Abstract
Background
Every year, at least one million children become ill with tuberculosis and around 200,000 children die. Xpert MTB/RIF and Xpert Ultra are World Health Organization (WHO)‐recommended rapid molecular tests that simultaneously detect tuberculosis and rifampicin resistance in adults and children with signs and symptoms of tuberculosis, at lower health system levels. To inform updated WHO guidelines on molecular assays, we performed a systematic review on the diagnostic accuracy of these tests in children presumed to have active tuberculosis.
Objectives
Primary objectives
• To determine the diagnostic accuracy of Xpert MTB/RIF and Xpert Ultra for (a) pulmonary tuberculosis in children presumed to have tuberculosis; (b) tuberculous meningitis in children presumed to have tuberculosis; (c) lymph node tuberculosis in children presumed to have tuberculosis; and (d) rifampicin resistance in children presumed to have tuberculosis
‐ For tuberculosis detection, index tests were used as the initial test, replacing standard practice (i.e. smear microscopy or culture)
‐ For detection of rifampicin resistance, index tests replaced culture‐based drug susceptibility testing as the initial test
Secondary objectives
• To compare the accuracy of Xpert MTB/RIF and Xpert Ultra for each of the four target conditions
• To investigate potential sources of heterogeneity in accuracy estimates
‐ For tuberculosis detection, we considered age, disease severity, smear‐test status, HIV status, clinical setting, specimen type, high tuberculosis burden, and high tuberculosis/HIV burden
‐ For detection of rifampicin resistance, we considered multi‐drug‐resistant tuberculosis burden
• To compare multiple Xpert MTB/RIF or Xpert Ultra results (repeated testing) with the initial Xpert MTB/RIF or Xpert Ultra result
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register, MEDLINE, Embase, Science Citation Index, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), Scopus, the WHO International Clinical Trials Registry Platform, ClinicalTrials.gov, and the International Standard Randomized Controlled Trials Number (ISRCTN) Registry up to 29 April 2019, without language restrictions.
Selection criteria
Randomized trials, cross‐sectional trials, and cohort studies evaluating Xpert MTB/RIF or Xpert Ultra in HIV‐positive and HIV‐negative children younger than 15 years. Reference standards comprised culture or a composite reference standard for tuberculosis and drug susceptibility testing or MTBDRplus (molecular assay for detection of Mycobacterium tuberculosis and drug resistance) for rifampicin resistance. We included studies evaluating sputum, gastric aspirate, stool, nasopharyngeal or bronchial lavage specimens (pulmonary tuberculosis), cerebrospinal fluid (tuberculous meningitis), fine needle aspirates, or surgical biopsy tissue (lymph node tuberculosis).
Data collection and analysis
Two review authors independently extracted data and assessed study quality using the Quality Assessment of Studies of Diagnostic Accuracy ‐ Revised (QUADAS‐2). For each target condition, we used the bivariate model to estimate pooled sensitivity and specificity with 95% confidence intervals (CIs). We stratified all analyses by type of reference standard. We assessed certainty of evidence using the GRADE approach.
Main results
For pulmonary tuberculosis, 299 data sets (68,544 participants) were available for analysis; for tuberculous meningitis, 10 data sets (423 participants) were available; for lymph node tuberculosis, 10 data sets (318 participants) were available; and for rifampicin resistance, 14 data sets (326 participants) were available. Thirty‐nine studies (80%) took place in countries with high tuberculosis burden. Risk of bias was low except for the reference standard domain, for which risk of bias was unclear because many studies collected only one specimen for culture.
Detection of pulmonary tuberculosis
For sputum specimens, Xpert MTB/RIF pooled sensitivity (95% CI) and specificity (95% CI) verified by culture were 64.6% (55.3% to 72.9%) (23 studies, 493 participants; moderate‐certainty evidence) and 99.0% (98.1% to 99.5%) (23 studies, 6119 participants; moderate‐certainty evidence). For other specimen types (nasopharyngeal aspirate, 4 studies; gastric aspirate, 14 studies; stool, 11 studies), Xpert MTB/RIF pooled sensitivity ranged between 45.7% and 73.0%, and pooled specificity ranged between 98.1% and 99.6%.
For sputum specimens, Xpert Ultra pooled sensitivity (95% CI) and specificity (95% CI) verified by culture were 72.8% (64.7% to 79.6%) (3 studies, 136 participants; low‐certainty evidence) and 97.5% (95.8% to 98.5%) (3 studies, 551 participants; high‐certainty evidence). For nasopharyngeal specimens, Xpert Ultra sensitivity (95% CI) and specificity (95% CI) were 45.7% (28.9% to 63.3%) and 97.5% (93.7% to 99.3%) (1 study, 195 participants).
For all specimen types, Xpert MTB/RIF and Xpert Ultra sensitivity were lower against a composite reference standard than against culture.
Detection of tuberculous meningitis
For cerebrospinal fluid, Xpert MTB/RIF pooled sensitivity and specificity, verified by culture, were 54.0% (95% CI 27.8% to 78.2%) (6 studies, 28 participants; very low‐certainty evidence) and 93.8% (95% CI 84.5% to 97.6%) (6 studies, 213 participants; low‐certainty evidence).
Detection of lymph node tuberculosis
For lymph node aspirates or biopsies, Xpert MTB/RIF pooled sensitivity and specificity, verified by culture, were 90.4% (95% CI 55.7% to 98.6%) (6 studies, 68 participants; very low‐certainty evidence) and 89.8% (95% CI 71.5% to 96.8%) (6 studies, 142 participants; low‐certainty evidence).
Detection of rifampicin resistance
Xpert MTB/RIF pooled sensitivity and specificity were 90.0% (67.6% to 97.5%) (6 studies, 20 participants; low‐certainty evidence) and 98.3% (87.7% to 99.8%) (6 studies, 203 participants; moderate‐certainty evidence).
Authors' conclusions
We found Xpert MTB/RIF sensitivity to vary by specimen type, with gastric aspirate specimens having the highest sensitivity followed by sputum and stool, and nasopharyngeal specimens the lowest; specificity in all specimens was > 98%. Compared with Xpert MTB/RIF, Xpert Ultra sensitivity in sputum was higher and specificity slightly lower. Xpert MTB/RIF was accurate for detection of rifampicin resistance. Xpert MTB/RIF was sensitive for diagnosing lymph node tuberculosis. For children with presumed tuberculous meningitis, treatment decisions should be based on the entirety of clinical information and treatment should not be withheld based solely on an Xpert MTB/RIF result. The small numbers of studies and participants, particularly for Xpert Ultra, limits our confidence in the precision of these estimates.
Plain language summary
Xpert tests for active tuberculosis in children
Why is improving the diagnosis of pulmonary tuberculosis important?
In 2018, at least one million children became ill with tuberculosis and around 200,000 died. When detected early and effectively treated, tuberculosis is largely curable. Xpert MTB/RIF and Xpert Ultra are World Health Organization‐recommended tests that simultaneously detect tuberculosis and rifampicin resistance in adults and children with tuberculosis symptoms. Rifampicin is an important anti‐tuberculosis drug. Not recognizing tuberculosis early may result in delayed diagnosis and treatment, severe illness, and death. A false tuberculosis diagnosis may result in anxiety and unnecessary treatment.
What is the aim of this review?
To determine the accuracy of tests in symptomatic children for diagnosing pulmonary tuberculosis, tuberculous meningitis, lymph node tuberculosis, and rifampicin resistance.
What was studied in this review?
Xpert MTB/RIF and Xpert Ultra, with results measured against culture and a composite reference standard (benchmarks), recognizing that neither reference is perfect in children.
What are the main results in this review?
A total of 49 studies were included. For pulmonary tuberculosis, we analysed 299 data sets including information describing nearly 70,000 children.
For a population of 1000 children:
Xpert MTB/RIF
‐ where 100 have pulmonary tuberculosis in sputum (by culture), 74 would be Xpert MTB/RIF‐positive, of whom 9 (12%) would not have tuberculosis (false‐positives); 926 would be Xpert MTB/RIF‐negative; and 35 (4%) would have tuberculosis (false‐negatives)
‐ where 100 have tuberculous meningitis (by culture), 86 would be Xpert MTB/RIF‐positive, of whom 59 (69%) would not have tuberculosis (false‐positives); 914 would be Xpert MTB/RIF‐negative; and 23 (3%) would have tuberculosis (false‐negatives)
‐ where 100 people have lymph node tuberculosis (by culture), 142 would be Xpert MTB/RIF‐positive, of whom 97 (68%) would not have lymph node tuberculosis (false‐positives); 858 would be Xpert MTB/RIF‐negative; and 5 (1%) would have lymph node TB (false‐negatives)
‐ where 100 have rifampicin resistance, 108 would have Xpert MTB/RIF‐rifampicin resistance detected, of whom 18 (17%) would not have rifampicin resistance (false‐positives); 892 would have Xpert MTB/RIF‐rifampicin resistance NOT detected; and 10 (1%) would have rifampicin resistance (false‐negatives)
Xpert Ultra
‐ where 100 have pulmonary tuberculosis in sputum (by culture), 100 would be Xpert Ultra‐positive, of whom 27 (27%) would not have tuberculosis (false‐positives); 900 would be Xpert Ultra‐negative; and 27 (3%) would have tuberculosis (false‐negatives)
How confident are we in the results of this review?
We are confident. We included many studies from different countries and settings and used two reference standards. Some studies included only children at referral centres or did not report the setting. Therefore, we could not assess how the tests would work in a primary care setting.
What children do the results of this review apply to?
Children with presumed pulmonary tuberculosis, tuberculous meningitis, lymph node tuberculosis, or rifampicin resistance.
What are the implications of this review?
The results of the review suggest Xpert tests have the potential to be used to detect tuberculosis and rifampicin resistance.
‐ The risk of missing a diagnosis of pulmonary tuberculosis confirmed by culture with Xpert MTB/RIF (in sputum) is low (4% of those whose Xpert MTB/RIF suggests they do not have tuberculosis) suggesting that only a small number of children with tuberculosis confirmed by culture will not receive treatment. The risk of wrongly diagnosing a child as having tuberculosis is slightly higher (12% of those whose Xpert MTB/RIF test suggests they do have tuberculosis). This may result in some of these children receiving unnecessary treatment.
‐ The risk of missing a diagnosis of rifampicin resistance with Xpert MTB/RIF is low (1% of those whose Xpert MTB/RIF suggests they do not have rifampicin resistance) suggesting that only a small number of children with tuberculosis will not receive the appropriate treatment. The risk of wrongly diagnosing a child as rifampicin resistance tuberculosis is higher (17% of those whose Xpert MTB/RIF test suggests they do have rifampicin resistance). This may result in some of these children receiving unnecessary treatment.
How up‐to‐date is this review?
To 29 April 2019.
Summary of findings
Background
Tuberculosis is one of the top 10 causes of death and the leading cause from a single infectious agent (above HIV/AIDS), causing an estimated 1.2 million deaths in 2018 (WHO Global TB Report 2019). Globally during that year, an estimated 10 million people developed tuberculosis disease, including around one million children younger than 14 years and 205,000 children who died of the disease (WHO Global TB Report 2019). Recent models that have been accepted and supported by the World Health Organization (WHO) suggest that there is substantial underreporting as well as under‐diagnosis of tuberculosis in children. Furthermore, tuberculosis‐associated deaths take a disproportionate toll among children: 253,000 deaths were estimated in 2016 in children younger than 15 years, accounting for 6.9% of the total deaths notified in that year; of these deaths, 80% occurred in children younger than five years of age (Dodd 2017; Jenkins 2017). Estimates suggest that most deaths among children occur in undiagnosed cases and represent a missed opportunity to start adequate treatment (Jenkins 2017).
Tuberculosis treatment for children follows the same principles as for adults, and the same drugs are used in most cases. The standard four‐drug combination regimen of isoniazid, rifampicin, pyrazinamide, and ethambutol given daily for a period of two months followed by isoniazid and rifampicin given daily for an additional four to six months is used for treatment of drug‐susceptible tuberculosis ‐ both pulmonary and extrapulmonary forms. Central nervous system tuberculosis is an exception in that treatment with isoniazid and rifampicin is extended to a total of 12 months. The recent introduction of paediatric fixed‐dose combinations with optimised dosing and taste masking has improved the efficiency of treatment (Wademan 2019). Treatment of drug‐resistant tuberculosis in children generally has better outcomes than in adults. Of note, in August 2018, the WHO released a rapid communication containing new recommendations for treatment of child tuberculosis, including the use of all‐oral regimens (Furin 2019; WHO 2018).
The diagnosis of child tuberculosis relies on a mix of clinical, epidemiological, radiological, and laboratory information. Child tuberculosis is typically paucibacillary (tuberculosis disease caused by a smaller number of bacteria), and young children cannot voluntarily produce sputum specimens (Marais 2005; Theart 2005). Hence, even under ideal clinical and laboratory conditions, only 30% to 40% of child tuberculosis cases are microbiologically confirmed (Dunn 2016). The probability of microbiological confirmation is increased in children with more severe or advanced disease (Marais 2006c; Marais 2006d). However, the diagnostic gap is perpetuated because conventional smear microscopy, which is of little value in diagnosing child tuberculosis, remains the most used and most widely available tuberculosis diagnostic method in low‐ and middle‐income countries. Further, the clinical skills and equipment needed for sputum induction and gastric aspiration are often not available in peripheral health clinics (Reid 2012). Tuberculosis culture methods have shown greater, yet highly variable, sensitivity in child tuberculosis (Chiang 2017; Frigati 2015); unfortunately, tuberculosis culture to support diagnosis is not widely available in high‐burden settings.
Xpert MTB/RIF represents a promising diagnostic modality for child tuberculosis. Since 2010, the WHO has recommended the use of Xpert MTB/RIF as the preferred initial microbiological test for people thought to have multi‐drug‐resistant tuberculosis or HIV‐associated tuberculosis (strong recommendation); this recommendation was extended to include children with presumed tuberculosis on the strength of evidence reported in adults (WHO 2011). In 2013 this guidance was updated with a recommendation specific to children, that is, that Xpert MTB/RIF should be used as the preferred initial diagnostic test for children thought to have multi‐drug‐resistant tuberculosis or HIV‐associated tuberculosis (strong recommendation; very low‐quality evidence) and as the initial diagnostic test for all children with presumptive tuberculosis (conditional recommendation acknowledging resource implications; very low‐quality evidence) (WHO 2013). At present, the WHO supports the use of Xpert MTB/RIF for diagnosis of child tuberculosis in the following four scenarios.
As the initial diagnostic test of choice, rather than conventional smear microscopy or culture (conditional recommendation acknowledging resource implications; very low‐quality evidence ‐ also called certainty of evidence).
For diagnosis in children suspected of having drug‐resistant tuberculosis or HIV‐associated tuberculosis (strong recommendation; very low‐quality evidence).
As a replacement test for culture in specific non‐respiratory specimens (lymph nodes and other tissues) for children presumed to have extrapulmonary tuberculosis (conditional recommendation; very low‐quality evidence).
As the preferred initial diagnostic in cerebrospinal fluid (CSF) for children suspected of having tuberculous meningitis (strong recommendation given the urgency of rapid diagnosis; very low‐quality evidence) (WHO 2014a).
The WHO does not currently recommend Xpert MTB/RIF for use with other specimen types such as stool. Further, existing guidelines acknowledge that all current recommendations regarding use of Xpert MTB/RIF in children rely on “very low‐certainty evidence” and are currently evolving with expansion of the use of Xpert MTB/RIF Ultra (Xpert Ultra) (WHO 2017).
A non‐inferiority analysis of Xpert Ultra compared to Xpert MTB/RIF found that Xpert Ultra has higher sensitivity than Xpert MTB/RIF, particularly in smear‐negative, culture‐positive specimens and in specimens from HIV‐positive patients. Xpert Ultra was also found to have accuracy that was at least as good as Xpert MTB/RIF for detection of rifampicin resistance. However, it was noted that Xpert Ultra may have reduced specificity in settings with high tuberculosis burden. Current WHO recommendations for the use of Xpert MTB/RIF now also apply to the use of Xpert Ultra as the initial diagnostic test for all adults and children with signs and symptoms of tuberculosis as well as to testing of selected extrapulmonary specimens (cerebrospinal fluid, lymph nodes, and tissue specimens). However, a negative test result does not exclude tuberculosis in children (WHO 2017). This systematic review estimated and compared the diagnostic accuracy of Xpert MTB/RIF and Xpert Ultra for children presumed to have pulmonary tuberculosis or specific forms of extrapulmonary tuberculosis.
Target condition being diagnosed
Tuberculosis is an infectious disease caused by bacteria within the Mycobacterium tuberculosis complex, most commonly Mycobacterium tuberculosis (M tuberculosis). Typically disseminated through the air, M tuberculosis predominantly affects the lungs, causing pulmonary tuberculosis, and less typically can cause disease in other organs of the body in extrapulmonary tuberculosis forms. Lymph node tuberculosis is the most common form of extrapulmonary tuberculosis in children, and tuberculous meningitis results in the highest morbidity and mortality. For this review, we limited evaluation of extrapulmonary tuberculosis to lymph node tuberculosis and tuberculous meningitis because other forms of extrapulmonary tuberculosis in children are less common, and because evidence evaluating Xpert (Xpert MTB/RIF and Xpert Ultra) as a diagnostic tool for other forms of extrapulmonary child tuberculosis is sparse.
The natural history of tuberculosis in children is distinct from that in adults due to more frequent progression to primary tuberculosis disease (Marais 2004). Children younger than five years are at particularly high risk of progression to tuberculous disease following infection, but the risk for older children and adolescents is also higher than in adults. Overall, it is estimated that 90% of tuberculous disease in young children occurs within one year of infection (Marais 2014). In addition to age, factors such as nutritional status, immune‐compromising conditions (e.g. HIV infection), BCG (bacillus Calmette–Guérin) vaccination status, and genetic susceptibility contribute to children’s risk of disease progression. Immediately following infection with M tuberculosis in a child, hematogenous spread (by way of the bloodstream) can occur. The period of highest risk for presentation with tuberculous meningitis and miliary tuberculosis is one to three months following primary infection. Children between six months and two years of age are at particularly high risk of these severe forms of tuberculous disease. Approximately 50% of children in this age range progress to tuberculous disease following infection, and 20% to 40% of those children will present with disseminated disease (Marais 2004; Marais 2014). Children younger than five years most commonly present with hilar lymph node forms of intrathoracic tuberculous disease. Older children and adolescents more commonly manifest adult‐type disease, including pleural tuberculosis and upper lobe consolidations (Marais 2004).
Laboratory confirmation of child tuberculosis disease is challenging for two reasons. First, child tuberculosis most commonly represents as a primary disease process, without the formation of cavities (Marais 2006a). The number of acid‐fast bacilli (the presence of acid‐fast bacilli on a sputum smear or other specimen often indicates tuberculous disease) present in forms of primary tuberculosis such as hilar lymph node or bronchial tuberculosis is substantially lower than the number present in a pulmonary cavity. Consequently, child tuberculosis is often referred to as ‘paucibacillary’, and it is more difficult to obtain the organisms needed to confirm disease via conventional smear or culture (Dunn 2016). Second, most children younger than six years of age lack the ability to expectorate sputum and are unable to voluntarily produce good‐quality specimens. Therefore, respiratory specimens are often obtained through sputum induction. As children swallow respiratory secretions, early‐morning gastric aspiration is another well‐established approach to specimen collection. In one study, the yield of three consecutive morning gastric aspirates was similar to the yield of one induced sputum specimen (Zar 2005). Nasopharyngeal aspiration for respiratory specimens is a less invasive mode of specimen collection (Zar 2012). Stool has also been studied as a child tuberculosis diagnostic specimen; although sensitivity has been lower than with traditional specimens, this specimen has great appeal because collection is non‐invasive and requires no training (Nicol 2013). Because laboratory diagnostics for tuberculosis perform poorly in children, algorithms involving signs, symptoms, tuberculosis exposure, HIV status, laboratory tests, and radiographic findings are commonly used to make a clinical diagnosis of child tuberculosis. However, these algorithms have been shown to perform differently across settings, and their sensitivity and specificity may be site‐specific (David 2017).
Index test(s)
The index tests in this review are Xpert MTB/RIF and Xpert Ultra (Cepheid Inc, Sunnyvale, CA, USA). Xpert MTB/RIF and Xpert Ultra are nucleic acid amplification tests (NAATs) that function as an automated, closed system that performs real‐time polymerase chain reaction (PCR). Specimens are processed using Xpert Sample Reagent and are incubated for 15 minutes, after which the processed samples are pipetted into the cartridge. These tests can be run by operators (such as laboratory technicians and nurses) with minimal technical expertise. Within two hours, the tests detect both live and dead M tuberculosis complex DNA and simultaneously recognize mutations in the M tuberculosis gene encoding the beta subunit of the RNA polymerase (rpoB) gene, which is the most common site of M tuberculosis mutations leading to rifampicin resistance. Xpert MTB/RIF and Xpert Ultra require an uninterrupted and stable electrical power supply, temperature control, and yearly calibration of the cartridge modules (WHO 2014b). The WHO has published extensive guidance and practical information on implementing the test (WHO 2014b).
There have been five generations of the cartridge: G1, G2, G3, G4, and Xpert Ultra. G1 to G4 cartridges initially improved the detection of tuberculosis and rifampicin resistance, but Xpert MTB/RIF sensitivity was still suboptimal in people with smear‐negative (which is often seen in children) and HIV‐associated tuberculosis. Xpert Ultra was developed in part to overcome this limitation and improve test sensitivity. There are limited data on the different sensitivity that Xpert Ultra offers as compared to the G4 cartridge; however, existing data suggest it may offer improved sensitivity for tuberculosis detection in hard‐to‐diagnose populations such as children, people living with HIV, and individuals with extrapulmonary tuberculosis (Dorman 2018; WHO 2017). To improve detection of M tuberculosis, Xpert Ultra incorporates two different multi‐copy amplification targets (IS6110 and IS1081). These revisions resulted in an approximately 1–log improvement in the lower limit of detection compared with Xpert MTB/RIF, including improved differentiation of certain silent mutations, improved detection of rifampicin resistance in mixed infections, and avoidance of false‐positive results for detection of rifampicin resistance in paucibacillary specimens (Chakravorty 2017). As mentioned above, Xpert Ultra also has decreased specificity compared to G4 and may be more likely to identify M tuberculosis DNA from prior episodes of tuberculosis disease, particularly in patients classified in the new ‘trace' category (Dorman 2018). Trace call corresponds to the lowest bacillary burden for M tuberculosis detection, as described below (WHO 2017). This Cochrane Review includes studies that used any of the Xpert generations in the diagnosis of tuberculosis (pulmonary tuberculosis, tuberculous meningitis, and lymph node tuberculosis) in children younger than 15 years.
Clinical pathway
Figure 1 presents an example of the clinical pathway and placement of the index tests. A careful clinical history of tuberculosis exposure and symptoms is the first step in the diagnostic pathway for child tuberculosis. Children with household or other close and persistent exposure to a person with tuberculosis are at increased risk of tuberculosis infection and resultant progression to tuberculous disease. All children with recent exposure to tuberculosis must be evaluated for clinical symptoms and for examination findings consistent with tuberculous disease. Additional testing depends on the context but may include chest radiography and a test of tuberculosis infection. Symptoms of tuberculosis disease are generally persistent for longer than two weeks and are unremitting (Marais 2005). The most common symptoms are cough, fever, decreased appetite, weight loss or failure to thrive, and fatigue or reduced playfulness. Symptoms of extrapulmonary tuberculosis are typically localized, and diagnostic findings are generally obtained from the site of disease (Figure 1). However, no symptom‐based diagnostic algorithms have been validated or have been shown to be reliable in multiple contexts. Symptom‐based diagnostic algorithms tend to perform poorly in children younger than three years and in HIV‐positive children ‐ two populations at high risk for disease progression (Marais 2006b).
1.

AFB: acid‐fast bacilli; CT: computed tomography; LAM: mycobacterial lipoarabinomannan antigen; MRI: magnetic resonance imaging; NAAT: nucleic acid amplification test; TB: tuberculosis; TST: tuberculin skin test.
The Clinical Pathway. Clinical suspicion of tuberculosis includes persistent cough, fever, weight loss or failure to thrive, lymphadenitis, irritability, lethargy, headache, vomiting or neurological symptoms, history of possible or confirmed exposure to M tuberculosis, increased risk for tuberculosis disease due to immunocompromising conditions. 1Availability of investigations and tests may be different in high‐ and low‐resource settings and may influence the approach to the diagnosis of child tuberculosis. 2Non‐microbiological confirmation of M tuberculosis does not exclude tuberculosis disease in children; therefore initiation of treatment should be considered empirically if other clinical indications are present. 3Mycobacterial culture results are rarely timely to aid the decision to initiate treatment but can confirm or refute clinical decision‐making if positive.
Unfortunately, no clinical examination features are specific to pulmonary tuberculosis in children. However, examination findings in extrapulmonary tuberculosis can be quite specific when identified. Clinicians should consider medical comorbidities that increase the risk for tuberculous disease and should modify diagnostic algorithms accordingly. HIV infection not only significantly increases risk of tuberculosis in the paediatric population, it also raises the risk of increased disease severity. HIV‐positive children, especially before effective antiretroviral therapy is established, often present with advanced tuberculosis such as disseminated disease and have high levels of immunosuppression, further complicating diagnosis and management.
Additional diagnostic imaging studies can assist in the diagnosis of pulmonary tuberculosis and nearly all forms of extrapulmonary tuberculosis. Tests of tuberculous infection, such as interferon gamma release assays or tuberculin skin tests, can also aid in establishing the probability of tuberculosis in a child but are not necessary to make the diagnosis. Diagnostic recommendations strongly suggest collecting appropriate specimens from suspected sites of involvement in both pulmonary and extrapulmonary tuberculosis for microbiological examination. The preferred specimen in pulmonary tuberculosis is sputum; however in young children who cannot expectorate, the specimen is commonly obtained via a gastric aspirate or induced sputum, and stool is increasingly used. To diagnose extrapulmonary tuberculosis, collection of samples targets the affected site of disease.
The purpose of Xpert MTB/RIF and Xpert Ultra testing is diagnosis of pulmonary and extrapulmonary tuberculosis and detection of rifampicin resistance. Results of Xpert can be used as a decision‐making tool in the following ways.
M tuberculosis detected/rifampicin resistance not detected: child would start treatment for drug‐sensitive tuberculosis.
M tuberculosis detected/rifampicin resistance detected: child would need further resistance testing and would start treatment for drug‐resistant tuberculosis according to country guidelines.
M tuberculosis not detected: a negative Xpert result does not rule out tuberculous disease; therefore, clinicians should still consider initiation of tuberculosis treatment in children with history and clinical or radiological features suggestive of tuberculosis disease despite a negative Xpert result. A negative Xpert result may also represent a true‐negative.
Possible consequences of a false‐positive and a false‐negative result may include the following.
False‐positives (FPs): children and their families would likely experience anxiety and morbidity caused by additional testing, unnecessary treatment, and possible adverse effects; as well as missed time at school, possible stigma associated with tuberculosis or a diagnosis of drug‐resistant tuberculosis, and the chance that a false‐positive may halt further diagnostic evaluation for other causes of illness. Families also experience unnecessary expense.
False‐negatives (FNs): would imply increased risk of morbidity and mortality and delayed start of treatment.
Alternative test(s)
Alternative approaches to Xpert for diagnosis of tuberculosis are still used extensively globally. Main tests include examination of smears for acid‐fast bacilli (tuberculosis bacteria) under a microscope (light microscopy, using the classical Ziehl‐Neelsen staining technique), fluorescence microscopy, and light‐emitting diode (LED)‐based fluorescence microscopy. The sensitivity of smear microscopy ranges from 0% to 10% in children (Kunkel 2016). Examination of histology specimens under a microscope following a tissue biopsy targets finding acid‐fast bacilli and granulomatous inflammation, frequently with caseous necrosis (necrotizing granulomas); however these options are seldom pursued to diagnose child tuberculosis in low‐resource settings due to the invasive nature of the procedures and the technical expertise required. Lipoarabinomannan (LAM) antigen is a lipopolysaccharide present in the mycobacterial cell wall that can be detected in the urine of people with tuberculous disease (Bjerrum 2019). This urine test offers potential advantages over sputum‐based testing due to ease of sample collection. The accuracy of urinary LAM detection is improved among people living with HIV with advanced immunosuppression (Bjerrum 2019;Nicol 2014; Shah 2016b). In two randomized trials, the use of lateral flow urine lipoarabinomannan assay (LF‐LAM) in HIV‐positive adult inpatients was shown to reduce mortality (Gupta‐Wright 2018; Peter 2016). Based on evidence from randomized trials and from a Cochrane Review (Bjerrum 2019), the WHO recommends that LF‐LAM (Alere Determine™ TB LAM Ag, Alere Inc, Waltham, MA, USA) was the only product available at the time of this recommendation and should be used to assist in the diagnosis of active tuberculosis in HIV‐positive adults, adolescents, and children. The full recommendations, which differ for inpatients and outpatients, are described at WHO Lateral flow LAM 2019. However, the evidence for LF‐LAM in children is limited and is primarily extrapolated from adults. A new urine‐based, point‐of‐care LAM test, Fujifilm SILVAMP TB LAM (FujiLAM, co‐developed by FIND, Geneva, Switzerland, and Fujifilm, Tokyo, Japan), for diagnosis of tuberculosis, is currently under investigation and has the potential to increase sensitivity in children (Broger 2019).
The quest for novel and more efficient technologies for diagnosis of tuberculosis is a cornerstone of current efforts to reduce the burden of disease worldwide. Over the past decade, unprecedented activity has focused on the development of new tools for diagnosis of extrapulmonary tuberculosis, largely supported by the engagement of global agencies. As a result, a strong pipeline of new tools for diagnosis of tuberculosis will complement the use of existing ones and will offer improved options (Boyle 2017).
Rationale
Timely and reliable diagnosis of tuberculosis in children remains challenging due to both difficulties in collecting sputum samples and the paucibacillary nature of the disease. As a result, undiagnosed cases of disease increase morbidity, mortality, and disease transmission in this key group.
We are aware of two systematic reviews that determined the diagnostic accuracy of Xpert MTB/RIF for tuberculosis in children. Wang 2015 (literature searched up to 28 October 2014) included 11 studies (3801 children) and found that Xpert MTB/RIF had pooled sensitivity and specificity (95% confidence interval) of 65% (61% to 69%) and 99% (98% to 99%) against a culture reference standard. Detjen 2015 (literature searched up to 6 January 2015) included 15 studies (3640 children). Similar to Wang 2015, Detjen 2015 found that sputum Xpert MTB/RIF had pooled sensitivity and specificity (95% credible interval) of 62% (51% to 73%) and 98% (97% to 99%) against culture.
In 2013, informed in part by the Detjen 2015 review, the WHO recommended the use of Xpert MTB/RIF for children as a front‐line test for diagnosis. In preparation for a WHO meeting to update recommendations on the use of molecular tests for active tuberculosis, we performed a Cochrane Review to update the literature, assess the accuracy of both Xpert MTB/RIF and Xpert Ultra, and address limitations noted in the prior reviews, in particular, the small number of included studies and the predominance of hospitalised children.
Objectives
Primary objectives
-
To determine the diagnostic accuracy of Xpert MTB/RIF and Xpert Ultra for (a) pulmonary tuberculosis in children presumed to have tuberculosis; (b) tuberculous meningitis in children presumed to have tuberculosis; (c) lymph node tuberculosis in children presumed to have tuberculosis; and (d) rifampicin resistance in children presumed to have tuberculosis
For tuberculosis detection, index tests were used as the initial test, replacing standard practice (i.e. smear microscopy or culture)
For detection of rifampicin resistance, index tests replaced culture‐based drug susceptibility testing as the initial test
Secondary objectives
To compare the accuracy of Xpert MTB/RIF and Xpert Ultra for each of the four target conditions
-
To investigate potential sources of heterogeneity in accuracy estimates
For tuberculosis detection, we considered age, disease severity, smear‐test status, HIV status, clinical setting, specimen type, high tuberculosis burden, and high tuberculosis/HIV burden
For detection of rifampicin resistance, we considered multi‐drug‐resistant tuberculosis burden
To compare multiple Xpert MTB/RIF or Xpert Ultra results (repeated testing) with the initial Xpert MTB/RIF or Xpert Ultra result
Methods
Criteria for considering studies for this review
Types of studies
We included cross‐sectional studies, cohort studies, and randomized controlled trials from all settings. We included randomized controlled trials that evaluated use of the test for patient health outcomes but also reported sensitivity and specificity. Although the study was a randomized trial for the purpose of determining the impact of the test versus a comparator (e.g. usual practice, another test) on health outcomes, the study design was a cross‐sectional design for the purpose of determining diagnostic accuracy for the index tests in this review. We included only studies from which we could extract or derive data on the index test being a true‐positive, a false‐positive, a true‐negative, or a false‐negative as measured against the reference standards specified below. We excluded case‐control studies and case reports. We used abstracts to identify published studies and included those that met the inclusion criteria.
Participants
We included studies that evaluated the index tests for pulmonary or extrapulmonary tuberculosis in HIV‐positive and HIV‐negative children aged 0 to 14 years presumed to have tuberculosis. Studies were eligible for inclusion if they described the use of Xpert MTB/RIF or Xpert Ultra on routine respiratory specimens such as expectorated or induced sputum and gastric and nasopharyngeal specimens. Gastric specimens could be obtained via gastric aspiration, lavage, or washing as described by study authors. We included studies that evaluated bronchoalveolar lavage specimens. In addition, we included studies evaluating stool specimens because tuberculosis bacilli are present in swallowed sputum and are recoverable from stool samples using Xpert MTB/RIF or Xpert Ultra. We also included studies that assessed several different specimen types.
Index tests
The index tests were Xpert MTB/RIF and Xpert Ultra.
Index test results are automatically generated, and the user is provided with a printable test result as follows.
MTB (M tuberculosis) DETECTED; Rif (rifampicin) resistance DETECTED.
MTB DETECTED; Rif resistance NOT DETECTED.
MTB DETECTED; Rif resistance INDETERMINATE.
MTB NOT DETECTED.
INVALID (the presence or absence of MTB cannot be determined).
ERROR (the presence or absence of MTB cannot be determined).
NO RESULT (the presence or absence of MTB cannot be determined).
Xpert Ultra incorporates a semi‐quantitative classification for results: trace, very low, low, moderate, and high. ‘Trace' corresponds to the lowest bacterial burden for detection of M tuberculosis (Chakravorty 2017). Although no rifampicin resistance result will be available for patients with trace results, a trace‐positive result is sufficient to initiate anti‐tuberculosis therapy in children or HIV‐positive patients, according to the WHO report (WHO 2017). Hence, we considered a trace result to mean M tuberculosis DETECTED.
Target conditions
The target conditions were active pulmonary tuberculosis; two forms of extrapulmonary tuberculosis ‐ lymph node tuberculosis and tuberculous meningitis; and rifampicin resistance.
Reference standards
For detection of pulmonary tuberculosis, tuberculous meningitis, and lymph node tuberculosis, we included two reference standards.
Culture: tuberculosis was defined as a positive culture on solid or liquid medium.
-
Composite reference standard: tuberculosis was defined as a positive culture or a clinical decision, based on clinical features, to initiate treatment for tuberculosis (i.e. clinically diagnosed tuberculosis). Clinical features might include cough longer than two weeks, fever, or weight loss; pneumonia that did not improve with antibiotics; or a history of close contact with an adult who had tuberculosis.
In the absence of information on tuberculosis treatment, for the composite reference standard, we accepted a study‐specific definition (i.e. a standardized definition of tuberculosis defined by the primary study authors), if available.
-
For the composite reference standard, when information about tuberculosis treatment was not available, we accepted the uniform research definition (Graham 2012; Graham 2015). In these situations, using the older definition (Graham 2012), we defined tuberculosis as:
confirmed, probable, and possible cases; and
non‐tuberculosis.
-
For the newer definition (Graham 2015), we used the categories tuberculosis confirmed and not confirmed.
In cases where a study‐specific definition for the composite references standard was applied, this was accepted as well.
A child was considered as ‘not tuberculosis' if the culture result was negative. In the absence of a culture result, a child was considered ‘not tuberculosis’ if an alternative diagnosis was established, his or her symptoms resolved without tuberculosis treatment, or he or she did not progress to tuberculosis disease after at least one month.
Children with unconfirmed tuberculosis were included among the true‐negative population when evaluated against a culture reference standard. In contrast, children who were not treated for tuberculosis, or who did not meet the study research definition for tuberculosis, were included in the true‐negative population when evaluated against a composite reference standard.
Regarding stool specimens (used for the diagnosis of pulmonary tuberculosis), we defined the reference standard as in MacLean 2019: (1) culture, or (2) Xpert MTB/RIF or Xpert Ultra performed on a routine respiratory specimen, such as sputum or gastric aspirate specimen. Stool Xpert MTB/RIF and stool Xpert Ultra were not included in the definition of the reference standard. In addition, none of the included studies used stool culture to verify pulmonary tuberculosis. For these reasons, we thought bias due to incorporation of the index test was unlikely. Hence, tuberculosis was defined as a positive culture or a positive Xpert MTB/RIF or a positive Xpert Ultra on a routine respiratory specimen.
Regarding stool specimens, we also included a composite reference standard as defined above.
Culture is generally considered the best reference standard for tuberculosis diagnosis. However, particularly in children with paucibacillary disease, tuberculosis is verified by culture in only 15% to 50% of cases, depending on disease severity, challenges of obtaining specimens, and resources (Graham 2015). Evaluation of multiple specimens, of the same or different types, may increase the yield of culture for confirming tuberculosis (Cruz 2012;Zar 2012). Therefore, we considered a higher‐quality reference standard to be one in which more than one specimen was used to confirm tuberculosis. We considered a lower‐quality reference standard to be one in which only one specimen was used for tuberculosis diagnosis. We reflected these considerations in the Quality Assessment of Studies of Diagnostic Accuracy ‐ Revised (QUADAS‐2), Reference Standard domain.
For rifampicin resistance, the reference standards were phenotypic drug susceptibility testing and MTBDRplus. MTBDRplus is a molecular line probe assay designed to detect the presence of multiple mutations causing resistance to isoniazid and rifampicin.
Search methods for identification of studies
We attempted to identify all relevant studies regardless of language or publication status (published, unpublished, in press, and in progress).
Electronic searches
We searched the following databases up to 29 April 2019 using the search terms and strategy described in Appendix 1.
Cochrane Infectious Diseases Group Specialized Register.
MEDLINE (OVID, from 1966).
Embase (OVID, from 1974).
Cumulative Index to Nursing and Allied Health Literature (CINAHL (EBSCOHost), from 1982).
Science Citation Index ‐ Expanded (from 1900), Conference Proceedings Citation Index ‐ Science (CPCI‐S, from 1990), from the Web of Science (Clarivate Analytics).
Scopus (Elsevier, from 1970).
We also searched ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP; www.who.int/trialsearch), and the International Standard Randomized Controlled Trials Number (ISRCTN) Registry (www.isrctn.com/) for trials in progress, up to 28 January 2020.
Searching other resources
We contacted researchers and experts in the field to identify additional eligible studies. We checked the references of relevant reviews and studies to identify additional studies.
Data collection and analysis
Selection of studies
Two review authors independently screened all titles and abstracts to identify potentially eligible studies. We then obtained the full‐text articles of potentially eligible studies, and two review authors independently assessed whether they should be included based on pre‐defined inclusion and exclusion criteria. We resolved disagreements by discussion or by consultation with a third review author if necessary. We contacted study authors for clarification of methods and other information as needed. We recorded and summarized reasons for excluding studies in a Characteristics of excluded studies table. We illustrated the study selection process in a PRISMA diagram (Moher 2009).
Data extraction and management
We designed a data extraction form and piloted it on two included studies (Appendix 2); we then finalized the form based on the pilot test. As above, two review authors independently extracted data using this data extraction form and discussed inconsistencies to achieve consensus. We consulted a third review author to resolve discrepancies as needed. We entered abstracted data into an Excel database on password‐protected computers (Excel 2013). We secured the data set in a cloud storage workspace (Dropbox), and we stored extracted data for future review updates. Selected details of data extraction are listed below.
Study details
Number of participants after screening for exclusion and inclusion criteria
Total number of children included in the analysis
Total number of specimens included with collection methods
Unit of sample collection: one specimen, multiple specimens, unknown, or unclear
Percentage (numerator/denominator) of children with prior tuberculosis
Target condition(s)? ‐ pulmonary tuberculosis, lymph node tuberculosis, tuberculous meningitis, rifampicin resistance
Patient characteristics and setting
Description of study population
Age: median, mean, range, and disaggregation into categories (0 to 4, 5 to 14)
Gender
HIV status
Smear status
Percentage and number of HIV‐positive or HIV‐negative participants, if both were included in the study
Type of respiratory specimen included: expectorated, induced, nasopharyngeal aspirate, gastric lavage, stool
Type of non‐respiratory specimen included: fine needle aspirate, lymph node biopsy, cerebrospinal fluid, multiple types, other, unknown
Number of cultures performed per child to exclude tuberculosis
Data on culture performance: number of contaminated cultures with respect to total cultures performed
Clinical setting: outpatient or inpatient or both
Description of radiographic findings
Information on tuberculosis burden in the country
We classified ‘country' as being high burden or not high burden for tuberculosis, tuberculosis/HIV, or multi‐drug‐resistant tuberculosis according to the WHO post‐2015 era classification (WHO Global TB Report 2019). A country could be classified as high burden for one, two, or all three of the high‐burden categories.
Index tests
Xpert cartridge: MTB/RIF or Ultra
Pretreatment processing procedure for specimens used for Xpert MTB/RIF or Xpert Ultra
Specimen condition: fresh, frozen, or both
Numbers of true‐positives, false‐positives, false‐negatives, and true‐negatives (see example table in Appendix 3)
Uninterpretable results for tuberculosis detection (invalid, error, or no result)
Indeterminate results for detection of rifampicin resistance
Reference standards
Details of culture ‐ solid or liquid
Composite reference standard ‐ description
Rifampicin resistance ‐ phenotypic drug susceptibility testing or MTBDRplus
For each target condition and specimen type, we considered one index test result per child. Hence, the primary unit of analysis was the patient. If studies evaluated more than one specimen type, we extracted data for each specimen. Hence, a study may have contributed more than one 2 × 2 table (data set) ‐ one for each type of specimen evaluated.
Assessment of methodological quality
We assessed the methodological quality of included studies using the QUADAS‐2 instrument, which we adapted for this review (Whiting 2011). The QUADAS‐2 tool consists of four domains: (1) patient selection, (2) index test(s), (3) reference standard(s), and (4) flow and timing. All domains are assessed for risk of bias, and the first three domains for concerns regarding applicability. We first developed guidance on how to appraise each signalling question within the domains and how to make the overall judgement for each domain. One review author piloted the tool with two of the included studies. We finalized the guidance based on experience gained from the pilot. The QUADAS‐2 tool with signalling questions tailored to this review is provided in Appendix 4. Two review authors independently completed QUADAS‐2. We resolved disagreements through discussion or by arbitration with a third review author when necessary. We have presented results of the quality assessment in the text, in tables, and in graphs.
Statistical analysis and data synthesis
We performed descriptive analyses of the included studies and presented their key characteristics in the Characteristics of included studies table. We presented individual study estimates of sensitivity and specificity graphically in forest plots and in receiver operating characteristics (ROC) space using Review Manager 5 (Review Manager 2014).
For detection of rifampicin resistance, we included children who:
were culture‐positive;
had a valid phenotypic drug susceptibility test (DST) result;
were Xpert tuberculosis‐positive; and
had a valid Xpert rifampicin result.
Sensitivity = Xpert rifampicin resistant/phenotypic or MTBDRplus DST rifampicin resistant. Specificity = Xpert rifampicin susceptible/phenotypic MTBDRplus DST rifampicin susceptible.
When data were sufficient, we performed meta‐analyses to estimate average sensitivities and specificities using a bivariate model (Chu 2006; Reitsma 2015). We used the bivariate model because the index tests, Xpert MTB/RIF, and Xpert Ultra all apply a common positivity criterion (Macaskill 2010). When we were unable to fit a bivariate model due to sparse data, few studies, or little observed variability in specificity, we simplified the model to a univariate random‐effects logistic regression model to pool sensitivity and specificity separately (Takwoingi 2015). For the analysis of Xpert MTB/RIF in the subgroup of smear‐positive children, we performed a univariate analysis of only sensitivity. We did this because studies or subgroups of smear‐positive children had few or zero false‐positives and true‐negatives; thus pooling specificity was not meaningful. We performed meta‐analyses using the meqrlogit command in Stata version 15 (Stata 15). We stratified all analyses by type of reference standard. For rifampicin resistance detection, we identified few studies evaluating Xpert MTB/RIF and zero studies evaluating Xpert Ultra. Therefore, we analysed all specimen types together.
We performed comparative meta‐analyses by restricting the analyses to only those studies that made direct comparisons between Xpert MTB/RIF and Xpert Ultra within the same participants. We performed comparative meta‐analyses using meta‐regression by including test type as a covariate in a bivariate model. We assessed model fit using likelihood ratio tests to compare models with and without the covariate terms. We calculated absolute differences in sensitivity and specificity using the bivariate model parameters. We obtained 95% confidence intervals and P values for the absolute differences using the delta method and Wald tests, respectively. We performed additional comparative analyses in which we compared the accuracy of Xpert MTB/RIF and Xpert Ultra for pulmonary tuberculosis on repeated testing versus a first test.
Approach to uninterpretable index test results
Xpert MTB/RIF and Xpert Ultra report an uninterpretable test result for unexpected results with any of the internal control measures of the assay. The uninterpretable rate for detection of tuberculosis is the number of tests classified as "invalid", "error", or "no result" divided by the total number of Xpert tests performed. The indeterminate rate for detection of rifampicin resistance is the number of tests classified as "MTB DETECTED; Rif resistance INDETERMINATE" divided by the total number of Xpert‐positive results. We had planned to estimate the pooled proportion of uninterpretable Xpert MTB/RIF and Xpert Ultra results for tuberculosis detection and indeterminate Xpert MTB/RIF and Xpert Ultra results for detection of rifampicin resistance. We were unable to perform the analyses owing to limited data, but we have summarized these results in a table along with the key characteristics of included studies.
Investigations of heterogeneity
We visually inspected forest and summary ROC plots for heterogeneity. When data allowed, we evaluated sources of heterogeneity using subgroup analyses and meta‐regression. We were interested in tests performed in key subgroups of children: age zero to four years, age 5 to 14 years, smear‐positive, smear‐negative, HIV‐positive, and HIV‐negative. For tuberculosis detection, we performed bivariate meta‐regression with the following potential sources of heterogeneity as a single covariate in the model.
High tuberculosis burden: yes or no.
High tuberculosis/HIV burden: yes or no.
Cultures used to verify tuberculosis: multiple or single.
Detection of rifampicin resistance
We had planned to perform bivariate meta‐regression while considering high multi‐drug‐resistant tuberculosis burden as a potential source of heterogeneity, but we were unable to perform this analysis owing to limited data.
Sensitivity analyses
Data were sufficient for sensitivity analyses to explore the effects of risk of bias items and study characteristics on pooled estimates of the accuracy of Xpert MTB/RIF. Such analyses were possible only for studies that used sputum as the specimen. We limited the meta‐analyses to the following.
Studies that used consecutive or random selection of participants.
Studies in which the reference standard results were interpreted without knowledge of the index test results.
Studies that included only untreated patients.
Studies that explicitly reported enrolling children 0 to 14 years old.
In addition, for studies in which gastric aspirate specimens and sputum specimens were collected, data were included with the sputum analyses within the main analyses; so we performed a sensitivity analysis excluding these studies.
Assessment of reporting bias
We did not formally assess reporting bias using funnel plots or regression tests because these have not been reported as helpful for diagnostic test accuracy studies (Macaskill 2010).
Assessment of certainty of the evidence
We assessed certainty of the evidence by using the GRADE approach for diagnostic studies (Balshem 2011; Schünemann 2008; Schünemann 2016). As recommended, we rated certainty of the evidence as high (not downgraded), moderate (downgraded by one level), low (downgraded by two levels), or very low (downgraded by more than two levels) based on five domains: risk of bias, indirectness, inconsistency, imprecision, and publication bias. For each outcome, certainty of the evidence started as high when high‐quality studies (cross‐sectional or cohort studies) enrolled participants with diagnostic uncertainty. If we found a reason for downgrading, we used our judgement to classify the reason as serious (downgraded by one level) or very serious (downgraded by two levels).
Three review authors (AWK, LGF, and KRS) discussed judgements and applied GRADE in the following way (Schünemann 2020a; Schünemann 2020b).
Risk of bias
We used QUADAS‐2 to assess risk of bias.
Indirectness
We assessed indirectness in relation to the population (including disease spectrum), setting, interventions, and outcomes (accuracy measures). We also used prevalence as a guide to whether there was indirectness in the population.
Inconsistency
GRADE recommends downgrading for unexplained inconsistency in sensitivity and specificity estimates. We carried out pre‐specified analyses to investigate potential sources of heterogeneity and downgraded when we could not explain inconsistency in accuracy estimates.
Imprecision
We considered a precise estimate to be one that would allow a clinically meaningful decision. We considered the width of the confidence interval (CI) and asked, “Would we make a different decision if the lower or upper boundary of the CI represented the truth?” In addition, we worked out projected ranges for true‐positive (TP), false‐negative (FN), true‐negative (TN), and false‐positive (FP) for a given prevalence of tuberculosis and made judgements on imprecision from these calculations.
Publication bias
We rated publication bias as undetected (not serious) for several reasons including comprehensiveness of the literature search and extensive outreach to tuberculosis researchers to identify studies.
Results
Results of the search
We identified 2174 records through database searches conducted up to 29 April 2019 and one additional record identified through other sources. After excluding duplicate records, we scrutinized the titles and abstracts of 835 records and excluded 701 records for relevance. We retrieved 134 articles and, after full‐text review, included 49 studies in the review (Anderson 2014; Andriyoko 2019; Atwebembeire 2016; Bacha 2017; Bates 2013; Bhatia 2016; Bholla 2016; Brent 2017; Bunyasi 2015; Causse 2011; Chipinduro 2017; Chisti 2014; Coetzee 2014; Das 2019; Gous 2015; Hanrahan 2018; Hasan 2017; Kasa Tom 2018; Kim 2015; LaCourse 2014; LaCourse 2018; Ligthelm 2011; Malbruny 2011; Marcy 2016; Moussa 2016; Myo 2018; Nhu 2013; Nicol 2011; Nicol 2013; Nicol 2018; Orikiriza 2018; Pang 2014; Rachow 2012; Reither 2015; Sabi 2018; Saini 2018; Sekadde 2013; Singh M 2016; Solomons 2015; Togun 2015; Tortoli 2012; Vadwai 2011; Walters 2014; Walters 2017a; Walters 2018a; Yin 2014; Zar 2012; Zar 2013; Zar 2019) See Characteristics of included studies. All studies were written in English. Figure 2 shows the flow of studies in the review. We recorded the excluded studies and reasons for their exclusion in the Characteristics of excluded studies table.
2.

Study flow diagram.
The 49 studies included one randomized trial, 20 cohort studies, and 28 cross‐sectional studies. Thirty‐nine studies (80%) took place in countries with high tuberculosis burden and 39 (80%) in countries with high TB/HIV burden. Most studies (30/49; 61%) used liquid culture for the reference standard (Table 5).
1. Key characteristics of included studies.
| Study | Index test | Reference standard | Study design | HIV status | Clinical setting | High TB burden | Specimens | Uninterpretable results for tuberculosis detection |
| Anderson 2014 | Xpert MTB/RIF | Culture, Composite | Cohort | Both | Both | Yes | Sputum | NR |
| Andriyoko 2019 | Xpert MTB/RIF | Culture | Cross‐sectional | NR | NR | Yes | Gastric aspirate specimen, stool, sputum | 6/40 (15%) stool, induced sputum or gastric aspirate specimen 1/30 (3%) |
| Atwebembeire 2016 | Xpert MTB/RIF | Culture | Cross‐sectional | Both | NR | No | Sputum | NR |
| Bacha 2017 | Xpert MTB/RIF | Culture, Composite | Cohort | Both | Both | Yes | Sputum | 3/455 (0.6%) |
| Bates 2013 | Xpert MTB/RIF | Culture | Cross‐sectional | Both | Inpatient | Yes | Sputum, gastric aspirate specimen | 17/930 (1.8%) |
| Bhatia 2016 | Xpert MTB/RIF | Culture, Composite | Cross‐sectional | NR | Inpatient | Yes | Cerebrospinal fluid | NR |
| Bholla 2016 | Xpert MTB/RIF | Culture | Cohort | Both | NR | Yes | Lymph node specimen | 0/70 (0%) |
| Brent 2017 | Xpert MTB/RIF | Culture, Composite | Cohort | Both | NR | Yes | Sputum | NR |
| Bunyasi 2015 | Xpert MTB/RIF | Culture | Randomized trial | HIV‐ | Inpatient | Yes | Sputum, gastric aspirate specimen | 47/4856 (0.9%) |
| Causse 2011 | Xpert MTB/RIF | Culture | Cross‐sectional | HIV‐ | Laboratory | No | Gastric aspirate specimen, cerebrospinal fluid | NR |
| Chipinduro 2017 | Xpert MTB/RIF | Culture, Composite | Cross‐sectional | Both | Outpatient | Yes | Sputum, stool | 1/218 (0.4%) induced sputum, 0/218 stool |
| Chisti 2014 | Xpert MTB/RIF | Culture, Composite | Cross‐sectional | NR | Inpatient | Yes | Gastric aspirate specimen, sputum | NR |
| Coetzee 2014 | Xpert MTB/RIF | Culture | Cross‐sectional | Both | Both | Yes | Lymph node | 1/110 (0.9%) |
| Das 2019 | Xpert MTB/RIF | Culture | Cross‐sectional | Both | Both | Yes | Gastric aspirate specimen, cerebrospinal fluid, lymph node, sputum | 2/181 (1%) |
| Gous 2015 | Xpert MTB/RIF | Culture | Cross‐sectional | NR | NR | Yes | Sputum | 27/467 (5.6%) |
| Hanrahan 2018 | Xpert MTB/RIF | Culture | Cohort | Both | Outpatient | Yes | Gastric aspirate specimen, nasopharyngeal specimen, sputum, stool | 15/114 (13%) stool, 1/57 (1.7%) gastric, 1/103 (.9%) IS |
| Hasan 2017 | Xpert MTB/RIF | Culture, Composite | Cross‐sectional | HIV‐ | Both | Yes | Gastric aspirate specimen, stool, sputum | NR |
| Kasa Tom 2018 | Xpert MTB/RIF | Composite | Cohort | Both | Inpatient | Yes | Gastric aspirate specimen, sputum | NR |
| Kim 2015 | Xpert MTB/RIF | Culture | Cross‐sectional | NR | Inpatient | No | Lymph node specimen | NR |
| LaCourse 2014 | Xpert MTB/RIF | Culture, Composite | Cross‐sectional | Both | Inpatient | No | Sputum | 0/300 (0%) |
| LaCourse 2018 | Xpert MTB/RIF | Culture, Composite | Cohort | HIV+ | Inpatient | Yes | Gastric aspirate specimen, sputum, stool | 2/150 (1.3%) |
| Ligthelm 2011 | Xpert MTB/RIF | Culture | Cross‐sectional | NR | Both | Yes | Lymph node specimen | 0/2 (0%) |
| Malbruny 2011 | Xpert MTB/RIF | Culture | Cross‐sectional | NR | Laboratory | No | Gastric aspirate specimen, cerebrospinal fluid, lymph node, sputum | NR |
| Marcy 2016 | Xpert MTB/RIF | Culture | Cohort | HIV+ | NR | Yes | Gastric aspirate specimen, nasopharyngeal specimen, sputum, stool | NR |
| Moussa 2016 | Xpert MTB/RIF | Culture, Composite | Cohort | HIV‐ | NR | No | Sputum, stool | 1/230 (.4%) |
| Myo 2018 | Xpert MTB/RIF | Culture, Composite | Cross‐sectional | Both | Inpatient | Yes | Gastric aspirate specimen | 4/231 (1.7%) |
| Nhu 2013 | Xpert MTB/RIF | Culture, Composite | Cross‐sectional | Both | Inpatient | Yes | Gastric aspirate specimen, sputum, cerebrospinal fluid | NR |
| Nicol 2011 | Xpert MTB/RIF | Culture, Composite | Cross‐sectional | Both | Inpatient | Yes | Sputum | NR |
| Nicol 2013 | Xpert MTB/RIF | Culture, Composite | Cohort | Both | Both | Yes | Sputum, stool | NR |
| Nicol 2018 | Xpert MTB/RIF and Xpert Ultra | Culture, Composite | Cohort | Both | Inpatient | Yes | Sputum | NR |
| Orikiriza 2018 | Xpert MTB/RIF | Culture, Composite | Cohort | Both | Both | No | Sputum, stool | 2/357 (0.5%) sputum, 1/64 (1.5%) stool |
| Pang 2014 | Xpert MTB/RIF | Culture, Composite | Cross‐sectional | Unk/NR | Inpatient | Yes | Gastric aspirate specimen | NR |
| Rachow 2012 | Xpert MTB/RIF | Culture, Composite | Cohort | Both | Both | Yes | Sputum | NR |
| Reither 2015 | Xpert MTB/RIF | Culture, Composite | Cohort | Both | NR | Yes | Sputum | NR |
| Sabi 2018 | Xpert MTB/RIF and Xpert Ultra | Culture, Composite | Cohort | Both | Both | Yes | Sputum | 3/520 (0.5%) |
| Saini 2018 | Xpert MTB/RIF | Culture, Composite | Cross‐sectional | HIV‐ | Inpatient | Yes | Bronchoalveolar lavage | NR |
| Sekadde 2013 | Xpert MTB/RIF | Culture | Cross‐sectional | Both | Both | No | Sputum | 2/250 (0.8%) |
| Singh M 2016 | Xpert MTB/RIF | Culture, Composite | Cross‐sectional | NR | Inpatient | Yes | Gastric aspirate specimen, sputum | NR |
| Solomons 2015 | Xpert MTB/RIF | Culture, Composite | Cross‐sectional | Both | Inpatient | Yes | Cerebrospinal fluid | NR |
| Togun 2015 | Xpert MTB/RIF | Culture, Composite | Cross‐sectional | HIV‐ | Outpatient | No | Sputum | NR |
| Tortoli 2012 | Xpert MTB/RIF | Culture | Cross‐sectional | NR | Laboratory | No | Gastric aspirate specimen, cerebrospinal fluid, lymph node specimen | 0/306 |
| Vadwai 2011 | Xpert MTB/RIF | Culture | Cross‐sectional | Both | Both | Yes | Cerebrospinal fluid | NR |
| Walters 2014 | Xpert MTB/RIF | Culture | Cross‐sectional | Both | Inpatient | Yes | Bronchoalveolar lavage | NR |
| Walters 2017a | Xpert MTB/RIF | Culture, Composite | Cohort | Both | Both | Yes | Gastric aspirate specimen, sputum, stool | 28/379 (7%) |
| Walters 2018a | Xpert MTB/RIF | Culture, Composite | Cohort | Both | Both | Yes | Gastric aspirate specimen, sputum, stool | 12/259 (5%) stool, 8/259 (3%) respiratory |
| Yin 2014 | Xpert MTB/RIF | Culture | Cross‐sectional | NR | Inpatient | Yes | Bronchoalveolar lavage | 4/255 (1.6%) |
| Zar 2012 | Xpert MTB/RIF | Culture, Composite | Cohort | Both | Inpatient | Yes | Nasopharyngeal specimen, sputum | NR |
| Zar 2013 | Xpert MTB/RIF | Culture, Composite | Cohort | Both | Outpatient | Yes | Nasopharyngeal specimen, sputum | 12/1754 (0.6%) |
| Zar 2019 | Xpert MTB/RIF and Xpert Ultra | Culture, Composite | Cohort | Both | Inpatient | Yes | Nasopharyngeal specimen, sputum | NR |
IS: induced sputum; NR: not reported; TB: tuberculosis.
For pulmonary tuberculosis, 299 data sets (68,544 participants) were available for analysis; for tuberculous meningitis, 10 data sets (423 participants) were available; for lymph node tuberculosis, 10 data sets (318 participants) were available; and for rifampicin resistance, 14 data sets (326 participants) were available.
Methodological quality of included studies
Pulmonary tuberculosis, tuberculous meningitis, and lymph node tuberculosis
Figure 3 and Figure 4 show risk of bias and applicability concerns for 49 studies that evaluated Xpert MTB/RIF and Xpert Ultra for detection of pulmonary tuberculosis, tuberculous meningitis, or lymph node tuberculosis.
3.
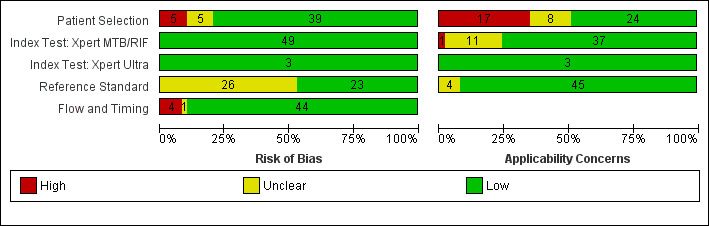
Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies.
4.
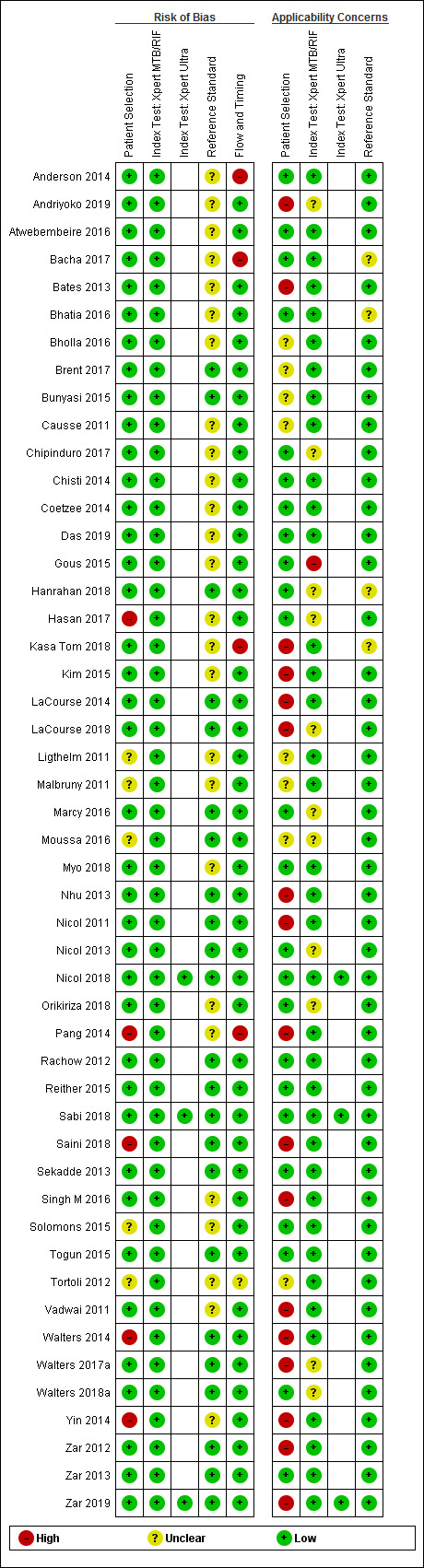
Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study.
In the Patient Selection domain, we considered 39 studies (80%) to have low risk of bias because studies enrolled a consecutive or random sample of eligible participants and avoided inappropriate exclusions. We considered five studies (10%) to have high risk of bias because they did not avoid inappropriate exclusions: one study enrolled participants whose sputum specimens were primarily or exclusively smear‐positive or smear‐negative (Pang 2014); three studies enrolled only participants with negative testing for tuberculosis before performance of bronchoalveolar lavage (Saini 2018; Walters 2014; Yin 2014); and one study enrolled patients using a convenience sample (Hasan 2017). In addition, we considered five studies to have unclear risk of bias because the manner of participant selection was not stated (Ligthelm 2011; Malbruny 2011; Moussa 2016; Solomons 2015; Tortoli 2012). With respect to applicability, we considered 24 studies (49%) to have low concern because participants in these studies were evaluated in primary care facilities, in local hospitals, or in both settings (Anderson 2014; Atwebembeire 2016; Bacha 2017; Bhatia 2016; Chipinduro 2017; Chisti 2014; Coetzee 2014; Das 2019; Gous 2015; Hanrahan 2018; Hasan 2017; Marcy 2016; Myo 2018; Nicol 2013; Nicol 2018; Orikiriza 2018; Rachow 2012; Reither 2015; Sabi 2018; Sekadde 2013; Solomons 2015; Togun 2015; Walters 2018a; Zar 2013). We considered 17 studies (34%) to have high concern because participants were evaluated exclusively as inpatients in tertiary care centres (Andriyoko 2019; Bates 2013; Kasa Tom 2018; Kim 2015; LaCourse 2014; LaCourse 2018; Nhu 2013; Nicol 2011; Pang 2014; Saini 2018; Singh M 2016; Vadwai 2011; Walters 2014; Walters 2017a; Yin 2014; Zar 2012; Zar 2019). We considered eight studies to have unclear concern because we could not be sure about concerns (Bholla 2016; Brent 2017; Bunyasi 2015; Causse 2011;Ligthelm 2011; Malbruny 2011; Moussa 2016; Tortoli 2012).
In the Index Test domain, we considered all studies to have low risk of bias. With respect to applicability, we considered 37 studies (76%) to have low concern and one study to have high concern because the ratio of sample reagent to specimen volume differed from that recommended by the manufacturer (Gous 2015). We also considered all 11 studies (22%) that evaluated stool specimens to have unclear concern because of the absence of an established protocol for stool processing before Xpert MTB/RIF testing (Andriyoko 2019; Chipinduro 2017; Hanrahan 2018; Hasan 2017; LaCourse 2018; Marcy 2016; Moussa 2016; Nicol 2013; Orikiriza 2018; Walters 2017a; Walters 2018a).
In the Reference Standard domain, we considered 23 studies to have low risk of bias and 26 studies (53%) to have unclear risk of bias because the ability of the reference standard to appropriately classify child tuberculosis was uncertain (Anderson 2014; Andriyoko 2019; Atwebembeire 2016; Bacha 2017; Bates 2013; Bhatia 2016; Bholla 2016; Causse 2011; Chipinduro 2017; Chisti 2014; Coetzee 2014; Das 2019; Gous 2015; Hasan 2017; Kasa Tom 2018; Kim 2015; Ligthelm 2011; Malbruny 2011; Myo 2018; Orikiriza 2018; Pang 2014; Singh M 2016; Solomons 2015; Tortoli 2012; Vadwai 2011; Yin 2014). With respect to applicability, we considered 45 studies to have low concern because speciation was performed, confirming M tuberculosis instead of other mycobacterial species, and four studies (8%) to have unclear concern because we could not tell whether speciation was performed (Bacha 2017; Bhatia 2016; Hanrahan 2018; Kasa Tom 2018).
In the Flow and Timing domain, we considered 44 studies (90%) to have low risk of bias because all participants were included in the analysis. We considered four studies to have high risk of bias because results of the index or reference tests were not available for many participants (Anderson 2014; Bacha 2017; Kasa Tom 2018; Pang 2014). We considered one study to have unclear risk of bias because we could not tell whether the index and reference tests were collected at appropriate intervals (Tortoli 2012).
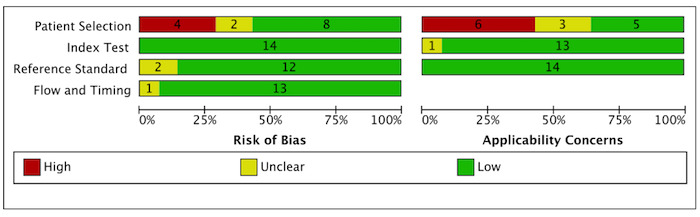
Rifampicin resistance
Figure 5 shows risk of bias and applicability concerns for 14 studies evaluating Xpert MTB/RIF for detection of rifampicin resistance.
5.
Risk of bias and applicability concerns graph for rifampicin resistance: review authors' judgements about each domain presented as percentages across included studies.
In the Patient Selection domain, we considered eight studies (57%) to have low risk of bias because studies enrolled a consecutive or random sample of eligible participants and avoided inappropriate exclusions (Bates 2013; Bholla 2016; Chipinduro 2017; Das 2019; Rachow 2012; Reither 2015; Zar 2012; Zar 2013). We considered four studies (29%) to have high risk of bias because studies did not avoid inappropriate exclusions and instead enrolled participants pre‐selected on the basis of their sputum specimens being smear‐negative or studies exclusively enrolled participants who had negative testing before bronchoalveolar lavage (Pang 2014; Saini 2018; Walters 2014; Yin 2014). We considered two studies (14%) to have unclear risk of bias because the manner of participant selection was not reported (Malbruny 2011; Tortoli 2012). With respect to applicability, we considered five studies (36%) to have low concern because participants in these studies were evaluated in primary care facilities, in local hospitals, or in both settings (Chipinduro 2017; Das 2019; Rachow 2012; Reither 2015; Zar 2013). We considered six studies to have high concern (43%) because participants were evaluated exclusively as inpatients in tertiary care centres (Bates 2013; Pang 2014; Saini 2018; Walters 2014; Yin 2014; Zar 2012). We considered the remaining three studies (21%) to have unclear concern because we could not be sure about concerns (Bholla 2016; Malbruny 2011; Tortoli 2012).
In the Index Test domain, we considered all studies to have low risk of bias. With respect to applicability, we considered 13 studies (93%) to have low concern and one study (7%) that evaluated stool specimens to have unclear concern because of the absence of an established protocol for stool processing before Xpert MTB/RIF testing (Chipinduro 2017).
In the Reference Standard domain, we considered eight studies (57%) to have low risk of bias because results of the reference standard were interpreted without knowledge of results of the index test (Bholla 2016; Chipinduro 2017; Rachow 2012; Reither 2015; Saini 2018; Tortoli 2012; Yin 2014; Zar 2012). We considered the remaining six studies (43%) to have unclear risk of bias because information about blinding was not reported (Bates 2013; Das 2019; Malbruny 2011; Pang 2014; Walters 2014; Zar 2013). With respect to applicability, we considered all studies to have low concern because in these studies, all specimens had already been speciated and identified as M tuberculosis.
In the Flow and Timing domain, we considered 12 studies (86%) to have low risk of bias because all participants were included in the analysis. We considered one study (7%) to have high risk of bias because all participants were not included in the analysis (Pang 2014). We considered one study (7%) to have unclear risk of bias because we could not tell if the index and reference tests were collected at appropriate intervals (Tortoli 2012).
Findings
I. Detection of pulmonary tuberculosis
Due to little observed variability in specificity and in the volume of analyses, we chose to present only forest plots, as such plots were more informative than corresponding summary receiver operator characteristics (SROC) plots.
I.A. Xpert MTB/RIF and Xpert Ultra for pulmonary tuberculosis
I.A.1. Xpert MTB/RIF in sputum ‐ induced or expectorated
Studies were conducted in Bangladesh, Gambia, India, Kenya, Malawi, South Africa, Tanzania, Uganda, Vietnam, Zambia, and Zimbabwe.
I.A.I.a. Culture reference standard
Twenty‐five studies (6812 participants) evaluated Xpert MTB/RIF in sputum specimens against culture (Anderson 2014; Atwebembeire 2016; Bacha 2017; Bates 2013; Brent 2017; Bunyasi 2015; Chipinduro 2017; Chisti 2014; Das 2019; Gous 2015; Hanrahan 2018; LaCourse 2014; Malbruny 2011; Nhu 2013; Nicol 2011; Nicol 2013; Orikiriza 2018; Rachow 2012; Reither 2015; Sekadde 2013; Singh M 2016; Togun 2015; Walters 2017a; Zar 2012; Zar 2013). Xpert MTB/RIF sensitivity ranged from 23% to 100%, and specificity from 87% to 100% (Figure 6). Two studies had no cases of tuberculosis, and so sensitivity was not estimable (Hanrahan 2018; Malbruny 2011). In sputum, Xpert MTB/RIF pooled sensitivity and specificity were as follows against culture (95% CI): 64.6% (55.3% to 72.9%) and 99.0% (98.1% to 99.5%).
6.
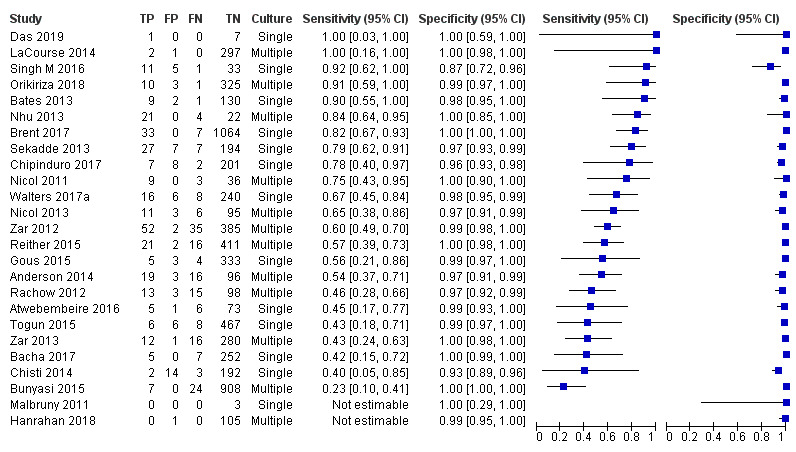
Forest plots of Xpert MTB/RIF sensitivity and specificity for pulmonary tuberculosis in sputum (culture reference standard). The squares represent the sensitivity and specificity of one study, the black line its confidence interval. FN: false‐negative; FP: false‐positive; TN: true‐negative; TP: true‐positive.
I.A.I.b. Composite reference standard
Seventeen studies (4382 participants) evaluated Xpert MTB/RIF in sputum specimens against a composite reference standard (Anderson 2014; Bacha 2017; Brent 2017; Chipinduro 2017; Chisti 2014; Hanrahan 2018; LaCourse 2014; Malbruny 2011; Nhu 2013; Nicol 2011; Nicol 2013; Rachow 2012; Reither 2015; Singh M 2016; Togun 2015; Zar 2012; Zar 2013) (Figure 7). Malbruny 2011 had no cases without tuberculosis, and so specificity was not estimable. In sputum, Xpert MTB/RIF pooled sensitivity and specificity were as follows against a composite reference standard (95% CI): 19.7% (12.1% to 30.4%) and 100% (99.8% to 100%) (Table 6).
7.
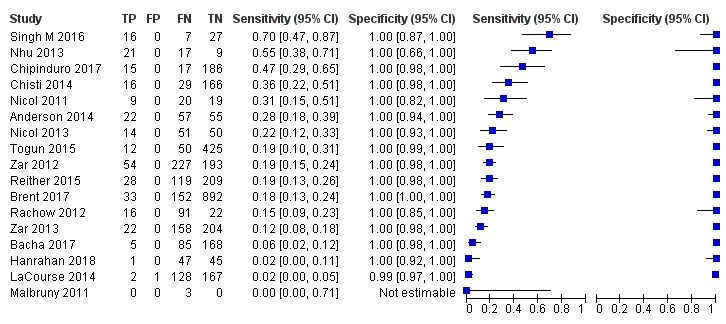
Forest plots of Xpert MTB/RIF sensitivity and specificity for pulmonary tuberculosis in sputum (composite reference standard). The squares represent the sensitivity and specificity of one study, the black line its confidence interval. FN: false‐negative; FP: false‐positive; TN: true‐negative; TP: true‐positive.
2. Diagnostic accuracy of Xpert MTB/RIF and Xpert Ultra for pulmonary tuberculosis and rifampicin resistance in children.
| Test, analysis group | Reference standard | Studies | Number of children (TB cases) | Sensitivity % (95% CI) | Specificity % (95% CI) | Positive predictive value % (95% CI)a | Negative predictive value % (95% CI)a |
| Xpert MTB/RIF, sputum | Culture | 23 | 6703 (493) | 64.6 (55.3 to 72.9) | 99.0 (98.1 to 99.5) | 88.2 (79.6 to 93.5) | 96.2 (95.1 to 97.0) |
| Xpert MTB/RIF, sputum | Composite | 16 | 4379 (1541) | 19.7 (12.1 to 30.4) | 100 (99.8 to 100) | 98.4 (89.2 to 99.8) | 91.8 (90.9 to 92.6) |
| Xpert Ultra, sputum | Culture | 3 | 697 (136) | 72.8 (64.7 to 79.6) | 97.5 (95.8 to 98.5) | 76.4 (65.6 to 84.6) | 97.7 (95.9 to 97.7) |
| Xpert Ultra, sputum | Composite | 3 | 753 (498) | 23.5 (20.0 to 27.4) | 99.2 (96.9 to 99.8) | 76.9 (45.3 to 93.0) | 92.1 (91.7 to 92.5) |
| Xpert MTB/RIF, gastric aspirate specimen | Culture | 14 | 3482 (273) | 73.0 (52.9 to 86.7) | 98.1 (95.5 to 99.2) | 81.0 (65.5 to 90.6) | 97.0 (94.5 to 98.4) |
| Xpert MTB/RIF, gastric aspirate specimen | Composite | 6 | 933 (461) | 31.7 (20.2 to 46.0) | 99.7 (97.1 to 100) | 91.7 (58.3 to 98.9) | 92.9 (91.6 to 94.0) |
| Xpert MTB/RIF, stool specimen | Culture | 11 | 1592 (174) | 61.5 (44.1 to 76.4) | 98.5 (97.0 to 99.2) | 81.7 (72.2 to 88.5) | 95.8 (93.8 to 97.3) |
| Xpert MTB/RIF, stool specimen | Composite | 10 | 1739 (879) | 16.3 (8.4 to 29.2) | 99.7 (97.8 to 100) | 87.4 (42.8 to 98.5) | 91.5 (90.5 to 92.4) |
| Xpert MTB/RIF, nasopharyngeal specimen | Culture | 4 | 1125 (144) | 45.7 (27.6 to 65.1) | 99.6 (98.9 to 99.8) | 92.6 (81.1 to 97.3) | 94.3 (92.0 to 95.9) |
| Xpert Ultra, nasopharyngeal specimen | Culture | 1 | 195 (35) | 45.7 (28.9 to 63.3) | 97.5 (93.7 to 99.3) | 67.0 (42.0 to 85.1) | 94.1 (92.2 to 95.6) |
| Xpert MTB/RIF, sputum, smear‐positiveb | Culture | 11 | 91 (88) | 97.8 (91.6 to 99.4) | − | − | − |
| Xpert MTB/RIF, sputum, smear‐negative | Culture | 12 | 3118 (184) | 58.9 (45.6 to 71.0) | 99.1 (97.1 to 99.7) | 88.4 (68.8 to 96.3) | 95.6 (94.0 to 96.8) |
| Xpert MTB/RIF, sputum, HIV‐positive | Culture | 10 | 642 (88) | 72.2 (59.9 to 81.8) | 99.4 (97.2 to 99.9) | 93.2 (74.0 to 98.5) | 97.0 (95.5 to 97.9) |
| Xpert MTB/RIF, sputum, HIV‐negative | Culture | 12 | 2784 (224) | 54.3 (43.5 to 64.7) | 99.3 (98.1 to 99.7) | 89.7 (80.5 to 94.9) | 95.1 (93.9 to 96.2) |
| Xpert MTB/RIF, gastric aspirate specimen, HIV‐positive | Culture | 3 | 634 (50) | 73.3 (54.9 to 86.1) | 98.5 (97.1 to 99.2) | 84.1 (72.7 to 91.3) | 97.1 (93.8 to 98.4) |
| Xpert MTB/RIF, stool specimen, HIV‐positive | Culture | 4 | 526 (53) | 69.8 (56.3 to 80.6) | 98.6 (96.1 to 99.5) | 84.7 (66.2 to 94.0) | 96.7 (95.1 to 97.8) |
| Xpert MTB/RIF, rifampicin resistance | Culture‐DST, MTBDRplus | 6 | 223 (20) | 90.0 (67.6 to 97.5) | 98.3 (87.7 to 99.8) | 85.7 (42.7 to 98.0) | 98.9 (95.9 to 99.7) |
CI: confidence interval; DST: drug susceptibility testing; MTBDRplus: molecular assay for detection of Mycobacterium tuberculosis and drug resistance; TB: tuberculosis.
aPredictive values were determined at a pre‐test probability of 10%. bWe performed a univariate meta‐analysis for this analysis group because in many studies, few or zero false‐positive and true‐negative values were reported.
I.B. Xpert Ultra in sputum
Studies were conducted in South Africa and Tanzania.
I.B.1.a. Culture reference standard
Three studies (697 participants) evaluated Xpert Ultra in sputum specimens against culture (Nicol 2018; Sabi 2018; Zar 2019). Xpert Ultra sensitivity ranged from 64% to 75%, and specificity from 97% to 100% (Figure 8). Xpert Ultra pooled sensitivity and specificity were as follows in sputum specimens (95% CI): 72.8% (64.7% to 79.6%) and 97.5% (95.8% to 98.5%) (Table 6).
8.
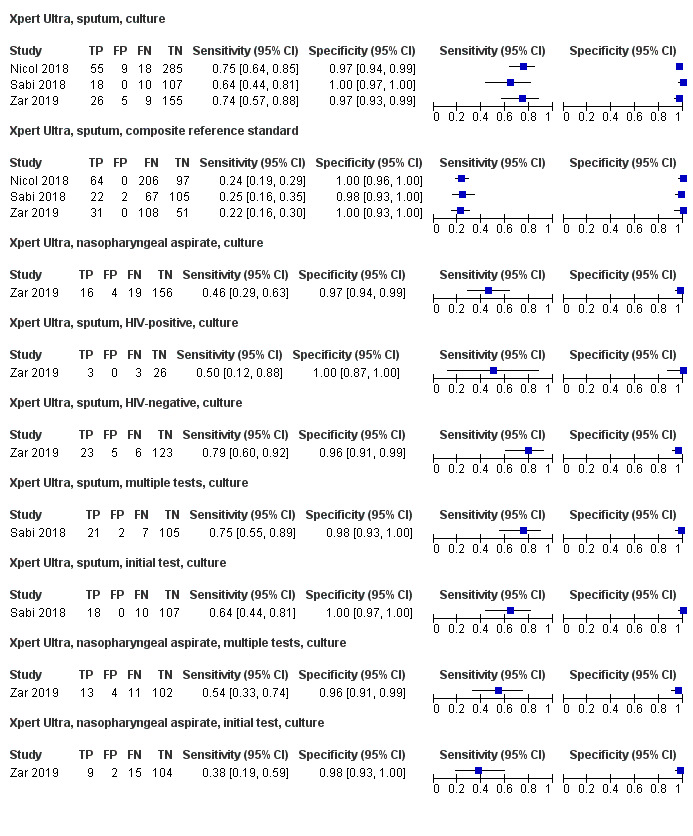
Forest plots of Xpert Ultra sensitivity and specificity for pulmonary tuberculosis by specimen type, reference standard, HIV status, and results of testing using multiple specimens compared to one specimen (culture reference standard). The squares represent the sensitivity and specificity of one study, the black line its confidence interval. FN: false‐negative; FP: false‐positive; TN: true‐negative; TP: true‐positive.
I.B.2.b. Composite reference standard
Three studies (753 participants) evaluated Xpert Ultra in sputum specimens against a composite reference standard (Nicol 2018; Sabi 2018; Zar 2019) (Figure 8). Xpert Ultra pooled sensitivity and specificity were as follows against a composite reference standard (95% CI): 23.5% (20.0% to 27.4%) and 99.2% (96.9% to 99.8%) (Table 6).
I.A.2. Xpert MTB/RIF in gastric aspirate specimens
Studies were conducted in Bangladesh, Burkina Faso, Cambodia, Cameroon, China, India, Italy, Kenya, Myanmar, Pakistan, South Africa, Spain, Vietnam, and Zambia.
I.A.2.a. Culture reference standard
Fifteen studies (3487 participants) evaluated Xpert MTB/RIF in gastric aspirate specimens against culture (Bates 2013; Bunyasi 2015; Causse 2011; Chisti 2014; Das 2019; Hanrahan 2018; Hasan 2017; LaCourse 2018; Malbruny 2011; Marcy 2016; Myo 2018; Nhu 2013; Pang 2014; Tortoli 2012; Walters 2017a (Figure 9). Malbruny 2011 had no cases with tuberculosis, and so sensitivity was not estimable. Xpert MTB/RIF sensitivity ranged from 0% to 100%, and specificity from 90% to 100%. In gastric aspirate specimens, Xpert MTB/RIF pooled sensitivity and specificity against culture were as follows (95% CI): 73.0% (52.9% to 86.7%) and 98.1% (95.5% to 99.2%) (Table 6).
9.
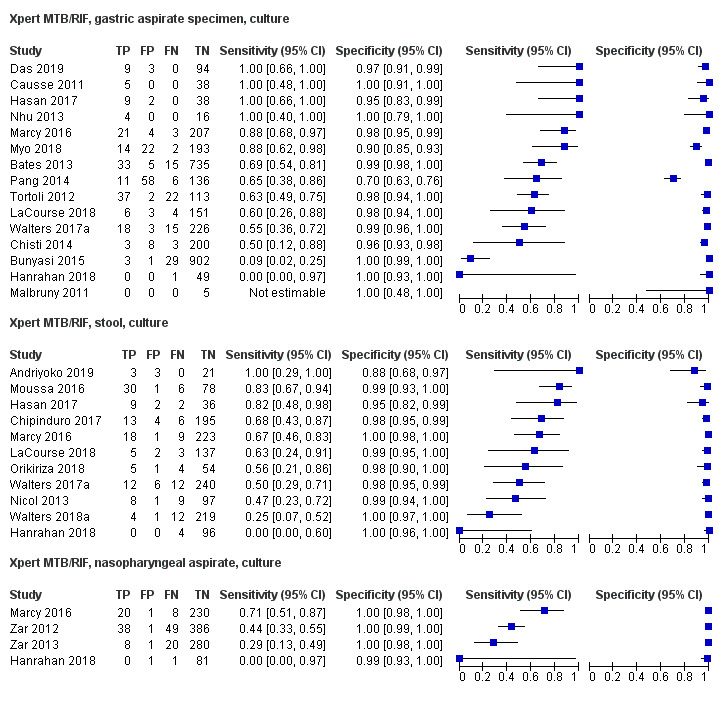
Forest plots of tests Xpert MTB/RIF sensitivity and specificity for pulmonary tuberculosis in gastric aspirate specimens, stool specimens, and nasopharyngeal aspirates (culture reference standard).The squares represent the sensitivity and specificity of one study, the black line its confidence interval. FN: false‐negative; FP: false‐positive; TN: true‐negative; TP: true‐positive.
I.A.2.b. Composite reference standard
Seven studies (948 participants) evaluated Xpert MTB/RIF in gastric aspirate specimens against a composite reference standard (Chisti 2014; Hasan 2017; Kasa Tom 2018; LaCourse 2018; ; Nhu 2013; Pang 2014). Nhu 2013 had no cases without tuberculosis, and so specificity was not estimable. In gastric aspirate specimens, Xpert MTB/RIF pooled sensitivity and specificity against a composite reference standard were as follows (95% CI): 31.7% (20.2% to 46.0%) and 99.7% (97.1% to 100%) (Table 6).
I.B.2. Xpert Ultra in gastric aspirate specimens
We did not identify any studies that evaluated Xpert Ultra in gastric aspirate specimens, against culture or against a composite reference standard.
I.A.3. Xpert MTB/RIF in stool specimens
Studies were conducted in Burkina Faso, Cambodia, Cameroon, Egypt, Indonesia, Kenya, Pakistan, South Africa, Uganda, Vietnam, and Zimbabwe.
1.A.3.a. Culture reference standard
Eleven studies (1592 participants) evaluated Xpert MTB/RIF in stool specimens against culture (Andriyoko 2019; Chipinduro 2017; Hanrahan 2018; Hasan 2017; LaCourse 2018; Marcy 2016; Moussa 2016; Nicol 2013; Orikiriza 2018; Walters 2017a; Walters 2018a) (Figure 9). In stool specimens, Xpert MTB/RIF pooled sensitivity and specificity against culture were as follows (95% CI): 61.5% (44.1% to 76.4%) and 98.5% (97.0% to 99.2%) (Table 6).
I.A.3.b. Composite reference standard
Ten studies (1739 participants) evaluated Xpert MTB/RIF in stool specimens against a composite reference standard (Chipinduro 2017; Hanrahan 2018; Hasan 2017; LaCourse 2018; Marcy 2016; Moussa 2016; Nicol 2013; Orikiriza 2018; Walters 2017a; Walters 2018a). In stool specimens, Xpert MTB/RIF pooled sensitivity and specificity against a composite reference standard were as follows (95% CI): 16.3% (8.4% to 29.2%) and 99.7% (97.8% to 100%) (Table 6).
I.B.3. Xpert Ultra in stool specimens
We did not identify any studies that evaluated Xpert Ultra in stool specimens against culture or a composite reference standard.
I.A.4. Xpert MTB/RIF in nasopharyngeal specimens
Studies were conducted in Burkina Faso, Cambodia, Cameroon, Vietnam, and South Africa.
1.A.4.a. Culture reference standard
Four studies (1125 participants) evaluated Xpert MTB/RIF in nasopharyngeal specimens against culture (Hanrahan 2018; Marcy 2016; Zar 2012; Zar 2013) (Figure 9). In nasopharyngeal specimens, Xpert MTB/RIF pooled sensitivity and specificity against culture were as follows (95% CI): 45.7% (27.6% to 65.1%) and 99.6% (98.9% to 99.8%) (Table 6).
I.A.4.b. Composite reference standard
For Xpert MTB/RIF, we did not extract data on nasopharyngeal specimens against a composite reference standard.
I.B.4. Xpert Ultra in nasopharyngeal specimens
1.B.4.a. Culture reference standard
One study evaluated Xpert Ultra in nasopharyngeal specimens against culture. Xpert Ultra sensitivity and specificity (95% CI) were 45.7% (28.9% to 63.3%) and 97.5% (93.7% to 99.3%), respectively (Zar 2019) (Figure 8).
I.B.4.b. Composite reference standard
For Xpert Ultra, we did not extract data on nasopharyngeal specimens against a composite reference standard.
I.C. Xpert Ultra versus Xpert MTB/RIF
Three studies compared Xpert Ultra and Xpert MTB/RIF in frozen sputum specimens against a reference standard of culture on a sputum specimen (Nicol 2018; Sabi 2018; Zar 2019). Each study mainly compared the tests head‐to‐head for the same participants. Xpert Ultra sensitivity (95% CI) was higher (70.5%, 62.7% to 77.45) than that of Xpert MTB/RIF (63.2%, 54.8% to 70.9%), and specificity lower (Xpert Ultra: 97.3%, 95.5% to 98.4% versus Xpert MTB/RIF: 99.2%, 97.8% to 99.7%). The absolute difference in sensitivity was not statistically significant, but test specificity was reduced with Xpert Ultra (P = 0.02) (Table 7).
3. Direct comparison of Xpert MTB/RIF and Xpert Ultra, sputum specimen (culture reference standard).
| Test, analysis group | Studies | Number of children (cases) | Sensitivity % (95% CI) | Specificity % (95% CI) |
| Xpert Ultra | 3 | 697 (146) | 70.5 (62.7 to 77.4) | 97.3 (95.5 to 98.4) |
| Xpert MTB/RIF | 3 | 609 (136) | 63.2 (54.8 to 70.9) | 99.2 (97.8 to 99.7) |
| Absolute difference | 7.31 (‐3.66 to 18.3), P = 0.19 | ‐1.88 (‐3.47 to ‐0.29), P = 0.02 |
CI: confidence interval.
Sensitivity and specificity estimates were determined with respect to culture.
I.D. Investigations of heterogeneity
I.D.1. Xpert MTB/RIF accuracy by smear status
I.D.1.a. Smear‐positive
Eleven studies (103 participants) evaluated Xpert MTB/RIF from sputum specimens in smear‐positive children against culture (Bacha 2017; Bates 2013; Brent 2017; Chipinduro 2017; LaCourse 2014; Nhu 2013; Nicol 2011; Rachow 2012; Sekadde 2013; Singh M 2016; Zar 2012) (Figure 10). In smear‐positive children, Xpert MTB/RIF pooled sensitivity in sputum specimens (95% CI) was 97.8% (91.6% to 99.4%) (Table 6).
10.

Forest plots of Xpert MTB/RIF sensitivity and specificity for pulmonary tuberculosis in sputum by smear status (culture reference standard). The squares represent the sensitivity and specificity of one study, the black line its confidence interval. FN: false‐negative; FP: false‐positive; TN: true‐negative; TP: true‐positive.
I.D.1.b. Smear‐negative
Thirteen studies (3121 participants) evaluated Xpert MTB/RIF from sputum specimens in smear‐negative children against culture (Bacha 2017; Bates 2013; Brent 2017; Chipinduro 2017; Chisti 2014; LaCourse 2014; Malbruny 2011; Nhu 2013; Nicol 2011; Rachow 2012; Sekadde 2013; Singh M 2016; Zar 2012) (Figure 10). Malbruny 2011 had no cases of tuberculosis, and so sensitivity was not estimable. For smear‐negative children, Xpert MTB/RIF pooled sensitivity and specificity (95% CI) in sputum specimens were 58.9% (45.6% to 71.0%) and 99.1% (97.1% to 99.7%) (Table 6).
I.D.2. Xpert MTB/RIF accuracy by HIV status
I.D.2.a. HIV‐positive sputum specimens
In HIV‐positive children, 11 studies (649 participants) evaluated Xpert MTB/RIF in sputum specimens (Anderson 2014; Bacha 2017; Bates 2013; Chipinduro 2017; LaCourse 2014; Nhu 2013; Nicol 2011; Nicol 2013; Rachow 2012; Sekadde 2013; Zar 2012) (Figure 11). LaCourse 2014 had no cases of tuberculosis, and so sensitivity was not estimable. Xpert MTB/RIF pooled sensitivity and specificity (95% CI) were 72.2% (59.9% to 81.8%) and 99.4% (97.2% to 99.9%) (Table 6).
11.
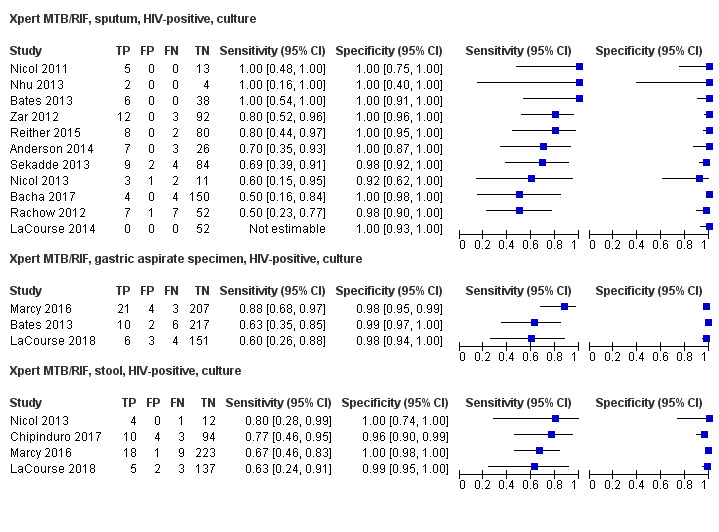
Forest plot of Xpert MTB/RIF sensitivity and specificity in sputum specimens, gastric aspirate specimens, and stool specimens in HIV‐positive children (culture reference standard). The squares represent the sensitivity and specificity of one study, the black line its confidence interval. FN: false‐negative; FP: false‐positive; TN: true‐negative; TP: true‐positive.
I.D.2.b. HIV‐negative sputum specimens
In HIV‐negative children, 12 studies (Anderson 2014; Bacha 2017; Bates 2013; Bunyasi 2015; LaCourse 2014; Nicol 2011; Nicol 2013; Rachow 2012; Reither 2015; Sekadde 2013; Togun 2015; Zar 2012) (2784 participants) evaluated Xpert MTB/RIF in sputum specimens. Xpert MTB/RIF pooled sensitivity and specificity (95% CI) were 54.3% (43.5% to 64.7%) and 99.3% (98.1% to 99.7%) (Table 6).
Restricting the analysis to studies that provided data for both HIV‐positive and HIV‐negative children within the same study did not make a difference in the accuracy estimates.
I.D.2.c. HIV‐positive gastric aspirate specimens
In HIV‐positive children, three studies (634 participants) evaluated Xpert MTB/RIF in gastric aspirate specimens against culture (Bates 2013; LaCourse 2018; Marcy 2016) (Figure 11). Xpert MTB/RIF pooled sensitivity and specificity (95% CI) were 73.3% (54.9% to 86.1%) and 98.5% (97.1% to 99.2%) (Table 6).
I.D.2.d. HIV‐positive stool specimens
In HIV‐positive children, four studies (526 participants) evaluated Xpert MTB/RIF in stool specimens against culture (Chipinduro 2017; LaCourse 2018; Marcy 2016; Nicol 2013) (Figure 11). Xpert MTB/RIF pooled sensitivity and specificity (95% CI) were 69.8% (56.3% to 80.6%) and 98.6% (96.1% to 99.5%) (Table 6).
I.D.3. Xpert MTB/RIF accuracy in children by age group
I.D.3.a. Sputum specimens ‐ induced
In children 5 to 14 years of age, five studies (627 participants) evaluated Xpert MTB/RIF in induced sputum specimens against culture (Chipinduro 2017; Nicol 2011; Rachow 2012; Sekadde 2013; Zar 2012). Xpert MTB/RIF pooled sensitivity and specificity (95% CI) were 80.5% (66.9% to 89.4%) and 98.2% (94.4% to 99.4%). In children birth to four years of age, seven studies (2062 participants) evaluated induced sputum specimens against culture (Bunyasi 2015; Chisti 2014; LaCourse 2014; Nicol 2011; Rachow 2012; Sekadde 2013; Zar 2012). Xpert MTB/RIF pooled sensitivity and specificity (95% CI) were 48.6% (32.5% to 65.0%) and 99.4% (96.7% to 99.9%). The absolute difference in sensitivity was statistically significant, at 31.9% (95% CI 11.7 to 52.2) (P = 0.002; Table 8).
4. Effects of potential sources of heterogeneity on the accuracy of Xpert MTB/RIF accuracy for pulmonary tuberculosis.
| Test, analysis group | Studies | Number of children (cases) | Sensitivity % (95% CI) | Specificity % (95% CI) |
| Age group | ||||
| Induced sputum, 5 to 14 years | 5 | 627 (65) | 80.5 (66.9 to 89.4) | 98.2 (94.4 to 99.4) |
| Induced sputum, 0 to 4 years | 7 | 2062 (143) | 48.6 (32.5 to 65.0) | 99.4 (96.7 to 99.9) |
| Absolute difference | 31.9 (11.7 to 52.2), P = 0.002 | ‐1.15 (‐3.49 to 1.20), P = 0.34 | ||
| Gastric aspirate specimen, 0 to 4 years | 4 | 1795 (77) | 43.0 (16.2 to 74.6) | 99.5 (97.0 to 99.9) |
| High tuberculosis burden | ||||
| Yes | 18 | 5162 (422) | 63.8 (53.5 to 73.0) | 99.1 (97.9 to 99.6) |
| No | 5 | 1466 (72) | 70.2 (46.9 to 86.3) | 98.8 (97.6 to 99.4) |
| Absolute difference | ‐6.42 (‐29.2 to 16.3), P = 0.58 | 0.35 (‐0.79 to 1.49), P = 0.55 | ||
| High TB/HIV burden | ||||
| Yes | 19 | 5824 (415) | 65.7 (55.0 to 75.1) | 99.2 (98.3 to 99.7) |
| No | 4 | 879 (79) | 59.5 (39.6 to 76.7) | 97.4 (93.8 to 98.9) |
| Absolute difference | 6.26 (‐15.7 to 28.2), P = 0.58 | 1.88 (‐0.51 to 4.26), P = 0.12 | ||
| Cultures used to verify tuberculosis | ||||
| Multiple | 11 | 3174 (312) | 61.0 (48.9 to 71.9) | 99.3 (98.4 to 99.7) |
| Single | 12 | 3439 (181) | 69.1 (56.6 to 79.3) | 98.6 (96.5 to 99.5) |
| Absolute difference | ‐8.05 (‐24.4 to 8.35), P = 0.34 | 0.67 (‐0.74 to 2.09), P = 0.35 | ||
CI: confidence interval; TB: tuberculosis.
Sensitivity and specificity estimates were determined with respect to culture.
I.D.3.b. Gastric aspirate specimens
In children birth to four years of age, four studies (1795 participants) evaluated Xpert MTB/RIF in gastric aspirate specimens against culture. Xpert MTB/RIF pooled sensitivity and specificity (95% CI) were 43.0% (16.2% to 74.6%) and 99.5% (97.0% to 99.9%) (Table 8).
I.D.4. Xpert MTB/RIF accuracy, effects of tuberculosis burden and TB/HIV burden
I.D.4.a. Countries with high tuberculosis burden
In countries with high tuberculosis burden, 18 studies (5162 participants) evaluated Xpert MTB/RIF in sputum against culture. Xpert MTB/RIF pooled sensitivity and specificity (95% CI) were 63.8% (53.5% to 73.0%) and 99.1% (97.9% to 99.6%). In countries not considered to be high burden, five studies (1466 participants) evaluated Xpert MTB/RIF in sputum against culture. Xpert MTB/RIF pooled sensitivity and specificity (95% CI) were 70.2% (46.9% to 86.3%) and 98.8% (97.6% to 99.4%). We did not find a significant difference in accuracy estimates (Table 8).
I.D.4.b. Countries with high TB/HIV burden
In countries with high TB/HIV burden, 19 studies (5824 participants) evaluated Xpert MTB/RIF in sputum against culture. Xpert MTB/RIF pooled sensitivity and specificity (95% CI) were 65.7% (55.0% to 75.1%) and 99.2% (98.3% to 99.7%). In countries not considered to be high TB/HIV burden, four studies (879 participants) evaluated Xpert MTB/RIF in sputum against culture. Xpert MTB/RIF pooled sensitivity and specificity (95% CI) were 59.5% (39.6% to 76.7%) and 97.4% (93.8% to 98.9%). We did not find a significant difference in accuracy estimates (Table 9).
5. Diagnostic accuracy of Xpert MTB/RIF and Xpert Ultra for tuberculous meningitis and lymph node tuberculosis.
| Test, analysis group |
Reference standard |
Studies | Number of children (TB cases) | Sensitivity % (95% CI) | Specificity % (95% CI) | Positive predictive value % (95% CI)a | Negative predictive value % (95% CI)a |
| Xpert MTB/RIF, CSF | Culture | 6 | 262 (28) | 54.0 (27.8 to 78.2) | 93.8 (84.5 to 97.6) | 49.1 (26.8 to 71.7) | 94.8 (91.1 to 97.1) |
| Xpert MTB/RIF, CSFb | Composite | 3 | 160 (94) | − | − | − | − |
| Xpert MTB/RIF, Lymph node | Culture | 6 | 210 (54) | 90.4 (55.7 to 98.6) | 89.8 (71.5 to 96.8) | 49.6 (23.7 to 75.7) | 98.8 (93.1 to 99.8) |
| Xpert MTB/RIF, Lymph nodeb | Composite | 3 | 107 (63) | − | − | − | − |
CI: confidence interval; CSF: cerebrospinal fluid; TB: tuberculosis.
aPredictive values were determined at a pre‐test probability of 10%. bWe did not perform a meta‐analysis owing to limited data.
I.D.5. Xpert MTB/RIF accuracy in sputum ‐ multiple specimens versus a single specimen used for the reference standard
We stratified studies that inoculated multiple sputum specimens on culture to verify pulmonary tuberculosis (higher‐quality reference standard) and those that inoculated a single sputum specimen on culture (lower‐quality reference standard) because we suspected that the former was likely to correctly classify more patients with tuberculosis (Figure 6). In comparison to studies that used a single culture (Xpert MTB/RIF pooled sensitivity (95% CI) 69.1% (56.6% to 79.3%) and specificity (95% CI) 98.6% (96.5% to 99.5%)), studies that used multiple cultures had lower sensitivity (95% CI), at 61.0% (48.9% to 71.9%) and similar specificity, at 99.3% (98.4% to 99.7%). We did not find a significant difference in accuracy estimates (Table 8).
II. Detection of tuberculous meningitis
II.A. Xpert MTB/RIF for tuberculous meningitis
Studies were conducted in France, India, Italy, Spain, South Africa, and Vietnam.
II. A.1. Culture reference standard
Eight studies (268 participants) evaluated Xpert MTB/RIF in cerebrospinal fluid against culture (Bhatia 2016; Causse 2011; Das 2019; Malbruny 2011; Nhu 2013; Solomons 2015; Tortoli 2012; Vadwai 2011) (Figure 12). Two of the studies had no cases of tuberculosis, and so sensitivity was not estimable (Causse 2011;Nhu 2013). Xpert MTB/RIF pooled sensitivity and specificity (95% CI) were 54.0% (27.8% to 78.2%) and 93.8% (84.5% to 97.6%) (Table 9).
12.
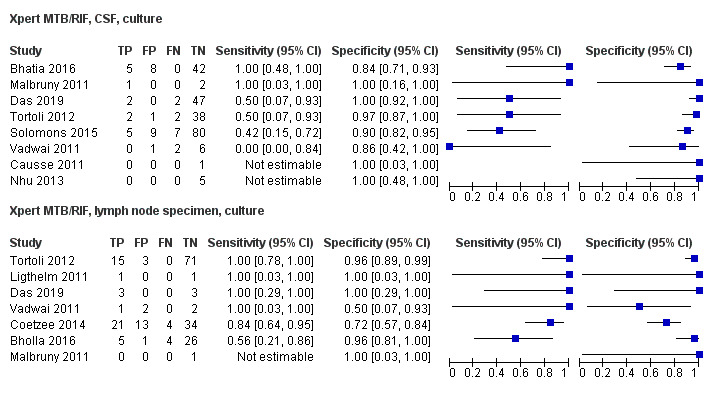
Forest plots of Xpert MTB/RIF sensitivity and specificity for tuberculous meningitis and lymph node tuberculosis (culture reference standard). The squares represent the sensitivity and specificity of one study, the black line its confidence interval. FN: false‐negative; FP: false‐positive; TN: true‐negative; TP: true‐positive.
II.A.2. Composite reference standard
Two studies evaluated Xpert MTB/RIF in cerebrospinal fluid against a composite reference standard. Sensitivities were 25% (95% CI 15 to 39) and 38% (95% CI 22 to 56) (Bhatia 2016; Solomons 2015); specificity was 100% in both studies. We did not perform a meta‐analysis owing to insufficient data.
II.B. Xpert Ultra for detection of tuberculous meningitis
We did not identify any studies that evaluated Xpert Ultra for tuberculous meningitis.
III. Detection of lymph node tuberculosis
III.A. Xpert MTB/RIF for detection of lymph node tuberculosis
Studies were conducted in India, Italy, South Africa, and Tanzania.
III.A.1. Culture reference standard
Seven studies (211 participants) evaluated Xpert MTB/RIF in lymph node specimens against culture (Bholla 2016; Coetzee 2014; Das 2019; Ligthelm 2011; Malbruny 2011; Tortoli 2012; Vadwai 2011) (Figure 12). Malbruny 2011 had no cases of tuberculosis, and so sensitivity was not estimable. Xpert MTB/RIF pooled sensitivity and specificity (95% CI) were 90.4% (55.7% to 98.6%) and 89.8% (71.5% to 96.8%) (Table 9).
III.A.2. Composite reference standard
Three studies (107 participants) evaluated Xpert MTB/RIF in lymph node specimens against a composite reference standard (Bholla 2016; Kim 2015; Ligthelm 2011). Ligthelm 2011 had no cases without tuberculosis, and so specificity was not estimable. In Bholla 2016, Xpert MTB/RIF sensitivity and specificity (95% CI) were 18% (9% to 30%) and 100% (4% to 100%), and in Kim 2015, sensitivity and specificity (95% CI) were 78% (52% to 94%) and 100% (87% to 100%). We did not perform a meta‐analysis owing to insufficient data.
III.B. Xpert Ultra for detection of lymph node tuberculosis
We did not identify any studies that evaluated Xpert Ultra for lymph node tuberculosis.
IV. Detection of rifampicin resistance
IV.A. Xpert MTB/RIF for detection of rifampicin resistance
Fourteen studies provided data on detection of rifampicin resistance (Bates 2013; Bholla 2016; Chipinduro 2017; Das 2019; Malbruny 2011; Pang 2014; Rachow 2012; Reither 2015; Saini 2018; Tortoli 2012; Walters 2014; Yin 2014; Zar 2012; Zar 2013) (Figure 13). Studies were conducted in China, India, South Africa, and Zambia. We were able to include only six studies in the meta‐analysis because the remaining eight studies did not have any cases of rifampicin resistance, and we could not estimate sensitivity (Bates 2013; Das 2019; Pang 2014; Saini 2018; Yin 2014; Zar 2012). All six studies (223 participants) evaluated only Xpert MTB/RIF and were conducted in countries with high multi‐drug‐resistant tuberculosis burden. These studies included sputum, gastric and bronchoalveolar lavage, and cerebrospinal fluid and lymph node specimens. Xpert MTB/RIF sensitivity for rifampicin resistance ranged from 67% to 100%, and specificity from 67% to 100%. Xpert MTB/RIF pooled sensitivity and specificity (95% CI) were 90.0% (67.6% to 97.5%) and 98.3% (87.7% to 99.8%) (Table 6).
13.
Forest plots of Xpert MTB/RIF sensitivity and specificity for rifampicin resistance. The squares represent the sensitivity and specificity of one study, the black line its confidence interval. FN: false‐negative; FP: false‐positive; TN: true‐negative; TP: true‐positive.
IV.B. Xpert Ultra for detection of rifampicin resistance
We identified one study that evaluated Xpert Ultra in nasopharyngeal specimens for rifampicin resistance verified by MGIT‐drug susceptibility testing and MTBDRplus as the reference standards (Zar 2019). Of 30 isolates tested, Xpert Ultra identified one isolate as rifampicin‐resistant, 22 isolates as rifampicin‐susceptible, and seven isolates as inconclusive. Among specimens with valid results, both sensitivity and specificity of Xpert Ultra for detection of rifampicin resistance were 100%.
Other analyses
Detection of pulmonary tuberculosis using more than one Xpert MTB/RIF or Xpert Ultra tests
Xpert MTB/RIF
We identified five studies that evaluated more than one Xpert MTB/RIF test (1935 participants) versus a single Xpert MTB/RIF test (1939 participants) in sputum specimens (Bunyasi 2015; Rachow 2012; Sabi 2018; Zar 2012; Zar 2013); one study (935 participants) that evaluated Xpert MTB/RIF in gastric aspirate specimens (Bunyasi 2015); one study (247 participants with multiple Xpert MTB/RIF tests and 236 with one Xpert MTB/RIF test) that evaluated Xpert MTB/RIF in stool specimens (Walters 2018a); and two studies (705 participants) that evaluated Xpert MTB/RIF in nasopharyngeal specimens (Zar 2012; Zar 2013) (Figure 14).
14.
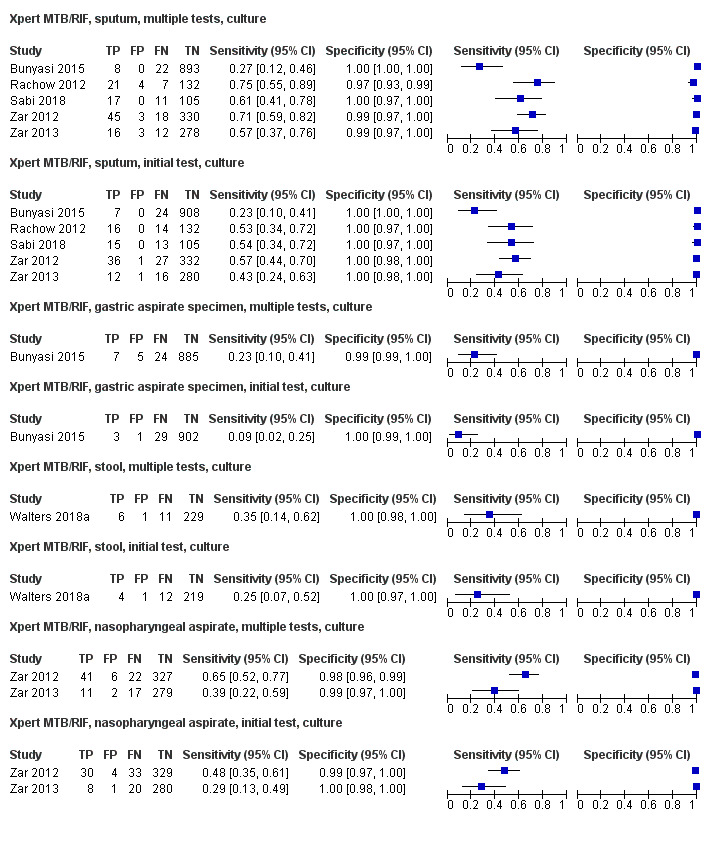
Forest plots of Xpert MTB/RIF sensitivity and specificity for pulmonary tuberculosis in various specimens, multiple Xpert MTB/RIF tests versus one Xpert MTB/RIF test. The squares represent the sensitivity and specificity of one study, the black line its confidence interval. FN: false‐negative; FP: false‐positive; TN: true‐negative; TP: true‐positive.
The absolute difference in pooled sensitivity (95% CI) for more than one Xpert MTB/RIF test versus a single Xpert MTB/RIF test by specimen type was 12.8% (‐6.78% to 32.3%; P = 0.20) in sputum specimens; 13.2% (‐4.64% to 31.1%; P = 0.15) in gastric aspirate specimens; 13.5% (‐9.50% to 36.5%; P = 0.25) in nasopharyngeal specimens; and 10.3% (‐20.8% to 41.4%; P = 0.52) in stool specimens. The absolute difference in pooled specificity (95% CI) for multiple Xpert MTB/RIF tests versus a single Xpert MTB/RIF test by specimen type was ‐0.34% (‐1.09% to 0.41%; P = 0.37) for sputum; ‐0.45% (‐0.99% to 0.09%; P = 0.10) for gastric aspirate specimens; ‐0.49% (‐1.63% to 0.66%; P = 0.40) for nasopharyngeal specimens; and 0.02% (‐1.21% to 1.25%; P =. 0.97) for stool specimens (Table 10).
6. Diagnostic accuracy of Xpert MTB/RIF and Xpert Ultra for pulmonary tuberculosis in children, comparison of repeated testing versus first test (culture reference standard).
| Test, analysis group | Studies | Number of children (TB cases) | Sensitivity %(95% CI) | Specificity %(95% CI) |
| More than 1 Xpert MTB/RIF, sputum | 5 | 1925 (177) | 59.1 (43.0 to 73.4) | 99.5 (97.7 to 99.9) |
| One Xpert MTB/RIF, sputum | 5 | 1939 (180) | 46.3 (35.0 to 57.9) | 99.9 (99.5 to 100) |
| Absolute difference | 12.8 (‐6.78 to 32.3), P = 0.20 | ‐0.34 (‐1.09 to 0.41), P = 0.37 | ||
| More than 1 Xpert Ultra, sputum | 1 | 135 (28) | 75.0 (55.1 to 89.3) | 98.1 (93.4 to 99.8) |
| One Xpert Ultra, sputum | 1 | 135 (28) | 64.3 (44.1 to 81.4) | 100 (96.6 to 100) |
| Absolute difference | 10.7 (‐13.2 to 34.6), P = 0.38 | ‐1.87 (‐4.44 to 0.70), P = 0.16 | ||
| More than 1 Xpert MTB/RIF, gastric aspirate specimen | 1 | 921 (31) | 22.6 (9.59 to 41.1) | 99.4 (98.7 to 99.8) |
| One Xpert MTB/RIF, gastric aspirate specimen | 1 | 935 (32) | 9.38 (1.98 to 25.0) | 99.9 (99.4 to 100) |
| Absolute difference | 13.2 (‐4.64 to 31.1), P = 0.15 | ‐0.45 (‐0.99 to 0.09), P = 0.10 | ||
| More than 1 Xpert MTB/RIF, stool specimen | 1 | 247 (17) | 35.3 (14.2 to 61.7) | 99.6 (97.6 to 100) |
| One Xpert MTB/RIF, stool specimen | 1 | 236 (16) | 25.0 (7.27 to 52.4) | 99.5 (97.5 to 100) |
| Absolute difference | 10.3 (‐20.8 to 41.4), P = 0.52 | 0.02 (‐1.21 to 1.25), P = 0.97 | ||
| More than 1 Xpert MTB/RIF, nasopharyngeal specimen | 2 | 705 (91) | 54.2 (36.1 to 71.3) | 98.7 (97.4 to 99.3) |
| One Xpert MTB/RIF, nasopharyngeal specimen | 2 | 705 (91) | 40.7 (27.9 to 54.9) | 99.2 (98.1 to 99.7) |
| Absolute difference | 13.5 (‐9.50 to 36.5), P = 0.25 | ‐0.49 (‐1.63 to 0.66), P = 0.40 | ||
| More than 1 Xpert Ultra, nasopharyngeal specimen | 1 | 130 (24) | 54.2 (32.8 to 74.4) | 96.2 (90.6 to 99.0) |
| One Xpert Ultra, nasopharyngeal specimen | 1 | 130 (24) | 37.5 (18.8 to 59.4) | 98.1 (93.4 to 99.8) |
| Absolute difference | 16.7 (‐11.1 to 44.5), P = 0.25 | ‐1.89 (‐6.34 to 2.57), P = 0.41 |
CI: confidence interval; TB: tuberculosis.
Xpert Ultra
We identified one study (135 participants) that evaluated Xpert Ultra in sputum specimens (Sabi 2018), and we identified another study (130 participants) that evaluated Xpert Ultra in nasopharyngeal specimens (Zar 2019) (Figure 8). The absolute difference in pooled sensitivity (95% CI) for more than one Xpert Ultra test versus a single Xpert Ultra test by specimen type was 10.7% (‐13.2% to 34.6%; P = 0.38) for sputum specimens and 16.7% (‐11.1% to 44.5%; P = 0.25) for nasopharyngeal specimens. The absolute difference in pooled specificity (95% CI) by specimen type was ‐1.87% (‐4.44% to 0.70%; P = 0.16) for sputum and ‐1.89% (‐6.34% to 2.57%; P = 0.41) for nasopharyngeal specimens (Table 10).
Uninterpretable index test results
Detection of tuberculosis, uninterpretable results
Few reported results were uninterpretable (i.e. invalid, error, or no result) for Xpert MTB/RIF and Xpert Ultra for detection of tuberculosis in the included studies. We summarized this information in Table 5.
Indeterminate results, rifampicin resistance
In 15 studies that reported indeterminate results for rifampicin resistance, few Xpert MTB/RIF results were indeterminate for detection of rifampicin resistance. Zero indeterminate results were reported in 11 studies (Bates 2013; Bholla 2016; Das 2019; Gous 2015; Ligthelm 2011; Malbruny 2011; Nhu 2013; Nicol 2011; Rachow 2012; Tortoli 2012; Sekadde 2013). Walters 2018a reported 10% (1/10) indeterminate results in stool specimens, and Zar 2012reported 3% (4/127) indeterminate results in nasopharyngeal and stool specimens.
Sensitivity analyses
We undertook sensitivity analyses by limiting inclusion in the meta‐analysis to the following,
Random or consecutive recruitment of participants.
Blinding of reference standard to index test results.
No pre‐treatment of participants.
Enrolment only of children aged 0 to 14 years.
Sputum only (excluding studies that also collected gastric aspirate specimens).
These sensitivity analyses made little difference to any of the findings (Table 11).
7. Sensitivity analyses for accuracy of Xpert MTB/RIF for detection of pulmonary tuberculosis, sputum specimen (culture reference standard).
| Analysis | Studies | Number of children (TB cases) | Sensitivity % (95% CI) | Specificity % (95% CI) |
| Random or consecutive recruitment of participants | 22 | 6485 (485) | 64.0 (54.4 to 72.6) | 99.1 (98.2 to 99.6) |
| Blinding of reference standard to index test results | 18 | 5796 (454) | 65.5 (55.3 to 74.4) | 99.0 (98.0 to 99.5) |
| No pretreatment of participants | 21 | 5877 (469) | 64.2 (54.8 to 72.7) | 99.1 (98.0 to 99.6) |
| Enrolled only children 0 to 14 years old | 20 | 5950 (437) | 65.5 (54.9 to 74.7) | 99.1 (98.1 to 99.6) |
| Sputum only (excluding studies that also collected gastric aspirate specimen) | 22 | 6653 (482) | 63.0 (53.8 to 71.3) | 99.2 (98.4 to 99.6) |
CI: confidence interval; TB: tuberculosis.
Discussion
Summary of main results
This systematic review summarizes the current literature and includes 49 unique studies on the accuracy of Xpert MTB/RIF and Xpert Ultra for pulmonary tuberculosis (TB), tuberculous meningitis, lymph node tuberculosis, and rifampicin resistance. Major findings from the review include the following.
Xpert MTB/RIF accuracy for pulmonary TB (culture reference standard)
In sputum specimen, Xpert MTB/RIF pooled sensitivity was 64.6% (95% CI 55.3% to 72.9%) (23 studies, 493 participants)
In gastric aspirate specimens, Xpert MTB/RIF pooled sensitivity was 73.0% (95% CI 52.9% to 86.7%) (14 studies, 273 participants)
In stool specimens, Xpert MTB/RIF pooled sensitivity was 61.5% (95% CI 44.1% to 76.4%) (11 studies, 174 participants)
In nasopharyngeal specimens, Xpert MTB/RIF pooled sensitivity was 45.7% (95% CI 27.6% to 65.1%) (4 studies, 144 participants)
In the above specimens, Xpert MTB/RIF pooled specificity was ≥ 98.1%
Xpert Ultra accuracy for pulmonary tuberculosis (culture reference standard)
In sputum specimens, Xpert Ultra pooled sensitivity was 72.8% (95% CI 64.7% to 79.6%) (3 studies, 136 participants)
In nasopharyngeal specimens, Xpert Ultra sensitivity was 45.7% (95% CI 28.9% to 63.3%) (1 study, 35 participants)
Xpert Ultra specificity was 97.5% in both specimen types
Xpert MTB/RIF accuracy for pulmonary TB (composite reference standard)
In sputum specimen, Xpert MTB/RIF pooled sensitivity was 19.7% (95% CI 12.1% to 30.4%) (16 studies, 1541 participants)
In gastric aspirate specimens, Xpert MTB/RIF pooled sensitivity was 31.7% (95% CI 20.2% to 46.0%) (6 studies, 461 participants)
In stool specimens, Xpert MTB/RIF pooled sensitivity was 16.3% (95% CI 8.4% to 29.2%) (10 studies, 879 participants)
In the above specimens, Xpert MTB/RIF specificity was ≥ 99.7%
Xpert Ultra accuracy for pulmonary tuberculosis (composite reference standard)
In sputum specimens, Xpert Ultra sensitivity was 23.5% (95% CI 20.0% to 27.4%) (3 studies, 498 participants)
Xpert MTB/RIF accuracy for tuberculous meningitis (culture reference standard)
In cerebrospinal fluid, Xpert MTB/RIF sensitivity and specificity were 54.0% (95% CI 27.8% to 78.2%) and 93.8% (95% CI 84.5% to 97.6%) (6 studies, 262 participants; 28 with tuberculosis)
We did not identify any studies of Xpert Ultra for tuberculous meningitis.
Xpert MTB/RIF accuracy for lymph node tuberculosis (culture reference standard)
In lymph node specimens, Xpert MTB/RIF sensitivity and specificity were 90.4% (95% CI 55.7% to 98.6%) and 89.8% (95% CI 71.5% to 96.8%) (6 studies, 210 participants; 54 with tuberculosis)
We did not identify any studies of Xpert Ultra for lymph node tuberculosis.
Rifampicin resistance
Xpert MTB/RIF sensitivity and specificity were 90.0% (95% CI 67.6% to 97.5%) and 98.3% (95% CI 87.7% to 99.8%) (6 studies, 223 participants; 20 with rifampicin resistance)
We did not identify studies of Xpert Ultra for detection of rifampicin resistance.
The main findings of the review are summarized in Table 1, Table 2, Table 3, and Table 4.
Summary of findings 1. Xpert MTB/RIF and Xpert Ultra for pulmonary tuberculosis in children.
| Review question: what is the diagnostic accuracy of Xpert MTB/RIF and Xpert Ultra for pulmonary tuberculosis in sputum in children with signs and symptoms of pulmonary tuberculosis? | |||||||
| Patients/population: children with presumed pulmonary tuberculosis | |||||||
| Index tests: Xpert MTB/RIF and Xpert Ultra | |||||||
| Role: an initial test | |||||||
| Threshold for index tests: an automated result is provided | |||||||
| Reference standard: culture | |||||||
| Types of studies: cross‐sectional and cohort studies | |||||||
| Setting: primary care facilities and local hospitals | |||||||
| Index test | Effect (95% Cl) | Number of participants (studies) | Test result | Number of results per 1000 patients tested(95% CI) | Certainty of the evidence (GRADE) | ||
| Prevalence 1% | Prevalence 10% | Prevalence 20% | |||||
| Xpert MTB/RIF | Pooled sensitivity 0.65 (95% CI 0.55 to 0.73) | 493 (23) | True‐positives | 6 (6 to 7) | 65 (55 to 73) | 129 (111 to 146) | ⨁⨁⨁ ◯ MODERATEa,b |
| False‐negatives | 4 (3 to 4) | 35 (27 to 45) | 71 (54 to 89) | ||||
| Pooled specificity 0.99 (95% CI 0.98 to 0.99) | 6119 (23) | True‐negatives | 980 (971 to 985) | 891 (883 to 896) | 792 (785 to 796) | ⨁⨁⨁ ◯ MODERATEc |
|
| False‐positives | 10 (5 to 19) | 9 (4 to 17) | 8 (4 to 15) | ||||
| Xpert Ultra | Pooled sensitivity 0.73 (95% CI 0.65 to 0.80) | 136 (3) | True‐positives | 7 (6 to 8) | 73 (65 to 80) | 146 (129 to 159) | ⨁⨁◯◯ LOWd,e |
| False‐negatives | 3 (2 to 4) | 27 (20 to 35) | 54 (41 to 71) | ||||
| Pooled specificity 0.97 (95% CI 0.96 to 0.98) | 551 (3) | True‐negatives | 960 (950 to 970) | 873 (864 to 882) | 776 (768 to 784) | ⨁⨁⨁⨁ HIGH |
|
| False‐positives | 30 (20 to 40) | 27 (18 to 36) | 24 (16 to 32) | ||||
CI: confidence interval.
Prevalence levels were suggested by the WHO Global Tuberculosis Programme.
aFor individual studies, sensitivity estimates ranged from 27% to 100%. We thought that differences in enrolment criteria (different populations targeted), disease severity, and different ages and settings could explain the heterogeneity. We did not downgrade for inconsistency. bEight studies (34%) had high or unclear concern about applicability because, in these studies, patients were enrolled from inpatient tertiary care centres, which could lead to the enrolment of children with more advanced disease. Of these studies, Nhu 2013 and Singh M 2016 had among the highest sensitivities. We downgraded one level for indirectness. cAs assessed by QUADAS‐2, 11 studies (47%) had unclear risk of bias based on the collection of a single culture to exclude tuberculosis. We downgraded one level for risk of bias. dTwo studies (66%) had high concern about applicability because, in these studies, patients were enrolled from inpatient tertiary care centres, which could lead to the enrolment of children with more advanced disease. We downgraded one level. eThe number of children with pulmonary tuberculosis contributing to this analysis for observed sensitivity was low. We downgraded one level for imprecision.
GRADE certainty of the evidence.
High: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
The results presented in this table should not be interpreted in isolation from the results of individual included studies contributing to each summary test accuracy measure.
Summary of findings 2. Xpert MTB/RIF for tuberculous meningitis in children.
| Review question: what is the diagnostic accuracy of Xpert MTB/RIF for tuberculous meningitis in CSF in children with signs and symptoms of tuberculous meningitis? | |||||
| Patients/population: children with presumed tuberculous meningitis | |||||
| Index tests: Xpert MTB/RIF | |||||
| Role: an initial test | |||||
| Threshold for index tests: an automated result is provided | |||||
| Reference standard: culture | |||||
| Types of studies: cross‐sectional and cohort studies | |||||
| Setting: inpatient | |||||
| Pooled sensitivity: 0.54 (95% CI 0.28 to 0.78) | Pooled specificity: 0.94 (95% CI 0.84 to 0.98) | |||||
| Test result | Number of results per 1000 patients tested (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | ||
| Prevalence 1% | Prevalence 5% | Prevalence 10% | |||
| True‐positives | 5 (3 to 8) | 27 (14 to 39) | 54 (28 to 78) | 28 (6) | ⨁◯◯◯ VERY LOWa,b,c,d |
| False‐negatives | 5 (2 to 7) | 23 (11 to 36) | 46 (22 to 72) | ||
| True‐negatives | 929 (837 to 966) | 891 (803 to 927) | 844 (761 to 878) | 213 (6) | ⨁⨁◯◯ LOWe,f |
| False‐positives | 61 (24 to 153) | 59 (23 to 147) | 56 (22 to 139) | ||
CI: confidence interval; CSF: cerebrospinal fluid.
Prevalence levels were suggested by the WHO Global Tuberculosis Programme.
aAs assessed by QUADAS‐2, three studies (50%) had low risk of bias and risk of bias was unclear for the remainder. We downgraded one level for risk of bias. bFor individual studies, sensitivity estimates ranged from 0% to 100%. We thought that differences in enrolment criteria (different populations targeted), disease severity, and setting could only in part explain heterogeneity. We downgraded one level for inconsistency. cThe setting was unclear or reflected a tertiary care inpatient setting in three studies (50%). However, this is reflective of where the target condition would typically be diagnosed; therefore we did not downgrade for indirectness. dThe number of children with tuberculous meningitis contributing to this analysis for observed sensitivity was low. We thought the 95% CI around false‐negatives and true‐positives would likely lead to different decisions, depending on which confidence limits are assumed. We downgraded one level for imprecision. eThe quality of the reference standard was unclear in three studies (50%). We downgraded one level for risk of bias. fWe thought the 95% CI around false‐positives and true‐negatives would likely lead to different decisions, depending on which confidence limits are assumed. We downgraded one level for imprecision.
GRADE certainty of the evidence.
High: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
The results presented in this table should not be interpreted in isolation from the results of individual included studies contributing to each summary test accuracy measure.
Summary of findings 3. Xpert MTB/RIF for lymph node tuberculosis in children.
| Review question: what is the diagnostic accuracy of Xpert MTB/RIF for lymph node tuberculosis in a lymph node specimen in children with signs and symptoms of lymph node tuberculosis? | |||||
| Patients/population: children with presumed lymph node tuberculosis | |||||
| Index tests: Xpert MTB/RIF | |||||
| Role: an initial test | |||||
| Threshold for index tests: an automated result is provided | |||||
| Reference standard: culture | |||||
| Types of studies: cross‐sectional and cohort studies | |||||
| Setting: inpatient | |||||
| Pooled sensitivity: 0.90 (95% CI 0.56 to 0.99) | Pooled specificity: 0.90 (95% CI 0.71 to 0.97) | |||||
| Test result | Number of results per 1000 patients tested (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | ||
|
Prevalence 1% Typically seen in |
Prevalence 5% Typically seen in |
Prevalence 10% Typically seen in |
|||
| True‐positives | 9 (6 to 10) | 45 (28 to 49) | 90 (56 to 99) | 68 (6) | ⨁◯◯◯ VERY LOWa,b,c |
| False‐negatives | 1 (0 to 4) | 5 (1 to 22) | 10 (1 to 44) | ||
| True‐negatives | 889 (708 to 958) | 853 (679 to 920) | 808 (644 to 871) | 142 (6) | ⨁⨁◯◯ LOWd,e,f |
| False‐positives | 101 (32 to 282) | 97 (30 to 271) | 92 (29 to 256) | ||
CI: confidence interval.
Prevalence levels were suggested by the WHO Global Tuberculosis Programme.
aAs assessed by QUADAS‐2, three studies (50%) had high risk of bias. We downgraded one level for risk of bias. bThree studies (50%) had high or unclear concern about applicability because, in these studies, patients were enrolled from inpatient tertiary care settings, which could lead to enrolment of children with more advanced disease. We did not downgrade for indirectness, as a more specialized centre for the diagnosis of extrapulmonary tuberculosis in children is expected. cThe number of children with lymph node tuberculosis contributing to this analysis for observed sensitivity was low. We thought the 95% CI around false‐negatives and true‐positives would likely lead to different decisions, depending on which confidence limits are assumed. We downgraded two levels for imprecision. dAs assessed by QUADAS‐2, all studies (100%) had unclear risk of bias relative to the quality of the reference standard. We downgraded one level for risk of bias. eFor individual studies, specificity estimates ranged from 50% to 100%. For most studies, specificity was ≥ 96%. We did not downgrade for inconsistency. fWe thought the 95% CI around false‐positives and true‐negatives would likely lead to different decisions, depending on which confidence limits are assumed. We downgraded one level for imprecision
GRADE certainty of the evidence.
High: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
The results presented in this table should not be interpreted in isolation from the results of individual included studies contributing to each summary test accuracy measure.
Summary of findings 4. Xpert MTB/RIF for rifampicin resistance in children.
| Review question: what is the diagnostic accuracy of Xpert MTB/RIF for rifampicin resistance in children with signs and symptoms of tuberculosis? | |||||
| Patients/population: children with presumed tuberculosis | |||||
| Index tests: Xpert MTB/RIF | |||||
| Role: an initial test | |||||
| Threshold for index tests: an automated result is provided | |||||
| Reference standard: phenotypic culture‐based drug susceptibility testing and MTBDRplus | |||||
| Types of studies: cross‐sectional and cohort studies | |||||
| Setting: inpatient | |||||
| Pooled sensitivity: 0.90 (95% CI 0.68 to 0.98) | Pooled specificity: 0.98 (95% CI 0.88 to 0.99) | |||||
| Test result | Number of results per 1000 patients tested (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | ||
| Prevalence 2% | Prevalence 10% | Prevalence 15% | |||
| True‐positives | 18 (14 to 20) | 90 (68 to 98) | 135 (102 to 147) | 20 (6) | ⨁⨁◯◯ LOWa |
| False‐negatives | 2 (0 to 6) | 10 (2 to 32) | 15 (3 to 48) | ||
| True‐negatives | 960 (862 to 970) | 882 (792 to 891) | 833 (748 to 842) | 203 (6) | ⨁⨁⨁◯ MODERATEb,c |
| False‐positives | 20 (10 to 118) | 18 (9 to 108) | 17 (8 to 102) | ||
CI: confidence interval.
Prevalence levels were suggested by the WHO Global Tuberculosis Programme.
aThe number of children with rifampicin resistance contributing to this analysis for observed sensitivity was low. We thought the 95% CI around false‐negatives and true‐positives would likely lead to different decisions, depending on which confidence limits are assumed. We downgraded two levels for imprecision. bFor individual studies, specificity estimates ranged from 67% in Saini 2018 to 100%. For most studies, specificity was ≥ 97%. We did not downgrade for inconsistency. cWe thought the 95% CI around false‐positives and true‐negatives would likely lead to different decisions, depending on which confidence limits are assumed. We downgraded one level for imprecision.
GRADE certainty of the evidence.
High: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
The results presented in this table should not be interpreted in isolation from the results of individual included studies contributing to each summary test accuracy measure.
Xpert MTB/RIF for pulmonary tuberculosis, sputum specimens
In theory, for a population of 1000 children, 100 of whom have pulmonary tuberculosis on culture, 74 would be Xpert MTB/RIF‐positive and 9 (12%) would not have tuberculosis (false‐positives); 926 would be Xpert MTB/RIF‐negative and 35 (4%) would have tuberculosis (false‐negatives) (Table 1).
Xpert Ultra for pulmonary tuberculosis, sputum specimens
In theory, for a population of 1000 children, 100 of whom have pulmonary tuberculosis on culture, 100 would be Xpert Ultra‐positive and 27 (27%) would not have tuberculosis (false‐positives); 900 would be Xpert Ultra‐negative and 27 (3%) would have tuberculosis (false‐negatives) (Table 1).
Xpert MTB/RIF for tuberculous meningitis, cerebrospinal fluid
In theory, for a population of 1000 children, 100 of whom have tuberculous meningitis on culture, 86 would be Xpert MTB/RIF‐positive and 59 (69%) would not have tuberculosis (false‐positives); 914 would be Xpert MTB/RIF‐negative and 23 (3%) would have tuberculosis (false‐negatives) (Table 2).
Xpert MTB/RIF for lymph node tuberculosis, lymph node specimens
In theory, for a population of 1000 children, 100 of whom have lymph node tuberculosis on culture, 142 would be Xpert MTB/RIF‐positive and 97 (68%) would not have lymph node tuberculosis (false‐positives); 858 would be Xpert MTB/RIF‐negative and 5 (1%) would have lymph node tuberculosis (false‐negatives) (Table 3).
Xpert MTB/RIF for detection of rifampicin resistance
In theory, for a population of 1000 children, 100 of whom have rifampicin resistance, 108 would have Xpert MTB/RIF‐rifampicin resistance detected and 18 (17%) would not have rifampicin resistance (false‐positives); 892 would have Xpert MTB/RIF‐rifampicin resistance NOT detected and 10 (1%) would have rifampicin resistance (false‐negatives) (Table 4).
Overall this review adds to the existing body of evidence on Xpert MTB/RIF and Xpert Ultra diagnostic accuracy in children. Most notable are the new data on performance of different specimen types that are now being introduced to improve access to diagnostic testing for tuberculosis in children. Further, this review expands on evidence supporting the increase in test sensitivity that is achieved through testing multiple specimens by Xpert MTB/RIF and Xpert Ultra. These findings provide new evidence by which to shape the development of global practice guidelines for the diagnosis of tuberculosis in children.
Specifically, our review demonstrated differing sensitivities in the different types of specimens. We think that these findings may in part be attributable to differences in the clinical setting and in the quality of the reference standard, mainly culture. With respect to the clinical setting, it is more common to collect gastric aspirate specimens in inpatient settings; further, these settings tend to include a higher number of children with advanced disease, which often has a higher microbiological yield (Marais 2006d). Thus, the higher sensitivity against a culture and composite reference standard for gastric aspirate specimens may in part be due to the inpatient setting and higher likelihood of advanced disease. Regarding the reference standard, some studies performed only one culture, and other studies more than one culture, to verify tuberculosis. We considered multiple cultures to be a higher‐quality reference standard. However, we did not observe a significant difference in Xpert MTB/RIF accuracy in sputum when multiple versus single cultures were used to verify pulmonary tuberculosis. In some studies from low‐income countries, only one culture was performed; this is consistent with standard practice in many countries with a high burden of tuberculosis. As has been previously documented, the sensitivity (95% CI) of Xpert MTB/RIF was nearly perfect in children with smear‐positive tuberculosis 97.8% (91.6% to 99.4%) and was still 58.9% (45.6% to 71.0%) in the smear‐negative subgroup. Data indicate clearly that the diagnostic accuracy of Xpert MTB/RIF is superior to that of sputum smear in children.
Against a composite reference standard, we found that Xpert MTB/RIF had a sensitivity of 19.7% and Xpert Ultra had a sensitivity of 23.5%. These results were higher then those reported in the prior review (Detjen 2015) and may reflect changes in the consensus definition or the broader sample of research sites included in this review. In adults, Xpert Ultra trace results may be more likely to reflect false‐positive results, particularly in patients with prior tuberculosis (Dorman 2018). Xpert Ultra trace results on sputum were reported in two studies (Nicol 2018; Sabi 2018). Twenty‐one trace results were obtained on frozen specimens, with 20 occurring in patients with confirmed or unconfirmed tuberculosis; one occurred in a patient with unlikely tuberculosis. Nasopharyngeal specimens specifically yielded nine trace results (Zar 2019), none of which were identified in patients unlikely to have tuberculosis. These limited data suggest that trace results in children indicate true disease and should be treated as such. More data are needed with regard to trace results, particularly on specimens other than sputum.
We found the sensitivity (95% CI) of stool Xpert MTB/RIF to be slightly lower at 61.5% (44.1% to 76.4%) than that of sputum Xpert MTB/RIF at 64.6% (95% CI 55.3% to 72.9%). Nonetheless, stool is a promising specimen for diagnosis because, unlike sputum, it is non‐invasive. Its greatest benefit may be seen in children younger than five years owing to the challenges of collecting specimens through sputum induction and gastric aspiration in this population. However, no studies evaluating Xpert MTB/RIF in stool provided disaggregated data for analyses in the youngest age groups. As in MacLean 2019, we noted the lack of standardized procedures for processing stool, with each study using a different approach.
When multiple specimens of the same type were tested, we noted an increase in Xpert MTB/RIF sensitivity for pulmonary tuberculosis in all specimen types. In comparison with testing an initial specimen, Xpert MTB/RIF specificity was similar when multiple specimens were tested.
In a head‐to‐head comparison of frozen sputum specimens, Xpert Ultra yielded higher sensitivity (70.5%) than Xpert MTB/RIF (63.2%) and lower specificity (Ultra: 97.3% versus Xpert MTB/RIF: 99.2%). Although in comparison with Xpert MTB/RIF, Ultra specificity was slightly decreased, the improvement in Xpert Ultra sensitivity may provide greater benefit than the harm associated with a slight reduction in Ultra specificity in the paediatric population.
In subgroup analyses, we found the sensitivity of Xpert MTB/RIF to be higher in HIV‐positive than HIV‐negative children. These findings were similar when we limited the analysis to studies that included both HIV‐positive and HIV‐negative children in the same study. We note that the number of cases in most studies was small: HIV‐positive group, median (interquartile range) = 8 (5 to 13), and HIV‐negative group, median (interquartile range) = 13 (5.5 to 26). We think random variation may in part explain these results, although they may also reflect the increased likelihood of presentation with severe tuberculosis in HIV‐positive children.
Regarding age, we found higher sensitivity of Xpert MTB/RIF in sputum from children 5 to 14 years old compared to children 0 to 4 years old. The sensitivity of Xpert MTB/RIF in gastric aspirate specimens from children 0 to 4 years old was similar to that in sputum. This has implications for clinical decision‐making in children 0 to 4 years of age, when a negative Xpert may provide less reassurance to the treating clinician, particularly due to higher risk for adverse tuberculosis outcomes in this population.
Although Xpert MTB/RIF sensitivity in lymph node specimens was 90%, specificity was lower than in sputum specimens (98%). Regarding the accuracy of the reference standard in lymph node specimens in particular, several factors may have contributed to false‐negative culture results, including inefficient specimen collection and overly harsh decontamination (Kohli 2018). As with pulmonary tuberculosis, the sensitivity of Xpert MTB/RIF for tuberculous meningitis is not adequate to withhold treatment based on the test result, and the entirety of the clinical information must be considered.
For detection of rifampicin resistance, we found Xpert MTB/RIF to have lower sensitivity (95% CI) at 90.0% (67.6% to 97.5%) than that in a Cochrane Review of Xpert MTB/RIF for pulmonary tuberculosis and rifampicin resistance in adults at 96% (94% to 97%) (Horne 2019). We note that in the current review, only 20 rifampicin‐resistant specimens contributed to the determination of sensitivity, whereas in Horne 2019, there were 1775 rifampicin‐resistant specimens. Compared to the current review, the sensitivity estimate in Horne 2019 was more precise. Specificities were similar (98%).
We identified one large pilot project in India that evaluated Xpert MTB/RIF for pulmonary tuberculosis in sputum verified by smear microscopy (Raizada 2015a). We did not include this study in our systematic review because the reference standard (smear) did not satisfy the criterion for inclusion. Results of this project demonstrated high sensitivity (1521/1530; 99.4%) of Xpert MTB/RIF against smear.
Strengths and weaknesses of the review
Completeness of evidence
The data set resulted from comprehensively searching numerous databases, handsearching references of included studies, and contacting investigators for additional evidence. We included all identified non‐English studies from which we could obtain accuracy data. However, we acknowledge that we may have missed some studies despite the comprehensive search and outreach to investigators. We included the two most common forms of extrapulmonary tuberculosis ‐ tuberculous meningitis and lymph node tuberculosis. Hence, the review does not include an evaluation of the accuracy of Xpert MTB/RIF and Xpert Ultra in less common forms of child tuberculosis.
Accuracy of the reference standards used
In a systematic review of diagnostic test accuracy studies, the reference standard is the best available test to determine the presence or absence of the target condition. In this review, we included two reference standards ‐ culture and a composite reference standard. Although culture is the best available microbiological reference standard, it is not a perfect reference standard for active tuberculosis in children owing to the paucibacillary nature of the disease. Some studies performed only one culture and others more than one culture to verify tuberculosis. We considered multiple cultures to be a higher‐quality reference standard. We also evaluated the accuracy of Xpert MTB/RIF and Xpert Ultra against a composite reference standard defined as (1) a positive culture or a clinician decision to treat for pulmonary or extrapulmonary tuberculosis, or (2) a standardized research definition of child tuberculosis. The accuracy of composite reference standards is also variable and limited (in most analyses, Xpert MTB/RIF and Ultra identified less than 30% of cases) but may reflect the paucibacillary nature of childhood tuberculosis, which is not taken into account when culture positivity is taken as the reference standard for comparison. If data on tuberculosis treatment were not provided, we accepted the uniform research definitions or the definition used by the primary study authors (study‐specific definition) for the composite reference standard. Therefore, clinical characteristics and component tests in the composite reference standard differed across studies, and these differences may have contributed to variation in accuracy estimates.
Quality of reporting of the included studies
We considered risk of bias to be low for the patient selection, index test, and flow and timing domains, and low or unclear for the reference standard domain, because some studies collected only a single specimen for culture. In general, studies were fairly well reported. When data were unclear, or when we needed additional information, we corresponded with many of the primary study authors. The quality of the studies was good, but for some specimen types and for Xpert Ultra, the numbers of studies and participants enrolled were small, limiting our ability to draw definitive conclusions in these circumstances.
Comparison with other systematic reviews
Our systematic review on the diagnostic accuracy of Xpert MTB/RIF and Xpert Ultra for tuberculosis in children builds on a prior systematic review on this topic ‐ Detjen 2015 ‐ and includes 34 additional studies on Xpert MTB/RIF published since that time, as well as emerging data on Xpert Ultra. For detection of pulmonary tuberculosis, Detjen 2015 found Xpert MTB/RIF sensitivity of 62% in sputum specimens and 66% in gastric aspirate specimens against culture. The current review found slightly higher Xpert MTB/RIF sensitivity estimates ‐ 65% in sputum specimens and 73% in gastric aspirate specimens. Another systematic review for pulmonary tuberculosis found Xpert MTB/RIF sensitivity of 65% in respiratory specimens (culture reference standard) (Wang 2015) ‐ similar to the pooled sensitivity estimate for sputum specimens in this review. Specificity in these reviews was similar to ours, at 98% to 99%.
We identified two additional systematic reviews on the diagnostic accuracy of Xpert MTB/RIF in stool specimens. MacLean 2019 found that stool Xpert MTB/RIF had a pooled sensitivity of 67% against a microbiological reference standard consisting of either culture or Xpert MTB/RIF on a respiratory specimen. Mesman 2019 found that stool Xpert MTB/RIF had a pooled sensitivity of 57% against culture on respiratory specimens and 60% against Xpert MTB/RIF on respiratory specimens. Our systematic review found stool Xpert MTB/RIF had a pooled sensitivity of 61.5% against a microbiological reference standard consisting of either culture or Xpert MTB/RIF on respiratory specimens ‐ findings consistent with those of the prior reviews. Specificity in both reviews was similar to ours, at 98% to 99%.
Applicability of findings to the review question
To assess the applicability of findings to the review question, we considered QUADAS‐2 domains for patient selection, index test, and reference standard. With respect to the patient selection domain, we considered many studies to have high or unclear risk of bias because either patients were evaluated exclusively as inpatients in a tertiary care setting or the clinical setting was not clearly reported. Therefore, our findings cannot definitively be applied to the primary care setting. Studies that take place in referral settings may include patients whose condition is more advanced or more difficult to diagnose than patients seen at lower levels of the health system. With respect to the index test, we considered most studies to have low concern about applicability. However, we considered applicability of the index test in stool to be unclear, as currently, there is not a standardized protocol for stool testing with Xpert MTB/RIF or Xpert Ultra. The applicability of Xpert Ultra in respiratory specimens comes with some uncertainty, as all data were reported in frozen specimens. With respect to the reference test domain, we considered most studies to have low concern about applicability.
Authors' conclusions
Implications for practice.
For detection of pulmonary tuberculosis, the sensitivity of Xpert Ultra was higher than that of Xpert MTB/RIF in sputum, suggesting that Xpert Ultra may detect an increased proportion of paucibacillary tuberculosis in children. Although in comparison with Xpert MTB/RIF, Ultra specificity was slightly decreased, the improvement in Xpert Ultra sensitivity may provide greater benefit than the harm associated with a slight reduction in Ultra specificity in the paediatric population. Nonetheless, owing to the small numbers of studies and participants in many of the analyses in this review, we advise caution in interpreting these results. In addition, we note that, in this review, all studies assessing the performance of Xpert Ultra evaluated the test in frozen specimens originally collected for testing Xpert MTB/RIF. Additional prospective studies on the performance of Xpert Ultra in fresh clinical specimens are needed. As always, the prevalence of tuberculosis in the test population will influence the predictive value of Xpert MTB/RIF and Xpert Ultra results.
Xpert MTB/RIF sensitivity (defined by culture) for pulmonary tuberculosis was variable across different specimen types, including sputum, gastric, stool, and nasopharyngeal specimens. The highest sensitivity was seen with gastric aspirate specimens, followed by sputum specimens, and the lowest in nasopharyngeal specimens. Specificity was high in all of these aforementioned specimens. Xpert MTB/RIF sensitivity may increase when multiple specimens of the same type are tested compared with a single specimen. Although not evaluated in this review, it is likely that collecting multiple specimens of different types will also increase Xpert MTB/RIF and Xpert Ultra sensitivity. However, considering the difficulties of collecting sputum specimens from children, as well as the limitations of culture to verify the diagnosis of tuberculosis, clinicians should consider collecting additional specimens, whenever possible the least invasive, for testing with Xpert MTB/RIF or Xpert Ultra. The lower sensitivity of the Xpert MTB/RIF test in children 0 to 4 years of age should prompt clinicians not only to collect multiple specimens but also to treat based on other clinical information in this vulnerable population.
Xpert MTB/RIF was accurate for detection of rifampicin resistance.
Xpert MTB/RIF was sensitive for lymph node tuberculosis. Regarding tuberculous meningitis, as with pulmonary tuberculosis, treatment decisions should be based on the entirety of clinical information, and treatment not withheld based solely on an Xpert MTB/RIF result.
Evidence in this review is based mainly on culture as the reference standard and accuracy calculated on the assumption that the reference standard is 100% sensitive and specific. Although culture is acceptable, it is an imperfect reference standard for child tuberculosis. Without a more accurate reference standard that has a limit of detection low enough to detect paucibacillary tuberculosis, the accuracy of novel diagnostic tests for tuberculosis in children will remain difficult to estimate. Despite the presence of a negative Xpert MTB/RIF or Xpert Ultra result, clinicians will still need to consider anti‐tuberculosis treatment in children with a high suspicion of tuberculosis or at high risk of a poor outcome.
Implications for research.
There are several areas for which additional research regarding the diagnostic accuracy of molecular tests in children is necessary. Studies are urgently needed that evaluate the accuracy of Xpert Ultra in gastric and stool specimens for pulmonary tuberculosis and extrapulmonary tuberculosis in children. Ideally, these studies would be prospective and would evaluate fresh specimens, rather than retrospective utilizing frozen specimens. Establishing the diagnostic accuracy of Xpert Ultra in extrapulmonary specimens is also an urgent need, particularly given the encouraging results regarding Xpert Ultra performance in cerebrospinal fluid obtained from adults.
Xpert MTB/RIF sensitivity estimates increased with multiple as compared to one specimen in a small number of studies; however, additional studies evaluating the combinatorial benefit of multiple specimen types are needed. Limited data suggest that the combination of non‐invasive specimens performs comparably with traditional gastric aspirate specimens or induced sputum specimens.
More research is needed to identify an improved reference standard that accurately defines tuberculosis in children. Additional operational and qualitative research is needed to determine the best approach to less invasive specimen collection. Implementation studies on a method of suction for nasopharyngeal aspiration that is appropriate for low‐skill or low‐resource environments are needed. Addtional operational research concerning the use of stool as a diagnostic specimen is needed. These studies should address integration into normal diagnostic clinical pathways, definition of laboratory protocols that successfully balance ease of implementation and diagnostic performance, and the impact of stool testing on patient‐important outcomes. There is a dearth of qualitative research identifying child and family preferences for and acceptability of comparative diagnostic approaches and specimen collection procedures.
We underscore the continued urgent need to develop new tools that accurately diagnose tuberculosis in children. Ideally, these new tools will be rapid, affordable, feasible, and acceptable to children and their parents.
History
Protocol first published: Issue 6, 2019 Review first published: Issue 8, 2020
Acknowledgements
The Academic Editors are Professor Gerry Davies (CIDG) and Dr Danielle van der Windt (DTA Group).
The CIDG editorial base is funded by UK aid from the UK government for the benefit of low‐ and middle‐income countries (project number 300342‐104). The views expressed do not necessarily reflect the UK government’s official policies.
We thank Vittoria Lutje (CIDG) for developing the search strategy. We also wish to acknowledge Ryan Vu (Rice University), who contributed to development of the protocol. In addition, we thank Mikashmi Kohli, who provided technical expertise, and Emily MacLean, who provided data on stool specimens; both are at McGill University. We thank Andrew DiNardo (Baylor College of Medicine) for technical assistance. We thank Gemma Villanueva and Hanna Bergman, both with Cochrane Response, who assisted with data entry. We also thank Aakshi Kalra (FIND), who provided data from a large‐scale Xpert MTB/RIF demonstration project conducted in India.
Development of the systematic review was in part made possible with financial support from the US Agency for International Development (USAID) administered by the World Health Organization (WHO) Global TB Programme, Switzerland. AK, LGF, YT, and AMM received funding from USAID to carry out the review.
Appendices
Appendix 1. Detailed search strategies
Database: Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R) <1946 to April 25, 2019>
Search Strategy:
Search Name: Cochrane Central Register of Controlled Trials
1 Mycobacterium tuberculosis/
2 Tuberculosis/ or "Tuberculosis, Multidrug‐Resistant"/ or Extensively Drug‐Resistant Tuberculosis/ or Tuberculosis, Pulmonary/ or Tuberculosis, Lymph Node/ or Tuberculosis, Meningeal/
3 (Tuberculosis or MDR‐TB or XDR‐TB or tuberculous).ti. or (Tuberculosis or MDR‐TB or XDR‐TB or tuberculous).ab.
4 ((extrapulmonary or lymph node* or mening* or pulmonary) and TB).ti. or ((extrapulmonary or lymph node* or mening* or pulmonary) and TB).ab.
5 1 or 2 or 3 or 4
6 Xpert*.ti. or Xpert*.ab.
7 (GeneXpert or cepheid).ti. or (GeneXpert or cepheid).ab.
8 (Xpert* and Ultra).mp.
9 (near* patient or near‐patient).ti. or (near* patient or near‐patient).ab.
10 6 or 7 or 8 or 9
11 5 and 10
12 (pediatric* or paediatric*).mp.
13 (child or children* or childhood or infan* or newborn or neonat* or toddler* or adolescen*).mp.
14 exp Child/ or exp Infant/ or Adolescent/ or exp Pediatrics/
15 12 or 13 or 14
16 11 and 15
20 19 and 16
Database: Embase 1947‐Present, updated daily
Search Strategy:
1 Mycobacterium tuberculosis.mp. or Mycobacterium tuberculosis/
2 lung tuberculosis/ or extrapulmonary tuberculosis/ or extensively drug resistant tuberculosis/ or multidrug resistant tuberculosis/ or drug resistant tuberculosis/ or tuberculosis/
3 (Tuberculosis or MDR‐TB or XDR‐TB or tuberculous).ti. or (Tuberculosis or MDR‐TB or XDR‐TB or tuberculous).ab.
4 ((extrapulmonary or lymph node* or mening* or pulmonary) and TB).ti. or ((extrapulmonary or lymph node* or mening* or pulmonary) and TB).ab.
5 1 or 2 or 3 or 4
6 Xpert*.ti. or Xpert*.ab.
7 (GeneXpert or cepheid).ti. or (GeneXpert or cepheid).ab.
8 (Xpert* and Ultra).mp.
9 (near* patient or near‐patient).ti. or (near* patient or near‐patient).ab.
10 6 or 7 or 8 or 9
11 5 and 10
12 (pediatric* or paediatric*).mp.
13 (child or children* or childhood or infan* or newborn or neonat* or toddler* or adolescen*).mp.
14 child/
15 infant/
16 adolescent/
17 pediatrics/
18 12 or 13 or 14 or 15 or 16 or 17
19 11 and 18
Search Name: Cochrane Central Register of Controlled Trials
Issue 4 of 12, April 2019
ID Search
#1 mycobacterium tuberculosis
#2 MeSH descriptor: [Mycobacterium tuberculosis] explode all trees
#3 Tuberculosis or MDR‐TB or XDR‐TB or tuberculous
#4 MeSH descriptor: [Tuberculosis, Pulmonary] explode all trees
#5 MeSH descriptor: [Tuberculosis] explode all trees
#6 MeSH descriptor: [Extensively Drug‐Resistant Tuberculosis] explode all trees
#7 MeSH descriptor: [Tuberculosis, Multidrug‐Resistant] explode all trees
#8 MeSH descriptor: [Tuberculosis, Lymph Node] explode all trees
#9 MeSH descriptor: [Tuberculosis, Meningeal] explode all trees
#10 ((extrapulmonary or lymph node* or mening* or pulmonary) and TB)
#11 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10
#12 Xpert* or GeneXpert or cepheid
#13 (Xpert* and Ultra)
#14 (near* patient or near‐patient)
#15 #12 or #13 or #14
#16 #11 and #15
#17 child or children* or childhood or infan* or newborn or neonat* or toddler* or adolescen*
#18 pediatric* or paediatric*
#19 #17 or #18
CINAHL (EBSCOHost)
# Query
S9 S7 AND S8
S8 "( child or children* or childhood or infan* or newborn or neonat* or toddler* or adolescen* ) OR ( pediatric* or paediatric* )"
S7 S3 AND S6
S6 S4 OR S5
S5 "Xpert MTB/RIF"
S4 "( Xpert* or GeneXpert or cepheid ) OR ( Xpert* and Ultra ) OR ( near* patient or near‐patient )"
S3 S1 OR S2
S2 "( XDR‐TB or MDR‐TB ) OR extrapulmonary tuberculosis"
S1 (MH "Mycobacterium Tuberculosis") OR (MH "Tuberculosis, Multidrug‐Resistant") OR (MH "Tuberculosis, Meningeal") OR (MH "Tuberculosis") OR "tuberculosis OR drug resistant tuberculosis OR Mycobacterium tuberculosis"
SCI‐EXPANDED, CPCI‐S (Web of Science)
# 7 #6 AND #5
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 6 TOPIC: (child or children* or childhood or infan* or newborn or neonat* or toddler* or adolescen*) OR TOPIC: (pediatric* or paediatric*)
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 5 #4 AND #1
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 4 #3 OR #2
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 3 TOPIC: (Xpert* Ultra or Cepheid or near* patient)
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 2 TOPIC: (Xpert* or Xpert MTB RIF)
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 1 TOPIC: (Tuberculosis or MDR‐TB or XDR‐TB or tuberculous) OR TOPIC: (mycobacterium tuberculosis) OR TOPIC: (((extrapulmonary or lymph node* or mening* or pulmonary) and TB))
Indexes=SCI‐EXPANDED, CPCI‐S Time span=All years
SCOPUS (Elsevier)
( TITLE‐ABS‐KEY ( ( tuberculosis OR mdr‐tb OR xdr‐tb OR tuberculous ) OR ( mycobacterium AND tuberculosis ) OR ( ( ( extrapulmonary OR lymph AND node* OR mening* OR pulmonary ) AND tb ) ) ) ) AND ( TITLE‐ABS‐KEY ( ( xpert* OR xpert AND mtb AND rif ) OR ( xpert* AND ultra OR cepheid OR near* AND patient ) ) ) AND ( TITLE‐ABS‐KEY ( ( child OR children* OR childhood OR infan* OR newborn OR neonat* OR toddler* OR adolescen* ) OR ( pediatric* OR paediatric* ) ) )
ClinicalTrials.gov, WHO ICTRP, and ISRCTN Registry: tuberculosis and Xpert* and children
Appendix 2. Data extraction form
| Diagnostic accuracy of Xpert in the diagnosis of child tuberculosis: data extraction form | |
| I. ID | |
| Study ID | First Name/Publication Year |
| First author | Name |
| Corresponding author | Name |
| Corresponding author email | |
| Was author contacted? | 1 – Yes 2 – No If yes, dates(s) |
| If yes, author response? | |
| Study data | 1 ‐ Published 2 ‐ In press 3 ‐ Ongoing |
| Title | |
| Year (of publication) | YYYY or 9 – Not reported |
| Year study start date | YYYY or 9 – Not reported |
| Language | 1 – English 2 – Other If other, specify: |
| II. Study details | |
| Country where study was conducted | |
| Country World Bank classification | 1 ‐ Low income
2 ‐ Middle income
3 ‐ High income 4 ‐ Low and high income 5 ‐ Other combination, describe |
| Country tuberculosis burden (WHO 2015) | 1 ‐ WHO tuberculosis high burden 2 ‐ WHO tuberculosis/HIV high burden 3 ‐ WHO MDR tuberculosis high burden 4 ‐ WHO tuberculosis + MDR tuberculosis high burden 5 ‐ WHO tuberculosis + HIV/tuberculosis high burden 6 ‐ WHO tuberculosis + HIV/tuberculosis + MDR tuberculosis high burden 7 ‐ Not a WHO high‐burden country 8 ‐ Both non‐high‐burden and high‐burden countries included 9 ‐ Other |
| Study design | 1 – Randomized controlled trial 2 – Cross‐sectional 3 – Cohort 4 – Other, specify 9 – Could not tell If other, describe: |
| Participant selection | 1 – Consecutive 2 – Random 3 – Convenience 7 – Other 9 – Unknown/Not reported |
| Direction of study data collection | 1 – Prospective 2 – Retrospective 9 – Unknown/Not reported |
| Inclusion criteria | 1 – Broad 2 – Rigorous 9 – Unknown/Not reported |
| Inclusion criteria for presumptive tuberculosis | 1 – Tuberculosis contact 2 – Cough 3 – Loss of weight 4 – Suggestive chest X‐ray 5 – Immunological evidence of tuberculosis infection (TST/IGRA) 6 ‐ Malnutrition 7 – HIV 8 ‐ Other, describe 9 – Unknown/Not reported |
| Describe inclusion criteria as in study | |
| Number included after recruitment by inclusion and exclusion criteria | Enter number or 9 – Unknown/Not reported |
| Total number of children included in systematic review analysis | Enter number or 9 – Unknown/Not reported |
| Total number of specimens included in analysis with collection method | Enter number or 9 – Unknown/Not reported |
| Unit of analysis (Xpert) | 1 – One specimen per patient 2 – Multiple specimens per patient 3 ‐ Unknown number of specimens per patient 9 – Unknown/Not reported Describe as written in study, if unclear: |
| Did the study include patients with previous tuberculosis history? | 1 – Yes 0 – No 9 – Unknown/Not reported |
| If so, what is the percentage? | Enter % and specify numerator/denominator |
| Target condition? Pulmonary tuberculosis? | 1 ‐ Yes 0 ‐ No |
| Target condition? Rifampicin resistance? | 1 ‐ Yes 0 ‐ No |
| Target condition? Lymph node tuberculosis? | 1 ‐ Yes 0 ‐ No |
| Target condition? Tuberculous meningitis? | 1 ‐ Yes 0 ‐ No |
| Comments about study design | |
| III. Patient characteristics and setting | |
| Description of study population (age, HIV info, etc.) | 1 – All enrolled 2 – All analysed 9 – Unknown/Not reported |
| Age: median, mean, range by months | Enter number or 9 – Unknown/Not reported |
| Gender | ##/total and % female |
| HIV status of participants | 0 – HIV‐ 1 – HIV+ 2 – Both HIV+/‐ 9 – Unknown/Not reported |
| If HIV‐positive participants included, what is the percentage? | % and specify numerator/denominator |
| Type of respiratory specimen included | 1 – All expectorated 2 – All induced 3 – All bronchoalveolar lavage 4 – All gastric lavage 5 – Nasopharyngeal aspirate 6 ‐ Stool 7 – Multiple types 8 – Other 9 – Unknown/Not reported If 7 or 8, describe types and record numbers: |
| Type of non‐respiratory specimen | 1 – Fine needle aspirate 2 – Lymph node biopsy 3 – Cerebrospinal fluid 4 – Multiple types 5 ‐ Other 9 ‐ Unknown/Not reported If 4 or 5, describe types and record numbers: |
| Were Xpert sample and culture obtained from same specimen? | 1 – Yes 0 – No 9 – Unknown/Not reported |
| Number of cultures used to exclude tuberculosis | Describe |
| Information on smear microscopy: was it used? | 1 – Yes 0 – No 9 – Unknown/Not reported |
| Type of microscopy used | 1 – Ziehl‐Neelsen
2 – Fluoresence microscopy
3 ‐ Light emitting diode‐based fluorescence microscopy 4 ‐ Multiple, describe: 9 – Unknown/Not reported |
| Smear type | 1 – Direct 2 – Concentrated (processed) 3 ‐ Both direct and concentrated 9 – Unknown/Not reported |
| Data on culture performance provided? | # of contaminated culture/Total # cultures performed or 9 ‐ Unknown/Not reported |
| Were patient‐important outcomes evaluated? (time to diagnosis, time to treatment, others) | 1 – Yes 2 – No 9 – Unknown/Not reported |
| Time to diagnosis? | Xpert: Culture: 9 – Unknown/Not reported Specify whether time from sample collection to diagnosis in lab or just turnaround time in lab |
| Time to treatment initiation | Xpert: Culture: 9 – Unknown/Not reported |
| Clinical setting, describe as written in the paper | 1 – Outpatient 2 – Inpatient 3 – Both outpatient and inpatient 4 – Other, specify 5 – Laboratory based 9 – Unknown/Not reported Describe as in paper: |
| Laboratory services level | 1 ‐ Central (reference) 2 ‐ Intermediate (regional) 3 ‐ Peripheral (microscopy centre, provincial hospital) 4 – Research laboratory 5 ‐ Other, specify |
| Where were Xpert tests performed? (tests generally available at different laboratory levels, although tests may overlap) Peripheral: acid‐fast bacilli (Ziehl‐Neelsen, Auramine‐rhodamine, Auramine‐O staining) and Xpert MTB/RIF Intermediate: peripheral laboratory tests and culture on solid media and line probe assay (LPA) from smear‐positive sputum Central: intermediate laboratory tests and culture on liquid media and DST (1st‐line and 2nd‐line anti‐tuberculosis drugs) on solid or in liquid media and LPA on positive cultures and rapid speciation tests | 1 ‐ Central (reference) 2 ‐ Intermediate (regional) 3 ‐ Peripheral (microscopy centre, provincial hospital) 4 ‐ Other, specify |
| Was Xpert run outside of a laboratory? | 1 ‐ Yes 0 ‐ No |
| Current treatment: were patients on treatment (defined as tuberculosis drugs for longer than 7 days) for the current tuberculosis episode? (note: may impact culture results) | 1 – Yes 2 – No 9 – Unknown/Not reported |
| If so, what is the percentage? | % Specify numerator/denominator |
| IV. Index test | |
| Xpert cartridge(s) evaluated | 1 ‐ Xpert only 2 ‐ Ultra only 3 ‐ Any combination Xpert and Ultra |
| Xpert platform: was Omni used? Unless Omni was explicitly described, assume standard platform | 1 – Yes, only Omni used for Xpert tests 2 – Yes, both Omni and standard platform used for Xpert tests 3 ‐ No |
| Pretreatment processing procedure for GeneXpert | 1 – None 2 – NALC‐NaOH 3 – NaOH (Petroff) 4 – Other 9 – Unknown/Not reported |
| For Xpert specimen, what was the condition of the specimen when tested? | 1 – Fresh 2 – Frozen 9 – Unknown/Not reported |
| Were uninterpretable (invalid error or no result) results reported for Xpert for tuberculosis detection? | 1 – Yes 9 – Unknown/Not reported If yes, describe numbers: |
| Were indeterminate results reported for Xpert for rifampicin resistance? | 1 – Yes 9 – Unknown/Not reported If yes, describe numbers: |
| V. Reference standard | |
| For tuberculosis detection, what reference standard(s) was used? Respiratory samples? | 1 – Solid culture (specify 1a) 2 – Liquid culture (specify 2a) 3 – Both solid and liquid culture (specify 1a and 2a) 9 – Unknown/Not reported 1a ‐ Solid culture LJ 7H10 7H11 Other 2a – Liquid culture MGIT 960 Other (specify): |
| For tuberculosis detection, what reference standard(s) was used? Lymph node? | 1 – Solid culture (specify 1a) 2 – Liquid culture (specify 2a) 3 – Both solid and liquid culture (specify 1a and 2a) 9 – Unknown/Not reported 1a ‐ Solid culture LJ 7H10 7H11 Other 2a – Liquid culture MGIT 960 Other (specify): |
| For tuberculosis detection, what reference standard(s) was used? Cerebrospinal fluid? | 1 – Solid culture (specify 1a) 2 – Liquid culture (specify 2a) 3 – Both solid and liquid culture (specify 1a and 2a) 9 – Unknown/Not reported 1a ‐ Solid culture LJ 7H10 7H11 Other (specify): 2a – Liquid culture MGIT 960 Other (specify): |
| Reference standard pulmonary tuberculosis: clinical | 1 ‐ Yes 0 – No Multiple answers, list: |
| If clinical, describe as in paper | |
| For rifampicin resistance detection, what reference standard(s) was used? Respiratory samples? |
1 – Solid culture (specify 1a) 2 – Liquid culture (specify 2a) 3 – Both solid and liquid culture (specify 1a and 2a) 4 – M tuberculosis DRplus 9 – Unknown/NR 1a ‐ Solid culture LJ 7H10 7H11 Other: Specify method (e.g. proportion): 2a – Liquid culture MGIT 960 Other (specify): |
| For rifampicin resistance detection, what reference standard(s) was used? Lymph node? | 1 – Solid culture (specify 1a) 2 – Liquid culture (specify 2a) 3 – Both solid and liquid culture (specify 1a and 2a) 4 – M tuberculosis DRplus 9 – Unknown/Not reported 1a ‐ Solid culture LJ 7H10 7H11 Other: Specify method (e.g. proportion): 2a – Liquid culture MGIT 960 Other (specify): |
| For rifampicin resistance detection, what reference standard(s) was used? Cerebrospinal fluid? | 1 – Solid culture (specify 1a) 2 – Liquid culture (specify 2a) 3 – Both solid and liquid culture (specify 1a and 2a) 4 – M tuberculosis DRplus 9 – Unknown/Not reported 1a ‐ Solid culture LJ 7H10 7H11 Other: Specify method (e.g. proportion): 2a – Liquid culture MGIT 960 Other (specify): |
| If information is available | |
| Is information on quality assurance of DST available in the study? | 1 – Yes 2 ‐ No 9 – Unknown/Not reported If yes, describe potential sources of bias |
DST: drug susceptibility testing; IGRA: Interferon‐gamma release assay; LJ: Löwenstein‐Jensen; MDR‐TB: multidrug‐resistant tuberculosis; MGIT: mycobacterial growth indicator tube; NALC: N‐acetyl‐L‐cysteine; NAOH: sodium hydroxide; TST: tuberculin skin test; WHO: World Health Organization.
Appendix 3. Example of 2 × 2 result table
| Pulmonary tuberculosis, Xpert MTB/RIF |
Tuberculosis, culture | |||
| Xpert MTB/RIF in sputum | Yes | No | Total | |
| Positive | ||||
| Negative | ||||
| Total | ||||
| Pulmonary tuberculosis, Xpert Ultra | Tuberculosis, culture | |||
| Xpert Ultra in sputum | Yes | No | Total | |
| Positive | ||||
| Negative | ||||
| Total | ||||
| Tuberculous meningitis | Tuberculosis, culture | |||
| Xpert MTB/RIF in CSF | Yes | No | Total | |
| Positive | ||||
| Negative | ||||
| Total | ||||
| Pulmonary tuberculosis | Tuberculosis, CRS | |||
| Xpert MTB/RIF in sputum | Yes | No | Total | |
| Positive | ||||
| Negative | ||||
| Total | ||||
CRS: composite reference standard; CSF: cerebrospinal fluid.
Appendix 4. QUADAS‐2 review‐specific guidance
Domain 1 ‐ Patient selection
Risk of bias: could the selection of patients have introduced bias?
Signalling question 1: was a consecutive or random sample of patients enrolled? We answered ‘yes' if the study enrolled a consecutive or random sample of eligible patients; ‘no' if the study selected patients by convenience; and ‘unclear' if the study did not report the manner of patient selection or if we could not tell.
Signalling question 2: did the study avoid inappropriate exclusions?
a. For pulmonary tuberculosis, we answered 'yes' for all studies because we did not think there were any inappropriate exclusions for children presumed to have pulmonary tuberculosis. For tuberculous meningitis and lymph node tuberculosis, we answered ‘no' if the study excluded specimens based on physical appearance (such as purulence) or a biochemical analysis (e.g. adenosine deaminase (ADA) or cell analysis). We answered ‘unclear' if we could not tell.
Applicability: are there concerns that the included patients and setting do not match the review question?
We are interested in how Xpert performs in patients who were evaluated as they would be in routine practice. Paediatric studies conducted in tertiary centres tend to include a larger number of children with advanced disease; therefore we answered ‘low concern' if patients were evaluated in local hospitals or primary care centres; ‘high concern' if patients were evaluated exclusively as inpatients in tertiary care centres; and ‘unclear concern' if the clinical setting was not reported or if information was insufficient to justify a decision. We also answered ‘unclear concern' if Xpert testing was done at a reference laboratory and the clinical setting was not reported because it is difficult to tell if a given reference laboratory provides services mainly to very sick patients (inpatients in tertiary care) or to patients with a broad spectrum of disease, including very sick patients and those with less severe disease (primary, secondary, and tertiary care).
Domain 2 ‐ Index test
Risk of bias: could the conduct or interpretation of the index test have introduced bias?
Signalling question 1: were the index test results interpreted without knowledge of results of the reference standard? We answered this question ‘yes' for all studies because Xpert MTB/RIF and Xpert Ultra test results are automatically generated, and the user is provided with printable test results; thus there is no room for subjective interpretation of test results.
Signalling question 2: if a threshold was used, was it pre‐specified? The threshold is pre‐specified in all versions of Xpert MTB/RIF and Xpert Ultra. We answered this question ‘yes' for all studies.
Applicability: are there concerns that the index test, its conduct, or its interpretation differs from the review question? Variations in test technology, execution, or interpretation may affect estimates of the diagnostic accuracy of a test. GeneXpert, the test device platform, simplifies molecular testing by fully integrating and automating the three processes (sample preparation, amplification, and detection) required for real‐time polymerase chain reaction (PCR)‐based molecular testing. All steps in the Xpert MTB/RIF and Xpert Ultra assays are completely automated and self‐contained following sample loading. Minimal training is required for operators such as laboratory technicians and nurses to run the index test.
For pulmonary tuberculosis, we answered ‘low concern' if the index test was performed as recommended by the manufacturer. For sputum specimens, we answered ‘unclear concern' if the ratio of the Xpert sample reagent: specimen volume was not 2:1 for a raw specimen or 3:1 for a centrifuged sediment, as recommended by the manufacturer, or if we could not tell (WHO 2014a). Central‐level laboratories use more highly trained staff than peripheral‐ and intermediate‐level laboratories or health facilities. However, we do not consider this to be a concern about applicability due to the minimal training required to run the index tests.
With respect to extrapulmonary specimens, the WHO has provided detailed information about processing steps in the ‘Xpert MTB/RIF implementation manual'. Technical and operational "how‐to" practical considerations. Annex 2 ‐ Standard operating procedure (SOP) for processing extrapulmonary specimens (CSF, lymph nodes, and other tissues) for "Xpert MTB/RIF assay”' (WHO 2014b). For extrapulmonary specimens, we answered ‘low concern' if the test was performed according to WHO standard operating procedures. We answered ‘high concern' if the test was performed in a way that deviated from these recommendations. We answered ‘unclear concern' if we could not tell.
Domain 3 ‐ Reference standard
Risk of bias: could the reference standard, its conduct, or its interpretation have introduced bias?
Signalling question 1.a: is a culture reference standard likely to correctly classify the target condition? For pulmonary tuberculosis, tuberculous meningitis, and lymph node tuberculosis, we anticipated that the vast majority of studies would perform culture. Culture is generally considered the best reference standard for tuberculosis diagnosis. However, particularly in children with paucibacillary disease, tuberculosis is verified by culture in only 15% to 50% of cases, depending on disease severity, challenges of obtaining specimens, and resources (Graham 2015). Evaluation of multiple specimens may increase the yield of culture for confirming tuberculosis (Cruz 2012;Zar 2012). We answered ‘yes' for studies using multiple specimens and ‘unclear' for studies using only one specimen.
Signalling question 1.b: is the composite reference standard likely to correctly classify the target condition? A composite reference standard aims to classify children who were not detected by culture. The definition of the composite reference standard is heterogeneous across studies. Irrespective of how tuberculosis was defined in the publications, we classified children as having tuberculosis if they were presumed to have tuberculosis and were started on anti‐tuberculosis treatment. For a composite reference standard, we answered ‘unclear’ for all studies.
For rifampicin resistance, we answered ‘yes' if a study used phenotypic culture‐based drug susceptibility testing or MTBDRplus as the reference standard. As this is an inclusion criterion for the review, we answered 'yes' for all studies.
Signalling question 2: were the reference standard results interpreted without knowledge of results of the index test? For pulmonary tuberculosis, tuberculous meningitis, and lymph node tuberculosis, we answered ‘yes' if the reference test provided an automated result (e.g. MGIT 960); blinding was explicitly stated; or it was clear that the reference standard was performed at a separate laboratory or performed by different people. We answered ‘no' if the study stated that the reference standard result was interpreted with knowledge of the Xpert test result. We answered ‘unclear' if we could not tell. For rifampicin resistance, we answered ‘yes' if the reference test provided an automated result (e.g. MGIT 960 SIRE); blinding was explicitly stated; or it was clear that the reference standard was performed at a separate laboratory or performed by different people. We answered ‘no' if the study stated that the reference standard result was interpreted with knowledge of the Xpert test result. We answered‘unclear' if we could not tell.
Applicability: are there concerns that the target condition as defined by the reference standard does not match the question?
For pulmonary tuberculosis, tuberculous meningitis, and lymph node tuberculosis, we answered ‘high concern' if the included studies did not differentiate Mycobacterium tuberculosis complex isolated in culture from other mycobacteria using any speciation technique; ‘low concern' if speciation was performed using any technique; and ‘unclear concern' if we could not tell.
For rifampicin resistance, we considered applicability to be of ‘low concern' for all studies because the method used (phenotypic culture‐based drug susceptibility testing or MTBDRplus) is appropriate.
Domain 4 ‐ Flow and timing
Risk of bias: could the patient flow have introduced bias?
Signalling question 1: was there an appropriate interval between the index test and the reference standard? We expected to find for most included studies that specimens for Xpert and culture were obtained at the same time when patients were evaluated for presumed tuberculosis. Even if there were a delay of several days between index test and reference standards, tuberculosis is a chronic disease, and we consider misclassification of disease status to be unlikely, as long as treatment was not initiated in the interim. We answered ‘yes' if the index test and the reference standard were performed at the same time, or if the time interval was less than or equal to seven days; ‘no' if the time interval was greater than seven days; and ‘unclear' if we could not tell.
Signalling question 2: did all patients receive the same reference standard? We answered ‘yes' if all patients received the same reference standard; ‘no' if all patients did not receive the same reference standard; and ‘unclear' if we could not tell.
Signalling question 3: were all patients included in the analysis? We determined the answer to this question by comparing the number of patients enrolled with the number of patients included in the 2 × 2 tables. We answered ‘yes' if the numbers matched, and ‘no' if there were patients enrolled in the study who were not included in the analysis. We answered ‘unclear' if we could not tell.
Judgements for risk of bias assessments for a given domain.
If we answered all signalling questions for a domain ‘yes', then judged risk of bias as ‘low'.
If we answered all or most signalling questions for a domain ‘no', then we judged risk of bias as ‘high'.
If we answered only one signalling question for a domain ‘no', we discussed further the risk of bias judgement.
If we answered all or most signalling questions for a domain ‘unclear', then we judged risk of bias as ‘unclear'.
If we answered only one signalling question for a domain ‘unclear', we discussed further the risk of bias judgement.
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
1. Test.
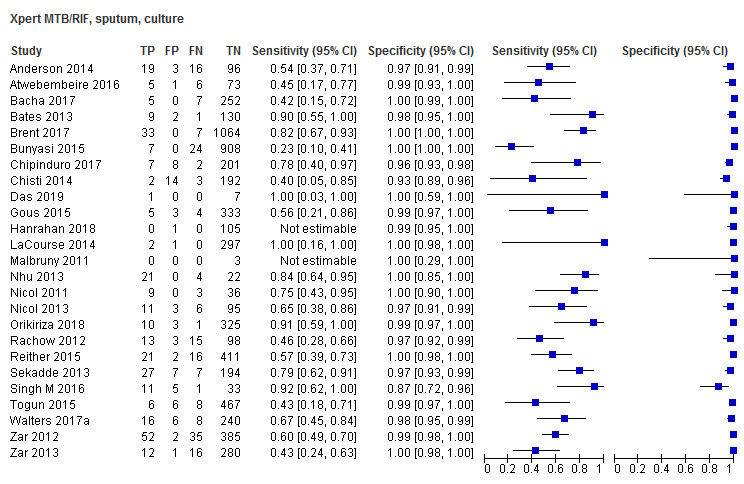
Xpert MTB/RIF, sputum, culture
2. Test.
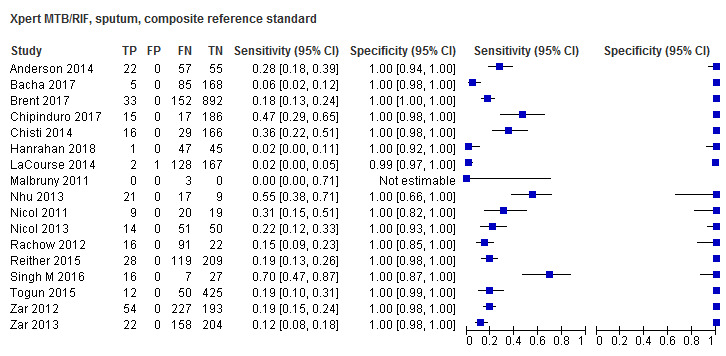
Xpert MTB/RIF, sputum, composite reference standard
3. Test.
Xpert Ultra, sputum, culture
4. Test.
Xpert Ultra, sputum, composite reference standard
5. Test.

Xpert MTB/RIF, gastric aspirate specimen, culture
6. Test.
Xpert MTB/RIF, gastric aspirate specimen, composite reference standard
7. Test.
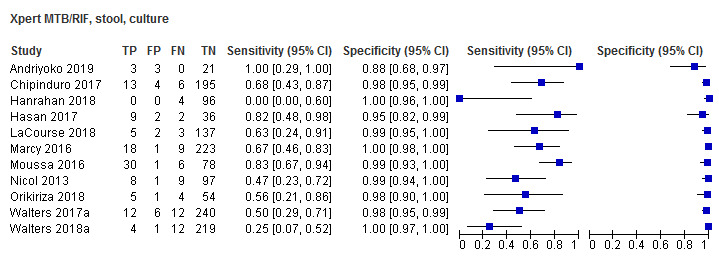
Xpert MTB/RIF, stool, culture
8. Test.

Xpert MTB/RIF, stool, composite reference standard
9. Test.

Xpert MTB/RIF, nasopharyngeal aspirate, culture
10. Test.

Xpert Ultra, nasopharyngeal aspirate, culture
11. Test.

Xpert MTB/RIF, sputum, culture, direct comparison
12. Test.
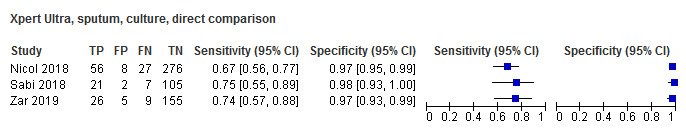
Xpert Ultra, sputum, culture, direct comparison
13. Test.

Xpert MTB/RIF, sputum, smear‐positive, culture
14. Test.
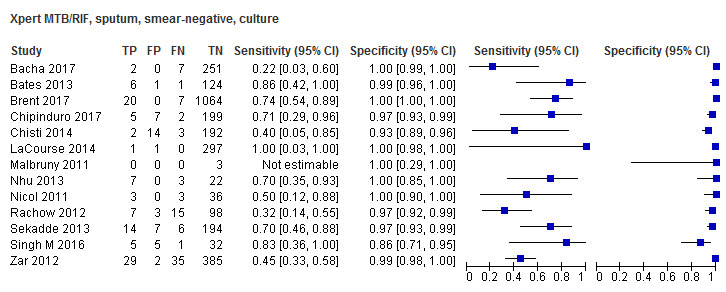
Xpert MTB/RIF, sputum, smear‐negative, culture
15. Test.
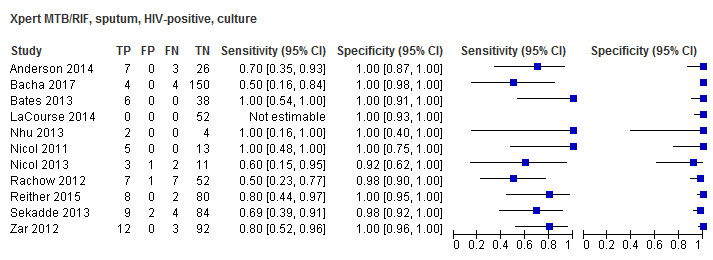
Xpert MTB/RIF, sputum, HIV‐positive, culture
16. Test.
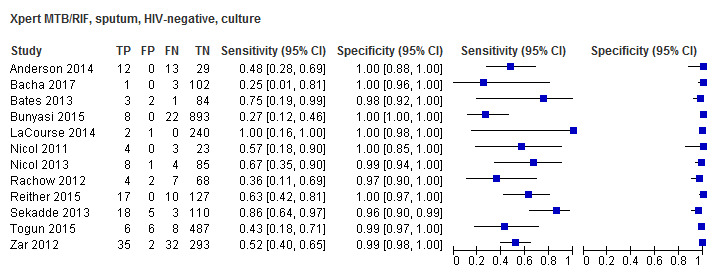
Xpert MTB/RIF, sputum, HIV‐negative, culture
17. Test.
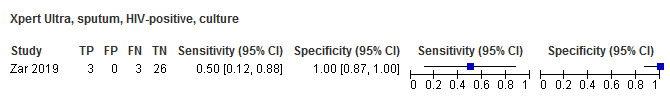
Xpert Ultra, sputum, HIV‐positive, culture
18. Test.

Xpert Ultra, sputum, HIV‐negative, culture
19. Test.

Xpert MTB/RIF, gastric aspirate specimen, HIV‐positive, culture
20. Test.
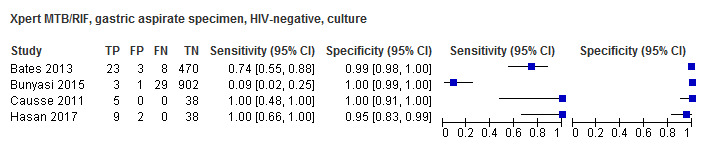
Xpert MTB/RIF, gastric aspirate specimen, HIV‐negative, culture
21. Test.
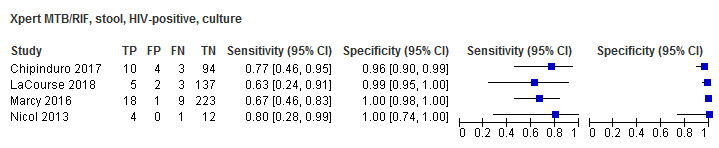
Xpert MTB/RIF, stool, HIV‐positive, culture
22. Test.
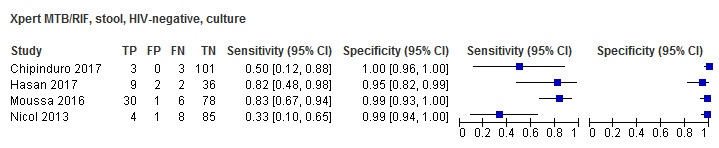
Xpert MTB/RIF, stool, HIV‐negative, culture
23. Test.
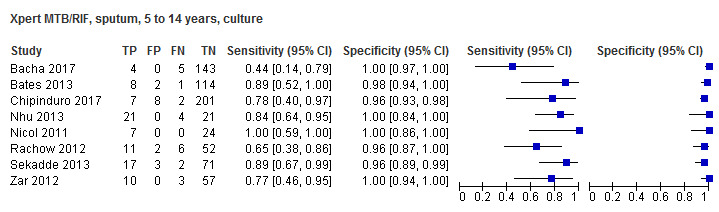
Xpert MTB/RIF, sputum, 5 to 14 years, culture
24. Test.
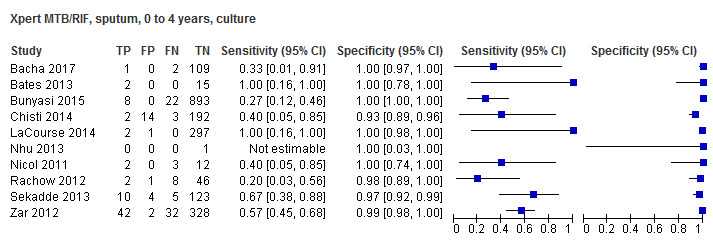
Xpert MTB/RIF, sputum, 0 to 4 years, culture
25. Test.
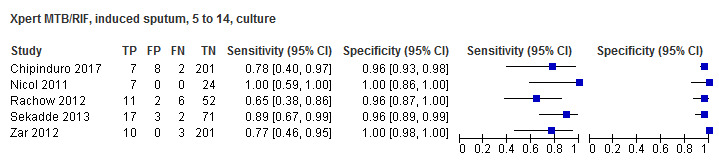
Xpert MTB/RIF, induced sputum, 5 to 14, culture
26. Test.
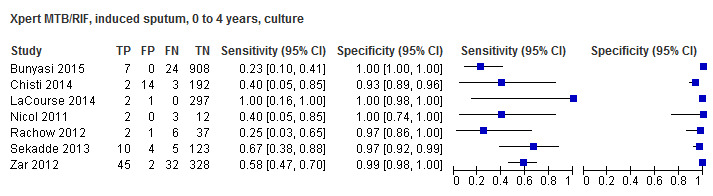
Xpert MTB/RIF, induced sputum, 0 to 4 years, culture
27. Test.
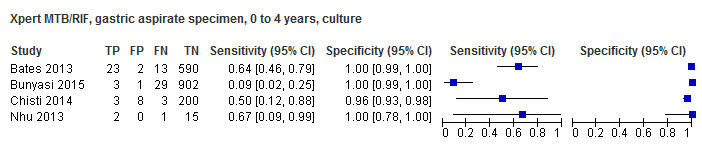
Xpert MTB/RIF, gastric aspirate specimen, 0 to 4 years, culture
28. Test.
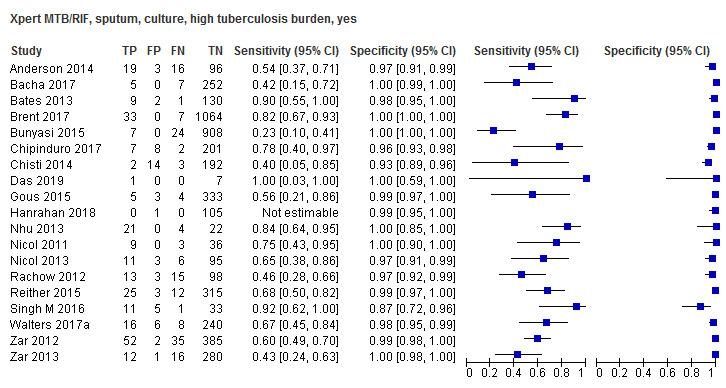
Xpert MTB/RIF, sputum, culture, high tuberculosis burden, yes
29. Test.
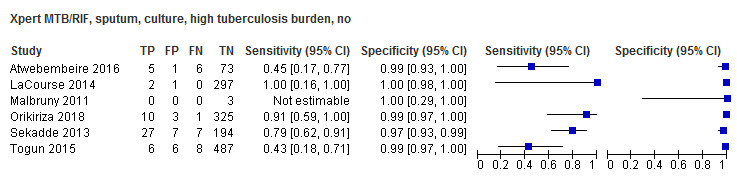
Xpert MTB/RIF, sputum, culture, high tuberculosis burden, no
30. Test.
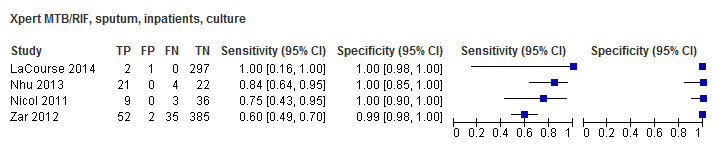
Xpert MTB/RIF, sputum, inpatients, culture
31. Test.
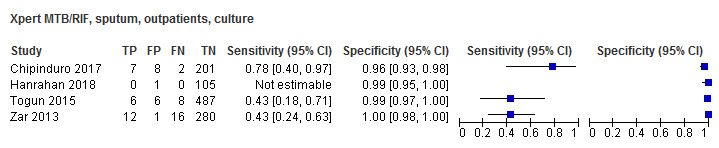
Xpert MTB/RIF, sputum, outpatients, culture
32. Test.
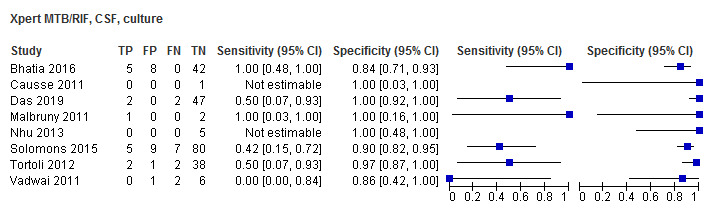
Xpert MTB/RIF, CSF, culture
33. Test.

Xpert MTB/RIF, CSF, composite reference standard
34. Test.
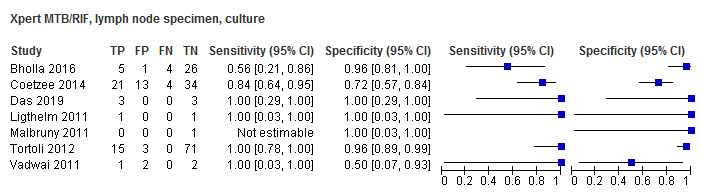
Xpert MTB/RIF, lymph node specimen, culture
35. Test.
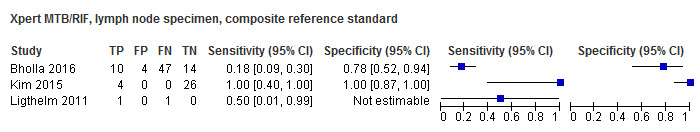
Xpert MTB/RIF, lymph node specimen, composite reference standard
36. Test.

Xpert MTB/RIF, rifampicin resistance, any specimen
37. Test.
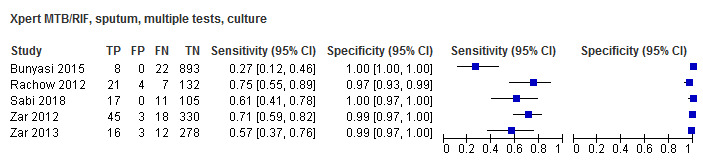
Xpert MTB/RIF, sputum, multiple tests, culture
38. Test.

Xpert MTB/RIF, sputum, initial test, culture
39. Test.
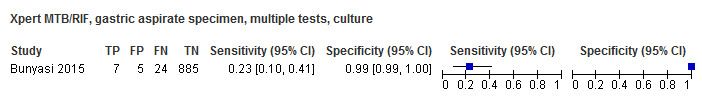
Xpert MTB/RIF, gastric aspirate specimen, multiple tests, culture
40. Test.
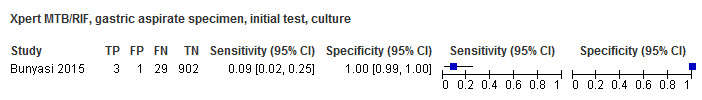
Xpert MTB/RIF, gastric aspirate specimen, initial test, culture
41. Test.

Xpert MTB/RIF, stool, multiple tests, culture
42. Test.

Xpert MTB/RIF, stool, initial test, culture
43. Test.

Xpert MTB/RIF, nasopharyngeal aspirate, multiple tests, culture
44. Test.

Xpert MTB/RIF, nasopharyngeal aspirate, initial test, culture
45. Test.

Xpert Ultra, sputum, multiple tests, culture
46. Test.

Xpert Ultra, sputum, initial test, culture
47. Test.

Xpert Ultra, nasopharyngeal aspirate, multiple tests, culture
48. Test.

Xpert Ultra, nasopharyngeal aspirate, initial test, culture
49. Test.
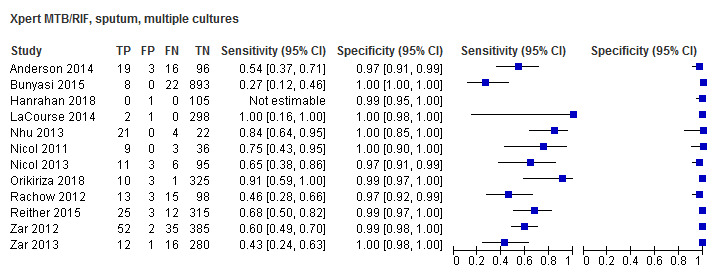
Xpert MTB/RIF, sputum, multiple cultures
50. Test.
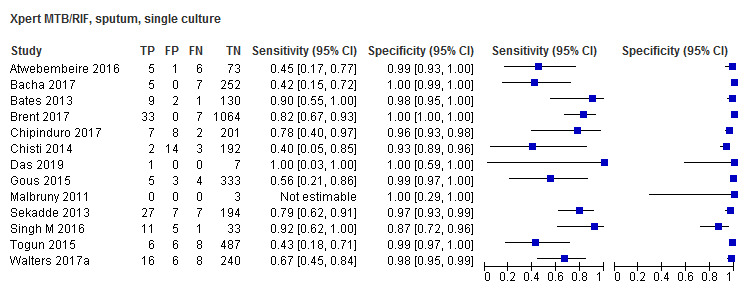
Xpert MTB/RIF, sputum, single culture
51. Test.

Xpert MTB/RIF, induced sputum, culture
52. Test.

Xpert MTB/RIF, gastric aspirate specimen, 5 to 14, culture
53. Test.
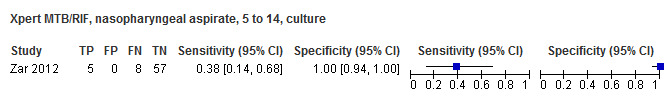
Xpert MTB/RIF, nasopharyngeal aspirate, 5 to 14, culture
54. Test.

Xpert MTB/RIF, nasopharyngeal aspirate, 0 to 4, culture
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anderson 2014.
| Study characteristics | |||
| Patient Sampling | Cohort study, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: persistent cough for > 2 weeks; pneumonia not responding to first‐line antibiotics; unexplained fever for > 2 weeks; unexplained progressive weight loss or failure to thrive for > 4 weeks; history of close TB contact; and/or a doctor’s clinical suspicion of TB for any other reason. Children with ≥ 1 of these features plus those referred for outpatient investigation for TB and children < 5 years old who were identified as household TB contacts of smear‐positive pulmonary TB were eligible for inclusion in the study Age: range for study population 0 to 180 months; culture‐confirmed cases: median (interquartile range) 37 (12 to 104) months Sex, female: 35% HIV infection: 40% Sample size included for analysis: 134 Clinical setting: inpatient and outpatient Laboratory level where index test was performed: not reported Country: Kenya World Bank income classification: middle income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 26% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis MGIT; composite reference standard; clinical reference standard (received tuberculosis treatment) |
||
| Flow and timing | 87% of enrolled children were excluded before selection for the array study for feasibility | ||
| Comparative | |||
| Notes | Primary aim of study was to evaluate a gene signature for TB diagnosis | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Andriyoko 2019.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: presumptive TB symptoms (no additional details) Age: median (IQR) 17 (5.5 to 78) months Sex: NR HIV infection: NR Sample size included for analysis: 27 Clinical setting: inpatient Laboratory level where index test was performed: intermediate Country: Indonesia World Bank income classification: middle income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 22% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis Xpert test on standard specimens (7 induced sputum and 20 gastric specimens) |
||
| Flow and timing | Index and reference test specimens were collected within 7 days | ||
| Comparative | |||
| Notes | No consensus has been reached on appropriate stool processing methods; this study used gravity separation | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Atwebembeire 2016.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: presumptive TB symptoms (no additional details) Age: 18% younger than 60 months; 82% 60 months or older Sex, female: 55% HIV infection: 31% Sample size included for analysis: 85 Clinical setting: inpatient Laboratory level where index test was performed: research Country: Uganda World Bank income classification: low income High TB burden country: no High TB/HIV burden country: yes High MDR‐TB burden country: no Prevalence of tuberculosis cases in the study: 13% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis MGIT; LJ |
||
| Flow and timing | Index and reference test specimens were collected within 7 days of each other | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Bacha 2017.
| Study characteristics | |||
| Patient Sampling | Cohort, consecutive, retrospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: clinicians used a combination of TB screening questions (e.g. history of known TB contact, failure to gain weight/weight loss, persistent cough, persistent fever, reduced activities, irritability) and clinical examination findings (e.g. malnutrition, lymphadenopathy, abnormal lung findings) based on national guidelines Age: mean (range) 60 (1 to 168) months Sex, female: 52% HIV infection: 54% Sample size included for analysis: 286 Clinical setting: inpatient and outpatient Laboratory level where index test was performed: research Country: Tanzania World Bank income classification: low income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: no Prevalence of tuberculosis cases in the study: 13% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis Microbiological confirmation (culture type not reported); composite reference standard; clinical reference standard (received tuberculosis treatment) |
||
| Flow and timing | Xpert and reference test performed within 7 days of each other | ||
| Comparative | |||
| Notes | All patients did not have a specimen collected for the microbiological reference standard | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Bates 2013.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional design, manner of selection not reported, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: suspected tuberculosis defined as having tuberculosis on the basis of a symptom‐and‐risk factor screen (≥ 1 of 5 factors: cough for longer than 2 weeks, weight loss, malnutrition, HIV, or tuberculosis contact) according to the Zambia National TB Programme and WHO guidelines
Age: median (IQR) 24 (12 to 74) months
Sex, female: not reported
HIV infection: 32%
Sample size included for analysis: 142 for expectorated sputum; 788 for gastric aspirate lavage
Clinical setting: inpatient
Laboratory level where index test was performed: university hospital laboratory (tertiary referral centre) Country: Zambia World Bank income classification: middle income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: no Prevalence of TB cases in the study: 5% |
||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Target condition: pulmonary tuberculosis Reference standard: MGIT |
||
| Flow and timing | Index and reference tests were collected simultaneously | ||
| Comparative | |||
| Notes | Only 1 culture was used to exclude TB, indicating an unclear ability of the reference standard to classify the target condition | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Bhatia 2016.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, manner of selection not reported, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: clinically suspected TBM (fever > 1 week, headache, vomiting, neck stiffness, convulsions, focal deficits, altered consciousness, history of TB contact) Age: median 59 months Sex, female: 41% HIV infection: not reported Sample size included for analysis: 55 Clinical setting: inpatient Laboratory level where index test was performed: regional Country: India World Bank income classification: middle income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 10% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Tuberculous meningitis MGIT; composite reference standard; clinical reference standard |
||
| Flow and timing | Index test and reference standard were collected simultaneously | ||
| Comparative | |||
| Notes | Only 1 culture was used to exclude TB, indicating an unclear ability of the reference standard to classify the target condition | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Bholla 2016.
| Study characteristics | |||
| Patient Sampling | Cohort, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: ≥ 1 palpable lymph nodes ≥ 1 cm persisting for longer than 4 weeks in spite of oral antibiotic therapy and a strong clinical suspicion or microbiological confirmation of mycobacterial infection Age: 0 to 60 months (59%); 61 to 204 months (41%) Sex, female: 39% HIV infection: 20% Sample size included for analysis: 75 Clinical setting: not reported Laboratory level where index test was performed: not reported Country: Tanzania World Bank income classification: low income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: no Prevalence of tuberculosis cases in the study: 9% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Lymph node tuberculosis MGIT; composite reference standard; clinical reference standard |
||
| Flow and timing | Index and reference tests performed simultaneously | ||
| Comparative | |||
| Notes | There was a high percentage of culture contamination (48%), and only 1 culture was used to exclude TB, indicating an unclear ability of the reference standard to classify the target condition | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Brent 2017.
| Study characteristics | |||
| Patient Sampling | Cohort, manner of selection not reported, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: children aged < 15 years were investigated for TB if ≥ 1 of the following features of suspected TB were present and they were not already on TB treatment; unexplained persistent cough for > 2 weeks; pneumonia not responding to first‐line antibiotics; unexplained fever for > 2 weeks; unexplained progressive weight loss or failure to thrive for > 4 weeks; history of close contact with a suspected or confirmed case of pulmonary TB; clinical suspicion of TB for any other reason Age, months: confirmed group median (IQR) 53 (21 to 112) Sex, female: confirmed group 39% HIV infection: confirmed group 28% Sample size included for analysis: 1442 Clinical setting: not reported Laboratory level where index test was performed: not reported Country: Kenya World Bank income classification: middle income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 4% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis MGIT; composite reference standard; clinical reference standard |
||
| Flow and timing | Index and reference tests were collected simultaneously | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Bunyasi 2015.
| Study characteristics | |||
| Patient Sampling | Randomized controlled trial, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: children were followed quarterly for ≥ 2 years to identify signs, symptoms, or household exposure that merited investigation for suspected TB disease, for example, weight loss in the preceding 2 months, cough for longer than 2 weeks without improvement, failure to thrive, conversion to a positive test of TB infection Age, months: median (IQR) 16.8 (12.0 to 22.1) Sex, female: 54% HIV infection: 0% Sample size included for analysis: 1020 Clinical setting: inpatient Laboratory level where index test was performed: not reported Country: South Africa World Bank income classification: middle income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 3% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis MGIT |
||
| Flow and timing | Index test and reference standard were collected simultaneously | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Causse 2011.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: presumptive tuberculosis Age, months: mean 43 Sex, female: not reported HIV infection: 0% Sample size included for analysis: 44 Clinical setting: not reported Laboratory level where index test was performed: central Country: Spain World Bank income classification: high income High TB burden country: no High TB/HIV burden country: no High MDR‐TB burden country: no Prevalence of tuberculosis cases in the study: not reported | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary TB; tuberculous meningitis Liquid culture (Middlebrook) |
||
| Flow and timing | Index and reference tests were collected simultaneously | ||
| Comparative | |||
| Notes | It is unclear whether those interpreting the reference test were blinded to results of the index test | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Chipinduro 2017.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, manner of selection not reported, unknown | ||
| Patient characteristics and setting | Presenting signs and symptoms: participants were children 5 to 16 years of age presenting with a chronic cough of 2 weeks and any of the classic signs and symptoms of TB, including weight loss, loss of appetite, persistent fever without an apparent cause, night sweats, or history of close contact with a TB index patient, defined as living in close proximity (sharing a room within a household) with an adult diagnosed with TB within the preceding 12 months Age, months: median 127 Sex, female: 56% HIV infection: 50% Sample size included for analysis: 218 Clinical setting: outpatient Laboratory level where index test was performed: not reported Country: Zimbabwe World Bank income classification: middle income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 9% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis Solid culture (LJ); composite reference standard; clinical reference standard |
||
| Flow and timing | Index test and reference standard were collected within pre‐specified interval | ||
| Comparative | |||
| Notes | Study evaluated Xpert MTB/RIF in stool and induced sputum; no consensus has been reached on the proper stool processing method for Xpert; this study used centrifugation | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Chisti 2014.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: respiratory symptoms (cough and/or respiratory distress) and radiological pneumonia Age, months: mean 12 Sex, female: not reported HIV infection: not reported Sample size included for analysis: 214 Clinical setting: inpatient Laboratory level where index test was performed: research laboratory Country: Bangladesh World Bank income classification: low income High TB burden country: yes High TB/HIV burden country: no High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 5% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis Solid culture (LJ); composite reference standard; clinical reference standard |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Coetzee 2014.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: all children < 13 years of age who were referred to the fine needle aspirate clinics or were admitted to the inpatient wards/clinics of both hospitals with persistent superficial lymphadenopathy and clinical suspicion of mycobacterial infection were included in the study Age, months: median 23 Sex, female: 40% HIV infection: 8% Sample size included for analysis: 72 Clinical setting: inpatient and outpatient Laboratory level where index test was performed: intermediate Country: South Africa World Bank income classification: middle income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 35% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Lymph node TB MGIT |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Das 2019.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: ≥ 1 of the following symptoms persisting for > 2 weeks without sustained improvement or resolution following appropriate treatment for other potential diagnoses (e.g. antibiotics for pneumonia; antimalarials for fever; nutritional support for failure to thrive): cough, fever, weight loss, or failure to thrive. Signs and symptoms of meningitis not responding to antibiotic treatment, with a subacute onset and/or raised intracranial pressure. Non‐painful enlarged lymph nodes without fistula formation Age, months: 45% 0 to 60, 55% 61 to 180 Sex, female: 40% HIV infection: 2% Sample size included for analysis: 171 Clinical setting: inpatient and outpatient Laboratory level where index test was performed: university hospital laboratory Country: India World Bank income classification: middle income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 10% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis; lymph node tuberculosis; tuberculosis meningitis Solid culture (LJ) |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Gous 2015.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: clinician suspicion of pulmonary tuberculosis Age, months: median 24 Sex, female: not reported HIV infection: not reported Sample size included for analysis: 345 Clinical setting: not reported Laboratory level where index test was performed: intermediate Country: South Africa World Bank income classification: middle income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 4% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis MGIT |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | Children younger than 5 years had small volumes of sputum collected via physiotherapy‐supported expectoration; Xpert was performed after "top up" of these small volumes with normal saline | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Hanrahan 2018.
| Study characteristics | |||
| Patient Sampling | Cohort, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: inclusion criteria for presumptive tuberculosis: (i) children 60 days to ≤ 10 years of age were eligible if they presented with ≥ 1 signs and symptoms of presumptive TB: persistent, non‐remitting, and unexplained cough for ≥ 2 weeks; (ii) unexplained weight loss > 5% in the last 3 months or failure to thrive, and not responding to nutritional rehabilitation (or antiretroviral treatment if HIV‐positive); (iii) persistent and unexplained fever for 1 week or longer; persistent, unexplained lethargy or decrease in playfulness/activity. Symptomatic child household contacts of an adult diagnosed with TB were also offered participation in the study regardless of symptom duration Age, months: median (IQR) 21.4 (12 to 43) Sex, female: 47% HIV infection: 18% Sample size included for analysis: 99 Clinical setting: outpatient Laboratory level where index test was performed: Central Country: South Africa World Bank income classification: Upper middle income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 3% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis MGIT; composite reference standard |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | Stool was included as a specimen for index testing; no protocol has been established for stool processing; the approach used in this study was centrifugation; method used for confirmation of M tuberculosis by culture was not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Hasan 2017.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, convenience, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: children younger than 15 years based on clinical symptoms, chest radiography, and Kenneth Jones score ≥ 5 Age, months: median (IQR) 82 (24 to 108) Sex, female: 22% HIV infection: 0% Sample size included for analysis: 50 Clinical setting: inpatient and outpatient Laboratory level where index test was performed: intermediate Country: Pakistan World Bank income classification: middle income High TB burden country: yes High TB/HIV burden country: no High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 18% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis MGIT; composite reference standard; clinical reference standard |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | The index test was performed on stool; no stool processing method for Xpert has been established; centrifugation was used in this study | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Kasa Tom 2018.
| Study characteristics | |||
| Patient Sampling | Cohort, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: children younger than 15 years with presumptive TB symptoms Age, months: median (IQR) 36 (17 to 72) Sex, female: 35% HIV infection: 18% Sample size included for analysis: 93 Clinical setting: inpatient Laboratory level where index test was performed: central Country: Papa New Guinea World Bank income classification: middle income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 95% composite | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis Composite reference standard |
||
| Flow and timing | Index and reference data were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | Only some children received the microbiological reference standard, so only a composite reference standard was applied | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Kim 2015.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, retrospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: children 0 to 18 years of age evaluated for extrapulmonary tuberculosis in whom Xpert MTB/RIF was used, as well as culture, and who were not on treatment for tuberculosis Age, months: not reported Sex, female: not reported HIV infection: not reported Sample size included for analysis: 30 Clinical setting: inpatient Laboratory level where index test was performed: regional Country: South Korea World Bank income classification: high income High TB burden country: no High TB/HIV burden country: no High MDR‐TB burden country: no Prevalence of tuberculosis cases in the study: 13% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Lymph node tuberculosis Composite reference standard |
||
| Flow and timing | Index and reference tests were performed within pre‐specified time period | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
LaCourse 2014.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: enrollees met WHO severe acute malnutrition criteria for children aged 6 to 60 months with weight‐for‐height z‐score ≤ 3 standard deviations below the median, mid‐upper arm circumference (MUAC) ≤ 115 mm, or bilateral pedal oedema Age, months: median (IQR0 18 (12 to 26) Sex, female: 51% HIV infection: 18% Sample size included for analysis: 300 Clinical setting: inpatient Laboratory level where index test was performed: research Country: Malawi World Bank income classification: low income High TB burden country: no High TB/HIV burden country: yes High MDR‐TB burden country: no Prevalence of tuberculosis cases in the study: 0.6% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis MGIT; composite reference standard; clinical reference standard |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
LaCourse 2018.
| Study characteristics | |||
| Patient Sampling | Randomized controlled trial, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: all children living with HIV who were hospitalized with an acute illness Age, months: median (IQR) 24 (13 to 58) Sex, female: 45% HIV infection: 100% Sample size included for analysis: 165 Clinical setting: inpatient Laboratory level where index test was performed: central Country: Kenya World Bank income classification: middle income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 6% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis MGIT; composite reference standard; clinical reference standard |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | No protocol for stool processing has been established; this study used the centrifugation method in a central laboratory | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Ligthelm 2011.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, manner of selection not reported, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: not reported Age, months: mean 36 Sex, female: not reported HIV infection: not reported Sample size included for analysis: 50 Clinical setting: inpatient and outpatient Laboratory level where index test was performed: central Country: South Africa World Bank income classification: middle income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 60% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Lymph node tuberculosis MGIT; composite reference standard |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Malbruny 2011.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, manner of selection not reported, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: clinically suspected tuberculosis Age, months: mean 88 Sex, female: not reported HIV infection: not reported Sample size included for analysis: 12 Clinical setting: laboratory Laboratory level where index test was performed: research laboratory Country: France World Bank income classification: high income High TB burden country: no High TB/HIV burden country: no High MDR‐TB burden country: no Prevalence of tuberculosis cases in the study: 8% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary TB, lymph node TB, tuberculous meningitis Solid culture; composite reference standard |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Marcy 2016.
| Study characteristics | |||
| Patient Sampling | Cohort, manner of selection not reported, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: children aged ≤ 13 years with suspicion of intrathoracic tuberculosis based on ≥ 1 of the following: persistent cough; persistent fever; failure to thrive, defined as recent deviation in the growth curve or weight‐for‐age z‐score < −2 standard deviations; failure of broad‐spectrum antibiotics for pulmonary infection; suggestive chest radiograph anomaly Age, months: median (IQR) 86 (49 to 85) Sex, female: 49% HIV infection: 100% Sample size included for analysis: 272 Clinical setting: not reported Laboratory level where index test was performed: not reported Country: Burkina Faso; Cambodia; Cameroon; Vietnam World Bank income classification: low and middle income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 11% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis MGIT and LJ; composite reference standard |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | No method for stool processing with Xpert has been established; this study used centrifugation | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Moussa 2016.
| Study characteristics | |||
| Patient Sampling | Cohort, manner of selection not reported, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: children older than 1 year and younger than 15 years with clinical signs of PTB Age, months: not reported Sex, female: 39% HIV infection: 0% Sample size included for analysis: 115 Clinical setting: not reported Laboratory level where index test was performed: intermediate Country: Egypt World Bank income classification: middle income High TB burden country: no High TB/HIV burden country: no High MDR‐TB burden country: no Prevalence of tuberculosis cases in the study: 31% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis Solid culture (LJ); composite reference standard; clinical reference standard |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | No method for stool processing before Xpert has been established; this study used centrifugation | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Myo 2018.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, manner of selection not reported, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: children 13 years of age or younger with suspected PTB were recruited into the study. PTB was suspected if a child had cough for ≥ 14 days and any of the following features; fever for 7 days, failure to thrive, unexplained loss of appetite, or lethargy Age, months: median 48 Sex, female: 47% HIV infection: 13% Sample size included for analysis: 231 Clinical setting: inpatient Laboratory level where index test was performed: central Country: Myanmar World Bank income classification: middle income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 7% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis Solid culture (LJ); composite reference standard; clinical reference standard |
||
| Flow and timing | Index and reference test specimens were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Nhu 2013.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: clinical suspicion of tuberculosis Age, months: median 106 Sex, female: not reported HIV infection: 10% Sample size included for analysis: 72 Clinical setting: inpatient Laboratory level where index test was performed: intermediate Country: Vietnam World Bank income classification: middle income High TB burden country: yes High TB/HIV burden country: no High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 30% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis and tuberculous meningitis MGIT; composite reference standard; clinical reference standard |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Nicol 2011.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: cough for longer than 14 days and 1 of the following: household contact infected with tuberculosis within previous 3 months, loss of weight or failure to gain weight in previous 3 months, positive skin test to purified protein derivative, or chest radiograph suggestive of pulmonary tuberculosis Age, months: median 72 Sex, female: not reported HIV infection: 37% Sample size included for analysis: 48 Clinical setting: inpatient Laboratory level where index test was performed: intermediate Country: South Africa World Bank income classification: middle income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 15% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis MGIT; composite reference standard; clinical reference standard |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Nicol 2013.
| Study characteristics | |||
| Patient Sampling | Cohort, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: children 0 to 14 years of age with cough lasting longer than 2 weeks and at least 1 of the following: (1) household tuberculosis contact in prior 3 months, (2) weight loss or failure to gain weight in previous 3 months, (3) positive tuberculin skin test, or (4) chest radiograph suggestive of PTB Age, months: median (IQR) 31 (19 to 57) Sex, female: not reported HIV infection: 15% Sample size included for analysis: 115 Clinical setting: outpatient and inpatient Laboratory level where index test was performed: intermediate Country: South Africa World Bank income classification: middle income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 15% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis MGIT; composite reference standard; clinical reference standard |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | No consensus has been reached about stool processing techniques on stool before Xpert; this study used centrifugation | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Nicol 2018.
| Study characteristics | |||
| Patient Sampling | Cohort, manner of selection not reported, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: children 0 to 14 years of age with cough lasting longer than 2 weeks and ≥ 1 of the following: (1) household tuberculosis contact in prior 3 months, (2) weight loss or failure to gain weight in previous 3 months, (3) positive tuberculin skin test, or (4) chest radiograph suggestive of PTB Age, months: median (IQR) 33 (15 to 74) Sex, female: 49% HIV infection: 19% Sample size included for analysis: 367 Clinical setting: inpatient and outpatient Laboratory level where index test was performed: research laboratory Country: South Africa World Bank income classification: middle income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 20% | ||
| Index tests | Xpert Ultra and Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis MGIT; composite reference standard; clinical reference standard |
||
| Flow and timing | Index and reference tests were performed within pre‐specified time period | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Orikiriza 2018.
| Study characteristics | |||
| Patient Sampling | Cohort, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: children 1 month to 14 years of age with presumptive TB defined as presence of ≥ 1 clinical sign suggestive of TB or TB contact history with abnormal chest X‐ray, or any child with chest X‐ray suggestive of TB Age, months: not reported Sex, female: 45% HIV infection: 30% Sample size included for analysis: 357 Clinical setting: inpatient and outpatient Laboratory level where index test was performed: intermediate Country: Uganda World Bank income classification: low income High TB burden country: no High TB/HIV burden country: yes High MDR‐TB burden country: no Prevalence of tuberculosis cases in the study: 4% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis Solid and liquid cultures (LJ and MGIT); composite reference standard; clinical reference standard |
||
| Flow and timing | Index and reference tests were obtained within pre‐specified time period | ||
| Comparative | |||
| Notes | No consensus method for stool processing before Xpert is known; this study used centrifugation | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Pang 2014.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: TB suspects had at least 1 of the following symptoms: cough for longer than 2 weeks, fever for longer than 2 weeks, weight loss, TB contact history, and radiological features Age, months: range 0 to 180 Sex, female: 39% HIV infection: not reported Sample size included for analysis: 211 Clinical setting: inpatient Laboratory level where index test was performed: central Country: China World Bank income classification: middle income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 8% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis MGIT; composite reference standard; clinical reference standard |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | Study included only smear‐negative participants | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Rachow 2012.
| Study characteristics | |||
| Patient Sampling | Cohort, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: children with 6 weeks to 14 years of clinical signs of tuberculosis were enrolled in the study and were prospectively followed up for a minimum of 12 months. All children had ≥ 1 of the following symptoms: persistent unremitting cough for.21 days; repeated episodes of fever within the last 21 days; weight loss or failure to thrive within the previous 3 months; or signs and symptoms suggestive of extrapulmonary tuberculosis Age, months: median (IQR) 70 (29 to 170) Sex, female: 48% HIV infection: 51% Sample size included for analysis: 129 Clinical setting: inpatient and outpatient Laboratory level where index test was performed: research laboratory Country: Tanzania World Bank income classification: low income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: no Prevalence of tuberculosis cases in the study: 22% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis Solid and liquid culture (LJ and MGIT); composite reference standard; clinical reference standard |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Reither 2015.
| Study characteristics | |||
| Patient Sampling | Cohort, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: ≥ 1 of the following inclusion criteria had to be met: persistent non‐remitting cough longer than 14 days not responding to a course of antibiotics; repeated episodes of fever within the last 14 days not responding to a course of antibiotics, after malaria has been excluded; weight loss or failure to thrive within previous 3 months Age, months: median (IQR) 67 (67 to 118) Sex, female: 51% HIV infection: 44% Sample size included for analysis: 356 Clinical setting: inpatient and outpatient Laboratory level where index test was performed: research laboratory Country: Uganda and Tanzania World Bank income classification: low income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: no Prevalence of tuberculosis cases in the study: 10% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis Solid and liquid culture (MGIT and LJ); composite reference standard; clinical reference standard |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Sabi 2018.
| Study characteristics | |||
| Patient Sampling | Cohort, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: children must have had 1 of the following symptoms: persistent non‐remitting cough longer than 14 days not responding to antibiotics; repeated episodes of fever within the last 14 days not responding to antibiotics, after malaria has been excluded; weight loss or failure to thrive during previous 3 months; signs and symptoms suggestive of extrapulmonary TB Age, months: median (IQR) 65 (18 to 120) Sex, female: 43% HIV infection: 52% Sample size included for analysis: 215 Clinical setting: inpatient and outpatient Laboratory level where index test was performed: research laboratory Country: Tanzania World Bank income classification: low income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: no Prevalence of tuberculosis cases in the study: 13% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis Solid and liquid culture (MGIT and LJ); composite reference standard; clinical reference standard |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | Ultra was performed on frozen specimens | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Saini 2018.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, manner of selection not reported, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: cough for longer than 2 weeks, fever, lethargy, contact with an adult patient with TB, positive tuberculin skin test, or chest radiograph consistent with TB. In addition, the patient needed to have negative testing by AFB smear and Xpert before moving to bronchoscopy for BAL Age, months: median (IQR) 120 (66 to 156) Sex, female: 53% HIV infection: 0% Sample size included for analysis: 41 Clinical setting: inpatient Laboratory level where index test was performed: central Country: India World Bank income classification: middle income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 27% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis MGIT; composite reference standard; clinical reference standard |
||
| Flow and timing | Participants were enrolled for BAL sample collection if they had probable TB and negative testing by AFB smear and Xpert by standard less invasive methods | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Sekadde 2013.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: persistent cough of 2 weeks or longer and 1 of the following: household TB contact, unexplained weight loss or failure to gain weight, unexplained fever for 2 weeks or longer Age, months: median (IQR) 36 (16 to 75) Sex, female: 46% HIV infection: 41% Sample size included for analysis: 235 Clinical setting: outpatient Laboratory level where index test was performed: central Country: Uganda World Bank income classification: low income High TB burden country: no High TB/HIV burden country: yes High MDR‐TB burden country: no Prevalence of tuberculosis cases in the study: 14% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis Solid and liquid culture (MGIT and LJ) |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Singh M 2016.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: ≥ 1 of the following: persistent fever and/or cough for ≥ 2 weeks, loss of weight/no weight gain, and history of contact with an infectious TB case Age, months: median (IQR) 64 (2 to 144) Sex, female: 38% HIV infection: not reported Sample size included for analysis: 50 Clinical setting: inpatient Laboratory level where index test was performed: intermediate Country: India World Bank income classification: middle income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 24% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis MGIT; composite reference standard; clinical reference standard |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | IS and GLA samples were combined; study was analysed as a sputum specimen | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Solomons 2015.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, manner of selection not reported, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: age 3 months to 13 years with clinical suspicion of meningitis, CSF sample collected for fluorescent auramine‐O microscopy, TB culture, Xpert assays Age, months: median (IQR) 36 (21 to 54) Sex, female: 44% HIV infection: 8% Sample size included for analysis: 101 Clinical setting: inpatient Laboratory level where index test was performed: intermediate Country: South Africa World Bank income classification: middle income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 12% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Tuberculous meningitis MGIT; composite reference standard; clinical reference standard |
||
| Flow and timing | Index and reference tests were collected in pre‐specified time period | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Togun 2015.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: unremitting cough ≥ 14 days and ≥ 1 of fever, weight loss or failure to thrive, malaise or fatigue, haemoptysis, night sweats, or enlarged cervical lymph nodes Age, months: median (IQR) 72 (36 to 108) Sex, female: 47% HIV infection: 0% Sample size included for analysis: 487 Clinical setting: outpatient Laboratory level where index test was performed: central Country: Gambia World Bank income classification: low income High TB burden country: no High TB/HIV burden country: no High MDR‐TB burden country: no Prevalence of tuberculosis cases in the study: 3% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis Solid and liquid culture (MGIT or LJ); composite reference standard; clinical reference standard |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Tortoli 2012.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, manner of selection not reported, retrospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: Not reported Age, months: median 84 Sex, female: not reported HIV infection: not reported Sample size included for analysis: 174 pulmonary tuberculosis; 89 LNTB; 43 TBM Clinical setting: laboratory Laboratory level where index test was performed: central Country: Italy World Bank income classification: high income High TB burden country: no High TB/HIV burden country: no High MDR‐TB burden country: no Prevalence of tuberculosis cases in the study: 22% PTB; 17% LNTB; 9% TBM | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | PTB, LNTB, TBM Solid and liquid culture (LJ and MGIT) |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Vadwai 2011.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: TBM symptoms included irritability, restlessness, neck stiffness, headache persistent for 2 to 3 weeks, vomiting, seizures, and changes in mental condition or behaviour. LNTB symptoms included enlargement of lymph nodes and mass formation in the neck Age, months: median (IQR) 66 (12 to 168) Sex, female: not reported HIV infection: 3% Sample size included for analysis: 14: 5 LNTB; 9 TBM Clinical setting: inpatient Laboratory level where index test was performed: central Country: India World Bank income classification: middle income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 20% LNTB and TBM | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Lymph node TB and tuberculous meningitis Solid and liquid culture (LJ and MGIT) |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Walters 2014.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, convenience, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: chronic respiratory symptoms, contact with a known TB source case or a reactive Mantoux skin test in combination with any of the following: severe life‐threatening intrathoracic large airway obstruction, radiographic evidence of complicated intrathoracic disease, or suspicion of drug‐resistant TB based on the susceptibility pattern of an adult source case; none of the bacteriological samples taken from the child to date had been positive Age, months: median (IQR) 16 (5 to 132) Sex, female: 43% HIV infection: 14% Sample size included for analysis: 14 Clinical setting: inpatient Laboratory level where index test was performed: intermediate Country: South Africa World Bank income classification: middle income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 60% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis MGIT; composite reference standard |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | Study on BAL samples | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Walters 2017a.
| Study characteristics | |||
| Patient Sampling | Cohort, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: 1 or more of cough ≥ 2 weeks, unexplained fever of ≥ 1 week, or poor growth or weight loss over preceding 3 months. Also included were children with any duration of cough, if 1 or more of the following were present: exposure to an identified TB source case in the past 12 months, positive tuberculin skin test if previously negative or unknown, or chest radiograph suggestive of TB as assessed by the study clinician. Infants younger than 3 months were also eligible if they had pneumonia unresponsive to appropriate antimicrobials, or unexplained and unresponsive sepsis syndrome Age, months: median (IQR) 16 (9 to 29) Sex, female: 48% HIV infection: 13% Sample size included for analysis: 379 Clinical setting: inpatient Laboratory level where index test was performed: intermediate Country: South Africa World Bank income classification: middle income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 18% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis MGIT; composite reference standard; clinical reference standard |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | No consensus has been reached for stool processing before Xpert; this study used centrifugation | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Walters 2018a.
| Study characteristics | |||
| Patient Sampling | Cohort, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: well‐defined TB symptoms or shorter history of cough with known TB exposure, positive tuberculin skin test, or chest radiographic changes of concern for tuberculosis Age, months: median (IQR) 16 (11 to 29) Sex, female: 44% HIV infection: 13% Sample size included for analysis: 244 Clinical setting: inpatient and outpatient Laboratory level where index test was performed: intermediate Country: South Africa World Bank income classification: middle income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 8% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis MGIT; composite reference standard; clinical reference standard |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | No consensus protocol for stool processing before Xpert is available; this study reported on stool filtration and swab processing | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Yin 2014.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, manner of selection not reported, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: children aged 18 years or younger were eligible for enrolment if they had been admitted to hospital, with suspected PTB, having a cough for longer than 2 weeks, and a chest radiograph suggesting the need for routine fibreoptic bronchoscopy Age, months: mean 73 Sex, female: 56% HIV infection: not reported Sample size included for analysis: 255 Clinical setting: inpatient Laboratory level where index test was performed: central Country: China World Bank income classification: middle income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 10% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis Solid and liquid culture (LJ and MGIT); composite reference standard; clinical reference standard |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | Xpert was performed on BAL samples | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Zar 2012.
| Study characteristics | |||
| Patient Sampling | Cohort, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: children 0 to 14 years with cough lasting longer than 2 weeks and at least 1 of the following: household tuberculosis contact in previous 3 months, weight loss or failure to gain weight in previous 3 months, positive tuberculin skin test, or chest radiograph suggestive of PTB Age, months: median (IQR) 19 (11 to 38) Sex, female: 45% HIV infection: 22% Sample size included for analysis: 474 Clinical setting: inpatient Laboratory level where index test was performed: intermediate Country: South Africa World Bank income classification: middle income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 17% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis MGIT; composite reference standard; clinical reference standard |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Zar 2013.
| Study characteristics | |||
| Patient Sampling | Cohort, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: children 0 to 14 years of age with cough lasting longer than 2 weeks and at least 1 of the following: household tuberculosis contact in previous 3 months, weight loss or failure to gain weight in previous 3 months, positive tuberculin skin test, or chest radiograph suggestive of PTB Age, months: median (IQR) 38 (21 to 57) Sex, female: 53% HIV infection: 8% Sample size included for analysis: 384 Clinical setting: outpatient Laboratory level where index test was performed: intermediate Country: South Africa World Bank income classification: middle income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 8% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis MGIT; composite reference standard; clinical reference standard |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Zar 2019.
| Study characteristics | |||
| Patient Sampling | Cohort, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: children 0 to 14 years of age with cough lasting longer than 2 weeks and at least 1 of the following: household tuberculosis contact in previous 3 months, weight loss or failure to gain weight in previous 3 months, positive tuberculin skin test, or chest radiograph suggestive of PTB Age, months: median (IQR) 23 (14 to 47) Sex, female: not reported HIV infection: 16% Sample size included for analysis: 195 Clinical setting: inpatient Laboratory level where index test was performed: intermediate Country: South Africa World Bank income classification: middle income High TB burden country: yes High TB/HIV burden country: yes High MDR‐TB burden country: yes Prevalence of tuberculosis cases in the study: 21% | ||
| Index tests | Xpert MTB/RIF | ||
| Target condition and reference standard(s) | Pulmonary tuberculosis MGIT; composite reference standard; clinical reference standard |
||
| Flow and timing | Index and reference tests were collected within pre‐specified time period | ||
| Comparative | |||
| Notes | Xpert Ultra test was performed on frozen specimens | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
AFB: acid‐fast bacillus.
BAL: bronchoalveolar lavage.
GLA: gastric lavage aspirate.
IQR: interquartile ratio.
IS: induced sputum.
LJ: Löwenstein‐Jensen.
LNTB: lymph node tuberculosis.
M tuberculosis: Mycobacterium tuberculosis.
MDR‐TB: multi‐drug‐resistant tuberculosis.
MGIT: mycobacteria growth indicator tube.
MTB/RIF: Mycobacterium tuberculosis complex and resistance to rifampin.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ali 2017 | Unable to separate paediatric data from adult data |
| Atashi 2017 | Adult population |
| Atehortúa Muñoz 2017 | Not a diagnostic accuracy study |
| Azevedo 2018 | Adult population |
| Ballif 2015 | Not a diagnostic accuracy study |
| Banada 2016 | Case‐control study |
| Biadglegne 2014 | Unable to separate paediatric data from adult data |
| Bojang 2016 | Unable to separate paediatric data from adult data |
| Che 2017 | Adult population |
| Cox 2014 | Adult population |
| Cross 2014 | Unable to separate paediatric data from adult data |
| Diallo 2016 | Unable to separate paediatric data from adult data |
| DiNardo 2016 | Not a diagnostic accuracy study |
| DiNardo 2018 | Index test not studied |
| Ejeh 2018 | Unable to separate paediatric data from adult data |
| Gautam 2018 | Unable to separate paediatric data from adult data |
| Gelalcha 2017 | Unable to separate paediatric data from adult data |
| Geleta 2015 | Adult population |
| Ghariani 2015 | Unable to separate paediatric data from adult data |
| Giang 2015 | Unable to extract data by sample type |
| Guajardo‐Lara 2018 | Insufficient data |
| Gulla 2019 | Not a diagnostic accuracy study |
| Hakim 2017 | Not a diagnostic accuracy study |
| Helb 2010 | Adult population |
| Horo 2017 | Unable to separate paediatric data from adult data |
| Huh 2014 | Adult population |
| Kuyinu 2018 | Inappropriate reference standard |
| Lopez 2019 | Index text not studied |
| Lu J 2017 | Screening for clinical tuberculosis before enrolment |
| Lu Y 2018 | Adult population |
| Malik 2018 | Not a diagnostic accuracy study |
| Marcy 2018 | Duplicate data for Marcy 2016 |
| Masenga 2017 | Unable to separate paediatric data from adult data |
| Mekonnen 2015 | Adult population |
| Memon 2018 | Clinical diagnosis of tuberculosis established at enrolment |
| Metaferia 2018 | Inappropriate reference standard |
| Mijovic 2018 | Not a diagnostic accuracy study |
| Modi 2016 | Index test not studied |
| Mulenga 2015 | Index test not studied |
| Naidoo 2016 | Not a diagnostic accuracy study |
| Nair 2016 | Not a diagnostic accuracy study |
| Nansumba 2016 | Index test not studied |
| Nataprawira 2016 | Not a diagnostic accuracy study |
| Ncube 2017 | Not a diagnostic accuracy study |
| Nduba 2015 | Index text not studied |
| Ngabonziza 2016 | Adult population |
| Ntinginya 2012 | Not a diagnostic accuracy study |
| Opota 2019 | Adult population |
| Pandey 2017 | Unable to separate paediatric data from adult data |
| Pink 2016 | Unable to separate paediatric data from adult data |
| Planting 2014 | Not a diagnostic accuracy study |
| Raizada 2014 | Inappropriate reference standard |
| Raizada 2015a | Inappropriate reference standard |
| Raizada 2015b | Inappropriate reference standard |
| Raizada 2018b | Inappropriate reference standard |
| Raizada 2018c | Inappropriate reference standard |
| Rathour 2019 | Screening for clinical tuberculosis before enrolment |
| Rebecca 2018 | Case‐control study |
| Rivera 2017 | Not a diagnostic accuracy study |
| Sabi 2016 | Not a diagnotic accuracy study |
| Sachdeva 2015 | Not a diagnostic accuracy study |
| Sanchini 2014 | Not a diagnostic accuracy study |
| Sander 2019 | Adult population |
| Sanjuan‐Jimenez 2015 | Adult population |
| Schumacher 2016 | Not a diagnostic accuracy study |
| Scott 2014 | Unable to separate paediatric data from adult data |
| Shah 2016a | Insufficient data |
| Shah 2018 | Not a diagnostic accuracy study |
| Shah 2019 | Case‐control study |
| Sharma 2015 | Unable to separate paediatric data from adult data |
| Sieiro 2018 | Unable to separate paediatric data from adult data |
| Singh 2015 | Clinical diagnosis of tuberculosis established at enrolment |
| Singh UB 2016 | Adult population |
| Solomons 2016 | Not a diagnostic accuracy study |
| Sureshbabu 2016 | Unable to separate paediatric data from adult data |
| Tadesse 2015 | Unable to separate paediatric data from adult data |
| Tafur 2018 | Not a diagnostic accuracy study |
| Tang 2017 | Adult population |
| Theron 2011 | Adult population |
| Triasih 2015 | Not a diagnostic accuracy study |
| Ullah 2017 | Unable to separate paediatric data from adult data |
| Walters 2012 | Insufficient data |
| Walters 2017b | Index text not studied |
| Walters 2018b | Not a diagnostic accuracy study |
| Zhang 2016 | Insufficient data |
Characteristics of ongoing studies [ordered by study ID]
ChiCTR1800015075.
| Study name | Diagnostic accuracy of Xpert MTB/RIF Ultra assay on diagnosing paediatric pulmonary tuberculosis |
| Target condition and reference standard(s) | Pulmonary tuberculosis |
| Index and comparator tests | Xpert Ultra |
| Starting date | 1 January 2018 |
| Contact information | Xuhui Liu; liuxuhui@shaphc.org |
| Notes |
NCT03831906.
| Study name | TB‐speed pneumonia |
| Target condition and reference standard(s) | Tuberculosis, pneumonia |
| Index and comparator tests | Xpert Ultra |
| Starting date | 20 March 2019 |
| Contact information | Aurelia Vessiere, PhD; aurelia.vessiere@u-bordeaux.fr |
| Notes |
MTB/RIF: Mycobacterium tuberculosis complex and resistance to rifampin.
TB: tuberculosis.
Differences between protocol and review
We defined the microbiological reference standard as culture only and did not include smear microscopy, which is less accurate. For stool, we accepted as a reference standard a positive result by Xpert MTB/RIF or Xpert Ultra in a sputum specimen. For the composite reference standard, when information about tuberculosis treatment was not available, we accepted the uniform research definition (Graham 2012; Graham 2015). In these situations, using the older definition (Graham 2012), we defined tuberculosis as (1) confirmed, probable, and possible cases, and (2) non‐tuberculosis. For the newer definition (Graham 2015), we used the categories tuberculosis confirmed and not confirmed. In cases where a study‐specific definition for the composite references standard was applied, this was accepted as well. For studies in which gastric aspirate specimens and sputum specimens were collected, data were included with the sputum analyses, and we performed a sensitivity analysis excluding these studies. We added MTBDRplus, a WHO‐recommended test, as a reference standard for rifampicin resistance. We had planned to estimate the pooled proportion of uninterpretable Xpert MTB/RIF and Xpert Ultra results for tuberculosis detection and indeterminate Xpert MTB/RIF and Xpert Ultra results for rifampicin resistance detection. However, we found few uninterpretable results reported. We have summarized these results in a table with the key characteristics of the included studies. We did not perform an indirect test comparison of Xpert MTB/RIF and Xpert Ultra because there were only three Ultra studies. These three studies also assessed Xpert MTB/RIF, and so we limited our analysis to a direct comparison. Owing to limited data, we were able to perform investigations of heterogeneity and sensitivity analyses only for Xpert MTB/RIF. Finally, as part of the analysis for the World Health Organization Molecular Diagnostics Guideline Development Group, we were asked to provide data on the diagnostic accuracy of multiple Xpert tests compared with a single Xpert test in children; this analysis has been added to the review.
Contributions of authors
AK, LFG, KRS, and AMM assessed articles for inclusion and extracted data.
AK and KRS entered data in RevMan.
AK, YT, KRS, and AMM analysed the data and interpreted the analyses. In particular, YT performed statistical analyses.
AK, LFG, YT, KRS, and AMM drafted the manuscript.
ME and AD provided critical comments on the manuscript.
All review authors read and approved the final manuscript draft.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK
External sources
-
Texas Children's Hospital (AK), USA
Pediatric Pilot Award and Global Health Innovation Award
-
Thrasher Foundation (AK), USA
Early Career Award
-
Department for International Development (DFID), UK
Project number 300342‐104
-
United States Agency for International Development (USAID), USA
Development of the systematic review was in part made possible with financial support from the USAID administered by the World Health Organization (WHO) Global TB Programme, Switzerland. AK, LGF, YT, and AMM received funding from USAID to carry out the review.
Declarations of interest
AK has conducted prior primary research on tuberculosis diagnostics. The Baylor College of Medicine Children's Foundation‐Swaziland, where Dr. Kay is based, received a discount from Cepheid on Xpert MTB/RIF Ultra cartridges for a tuberculosis case finding programme. The Baylor College of Medicine Children’s Foundation‐Swaziland is separate from Baylor College of Medicine (AK's employer).
LGF has no known conflicts of interest.
YT has no known conflicts of interest.
ME has no known conflicts of interest.
AD has conducted prior primary research on tuberculosis diagnostics and has no known conflicts of interest.
KRS has received financial support for the preparation of systematic reviews and educational materials, consultancy fees from FIND (for the preparation of systematic reviews), honoraria, and travel support to attend WHO guidelines meetings.
AMM has conducted prior primary research on tuberculosis diagnostics and has no known conflicts of interest.
These authors contributed equally
These authors contributed equally
New
References
References to studies included in this review
Anderson 2014 {published data only}
- Anderson S, Kaforou M, Brent AJ, Wright VJ, Banwell CM, Chagaluka G, et al. Diagnosis of childhood tuberculosis and host RNA expression in Africa. New England Journal of Medicine 2014;370(18):1712-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andriyoko 2019 {published data only}
- Andriyoko B, Janiar H, Kusumadewi R, Klinkenberg E, Haas P, Tiemersma E. Simple stool processing method for the diagnosis of pulmonary tuberculosis using GeneXpert MTB/RIF. European Respiratory Journal 2019;53(3):pii: 1801832. [DOI: 10.1183/13993003.01832-2018] [DOI] [PubMed] [Google Scholar]
Atwebembeire 2016 {published data only}
- Atwebembeire J, Orikiriza P, Bonnet M, Atwine D, Katawera V, Nansumba M, et al. Xpert® MTB/RIF for detection of Mycobacterium tuberculosis from frozen string and induced sputum sediments. International Journal of Tuberculosis and Lung Disease 2016;20(8):1113-7. [DOI] [PubMed] [Google Scholar]
Bacha 2017 {published data only}
- Bacha JM, Ngo K, Clowes P, Draper HR, Ntinginya EN, DiNardo A, et al. Why being an expert - despite Xpert - remains crucial for children in high TB burden settings. BMC Infectious Diseases 2017;17(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bates 2013 {published data only}
- Bates M, O'Grady J, Maeurer M, Tembo J, Chilukutu L, Chabala C, et al. Assessment of the Xpert MTB/RIF assay for diagnosis of tuberculosis with gastric lavage aspirates in children in sub-Saharan Africa: a prospective descriptive study. Lancet. Infectious Diseases 2013;13(1):36-42. [DOI] [PubMed] [Google Scholar]
Bhatia 2016 {published data only}
- Bhatia R, Dayal R, Jindal S, Agarwal D, Goyal A. GeneXpert for diagnosis of tubercular meningitis. Indian Journal of Pediatrics 2016;83(11):1353-5. [DOI] [PubMed] [Google Scholar]
Bholla 2016 {published data only}
- Bholla M, Kapalata N, Masika E, Chande H, Jugheli L, Sasamalo M, et al. Evaluation of Xpert® MTB/RIF and Ustar EasyNATTM TB IAD for diagnosis of tuberculous lymphadenitis of children in Tanzania: a prospective descriptive study. BMC Infectious Diseases 2016;16:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brent 2017 {published data only}
- Brent AJ, Mugo D, Musyimi R, Mutiso A, Morpeth SC, Levin M, et al. Bacteriological diagnosis of childhood TB: a prospective observational study. Scientific Reports 2017;7(1):11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bunyasi 2015 {published data only}
- Bunyasi EW, Tameris M, Geldenhuys H, Schmidt BM, Luabeya AKK, Mulenga H, et al. Evaluation of Xpert® MTB/RIF assay in induced sputum and gastric lavage samples from young children with suspected tuberculosis from the MVA85A TB vaccine trial. PLoS One 2015;10(11):e0141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Causse 2011 {published data only}
- Causse M, Ruiz P, Gutiérrez-Aroca JB, Casal M. Comparison of two molecular methods for rapid diagnosis of extrapulmonary tuberculosis. Journal of Clinical Microbiology 2011;49(8):3065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chipinduro 2017 {published data only}
- Chipinduro M, Mateveke K, Makamure B, Ferrand RA, Gomo E. Stool Xpert® MTB/RIF test for the diagnosis of childhood pulmonary tuberculosis at primary clinics in Zimbabwe. International Journal of Tuberculosis and Lung Disease 2017;21(2):161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chisti 2014 {published data only}
- Chisti MJ, Graham SM, Duke T, Ahmed T, Ashraf H, Faruque AS, et al. A prospective study of the prevalence of tuberculosis and bacteraemia in Bangladeshi children with severe malnutrition and pneumonia including an evaluation of Xpert MTB/RIF assay. PLoS One 2014;9(4):e93776. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coetzee 2014 {published data only}
- Coetzee L, Nicol MP, Jacobson R, Schubert PT, Helden PD, Warren RM, et al. Rapid diagnosis of pediatric mycobacterial lymphadenitis using fine needle aspiration biopsy. Pediatric Infectious Disease Journal 2014;33(9):893-6. [DOI] [PubMed] [Google Scholar]
Das 2019 {published data only}
- Das A, Anupurba S, Mishra OP, Banerjee T, Tripathi R. Evaluation of Xpert MTB/RIF Assay for diagnosis of tuberculosis in children. Journal of Tropical Pediatrics 2019;65(1):14-20. [DOI] [PubMed] [Google Scholar]
Gous 2015 {published data only}
- Gous N, Scott LE, Khan S, Reubenson G, Coovadia A, Stevens W. Diagnosing childhood pulmonary tuberculosis using a single sputum specimen on Xpert MTB/RIF at point of care. South African Medical Journal 2015;105(12):1044-8. [DOI] [PubMed] [Google Scholar]
Hanrahan 2018 {published data only}
- Hanrahan CF, Dansey H, Mutunga L, France H, Omar SV, Ismail N, et al. Diagnostic strategies for childhood tuberculosis in the context of primary care in a high-burden setting: the value of alternative sampling methods. Paediatrics and International Child Health 2018;39(2):88-94. [DOI] [PubMed] [Google Scholar]
Hasan 2017 {published data only}
- Hasan Z, Shakoor S, Arif F, Mehnaz A, Akber A, Haider M, et al. Evaluation of Xpert MTB/RIF testing for rapid diagnosis of childhood pulmonary tuberculosis in children by Xpert MTB/RIF testing of stool samples in a low resource setting. BMC Research Notes 2017;10(1):473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kasa Tom 2018 {published data only}
- Kasa Tom S, Welch H, Kilalang C, Tefuarani N, Vince J, Lavu E, et al. Evaluation of Xpert MTB/RIF assay in children with presumed pulmonary tuberculosis in Papua New Guinea. Paediatrics and International Child Health 2018;38(2):97-105. [DOI] [PubMed] [Google Scholar]
Kim 2015 {published data only}
- Kim YW, Kwak N, Seong MW, Kim EC, Yoo CG, Kim YW, et al. Accuracy of the Xpert® MTB/RIF assay for the diagnosis of extra-pulmonary tuberculosis in South Korea. International Journal of Tuberculosis and Lung Disease 2015;19(1):81-6. [DOI] [PubMed] [Google Scholar]
LaCourse 2014 {published data only}
- LaCourse SM, Chester FM, Preidis G, McCrary LM, Arscott-Mills T, Maliwichi M, et al. Use of Xpert for the diagnosis of pulmonary tuberculosis in severely malnourished hospitalized Malawian children. Pediatric Infectious Disease Journal 2014;33(11):1200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
LaCourse 2018 {published data only}
- LaCourse SM, Pavlinac PB, Cranmer LM, Njuguna IN, Mugo C, Gatimu J, et al. Stool Xpert MTB/RIF and urine lipoarabinomannan for the diagnosis of tuberculosis in hospitalized HIV-infected children. AIDS (London, England) 2018;32(1):69-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ligthelm 2011 {published data only}
- Ligthelm LJ, Nicol MP, Hoek KGP, Jacobson R, Helden PD, Marais BJ, et al. Xpert MTB/RIF for rapid diagnosis of tuberculous lymphadenitis from fine-needle-aspiration biopsy specimens. Journal of Clinical Microbiology 2011;49(11):3967-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Malbruny 2011 {published and unpublished data}
- Malbruny B, Le Marrec G, Courageux K, Leclercq R, Cattoir V. Rapid and efficient detection of Mycobacterium tuberculosis in respiratory and non-respiratory samples. International Journal of Tuberculosis Lung Disease 2011;15(4):553-5. [DOI] [PubMed] [Google Scholar]
Marcy 2016 {published data only}
- Marcy O, Ung V, Goyet S, Borand L, Msellati P, Tejiokem M, et al. Performance of Xpert MTB/RIF and alternative specimen collection methods for the diagnosis of tuberculosis in HIV-Infected children. Clinical Infectious Diseases 2016;62(9):1161-8. [DOI] [PubMed] [Google Scholar]
Moussa 2016 {published data only}
- Moussa HS, Bayoumi FS, Mohamed AMA. Gene Xpert for direct detection of Mycobacterium tuberculosis in stool specimens from children with presumptive pulmonary tuberculosis. Annals of Clinical and Laboratory Science 2016;46(2):198-203. [PubMed] [Google Scholar]
Myo 2018 {published data only}
- Myo K, Zaw M, Swe TL, Kyaw YY, Thwin T, Myo TT, et al. Evaluation of Xpert® MTB/RIF assay as a diagnostic test for pulmonary tuberculosis in children in Myanmar. International Journal of Tuberculosis and Lung Disease 2018;22(9):1051-5. [DOI] [PubMed] [Google Scholar]
Nhu 2013 {published data only}
- Nhu NT, Ha DT, Anh ND, Thu DD, Duong TN, Quang ND, et al. Evaluation of Xpert MTB/RIF and MODS assay for the diagnosis of pediatric tuberculosis. BMC Infectious Diseases 2013;13:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nicol 2011 {published data only}
- Nicol MP, Workman L, Isaacs W, Munro J, Black F, Eley B, et al. Accuracy of the Xpert MTB/RIF test for the diagnosis of pulmonary tuberculosis in children admitted to hospital in Cape Town, South Africa: a descriptive study. Lancet. Infectious Diseases 2011;11(11):819-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nicol 2013 {published data only}
- Nicol MP, Spiers K, Workman L, Isaacs W, Munro J, Black F, et al. Xpert MTB/RIF testing of stool samples for the diagnosis of pulmonary tuberculosis in children. Clinical Infectious Diseases 2013;57(3):e18-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nicol 2018 {published data only}
- Nicol MP, Workman L, Prins M, Bateman L, Ghebrekristos Y, Mbhele S, et al. Accuracy of Xpert MTB/RIF Ultra for the diagnosis of pulmonary tuberculosis in children. Paediatric Infectious Disease Journal 2018;37(10):e261-3. [DOI] [PubMed] [Google Scholar]
Orikiriza 2018 {published data only}
- Orikiriza P, Nansumba M, Nyehangane D, Bastard M, Mugisha IT, Nansera D, et al. Xpert MTB/RIF diagnosis of childhood tuberculosis from sputum and stool samples in a high TB-HIV-prevalent setting. European Journal of Clinical Microbiology & Infectious Diseases 2018;37(8):1465-73. [DOI] [PubMed] [Google Scholar]
Pang 2014 {published data only}
- Pang Y, Wang Y, Zhao S, Liu J, Zhao Y, Li H. Evaluation of the Xpert MTB/RIF assay in gastric lavage aspirates for diagnosis of smear-negative childhood pulmonary tuberculosis. Paediatric Infectious Disease Journal 2014;33(10):1047-51. [DOI] [PubMed] [Google Scholar]
Rachow 2012 {published data only}
- Rachow A, Clowes P, Saathoff E, Mtafya B, Michael E, Ntinginya EN, et al. Increased and expedited case detection by Xpert MTB/RIF assay in childhood tuberculosis: a prospective cohort study. Clinical Infectious Diseases 2012;54(10):1388-96. [DOI] [PubMed] [Google Scholar]
Reither 2015 {published data only}
- Reither K, Manyama C, Clowes P, Rachow A, Mapamba D, Steiner A, et al. Xpert MTB/RIF assay for diagnosis of pulmonary tuberculosis in children: a prospective, multi-centre evaluation. Journal of Infection 2015;70(4):392-9. [DOI] [PubMed] [Google Scholar]
Sabi 2018 {published data only}
- Sabi I, Rachow A, Mapamba D, Clowes P, Ntinginya NE, Sasamalo M, et al. Xpert MTB/RIF Ultra assay for the diagnosis of pulmonary tuberculosis in children: a multicentre comparative accuracy study. Journal of Infection 2018;77(4):321-7. [DOI] [PubMed] [Google Scholar]
Saini 2018 {published data only}
- Saini I, Mukherjee A, Gautam H, Singla M, Jat KR, Lodha R, et al. Diagnostic yield of Xpert MTB/RIF in bronchoalveolar lavage in children with probable pulmonary tuberculosis. Indian Pediatrics 2018;55(12):1062-5. [PubMed] [Google Scholar]
Sekadde 2013 {published data only}
- Sekadde MP, Wobudeya E, Joloba ML, Ssengooba W, Kisembo H, Bakeera-Kitaka S, et al. Evaluation of the Xpert MTB/RIF test for the diagnosis of childhood pulmonary tuberculosis in Uganda: a cross-sectional diagnostic study. BMC Infectious Diseases 2013;13:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh M 2016 {published data only}
- Singh M, Sethi GR, Mantan M, Khanna A, Hanif M. Xpert® MTB/RIF assay for the diagnosis of pulmonary tuberculosis in children. International Journal of Tuberculosis and Lung Disease 2016;20(6):839-43. [DOI] [PubMed] [Google Scholar]
Solomons 2015 {published data only}
- Solomons RS, Visser DH, Friedrich SO, Diacon AH, Hoek KG, Marais BJ, et al. Improved diagnosis of childhood tuberculous meningitis using more than one nucleic acid amplification test. International Journal of Tuberculosis and Lung Disease 2015;19(1):74-80. [DOI] [PubMed] [Google Scholar]
Togun 2015 {published data only}
- Togun TO, Egere U, Sillah AK, Ayorinde A, Mendy F, Tientcheu L, et al. Contribution of Xpert® MTB/RIF to the diagnosis of pulmonary tuberculosis among TB-exposed children in The Gambia. International Journal of Tuberculosis and Lung Disease 2015;19(9):1091-7, i-ii. [DOI] [PubMed] [Google Scholar]
Tortoli 2012 {published data only}
- Tortoli E, Russo C, Piersimoni C, Mazzola E, Dal Monte P, Pascarella M, et al. Clinical validation of Xpert MTB/RIF for the diagnosis of extrapulmonary tuberculosis. European Respiratory Journal 2012;40(2):442-7. [DOI] [PubMed] [Google Scholar]
Vadwai 2011 {published data only}
- Vadwai V, Boehme C, Nabeta P, Shetty A, Alland D, Rodrigues C. Xpert MTB/RIF: a new pillar in diagnosis of extrapulmonary tuberculosis? Journal of Clinical Microbiology 2011;49(7):2540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walters 2014 {published data only}
- Walters E, Goussard P, Bosch C, Hesseling AC, Gie RP. GeneXpert MTB/RIF on bronchoalveolar lavage samples in children with suspected complicated intrathoracic tuberculosis: a pilot study. Pediatric Pulmonology 2014;49(11):1133-7. [DOI] [PubMed] [Google Scholar]
Walters 2017a {published data only}
- Walters E, Zalm MM, Palmer M, Bosch C, Demers AM, Draper H, et al. Xpert MTB/RIF on stool is useful for the rapid diagnosis of tuberculosis in young children with severe pulmonary disease. Pediatric Infectious Disease Journal 2017;36(9):837-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walters 2018a {published data only}
- Walters E, Scott L, Nabeta P, Demers AM, Reubenson G, Bosch C, et al. Molecular detection of Mycobacterium tuberculosis from stools in young children by use of a novel centrifugation-free processing method. Journal of Clinical Microbiology 2018;56(9):pii: e00781-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yin 2014 {published data only}
- Yin QQ, Jiao WW, Han R, Jiao AX, Sun L, Tian JL, et al. Rapid diagnosis of childhood pulmonary tuberculosis by Xpert MTB/RIF assay using bronchoalveolar lavage fluid. BioMed Research International 2014;2014:310194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zar 2012 {published data only}
- Zar HJ, Workman L, Isaacs W, Munro J, Black F, Eley B, et al. Rapid molecular diagnosis of pulmonary tuberculosis in children using nasopharyngeal specimens. Clinical Infectious Diseases 2012;55(8):1088-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zar 2013 {published data only}
- Zar HJ, Workman L, Isaacs W, Dheda K, Zemanay W, Nicol MP. Rapid diagnosis of pulmonary tuberculosis in African children in a primary care setting by use of Xpert MTB/RIF on respiratory specimens: a prospective study. Lancet. Global Health 2013;1(2):e97-104. [DOI] [PubMed] [Google Scholar]
Zar 2019 {published data only}
- Zar HJ, Workman LJ, Prins M, Bateman LJ, Mbhele SP, Whitman CB, et al. Tuberculosis diagnosis in children using Xpert Ultra on different respiratory specimens. American Journal of Respiratory and Critical Care Medicine 2019;200(12):1531-8. [DOI: 10.1164/rccm.201904-0772OC] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ali 2017 {published data only}
- Ali RH, Ibrahim NY, Elegail AMA, Eltohami NAM, Ebraheem RSM, Ahmed SFM, et al. Evaluation of GeneXpert MTB/RIF and line probe assay for rapid diagnosis of Mycobacterium tuberculosis in Sudanese pulmonary TB patients. Asian Pacific Journal of Tropical Disease 2017;7(7):426-9. [Google Scholar]
Atashi 2017 {published data only}
- Atashi S, Izadi B, Jalilian S, Madani SH, Farahani A, Mohajeri P. Evaluation of GeneXpert MTB/RIF for determination of rifampicin resistance among new tuberculosis cases in west and northwest Iran. New Microbes and New Infections 2017;19:117-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Atehortúa Muñoz 2017 {published data only}
- Atehortúa Muñoz SL, Muñoz JR, Cárdenas Moreno SV, Ferreira CA, Cornejo Ochoa JW. Xpert MTB/RIF as a diagnostic tool in a cohort of children under 15 years of age with clinical suspicion of pulmonary tuberculosis in a hospital of high complexity in Medellin [Xpert MTB/RIF® como herramienta diagnóstica en una cohorte de niños menores de 15 años con sospecha clínica de tuberculosis pulmonar en un hospital de alta complejidad de Medellín]. Infectio 2017;21(1):25-31. [Google Scholar]
Azevedo 2018 {published data only}
- Azevedo RG, Dinallo FS, De Laurentis LS, Boulware DR, Vidal JE. Xpert MTB/RIF® assay for the diagnosis of HIV-related tuberculous meningitis in Sao Paulo, Brazil. International Journal of Tuberculosis and Lung Disease 2018;22(6):706-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ballif 2015 {published data only}
- Ballif M, Renner L, Claude Dusingize J, Leroy V, Ayaya S, Wools-Kaloustian K, et al. Tuberculosis in pediatric antiretroviral therapy programs in low- and middle-income countries: diagnosis and screening practices. Journal of the Pediatric Infectious Diseases Society 2015;4(1):30-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Banada 2016 {published data only}
- Banada PP, Naidoo U, Deshpande S, Karim F, Flynn JL, O'Malley M, et al. A novel sample processing method for rapid detection of tuberculosis in the stool of pediatric patients using the Xpert MTB/RIF assay. PLoS One 2016;11(3):e0151980. [DOI] [PMC free article] [PubMed] [Google Scholar]
Biadglegne 2014 {published data only}
- Biadglegne F, Mulu A, Rodloff AC, Sack U. Diagnostic performance of the Xpert MTB/RIF assay for tuberculous lymphadenitis on fine needle aspirates from Ethiopia. Tuberculosis (Edinburgh, Scotland) 2014;94(5):502-5. [DOI] [PubMed] [Google Scholar]
Bojang 2016 {published data only}
- Bojang AL, Mendy FS, Tientcheu LD, Otu J, Antonio M, Kampmann B, et al. Comparison of TB-LAMP, GeneXpert MTB/RIF and culture for diagnosis of pulmonary tuberculosis in The Gambia. Journal of Infection 2016;72(3):332-7. [DOI] [PubMed] [Google Scholar]
Che 2017 {published data only}
- Che NY, Huang SJ, Ma Y, Han Y, Liu ZC, Zhang C, et al. Comparison of histological, microbiological, and molecular methods in diagnosis of patients with TBLN having different anti-TB treatment background. Biomedical and Environmental Sciences 2017;30(6):418-25. [DOI] [PubMed] [Google Scholar]
Cox 2014 {published data only}
- Cox HS, Mbhele S, Mohess N, Whitelaw A, Muller O, Zemanay W, et al. Impact of Xpert MTB/RIF for TB diagnosis in a primary care clinic with high TB and HIV prevalence in South Africa: a pragmatic randomised trial. PLoS Medicine 2014;11(11):e1001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cross 2014 {published data only}
- Cross GB, Coles K, Nikpour M, Moore OA, Denholm J, McBryde ES, et al. TB incidence and characteristics in the remote gulf province of Papua New Guinea: a prospective study. BMC Infectious Diseases 2014;14:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diallo 2016 {published data only}
- Diallo AB, Kollo AI, Camara M, Lo S, Ossoga GW, Mbow M, et al. Performance of GeneXpert MTB/RIF in the diagnosis of extrapulmonary tuberculosis in Dakar: 2010-2015 [Performance du GeneXpert MTB/RIF ® dans le diagnostic de la tuberculose extra-pulmonaire à Dakar: 2010-2015]. Pan African Medical Journal 2016;25:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
DiNardo 2016 {published data only}
- DiNardo AR, Detjen A, Ustero P, Ngo K, Bacha J, Mandalakas AM. Culture is an imperfect and heterogeneous reference standard in pediatric tuberculosis. Tuberculosis (Edinburgh, Scotland) 2016;101S:S105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
DiNardo 2018 {published data only}
- DiNardo AR, Kay AW, Maphalala G, Harris NM, Fung C, Mtetwa G, et al. Diagnostic and treatment monitoring potential of a stool-based quantitative polymerase chain reaction assay for pulmonary tuberculosis. American Journal of Tropical Medicine and Hygiene 2018;99(2):310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ejeh 2018 {published data only}
- Ejeh EF, Undiandeye A, Akinseye VO, Okon KO, Kazeem HM, Kudi CA, et al. Diagnostic performance of GeneXpert and Ziehl-Neelson microscopy in the detection of tuberculosis in Benue State, Nigeria. Alexandria Journal of Medicine 2018;54(4):529-33. [Google Scholar]
Gautam 2018 {published data only}
- Gautam H, Agrawal SK, Verma SK, Singh UB. Cervical tuberculous lymphadenitis: clinical profile and diagnostic modalities. International Journal of Mycobacteriology 2018;7(3):212-6. [DOI] [PubMed] [Google Scholar]
Gelalcha 2017 {published data only}
- Gelalcha AG, Kebede A, Mamo H. Light-emitting diode fluorescent microscopy and Xpert MTB/RIF® assay for diagnosis of pulmonary tuberculosis among patients attending Ambo hospital, west-central Ethiopia. BMC Infectious Diseases 2017;17(1):613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Geleta 2015 {published data only}
- Geleta DA, Megerssa YC, Gudeta AN, Akalu GT, Debele MT, Tulu KD. Xpert MTB/RIF assay for diagnosis of pulmonary tuberculosis in sputum specimens in remote health care facility. BMC Microbiology 2015;15:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghariani 2015 {published data only}
- Ghariani A, Jaouadi T, Smaoui S, Mehiri E, Marouane C, Kammoun S, et al. Diagnosis of lymph node tuberculosis using the GeneXpert MTB/RIF in Tunisia. International Journal of Mycobacteriology 2015;4(4):270-5. [DOI] [PubMed] [Google Scholar]
Giang 2015 {published data only}
- Giang DC, Duong TN, Ha DTM, Nhan HT, Wolbers M, Nhu NTQ, et al. Prospective evaluation of GeneXpert for the diagnosis of HIV-negative pediatric TB cases. BMC Infectious Diseases 2015;15:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guajardo‐Lara 2018 {published data only}
- Guajardo-Lara CE, Saldaña-Ramírez MI, Hernández-Galván NN, Dimas-Adame MA, Ayala-Gaytán JJ, Valdovinos-Chávez SB. MGIT and other methods for diagnosing tuberculosis in a private hospital system with low incidence [MGIT y otros métodos para diagnosticar tuberculosis en un sistema hospitalario privado con baja incidencia]. Revista Médica del Instituto Mexicano del Seguro Social 2018;56(2):158-62. [PubMed] [Google Scholar]
Gulla 2019 {published data only}
- Gulla KM, Gunathilaka G, Jat KR, Sankar J, Karan M, Lodha R, et al. Utility and safety of endobronchial ultrasound-guided transbronchial needle aspiration and endoscopic ultrasound with an echobronchoscope-guided fine needle aspiration in children with mediastinal pathology. Pediatric Pulmonology 2019;54(6):881-5. [DOI] [PubMed] [Google Scholar]
Hakim 2017 {published data only}
- Hakim J, Musiime V, Szubert AJ, Mallewa J, Siika A, Agutu C, et al. Enhanced prophylaxis plus antiretroviral therapy for advanced HIV infection in Africa. New England Journal of Medicine 2017;377(3):233-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Helb 2010 {published data only}
- Helb D, Jones M, Story E, Boehme C, Wallace E, Ho K, et al. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. Journal of Clinical Microbiology 2010;48(1):229-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Horo 2017 {published data only}
- Horo K, N'Guessan R, Koffi MO, Kouamé-N'Takpé N, Koné A, Samaké K, et al. Use of the Xpert® MTB/RIF test in routine screening of new cases of pulmonary tuberculosis in an endemic area. Revue des Maladies Respiratoires 2017;4(7):749-57. [DOI] [PubMed] [Google Scholar]
Huh 2014 {published data only}
- Huh HJ, Jeong B-H, Jeon K, Koh W-J, Ki C-S, Lee NY. Performance evaluation of the Xpert MTB/RIF assay according to its clinical application. BMC Infectious Diseases 2014;14:589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuyinu 2018 {published data only}
- Kuyinu YA, Odugbemi BA, Salisu-Olatunji SO, Adepoju FO, Odusanya OO. Characteristics of Mycobacterium tuberculosis positive patients screened for drug-resistant tuberculosis at a tertiary health facility in Lagos, Nigeria. Journal of the National Medical Association 2018;110(1):88-91. [DOI] [PubMed] [Google Scholar]
Lopez 2019 {published data only}
- Lopez AL, Aldaba JG, Morales-Dizon M, Sarol JN, Daag JV, Ama MC, et al. Urine Xpert MTB/RIF for the diagnosis of childhood tuberculosis. International Journal of Infectious Diseases 2019;79:44-6. [DOI] [PubMed] [Google Scholar]
Lu J 2017 {published data only}
- Lu J, Li H, Dong F, Shi J, Yang H, Han S, et al. The feasibility of Xpert MTB/RIF testing to detect rifampicin resistance among childhood tuberculosis for prevalence surveys in Northern China. BioMed Research International 2017;2017:5857369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu Y 2018 {published data only}
- Lu Y, Zhu Y, Shen N, Tian L, Sun Z. Evaluating the diagnostic accuracy of the Xpert MTB/RIF assay on bronchoalveolar lavage fluid: a retrospective study. International Journal of Infectious Diseases 2018;71:14-9. [DOI] [PubMed] [Google Scholar]
Malik 2018 {published data only}
- Malik AA, Amanullah F, Codlin AJ, Siddiqui S, Jaswal M, Ahmed JF, et al. Improving childhood tuberculosis detection and treatment through facility-based screening in rural Pakistan. International Journal of Tuberculosis and Lung Disease 2018;22(8):851-7. [DOI] [PubMed] [Google Scholar]
Marcy 2018 {published data only}
- Marcy O, Tejiokem M, Msellati P, Truong Huu K, Do Chau V, Tran Ngoc D, et al. Mortality and its determinants in antiretroviral treatment-naive HIV-infected children with suspected tuberculosis: an observational cohort study. Lancet HIV 2018;5(2):e87-95. [DOI] [PubMed] [Google Scholar]
Masenga 2017 {published data only}
- Masenga SK, Mubila H, Hamooya BM. Rifampicin resistance in mycobacterium tuberculosis patients using GeneXpert at Livingstone Central Hospital for the year 2015: a cross sectional explorative study. BMC Infectious Diseases 2017;17(1):640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mekonnen 2015 {published data only}
- Mekonnen F, Tessema B, Moges F, Gelaw A, Eshetie S, Kumera G. Multidrug resistant tuberculosis: prevalence and risk factors in districts of Metema and West Armachiho, Northwest Ethiopia. BMC Infectious Diseases 2015;15:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Memon 2018 {published data only}
- Memon SS, Sinha S, Sharma SK, Kabra SK, Lodha R, Soneja M. Diagnostic accuracy of Xpert Mtb/Rif assay in stool samples in intrathoracic childhood tuberculosis. Journal Tuberculosis and Therapeutics 2018;3(2):115. [Google Scholar]
Metaferia 2018 {published data only}
- Metaferia Y, Seid A, Fenta GM, Gebretsadik D. Assessment of extrapulmonary tuberculosis using Gene Xpert MTB/RIF assay and fluorescent microscopy and its risk factors at Dessie referral hospital, Northeast Ethiopia. BioMed Research International 2018;2018:8207098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mijovic 2018 {published data only}
- Mijovic H, Al-Nasser Y, Al-Rawahi G, Roberts A. Experience with using rapid molecular testing in diagnosing pulmonary and extra-pulmonary pediatric tuberculosis in a non-endemic setting - a retrospective case series. Paediatrics and Child Health (Canada) 2018;23(Suppl 1):e44-5. [Google Scholar]
Modi 2016 {published data only}
- Modi S, Cavanaugh JS, Shiraishi RW, Alexander HL, McCarthy KD, Burmen B, et al. Performance of clinical screening algorithms for tuberculosis intensified case finding among people living with HIV in Western Kenya. PLoS One 2016;11(12):e0167685. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mulenga 2015 {published data only}
- Mulenga H, Tameris MD, Luabeya KKA, Geldenhuys H, Scriba TJ, Hussey GD, et al. The role of clinical symptoms in the diagnosis of intrathoracic tuberculosis in young children. Pediatric Infectious Disease Journal 2015;34(11):1157-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Naidoo 2016 {published data only}
- Naidoo P, Dunbar R, Lombard C, du Toit E, Caldwell J, Detjen A, et al. Comparing tuberculosis diagnostic yield in smear/culture and Xpert MTB/RIF-based algorithms using a non-randomised stepped-wedge design. PLoS One 2016;11(3):e0150487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nair 2016 {published data only}
- Nair SA, Raizada N, Sachdeva KS, Denkinger C, Schumacher S, Dewan P, et al. Factors associated with tuberculosis and rifampicin-resistant tuberculosis amongst symptomatic patients in India: a retrospective analysis. PLoS One 2016;11(2):e0150054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nansumba 2016 {published data only}
- Nansumba M, Kumbakumba E, Orikiriza P, Muller Y, Nackers F, Debeaudrap P, et al. Detection yield and tolerability of string test for diagnosis of childhood intrathoracic tuberculosis. Paediatric Infectious Disease Journal 2016;35(2):146-51. [DOI] [PubMed] [Google Scholar]
Nataprawira 2016 {published data only}
- Nataprawira HM, Ruslianti V, Solek R, Hawani D, Mianti M, Anggraeni R, et al. Outcome of tuberculous meningitis in children: the first comprehensive retrospective cohort study in Indonesia. International Journal of Tuberculosis and Lung Disease 2016;20(7):909-14. [DOI] [PubMed] [Google Scholar]
Ncube 2017 {published data only}
- Ncube RT, Takarinda KC, Zishiri C, den Boogaard W, Mlilo N, Chiteve C, et al. Age-stratified tuberculosis treatment outcomes in Zimbabwe: are we paying attention to the most vulnerable? Public Health Action 2017;7(3):212-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nduba 2015 {published data only}
- Nduba V, Hoog AH, Mitchell E, Onyango P, Laserson K, Borgdorff M. Prevalence of tuberculosis in adolescents, western Kenya: implications for control programs. International Journal of Infectious Diseases 2015;35:11-7. [DOI] [PubMed] [Google Scholar]
Ngabonziza 2016 {published data only}
- Ngabonziza JCS, Ssengooba W, Mutua F, Torrea G, Dushime A, Gasana M, et al. Diagnostic performance of smear microscopy and incremental yield of Xpert in detection of pulmonary tuberculosis in Rwanda. BMC Infectious Diseases 2016;16(1):660. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ntinginya 2012 {published data only}
- Ntinginya EN, Squire SB, Millington KA, Mtafya B, Saathoff E, Heinrich N, et al. Performance of the Xpert® MTB/RIF assay in an active case-finding strategy: a pilot study from Tanzania. International Journal of Tuberculosis and Lung Disease 2012;16(11):1468-70. [DOI] [PubMed] [Google Scholar]
Opota 2019 {published data only}
- Opota O, Zakham F, Mazza-Stalder J, Nicod L, Greub G, Jaton K. Added value of Xpert MTB/RIF Ultra for diagnosis of pulmonary tuberculosis in a low-prevalence setting. Journal of Clinical Microbiology 2019;57(2):pii: e01717-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pandey 2017 {published data only}
- Pandey P, Pant ND, Rijal KR, Shrestha B, Kattel S, Banjara MR, et al. Diagnostic accuracy of GeneXpert MTB/RIF assay in comparison to conventional drug susceptibility testing method for the diagnosis of multidrug-resistant tuberculosis. PLoS One 2017;12(1):e0169798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pink 2016 {published data only}
- Pink F, Brown TJ, Kranzer K, Drobniewski F. Evaluation of Xpert MTB/RIF for detection of Mycobacterium tuberculosis in cerebrospinal fluid. Journal of Clinical Microbiology 2016;54(3):809-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Planting 2014 {published data only}
- Planting NS, Visser GL, Nicol MP, Workman L, Isaacs W, Zar HJ. Safety and efficacy of induced sputum in young children hospitalised with suspected pulmonary tuberculosis. International Journal of Tuberculosis and Lung Disease 2014;18(1):8-12. [DOI] [PubMed] [Google Scholar]
Raizada 2014 {published data only}
- Raizada N, Sachdeva KS, Nair SA, Kulsange S, Gupta RS, Thakur R, et al. Enhancing TB case detection: experience in offering upfront Xpert MTB/RIF testing to pediatric presumptive TB and DR TB cases for early rapid diagnosis of drug sensitive and drug resistant TB. PLoS One 2014;9(8):e105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raizada 2015a {published data only}
- Raizada N, Sachdeva KS, Swaminathan S, Kulsange S, Khaparde SD, Nair SA, et al. Piloting upfront Xpert MTB/RIF testing on various specimens under programmatic conditions for diagnosis of TB & DR-TB in paediatric population. PLoS One 2015;10(10):e0140375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raizada 2015b {published data only}
- Raizada N, Sachdeva KS, Sreenivas A, Kulsange S, Gupta RS, Thakur R, et al. Catching the missing million: experiences in enhancing TB & DR-TB detection by providing upfront Xpert MTB/RIF testing for people living with HIV in India. PLoS One 2015;10(2):e0116721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raizada 2018b {published data only}
- Raizada N, Khaparde SD, Salhotra VS, Rao R, Kalra A, Swaminathan S, et al. Accelerating access to quality TB care for pediatric TB cases through better diagnostic strategy in four major cities of India. PLoS One 2018;13(2):e0193194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raizada 2018c {published data only}
- Raizada N, Khaparde SD, Rao R, Kalra A, Sarin S, Salhotra VS, et al. Upfront Xpert MTB/RIF testing on various specimen types for presumptive infant TB cases for early and appropriate treatment initiation. PLoS One 2018;13(8):e0202085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rathour 2019 {published data only}
- Rathour JS, Mantan M, Khanna A, et al. Evaluation of GENE XPERT assay in extrapulmonary tuberculosis in children. Journal of Evolution of Medical and Dental Sciences 2019;8(1):76-80. [Google Scholar]
Rebecca 2018 {published data only}
- Rebecca B, Chacko A, Verghese V, Rose W. Spectrum of pediatric tuberculosis in a tertiary care setting in South India. Journal of Tropical Pediatrics 2018;64(6):544-7. [DOI] [PubMed] [Google Scholar]
Rivera 2017 {published data only}
- Rivera VR, Jean-Juste MA, Gluck SC, Reeder HT, Sainristil J, Julma P, et al. Diagnostic yield of active case finding for tuberculosis and HIV at the household level in slums in Haiti. International Journal of Tuberculosis and Lung Disease 2017;21(11):1140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sabi 2016 {published data only}
- Sabi I, Kabyemera R, Mshana SE, Kidenya BR, Kasanga G, Gerwing-Adima LE, et al. Pulmonary TB bacteriologically confirmed by induced sputum among children at Bugando Medical Centre, Tanzania. International Journal of Tuberculosis and Lung Disease 2016;20(2):228-34. [DOI] [PubMed] [Google Scholar]
Sachdeva 2015 {published data only}
- Sachdeva KS, Raizada N, Sreenivas A, Van't Hoog AH, Van Den Hof S, Dewan PK, et al. Use of Xpert MTB/RIF in decentralized public health settings and its effect on pulmonary TB and DR-TB case finding in India. PLoS One 2015;10(5):e0126065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanchini 2014 {published data only}
- Sanchini A, Fiebig L, Drobniewski F, Haas W, Richter E, Katalinic-Jankovic V, et al. Laboratory diagnosis of paediatric tuberculosis in the European Union/European Economic Area: analysis of routine laboratory data, 2007 to 2011. Eurosurveillance 2014;19(11):pii: 20744. [DOI] [PubMed] [Google Scholar]
Sander 2019 {published data only}
- Sander MS, Laah SN, Titahong CN, Lele C, Kinge T, Jong BC, et al. Systematic screening for tuberculosis among hospital outpatients in Cameroon: the role of screening and testing algorithms to improve case detection. Journal of Clinical Tuberculosis and Other Mycobacterial Diseases 2019;15:100095. [DOI: 10.1016/j.jctube.2019.100095] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanjuan‐Jimenez 2015 {published data only}
- Sanjuan-Jimenez R, Toro-Peinado I, Bermudez P, Colmenero JD, Morata P. Comparative study of a real-time PCR assay targeting senX3-regX3 versus other molecular strategies commonly used in the diagnosis of tuberculosis. PLoS One 2015;10(11):e0143025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schumacher 2016 {published data only}
- Schumacher SG, Smeden M, Dendukuri N, Joseph L, Nicol MP, Pai M, et al. Diagnostic test accuracy in childhood pulmonary tuberculosis: a Bayesian latent class analysis. American Journal of Epidemiology 2016;184(9):690-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scott 2014 {published data only}
- Scott LE, Beylis N, Nicol M, Nkuna G, Molapo S, Berrie L, et al. Diagnostic accuracy of Xpert MTB/RIF for extrapulmonary tuberculosis specimens: establishing a laboratory testing algorithm for South Africa. Journal of Clinical Microbiology 2014;52(6):1818-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2016a {published data only}
- Shah I, Gupta Y. Xpert MTB/RIF for diagnosis of tuberculosis and drug resistance in Indian children. Indian Pediatrics 2016;53(9):837-8. [DOI] [PubMed] [Google Scholar]
Shah 2018 {published data only}
- Shah MA, Shah I. Increasing prevalence of pediatric drug-resistant tuberculosis in Mumbai, India, and Its outcome. Pediatric Infectious Disease Journal 2018;37(12):1261-3. [DOI] [PubMed] [Google Scholar]
Shah 2019 {published data only}
- Shah I, Bhamre R, Shetty NS. Accuracy of Xpert® Mycobacterium tuberculosis/rifampicin assay in diagnosis of pulmonary tuberculosis. Infectious Diseases 2019;51(7):550-3. [DOI] [PubMed] [Google Scholar]
Sharma 2015 {published data only}
- Sharma SK, Kohli M, Yadav RN, Chaubey J, Bhasin D, Sreenivas V, et al. Evaluating the diagnostic accuracy of Xpert MTB/RIF assay in pulmonary tuberculosis. PLoS One 2015;10(10):e0141011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sieiro 2018 {published data only}
- Sieiro TLA, Aurílio RB, Soares ECC, Chiang SS, Sant Anna CC. The role of the Xpert MTB/RIF assay among adolescents suspected of pulmonary tuberculosis in Rio de Janeiro, Brazil. Revista da Sociedade Brasileira de Medicina Tropical 2018;51(2):234-6. [DOI] [PubMed] [Google Scholar]
Singh 2015 {published data only}
- Singh S, Singh A, Prajapati S, Kabra SK, Lodha R, Mukherjee A, et al. Xpert MTB/RIF assay can be used on archived gastric aspirate and induced sputum samples for sensitive diagnosis of paediatric tuberculosis. BMC Microbiology 2015;15:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh UB 2016 {published data only}
- Singh UB, Pandey P, Mehta G, Bhatnagar AK, Mohan A, Goyal V, et al. Genotypic, phenotypic and clinical validation of GeneXpert in extra-pulmonary and pulmonary tuberculosis in India. PLoS One 2016;11(2):e0149258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Solomons 2016 {published data only}
- Solomons RS, Visser DH, Marais BJ, Schoeman JF, Furth AM. Diagnostic accuracy of a uniform research case definition for TBM in children: a prospective study. International Journal of Tuberculosis and Lung Disease 2016;20(7):903-8. [DOI] [PubMed] [Google Scholar]
Sureshbabu 2016 {published data only}
- Sureshbabu R, Lakshmi Murali A, Palaniswamy M. Molecular diagnosis of drug resistance tuberculosis In the Districts Of Tamilnadu. International Journal of Pharma and Bio Sciences 2016;7(4):B42-B6. [Google Scholar]
Tadesse 2015 {published data only}
- Tadesse M, Abebe G, Abdissa K, Aragaw D, Abdella K, Bekele A, et al. GeneXpert MTB/RIF Assay for the diagnosis of tuberculous lymphadenitis on concentrated fine needle aspirates in high tuberculosis burden settings. PLoS One 2015;10(9):e0137471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tafur 2018 {published data only}
- Tafur KT, Coit J, Leon SR, Pinedo C, Chiang SS, Contreras C, et al. Feasibility of the string test for tuberculosis diagnosis in children between 4 and 14 years old. BMC Infectious Diseases 2018;18:574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tang 2017 {published data only}
- Tang T, Liu F, Lu X, Huang Q. Evaluation of GeneXpert MTB/RIF for detecting Mycobacterium tuberculosis in a hospital in China. Journal of International Medical Research 2017;45(2):816-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Theron 2011 {published data only}
- Theron G, Peter J, Zyl-Smit R, Mishra H, Streicher E, Murray S, et al. Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in a high HIV prevalence setting. American Journal of Respiratory and Critical Care Medicine 2011;184(1):132-40. [DOI] [PubMed] [Google Scholar]
Triasih 2015 {published data only}
- Triasih R, Robertson C, Duke T, Graham SM. Risk of infection and disease with Mycobacterium tuberculosis among children identified through prospective community-based contact screening in Indonesia. Tropical Medicine & International Health 2015;20(6):737-43. [DOI] [PubMed] [Google Scholar]
Ullah 2017 {published data only}
- Ullah I, Javaid A, Masud H, Ali M, Basit A, Ahmad W, et al. Rapid detection of Mycobacterium tuberculosis and rifampicin resistance in extrapulmonary tuberculosis and sputum smear negative pulmonary suspects using Xpert MTB/RIF. Journal of Medical Microbiology 2017;66(4):412-8. [DOI] [PubMed] [Google Scholar]
Walters 2012 {published data only}
- Walters E, Gie RP, Hesseling AC, Friedrich SO, Diacon AH, Gie RP. Rapid diagnosis of pediatric intrathoracic tuberculosis from stool samples using the Xpert MTB/RIF assay: a pilot study. Pediatric Infectious Disease Journal 2012;31(12):1316. [DOI] [PubMed] [Google Scholar]
Walters 2017b {published data only}
- Walters E, Demers AM, Zalm MM, Whitelaw A, Palmer M, Bosch C, et al. Stool culture for diagnosis of pulmonary tuberculosis in children. Journal of Clinical Microbiology 2017;55(12):3355-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walters 2018b {published data only}
- Walters E, Zalm MM, Demers AM, Whitelaw A, Palmer M, Bosch C, et al. Specimen pooling as a diagnostic strategy for microbiologic confirmation in children with intrathoracic tuberculosis. Paediatric Infectious Disease Journal 2018;38(6):e128-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2016 {published data only}
- Zhang AM, Li F, Liu XH, Xia L, Lu SH. [Application of Gene Xpert Mycobacterium tuberculosis DNA and resistance to rifampicin assay in the rapid detection of tuberculosis in children]. Chinese Journal of Pediatrics [Zhonghua er ke za zhi] 2016;54(5):370-4. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ChiCTR1800015075 {unpublished data only}
- ChiCTR1800015075. Diagnostic accuracy of Xpert MTB/RIF Ultra assay on diagnosing pediatric pulmonary tuberculosis. chictr.org.cn/showprojen.aspx?proj=25233 (study execute time 1 January 2018). [ChiCTR1800015075]
NCT03831906 {unpublished data only}
- NCT03831906. TB-speed pneumonia [Impact of systematic early tuberculosis (TB) detection using Xpert MTB/RIF Ultra in children with severe pneumonia in high tuberculosis burden countries]. clinicaltrials.gov/ct2/show/NCT03831906 (first posted 6 February 2019). [NCT03831906]
Additional references
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401-6. [DOI] [PubMed] [Google Scholar]
Bjerrum 2019
- Bjerrum S, Schiller I, Dendukuri N, Kohli M, Nathavitharana RR, Zwerling AA, et al. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in people living with HIV. Cochrane Database Systematic Reviews 2019;10(10):CD011420. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyle 2017
- Boyle D. Tuberculosis Diagnostics Technology and Market Landscape. 5th edition. Vernier: World Health Organization Unitaid Secretariat, 2017. [Google Scholar]
Broger 2019
- Broger T, Sosse B, Du Toit E, Kerkhoff AD, Schutz C, Reipold EI, et al. Novel lipoarabinomannan point-of-care tuberculosis test for people with HIV: a diagnostic accuracy study. Lancet. Infectious Diseases 2019;19(8):852-61. [DOI] [PMC free article] [PubMed]
Chakravorty 2017
- Chakravorty S, Simmons AM, Rowneki M, Parmar H, Cao Y, Ryan J, et al. The new Xpert MTB/RIF Ultra: improving detection of Mycobacterium tuberculosis and resistance to rifampin in an assay suitable for point-of-care testing. mBio 2017;8(4):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chiang 2017
- Chiang SS, Swanson DS, Starke JR. New diagnostics for childhood tuberculosis. Infectious Disease Clinics of North America 2015;29(3):477-502. [DOI] [PubMed] [Google Scholar]
Chu 2006
- Chu H, Cole SR. Bivariate meta-analysis of sensitivity and specificity with sparse data: a generalized linear mixed model approach. Journal of Clinical Epidemiology 2006;59(12):1331-2. [DOI] [PubMed] [Google Scholar]
Cruz 2012
- Cruz AT, Revell PA, Starke JR. Gastric aspirate yield for children with suspected pulmonary tuberculosis. Journal of the Pediatric Infectious Diseases Society 2012;2(2):171-4. [DOI] [PubMed] [Google Scholar]
David 2017
- David SG, Lovero KL, Pombo-March MFB, Abreu TG, Ruffino-Netto A, Kritski AL, et al. A comparison of tuberculosis diagnostic systems in a retrospective cohort of HIV-infected children in Rio de Janeiro, Brazil. International Journal of Infectious Diseases 2017;59:150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Detjen 2015
- Detjen AK, DiNardo AR, Leyden J, Steingart KR, Menzies D, Schiller I, et al. Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in children: a systematic review and meta-analysis. Lancet Respiratory Medicine 2015;3(6):451-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dodd 2017
- Dodd PJ, Yuen CM, Sismanidis C, Seddon JA, Jenkins HE. The global burden of tuberculosis mortality in children: a mathematical modelling study. Lancet Global Health 2017;5(9):e898-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dorman 2018
- Dorman SE, Schumacher SG, Alland D, Nabeta P, Armstrong DT, King B, et al. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infectious Diseases 2018;18(1):76-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dropbox [Computer program]
- Dropbox. San Francisco: Dropbox Inc, 2007.
Dunn 2016
- Dunn JJ, Starke JR, Revell PA. Laboratory diagnosis of Mycobacterium tuberculosis infection and disease in children. Journal of Clinical Microbiology 2016;54(6):1434-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Excel 2013 [Computer program]
- Microsoft Excel Software. Version 16.16.4. Seattle: Microsoft Corporation, 2013.
Frigati 2015
- Frigati L, Maskew M, Workman L, Munro J, Andronikou S, Nicol MP, et al. Predictors of culture-confirmed pulmonary tuberculosis in children in a high tuberculosis and HIV prevalence area. Pediatric Infectious Disease Journal 2015;34(9):e206-10. [DOI] [PubMed] [Google Scholar]
Furin 2019
- Furin J. Advances in the diagnosis, treatment, and prevention of tuberculosis in children. Expert Review of Respiratory Medicine 2019;13(3):301-11. [DOI] [PubMed] [Google Scholar]
Graham 2012
- Graham SM, Ahmed T, Amanullah F, Browning R, Cardenas V, Casenghi M, et al. Evaluation of tuberculosis diagnostics in children: 1. Proposed clinical case definitions for classification of intrathoracic tuberculosis disease. Consensus from an expert panel. Journal of Infectious Diseases 2012;205(Suppl 2):S199-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Graham 2015
- Graham SM, Cuevas LE, Jean-Philippe P, Browning R, Casenghi M, Detjen AK. Clinical case definitions for classification of intrathoracic tuberculosis in children: an update. Clinical Infectious Diseases 2015;61(Suppl 3):S179-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta‐Wright 2018
- Gupta-Wright A, Corbett EL, Oosterhout JJ, Wilson D, Grint D, Alufandika-Moyo M, et al. Rapid urine-based screening for tuberculosis in HIV-positive patients admitted to hospital in Africa (STAMP): a pragmatic, multicentre, parallel-group, double-blind, randomised controlled trial. Lancet 2018;392:292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Horne 2019
- Horne DJ, Kohli M, Zifodya JS, Schiller I, Dendukuri N, Tollefson D, et al. Xpert MTB/RIF and Xpert MTB/RIF Ultra for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database of Systematic Reviews 2019;7(6):CD009593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jenkins 2017
- Jenkins HE, Yuen CM, Rodriguez CA, Nathavitharana RR, McLaughlin MM, Donald P, et al. Mortality in children diagnosed with tuberculosis: a systematic review and meta-analysis. Lancet Infectious Diseases 2017;17(3):285-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kohli 2018
- Kohli M, Schiller I, Dendukuri N, Dheda K, Denkinger CM, Schumacher SG, et al. Xpert® MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance. Cochrane Database of Systematic Reviews 2018, Issue 8. Art. No: CD012768. [DOI: 10.1002/14651858.CD012768.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kunkel 2016
- Kunkel A, Abel Zur Wiesch P, Nathavitharana RR, Marx FM, Jenkins HE, Cohen T. Smear positivity in paediatric and adult tuberculosis: systematic review and meta-analysis. BMC Infectious Diseases 2016;16(282):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Macaskill 2010
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10. Analysing and presenting results. In: Deeks JJ, Bossuyt PM, Gatsonis C, editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0.0. Cochrane, 2013. Available from srdta.cochrane.org.
MacLean 2019
- MacLean E, Sulis G, Denkinger CM, Johnston JC, Pai M, Khana FA. Diagnostic accuracy of stool Xpert MTB/RIF for detection of pulmonary tuberculosis in children: a systematic review and meta-analysis. Journal of Clinical Microbiology 2019;57(6):e02057-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marais 2004
- Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Obihara CC, Starke JJ, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. International Journal of Tuberculosis and Lung Disease 2004;8(4):392-402. [PubMed] [Google Scholar]
Marais 2005
- Marais BJ, Gie RP, Obihara CC, Hesseling AC, Schaaf HS, Beyers N. Well defined symptoms are of value in the diagnosis of childhood pulmonary tuberculosis. Archives of Disease in Children 2005;90(11):1162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marais 2006a
- Marais BJ, Gie RP, Hesseling AC, Schaaf HS, Enarson DA, Beyers N. Radiographic signs and symptoms in children treated for tuberculosis: possible implications for symptom-based screening in resource-limited settings. Pediatric Infectious Disease Journal 2006;25(3):237-40. [DOI] [PubMed] [Google Scholar]
Marais 2006b
- Marais BJ, Gie RP, Hesseling AC, Schaaf HS, Lombard C, Enarson DA, et al. A refined symptom-based approach to diagnose pulmonary tuberculosis in children. Pediatrics 2006;118(5):e1350-9. [DOI] [PubMed] [Google Scholar]
Marais 2006c
- Marais BJ, Gie RP, Schaaf HS, Hesseling AS, Enarson DA, Beyers N. The spectrum of disease in children treated for tuberculosis in a highly endemic area. International Journal of Tuberculosis and Lung Disease 2006;10:732–8. [PubMed] [Google Scholar]
Marais 2006d
- Marais BJ, Gie RP, Hesseling AC, Schaaf HS, Enarson DA, Beyers N. The bacteriologic yield in children with intrathoracic tuberculosis. Clinical Infectious Diseases 2006;42:69-71. [DOI] [PubMed] [Google Scholar]
Marais 2014
- Marais BJ, Schaaf HS. Tuberculosis in children. Cold Spring Harbor Perspectives in Medicine 2014;4:1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mesman 2019
- Mesman AW, Rodriguez C, Ager E, Coit J, Trevisi L, Franke MF. Diagnostic accuracy of molecular detection of Mycobacterium tuberculosis in pediatric stool samples: a systematic review and meta-analysis. Tuberculosis 2019;119:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 6;7:e1000097. [DOI: 10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nicol 2014
- Nicol MP, Allen V, Workman L, Isaacs W, Munro J, Pienaar S, et al. Urine lipoarabinomannan testing for diagnosis of pulmonary tuberculosis in children: a prospective study. Lancet Global Health 2014;2(5):e278-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peter 2016
- Peter JG, Zijenah LS, Chanda D, Clowes P, Lesosky M, Gina P, et al. Effect on mortality of point-of-care, urine-based lipoarabinomannan testing to guide tuberculosis treatment initiation in HIV-positive hospital inpatients: a pragmatic, parallel-group, multicountry, open-label, randomised controlled trial. Lancet 2016;387(10024):1187-97. [DOI] [PubMed] [Google Scholar]
Reid 2012
- Reid MJ, Saito S, Fayorsey R, Carter RJ, Abrams EJ. Assessing capacity for diagnosing tuberculosis in children in sub-Saharan African HIV care settings. International Journal of Tuberculosis and Lung Disease 2012;16(7):924-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reitsma 2015
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58:982‐90. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, Cochrane Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, Cochrane, 2014.
Schünemann 2008
- Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ 2008;336(7653):1106-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2016
- Schünemann HJ, Mustafa R, Brozek J, Santesso N, Alonso-Coello P, Guyatt G, et al. GRADE Working Group. GRADE Guidelines: 16. GRADE evidence to decision frameworks for tests in clinical practice and public health. Journal of Clinical Epidemiology 2016;76:89-98. [DOI: 10.1016/j.jclinepi.2016.01.032] [DOI] [PubMed] [Google Scholar]
Schünemann 2020a
- Schünemann HJ, Mustafa R, Brozek J, Steingart KR, Leeflang M, Murad MH, et al. GRADE guidelines: 21 part 1. Study design, risk of bias and indirectness in rating the certainty across a body of evidence for test accuracy. Journal of Clinical Epidemiology 2020;122:129-41. [DOI] [PubMed] [Google Scholar]
Schünemann 2020b
- Schünemann HJ, Mustafa R, Brozek J, Steingart KR, Leeflang M, Murad MH, et al. GRADE guidelines: 21 part 2. Inconsistency, Imprecision, publication bias and other domains for rating the certainty of evidence for test accuracy and presenting it in evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2020;122:142-52. [DOI] [PubMed] [Google Scholar]
Shah 2016b
- Shah M, Hanrahan C, Wang ZY, Dendukuri N, Lawn SD, Denkinger CM, et al. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in HIV-positive adults. Cochrane Database of Systematic Reviews 2016, Issue 5. Art. No: CD011420. [DOI: 10.1002/14651858.CD011420.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stata 15 [Computer program]
- Stata Statistical Software. StataCorp, Version 15. College Station: StataCorp LP, 2017.
Takwoingi 2015
- Takwoingi Y, Guo B, Riley RD, Deeks JJ. Performance of methods for meta-analysis of diagnostic test accuracy with few studies or sparse data. Statistical Methods in Medical Research 2015;0:1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Theart 2005
- Theart AC, Marais BJ, Gie RP, Hesseling AC, Beyers N. Criteria used for the diagnosis of childhood tuberculosis at primary health care level in a high-burden, urban setting. International Journal of Tuberculosis and Lung Disease 2005;9(11):1210-4. [PubMed] [Google Scholar]
Wademan 2019
- Wademan DT, Busakwe L, Nicholson TJ, Zalm M, Palmer M, Workman J, et al. Acceptability of a first-line anti-tuberculosis formulation for children: qualitative data from the SHINE trial. International Journal of Tuberculosis and Lung Disease 2019;12:1263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2015
- Wang XW, Pappoe F, Huang Y, Cheng XW, Xu DF, Wang H, et al. Xpert MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in children: a meta-analysis. Clinical Laboratory 2015;61:1775-85. [DOI] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529-36. [DOI] [PubMed] [Google Scholar]
WHO 2011
- World Health Organization. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system for the diagnosis of pulmonary and extrapulmonary TB in adults and children. apps.who.int/iris/handle/10665/112472 (accessed prior to 29 April 2019). [PubMed]
WHO 2013
- World Health Organization. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children. apps.who.int/iris/bitstream/handle/10665/112472/9789241506335_eng.pdf?sequence=1 (accessed prior to 29 April 2019).
WHO 2014a
- World Health Organization. Guidance for national tuberculosis programmes on the management of tuberculosis in children. apps.who.int/medicinedocs/documents/s21535en/s21535en.pdf (accessed prior to 29 April 2019).
WHO 2014b
- World Health Organization. Xpert MTB/RIF implementation manual. Technical and operational ‘how-to’ practical considerations. apps.who.int/iris/bitstream/10665/112469/1/9789241506700_eng.pdf 2014 (accessed prior to 29 April 2019).
WHO 2015
- World Health Organization. Use of high burden country lists for TB by WHO in the post-2015 era. www.who.int/tb/publications/global_report/high_tb_burdencountrylists2016-2020.pdf?ua=1 (accessed prior to 29 April 2019).
WHO 2017
- World Health Organization. WHO Meeting Report of a Technical Expert Consultation: non-inferiority analysis of Xpert MTB/RIF Ultra compared to Xpert MTB/RIF. apps.who.int/iris/bitstream/10665/254792/1/WHO-HTM-TB-2017.04-eng.pdf (accessed prior to 29 April 2019).
WHO 2018
- World Health Organization. Rapid Communication: key changes to treatment of multidrug- and rifampicin-resistant tuberculosis (MDR/RR-TB). August 2018. www.who.int/tb/publications/2018/rapid_communications_MDR/en/ (accessed 5 March 2019).
WHO Global TB Report 2019
- World Health Organization. Global Tuberculosis Report 2019. Geneva: World Health Organization, 2019. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
WHO Lateral flow LAM 2019
- World Health Organization. Lateral flow urine lipoarabinomannan assay (LF-LAM) for the diagnosis of active tuberculosis in people living with HIV. Policy update 2019. Geneva: World Health Organization, 2019. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
Zar 2005
- Zar HJ, Hanslo D, Apolles P, Swingler G, Hussey G. Induced sputum versus gastric lavage for microbiological confirmation of pulmonary tuberculosis in infants and young children: a prospective study. Lancet 2005;365(9454):130-4. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kay 2019
- Kay AW, González Fernández L, Takwoingi Y, Eisenhut M, Vu RD, Steingart KR, Detjen AK, Mandalakas AM. Xpert MTB/RIF and Xpert MTB/RIF Ultra assays for active tuberculosis and rifampicin resistance in children. Cochrane Database of Systematic Reviews 2019, Issue 6. Art. No: CD013359. [DOI: 10.1002/14651858.CD013359] [DOI] [PMC free article] [PubMed] [Google Scholar]


