Abstract
Background
Telephone communication is increasingly being accepted as a useful form of support within health care. There is some evidence that telephone support may be of benefit in specific areas of maternity care such as to support breastfeeding and for women at risk of depression. There is a plethora of telephone‐based interventions currently being used in maternity care. It is therefore timely to examine which interventions may be of benefit, which are ineffective, and which may be harmful.
Objectives
To assess the effects of telephone support during pregnancy and the first six weeks post birth, compared with routine care, on maternal and infant outcomes.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (23 January 2013) and reference lists of all retrieved studies.
Selection criteria
We included randomised controlled trials, comparing telephone support with routine care or with another supportive intervention aimed at pregnant women and women in the first six weeks post birth.
Data collection and analysis
Three review authors independently assessed studies identified by the search strategy, carried out data extraction and assessed risk of bias. Data were entered by one review author and checked by a second. Where necessary, we contacted trial authors for further information on methods or results.
Main results
We have included data from 27 randomised trials involving 12,256 women. All of the trials examined telephone support versus usual care (no additional telephone support). We did not identify any trials comparing different modes of telephone support (for example, text messaging versus one‐to‐one calls). All but one of the trials were carried out in high‐resource settings. The majority of studies examined support provided via telephone conversations between women and health professionals although a small number of trials included telephone support from peers. In two trials women received automated text messages. Many of the interventions aimed to address specific health problems and collected data on behavioural outcomes such as smoking cessation and relapse (seven trials) or breastfeeding continuation (seven trials). Other studies examined support interventions aimed at women at high risk of postnatal depression (two trials) or preterm birth (two trials); the rest of the interventions were designed to offer women more general support and advice.
For most of our pre‐specified outcomes few studies contributed data, and many of the results described in the review are based on findings from only one or two studies. Overall, results were inconsistent and inconclusive although there was some evidence that telephone support may be a promising intervention. Results suggest that telephone support may increase women's overall satisfaction with their care during pregnancy and the postnatal period, although results for both periods were derived from only two studies. There was no consistent evidence confirming that telephone support reduces maternal anxiety during pregnancy or after the birth of the baby, although results on anxiety outcomes were not easy to interpret as data were collected at different time points using a variety of measurement tools. There was evidence from two trials that women at high risk of depression who received support had lower mean depression scores in the postnatal period, although there was no clear evidence that women who received support were less likely to have a diagnosis of depression. Results from trials offering breastfeeding telephone support were also inconsistent, although the evidence suggests that telephone support may increase the duration of breastfeeding. There was no strong evidence that women receiving telephone support were less likely to be smoking at the end of pregnancy or during the postnatal period.
For infant outcomes, such as preterm birth and infant birthweight, overall, there was little evidence. Where evidence was available, there were no clear differences between groups. Results from two trials suggest that babies whose mothers received support may have been less likely to have been admitted to a neonatal intensive care unit (NICU), although it is not easy to understand the mechanisms underpinning this finding.
Authors' conclusions
Despite some encouraging findings, there is insufficient evidence to recommend routine telephone support for women accessing maternity services, as the evidence from included trials is neither strong nor consistent. Although benefits were found in terms of reduced depression scores, breastfeeding duration and increased overall satisfaction, the current trials do not provide strong enough evidence to warrant investment in resources.
Plain language summary
Telephone support for women during pregnancy and up to six weeks after the birth
Telephone support may be of benefit to women with particular problems during pregnancy and in the first six weeks after the birth of the baby but it is not clear which interventions may be helpful, which are ineffective, and which may be harmful.
Telephone communication is increasingly being accepted as a useful form of support within health care, with many telephone‐based interventions currently being used in maternity care.
In this review we have included results from 27 randomised trials with more than 12,000 women. All of the trials examined telephone support versus usual care (no additional telephone support). In two trials women received automated text messages. We did not identify any trials comparing different types of telephone support (for example, text messaging versus one‐to‐one calls). All but one of the trials were carried out in high‐resource settings. The majority of studies examined support provided via telephone conversations between women and health professionals although a small number of trials included telephone support from peers. Many of the results described in the review are based on findings from only one or two studies. Overall, results were inconsistent and inconclusive. Telephone support may increase women's overall satisfaction with their care during pregnancy and the postnatal period; although results for both periods were from only two studies. There was no consistent evidence confirming that telephone support reduces anxiety during pregnancy or after the birth of the baby. Evidence from two trials showed that women who received support had lower average depression scores in the postnatal period but without clear evidence that women who were supported were less likely to have a diagnosis of depression. Results from trials encouraging breastfeeding through telephone support were also inconsistent, although there was some evidence that telephone support may increase the duration of breastfeeding. There was no strong evidence that women receiving telephone support were less likely to be smoking at the end of pregnancy or during the postnatal period.
For infant outcomes, such as preterm birth and infant birthweight, overall, there was little evidence. Where evidence was available, there were no clear differences between groups.
There remains uncertainty regarding the benefit of telephone support and despite some encouraging findings, there is insufficient evidence to recommend routine telephone support for women accessing maternity services.
Background
Telephone interventions as part of health services and M‐health (i.e. health‐service provision via mobile communication technologies) (Vital Wave Consulting 2009), have grown in popularity, reaching those who, previously, may not have been reached. M‐health is particularly important in a low‐resourced setting where women are isolated by geographical location, and often have to travel long distances to access health‐professional support. Approximately 64% of all mobile phone users are resident in low‐resourced settings (United Nations 2007). Furthermore, estimates show that by 2012, half of all individuals in remote areas of the world will have mobile phones (Vital Wave Consulting 2009).
The use of telephone communication as a means of providing support in health care is not new; the first report appeared in the Lancet in 1897 when a doctor used communication via the telephone to diagnose a child with croup (Fosarelli 1983). Over a decade ago researchers suggested that the telephone was one of the most under‐utilised resources in health care (Latimer 1998; Oda 1995). However, telephone communication is increasingly being accepted as a useful form of support within health care (Wootton 2001; Wyatt 2001). The boom in mobile phone technology, in particular, has enhanced this acceptance, leading to its use in a number of healthcare settings.
Within maternity care, telephone support has been provided in the antenatal and postnatal periods. In the antenatal period telephone support has been used to support women in different ways including: to assist pregnant women with smoking cessation (Solomon 2005); to support women at risk of preterm birth (Moore 2004) and as a means of conducting maternity triage (Kennedy 2007). The potential psychosocial benefits of telephone support for pregnant women have also been explored (Bullock 1995), offering some confirmation of benefit.
In the postnatal period telephone ‘hot lines’ have grown in popularity, partly in response to early hospital discharge policies, in an attempt to provide continuity and support to parents (Rush 1991; Siegel 1992). These ‘hotlines’ appear to be valued by women, particularly for advice on breastfeeding and newborn care (Osman 2010). Some of these services were established exclusively as means of providing breastfeeding support (Chamberlain 2005; Wang 2008); others focused on mothers who were considered to be at risk of complications, for example, following caesarean birth (David 2010).
Description of the intervention
Telephone support presents itself in different ways. Support may be passive, whereby support is only available when requested, or it may be proactively offered. Scheduled and unscheduled telephone interactions have also been reported (Knight 2010). The medium for the support may be text messaging (Jareethum 2008) or verbal communication. Furthermore, support may be offered by a healthcare professional or a lay person. Telephone support may target a particular sample population, with the commonality of a particular medical condition, e.g. diabetes, or it may be used in health promotion, e.g. to support smoking cessation.
An earlier systematic review of telephone support for pregnant and postnatal women included 14 randomised controlled trials involving 8037 women (Dennis 2008) The authors reviewed telephone support interventions in which the primary focus was smoking, preterm birth, low birthweight, breastfeeding, or postpartum depression. Although there were methodological weaknesses in some of the reported trials, the review authors concluded that proactive telephone support may (a) assist in preventing smoking relapse, (b) play a role in preventing low birthweight, (c) increase breastfeeding duration and exclusivity, and (d) decrease postpartum depression symptoms. None of the telephone interventions were effective in improving preterm birth or smoking cessation rates.
Why it is important to do this review
Telephones are now an integral tool in mother and health‐professional communication. Given the increase in telephone communications, coupled with extensive global resource deficits, this trend it likely to continue. Although an earlier systematic review (Dennis 2008) provided some evidence of benefit in specific areas of maternity care, there is a plethora of telephone‐based interventions currently being used in maternity care. It is therefore timely to build on previous assessments to examine which interventions may be of benefit, which are ineffective, and which may be harmful.
Objectives
The primary objective is to assess the effects of telephone support during pregnancy and the first six weeks post birth, compared with routine care, on maternal and infant outcomes.
The secondary objective is to compare the effect of different types of telephone support, on maternal and infant outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We considered all published and unpublished randomised controlled trials, comparing telephone support with routine care or with another supportive intervention. We also considered cluster‐randomised trials. Quasi‐randomised trials and cross‐over studies were excluded.
Types of participants
Pregnant women and postnatal women in the first six weeks post birth.
Types of interventions
All interventions aimed at supporting women by using telephones, whether for general support/information or for a specific medical/social reason (e.g. diabetes, smoking). We have included studies where the intervention is introduced in pregnancy or in the first six weeks post birth, or both. The intervention may, or may not, have extended from the antenatal to postnatal period. Interventions may have been in any setting and delivered by healthcare staff, peer supporters or using automated messaging.
We planned to make the following comparisons.
Telephone support versus any other supportive intervention, or no telephone support.
Verbal telephone support versus text support.
Types of outcome measures
Primary outcomes
Maternal satisfaction with support during pregnancy and the first six months postpartum (as defined by trial authors).
Maternal anxiety (measures as defined by trial authors, e.g. Hospital Anxiety and Depression Scale).
Secondary outcomes
Maternal outcomes
Mother‐infant attachment.
General health (e.g. as defined by standardised measures such as general health questionnaires).
Mortality and serious morbidity (e.g. perineal haematoma or deep surgical infection).
Health service utilisation (presentation/attendance at clinics, accident and emergency departments or general practices).
Postpartum depression (measures as defined by author, e.g. the Edinburgh Postnatal Depression Scale (EPDS)).
Positive behaviour change (as defined by trial authors, e.g. smoking reduction).
Infant outcomes
Preterm birth/low birthweight.
Breastfeeding duration (exclusive or combined feeding).
Infant developmental measures (physical and cognitive as defined by trial authors).
Neonatal/infant mortality.
Major neonatal/infant morbidity (as defined by trial authors, e.g. prolonged admission to special care baby unit).
Service
Intervention cost.
Search methods for identification of studies
Electronic searches
We contacted the Trials Search Co‐ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (23 January 2013).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We included abstracts provided sufficient data and methodological detail were available. Where necessary, we contacted the authors of abstracts to obtain further information.
Searching other resources
We searched the reference lists of all retrieved trial reports.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Three review authors (T Lavender (TL), S Milan (SM) and R Smyth (RS)) independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion with the whole team.
Data extraction and management
We designed a form to extract data. For eligible studies, three review authors (T Dowswell (TD), R Smyth (RS) and S Milan (SM)) extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the remaining authors. TD entered data into Review Manager software (RevMan 2012) and data were checked for accuracy by RS.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Three review authors (TD, SM, RS) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving the remaining authors.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence and determined whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
It is very difficult to blind staff and participants to randomisation group for this type of intervention. Theoretically, it may be possible to randomise participants to two different phone interventions with one of them designated as the control intervention. We have described for each included study the methods used, if any, to attempt to blind study participants or staff from knowledge of which intervention a participant received, and we have noted where any information was provided on the success of blinding. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
It may be possible to blind outcome assessors, and we have described for each included study the methods used, if any, to blind them from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We have described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We state whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or was supplied by the trial authors, we have re‐included missing data in our analyses.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated' analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting bias (checking for reporting bias)
We have described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other sources of bias (checking for bias due to problems not covered by (1) to (5) above)
We have also described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We have made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions(Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses. In this version of the review, too few studies contributed data to allow this planned analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we have presented results as summary risk ratio (RR) with 95% confidence intervals (CIs).
Continuous data
For continuous data, we used the mean difference (MD) if outcomes were measured in the same way between trials. We used the standardised mean difference (SMD) to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We planned to include cluster‐randomised trials in the analyses along with individually‐randomised trials. In this version of the review we identified one cluster‐randomised trials that was eligible for inclusion. The author reported adjusted data for this trial and these data are presented in an additional table (Table 1).
1. Mobile phone intervention and skilled attendance at the birth.
| OUTCOME | Intervention (Total 1311 women) | Control (Total 1239 women) | Adjusted Odds Ratio (OR) |
| Skilled attendant at the birth (all) Unadjusted data |
766 (60%) | 560 (47%) | |
| Skilled attendant at the birth (rural residence) OR adjusted to take account of cluster‐design effect |
317 (43%) | 313 (44%) | 0.85 (95% CI 0.42 to 1.71) |
| Skilled attendant at the birth (urban residence) OR adjusted to take account of cluster‐design effect |
449 (82%) | 247 (50%) | 5.73 (95% CI 1.51 to 21.81) P < 0.01 |
CI: confidence interval
In updates of the review if more such trials are included, we will adjust their sample sizes using the methods described in the Cochrane Handbook using an estimate of the intra‐cluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
We have not included cross‐over trials as these are not an appropriate study design for the interventions in this review.
Other unit of analysis issues
For studies including multiple pregnancies, we have treated the infants as independent and noted the effects of estimates of CIs in the review.
For studies using one or more treatment groups (multi‐arm studies), where appropriate, we combined groups to create a single pair‐wise comparison using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
For included studies, we noted levels of attrition. For primary outcomes, we planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis. In this version of the review, too few studies contributed data to allow this planned analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we have attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² is greater than 30% and either T² is greater than zero, or there is a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In this version of the review insufficient studies contributed data to allow us to explore possible reporting biases. In updates, if more data become available, for those outcomes where there are 10 or more studies in the meta‐analysis we plan to investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2012). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies estimate the same underlying treatment effect: i.e. where trials examined the same intervention, and the trials’ populations and methods were judged sufficiently similar. If we considered that there was clinical heterogeneity sufficient to expect that the underlying treatment effects would differ between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary provided that an average treatment effect was considered clinically meaningful. If the average treatment effect was not considered to be clinically meaningful, we did not combine trials.
Where we used random‐effects analyses, we have presented the results as the average treatment effect with its 95% CI, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
If we had identified substantial heterogeneity, we planned to investigate it using subgroup analyses and sensitivity analyses.
We planned to carry out the following subgroup analyses.
Younger women versus older women (as defined by trial authors).
Low‐resource versus high‐resource settings.
Primigravidae versus multigravidae.
Women as the active initiators of support versus women as the passive recipients of support.
Health‐professional support versus lay support.
Timing and duration of telephone‐based support.
We planned to use the following outcomes in subgroup analysis: maternal satisfaction and maternal anxiety/stress. In this version of the review, due to lack of data we did not carry out this planned analysis. In updates if more data become available, we will assess differences between subgroups by interaction tests.
Sensitivity analysis
We planned to carry out sensitivity analyses as appropriate to evaluate the effect of trial quality; in this version of the review we did not carry out this analysis due to the paucity of data for most outcomes.
Results
Description of studies
Results of the search
The search of the Cochrane Pregnancy and Childbirth Group's Trials Register (23 January 2013) identified 86 reports representing 62 different studies (several studies resulted in more than one publication). Altogether, 29 trials met the inclusion criteria, 29 studies were excluded, and four trials are still ongoing (Dennis 2012; Evans 2012; Moniz 2012; Patel 2011).
Included studies
Twenty‐nine trials met the inclusion criteria for the review. The trials all included pregnant women or women in the early postpartum period (up to six weeks postpartum) and examined interventions that involved telephone support. Trials that included telephone support as one part of more complex interventions were only included if we judged that the telephone support was the key difference between women in the intervention and control groups, or if women in control groups received all other parts of complex interventions apart from telephone support. Two trials, which were otherwise eligible for inclusion, did not report results by randomisation group or the data were not presented in a way that allowed us to include results in the data and analysis tables (Parker 2007; Stotts 2002). We have set out more information about these trials in Characteristics of included studies tables but these studies are not discussed further in the results below. Results are therefore based on 27 trials including a total of more than 12000 women which contribute data to the review.
The included trials were published between 1982 and 2012; 13 of the trials were carried out in the USA (Boehm 1996; Bullock 2009; Bunik 2010; Di Meglio 2010; Donaldson 1988; Ershoff 1999; Ferrara 2011; Little 2002; McBride 1999; Moore 1998; Pugh 2002; Rasmussen 2011; Rigotti 2006), five in Canada (Bloom 1982; Dennis 2002; Dennis 2009; Johnson 2000; Mongeon 1995), two in Australia (Bryce 1991; Milgrom 2011) two in England (Naughton 2012; Smith 2008) and one each in Thailand (Jareethum 2008), New Zealand (Bullock 1995), Italy (Simonetti 2012), Zanzibar (Lund 2012) and Scotland (Hoddinott 2012).
All of the trials recruited women during pregnancy or the early postpartum period. Many of the trials recruited women from high‐risk groups (e.g. women at high risk of depression, or women who were smokers) and the intervention was specifically designed to address the risk factor.
Interventions
Nine of the trials were designed to support breastfeeding women (Bloom 1982; Bunik 2010; Dennis 2002; Di Meglio 2010; Hoddinott 2012; Mongeon 1995; Pugh 2002; Rasmussen 2011; Simonetti 2012). In all but the Rasmussen 2011 trial, women were recruited after the birth of the baby and interventions took place during the postnatal period. In the trials by Bloom 1982, Bunik 2010, Hoddinott 2012, Rasmussen 2011 and Simonetti 2012 the telephone support intervention was carried out by healthcare professionals (nurses, midwives or lactation consultants), whereas in the Dennis 2002, Di Meglio 2010 and Mongeon 1995 trials, the telephone intervention was delivered by trained volunteers (in the Di Meglio 2010 study both participants and volunteers were under 20 years of age). In the Pugh 2002 trial telephone support was from both nurses and peer counsellors.
Six studies aimed to encourage women to quit smoking, or to prevent smoking relapse (Bullock 2009; Ershoff 1999; Johnson 2000; McBride 1999; Naughton 2012; Rigotti 2006). In three of these studies the interventions were provided during pregnancy only (Bullock 2009; Ershoff 1999; Naughton 2012), while in McBride 1999 and Rigotti 2006 telephone support started in pregnancy and continued after the birth of the baby. Johnson 2000 focused on the prevention of smoking relapse in the postnatal period. In the Naughton 2012 trial, women received automated text messages encouraging smoking cessation, whereas in the other trials telephone support was from health professionals (nurses or trained counsellors).
Two trials focused specifically on women at high risk of postnatal depression (Dennis 2009; Milgrom 2011). In both cases the telephone support intervention was delivered by health professionals. In the Dennis 2009 trial women received the support intervention during the postnatal period only. Participants in the Milgrom 2011 study were assessed during pregnancy and those at high risk of depression and randomised to the intervention group received supportive telephone calls from a psychologist during pregnancy and the early postpartum period.
Two studies focused on women who were at high risk of preterm birth (Boehm 1996; Bryce 1991) and in both of these trials women received phone calls during pregnancy from trained staff. In the Boehm 1996 trial calls were made daily to assess symptoms and provide support. In the Bryce 1991 trial, women received supportive phone calls between antenatal visits, which aimed to provide emotional support rather than education.
Ferrara 2011 recruited women at high risk of gestational diabetes and the intervention aimed to increase exercise, encourage a healthy diet and promote breastfeeding; the intervention was delivered by professionals including dieticians and lactation consultants.
Six of the studies examined more general telephone support interventions. In the trial by Jareethum 2008, women received text messages giving advice on health in pregnancy and signs and symptoms which were tailored for gestational age. In the trial by Moore 1998, women aged under 18 or at high risk received general advice from a nurse on health during pregnancy. Little 2002 also focused on high‐risk women with support during the antenatal period only from nurses. Similarly, Smith 2008 examined telephone support from midwives during the antenatal period. In the Bullock 1995 trial, women received general advice and support during both the antenatal and postnatal periods from trained volunteers. In a cluster‐randomised trial in Zanzibar, women in the intervention group received automated mobile phone messages tailored to gestational age; messages provided general health education and encouraged women to attend antenatal care appointments and to seek skilled attendance for the birth (Lund 2012). Finally, the Donaldson 1988 trial focused on the early postnatal period, and again women received general advice and support from nurse educators.
Excluded studies
Several other studies were considered but excluded for various reasons. These included recruitment to the trial beyond six weeks postpartum (Dennis 2003; Edwards 1997; Fjeldsoe 2010; Kersten‐Alvarez 2010; van Doesum 2008), telephone support not being the trial intervention (Bartholomew 2011; Brooten 1994; Haider 1997; Janssen 2006; Langer 1993; Lewis 2011; Sink 2001), or only a small component of the overall intervention (Brooten 2001; Frank 1986; Katz 2011; Norbeck 1996; Oakley 1990), or the telephone support was provided to both study groups (Alemi 1996; Gagnon 2002; Iams 1988). Trials that evaluated the frequency of telephone support (Rush 1991), or as a screening tool (Steel O'Conner 2003), or the intervention included several elements in addition to telephone support (Gjerdingen 2009) were also excluded. In addition, we excluded studies based on trial methodology; two were quasi‐randomised (Jang 2008; Lando 2001), one used alternate allocation (Chen 1993), and the remaining trial was not randomised (Ershoff 2000). The two remaining reports were trial protocols (Caramlau 2011; Stomp‐van den Berg 2007). Details of excluded studies are given in the Characteristics of excluded studies tables.
Risk of bias in included studies
Overall, the studies were of mixed methodological quality. We have summarised findings for risk of bias for each bias domain in Figure 1, and in Figure 2 we have set out 'Risk of bias' assessments for each included study.
1.
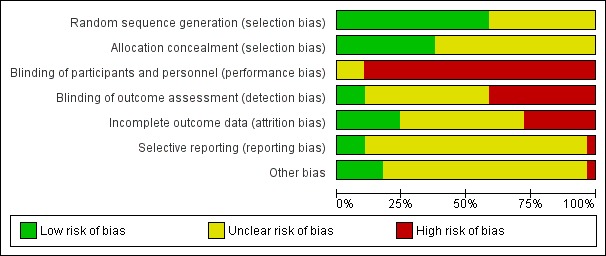
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
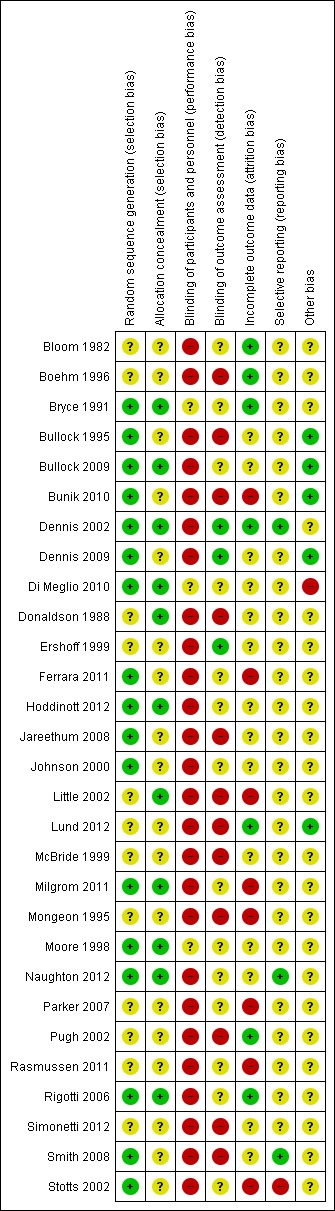
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We assessed that 16 of the 27 trials used methods that were of low risk of bias for generation of the randomisation sequence (Bryce 1991; Bullock 1995; Bullock 2009; Bunik 2010; Dennis 2002; Dennis 2009; Di Meglio 2010; Ferrara 2011; Hoddinott 2012; Jareethum 2008; Johnson 2000; Milgrom 2011; Moore 1998; Naughton 2012; Rigotti 2006; Smith 2008). These trials used methods such as computer‐generated randomisation, random number tables, or web‐based or telephone external randomisation services. The remaining trials used methods that were either not described or descriptions were not clear (Bloom 1982; Boehm 1996; Donaldson 1988; Ershoff 1999; Little 2002; Lund 2012; McBride 1999; Mongeon 1995; Pugh 2002; Rasmussen 2011; Simonetti 2012). No studies used methods that we assessed as inadequate.
Eleven studies used methods of concealing group allocation at the point of randomisation, which we judged were at low risk of bias such as allocations concealed in opaque, sealed and numbered envelopes or external services (Bryce 1991; Bullock 2009; Dennis 2002; Di Meglio 2010; Donaldson 1988; Hoddinott 2012; Little 2002; Milgrom 2011; Moore 1998; Naughton 2012; Rigotti 2006). The remaining studies did not describe methods or methods were unclear with regard to risk of bias for this domain.
Blinding
Blinding women or those delivering support for this type of intervention is not easy to achieve, and this lack of blinding may have an important impact depending on what sort of outcomes were measured, and for self‐report measures there may have been a high risk of response bias. For outcome assessment, blinding may be more feasible. Twelve of the studies did not mention any attempt to blind those collecting outcome data (Boehm 1996; Bullock 1995; Bunik 2010; Donaldson 1988; Jareethum 2008; Little 2002; Lund 2012; McBride 1999; Mongeon 1995; Pugh 2002; Simonetti 2012, Smith 2008). In 11 studies, trial authors said that outcome assessors were blind to group allocation (Bloom 1982; Bryce 1991; Dennis 2002; Dennis 2009; Di Meglio 2010; Ferrara 2011; Hoddinott 2012; Johnson 2000; Milgrom 2011; Moore 1998; Rasmussen 2011). However, it is possible that women revealed their allocation to interviewers, and so it is difficult to judge whether these attempts at blinding were successful in practice. In only one trial did the author describe attempts to test the effectiveness of blinding, and in this case it appeared that most of those collecting outcome data were unaware of randomisation group (Dennis 2009). In four trials where the primary outcome was smoking cessation outcome assessment was by cotinine analysis, and it is likely that staff carrying out tests were blind to group allocation (Bullock 2009; Ershoff 1999; Naughton 2012; Rigotti 2006).
Incomplete outcome data
Follow‐up was a problem in several of the included trials. In seven studies sample attrition was low and most of those randomised provided outcome data (Boehm 1996; Bloom 1982; Bryce 1991; Dennis 2002; Lund 2012; Pugh 2002; Rigotti 2006). In 14 trials the impact of sample attrition was not clear (Bullock 1995; Bullock 2009; Dennis 2009; Di Meglio 2010; Donaldson 1988; Ershoff 1999; Hoddinott 2012; Jareethum 2008; Johnson 2000; McBride 1999; Moore 1998; Naughton 2012; Simonetti 2012; Smith 2008). For some outcomes even relatively low attrition can mean that results are difficult to interpret (Higgins 2011). Sample attrition is not generally random, and it is possible that women at most risk of poor outcomes were less likely to respond (e.g. women at risk of depression, or who continue smoking, or abandon breast feeding). In four of the trials there were high levels of attrition, or there were missing data for some outcomes; in the Bunik 2010 trial, women in the intervention group who did not receive the intervention as planned were excluded from the analysis (27% loss to follow‐up). For some outcomes only 71/175 of the women randomised provided data in the trial by Little 2002. In the Rasmussen 2011 study loss to follow‐up was 20%, in Mongeon 1995 there were 30% missing data for some outcomes, and finally in the Milgrom 2011 trial, complete data were available for only 62% of those randomised, although there was an intention‐to‐treat analysis for the primary outcome. In Ferrara 2011, the loss in the intervention and control groups was not balanced (10% of controls were lost to follow‐up compared with 20% of the intervention group).
Selective reporting
Assessing outcome reporting bias is very difficult without access to study protocols and most of the studies were assessed as unclear for outcome reporting bias. Several authors provided us with additional information or data (Bullock 2009; Dennis 2009; Hoddinott 2012; Little 2002; Lund 2012; Milgrom 2011; Rigotti 2006; Smith 2008).
Other potential sources of bias
There were no obvious other sources of bias in most of the studies. Some baseline imbalance between groups was reported by Dennis 2002, Hoddinott 2012, Little 2002, Rasmussen 2011 and Rigotti 2006; even where baseline imbalance was not statistically significant it may affect the interpretation of results. In the trials by Bunik 2010, Di Meglio 2010, Milgrom 2011 and Smith 2008, it was reported that many women in the intervention group did not receive, or received only a small part of the intervention; this again means that it was difficult to interpret any differences, or lack of differences identified between groups.
Effects of interventions
Telephone support versus any other supportive intervention, or no telephone support (27 studies with 12,256 women)
All of the studies included in the review compared telephone support with routine care/no telephone support. We have included results from 24 studies in the meta‐analyses, results from a cluster‐randomised trial are set out in an additional table (Lund 2012), and results from a further two studies are discussed in the text (Bunik 2010; Di Meglio 2010).
Primary outcomes
Maternal satisfaction with support during pregnancy and the first six months postpartum (as defined by trial authors)
Two studies reported mean satisfaction scores with care during pregnancy; Little 2002 and Jareethum 2008 reported scores for overall satisfaction with care, although in the Jareethum 2008 trial women were not asked about their care until after the birth. Compared with those receiving telephone support, women in the control group had lower mean levels of satisfaction (standardised mean difference (SMD) 1.16, 95% confidence interval (CI) 0.79 to 1.54; two studies with 132 women) (Analysis 1.1). In addition, Mongeon 1995 reported the number of women in each group who said they were not satisfied with their care; there were no clear differences between groups (risk ratio (RR) 0.84, 95% CI 0.43 to 1.64; one study with 181 women) (Analysis 1.2).
1.1. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 1 Maternal satisfaction with support during pregnancy.
1.2. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 2 Maternal satisfaction with support in postnatal period (number feeling they were not supported).
Two trials reported mean scores for satisfaction with support in the postnatal period; women who received telephone support had higher mean satisfaction scores (SMD 0.54, 95% CI 0.17 to 0.91; two studies with 119 women) (Analysis 1.3).
1.3. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 3 Maternal satisfaction with support in postnatal period.
Maternal anxiety (measures as defined by trial authors)
Mean scores for anxiety during pregnancy were reported in two studies (Jareethum 2008; Smith 2008), although women rated their anxiety during pregnancy during the early postpartum period in the Jareethum 2008 study, which makes results from this trial difficult to interpret. There were no clear differences between groups for this outcome (SMD ‐0.09, 95% CI 0.29 to 0.11; two studies with 386 women) (Analysis 1.4).
1.4. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 4 Maternal anxiety in pregnancy.
The number of women with anxiety in the postnatal period (12 weeks postpartum) was reported in two trials (Dennis 2009; Milgrom 2011); there were no clear differences between those receiving the telephone intervention versus controls (average RR 0.50, 95% CI 0.17 to 1.46; two studies with 702 women) (Analysis 1.5). Anxiety was defined in different ways in these two trials. Dennis 2009 reported the number of women with STAI (State/Trait Axiety Inventory) scores greater than 44, whereas Milgrom 2011 reported on the number with DASS (Depression and Anxiety Short Scale) anxiety scores greater than, or equal to eight. These differences in measurement tools may account for the high statistical heterogeneity observed for this outcome (I2 = 69%) (Analysis 1.5). Mean scores for maternal anxiety in the postnatal period were reported in three trials; again, there were differences between studies in measurement tools and when outcomes were recorded. Anxiety scores were, on average, slightly lower in the intervention group (SMD ‐0.15, 95% CI ‐0.27 to ‐0.02; three studies with 952 women) (Analysis 1.6).
1.5. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 5 Maternal anxiety (number of women with anxiety) at last postpartum assessment.
1.6. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 6 Maternal anxiety in postnatal period.
One study reported on the number of women with high scores (260 or more) on the Parenting Stress Index at three months postpartum. Women who had received telephone support were less likely to have high stress scores; the difference between groups approached statistical significance (RR 0.30, 95% CI 0.09 to 1.00, one study with 94 women, P = 0.05) (Analysis 1.7).
1.7. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 7 Parenting stress: high score on parenting stress index at 12 weeks postpartum.
Secondary outcomes
Maternal outcomes
Mother‐infant attachment
This outcome was not reported in any of the included trials.
General health (e.g. as defined by standardised measures such as general health questionnaires)
This outcome was reported in one of the included trials; Donaldson 1988 collected data on the number of women rating their general health as good or very good at six weeks postpartum. The majority of women in both groups reported good general health (RR 0.93, 95% CI 0.72 to 1.21, one study with 37 women) (Analysis 1.8).
1.8. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 8 General health: general health at 8 weeks postpartum rated as good or very good.
Mortality and serious morbidity (e.g. perineal haematoma or deep surgical infection)
This outcome was not reported in any of the included trials.
Health service utilisation (presentation/attendance at clinics, accident and emergency departments or general practices)
Four studies reported maternal health service utilisation during pregnancy, the birth, or the postnatal period. Studies focused on different aspects of care and many of the data on particular outcomes were derived from only one or two studies. Overall, there was no strong evidence of differences between groups for health service utilisation.
Boehm 1996 and Smith 2008 reported the mean number of antenatal visits; there were no clear differences between women receiving or not receiving telephone support (mean difference (MD) 0.24. 95% CI ‐0.26 to 0.74; two studies 563 women) (Analysis 1.10).
1.10. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 10 Health service utilisation: mean number of antenatal visits.
Smith 2008 reported on antenatal hospital admissions; there were no clear differences between groups (RR 1.61, 95% CI 0.95 to 2.75; one study with data for 554 women) (Analysis 1.11).
1.11. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 11 Health service utilisation: admission to hospital during pregnancy.
Boehm 1996 provided data on mean length of hospital stay at the time of the birth; there was no strong evidence that the average length of stay varied by randomisation group (MD 0.81, 95% CI ‐1.56 to 3.18; one study with 42 women) (Analysis 1.12).
1.12. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 12 Health service utilisation: maternal length of hospital stay.
The mean number of contacts with community midwives and health visitors up to eight weeks postpartum were described by Hoddinott 2012. There was no significant evidence of differences in the number of contacts with either type of health professional (MD ‐0.40, 95 % CI ‐1.46 to 0.66, and MD ‐0.50, 95% CI ‐1.33 to 0.33, respectively; one study with data for 58 women) (Analysis 1.13).
1.13. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 13 Health service utilisation up to 8 weeks postpartum (mean contacts).
Dennis 2009 did not reveal statistically significant differences between women in the two randomised groups in terms of the mean number of health service contacts up to six months postpartum (MD ‐ 0.03, 95% CI ‐0.28 to 0.22; one study with 600 women) (Analysis 1.14).
1.14. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 14 Health service utilisation in postnatal period (last assessment up to 6 months) Mean number of contact.
Postpartum depression (measures as defined by author, e.g. the Edinburgh Postnatal Depression Scale (EPDS))
Dennis 2009 reported on the number of women with a diagnosis of depression at three months postpartum following a telephone intervention to support women at high risk of depression; there was no clear difference between groups (RR 0.65, 95% CI 0.34 to 1.23; one study, data for 612 women) (Analysis 1.15). The number of women with scores on the EPDS greater than 12, and those with scores of 14 or more on the Becks Depression scale, (both indicating a high risk of depression) were reported by Dennis 2009 and Milgrom 2011 respectively. Pooled results suggest that women receiving telephone support interventions were less likely to have scores above the cut‐offs denoting high risk at three months postpartum (RR 0.51, 95% CI 0.37 to 0.70; two studies with data for 701 women) (Analysis 1.16). Dennis 2009 also reported mean scores on the EPDS at three months, and average scores were lower in the group receiving telephone support (MD ‐ 0.96, 95% CI ‐1.75 to ‐0.17; one study 612 women) (Analysis 1.17).
1.15. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 15 Postnatal depression: clinical diagnosis of depression at 3 months postpartum.
1.16. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 16 Postnatal depression symptoms (high risk on scale) at 3 months postpartum.
1.17. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 17 Postnatal depression symptoms (mean score on EPDS) at 3 months postpartum.
Positive behaviour change (as defined by trial authors, e.g. smoking reduction)
Trials reported on several different types of behavioural change following telephone support interventions. Broadly, depending on the focus of the intervention, behaviour change was examined for smoking (cessation or relapse); breastfeeding (any or exclusive breastfeeding), alcohol consumption and general lifestyle changes (e.g. increase in exercise).
Seven trials reported on one or more outcomes relating to smoking (Bullock 1995; Bullock 2009; Ershoff 1999; Johnson 2000; McBride 1999; Naughton 2012; Rigotti 2006). Cotinine‐validated smoking cessation in pregnancy was reported in four trials; there was no strong evidence that women receiving telephone support interventions were less likely to be smoking at the end of pregnancy (RR 1.12, 95% CI 0.87 to 1.44; four trials with data for 1361 women) (Analysis 1.18). Similarly, there was no strong evidence that interventions reduced the number of women themselves reporting that they had stopped smoking at the end of pregnancy (RR 1.08, 95% CI 0.95 to 1.23; four studies with data for 1638 women) (Analysis 1.19). Two trials reported cotinine‐validated results for women who had stopped smoking (or had not relapsed) in the early postpartum period; there was no strong evidence that the intervention was effective (RR 0.90, 95% CI 0.62 to 1.32, two studies with 949 women) (Analysis 1.20); similarly, self‐reported smoking cessation was not significantly different in women receiving or not receiving telephone support (RR 1.28, 95% CI 0.94 to 1.73, two studies with data for 670 women) (Analysis 1.21).
1.18. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 18 Positive behaviour change: stopped smoking by the end of pregnancy (cotinine validated).
1.19. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 19 Positive behaviour change: stopped/not smoking by the end of pregnancy (self‐report).
1.20. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 20 Positive behaviour change: stopped smoking at last postpartum assessment (cotinine validated).
1.21. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 21 Positive behaviour change: stopped smoking at last postpartum assessment (self‐report).
Eight trials reported outcomes relating to breastfeeding (any, and or exclusive breastfeeding) (Bloom 1982; Dennis 2002; Ferrara 2011; Hoddinott 2012; Mongeon 1995; Pugh 2002; Rasmussen 2011; Simonetti 2012). There was no clear evidence that interventions had a positive effect on the number of women breastfeeding at six weeks postpartum although results were inconsistent between trials (average RR 0.98, 95% CI 0.86 to 1.12, five trials with 735 women, I2 69%) (Analysis 1.22). At six months postpartum it appeared that results favoured the intervention group, with those women receiving telephone support being more likely to be still breastfeeding (RR 1.21, 95% CI 1.06 to 1.38, five trials with 691 women) (Analysis 1.23).
1.22. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 22 Any breastfeeding at up to 6 weeks postpartum.
1.23. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 23 Any breastfeeding up to 6 months postpartum.
Four trials examined exclusive breastfeeding at four to eight weeks postpartum. Three of the four trials reported results favouring the group receiving telephone support; however, results were inconsistent and there was high heterogeneity for this outcome. Pooled results showed no statistically significant difference between groups (average RR 1.27, 95% CI 0.88 to 1.83, four trials with 465 women) (Analysis 1.24). Three trials examined exclusive breastfeeding at three to six months postpartum; pooled results showed a statistically significant difference between groups, with women who had received the support intervention being more likely to be exclusively breastfeeding (RR 1.51, 95% CI 1.19 to 1.93, three trials with 411 women) (Analysis 1.25).
1.24. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 24 Exclusive breastfeeding at 4‐8 weeks.
1.25. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 25 Exclusive breastfeeding at 3 ‐6 months.
Bloom 1982 reported that mean breastfeeding duration was 7.6 days longer for women receiving the telephone intervention and the difference between groups approached statistical significance (5% CI 0.06 to 15.14, P = 0.05, 99 women) (Analysis 1.26). Duration of breastfeeding was also measured by Di Meglio 2010 who reported that "duration did not differ significantly between the intervention group and the control group (median, 77 versus 35 days; hazard ratio 0.71, 95% CI 0.39 to 1.30, P = 0.26)." (Data not shown in data and analysis tables.) Bunik 2010 also reported breastfeeding duration and stated that by one month postpartum 71% of women in both the intervention and control group had introduced infant formula (data not shown).
1.26. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 26 Mean breastfeeding duration (any breastfeeding) in days.
Dennis 2002 reported mean scores for women's satisfaction with their experience of breastfeeding their babies (this was not one of our pre‐specified outcomes); women receiving the intervention were not shown to be significantly more satisfied (MD 0.83, 95% CI ‐0.60 to 2.26, 256 women) (Analysis 1.38).
1.38. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 38 Non prespecified outcome: satisfaction with infant feeding experience.
Bullock 1995 reported the number of women who were not consuming alcohol in late pregnancy; there were no clear differences between the intervention and control groups in the number of women who reported that they had not consumed any alcohol in the last month (RR 0.95, 95% CI 0.75 to 1.20, 122 women) (Analysis 1.27). Ferrara 2011 provided data on the number of women who achieved weight goals at six months postpartum; there was no strong evidence that the intervention had a positive effect (RR 1.20, 95% CI 0.67 to 2.17, 189 women) (Analysis 1.28).
1.27. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 27 Positive behaviour change: not drinking in the last month (late pregnancy).
1.28. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 28 Positive behavioural change: women meeting postpartum weight goals at 6 months postpartum.
Infant outcomes
Preterm birth/low birthweight
Four studies provided data on the number of preterm births (before 37 weeks' gestation). Although the intervention was associated with a decrease in the number of preterm births the difference between groups was not statistically significant (RR 0.91, 95% CI 0.77 to 1.08, 3992 women) (Analysis 1.29). Boehm 1996 reported the mean gestational age at delivery which was identical in the two groups (MD 0.00, 95% CI ‐1.49 to 1.49, 42 women) (Analysis 1.30).
1.29. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 29 Preterm birth < 37 weeks.
1.30. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 30 Mean gestational age at delivery.
Three trials provided data on the number of low birthweight babies (less than 2500 g); and while results slightly favoured the intervention group, the difference was not statistically significant (RR 0.90, 95% CI 0.76 to 1.07, 3862 women) (Analysis 1.31). There were no clear differences between groups for mean infant birthweight (MD ‐42.11g, 95% CI ‐130.36 to 46.14, two trials with 592 women) (Analysis 1.32).
1.31. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 31 Low birthweight < 2500 g.
1.32. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 32 Mean birthweight.
Infant developmental measures (physical and cognitive as defined by trial authors)
Outcomes relating to infant development were not reported in included trials.
Neonatal/infant mortality and major neonatal/infant morbidity (as defined by trial authors, e.g. prolonged admission to special care baby unit)
Only one trial provided data on neonatal and infant mortality; the number of deaths was higher where woman had received telephone support but the difference between groups was not statistically significant (RR 1.40, 95% CI 0.82 to 2.42, 1884 women) (Analysis 1.34).
1.34. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 34 Neonatal/infant mortality..
Two studies reported on admissions to neonatal intensive care units; there appeared to be fewer admissions if women had received telephone support (0.71, 95% CI 0.52 to 0.97; two studies, 2403 women) (Analysis 1.35).
1.35. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 35 Major neonatal/infant morbidity/admission to NICU.
Service outcome
Intervention cost
This outcome was not reported.
Non‐prespecified outcomes
One study used a cluster‐randomised design, and 24 healthcare facilities in Zanzibar were randomised (Lund 2012). The study reported results for 2550 women. The primary outcome in this study was the number of women with skilled attendance at the birth. Results for this outcome are set out in Table 1. Overall, 60% of women in the group receiving the mobile phone support intervention had skilled help at the birth compared with 47% of women in the control group (unadjusted data). The difference between groups was almost all due to the increased number of women in the intervention group living in urban areas having skilled attendance; the intervention did not seem to make much difference for women living in rural areas where more than half of the women in both groups had no skilled help at the birth. Other outcomes from this trial will be reported in future papers and we hope to include them in updates of the review.
Other non‐prespecified outcomes
Three studies reported on infant health service utilisation. Boehm 1996 reported on infant length of hospital stay following the birth; there were no clear differences between groups (MD 0.80, 95% CI ‐0.31 to 1.91, 42 women) (Analysis 1.36). Pugh 2002 described the number of infant healthcare visits for a sample of 41 women; the babies of women who received telephone support were reported to receive a mean of 1.4 fewer healthcare visits (3.6 versus five visits, 95% CI ‐2.57 to ‐0.23) (Analysis 1.37). Bunik 2010 reported that by one month postpartum, similar numbers of babies in the intervention and control group had attended well‐baby clinic visits, however, 25% of babies in the intervention group and 36% in the control group had had at least one sick visit (data not shown).
1.36. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 36 Non‐prespecified: infant length of hospital stay.
1.37. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 37 Non prespecified: mean number of infant healthcare visits.
Boehm 1996 reported on the diagnosis of preterm labour; there was no evidence of differences between groups (RR 1.00, 95% CI 0.50 to 2.01) (Analysis 1.40).
1.40. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 40 Non prespecified outcome: diagnosis of preterm labour.
Three studies reported the number of women having caesarean births; overall numbers were similar in intervention and control groups (RR 1.06, 95% CI 0.90 to 1.34, 2480 women) (Analysis 1.39).
1.39. Analysis.
Comparison 1 Telephone support versus any other supportive intervention, or no telephone support, Outcome 39 Caesarean section.
Discussion
Summary of main results
In this review we have included data from 27 randomised trials with more than 12,000 women. All of the trials examined telephone support versus usual care (no additional telephone support). We did not identify any trials comparing different modes of telephone support (for example, text messaging versus one‐to‐one calls). All but two of the trials were carried out in high‐resource settings. The majority of studies examined support provided via telephone conversations between women and health professionals although a small number of trials included telephone support from peers. In three trials women received automated text messages. Many of the interventions aimed to address specific health problems and collected data on behavioural outcomes such as smoking cessation and relapse (seven trials) or breastfeeding continuation (eight trials). Other studies examined support interventions aimed at women at high risk of postnatal depression (two trials) or preterm birth (two trials); the rest of the interventions were designed to offer women more general support and advice.
For most of our pre‐specified outcomes few studies contributed data, and many of the results described in the review are based on findings from only one or two studies. Overall, results were inconsistent and inconclusive although there was some evidence that telephone support may be a promising intervention. Results suggest that telephone support may increase women's overall satisfaction with their care during pregnancy and the postnatal period; although results for both periods were derived from only two studies. There was no consistent evidence confirming that telephone support reduces maternal anxiety during pregnancy or after the birth of the baby although results on anxiety outcomes were not easy to interpret as data were collected at different time points and using a variety of measurement tools. One trial with a small sample size suggested that support may reduce parenting stress, although the difference between groups was not statistically significant. There was evidence from two trials that women who received support had lower mean depression scores in the postnatal period although there was no clear evidence that women who were supported were less likely to have a diagnosis of depression. Results from trials encouraging breastfeeding through telephone support were also inconsistent, although for longer‐term breastfeeding outcomes (up to six months postpartum), results suggested that women receiving telephone support were more likely to continue any breastfeeding and exclusive breastfeeding for longer. There was no strong evidence that women receiving telephone support interventions were less likely to be smoking at the end of pregnancy or during the postnatal period, nor was it clear that interventions reduced smoking relapse.
For infant outcomes, such as preterm birth and infant birthweight, there was little evidence overall. Where evidence was available, there were no clear differences between groups. Results from two trials suggest that babies whose mothers received support may have been less likely to have been admitted to neonatal intensive care unit, although it is not easy to understand the mechanisms underpinning this finding.
Results from one cluster‐randomised trial examined whether women receiving a mobile phone intervention were more likely to have skilled attendance at the birth and for women living in urban areas results favoured the intervention group; although this was not one of our prespecified outcomes skilled attendance at the birth may have an impact on both maternal and infant health outcomes. We hope to include further results from this trial in updates of the review.
Based on the limited evidence from the 27 trials that provided data for this review, there remains uncertainty regarding the effectiveness of telephone support.
Overall completeness and applicability of evidence
The studies included in this review were from a variety of countries; however, all but two were from a high‐resourced setting. This limits the applicability of the findings, particularly as it is conceivable that telephone support has the potential for greater impact in environments where health services are unavailable or hard to access. In the postnatal period, for example, this may change the intervention from supplementary support to the only support, in some environments.
As mentioned previously, the review included trials with a variety of aims; some offering general support and others intent on improving behaviour. However, even within these trial groupings, there were variations in the choice of outcomes measured, when they were measured, and the ways in which they were measured. This made it difficult to draw meaningful conclusions. Moreover, important clinical outcomes, such as serious maternal morbidity and mother‐infant attachment, were absent from the included trials.
We had hoped to compare conversational telephone support with the use of text or instant messaging. We were unable to find any randomised controlled trials that had compared alternative modes.
Quality of the evidence
The overall methodological quality of the studies included in the review was mixed; approximately half of the trials used methods to randomise women to experimental and control groups using methods that we judged were at low risk of bias. None of the trials achieved effective blinding of women or those providing care; in four of the trials looking at smoking cessation cotinine analysis was carried out to confirm smoking status and this would be likely to be at low risk of bias for this particular outcome. However, for most outcomes there was a high risk of bias associated with the lack of blinding. Even where authors reported that outcome assessors were blinded it is possible that women revealed their allocation; only one trial author reported attempts to assess the success of blinding outcome assessors. The overall impact of sample attrition was difficult to assess; for most of the trials we judged that attrition was unclear, or that results were at high risk of bias due to loss to follow‐up.
Most of the results of this review are derived from one or two studies and several of the studies had small sample sizes; we were therefore unable to pool many of the data in meta‐analyses. There was a lack of consistency between studies in terms of the outcomes reported, and the time and way in which outcomes were measured. In addition, there was considerable diversity in terms of the aims of interventions and the way they were delivered. These differences mean that for any one outcome there were few data and most of our results were inconclusive.
Potential biases in the review process
We are aware that the review process itself may introduce bias. We took various steps to reduce bias; at least two review authors independently carried out data extraction and assessed risk of bias. If study methods or results were unclear, we attempted to contact trial authors and several authors provided additional data or clarified study methods.
Agreements and disagreements with other studies or reviews
This study builds on an earlier review by Dennis 2008, which had similar inclusion criteria to this review, and included 14 trials. We excluded some of the studies that were included by Dennis 2008 because we were unconvinced that the telephone support made a substantial contribution to the intervention being assessed (e.g. Brooten 2001; Frank 1986). Nevertheless, the findings were not dissimilar. Both reviews suggest potential benefits without any evidence of harm, and both reviews recommend the need for further research in this area.
Authors' conclusions
Implications for practice.
Despite some encouraging findings, there is insufficient evidence to recommend routine telephone support for women accessing maternity services, as the evidence from included trials is neither strong nor consistent. Although benefits were found in terms of reduced depression scores and increased overall satisfaction, the current trials do not provide strong enough evidence to warrant investment in resources.
Implications for research.
This review has highlighted the need for further research to assess the effects of telephone support during pregnancy and the first six weeks post birth. Further research is required to assess its application for general support of women, and for those with specific needs, such as high risk of preterm birth.
The review has raised a number of questions regarding the actual intervention. Further research is needed to explore the optimum intervention, in terms of mode of delivery (text or conversation), style of support (proactive or reactive), deliverer (health professional, trained volunteer, automated), frequency, duration and timing of delivery. A clear audit trail of intervention development should be apparent; this should include consumer input.
Standardisation of outcomes in future trials would aid synthesis and transferability. Important outcomes were absent from existing trials (maternal mortality/severe morbidity, maternal‐infant attachment and infant development); these should be considered in future studies. Furthermore, there was no information on the costs associated with telephone support; future studies should include cost effectiveness as an integral part of the trial design.
Despite a plethora of m‐health programmes implementing mobile phone support, particularly in low‐resourced settings, many of these have not yet been subjected to randomised controlled trials. This is a particular area of need.
Acknowledgements
We acknowledge an earlier version of this protocol by Cindy‐Lee Dennis and Debra Creedy.
As part of the pre‐publication editorial process, this review has been commented on by four peers (an editor and three referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pregnancy and Childbirth Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. Telephone support versus any other supportive intervention, or no telephone support.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal satisfaction with support during pregnancy | 2 | 132 | Std. Mean Difference (IV, Fixed, 95% CI) | 1.16 [0.79, 1.54] |
| 2 Maternal satisfaction with support in postnatal period (number feeling they were not supported) | 1 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.43, 1.64] |
| 3 Maternal satisfaction with support in postnatal period | 2 | 119 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.54 [0.17, 0.91] |
| 4 Maternal anxiety in pregnancy | 2 | 386 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.29, 0.11] |
| 5 Maternal anxiety (number of women with anxiety) at last postpartum assessment | 2 | 702 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.17, 1.46] |
| 6 Maternal anxiety in postnatal period | 3 | 952 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.27, ‐0.02] |
| 7 Parenting stress: high score on parenting stress index at 12 weeks postpartum | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.09, 1.00] |
| 8 General health: general health at 8 weeks postpartum rated as good or very good | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.72, 1.21] |
| 9 Mortality and serious morbidity | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Health service utilisation: mean number of antenatal visits | 2 | 563 | Mean Difference (IV, Fixed, 95% CI) | 0.24 [‐0.26, 0.74] |
| 11 Health service utilisation: admission to hospital during pregnancy | 1 | 554 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [0.95, 2.75] |
| 12 Health service utilisation: maternal length of hospital stay | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.81 [‐1.56, 3.18] |
| 13 Health service utilisation up to 8 weeks postpartum (mean contacts) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.1 Contacts with community midwife | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.46, 0.66] |
| 13.2 Contacts with health visitor | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.33, 0.33] |
| 14 Health service utilisation in postnatal period (last assessment up to 6 months) Mean number of contact | 1 | 600 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.28, 0.22] |
| 15 Postnatal depression: clinical diagnosis of depression at 3 months postpartum | 1 | 612 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.34, 1.23] |
| 16 Postnatal depression symptoms (high risk on scale) at 3 months postpartum | 2 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.37, 0.70] |
| 17 Postnatal depression symptoms (mean score on EPDS) at 3 months postpartum | 1 | 612 | Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐1.75, ‐0.17] |
| 18 Positive behaviour change: stopped smoking by the end of pregnancy (cotinine validated) | 4 | 1361 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.87, 1.44] |
| 19 Positive behaviour change: stopped/not smoking by the end of pregnancy (self‐report) | 4 | 1638 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.95, 1.23] |
| 20 Positive behaviour change: stopped smoking at last postpartum assessment (cotinine validated) | 2 | 949 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.62, 1.32] |
| 21 Positive behaviour change: stopped smoking at last postpartum assessment (self‐report) | 2 | 670 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.94, 1.73] |
| 22 Any breastfeeding at up to 6 weeks postpartum | 5 | 735 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.86, 1.12] |
| 23 Any breastfeeding up to 6 months postpartum | 5 | 691 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.06, 1.38] |
| 24 Exclusive breastfeeding at 4‐8 weeks | 4 | 465 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.88, 1.83] |
| 25 Exclusive breastfeeding at 3 ‐6 months | 3 | 411 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.19, 1.93] |
| 26 Mean breastfeeding duration (any breastfeeding) in days | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 7.60 [0.06, 15.14] |
| 27 Positive behaviour change: not drinking in the last month (late pregnancy) | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.75, 1.20] |
| 28 Positive behavioural change: women meeting postpartum weight goals at 6 months postpartum | 1 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.67, 2.17] |
| 29 Preterm birth < 37 weeks | 4 | 3992 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.77, 1.08] |
| 30 Mean gestational age at delivery | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.49, 1.49] |
| 31 Low birthweight < 2500 g | 3 | 3862 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.76, 1.07] |
| 32 Mean birthweight | 2 | 592 | Mean Difference (IV, Fixed, 95% CI) | ‐42.11 [‐130.36, 46.14] |
| 33 Infant developmental measures | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 34 Neonatal/infant mortality. | 1 | 1884 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.82, 2.42] |
| 35 Major neonatal/infant morbidity/admission to NICU | 2 | 2403 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.52, 0.97] |
| 36 Non‐prespecified: infant length of hospital stay | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐0.31, 1.91] |
| 37 Non prespecified: mean number of infant healthcare visits | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐1.4 [‐2.57, ‐0.23] |
| 38 Non prespecified outcome: satisfaction with infant feeding experience | 1 | 256 | Mean Difference (IV, Fixed, 95% CI) | 0.83 [‐0.60, 2.26] |
| 39 Caesarean section | 3 | 2480 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.90, 1.24] |
| 40 Non prespecified outcome: diagnosis of preterm labour | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.50, 2.01] |
Comparison 2. Verbal telephone support versus text support.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal satisfaction with support during pregnancy and the first six months postpartum | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Maternal anxiety | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Mother‐infant attachment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 General health | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Mortality and serious morbidity | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Health service utilisation | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Postpartum depression | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Positive behaviour change | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Preterm birth/low birthweight | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding duration | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Infant developmental measures | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Neonatal/infant mortality. | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Major neonatal/infant morbidity | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Intervention cost | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bloom 1982.
| Methods | RCT. 3 groups of primiparous breastfeeding mothers; 51 in the experimental and 49 in the control group. The study involved a 3rd group of formula‐feeding mothers n = 57; this non‐randomised group is not included in the results in the review. | |
| Participants | Setting: hospital in Halifax, Nova Scotia, Canada. 100 women recruited 3 days after birth – all interviewed by same female research assistant (nurse who had breastfed own child). Inclusion criteria: primiparous breastfeeding mothers. Exclusion criteria: multiparous mothers excluded from study to remove effects of previous breastfeeding experience. Also excluded were mothers with baby below 2500 g at birth or where Apgar at 5 minutes was below 5, those who had operant deliveries or twins, those relinquishing their infants, single mothers and those who did not speak/understand English. |
|
| Interventions | All breastfeeding women received pamphlet on breastfeeding technique and infant behaviour. Experimental group (51) received 3 telephone calls at 1‐week intervals beginning 10 days postpartum (to offer support to breastfeeding mother). All calls were made by same nurse and were described as friendly 5‐10 minute conversations asking about experiences with her baby and breastfeeding. Advice on breastfeeding and infant behaviour in general was offered. Problems described and changes of feeding practice were recorded. Mothers expressing special concern or requesting medical advice were reassured and referred to nurse (specialised in breastfeeding guidance) at the maternity hospital. During the first call mothers were asked if they had found the information pamphlet helpful. Calls were discontinued when a mother stopped breastfeeding Control group: (49 women). All breastfeeding mothers received pamphlet on breastfeeding technique and infant behaviour. |
|
| Outcomes | At 6 weeks postpartum a second female interviewer called (unaware of which group allocated to or changes with feeding practice) for 10‐minute interview to assess mothers adjustment to the first few weeks at home with baby and aspects of the infant's behaviour. Reports: ‘the average duration of breastfeeding was extended one week for the experimental group (mean 28.6 days v 21 days P = 0.05)’. (SD was not reported so we imputed a SD based on the P value given in the paper P = 0.05). On page 12 authors refer to Fig 1 but there are no figures in the paper. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised – no further detail provided. |
| Allocation concealment (selection bias) | Unclear risk | Study described as randomised – no further detail provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of women and staff providing care not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Second nurse collecting outcome data was reported to be blinded to allocation but it is unclear how effective this would have been in practice as it may have become quickly apparent in conversation with a mother which group she had been allocated to. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 woman lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Most of the results were not comparisons of the randomised groups. It was not clear when women were followed up. It was reported that there were telephone interviews at 6 weeks but ‐ mean duration of breastfeeding in the intervention and control groups was reported but no SD was reported. Also if follow‐up was at 6 weeks postpartum; reporting the mean suggests that all women had discontinued breastfeeding by 6 weeks but elsewhere it was stated that 25% of the breastfeeding control group changed to formula feeding within 6 weeks, which suggests that most women were still breastfeeding. |
| Other bias | Unclear risk | Results were confusing. Samples from 2 different studies were compared, randomised and non‐randomised women were compared. Results were not reported in full. |
Boehm 1996.
| Methods | 2‐arm RCT with individual randomisation (a 2nd non‐randomised control group have not been included in the analysis). | |
| Participants | Setting: 42 women at high risk of preterm birth attending 2 hospitals in the USA between 1989 to 1993; recruitment from 20 weeks’ gestation. Inclusion criteria: 18 years or more, previous preterm birth (< 37 weeks and not related to incompetent cervix, multiple gestation, pregnancy‐induced hypertension or intrauterine growth retardation), 20 weeks' gestation or more and absence of maternal conditions that might lead to early delivery (e.g. diabetes or drug abuse), telephone access at home. |
|
| Interventions | Intervention group (21 women) in addition to usual care, daily telephone calls from recruitment to delivery from the research nurse, women were asked about symptoms and opportunity for women to discuss any concerns. Control group (21 women) usual care which included 24 hour phone access help line if they had any problems and prenatal visits at least fortnightly from 20 weeks to delivery. |
|
| Outcomes | Diagnosis and treatment of preterm labour, number of prenatal visits, mode of delivery, mean length of hospital stay for mother and baby, gestational age at delivery and infant birthweight. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It appeared that all women randomised were followed up. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | Groups appeared comparable at baseline. |
Bryce 1991.
| Methods | RCT (Zelen design), 2 arms with individual randomisation. | |
| Participants | Setting: women attending 3 public hospitals of obstetrician or GP antenatal clinics in Perth, Western Australia. 1970 recruited and randomised. Inclusion criteria: women were enrolled at their first antenatal visit if they had a history of preterm birth (< 2500 g), perinatal death, 3 or more first trimester miscarriages or more than 1 second trimester (12‐19 weeks) miscarriages or antenatal haemorrhage in previous pregnancy. Exclusion criteria: non‐English speaking, more than 25 weeks’ gestation or if the fetus was dead. |
|
| Interventions | Intervention (983 women). In addition to usual care women received support during pregnancy by midwives. Women received additional home visits (at roughly 4‐6 weeks intervals) and between visits midwives telephone women to provide general support and empathy rather than education or antenatal checks. It was not clear how many phone calls women received. Control (987 women) women received usual antenatal care with no additional home visits or phone calls. |
|
| Outcomes | Primary outcome was incidence of preterm birth. Other outcomes included mode of delivery, and stillbirth and neonatal death. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation (block size not stated) carried out by computer programmer. |
| Allocation concealment (selection bias) | Low risk | Zelen method. Women were randomised before they consented. Outcomes for women who refused consent or could not be followed up were analysed along with women who received the intervention. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Staff providing antenatal care were reported to be blind to group allocation. Women and those delivering the intervention would not be blind. The impact of lack of blinding on outcomes assessed was not clear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not clear if outcome assessment was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Zelen randomisation method 93/983 in the intervention group either refused consent or not available to follow‐up but were analysed by ITT. There were very little missing outcome data for primary outcome. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | Groups appeared comparable at baseline. |
Bullock 1995.
| Methods | RCT, 2 arms with individual randomisation. | |
| Participants | Setting: 131 women recruited from outpatient department of a large public maternity hospital, general practice surgeries and self‐referral to the study. Recruitment between March 1993 and December 1993 Inclusion criteria: women at less than 20 weeks' gestation, either single or in a relationship where partner was unemployed. Exclusion criteria: being unable to access a telephone (no women were in this category). |
|
| Interventions | Intervention group: 65 women. Women in both the intervention and control groups received a package of educational materials. In addition women in the intervention group received a weekly telephone call from a trained volunteer. Calls started from initial assignment to intervention group until 12 weeks after baby was born. 19 women volunteers delivered the weekly calls (received training in healthy mothers/healthy babies, research methods, communication techniques, and general information about normal occurrences in the antenatal and postnatal period. Calls included questions about the pregnancy and health behaviours such as alcohol and drug use, smoking and number of meals a day and in last week and when woman was going for next antenatal appointment, and whether she had felt stressed in the last week. Each volunteer contacted between 2‐6 women. Control group: given a package of publicly available educational material on healthy behaviours during pregnancy. |
|
| Outcomes | All interviewed (by the PI) 4 times during study: at baseline, 34 weeks' gestation, 6 weeks and 12 weeks' postnatal. To assess stress level, social support from partner, social support from family and friends, self‐esteem, anxiety, depression and a variety of health behaviours as well as demographic factors. In this report of the trial only baseline and 34 weeks data were included. | |
| Notes | Data are reported as baseline and late pregnancy means and P values but not SEMs. We have contacted the author to see if the corresponding SDs (or SEMs) are available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated assignment’ in balanced blocks of 50. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 131 women were randomised and 122 followed up; 3 women were lost from the control group and 6 from the intervention group. It was not clear whether there were further missing data. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. Mean scores were provided with no SDs which meant that results were difficult to interpret. |
| Other bias | Low risk | At baseline there were no differences in stress, social support, self‐esteem, STAI and depression indices. |
Bullock 2009.
| Methods | RCT. Factorial design. Block randomisation into 4 groups. Randomisation at the individual level. | |
| Participants | Setting: reported as: ‘Women (N = 695) attending 21 rural Women Infant and Children Nutritional Supplement (WIC) clinics in a Midwest state’ They were recruited ‘between January 2002 and October 2005'. Inclusion criteria: reported as: Women ‘smoking at least 1 cigarette per day, spoke English, and were 18 years or older and less than 24 weeks gestation’. Exclusion criteria: reported as ‘The most frequent reasons for ineligibility were age (below 18 years) and spontaneous abortion prior to the home visit’. |
|
| Interventions | 4 study groups (695 randomised and included in ITT analysis). For analysis in this review groups 1 and 2 (telephone support with or without booklet) were treated as the intervention group and 3 and 4 (no telephone support, with or without booklet) as the control group. 1. (170 women) telephone support plus booklet. Telephone support was by trained nursing staff. The intervention was scheduled weekly telephone calls at a time convenient to women. The purpose of the call was general support (information and emotional support) and smoking cessation messages were only offered when the nurses thought women would be receptive to them. Women also had access to 24‐hour nurse support for any additional support needed. The intervention was designed to be responsive to individual support needs. Women also received 8 booklets (mailed weekly) with smoking cessation messages. (BEEP intervention = Behavioral Education Enhancement of Pregnancy) Nurses delivering intervention received 80 hours training on the intervention and the study design. 2. (175 women) As 1 but telephone support intervention only (no booklets). 3. As group 1 but booklet only (179). 4. No intervention (171). |
|
| Outcomes | The main outcome was smoking cessation (salivary cotinine of 30 ng/mL or less). Samples were taken monthly during pregnancy and 6 weeks postpartum. Self‐report data on smoking at 28‐32 weeks and 6 weeks postpartum. (Information on stress and mental health was also collected at these time points but results are not reported.) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported as: 'Random assignments were prepared individually for each nurse, and assignments were computer generated in blocks of 40 for an evenly distributed workload'. |
| Allocation concealment (selection bias) | Low risk | Reported as: ‘At the completion of the interview, the baseline assessment, the nurse opened an opaque, sealed envelope, prepared by the PI that contained the study group assignment’. It was not clear that all envelopes were accounted for. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and clinical staff were not blinded and collection of self‐reported outcomes was not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Cotinine analysis was blinded: ‘The laboratory was blind to study group assignment while running the cotinine analyses. The assistants who collected the monthly saliva sample may or may not have been blinded to the study group but the rule was to treat all the women the same way. The nurses who collected samples when they conducted the follow‐up interviews in late pregnancy and 6‐weeks postdelivery were aware of the study group assignment’. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Relatively few women dropped out, however, there was a loss of cotinine samples (balanced across groups) for 165 women. An ITT analysis was carried out for the whole sample for the primary outcome, but other analysis was for 530 women. Complete data were available for 476 women (missing data were also relatively evenly spread across groups). |
| Selective reporting (reporting bias) | Unclear risk | It was not clear whether the intervention aimed to address stress and mental health. Although data were collected on a range of measures of stress and mental health findings for these outcomes were not reported. |
| Other bias | Low risk | Groups appeared comparable at baseline. No other bias apparent. |
Bunik 2010.
| Methods | RCT, 2‐arm trial (with block randomisation). Randomisation at the individual level. | |
| Participants | Setting: ‘primiparous women who delivered a term infant at an urban safety‐net hospital in Denver Colorado’ Recruited February 2005 to May 2006. The study hospital provided services to a predominantly Hispanic “medically underserved” population. 341 women randomised. Inclusion criteria: ‘women 18 years or older who delivered a healthy, term, singleton infant, and who were willing to consider breastfeeding. Exclusion criteria: ‘if their primary language was not English or Spanish, if they had medical complications that interfered with breastfeeding, and if they required a hospital stay longer than 72 hours for vaginal deliveries or longer than 96 hours for cesarean section, or if their infant had medical problems that required admission to the intensive care nursery or hospitalisation for more than 72 hours'. |
|
| Interventions | Intervention group: (161 women) the intervention was daily telephone support from the day following hospital discharge until 2 weeks postpartum. The intervention was by trained nurses following a specific protocol covering advantages and disadvantages of breastfeeding, cultural issues, technique, and discussion of problems with referral for any lactation or medical problems. Control group: (180 women) all women received usual hospital care which included pamphlets on breastfeeding, a breast pump, lanolin cream and a water bottle and usual discharge care with commercial discharge packs. Usual care also included scheduled healthcare visits at 3‐5 days and at 2 weeks at the local community health centre |
|
| Outcomes | Duration and exclusivity of breastfeeding, maternal satisfaction with feeding, rationale for discontinuing breastfeeding and healthcare utilisation. Maternal confidence assessed at 3 months. Assessed by maternal report over telephone at 1, 3 and 6 months postpartum. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation. |
| Allocation concealment (selection bias) | Unclear risk | ‘allocation assignment was not blinded and was done using sequentially numbered opaque sealed envelopes.’ |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 341 women were randomised. At 1 month there approximately 8% loss to follow‐up. By 6 months 27% loss. 73% were described as included in the analyses; women in the intervention group that did not receive the intervention as planned were not included. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study reports. |
| Other bias | Low risk | Groups appear well balanced at baseline. |
Dennis 2002.
| Methods | RCT, 2‐arm trial with individual randomisation. | |
| Participants | Setting: 2 hospitals (described as semi‐urban) near Toronto, Canada. Recruitment Sept 1997 to June 1998. 258 women randomised. Inclusion criteria: reported as ‘Eligible participants were all in‐hospital, primiparous, breast‐feeding women who were at least 16 years of age, able to speak English, had a singleton birth at 37 weeks’ gestation or later and resided in the surrounding region accessible by a local telephone call‘. “The majority of the 256 participants were married (91.4%), identified themselves as Canadian (73%), completed post‐secondary education (74.7%), and had an annual household income greater than $40,000 Canadian (77.8%). The mean age was 29.0 years (SD = 4.68) ranging from 17”. Exclusion criteria: reported as ‘Mothers were excluded if they had a factor that could significantly interfere with breast‐feeding, such as serious maternal illness, infant congenital abnormality or an infant in the special care nursery who would not be discharged home with the mother. We also excluded mothers if they had enrolled prenatally with the participating volunteer breast‐feeding organization'. |
|
| Interventions | Reported as: ‘conventional care plus telephone‐based support, initiated within 48 hours after hospital discharge, from a woman experienced with breast‐feeding who attended a 2.5‐hour orientation session’. Intervention group: (132 women allocated to intervention) usual care plus telephone support from peer volunteers to promote breastfeeding. Peer supporters were recruited by a local voluntary organisation specifically for the trial and received training. Volunteers had breastfeeding experience, positive attitudes about breastfeeding and completed 2.5 hours training session and provided with handbook with information and details of referral contacts. Volunteers were paired with women and asked to contact them by telephone within 48 hours of hospital discharge. The number and timing of subsequent telephone contacts were not prescribed (volunteer logs suggested approximately 5 contacts lasting an average of 16 minutes). Most contacts were initiated by the volunteers. The purpose of the calls was to provide information, feedback and emotional support. Control group: (126 women) usual care which included hospital and community support by nursing and medical staff, access by telephone to a breastfeeding support line, access to a hospital lactation consultant clinic. |
|
| Outcomes | Outcome data collected at baseline, then at 4, 8, and 12 weeks postpartum. Primary outcome – self‐reported breastfeeding in the 24 hours before the 12‐week telephone interview. Secondary outcomes, breastfeeding duration (any, exclusive) maternal satisfaction with feeding, problems with breastfeeding, health service utilisation, perceptions re peer support. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported as: ‘Randomization was achieved using consecutively numbered, sealed, opaque envelopes containing randomly generated numbers constructed by a biostatistician who was not involved in the recruitment process'. |
| Allocation concealment (selection bias) | Low risk | Reported as: ‘Randomization was achieved using consecutively numbered, sealed, opaque envelopes containing randomly generated numbers constructed by a biostatistician who was not involved in the recruitment process'. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and staff would not be blind to intervention. Outcome data were reported by women in telephone interviews. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome data collected by research assistants reported to be blind to allocation (it was not clear whether blinding was successful). Data entry also by blinded investigators. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 258 women were randomised and all but 2 (both in the control group) were followed up at all 3 data collection points.The authors stated that analysis was according to ITT with women analysed according to allocation whether or not they received the intended intervention. Secondary analysis looked at treatment effect according to the intervention received. |
| Selective reporting (reporting bias) | Low risk | Additional data provided by the author. |
| Other bias | Unclear risk | There was some baseline imbalance; there was a significant difference between 2 groups on ‘Decided to breast‐feed before pregnancy’ (with more doing so in peer support than in control group) and fewer women in the peer support group than in the control group had a cesarean section (18.9% v. 27.4%); 'although this difference is not statistically significant, it is clinically important’. |
Dennis 2009.
| Methods | Multi‐site RCT, 2 arms with individual randomisation. | |
| Participants | Setting: reported as: “Women were recruited from seven large health regions and their corresponding public health departments across Ontario, Canada, between November 2004 and September 2006.” 701 women randomised. Inclusion criteria: women were assessed at routine telephone call by public health nurse 24‐48 hours after hospital discharge after birth. Participants were women approximately 2 weeks postpartum scoring more than 9 on the EPDS ‘Eligible participants were all new mothers about two weeks postpartum or less who were at least 18 years of age, able to speak English, had a live birth, and were discharged home from hospital'. Exclusion criteria: described as: ‘We excluded women whose babies were not discharged home with the mother and women who were currently taking antidepressant or antipsychotic drugs'. |
|
| Interventions | Intervention group: usual care and peer support (n = 349), Reported as ‘Proactive individualised telephone based peer (mother to mother) support, initiated within 48‐72 hours of randomisation, provided by a volunteer recruited from the community who had previously experienced and recovered from self reported postnatal depression and attended a 4‐hour training session.’The volunteers were asked to make a minimum of 4 contacts and to interact and refer to other agencies as necessary. Women received a mean of 8.8 (SD 6.0) contacts (but this included answer machine contacts). 7% of contacts were initiated by the participants. Control group: usual care (n = 352) women could proactively seek services from public health nurses, doctors, and other community resources. |
|
| Outcomes | Reported as: ‘Main outcome measures Edinburgh postnatal depression scale, structured clinical interview‐depression, state‐trait anxiety inventory (STAI), UCLA loneliness scale, and use of health services’. Also maternal satisfaction with intervention. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported as ‘Randomisation was centrally controlled with a web based randomisation service (www.randomize.net), with stratification based on self reported history of depression, a known risk factor for postnatal depression’. |
| Allocation concealment (selection bias) | Unclear risk | Although a centralised external randomisation service was used it was not clear what happened at the point of randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women and staff delivering the intervention were not blind to group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reported as ‘Research nurses blinded to group allocation telephoned all participants at 12 and 24 weeks postpartum to assess trial outcomes. At 12 weeks, women in the intervention group answered questions regarding their experience with the peer volunteer via a mailed questionnaire. While trial participants and peer volunteers could not be blinded to group allocation, health professionals and service providers of standard community postpartum care were not informed of any mother’s participation in the trial or group allocation.’ ‘To assess for blinding of the outcome assessor, at the end of the interview the data collection nurses indicated whether they thought the participant was in the control group or the intervention group or they did not know. At the 12 week interview, the data collection nurses had no opinion regarding which group 595 (97%) women were allocated to; a similar rate was found at the 24 week interview (n = 588, 98%).’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Of 349 women randomised to the intervention group, 297 (85.1%) were followed up at 12 weeks and 289 (82.8%) at 12 weeks; for controls 352 randomised, 316 (89.8%) were followed up at 12 weeks and 311 (88.3) at 24 weeks. Fewer women in the intervention group were followed up at 24 weeks. It is possible that women lost to follow‐up were more likely to be depressed. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Low risk | Authors reported that ‘There were no clinically important differences between the two groups'. Other bias not apparent. |
Di Meglio 2010.
| Methods | RCT, 2 arms with individual randomisation. | |
| Participants | Setting: recruited from 2 Rochester NY USA hospitals between Sept 1996 to June 1997. Women were approached 12‐36 hours after vaginal or 24‐48 hours after caesarean delivery. 78 mothers randomly assigned (38 receiving telephone peer support and 40 not receiving this support). Inclusion criteria: breastfeeding single infant. Aged under 20 years. No contraindications to breastfeeding and uncomplicated postpartum course. For infant: gestation age above 36 weeks, weight above 2000 g, not in ICU or special care unit for more than 6 hours, no anomalies that would interfere with nursing (cleft lip or palate) and discharged home with mother. |
|
| Interventions | Intervention group: (40 women) peer telephone support 2, 4 and 7 days post discharge and then at 2, 3, 4 and 5 weeks post discharge. Peers introduced themselves and asked about breastfeeding experience and gave mother telephone number so she could also call them. 5 adolescents who had recently breastfed were trained* to give support. *Peer counselor training programme – 10 2 hour sessions developed and delivered by La Leche League leaders. They were advised to refer the young women who were experiencing problems for breastfeeding information or to their physician. Intervention based on breastfeeding promotion information disseminated by the Women Infants and Children (WIC) programme. An independent interviewer called all mothers weekly to determine feeding patterns – every week for 5‐10 minute interviews for 4 weeks and then every two weeks for 4 weeks, and then once a month until breastfeeding stopped to review infant feeding. At 1, 2, 3, 4, 6 and 8 weeks postpartum (for completing this they received a 25$ mall certificate). Peer counsellors and the mothers in intervention group could meet at monthly pizza parties. Control group: (38 women) Women received standard care (no telephone support from peers) both intervention and control groups had access to other care including paediatric care and hospital lactation consultants. |
|
| Outcomes | Primary: breastfeeding duration (i.e. age when breastfeeding ceased). Secondary: exclusive breastfeeding duration (i.e. age when supplement introduced – water, juice, vitamins or formula). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Sealed numbered envelopes. Only PI aware of group assignment and had no contact with participants. Envelopes were sequentially opened as participants were recruited. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Reported as ‘Subjects blinded to research hypothesis and group assignment’ . However, mothers were aware of role of peer support adolescents in the study – and were therefore probably aware of the purpose of the study. It was not clear whether blinding was successful. Peer supporters were blinded to research hypothesis and group assignment, but again it was not clear whether attempted blinding was successful. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Reported as ‘Interviewer blinded to research hypothesis and group assignment'. It is unclear how effective this attempt at blinding may have been given that participant and individuals giving peer support were aware peer support was being given and the interviewer in weekly conversation with the participant may have become aware that some individuals had received support and some had not. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 11 (of the 78) dropped out immediately. 54 were contacted at 8 weeks (end point of participation incentive) In 13 who could not be contacted 8 had already discontinued breastfeeding and 5 were lost to follow‐up. A further 16 dropped out between 8 and 37 weeks. 46 were successfully followed to breastfeeding cessation (22 in intervention and 24 in control group). Authors state that an ITT analysis was carried out but this was not clear. |
| Selective reporting (reporting bias) | Unclear risk | The analysis is presented in diagrams (Kaplan‐Meier Curves) and are not simple to interpret. Assessment from published study report. |
| Other bias | High risk | There was very poor compliance with possibly only half of the intervention group receiving the intervention. There was no apparent baseline imbalance between groups. |
Donaldson 1988.
| Methods | RCT. 2‐arm trial with individual randomisation/ | |
| Participants | Setting: University of California Medical Centre, USA January to July 1987 39 low risk primiparous women randomised to 6 weekly ‘nurse‐initiated postpartum telephone contacts following hospital discharge’ (n = 19) versus standard postpartum nursing care (n = 20). Inclusion criteria: low‐risk women experiencing a ‘normal’ childbirth. Primiparous women aged between 18 and 38 years, able to speak and read English, healthy term infant, currently living with father of baby, access to telephone. Exclusion criteria: infant with anomaly, maternal or infant illness requiring hospitalisation beyond normal 1 or 2 day post delivery, maternal history of psychiatric disorder/substance abuse, maternal report of current significant distress or unable to receive telephone contacts. |
|
| Interventions | Intervention group (19 women) 6 weekly nurse‐initiated postpartum telephone contacts following hospital discharge’. For ‘educational and supportive postpartum follow‐up’. Delivered by a single staff clinical nurse specialist. Most calls lasted 15‐30 minutes. Control: (20 women) standard postpartum nursing care which included nurse education prior to discharge from hospital. |
|
| Outcomes | Maternal postpartum adaptation (maternal reported mood disturbance, maternal sense on competence as a parent, maternal developmental expectations). Data collected at 8 weeks postpartum using mailed self‐report questionnaires: Profile of Mood States (POMS) Parental sense of competence scale (PSOC) and Developmental Expectations (DE). Mailed to mother at 6 weeks to be completed in own home – instructed to complete within 5 days of infant at 8 weeks of age. Data also included: mothers feelings about being a mother, perceived greatest concerns, mothers rating of general health/infant health. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentions table of random numbers – although process described as ‘subjects alternately assigned to each group based on their sequence of induction’. |
| Allocation concealment (selection bias) | Low risk | Sealed and numbered envelopes (those inducting participants were blind to group assignment) and allocation known only when envelopes opened. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Described as unmasked. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 46 recruited and 7 lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Not apparent. |
| Other bias | Unclear risk | Recruitment insufficient so study did not have the power to detect differences between groups. No differences between groups for demographic and baseline variables. |
Ershoff 1999.
| Methods | 3‐arm trial with individual randomisation. | |
| Participants | Setting: ‘Between November 1996 and June 1997 in a prospective, randomised trial we recruited women initiating prenatal care at Kaiser Permanente Southern California (KPSC)’. Inclusion criteria: 390 women. Women who had a prepregnancy smoking rate of 7 or more cigarettes per week and had not quit by recruitment, aged 18 or more, who had begun prenatal care by 26 weeks’ gestation. Exclusion criteria: reported as ‘women under the age of 18 (n = 59) and those beginning prenatal care beyond the 26th week of pregnancy (n = 69)'. |
|
| Interventions | 3 study arms. Intervention focusing on smoking cessation. 1. 131 (controls) Booklet giving advice on stopping smoking. 2. 133 (booklet plus computer). Booklet with access to an interactive telephone computer programme free of charge. (Only 25 women accessed the service). 3. 126 (booklet plus motivational interviewing). Women in this group (intervention) had counselling from trained nurse educators. Counsellors were asked to complete 4‐6 calls of 10‐15 minutes duration with each woman assigned to them. Weekly calls were recommended but this was at the discretion of the nurses. For this review we have used data for groups 1 (control) and 3 (intervention) only. |
|
| Outcomes | Reported as ‘Biochemically confirmed abstinence measured by level of cotinine in urine samples obtained during a routine prenatal visit at approximately the 34th week of pregnancy. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women and clinical staff delivering the intervention were not blind to group allocation although it was reported that other care providers were blind to study participation and group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical analysis for primary outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Of 390 women randomised, cotinine analysis was carried out for 332 (85%). Follow‐up interview data were available for 285 women. |
| Selective reporting (reporting bias) | Unclear risk | The way results were reported in tables was confusing and several results were for subsamples. |
| Other bias | Unclear risk | No baseline imbalance apparent. Ther were considerable discrepancies between cotinine‐validated quit rates and self‐report measures. |
Ferrara 2011.
| Methods | Pilot RCT, 2 arms with individual randomisation. | |
| Participants | Setting: 197 women receiving care through Kaiser Permanente Medical Care Program in Northern California USA. The study was carried out between 2005‐2008. Inclusion criteria; 96 women diagnosed with GDM. Exclusion criteria: aged under 18 years, multiple gestation, diabetic retinopathy, high‐risk pregnancy (drug or alcohol abuse, chronic illness, thyroid disease or pregnancy complications), non‐English speaker. |
|
| Interventions | Intervention group (96 women) a complex lifestyle intervention including telephone support encouraging changes in diet and exercise and promoting breastfeeding. During pregnancy women had individual counselling from a dietician and this was followed by 2 telephone counselling contacts to encourage women to comply with IOM guidelines on weight gain and to engage in moderate intensity exercise. Women also received written information on diet. Towards the end of pregnancy women were referred to a lactation consultant who then scheduled between 1‐4 telephone calls during the first 6 weeks after delivery to encourage breastfeeding. During the postpartum period women had 8‐16 sessions on diet and exercise, 2 face‐to‐face with dieticians the rest over the telephone which encouraged women to exercise and reduce fat intake so as to regain their pre‐pregnancy weight or reduce weight if they were overweight. Women were given diaries to monitor their exercise. Usual care: 101 women received printed educational materials on GDM. |
|
| Outcomes | Breastfeeding at 6 weeks and 7 months postpartum, reported amount of calories from fat and change in physical activity, number of women exceeding IOM weight gain goals at 6 weeks and 7 and 12 months postpartum. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer randomisation program. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was stated that research assistants collecting outcome data were unaware of group assignment. It was not clear if attempted blinding was successful. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In the intervention group 20% were lost to follow‐up by 12 months postpartum compared with 10% in the control group. It was not clear why loss was greater in the intervention group. |
| Selective reporting (reporting bias) | Unclear risk | Assessed from published study report. |
| Other bias | Unclear risk | Results were not consistent over time so some results were difficult to interpret. Groups appeared balanced at baseline. |
Hoddinott 2012.
| Methods | Pilot RCT, 2 arms with individual randomisation. | |
| Participants | Setting: 69 women admitted to a maternity hospital in Scotland between July and October 2010. Inclusion criteria: women living in the 3 most disadvantaged postcode areas served by the hospital and giving the baby some breast milk at hospital discharge. Exclusion criteria: women under 16 or with serious medical or psychiatric problems or insufficient English to communicate by telephone. |
|
| Interventions | Intervention group: (35 women) proactive phone calls from hospital discharge up to 14 days by a member of the feeding team. The median number of calls per women was 8 calls and the median length was 3 minutes. The calls were to provide support. Women could also initiate calls themselves. Control group: (34 women) reactive phone calls made by women up to 14 days pp. Only 1 woman called for advice. |
|
| Outcomes | Breastfeeding or exclusive breastfeeding at 6‐8 weeks. Satisfaction with care. Cost of intervention and service utilisation. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by Internet randomisation sequence service set up by an independent statistician. Stratified by parity. |
| Allocation concealment (selection bias) | Low risk | By independent statistician. Women were only aware of allocation if they received calls. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women would be aware of calls and staff providing care may have been informed by women if they received calls. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Follow‐up at 6 weeks by researcher who was reported to be blind of study allocation and had no other contact with the women. Effect on outcomes of partial blinding unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 69 women were randomised and 11 lost to follow‐up. It was stated that an ITT analysis was carried out for women with complete data at follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Pilot study. Assessment from published study report. |
| Other bias | Unclear risk | Women in the intervention group were on average 1 year older and more likely to be living in the most disadvantaged areas and hospital stays were slightly longer, otherwise groups were comparable. |
Jareethum 2008.
| Methods | RCT 2 arms with individual randomisation. | |
| Participants | Setting: 68 healthy pregnant women who attended the antenatal clinic and delivered at a Bangkok hospital May‐October 2007. Inclusion criteria: age over 18 years old, no medical diseases or obstetric complications, singleton pregnancy, dating confirmed by ultrasound, gestational age less than 28 weeks at recruitment. All participants had their own mobile phone and could receive and understand short message service (SMS) messages. Exclusion criteria: pregnant women who aborted before 28 weeks of gestation or changed to deliver at another hospital were excluded after randomisation. |
|
| Interventions | Not clear how many randomised to intervention and control groups. At follow‐up 32 in the intervention group and 29 in the control group. Intervention group: SMS via mobile phone for prenatal support. Reported as: ‘two SMS messages per week from 28 weeks of gestation until giving birth’. Added to same standard care as control. Message contained information and warnings relating to abnormal symptoms which, if the pregnant woman had, would require that they consult the doctor. The SMS messages were appropriate to the women’s gestational age. Control group: standard care. 'Both groups received the same antenatal and perinatal care’. |
|
| Outcomes | Satisfaction with antenatal and perinatal care (VAS) also anxiety scores (not clear how measured), gestational age at delivery, preterm delivery, birthweight and mode of delivery. Information was collected on the postnatal ward (therefore assessment of antenatal anxiety would be retrospective). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported as ‘table of random numbers’. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned. Women would be aware of intervention and most outcomes were self‐reported. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned. Most outcomes self‐reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 68 women randomised, 61 followed up. It was not clear that loss was balanced across groups. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | Groups were reported to be comparable at baseline. |
Johnson 2000.
| Methods | RCT, 2 arms with individual randomisation. | |
| Participants | Setting: 254 women in 5 hospitals in Canada (unclear). Inclusion criteria: reported as ‘Women who gave birth at one of 5 hospitals who: identified herself as a smoker before pregnancy; quit smoking once aware of pregnancy or in attempting to become pregnant; ceased smoking for at least 6 weeks before delivery; if smoking occurred in 6 week period immediately before delivery smoked on fewer than 6 occasions; gave birth to a healthy infant not requiring hospitalisation beyond discharge of the mother; planned to remain in hospital for at least 24 hours; able to read and comprehend English; could be contacted by telephone’. |
|
| Interventions | Intervention group: 125 women in treatment group included in analysis. Postpartum counselling intervention to prevent smoking relapse. The intervention was provided one‐to‐one by specially recruited and trained nurses. Women received initial counselling in hospital along with pamphlets and no smoking signs for their homes. There were then 8 postnatal phone calls (weekly during the first month, then fortnightly) which lasted between 1 and 20 minutes. Only 25% of the intervention group received all 8 calls. Control group: 126 women in the analysis. Received usual care (did not include any information about effects of smoking or prevention of smoking relapse). |
|
| Outcomes | Self‐report smoking status, Bedfont EC50 Smokerlyzers, Carbon monoxide (CO) readings of 10 or more parts per million (ppm). Continuous abstinence: complete avoidance of smoking during the entire 6‐month period. Smoking cessation self‐efficacy was measured by the Smoking Abstinence Self Efficacy Scale. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation (in groups of 50) randomisation via ‘computer software package’. |
| Allocation concealment (selection bias) | Unclear risk | Unclear “identification numbers randomly assigned to two groups”. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | At 6 months after delivery both groups were interviewed by research assistants in own home who had not delivered the intervention and who were reported to be blind to group assignment. Carbon monoxide in expired air was also measured for some women. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Follow‐up interviews actually occurred between 24 and 50 weeks after baseline data collection. 11 interviews conducted away from woman’s home and 19 were over telephone (so breathalyzer not completed. 6 from control and 4 from treatment refused to participate. They (the 10) were coded as failing to maintain abstinence as daily smokers – self‐efficacy scores were not imputed for these 10 women. 3 women excluded from analysis: reported as: ‘ 1 assigned to control group but inadvertently given intervention and 3 were assigned to treatment group but did not receive any telephone contacts’. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | It was reported that smoking related variables were similar in the 2 groups at baseline. |
Little 2002.
| Methods | RCT, 2 arms, randomisation by individual. | |
| Participants | Setting: study in the USA. Reported as ‘Those taking part in the study were high‐risk pregnant women who obtained prenatal care from two large obstetric clinics and delivered at a level‐3 tertiary center’. Also reported ‘The first referral to the study was made in mid‐March 1999, and the first delivery was in late November 1999. The last delivery was in mid‐March 2001’. 175 women randomised. Inclusion criteria: described as ‘high‐risk pregnant women who obtained prenatal care from two large obstetric clinics and delivered at a level‐3 tertiary center’, women were ‘primarily of minority cultural and racial backgrounds’ and predominantly low‐income. Only those with singletons were retained in final analysis regarding infant gestational age, infant birthweights, and costs. However, it appears that both those with singletons and twins were retained in final analysis of satisfaction. |
|
| Interventions | Intervention group (91 women): ‘The nurse case managers contacted the patients in the case‐managed group every 7 to 14 days to assess their pregnancy status and offer support and teaching related to pregnancy and their diagnoses.' The patients could contact the nurse case managers with non‐emergent questions or concerns. Appropriate written educational materials were sent after the initial assessment contact and throughout the pregnancy. Contacts were made by the nurse case managers, as appropriate, to the patient’s health care providers (physicians and home care agencies) to obtain information regarding the pregnancy status and/or plan of treatment, and to initiate and/or follow‐up on home care services provided to the woman. A final contact was made after delivery to obtain delivery information and to complete a postpartum mother/infant assessment. ‘The treatment group participants were educated in the signs and symptoms of preterm labor, importance of good hydration throughout the pregnancy, and self‐monitoring of fetal movement. They were encouraged to maintain good prenatal care by keeping their regular clinic appointments and to report concerns or questions to their health care providers. They were also encouraged to partner in decision‐making regarding their own care. In certain cases the nurse case manager was able to assure that the participant obtained more thorough nutrition education’. Control group: 84 women. Standard care. The control group was contacted twice during the study, once to complete the initial pregnancy risk screening and a final contact to gather delivery information and to complete a postpartum mother/infant assessment. No teaching or written educational materials were provided, and no contacts were made to healthcare providers involved in the control patients’ care. |
|
| Outcomes | Gestational age at delivery, birthweight, costs, satisfaction and mode of delivery. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was reported that the study administrator randomly assigned women. |
| Allocation concealment (selection bias) | Low risk | Additional information from the author: For our study, we employed a nurse who would attend the clinic to explain the study to Medicaid patients and she would enrol them if they were interested in participating. She was not a case manager with ROSEBUD and was not involved in assignment of the patients to the ROSEBUD case managers. Our administrative assistant (not a nurse) would assign these patients randomly to the nurses who would be their ROSEBUD case managers. The treatment group patients were assigned to 1 of 2 nurses and a third nurse followed the control group… there was no involvement with the nurse enrolling the patients and also assigning them. We kept these 2 areas completely separate and the information about the patients was not known to the case manager nurses prior to being randomly assigned." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described. Different nurses were involved in the care of intervention and control group women. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 175 women were randomised. Women who were difficult to contact, expecting twins or not available to follow‐up were not included in the analysis. For patient outcomes relating to satisfaction there were data for only 71 women. |
| Selective reporting (reporting bias) | Unclear risk | This study is very complicated in terms of reporting (largely because of lack of balance between groups); all results were adjusted for a number of related variables. |
| Other bias | Unclear risk | Some baseline imbalance. ‘The case management group (N = 61) and control group (N = 50) did not differ significantly in demographic characteristics, but authors report: ‘The treatment group had significantly larger proportions of patients with anemia, obesity, symptoms of preterm labor, and undiagnosed vaginal bleeding in pregnancy. The control group had significantly more patients who were substance abusers and more patients in the control group had poor prenatal care, but not significantly so. The treatment group contained more who were experiencing stress in their environments. Treatment group participants were more likely to have abnormal ultrasound results and to report decreased fetal movement. The groups did not differ significantly on such medical risk factors as mental illness, cardiac disease, chronic hypertension, gestational diabetes, or insulin dependent diabetes mellitus’. With respect to birthweight analysis they note ‘A multiple analysis of variance with covariates was performed, controlling for maternal obesity and NICU admission, as well as study group, gestational age at referral, and number of preterm births. Similarly, with respect to gestational age at delivery as they note ‘A multivariate analysis of variance and covariance also was performed on gestational age at delivery, controlling for study group (treatment or control), maternal age, gestational age at referral, number of preterm births, and admission to the NICU.' |
Lund 2012.
| Methods | Cluster‐randomised trial (24 primary healthcare clinics randomised) in Zanzibar. | |
| Participants | (24 healthcare facilities randomised in 2009‐10) 2550 women attending for antenatal care at participating primary healthcare facilities. Women were recruited at their first antenatal appointment and were followed until 6 weeks postpartum. All pregnant women were eligible for inclusion. | |
| Interventions | Mobile phone intervention to encourage women to seek skilled attendants for the birth. All women received routine care which included at least four antenatal visits, offer of skilled attendant for the birth and at least one postnatal visit within 48 hours of the birth for women not delivering in healthcare facilities. Mobile phone intervention (12 centres, 1351 women recruited). Women in the intervention group with phones received automated short mobile phone health education messages and mobile phone vouchers to allow women to call healthcare staff. All women received free mobile phone vouchers irrespective of whether or not they owned a phone (38% had their own phone). Messages encouraged women to attend antenatal care appointments, and to seek skilled attendance for the birth. Messages were tailored to gestational age. Midwives, ambulance drivers and healthcare staff were provided with phones. Control group: (12 centres, 1286 women) usual care. |
|
| Outcomes | The number of women with skilled attendants at the birth. maternal death. | |
| Notes | We contacted the author of this trial in January 2012 for more information to allow us to adjust data to take account of cluster‐ design effect. We have included adjusted results for this trial in an additional table. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Simple random allocation. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women and staff were not blind to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were available for women at all centres (24) randomised. Of 2637 women recruited 82 were not included in the analysis (no end of study data). |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. More data are available from this trial (future publications). |
| Other bias | Low risk | Analysis accounted for cluster‐ design effect. No evidence of baseline imbalance between groups. |
McBride 1999.
| Methods | RCT, 3 arms with individual randomisation. | |
| Participants | Setting: 1007 women randomised. Women were stratified by site (2 sites) and baseline smoking status (2 levels: current smoker vs recent quitter). ‘The clinical sites for the trial were Group Health Cooperative of Puget Sound (Seattle) and Park‐Nicollet of Minnesota. Group Health Cooperative is a health maintenance organisation that serves over 450 000 enrollees in western Washington State. Park‐ Nicollet is a multispecialty group practice with a patient base that is 60% health maintenance organisation and 40% traditional fee‐for‐service’. Inclusion criteria: reported as ‘Women were eligible for the intervention trial if they completed the baseline survey, were fewer than 20 weeks pregnant, and reported being a current smoker or a recent quitter (had been a smoker in the 30 days before pregnancy but had quit by the time of the baseline survey)’. Exclusion criteria: ‘women (Seattle n = 60; Minnesota n = 28) subsequently miscarried between baseline and the 28‐week follow‐up.’ And ‘22 women in Seattle were mailed the wrong intervention materials; they were also excluded'. |
|
| Interventions | 3‐arm trial.
(In this review data for groups 2 and 3 (telephone support) have been combined.) |
|
| Outcomes | The proportion of women who reported not having smoked in the previous 7 days at the 28‐week follow‐up. 3 postpartum outcomes were assessed: postpartum relapse, the proportion of women who were abstinent at 28 weeks but had returned to smoking; postpartum cessation, the proportion who had been smoking at the 28‐week follow‐up but had not smoked any cigarettes in the 7 days preceding the postpartum follow‐up; and postpartum 7‐day prevalent abstinence, the proportion of all women randomised who had not smoked any cigarettes in the 7 days preceding the postpartum follow‐up. For women who reported stopping smoking there was saliva cotinine analysis although results were not reported (64%‐78% of women who reported stopping smoking returned saliva samples – there was no ITT reported). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified randomisation, but it was not clear how this was carried out. |
| Allocation concealment (selection bias) | Unclear risk | Stratified randomisation, but it was not clear how this was carried out. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The staff recruiting women and carrying out telephone follow‐up were not involved in delivering the intervention or other care. Women and smoking counsellors would be aware of the intervention and the main outcome was a self‐report measure. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The staff recruiting women and carrying out telephone follow‐up were not involved in delivering the intervention or other care. Women and smoking counsellors would be aware of the intervention and the main outcome was a self‐report measure. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were some post‐randomisation exclusions. Of 1007 randomised, 88 miscarried and were excluded, and 22 women were mailed the wrong information and were excluded. 897 women were followed up. Response rates were good with 80% of women responding at all 4 data collection points. |
| Selective reporting (reporting bias) | Unclear risk | Results for cotinine analysis were not reported. Results were difficult to interpret as many were for subgroups (e.g. for women who had reported stopping at a given time point) and denominators were not clear). |
| Other bias | Unclear risk | Groups were similar at baseline although there were some differences in sample characteristics at the two sites and the analysis was adjusted to take account of these differences. |
Milgrom 2011.
| Methods | 2‐arm trial with individual randomisation. | |
| Participants | Setting: 143 women were recruited from 2 hospitals in Melbourne, Australia when 20–32 weeks pregnant’. Inclusion criteria: women were assessed for depression and women with high scores and a proportion of those with low scores were recruited (13 or more on EPDS or the Risk Assessment Checklist). 143 women recruited; 43 with high scores and 100 with low. Exclusion criteria: reported as ‘1) inability to understand written English, 2) presence of psychotic symptoms, 3) extreme levels of distress requiring crisis management, or 4) stage of pregnancy >32 weeks gestation’. |
|
| Interventions | Intervention group (43) women received the Towards Parenthood intervention in addition to community networking. The intervention consisted of a self‐help workbook which women read each week (8 sessions) and then discussed the content with a psychologist or trainee psychologist in a weekly telephone support session. The postnatal unit was completed 6 weeks following the birth. Telephone calls lasted approximately half an hour and allowed for tailored discussion and problem‐solving around the unit content. Telephone calls were made by the therapist at a regular prearranged time each week. Psychologists followed structured session prompts and kept detailed notes. 8 telephone sessions were scheduled (covering 9 workbook units). However there was considerable variability in terms of number of sessions that the mothers received; ‘only 56/100 women in the intervention attended one or more sessions, and of these only 46 attended five or more sessions’. Control group (100 women): routine care. Community networking was implemented as in the intervention condition. Women in routine care were sent the Towards Parenthood workbook post‐study. |
|
| Outcomes | It was hypothesised that the intervention would be beneficial in terms of: 1) less depression, (Beck depression index II); 2) less anxiety, (Depression and anxiety Short scale (DASS)); 3) less parenting dysfunction; 4) less general stress. Outcomes were measured at 12 weeks postpartum. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ‘Women were allocated to either intervention or routine care via a coded, double‐blinded, variable‐length permuted block randomised treatment allocation schedule produced by an independent person prior to commencement. The schedule was stratified for screening score (high or low), to ensure a balanced representation across treatments, and was administered by a hospital administrator blind to coding’. |
| Allocation concealment (selection bias) | Low risk | See above. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was described as a double‐blind study but women and psychologists would be aware of the intervention and most of the measures were self‐report so it is difficult to assess the impact of lack of blinding on outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | This was described as a double‐blind study but women and psychologists would be aware of the intervention and most of the measures were self‐report so it is difficult to assess the impact of lack of blinding on outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was considerable loss to follow‐up. Of 143 randomised, observed data were available for only 89 (62%). An ITT analysis was carried out where scores were imputed for missing data using “maximum likelihood methods”. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | Given the low compliance it was difficult to draw conclusions about effectiveness. There was some baseline imbalance with more women in the intervention group with post‐high school education but level of education was reported not to be associated with outcomes. |
Mongeon 1995.
| Methods | RCT, 2 arms with individual randomisation. | |
| Participants | Setting: 200 women attending prenatal care at primary care clinics in Quebec, Canada. Inclusion criteria: women planning to breastfeed for the first time (first time mothers or women who had not previously breastfed). |
|
| Interventions | Intervention group (100 women) usual care plus visits and phone calls by peer volunteers. Volunteers were woman who had previously breastfed and each volunteer contacted 1 to 3 women. Volunteers were trained and supervised and visited women at home once during the first month postpartum, then weekly phone calls for 6 weeks then fortnightly phone calls until the baby was 5 months old or until breastfeeding discontinuation. Control group (100 women) usual care involved a home visit from a primary care nurse and then other visits and phone calls as required. |
|
| Outcomes | Baseline data collected at 32 weeks' gestation and follow‐up was at 6 months postpartum. Breastfeeding at 1, 2, 3, 4, 5 and 6 months after the birth, breastfeeding problems and women’s views about breastfeeding. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described, (translated as “shared randomly in two groups by drawing numbered papers"). |
| Allocation concealment (selection bias) | Unclear risk | Not described, (translated as “shared randomly in two groups by drawing numbered papers"). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Most women were available for follow‐up (6/200 lost to follow‐up). More than 30% missing for some outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Not clear (assessment from translation notes). |
| Other bias | Unclear risk | Not clear (assessment from translation notes). |
Moore 1998.
| Methods | RCT 2‐arm trial with individual randomisation. Analysis was stratified by race and age. | |
| Participants | Setting: 1554 women randomised attending community clinics in North Carolina USA. Inclusion criteria: Black women (all ages) white and other women at high risk of preterm birth and white and other women aged 18 or younger. English speaking, access to telephone with gestational age 22‐32 weeks at entry. |
|
| Interventions | Intervention group: ‘Women in the intervention group received telephone calls from a registered nurse, one or two times weekly from 24 weeks’ through 37 weeks’ gestation'.'Nurse telephone calls were made on the schedule to which nurse and subject agreed, from the time of the home visit (22–32 weeks’ gestation) until the 37th week. The timing of the calls was designed to suit the woman’s convenience. The goal was three telephone contacts per week. Women without telephones could contact their primary nurse via the nurse’s pager when they were near a telephone. The nurse returned the call immediately. Although no formal script was followed, each telephone call addressed three major areas: assessment of health status (perception of uterine contractions and other pregnancy changes, color of urine as an assessment of hydration, number of meals eaten, number of cigarettes smoked, alcohol and drug use, and ingestion of a prenatal vitamin capsule on the previous day); recommendations based on assessment; and discussion of any additional issues important to the mother. The duration of each call was recorded. Three experienced nurses, hired specifically for the telephone intervention study, conducted the intake interviews and all of the telephone intervention'. ‘Women in the intervention group received additional instruction about the signs of preterm labor, and a schedule was established for the time and frequency of telephone calls they would receive from the nurse, as well as instructions about contacting the nurse by telephoning her pager number’. Women were given a $25 dollar gift voucher if they returned their assessment and remained in contact with the nurse. Control group: standard care. Women were given a booklet about preventing preterm birth which was available in the clinic and routine care. They were given a $10 gift voucher for completing assessments. |
|
| Outcomes | LBW or preterm births. Cost data reported for a subsample. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation table. |
| Allocation concealment (selection bias) | Low risk | Reported as; ‘Random assignment was directed by the biostatistician. The words “phone” for the intervention group and “book” for the control group were placed in opaque envelopes that were numbered and sealed by the biostatistician’. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Women were not blinded; it was reported that clinical staff were blind to intervention but it was not clear if this was successful. ‘Clinic personnel, including attending physicians, residents, nurses, and others were blinded to group assignment. If a referral to clinic, the labor suite, or another agency was needed, the nurse instructed the patient to make the call and then ascertained that the call was made’. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Data collection was done by an additional nurse hired specifically for this task. She did not participate in the intervention, was not present at any meetings during which patients were discussed, and was blinded to group assignment.’ It is not clear how effective the blinding would actually be in practice. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 121 women randomised; 7.8% changed healthcare providers, moved from the community, or had multiple gestation pregnancies. It was stated that analysis was on an ITT basis. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | There were no clear differences between groups at baseline; analysis was stratified by age and race. |
Naughton 2012.
| Methods | RCT, 2 arms with individual randomisation. | |
| Participants | Setting: 7 National Health Service Trusts in England between 2008‐9. 207 women randomised. Inclusion criteria: women recruited at booking visit by midwives. Gestational age less than 21 weeks, 16 years of age or more, smoked 7 or more cigarettes per week, had use of mobile telephone and could understand written English. |
|
| Interventions | Intervention group: (MiQuit) women were mailed an individually tailored four page colour self‐help leaflet by post, 3 days later automated phone text messages were sent. Women could be sent 0‐2 texts each day randomly . The system sent out approximately 80 texts over the 11‐week intervention period to each woman. Women could request instant response supportive texts at any time and could stop texts (102 women). Comparison intervention: women were mailed a non‐individually tailored self‐help leaflet but received no phone intervention (105 women). |
|
| Outcomes | Main outcomes were acceptability and self‐efficacy scores. Women were also assessed at 3, 7 and 12 weeks and reported smoking behaviour (7 day point prevalence). Women who reported quitting at 12 weeks were asked to mail saliva sample for cotinine validation. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified (by baseline smoking rate) block randomisation (block size 2) “Generation of the randomization tables and allocation of participants were implemented in a computer program and managed by [investigator] who had no contact with participants". |
| Allocation concealment (selection bias) | Low risk | “Generation of the randomization tables and allocation of participants were implemented in a computer program and managed by {investigator} who had no contact with participants.” Allocation sequence was concealed from staff providing care and staff carrying out data collection. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women would not be blind to intervention. It was reported that clinical staff and staff collecting outcome data were blind but women may have revealed allocation (24% in the intervention group thought the texts were annoying and 26% thought they got too many texts and may have revealed this before the final assessment). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Cotinine validation of reported quit rates would reduce detection bias for this outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 207 women were randomised and 174 completed assessment at 12 weeks (84%). There were missing data for some outcomes and less than half of the women who reported quitting smoking had this confirmed by cotinine validation). |
| Selective reporting (reporting bias) | Low risk | (Supplementary information available.) No obvious outcome reporting bias. |
| Other bias | Unclear risk | Groups appeared similar at baseline apart form more participants in the control group had smoked in a previous pregnancy. Authors reported carrying out sensitivity analysis adjusted for this. |
Parker 2007.
| Methods | RCT. 3‐arm trial with individual randomisation. | |
| Participants | Setting:1065 women attending for antenatal care at 22 urban clinics in Rhode island, Connecticut and Massachusetts, USA. 80% insured by Medicaid. Inclusion criteria: women who were identified as smokers (smoked at least 1 puff of a cigarette in the previous 30 days), no more than 26 weeks’ gestation, have access to a phone and able to speak English or Spanish. |
|
| Interventions | 3 groups.
|
|
| Outcomes | Outcomes included reported and cotinine‐validated quit rates at 32 weeks and at 6 weeks and 6 months postpartum. Information on process variables and cost of the programme were also collected. The published paper does not report results by randomisation group and we were not able to include them in the analysis. | |
| Notes | We contacted the author for more information on results and are awaiting a response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as “randomly assigned”. |
| Allocation concealment (selection bias) | Unclear risk | Described as “randomly assigned”. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women and staff would be aware of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Reported smoking cessation was cotinine validated (but results by randomisation group not reported in this paper). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | By 6 months 688 (65%) of the 1065 randomised were available to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Results by randomisation group not reported in this paper.We contacted the author for more information on results and are awaiting a response. |
| Other bias | Unclear risk | Results focused on feasibility and process outcomes. |
Pugh 2002.
| Methods | RCT 2 arms with individual randomisation. | |
| Participants | Setting: the trial was conducted between April 1999 and February 2000 in a large hospital in the USA. Inclusion criteria 41 women on low incomes (receiving financial medical assistance support). Women were recruited in hospital after the birth of their baby. |
|
| Interventions | Women in both groups received breastfeeding support from hospital nurses and all women had access to telephone advice. Women whose babies were born on weekdays were visited by a lactation consultant. Intervention group (21 women) women received extra visits from the community health nurse or one of the peer counsellor team daily while they were in hospital then at home during weeks 1,2 and 4. In addition peer counsellors provided telephone support twice weekly until the babies were 8 weeks old and then weekly until the baby was 6 months old (even if the mother had stopped breastfeeding). Control group (20 women) Usual care. |
|
| Outcomes | Duration of breastfeeding, duration of exclusive breastfeeding, costs of care, health service utilisation. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | "assigned randomly by a sealed envelope technique.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No women appeared to be lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Not clear, assessment from published study report. |
| Other bias | Unclear risk | Not apparent. |
Rasmussen 2011.
| Methods | 2 separate randomised trials are described in the same paper. Here we have included details of 1 of these trials examining postnatal telephone support. 2‐arm RCT with individual randomisation. | |
| Participants | Setting: hospital in a rural area of New York, USA with established health promotion and support for breastfeeding. May 2006‐Feb 2007. Inclusion criteria: (50 women randomised) reported as ‘infant delivered at term and within the period of study data collection, ever put to the breast, not injured during delivery, and not placed in foster care or cared for elsewhere; mother still available for telephone contact... and those with a prepregnancy BMI > 29 kg/m2 were approached during one of their prenatal visits by a research assistant for the study. Women were enrolled in the studies if they intended to breastfeed, had no history of breast surgery, resided near Bassett Healthcare (Cooperstown), were least 19 years old, and were carrying a singleton fetus 35 weeks of gestational age at the time of enrolment'. |
|
| Interventions | Participants in both groups received a telephone call during pregnancy from a lactation consultant, in the intervention group (targeted‐care group) the pregnancy call was more detailed and women were asked about knowledge and expectations about breastfeeding and were given practical information. After the birth nurses encouraged women to get up and move around and encouraged visitors to give the woman privacy for breastfeeding. At 24 and 72 hours after hospital discharge women received additional calls from the lactation consultant and gave standardised advice and responded to questions and addressed any other issues that came up. If necessary the lactation consultant could arrange to visit the woman. Intervention group: 25 women randomised, 5 excluded post randomisation, 20 followed up (In the event only approximately half of the women received the scheduled telephone calls 11). Control group: 25 women randomised, 5 excluded post randomisation, 20 followed up. (Only 11 women in the control group received the pregnancy phone call.) |
|
| Outcomes | Reported initiation of breastfeeding (still breastfeeding at 4 days after the birth), breastfeeding (any, 30 and 90 days postpartum) and exclusive breastfeeding (7 and 30 days). Duration of exclusive or any breastfeeding after hospital discharge. Outcome data collected in telephone interviews with women. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women and care providers were not blinded and would be aware of receiving the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was stated that the research assistants recruiting women and carrying out interviews were not aware of participants assigned groups. Women may have disclosed this information. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 50 women were randomised and 10 excluded post‐randomisation (20%). It was stated that an ITT analysis was carried out but this was not for all women only those 40 that remained eligible and were followed up. Women were analysed by intervention group whether or not they received the intervention and large numbers of women (almost half) did not receive the scheduled phone calls (11 women in intervention and 11 in control received study phone calls). |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | There was some baseline imbalance; 'There were no statistically significant differences between treatment groups in the initial characteristics of mother–infant dyads.... However, women in the targeted‐care group in BIBS 1 tended ( P < 0.07) to have a higher prepregnancy BMI as well as a higher ( P < 0.051) BMI at delivery than those in the usual‐care group'. |
Rigotti 2006.
| Methods | RCT, 2‐arm trial with individual randomisation. | |
| Participants | Setting: 442 pregnant smokers referred by Massachusetts prenatal providers. Pregnant smokers were identified from 2 sources: 1) Tufts Health Plan, a non‐profit Massachusetts‐based network‐model managed care organisation, and 2) a group of community‐based prenatal care practices. Recruitment 2001‐2004. Inclusion criteria: ‘Pregnant women identified as current cigarette smokers at a prenatal care visit were recruited if they had smoked at least 1 cigarette in the past 7 days, were 18 years of age or older, at 26 weeks or less of gestation, willing to consider altering their smoking during the pregnancy, reachable by telephone, English‐ speaking, and expected to live in New England for the next year. Exclusion criteria: ‘The major reasons for ineligibility were referral after 26 weeks of gestation, miscarriage, and patient denial of smoking in past week.’ Women who miscarried were excluded post randomisation. |
|
| Interventions | Intervention group: (220 women) best practice care (mailed pregnancy tailored smoking cessation booklet) and care providers sent ACOG smoking cessation guideline and reminder to address smoking at participants’ visits and a brief telephone call by a trained counsellor of about 5 minutes to discuss smoking, In addition women in the intervention group received a series of phone calls and additional mailed material. Each women had a counsellor offering up to 90 minutes of phone counselling during pregnancy and 15 minutes in the postpartum period. The counselling was tailored to women’s readiness to quit and focused on encouraging smoking reduction or quitting using motivational interviewing or cognitive behavioural strategies to increase sense of self‐efficacy and social support. Participants received a mean of 5 calls (range 0‐20) on average 4 during pregnancy (total duration mean 63 minutes) and 1 postpartum (mean 5 minutes). Control group: (222 women) best practice care (mailed pregnancy tailored smoking cessation booklet) and care providers sent ACOG smoking cessation guideline and reminder to address smoking at participants’ visits and a brief telephone call by a trained counsellor of about 5 minutes to discuss smoking; smokers who requested further help were referred to a 'quitline'. |
|
| Outcomes | Cotinine‐validated and self‐report quit rates at late pregnancy (after 28 weeks) and at 3 months postpartum. Women also reported number of attempts at quitting and reduction of 50% in number of cigarettes smoked. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were assigned to treatment conditions using a computer‐generated randomisation list arranged in balanced blocks of 4 and stratified by referral source (Tufts Health Plan versus community practice). |
| Allocation concealment (selection bias) | Low risk | ‘The list was contained in the computer‐assisted interviewing application used for the study and accessible only to the application’s developer. Counselors could not view the list; the application revealed the next assignment only after the smoker had consented to participate in the study.' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women and counsellors would be aware of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Research assistants rather than counsellors collected self‐report data at telephone follow‐up (it was not clear whether they would be aware of randomisation group). Self‐report of quitting was validated by mailed saliva samples for cotinine analysis (low risk for outcome detection bias for primary outcome). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | For the primary outcome there was an ITT analysis with those women not followed up or not mailing saliva samples assumed to be still smoking. Only 23 women who had miscarried were excluded from this analysis. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study paper. |
| Other bias | Unclear risk | Some baseline imbalance: ‘Partner smokes’ (borderline significantly more in Intervention group than in control) and ‘Made quit attempt in this pregnancy’(more in Intervention group than in control) ‘Nulliparous’ (borderline significantly more in Intervention group than in control) and weeks in gestation (borderline significantly higher in Intervention group than in control, support for quitting from partner more in Intervention group than in control). |
Simonetti 2012.
| Methods | RCT, 2 arms with individual randomisation. | |
| Participants | Setting: 114 primiparous women delivering in an Italian maternity ward in 2 months in 2009. Inclusion criteria: healthy primiparous women intending to breastfeed. Exclusion criteria: women with preterm or low birthweight babies, or with baby admitted to NICU or women with any condition that may impede breastfeeding. |
|
| Interventions | Intervention group: 55 women. Women received telephone calls during the first 6 weeks postpartum at least weekly. The calls were to provide information on breastfeeding, promote exclusive breastfeeding and to discuss any problems. Control group: 59 women received usual care which involved visits to the doctor at 1, 3 and 5 months postpartum. Both groups received breastfeeding education as part of routine antenatal care. |
|
| Outcomes | Exclusive breastfeeding at 1, 3 and 5 months following delivery. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | "randomly assigned." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned. Women and staff would be aware of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 114 women were enrolled. It was stated that 4 women in the intervention group were excluded post randomisation as they did not meet inclusion criteria (it was not clear when women were randomised). |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | There did not appear to be differences between the groups at baseline. The description of study methods was very brief. |
Smith 2008.
| Methods | RCT, 3 arms with individual randomisation. | |
| Participants | 840 women randomised 536 randomised to arms included in analysis in this review. Low‐risk nulliparous women. Setting not described (UK). Women recruited between 2004‐2007. Inclusion criteria: Low‐risk nulliparous women. |
|
| Interventions | 3‐arm trial. We have used data for groups 1 and 2 only in this review.
|
|
| Outcomes | Number of scheduled and unscheduled visits to clinic, STAI at 36 weeks and 6 weeks postpartum. Satisfaction with antenatal care. Other outcomes included social support, pre‐eclampsia and infant birthweight. | |
| Notes | Additional unpublished data were provided by the author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Author reported that sequence generation was by a web‐based computer package. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 41/565 (7%) lost to follow‐up but there were high levels of missing data for some outcomes. Analysis according to randomised group although many women in the telephone support group did not receive the intervention as planned. |
| Selective reporting (reporting bias) | Low risk | Brief conference abstract, additional information provided by the author. |
| Other bias | Unclear risk | Many of the women in the telephone support group did not receive the intervention as planned. (28.7% received all three scheduled calls, 30.1% 2 calls, 22.3% 1 call and 18.8% none of the planned calls). |
Stotts 2002.
| Methods | RCT. 2‐arm trial with individual randomisation. | |
| Participants | 269 women (134 in intervention and 135 in control) Setting: ‘The sample was drawn from 21 satellite locations of three large multispecialty clinics in the Houston and Dallas metropolitan areas’. Inclusion criteria: current smokers at 28 weeks. Reported as ‘Preliminary eligibility was based on fluency in English, age (18 years or older), smoking frequency (at least 5 cigarettes per week prior to pregnancy), and gestational age at first prenatal visit (before or at 20 weeks)’. Exclusion criteria: non‐smokers and ex‐smokers. |
|
| Interventions | 1‐to‐1 intervention group. (134 women) Usual care plus 5 newsletters and a video for women and their partners on healthy behaviours (including weight loss and smoking). Newsletters mailed at 2‐week intervals from 39th‐40th week (i.e. mainly postnatal). Women also received one 20‐30 minute telephone counselling call using motivational interviewing strategies within 2 weeks of the 28 weeks’ assessment. They were then mailed a personal feedback letter and then a second telephone counselling intervention was carried out a few days later. Counselling by trained nurse educators. The control group (135 women). Usual care included a 3‐5 minute counselling session on smoking cessation in pregnancy and were given the first in a series of self‐help booklets at 28 weeks. The remaining 7 booklets in the series were mailed to women weekly thereafter. (It was not clear whether women in the control group also received the video and more general pregnancy information; we have contacted the author for more information.) |
|
| Outcomes | Reported as ‘Data for the present study were collected at the first prenatal visit (intake), during the 28th and 34th weeks of pregnancy, and 6 weeks and 3 and 6 months postpartum’. 34th week cotinine testing, and postpartum telephone interviews conducted at 6 weeks and 3 and 6 months for reported smoking behaviour (quit or “light” smoking). |
|
| Notes | Raw data were not reported for most outcomes. We have contacted the author for more information and are awaiting a response. Cotinine data for a subset only. Very high attrition. Data from this study have not been included in the data and analysis tables. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number list. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Most outcomes are self‐reported. Women and care providers were not blind to intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Urine samples were collected anonymously on 175 of the 269 randomised women during clinic visits and analysed for cotinine. The only markers attached to the cotinine samples were a coded ID number that indicated experimental group status and the amount of intervention received. These women (Intervention n = 84; Control n = 82) represent the Anonymous Cotinine Subsample.’. High risk of detection bias for self‐report outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Urine samples were collected on only 175 of the 269 randomised women (40%). Postpartum follow‐up subsample: These analyses were performed on those who consented to the postpartum follow‐up study (n = 166) and not the total randomised sample (62% followed up). No ITT analysis. Much of the analysis was for women who had received all or part of the intervention (62% of the experimental group). |
| Selective reporting (reporting bias) | High risk | No ITT analysis and outcomes were reported in the postpartum period for light as well as non‐smokers (not smoking most of the time but have a few puffs now and again – not clear what this means). It was reported that very few women reported no smoking at 6 weeks, 3 and 6 months pp (12,11, and 11 women not reported by randomisation group). |
| Other bias | Unclear risk | Initial comparisons of demographic variables revealed no differences between the experimental and control groups’. However, table 2 reveals a significant difference on Smoking before pregnancy (No. of cigarettes per week) – there is a higher incidence of smoking more than 61 a week in the intervention group. Figures on reported smoking in the postnatal period were adjusted for baseline behaviour. (Not clear how this was done.) |
ACOG: American College of Obstetricians and Gynecologists BMI: body mass index EPDS: Edinburgh Postnatal Depresssion Scale GDM: gestational diabetes mellitus GP: General Practitioner ICU: intensive care unit IOM: Institute of Medicine ITT: intention‐to‐treat LBW: low birthweight NICU: neonatal intensive care unit PI: principal investigator RCT: randomised controlled trial SD: standard deviation SEM: standard error of the mean STAI: State Trait Anxiety Inventory VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alemi 1996 | Both intervention and control groups received telephone support. |
| Bartholomew 2011 | This study describes 2 methods of blood glucose monitoring in pregnancy; telephone reporting is compared with a new methods involving plugging the glucometer into a cell telephone which relays results to a website for clinical staff to review. There is no support intervention. |
| Brooten 1994 | The purpose of the study was to examine the average time nurses spent planning for early hospital discharge and providing home care to women who delivered by unplanned caesarean birth, therefore excluded. |
| Brooten 2001 | Telephone support is only a small component of the intervention (prenatal care in the women's homes which included teaching, counselling, telephone outreach, daily telephone availability, postpartum home visit by nurse specialist with physician backup). The study was excluded as the intervention included other elements as well as telephone support and therefore results may be misleading. |
| Caramlau 2011 | Publication is a trial protocol. |
| Chen 1993 | Randomisation alternate allocation (in order of woman's availability to join trial) therefore excluded. |
| Dennis 2003 | The study included women randomised to the intervention between 8 and 12 weeks postpartum, therefore excluded. |
| Edwards 1997 | The study included women randomised to the intervention 3 months postpartum, therefore excluded. |
| Ershoff 2000 | This was a quasi‐experimental historical design study and therefore excluded. |
| Fjeldsoe 2010 | The study included women randomised to the intervention up to 12 months postpartum, therefore excluded. |
| Frank 1986 | Telephone support is only a small component of the intervention ‐ single telephone contact at 4 months. The study was excluded as the intervention included other elements as well as telephone support and therefore results may be misleading. |
| Gagnon 2002 | All women received telephone support (nurse telephone contact at 48 hours post birth), women were then randomised to nurse visit at 3 to 4 days postpartum in either the woman's home by the community nurse (intervention) or by the hospital nurse in the hospital clinic (control). |
| Gjerdingen 2009 | This study was excluded as the intervention included several elements as well as telephone support, and the intervention mainly took place after 6 weeks postpartum. The trial focused on women screened as being at high risk of postnatal depression during a well‐child clinic visit. Women were recruited at up to a month postpartum and the intervention began within 1 or 2 weeks and continued up to 9 months (i.e. the intervention predominantly took place after 6 weeks postpartum). The intervention was complex and included referral to the primary care provider with a recommendation for treatment either with anti‐depressants and/or referral for psychotherapy. Care professionals received decision‐support and educational materials. Women were followed up by telephone by a case manager. Women also received educational material. Women in the control group were informed of the depression diagnosis and referred to their primary care provider for usual care (at the discretion of the care provider). |
| Haider 1997 | Telephone calls were not the intervention. |
| Iams 1988 | Both intervention and control groups received telephone support. |
| Jang 2008 | This was a quasi‐experimental study and therefore excluded. |
| Janssen 2006 | The study aimed at evaluating telephone (the current standard of care) with home‐based triage. Triage comprised advice (either over the telephone or at home) as to when to come into hospital at term. The study was excluded as the intervention compared two types of triage. |
| Katz 2011 | This study examined a complex intervention aimed at adolescent and teenaged pregnant women. The intervention started early in pregnancy and continued for 18 months postpartum (i.e. the intervention predominantly occurred after pregnancy). The intervention included a range of approaches including telephone support, provision of written materials, face‐to‐face meetings with a counsellor and lunchtime meetings every 3 months throughout the intervention period. The study was excluded as the intervention included other elements as well as telephone support. |
| Kersten‐Alvarez 2010 | The study included women randomised to the intervention on average 6 months postpartum. therefore excluded. |
| Lando 2001 | This was a quasi‐experimental effectiveness study and therefore excluded. |
| Langer 1993 | Telephone calls were not the intervention. |
| Lewis 2011 | The purpose of this study was to evaluate strategies for recruiting pregnant postpartum women into trials. |
| Norbeck 1996 | Telephone support is only a small component of the intervention ‐ social support intervention (4 standard face‐to‐face sessions 1‐ interview providing support on three problem areas; 2‐ video‐tape; 3 and 4 ‐ focused on relationships that foster self‐esteem. Sessions occurred approximately every 2 weeks and telephone contact was maintained in between). The study was excluded as the intervention included other elements as well as telephone support and therefore results may be misleading. |
| Oakley 1990 | Telephone support is only a small component of the intervention ‐ social support intervention (3 home visits carried out 14, 20 and 28 weeks' gestation, plus 2 telephone contacts or brief home visits in between those times, midwives on‐call to the mothers 24 hours/day, semi‐structured interviews to provide a basis for flexible and open‐ended communication between midwives and mothers, midwives provided topic specific information to mothers) The study was excluded as the intervention included other elements as well as telephone support and therefore results may be misleading. |
| Rush 1991 | No clinical outcomes reported. Trial evaluated the frequency of use of a hospital telephone line for new parents. |
| Sink 2001 | Telephone calls were not the intervention. |
| Steel O'Conner 2003 | The study examined postpartum home visits or screening telephone call. The telephone intervention comprised of a screening call to new mothers on the first day following discharge from hospital. The content of the call was structured to elicit the mothers concerns in the areas of infant feeding, her baby's general health and her emotional status. A home visit was made if either the mother or clinician identified a need. The study was excluded a the telephone calls were used as a screening tool. |
| Stomp‐van den Berg 2007 | Publication is a trial protocol. |
| van Doesum 2008 | The study included women randomised to the intervention up to 12 months postpartum, therefore excluded. |
Characteristics of ongoing studies [ordered by study ID]
Dennis 2012.
| Trial name or title | The effect of telephone‐based interpersonal psychotherapy for treatment of postpartum depression. |
| Methods | RCT. 2 arms. Individual randomisation. |
| Participants | 240 women between 2 and 24 weeks postpartum with a diagnosis of major depression. |
| Interventions | Standard care plus telephone‐based interpersonal psychotherapy by a trained nurse (12 sessions) compared with standard care alone. |
| Outcomes | Postpartum depression, depressive symptomatology, anxiety, couple adjustment and health service use. |
| Starting date | 212 participants enrolled by March, 2012. |
| Contact information | Cindy‐Lee Dennis cindylee.dennis@utoronto.ca |
| Notes |
Evans 2012.
| Trial name or title | The Text4baby case study. |
| Methods | Pilot RCT. |
| Participants | Pregnant women attending for first prenatal care appointment. Planned recruitment 249 women. |
| Interventions | Usual care plus mobile phone text messages during pregnancy and the postnatal period (general health promotion, healthcare utilisation, nutrition, smoking, 130 prenatal messages and similar number for the postnatal period) compared with usual care alone. |
| Outcomes | Gestational age at delivery, antenatal hospital admissions, attendance at antenatal care, body mass index, weight gain in pregnancy, infant birthweight. |
| Starting date | Not clear, planned completion of recruitment "late 2012". |
| Contact information | wdevans@gwu.edu |
| Notes |
Moniz 2012.
| Trial name or title | Influenza and text messaging in pregnancy. |
| Methods | RCT, 2 arms with individual randomisation. |
| Participants | Estimated recruitment 250 women less than 28 weeks' gestation aged between 14‐50 and willing to receive text messages and no contraindications to receiving the influenza vaccine. |
| Interventions | Text messages re general health in pregnancy plus messages on influenza vaccination from enrolment until delivery compared with text messages on general health alone. |
| Outcomes | Influenza vaccination uptake and knowledge re influenza in pregnancy. |
| Starting date | September 2010. |
| Contact information | Michelle Moniz, University of Pittsburgh, USA. |
| Notes |
Patel 2011.
| Trial name or title | "Effectiveness of cell phone couseling to improve breast feeding indicators." |
| Methods | Cluster‐randomised trial. |
| Participants | 1036 women between 32‐36 weeks' gestation attending antenatal clinic at 2 hospitals serving socio‐economically disadvantaged women in India. |
| Interventions | Breastfeeding counselling by phone starting during pregnancy and up to 6 months postpartum compared with hospital counselling. |
| Outcomes | Exclusive breastfeeding up to 26 weeks postpartum, infant hospitalisation, immunisation uptake. |
| Starting date | January 2010. |
| Contact information | dr_apatel@yahoo.com |
| Notes |
RCT: randomised controlled trial
Differences between protocol and review
In the protocol we planned to consider all published and unpublished randomised controlled trials and quasi‐randomised controlled trials, however, given that quasi‐randomisation methods are at high risk of bias, and there have now been a relatively large number of randomised trials published in this area, we decided to focus on published and unpublished randomised controlled trials only.
In this version of the review (2013) we have included a number of non pre‐specified outcomes. In any future updates, we will include these as secondary outcomes if further data become available.
Contributions of authors
Tina Lavender and Yana Richens conceived and designed the review.
Tina Lavender and Yana Richens wrote the first draft of the protocol.
Tina Lavender, Yana Richens and Stephen Milan contributed to the drafts of the protocol.
Stephen Milan, Therese Dowswell and Rebecca Smyth carried out data extraction and assessed risk of bias.
Therese Dowswell set up the analysis and entered data and these were checked by Rebecca Smyth.
Tina Lavender, Rebecca Smyth and Therese Dowswell drafted the results and discussion sections of the review, and Stephen Milan and Yana Richens commented on drafts.
Sources of support
Internal sources
University of Manchester, UK.
University College London Hospitals, UK.
The University of Liverpool, UK.
External sources
-
National Institute for Health Research, UK.
NIHR Programme of centrally‐managed pregnancy and childbirth systematic reviews of priority to the NHS and users of the NHS: 10/4001/02
Declarations of interest
None known.
New
References
References to studies included in this review
Bloom 1982 {published data only}
- Bloom K, Goldbloom RB, Robinson SC, Stevens FE. II. Factors affecting the continuance of breast feeding. Acta Paediatrica Scandinavica 1982;71(Suppl 300):9‐14. [PubMed] [Google Scholar]
Boehm 1996 {published data only}
- Boehm MD, Glass CA, Reed GW. Prevention of preterm birth: role of daily telephone contact. Journal of Reproductive Medicine 1996;41:595‐601. [PubMed] [Google Scholar]
Bryce 1991 {published data only}
- Bryce RL, Stanley FJ, Garner JB. Randomized controlled trial of antenatal social support to prevent preterm birth. British Journal of Obstetrics and Gynaecology 1991;98:1001‐8. [DOI] [PubMed] [Google Scholar]
Bullock 1995 {published data only}
- Bullock LF, Wells JE, Duff GB, Hornblow AR. Telephone support for pregnant women: outcome in late pregnancy. New Zealand Medical Journal 1995;108(1012):476‐8. [PubMed] [Google Scholar]
Bullock 2009 {published data only}
- Bullock L, Everett KD, Mullen PD, Geden E, Longo DR, Madsen R. Baby BEEP: A randomized controlled trial of nurses’ individualized social support for poor rural pregnant smokers. Maternal and Child Health Journal 2009;13(3):395‐406. [DOI] [PubMed] [Google Scholar]
- Bullock LF, Everett KD, Mullen PD. Baby beep: a randomized clinical trial of smoking cessation for low‐income rural pregnant women using nurse‐delivered social support. Annals of Behavioral Medicine 2008;35:S99. [Google Scholar]
Bunik 2010 {published data only}
- Bunik M, Beaty B, Dickinson M, Shobe P, Kempe A, O'Connor ME. Early formula supplementation in breastfeeding mothers: how much is too much for BF duration success?. Breastfeeding Medicine 2007;2(3):184. [Google Scholar]
- Bunik M, Shobe P, Crane L, Kempe A. Low‐income Latina mothers' perspectives on breastfeeding issues and participation in a telephone based support intervention. Breastfeeding Medicine 2007;2(3):184. [Google Scholar]
- Bunik M, Shobe P, O'Connor ME, Beaty B, Langendoerfer S, Crane L, et al. Are 2 weeks of daily breastfeeding support insufficient to overcome the influences of formula?. Academic Pediatrics 2010;10(1):21‐8. [DOI] [PubMed] [Google Scholar]
- Bunik M, Shobe P, O'Connor ME, Beaty B, Langendoerfer S, Crane L, et al. Randomized controlled trial to evaluate a telephone support intervention for breastfeeding in low‐income Latina mothers. Breastfeeding Medicine 2007;2(3):183. [Google Scholar]
- Bunik M, Shobe P, O'Connor ME, Beaty B, Langendoerfer S, Crane L, et al. Telephone support intervention for breastfeeding in low‐income Latina mothers. Pediatric Academic Societies Annual Meeting; 2007 May 5‐8; Toronto, Canada. 2007.
Dennis 2002 {published data only}
- Dennis CL. A randomized controlled trial evaluating the effect of peer (mother‐to‐mother) support on breastfeeding duration among primiparious women. Dissertation: University of Toronto.
- Dennis CL, Hodnett E, Gallop R, Chalmers B. The effect of peer support on breast‐feeding duration among primiparous women: a randomized controlled trial [See comments]. CMAJ: Canadian Medical Association Journal 2002;166(1):21‐8. [PMC free article] [PubMed] [Google Scholar]
Dennis 2009 {published data only}
- Dennis CL, Hodnett E, Kenton L, Weston J, Zupancic J, Stewart DE, et al. Effect of peer support on prevention of postnatal depression among high risk women: multisite randomised controlled trial. BMJ 2009;338:a3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukhovny D, Dennis CL, Hodnett E, Kenton L, Weston J, Stewart DE, et al. Prospective economic evaluation of a peer support intervention for prevention of postpartum depression among high risk women in Ontario, Canada. American Academy of Pediatrics Annual Meeting; 2010 October 2‐5; San Francisco, California, USA. 2010. [DOI] [PubMed]
- Dukhovny D, Dennis CL, Hodnett E, Kenton L, Weston J, Stewart DE, et al. Prospective economic evaluation of a peer support intervention for prevention of postpartum depression amongst high risk women. Pediatric Academic Societies' 2010 Annual Meeting; 2010 May 1‐4; Vancouver, Canada. 2010.
Di Meglio 2010 {published data only}
- Meglio GD, McDermott MP, Klein JD. A randomized controlled trial of telephone peer support's influence on breastfeeding duration in adolescent mothers. Breastfeeding Medicine 2010;5:41‐7. [DOI] [PubMed] [Google Scholar]
Donaldson 1988 {published data only}
- Donaldson NE. Effect of telephone postpartum follow‐up: a clinical trial [thesis]. San Francisco: University of California, 1988. [Google Scholar]
Ershoff 1999 {published data only}
- Ershoff DH, Quinn VP, Boyd NR, Stern J, Gregory M, Wirtschafter D. The Kaiser Permanente prenatal smoking‐cessation trial When more isn't better, what is enough?. American Journal of Preventive Medicine 1999;17(3):161‐8. [DOI] [PubMed] [Google Scholar]
Ferrara 2011 {published data only}
- Ferrara A, Hedderson MM, Albright CL, Ehrlich SF, Quesenberry CP Jr, Peng T, et al. A pregnancy and postpartum lifestyle intervention in women with gestational diabetes mellitus reduces diabetes risk factors: a feasibility randomized control trial. Diabetes Care 2011;34(7):1519‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoddinott 2012 {published data only}
- Hoddinott P, Craig L, MacLennan G, Boyers D, Vale L. Process evaluation for the FEeding Support Team (FEST) randomised controlled feasibility trial of proactive and reactive telephone support for breastfeeding women living in disadvantaged areas. BMJ Open 2012;2(2):e001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoddinott P, Craig L, MacLennan G, Boyers D, Vale L. The FEeding Support Team (FEST) randomised, controlled feasibility trial of proactive and reactive telephone support for breastfeeding women living in disadvantaged areas. BMJ Open 2012;2(2):e000652. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jareethum 2008 {published data only}
- Jareethum R, Titapant V, Chantra T, Sommai V, Chuenwattana P, Jirawan C. Satisfaction of healthy pregnant women receiving short message service via mobile phone for prenatal support: a randomized controlled trial. Journal of the Medical Association of Thailand 2008;91(4):458‐63. [PubMed] [Google Scholar]
Johnson 2000 {published data only}
- Johnson JL, Ratner PA, Bottorff JL, Hall W, Dahinte S. Preventing smoking relapse in postpartum women. Nursing Research 2000;49(1):44‐52. [DOI] [PubMed] [Google Scholar]
Little 2002 {published data only}
- Little M, Saul G, Testa K, Gaziano C. The influence of telephonic nursing care coordination on patient satisfaction in a predominantly low‐income, high‐risk pregnancy population. Lippincott's Case Management 2002;7:15‐23. [DOI] [PubMed] [Google Scholar]
- Little M, Saul GD, Testa K, Gaziano C. Improving pregnancy outcome and reducing avoidable clinical resource utilization through telephonic perinatal care coordination. Lippincott's Case Management 2002;7:103‐12. [DOI] [PubMed] [Google Scholar]
Lund 2012 {published data only}
- Lund S, Hemed M, Nielsen BB, Said A, Said K, Makungu MH, et al. Mobile phones as a health communication tool to improve skilled attendance at delivery in Zanzibar: A cluster‐randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2012;119(10):1256‐64. [DOI] [PubMed] [Google Scholar]
McBride 1999 {published data only}
- McBride CM, Curry SJ, Lando HA, Pirie PL, Grothaus LC, Nelson JC. Prevention of relapse in women who quit smoking during pregnancy. American Journal of Public Health 1999;89(5):706‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Milgrom 2011 {published data only}
- Milgrom J. Toward parenthood: delivering an antenatal self‐help intervention with telephone support for depression, anxiety and parenting difficulties ‐ facilitating the perinatal health journey. Australian New Zealand Clinical Trials Registry (www.anzctr.org.au) (accessed 19 February 2008).
- Milgrom J, Schembri C, Ericksen J, Ross J, Gemmill AW. Towards parenthood: an antenatal intervention to reduce depression, anxiety and parenting difficulties. Journal of Affective Disorders 2011;130(3):385‐94. [DOI] [PubMed] [Google Scholar]
Mongeon 1995 {published data only}
- Mongeon M, Allard R. A controlled study with regular telephonic support given by volunteers on the progress and outcome of breast‐feeding [Essai controle d'un soutien telephonique regulier donne par une benevole sur le deroulement et l'issue de l'allaitement .]. Canadian Journal of Public Health 1995;86:124‐7. [PubMed] [Google Scholar]
Moore 1998 {published data only}
- Moore ML, Ketner M, Walsh K, Wagoner S. Listening to women at risk for preterm birth. Maternal‐Child Nursing Journal 2004;29(6):391‐7. [DOI] [PubMed] [Google Scholar]
- Moore ML, Meis PJ, Ernest JM. Reducing low birthweight birth through intensive nursing intervention via telephone. American Journal of Obstetrics and Gynecology 1994;170:383. [Google Scholar]
- Moore ML, Meis PJ, Ernest JM, Wells HB, Zaccaro DJ, Terrell T. A randomized trial of nurse intervention to reduce preterm and low birth weight births. Obstetrics & Gynecology 1998;91(5 Pt 1):656‐61. [DOI] [PubMed] [Google Scholar]
- Muender MM, Moore ML, Chen GJ, Sevick MA. Cost‐benefit of a nursing telephone intervention to reduce preterm and low‐birthweight births in an african american clinic population. Preventive Medicine 2000;30(4):271‐6. [DOI] [PubMed] [Google Scholar]
Naughton 2012 {published data only}
- Naughton F, Prevost AT, Gilbert H, Sutton S. Randomized controlled trial evaluation of a tailored leaflet and SMS text message self‐help intervention for pregnant smokers (MiQuit). Nicotine and Tobacco Research 2012;14(5):569‐77. [DOI] [PubMed] [Google Scholar]
Parker 2007 {published data only}
- Parker DR, Windsor RA, Roberts MB, Hecht J, Hardy NV, Strolla LO, et al. Feasibility, cost, and cost‐effectiveness of a telephone‐based motivational intervention for underserved pregnant smokers. Nicotine & Tobacco Research 2007;9(10):1043‐51. [DOI] [PubMed] [Google Scholar]
Pugh 2002 {published data only}
- Pugh LC, Milligan RA, Frick KD, Spatz D, Bronner Y. Breastfeeding duration, costs, and benefits of a support program for low‐income breastfeeding women. Birth 2002;29(2):95‐100. [DOI] [PubMed] [Google Scholar]
Rasmussen 2011 {published data only}
- Rasmussen KM, Dieterich CM, Zelek ST, Altabet JD, Kjolhede CL. Interventions to increase the duration of breastfeeding in obese mothers: the Bassett Improving Breastfeeding Study. Breastfeeding Medicine 2011;6:69‐75. [DOI] [PubMed] [Google Scholar]
Rigotti 2006 {published data only}
- Rigotti N, Park E, Regan S, Chang Y, Perry K, Loudin B, et al. Efficacy of proactive telephone counseling for pregnant smokers: a randomized trial. Society for Research on Nicotine and Tobacco 12th Annual Meeting; 2006 February 15‐18, Orlando, Florida, USA 2006:22.
- Rigotti NA, Park ER, Regan S, Chang Y, Perry K, Loudin B, et al. Efficacy of telephone counseling for pregnant smokers. A randomized controlled trial. Obstetrics and Gynecology 2006;108:83–92. [DOI] [PubMed] [Google Scholar]
Simonetti 2012 {published data only}
- Simonetti V, Palma E, Giglio A, Mohn A, Cicolini G. A structured telephonic counselling to promote the exclusive breastfeeding of healthy babies aged zero to six months: a pilot study. International Journal of Nursing Practice 2012;18(3):289‐94. [DOI] [PubMed] [Google Scholar]
Smith 2008 {published and unpublished data}
- Smith VJ, Hewison J, McParlin C, Steen IN, Deverill M, Robson SC. Antenatal support and reassurance for low‐risk nulliparous women. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93(Suppl 1):Fa73. [Google Scholar]
- Snaith V. Support and reassurance in antenatal care. Current Controlled Trials (www.controlled‐trials.com/mrct) (accessed 21 March 2006).
Stotts 2002 {published data only}
- Stotts AL, DiClemente CC, Dolan‐Mullen P. One‐to‐one. A motivational intervention for resistant pregnant smokers. Addictive Behaviors 2002;27:275‐92. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alemi 1996 {published data only}
- Alemi F, Stephens RC, Javalghi RG, Dyches H, Butts J, Ghadiri A. A randomized trial of a telecommunications network for pregnant women who use cocaine. Medical Care 1996;34(10):OS10‐20. [DOI] [PubMed] [Google Scholar]
Bartholomew 2011 {published data only}
- Bartholomew ML, Church K, Graham G, Burlingame J, Zalud I, Sauvage L, et al. Managing diabetes in pregnancy using cell phone/internet technology. American Journal of Obstetrics and Gynecology 2011;204(1 Suppl):S113‐4. [Google Scholar]
Brooten 1994 {published data only}
- Brooten D, Knapp H, Borucki L, Jacobsen B, Finkler S, Arnold L, et al. Early discharge and home care after unplanned cesarean birth: nursing care time. Journal of Obstetric, Gynecologic and Neonatal Nursing 1996;25(7):595‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooten D, Roncoli M, Finkler S, Arnold L, Cohen A, Mennuti M. A randomized trial of early hospital discharge and home follow‐up of women having cesarean birth. Obstetrics & Gynecology 1994;84:832‐8. [PMC free article] [PubMed] [Google Scholar]
Brooten 2001 {published data only}
- Brooten D, Youngblut JM, Brown L, Finkler SA, Neff DF, Madigan E. A randomized trial of nurse specialist home care for women with high‐risk pregnancies: outcomes and costs [Erratum appears in American Journal of Management Care 2001 Sep;7(9):855]. American Journal of Managed Care 2001;7(8):793‐803. [PMC free article] [PubMed] [Google Scholar]
Caramlau 2011 {published data only}
- Caramlau I, Barlow J, Sembi S, McKenzie‐McHarg K, McCabe C. Mums 4 Mums: structured telephone peer‐support for women experiencing postnatal depression. Pilot and exploratory RCT of its clinical and cost effectiveness. Trials 2011;12:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 1993 {published data only}
- Chen CH. Effects of home visits and telephone contacts on breastfeeding compliance in Taiwan. Maternal‐Child Nursing Journal 1993;21(3):82‐90. [PubMed] [Google Scholar]
Dennis 2003 {published data only}
- Dennis CL. The effect of peer support on postpartum depression: a pilot randomized controlled trial. Canadian Journal of Psychiatry ‐ Revue Canadienne de Psychiatrie 2003;48(2):115‐24. [DOI] [PubMed] [Google Scholar]
Edwards 1997 {published data only}
- Edwards NC, Sims‐Jones N. A randomized controlled trial of alternative approaches to community follow‐up for postpartum women. Canadian Journal of Public Health. Revue Canadienne de Sante Publique 1997;88:123‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ershoff 2000 {published data only}
- Ershoff DH, Quinn VP, Boyd NR, Stern J, Gregory M, Wirtschafter D. The Kaiser Permanente prenatal smoking cessation trial: when more isn't better, what is enough?. Tobacco Control 2000;9 Suppl 3:iii60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fjeldsoe 2010 {published data only}
- Fjeldsoe BS, Miller YD, Marshall AL. MobileMums: a randomized controlled trial of an SMS‐based physical activity intervention. Annals of Behavioral Medicine 2010;39(2):101‐11. [DOI] [PubMed] [Google Scholar]
Frank 1986 {published data only}
- Frank DA, Wirtz SJ, Sorenson JR, Heeren R. Duration of breast‐feeding among low‐income women: a randomized trial of the effects of commercial hospital discharge packs and hospital‐based telephone counseling. American Journal of Diseases of Children 1986;140:311. [Google Scholar]
- Frank DA, Wirtz SJ, Sorenson JR, Heeren T. Commercial discharge packs and breast‐feeding counseling: effects on infant‐feeding practices in a randomized trial. Pediatrics 1987;80:845‐54. [PubMed] [Google Scholar]
Gagnon 2002 {published data only}
- Gagnon AJ, Dougherty G, Jimenez V, Leduc N. Randomized trial of postpartum care after hospital discharge. Pediatrics 2002;109(6):1074‐80. [DOI] [PubMed] [Google Scholar]
- Gagnon AJ, Edgar L, Kramer MS, Papegeorgiou A, Waghorn K, Klein MC. A randomized trial of a program of early postpartum discharge with nurse visitation. American Journal of Obstetrics and Gynecology 1997;176:205‐11. [DOI] [PubMed] [Google Scholar]
Gjerdingen 2009 {published data only}
- Gjerdingen D, Crow S, McGovern P, Miner M, Center B. Stepped care treatment of postpartum depression: impact on treatment, health, and work outcomes. Journal of the American Board of Family Medicine: JABFM 2009;22(5):473‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haider 1997 {published data only}
- Haider R, Islam A, Hamadani J, Amin NJ, Kabir I, Malek MA, et al. Breast‐feeding counseling in a diarrhoeal disease hospital [Orientacion sobre lactancia materna en un hospital para enfermedades diarreicas]. Pan American Journal of Public Health 1997;1:355‐61. [Google Scholar]
Iams 1988 {published data only}
- Iams JD, Johnson FF, O'Shaughnessy RW. A prospective random trial of home uterine activity monitoring in pregnancies at increased risk of preterm labor. American Journal of Obstetrics and Gynecology 1988;159:595‐603. [DOI] [PubMed] [Google Scholar]
Jang 2008 {published data only}
- Jang GJ, Kim SH, Jeong KS. Effect of postpartum breast‐feeding support by nurse on the breast‐feeding prevalence. Taehan Kanho Hakhoe Chi 2008;38(1):172‐9. [DOI] [PubMed] [Google Scholar]
Janssen 2006 {published data only}
- Janssen PA, Still DK, Klein MC, Singer J, Carty EA, Liston RM, et al. Early labor assessment and support at home versus telephone triage: a randomized controlled trial. Obstetrics & Gynecology 2006;108(6):1463‐9. [DOI] [PubMed] [Google Scholar]
Katz 2011 {published data only}
- Katz KS, Rodan M, Milligan R, Tan S, Courtney L, Gantz M, et al. Efficacy of a randomized cell phone‐based counseling intervention in postponing subsequent pregnancy among teen mothers. Maternal & Child Health Journal 2011;15 Suppl 1:S42‐53. [DOI] [PubMed] [Google Scholar]
Kersten‐Alvarez 2010 {published data only}
- Kersten‐Alvarez LE, Hosman CM, Riksen‐Walraven JM, Doesum KT, Hoefnagels C. Long‐term effects of a home‐visiting intervention for depressed mothers and their infants. Journal of Child Psychology and Psychiatry 2010;51(10):1160‐70. [DOI] [PubMed] [Google Scholar]
Lando 2001 {published data only}
- Lando HA, Valanis BG, Lichtenstein E, Curry SJ, McBride CM, Pirie PL, et al. Promoting smoking abstinence in pregnant and postpartum patients: a comparison of 2 approaches. American Journal of Managed Care 2001;7:685‐93. [PubMed] [Google Scholar]
Langer 1993 {published data only}
- Langer A, Campero L, Garcia C, Reynoso S. Effects of psychosocial support during labour and childbirth on breastfeeding, medical interventions, and mothers' wellbeing in a Mexican public hospital: a randomised clinical trial. British Journal of Obstetrics and Gynaecology 1998;105:1056‐63. [DOI] [PubMed] [Google Scholar]
- Langer A, Farnot U, Garcia C, Barros F, Victora C, Belizan JM, et al. The Latin American trial of psychosocial support during pregnancy: effects on mother's wellbeing and satisfaction Latin American Network For Perinatal and Reproductive Research (LANPER). Social Sciences and Medicine 1996;42(11):1589‐97. [DOI] [PubMed] [Google Scholar]
- Langer A, Garcia C, Leis T, Reynoso S, Hernandez B. Pyschosocial support as a strategy to promote the health of the newborn. Revista de Investigacion Clinica 1993;45:317‐28. [PubMed] [Google Scholar]
- Langer A, Victora C, Victora M, Barros F, Farnot U, Belizan J, et al. The Latin American trial of psychosocial support during pregnancy: a social intervention evaluated through an experimental design. Social Sciences and Medicine 1993;36:495‐507. [DOI] [PubMed] [Google Scholar]
Lewis 2011 {published data only}
- Lewis B, Avery M, Gjerdingen D, Sirard J. Innovative methods for recruiting pregnant and postpartum women for behavioral intervention trials. Annals of Behavioral Medicine 2011;41 Suppl 1:S114. [Google Scholar]
Norbeck 1996 {published data only}
- Norbeck JS, DeJoseph JF, Smith RT. A randomized trial of an empirically‐derived social support intervention to prevent low birthweight among African American women. Social Sciences and Medicine 1996;43(6):947‐54. [DOI] [PubMed] [Google Scholar]
Oakley 1990 {published data only}
- Oakley A, Rajan L. The social support and pregnancy outcome study. Advances in the Prevention of Low Birthweight: an International Symposium; 1988 May 8‐11; Cape Cod, Massachusetts, USA. 1988:123‐38.
- Oakley A, Rajan L, Grant AM. Social support and pregnancy outcome. British Journal of Obstetrics and Gynaecology 1990;97:155‐62. [DOI] [PubMed] [Google Scholar]
Rush 1991 {published data only}
- Rush JP, Kitch TL. A randomized, controlled trial to measure the frequency of use of a hospital telephone line for new parents. Birth 1991;18:193‐7. [DOI] [PubMed] [Google Scholar]
Sink 2001 {published data only}
- Sink KK. Perceptions, informational needs, and feelings of competency of new parents [thesis]. Michigan: University of Michigan, 2001. [Google Scholar]
Steel O'Conner 2003 {published data only}
- Steel O'Connor KO, Mowat DL, Scott HM, Carr PA, Dorland JL, Young Tai KF. A randomized trial of two public health nurse follow‐up programs after early obstetrical discharge: an examination of breastfeeding rates, maternal confidence and utilization and costs of health services. Canadian Journal of Public Health. Revue Canadienne de Sante Publique 2003;94(2):98‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stomp‐van den Berg 2007 {published data only}
- Stomp‐van den Berg SG, Poppel MN, Hendriksen IJ, Bruinvels DJ, Uegaki K, Bruijne MC, et al. Improving return‐to‐work after childbirth: design of the mom@work study, a randomised controlled trial and cohort study. BMC Public Health. 7:43, 2007. 2007;7:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uegaki K, Stomp‐van den Berg SG, Bruijne MC, Poppel MN, Heymans MW, Mechelen W, et al. Cost‐utility analysis of a one‐time supervisor telephone contact at 6‐weeks post‐partum to prevent extended sick leave following maternity leave in The Netherlands: results of an economic evaluation alongside a randomized controlled trial. BMC Public Health 2011;11:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Doesum 2008 {published data only}
- Doesum KT, Riksen‐Walraven JM, Hosman CM, Hoefnagels C. A randomized controlled trial of a home‐visiting intervention aimed at preventing relationship problems in depressed mothers and their infants. Child Development 2008;79(3):547‐61. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Dennis 2012 {published data only}
- Dennis CL, Ravitz P, Grigoriadis S, Jovellanos M, Hodnett E, Ross L, et al. The effect of telephone‐based interpersonal psychotherapy for the treatment of postpartum depression: study protocol for a randomized controlled trial. Trials [Electronic Resource] 2012;13:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Evans 2012 {published data only}
- Evans WD, Abroms LC, Poropatich R, Nielsen PE, Wallace JL. Mobile health evaluation methods: the Text4baby case study. Journal of Health Communication 2012;17 Suppl 1:22‐9. [DOI] [PubMed] [Google Scholar]
Moniz 2012 {published data only}
- Moniz M. Text messaging for preventative health during pregnancy; improving influenza vaccination rates in pregnancy: a randomized controlled trial of text messaging to increase vaccine uptake. http://clinicaltrials.gov/show/NCT01248520 2012. [DOI] [PubMed]
Patel 2011 {published data only}
- Patel A. Effectiveness of cell phone counseling to improve breast feeding indicators. Clinical Trials Registry ‐ India (http://ctri.nic.in) (accessed 2 August 2011) 2011.
- Patel A. Effectiveness of cell phone counseling to improve breast feeding indicators. http://clinicaltrials.gov/ct2/show/NCT01383070 2012.
Additional references
Chamberlain 2005
- Chamberlain B, Merewood A, Malone SKLC, Philipp BL. Calls to an inner‐city hospital breastfeeding telephone support line. Journal of Human Lactation 2005;21:53‐8. [DOI] [PubMed] [Google Scholar]
David 2010
- David S, Fenwick J, Bayes S, Martin T. A qualitative analysis of the content of telephone calls made by women to a dedicated ‘Next Birth After Caesarean’ antenatal clinic. Women & Birth 2010;23(4):166‐71. [DOI] [PubMed] [Google Scholar]
Dennis 2008
- Dennis CL, Kingston D. A systematic review of telephone support for women during pregnancy and the early postpartum period. Journal of Obstetric, Gynecologic, and Neonatal Nursing: JOGNN 2008;37:301‐14. [DOI] [PubMed] [Google Scholar]
Fosarelli 1983
- Fosarelli PD. The telephone in pediatric medicine: a review. Clinical Pediatrics 1983;22(4):293‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kennedy 2007
- Kennedy S. Telephone triage in maternity care. RCM Midwives 2007;10(10):478‐80. [PubMed] [Google Scholar]
Knight 2010
- Knight K, Endacott R, Kenny A. Ambiguous and arbitrary: the role of telephone interactions in rural health service delivery. Australian Journal of Primary Health 2010;16(2):126‐31. [DOI] [PubMed] [Google Scholar]
Latimer 1998
- Lattimer V, George S, Thompson F, Thomas E, Mullee M, Turnbull J, et al. Safety and effectiveness of nurse telephone consultation in out of hours primary care: randomised controlled trial. BMJ 1998;317:1054‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2004
- Moore ML, Ketner M, Walsh K, Wagoner S. Listening to women at risk for preterm birth: their perceptions of barriers to effective care and nurse telephone interventions. MCN: The American Journal of Maternal Child Nursing 2004;29(6):391‐7. [DOI] [PubMed] [Google Scholar]
Oda 1995
- Oda DS, Heilbron DC, Taylor HJ. A preventive child health program: the effect of telephone and home visits by public health nurses. American Journal of Public Health 1995;85(6):854‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Osman 2010
- Osman H, Chaaya M, Zein L, Naassan G, Wick L. What do first‐time mothers worry about? A study of usage patterns and content of calls made to a postpartum support telephone hotline. BMC Public Health 2010;10:611. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Siegel 1992
- Siegel SB. Telephone follow‐up programs as creative nursing interventions. Pediatric Nursing 1992;18:86‐9. [PubMed] [Google Scholar]
Solomon 2005
- Solomon LJ, Flynn BS. Telephone support for pregnant smokers who want to stop smoking. Health Promotion Practice 2005;6(1):105‐8. [DOI] [PubMed] [Google Scholar]
United Nations 2007
- Department of Economic and Social Affairs of the United Nations. Mobile Applications on Health and Learning. Compendium of ICT Applications on Electronic Government: New York. Vol. 1, New York: United Nations, 2007. [Google Scholar]
Vital Wave Consulting 2009
- Vital Wave Consulting. mHealth for Development: The Opportunity of Mobile Technology for Healthcare in the Developing World. Washington, D.C. and Berkshire, UK: UN Foundation‐Vodafone Foundation Partnership, 2009. [Google Scholar]
Wang 2008
- Wang SF, Chen CH, Cehn CH. Related factors in using a free breastfeeding hotline service in Taiwan. Journal of Clinical Nursing 2008;17:949‐56. [DOI] [PubMed] [Google Scholar]
Wootton 2001
- Wootton R. Recent advances: telemedicine. BMJ 2001;323:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wyatt 2001
- Wyatt JC, Keen J. The new NHS information technology strategy. Technology will change practice. BMJ 2001;322:1378‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Lavender 2011
- Lavender T, Richens Y, Milan SJ, Smyth RMD. Telephone support for women during pregnancy and the first six weeks postpartum. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD009338] [DOI] [PMC free article] [PubMed] [Google Scholar]


