Abstract
Background
Non‐specific low back pain is a major health problem worldwide. Interventions based on exercises have been the most commonly used treatments for patients with this condition. Over the past few years, the Pilates method has been one of the most popular exercise programmes used in clinical practice.
Objectives
To determine the effects of the Pilates method for patients with non‐specific acute, subacute or chronic low back pain.
Search methods
We conducted the searches in CENTRAL, MEDLINE, EMBASE, CINAHL, PEDro and SPORTDiscus from the date of their inception to March 2014. We updated the search in June 2015 but these results have not yet been incorporated. We also searched the reference lists of eligible papers as well as six trial registry websites. We placed no limitations on language or date of publication.
Selection criteria
We only included randomised controlled trials that examined the effectiveness of Pilates intervention in adults with acute, subacute or chronic non‐specific low back pain. The primary outcomes considered were pain, disability, global impression of recovery and quality of life.
Data collection and analysis
Two independent raters performed the assessment of risk of bias in the included studies using the 'Risk of bias' assessment tool recommended by The Cochrane Collaboration. We also assessed clinical relevance by scoring five questions related to this domain as 'yes', 'no' or 'unclear'. We evaluated the overall quality of evidence using the GRADE approach and for effect sizes we used three levels: small (mean difference (MD) < 10% of the scale), medium (MD 10% to 20% of the scale) or large (MD > 20% of the scale). We converted outcome measures to a common 0 to 100 scale when different scales were used.
Main results
The search retrieved 126 trials; 10 fulfilled the inclusion criteria and we included them in the review (a total sample of 510 participants). Seven studies were considered to have low risk of bias, and three were considered as high risk of bias.
A total of six trials compared Pilates to minimal intervention. There is low quality evidence that Pilates reduces pain compared with minimal intervention, with a medium effect size at short‐term follow‐up (less than three months after randomisation) (MD ‐14.05, 95% confidence interval (CI) ‐18.91 to ‐9.19). For intermediate‐term follow‐up (at least three months but less than 12 months after randomisation), two trials provided moderate quality evidence that Pilates reduces pain compared to minimal intervention, with a medium effect size (MD ‐10.54, 95% CI ‐18.46 to ‐2.62). Based on five trials, there is low quality evidence that Pilates improves disability compared with minimal intervention, with a small effect size at short‐term follow‐up (MD ‐7.95, 95% CI ‐13.23 to ‐2.67), and moderate quality evidence for an intermediate‐term effect with a medium effect size (MD ‐11.17, 95% CI ‐18.41 to ‐3.92). Based on one trial and low quality evidence, a significant short‐term effect with a small effect size was reported for function (MD 1.10, 95% CI 0.23 to 1.97) and global impression of recovery (MD 1.50, 95% CI 0.70 to 2.30), but not at intermediate‐term follow‐up for either outcome.
Four trials compared Pilates to other exercises. For the outcome pain, we presented the results as a narrative synthesis due to the high level of heterogeneity. At short‐term follow‐up, based on low quality evidence, two trials demonstrated a significant effect in favour of Pilates and one trial did not find a significant difference. At intermediate‐term follow‐up, based on low quality evidence, one trial reported a significant effect in favour of Pilates, and one trial reported a non‐significant difference for this comparison. For disability, there is moderate quality evidence that there is no significant difference between Pilates and other exercise either in the short term (MD ‐3.29, 95% CI ‐6.82 to 0.24) or in the intermediate term (MD ‐0.91, 95% CI ‐5.02 to 3.20) based on two studies for each comparison. Based on low quality evidence and one trial, there was no significant difference in function between Pilates and other exercises at short‐term follow‐up (MD 0.10, 95% CI ‐2.44 to 2.64), but there was a significant effect in favour of other exercises for intermediate‐term function, with a small effect size (MD ‐3.60, 95% CI ‐7.00 to ‐0.20). Global impression of recovery was not assessed in this comparison and none of the trials included quality of life outcomes. Two trials assessed adverse events in this review, one did not find any adverse events, and another reported minor events.
Authors' conclusions
We did not find any high quality evidence for any of the treatment comparisons, outcomes or follow‐up periods investigated. However, there is low to moderate quality evidence that Pilates is more effective than minimal intervention for pain and disability. When Pilates was compared with other exercises we found a small effect for function at intermediate‐term follow‐up. Thus, while there is some evidence for the effectiveness of Pilates for low back pain, there is no conclusive evidence that it is superior to other forms of exercises. The decision to use Pilates for low back pain may be based on the patient's or care provider's preferences, and costs.
Plain language summary
Pilates for low back pain
Review question
To determine the effects of the Pilates method for patients with non‐specific acute, subacute or chronic low back pain.
Background
Low back pain is an important health problem around the world. One of the most common treatments is exercise and in recent years Pilates has been a common option for treating low back pain.
Search date
We conducted searches up to March 2014. We updated the search in June 2015 but these results have not yet been incorporated.
Study characteristics
This review included 10 studies and 510 patients. All studies included a similar population of people with non‐specific low back pain. The studies only included participants with chronic low back pain. The duration of the treatment programmes in the included trials ranged from 10 days to 90 days. The duration of follow‐up varied from four weeks to six months. None of the included studies measured follow‐up beyond six months. The sample sizes ranged from 17 to 87 participants.
Key results
The included studies demonstrated that Pilates is probably more effective than minimal intervention in the short and intermediate term for pain and disability outcomes, and more effective than minimal intervention for improvement in function and global impression of recovery in the short term. Pilates is probably not more effective than other exercises for pain and disability in the short and intermediate term. For function, other exercises were more effective than Pilates at intermediate‐term follow‐up, but not at short‐term follow‐up. Thus, while there is some evidence for the effectiveness of Pilates for low back pain, there is no conclusive evidence that it is superior to other forms of exercise. Minor or no adverse events were reported for the interventions in this review.
Quality of evidence
The overall quality of the evidence in this review ranged from low to moderate.
Summary of findings
Background
Non‐specific low back pain (LBP) is a highly prevalent condition (Hoy 2012), which is associated with disability and work absenteeism worldwide (Waddell 2004). Recent prognostic studies have concluded that around 40% of patients with acute LBP will not recover within three months (Costa 2012; Henschke 2008), and of these only 40% will recover during the following 12 months (Costa 2009; Costa 2012). Not surprisingly, the costs associated with LBP and related disability are enormous, causing a major economic burden for patients, governments and health insurance companies (Dagenais 2008).
Exercise therapy is probably the most commonly used intervention for the treatment of patients with chronic non‐specific LBP. Exercise has a plausible biological rationale and low cost, and it has been recommended in most of the clinical practice guidelines for chronic LBP (Chou 2007; Delitto 2012; European Guidelines 2006), as well as by important systematic reviews on this topic (Hayden 2005; Hayden 2007). These reviews and guidelines have typically reported the effects of exercise in general, but not separately the effects of different approaches to exercise. However, the exercise programmes now used for low back pain vary enormously, for example hydrotherapy, walking programmes, behavioural approaches such as graded activity and graded exposure, and mind‐body exercises such as yoga and Tai Chi. To guide the treatment choice of both the clinician and patient it would be useful to have separate evidence on the effectiveness of the most popular approaches to exercise.
One type of exercise programme that has been increasingly used for patients with LBP over the last decade is the Pilates method (Musculino 2004; Queiroz 2005; Rydeard 2006). Pilates exercises were developed by Joseph Pilates in the 1920s and this method was originally named 'centrology' (Anderson 2000). These exercises can be performed with or without specialised equipment following six basic principles: centering, concentration, control, precision, flow and breathing (Wells 2012). The effectiveness of the Pilates approach has been tested in a few randomised controlled trials (Curnow 2009; Fonseca 2009; Gladwell 2006; Rydeard 2006; Wajswelner 2012). Our aim was to perform the first Cochrane systematic review on this topic in order to provide accurate and robust information on the effectiveness of the Pilates approach for low back pain, compared to no intervention, placebo or other types of interventions.
Description of the condition
Low back pain is defined as pain or discomfort localised below the ribs and above the gluteal crease (where the upper leg meets the buttock), with or without referred leg pain (European Guidelines 2006). Non‐specific LBP is the most common and can be defined as LBP without any known specific cause or pathology, such as nerve root compromise or serious spinal pathology (i.e. fracture, cancer and inflammatory diseases). Low back pain is often classified in three stages (acute, subacute and chronic) according to its duration and this provides some information to the clinician with regards to treatment and prognosis. Acute LBP is usually defined as an episode persisting for less than six weeks; subacute as LBP persisting for between six and 12 weeks, and chronic as LBP persisting for 12 weeks or longer (European Guidelines 2006). For the purposes of this review, we included studies that recruited patients with non‐specific LBP of any duration, but we analysed them separately (if applicable).
Description of the intervention
The Pilates method was developed by Joseph Hubertus Pilates and consists of comprehensive body conditioning, which aims to develop better body awareness and improved posture (Queiroz 2005; Rydeard 2006). Pilates exercises mainly involve isometric contractions (i.e. contraction without joint movement) of the core muscles, which make up the muscular centre responsible for the stabilisation of the body, both while it is moving or at rest. Pilates became popular as a treatment for low back pain long after Joseph Pilates died. Traditional Pilates exercises follow six basic principles: centering (i.e. tightening the 'powerhouse' (trunk muscles)), concentration (i.e. cognitive attention while performing the exercises), control (i.e. postural management while performing the exercises), precision (i.e. accuracy of exercise technique), flow (i.e. smooth transition of movements within the exercise sequence) and breathing in co‐ordination with the exercises (Wells 2012). A recent systematic review of Pilates exercises concluded that another principle should be added whenever these exercises are used in the treatment of LBP, which is posture (Wells 2012). Pilates exercises are usually prescribed by certified instructors. The exercises are considered to be similar to spinal stabilisation exercises (also known as motor control exercises); however, they do not involve conscious activation of specific muscles in the manner often used in spinal stabilisation exercises. During dynamic exercises in Pilates, co‐contraction of the multifidus (a deep back muscle), transversus abdominis (a deep abdominal muscle), pelvic floor and diaphragm muscles is observed. The goal of the co‐contraction of these muscles is to reduce joint compression and alter pelvic tilt (Bryan 2003; Gladwell 2006).
The Pilates method includes several stretching and strengthening exercises, which can be divided into two categories: mat Pilates (exercises performed on the ground, without any specific equipment) and exercises with the Pilates apparatus. The first exercises developed by Pilates were performed on the ground; he then created a series of apparatus on which to perform exercises against resistance provided by springs and pulleys (Musculino 2004; Queiroz 2005). The reported benefits of Pilates exercises include improvements in strength, range of motion, co‐ordination, balance, muscle symmetry, flexibility, proprioception (awareness of posture), body definition and general health (Bryan 2003; Gladwell 2006). The exercises are adapted to the condition of the patient and difficulty is gradually increased while respecting individual abilities and characteristics. The springs and pulleys of each apparatus can be used to make the exercises easier or more difficult to perform.
How the intervention might work
One biological rationale for how Pilates exercises might work is based upon the idea that stability and control of spinal muscles are altered in people with LBP (Hodges 1996). Two motor control impairments are proposed to occur in people with LBP: first the onset of activity of deep muscles such as the multifidus and transversus abdominis is delayed when the stability of the spine is challenged in dynamic tasks (Rackwitz 2006). Second, patients with LBP tend to compensate for this lack of stability by increasing the activity of superficial muscles (Hodges 1996; Rackwitz 2006), which increases the stiffness of the spine. The exercises advocated by the Pilates approach aim to target these two factors (i.e. improving the stability of the spine by improving the motor control of the deep muscles and reducing the activity of superficial muscles), as well as to improve posture and body awareness. These factors have the potential to improve pain, disability and quality of life in patients with LBP.
Why it is important to do this review
Over the last decade, the popularity of the Pilates method as an intervention for patients with LBP and other musculoskeletal conditions has steadily increased worldwide. There are published trials (Curnow 2009; Fonseca 2009; Gladwell 2006; Rajpal 2008; Rydeard 2006) and systematic reviews (Lim 2011; Miyamoto 2013; Posadzki 2011; Wells 2014) available on this topic. However, we are aware of new existing trials on this topic. Therefore, a well‐conducted systematic review is needed to better inform clinicians, patients and policy‐makers about the effectiveness of this intervention in patients with non‐specific LBP.
Objectives
To determine the effects of the Pilates method for patients with non‐specific acute, subacute or chronic low back pain.
Methods
Criteria for considering studies for this review
Types of studies
We only included randomised controlled trials in this review. We did not consider trials that used quasi‐random allocation procedures in this review to avoid biased estimates of treatment effects across the included studies (Higgins 2011).
Types of participants
Inclusion criteria:
Adult participants aged 16 or older with acute, subacute or chronic non‐specific LBP.
Patients recruited from primary, secondary or tertiary care; these patients could be either seeking care for back pain or recruited from the community.
Exclusion criteria:
Patients with any contraindication to exercise therapy.
Pregnancy.
Patients with serious spinal pathology (i.e. cancer, fracture, cauda equina syndrome and inflammatory diseases).
Trials that included more than 5% of participants with evidence of nerve root compromise.
Types of interventions
We considered any type of exercise therapy that followed the Pilates method. We judged trials to have evaluated Pilates when at least one of the following criteria was met:
The study explicitly stated that the intervention was based upon the Pilates principles (i.e. centering, concentration, control, precision, flow, breathing and posture) or at least three of these elements (Wells 2012).
The therapists who provided the interventions had previous training in Pilates exercises or the therapists were described as certified Pilates instructors.
Types of outcome measures
We included any type of clinically relevant measure that could be considered patient‐centred. We did not consider physiological and biomechanical variables (e.g. range of motion, motor control, muscle endurance) for this review.
Primary outcomes
Pain intensity measured by any reliable and valid self report outcome measure.
Disability measured by any reliable and valid self report outcome measure.
Global impression of recovery measured by any reliable and valid type of Global Perceived Effect Scale.
Quality of life (measured by any reliable and valid instrument).
Secondary outcomes
Return to work (measured by any reliable and valid instrument).
Adverse effects.
Search methods for identification of studies
Electronic searches
We searched for randomised controlled trials from the following electronic databases without restrictions on language or date of publication. We used the search strategies developed by the Cochrane Back Review Group. We searched all databases from the date of their inception to March 2014.
CENTRAL (Cochrane Central Register of Controlled Trials, The Cochrane Library, which contains the Back Group Trials Register) (Appendix 1).
MEDLINE (OvidSP, 1946 to March Week 2 2014) and MEDLINE In‐Process & Other Non‐Indexed Citations (OvidSP, March 24, 2014) (Appendix 2).
EMBASE (OvidSP, 1980 to 2014 Week 12) (Appendix 3).
CINAHL (Cumulative Index to Nursing and Allied Health Literature, EBSCO) (Appendix 4).
PEDro (Physiotherapy Evidence Database) (Appendix 5).
SPORTDiscus (EBSCO) (Appendix 6).
All databases were previously searched in March 2013. For the March 2014 search, we added MEDLINE In‐Process and Other Non‐Indexed Citations, we updated the EMBASE study design filter, we added a new term to the CINAHL strategy and we searched new fields in PEDro. Details can be found in the Appendices.
We performed an updated search in June 2015. We added one eligible study to the awaiting classification section and we will incorporate this in the next review update.
Searching other resources
We also searched the reference lists of eligible papers as well as trial registry websites: Australian and New Zealand Clinical Trials Registry (ANZCTR), National Research Registry, ClinicalTrials.gov, metaRegister of Controlled Trials (mRCT), Brazilian Registry of Clinical Trials (ReBEC) and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP). The search strategies for ClinicalTrials.gov and WHO ICTRP are described in Appendix 7.
Data collection and analysis
Selection of studies
Two pairs of review authors (CMNC and LCMC, BTS and TPY) independently screened titles and abstracts for potentially eligible studies. We used full‐text papers to determine the final inclusion in the review. We resolved disagreements between review authors through discussion or by the arbitration of a third review author (LOPC or CM) when consensus could not be reached. We included only full‐text papers, written in any language, regardless of the date of publication. We included papers written in English, Portuguese, Spanish, Italian and Dutch as the review team includes authors who are able to read these languages. We sent all remaining papers that were written in languages other than these to translators. We also scanned the reference lists from previous published reviews on Pilates as well as the reference lists from the eligible randomised trials.
Data extraction and management
Two independent review authors (TPY and BTS) extracted the following data from each of the eligible papers using a standardised data extraction form (Appendix 8). We resolved disagreements between review authors through discussion or by the arbitration of a third review author (CM).
Bibliometric data (authors, year of publication, language).
Study characteristics (study design, sample size, description of the sample, country, recruitment modality, funding).
Characteristics of the participants (gender, age, duration of symptoms, severity of the condition at baseline).
Description of the interventions (both experimental and control interventions), including dose (number of sessions, duration of each session of treatment, etc) and co‐interventions.
Duration of follow‐up assessments.
Outcomes assessed (converted to a common 0 to 100 scale when different scales were used).
Study results.
Time periods for outcome assessment: short‐term (less than three months after randomisation), intermediate‐term (at least three months but less than 12 months after randomisation) and long‐term (12 months or more after randomisation) follow‐up. When there were multiple time points that fell within the same category we used the one that was closer to the end of the treatment, six months and 12 months.
We pilot tested the data extraction form using two RCTs on back pain.
Assessment of risk of bias in included studies
We assessed the risk of bias in the included studies using the 'Risk of bias' assessment tool as recommended by The Cochrane Collaboration (Higgins 2011) and the Cochrane Back Review Group (Furlan 2009) (Appendix 9).
Two review authors (BTS and TPY) independently performed this 'Risk of bias' assessment and resolved possible disagreements between review authors by discussion, or arbitration by a third review author (CM) when consensus could not be reached. We scored each of the 12 items of the 'Risk of bias' assessment as 'high', 'low' or 'unclear' risk. We defined a study with an overall low risk of bias as having low risk of bias on six or more of these items.
We assessed clinical relevance by scoring five questions related to this domain as 'yes', 'no' or 'unclear' (Appendix 10). Two independent authors performed this and resolved possible disagreements by discussion, or arbitration by a third review author when consensus could not be reached.
Measures of treatment effect
We expected to deal mostly with continuous outcome measures to determine the treatment effect, such as pain intensity, disability or quality of life scales. For all continuous outcomes we quantified the treatment effects with the mean difference (MD). To accommodate the different scales used for these outcomes, we converted outcomes to a common 0 to 100 scale. We also expected to encounter dichotomous outcomes such as recovery or return to work and in such cases we calculated the risk ratios (RR) for experiencing the positive outcome. We used effect sizes and 95% confidence intervals (CI) as a measure of treatment effect. We considered between‐group differences of at least 20% as clinically important (Ostelo 2008). We used Review Manager 5 for all analyses. For effect sizes, we defined three levels as: small (MD < 10% of the scale), medium (MD 10% to 20% of the scale) or large (MD > 20% of the scale) (Rubinstein 2011) (Appendix 10).
Unit of analysis issues
We did not encounter any cross‐over or cluster‐randomised trials. To deal with repeated observations on participants we followed the advocated strategy of defining the outcomes (already stated previously) as well as the time points a priori (Higgins 2011). The time points were short‐term (less than three months after randomisation), intermediate‐term (at least three months but less than 12 months after randomisation) and long‐term (12 months or more after randomisation) follow‐up. When there were multiple time points that fell within the same category we used the one that was closer to the end of the treatment (short‐term), six months (intermediate‐term) and 12 months (long‐term). If studies included multiple treatment arms, we formed multiple treatment comparisons but if there was a shared group we split this in order to be able to include two (reasonably independent) comparisons.
Dealing with missing data
Firstly, review authors emailed the authors of each study requesting any necessary data that was not comprehensively reported in the manuscript. In cases where data were reported as a median and interquartile range (IQR), we assumed that the median is equivalent to the mean and the width of the IQR is equivalent to 1.35 times the standard deviation (Higgins 2011). We also estimated data from graphs in cases where this information was not presented in tables or text. If any information regarding standard deviations was missing, we calculated these from confidence intervals or standard errors (if available) of the same study. Finally, if no measure of variability was presented anywhere in the text, we estimated the standard deviation from the most similar trial in the review, taking the risk of bias of individual studies into consideration.
Assessment of heterogeneity
The assessment of heterogeneity was based upon visual inspections of the forest plots (e.g. overlapping confidence intervals) and more formally by the Chi2 test and the I2 statistic as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We combined results in a meta‐analysis using a random‐effects model if I2 < 50%. If substantial heterogeneity was present, we did not combine the results but instead presented them as a narrative synthesis. If I2 values were slightly higher than 50% but we identified no clear heterogeneity by visual inspection, we combined the results into a meta‐analysis.
Assessment of reporting biases
We performed comprehensive searches in order to reduce the possibility of reporting biases. We also planned to generate funnel plots (if we retrieved at least 10 trials) in order to determine possible reporting biases.
Data synthesis
We combined the results from individual trials through meta‐analysis. This pooling of the data was dependent on the level of statistical heterogeneity of the retrieved studies. We combined results in a meta‐analysis using a random‐effects model if the I2 value was less than 50%. If substantial statistical heterogeneity was present, we did not quantitatively pool the results but presented them as a narrative synthesis. If the I2 value was slightly higher than 50% but no clear clinical heterogeneity was detected by visual inspection we combined the results in a meta‐analysis.
Regardless of whether there were sufficient data available to use quantitative analyses to summarise the data, we assessed the overall quality of the evidence for each outcome. To accomplish this, we used the GRADE approach, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and adapted in the updated Cochrane Back Review Group method guidelines (Furlan 2009). Factors that may decrease the quality of the evidence are: study design and risk of bias (downgraded if more than 25% of the participants were from studies with a high risk of bias); inconsistency of results (downgraded if significant heterogeneity was present by visual inspection or if the I² value was greater than 50%); indirectness (generalisability of the findings; downgraded if more than 50% of the participants were outside the target group); imprecision (downgraded if fewer than 400 participants were included in the comparison for continuous data and there were fewer than 300 events for dichotomous data (Mueller 2007)) and other factors (e.g. reporting bias, publication bias). We considered single studies with fewer than 400 participants for continuous or dichotomous outcomes inconsistent and imprecise, providing 'low quality evidence', which could be downgraded to 'very low quality evidence' if there were further limitations on the quality of evidence (Rubinstein 2012). We reduced the quality of the evidence for a specific outcome by a level, according to the performance of the studies against these five factors and we described them as follows.
High quality evidence: there are consistent findings among at least 75% of RCTs with low risk of bias, consistent, direct and precise data and no known or suspected publication biases. Further research is unlikely to change either the estimate or our confidence in the results.
Moderate quality evidence: one of the domains is not met. Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality evidence: two of the domains are not met. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality evidence: three of the domains are not met. We are very uncertain about the results.
No evidence: no RCTs were identified that addressed this outcome.
Subgroup analysis and investigation of heterogeneity
We stratified some of the analyses based upon a number of factors when necessary (Higgins 2011):
types of control groups (e.g. minimal intervention, placebo, another type of treatment, wait‐and‐see groups);
duration of follow‐up (i.e. short‐term, intermediate‐term and long‐term);
risk of bias (i.e. low and high risk of bias studies.
Sensitivity analysis
We did not plan to perform any sensitivity analyses as we anticipated that the number of studies and comparisons would be low. This turned out to be the case.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
The search retrieved 126 trials, of which nine fulfilled the inclusion criteria and were included in the review (a total pooled sample of 510 participants) (Figure 1).
1.
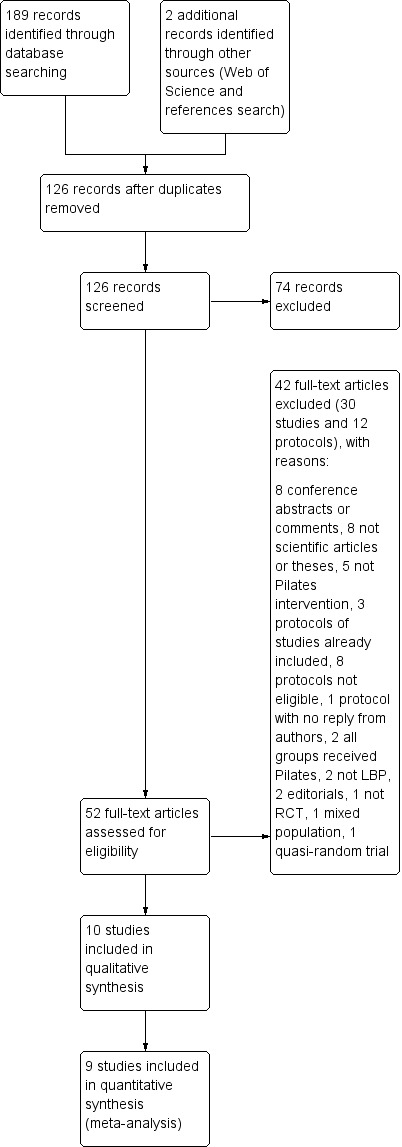
Study flow diagram.
A search for unpublished trials in ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) search portal revealed 13 records of trials evaluating Pilates for low back pain (LBP). Three records were for studies already included in this review (Marshall 2013; Miyamoto 2013; Wajswelner 2012) and eight were ineligible (three compared different forms of Pilates (NCT01533805, PACTR201211000443397, RBR‐7tyg5j), two were from populations not included in this review (e.g. military settings, children) (ACTRN12607000471482, NCT01711203), one included both groups performing Pilates exercise (NCT01919268), one included cervical pain (NCT01999283) and one is not a RCT (ACTRN12609000927224). Two registered trials appeared potentially eligible for this review (NCT01502059 and RBR‐7yhzym). We were able to find the published study for one of these records (NCT01502059) and we included the study in the review (Natour 2014). We did not find any publicly available report for the other record (RBR‐7yhzym), and the authors did not reply to our email contact attempts. For the additional updated search, one study fulfilled the inclusion criteria (Anand 2014) and we added it to the awaiting classification section to be incorporated in the next review update.
The 10 trials included in the review were conducted in five different countries: three were conducted in Australia (Brooks 2012; Marshall 2013; Wajswelner 2012), three in Brazil (Fonseca 2009; Miyamoto 2013; Natour 2014), two in the United Kingdom (Gladwell 2006; Quinn 2011), and one in each of Hong Kong (Rydeard 2006), and India (Rajpal 2008). All trials were published in English.
Included studies
A total of 510 participants were enrolled in the 10 included trials, of which we included data from 478 participants in the meta‐analyses. The study sample sizes ranged from 17 to 87 participants (median (IQR) = 41 (31.5)). One study was not included in the meta‐analysis because we found substantial heterogeneity in the comparison in which this study was included (Rajpal 2008).
The assessment of clinical relevance for each study is described in Table 3.
1. Clinical Relevance Assessment for Each Study.
| Studies/criteria | Are the patients described in detail so that you can decide whether they are comparable to those that you see in your practice? | Are the interventions and treatment settings described well enough so that you can provide the same for your patients? | Were all clinically relevant outcomes measured and reported? | Is the size of the effect clinically important?* | Are the likely treatment benefits worth the potential harms? |
| Brooks 2012 | Yes | Yes | Yes | No | Yes |
| Fonseca 2009 | Yes | Yes | No | No | Yes |
| Gladwell 2006 | Yes | Yes | Yes | No | Yes |
| Marshall 2013 | Yes | Yes | Yes | No | Yes |
| Miyamoto 2013 | Yes | Yes | Yes | Yes1 | Yes |
| Natour 2014 | Yes | Yes | Yes | Yes1 | Yes |
| Quinn 2011 | Yes | Yes | Yes | Yes2 | Yes |
| Rajpal 2008 | No | Yes | No | No | Yes |
| Rydeard 2006 | Yes | Yes | Yes | No | Yes |
| Wajswelner 2012 | Yes | Yes | Yes | No | Yes |
*Clinical importance: consider 30% on VAS/NRS for pain intensity as clinically significant, and 2 to 3 points (or 8% to 12%) on the Roland‐Morris Disability Questionnaire for disability.
1Disability (short and intermediate‐term).
2Disability (short‐term).
Types of studies
We identified the following comparisons in this review: (i) six trials compared the Pilates method with minimal intervention or no intervention (Fonseca 2009; Gladwell 2006; Miyamoto 2013; Quinn 2011; Rydeard 2006; Natour 2014), and (ii) four trials compared the Pilates method with other types of exercises, including general exercise (Brooks 2012; Marshall 2013; Wajswelner 2012), and the McKenzie method (Rajpal 2008). We did not include studies evaluating two types of Pilates (e.g. mat Pilates versus equipment‐based Pilates) as the aim of this review was to provide evidence on the effectiveness of the Pilates method for low back pain; thereby we focused our comparisons on no intervention, placebo or other interventions.
Study population
Most participants in the included trials were middle‐aged (mean: 38 years), ranging from 22 to 50 years of age. Two trials included only women participants (Quinn 2011; Rajpal 2008), and all the other trials included both men and women. All trials included exclusively chronic participants (low back pain persisting for 12 weeks or more), except for one trial that included participants with LBP for at least six weeks (Rydeard 2006).
Technique: number and duration of treatments
The duration of the treatment programmes in the included trials ranged from 10 days to 90 days. One trial provided treatment twice a week for a total of 90 days (Natour 2014). In four trials, the participants received an eight‐week programme. In two of the four trials the frequency of treatment was three times per week (Brooks 2012; Marshall 2013), one trial provided treatment twice a week (Fonseca 2009), and the other one provided treatment once a week (Quinn 2011). In three trials the treatment duration was six weeks, with two trials evaluating treatment provided twice a week (Miyamoto 2013; Wajswelner 2012), and one trial evaluating treatment delivered once a week (Gladwell 2006). Two trials performed the treatment for four weeks, one included daily sessions (Rajpal 2008), and the other provided treatment three times per week (Rydeard 2006). The duration of all sessions was approximately one hour for all included studies. The mean number of sessions in the included studies was 15.3, ranging from six to 30 sessions.
Primary outcomes
Pain intensity
All studies measured pain intensity. In most cases, pain intensity was measured with a visual analogue scale (VAS) or numerical rating scale (NRS), and one study used the 0 to 5 point Roland Morris pain rating visual analogue scale (RMVAS) (Gladwell 2006). We converted all scales to a 0 to 100‐point scale.
Disability
Seven studies measured disability (Brooks 2012; Gladwell 2006; Marshall 2013; Miyamoto 2013; Quinn 2011; Rydeard 2006; Wajswelner 2012). Four studies measured disability with the Roland Morris Disability Questionnaire (Miyamoto 2013; Quinn 2011; Rydeard 2006, Natour 2014). Three studies used the Oswestry Disability Index for measuring disability (Brooks 2012; Gladwell 2006; Marshall 2013), and one study used the Quebec Disability scale (Wajswelner 2012). We converted all scales to a 0 to 100‐point scale.
Global impression of recovery
One study measured global impression of recovery using a Global Perceived Effect Scale (Miyamoto 2013).
Quality of life
Two studies measured quality of life, but the data from the physical and mental components were not available in the text and the authors did not provide this information on request (Natour 2014; Wajswelner 2012).
Secondary outcomes
We considered return to work and adverse effects as secondary outcomes in this review; however, none of the included studies reported these outcomes.
Other outcomes
Function
Two studies measured function using the Patient Specific Functional Scale (Miyamoto 2013; Wajswelner 2012).
Follow‐up
All studies measured short‐term follow‐up, which varied from four to eight weeks. Three studies measured intermediate follow‐up, from three to six months (Marshall 2013; Miyamoto 2013; Wajswelner 2012). None of the included studies measured follow‐up beyond six months.
Excluded studies
We excluded 42 studies in the full‐text assessment for eligibility (30 full text articles and 12 protocols). Of the 30 full text articles excluded, eight were conference abstracts, presentations or comments (Anderson 2006; Boden 2010; Cámara 2011; Kennedy 2012; Natour 2011; O'Brien 2006; SeQueira 2010; Xue‐Qiang 2013); five were magazine articles (Jaecks 2004; Kagan 2008; Parker 2010; Robinson 2007; Sparrowe 2007), two were theses (Anderson 2005; Gagnon 2005), and one was an opinion piece (Ickes 2007). In five studies, the intervention was not Pilates exercise (Mehling 2005; Rasmussen‐Barr 2003; Sherman 2010; Tekur 2008; Tilbrook 2011). In two studies both groups received Pilates or different forms of Pilates were tested (Curnow 2009; da Luz 2014). Two studies did not recruit LBP patients (Alves 2012; Phrompaet 2011), and two were editorials (McNeill 2009; McNeill 2010). One study included a mixed population of healthy participants and those who had LBP (Hides 2012), one study used a quasi‐random allocation procedure (Donzelli 2006), and one was a case report study (Blum 2002). Of the 12 protocols excluded, three records were for studies already included in this review (Marshall 2013; Miyamoto 2013; Wajswelner 2012) and eight were ineligible (NCT01533805;PACTR201211000443397;RBR‐7tyg5j; ACTRN12607000471482; NCT01711203; NCT01919268; NCT01999283; ACTRN12609000927224), and for one report we did not find any publicly available report (RBR‐7yhzym) and the authors did not reply to our email contact attempts.
Risk of bias in included studies
The results from the 'Risk of bias' assessment for the individual studies are summarised in Figure 2. In total, we considered 70% of the studies to have a low risk of bias, which represents 83.7% of all participants.
2.
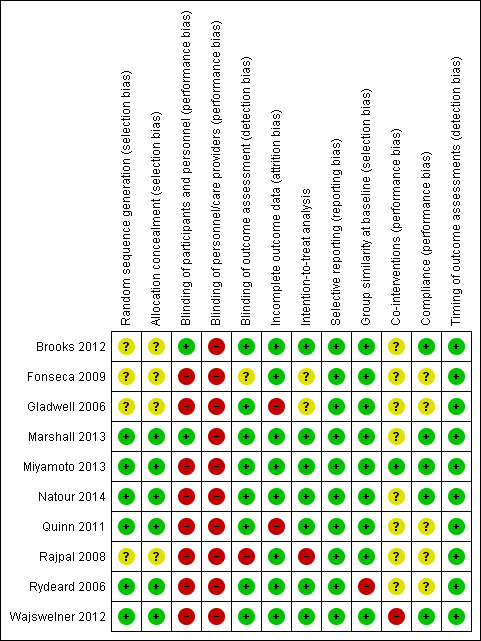
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
More than half of the included trials met the criteria for allocation (Marshall 2013; Miyamoto 2013; Natour 2014; Quinn 2011; Rydeard 2006; Wajswelner 2012). In four trials there was no information about the randomisation and allocation procedures (Brooks 2012; Fonseca 2009; Gladwell 2006; Rajpal 2008).
Blinding
One trial blinded both participants and assessors (Brooks 2012). One trial blinded only the participants (Marshall 2013), and seven trials blinded only the assessor (Gladwell 2006; Miyamoto 2013; Natour 2014; Quinn 2011; Rydeard 2006; Wajswelner 2012). In one trial the information about blinding was unclear (Fonseca 2009), and one trial did not blind both the assessor and patients (Rajpal 2008). Presumably, blinding of therapists was not possible for the intervention proposed.
Incomplete outcome data
A total of eight trials provided adequate information about missing data and were able to keep these below 20% for short and intermediate‐term outcomes, though none of the trials report long‐term follow‐up (Brooks 2012; Fonseca 2009; Marshall 2013; Miyamoto 2013; Natour 2014; Rajpal 2008; Rydeard 2006; Wajswelner 2012). Two trials exceeded the maximum of 20% withdrawals, with about 30% for both trials (Gladwell 2006; Quinn 2011).
Selective reporting
Published or registered protocols were available for four trials (Marshall 2013; Miyamoto 2013; Natour 2014; Wajswelner 2012); all were registered at the Australian and New Zealand Clinical Trials Registry. For one trial the protocol was also published (Miyamoto 2013). Trials for which it was not possible to find the protocols, but it was clear that all expected outcomes were included or were reported in a pre‐specified way, fulfilled this criterion. We considered all included studies at low risk of bias for this criterion.
Other potential sources of bias
Publication bias
We did not assess publication bias with funnel plots because too few studies were included in the meta‐analysis.
Effects of interventions
for the main comparison.
| Pilates compared with minimal intervention for low back pain | ||||||
|
Patient or population: patients with low back pain Settings: primary or tertiary care Intervention: Pilates Comparison: minimal intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Minimal intervention | Pilates | |||||
|
Pain NRS: scale from 0 to 100 (worse pain) Follow‐up: short‐term (less than 3 months from randomisation) |
The mean pain at short‐term follow‐up ranged across control groups from 33.9 to 52 points |
The mean pain at short‐term follow‐up in the intervention groups was
14.05 lower (18.9 to 9.2 lower) |
Mean difference ‐14.05 (‐18.91 to ‐9.19) | 265 participants (6 studies) | ⊕⊕⊝⊝ low1,2 | This is a moderate effect that is clinically relevant in this patient group |
|
Pain NRS: scale from 0 to 100 (worse pain) Follow‐up: intermediate‐term (more than 3 months and less than 12 months) |
The mean pain at intermediate‐term follow‐up ranged across control groups from 53 to 58.3 points |
The mean pain at intermediate‐term follow‐up in the intervention group was 10.5 lower (18.5 to 2.6 lower) |
Mean difference ‐10.54 (‐18.46 to ‐2.62) | 146 participants (2 studies) |
⊕⊕⊕⊝ moderate1 | This is a moderate effect that is clinically relevant in this patient group |
|
Disability Multiple scales: scale from 0 to 100 (worse disability) Follow‐up: short‐term (less than 3 months from randomisation) |
The mean disability at short‐term follow‐up ranged across control groups from 13.3 to 44.1 points |
The mean disability at short‐term follow‐up in the intervention groups was 7.95 lower (13.2 to 2.7 lower) |
Mean difference ‐7.95 (‐13.23 to ‐2.67) | 248 participants (5 studies) |
⊕⊕⊝⊝ low1,4 | This is a small effect that may be clinically relevant in this patient group |
|
Disability Multiple scales: scale from 0 to 100 (worse disability) Follow‐up: intermediate‐term (more than 3 months and less than 12 months) |
The mean disability at intermediate‐term follow‐up ranged across control groups from 27.9 to 44.4 points |
The mean disability at intermediate‐term follow‐up in the intervention groups was
11.2lower (18.4 to 3.9 lower) |
Mean difference ‐11.17 (‐18.41 to ‐3.92) | 146 participants (2 study) |
⊕⊕⊕⊝ moderate1 | This is a moderate effect that is clinically relevant in this patient group |
|
Function Patient Specific Functional Scale: used in a 11‐point scale from 0 to 10 (greater functional ability) Follow‐up: short‐term (less than 3 months from randomisation) |
The mean function at short‐term follow‐up in the control group was 6.4 points |
The mean function at short‐term follow‐up in the intervention group was 1.1 higher (0.2 to 2.0 higher) |
Mean difference 1.10 (0.23 to 1.97) | 86 participants (1 study) |
⊕⊕⊝⊝ low1,3 | This is a small effect that may be clinically relevant in this patient group (results from 1 single study) |
|
Function Patient Specific Functional Scale: used in a 11‐point scale from 0 to 10 (greater functional ability) Follow‐up: intermediate‐term (more than 3 months and less than 12 months) |
The mean function at intermediate‐term follow‐up in the control group was 6.1 points |
The mean function at intermediate‐term follow‐up in the intervention group was 0.8 higher (0.0 lower to 1.6 higher) |
Mean difference 0.80 (‐0.00 to 1.60) | 86 participants (1 study) |
⊕⊕⊝⊝ low1,3 | The difference is not statistically or clinically significant (results from 1 single study) |
|
Global impression of recovery Global Perceived Effect Scale: scale from ‐5 to +5 (greater recovery) Follow‐up: short‐term (less than 3 months from randomisation) |
The mean global impression of recovery at short‐term follow‐up in the control group was 1.7 points |
The mean global impression of recovery at short‐term follow‐up in the intervention group was 1.5 higher (0.7 to 2.3 higher) |
Mean difference 1.50 (0.70 to 2.30) | 86 participants (1 study) |
⊕⊕⊝⊝ low1,3 | This is a small effect that may be clinically relevant in this patient group (results from 1 single study) |
|
Global impression of recovery Global Perceived Effect Scale: scale from ‐5 to +5 (greater recovery) Follow‐up: intermediate‐term (more than 3 months and less than 12 months) |
The mean global impression of recovery at intermediate‐term follow‐up in the control group was 1.7 points |
The mean global impression of recovery at intermediate‐term follow‐up in the intervention group was 0.7 higher (0.1 lower to 1.5 higher) |
Mean difference 0.70 (‐0.11 to 1.51) | 86 participants (1 study) |
⊕⊕⊝⊝ low1,3 | The difference is not statistically or clinically significant (results from 1 single study) |
| Adverse events | See comment | See comment | Not estimable | See comment | Only 1 included trial assessed adverse events and none were reported | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one level due to imprecision (fewer than 400 participants, total).
2 Downgraded one level due to risk of bias (> 25% of the participants were from studies with a high risk of bias).
3Downgraded one level due to clear inconsistency of results.
4Downgraded one level due to inconsistency (I² > 50%).
2.
| Pilates compared with other exercises for low back pain | ||||||
|
Patient or population: participants with low back pain Settings: primary and tertiary care Intervention: Pilates Comparison: other exercises | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other exercises | Pilates | |||||
|
Pain NRS: scale from 0 to 100 (worse pain) Follow‐up: short‐term (less than 3 months from randomisation) |
Not estimated | Not estimated | Not estimated | 181 participants (3 studies) |
⊕⊕⊝⊝ low1,2 | Pooled results not estimated due to high heterogeneity |
|
Pain NRS: scale from 0 to 100 (worse pain) Follow‐up: intermediate‐term (more than 3 months and less than 12 months) |
Not estimated | Not estimated | Not estimated | 151 participants (2 studies) |
⊕⊕⊝⊝ low1,2 | Pooled results not estimated due to high heterogeneity |
|
Disability Multiple scales: scale from 0 to 100 (worse disability) Follow‐up: short‐term (less than 3 months from randomisation) |
The mean disability at short‐term follow‐up ranged across control groups from 17.1 to 20 points |
The mean disability at short‐term follow‐up in the intervention groups was
3.3 lower (6.8 lower to 0.2 higher) |
Mean difference ‐3.29 (‐6.82 to 0.24) | 149 participants (2 studies) |
⊕⊕⊕⊝ moderate1 | The difference is not statistically or clinically significant |
|
Disability Multiple scales: scale from 0 to 100 (worse disability) Follow‐up: intermediate‐term (more than 3 months and less than 12 months) |
The mean disability at intermediate‐term follow‐up ranged across control groups from 13 to 18.1 points |
The mean disability at intermediate‐term follow‐up in the intervention groups was
0.9 lower (5.0 lower to 3.2 higher) |
Mean difference ‐0.91 (‐5.02 to 3.20) | 151 participants (2 studies) |
⊕⊕⊕⊝ moderate1 | The difference is not statistically or clinically significant |
|
Function Patient Specific Functional Scale: scale from 0 to 30 (greater functional ability) Follow‐up: short‐term (less than 3 months from randomisation) |
The mean function at short‐term follow‐up in the control group was 18.9 points |
The mean function at short‐term follow‐up in the intervention group was 0.1 lower (2.4 lower to 2.6 higher) |
Mean difference 0.10 (‐2.44 to 2.64) | 87 participants (1 study) |
⊕⊕⊝⊝ low1,3 | The difference is not statistically or clinically significant (results from 1 single study) |
|
Function Patient Specific Functional Scale: scale from 0 to 30 (greater functional ability) Follow‐up: intermediate‐term (more than 3 months and less than 12 months) |
The mean function at intermediate‐term follow‐up in the control group was 22.8 points |
The mean function at intermediate‐term follow‐up in the intervention group was 3.6 lower (7 to 0.2 lower) |
Mean difference ‐3.60 (‐7.00 to ‐0.20) | 87 participants (1 study) |
⊕⊕⊝⊝ low1,3 | This is a small effect that may be clinically relevant in this patient group (results from 1 single study) |
| Adverse events | See comment | See comment | Not estimable | See comment | 1 trial assessed adverse events and reported minor events | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one level due to imprecision (fewer than 400 participants, total).
2Downgraded one level due to inconsistency (I² > 50%).
3Downgraded one level due to clear inconsistency of results.
See: Table 1 for the effect of Pilates versus minimal intervention, and Table 2 for the effect of Pilates versus other exercises.
Effect of Pilates versus minimal intervention
We included a total of six trials in the meta‐analysis (Fonseca 2009; Gladwell 2006; Miyamoto 2013; Natour 2014; Quinn 2011; Rydeard 2006); four trials with low risk of bias (Miyamoto 2013; Natour 2014; Quinn 2011; Rydeard 2006) and two with high risk of bias (Fonseca 2009; Gladwell 2006). Most of the trials included in the comparison of Pilates with minimal intervention had small sample sizes (ranging from 17 to 86 participants).
Primary outcomes
For pain intensity, based on six trials, there is low quality evidence (downgraded due to imprecision and risk of bias) that Pilates reduces pain compared with minimal intervention at short‐term follow‐up, with a medium effect size (mean difference (MD) –14.05, 95% confidence interval (CI) –18.91 to –9.19; P value < 0.001) (Analysis 1.1). At intermediate‐term follow‐up, two trials, Miyamoto 2013 and Natour 2014, provided moderate quality evidence (downgraded due to imprecision) that Pilates reduces pain compared with minimal intervention, with a medium effect size (MD –10.54, 95% CI –18.54 to –2.62) (Analysis 1.1; Figure 3).
1.1. Analysis.
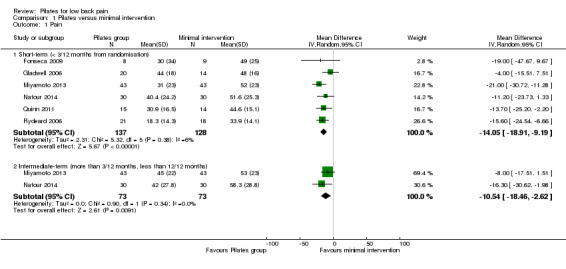
Comparison 1 Pilates versus minimal intervention, Outcome 1 Pain.
3.
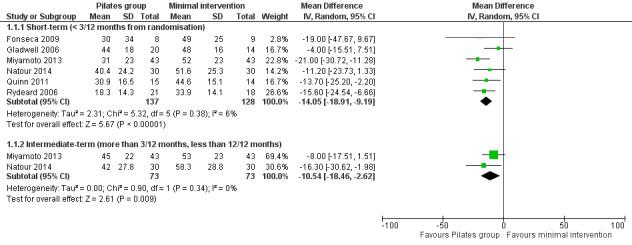
Forest plot of comparison: 1 Pilates versus minimal intervention, outcome: 1.1 Pain.
We considered disability for the meta‐analysis as we did not find considerable heterogeneity (I2 = 56%), but we downgraded the quality of the evidence due to inconsistency (Borenstein 2009; Higgins 2011). Based on five trials, there is low quality evidence (downgraded due to imprecision and inconsistency) that Pilates improves disability at short‐term follow‐up compared with minimal intervention, with a small effect size (MD –7.95, 95% CI –13.23 to –2.67; P value = 0.003) (Analysis 1.2). At intermediate‐term follow‐up, two trials, Miyamoto 2013 and Natour 2014, provided moderate quality evidence (downgraded due to imprecision) of a significant effect in favour of Pilates, with a medium effect size (MD –11.17, 95% CI –18.41 to –3.92) (Analysis 1.2).
1.2. Analysis.
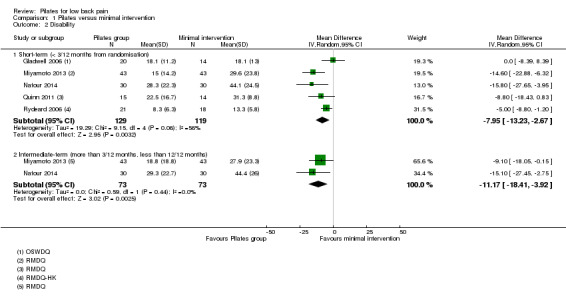
Comparison 1 Pilates versus minimal intervention, Outcome 2 Disability.
Based on one trial, Miyamoto 2013, and low quality evidence (downgraded due to imprecision and inconsistency), we found a significant short‐term effect, with a small effect size for global impression of recovery (MD 1.50, 95% CI 0.70 to 2.30) (Analysis 1.4), but not for intermediate‐term follow‐up. One trial (Natour 2014) evaluated quality of life but the estimates for the physical and mental components were not available in the publication and the authors did not provide this information on request.
1.4. Analysis.
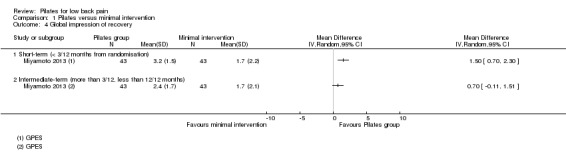
Comparison 1 Pilates versus minimal intervention, Outcome 4 Global impression of recovery.
Secondary outcomes
Only one trial assessed adverse events, but none were reported (Miyamoto 2013). None of the included trials evaluated return to work.
Other outcomes
Based on one trial, Miyamoto 2013, and low quality evidence (downgraded due to imprecision and inconsistency), there is a significant short‐term effect, with a small effect size, for function (MD 1.10, 95% CI 0.23 to 1.97) (Analysis 1.3). No differences was found for an intermediate‐term follow‐up.
1.3. Analysis.
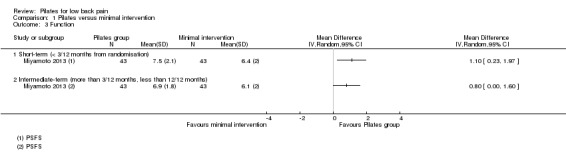
Comparison 1 Pilates versus minimal intervention, Outcome 3 Function.
Effect of Pilates versus other exercises
Four trials were included in this comparison (Brooks 2012; Marshall 2013; Rajpal 2008; Wajswelner 2012), and three were included in the meta‐analysis (Brooks 2012; Marshall 2013; Wajswelner 2012). Two trials compared Pilates with general exercise (Brooks 2012; Wajswelner 2012), and one trial compared Pilates with stationary cycling exercise (Marshall 2013). Most of the trials included in the comparisons between Pilates and other exercises had small sample sizes (ranging from 32 to 87 participants).
Primary outcomes
Due to the high level of heterogeneity, we did not combine the results for pain intensity at short‐term and intermediate‐term follow‐up in a meta‐analysis (I2 = 74% for short‐term and I2 = 86% for intermediate‐term follow‐up), but we report these descriptively. For pain intensity, based on low quality evidence (downgraded due to imprecision and inconsistency), at short‐term follow‐up two trials demonstrated significant effect in favour of Pilates (Brooks 2012; Rajpal 2008), and one trial did not find significant difference (Wajswelner 2012). At intermediate‐term follow‐up, based on low quality evidence (downgraded due to imprecision and inconsistency), one trial reported a significant effect in favour of Pilates (Marshall 2013), and one trial reported a non‐significant difference in pain intensity (Wajswelner 2012).
In the meta‐analysis for disability there is moderate quality evidence (downgraded due to imprecision) that there is no significant difference between Pilates and other exercise at short‐term (MD –3.29, 95% CI –6.82 to 0.24) or intermediate‐term follow‐up (MD –0.91, 95% CI –5.02 to 3.20), based on two studies for each comparison (Analysis 2.2; Figure 4). One trial (Wajswelner 2012) evaluated quality of life but the estimates for the physical and mental components were not available in the publication and the authors did not provide this information on request.
2.2. Analysis.
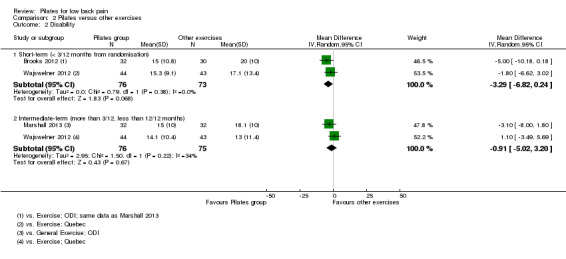
Comparison 2 Pilates versus other exercises, Outcome 2 Disability.
4.
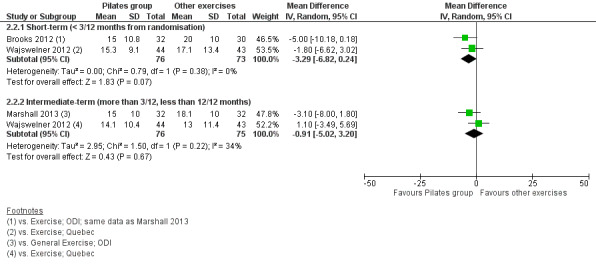
Forest plot of comparison: 2 Pilates versus other exercises, outcome: 2.2 Disability.
Secondary outcomes
One trial reported minor adverse events in both groups (Wajswelner 2012). In the Pilates group two participants reported minor shoulder pain and one reported knee pain, but they were all able to continue the exercises. In the general exercise group, two participants reported back spasms but were able to continue the programme, and two reported worsening back pain causing them to cease the exercise. None of the included trials evaluated return to work.
Other outcomes
Based on low quality evidence (downgraded due to imprecision and inconsistency) from one trial (Wajswelner 2012), there was no significant difference between Pilates and other exercises in function at short‐term follow‐up (MD 0.10, 95% CI ‐2.44 to 2.64). However, there was a significant effect in favour of other exercises (general exercise) in intermediate‐term function, with a small effect size (MD ‐3.60, 95% CI ‐7.00 to ‐0.20) (Analysis 2.3).
2.3. Analysis.
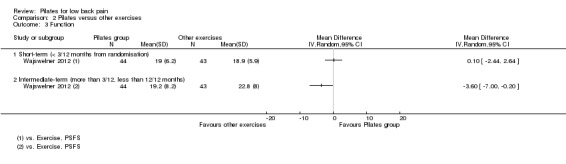
Comparison 2 Pilates versus other exercises, Outcome 3 Function.
Discussion
Summary of main results
The evidence on the effectiveness of Pilates for chronic non‐specific low back pain (LBP) is of low to moderate quality primarily because there are only a few small studies (range 17 to 87 participants). Of the 15 estimates of treatment effect we provide in this review, only eight are based on more than one study. None of the trials reported long‐term outcomes, which would be important to consider for patients with chronic LBP. In addition, we did not find any studies that investigated the effectiveness of Pilates for acute and subacute LBP.
A total of six trials (n = 265 participants) compared Pilates to minimal intervention. At short‐term follow‐up Pilates is more effective than minimal intervention for improvement in pain intensity, disability, function and global impression of recovery. At intermediate‐term follow‐up Pilates led to better pain intensity and disability outcomes, but was not superior to minimal intervention in terms of function and global impression of recovery. The effect sizes varied from small to medium for this comparison.
Four trials (n = 245 participants) compared Pilates to other exercises. Pilates appears not to be more effective than other exercises for pain intensity and disability outcomes. For function, one study found a small significant effect at intermediate‐term, but not at short‐term follow‐up.
Pilates appears to be an effective treatment compared to minimal intervention, but when compared to other types of exercises the effect sizes tend to be smaller or no difference in effectiveness is observed. This is in accordance with clinical practice guidelines (European Guidelines 2006) and previous reviews of exercise for low back pain (Hayden 2005), which recommend exercise therapy for patients with low back pain but note that there seems to be no clear difference in effectiveness between the various forms of exercise. We did not find any studies that reported return to work. Only two trials assessed adverse events: one trial found minor adverse effects in the Pilates group (shoulder and knee pain) (Wajswelner 2012); another trial did not find any adverse events (Miyamoto 2013).
Overall completeness and applicability of evidence
The trials included in this review were conducted in Australia, South America, Europe or Asia, with adult participants from primary or tertiary care with non‐specific low back pain for at least 12 weeks in most trials. The care providers were all experienced instructors or physiotherapists trained in the Pilates method, except for one trial that did not provide information about the care provider (Rajpal 2008). Therefore, we can generalise the results of this review to a range of settings. Regarding our clinical relevance assessment, most trials included provided a clear description of the patients, outcomes and interventions used. However, none of the trials found a clinically important effect size for pain intensity and only three reported a clinically important effect for disability (Miyamoto 2013; Natour 2014; Quinn 2011).
Quality of the evidence
In general, most included trials demonstrated a low risk of bias (427 participants were from studies with low risk of bias out of 510 participants in total). The most affected items were blinding of participants and care providers, which is understandable for exercise intervention trials. However, only 10 trials could be included in this review, which compromises the quality of the evidence provided. Also, the sample sizes in general were small (ranging from 17 to 87 participants); therefore, our results cannot be considered robust.
Potential biases in the review process
The main limitation of this review is the low number of trials and small sample sizes per comparison, outcome and follow‐up period, which prevented us from conducting a sensitivity analyses. An additional limitation is the potential for publication bias in the trials included. In this review, it was not possible to assess publication bias using funnel plots as too few studies were included. However, by inspecting registries we found one completed trial (from 2011) that was not yet published, which may indicate potential publication bias. Moreover, the source of funding should be considered due to potential financial conflicts from industry‐sponsored research (Bekelman 2003; Okike 2008). One trial received funding from a Pilates clinic to conduct the study (Wajswelner 2012). The remaining trials were not funded.
Finally, we found eight conference abstracts and for these we could not find a full publication. They were therefore not included in the analysis. We also did not include two theses. As unpublished studies are more likely to report negative findings, it is possible that the review's conclusions are overly optimistic.
Agreements and disagreements with other studies or reviews
In general, the results of this review are reasonably consistent with previous reviews regarding pain intensity and disability outcomes (Lim 2011; Miyamoto 2013; Wells 2014). In the most recent review, the authors reported a statistically significant short‐term effect for pain intensity and disability compared to usual care and/or physical activity (Wells 2014). For the comparison with other forms of exercises, the results were conflicting. The results of this review are partially consistent with our findings. The key limitation of this review is that the authors did not perform a meta‐analysis, limiting the comparison with our review.
In the 2013 review of Miyamoto et al the authors found a small short‐term effect on pain intensity and disability when compared to minimal intervention but not compared to other types of exercises (Miyamoto 2013). This is consistent with our review although we mostly found medium effect sizes for the comparison with minimal intervention and we considered the results for pain intensity compared to other exercises too heterogeneous to be combined in a meta‐analysis. The 2011 review of Lim et al only found a small significant effect on pain intensity in the short term compared to minimal intervention but not on disability (Lim 2011). This previous review did not find any significant effect for the comparison with other exercises; however, the authors included only one trial (Donzelli 2006) and one thesis (Gagnon 2005) in this comparison. Another systematic review concluded that no definite conclusions can be drawn except that further better quality research is needed (Posadzki 2011). The authors only had four trials available in their review, each one with a different control group, making any comparison or conclusions difficult.
Authors' conclusions
Implications for practice.
No definite conclusions or recommendations can be made as we did not find any high quality evidence for any of the treatment comparisons, outcomes or follow‐up periods investigated. However, there is low to moderate quality evidence that Pilates is more effective than minimal intervention in the short and intermediate term as the benefits were consistent for pain intensity and disability, with most of the effect sizes being considered medium. It was less clear whether Pilates was more effective than other exercises for pain intensity, disability and function as the results across outcomes were contradictory. However, a small effect favouring other exercises was found for function at intermediate‐term follow‐up. The decision to use Pilates for chronic low back pain may be based on the patient's or care provider's preferences, and costs.
Implications for research.
There is an urgent need for large, high quality trials to evaluate Pilates for low back pain. Most trials included fewer than 40 participants in total (Fonseca 2009; Gladwell 2006; Quinn 2011; Rajpal 2008; Rydeard 2006), or were unregistered (Brooks 2012; Fonseca 2009; Gladwell 2006; Quinn 2011; Rajpal 2008; Rydeard 2006). None of the trials included long‐term follow‐up. In addition, including an economic evaluation alongside a clinical trial of the Pilates method would be useful to guide clinical choices between competing treatment options. There is one study in the awaiting classification section for the next update of this review, which will contribute to the results of this review in the future.
Acknowledgements
Tiê Parma Yamato is supported by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), Brazil. Chris Maher is supported by National Health and Medical Research Council of Australia. Bruno Tirotti Saragiotto is supported by CNPQ (Conselho Nacional de Desenvolvimento Científico e Tecnológico), Brazil.
Appendices
Appendix 1. CENTRAL search strategy
Last searched 24 March 2014
#1 MeSH descriptor: [Back Pain] explode all trees
#2 dorsalgia
#3 backache
#4 lumbar next pain OR coccyx OR coccydynia OR sciatica OR spondylosis
#5 MeSH descriptor: [Sciatica] explode all trees
#6 MeSH descriptor: [Spine] explode all trees
#7 MeSH descriptor: [Spinal Diseases] explode all trees
#8 lumbago OR discitis OR disc near degeneration OR disc near prolapse OR disc near herniation
#9 spinal fusion
#10 spinal neoplasms
#11 facet near joints
#12 MeSH descriptor: [Intervertebral Disk] explode all trees
#13 postlaminectomy
#14 arachnoiditis
#15 failed near back
#16 MeSH descriptor: [Cauda Equina] explode all trees
#17 lumbar near vertebra*
#18 spinal near stenosis
#19 slipped near (disc* or disk*)
#20 degenerat* near (disc* or disk*)
#21 stenosis near (spine or root or spinal)
#22 displace* near (disc* or disk*)
#23 prolap* near (disc* or disk*)
#24 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23
#25 Pilates (Word variations have been searched)
#26 MeSH descriptor: [Exercise Movement Techniques] explode all trees
#27 #25 or #26
#28 #24 and #27 in Trials
Appendix 2. MEDLINE search strategy
Last searched 25 March 2014
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab,ti.
drug therapy.fs.
randomly.ab,ti.
trial.ab,ti.
groups.ab,ti.
or/1‐8 (3329089)
(animals not (humans and animals)).sh.
9 not 10
dorsalgia.ti,ab.
exp Back Pain/
backache.ti,ab.
(lumbar adj pain).ti,ab.
coccyx.ti,ab.
coccydynia.ti,ab.
sciatica.ti,ab.
exp sciatic neuropathy/
spondylosis.ti,ab.
lumbago.ti,ab.
back disorder$.ti,ab.
(disc adj degeneration).ti,ab.
(disc adj prolapse).ti,ab.
(disc adj herniation).ti,ab.
(failed adj back).ti,ab.
or/12‐26
11 and 27
Exercise Movement Techniques/
pilates.mp.
29 or 30
28 and 31
Appendix 3. EMBASE search strategy
Last searched 24 March 2014; in the previous search March 2013, line 31 read 14 and 30
Clinical Article/
exp Clinical Study/
Clinical Trial/
Controlled Study/
Randomized Controlled Trial/
Major Clinical Study/
Double Blind Procedure/
Multicenter Study/
Single Blind Procedure/
Phase 3 Clinical Trial/
Phase 4 Clinical Trial/
crossover procedure/
placebo/
or/1‐13
allocat$.mp.
assign$.mp.
blind$.mp.
(clinic$ adj25 (study or trial)).mp.
compar$.mp.
control$.mp.
cross?over.mp.
factorial$.mp.
follow?up.mp.
placebo$.mp.
prospectiv$.mp.
random$.mp.
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp.
trial.mp.
(versus or vs).mp.
or/15‐29
14 or 30
exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
human/ or normal human/ or human cell/
32 and 33
32 not 34
31 not 35
dorsalgia.mp.
back pain.mp.
exp BACKACHE/
(lumbar adj pain).mp.
coccyx.mp.
coccydynia.mp.
sciatica.mp.
exp ISCHIALGIA/
spondylosis.mp.
lumbago.mp.
back disorder$.ti,ab.
or/37‐47
36 and 48
pilates/
pilates.mp.
50 or 51
49 and 52
Appendix 4. CINAHL search strategy
Last searched 25 March 2014; line S49 "pilates" was added
S51 S28 AND S47 AND S50
S50 S48 OR S49
S49 "pilates"
S48 (MH "Pilates")
S47 S34 or S42 or S46
S46 S43 or S44 or S45
S45 lumbago
S44 (MH "Spondylolisthesis") OR (MH "Spondylolysis")
S43 (MH "Thoracic Vertebrae")
S42 S35 or S36 or S37 or S38 or S39 or S40 or S41
S41 lumbar N2 vertebra
S40 (MH "Lumbar Vertebrae")
S39 coccydynia OR back disorder*
S38 "coccyx"
S37 sciatica
S36 (MH "Sciatica")
S35 (MH "Coccyx")
S34 S29 or S30 or S31 or S32 or S33
S33 lumbar N5 pain
S32 lumbar W1 pain
S31 backache
S30 (MH "Back Pain+")
S29 "dorsalgia"
S28 S26 NOT S27
S27 (MH "Animals")
S26 S7 or S12 or S19 or S25
S25 S20 or S21 or S22 or S23 or S24
S24 volunteer*
S23 prospectiv*
S22 control*
S21 followup stud*
S20 follow‐up stud*
S19 S13 or S14 or S15 or S16 or S17 or S18
S18 (MH "Prospective Studies+")
S17 (MH "Evaluation Research+")
S16 (MH "Comparative Studies")
S15 "latin square"
S14 (MH "Study Design+")
S13 (MH "Random Sample+")
S12 S8 or S9 or S10 or S11
S11 random*
S10 "placebo*"
S9 (MH "Placebos")
S8 (MH "Placebo Effect")
S7 S1 or S2 or S3 or S4 or S5 or S6
S6 triple‐blind
S5 single‐blind
S4 double‐blind
S3 clinical W3 trial
S2 "randomi?ed controlled trial*"
S1 (MH "Clinical Trials+")
Appendix 5. PEDro search strategy
Last searched 25 March 2014
Abstract and Title: pilates and back pain
Previous search 21 March 2013
Abstract and Title: Pilates AND Body Part: Lumbar spine, sacroiliac joint or pelvis AND Method: Clinical trial
Appendix 6. SPORTDiscus search strategy
Last searched 24 March 2014
S21 S10 AND S17 AND S20
S20 S18 OR S19
S19 DE "PILATES method"
S18 TX pilates
S17 S11 OR S12 OR S13 OR S14 OR S15 OR S16
S16 DE "LUMBAR vertebrae" OR DE "LUMBOSACRAL region"
S15 DE "SCIATICA"
S14 TX backache
S13 TX sciatica
S12 TX low back pain
S11 DE "BACKACHE"
S10 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9
S9 TX single blind
S8 TX random allocation
S7 SU randomized controlled trial
S6 SU clinical trials
S5 TX clinical trials
S4 TX placebo
S3 TX controlled clinical trial
S2 TX double blind
S1 TX randomi?ed controlled trial
Appendix 7. ClinicalTrials.gov and WHO ICTRP search strategy
Last searched 25 March 2014
Basic search: pilates and back pain
Previous search 21 March 2013
ClinicalTrials.gov: Search: Pilates AND Condition: back pain
WHO ICTRP: Title: Pilates AND Condition: back pain
Appendix 8. Data extraction forms
Reviewer: ____________________________________________________________
First author: ____________________________________________________
Year: __________________________________________________________
Citation (journal, volume, pages): ___________________________________
Eligibility: (tick the relevant box)
| Criterion | Yes | No | Uncertain |
| RCT | |||
| Non‐specific low back pain (LBP) | |||
| At least one relevant outcome measure | |||
| Pilates intervention |
Description of interventions in each group
(# of treatment session, session duration, program duration, co‐interventions)
1. _______________________________________________________________________________
___________________________________________________________________________________
2. _______________________________________________________________________________
___________________________________________________________________________________
3. _______________________________________________________________________________
___________________________________________________________________________________
4. _______________________________________________________________________________
____________________________________________________________________________________
Details of the included randomised controlled trials
| Authors (year) | Patients | Interventions | Duration of Pilates intervention | Outcomes | Risk of Bias score |
Continuous outcomes
| Study: |
# 1 ‐ Pilates | # 2‐ | # 3‐ | ||||||
| Mean | SD | N | Mean | SD | N | Mean | SD | N | |
| Outcome #1 |
|||||||||
| Baseline |
|||||||||
| Short term (< 3/12 from randomisation) |
|||||||||
| Intermediate (greater than 3/12, less than 12/12) |
|||||||||
| Long term (greater than 12/12) |
|||||||||
| Outcome #2 |
|||||||||
| Baseline |
|||||||||
| Short‐term (< 3/12 from randomisation) |
|||||||||
| Intermediate‐term (greater than 3/12, less than 12/12) |
|||||||||
| Long‐term (greater than 12/12) |
|||||||||
| Outcome #3 |
|||||||||
| Baseline |
|||||||||
| Short‐term (< 3/12 from randomisation) |
|||||||||
| Intermediate‐term (greater than 3/12, less than 12/12) |
|||||||||
| Long‐term (greater than 12/12) |
|
||||||||
Appendix 9. Criteria for assessing risk of bias for internal validity (Higgins 2011)
Random sequence generation (selection bias)
Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence
There is a low risk of selection bias if the investigators describe a random component in the sequence generation process such as: referring to a random number table, using a computer random number generator, coin tossing, shuffling cards or envelopes, throwing dice, drawing of lots, minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random).
There is a high risk of selection bias if the investigators describe a non‐random component in the sequence generation process, such as: sequence generated by odd or even date of birth, date (or day) of admission, hospital or clinic record number; or allocation by judgement of the clinician, preference of the participant, results of a laboratory test or a series of tests, or availability of the intervention.
Allocation concealment (selection bias)
Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment
There is a low risk of selection bias if the participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; or sequentially numbered, opaque, sealed envelopes.
There is a high risk of bias if participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; or other explicitly unconcealed procedures.
Blinding of participants
Performance bias due to knowledge of the allocated interventions by participants during the study
There is a low risk of performance bias if blinding of participants was ensured and it was unlikely that the blinding could have been broken; or if there was no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding.
Blinding of personnel/care providers (performance bias)
Performance bias due to knowledge of the allocated interventions by personnel/care providers during the study
There is a low risk of performance bias if blinding of personnel was ensured and it was unlikely that the blinding could have been broken; or if there was no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding.
Blinding of outcome assessor (detection bias)
Detection bias due to knowledge of the allocated interventions by outcome assessors
There is low risk of detection bias if the blinding of the outcome assessment was ensured and it was unlikely that the blinding could have been broken; or if there was no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding, or:
for patient‐reported outcomes in which the patient was the outcome assessor (e.g. pain, disability): there is a low risk of bias for outcome assessors if there is a low risk of bias for participant blinding (Boutron 2005);
for outcome criteria that are clinical or therapeutic events that will be determined by the interaction between patients and care providers (e.g. co‐interventions, length of hospitalisation, treatment failure), in which the care provider is the outcome assessor: there is a low risk of bias for outcome assessors if there is a low risk of bias for care providers (Boutron 2005);
for outcome criteria that are assessed from data from medical forms: there is a low risk of bias if the treatment or adverse effects of the treatment could not be noticed in the extracted data (Boutron 2005).
Incomplete outcome data (attrition bias)
Attrition bias due to amount, nature or handling of incomplete outcome data
There is a low risk of attrition bias if there were no missing outcome data; reasons for missing outcome data were unlikely to be related to the true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data were balanced in numbers, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, the plausible effect size (difference in means or standardised difference in means) among missing outcomes was not enough to have a clinically relevant impact on observed effect size, or missing data were imputed using appropriate methods (if dropouts are very large, imputation using even "acceptable" methods may still suggest a high risk of bias) (van Tulder 2003). The percentage of withdrawals and dropouts should not exceed 20% for short‐term follow‐up and 30% for long‐term follow‐up and should not lead to substantial bias (these percentages are commonly used but arbitrary, not supported by literature) (van Tulder 2003).
Selective reporting (reporting bias)
Reporting bias due to selective outcome reporting
There is low risk of reporting bias if the study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way, or if the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon).
There is a high risk of reporting bias if not all of the study's pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Group similarity at baseline (selection bias)
Bias due to dissimilarity at baseline for the most important prognostic indicators.
There is low risk of bias if groups are similar at baseline for demographic factors, value of main outcome measure(s), and important prognostic factors (examples in the field of back and neck pain are duration and severity of complaints, vocational status, percentage of patients with neurological symptoms) (van Tulder 2003).
Co‐interventions (performance bias)
Bias because co‐interventions were different across groups
There is low risk of bias if there were no co‐interventions or they were similar between the index and control groups (van Tulder 2003).
Compliance (performance bias)
Bias due to inappropriate compliance with interventions across groups
There is low risk of bias if compliance with the interventions was acceptable, based on the reported intensity/dosage, duration, number and frequency for both the index and control intervention(s). For single‐session interventions (e.g. surgery), this item is irrelevant (van Tulder 2003).
Intention‐to‐treat‐analysis
There is low risk of bias if all randomised patients were reported/analysed in the group to which they were allocated by randomisation.
Timing of outcome assessments (detection bias)
Bias because important outcomes were not measured at the same time across groups
There is low risk of bias if all important outcome assessments for all intervention groups were measured at the same time (van Tulder 2003).
Other bias
Bias due to problems not covered elsewhere in the table
There is a low risk of bias if the study appears to be free of other sources of bias not addressed elsewhere (e.g. study funding).
Appendix 10. Assessing the clinical relevance
Are the patients described in detail so that you can decide whether they are comparable to those that you see in your practice?
Are the interventions and treatment settings described well enough so that you can provide the same for your patients?
Were all clinically relevant outcomes measured and reported?
Is the size of the effect clinically important?
Are the likely treatment benefits worth the potential harms?
Data and analyses
Comparison 1. Pilates versus minimal intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Short‐term (< 3/12 months from randomisation) | 6 | 265 | Mean Difference (IV, Random, 95% CI) | ‐14.05 [‐18.91, ‐9.19] |
| 1.2 Intermediate‐term (more than 3/12 months, less than 12/12 months) | 2 | 146 | Mean Difference (IV, Random, 95% CI) | ‐10.54 [‐18.46, ‐2.62] |
| 2 Disability | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Short‐term (< 3/12 months from randomisation) | 5 | 248 | Mean Difference (IV, Random, 95% CI) | ‐7.95 [‐13.23, ‐2.67] |
| 2.2 Intermediate‐term (more than 3/12 months, less than 12/12 months) | 2 | 146 | Mean Difference (IV, Random, 95% CI) | ‐11.17 [‐18.41, ‐3.92] |
| 3 Function | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Short‐term (< 3/12 months from randomisation) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Intermediate‐term (more than 3/12 months, less than 12/12 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Global impression of recovery | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Short‐term (< 3/12 months from randomisation) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Intermediate‐term (more than 3/12, less than 12/12 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Pilates versus other exercises.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Short‐term (< 3/12 months from randomisation) | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Intermediate‐term (more than 3/12, less than 12/12 months) | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Disability | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Short‐term (< 3/12 months from randomisation) | 2 | 149 | Mean Difference (IV, Random, 95% CI) | ‐3.29 [‐6.82, 0.24] |
| 2.2 Intermediate‐term (more than 3/12, less than 12/12 months) | 2 | 151 | Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐5.02, 3.20] |
| 3 Function | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Short‐term (< 3/12 months from randomisation) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Intermediate‐term (more than 3/12, less than 12/12 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
Comparison 2 Pilates versus other exercises, Outcome 1 Pain.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brooks 2012.
| Methods | Randomised controlled trial | |
| Participants | 64 participants with low back pain Settings: not reported Country: Australia Inclusion criteria: men and women aged between 18 and 50 years, with ongoing recurrent LBP (> 12 weeks) located between the costal margins and inferior gluteal folds Exclusion criteria: presence of a severe postural abnormality, pain radiating below the knee, known lumbar disc hernia or fracture, history of back surgery, diagnosed inflammatory joint disease, known severe osteoporosis, known metabolic or neuromuscular disease, or recent (< 3 months) participation in an exercise programme or any form of therapeutic treatment (i.e. manipulation, mobilisation, massage) |
|
| Interventions | 1. Specific trunk exercise group (Pilates): the specific programme was based on a Pilates training model, which incorporated skilled contraction techniques, general trunk focused strengthening exercise, whole‐body movements, and stretching of the trunk and hip musculature. It was thought that this model of training best represented the most utilised components of specific trunk exercise rehabilitation programmes for LBP, with a strong focus on the use of skilled contraction techniques (abdominal drawing‐in and abdominal bracing) 2. General exercise group: the general exercise programme was indoor stationary cycle training. Intensity of effort within each component was based on combinations of heart rate training zones (based on percentage of maximal heart rate) and rate of perceived exertion scales All participants were required to attend exercise classes 3 times per week for a total of 8 weeks. Every exercise class was for a duration of 50 to 60 minutes and was supervised, with a participant‐to‐instructor ratio of 10:1. Exercise classes for the 2 groups were administered in different training rooms to minimise the likelihood of contact between participants in the different groups. Instructors for the exercise classes were trained and experienced (minimum 5 years) in a particular intervention only and had no contact with participants or instructors from the different group. Instructors for the exercise groups had no involvement in the recruitment, allocation or assessment of participants in the trial |
|
| Outcomes | 1. Self rated disability: Oswestry Disability Index (ODI) 2. Pain: using a 100 mm visual analogue scale (VAS) (left anchor "no pain at all", right anchor "worst pain imaginable") for back pain experienced in the last week, and current back pain (VAS) |
|
| Notes | No funding was received in support of this work
"No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript." Adverse events: not evaluated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Review authors' comment: the sequence generation procedure or the method of allocation were not mentioned. The title, abstract and flowchart indicate that it is a RCT |
| Allocation concealment (selection bias) | Unclear risk | Reviewers comment: The sequence generation procedure or the method of allocation were not mentioned. The title, abstract, and flowchart indicate that it is a RCT |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "To control for expectation bias, participants were blinded to the use of different modalities in the trial by being informed that they were volunteering for an exercise trial to investigate how exercise programs work for people with chronic LBP." |
| Blinding of personnel/care providers (performance bias) All outcomes | High risk | No mention of any attempts to blind the care providers |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Self‐report outcome measures and APAs were assessed before and after the 8‐week intervention by a blinded assessor." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The percentage of withdrawals and dropouts was within the acceptable range |
| Intention‐to‐treat analysis | Low risk | "Analysis of self‐report data (ODI and VAS scores) was conducted by “intention to treat” (i.e., all available data from all randomised participants were analysed in the group to which the participant was allocated)." |
| Selective reporting (reporting bias) | Low risk | It was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics, based on the Table 2 |
| Co‐interventions (performance bias) | Unclear risk | Not mentioned |
| Compliance (performance bias) | Low risk | Compliance was acceptable, based on the reported intensity/dosage, duration, number and frequency for both the intervention and control groups |
| Timing of outcome assessments (detection bias) | Low risk | All important outcomes for both groups were measured at the same time |
Fonseca 2009.
| Methods | Randomised controlled trial | |
| Participants | 17 participants with chronic low back pain Settings: waiting list for physiotherapeutic treatment (intervention group), and students and staff of the University (control group) Country: Brazil Inclusion criteria: independent gait execution without the use of any support device (crutch, walking stick, etc), complaints of low back pain for at least 6 months (low back group), no complaints of low back pain or musculoskeletal pain (control group), and aged between 18 and 59 years old Exclusion criteria: neurological disease, major visual deficits, true leg‐length discrepancy greater than 2 cm, and history of ankylosing spondylitis, disc herniation, tumour, infection or fracture, cauda equina syndrome, spine‐fusion surgery or any lower extremity orthopaedic surgery within 1 year of the beginning of the study |
|
| Interventions | 1. Pilates group: performed 15 sessions of Pilates exercises, 2 sessions per week. The sessions lasted for an hour and were performed individually. The exercise programme was taught by a certified Pilates instructor. The programme of exercises consisted of 4 stages: (i) isolated contraction training of the core muscles; (ii) co‐contraction of the core muscles, that is, simultaneous contraction of the transversus abdominis, multifidus and pelvic‐floor muscles; (iii) co‐contraction of the core muscles combined with limb movements, keeping the spine static; and (iv) co‐contraction of the core muscles during dynamic functional movements of the trunk. The participants were instructed to recruit the core muscles during the exercises and not to substitute them for global muscles. The exercise programme consisted of basic‐level Pilates exercises, progressing from positions with low loads (supine position, prone position and side‐lying) to more functional body positions with gradually increasing external loads (box and sitting positions). The participants were instructed to maintain aligned and symmetrical posture of the spine and limbs and to perform the exercises with the spine neutral. The exercises were taught by appropriate verbal instructions given by the instructor. The participants were instructed to inform the instructor if they experienced any pain, discomfort, cramps or inability to maintain the contraction of the core muscles or neutral spine. In those cases, the exercise was interrupted and, if necessary, modified by decreasing lever lengths for any individual participants who found the particular exercise too challenging to enable them to maintain a neutral spine. If participants felt loss of control for a movement, they were advised to go back to a base position for that particular exercise. Until the seventh session, homework was assigned so that co‐contraction of the core muscles would become automatic and efficient. Those exercises were indicated to be performed once a day. From the eighth session on, the participants were encouraged to activate these muscles regularly during daily activities (while walking, watching TV, etc) 2. No Pilates group: continued with their normal activities and did not undergo any other type of treatment aside from medication taken for conditions not related to the study |
|
| Outcomes | 1. Pain: visual analogue scale (VAS) | |
| Notes | No statement about conflicts of interest or funding provided Adverse events: not evaluated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Review authors' comment: the sequence generation procedure or the method of allocation were not mentioned. The title, abstract and flowchart indicate that it is a RCT |
| Allocation concealment (selection bias) | Unclear risk | Review authors' comment: the sequence generation procedure or the method of allocation were not mentioned. The title, abstract and flowchart indicate that it is a RCT |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempts to blind the participants |
| Blinding of personnel/care providers (performance bias) All outcomes | High risk | No mention of any attempts to blind the care providers |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study is described as a single‐blind RCT, but there is not enough information to know who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Unclear, but the text suggest that there were no drop‐outs |
| Intention‐to‐treat analysis | Unclear risk | No information about intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | It was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | Low risk | "There were no significant differences between the Pilates group and the no‐Pilates group in baseline data for age, height, weight, visual analogue pain scale (Pilates = 5.9 ± 2.0 and no Pilates = 6.1 ± 1.8), and present pain intensity (Pilates = 2.8 ± 1.5 and no Pilates = 2.0 ± 0.7)." |
| Co‐interventions (performance bias) | Unclear risk | Not mentioned |
| Compliance (performance bias) | Unclear risk | There was insufficient information about the control group |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Gladwell 2006.
| Methods | Single‐blind randomised controlled trial | |
| Participants | 49 participants who had had non‐specific chronic low back pain for more than 12 weeks (located below the scapulae and above the cleft of the buttocks) Settings: individuals living within the Colchester region were offered the chance to participate in this study via posters and letters given to local doctors' clinics and via e‐mailed information to staff and students at the local university Country: United Kingdom Inclusion criteria: chronic low back pain for at least 12 weeks not attributable to any specific pathology (see exclusions) located below the scapulae and above the cleft of the buttocks. Aged between 18 and 60 years old. Patient able to travel independently. Patient is otherwise medically fit to perform physical training and able to consent and understand what the study entails Exclusion criteria: back pain attributed to any specific pathology: e.g. disc herniation, tumour, infection or fracture, osteoporosis, structural deformity, inflammatory disorder, radicular syndrome or cauda equina. Patient is unable to walk without a walking aid. Patient already involved in regular Pilates classes. Constant or severe back pain judged on clinical grounds due to nerve root irritation. Major surgery within the past year |
|
| Interventions | 1. Pilates group: performed 6 1‐hour classes of Pilates exercise (maximum class size = 12), 1 class per week. The Pilates exercise programme was taught by a certified Pilates Institute Instructor. In the first class, the basic principles of Pilates were explained and a handout was provided to participants for home reading. Basic principles were reiterated at the beginning of every class throughout the intervention period with an increasing portfolio of relevant Pilates techniques. In each 1‐hour class, an educational aspect was provided followed by specific modified Pilates exercises. Educational aspects included posture check (including neutral spine and pelvis), recruitment of "core muscles" and encouragement not to substitute from global muscles; all aspects were completed during controlled breathing. The exercises were "cued" by appropriate verbal instructions given by the instructor. All exercises started at the base level and were progressed by incorporating limb movement, when participants were able to maintain control of the spine. Additional exercises were also added during each session. The exercises taught within a class were also repeated individually during 2 30‐minute sessions each week performed at home without supervision. No progression of exercises was made during home sessions. Compliance with home‐based exercises was recorded in a diary 2. Control group: continued with their normal activities and pain relief |
|
| Outcomes | 1. Pain: Roland Morris pain rating visual analogue scale (RMVAS) 2. Disability: the Oswestry Low‐Back Pain Disability Questionnaire (OSWDQ) |
|
| Notes | No statement about conflicts of interest or funding provided Adverse events: not evaluated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Review authors' comment: the sequence generation procedure or the method of allocation were not mentioned. The title, abstract and flowchart indicate that it is a RCT |
| Allocation concealment (selection bias) | Unclear risk | Review authors' comment the sequence generation procedure or the method of allocation were not mentioned. The title, abstract and flowchart indicate that it is a RCT |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempts to blind the participants |
| Blinding of personnel/care providers (performance bias) All outcomes | High risk | No mention of any attempts to blind the care providers |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "...a functional assessment were performed by an assessor blinded to the allocation of individuals to the two groups" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts exceeded 20% |
| Intention‐to‐treat analysis | Unclear risk | No information about intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | It was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics, based on Table 3 |
| Co‐interventions (performance bias) | Unclear risk | Not mentioned |
| Compliance (performance bias) | Unclear risk | There was not enough data for the control group |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Marshall 2013.
| Methods | Single‐centre, single‐blind, randomised controlled study conducted in Sydney, Australia | |
| Participants | 64 participants with chronic low back pain Settings: recruited from the community via newspaper advertising, letterbox leaflets, advertisements placed on community notice boards and word‐of‐mouth communication Country: Australia Inclusion criteria: males and females aged 18 to 50 years with ongoing recurrent LBP (> 12 weeks) located between the costal margins and inferior gluteal folds Exclusion criteria: presence of a postural abnormality contributing to the diagnosis (as presented in medical notes summarising orthopaedic surgeon or physiotherapist diagnosis; as accredited exercise physiologists it was not within the industry scope of practice for the researchers in this study to specifically diagnose abnormalities. If a postural abnormality was observed, for example, scoliosis more than 20°, participants were referred to local medical practitioners for confirmation and thus exclusion from the trial). Further exclusion criteria were pain radiating below the knee, known history of or currently symptomatic lumbar disc hernia or fracture (60% of participants had undergone magnetic resonance imaging and/or radiography in the last 2 years), history of back surgery, diagnosed inflammatory joint disease, known severe osteoporosis, known metabolic or neuromuscular disease (as assessed by American College of Sports Medicine pre‐exercise screening tool for cardiovascular risk factors prior to entry into an exercise programme), pregnancy, recent (< 3 months) participation in an exercise programme or any form of physical treatment (i.e. manipulation, mobilisation massage) |
|
| Interventions | 1. Specific trunk exercise group: performed Pilates exercise. Pilates has been described as a system of mind‐body exercises requiring core stability, strength and flexibility, with attention to muscle control, posture and breathing 2. Stationary cycling exercise group: participants were informed that they were performing a style of cycling known as Pilates Pedal to reduce any expectation bias as to why they were prescribed an exercise programme that did not include specific trunk exercise. Intensity of effort within each component was based on combinations of heart rate training zones (based on % of maximal heart rate) and rate of perceived exertion scales All participants were required to attend exercise classes 3 times per week for a total of 8 weeks. Every exercise class was between 50 and 60 minutes duration, and was supervised with a participant to instructor ratio of 10:1. Exercise classes for the 2 groups were administered in different training rooms to minimise the likelihood of contact between participants. Instructors were trained and experienced (> 5 years) in that intervention only, and had no contact with participants or instructors from the different group |
|
| Outcomes | 1. Pain: 10 cm VAS with "no pain" on the left side and "worst pain" on the right side 2. Disability: Oswestry Low Back Pain Disability Index (ODI) |
|
| Notes | No funds were received in support of this work
No relevant financial activities outside the submitted work Adverse events: not evaluated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was conducted by a researcher with no involvement in the assessment protocols or training programs. Participants were randomly assigned in blocks of 8 with equal number of participants assigned to each group." |
| Allocation concealment (selection bias) | Low risk | "The allocation sequence was concealed from researchers involved in enrolling and assessing participants." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "To reduce expectation bias, participants were blinded to the use of different modalities in the trial. Participants were informed that they were volunteering for a study to investigate how exercise programs work for people with LBP." |
| Blinding of personnel/care providers (performance bias) All outcomes | High risk | No mention of any attempts to blind the care providers |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Self‐report questionnaires were completed by participants at baseline, 8 weeks, and 6 months, and were processed by blinded research assistants." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The percentage of withdrawals and dropouts was within the acceptable range |
| Intention‐to‐treat analysis | Low risk | "Data were analysed using SPSS with "intention‐to‐treat" principles (i.e. all available data collected from all randomised participants were analysed in the group to which the participant was allocated)." |
| Selective reporting (reporting bias) | Low risk | It was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics, based on Table 2 |
| Co‐interventions (performance bias) | Unclear risk | Not mentioned |
| Compliance (performance bias) | Low risk | Compliance was acceptable, based on the reported intensity/dosage, duration, number and frequency for both the intervention and control groups |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Miyamoto 2013.
| Methods | Randomised controlled trial carried out at the outpatient physical therapy department of (Universidade Cidade de Sao Paulo, Sao Paulo, Brazil) | |
| Participants | 86 participants with chronic low back pain Settings: patients who responded to an advertisement placed in a regional newspaper and on the university website Country: Brazil Inclusion criteria: the study included patients with chronic non‐specific low back pain with a duration of at least 3 months and aged between 18 to 60 years Exclusion criteria: any contraindication for physical exercise (assessed with the Physical Activity Readiness Questionnaire), previous regular Pilates method training, pregnancy, serious spinal pathologies, previous or scheduled spine surgery, low back pain due to nerve root compromise, physical therapy treatment for CLBP in the previous 6 months, and inability to write or speak in Portuguese |
|
| Interventions | 1. Booklet group: the participants allocated to the booklet group received an educational booklet containing information about the anatomy of the spine and pelvis, low back pain and recommendations regarding posture and movements involved in activities of daily living. The participants in this group did not receive additional exercise, and they were instructed not to undergo treatment elsewhere during the period of the study. However, they had direct access to the physical therapist overseeing the intervention and, over the next 6 weeks, they received twice‐weekly telephone calls for clarification regarding the booklet instructions 2. Pilates group (Modified Pilates Exercise + Educational Programme): participants allocated to the Pilates group received the same educational booklet in the first session of treatment. In addition to the educational booklet, they received an individual, supervised treatment using the modified Pilates method. The Pilates group received a 1‐hour session, twice a week, over 6 weeks. These exercises followed the traditional Pilates principles of centering (contracting deep trunk muscles known as "power house muscles"), concentration, control, precision, flow and breathing. All exercises aimed at improving breathing, core stability, motor control, posture, flexibility and mobility with the spine in a neutral position. At the beginning of all treatment sessions, 5 warm‐up exercises were performed. These exercises were aimed at improving spine and pelvis mobility. Then participants received the modified Pilates protocol that was based on 8 exercises aimed at improving breathing associated with core stability, posture, strengthening of specific muscles (such as abdominal wall muscles, multifidus, gluteal muscles, and hip flexors, extensors, adductors and abductors), and flexibility of the lower limbs and spinal muscles in all planes of movement. The number of repetitions for each exercise was individualised for each patient and ranged from 5 to 10 repetitions. These exercises were tailored individually and progressed in difficulty in 3 levels (basic, intermediate and advanced). The physical therapist who provided the intervention was a certified Pilates instructor with 3 years of clinical experience |
|
| Outcomes | 1. Pain Intensity: 0 to 10 Pain Numeric Rating Scale (NRS) 2. Disability: 0 to 24 Roland‐Morris Disability Questionnaire (RMDQ) 3. Global Impression of Recovery: ‐5 to +5 Global Perceived Effect Scale 4. Function: Patient‐Specific Functional scale (PSFS) |
|
| Notes | No statement about conflicts of interest or funding provided Adverse events: no adverse events were observed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Simple randomisation was conducted using Microsoft Excel for Windows software (Microsoft Corporation, Redmond, Washington) by a researcher who was not involved in participant recruitment." |
| Allocation concealment (selection bias) | Low risk | "The allocation sequence was generated by one of the authors who was not involved with participant recruitment and treatment. Allocation was concealed by using consecutively numbered, sealed, opaque envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "it was not possible to blind the participants and the therapist involved in the study." |
| Blinding of personnel/care providers (performance bias) All outcomes | High risk | "it was not possible to blind the participants and the therapist involved in the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "...a previously trained, blinded assessor conducted an evaluation..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The percentage of withdrawals and dropouts was within the acceptable range |
| Intention‐to‐treat analysis | Low risk | "The analyses followed the intention‐to‐treat principles" |
| Selective reporting (reporting bias) | Low risk | It was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics, described in Table 2 |
| Co‐interventions (performance bias) | Low risk | Balanced for both groups |
| Compliance (performance bias) | Low risk | Compliance was acceptable, based on the description for both groups |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Natour 2014.
| Methods | Randomised, controlled clinical trial | |
| Participants | 60 patients were selected Settings: not reported Country: Brazil Inclusion criteria: diagnosis of chronic low back pain (defined as pain between the lower rib cage and gluteal folds for more than 12 months); non‐specific low back pain characterised by the absence of signs of a serious underlying condition (such as cancer, infection or cauda equina syndrome), spinal stenosis or radiculopathy, or another specific spinal cause (such as vertebral compression fracture or ankylosing spondylitis), pain that becomes accentuated with physical effort and is relieved with rest; male or female; aged 18 to 50 years; pain between four and seven on a 10 cm visual analogue scale; and agreement to participate in the study Exclusion criteria: diagnosis of low back pain due to other causes; fibromyalgia; prior spine surgery; lawsuit; having initiated or changed regular physical activity in the previous 3 months; body mass index > 30; and having undergone treatment with physical therapy or acupuncture in the previous 3 months |
|
| Interventions | 1. Experimental group: patients maintained medical treatment with the use of a non‐steroidal anti‐inflammatory drug and underwent treatment with the Pilates method 2. Control group: patients continued medical treatment with the use of a non‐steroidal anti‐inflammatory drug and did not undergo any other intervention | |
| Outcomes | 1. Pain: measured with the patient indicating his/her current level of pain by marking a point on a 10 cm VAS
2. Function: measured with the Roland‐Morris questionnaire
3. Quality of life: measured with the SF‐36 4. Satisfaction with treatment: measured with a Likert scale used to determine patient satisfaction with the treatment (patients answered the question 'How do you feel today in comparison with your last evaluation?', for which the options were 'much better', 'a little better', 'the same', 'a little worse' and 'much worse') 5. Flexibility: measured with a sit and reach test, which is the maximal distance achieved in the Wells bench 6. Non‐steroidal anti‐inflammatory drug intake; the sodium diclofenac intake was recorded on a chart supplied to each patient |
|
| Notes | The authors declare that there is no conflict of interest This study was funded by grants provided by Fundacao Amparo a Pesquisa do Estado de Sao Paulo (2007/53423‐5) Adverse events: not evaluated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomised using an electronically generated randomisation table" |
| Allocation concealment (selection bias) | Low risk | "Sealed, opaque envelopes were used to ensure the confidentiality of the assignment. The envelopes were stored in a locked cupboard and only opened after the initial evaluation by an individual who did not participate in the study." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "One limitation of this study is that the treatment provider and participants could not be blinded to the interventions." |
| Blinding of personnel/care providers (performance bias) All outcomes | High risk | "One limitation of this study is that the treatment provider and participants could not be blinded to the interventions." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "An examiner blind to the assignment of the patients performed all evaluations." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The percentage of withdrawals and dropouts was within the acceptable range |
| Intention‐to‐treat analysis | Low risk | "Data for all patients were evaluated with intention‐to‐treat analysis" |
| Selective reporting (reporting bias) | Low risk | It was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics, based on the Table 1 |
| Co‐interventions (performance bias) | Unclear risk | Not mentioned |
| Compliance (performance bias) | Low risk | Compliance was acceptable, based on the description for both groups |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Quinn 2011.
| Methods | Single‐blinded randomised controlled trial | |
| Participants | 29 participants with chronic low back pain Settings: patients previously seeking care at the hospital Country: United Kingdom Inclusion criteria: participants aged between 18 and 60 years, with chronic LBP (> 3 months duration) with no pain radiating below the knee and willing to attend Pilates classes for 8 weeks. Participants also had to have some residual pain (VAS > 18 mm) and have failed the Sahrmann Abdominal Test for core stability Exclusion criteria: significant other co‐morbidity such as unstable cardiovascular system, uncontrolled epilepsy, Modified Zung Depression Index score > 33/6914 or significant pain in other joints which would affect their ability to participate in class. Participants were also excluded if they were pregnant, had spinal surgery in the past 12 months or were diagnosed with significant disc prolapse on MRI, severe scoliosis, inflammatory low back pain or had a high level of disability (Roland Morris Disability Questionnaire < 16/24) |
|
| Interventions | 1. Pilates group: the 1‐hour class consisted of modified mat‐based Pilates exercises and was based on a Body Control Pilates exercise programme used by a previous study. All classes were run by the chief investigator who was a chartered physiotherapist and a qualified Body Control Pilates instructor. Participants in the intervention group were also advised to complete 15 minutes of Pilates exercise 5 days of the week at home. Compliance with home‐based exercise was monitored by a self recorded diary 2. Control group: participants in the control group received no further intervention for the 8‐week period |
|
| Outcomes | 1. Pain: visual analogue scale (VAS) 2. Disability: Roland Morris Disability Questionnaire (RMDQ) |
|
| Notes | No statement about conflicts of interest or funding provided Adverse events: not evaluated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation and concealed allocation was carried out using sequentially numbered, opaque sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | "Randomisation and concealed allocation was carried out using sequentially numbered, opaque sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The study design did not permit blinding of the participants or the treating physiotherapist" |
| Blinding of personnel/care providers (performance bias) All outcomes | High risk | "The study design did not permit blinding of the participants or the treating physiotherapist" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "...Baseline and final outcome measures of subjects participating in the study were recorded at a separate appointment by another physiotherapist (LB) who was blinded to group allocation and was not involved in providing treatment..." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts exceed 20%. |
| Intention‐to‐treat analysis | Low risk | "Groups were analysed on an intention to treat basis. All subjects were included to avoid bias by omitting non compliers." |
| Selective reporting (reporting bias) | Low risk | It was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics, based on the Table 1 |
| Co‐interventions (performance bias) | Unclear risk | Not mentioned |
| Compliance (performance bias) | Unclear risk | There were insufficient data for the control group |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Rajpal 2008.
| Methods | Randomised controlled trial performed in the outpatient department of Sardar Bhagwan Singh Post Graduate Institute of Biomedical Sciences and Research, Balawala, Dehradun | |
| Participants | 40 female participantsaged 20 to 30 years with low back pain Settings: not reported Country: India Inclusion criteria: patients with postural low back pain for 3 months; female; in the age range 20 to 30 years; standing pelvic tilt angle of 9º or more; reduced abdominal muscle strength Exclusion criteria: sciatica or any neurological deficit; soft tissue injuries; spinal fractures; disc prolapse; back pain due to structural deformity, infection, tumour |
|
| Interventions | 1. Pilates group: participants were given Pilates exercises for 1 month. The exercises were done 10 times with 10 seconds hold in between, daily. The participants were made to lie in crook lying with hip and knee flexed. In this position, the lumbar spine is neither arched up nor flattened against the floor, but is aligned normally with a small gap between the floor and the back. The participants were asked to breathe in deeply and relax all the stomach muscles. While breathing out, the participant draws the lower abdomen inwards as if the umbilicus goes backwards and upwards.The contraction was held for 10 seconds and then relaxed. This exercise was done 10 times daily for 10 days. The participants were made to lie in quadruped/4‐point kneeling position and were allowed to do the same contractions for 10 times daily for next 10 days. The participants were made to sit on an exercise ball with both hands over the pelvis and were made to perform the same contractions and, along with that, were made to extend their leg simultaneously. This exercise was performed 10 times daily for the next 10 days 2. McKenzie group: participants were taught postural correction and re‐education. The participants were told that as a person sits, the spine sooner or later takes a relaxed posture and the lumbar spine moves into a fully flexed position that places stress over the various ligamentous structures. This position is painful if maintained for longer period. The participants were taught how to obtain and maintain the sitting posture for longer periods. To obtain the correct sitting posture, this includes 'slouch‐overcorrect' procedure. The participants were made to sit slouched on a backless chair or stool, allowing the lumbar spine to rest on the ligaments in the fully flexed position and permit head and chin to protrude. Then, slowly moved into the erect sitting posture with the lordosis at its maximum and the head held directly over the spine with the chin pulled up. This sequence was repeated for 3 times daily, 15 to 20 times at each session Once they had mastered this procedure, they were advise to follow this procedure whenever they feel pain and maintain the position. To maintain the correct sitting position, the participants were taught about maintaining the lumbar lordosis in 2 ways: a) actively by conscious control of the lordosis, when sitting on a chair without back rest; and b) passively by using the lumbar support, when sitting on a seat with a back rest. The lumbar roll was used to hold the lumbar spine in a good position while prolonged sitting. The roll was placed at or just above the belt line (area of L3 and L4 vertebrae). This procedure was repeated for 3 times daily, 15 to 20 times at each session. The participants were made to stand and moving the lower part of the spine backwards by tightening the abdominal muscles and tilting the pelvis posteriorly, while at the same time move the upper spine forwards and raising the chest. This procedure was repeated for 3 times daily, 15 to 20 times at each session |
|
| Outcomes | 1. Pain: visual analogue scale (VAS) | |
| Notes | No statement about conflicts of interest or funding provided Adverse events: not evaluated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Review authors' comment: the sequence generation procedure or the method of allocation were not mentioned. The title, abstract and flowchart indicate that it is a RCT |
| Allocation concealment (selection bias) | Unclear risk | Review authors' comment: the sequence generation procedure or the method of allocation were not mentioned. The title, abstract and flowchart indicate that it is a RCT |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempts to blind the patients |
| Blinding of personnel/care providers (performance bias) All outcomes | High risk | No mention of any attempts to blind the care providers |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of any attempts to blind the assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The percentage of withdrawals and dropouts was within the acceptable range |
| Intention‐to‐treat analysis | High risk | Not mentioned |
| Selective reporting (reporting bias) | Low risk | It was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics, based on the Table 1 |
| Co‐interventions (performance bias) | Unclear risk | Not mentioned |
| Compliance (performance bias) | Unclear risk | There are not enough data |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Rydeard 2006.
| Methods | Randomised controlled trial | |
| Participants | 39 participants with chronic low back pain Settings: participants were recruited through notices posted to private and public physicians' and physiotherapists' offices, notices posted to local sports clubs and universities, and by advertisement in an English‐language newspaper Country: Hong Kong Inclusion criteria: physically active adults between 20 and 55 years old, living in Hong Kong, with longstanding, persistent LBP (with or without leg pain) of greater than 6 weeks duration or recurring LBP (with at least 2 painful incidences per year) of sufficient intensity to restrict functional activity in some manner Exclusion criteria: participants were excluded from the study if they were pregnant, had a past history of spinal surgery or spinal fracture, were diagnosed with inflammatory joint disease, systemic metabolic disorder, rheumatic disease or chronic pain syndrome, showed evidence of overt neurological compromise or acute inflammatory process, or had difficulty understanding written or spoken English |
|
| Interventions | 1. Specific exercise training group (SETG): the SETG received a treatment protocol consisting of training in specialised (Pilates) exercise apparatus in the clinic for 3 1‐hour sessions per week, and training in a 15‐minute home programme performed 6 days per week for 4 weeks.The standardised, progressive treatment protocol addressed targeted muscle activation strategies throughout a variety of movement patterns involving hip extension. The participant was required to consciously recruit specific muscles ‐ the deep anterolateral abdominals (with co‐activation of the pelvic floor and lumbar multifidus), followed by activation of the gluteus maximus muscles. Static postures were initially trained, followed by training a variety of movement patterns to stress the lumbar‐pelvic region and involving hip extension. The training was progressed on the Pilates Reformer over the 4‐week period as tolerated. Initially movements were practised using weight‐bearing patterns in supine, with the lumbar spine in the neutral position. Gradually more upright postures and controlled movement of the lumbar‐pelvic region out of neutral posture were incorporated. Prescribed movements were performed slowly, smoothly and without pain. Individualised facilitation strategies were provided by the physiotherapist to correct technique, control speed, assist appropriate muscle activation or modify the exercise or the progression to suit the participants' needs The home treatment protocol consisted of 2 parts: (1) floor exercises to specifically activate the deep anterolateral abdominals and local stability synergists and the gluteus maximus muscle by moving the leg in a manner similar to that utilised on the apparatus; and (2) skill drills in which difficult tasks were broken down into movement components and practised in isolation incorporating correct abdominal and gluteal control 2. Control group (CG): no specific exercise training and continued with usual care, defined as consultation with a physician and other specialists and healthcare professionals as necessary. They were not restricted from seeking any other treatment if they so wished. Participants were instructed to continue to do what they were previously doing, including regular physical activity |
|
| Outcomes | 1. Pain: 101‐point numerical rating scale (NRS‐101) 2. Disability: Roland Morris Disability questionnaire, Chinese version validated in a Hong Kong Chinese population (RMDQ‐HK) |
|
| Notes | No statement about conflicts of interest or funding provided Adverse events: not evaluated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects randomly pulled a card from a box of concealed pre‐marked cards to obtain assignment to either the specific exercise group or control group." |
| Allocation concealment (selection bias) | Low risk | "Subjects randomly pulled a card from a box of concealed pre‐marked cards to obtain assignment to either the specific exercise group or control group." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempts to blind the patients |
| Blinding of personnel/care providers (performance bias) All outcomes | High risk | No mention of any attempts to blind the care providers |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Data collection monitored both pain intensity and functional status and included 2 self‐report questionnaires administered by the research assistant, an independent physiotherapist investigator blinded to group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The percentage of withdrawals and dropouts was within the acceptable range |
| Intention‐to‐treat analysis | Low risk | Intention‐to‐treat analysis was used to analyse the data |
| Selective reporting (reporting bias) | Low risk | It was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | High risk | Differences at baseline regarding pain and disability |
| Co‐interventions (performance bias) | Unclear risk | Unclear, but it seems that co‐interventions were not avoided |
| Compliance (performance bias) | Unclear risk | There were insufficient data for the control group |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Wajswelner 2012.
| Methods | Parallel, single‐assessor blinded randomised controlled trial | |
| Participants | 87 participants with chronic low back pain Settings: participants were recruited from the community via local newspaper advertisements in 2 inner suburban areas of Melbourne, Australia, and via e‐mail news items at the University of Melbourne Country: Australia Inclusion criteria: aged between 18 and 70 years, symptoms of pain or stiffness in the lower back with or without lower limb symptoms on most days of the week for more than 3 months (defined as chronic), average pain score in the past week at telephone screening Q4 on an 11‐point numeric rating scale (0 = no pain and 10 = worst pain possible), and good understanding of written and spoken English Exclusion criteria: spinal surgery; fever, infection, night sweats or rigours; unexplained weight loss or loss of appetite; history of cancer or malignancy; cauda equina lesion, loss of bladder or bowel control, or saddle paraesthesia; pregnancy or the possibility of pregnancy in the next 6 months; spinal fractures or diagnosed osteoporosis; spinal inflammatory disease such as ankylosing spondylitis, rheumatoid arthritis; co‐morbidities that would prevent exercise; previous participation in a clinical Pilates programme or other regular therapeutic back exercise programme in the last 6 months; inability to comply with trial requirements; or compensable back pain |
|
| Interventions | 5 musculoskeletal physiotherapists located in 2 accredited private practices and with expertise in the exercise‐based treatment of CLBP prescribed and supervised both treatments. Both interventions comprised an initial 1‐hour individual session with the physiotherapist whereby an exercise programme was prescribed. The therapist could use up to 2 further 30‐minute individual sessions to ensure that the participant could perform all exercises safely and effectively. After this, the participant attended group exercise sessions (maximum number of 4 people) at one of the trial clinics twice a week (60 minutes) for the 6‐week duration of the programme. All sessions were supervised by one of the project physiotherapists. Participants were also requested to perform a smaller number of daily home exercises 1. Pilates group: the clinical Pilates group received a tailor‐made, direction‐specific exercise programme prescribed by a physiotherapist based on history, aggravating factors and physical examination. The clinical Pilates exercise programme was a series of exercises performed on the reformer and trapeze equipment. The equipment both supports the patient and guides the direction and type of movement required for the prescribed exercises. The exercises were designed to work the patient in a specific direction, for example, flexion, extension, neutral or to the left or right side. Common to all exercises were concepts of finding and maintaining the spinal comfort range, exercise movement precision, breathing control, correct postural alignment, central trunk stability, smoothness of movement and complete range of motion. The clinical Pilates programme consisted of 6 to 12 of the equipment‐based exercises plus 1 to 4 home‐based clinical Pilates exercises using the floor or simple props such as a chair or wall. 2. General exercise group: participants of the general exercise group were taught a standardised generic set of exercises traditionally used by physiotherapists for the management of CLBP. These exercises were chosen via consensus of 7 musculoskeletal physiotherapists with expertise in exercise prescription as well as from previous studies of general exercise programmes for CLBP. The exercises included stationary bike, leg stretches, upper body weights, theraband, Swiss ball and floor exercises that were multidirectional and nonspecific in nature. 4 daily home exercises were given to the general exercise group participants |
|
| Outcomes | 1. Pain: numerical rating scale 0 to 10 2. Disability: Quebec scale 3. Quality of life: SF‐36 4. Function: Patient Specific Functional Scale |
|
| Notes | Funding for this trial was provided by Mr. Craig Phillips of DMA Clinical Pilates Physiotherapy in South Yarra, Melbourne, Victoria, Australia, and Mr. Marcus Pain of Back in Motion Physiotherapy in Brunswick, Melbourne, Victoria, Australia Henry Wajswelner works at a physiotherapy and Pilates clinic that uses clinical Pilates exercises to treat patients. He also teaches clinical Pilates to other physiotherapists Adverse events: in the Pilates group, 2 participants reported minor shoulder pain, and 1 experienced knee pain. In the general exercise group, 2 participants reported worsening back pain, and 2 experienced some back spasms |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "participants were randomly allocated in permuted blocks of six and eight, stratified by age (18–35, 35–55, and 55–70 years) and gender, to either the clinical Pilates group or the general exercise group. The randomisation sequence was generated a priori using a computer program by an independent investigator." |
| Allocation concealment (selection bias) | Low risk | "Allocation was sealed in opaque and consecutively numbered envelopes held centrally. An independent administrator opened the envelopes in sequence and then revealed the group allocation to the physiotherapist just before the participant presented for treatment." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempts to blind the patients |
| Blinding of personnel/care providers (performance bias) All outcomes | High risk | No mention of any attempts to blind the care provider |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | There was blinding of the assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The percentage of withdrawals and dropouts was within the acceptable range |
| Intention‐to‐treat analysis | Low risk | "The primary analysis was by intention‐to‐treat and was performed in a blinded manner." |
| Selective reporting (reporting bias) | Low risk | It was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics, reported in Table 1 |
| Co‐interventions (performance bias) | High risk | There were few reported co‐interventions in the study |
| Compliance (performance bias) | Low risk | Compliance was acceptable, based on the description for both groups |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
CLBP: chronic low back pain LBP: low back pain MRI: magnetic resonance imaging RCT: randomised controlled trial VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12607000471482 | Population not eligible for this review |
| ACTRN12609000927224 | Not RCT |
| Alves 2012 | Did not recruit LBP patients |
| Anderson 2005 | Thesis |
| Anderson 2006 | Conference abstract |
| Blum 2002 | Case report |
| Boden 2010 | Conference presentation |
| Curnow 2009 | Both groups received Pilates |
| Cámara 2011 | Conference abstract |
| da Luz 2014 | 2 different forms of Pilates were tested |
| Donzelli 2006 | Used a quasi‐random procedure |
| Gagnon 2005 | Thesis |
| Hides 2012 | Mixed population of healthy participants and those who have LBP |
| Ickes 2007 | Opinion piece |
| Jaecks 2004 | Magazine article |
| Kagan 2008 | Magazine article |
| Kennedy 2012 | Conference abstract |
| McNeill 2009 | Editorial |
| McNeill 2010 | Editorial |
| Mehling 2005 | The intervention was not Pilates exercise |
| Natour 2011 | Conference abstract |
| NCT01533805 | Compared different forms of Pilates |
| NCT01711203 | Population not eligible for this review |
| NCT01919268 | Both groups performed Pilates exercise |
| NCT01999283 | Included patients with cervical pain |
| O'Brien 2006 | Conference abstract |
| PACTR201211000443397 | Compared different forms of Pilates |
| Parker 2010 | Magazine article |
| Phrompaet 2011 | Did not recruit LBP patients |
| Rasmussen‐Barr 2003 | The intervention was not Pilates exercise |
| RBR‐7tyg5j | Compared different forms of Pilates |
| Robinson 2007 | Magazine article |
| SeQueira 2010 | Conference abstract |
| Sherman 2010 | The intervention was not Pilates exercise |
| Sparrowe 2007 | Magazine article |
| Tekur 2008 | The intervention was not Pilates exercise |
| Tilbrook 2011 | The intervention was not Pilates exercise |
| Xue‐Qiang 2013 | Conference abstract |
LBP: low back pain
Characteristics of studies awaiting assessment [ordered by study ID]
Anand 2014.
| Methods | Randomised controlled trial |
| Participants | 52 participants with chronic low back pain Settings: not reported Country: India Inclusion criteria: participants with low back pain of not more than 5 on a visual analogue scale (moderate pain level), age range from 18 years to 60 years, both sexes, pain of more than 3 months duration, doing normal ADL activity, working population (since they do their routine activity), BMI within normal limit, not taking part in any of the research studies and not receiving physiotherapy for the past 2 months (to avoid a carry‐over effect) and no psychological or yellow flag participants Exclusion criteria: participants with prolapsed intervertebral disc, radiating pain, stenosis, severe spondylosis and spondylolisthesis, cardiovascular problems, tumours, infection or fracture, osteoporosis, radicular syndrome, inflammatory disorder, structural deformity not optimal for exercises or psychologically unstable |
| Interventions | 1. Experimental group: underwent modified Pilates‐based exercises for 45 minutes, prior to the modified Pilates‐based exercises session; the general flexibility exercises were given for 15 minutes. The modified Pilates‐based exercises included modified side kick, modified one leg stretch, modified shoulder bridge, the hundred (base level modification), swimming (a modification from a 4‐point base), modified swan dive, modified roll up, modified spine twist, double arm stretch, modified one leg circle. The flexibility exercises included the gluteus, hip flexors and quadriceps and hamstrings stretches were encouraged 2. Control group: participants received therapeutic exercises for 45 minutes, prior to the therapeutic exercises session; the general flexibility exercises were given for 15 minutes. The back exercises includes pelvic bridging, prone straight leg raise, prone cobra and prone arm rise (unilateral initially and bilateral later), dynamic strengthening exercises, stationary bicycle and Swiss ball co‐ordination exercises |
| Outcomes | 1. Self rated disability: Oswestry Disability Index (ODI) 2. Pain: using a visual analogue scale (VAS) |
| Notes | No funding was received in support of this work Adverse events: not evaluated |
ADL: activities of daily living BMI: body mass index
Characteristics of ongoing studies [ordered by study ID]
RBR‐7yhzym.
| Trial name or title | Comparison between Pilates and conventional physical therapy for treatment of patients with nonspecific chronic low back pain: randomized controlled trial |
| Methods | Clinical trial, 2 arms, randomized controlled, single blind |
| Participants | Individuals diagnosed with chronic low back pain Inclusion criteria: medical diagnosis of chronic low back pain, aged 18 to 55 years Exclusion criteria: protrusion of intervertebral disc Scoliosis, Spondylolisthesis, previous spine surgery, radicular symptoms (leg pain, loss of sensation and reflexes), inflammatory diseases, rheumatic diseases, Cancer and pregnancy |
| Interventions | Pilates method (mat and apparatus) is the experimental group (n=31). The activities will be practised twice a week, for 45 minutes (morning or afternoon, according to the convenience of the participant). The exercises are those that strengthen the abdominal and and paraspinal muscles, the mobility of the spine and stretching of the muscle chains and move on to trunk exercises and balance. The control group (n=31) will undergo conventional physical therapy(electrotherapy, heat, strength training, stretching, mobilisation and patient education) also twice a week, for 45 minutes (morning or afternoon, according to the convenience of the participant) |
| Outcomes | Primary outcomes: functionality and pain. Secondary outcomes: flexibility, muscle strength, fatigue and muscle endurance. |
| Starting date | 30/06/2011 |
| Contact information | Full name: Jefferson Rosa Cardoso Address: Av. Robert Kock 60 City: Londrina / Brazil Zip Code: 86038‐440 Telephone: (43) 3371.2649 E‐mail: jeffcar@uel.br Affiliation: Universidade Estadual de Londrina |
| Notes |
Differences between protocol and review
There were some differences between the protocol and review in three subsections of Data collection and analysis:
In Selection of studies, the screening for potentially eligible studies was conducted by two pairs of review authors instead of two review authors as stated in the protocol.
In Measures of treatment effect, we had pre‐specified in our protocol that for different scales we were going to quantify effect using the standardised mean difference (SMD) and the mean difference (MD) for studies using the same scale. However, we decided to quantify the effects of treatments using the MD for all continuous outcomes. If different scales were used we converted the scales to a 0 to 100 point scale.
We did not perform a sensitivity analysis by excluding trials where the definition of the intervention was not clear because all definitions of Pilates exercise were consistent with our criteria.
We did not perform a subgroup analysis for duration of symptoms as all trials included chronic patients.
In Assessment of heterogeneity, we included an acceptable range of the I2 value (< 50%) to combine the results in a meta‐analysis when no clear heterogeneity was identified by visual inspection. We used the 50% cut‐off as I2 values above this value may represent substantial heterogeneity.
The approach to GRADE has been clarified further since the protocol with more detail about downgrading.
We found three potentially eligible studies in trial registries reported as completed at least two years ago, for which no publicly available report was found. Additionally, we were unable to contact the authors for these trials. Thus, we considered that there was a possibility of publication bias in this review and downgraded all studies regarding publication bias for the analysis of quality of evidence (GRADE). This condition was not mentioned previously in the protocol.
Two studies measured quality of life, but the data from the physical and mental components were not available in the text and the authors did not provide this information on request (Natour 2014; Wajswelner 2012), so we were unable to analyse this primary outcome cited in the protocol.
We did not find any studies that reported return to work, so we were unable to analyse this secondary outcome cited in the protocol.
Contributions of authors
Conception, design and drafting of the protocol: Leonardo OP Costa, Luciola da Cunha Menezes Costa and Cristina Maria Nunes Cabral.
Critical revision of the protocol for important intellectual content: Chris G Maher, Raymond Ostelo and Mark Hancock.
Final approval of the protocol: all authors.
Collection and assembly of data: Tiê P Yamato, Bruno T Saragiotto.
Analysis and interpretation of the data: Tiê P Yamato, Bruno T Saragiotto and Chris G Maher.
Drafting of the article: Tiê P Yamato, Bruno T Saragiotto and Chris G Maher.
Critical revision of the article for important intellectual content: Leonardo OP Costa, Luciola da Cunha Menezes Costa, Chris G Maher, Raymond Ostelo and Mark Hancock.
Final approval of the article: all authors.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
Tiê P Yamato has no conflict of interest.
Christopher G Maher has no conflict of interest.
Bruno T Saragiotto has no conflict of interest.
Mark J Hancock has no conflict of interest.
Raymond WJG Ostelo has no conflict of interest.
Cristina MN Cabral conducted two randomised controlled trials that use Pilates as an intervention for patients with chronic non‐specific low back pain and she is also author of one of the included trials (Miyamoto 2013).
Luciola da C Menezes Costa has no conflict of interest.
Leonardo OP Costa conducted two randomised controlled trials that use Pilates as an intervention for patients with chronic non‐specific low back pain and he is also author of one of the included trials (Miyamoto 2013).
New
References
References to studies included in this review
Brooks 2012 {published and unpublished data}
- Brooks C, Kennedy S, Marshall PWM. Specific trunk and general exercise elicit similar changes in anticipatory postural adjustments in patients with chronic low back pain. Spine 2012;37(25):E1543‐50. [DOI] [PubMed] [Google Scholar]
Fonseca 2009 {published and unpublished data}
- Fonseca JL, Magini M, Freitas TH. Laboratory gait analysis in patients with low back pain before and after a Pilates intervention. Journal of Sport Rehabilitation 2009;18:269‐82. [DOI] [PubMed] [Google Scholar]
Gladwell 2006 {published and unpublished data}
- Gladwell V, Head S, Haggar M, Beneke R. Does a program of Pilates improve chronic non‐specific low back pain?. Journal of Sport Rehabilitation 2006;15:338‐50. [Google Scholar]
Marshall 2013 {published data only}
- Marshall PWM, Kennedy S, Brooks C, Lonsdale C. Pilates exercise or stationary cycling for chronic nonspecific low back pain: does it matter? A randomized controlled trial with 6‐month follow‐up. Spine 2013;38(15):952‐9. [DOI] [PubMed] [Google Scholar]
Miyamoto 2013 {published and unpublished data}
- Miyamoto GC, Costa LOP, Galvanin T, Cabral CMN. Efficacy of the addition of modified Pilates exercise to a minimal intervention in patients with chronic low back pain: a randomized controlled trial. Physical Therapy 2013;93:310‐20. [DOI] [PubMed] [Google Scholar]
Natour 2014 {published data only}
Quinn 2011 {published and unpublished data}
- Quinn K, Barry S, Barry L. Do patients with chronic low back pain benefit from attending Pilates classes after completing conventional physiotherapy treatment?. Physiotherapy Ireland 2011;32(1):5‐12. [Google Scholar]
Rajpal 2008 {published and unpublished data}
- Rajpal N, Arora M, Chauhan V. A study on efficacy of Pilates & Pilates & McKenzie exercise in postural low back pain ‐ a rehabilitative protocol. Physiotherapy and Occupational Therapy Journal 2008;1(1):33‐56. [Google Scholar]
Rydeard 2006 {published and unpublished data}
- Rydeard R, Leger A, Smith D. Pilates‐based therapeutic exercise: effect on subjects with nonspecific chronic low back pain and functional disability: a randomized controlled trial. Journal of Orthopaedic & Sports Physical Therapy 2006;36(7):472‐84. [DOI] [PubMed] [Google Scholar]
Wajswelner 2012 {published and unpublished data}
- Wajswelner H, Metcalf B, Bennell K. Clinical Pilates versus general exercise for chronic low back pain: randomized trial. Medicine & Science in Sports & Exercise 2011;44(7):1197‐205. [DOI: 10.1249/MSS.0b013e318248f665] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12607000471482 {published data only}
- ACTRN12607000471482. Short‐term effects of clinical pilates on pain and function in Australian Defence Force members with chronic low back pain: A feasibility study. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=82279 accessed 23 June 2015.
ACTRN12609000927224 {published data only}
- ACTRN12609000927224. Pilates and the effectiveness of the transversus abdominis in the individuals with low back pain. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12609000927224 accessed 23 June 2015.
Alves 2012 {published data only}
- Alves de Araujo ME, Bezerra da Silva E, Bragade Mello D, Cader SA, Shiguemi Inoue Salgado A, Dantas EH. The effectiveness of the Pilates method: reducing the degree of non‐structural scoliosis, and improving flexibility and pain in female college students. Journal of Bodywork and Movement Therapies 2012;16:191‐8. [DOI] [PubMed] [Google Scholar]
Anderson 2005 {published data only}
- Anderson BD. Randomized Clinical Trial Comparing Active Versus Passive Approaches to the Treatment of Recurrent and Chronic Low Back Pain [Thesis]. Miami, FL: University of Miami, 2005. [Google Scholar]
Anderson 2006 {published data only}
- Anderson BD, Butler MN, Roach KE. OPL44 ‐ A randomized controlled study examining the effects of Pilates on pain and disability in subjects with chronic and recurrent low back pain (CSM 2006 Orthopaedic Section Platform Presentations). Journal of Orthopaedic & Sports Physical Therapy 2006;36:A18. [Google Scholar]
Blum 2002 {published data only}
- Blum CL. Chiropractic and Pilates therapy for the treatment of adult scoliosis. Journal of Manipulative and Physiological Therapeutics 2002;25:E3. [DOI] [PubMed] [Google Scholar]
Boden 2010 {published data only}
- Boden Scott D. Spinescope. Seminars in Spine Surgery 2010;22:103‐8. [Google Scholar]
Cámara 2011 {published data only}
- Montero CJ, Sierra SE, Monteagudo SAM, López FJ, López LA, Barco PM. T643 ‐ Active stretching based on Pilates against passive analytical hamstring stretching in subacute and chronic non‐specific low back pain. Pilot trial. European Journal of Pain Supplements 2011;5:93. [Google Scholar]
Curnow 2009 {published data only}
- Curnow D, Cobbin D, Wyndham J, Boris Choy ST. Altered motor control, posture and the Pilates method of exercise prescription. Journal of Bodywork and Movement Therapies 2009;13:104‐11. [DOI] [PubMed] [Google Scholar]
da Luz 2014 {published data only}
- Luz MA Jr, Costa LO, Fuhro FF, Manzoni AC, Oliveira NT, Cabral CM. Effectiveness of mat Pilates or equipment‐based Pilates exercises in patients with chronic nonspecific low back pain: a randomized controlled trial. Physical Therapy 2014;94:623‐31. [DOI] [PubMed] [Google Scholar]
Donzelli 2006 {published data only}
- Donzelli S, Domenica F, Cova AM, Galletti R, Giunta N. Two different techniques in the rehabilitation treatment of low back pain: a randomized controlled trial. Europa Medicophysica 2006;42:205‐10. [PubMed] [Google Scholar]
Gagnon 2005 {published data only}
- Gagnon LH. Efficacy of Pilates Exercises as Therapeutic Intervention in Treating Patients with Low Back Pain [Thesis]. Knoxville, TN: University of Tennessee, 2005. [Google Scholar]
Hides 2012 {published data only}
- Hides JA, Stanton WR, Mendis MD, Gildea J, Sexton MJ. Effect of motor control training on muscle size and football games missed from injury. Medicine & Science in Sports & Exercise 2012;44:1141‐9. [DOI] [PubMed] [Google Scholar]
Ickes 2007 {published data only}
- Ickes DM. Improving postural awareness. The Interdisciplinary Journal of Rehabilitation 2007;20:18‐9. [PubMed] [Google Scholar]
Jaecks 2004 {published data only}
- Jaecks KS. Benefits of Pilates... Julie Kagan's "Pilates" article. RDH 2004;24:12. [Google Scholar]
Kagan 2008 {published data only}
- Kagan J. Mind your body to work without pain: movement is the key to keeping your body healthy and preventing musculoskeletal disorders. RDH 2008;28:32. [Google Scholar]
Kennedy 2012 {published data only}
- Kennedy S. Exercise rehabilitation programs for chronic non‐specific low back pain: a comparison of Pilates exercise and general aerobic exercise (Conference: Be Active 2012 Sydney). Journal of Science and Medicine in Sport 2012;15:S80. [Google Scholar]
McNeill 2009 {published data only}
- McNeill W. From journal page to treatment space. Journal of Bodywork and Movement Therapies 2009;13:93‐7. [DOI] [PubMed] [Google Scholar]
McNeill 2010 {published data only}
- McNeill W. About prevention. Journal of Bodywork and Movement Therapies 2010;14:289‐93. [DOI] [PubMed] [Google Scholar]
Mehling 2005 {published data only}
- Mehling WE, Hamel KA, Acree M, Byl N, Hecht FM. Randomized, controlled trial of breath therapy for patients with chronic low‐back pain. Alternative Therapies in Health and Medicine 2005;11:44‐52. [PubMed] [Google Scholar]
Natour 2011 {published data only}
- Natour J, Baptista AS, Cazotti LA, Ribeiro LHC, Jones A. Pilates to treat chronic non‐specific low back pain. Arthritis and Rheumatism (Abstracts of the American College of Rheumatology/Association of Rheumatology Health Professionals, Chicago) 2011;63:4‐9. [Google Scholar]
NCT01533805 {published data only}
- NCT01533805. Effects of Different Techniques of the Method Pilates in Chronic Low Back Pain. https://clinicaltrials.gov/ct2/show/NCT01533805 accessed 23 June 2015.
NCT01711203 {published data only}
- NCT01711203. The Addition of a Pilates Program for Short‐Term Improvements in Patients With Spondylolysis or Spondylolisthesis. https://clinicaltrials.gov/ct2/show/NCT01711203 accessed 23 June 2015.
NCT01919268 {published data only}
- NCT01919268. Addition of the Interferential Current to the Pilates Method in the Treatment of Chronic Nonspecific Low Back Pain. https://clinicaltrials.gov/ct2/show/NCT01919268 accessed 23 June 2015.
NCT01999283 {published data only}
- NCT01999283. Effect of Group Pilates and Yoga Exercise Classes for Chronic Cervical Pain (Pilates/Yoga). https://clinicaltrials.gov/ct2/show/NCT01999283 accessed 23 June 2015.
O'Brien 2006 {published data only}
- O'Brien M, Meldrum D. Randomised, controlled trial comparing physiotherapy and Pilates in the treatment of ordinary low back pain (Rehabilitation and Therapy Research Society Second Annual Conference). Physical Therapy Reviews 2006;11:224‐5. [Google Scholar]
PACTR201211000443397 {published data only}
- PACTR201211000443397. The effects of supervised versus non‐supervised pilates mat exercises on non‐specific chronic lower back pain. http://www.pactr.org/ATMWeb/appmanager/atm/atmregistry?dar=true&tNo=PACTR201211000443397 accessed 23 June 2015.
Parker 2010 {published data only}
- Parker SS. The Pilates approach to back pain: simple exercises can help dental professionals prevent and treat back pain. Dimensions of Dental Hygiene 2010;8:52‐3. [Google Scholar]
Phrompaet 2011 {published data only}
- Phrompaet S, Paungmali A, Pirunsan U, Sitilertpisan P. Effects of Pilates training on lumbo‐pelvic stability and flexibility. Asian Journal of Sports Medicine 2011;2:16‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rasmussen‐Barr 2003 {published data only}
- Rasmussen‐Barr E, Nilsson‐Wikmar L, Arvidsson I. Stabilizing training compared with manual treatment in sub‐acute and chronic low‐back pain. Manual Therapy 2003;8:233‐41. [DOI] [PubMed] [Google Scholar]
RBR‐7tyg5j {published data only}
- RBR‐7tyg5j. Effectiveness of the Pilates method performed in the equipament or mat Pilates in the treatment of chronic non‐specific low back pain. http://www.ensaiosclinicos.gov.br/rg/RBR‐7tyg5j/ accessed 23 June 2015.
Robinson 2007 {published data only}
- Robinson L. Body control Pilates. Talkback Magazine 2007:16‐8.
SeQueira 2010 {published data only}
- SeQueira EJ, Cornell PJ, Richards S. Modified Pilates exercises for low back pain: do they help? (Conference: Rheumatology 2010). Rheumatology. 2010.
Sherman 2010 {published data only}
- Sherman KJ, Cherkin DC, Cook AJ, Hawkes RJ, Deyo RA, Wellman R, et al. Comparison of yoga versus stretching for chronic low back pain: protocol for the Yoga Exercise Self‐care (YES) trial. Trials 2010;11:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sparrowe 2007 {published data only}
- Sparrowe L. Health in motion. Reestablish your core values: the pain may be in your back, but the solution lies in your belly. Alternative Medicine Magazine 2007:31‐3.
Tekur 2008 {published data only}
- Tekur P, Singphow C, Nagendra H R, Raghuram N. Effect of short‐term intensive yoga program on pain, functional disability and spinal flexibility in chronic low back pain: a randomized control study. Journal of Alternative and Complementary Medicine 2008;14:637‐44. [DOI] [PubMed] [Google Scholar]
Tilbrook 2011 {published data only}
- Tilbrook HE, Cox H, Hewitt CE, Kang'ombe AR, Chuang LH, Jayakody S, et al. Yoga for chronic low back pain: a randomized trial. Annals of Internal Medicine 2011;155:569‐78. [DOI] [PubMed] [Google Scholar]
Xue‐Qiang 2013 {published data only}
- Xue‐Qiang W, Jie‐Jiao Z, Pei‐Jie C. Clinical Pilates versus general exercise for chronic low back pain. Medicine & Science in Sports & Exercise 2013;45:603. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Anand 2014 {published data only}
- Anand UA, Caroline PM, Arun B, Gomathi GL. A study to analyse the efficacy of modified Pilates based exercises and therapeutic exercises in individuals with chronic non specific low back pain: a randomized controlled trial. International Journal of Physiotherapy and Research 2014;2:525‐9. [Google Scholar]
References to ongoing studies
RBR‐7yhzym {published data only}
- RBR‐7yhzym. Comparison between Pilates and conventional physical therapy for treatment of patients with nonspecific chronic low back pain: randomized controlled trial. http://www.ensaiosclinicos.gov.br/rg/RBR‐7yhzym/ accessed 23 June 2015.
Additional references
Anderson 2000
- Anderson BD, Spector A. Introduction to Pilates‐based rehabilitation. Orthopaedic Physical Therapy Clinics of North America 2000;9:395‐410. [Google Scholar]
Bekelman 2003
- Bekelman JE, Li Y, Gross CP. Scope and impact of financial conflicts of interest in biomedical research: a systematic review. JAMA 2003;289:454‐65. [DOI] [PubMed] [Google Scholar]
Borenstein 2009
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta‐Analysis. 1st Edition. Vol. 1, Chichester: John Wiley & Sons, Ltd, 2009. [Google Scholar]
Boutron 2005
- Boutron I, Moher D, Tugwell P, Giraudeau B, Poiraudeau S, Nizard R, et al. A checklist to evaluate a report of a non pharmacological trial (CLEAR NPT) was developed using consensus. Journal of Clinical Epidemiology 2005;58(12):1233‐40. [DOI] [PubMed] [Google Scholar]
Bryan 2003
- Bryan M, Hawson S. The benefits of Pilates exercise in orthopaedic rehabilitation. Techniques in Orthopaedics 2003;18(1):126‐9. [Google Scholar]
Chou 2007
- Chou R, Qaseem A, Snow V, Casey D, Cross JT, Shekelle P, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Annals of Internal Medicine 2007;147(7):478‐91. [DOI] [PubMed] [Google Scholar]
Costa 2009
- Costa LCM, Maher CG, McAuley JH, Hancock MJ, Herbert RD, Refshauge KM, et al. Prognosis for patients with chronic low back pain: inception cohort study. BMJ 2009;339(7725):b3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Costa 2012
- Costa LCM, Maher CG, Hancock M, McAuley JH, Herbert RD, Costa LOP. The prognosis of acute and persistent low back pain: a meta‐analysis. Canadian Medical Association Journal 2012;184(11):e613‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dagenais 2008
- Dagenais DC, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. The Spine Journal 2008;8(1):8‐20. [DOI] [PubMed] [Google Scholar]
Delitto 2012
- Delitto A, George SZ, Dillen LR, Whitman JM, Sowa G, Shekelle P, et al. Orthopaedic Section of the American Physical Therapy, Association. Low back pain. Journal of Orthopaedic & Sports Physical Therapy 2012;42(4):A1‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
European Guidelines 2006
- COST Action B13 working group. European Guidelines for the Management of Non‐Specific Low Back Pain. European Spine Journal 2006;15(Suppl 2):S1‐S300. [Google Scholar]
Furlan 2009
- Furlan AD, Pennick V, Bombardier C, Tulder M. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine 2009;34(18):1929‐41. [DOI] [PubMed] [Google Scholar]
Hayden 2005
- Hayden J, Tulder MV, Malmivaara A, Koes BW. Exercise therapy for treatment of non‐specific low back pain. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD000335.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hayden 2007
- Hayden JA, Tulder MW, Tomlinson G. Systematic review: strategies for using exercise therapy to improve outcomes in chronic low back pain. Annals of Internal Medicine 2005;142(9):776‐85. [DOI] [PubMed] [Google Scholar]
Henschke 2008
- Henschke N, Maher CG, Refshauge KM, Herbert RD, Cumming RG, Bleasel J, et al. Prognosis in patients with recent onset low back pain in Australian primary care: inception cohort study. BMJ 2008;337(7662):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hodges 1996
- Hodges PW, Richardson CA. Inefficient muscular stabilization of the lumbar spine associated with low back pain. A motor control evaluation of transversus abdominis. Spine 1996;21(22):2640‐50. [DOI] [PubMed] [Google Scholar]
Hoy 2012
- Hoy D, Bain C, Williams G, March L, Brooks P, Blyth F, et al. A systematic review of the global prevalence of low back pain. Arthritis & Rheumatology 2012;64(6):2028‐37. [DOI] [PubMed] [Google Scholar]
Lim 2011
- Lim EC, Poh RL, Low AY, Wong WP. Effects of Pilates‐based exercises on pain and disability in persistent nonspecific low back pain: a systematic review with meta‐analysis. Journal of Orthopaedic & Sports Physical Therapy 2011;41(2):70‐80. [DOI] [PubMed] [Google Scholar]
Mueller 2007
- Mueller PS, Montori VM, Bassler D, Koenig BA, Guyatt GH. Ethical issues in stopping randomized trials early because of apparent benefit.. Annals of Internal Medicine 2007;146(12):878‐81. [DOI] [PubMed] [Google Scholar]
Musculino 2004
- Musculino JE, Cipriani S. Pilates and the "powerhouse"‐ I. Journal of Bodywork and Movement Therapies 2004;8(1):15‐24. [Google Scholar]
NCT01502059
- NCT01502059. Pilates to Treat Low Back Pain (PTLBP). https://clinicaltrials.gov/ct2/show/NCT01502059 accessed 23 June 2015. [NCT01502059]
Okike 2008
- Okike K, Kocher MS, Mehlman CT, Bhandari M. Industry‐sponsored research. Injury 2008;39:666‐80. [DOI] [PubMed] [Google Scholar]
Ostelo 2008
- Ostelo RW, Deyo RA, Stratford P, Waddell G, Croft P, Korff M, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine 2008;33(1):90‐4. [DOI] [PubMed] [Google Scholar]
Posadzki 2011
- Posadzki P, Lizis P, Hagner‐Derengowska M. Pilates for low back pain: a systematic review. Complementary Therapy Clinical Practice 2011;17(2):85‐9. [DOI] [PubMed] [Google Scholar]
Queiroz 2005
- Queiroz BC, Cagliari MF, Amorim CF, Sacco IC. Muscle activation during four Pilates core stability exercises in quadrupled position. Archives of Physical Medicine and Rehabilitation 2010;91(1):86‐92. [DOI] [PubMed] [Google Scholar]
Rackwitz 2006
- Rackwitz B, Bie R, Limm H, Garnier K, Ewert T, Stucki G. Segmental stabilizing exercises and low back pain. What is the evidence? A systematic review of randomized controlled trials. Clinical Rehabilitation 2006;20(7):553‐67. [DOI] [PubMed] [Google Scholar]
Rubinstein 2011
- Rubinstein SM, Middelkoop M, Assendelft WJJ, Boer MR, Tulder MW. Spinal manipulative therapy for chronic low‐back pain. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD008112.pub2] [DOI] [PubMed] [Google Scholar]
Rubinstein 2012
- Rubinstein SM, Terwee CB, Assendelft WJJ, Boer MR, Tulder MW. Spinal manipulative therapy for acute low‐back pain. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD008880.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Tulder 2003
- Tulder M, Furlan A, Bombardier C, Bouter L, Editorial Board Cochrane Back Review Group. Updated method guidelines for systemic reviews in the Cochrane Collaboration Back Review Group. Spine 2003;28(12):1290‐9. [DOI] [PubMed] [Google Scholar]
Waddell 2004
- Waddell G. The Back Pain Revolution. Churchill Livingstone, 2004. [Google Scholar]
Wells 2012
- Wells C, Kolt GS, Bialocerkowski A. Defining Pilates exercise: a systematic review. Complementary Therapy Medicine 2012;20(4):253‐62. [DOI] [PubMed] [Google Scholar]
Wells 2014
- Wells C, Kolt GS, Marshall P, Hill B, Bialocerkowski A. The effectiveness of Pilates exercise in people with chronic low back pain: a systematic review. PloS One 2014;9(7):e100402. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Costa 2012b
- Costa LOP, Hancock M, Maher CG, Ostelo RWJG, Cabral CMN, Menezes Costa LD. Pilates for low‐back pain. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD010265] [DOI] [Google Scholar]


