Abstract
Background
Gender dysphoria is described as a mismatch between an individual's experienced or expressed gender and their assigned gender, based on primary or secondary sexual characteristics. Gender dysphoria can be associated with clinically significant psychological distress and may result in a desire to change sexual characteristics. The process of adapting a person's sexual characteristics to their desired sex is called ‘transition.'
Current guidelines suggest hormonal and, if needed, surgical intervention to aid transition in transgender women, i.e. persons who aim to transition from male to female. In adults, hormone therapy aims to reverse the body's male attributes and to support the development of female attributes. It usually includes estradiol, antiandrogens, or a combination of both. Many individuals first receive hormone therapy alone, without surgical interventions. However, this is not always sufficient to change such attributes as facial bone structure, breasts, and genitalia, as desired. For these transgender women, surgery may then be used to support transition.
Objectives
We aimed to assess the efficacy and safety of hormone therapy with antiandrogens, estradiol, or both, compared to each other or placebo, in transgender women in transition.
Search methods
We searched MEDLINE, the Cochrane Central Register of Controlled Trials (CENTRAL), Embase, Biosis Preview, PsycINFO, and PSYNDEX. We carried out our final searches on 19 December 2019.
Selection criteria
We aimed to include randomised controlled trials (RCTs), quasi‐RCTs, and cohort studies that enrolled transgender women, age 16 years and over, in transition from male to female. Eligible studies investigated antiandrogen and estradiol hormone therapies alone or in combination, in comparison to another form of the active intervention, or placebo control.
Data collection and analysis
We used standard methodological procedures expected by Cochrane to establish study eligibility.
Main results
Our database searches identified 1057 references, and after removing duplicates we screened 787 of these. We checked 13 studies for eligibility at the full text screening stage. We excluded 12 studies and identified one as an ongoing study. We did not identify any completed studies that met our inclusion criteria. The single ongoing study is an RCT conducted in Thailand, comparing estradiol valerate plus cyproterone treatment with estradiol valerate plus spironolactone treatment. The primary outcome will be testosterone level at three month follow‐up.
Authors' conclusions
We found insufficient evidence to determine the efficacy or safety of hormonal treatment approaches for transgender women in transition. This lack of studies shows a gap between current clinical practice and clinical research. Robust RCTs and controlled cohort studies are needed to assess the benefits and harms of hormone therapy (used alone or in combination) for transgender women in transition. Studies should specifically focus on short‐, medium‐, and long‐term adverse effects, quality of life, and participant satisfaction with the change in male to female body characteristics of antiandrogen and estradiol therapy alone, and in combination. They should also focus on the relative effects of these hormones when administered orally, transdermally, and intramuscularly. We will include non‐controlled cohort studies in the next iteration of this review, as our review has shown that such studies provide the highest quality evidence currently available in the field. We will take into account methodological limitations when doing so.
Plain language summary
Does hormone therapy help transgender women undergoing gender reassignment to transition?
Background
Transgender women may feel that they have been born in a body with the wrong sexual characteristics. This may result in significant psychological distress (gender dysphoria) and the desire to adapt their male physical and sexual characteristics to be more consistent with their experienced female gender. This is a process called transition. If measures to aid transition are not taken, this can result in greater psychological distress. One of the medical treatments given to help transgender women with male bodies to achieve transition is synthetic female hormones. These hormones can be taken by mouth, absorbed through the skin or injected into muscle.
Study characteristics
We looked for randomised controlled trials (RCTs) that included transgender women (age 16 and over) in transition from male to female. RCTs are a type of research study that can reduce the possibility of several types of bias. To be included in this review, studies needed to compare different hormone treatments used to support transgender women to transition (oestrogen alone, testosterone blockers alone, or oestrogen in combination with testosterone blockers), or compare these hormone treatments to placebos (fake or dummy treatments that appear to be the same as the actual treatment, but have no medical effects). We wanted to see whether hormone treatments help transgender women to make a transition that they are happy with. We also wanted to look at whether there were any health risks of the treatment.
Key results
We searched for studies up to 19 December 2019. We were unable to find any relevant completed studies that we could include. We did find one ongoing study that aimed to recruit all of the people taking part in the study by the end of 2020. This study is comparing the effects of estradiol valerate plus cyproterone treatment with estradiol valerate plus spironolactone treatment in transitioning transgender women in Thailand.
Quality of evidence
Our review found no RCTs that looked at whether hormone therapies are effective and safe when used to help transgender women to transition. Therefore, high‐quality RCTs are needed to research these questions.
Background
Description of the condition
There is a growing trend towards de‐psychopathologisation of transgenderism (Drescher 2014; ATME 2015). There is an emerging consensus that transgenderism is not a psychiatric disorder (WPATH 2011). For instance, the 11th Revision of the International Classification of Diseases (ICD‐11) (WHO 2018) no longer classifies transgenderism as a behavioural and personality disorder, but has instead drafted the term "gender incongruence" to describe gender dysphoria.
In contrast, the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM–5) (DSM‐5 2013) describes gender dysphoria as a "marked incongruence between one’s experienced/expressed gender and assigned gender, of at least six months duration, as manifested by at least two of the following" characteristics:
A marked incongruence between one’s experienced/expressed gender and primary and/or secondary sex characteristics (or, in young adolescents, the anticipated secondary sex characteristics);
A strong desire to be rid of one’s primary and/or secondary sex characteristics because of a marked incongruence with one’s experienced/expressed gender (or, in young adolescents, a desire to prevent the development of the anticipated secondary sex characteristics);
A strong desire for the primary and/or secondary sex characteristics of the other gender;
A strong desire to be of the other gender (or some alternative gender different from one’s assigned gender);
A strong desire to be treated as the other gender (or some alternative gender different from one’s assigned gender);
A strong conviction that one has the typical feelings and reactions of the other gender (or some alternative gender different from one’s assigned gender).
Gender dysphoria has been defined as associated with "clinically significant distress or impairment in social, occupational or other important areas of functioning" (Zucker 2016), which may lead to substantial suffering in affected people (Deutsch 2016a; Soll 2018). Gender dysphoria may result in the desire to modify one's physical and sexual characteristics to be consistent with those of the experienced gender. This process of adaptation is called transition.
The treatments applied in transition differ from those used for maintenance of the new sexual characteristics. Currently, there is uncertainty about the value of hormone therapy as a sole intervention, or when combined with surgery, for transition from male to female. This Cochrane Review specifically focuses on ‘transgender women in transition from male to female,' a definition that includes biological males aiming to adapt their sexual characteristics to be consonant with those of females.
A meta‐analysis that analyzed 21 studies on the prevalence of gender dysphoria (of which 12 studies contained evaluable data) estimated an overall prevalence of transgender women with gender dysphoria at 6.8 per 100,000 individuals (Arcelus 2015).
Description of the intervention
Current guidelines suggest hormonal and, if needed, surgical treatment of gender dysphoria in transgender women (WPATH 2011). Hormone therapy aims to suppress the development of, or to reverse, male attributes that have already developed. At the same time, hormones aim to develop female attributes. However, where male characteristics have already developed in adult males, such as in the bone structure of the face, hormones are not effective. Other treatments, such as surgery, would be required to change these (WPATH 2011).
The guidelines of the Endocrine Society working group suggest treatment with both oestrogens and antiandrogens (Hembree 2017). Oestrogens can be administered as either oral oestrogen, absorbed through transdermal estradiol patches, or by injection of estradiol valerate or estradiol cypionate. The application frequency differs depending on the patient’s reaction to the agent and the administration regimen; it could be multiple times per day or once every two weeks. Meanwhile, antiandrogens such as spironolactone or cyproterone acetate (CPA) are commonly taken orally. Additionally, it is possible to block male puberty by treatment with gonadotropin‐releasing hormone (GnRH) agonist injections (Hembree 2017).
While not every transgender woman undergoes hormone therapy in her transition, this intervention is still widely used (Hembree 2017). We know of no studies identifying the ratio of patients who undergo hormone therapy, nor do we know of studies investigating how much time passes between the start of transition (the decision to transition) and the start of hormone therapy. We are not aware of any studies on how often antiandrogens are being prescribed in addition to or instead of 17‐beta‐estradiol, how often they are being taken, or which kinds of androgens are in use besides CPA and spironolactone.
How the intervention might work
Several hormonal substances and combinations are used clinically for hormone therapy in transitioning women. CPA is a progestin, steroidal anti‐androgen and anti‐gonadotropin that blocks the receptors for testosterone (T) and dihydrotestosterone (DHT), and thereby prevents these steroidal hormones from exerting their androgenic effects. Hence, it stops processes like body hair growth, hair loss on the head, male body fat distribution and others (Figg 2010; WPATH 2011). According to the World Professional Association for Transgender Health (WPATH) guidelines, it is possible to suppress puberty with GnRH analogues or progestins such as medroxyprogesterone (WPATH 2011).
Spironolactone acts as a weak androgen receptor antagonist (Wenqing 2005). It also causes an increase in oestradiol levels (Thompson 1993), so that further virilisation is prevented and feminisation occurs (WPATH 2011).
17‐beta‐estradiol is used to feminise the external appearance (WPATH 2011). It binds to oestrogen receptors and thus ensures gene expression, which in turn feminises appearance (Hye‐Rim 2012). In addition, estradiol suppresses gonadal testosterone production via the control systems of the hypothalamus (Hayes 2000).
Feminisation therapy aims to adapt the physical appearance and experience of the male body to that of a female body, by inducing breast growth, softening facial features, and inducing other physical changes commonly considered to comprise a feminine appearance (WPATH 2011). For this purpose, oral or transdermal oestrogen is recommended, and therapy with oestrogen in combination with antiandrogens is most common. Co‐treatment with antiandrogens minimises the required dose of oestrogen, and thereby reduces the potential risks of oestrogen identified in previous studies (Schürmeyer 1986; Prior 1989). Some antiandrogens are approved by WPATH, such as spironolactone, cyproterone acetate, GnRH analogues like goserelin, and 5‐alpha‐reductase inhibitors like finasteride (WPATH 2011).
Why it is important to do this review
Antiandrogens like CPA and spironolactone are prescribed to transgender women in transition by clinicians, including gynaecologists and endocrinologists (Schneider 2006; Flütsch 2015), and they are commonly considered to be valuable drugs to support transition (WPATH 2011; Hembree 2017). However, clinical evidence suggests that taking these drugs can result in adverse events; for example, CPA has significant potential for causing depression and for worsening depressive symptoms (Seal 2012). There is also some concern that CPA can lead to other psychiatric, neurological, and metabolic disorders (Griard 1978; Ramsay 1990; Oberhammer 1996; Giltay 2000; Calderón 2009; Bessone 2015). The most common adverse effects of spironolactone are hyperkalaemia, dehydration and hyponatraemia (Greenblatt 1973). Furthermore, spironolactone might have an influence on feelings of anxiety (Fox 2016).
Other studies from the 1980s and 90s reported that there were adverse effects from high‐dose estradiol, but these studies used ethinyl estradiol or equine premarin (equine estradiol) instead of bioidentical 17‐beta‐estradiol; and used progestins, instead of bioidentical progesterone. This may have contributed to the adverse effect profile of these specific treatments (Prior 1989). Unlike the bioidentical alternatives used today (hormone preparations made from plant sources that are similar or identical to human hormones), substances administered in the past (e.g. equine oestrogens, ethinyl estradiol) were associated with more diverse adverse effects like thrombophilia, cardiovascular problems, breast and prostate cancer, as well as liver, adrenal gland and neural dysfunction (Griard 1978; Calderón 2009; Asscheman 2011). The health risks attributed to estradiol doses high enough to suppress androgens have not been found in the parenteral or transdermal application of bioidentical estradiol (Hembree 2017). Thus, it is unclear why those estradiol doses should be kept low in order to make the addition of androgen antagonists like CPA or spironolactone necessary.
In light of discussions among experts (Seal 2012; Wierckx 2014), and current recommendations for hormonal gender affirmation treatment (WPATH 2011) (which are strongly based on the values and preferences of health consumers), it is necessary to review the evidence from trials that show results for outcomes such as feminisation, satisfactory sexual function, reduced gender dysphoria, and improved quality of life (e.g. Murad 2010).
In 2017, the overall quality of evidence relating to these outcomes was classified as low (Hembree 2017). In 2011, WPATH summarised the situation as follows. "There is a need for further research on the effects of hormone therapy without surgery, and without the goal of maximum physical feminisation or masculinisation" (WPATH 2011). It is necessary to determine whether subsequent trials have provided additional evidence for efficacy, or whether there is still a lack of evidence for these desired outcomes.
Objectives
We aimed to assess the efficacy and safety of hormone therapy with antiandrogens, estradiol, or both, compared to each other or placebo, in transgender women in transition.
Methods
Criteria for considering studies for this review
Types of studies
We aimed to include randomised controlled trials (RCTs), quasi‐RCTs and controlled cohort studies.
We chose to include quasi‐RCTs and cohort studies due to the low prevalence of the condition and the consequent current scarcity of RCTs (WPATH 2011).
Types of participants
We aimed to include studies that enrolled transgender women, age 16 years and over, in transition from male to female. Transitioning is defined as the process of changing one's gender profile or sexual characteristics (or both) to accord with one's sense of gender identity (WPATH 2011). Transition as a concept thus encompasses several aspects, e.g. social, psychological, or physical aspects, or a combination of these. There is consistency in the literature on when the transition begins: namely, with the decision to change a person's gender assignment (Brown 1996). However, we did not differentiate among any supposed phases of the respective types of transitions. Depending on the personal situation, the process of transition (which may include the decision to transition, gathering of information, gathering of experience, medical treatment and change of social role), can take very different periods of time, usually several months to years. Therefore, it is difficult to distinguish certain 'phases' of this process. When focusing on hormone therapy, the transition term can be more precisely defined. The transition process lasts as long as patients are in the process of changing their sexual characteristics (WPATH 2011). We aimed to include studies with participants age 16 years and older because, according to currently applied guidelines, this is the age when patients start being treated with hormone therapy. Patients below this age are usually being treated with puberty blockers, which are outside the scope of this review (WPATH 2011).
Types of interventions
We considered studies evaluating hormone‐based interventions only, excluding those that examined combined hormonal and either psychological or surgical treatments. We aimed to include studies reporting treatment with the following experimental interventions.
Antiandrogens (cyproterone acetate or spironolactone) and estradiol
Antiandrogens (cyproterone acetate or spironolactone) alone
Estradiol alone
For the above interventions, we considered all types of administration: oral, sublingual, transdermal, subdermal and intramuscular. For estradiol, we also considered bioidentical 17‐beta‐estradiol, as well as synthetic derivatives.
We aimed to include the following comparator interventions.
Any of the active interventions listed above
Placebo
Although we consider placebo‐controlled studies to be unethical (Bostick 2008), we made them eligible for inclusion in this review so that we could consider the evidence in its entirety. We did not consider interventions consisting purely of psychological treatment, spiritual support, or conversion therapy.
Types of outcome measures
For studies with repeated follow‐up (i.e. reporting of outcomes at multiple time points), we regarded follow‐up at three to six months as short term, six months to two years as medium term, and more than two years as long term (WPATH 2011).
We intended to include in the descriptive section of the review all studies that met the criteria for type of study, participants, intervention and comparator, regardless of outcomes reported or missing data.
Primary outcomes
Quality of life (QoL) as measured by validated generic instruments, e.g. Quality of Life Inventory (QOLI) (Frisch 2005); or specific instruments, e.g. for body image, the Body Image Quality of Life Inventory (BIQLI) (Cash 2004); or for sexual life the Sexual Satisfaction Scale for Women (SSS‐W) (Meston 2005).
Satisfaction with change of male to female body characteristics, as measured with validated instruments
Adverse events specific to hormone therapy, including serious adverse events
Secondary outcomes
Severity of gender dysphoria/gender incongruence, e.g. as measured with the Utrecht Gender Dysphoria Scale (UGDS) (Schneider 2016)
-
Measures of specific body changes, including:
breast size, e.g. by measurement of bust girth;
skin thickness, e.g. by echographic measurement (Laurent 2007);
skin sebum production, e.g. as measured by three‐hour sebum collection with absorbent paper (Downing 1981; Giltay 2008; Ezerskaia 2016); and
hair growth, including hair density, diameter, growth rate and anagen/telogen ratio (Giltay 2000; Hoffmann 2013).
Incidence or severity of depression.
We did not include surrogate outcomes, such as serum hormone levels (e.g. 17‐beta‐estradiol or testosterone). While these measures can help with monitoring the progress of hormone therapy, they are of little interest of themselves, especially since individuals require varying levels of these hormones to achieve a certain level of feminisation (Gooren 2017).
Search methods for identification of studies
Electronic searches
We searched the following electronic databases for relevant trials up to 19 December 2019 with no restrictions based on language of publication, date of publication, or publication status:
MEDLINE via PubMed
Cochrane Central Register of Controlled Trials (CENTRAL)
Embase
Biosis Preview
PsycINFO
PSYNDEX
Our search strategy is outlined in Appendix 1. We have successfully tested the screening methods for abstracts and titles.
Searching other resources
Had we identified any eligible studies through the electronic searches above we would have searched the reference lists of these in order to find additional relevant studies. We also searched the scientific abstracts of the last two meetings of each of the following organisations:
American Association of Clinical Endocrinologists
American Society of Andrology
Berufsverband der deutschen Endokrinologen (Professional Association of the German Endocrinologists)
Berufsverband der Frauenärzte e.V. (Professional Association of the Gynaecologists)
Dachverband Reproduktionsbiologie und Medizin e.V. (Federal Association Reproductive Biology and Medicine)
Deutsche Gesellschaft für Endokrinologie (German Society for Endocrinology)
Deutsche Gesellschaft für Gynäkologie und Geburtshilfe (German Society for Gynaecology and Obstretics)
Endocrine Society
European Society of Gynaecological Oncology
European Thyroid Association
Nordrhein‐Westfälische Gesellschaft für Endokrinologie und Diabetologie (North Rhine‐Westphalian Society for Endocrinology and Diabetology)
Royal College of Obstetricians and Gynaecologists
Society for Endocrinology
Society for Gynaecologic Investigation
We also searched the following grey literature databases:
The New York Academy of Medicine Grey Literature Report (www.greylit.org/)
OAIster (www.oclc.org/oaister.en.html)
OpenGrey (www.opengrey.eu/)
Finally, in order to identify completed but unpublished or ongoing studies, we searched the following trial registries.
ClinicalTrials.gov (www.clinicaltrials.gov/)
metaRegister of Controlled Trials (mRCT; www.controlledtrials.com/mrct/)
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) Search Portal (www.who.int/trialsearch/)
Drugs@FDA (www.accessdata.fda.gov/scripts/cder/drugsatfda/)
European Public Assessment Reports (EPAR; www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/landing/epar_search.jsp)
We contacted fifteen manufacturers of hormonal agents and experts in the field to identify unpublished or ongoing trials.
Data collection and analysis
Selection of studies
We used the reference management tool Covidence to identify and remove potential duplicate records of relevant studies (www.covidence.org). Two review authors (AKU and MHE) independently scanned titles and abstracts of the remaining records to compile a list of potential papers to potentially be included in the review. After this, the same review authors investigated the references in detail (as full text articles or matched records to studies), and categorised these as ‘included studies,' ‘excluded studies,' ‘studies awaiting classification' and ‘ongoing studies.' We executed this task in accordance with the criteria provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). If there had been discrepancies or if a consensus could not be reached, a third review author would have adjudicated (CHA). There were no disagreements that could not be thus resolved. Had this been the case, we would have designated the study as ‘awaiting classification' and contacted the study authors for clarification. We listed studies excluded during the full text review stage, and documented the reasons for exclusion in Characteristics of excluded studies. We included an adapted PRISMA flow diagram outlining the study selection process (Moher 2009) (Figure 1).
1.
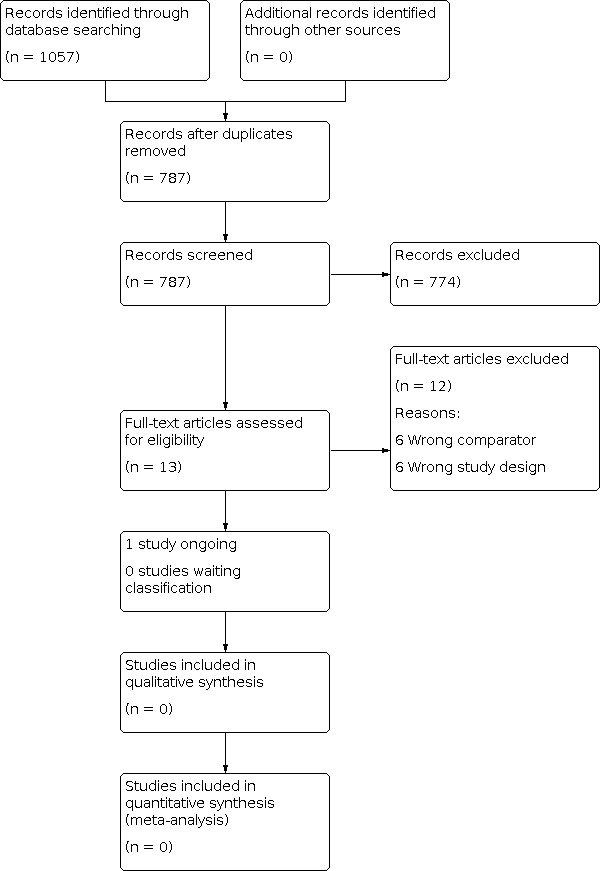
Study Flow Diagram
Data extraction and management
If we had found relevant studies, two review authors (AKU and MHE) would have extracted data from all studies deemed eligible for inclusion independently, with the help of a standardized data extraction form that would have been pilot tested according to Chapter 7 of the Cochrane Handbook (Higgins 2011a). We have used Google Spreadsheets to manage all data gathered.
We would have collected data on the following items:
General information on the study: first author, date of publication, study dates, publication type (full text article, abstract, unpublished), citation.
Study methods: study design (e.g. parallel, factorial), number of study arms, study setting (single institution, multi‐centre national, multi‐centre international), study location, and length of follow‐up.
Participant characteristics: study inclusion/exclusion criteria, age (mean/median with range), ethnic distribution, number of participants randomised and included in analysis, participants lost to follow‐up.
Interventions: type of hormonal agents (for example CPA, estradiol, progesterone, spironolactone), dose, administration route, dosing schedule and any other associated therapies. We would have extracted data on the sample size for each intervention group.
Outcomes: definition and method of assessment for each outcome (including the adverse event classification system used in individual studies), as well as any relevant subgroups. We would have extracted the number of events and participants per treatment group for dichotomous outcomes. We would also extract the mean, standard deviation or median and range, and number of participants per treatment group for continuous outcomes.
Study funding sources.
Declarations of potential conflicts of interest reported by study authors.
For each included study, we would have extracted the outcome data relevant for this review, and which would be required for the calculation of summary statistics and measures of variance. If there had been disagreements, we would have resolved them by discussion. If necessary, we would have consulted a third review author (CHA). We provided key information about potentially relevant ongoing studies, including trial identifiers, in the table of Characteristics of ongoing studies. We would have attempted to contact authors of included studies to obtain missing key data if needed.
Assessment of risk of bias in included studies
If relevant studies had been found, two review authors (AKU and MHE) would have examined all included studies to assess risk of bias (assessment of methodological quality) independently. We would have used the Cochrane 'Risk of bias' tool for assessing risk of bias in RCTs, as described in the Cochrane Handbook (Higgins 2011b). We would have resolved disagreements by consensus or by consulting a third review author (CHA). Our summary judgement would have included a rating (low, high or unclear risk of bias) for each domain (Higgins 2011b). We would have assessed the risk of bias for the following domains:
Random sequence generation
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data
Selective reporting
Other bias
We would have evaluated the risks of performance bias (blinding of participants and personnel) and detection bias (blinding of outcome assessment) separately for each outcome.
For any relevant cohort studies we would have used the ROBINS‐I tool to assess risk of bias (Sterne 2016). We would have assessed each individual study in accordance with the guidance, documenting the results using a spreadsheet and providing details in ‘Risk of bias' tables. We would have documented the reasons for our judgements, and would have included relevant quotations from the full‐text articles or from information about the study provided by authors in the notes section of the 'Risk of bias' tables. We would have summarised the risk of bias across domains for each primary outcome in every included study, as well as across studies and domains for each primary outcome.
Measures of treatment effect
Dichotomous data
We planned to summarise dichotomous data using risk ratios (RRs), reported with 95% confidence intervals (CIs).
Continuous data
For continuous outcomes with a standard measure, we would have summarised the obtained data as mean differences (MDs) with 95% CIs. For continuous outcomes without a standard measure, we would have summarised data as standardized mean differences (SMDs) with 95% CIs. Alternatively, if the mean value and variance were missing, we would have estimated them using the methods described in Hozo 2005, which allows estimations for mean value and variance of a sample when only the median, range and size of the sample are known. We would also have considered the guidance in the Cochrane Handbook where appropriate (Higgins 2011c).
Unit of analysis issues
We planned to treat recurring events in individual participants as single events occurring in one participant (e.g. three episodes of major depressive disorder in one participant would have been recorded as one participant with major depressive disorder). We did not expect to include studies with interventions delivered at the cluster level.
Dealing with missing data
For studies with missing data, we would have followed the recommendations of the Cochrane Handbook (Higgins 2011d). We would have collected dropout rates for each study group and would have reported these in the ‘Risk of bias' table. Our preferred option would have been to contact study authors in cases of missing data or statistics that were not due to participant dropout (e.g. missing statistics such as standard deviation (SD)). If missing outcome data were not provided, then we would have attempted to impute data where possible and appropriate, and conduct sensitivity analyses to assess the effect of this on the analysis. However, where imputation is not appropriate, we would not have included the study in the respective meta‐analysis, and would have discussed the potential impact of this in the text of the review. In the case of participants lost to follow‐up, we would have performed meta‐analyses on an intention‐to‐treat basis. We would have performed sensitivity analyses, excluding studies with missing outcome data, to evaluate the impact of missing data. We would have discussed the potential impact of missing data on review findings in the ‘Discussion' section of the full review, using a summary table if appropriate.
Assessment of heterogeneity
We would have compared the characteristics of included studies to identify heterogeneity of content or methodology, and to determine the feasibility of performing a meta‐analysis. We would have deemed meta‐analyses unsuitable in cases where there was substantial content‐related or methodological heterogeneity across studies. Instead, we would have used a narrative approach to data synthesis. Had meta‐analyses been deemed appropriate, we would have assessed statistical heterogeneity by visually inspecting the scatter of individual study effect estimates on forest plots and by calculating the I2 statistic (Higgins 2011c), which gives the percentage of variability in effect estimations that can be attributed to heterogeneity rather than to chance. We would have considered an I2 of more than 50% to represent substantial heterogeneity. In the case of statistical heterogeneity, we would have conducted the prespecified subgroup and sensitivity analyses described below to investigate the source.
Assessment of reporting biases
If we had included 10 or more studies that investigated the same outcome, we would have used funnel plots to assess small‐study effects and publication bias. Given that several explanations are possible for funnel plot asymmetry, we would have interpreted results carefully (Sterne 2011).
Data synthesis
Had we identified any eligible studies, we would have provided a narrative summary of the included studies. We would also have conducted meta‐analyses of RCTs for all relevant outcomes, where possible, using data from studies that 1) compared the actual hormone therapy‐relevant agents or combinations of agents to placebo, and 2) compared the actual hormone therapy‐relevant agents or combinations of agents to other hormone therapy‐agents or combinations of agents. Studies comparing two variations on the intervention would have been pooled separately to studies comparing the intervention to placebo. However, if there had been significant variability in the definition of outcomes across trials, we would have decided not to pool data.
Had we conducted meta‐analyses, we would have used the Mantel‐Haenszel approach to combine dichotomous data and calculate RRs with 95% CIs (Higgins 2011c). For continuous outcomes (e.g. quality of life) we would have calculated MDs or SMDs, with 95% CIs, using the inverse variance approach. Had studies reported the same outcome measure but some studies had reported data on the change from baseline (e.g. mean values and standard deviations) and others for final measurements of outcomes, they would have been placed in subgroups in the meta‐analysis and pooled according to guidance in the Cochrane Handbook (Higgins 2011c).
For meta‐analyses, we would have used a random‐effects model, expecting the true effects to be related, but not the same, across all studies. We would have interpreted random‐effects meta‐analyses with due consideration of the whole distribution of effects, ideally by presenting a prediction interval (Higgins 2009). A prediction interval specifies a predicted range for the true treatment effect in an individual study (Riley 2011). In addition, we would have performed statistical analyses according to the statistical guidelines contained in the Cochrane Handbook (Higgins 2011c).
We would have summarised outcome data from cohort studies (e.g. change scores) narratively.
Subgroup analysis and investigation of heterogeneity
Wherever possible, we would have considered subgroup analyses that are structured by the following characteristics.
Type of application of intervention (oral, transdermal, intramuscular, subcutaneous)
Orchiectomy before or during hormone therapy
The justification for these analyses is as follows. Pharmacokinetic mechanisms lead to significant differences in the absorption and metabolism of an active substance depending on the type of application. Therefore, we would, if possible, have formed appropriate subgroups based on the application method of the intervention. Also, patients who have undergone an orchiectomy could have different outcomes than those patients without orchiectomy (Defreyne 2017).
Sensitivity analysis
We would have conducted sensitivity analyses to investigate any potential effect of removing studies judged to be at high risk of bias from meta‐analyses. We would have classified studies as being at high risk of bias overall if one or more domains were judged to be at high risk. If appropriate, we would also have conducted sensitivity analyses excluding studies with missing outcome data, or where missing data have been imputed by the review author team. We would also have conducted a sensitivity analysis to compare a fixed‐effect model to a random effects model where the studies in a meta‐analysis appear more homogeneous than expected.
Summary of findings and assessment of the certainty of the evidence\
Following standard Cochrane methodology, had we identified any included studies, we would have created a 'Summary of findings' table for all three primary outcomes. Also following standard Cochrane methodology, we would have used the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome, and to draw conclusions about the quality of evidence within the text of the review.
Results
Description of studies
Results of the search
We conducted our searches on 18 January 2019 and updated them on 19 December 2019. Through the database searches, we identified a total of 1057 references. After removing duplicates, we screened the titles and abstracts of 787 references. Through this screening, we identified 13 studies to assess as full text articles. We fully inspected these articles, and excluded 12 studies. The remaining study was still ongoing. Therefore, we did not include any studies in this review (Figure 1).
Of the manufacturers and experts in the field whom we contacted,15 responded but did not report any additional studies.
Included studies
None of the reports retrieved met the inclusion criteria for this review. Suggestions for future studies are given in Table 1
1. Suggested design of future studies.
| Methods | RCT or controlled cohort study |
| Participants | Transgender women experiencing gender dysphoria, in transition N* Age: from the age of 16 years |
| Intervention |
All types of administration: oral, sublingual, transdermal, subdermal and intramuscular. For estradiol and bioidentical 17‐beta‐estradiol, as well as synthetic derivatives. |
| Comparator | Any of the active interventions listed above |
| Outcomes | Primary outcomes
|
| Notes | * Size of study with sufficient power to detect a ~ 10% difference between the two groups for primary outcome |
Excluded studies
We excluded all 12 of the full‐text articles that we had assessed for eligibility, either because they used an ineligible comparator or because they used an ineligible study design. See Characteristics of excluded studies for further details.
Ongoing studies
We identified one ongoing RCT in Thailand, comparing spironolactone with CPA (Krasean 2019). This study started in April 2019. We describe this study in Characteristics of ongoing studies.
Risk of bias in included studies
As no studies met the inclusion criteria, it was not possible to assess risk of bias.
Effects of interventions
As no studies met the inclusion criteria, we were unable to calculate any effects of the interventions.
Discussion
Summary of main results
No study met the inclusion criteria for this review. A total of 13 potentially eligible studies were identified, but ultimately all but one was excluded after we assessed the full text articles. The one remaining RCT is ongoing, and we are awaiting its publication (Krasean 2019). We conducted a comprehensive search to identify eligible studies for inclusion in this review. Despite more than four decades of ongoing efforts to improve the quality of hormone therapy for women in transition, we found that no RCTs or suitable cohort studies have yet been conducted to investigate the efficacy and safety of hormonal treatment approaches for transgender women in transition.
Overall completeness and applicability of evidence
The evidence is incomplete because no studies met the inclusion criteria for the review. This lack of studies shows a gap between current clinical practice and clinical research, which has been repeatedly emphasised (Hembree 2009; Hembree 2017). If hormone therapy is highly valued in the treatment of gender dysphoria (Hembree 2009; WPATH 2011; Hembree 2017), then this raises the question: why are there no RCTs or appropriate cohort studies for this clinical condition? There is also an ethical need for research into the efficacy and safety of hormone therapy, particularly comparing combination therapy with CPA/estradiol and spironolactone/estradiol to monotherapy with estradiol alone. In view of the reported but rather alarming side‐effect profiles of CPA and spironolactone in other populations (De Bastos 2014; Khan 2016; PG12 2019), long‐term clinical studies that aim to achieve adequate outcomes are urgently needed for the population of transgender women in transition.The lack of reliable data on hormone therapy for transitioning transgender women should encourage the development of well‐planned RCTs and cohort studies to evaluate widespread empirical practice in the treatment of gender dysphoria.
The most common reason for the exclusion of studies from this review was the lack of a control group. We excluded some studies because they did not meet the eligibility requirements for study design (e.g. case series or case‐control studies). Further, interventions were not clearly defined.
Among guideline developers in the field of transgender medicine, it has been discussed in recent years why the available evidence remains limited (Deutsch 2016aReilly 2019). Deutsch 2016a has identified three main reasons, which they believe have hindered the development of evidence based healthcare guidelines. Firstly, a lack of research funding and institutional stigma means that the evidence currently centres around less robust study designs, such as retrospective studies, case series, and individual case reports (Bockting 2016Reisner 2016a); secondly variation in the collection of gender identity data in observational data sets makes it difficult to identify relevant populations and monitor their health outcomes (Deutsch 2013Bauer 2009); and finally, academic programmes focused on transgender medicine are in their infancy and few exist (Reisner 2016b), meaning there is a general lack of research and training on this topic.
Against this background, methodological problems such as inconsistent and missing comparison groups, uncontrolled confounding factors, small sample size, short follow‐up time and difficulties in recording and evaluating a broad spectrum of health outcomes (physical and mental health, social functioning and QoL) have become apparent in hormone therapy (Deutsch 2016b). The performance of RCTs is controversial, especially with regard to placebo studies, and ethical and methodological objections have been raised (e.g. violation of the principle of equipoise, Miller 2003). However, the positive research potential of active‐controlled RCTs is acknowledged, in order to compare different types, dosages and methods of administration of active treatments. Overall, there is a trend in the discussion to favour not only RCTs and quasi‐RCTs, but also high‐quality cohort studies conducted in a network of health centres, hospitals and practices (Deutsch 2016a; Deutsch 2016b).
Quality of the evidence
We could not appraise the quality of the evidence because no studies met our review's inclusion criteria.
Potential biases in the review process
We consider our search to have been consistent and comprehensive (including the fifteen contacts with manufacturers and experts in the field). At each stage, the review authors independently applied the inclusion criteria before comparing their judgements. Reliability testing was performed in the screening phase. Even though we were unable to test for publication bias, we think it is unlikely that there are studies that have been conducted but remained unpublished. The experts in the field we interviewed believed that there was a general lack of research activity by treatment manufacturers, and considered it very likely that no phase IV studies have ever been conducted in this population. For example, one expert stated that there was probably "nothing to be kept secret."
Agreements and disagreements with other studies or reviews
There are currently no systematic reviews in the Cochrane Library that evaluate the effectiveness of hormone therapy for transgender women in transition, nor are there systematic reviews that evaluate the clinical and economic impact of hormone therapy on transgender women in transition. The Endocrine Society's 2009 and 2017 guidelines addressed endocrine treatment of gender‐dysphoric/gender‐incongruent persons (Hembree 2009; Hembree 2017). The literature search included in these guidelines did not identify any RCTs of hormone therapy in transitioning transgender women. In the context of the preparation of UK National Health Service (NHS) guidelines (PG12 2019), the NHS Guideline Panel also found no RCTs. However, PG12 2019 includes a recommendation for the prescription of hormone therapy for transitioning transgender women.
Of the potentially relevant studies we excluded, some reported on relevant questions. Asscheman 2011 focused on the important outcome of mortality. Fisher 2016 investigated the important relationship between hormone therapy‐related body changes and psychobiological well‐being. Giltay 2000 focused on body related outcomes such as hormone therapy's effects on the skin (hair growth rate, density, and shaft diameter by image analysis; and sebum production). Toorians 2003 focused on the outcomes of different interventions (estradiol alone compared with combination therapy estradiol and antiandrogens). Miles 2006 was based on a cross‐over design with the intention of comparing groups of individuals on and off oestrogen. Due to the reported deficits, we excluded these studies, although they addressed important questions.
Authors' conclusions
Implications for practice.
We found insufficient evidence to determine the efficacy or safety of hormonal treatment approaches (estradiol alone or in combination with cyproterone acetate or spironolactone) for transgender women in transition. The evidence is very incomplete, demonstrating a gap between current clinical practice and clinical research.
Implications for research.
This systematic review has shown that well‐designed, sufficiently robust randomised controlled trials (RCTs) and controlled‐cohort studies do not exist, and are needed, to assess the benefits and harms of hormone therapies (used alone or in combination) for transgender women in transition. The following questions should be addressed via RCTs and cohort studies:
What are the short‐, medium‐, and long‐term effects (including adverse effects, benefits, and prognoses) of estradiol therapy alone, as opposed to combination therapy using estradiol together with cyproterone acetate or spironolactone?
What is the short‐, medium‐, and long‐term clinical efficacy of hormone therapy when applied orally, transdermally, and intramuscularly?
Table 1 presents design components that we suggest could be used in future studies. Studies should be structured and reported according to the CONSORT Statement or the STROBE Statement in order to improve the quality of reporting on efficacy and to obtain better reports on harms in clinical research (von Elm 2007; Schulz 2010). There is an urgent need for research in this area, not least for ethical reasons.
We will include non‐controlled cohort studies in the next iteration of this review, as this review has demonstrated that this is the highest quality evidence currently available in the field. We will take methodological limitations into account when doing so.
History
Protocol first published: Issue 10, 2018 Review first published: Issue 11, 2020
Acknowledgements
We would like to acknowledge the support of Dr. Jonathan Livingstone‐Banks, Dr. Nicola Lindson, and Dr. Paul Aveyard from the Tobacco Addiction Group, as well as Dr. Erik von Elm from Cochrane Switzerland, whom we consulted in preparing this review.
We greatly appreciate Dr. Alissa Jones Nelson’s support in reviewing the spelling and grammar of this review. We also gratefully acknowledge peer review comments from Igor Grabovac, Department of Social and Preventive Medicine, Centre for Public Health, Medical University of Vienna, Vienna, Austria and Dr. Barbara Nussbaumer‐Streit, Cochrane Austria, Department for Evidence‐based Medicine and Evaluation, Danube‐University Krems, Krems, Austria, and consumer review comments from Sarah Stephenson‐Hunter.
Appendices
Appendix 1. OvidSP search strategy
| Search | Query |
| #1 | (transsexual* OR transgender OR "gender dysphoria" OR transident* OR "trans women" OR "trans woman").mp. |
| #2 | ("cyproterone acetate" OR CPA OR androcur).mp. or cyproterone Acetate/ |
| #3 | (spironolactone OR Aldactone OR Jenaspiron OR Osyrol OR Spirobene OR Verospiron OR Xenalon).mp. or spironolactone/ |
| #4 | (estradiol* OR oestradiol* OR estrifam OR gynocadin OR neofollin OR lenzetto).mp. or Estradiol/ |
| #5 | 2 OR 3 OR 4 |
| #6 | 1 AND 5 |
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Asscheman 2011 | Mortality rates in transgender people receiving long‐term cross‐sex hormones. A cohort study. Adequate controls are missing. Interventions are not clearly defined |
| Colizzi 2015 | Increased prevalence of metabolic syndrome among individuals with gender dysphoria treated by cross‐sex hormonal treatment. Study without adequate comparator group. |
| Fighera 2018 | Hormone therapy has been associated with changes in bone and lean/fat mass. This study assessed bone mineral density, appendicular lean mass, and total fat mass in transwomen undergoing cross‐sex hormone therapy. Study without adequate comparator group. |
| Fisher 2014 | This study aimed to assess differences in body uneasiness and psychiatric symptoms between gender dysphoria clients taking hormone therapy and those not taking hormones (no hormone therapy). A second aim was to assess whether length of hormone treatment and daily dose provided an explanation for levels of body uneasiness and psychiatric symptoms. Cross‐sectional design. |
| Fisher 2016 | The objective of the study was to assess whether hormone therapy‐related body changes affect psychobiological well‐being in gender dysphoria. Study without adequate comparator group. |
| Giltay 2000 | Hormone therapy effects on the skin (hair growth rate, density, and shaft diameter by image analysis; and sebum production) of transsexual patients receiving cross‐sex hormones. It is a case series, adequate controls are missing. |
| Haraldsen 2005 | Hormone therapy effects on cognitive performance. Study without adequate comparator group. |
| Haraldsen 2007 | The effects of cross‐sex hormones on bone metabolism (bone mineral density, total body fat, total lean body mass) in patients with early onset gender identity disorder. Study without adequate comparator group. |
| Miles 2006 | The study was designed to examine associations between oestrogen and cognition (memory, including visual, spatial, object and location memory, other cognitive abilities that show reliable sex differences, including verbal and visual‐spatial abilities, and mood variables). The cross‐over design used was comparative, but did not used randomization or quasi‐randomisation. |
| Schlatterer 1998 | This follow‐up study was carried out to validate the effectiveness of cross‐gender hormone therapy embedded in a multistep treatment concept for transgender patients. Study without adequate comparator group. This study lacks adequate controls. |
| Toorians 2003 | To find an explanation for the different thrombotic risks of oral ethinyl estradiol and transdermal 17‐beta‐estradiol use, the researchers compared the effects of treatment of male‐to‐female transgender people with cyproterone acetate only, and with cyproterone acetate in combination with transdermal 17‐beta‐estradiol, oral ethinyl estradiol, or oral 17‐beta‐estradiol on a number of haemostatic variables. There is no adequate control group. |
| Van Goozen 1995 | Effects of sex hormones to the establishment of gender differences in behaviour, a large battery of tests on aggression, sexual motivation and cognitive functioning was administered twice: shortly before and three months after the start of cross‐sex hormone treatment. The study does not have an adequate comparator group. |
Characteristics of ongoing studies [ordered by study ID]
Krasean 2019.
| Study name | Anti‐androgenic effects comparison between cyproterone acetate and spironolactone in transgender women: a randomised controlled trial (Trial ID: TCTR20190404001) |
| Methods | Allocation: randomised Study design: randomised controlled trial Control: active Study endpoint classification: efficacy study Intervention model: Parallel Number of arms: 2 Masking: double blind (Masked roles: participant caregiver, investigator) Primary purpose: treatment Study phase: phase 4 |
| Participants | Gender: male Age limit: minimum 18 years: maximum 40 years Condition: Gender dysphoria patients diagnosed from DSM V Male to female transgender Not undergone orchidectomy No psychological disease or mental disability |
| Interventions | Arm 1: Intervention name: cyproterone acetate Type: active comparator Classification: drug Descriptions: participants (gender dysphoria patients) will receive estradiol valerate (4 mg daily) combined with cyproterone acetate (25 mg daily) for cross‐sex hormone treatment. Arm: 2 Intervention name: spironolactone Type: experimental Classification: drug Descriptions: participants (gender dysphoria patients) will be received estradiol valerate (4 mg daily) combined with spironolactone (100 mg daily) for cross‐sex hormone treatment. |
| Outcomes | Primary outcome(s): Outcome name: testosterone level Measurement: Electrochemiluminescent Immunoassay (ECLIA) of total testosterone level Time point: three months after intervention Safety issue: no Key secondary outcomes: Outcome name: physical and metabolic changes Measurement: physical examination, metabolic profile parameters Time point: three months after intervention Safety Issue: no |
| Starting date | April 3, 2019 (estimated end date: June 16, 2020) |
| Contact information | Contact: Krasean Panyakhamlerd Degree: Assoc. Prof. Phone: 0926536415 Email: krasean@hotmail.com Postal Address: 1873 Rama 4 Road, Patumwan State/Province: Bangkok Postal Code: 10400 Country: Thailand |
| Notes | Source(s) of monetary or material supports: Ratchadapisek Sompoch Fund, Faculty of Medicine, Chulalongkorn University Declarations of interest not reported |
Contributions of authors
All authors contributed to the Abstract, Background, Methods, Results, Discussion, and Authors' conclusions. Claudia Haupt, Alexia Kutschmar and Miriam Henke conducted the study selection.
Declarations of interest
Claudia Haupt declares no competing interest.
Miriam Henke declares no competing interest.
Alexia Kutschmar declares no competing interest.
Birgit Hauser (BH) declares no competing interest. BH is a clinical practitioner in private practice, who also prescribes hormone therapy.
Sandra Baldinger declares no competing interest.
Sarah Rafaela Saenz declares no competing interest.
Gerhard Schreiber declares no competing interest.
None of the review authors' incomes depends on the prescription of drugs. The review authors did not receive any financial support for this project, but paid for all related expenses themselves. They worked voluntarily and free of charge.
New
References
References to studies excluded from this review
Asscheman 2011 {published data only}
- Asscheman H, Giltay EJ, Megens JA, Ronde WP, Trotsenburg MA, Gooren LJ. A long-term follow-up study of mortality in transsexuals receiving treatment with cross-sex hormones. European Journal of Endocrinology 2011;164(4):635-42. [DOI: ] [DOI] [PubMed] [Google Scholar]
Colizzi 2015 {published data only}
- Colizzi M, Costa R, Scaramuzzi F. Concomitant psychiatric problems and hormonal treatment induced metabolic syndrome in gender dysphoria individuals: A 2 year follow-up study. Journal of Psychosomatic Research 2015;78:399–406. [DOI: 10.1016/j.jpsychores.2015.02.001] [DOI] [PubMed] [Google Scholar]
Fighera 2018 {published data only}
- Fighera TM, da Silva E, Lindenau JD, Spritzer PM. Impact of cross-sex hormone therapy on bone mineral density and body composition in transwomen. Clinical Endocrinology 2018;88(6):856-862. [DOI: 10.1111/cen.13607] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fisher 2014 {published data only}
- Fisher AD, Castellini G, Bandini E, Casale H, Fanni E, Benni L, et al. Cross-sex hormonal treatment and body uneasiness in individuals with gender dysphoria. International Society for Sexual Medicine 2014;11:709–19. [DOI] [PubMed] [Google Scholar]
Fisher 2016 {published data only}
- Fisher AD, Castellini G, Ristori J, Casale H, Cassioli E, Sensi C, et al. Cross-sex hormone treatment and psychobiological changes in transsexual persons: two-year follow-up data. The Journal of Clinical Endocrinology and Metabolism 2016;101:0000-0000. [DOI] [PubMed] [Google Scholar]
Giltay 2000 {published data only}
- Giltay EJ, Gooren L. Effects of sex steroid deprivation/administration on hair growth and skin sebum production in transsexual males and females. The Journal of Clinical Endocrinology & Metabolism 2000;85(8):2913-21. [DOI: 10.1210/jc.85.8.2913] [DOI] [PubMed] [Google Scholar]
Haraldsen 2005 {published data only}
- Haraldsen IR, Egeland T, Haug E, Finset A, Opjordsmoen S. Cross-sex hormone treatment does not change sex-sensitive cognitive performance in gender identity disorder patients. Psychiatry Research 2005;137:161-74. [10.1016/j.psychres.2005.05.014] [DOI] [PubMed] [Google Scholar]
Haraldsen 2007 {published data only}
- Haraldsen IR, Haug E, Falch J, Egeland T, Opjordsmoen S. Cross-sex pattern of bone mineral density in early onset gender identity disorder. Hormones and Behavior 2007;52:334-43. [DOI: 10.1016/j.yhbeh.2007.05.012] [DOI] [PubMed] [Google Scholar]
Miles 2006 {published data only}
- Miles C, Green R, Hines M. Estrogen treatment effects on cognition, memory and mood in male-to-female transsexuals. Hormones and Behavior 2006;50:708-17. [DOI: 10.1016/j.yhbeh.2006.06.008] [DOI] [PubMed] [Google Scholar]
Schlatterer 1998 {published data only}
- Schlatterer K, Auer DP, Yassouridis A, Von Werder K, Stalla GK. Transsexualism and osteoporosis. Archives of Sexual Behavior 1998;27(5):475-92. [0004-0002/98/1000-0475] [DOI] [PubMed] [Google Scholar]
Toorians 2003 {published data only}
- Toorians AW, Thomassen MCLGD, Zweegman S, Magdeleyns EJP, Tans G, Gooren L, et al. Venous thrombosis and changes of hemostatic variables during cross-sex hormone treatment in transsexual people. The Journal of Clinical Endocrinology & Metabolism 2003;88(12):5723-29. [DOI: 10.1210/jc.2003-030520] [DOI] [PubMed] [Google Scholar]
Van Goozen 1995 {published data only}
- Van Goozen SHM. Gender differences in behaviour: activating effects of cross-sex hormones. Psychoneuroendocrinology 1995;20(4):343-63. [DOI: 10.1016/0306-4530%2894%2900076-X] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Krasean 2019 {published data only}
Additional references
Arcelus 2015
- Arcelus J, Bouman WP, Van Den Noortgate W, Claes L, Witcomb G, Fernandez-Aranda F. Systematic review and meta-analysis of prevalence studies in transsexualism. European Psychiatry 2015;30(6):807-15. [DOI] [PubMed] [Google Scholar]
ATME 2015
- Aktion Transsexualität und Menschenrecht eV (ATME). Alternative recommendations for treatment in the presence of so-called "sex/gender variance". Medicine and psychotherapy without gender stereotyping. [STUTTGARTER ERKLÄRUNG - Alternative Handlungsempfehlungen bei geschlechtlichen Normvariationen]. In: In: v. Schreiber G editor(s). Transsexualität in Theologie und Neurowissenschaften ‐ Ergebnisse, Kontroversen, Perspektiven. Vol. 1. Berlin: De Gruyter, 2015:77-8. [ISBN: 978-3110440805] [Google Scholar]
Bauer 2009
- Bauer GR, Hammond R, TraversR, Kaay M, Hohenadel KM, Boyce M. “I don't think this is theoretical; this is our lives”: how erasure impacts health care for transgender people. Journal of the Association of Nurses in AIDS Care 2009;20(5):348-61. [DOI: 10.1016/j.jana.2009.07.004] [DOI] [PubMed] [Google Scholar]
Bessone 2015
- Bessone F, Lucena MI, Roma MG, Stephens C, Medina-Cáliz I, Frider B, et al. Cyproterone acetate induces a wide spectrum of acute liver damage including corticosteroid-responsive hepatitis: report of 22 cases. Liver International: Official Journal of the International Association for the Study of the Liver. 2015;36(2):302-10. [DOI: ] [DOI] [PubMed] [Google Scholar]
Bockting 2016
- Bockting W, Coleman E, Deutsch MB, Guillamon A, Meyer I, Meyer III W, et al. Adult development and quality of life of transgender and gender nonconforming people. Current Opinion in Endocrinology, Diabetes, and Obesity 2016;23(2):188. [DOI: 10.1097/MED.0000000000000232 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bostick 2008
- Bostick NA, Sade R, Levine MA, Stewart Jr DM. Placebo use in clinical practice: report of the American Medical Association Council on Ethical and Judicial Affairs. The Journal of Clinical Ethics 2008;19(1):58-61. [PMID: ] [PubMed] [Google Scholar]
Brown 1996
- Brown ML, Rounsley CA. True Selves: Understanding Transsexualism - For Families, Friends, Coworkers, and Helping Professionals. 1 edition. Vol. 1. San Francisco: Jossey-Bass, 1996. [ISBN: 0-7879-6702-5] [Google Scholar]
Calderón 2009
Cash 2004
- Cash TF, Jakatdar TA, Williams EF. The body image quality of life inventory: further validation with college men and women. Body Image 2004;1(3):279–87. [DOI: ] [DOI] [PubMed] [Google Scholar]
De Bastos 2014
- Bastos M, Stegeman B, Rosendaal F. Combined oral contraceptives: venous thrombosis. Cochrane Database of Systematic Reviews 2014;3(Issue ID 2351):Art. No.: CD010813. [DOI: 10.1002/14651858.CD010813.pub2.] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Defreyne 2017
- Defreyne J, Nota N, Pereira C, Schreiner T, Fisher AD, den Heijer M, et al. Transient elevated serum prolactin in trans women is caused by cyproterone acetate treatment. LGBT Health 2017;4(5):328-36. [DOI: ] [DOI] [PubMed] [Google Scholar]
Deutsch 2013
- Deutsch MB, Green J, Keatley J, Mayer G, Hastings J, Hall AM, et al. Electronic medical records and the transgender patient: recommendations from the World Professional Association for Transgender Health. Journal of the American Medical Informatics Association 2013;20(4):700-3. [10.1136/amiajnl-2012-001472] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deutsch 2016a
- Deutsch M. Guidelines for the primary and gender-affirming care of transgender and gender nonbinary people. Center of Excellence for Transgender Health June 17th 2016;2nd Edition.
Deutsch 2016b
- Deutsch MB, Radix A, Reisner S. What’s in a guideline? Developing collaborative and sound research designs that substantiate best practice recommendations for transgender health care. AMA Journal of Ethics 2016;18(11):1098-106. [DOI: 10.1001/journalofethics.2016.18.11.stas1-1611] [DOI] [PMC free article] [PubMed] [Google Scholar]
Downing 1981
- Downing DT, Stewart ME, Strauss JS. Estimation of sebum production rates in man by measurement of the squalene content of skin biopsies. Journal of Investigative Dermatology 1981;77(4):358-60. [PMID: ] [DOI] [PubMed] [Google Scholar]
Drescher 2014
- Drescher J. Controversies in gender diagnoses. LGBT Health 2014;1(1):10-14. [DOI: ] [DOI] [PubMed] [Google Scholar]
DSM‐5 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5), Fifth edition. 1 edition. Vol. 1. Göttingen: Hogrefe, 2013. [ISBN: 9783801725990] [Google Scholar]
Ezerskaia 2016
- Ezerskaia A, Pereira SF, Urbach HP, Verhagen R, Varghese B. Infrared spectroscopic measurement of skin hydration and sebum levels and comparison to corneometer and sebumeter. Proceedings SPIE 2016;9887(98872G):552-6. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Figg 2010
- Figg W, Chau CH, Cindy H, Small EJ. Drug Management of Prostate Cancer. 1 edition. New York: Springer, 2010. [ISBN: 978-1-60327-829-4] [Google Scholar]
Flütsch 2015
- Flütsch N. Endocrinological treatment of the gender dysphoria in people with gender incongruence. [Endokrinologische Behandlung der Geschlechtsdysphorie bei Menschen mit Geschlechtsinkongruenz]. Journal of Clinical Endocrinology and Metabolism 2015;8(2):42-8. [Google Scholar]
Fox 2016
- Fox LC, Davies DR, Scholl JL, Watt MJ, Forster GL. Differential effects of glucocorticoid and mineralocorticoid antagonism on anxiety behavior in mild traumatic brain injury. Behavioural Brain Research 2016;312:362-5. [DOI: ] [DOI] [PubMed] [Google Scholar]
Frisch 2005
- Frisch MB, Clark MP, Rouse SV, Rudd MD, Paweleck JK, Greenstone A, et al. Predictive and treatment validity of life satisfaction and the quality of life inventory. Assessment 2005;12(1):66-78. [DOI: ] [DOI] [PubMed] [Google Scholar]
Giltay 2008
- Giltay EJ, Bunck MC, Gooren L, Zitman FG, Diamant M, Teerlink T. Effects of sex steroids on the neurotransmitter-specific aromatic amino acids phenylalanine, tyrosine, and tryptophan in transsexual subjects. Neuroendocrinology 2008;88(2):103-10. [DOI: ] [DOI] [PubMed] [Google Scholar]
Gooren 2017
- den Heijer M, Bakker A, Gooren L. Long term hormonal treatment for transgender people. The BMJ 2017;359(j5027):n/a. [DOI: ] [DOI] [PubMed] [Google Scholar]
Greenblatt 1973
- Greenblatt DJ, Koch-Weser J. Adverse reactions to spironolactone. JAMA 1973;225(1):40-3. [DOI: ] [DOI] [PubMed] [Google Scholar]
Griard 1978
- Griard J, Bühler U, Zuppinger K, Haas HG, Staub JJ, Wyss HI. Cyproterone acetate and ACTH adrenal function. The Journal of Clinical Endocrinology and Metabolism 1978;47(3):581-6. [DOI: ] [DOI] [PubMed] [Google Scholar]
Hayes 2000
- Hayes FJ, Seminara SB, Decruz S, Boepple PA, Crowley WF Jr. Aromatase inhibition in the human male reveals a hypothalamic site of estrogen feedback. The Journal of Clinical Endocrinology & Metabolism 2000;85(9):3027-35. [DOI: ] [DOI] [PubMed] [Google Scholar]
Hembree 2009
- Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, Gooren LJ, Meyer III WJ, Spack NP, et al. Endocrine treatment of transsexual persons: an endocrine society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 2009;94(9):3132–54. [DOI: 10.1210/jc.2009-0345] [DOI] [PubMed] [Google Scholar]
Hembree 2017
- Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, Gooren L, Hannema SE, Meyer III WJ, et al. Endocrine treatment of transsexual persons: an Endocrine Society clinical practice guideline. The Journal of Clinical Endocrinology & Metabolism 2017;102(11):3869-903. [DOI: ] [DOI] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. Journal of the Royal Statistical Society: Series A (Statistics in Society) 2009;172(1):137-59. [DOI: 172(1):137–159] [DOI] [PMC free article] [PubMed]
Higgins 2011a
- Higgins JPT, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Deeks JJ, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Deeks JJ. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Altman DG, Sterne JAC, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
- Higgins JPT, Deeks JJ. Chapter 9: Analysing data and undertaking meta-analyses. In: Deeks JJ, Higgins JPT, Altman DG, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011d
- Higgins JPT, Deeks JJ. Chapter 16: General principles for dealing with missing data. In: Higgins JPT, Deeks JJ, Altman DG, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoffmann 2013
- Hoffman R. TrichoScan: a novel tool for the analysis of hair growth in vivo. Journal of Investigative Dermatology Symposium Proceedings 2003;8(1):109-15. [DOI: ] [DOI] [PubMed] [Google Scholar]
Hozo 2005
- Hozo SP, Djulbegovic B, Iztok I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;13(5). [DOI: ] [DOI] [PMC free article] [PubMed]
Hye‐Rim 2012
- Hye-Rim L, Tae-Hee K, Kyung-Chul C. Functions and physiological roles of two types of estrogen receptors, ERα and ERβ, identified by estrogen receptor knockout mouse. Laboratory Animal Research 2012;28(2):71-6. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2016
- Khan O, Mashru A. The efficacy, safety and ethics of the use of testosterone-suppressing agents in the management of sex offending. Current Opinion in Endocrinology, Diabetes and Obesity 2016;23(3):271-8. [DOI: 10.1097/MED.0000000000000257] [DOI] [PubMed] [Google Scholar]
Laurent 2007
- Laurent A, Mistretta F, Bottigioli D, Dahel K, Goujon C, Nicolas JF, et al. Echographic measurement of skin thickness in adults by high frequency ultrasound to assess the appropriate microneedle length for intradermal delivery of vaccines. Vaccine 2007;25(34):6423-30. [DOI: 25(34):6423-6430] [DOI] [PubMed]
Meston 2005
- Meston C, Trapnell P. Development and validation of a five-factor sexual satisfaction and distress scale for women: the Sexual Satisfaction Scale for Women (SSS-W). The Journal of Sexual Medicine 2005;2(1):66-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 2003
- Miller FG, Brody H. A critique of clinical equipoise: therapeutic misconception in the ethics of clinical trials. Hastings Center Report 2003;33(3):19-28. [PMID: 12854452] [PubMed] [Google Scholar]
Moher 2009
Murad 2010
Oberhammer 1996
- Oberhammer F, Nagy P, Tiefenbacher R, Fröschl G, Bouzahzah B, Thorgeirsson SS, et al. The antiandrogen cyproterone acetate induces synthesis of transforming growth factor beta 1 in the parenchymal cells of the liver accompanied by an enhanced sensitivity to undergo apoptosis and necrosis without inflammation. Hepatology 1996;23(2):329-37. [DOI: ] [DOI] [PubMed] [Google Scholar]
PG12 2019
- Sullivan C, Dean J. Prescribing Guideline PG12 Pharmacological Treatment of Gender Dysphoria. Devon Partnership NHS Trust 2019.
Prior 1989
- Prior CJ, Vigna YM, Watson D. Spironolactone with physiological female steroids for presurgical therapy of male-to-female transsexualism. Archives of Sexual Behavior 1989;18(1):49-57. [DOI: 18(1):49-57] [DOI] [PubMed] [Google Scholar]
Ramsay 1990
- Ramsay ID, Rushton DH. Reduced serum vitamin B12 levels during oral cyproterone-acetate and ethinyl-oestradiol therapy in women with diffuse androgen-dependent alopecia. Clinical and Experimental Dermatology 1990;15(4):277-81. [DOI: ] [DOI] [PubMed] [Google Scholar]
Reilly 2019
- Reilly ZP, Fruhauf TF, Martin SJ. Barriers to evidence-based transgender care: knowledge gaps in gender-affirming hysterectomy and oophorectomy. Obstetrics & Gynecology 2019;134(4):714-17. [DOI: 10.1097/AOG.0000000000003472 ] [DOI] [PubMed] [Google Scholar]
Reisner 2016a
- Reisner SL, Deutsch MB, Bhasin S, Bockting W, Brown GR, Feldman J, et al. Advancing methods for US transgender health research. Current opinion in endocrinology, diabetes, and obesity 2016;23(2):198. [DOI: 10.1097/MED.0000000000000229] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reisner 2016b
- Reisner SL, Radix A, Deutsch MB. Integrated and gender-affirming transgender clinical care and research. Journal of acquired immune deficiency syndromes 1999;72(3):235. [DOI: 10.1097/QAI.0000000000001088] [DOI] [PMC free article] [PubMed] [Google Scholar]
Riley 2011
Schneider 2006
- Schneider H, Stalla G. Hormonal Therapy [Hormonelle Therapie]. In: Therapieleitfaden Transsexualität. 1 edition. Bremen: Uni-Med Science, 2006:85-9. [ISBN: 3-89599-888-5] [Google Scholar]
Schneider 2016
- Schneider C, Cerwenka S, Nieder TO, Briken P, Cohen-Kettenis PT, Cuypere G, et al. Measuring gender dysphoria: a multicenter examination and comparison of the Utrecht gender dysphoria scale and the gender identity/gender dysphoria questionnaire for adolescents and adults. Archives of Sexual Behavior 2013;45(3):551-8. [DOI: ] [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. The BMJ 2010;340:698-702. [DOI: 10.1136/bmj.c332] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schürmeyer 1986
Seal 2012
- Seal LJ, Franklin S, Richards C, Shishkareva A, Sinclaire C, Barret J. Predictive markers for mammoplasty and a comparison of side effect profiles in transwomen taking various hormonal regimens. The Journal of Clinical Endocrinology & Metabolism 2012;97(12):4422-8. [DOI: ] [DOI] [PubMed] [Google Scholar]
Soll 2018
- Soll BM, Robles-García R, Brandelli-Costa A, Mori D, Mueller A, Vaitses-Fontanari AM, et al. Gender incongruence: a comparative study using ICD-10 and DSM-5 diagnostic criteria. Brazilian Journal of Psychiatry 2018;40(2):174-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
Sterne 2016
- Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI: 10.1136/bmj.i4919] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thompson 1993
- Thompson DF, Carter JR. Drug-induced gynecomastia. Pharmacotherapy Jan-Feb 1993;13(1):37-45. [PMID: ] [PubMed] [Google Scholar]
von Elm 2007
- Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies.. Annals of Internal Medicine 2007;147(8):573-7. [DOI: 10.7326/0003-4819-147-8-200710160-00010] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wenqing 2005
- Wenqing G, Bohl CE, Dalton JT. Chemistry and structural biology of androgen receptor. Chemical Reviews 2005;105(9):3352-70. [DOI: 10.1021/cr020456u] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2018
- World Health Organisation. International classification of diseases for mortality and morbidity statistics (11th Revision). icd.who.int/en/ (accessed 30 October 2020).
Wierckx 2014
- Wierckx K, Caenegem E, Schreiner T, Haraldsen I, Fisher AD, Toye K, et al. Cross-sex hormone therapy in trans persons is safe and effective at short-time follow-up: results from the European network for the investigation of gender incongruence. International Society for Sexual Medicine 2014;11(8):1999-2011. [DOI: 10.1111/jsm.12571] [DOI] [PubMed] [Google Scholar]
WPATH 2011
- Coleman E, Bockting W, Botzer M, Cohen-Kettenis P, DeCuypere G, Feldman J, et al. Standards of care for the health of transsexual, transgender, and gender-nonconforming people version 7. International Journal of Transgenderism 2011;13(4):165–232. [DOI: 10.1080/15532739.2011.700873] [DOI] [Google Scholar]
Zucker 2016
- Zucker KJ. The DSM-5 diagnostic criteria for gender dysphoria. In: Trombetta C, Liguori G, Bertolotto M, editors(s). Management of Gender Dysphoria - A Multidisciplinary Approach. First edition. Vol. 1. Mailand: Springer, 2016:33-7. [ISBN: 978-88-470-5695-4] [Google Scholar]
References to other published versions of this review
Haupt 2018
- Haupt C, Henke M, Kutschmar A, Hauser B, Baldinger S, Schreiber G. Antiandrogens or estradiol treatments or both during hormone replacement therapy in transitioning transgender women. Cochrane Database of Systematic Reviews 2018, Issue 10. Art. No: CD013138. [DOI: 10.1002/14651858.CD013138] [DOI] [PMC free article] [PubMed] [Google Scholar]


