||
| Narciso 1983 | |||
| Peltola 1989 | |||
| Steele 1983 | |||
| Wells 1984 |
Sensitivity analysis
We carried out a sensitivity analysis for each outcome as well as on the composite outcome of treatment failure. We defined treatment failure as the presence of one or more of the primary outcomes, that is death, disabling sequelae or severe deafness (defined above under Types of outcome measures) at the end of follow‐up. Combining the results, we included all studies in our primary analysis. Next, as intended in our protocol, we set out to examine studies from high‐income and low‐ to middle‐income countries separately as a secondary analysis. We conducted both overall and organism‐specific analyses.
There may be a view that the assumption of a fixed‐effect model is not correct because the studies from low‐ to middle‐income and high‐income countries may not be measuring the same underlying effects, even though the test for homogeneity was statistically non‐significant. To examine whether the results were sensitive to this assumption, we examined the effect using a random‐effects model. We did not find any important differences in the results.
Results
Description of studies
Results of the search
In this 2011 update we retrieved 104 records when searching the electronic databases. We excluded one new trial (Wazib 2008).
Death was reported clearly in all studies. Six studies had no deaths in any group. Deaths were more common in studies in low‐ to middle‐income countries. In studies from high‐income countries, there were seven deaths in each arm: among 254 patients in cephalosporins and 256 patients in conventional arms; whereas there were 37 deaths among 409 patients in the cephalosporin group and 36 deaths among 382 patients in the conventional group in low‐ to middle‐income countries. The reporting of outcomes other than death was inconsistent across studies. Deafness was classified as mild, moderate and severe in some, while not in others. It was difficult to determine whether the deafness reported in the studies interfered with usual activities, the outcome we planned to analyse. We took moderate hearing loss, even in one ear, as interfering with usual activities. For disability (other than deafness) there was no consistency in reporting. Some studies reported incidence of various neurological sequelae, but the number of participants involved was not clear. There were participants with more than one sequela and therefore, we could not extract the analysable data on disability.
Details of each study are given in the Characteristics of included studies table.
Included studies
Nineteen trials with 1496 participants met the inclusion criteria (Characteristics of included studies table). The empirical experimental treatment was ceftriaxone in 16, cefotaxime in two and ceftazidime in one trial. The empirical control treatment was ampicillin plus chloramphenicol in nine, ampicillin plus chloramphenicol plus gentamicin in three, benzylpenicillin plus chloramphenicol in two, ampicillin alone in two, benzylpenicillin alone in two, and oily injection of chloramphenicol in one trial.
The minimum age was one month (post‐neonatal), except in two studies (Congeni 1984; Steele 1983) that had a minimum age of seven days and 14 days, respectively. Only four studies included adults, of which two were restricted to participants older than 15 years of age. Ten studies were from low‐ to middle‐income countries and nine from high‐income countries.
Thirteen studies continued the same antibiotic as the empirical therapy throughout the course of treatment, but in four studies the culture report determined the antibiotic regimen following the initial empiric therapy; but changes (only in the control group) were within the conventional regimens. In one study (Peltola 1989) two participants in the chloramphenicol group had the addition of cefotaxime; one participant in the cefotaxime group had the addition of ampicillin; and four participants in the ceftriaxone group had the addition of chloramphenicol, ampicillin or penicillin. Thus, there has been little contamination (crossover) between the experimental and control groups. One study (Nathan 2005) reported a single injection of ceftriaxone (100 mg/kg to a maximum of 4 g in one intramuscular dose) or oily chloramphenicol (100 mg/kg to a maximum of 3 g in one intramuscular dose).
The follow‐up period was confined to 72 hours in one study, hospital stay in two studies, one to two weeks in four, more than one month in eight, and not mentioned in four studies. Five studies had a follow‐up of three months or more. Peltola (Peltola 1989) had a follow‐up of 12 months.
Excluded studies
The Characteristics of excluded studies table gives reasons for exclusion. Briefly, the studies did not qualify because two were only pharmacokinetic studies, five were non‐RCTs, four were narrative review articles, two had ineligible participants, 12 did not have the desired interventions (for example, six did not have an arm with third generation cephalosporins) and one was a duplicate publication.
Risk of bias in included studies
We considered concealment of randomisation, blinding, completeness of follow‐up and ITT analysis.
Allocation
None of the studies described in detail the method to conceal the randomisation process. One study (Nathan 2005) described use of a computer for sequence generation and sealed envelopes for treatment allocation. One study (Peltola 1989) mentioned using the telephone to randomly allocate the participants. The recruiting person assessed eligible participants, telephoned the person holding the randomisation sequence for allocation, registered the participant and secured the allocation. Probably all cases were randomised by telephone, and thus the process was adequately concealed. Concealment might not be a major issue if consecutive participants eligible for inclusion in the studies were randomised, but it was unclear whether this was indeed the case in the studies.
Blinding
No study had drug packs similar in appearance for the treatment and control arms. Blinding was mentioned in only one study and the term used was "single‐blinded". It is not clear who was blinded and how, but most likely the participants were blinded. Blinding may not be relevant for measuring the fatal outcomes, and probably deafness by audiometry, but measurement of other outcomes may be influenced by the lack of blinding.
Incomplete outcome data
No study clearly stated that there were no losses to follow‐up. This raises concern whether the losses to follow‐up were excluded from data analysis. However, for mortality this is unlikely to be a major issue because nearly all deaths in this disease occur in hospital.
Selective reporting
We assessed the selective reporting of data by comparing the outcomes listed in the methods section of the trials and the outcomes listed in the results.
Other potential sources of bias
We assessed other sources that could potentially bias the results. Possible sources included design‐specific risk of bias, early stopping, baseline imbalance and inappropriate administration of a co‐intervention.
Completeness of follow‐up
No study clearly stated that there were no losses to follow‐up, but the number analysed and randomised were the same in 15 studies.
ITT analysis
Only one study (Nathan 2005) clearly mentioned that the ITT principle was used in the analysis. Most studies excluded participants from analysis if they turned out to have aseptic meningitis. If this was done irrespective of and without knowledge of the outcome, it may not create a significant bias. However, the studies do not report this clearly.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Initially we considered 52 studies. Two review authors (KP, TS) applied the inclusion and exclusion criteria. The observed agreement for inclusion and exclusion was 88% and the kappa coefficient was 0.76, indicating an excellent level of chance‐corrected agreement. We resolved disagreements through discussion. Nineteen studies were included and 33 excluded.
The agreement between the two review authors (TS, TJ) on methodological quality of the studies was also high (observed agreement 89% for adequate description of method randomisation and 100% for blinding); the corresponding kappa coefficient for randomisation was 0.72 and weighted kappa coefficient for blinding was 1.0. The main observation with regards to the quality of the studies was that this was not adequately assessable from the reports. The Peltola 1989 study did seem to fulfil most of the methodological criteria. However, the other 18 studies were also given the benefit of the doubt and included in the analysis.
We included 19 trials, involving 1496 participants in the analysis. The ages ranged from one week to 59 years, but only four studies included adults and only two studies were restricted to adults. The duration of illness before the start of treatment was less than two days in two studies; two to four days in seven; more than four days in one; and not mentioned in eight studies. There was no heterogeneity of results among the studies in any outcome except diarrhoea (see below). The route of administration of antibiotics was parenteral in 17 studies (intravenous in 15 and intramuscular in two studies). The remaining two studies started with the intravenous route and changed over to oral (in the conventional antibiotic group) after the participants improved.
Most of the included studies did not mention the rates of antimicrobial resistance of the isolates. We found only two studies giving this information. Jacobs 1985 reported 29 isolates of H. influenzae, of which 34% were resistant to ampicillin, none were resistant to chloramphenicol or cefotaxime; eight isolates of S. pneumoniae, of which one was "relatively resistant" to penicillin (MIC 0.8 mcg/ml); and eight isolates of N. meningitidis, of which all were sensitive to cefotaxime. Aronoff 1984 reported two isolates of N. meningitidis; both were susceptible to ampicillin, chloramphenicol and ceftriaxone. He also reported one isolate of S. pneumoniae, which was susceptible to ceftriaxone. Overall, there was limited information on sensitivity of the isolates.
Death
The total number of deaths was 52 (out of 750) in the cephalosporin group and 50 (out of 746) in the conventional antibiotic group. There was no statistically significant difference in the risk of death between the groups (RD 0%; 95% CI ‐3% to 2%). However, the 95% CI indicated that a small difference, of 3% less or 2% more mortality in the cephalosporin group compared to the conventional antibiotic group, cannot be ruled out with 95% certainty.
Deafness
This was present in 21 (out of 247) in the cephalosporin group and 30 (out of 254) in the conventional antibiotic group. There was no statistically significant difference in the risk of deafness between the groups (RD ‐4%; 95% CI ‐9% to 1%). This result should be read with caution because this outcome is lower in hierarchy than mortality. In a disease with mortality, a treatment may reduce incidence of deafness by allowing (or causing) more deaths in that arm, thus leaving less people to report deafness. For this reason, death and deafness need to be combined as one outcome, 'treatment failure' (see next outcome).
Treatment failure
We considered treatment failure as the presence of either death or deafness (other disabling sequelae were not reported clearly enough to be included under this outcome). This analysis was done for the reason given above and to increase power for the analysis. The total number of treatment failures was 63 (out of 750) in the cephalosporin group and 65 (out of 746) in the conventional antibiotic group. There was no statistically significant difference in the risk of treatment failure between the groups (RD ‐1%; 95% CI ‐4% to 2%).
Culture positivity of CSF after 10 to 48 hours
Culture positivity of CSF after 10 to 48 hours was reported in 12 studies. It was seen in a total 14 (out of 228) in the cephalosporin group and 25 (out of 214) in the conventional antibiotic group. There was a statistically significant difference in the risk of culture positivity of CSF after 10 to 48 hours between the groups (RD ‐6%; 95% CI ‐11% to 0%).
Neutropaenia
Neutropaenia was reported in 10 trials. The total number with neutropaenia was 7 (out of 240) in the cephalosporin group and 12 (out of 217) in the conventional antibiotic group. There was no statistically significant difference in the risk of neutropaenia between the groups (RD ‐3%; 95% CI ‐7% to 2%).
Diarrhoea
Diarrhoea was reported in 10 trials. The total number of diarrhoeal incidents was 76 (out of 369) in the cephalosporin group and 45 (out of 361) in the conventional antibiotic group. There was a statistically significant difference in the risk of diarrhoea between the groups (RD 8%; 95% CI 3% to 13%).
Skin rash
Skin rash was reported in seven trials. The total number of participants with skin rash was 1 (out of 249) in the cephalosporin group and 4 (out of 219) in the conventional group. There was no statistically significant difference in the risk of skin rash between the groups (RD ‐1%; 95% CI ‐4% to 2%).
Subgroup analyses
The results of analysis according to the causative organism also did not reveal any difference between the two regimens, though the data were sparse. The organism‐wise data could be extracted for death only. The RD for H. influenzae was 1% (95% CI ‐5% to 6%); for S. pneumoniae RD ‐2% (95% CI ‐18% to 14%); and for N. meningitis RD 0% (95% CI ‐5% to 4%). The data for S. pneumoniae were particularly sparse with wide CIs and therefore, need to be read with caution.
Separate analyses for low‐ to middle‐income and high‐income countries yielded very similar results. Again, subgrouping of the studies, as expected, reduced the power of the analysis to exclude clinically important differences. An income‐based classification of countries where studies were conducted is summarised in Table 6.
Discussion
We have conducted a systematic review using Cochrane methodologies to address the question of whether there is any difference in the effectiveness and safety between third generation cephalosporins and conventional antibiotic treatments in patients with community‐acquired ABM.
Summary of main results
Our results essentially show no important difference between the cephalosporins and conventional antibiotics groups in any of the outcomes, except for culture positivity at 10 to 48 hours and diarrhoea. However, absence of evidence of difference may not be taken as evidence of absence of a difference. A small difference between the two groups in death, deafness or treatment failure cannot be ruled out, but we doubt if the small difference would be considered clinically important by most physicians.
Overall completeness and applicability of evidence
Agreement between the review authors in the selection of studies, quality assessment and data extraction was high. Two review authors (KP, AK) resolved all disagreements through discussion or by referral to a third review author (PKG). This provides confidence in the control of bias in the reviewing process. The main problem we faced during data extraction was high variability in the way disability was defined or assessed. Multiple sequelae in the same person were counted separately, thus the number of patients with one or more disabilities could not be extracted. This rendered disability data non‐analysable. However, deafness was recorded consistently, though not in a uniform fashion. We feel that there is a need to standardise the outcome definitions and assessment methods, particularly for non‐fatal outcomes in meningitis studies.
Quality of the evidence
We included 19 studies in our meta‐analysis; there may be some unpublished studies. The inclusion of such studies may have enhanced the power of analysis but there is no reason to believe that they would have significantly different results. The funnel plot (Figure 1) does not reveal any asymmetry, indicating that publication bias is unlikely to be significant. Many studies included in the analysis suggest uncertainty regarding their quality. How much of this is a reflection of the style of reporting or editorial restrictions in the form and length of papers, and how much is due to the actual methodological quality, is difficult to say. But the striking similarity in results across the different studies suggests that the conclusions are robust.
1.
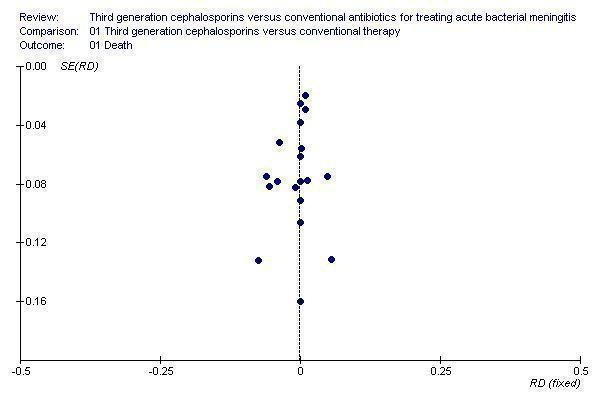
Funnel plot showing symmetry of risk difference (RD) against the standard error (SE) in individual studies
There are only two statistically significant differences between the two groups in this review. Culture positivity after 10 to 48 hours is reduced with the use of third generation cephalosporins, indicating rapid sterilisation of the CSF with this group of drugs. However, only two studies (Congeni 1984; Odio 1986) are driving the statistical significance, the rest show practically no difference in sterilisation of CSF. Moreover, this is an intermediate outcome. If the final outcome is no different, the two antibiotic regimens may be considered equally effective. To show that the two regimens are not different requires adequate power (this point is discussed below under 'limitations'). Diarrhoea was more frequent in the cephalosporins group. However, no study reported that this was uncontrollable or associated with Clostridium difficile (C. difficile), colitis or septicaemia. Therefore, this is unlikely to determine the choice of antibiotics. It may be worth mentioning that some evidence is emerging to suggest that antibiotic‐associated diarrhoea can be prevented with the use of probiotics (Johnston 2007; Pillai 2008). Whether or not this applies to meningitis patients being treated with ceftriaxone and cefotaxime is a topic worthy of research.
The data suggest that third generation cephalosporins and the combination of ampicillin‐chloramphenicol or chloramphenicol alone can be used as alternative empirical therapies for ABM, but this may not hold true as bacteria increasingly develop resistance to ampicillin and chloramphenicol. There is substantial evidence to suggest that an increasing proportion of H. influenzae and S. pneumoniae isolated from meningitis cases are resistant to ampicillin and chloramphenicol. This suggests that third generation cephalosporins should always be the first choice for empirical therapy for meningitis in the post‐neonatal period. The generalisability of the resistance pattern (studied mostly in urban centres) to rural and remote districts of low‐ to middle‐income countries is questionable. Non‐availability of these antibiotics and infrequent usage may limit the development of such resistance in remote and rural areas. Also, what if third generation cephalosporins are not available or affordable? This situation is real in many parts of low‐ to middle‐income countries. Despite all efforts by national and international agencies, the situation is unlikely to be resolved over the next few decades. Practitioners may have the option of using conventional antibiotics or no antibiotics. They run the risk of interpreting the literature that conventional antibiotics are as useless as no antibiotic at all. We think our review provides support for continued use of ampicillin‐chloramphenicol in such situations. On the other hand, in Africa there is uncertainty about continued production of oily chloramphenicol injections, a commonly used drug during epidemics of meningitis (Nathan 2005). In cases of non‐availability of this drug, a single intramuscular injection of ceftriaxone with close follow up is an alternative.
The study by Peltola (Peltola 1989) probably deserves special mention. This is the only study that allowed comparison between ceftriaxone, cefotaxime, ampicillin‐chloramphenicol, and chloramphenicol separately. It had the best methodological quality and longest follow up. Its conclusions clearly show that intravenous chloramphenicol alone should never be used, though this may not apply to intramuscular injections of oily chloramphenicol (the study by Nathan 2005 shows similar efficacy between intramuscular injection of oily chloramphenicol and ceftriaxone). Also, that there is little to chose between ceftriaxone, cefotaxime and ampicillin on the basis of efficacy. But most of the data is on the combination of ampicillin and chloramphenicol. In addition, many strains resistant to ampicillin are sensitive to chloramphenicol, and vice‐versa. Hence the conventional regimen, whenever used, should consist of the ampicillin‐chloramphenicol combination, to which the resistance prevalence in low‐ to middle‐income countries is below 10% (Reis 2002).
Antibacterial resistance and impact on outcome
There are several papers reporting the increasing trend in the proportion of H. influenzae, S. pneumoniae and N. meningitidis showing resistance to penicillin and chloramphenicol (Enting 1996; Mwangi 2002; Tristram 2007; Wasfy 2005). Thus, use of conventional antibiotics may be associated with higher mortality and morbidity. In fact, H. influenzae and S. pneumoniae have even developed resistance to cephalosporins. This has prompted many experts to advocate the use of combination vancomycin and third generation cephalosporins as the empiric treatment for bacterial meningitis. However, in cases where the isolates turn out to be sensitive to conventional antibiotics, our review indicates that the outcome with third generation cephalosporins and conventional antibiotics is likely to be the same.
Limitations of the review
Power
This review did not find any difference between the two antibiotic regimens. However, we have to assess the risk of fallacy: "absence of evidence of difference is not evidence of absence of difference''. This fallacy confronts any result or conclusion favouring equivalence. The power necessary to show equivalence theoretically requires infinite sample sizes. In other words, no sample size is large enough to show statistical equivalence. Clinicians will accept two interventions as reasonably equivalent if the 95% CI rules out a clinically important difference, implying that the review had sufficient power to permit clinical equivalence. Here there is room for difference of opinion. What is acceptable to one clinician may not be so for another. The 95% CI of the summary RD for death and severe deafness, in our opinion, excludes any important difference, but a contrary opinion may also be reasonable.
Unclear concealment
Usually, a lack of clear concealment is associated with bias towards showing a difference between two arms, which is not the case in this review. However, if the general impression has been that third generation cephalosporins would be a better choice than conventional therapy, then unclear concealment may have led to a tendency to treating the most severely ill patients with third generation cephalosporins. Following this example, unclear concealment could result in a bias towards no difference.
Selection bias
The results probably apply only to children, because there were only three studies with adults; all the remaining studies had participants younger than 15 years old. Also, the mortality observed in these studies (3% in high income countries and 13% in low‐ to middle‐income countries) is low compared to reports in some case series (10% to 30%). This raises concerns about whether the study sample is representative of meningitis cases. If less serious patients are over represented in the studies, the findings may not generalise to all meningitis cases. Any difference between treatments is more likely to be detectable in seriously ill patients and thus the studies might have underestimated any difference which might exist.
Time of the studies
All the studies except for three were conducted in the 1980s. The results might be due to less prevalence of resistance to conventional antibiotics at that time. The same results may not apply now. It should be stressed that the recent studies mainly treated meningococcal meningitis and so the relevance of this review in 2007 may be questioned. When so much has been written about increasing resistance to ampicillin, chloramphenicol, and even to ceftriaxone and cefotaxime, what purpose does this review serve? We believe the review does serve a purpose. In some low‐ to middle‐income countries, one of the cephalosporins or conventional antibiotics may not be available or affordable. In Africa, there is uncertainty about continued production of oily chloramphenicol, whereas ceftriaxone injections may be available. We know that most people in other low‐ to middle‐income countries, where both groups of antibiotics are available, have to spend out‐of‐pocket for all treatments. We also know that poor people cannot afford to buy third generation cephalosporins. In many hospitals in low‐ to middle‐income countries, ampicillin and chloramphenicol may be freely available but not ceftriaxone or cefotaxime. We do not believe that the situation will change for several years. This review provides support for combination ampicillin‐chloramphenicol as an alternative to third generation cephalosporins when the latter is not available or affordable.
Lack of ITT analysis
The included studies (except Nathan 2005) did not use an ITT analysis. This may have introduced a different degree of bias in the results of different studies. Usually the resulting bias in studies overestimates difference between the two arms. As the final results do not show any difference, this may not be a matter of great concern.
Overall risk of bias is represented in Figure 2 and Figure 3.
2.
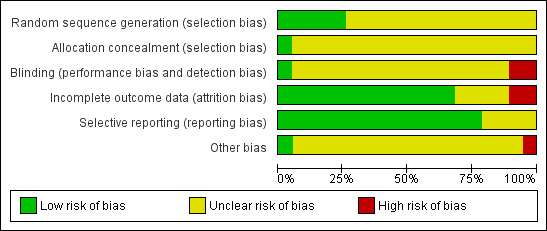
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
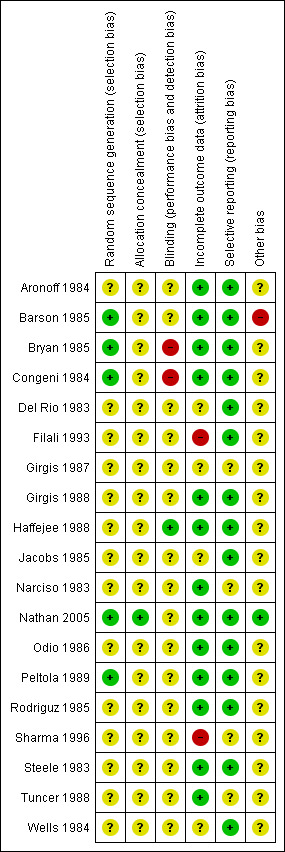
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Potential biases in the review process
Two review authors (KP, AK) independently assessed trial selection, quality assessment and data extraction, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We did this in order to avoid bias in the reviewing process. Our searches were reasonably comprehensive and we believe they identified all relevant studies. The funnel plot did not reveal any asymmetry which indicates that publication bias was unlikely.
Agreements and disagreements with other studies or reviews
We are not aware of any other systematic review on this topic, but recent recommendations for the treatment of acute pyogenic meningitis favour third generation cephalosporins, thus indicating that those countries where conventional antibiotics are becoming unavailable (for example some African countries), can switch over to using third generation cephalosporin.
Authors' conclusions
Implications for practice.
Although the review shows no clinically important difference between ceftriaxone and cefotaxime versus conventional antibiotics, the studies were conducted two decades ago and may not apply to current routine practice. Whenever one of the third generation cephalosporins or conventional antibiotics are not available or affordable, the other can be used as an alternative to start initial empirical treatment in ABM. The subsequent course of action may depend on the culture and sensitivity report and the response of the patients to such therapy. However, if intravenous chloramphenicol is used, it should be used in combination with ampicillin.
Implications for research.
Studies are needed to evaluate whether newer recommended regimens are superior to those already in practice in areas showing increasing prevalence of resistance to the antibiotics in use. For example, whether vancomycin plus ceftriaxone is better than ceftriaxone alone. The antimicrobial resistance pattern against various antibiotics needs to be determined in rural and remote areas of low‐ to middle‐income as well as in high income countries. This would help to determine the generalisability of changing recommendations due to increasing prevalence of antibiotic resistance observed in urban areas and high income countries.
What's new
| Date | Event | Description |
|---|---|---|
| 27 May 2013 | Review declared as stable | We do not think any new trials will appear in the literature that fulfills the inclusion criteria for this review. Current guidelines for choice of antibiotics universally recommend third generation cephalosporin (+ vancomycin) as an empiric therapy in community‐acquired bacterial meningitis and has become ‘standard of care’. This is based on the emergence of antibiotic resistance of common aetiologic organisms. Thus, any trial with one arm receiving older (conventional) antibiotics, such as penicillin or chloramphenicol will probably be considered unethical. Hence, we do not think any new trial of these interventions will appear in the literature. We also conducted a search on ‘PubMed’ from March 2006 to May 2013 using keywords ‘meningitis’ AND ‘cephalosporin’ OR ‘ceftraxone’ OR ‘cefotaxime’ OR ‘ceftazidime’ OR ‘cefmenoxime’ OR ‘cefodizime’ OR ‘ceftizoxime’ OR ‘moxalactam’ OR ‘third generation cephalosporin’ AND ‘conventional antibiotics’ or ‘ampicillin’ OR ‘penicillin’ OR ‘ampicillin‐chloramphenicol’ without any language or publication restrictions. We retrieved 627 hits. After reading the titles we excluded 600 trials and after reading the abstracts we excluded 27 trials. We did not find any new study that fulfilled our inclusion criteria. |
History
Protocol first published: Issue 4, 1999 Review first published: Issue 2, 2004
| Date | Event | Description |
|---|---|---|
| 7 April 2011 | New search has been performed | Searches conducted. No new trials were identified for inclusion. One trial was excluded in this update (Wazib 2008). Our conclusions remain unchanged |
| 28 June 2008 | Amended | Converted to new review format. |
| 21 August 2007 | New search has been performed | In this 2007 updated review, a new study from Niger has been added to the review. Overall conclusions remain the same. |
Acknowledgements
We acknowledge the infrastructure support provided by the All India Institute of Medical Sciences, New Delhi; the College of Medicine & Medical Sciences; and The Arabian Gulf University, Bahrain. The review authors gratefully acknowledge Dr Mark Coulthard's contribution in developing the protocol and finding the studies; and Dr Nitin Jain for his contribution in the first publication of the review. Finally, we wish to thank the following people for commenting on the updated draft review: Liz Dooley, Sarah Thorning, Alison Thomas, Mette Nørgaard, Xavier Sáez‐Llorens, Terry Neeman and Diederik van de Beek.
Appendices
Appendix 1. MEDLINE and CENTRAL search strategy
MEDLINE (OVID) 1 exp Meningitis/ 2 (bacteria* adj2 meningit*).tw. 3 1 or 2 4 exp Cephalosporins/ 5 cephalosporin*.tw,nm. 6 Ceftriaxone/ 7 ceftriaxon*.tw,nm. 8 exp Cefotaxime/ 9 cefotaxim*.tw,nm. 10 Ceftazidime/ 11 ceftazidim*.tw,nm. 12 Cefmenoxime/ 13 cefmenoxim*.tw,nm. 14 Ceftizoxime/ 15 ceftizoxim*.tw,nm. 16 Moxalactam/ 17 moxalactam*.tw,nm. 18 cefodizim*.tw,nm. 19 or/4‐18 20 3 and 19
Appendix 2. EMBASE search strategy
EMBASE (EMBASE.com) 13. #9 AND #12 12. #10 OR #11 11. random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR 'cross‐over':ab,ti OR volunteer*:ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR ((singl* OR doubl*) NEAR/2 (blind* OR mask*)):ab,ti 10. 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp 9. #3 AND #8 8. #4 OR #5 OR #6 OR #7 7. ceftriaxone:ab,ti OR cefotaxime:ab,ti OR ceftazidime:ab,ti OR cefmenoxime:ab,ti OR ceftizoxime:ab,ti OR latamoxef:ab,ti OR moxalactam:ab,ti OR cefodizime:ab,ti 6. 'ceftriaxone'/de OR 'cefotaxime'/de OR 'ceftazidime'/de OR 'cefmenoxime'/de OR 'ceftizoxime'/de OR 'latamoxef'/de OR 'cefodizime'/de 5. cephalosporin*:ab,ti 4. 'cephalosporin derivative'/exp 3. #1 OR #2 2. (meningit* NEAR/2 bacteria*):ab,ti 1. 'meningitis'/exp
Appendix 3. Details of previous search
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2007, issue 1) which contains the Cochrane Acute Respiratory Infections Group's Specialised Register; MEDLINE (January 1966 to March 2007); and EMBASE (January 1974 to March 2007). We also searched the reference list of review articles and book chapters, and contacted experts for any unpublished trials. We imposed no language or publication restrictions.
We searched MEDLINE and CENTRAL using the following keywords and MeSH terms. We searched the MEDLINE terms in combination with the highly sensitive search strategies designed by The Cochrane Collaboration for identifying RCTs (Dickersin 1994). We searched EMBASE using an amended version of this strategy.
MEDLINE (OVID) 1 exp MENINGITIS/ 2 exp Meningitis, Bacterial/ 3 (bacteria$ adj meningit$).mp. 4 or/1‐3 5 exp CEPHALOSPORINS/ 6 cephalosporin$.mp. 7 exp CEFTRIAXONE/ 8 exp CEFOTAXIME/ 9 exp CEFTAZIDIME/ 10 exp CEFMENOXIME/ 11 exp CEFTIZOXIME/ 12 exp MOXALACTAM/ 13 (ceftraxone or cefotaxime or ceftazidime or cefmenoxime or cefodizime or ceftizoxime or moxalactam).mp. 14 or/5‐13 15 4 and 14
Data and analyses
Comparison 1. Third generation cephalosporins versus conventional therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 19 | 1496 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.03, 0.02] |
| 2 Deafness | 10 | 501 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.04 [‐0.09, 0.01] |
| 3 Culture positive 10 to 48 hours after start of treatment | 12 | 442 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.06 [‐0.11, ‐0.00] |
| 4 Diarrhoea | 12 | 750 | Risk Difference (M‐H, Fixed, 95% CI) | 0.08 [0.03, 0.13] |
| 5 Neutropenia | 10 | 472 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.07, 0.02] |
| 6 Skin rash | 8 | 533 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.04, 0.01] |
| 7 Treatment failure (either death or deafness) | 19 | 1496 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.04, 0.02] |
1.1. Analysis.

Comparison 1 Third generation cephalosporins versus conventional therapy, Outcome 1 Death.
1.2. Analysis.

Comparison 1 Third generation cephalosporins versus conventional therapy, Outcome 2 Deafness.
1.3. Analysis.

Comparison 1 Third generation cephalosporins versus conventional therapy, Outcome 3 Culture positive 10 to 48 hours after start of treatment.
1.4. Analysis.

Comparison 1 Third generation cephalosporins versus conventional therapy, Outcome 4 Diarrhoea.
1.5. Analysis.

Comparison 1 Third generation cephalosporins versus conventional therapy, Outcome 5 Neutropenia.
1.6. Analysis.

Comparison 1 Third generation cephalosporins versus conventional therapy, Outcome 6 Skin rash.
1.7. Analysis.

Comparison 1 Third generation cephalosporins versus conventional therapy, Outcome 7 Treatment failure (either death or deafness).
Comparison 2. Cephalosporins versus conventional therapy for H. influenzae meningitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 10 | 318 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.05, 0.06] |
2.1. Analysis.

Comparison 2 Cephalosporins versus conventional therapy for H. influenzae meningitis, Outcome 1 Death.
Comparison 3. Third generation cephalosporins versus conventional antibiotics for S. pneumoniae meningitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 11 | 129 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.18, 0.14] |
3.1. Analysis.

Comparison 3 Third generation cephalosporins versus conventional antibiotics for S. pneumoniae meningitis, Outcome 1 Death.
Comparison 4. Third generation cephalosporins versus conventional therapy for meningococcal meningitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 13 | 477 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.05, 0.04] |
4.1. Analysis.

Comparison 4 Third generation cephalosporins versus conventional therapy for meningococcal meningitis, Outcome 1 Death.
Comparison 5. Third generation cephalosporins versus conventional antibiotics (high and low/middle‐income countries).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 19 | 1301 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.03, 0.02] |
| 1.1 High income countries | 9 | 510 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.03, 0.04] |
| 1.2 Low/middle income countries | 10 | 791 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.05, 0.03] |
| 2 Deafness | 10 | 501 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.04 [‐0.09, 0.01] |
| 2.1 Developed countries | 6 | 380 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.05 [‐0.11, 0.01] |
| 2.2 Developing countries | 4 | 121 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.09, 0.08] |
| 3 Treatment failure | 19 | 1301 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.04, 0.02] |
| 3.1 Developed countries | 9 | 510 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.06, 0.03] |
| 3.2 Developing countries | 10 | 791 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.05, 0.03] |
5.1. Analysis.

Comparison 5 Third generation cephalosporins versus conventional antibiotics (high and low/middle‐income countries), Outcome 1 Death.
5.2. Analysis.

Comparison 5 Third generation cephalosporins versus conventional antibiotics (high and low/middle‐income countries), Outcome 2 Deafness.
5.3. Analysis.

Comparison 5 Third generation cephalosporins versus conventional antibiotics (high and low/middle‐income countries), Outcome 3 Treatment failure.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aronoff 1984.
| Methods | Method of randomisation unclear; unclear blinding status; ITT unclear | |
| Participants | Age 0.17 to 8.75 years (mean 2.46 years); both sexes; duration of illness before onset of therapy not clear | |
| Interventions | Experimental arm: ceftriaxone; control arm: ampicillin‐chloramphenicol | |
| Outcomes | Death; sensorineural deafness; hydrocephalus and blindness | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study did not address this |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Insufficient information |
Barson 1985.
| Methods | Computer‐generated random numbers used for randomisation, but concealment unclear; blinding status unclear; 4 patients excluded from analysis | |
| Participants | Age 0.2 to 5 years; both sexes; duration of illness before therapy 2.4 days; number comatosed not mentioned | |
| Interventions | Experimental arm: ceftriaxone; control arm: ampicillin‐chloramphenicol for 10 days | |
| Outcomes | Death; sensorineural deafness; seizures and cranial nerve palsies | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study did not address this |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | High risk | 2 patients developed absolute granulocytopenia while receiving chloramphenicol and switched to ampicillin group |
Bryan 1985.
| Methods | Allocation using random number allocation table Unclear concealment No blinding No ITT analysis | |
| Participants | Age > 2 months (94% 2 months to 17 years) Both sexes Clinically diagnosed ABM (pts evaluated if: (a) survived ≥ 6 hrs (b) had CSF culture or gram stain proof of bacterial meningitis, or (c) > 10,000 leukocytes/mm3 of CSF (predominantly polymorphonuclear leukocytes), protein concentration of > 250 mg/dl and glucose concentration of = 20 mg/dl) Patients with prior antibiotic usage were excluded from evaluation | |
| Interventions | Experimental arm: ceftriaxone (100 mg/kg i.v. loading dose followed by 80 mg/kg every 24 hrs) Control arm: ampicillin (75 mg/kg i.v. loading dose followed by 50 mg/kg every 4 hours) and chloramphenicol (50 mg/kg i.v. loading dose followed by 25 mg/kg every 6 hrs) Duration of treatment: at least 10 days except in meningococcal group who were treated for 7 days | |
| Outcomes | Death Neurological sequelae during treatment and at the end of a variable follow‐up period of a max of 1 month Time for CSF sterility Duration of fever Adverse effects to ceftriaxone recorded were anaemia, transient mild neutropenia, diarrhoea and rash Adverse effects in the control arm recorded were anaemia, transient moderate neutropenia and diarrhoea | |
| Notes | Study done in Brazil. 10/36 evaluated were culture negative | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Insufficient information |
Congeni 1984.
| Methods | Allocation using computer‐generated randomisation tables. Unclear concealment No blinding No ITT analysis | |
| Participants | Age range: 1 day to 15 years Both sexes Criteria for diagnosis not mentioned | |
| Interventions | Experimental arm: ceftriaxone (50 mg/kg i.v. every 12 hrs) Control arm: ampicillin (200 to 400 mg/kg day i.v. in 4 divided doses) and chloramphenicol (75 mg/kg/day i.v. 6 hrly). Neonates were given gentamycin 2.5 mg/kg i.v. 8 hrly 1 patient in conventional therapy group received nafcillin as the agent Staphylococcus epidermidis (S. epidermidis) was not susceptible to ampicillin or chloramphenicol Duration of treatment not specified | |
| Outcomes | Death Complications and sequelae during the period of treatment Time for CSF sterility Duration of fever Potential adverse effects in ceftriaxone group recorded were transient eosinophilia, transient neutropenia, anaemia and mild diarrhoea Adverse effect recorded in conventional therapy group was mild diarrhoea | |
| Notes | Study done in the USA Culture negative were excluded from the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Insufficient Information |
Del Rio 1983.
| Methods | Random allocation, but method of randomisation and concealment not mentioned
Unclear blinding status No ITT analysis |
|
| Participants | Patients above 6 weeks of age (66/78 < 2 years). Patients with suspected or definite meningitis enrolled. Those with positive cultures and/or characteristic CSF findings were subsequently analysed. (Criteria for CSF findings not mentioned) | |
| Interventions | Experimental arm: ceftriaxone (75 mg/kg i.v. loading dose followed by 50 mg/kg/dose i.v. 12 hrly) Control arm: ampicillin (200 mg/kg/day i.v. 6 hrly) and chloramphenicol (100 mg/kg/day i.v. 6 hrly) Duration of therapy: 7 days in both groups for meningococcal meningitis and 10 days for others | |
| Outcomes | Death Sensorineural deafness (defined as > 30 dB hearing loss) Other disabilities Duration of fever Duration of follow‐up: 1 to 5 months Adverse effects noted in ceftriaxone group were diarrhoea, arthritis, phlebitis, raised SGOT and drug fever Adverse effects noted in conventional therapy group were diarrhoea, arthritis and drug fever | |
| Notes | Study done in the USA 72/92 enrolled were culture positive | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Study did not address this |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | insufficient information |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Insufficient information |
Filali 1993.
| Methods | Random allocation, but method of randomisation and concealment not mentioned Unclear blinding status ITT analysis (?) | |
| Participants | Age more than 16 yrs (mean age 28.9 years) Both sexes (more males in control group than in experimental group) Diagnosis of meningococcal meningitis based on a positive CSF culture or detection of meningococcal polysaccharide antigen in CSF. Also, if a patient presented with the characteristic clinical findings of meningitis and purulent CSF during an epidemic, a diagnosis of meningococcal meningitis was accepted | |
| Interventions | Experimental arm: ceftriaxone (2 g i.v. daily for 2 days) Control arm: penicillin G (300,000 IU/kg/day 4 hrly for 6 days) 1/16 patients randomised to ceftriaxone group had to be given ceftriaxone for 7 days due to severity of disease | |
| Outcomes | Death
Neurological sequelae
Duration of coma
Duration of fever Duration of follow‐up: 2 months after discharge No adverse effects noted in either group |
|
| Notes | Study conducted in Morocco | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study did not address this |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No adverse effects noted in either group |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Insufficient information |
Girgis 1987.
| Methods | Method of randomisation not described; unclear blinding status; only 30 out of 50 enrolled were analysed | |
| Participants | Age 16 to 30 years; both sexes; duration of illness before therapy less than 4 days; nearly two‐third comatosed in each group | |
| Interventions | Experimental arm: ceftriaxone (100 mg/kg/24 hrs i.m. to children i.v. to adults) Control arm: ampicillin (160 mg/kg/day 6 hrly and chloramphenicol (100 mg/kg/day 6 hrly Duration of therapy according to response | |
| Outcomes | Death; duration of fever | |
| Notes | Study conducted in Egypt | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study did not address this |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Girgis 1988.
| Methods | Random allocation but method of randomisation and concealment not mentioned Unclear blinding status ITT analysis (?) | |
| Participants | Age range: 5 months to 28 years Both sexes; patients with signs and symptoms of meningitis randomised. Patients from whom organisms were isolated in CSF were included in final analysis | |
| Interventions | Experimental arm: ceftriaxone (100 mg/kg/24 hrs i.m. to children i.v. to adults) Control arm: ampicillin (160 mg/kg/day 6 hrly i.m. to children i.v. to adults) and chloramphenicol (100 mg/kg/day 6 hrly i.m. to children i.v. to adults) Duration of therapy not specified | |
| Outcomes | Death Days to become fully alert Duration of fever Duration of follow‐up not mentioned Adverse effects recorded were mild diarrhoea and cramps, nausea in either group | |
| Notes | Study conducted in Egypt Only those included in analysis from whom organisms were isolated from CSF | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study did not address this |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Insufficient information |
Haffejee 1988.
| Methods | Random allocation but method of randomisation and concealment not mentioned Single blinding mentioned but method of blinding not clear No ITT analysis | |
| Participants | Age range: 1 month to 9 years Both sexes Diagnosed bacterial meningitis on the basis of Gram stain of CSF and/or CSF culture | |
| Interventions | Experimental arm: cefotaxime (100 to 200 mg/kg/d i.v. for initial 3 to 5 days followed by i.m. for the rest of the duration in 2 or 3 divided doses) Control arm: penicillin G (0.5 ‐ 1 million I.U. 6 hourly i.v. for initial 3 to 5 days followed by i.m. for the rest of the duration) and chloramphenicol (80 to 100 mg/kg/day orally in 3 to 4 divided doses) Sulphadiazine (100 mg/kg/day orally) was also given to 8/15 analysed in the control arm | |
| Outcomes | Death Sensorineural deafness Other disabilities Duration of fever Mean duration of follow‐up: 27.1 months Adverse effects noted in cefotaxime group were diarrhoea, neutropenia, anaemia and thrombocytosis Adverse effects noted in conventional therapy group were all the above plus thrombocytopaenia | |
| Notes | Study conducted in South Africa Sulfadiazine used in first 18 months of 52 month‐long trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Insufficient information |
Jacobs 1985.
| Methods | Random allocation, but method and concealment unclear Unclear blinding status No ITT analysis | |
| Participants | Age range: 1 week to 16 years Both sexes Criteria for diagnosis not specified | |
| Interventions | Experimental arm: cefotaxime (50 mg/kg/dose i.v. 6 hrly) Control arm: ampicillin (50 to 100 mg/kg/dose i.v. 6 hrly) plus chloramphenicol or gentamicin Chloramphenicol was given 25 mg/kg/dose i.v. 6 hrly Gentamycin for neonates was given in a dose of 2.5 mg/kg/dose 8 to 12 hrly Mean duration of cefotaxime therapy: 11.1 days Mean duration of conventional therapy: 11.9 days | |
| Outcomes | Death Sensorineural deafness other sequelae Duration of fever Minimum duration of follow‐up: 3 months Adverse effects in cefotaxime group: diarrhoea Adverse effects in conventional therapy group: acute tubular necrosis | |
| Notes | Study conducted in the USA Culture negative patients were excluded from evaluation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Study did not address this |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Insufficient information |
Narciso 1983.
| Methods | Random allocation, but method and concealment unclear Unclear blinding status ITT analysis | |
| Participants | Age: mean age 46 years (all adults) Sex distribution not clear Criteria for diagnosis not specified | |
| Interventions | Experimental arm: ceftriaxone (80 to 100 mg/kg/day i.v. 12 hrly) Control arm: ampicillin (110 mg/kg/day i.v. 8 hrly) Dosages were halved in both groups when meningeal symptoms disappeared Duration of treatment: treatment continued until cells in CSF became normal | |
| Outcomes | Death Duration of coma Duration of fever Duration of follow‐up: until discharge | |
| Notes | Study done in Italy 7/10 were culture positive | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Study did not address this |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Nathan 2005.
| Methods | Allocation using computer‐generated list and sealed envelopes | |
| Participants | Age: median age 7 years (all patients > 2 months) Both sexes Patients who had suspected meningitis on the basis of fever in last 24 hrs/ sudden onset of fever along with at least one of the following: neck stiffness, impaired consciousness, or petechial rash (> 1 yr); or bulging fontanel, axial hypotonia, upwardly gazing or petechial rash (< 1 year) | |
| Interventions | Experimental arm : ceftriaxone (100 mg/kg/day i.m. up to a maximum dose of 4 g; 2nd dose of 75 mg/kg at 24 to 48 hrs. In case of clinical failure. Control arm: oily suspension of chloramphenicol (100 mg/kg/day i.m. up to a max. dose of 3 g; 2nd dose of 100 mg/kg in case of clinical failure at 24 to 48 hrs | |
| Outcomes | Death Overall treatment failure (death or clinical failure at 72 h defined as state of consciousness remaining severely altered, no improvement in the state of consciousness since 0 hrs, repeated or persistent convulsions, worsened neurological symptoms since 0 hrs, axillary temperature > 38.5C) Neurological sequelae at 72 h | |
| Notes | Study done in eastern Niger for 1 month during a meningitis epidemic | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Study did not address this |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Odio 1986.
| Methods | Patients were assigned treatment from a list of randomised therapy assignments Concealment unclear Unclear blinding status No ITT analysis | |
| Participants | Age range: 2 months to 10.5 years Both sexes Pts who had suspected bacterial meningitis on the basis of identification of microbes on gram stained smear of CSF or proved bacterial meningitis were eligible for the study However, patients with culture proved bacterial meningitis were enrolled and evaluated | |
| Interventions | Experimental arm: cefotaxime (50 mg/kg/dose i.v. 6 hrly for at least 10 days (mean duration 12.7 days) Control arm: ampicillin (50 mg/kg/dose i.v. 6 hrly for first 5 days) and chloramphenicol (25 mg/kg/dose i.v. 6 hrly for 5 days followed by oral administration for at least 10 days). Mean duration of therapy in control group was 11.7 days | |
| Outcomes | Death Sensory sequelae including visual auditory and co‐ordination problems Inability to perform ADL Developmental abnormalities Duration of fever Other sequelae Duration of follow‐up: at least 4 months Adverse effects noted in the experimental group were diarrhoea, thrombocytosis, neutropaenia, skin rash, prolonged fever, elevated ALT, elevated blood urea nitrogen and hyperkalaemia Adverse effects noted in conventional therapy group were diarrhoea, neutropenia, prolonged fever and elevated ALT | |
| Notes | Study conducted in Costa Rica 16/42 in experimental arm and 18/43 in conventional therapy group had already received previous antibiotics Neonates were initially included in the study but later excluded from analysis Pts were evaluated for sequelae at the time of discharge and at the end of 4 months or longer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study did not address this |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Insufficient information |
Peltola 1989.
| Methods | Randomised into 4 treatment groups using a list Concealment Blinding unclear No ITT analysis | |
| Participants | Age range: 3 months to 15 years Both sexes Patients diagnosed having ABM if CSF culture was positive or for culture negative cases, presence of signs and symptoms, total leucocyte count > 100 /microlitre and 1 or more of the following: a positive blood culture; bacteria in CSF by gram stain; or positive serology Also, if the patient presented with a characteristic clinical picture and showed high CSF leucocyte count, serum C‐reactive protein of 20 mg/L or above, or CSF lactate ≥ 3 mg/L | |
| Interventions | 4 treatment groups as under: 1) chloramphenicol (100 mg/kg/day i.v. in 4 divided doses) 2) ampicillin (250 mg/kg/day i.v. in 4 divided doses 3) cefotaxime (150 mg/kg/day in 4 divided doses 4) ceftriaxone (100 mg/kg/day i.v. once daily) The first dose of all drugs was increased by 50% All drugs were given for 7 days | |
| Outcomes | Death Sensorineural deafness Recurrence of bacterial meningitis Duration of consciousness impairment Duration of fever Duration of follow‐up: 12 months after discharge Adverse effects noted in the ceftriaxone group were diarrhoea, gall bladder precipitate Diarrhoea was also noted in the other groups | |
| Notes | Study conducted in Finland 183/191 culture positive initially | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Study did not address this |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Insufficient information |
Rodriguz 1985.
| Methods | Random allocation but method and concealment unclear Unclear blinding status ITT analysis | |
| Participants | Children over 1 month of age (84% ≤ 3 yrs) Both sexes Criteria for diagnosis of ABM not mentioned | |
| Interventions | Experimental group: ceftazidime (150 mg/kg/day i.v. in three doses for mean duration of 10.2 days) Control group: ampicillin (400 mg/kg/d i.v. 6 hrly) and chloramphenicol (75‐100 mg/kg/d ay 6 hrly for a mean duration of 9.5 days) | |
| Outcomes | Death Other sequelae (hearing loss not assessed) Duration of fever Days until asymptomatic Duration of follow‐up not mentioned Adverse effects recorded in ceftazidime group were diarrhoea, drug fever and leukopenia/anaemia requiring blood transfusion | |
| Notes | Study done in Dominican Republic | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Study did not address this |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Insufficient information |
Sharma 1996.
| Methods | Random allocation mentioned, but method and concealment not clear Unclear blinding status ITT analysis | |
| Participants | Age range 5 months to 5 years Both sexes Diagnosed ABM on clinical and CSF grounds (Clinical features: fever of acute onset, vomiting, convulsion, bulging fontanel in infants CSF features: cell count > 100 with > 60% polymorphs Latex agglutination test was also done | |
| Interventions | Experimental arm: ceftriaxone (50 mg/kg/day i.m. in a single dose for 7 days) Control arm: chloramphenicol (100 mg/kg/day i.v. 6 hrly for 14 days) and benzylpenicillin (200,000 IU/kg/day 6 hourly for 14 days) | |
| Outcomes | Deaths Duration of fever Adverse outcome events not mentioned No data on sequelae Followed till discharge | |
| Notes | Study done in Nepal No culture data provided Organisms identified by CSF agglutination test | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Study did not address this |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adverse outcome events not mentioned; no data on sequelae |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Steele 1983.
| Methods | Random allocation mentioned, but method and concealment not clear Unclear blinding status ITT analysis | |
| Participants | Age range: 14 days to 14 years Both sexes Patients with culture positive bacterial meningitis | |
| Interventions | Experimental arm: ceftriaxone (100 mg/kg/day i.v. 12 hrly; i.m. route was used in some in last days of treatment) Control arm: ampicillin (200 to 400 mg/kg/day i.v. 6 hrly) and chloramphenicol (100 mg/kg/day i.v. 6 hrly; orally in some in last days of treatment) Duration of treatment in both the groups was 7 days for N. meningitidis; 10 days for H. influenzae; 14 days for S. pneumoniae; 21 days for other organisms | |
| Outcomes | Death Sensorineural deafness Other disabilities Duration of fever Duration of follow‐up: 3 months after treatment Adverse effects noted in the ceftriaxone group were diarrhoea (defined as > 20 mg/kg/day stool) and anaemia Adverse effects noted in the control group were the same | |
| Notes | Study done in the USA Also gives data on ceftriaxone pharmacokinetics as well as susceptibility | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Study did not address this |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Insufficient information |
Tuncer 1988.
| Methods | Random allocation mentioned but method and concealment not clear Unclear blinding status ITT analysis | |
| Participants | Age range: 1 month to 12 years Both sexes Patients having meningococcal meningitis diagnosed on clinical and CSF grounds Criteria for diagnosis: 1) meningitis with or without purpura; 2) isolation of N. meningitidis from blood or CSF culture; or 3) positively stained smear of CSF for gram negative diplococci | |
| Interventions | Experimental arm: ceftriaxone (80 to 100 mg/kg/day i.v. in a single dose for 4 days) Control arm: penicillin G (500,000 IU/kg/day i.v. 4 hourly for 5 days | |
| Outcomes | Deaths Duration of fever Reccurrence of disease within 6 months Duration of follow‐up: max. of 6 months | |
| Notes | Study done in Turkey 33% of patients had meningococcaemia without meningitis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Study did not address this |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Wells 1984.
| Methods | Random allocation mentioned, but method and concealment not clear Unclear blinding status No ITT analysis | |
| Participants | Age range: 0.25 to 200 months Both sexes Pts. with clinically suspected meningitis and purulent CSF initially randomised Those who were later shown to be culture proven viral meningitis or having sterile CSF were excluded from the analysis | |
| Interventions | Experimental arm: cefotaxime (50 mg/kg/dose i.v. 6 hrly for 10 to 14 days) Control arm: ampicillin (50 to 100 mg/kg/dose i.v. 6 hrly for 10 to 14 days) plus chloramphenicol (25 mg/kg/dose 6 hrly i.v. for 10 to 14 days) or gentamycin (2.5 mg/kg/dose 8 to 12 hrly for 10 to 14 days for children < 1 month of age) | |
| Outcomes | Death Sensorineural deafness Other disabilities Patients followed up until discharge No adverse effects noted in the cefotaxime group. In the conventional therapy group ATN was recorded as a potential adverse effect | |
| Notes | Study done in USA; 30/37 randomised had culture positive for bacteria No mention about sensorineural deafness in the result section, but in the methodology section they say they have evaluated hearing at discharge | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Study did not address this |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Insufficient information |
ABM: acute bacterial meningitis ADL: activity of daily living ALT: alanine transaminase ATN: acute tubular necrosis CSF: cerebrospinal fluid dB: decibel hrs: hours i.v.: intravenous i.m.: intramuscular Pts: patients SGOT: serum glutamic oxaloacetic transaminase yrs: years
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chartrand 1984 | In some patients ampicillin and moxalactam were combined |
| Donma 1992 | This is a review article |
| Dutta 1979 | There is no group with third generation cephalosporin |
| Feldstein 1987 | This is a review article |
| Hohl 1990 | This is a pharmacokinetic study |
| Kaplan 1988a | This is a review article |
| Kavaliotis 1989 | There is no conventional therapy arm |
| Kobayashi 1981 | A non‐comparative study |
| Kumar 1993 | There is no third generation cephalosporin arm |
| Lapointe 1984 | Not a randomised study |
| Macfarlane 1979 | There is no third generation cephalosporin arm |
| Marks 1986 | There is no third generation cephalosporin arm |
| Martin 1990 | There is no conventional antibiotic arm |
| Ngu 1987 | Not a randomised study |
| Pecoul 1991 | There is no third generation cephalosporin arm |
| Pfister 1989 | Patients had neuroborreliosis |
| Powell 1990 | This is not a study comparing antibiotics; a study of fluid intake |
| Reed 1983 | A pharmacokinetic study |
| Rodriguez 1985 | This study contains the same data as published later by Rodriguez in 1986, which is included |
| Schaad 1990 | A study comparing ceftriaxone and cefuroxime, not conventional antibiotics |
| Steele 1984 | No data presented |
| Tessin 1989 | Ampicillin was added to ceftazidime arm |
| Tetanye E, 1990 | There is no third generation cephalosporin arm |
| Vallejo 1991 | A retrospective study |
| Wazib 2008 | Non‐randomised trial |
| Yogev 1986 | A non‐comparative study |
| Zavala 1988 | The meningitis was not community‐acquired |
Contributions of authors
Kameshwar Prasad (KP) conceptualised the review, developed and wrote the protocol, contributed to the selection of the studies, extraction of data, entry of data and wrote the final draft of the review. He also conducted the inter‐observer agreement analysis and wrote the update of this review.
Amit Kumar (AK) and Tarun Singhal (TS) contributed to the selection of studies, extraction of data, entry of data and review of the final draft. AK and TS entered the data for this update. PK Gupta (PKG) contributed to entry of data, prepared tables for agreement analysis and provided valuable comments on the final draft of the review. He participated in the selection of new studies during the update.
Sources of support
Internal sources
All India Institute of Medical Sciences, New Delhi, India.
External sources
No sources of support supplied
Declarations of interest
None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Aronoff 1984 {published data only}
- Aronoff SC, Reed MD, O'Brien CA, Blumer JL. Comparison of the efficacy and safety of ceftriaxone to ampicillin‐chloramphenicol in the treatment of childhood meningitis. Journal of Antimicrobial Chemotherapy 1984;13(2):143‐51. [DOI] [PubMed] [Google Scholar]
Barson 1985 {published data only}
- Barson WJ, Miller MA, Brady MT, Powell DA. Prospective comparative trial of ceftriaxone vs conventional therapy for treatment of bacterial meningitis in children. Pediatrics Infectious Diseases Journal 1985;4(4):362‐8. [DOI] [PubMed] [Google Scholar]
Bryan 1985 {published data only}
- Bryan JP, Rocha H, Silva HR, Taveres A, Sande MA, Scheld WM. Comparison of ceftriaxone and ampicillin plus chloramphenicol for the therapy of acute bacterial meningitis. Antimicrobial Agents and Chemotherapy 1985;28(3):361‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Congeni 1984 {published data only}
- Congeni BL. Comparison of cefotaxime and traditional therapy of bacterial meningitis. Antimicrobial Agents and Chemotherapy 1984;25(1):40‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Del Rio 1983 {published data only}
- Rio MA, Chrane D, Shelton S, McCraken GH Jr, Nelson JD. Ceftriaxone versus ampicillin and chloramphenicol for treatment of bacterial meningitis. Lancet 1983;1(8336):1241‐4. [DOI] [PubMed] [Google Scholar]
Filali 1993 {published data only}
- Filali KM, Noun M, Chakib A, Zahraoui M, Himmich H. Ceftriaxone versus Penicillin G in the short term treatment of meningococcal meningitis in adults. European Journal of Clinical Microbiology and Infectious Diseases 1993;12(10):766‐8. [DOI] [PubMed] [Google Scholar]
Girgis 1987 {published data only}
- Girgis NI, Abu El‐Ella AH, Farid Z, Woody JN, Lissner C. Ceftriaxone compared with a combination of ampicillin and chloramphenicol in the treatment of bacterial meningitis in adults. Drugs in Experimental and Clinical Research 1987;XIII(8):497‐500. [PubMed] [Google Scholar]
Girgis 1988 {published data only}
- Girgis NI, Abu El‐Ella AH, Farid Z, Haberberger RL, Woody JN. Ceftriaxone alone compared to ampicillin and chloramphenicol in the treatment of bacterial meningitis. Chemotherapy 1988;34(Suppl 1):16‐20. [DOI] [PubMed] [Google Scholar]
Haffejee 1988 {published data only}
- Haffejee IE. Cefotaxime versus penicillin‐chloramphenicol in purulent meningitis: a controlled single‐blind clinical trial. Annals of Tropical Pediatrics 1988;8(4):225‐9. [DOI] [PubMed] [Google Scholar]
Jacobs 1985 {published data only}
- Jacobs RF, Wells TG, Steele RW, Yamauchi T. A prospective randomized comparison of cefotaxime vs ampicillin and chloramphenicol for bacterial meningitis in children. Journal of Pediatrics 1985;107(1):129‐33. [DOI] [PubMed] [Google Scholar]
Narciso 1983 {published data only}
- Narciso P, Mori P, Giannuzzi R, Tocci G, Visco G. Ceftriaxone versus ampicillin therapy for purulent meningitis in adults. Drugs in Experimental and Clinical Research 1983;IX(10):717‐9. [Google Scholar]
Nathan 2005 {published data only}
- Nathan N, Borel T, Djibo A, Evans D, Djibo S, Corty JF, et al. Ceftriaxone as effective as long‐acting chloramphenicol in short‐course treatment of meningococcal meningitis during epidemics: a randomised non‐inferiority study. Lancet 2005;366(9482):308‐13. [DOI] [PubMed] [Google Scholar]
Odio 1986 {published data only}
- Odio CM, Faingezicht I, Salas JL, Guevara J, Mohs E, McCracken GH Jr. Cefotaxime vs conventional therapy for the treatment of bacterial meningitis of infants and children. Pediatric Infectious Diseases Journal 1986;5(4):402‐7. [DOI] [PubMed] [Google Scholar]
Peltola 1989 {published data only}
- Peltola H, Anttila M, Renkonen OV, Finnish Study Group. Randomised comparison of chloramphenicol, ampicillin, cefotaxime, and ceftriaxone for childhood bacterial meningitis. Lancet 1989;8650(1):1281‐7. [DOI] [PubMed] [Google Scholar]
Rodriguz 1985 {published data only}
- Rodriguez WJ, Puig JR, Khan WN, Feris J, Gold BG, Sturla C. Ceftazidime vs. standard therapy for pediatric meningitis: therapeutic, pharmacologic and epidemiologic observations. Pediatric Infectious Diseases Journal 1986;5(4):408‐15. [DOI] [PubMed] [Google Scholar]
Sharma 1996 {published data only}
- Sharma PR, Adhikarai RK, Joshi MP, Lal M, Chodon T, Pokhrel BM. Intravenous chloramphenicol plus penicillin versus intramuscular ceftriaxone for the treatment of pyogenic meningitis in Nepalese children (letter to the editor). Tropical Doctor 1996;26(2):84‐5. [DOI] [PubMed] [Google Scholar]
Steele 1983 {published data only}
- Steele RW, Bradsher RW. Comparison of ceftriaxone with standard therapy for bacterial meningitis. Journal of Pediatrics 1983;103(1):138‐41. [DOI] [PubMed] [Google Scholar]
Tuncer 1988 {published data only}
- Tuncer MA, Gur I, Ertem U, Ece A, Turkmen S, Deniz B, et al. Once daily ceftriaxone for meningococcemia and meningococcal meningitis. Pediatric Infectious Diseases Journal 1988;7(10):711‐3. [DOI] [PubMed] [Google Scholar]
Wells 1984 {published data only}
- Wells TG, Trang JM, Brown AL, Marmer BC, Jacobs RF. Cefotaxime therapy of bacterial meningitis in children. Journal of Antimicrobial Chemotherapy 1984;14(Suppl B):81‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Chartrand 1984 {published data only}
- Chartrand SA, Marks MI, Scribner RK, Johnston JT, Frederick DF. Moxalactam therapy of Haemophilus influenzae type b meningitis in children. Journal of Pediatrics 1984;104(3):454‐9. [DOI] [PubMed] [Google Scholar]
Donma 1992 {published data only}
- Donma MM, Donma O. Cephalosporins in childhood bacterial meningitis. Journal of the Singapore Paediatric Society 1992;34(3‐4):141‐7. [PubMed] [Google Scholar]
Dutta 1979 {published data only}
- Dutta AK, Sehgal H, Khandpur SC. Evaluation of ampicillin in the treatment of acute bacterial meningitis in children. Indian Pediatrics 1979;16(3):239‐43. [PubMed] [Google Scholar]
Feldstein 1987 {published data only}
- Feldstein TJ, Uden DL, Larson TA. Cefotaxime for treatment of gram‐negative bacterial meningitis in infants and children. Pediatric Infectious Diseases Journal 1987;6(5):471‐5. [DOI] [PubMed] [Google Scholar]
Hohl 1990 {published data only}
- Hohl P, Martin E, Kayser FH. Short course single daily ceftriaxone monotherapy for acute bacterial meningitis in children: results of a Swiss multicenter study. Part II: Bacteriological results. Infection 1990;18(2):78‐82. [DOI] [PubMed] [Google Scholar]
Kaplan 1988a {published data only}
- Kaplan SL, Mason SK, Mason EO Jr, Murphy M, Smith EO. Follow‐up of prospective randomized trial of ampicillin or chloramphenicol versus moxalactam treatment of Haemophilus influenzae type b meningitis. Journal of Pediatrics 1988;112(5):795‐8. [DOI] [PubMed] [Google Scholar]
Kavaliotis 1989 {published data only}
- Kavaliotis J, Manios SG, Kansouzidou A, Danielidis V. Treatment of childhood bacterial meningitis with ceftriaxone once daily: open, prospective, randomized, comparative study of short‐course versus standard‐length therapy. Chemotherapy 1989;35(4):296‐303. [DOI] [PubMed] [Google Scholar]
Kobayashi 1981 {published data only}
- Kobayashi Y, Morikawa Y, Haruta T, Fujii R, Meguro H, Hori M. Clinical evaluation of cefotaxime in the treatment of purulent meningitis in children. Japanese Journal of Antibiotics 1981;34(6):946‐54. [PubMed] [Google Scholar]
Kumar 1993 {published data only}
- Kumar P, Verma IC. Antibiotic therapy for bacterial meningitis in children in developing countries. Bulletin of the World Health Organization 1993;71(2):183‐8. [PMC free article] [PubMed] [Google Scholar]
Lapointe 1984 {published data only}
- Lapointe JR, Beliveau C, Chicoine L, Joncas JH. A comparison of ampicillin‐cefotaxime and ampicillin‐chloramphenicol in childhood bacterial meningitis: an experience in 55 patients. Journal of Antimicrobial Chemotherapy 1984;14(B):167‐80. [DOI] [PubMed] [Google Scholar]
Macfarlane 1979 {published data only}
- Macfarlane JT, Anjorin FI, Cleland PG, Hassan‐King M, Tor‐Agbidye S, Wali SS. Single injection treatment of meningococcal meningitis. 1. Long‐acting penicillin. Transactions of the Royal Society of Tropical Medicine and Hygiene 1979;73(6):693‐7. [DOI] [PubMed] [Google Scholar]
Marks 1986 {published data only}
- Marks WA, Stutman HR, Marks MI, Abramson JS, Ayoub EM, Chartrand SA, et al. Cefuroxime versus ampicillin plus chloramphenicol in childhood bacterial meningitis: a multicenter randomized controlled trial. Journal of Pediatrics 1986;109(1):123‐30. [DOI] [PubMed] [Google Scholar]
Martin 1990 {published data only}
- Martin E, Hohl P, Guggi T, Kayser FH, Fernex M. Short course single daily ceftriaxone monotherapy for acute bacterial meningitis in children: results of a Swiss multicenter study. Part I: Clinical results. Infection 1990;18(2):70‐7. [DOI] [PubMed] [Google Scholar]
Ngu 1987 {published data only}
- Ngu J, Youmbissi T. A comparative study with ceftriaxone (Rocephin) versus ampicillin and chloramphenicol in children with bacterial meningitis. Chemioterapia 1987;6(Suppl 2):417‐8. [PubMed] [Google Scholar]
Pecoul 1991 {published data only}
- Pecoul B, Varaine F, Keita M, Soga G, Djibo A, Soula G, et al. Long‐acting chloramphenicol versus intravenous ampicillin for treatment of bacterial meningitis. Lancet 1991;338(8771):862‐6. [DOI] [PubMed] [Google Scholar]
Pfister 1989 {published data only}
- Pfister HW, Preac‐Mursic V, Wilske B, Einhaupl KM. Cefotaxime vs penicillin G for acute neurologic manifestations in Lyme borreliosis. A prospective randomized study. Archives of Neurology 1989;46(11):1190‐4. [DOI] [PubMed] [Google Scholar]
Powell 1990 {published data only}
- Powell KR, Sugarman LI, Eskenazi AE, Woodin KA, Kays MA, McCormick KL, et al. Normalization of plasma arginine vasopressin concentrations when children with meningitis are given maintenance plus replacement fluid therapy. Journal of Pediatrics 1990;117(4):515‐22. [DOI] [PubMed] [Google Scholar]
Reed 1983 {published data only}
- Reed MD, Rekate HL, Aronoff SC, Myers CM, Blumer JL. Single‐dose plasma and cerebrospinal fluid pharmacokinetics of ceftriaxone in infants and children. Clinical Pharmacology 1983;2(6):558‐63. [PubMed] [Google Scholar]
Rodriguez 1985 {published data only}
- Rodriguez WJ, Khan WN, Gold B, Feris J, Puig J, Sturla C. Ceftazidime in the treatment of meningitis in infants and children over one month of age. American Journal of Medicine 1985;79(2A):52‐5. [DOI] [PubMed] [Google Scholar]
Schaad 1990 {published data only}
- Schaad UB, Suter S, Gianella‐Borradori A, Pfenninger J, Auckenthaler R, Bernath O, et al. A comparison of ceftriaxone and cefuroxime for the treatment of bacterial meningitis in children. New England Journal of Medicine 1990;322(3):141‐7. [DOI] [PubMed] [Google Scholar]
Steele 1984 {published data only}
- Steele RW. Ceftriaxone therapy of meningitis and serious infections. American Journal of Medicine 1984;77(4C):50‐3. [PubMed] [Google Scholar]
Tessin 1989 {published data only}
- Tessin I, Trollfors B, Thiringer K, Thorn Z, Larsson P. Concentrations of ceftazidime, tobramycin and ampicillin in the cerebrospinal fluid of newborn infants. European Journal of Pediatrics 1989;148(7):679‐81. [DOI] [PubMed] [Google Scholar]
Tetanye E, 1990 {published data only}
- Tetanye E, Yondo D, Bernard‐Bonnin AC, Tchokoteu PF, Kago I, Ndayo M. Initial treatment of bacterial meningitis in Yaounde, Cameroon: theoretical benefits of the ampicillin‐chloramphenicol combination versus chloramphenicol alone. Annals of Tropical Pediatrics 1990;10(3):285‐91. [DOI] [PubMed] [Google Scholar]
Vallejo 1991 {published data only}
- Vallejo JG, Kaplan SL, Mason EO Jr. Treatment of meningitis and other infections due to ampicillin‐resistant Haemophilus influenzae type b in children. Reviews of Infectious Diseases 1991;13(2):197‐200. [DOI] [PubMed] [Google Scholar]
Wazib 2008 {published data only}
- Wazib A, Hossain MZ, Hanan R, Alam MB. A comparative study of benzylpenicillin versus ceftriaxone in the treatment of adult pyogenic meningitis. Journal of Dhaka Medical College 2008;17(2):59‐61. [Google Scholar]
Yogev 1986 {published data only}
- Yogev R, Shulman ST, Chadwick EG, Davis AT, Glogowski W. Once daily ceftriaxone for central nervous system infections and other serious pediatric infections. Pediatric Infectious Diseases 1986;5(3):298‐303. [DOI] [PubMed] [Google Scholar]
Zavala 1988 {published data only}
- Zavala I, Barrera E, Nava A. Ceftriaxone in the treatment of bacterial meningitis in adults. Chemotherapy 1988;34(1):47‐52. [DOI] [PubMed] [Google Scholar]
Additional references
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309(6964):1286‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Enting 1996
- Enting RH, Spanjaard L, Beek D, Hensen EF, Gans J, Dankert J. Antimicrobial susceptibility of Haemophilus influenzae, Neisseria meningitidis and Streptococcus pneumoniae isolates causing meningitis in The Netherlands, 1993‐1994. Journal of Antimicrobial Chemotherapy 1996;38(5):777‐86. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2011.
Higgins 2011b
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Johnston 2007
- Johnston BC, Supina AL, Ospina M, Vohra S. Probiotics for the prevention of pediatric antibiotic‐associated diarrhea. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD004827.pub2] [DOI] [PubMed] [Google Scholar]
Kaplan 1988b
- Kaplan SL, Fishman MA. Update on bacterial meningitis. Journal of Child Neurology 1988;3(2):82‐3. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2011.
Mwangi 2002
- Mwangi I, Berkley J, Lowe B, Peshu N, Marsh K, Newton CRJC. Acute bacterial meningitis in children admitted to a rural Kenyan Hospital: increasing antibiotic resistance and outcome. Pediatric Infectious Disease Journal 2002;21(11):1042‐8. [DOI] [PubMed] [Google Scholar]
Pillai 2008
- Pillai A, Nelson RL. Probiotics for treatment of Clostridium difficile associated colitis in adults. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD004611.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reis 2002
- Reis JN, Lima JB, Ribeiro GS, Corderio SM, Salgado K, Reis MG, et al. Antimicrobial resistance in Haemophilus influenzae isolated during population‐based surveillance for meningitis in Salvador, Brazil. Antimicrobial Agents and Chemotherapy 2002;46(11):3641‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2011.
Sutcliffe1988
- Sutcliffe EM, Jones DM, El‐Sheikh S, Percival A. Penicillin insensitive meningococci in the UK. Lancet 1988;3(8586):82‐93. [DOI] [PubMed] [Google Scholar]
Tristram 2007
- Tristram S, Jacobs MR, Appelbaum PC. Antimicrobial resistance in Haemophilus influenzae. Clinical Microbiology Reviews 2007;20(2):368‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wasfy 2005
- Wasfy MO, Pimentel G, Abdel‐Mansoud M, Russell KL, Barrozo CP, Klena JD, et al. Antimicrobial susceptibility and serotype distribution of Streptococcus pneumoniae causing meningitis in Egypt, 1998‐2003. Journal of Antimicrobial Chemotherapy 2005;55(6):958‐64. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Prasad 2004
- Prasad K, Singhal T, Jain N, Gupta PK. Third generation cephalosporins versus conventional antibiotics for treating acute bacterial meningitis. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD001832.pub3] [DOI] [PubMed] [Google Scholar]


