Abstract
Background
This review is one of six looking at the primary medical management options for patients with chronic rhinosinusitis.
Chronic rhinosinusitis is common and is characterised by inflammation of the lining of the nose and paranasal sinuses leading to nasal blockage, nasal discharge, facial pressure/pain and loss of sense of smell. The condition can occur with or without nasal polyps. Nasal saline irrigation is commonly used to improve patient symptoms.
Objectives
To evaluate the effects of saline irrigation in patients with chronic rhinosinusitis.
Search methods
The Cochrane ENT Information Specialist searched the ENT Trials Register; Central Register of Controlled Trials (CENTRAL 2015, Issue 9); MEDLINE; EMBASE; ClinicalTrials.gov; ICTRP and additional sources for published and unpublished trials. The date of the search was 30 October 2015.
Selection criteria
Randomised controlled trials (RCTs) with a follow‐up period of at least three months comparing saline delivered to the nose by any means (douche, irrigation, drops, spray or nebuliser) with (a) placebo, (b) no treatment or (c) other pharmacological interventions.
Data collection and analysis
We used the standard methodological procedures expected by Cochrane. Our primary outcomes were disease‐specific health‐related quality of life (HRQL), patient‐reported disease severity and the commonest adverse event ‐ epistaxis. Secondary outcomes included general HRQL, endoscopic nasal polyp score, computerised tomography (CT) scan score and the adverse events of local irritation and discomfort. We used GRADE to assess the quality of the evidence for each outcome; this is indicated in italics.
Main results
We included two RCTs (116 adult participants). One compared large‐volume (150 ml) hypertonic (2%) saline irrigation with usual treatment over a six‐month period; the other compared 5 ml nebulised saline twice a day with intranasal corticosteroids, treating participants for three months and evaluating them on completion of treatment and three months later.
Large‐volume, hypertonic nasal saline versus usual care
One trial included 76 adult participants (52 intervention, 24 control) with or without polyps.Disease‐specific HRQL was reported using the Rhinosinusitis Disability Index (RSDI; 0 to 100, 100 = best quality of life). At the end of three months of treatment, patients in the saline group were better than those in the placebo group (mean difference (MD) 6.3 points, 95% confidence interval (CI) 0.89 to 11.71) and at six months there was a greater effect (MD 13.5 points, 95% CI 9.63 to 17.37). We assessed the evidence to be of low quality for the three months follow‐up and very low quality for the six months follow‐up.
Patient‐reported disease severity was evaluated using a "single‐item sinus symptom severity assessment" but the range of scores is not stated, making it impossible for us to determine the meaning of the data presented.
No adverse effects data were collected in the control group but 23% of participants in the saline group experienced side effects including epistaxis.
General HRQL was measured using SF‐12 (0 to 100, 100 = best quality of life). No difference was found after three months of treatment (low quality evidence) but at six months there was a small difference favouring the saline group, which may not be of clinical significance and has high uncertainty (MD 10.5 points, 95% CI 0.66 to 20.34) (very low quality evidence).
Low‐volume, nebulised saline versus intranasal corticosteroids
One trial included 40 adult participants with polyps. Our primary outcome of disease‐specific HRQL was not reported. At the end of treatment (three months) the patients who had intranasal corticosteroids had less severe symptoms (MD ‐13.50, 95% CI ‐14.44 to ‐12.56); this corresponds to a large effect size. We assessed the evidence to be of very low quality.
Authors' conclusions
The two studies were very different in terms of included populations, interventions and comparisons and so it is therefore difficult to draw conclusions for practice. The evidence suggests that there is no benefit of a low‐volume (5 ml) nebulised saline spray over intranasal steroids. There is some benefit of daily, large‐volume (150 ml) saline irrigation with a hypertonic solution when compared with placebo, but the quality of the evidence is low for three months and very low for six months of treatment.
Plain language summary
Saline irrigation for chronic rhinosinusitis
Review question
We reviewed the evidence for the benefits and harms of nasal saline irrigation in patients with chronic rhinosinusitis.
Background
Chronic rhinosinusitis is a common condition that is defined as inflammation of the nose and paranasal sinuses (a group of air‐filled spaces behind the nose, eyes and cheeks). Patients with chronic rhinosinusitis experience at least two or more of the following symptoms for at least 12 weeks: blocked nose, discharge from their nose or runny nose, pain or pressure in their face and/or a reduced sense of smell (hyposmia). Some people will also have nasal polyps, which are grape‐like swellings of the normal nasal lining inside the nasal passage and sinuses.
Nasal irrigation (also know as nasal douche, wash or lavage) is a procedure that rinses the nasal cavity with isotonic or hypertonic saline (salt water) solutions. The patient instils saline into one nostril and allows it to drain out of the other nostril, bathing the nasal cavity. Saline nasal irrigation can be performed with low positive pressure from a spray, pump or squirt bottle, with a nebuliser or with gravity‐based pressure using a vessel with a nasal spout, such as a 'neti pot'. This therapy is available over the counter and is used as a standalone or add‐on treatment by many patients with chronic rhinosinusitis.
Study characteristics
We included two randomised controlled trials with a total of 116 adult participants in this review. One compared large‐volume (150 ml) hypertonic saline irrigation with usual treatment over a six‐month period. The other compared 5 ml of nebulised saline twice a day with intranasal corticosteroids, treating participants for three months and evaluating them on completion of treatment and three months later. Both of these studies had important limitations in their methodology and we considered them to have a high risk of bias.
Key results and quality of the evidence
Large‐volume, hypertonic nasal saline versus usual care
In the small trial of 76 participants our primary outcome of 'disease‐specific health‐related quality of life' was reported using a 0‐ to 100‐point scale. At the end of three months of treatment, patients in the saline group were better than those in the placebo group and at six months of treatment there was a greater effect. We assessed the evidence to be of low quality for the three months follow‐up and very low quality for the six months follow‐up.
Patient‐reported disease severity was also evaluated but the trialists did not state the range of scores used, which made it impossible for us to determine the meaning of the data presented.
No adverse effects data were collected in the control group but 23% of participants in the saline group experienced side effects including nosebleeds (epistaxis).
General health‐related quality of life was also measured in this study. No difference was found after three months of treatment but at six months there was a small difference (although the result is uncertain). We assessed the evidence to be of low quality.
Low‐volume, nebulised saline versus intranasal corticosteroids
One small trial had 20 patients in each of the two arms being compared. Our primary outcome of disease‐specific health‐related quality of life was not reported. At the end of treatment (three months) there was an improvement in symptoms.
Conclusions
The two studies were very different in terms of included populations, interventions and comparisons and so it is therefore difficult to draw conclusions for practice. The evidence suggests that there was no benefit of a low‐volume (5 ml) nebulised saline spray over intranasal steroids, but there may be some benefit of daily, large‐volume (150 ml) saline irrigation with a hypertonic solution compared with placebo, although the quality of the evidence was low for three months and very low for six months of treatment.
Summary of findings
Background
Description of the condition
Chronic rhinosinusitis is defined as inflammation of the nose and paranasal sinuses characterised by two or more symptoms, one of which must be nasal blockage/obstruction/congestion or nasal discharge (anterior/posterior nasal drip). The other possible symptoms include facial pain/pressure, reduction or loss of sense of smell (in adults) or cough (in children). Symptoms must have continued for at least 12 weeks. In addition people must have either mucosal changes within the ostiomeatal complex and/or sinuses as evidenced by a computerised tomography (CT) scan and/or endoscopic signs of at least one of the following: nasal polyps, mucopurulent discharge primarily from middle meatus or oedema/mucosal obstruction primarily in the middle meatus (EPOS 2012).
Chronic rhinosinusitis represents a common source of ill health; 11% of UK adults reported chronic rhinosinusitis symptoms in a worldwide population study (Hastan 2011). Symptoms, including nasal obstruction, nasal discharge, facial pain, anosmia and sleep disturbance, have a major impact on quality of life, reportedly greater in several domains of the SF‐36 than angina or chronic respiratory disease (Gliklich 1995). Acute exacerbations, inadequate symptom control and respiratory disease exacerbation are common. Complications are rare, but may include visual impairment and intracranial infection.
Two major phenotypes of chronic rhinosinusitis have been identified based on the presence or absence of nasal polyps on examination. Nasal polyps are tumour‐like hyperplastic swellings of the nasal mucosa, most commonly originating from within the ostiomeatal complex (Larsen 2004). Chronic rhinosinusitis with nasal polyps (CRSwNP) is diagnosed when polyps are seen (on direct or endoscopic examination) bilaterally in the middle meatus. The acronym CRSsNP is used for the condition in which no polyps are present.
Although the aetiology of chronic rhinosinusitis is not fully understood, it may involve abnormalities in the host response to irritants, commensal and pathogenic organisms and allergens, obstruction of sinus drainage pathways, abnormalities of normal mucociliary function, loss of the normal mucosal barrier or infection. Two typical profiles may be observed with respect to inflammatory mediators; in eosinophilic chronic rhinosinusitis, which is typically associated with nasal polyps, high levels of eosinophils, immunoglobulin E (IgE) and interleukin (IL)‐5 may be found, while in neutrophilic chronic rhinosinusitis, more often associated with chronic rhinosinusitis without polyps, neutrophils predominate, with elevated interferon (IFN) gamma, IL‐8 and tumour necrosis factor (TNF) (EPOS 2012).
While treatment decisions should be made based on an understanding of the patient's chronic rhinosinusitis phenotype and likely aetiology, in practice treatment may be initiated without knowledge of the polyp status, particularly in primary care. This review (and most of its companion reviews) consider patients with and without polyps together in the initial evaluation of treatment effects. However, subgroup analyses explore the potential differences between them.
The most commonly used interventions for chronic rhinosinusitis are used either topically (sprayed into the nose) or systemically (by mouth) and include steroids, antibiotics and saline.
Description of the intervention
Nasal irrigation (also know as nasal douche, wash or lavage) is a procedure that rinses the nasal cavity with isotonic or hypertonic saline (salt water) solutions. It is performed by instilling saline into one nostril and allowing it to drain out of the other nostril, bathing the nasal cavity. Saline nasal irrigation can be performed with low positive pressure from a spray, pump or squirt bottle, with a nebuliser or with gravity‐based pressure using a vessel with a nasal spout, such as a neti pot. This therapy is available over the counter and is used as a standalone or adjunct treatment by many patients with chronic rhinosinusitis.
How the intervention might work
The exact mechanism of action of saline nasal irrigation is unknown. Saline nasal irrigation may improve nasal mucosa function through several physiological effects, including direct cleansing of mucus (mucus is a potential medium for bacterial growth; saline thins mucus and helps to clear it out); removal of antigens, biofilm or inflammatory mediators (thereby resolving inflammation); and improved mucociliary function (as suggested by increased ciliary beat frequency; Brown 2004). Both the method of irrigation and the tonicity (concentration) of the saline solution may have an impact on its effectiveness.
Why it is important to do this review
Nasal saline irrigation has been adopted widely based on the presumption that it is safe, cheap and widely available. A 2007 Cochrane review assessed this intervention (Harvey 2007). However, this previous review had broad inclusion criteria, including patients with very broadly defined chronic rhinosinusitis. In this new review, which replaces the original, we have adopted a stricter definition of chronic rhinosinusitis and aim not only to evaluate the overall effectiveness of nasal saline irrigation but also, where possible, that of various methods of delivery and concentrations. We have looked at the benefits and harms of nasal saline compared with no treatment or 'placebo' and other treatments for chronic rhinosinusitis, and its effects as an adjunct treatment in patients with chronic rhinosinusitis who are also using other treatments, such as intranasal corticosteroids, oral corticosteroids, antibiotics or combinations.
This review is one of a suite of Cochrane reviews looking at common management options for patients with chronic rhinosinusitis (Chong 2016a; Chong 2016b; Head 2016a; Head 2016b; Head 2016c), and we use the same outcome measures across the reviews. We have not included studies designed to evaluate interventions in the immediate peri‐surgical period, which are focused on assessing the impact of the intervention on the surgical procedure or on modifying the post‐surgical results (preventing relapse).
Objectives
To evaluate the effects of saline irrigation in patients with chronic rhinosinusitis.
Methods
Criteria for considering studies for this review
Types of studies
We included studies with the following design characteristics:
randomised controlled trials, including cluster‐randomised trials and quasi‐randomised trials (cross‐over trials were only to be included if the data from the first phase were available); and
patients were followed up for at least two weeks.
We excluded studies with the following design characteristics:
randomised patients by side of nose (within‐patient controlled) because it is difficult to ensure that the effects of any of the interventions considered can be localised; or
perioperative studies, where the sole purpose of the study was to investigate the effect of the intervention on surgical outcome.
Types of participants
Patients with chronic rhinosinusitis, whether with or without polyps.
We excluded studies that included a majority of patients with:
cystic fibrosis;
allergic fungal sinusitis/eosinophilic fungal/mucinous rhinosinusitis;
aspirin‐exacerbated respiratory disease;
a history of surgery for nasal polyps within six weeks of entry to the study.
Types of interventions
Saline, as an active treatment, delivered to the nose by any means (douche, irrigation, drops, spray or nebuliser, using an intermittent, continuous or pulsed strategy).
The comparators were: no treatment or placebo or other standard treatments such as intranasal corticosteroids, short‐course oral steroids and/or antibiotics.
There are other additives, such as xylitol, antibacterials and surfactants, which can be added to nasal saline irrigation, and there are also other formulations, such as lactated Ringer's solution. We have not included these in this review.
The main comparison pairs were:
nasal saline versus no treatment/placebo;
nasal saline plus intranasal corticosteroids versus placebo or no treatment plus intranasal corticosteroids.
Other possible comparison pairs included:
nasal saline versus intranasal corticosteroids;
nasal saline type A versus other types/delivery methods/volumes of nasal irrigation;
hypertonic versus isotonic saline.
This review is part of a larger series of six reviews for the treatment of chronic rhinosinusitis:
Intranasal steroids versus placebo or no intervention for chronic rhinosinusitis (Chong 2016a).
Different types of intranasal steroids for chronic rhinosinusitis (Chong 2016b). This review compares different classes, doses and delivery methods of intranasal corticosteroids for chronic rhinosinusitis.
Short‐course oral steroids alone for chronic rhinosinusitis (Head 2016a). This review compares short‐course oral steroids alone with placebo or no intervention, or against other pharmacological interventions such as antibiotics or nasal saline irrigation.
Short‐course oral steroids as an adjunct therapy for chronic rhinosinusitis (Head 2016b). This review compares oral steroids where they have been used as add‐on therapy to other treatments for chronic rhinosinusitis (such as intranasal corticosteroids, antibiotics or saline solution).
Saline irrigation for chronic rhinosinusitis (this review). This review compares nasal saline irrigation for chronic rhinosinusitis with both placebo/no intervention and with intranasal corticosteroids, short‐course oral steroids or antibiotics.
Systemic and topical antibiotics for chronic rhinosinusitis (Head 2016c). This review compares both topical and systemic antibiotics with placebo/no treatment, two different antibiotics with each other and antibiotics with intranasal corticosteroids.
Types of outcome measures
We analysed the following outcomes in the review, but we did not use them as a basis for including or excluding studies.
Primary outcomes
Health‐related quality of life, using disease‐specific health‐related quality of life scores, such as the Sino‐Nasal Outcome Test‐22 (SNOT‐22), Rhinosinusitis Outcome Measures‐31 (RSOM‐31) and SNOT‐20.
Disease severity, as measured by patient‐reported symptom score (such as the Chronic Sinusitis Survey (CSS) questionnaire and visual analogue scales). In the absence of validated symptom score data, we reported patient‐reported individual symptom scores for the following symptoms: nasal obstruction/blockage/congestion, nasal discharge (rhinorrhoea), facial pressure/pain, loss of sense of smell (adults), cough (children).
Significant adverse effect: epistaxis.
Secondary outcomes
Health‐related quality of life, using generic quality of life scores, such as the SF‐36, EQ‐5D and other well‐validated instruments.
Other local adverse effects: local irritation.
Other local adverse effects: discomfort.
Endoscopic score (depending on population, either nasal polyps size score or endoscopy score, e.g. Lund‐Mackay/Lund‐Kennedy).
Computerised tomography (CT) scan score (e.g. Lund‐Mackay).
We grouped outcome measures into these time periods: three to less than six months, six to 12 months and more than 12 months. For adverse events, we analysed data from the longest time periods.
The adverse events that we aimed to collect from studies including one of the various comparators listed above were the same as those adverse events identified in the methods section of the companion reviews assessing the effects of those interventions as primary treatments. For example, for studies in which all participants received intranasal corticosteroids, the list of adverse events also included those specifically for intranasal corticosteroids as found in Chong 2016a and Chong 2016b.
Search methods for identification of studies
The Cochrane ENT Information Specialist conducted systematic searches for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions. The date of the search was 30 October 2015.
Electronic searches
The Information Specialist searched:
the Cochrane Register of Studies ENT Trials Register (searched 30 October 2015);
the Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 9);
-
Ovid MEDLINE (1946 to October week 4 2015);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations) (searched 30 October 2015);
PubMed (as a top up to searches in Ovid MEDLINE) (searched 30 October 2015);
Ovid EMBASE (1974 to 30 October 2015);
ClinicalTrials.gov, www.clinicaltrials.gov (search via the Cochrane Register of Studies) (searched 30 October 2015);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (searched 30 October 2015);
Google Scholar (searched 30 October 2015).
The Information Specialist modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011). Search strategies for major databases including CENTRAL are provided in Appendix 1.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, the Information Specialist searched PubMed, The Cochrane Library and Google to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials.
Data collection and analysis
Selection of studies
At least two review authors independently screened all titles and abstracts of the studies obtained from the database searches to identify potentially relevant studies. At least two review authors evaluated the full text of each potentially relevant study to determine if it met the inclusion and exclusion criteria for this review.
We resolved any differences by discussion and consensus, with the involvement of a third author for clinical and/methodological input where necessary.
Data extraction and management
Two review authors independently extracted data from each study using a standardised data collection form (see Appendix 2). Whenever a study had more than one publication, we retrieved all publications to ensure complete extraction of data. Where there were discrepancies in the data extracted by different review authors, we checked these against the original reports and resolved differences by discussion and consensus, with the involvement of a third author or a methodologist where appropriate. We contacted the original study authors for clarification or for missing data whenever possible. If differences were found between publications of a study, we contacted the original authors for clarification. We used data from the main paper(s) if no further information was found.
We included key characteristics of the studies, such as study design, setting, sample size, population and how outcomes were defined or collected in the studies. In addition, we also collected baseline information on prognostic factors or effect modifiers. For this review, this included:
presence or absence of nasal polyps;
baseline nasal polyps score;
whether the patient has had previous sinus surgery.
For the outcomes of interest to the review, we extracted the findings of the studies on an available case analysis basis; i.e. we included data from all patients available at the time points based on the treatment randomised whenever possible, irrespective of compliance or whether patients had received the treatment as planned.
In addition to extracting pre‐specified information about study characteristics and aspects of methodology relevant to risk of bias, we extracted the following summary statistics for each trial and each outcome:
For continuous data: the mean values, standard deviations and number of patients for each treatment group. Where endpoint data were not available, we extracted the values for change from baseline. We analysed data from measurement scales such as SNOT‐22 and EQ‐5D as continuous data.
For binary data: the numbers of participants experiencing an event and the number of patients assessed at the time point.
For ordinal scale data: if the data appeared to be approximately normally distributed or if the analysis that the investigators performed suggested parametric tests were appropriate, then we treated the outcome measures as continuous data. Alternatively, if data were available, we planned to convert into binary data.
We prespecified the time points of interest for the outcomes in this review. While studies may report data at multiple time points, we only extracted the longest available data within the time points of interest. For example, for 'short' follow‐up periods, our time point was defined as 'three to six months' post‐randomisation. If a study had reported data at three, four and six months, we only extracted and analysed the data for the six‐month follow‐up.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias of each included study. We followed the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011), and we used the Cochrane 'Risk of bias' tool. With this tool we assessed the risk of bias as 'low', 'high' or 'unclear' for each of the following six domains:
sequence generation;
allocation concealment;
blinding of participants, personnel and outcome assessment;
incomplete outcome data;
selective reporting;
other sources of bias.
Measures of treatment effect
We summarised the effects of dichotomous outcomes (e.g. proportion of patients with symptom resolution) as risk ratios (RR) with CIs. For the key outcomes that we presented in the 'Summary of findings' table, we also expressed the results as absolute numbers based on the pooled results and compared to the assumed risk. We also planned to calculate the number needed to treat to benefit (NNTB) using the pooled results. The assumed baseline risk is typically either (a) the median of the risks of the control groups in the included studies, this being used to represent a 'medium risk population' or, alternatively, (b) the average risk of the control groups in the included studies is used as the 'study population' (Handbook 2011). If a large number of studies were available, and where appropriate, we had also planned to present additional data based on the assumed baseline risk in (c) a low‐risk population and (d) a high‐risk population.
For continuous outcomes, we expressed treatment effects as a mean difference (MD) with standard deviation (SD) or as standardised mean difference (SMD) if different scales had been used to measure the same outcome. We planned to provide a clinical interpretation of the SMD values.
Unit of analysis issues
This review did not use data from phase II of cross‐over studies or from studies where the patient was not the unit of randomisation, i.e. studies where the side (right versus left) was randomised.
If we had found cluster‐randomised trials, we would have analysed these according to the methods in section 16.3.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011).
Dealing with missing data
We contacted study authors via email whenever the outcome of interest was not reported, if the methods of the study suggested that the outcome had been measured. We did the same if not all data required for meta‐analysis had been reported, unless the missing data were standard deviations. If standard deviation data were not available, we approximated these using the standard estimation methods from P values, standard errors or 95% CIs if these were reported as detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011). If it was impossible to estimate these, we contacted the study authors.
Apart from imputations for missing standard deviations, we conducted no other imputations. However, we had to carry out calculations relating to disease severity (reported as symptom scores) as most of the data were not measured using validated instruments nor reported in a way that was comparable across studies (see 'Imputing total symptom scores' below).
We extracted and analysed all data using the available case analysis method.
Imputing total symptom scores
Where a paper did not present information for the total disease severity in terms of patient‐reported symptom scores but did present data for the results of individual symptoms, we used these to calculate a total symptom score. In addition, some studies used instruments that were not validated for patients with chronic rhinosinusitis and contained many additional symptoms not relevant to chronic rhinosinusitis. Whenever study reports provided sufficient information to cover the important domains related to the EPOS criteria for diagnosing chronic rhinosinusitis (EPOS 2012), we added up these individual scores. These EPOS 2012 criteria for chronic rhinosinusitis require at least two symptoms. One of the symptoms must be either nasal blockage or nasal discharge, and the other symptoms can include facial pressure/pain, loss of sense of smell (for adults) or cough (for children). Where a mean change or final value for individual symptoms was provided we summed these to calculate an overall summed mean for the total score. We calculated standard deviations for the total mean score as if the symptom data were an independent, random variable that was normally distributed. We acknowledge that there is likely to be a degree of correlation between the individual symptoms, however we used this process because the magnitude of correlation between the individual symptoms is not currently well understood (no evidence found). If the correlation is high, the summation of variables as discrete variables is likely to give a conservative estimate of the total variance of the summed final score. If the correlation is low, this method of calculation will underestimate the standard deviation of the total score. However, the average patient‐reported symptom scores have a correlation coefficient of about 0.5; if this is also applicable to chronic rhinosinusitis symptoms, the method used should have minimal impact (Balk 2012). As this method of calculation does not take into account weighting of different symptoms (no evidence found), we downgraded all the disease severity outcomes for lack of use of validated scales whenever this occurred.
However, the studies found in this review did not report data in a way that required imputation to calculate total symptom scores and we did not need to use this method.
Assessment of heterogeneity
We assessed clinical heterogeneity (which may be present even in the absence of statistical heterogeneity) by examining the included trials for potential differences between studies in the types of participants recruited, interventions or controls used and the outcomes measured.
We assessed statistical heterogeneity by visually inspecting the forest plots and by considering the Chi² test (with a significance level set at P value < 0.10) and the I² statistic, which calculates the percentage of variability that is due to heterogeneity rather than chance, with I² values over 50% suggesting substantial heterogeneity (Handbook 2011).
Assessment of reporting biases
We assessed reporting bias as between‐study publication bias and within‐study outcome reporting bias.
Outcome reporting bias (within‐study reporting bias)
We assessed within‐study reporting bias by comparing the outcomes reported in the published report against the study protocol, whenever this could be obtained. If the protocol was not available, we compared the outcomes reported to those listed in the methods section. If results are mentioned but not reported adequately in a way that allows analysis (e.g. the report only mentions whether the results were statistically significant or not), bias in a meta‐analysis is likely to occur. We sought further information from the study authors. If no further information could be found, we noted this as being a 'high' risk of bias. There was frequently insufficient information to judge the risk of bias; we noted this as an 'unclear' risk of bias (Handbook 2011).
Publication bias (between‐study reporting bias)
We planned to assess funnel plots if sufficient trials (more than 10) had been available for an outcome. If we observed asymmetry of the funnel plot, we planned to conduct more formal investigation using the methods proposed by Egger 1997.
Data synthesis
We conducted all meta‐analyses using Review Manager 5.3 (RevMan 2014). For dichotomous data, we planned to analyse treatment differences as a risk ratio (RR) calculated using the Mantel‐Haenszel methods. We planned to analyse time‐to‐event data using the generic inverse variance method.
For continuous outcomes, if all the data were from the same scale, we planned to pool mean values obtained at follow‐up with change outcomes and report this as a MD. However, if the SMD had to be used as an effect measure, we would not have pooled change and endpoint data.
When statistical heterogeneity is low, random‐effects versus fixed‐effect methods yield trivial differences in treatment effects. However, when statistical heterogeneity is high, the random‐effects method provides a more conservative estimate of the difference.
Subgroup analysis and investigation of heterogeneity
We planned to conduct some subgroup analyses regardless of whether statistical heterogeneity was observed, as these are widely suspected to be potential effect modifiers. For this review, this included:
phenotype of patients: whether patients have chronic rhinosinusitis without nasal polyps, chronic rhinosinusitis with nasal polyps, a mixed group or the status of polyps is not known or not reported. We planned to undertake this subgroup analysis because although there appears to be a considerable overlap between the two forms of chronic rhinosinusitis with regards to inflammatory profile, clinical presentation and effect of treatment (Cho 2012; DeMarcantonio 2011; Ebbens 2010; EPOS 2007; Ragab 2004; Ragab 2010; van Drunen 2009), there is some evidence that points to differences in the respective inflammatory profiles (Kern 2008; Keswani 2012; Tan 2011; Tomassen 2011; Zhang 2008; Zhang 2009), and potentially even differences in treatment outcome (Ebbens 2011). Sinus penetration of irrigation fluids differs in patients with and without polyps, and according to whether previous sinus surgery has been conducted (Brown 2004).
We planned to present the main analyses of this review according to the subgroups of phenotypes of chronic rhinosinusitis. We planned to present all other subgroup analysis results in tables.
When studies had a mixed group of patients, we planned to analyse the study as one of the subgroups (rather than as a mixed group) if more than 80% of patients belonged to one category. For example, if 81% of patients had chronic rhinosinusitis without nasal polyps, we would have analysed the study as that subgroup.
In addition to the subgroups above, we planned to conduct the following subgroup analyses in the presence of statistical heterogeneity:
patient age (children versus adults);
dose (volume or frequency);
tonicity;
duration of treatment;
method of delivery.
Sensitivity analysis
We planned to carry out sensitivity analyses to determine whether the findings were robust to the decisions made in the course of identifying, screening and analysing the trials. We planned to conduct sensitivity analysis for the following factors, whenever possible:
impact of model chosen: fixed‐effect versus random‐effects model;
risk of bias of included studies: excluding studies with high risk of bias (we defined these as studies that had a high risk of allocation concealment bias and a high risk of attrition bias (overall loss to follow‐up of 20%, differential follow‐up observed);
how outcomes were measured: we planned to investigate the impact of including data where the validity of the measurement is unclear.
If any of these investigations had found a difference in the size of the effect or heterogeneity, we would have mentioned this in the Effects of interventions section.
GRADE and 'Summary of findings' table
We used the GRADE approach to rate the overall quality of evidence using the GDT tool (http://www.guidelinedevelopment.org/) for the main comparison pairs listed in the Types of interventions section. The quality of evidence reflects the extent to which we are confident that an estimate of effect is correct and we applied this in the interpretation of results. There are four possible ratings: 'high', 'moderate', 'low' and 'very low'. A rating of 'high' quality evidence implies that we are confident in our estimate of effect and that further research is very unlikely to change our confidence in the estimate of effect. A rating of 'very low' quality implies that any estimate of effect obtained is very uncertain.
The GRADE approach rates evidence from RCTs that do not have serious limitations as high quality. However, several factors can lead to the downgrading of the evidence to moderate, low or very low. The degree of downgrading is determined by the seriousness of these factors:
study limitations (risk of bias);
inconsistency;
indirectness of evidence;
imprecision;
publication bias.
The 'Summary of findings' table presents only the seven top priority outcomes (disease‐specific HRQL, disease severity score, adverse effects and generic quality of life score). We did not include the outcomes of endoscopic score and CT scan score in the 'Summary of findings' table.
Results
Description of studies
Results of the search
The searches retrieved a total of 1214 references after removal of duplicates. We screened titles and abstracts and subsequently removed 1175 references. We assessed 39 full texts for eligibility. We excluded 28 studies (32 references), with reasons. We included two studies. We identified four references to ongoing studies. There are no studies awaiting assessment.
A flow chart of study retrieval and selection is provided in Figure 1.
1.
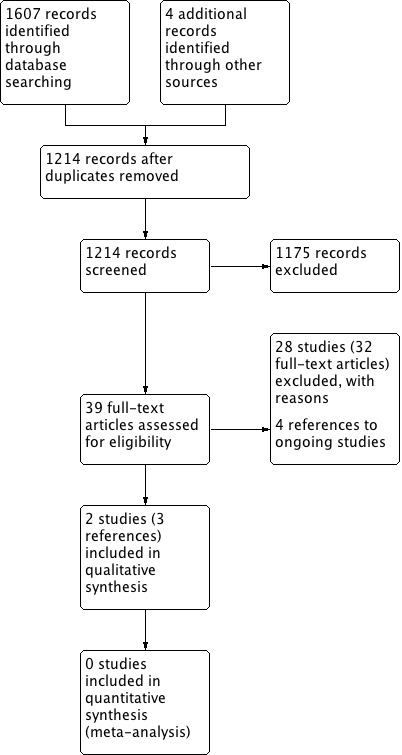
Process for sifting search results and selecting studies for inclusion.
Included studies
Two studies met the criteria for inclusion (Cassandro 2015; Rabago 2002). See Characteristics of included studies for full details.
Design and sample sizes
Both studies were non‐blinded randomised controlled trials.
One study treated and followed up patients for a total of six months (Rabago 2002), whereas the other treated patients for three months and then followed them up for a further three months (Cassandro 2015).
The two studies were small, recruiting 76 (Rabago 2002) and 80 participants (Cassandro 2015). Only 40 participants in the Cassandro study (20 in each intervention arm) received relevant interventions for this review. Rabago 2002 randomised patients in a 2:1 ratio: there were 52 participants in the saline group and 24 participants in the control group.
Setting
Rabago 2002 took place in the USA and included participants who were mostly (about 80%) from primary care settings. Cassandro 2015 was conducted in Italy, in a secondary care setting.
Participants
Both studies included adults (18 to 65 years old). Cassandro 2015 had almost equal numbers of male and female participants, whereas the participants in Rabago 2002 were predominantly female: 75% in the control group and 71% in the intervention group. There were few smokers in Rabago 2002 (4% and 1% of the control and intervention participants), whereas half of the population of Cassandro 2015 were smokers.
Rabago 2002 recruited patients by screening the billing databases for the University of Wisconsin primary care and ENT practices for billing codes of acute and chronic sinusitis (using the International Classification of Diseases, (ICD), 9th revision codes of ICD 461 and ICD 473 respectively). Adult patients with at least two episodes of acute sinusitis or one episode of chronic sinusitis per year for two consecutive years (n = 602) were sent a letter explaining the study and inviting participation. This definition of chronic rhinosinusitis is different from that agreed in EPOS 2012.
Cassandro 2015 defined chronic rhinosinusitis as a duration of 12 weeks of at least two of the following nasal symptoms: inflammation of the nose and paranasal sinuses, nasal obstruction, postnasal drip, sneezing, cough, olfactory disturbance, facial pain, snoring and nasal dryness. Although the authors state that this was "in accordance with current clinical guidelines", it is unclear to which clinical guidelines they are referring. Inflammation, sneezing, cough, snoring and nasal dryness do not form part of the EPOS definition of chronic rhinosinusitis.
Interventions
Nasal saline (hypertonic, 2%, large‐volume, 150 ml) versus no intervention
In Rabago 2002, participants in the intervention group were instructed to irrigate the nose daily for six months with the SinuCleanse nasal cup: 150 ml through each nostril of a solution containing 2.0% saline buffered with baking soda. The solution of one heaped teaspoon of canning salt, one‐half teaspoon of baking soda and one pint of tap water was freshly mixed by the patient every one to two days.
Control participants continued with treatment of sinus disease in their usual manner. All participants were telephoned at two weeks to assess initial compliance with study protocols and thereafter if assessment instruments were not returned promptly.
There is no description of what the allowed concurrent interventions were. However, the study collected data for antibiotics and "nasal spray" use every two weeks and noted that the use of antibiotics and nasal sprays ("percentage of 2 week blocks" when these treatments were used) was about two times higher in the control group.
Intranasal corticosteroids versus nasal saline (nebulised, 5 ml)
Cassandro 2015 comprised four groups, two of which are relevant to this review:
nebulised saline: aerosol therapy (NEBULA, Air Liquide Medical Systems Italy) with 5 ml of saline twice daily;
intranasal corticosteroid spray: mometasone furoate nasal spray 200 µg twice daily.
Outcomes
Rabago 2002 assessed patients with questionnaires at baseline and at 1.5, three and six months. At the six‐month assessment participants were shown their baseline answers for comparison, but not at 1.5 and three months. Compliance with nasal irrigation was recorded in a daily diary.
Disease‐specific health‐related quality of life (HRQL) was measured using the RSDI (Rhinosinusitis Disability Index), a validated disease‐specific instrument assessing quality of life in emotional, functional and physical domains.
Quality of life was also measured using the general health assessment Medical Outcomes Survey Short Form (SF‐12).
Overall sinus symptom severity was measured with a Single‐Item Symptom Severity Assessment (SIA) on a Likert scale (range not specified) (there is no indication this was validated).
The presence or absence of sinus symptoms (headache, congestion, facial pressure, facial pain, nasal discharge), antibiotic and nasal spray use, and overall satisfaction with use was measured using an "exit questionnaire" at end of the study (six months).
This study did not use endoscopy or CT scans, either at baseline or as an outcome measure.
Cassandro 2015 assessed patients before therapy, at one month and at three months following treatment initiation and at three months following its cessation.
Assessment of symptoms was recorded by the patient and guardian using a validated 10 cm visual analogue scale (VAS). However, it is unclear how these were eventually scored and analysed.
Nasal endoscopy, using a modified Lund‐Mackay score, by two otorhinolaryngologists.
Axial and coronal computed tomography (CT) scans of the nose and paranasal sinuses were scored using the Lund‐Kennedy score.
Source of funding and conflict of interest
Declarations of interest were not provided in either report.
Rabago 2002 reported that the study was supported by the Small Grant Program from the Department of Family Medicine, University of Wisconsin, Madison. Cassandro 2015 did not report the source of funding, but noted that they received editorial assistance, which was "sponsored by IBSA". IBSA is the manufacturer of nebulised sodium hyaluronate, which was included in the treatment arms not considered for this review.
Excluded studies
We excluded 28 studies (32 references) after reading the full‐text articles. Further details of the reasons for exclusion can be found in the Characteristics of excluded studies tables.
Of these studies we excluded 20 due to the interventions or comparisons within the studies, which did not meet the inclusion criteria for this review (ACTRN12615000154505; Bachmann 2000; Cho 2010; Cho 2015; Desrosiers 2001; Friedman 2006; Friedman 2012; Heatley 2000; Hunninghake 2012; NCT02097576; NCT00924404; NCT01700725; Ottaviano 2011; Passali 2007; Passali 2008; Passali 2008a; Pynnonen 2007; Salami 2000; Taccariello 1999; Wendeler 1997). Interventions used in studies that we excluded from the review included the use of irrigation with xylitol, thermal waters and homeopathic remedies, as well as the use of reflexology and antifungal agents.
We excluded five studies based on the included population. One study only included people who underwent surgery within the month prior to the trial (Jiang 2014). In four studies the population included had perennial or seasonal allergic rhinitis (Cordray 2005; Garavello 2003; Garavello 2005; Rogkakou 2005). It should be noted that all of these trials were included in the previous Cochrane review (Harvey 2007), because the inclusion criteria for patients comprised a wider population.
Three studies included comparisons that were valid and all other aspects of the trial appeared to meet the inclusion criteria, with the exception of the duration of treatment and follow‐up (Culig 2010; Shoseyov 1998; Ural 2009). The minimum duration of follow‐up was set at three months. These studies followed up patients for 10 days (Ural 2009), 15 days (Culig 2010), and two months (Shoseyov 1998). Ural 2009 was conducted in patients with chronic rhinosinusitis, acute rhinosinusitis, allergic rhinitis and in healthy volunteers to study the impact of saline irrigation on mucociliary clearance. Culig 2010 compared hypertonic versus isotonic seawater sprays, whereas Shoseyov 1998 treated children with maxillary sinusitis with four weeks of hypertonic versus isotonic saline and followed them up for another four weeks.
Ongoing studies
We identified four papers reporting ongoing studies (ISRCTN88204146; NCT00335309; NCT02582099; TCTR20140323002). Three of the trials are in adults with chronic rhinosinusitis. One trial aims to compare saline irrigation, steam inhalation or a combination of both daily for six months (ISRCTN88204146). NCT00335309 compares nasal saline irrigation with no irrigation as an adjunct to antibiotic treatment. Treatment will be for 10 days but personal communication from the authors confirmed that the follow‐up period will be for one year. TCTR20140323002 is a study that aims to compare "warm saline irrigation" with "placebo", although it is unclear what the placebo is and no contact with the study authors could be established.
The last study, conducted in children with chronic rhinosinusitis and/or recurrent acute/subacute sinusitis, compares antibiotics (gentamicin) with normal saline. It is due to be completed in December 2016, although the primary outcome measure appears to be recurrence of sinusitis in a one‐year follow‐up period so it may not assess an outcome of specific interest to this review.
Risk of bias in included studies
See Figure 2 for a 'Risk of bias' graph (our judgements about each risk of bias item presented as percentages across all included studies) and Figure 3 for a 'Risk of bias' summary (our judgements about each risk of bias item for each included study).
2.
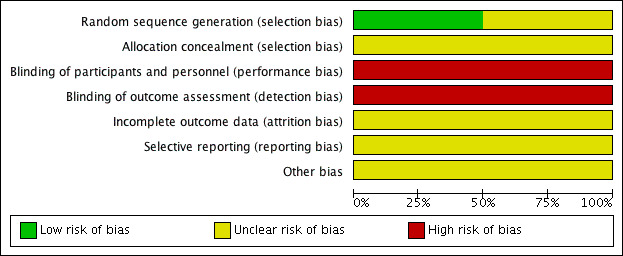
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
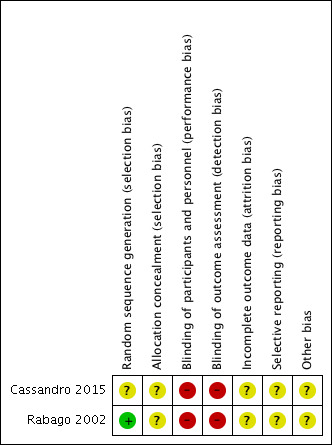
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
Cassandro 2015 merely states that patients were "randomly" assigned to their treatment groups. The risk of bias is unclear.
We considered Rabago 2002 to be at low risk of bias for sequence generation. "The randomization scheme was prepared by the Investigational Drug Services of the University of Wisconsin Hospital and Clinics. Subjects were stratified by smoking status and then randomized by using an approximate 2:1 block design, with 10 subjects per block. Therefore 68% of subjects were assigned to the experimental group and 32% to the control group."
Allocation concealment
Cassandro 2015 provided no description of allocation concealment, therefore the risk of bias is unclear.
In Rabago 2002, one of the investigators "facilitated each informational meeting of 1 to 6 persons". "Sealed envelopes containing the patient's randomized group assignment were distributed to subjects in the order they entered the room. The group assignment was unknown to the investigator. Subjects broke the seal and learned their assignment". Although there seemed to be an attempt to conceal allocation, we rated this as unclear risk of bias because we were unsure whether the use of randomisation blocks and a 2:1 design block could have affected concealment. Moreover, all the patients who were referred from the ENT clinic were allocated into the saline group.
Blinding
Performance bias
Neither Cassandro 2015 nor Rabago 2002 blinded participants to the type of treatments received. Therefore, we considered the risk of bias to be high.
Detection bias
Most outcomes were subjective and in both studies patients were not blinded to the treatment received (Cassandro 2015; Rabago 2002).
In Rabago 2002, patients were also allowed to see their baseline results when they were asked to complete their questionnaires at the final (six‐month) follow‐up, so that they could compare how they felt at the beginning versus the end of the study. It also seemed that only the participants in the active intervention group were asked about side effects. Persons managing and analysing the data also saw unblinded data but had no contact with participants.
Incomplete outcome data
Cassandro 2015 did not mention any loss to follow‐up, or participants not receiving interventions as intended. Therefore we considered this as an unclear risk of bias.
Rabago 2002 provided clear reporting on the people who dropped out, compared the drop‐outs against those who remained in the study and attempted to telephone some patients who dropped out to ascertain the reasons. The use of multiple regression to impute the missing values and inclusion of all patients using the intention‐to‐treat model was clearly specified. We considered the risk of attrition bias to be unclear. Although drop‐outs were not high (12% in the saline group, 4% in the control group), the proportion was larger in the treatment group. The baseline RSDI is also about 10 points higher in the drop‐out group ‐ these are the patients who were less unwell at baseline.
Selective reporting
The overall risk of selective reporting bias is unclear in both studies.
Rabago 2002 clearly reported all effectiveness outcomes and we found no reason to suspect deviation from the planned analysis. However, adverse events seemed to be collected only in the intervention group and they reported the total number of people with events.
In Cassandro 2015, effectiveness outcomes also seemed to be reported as stated in the methods section, except for CT scan score where it was stated that all groups showed improvement compared to the saline group. However, they did not describe how the scores were added up or analysed. There was no description in the methods of how adverse events were to be collected.
Other potential sources of bias
Use of validated outcome measures
Cassandro 2015 used 10 cm visual analogue scales (VAS). They stated that "the 10‐cm VAS we used consisted of a statistically validated questionnaire that the patient filled out, answering the question 'how troublesome are your symptoms of rhinosinusitis?' is used. The answers range from 0 (not troublesome) to 10 (worst thinkable troublesome)...". However, they did not report fully on how these scores were added up and analysed.
Rabago 2002 used validated scales for quality of life measures: RSDI for disease‐specific quality of life and SF‐12 for generic quality of life. They made some minor amendment to some RSDI items to clarify that they were referring to the chronic rhinosinusitis symptoms and this should not have a big impact on its validity. There is less clarity on the validity and discriminant validity of the Single Item Assessment "Likert scale" ‐ the range of the scale was not reported.
Baseline characteristics
In Rabago 2002, baseline risks appeared balanced but all the ENT clinic participants ended up in the intervention group. Baseline risk also appears balanced in Cassandro 2015 and is clearly reported.
Effects of interventions
Summary of findings for the main comparison. Nasal saline versus usual care.
| Nasal saline (hypertonic) versus usual care for chronic rhinosinusitis | |||||
| Patient or population: chronic rhinosinusitis Setting: most patients recruited from primary care Intervention: nasal saline, hypertonic (2%), large‐volume (150 ml), used every day Comparison: usual treatment | |||||
|
Outcomes № of participants (studies) |
Anticipated absolute effects* (95% CI) | Quality | What happens | ||
| Without nasal saline | With nasal saline (hypertonic, 2%, large‐volume, 150 ml) | Difference | |||
| Disease‐specific HRQL ‐ measured as change from baseline using the RSDI (range 0 to 100) at 3 months follow‐up
Higher score = better № of participants: 76 (1 RCT) |
The mean change from baseline was 7.7 points | The mean change from baseline was 14 points | The mean disease‐specific HRQL score was 6.3 points higher (0.89 higher to 11.71 higher) than the usual treatment group | ⊕⊕⊝⊝ LOW 1 2 3 | People who used nasal saline irrigation had better quality of life (moderate effect size) |
| Disease‐specific HRQL ‐ measured as change from baseline using the RSDI (range 0 to 100) at 6 months follow‐up
Higher score = better № of participants: 76 (1 RCT) |
The mean change from baseline was 0.9 points | The mean change from baseline was 14.4 points | The mean disease‐specific HRQL score was 13.5 higher (9.63 higher to 17.37 higher) than the usual treatment group | ⊕⊕⊝⊝ VERY LOW 1 2 4 | People who used nasal saline irrigation had better quality of life (large effect size) |
| Disease severity ‐ measured as change using a single‐item score (range not known) at 3 months follow‐up
Lower score = better № of participants: 76 (1 RCT) |
The mean change from baseline was ‐0.3 | The mean change from baseline was ‐1.2 | The mean change in disease severity score was 0.9 points lower (1.45 lower to 0.35 lower) than the usual treatment group | ⊕⊕⊝⊝ LOW 1 2 3 | People who used nasal saline irrigation seemed to report less severe symptoms (moderate effect size) |
| Disease severity measured as change using a single‐item score (range not known) at 6 months follow‐up
Lower score = better № of participants: 76 (1 RCT) |
The mean change from baseline was ‐0.005 | The mean change from baseline was ‐1.6 | The mean change in disease severity score was 1.59 points lower (2.15 lower to 1.04 lower) than the usual treatment group | ⊕⊕⊝⊝ VERY LOW 1 2 4 | People who used nasal saline irrigation seemed to report less severe symptoms (large effect size) |
| Generic HRQL ‐ measured using the SF‐12 (range 0 to 100) at 3 months follow‐up
Higher score = better № of participants: 76 (1 RCT) |
The mean score was 2.9 points | The mean score was 8.2 points | The mean generic HRQL ‐ measured using SF‐12 (range 0 to 100) at 3 months follow‐up in the intervention group was 5.3 points higher (4.38 lower to 14.98 higher) than the usual treatment group | ⊕⊕⊝⊝ LOW 1 2 3 | It was unclear whether there was a difference between groups in generic HRQL |
| Generic HRQL ‐ measured using the SF‐12 (range 0 to 100) at 6 months follow‐up
Higher score = better № of participants: 76 (1 RCT) |
The mean score was 2.2 points | The mean score was 12.7 points | The mean generic HRQL ‐ measured using SF‐12 (range 0 to 100) at 6 months follow‐up was 10.5 points higher (0.66 higher to 20.34 higher) than the usual treatment group | ⊕⊕⊝⊝ VERY LOW 1 2 4 | It was unclear whether there was a difference between groups in generic HRQL |
| Adverse events | Outcome was collected only in the saline group. "Ten subjects (23%) experienced side effects; 8 identified nasal irritation, nasal burning, tearing, nosebleeds, headache, or nasal drainage as occurring but 'not significant'. Two subjects (3%) identified nasal burning, irritation, and headache as 'significant'." | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HRQL: health‐related quality of life; RCT: randomised controlled trial; RSDI: Rhinosinusitis Disability Index | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1Open‐label study. Outcomes are subjective and reported by patients. 2Sample sizes are small and the study was randomised in a 2:1 manner. 3Most of the patients were recruited from primary care. This has good applicability to most patients. 4Patients were shown their results from baseline at the six‐month follow‐up, before they filled out the questionnaire.
Summary of findings 2. Intranasal corticosteroids versus nasal saline.
| Intranasal corticosteroids versus nasal saline for chronic rhinosinusitis | ||||||
| Patient or population: chronic rhinosinusitis Setting: secondary care Intervention: intranasal corticosteroids daily Comparison: nasal saline, nebulised, small‐volume (5 ml) used every day | ||||||
|
Outcomes № of participants (studies) |
Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Quality | What happens | ||
| With nasal saline (nebulised, small‐volume) | With intranasal corticosteroids | Difference | ||||
| Disease‐specific quality of life | — | Outcome not measured or reported in the study | ||||
| Disease severity ‐ overall score (range not known) at 3 months follow‐up (end of treatment)
Higher score = worse № of participants: 40 (1 RCT) |
— | The mean score was 6.6 points | The mean score was 20.1 points | The mean disease severity ‐ overall score (range not known) ‐ at 3 months follow‐up (end of treatment) in the intervention group was 13.5 lower (14.44 lower to 12.56 lower) | ⊕⊝⊝⊝ VERY LOW 1 2 | Patients on intranasal corticosteroids seemed to have less severe symptoms by the end of treatment (large effect size) |
| Disease severity ‐ overall score (range not known) at 6 months follow‐up (3 months after end of treatment)
Higher score = worse № of participants: 40 (1 RCT) |
— | The mean score was 13.19 points | The mean score was 20.9 points | The mean disease severity ‐ overall score (range not known) ‐ at 6 months follow‐up (3 months after end of treatment) in the intervention group was 7.71 lower (8.72 lower to 6.7 lower) | ⊕⊝⊝⊝ VERY LOW 1 2 | Patients on intranasal corticosteroids seemed to continue having less severe symptoms 3 months after treatment was stopped (large effect size) |
| Adverse events ‐ epistaxis № of participants: 40 (1 RCT) |
RR 2.00 (0.20 to 20.33) | Study population | ⊕⊝⊝⊝ VERY LOW 1 3 | More people on intranasal corticosteroids could have epistaxis than those on nebulised saline | ||
| 50 per 1000 | 100 per 1000 | 50 more per 1000 with INCS (80 fewer to 1933 more) | ||||
| Adverse events ‐ local irritation № of participants: 40 (1 RCT) |
RR 3.00 (0.13 to 69.52) | Study population | ⊕⊝⊝⊝ VERY LOW 1 3 | More patients on intranasal corticosteroids could have local irritation compared to those on nebulised saline | ||
| No events with nasal saline reported | 50 per 1000 | Not estimable | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; INCS: intranasal corticosteroids; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Open‐label study. Outcomes assessed were subjective. Method of sequence generation and allocation concealment unclear. 2Very small study. Could be susceptible to small study effects (overestimation of effect sizes). 3Number of events and participants too small to estimate this precisely.
Where the range of scales and the values for minimal important differences (MID) were unclear, we used standardised mean difference (SMD) to estimate the effect sizes. As suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011), we used standard rules of thumb in the interpretation of effect sizes (SMD or Cohen's effect size of < 0.41 = small, 0.40 to 0.70 = moderate, > 0.70 = large) (Cohen 1998). Established scales such as the SF‐12 may have other rules of thumb to estimate the minimal important difference (MID = 0.5 SMD) and we use those to guide our interpretation whenever available (Jaeschke 1989; Revicki 2008).
Comparison 1: Nasal saline (hypertonic, 2%, large‐volume, 150 ml) versus usual care
We found only one study for this comparison, with 76 participants (Rabago 2002). Assessment of the effectiveness of the intervention for the outcomes presented below should take into account the higher "percentage of 2 week blocks" when patients had used either antibiotics or "nasal sprays" (or both) during the six‐month study period. In the control group antibiotics were used in an average of 20% of the two‐week blocks and nasal sprays were used in 8%. These numbers were halved in the nasal saline group.
Primary outcomes
1. Health‐related quality of life, using disease‐specific health‐related quality of life scores
Rhinosinusitis Disability Index (RSDI) scores were used to measure this outcome (scale 0 to 100, higher score = better overall quality of life). The change in mean RSDI from baseline among treated participants was greater (better) with nasal saline than controls at three and six months (Analysis 1.1).
1.1. Analysis.
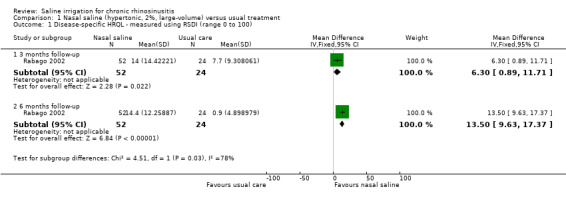
Comparison 1 Nasal saline (hypertonic, 2%, large‐volume) versus usual treatment, Outcome 1 Disease‐specific HRQL ‐ measured using RSDI (range 0 to 100).
At three months of follow‐up, the mean difference (MD) in change between baseline and three months between the groups was 6.30 (95% confidence interval (CI) 0.89 to 11.71; 76 participants). This corresponds to a moderate effect size. The quality of the evidence for this outcome was low, because the study was small and unblinded.
At six months follow‐up, the MD in change between baseline scores and six months between the groups was 13.50 (95% CI 9.63 to 17.37; 76 participants). This corresponds to a large effect size. The quality of the evidence was very low. In addition, this was a small, unblinded study and participants were shown their baseline ratings when filling out the questionnaires.
2. Disease severity, as measured by patient‐reported symptom score
This was measured using a "Likert scale" (range not described), with higher scores indicating more severe symptoms. Patients were asked "please evaluate the overall severity of your sinus symptoms since you enrolled in the study" (Analysis 1.2).
1.2. Analysis.
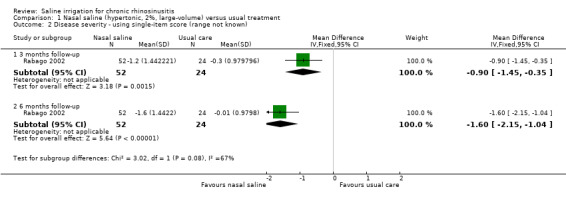
Comparison 1 Nasal saline (hypertonic, 2%, large‐volume) versus usual treatment, Outcome 2 Disease severity ‐ using single‐item score (range not known).
At three months follow‐up, the MD was ‐0.90 (95% CI ‐1.45 to ‐0.35; 76 participants). This corresponds to a moderate to large effect size.
At six months of follow‐up, the MD was ‐1.59 (95% CI ‐2.15 to ‐1.04; 76 participants). This corresponds to a large effect size.
The quality of the evidence is very low for the reasons stated earlier and the validity of the scale is unclear.
3. Significant adverse effect: epistaxis
Adverse effects were not collected for the control group. In the nasal saline group, "Ten subjects (23%) experienced side effects; 8 identified nasal irritation, nasal burning, tearing, nosebleeds, headache, or nasal drainage as occurring but not significant." Two out of 46 participants (4%) identified nasal burning, irritation and headache as "significant".
Secondary outcomes
1. Health‐related quality of life, using generic quality of life scores
General health‐related quality of life (HRQL) was measured using the SF‐12 (Analysis 1.3). The range of this score is 0 to 100, with higher scores indicating better quality of life.
1.3. Analysis.
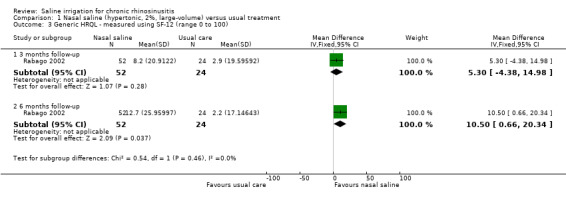
Comparison 1 Nasal saline (hypertonic, 2%, large‐volume) versus usual treatment, Outcome 3 Generic HRQL ‐ measured using SF‐12 (range 0 to 100).
At three months follow‐up (end of treatment), the MD in change from baseline between the two groups was 5.30 (95% CI ‐4.38 to 14.98; 76 participants). The effect size (SMD 0.26, 95% CI ‐0.23 to 0.74) is smaller than the commonly accepted threshold of 0.5 SMD for a minimal important difference (MID) on the SF‐12.
At six months follow‐up the MD in change from baseline between the two groups was 10.50 (95% CI 0.66 to 20.34; 76 participants). The effect size (SMD 0.44, 95% CI ‐0.05 to 0.93) is less than the commonly accepted MID threshold.
The quality of the evidence is low.
2. Other local adverse effects: local irritation
As reported above.
3. Other local adverse effects: discomfort
As reported above.
4. Endoscopic score (including nasal polyps score)
This was not assessed in the study.
5. Computerised tomography (CT) scan score
This was not assessed in the study.
Comparison 2: Intranasal corticosteroids versus nasal saline (nebulised, 5 ml)
We found only one small study with 20 participants in each intervention arm (Cassandro 2015).
1. Health‐related quality of life, using disease‐specific health‐related quality of life scores
This outcome was not reported in the study.
2. Disease severity, as measured by patient‐reported symptom score
This was measured using a 10 cm visual analogue "Likert scale" (range not described), with higher scores indicating more severe symptoms. Patients were asked "how troublesome are your symptoms of rhinosinusitis". The range was from 0 (not troublesome) to 10 (worst thinkable troublesome). They mentioned assessing nasal obstruction, nasal discharge, postnasal drip, sneezing, cough, olfactory disturbance, facial pain, snoring and nasal dryness. The study reported an overall score, but it was unclear which symptoms were included in the analysis (i.e. whether this is a total score for all symptoms measured) and therefore the scale range is not known.
At three months follow‐up (end of treatment), the MD was ‐13.50 (95% CI ‐14.44 to ‐12.56; 40 participants), with less severe symptoms in the intranasal corticosteroids group. This corresponds to a large effect size (SMD ‐8.71, 95% CI ‐10.81 to ‐6.60).
At six months follow‐up (three months after end of treatment), the MD was ‐7.71 (95% CI ‐8.72 to ‐6.70; 40 participants) with less severe symptoms in the intranasal corticosteroids group. This corresponds to a large effect size (SMD ‐4.63, 95% CI ‐5.87 to ‐3.40).
The quality of the evidence is very low due to the lack of blinding for a subjective outcome, the unclear validity and range of the scale, and the very small sample size.
In addition to the symptom score, the study also assessed patients every two weeks for individual symptoms. Patients on nasal saline had fewer "2‐week blocks" with nasal congestion, sinus headache and frontal pain. The results are shown in Analysis 1.4.
1.4. Analysis.
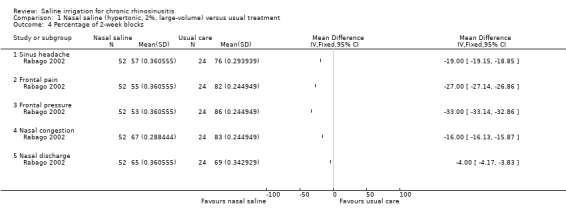
Comparison 1 Nasal saline (hypertonic, 2%, large‐volume) versus usual treatment, Outcome 4 Percentage of 2‐week blocks.
3. Significant adverse effect: epistaxis
The risk ratio (RR) for epistaxis was 2.00 (95% CI 0.20 to 20.33; 40 participants), but the evidence is inconclusive due to the very small sample size (very low quality evidence) (Analysis 2.2). The intranasal corticosteroids group (2/20) had epistaxis compared with the nasal saline group (1/20).
2.2. Analysis.
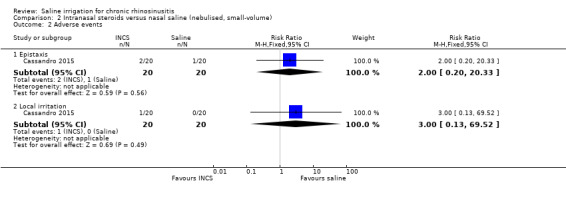
Comparison 2 Intranasal steroids versus nasal saline (nebulised, small‐volume), Outcome 2 Adverse events.
Secondary outcomes
1. Health‐related quality of life, using generic quality of life scores
This outcome was not reported in the study.
2. Other local adverse effects: local irritation
More patients in the intranasal corticosteroids group (1/20) reported local irritation compared with the nasal saline group (0/20); the RR was 3.00 (95% CI 0.13 to 69.52; 40 participants), but the evidence is inconclusive due to the very small sample size (very low quality evidence) (Analysis 2.2).
3. Other local adverse effects: discomfort
This was not reported as an adverse event.
4. Endoscopic score (including nasal polyps score)
The endoscopic scores were rated using a modified Lund‐Mackay scale. The range of this modified score was not reported. The results are shown graphically in Analysis 2.3. The study indicated that a lower score corresponds with an improvement.
2.3. Analysis.
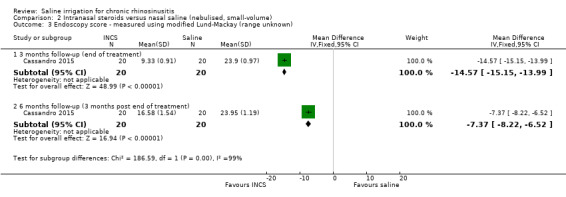
Comparison 2 Intranasal steroids versus nasal saline (nebulised, small‐volume), Outcome 3 Endoscopy score ‐ measured using modified Lund‐Mackay (range unknown).
At three months follow‐up (end of treatment), the MD was ‐14.57 (95% CI ‐15.15 to ‐13.99; 40 participants), favouring the intranasal corticosteroids group.
At six months follow‐up (three months after end of treatment), the MD was ‐7.37 (95% CI ‐8.22 to ‐6.52; 40 participants), favouring the intranasal corticosteroids group.
The quality of the evidence is low due to the small effect sizes and lack of blinding. It is also unclear whether modification of the scale affected its validity.
5. Computerised tomography (CT) scan score
This was assessed in the study but not fully reported. The study only stated that CT scans in all groups showed improvement compared to the saline group.
Discussion
Summary of main results
We found only two very small, open‐label studies with important limitations (Cassandro 2015; Rabago 2002).
One of the studies investigated adding daily, large‐volume (150 ml) hypertonic (2%) nasal saline irrigation to usual care (Rabago 2002). This study used validated quality of life outcomes and found moderate to large effects in improvement of disease‐specific (Rhinosinusitis Disability Index ‐ RSDI) and generic (SF‐12) quality of life measures after three and six months of treatment, respectively. This improvement was observed despite higher usage of antibiotics and "nasal sprays" in the control group.
The other study compared intranasal corticosteroids versus nasal saline nebulisation administered twice a day (Cassandro 2015). This was not as effective as intranasal corticosteroids for the outcomes of disease severity and endoscopy score that were measured.
Overall completeness and applicability of evidence
We found little evidence on whether nasal saline is effective. The two studies included were very different and had different control groups; this makes comparison difficult. One study used high‐volume nasal saline irrigation (150 ml daily) with a hypertonic saline solution in patients from primary care and might have included both chronic and acute rhinosinusitis patients (Rabago 2002), whereas the other study compared low‐volume nasal saline (5 ml nebulised spray) with intranasal corticosteroids in people with chronic rhinosinusitis in secondary care (Cassandro 2015).
There are three aspects of nasal saline irrigation that are important to consider and for which we did not identify evidence during this review: the volume of the irrigation, the method of delivery and the tonicity of the solution used. With regards to volume it is unclear whether there is a minimum volume that could be considered to be irrigation. We found no studies that investigated whether high‐volume irrigation (such as the 150 ml daily used in Rabago 2002) improves patient symptoms better than low‐volume irrigation. The volume administered will also be directly linked to the method of delivery of irrigation to the nose and this is an aspect that has not been studied. There are many widely available commercial products that have different delivery methods, from a nebulised spray that provides a 'mist' of saline solution likely to have the effect of moistening the inside of the nostrils, to products where a sachet is mixed with water and then put into a bottle that has been designed to aid in the delivery of saline to the sinuses. We found no evidence to compare these. Lastly the tonicity of the solution: isotonic saline is reported to improve mucociliary clearance, most likely through mechanical cleaning, while it has been proposed that hypertonic saline solutions may have an effect by decreasing oedema and increasing mucociliary clearance through stimulation of ciliary beat frequency, thinning of mucus and suppression of inflammation (Ural 2009). There were no included studies that directly compared hypertonic and isotonic nasal saline solutions. Rabago 2002 used a hypertonic solution and there is no information regarding the tonicity of the nasal spray used in Cassandro 2015.
The advantage of nasal saline irrigation solutions is that they are very accessible for patients, who may feel empowered by using them (Rabago 2006). Solutions can be made and administered at home and there are resources to help guide technique (such as http://www.fammed.wisc.edu/nasal‐irrigation/). The adverse effects of using nasal saline irrigation were not well reported in the included trials, but based on these studies they are not likely to be severe. Patients in a qualitative study have reported an initial fear of having solution in the nasal cavity and an unpleasant sensation during the irrigation process, however these were often overcome with education and coached practice on nasal irrigation techniques (Rabago 2006).
Quality of the evidence
We downgraded the quality of the evidence for effectiveness because it is drawn from only one very small study for each comparison. These studies had important methodological limitations, which put them at high risk of bias. Although one of the studies used validated scales for quality of life, it was uncertain whether the larger effects observed at six months (compared to three months) were biased by the fact that patients were shown their scores at baseline (Rabago 2002). Cassandro 2015 used a 0 to 10 mm visual analogue scale to score individual symptom severity, but they did not provide any descriptions of how these were added up or scored, and there was no information on the range of the scale.
Adverse events were collected only from the treatment arm in Rabago 2002, making a comparison with the control group impossible. As sample size of only 20 patients in each arm in Cassandro 2015 means that it is unlikely to provide any precise data to estimate the risks of adverse events.
Potential biases in the review process
We imputed the standard deviations using standard methods, based on the standard errors reported in Rabago 2002. This accuracy of this estimation could be affected by the small sample sizes.
Rabago 2002 was a study that was primarily conducted in patients seen in primary care. This study did not use the EPOS diagnostic criteria (EPOS 2012), but included patients who had two consecutive years where they had at least one episode of chronic rhinosinusitis and two episode of acute rhinosinusitis per year. The symptom outcome of the study suggested that patients in the control group had about 80% of the two‐week blocks with most chronic rhinosinusitis symptoms. We decided to include this study because we thought that this was representative of the population presenting to primary care, although it should be noted that they are a heterogenous group that includes acute rhinosinusitis and may not represent chronic rhinosinusitis patients according to clear definitions such as EPOS 2007 and EPOS 2012.
Agreements and disagreements with other studies or reviews
This review only includes patients with chronic rhinosinusitis and includes fewer studies than many other similar reviews on saline (which have included studies of other groups of patients, such as allergic rhinitis patients, and have often included non‐randomised trials). We conducted the review as part of a series of reviews looking at the effectiveness of non‐surgical interventions for chronic rhinosinusitis (Chong 2016a; Chong 2016b; Head 2016a; Head 2016b; Head 2016c). In the four reviews on intranasal corticosteroids and oral corticosteroids, only two small studies specifically did not allow the use of nasal saline irrigation (Kirtsreesakul 2012; Vlckova 2009). It is unclear whether nasal saline was used widely in other studies.
The previous Cochrane review on this topic included patients suffering from rhinitis with seasonal exacerbations, perennial rhinitis, recurrent acute sinusitis in patients with ongoing symptoms between exacerbations and chronic rhinosinusitis (Harvey 2007). This review included eight studies and was only able to draw similar conclusions to this review: "The beneficial effect of saline appears to outweigh the drawbacks for the majority of patients. Topical saline could be included as a treatment adjunct for managing the symptoms of chronic rhinosinusitis and conditions producing chronic sinonasal symptoms. There is no evidence that saline is more effective than active agents. There is evidence that hypertonic solutions improve mucociliary clearance (Talbot 1997; Bachmann 2000). The effect on symptoms is less evident. There may be some added clinical benefit but it is balanced against patient tolerance. No information can be provided regarding the delivery type, dosage frequency or volume."
Authors' conclusions
Implications for practice.
This review includes two studies, which are very different in terms of their included populations, interventions and comparisons. It is therefore difficult to draw conclusions for practice. The evidence suggests there is no benefit of a low‐volume (5 ml) nebulised saline spray over intranasal steroids, but there is some benefit of daily, large‐volume (150 ml) saline irrigation with a hypertonic solution compared with placebo, although the quality of the evidence was low. No information can be provided on the tonicity, volume, delivery method, frequency or duration of use. Nasal saline irrigations are easy for patients to administer and are unlikely to cause severe adverse events. Patients may feel empowered through the use of topical saline irrigation, although this must be balanced against patient tolerance.
Implications for research.
As of October 2015, we found only two very small, open‐label studies of nasal saline irrigation in people with chronic rhinosinusitis. There is low‐quality evidence (we are uncertain about the estimates) to suggest that, for people with chronic rhinosinusitis, a large‐volume (150 ml) nasal saline irrigation intervention is effective in improving patients' quality of life and symptoms compared to usual care. This improvement was observed despite the higher usage of antibiotics and "nasal sprays" in the control group. There is very low‐quality evidence to suggest that nasal saline nebulisation is not as effective as intranasal corticosteroids. These studies had important methodological limitations, which puts them at high risk of bias. The quality of the evidence for adverse effects is very low due to inadequate reporting methods and small study sizes.
We considered the potential for future research into the use of nasal saline and feel that this area of research might not be prioritised above research for other standard interventions as identified by the other reviews in this suite (Chong 2016a; Chong 2016b; Head 2016a; Head 2016b; Head 2016c). If research is carried out, open questions remain about the use of nasal saline irrigation in patients with chronic rhinosinusitis, including the optimal volume of irrigation, delivery methods and tonicity of the solutions used. In addition, the use of nasal saline solution as an adjunct to other standard treatments also could be considered. Any trial undertaken, however, should be designed as a randomised controlled trial, including patients with chronic rhinosinusitis diagnosed using the EPOS 2012 criteria and include both patients with and without nasal polyps (stratified randomisation by subgroup). Future trials of saline irrigation for chronic rhinosinusitis should focus on clinically relevant treatment comparisons; their design should allow for comparison of different compositions of saline solutions, tonicity, volume or delivery methods. The intervention and follow‐up should be carried out for at least three or six months, since saline is used as a long‐term treatment for a chronic condition.
This review is one of a suite of reviews of medical treatments for chronic rhinosinusitis, each of which features its own research recommendations. Across all reviews, key features of future research are as follows:
Trials should be adequately powered and imbalances in prognostic factors (for example, prior sinus surgery) must be accounted for in the statistical analysis.
Study participants should be diagnosed with chronic rhinosinusitis using the EPOS 2012 criteria and should primarily be recruited based on their symptoms. Different patient phenotypes (that is, those with and without nasal polyps) should be recognised and trials should use stratified randomisation within these subgroups or focus on one or other of the phenotypes.
Studies should focus on outcomes that are important to patients and use validated instruments to measure these. Validated chronic rhinosinusitis‐specific health‐related quality of life questionnaires exist, for example the Sino‐Nasal Outcome Test‐22 (SNOT‐22). Patients may find dichotomised outcomes easiest to interpret; for example the percentage of patients achieving a minimal clinically important difference (MCID) or improvement for that outcome. Such MCIDs or cut‐off points should be included in the study protocol and clearly outlined in the methods section.
Trials and other high‐quality studies should use consistent outcomes and adhere to reporting guidelines, such as CONSORT, so that results can be compared across future trials. The development of a standardised set of outcomes, or core outcome set, for chronic rhinosinusitis, agreed by researchers, clinicians and patients, will facilitate this process.
Acknowledgements
This project is one of a suite of reviews on the medical treatment of chronic rhinosinusitis, funded by the National Institute for Health Research (award reference 14/174/03).
This project was also supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to Cochrane ENT. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
We would like to express our thanks to the external peer reviewer, Professor Wytske Fokkens, the consumer referee Joan Blakley and the Cochrane ENT editors for their detailed and insightful comments, which helped to strengthen this review. Thank you also to acting Co‐ordinating Editor, Professor Richard Harvey, for his oversight of this publication.
We are indebted to J. Yoon Irons for translating and excluding primary studies for this review.
The authors are grateful for the assistance provided by Jenny Bellorini and Samantha Faulkner, with editorial support and searching for studies. We are also grateful for the contribution from Lynda Ware for her assistance in abstract screening.
Appendices
Appendix 1. Search strategies
| CENTRAL | Ovid MEDLINE |
| #1 MeSH descriptor: [Sinusitis] explode all trees #2 MeSH descriptor: [Rhinitis] this term only #3 MeSH descriptor: [Rhinitis, Atrophic] this term only #4 MeSH descriptor: [Rhinitis, Vasomotor] this term only #5 MeSH descriptor: [Paranasal Sinus Diseases] this term only #6 MeSH descriptor: [Paranasal Sinuses] explode all trees #7 rhinosinusitis or nasosinusitis or pansinusitis or ethmoiditis or sphenoiditis #8 kartagener* near syndrome* #9 inflamm* near sinus* #10 (maxilla* or frontal*) near sinus* #11 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 #12 MeSH descriptor: [Chronic Disease] explode all trees #13 MeSH descriptor: [Recurrence] explode all trees #14 chronic or persis* or recurrent* #15 #12 or #13 or #14 #16 #11 and #15 #17 CRSsNP #18 (sinusitis or rhinitis) near (chronic or persis* or recurrent*) #19 #16 or #17 or #18 #20 MeSH descriptor: [Nasal Polyps] explode all trees #21 MeSH descriptor: [Nose] explode all trees #22 MeSH descriptor: [Nose Diseases] explode all trees #23 #21 or #22 #24 MeSH descriptor: [Polyps] explode all trees #25 #23 and #24 #26 (nose or nasal or rhino* or rhinitis or sinus* or sinonasal) near (papilloma* or polyp*) #27 rhinopolyp* or CRSwNP #28 #19 or #20 or #25 or #26 or #27 #29 MeSH descriptor: [Solutions] this term only #30 MeSH descriptor: [Hypertonic Solutions] this term only #31 MeSH descriptor: [Saline Solution, Hypertonic] this term only #32 MeSH descriptor: [Hypotonic Solutions] explode all trees #33 MeSH descriptor: [Isotonic Solutions] explode all trees #34 MeSH descriptor: [Sodium Chloride] explode all trees #35 MeSH descriptor: [Mineral Waters] explode all trees #36 MeSH descriptor: [Seawater] explode all trees #37 saline or "sodium chloride" or saltwater or hypertonic* or hypotonic* or isotonic* or hypersaline or "sea water" or seawater or ((salt* or thermal or mineral or sulfur* or bromic or iodic* or bromide or iodine or bromine) and (water* or solution*)) #38 #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 #39 MeSH descriptor: [Therapeutic Irrigation] this term only #40 MeSH descriptor: [Nasal Lavage] explode all trees #41 MeSH descriptor: [Administration, Inhalation] explode all trees #42 MeSH descriptor: [Administration, Intranasal] explode all trees #43 MeSH descriptor: [Nasal Sprays] explode all trees #44 douch* or spray* or lavag* or wash* or rinse* or rinsing or irrigat* or pulsed or nebulise* or aerosol* or buffer* or atomis* or atomiz* or (squeeze and bottle) #45 intranasal or inhalation* or irrigator #46 #39 or #40 or #41 or #42 or #43 or #44 or #45 or #45 #47 #38 and #46 #48 sterimar or NeilMed or nasaline or navage or marimer or physiomer or Emcur or "simply saline" or "nasal mist" or ayr or salex or "otrovin saline" or ISCS or Prorhinel or SSBI #49 (nasal or intranasal or sinus or nose or sinonasal) near/3 (irrigation* or rinsing or rinse* or wash* or lavage or douch* or hygiene) #50 MeSH descriptor: [Mineral Waters] explode all trees and with qualifier(s): [Therapeutic use ‐ TU] #51 #47 or #48 or #49 or #50 #52 #28 and #53 |
1 exp Sinusitis/ 2 paranasal sinus diseases/ or rhinitis/ or rhinitis, atrophic/ or rhinitis, vasomotor/ 3 exp Paranasal Sinuses/ 4 (rhinosinusitis or nasosinusitis or pansinusitis or ethmoiditis or sphenoiditis).ab,ti. 5 (kartagener* adj3 syndrome*).ab,ti. 6 (inflamm* adj5 sinus*).ab,ti. 7 ((maxilla* or frontal*) adj3 sinus*).ab,ti. 8 1 or 2 or 3 or 4 or 5 or 6 or 7 9 exp chronic disease/ 10 exp Recurrence/ 11 (chronic or persis* or recurrent*).ab,ti. 12 9 or 10 or 11 13 8 and 12 14 CRSsNP.ab,ti. 15 ((sinusitis or rhinitis) adj3 (chronic or persis* or recurrent*)).ab,ti. 16 13 or 14 or 15 17 exp Nasal Polyps/ 18 exp Nose/ or exp Nose Diseases/ 19 exp Polyps/ 20 18 and 19 21 ((nose or nasal or rhino* or rhinitis or sinus* or sinonasal) adj3 (papilloma* or polyp*)).ab,ti. 22 (rhinopolyp* or CRSwNP).ab,ti. 23 16 or 17 or 20 or 21 or 22 24 Solutions/ 25 Hypertonic Solutions/ 26 exp Sodium Chloride/ 27 Saline Solution, Hypertonic/ 28 exp Hypotonic Solutions/ 29 exp Mineral Waters/ 30 exp Isotonic Solutions/ 31 exp Seawater/ 32 (saline or "sodium chloride" or saltwater or hypertonic* or hypotonic* or isotonic* or hypersaline or "sea water" or seawater or ((salt* or thermal or mineral or sulfur* or bromic or iodic* or bromide or iodine or bromine) and (water* or solution*))).ab,ti. 33 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 34 Therapeutic Irrigation/ 35 exp Nasal Lavage/ 36 exp administration, inhalation/ or exp administration, intranasal/ 37 exp Nasal Sprays/ 38 Aerosols/ 39 (douch* or spray* or lavag* or wash* or rinse* or rinsing or irrigat* or pulsed or nebulise* or aerosol* or buffer* or atomis* or atomiz* or (squeeze and bottle)).ab,ti. 40 (intranasal or inhalation* or irrigator).ab,ti. 41 34 or 35 or 36 or 37 or 38 or 39 or 40 42 ((nasal or intranasal or sinus or nose or sinonasal) adj3 (irrigation* or rinsing or rinse* or wash* or lavage or douch* or hygiene)).ab,ti. 43 (sterimar or NeilMed or nasaline or navage or marimer or physiomer or Emcur or "simply saline" or "nasal mist" or ayr or salex or "otrovin saline" or ISCS or Prorhinel or SSBI).ab,ti. 44 exp Mineral Waters/tu [Therapeutic Use] 45 41 or 42 or 43 or 44 46 23 and 45 |
| Ovid EMBASE | Trial registries (via CRS) |
| 1 exp sinusitis/ or paranasal sinus disease/ 2 atrophic rhinitis/ or chronic rhinitis/ or rhinosinusitis/ or vasomotor rhinitis/ 3 exp paranasal sinus/ 4 (rhinosinusitis or nasosinusitis or pansinusitis or ethmoiditis or sphenoiditis).tw. 5 (kartagener* adj3 syndrome*).tw. 6 (inflamm* adj5 sinus*).tw. 7 ((maxilla* or frontal*) adj3 sinus*).tw. 8 1 or 2 or 3 or 4 or 5 or 6 or 7 9 exp chronic disease/ 10 exp recurrent disease/ 11 (chronic or persis* or recurrent*).tw. 12 9 or 10 or 11 13 8 and 12 14 CRSsNP.tw. 15 ((sinusitis or rhinitis) adj3 (chronic or persis* or recurrent*)).tw. 16 13 or 14 or 15 17 exp nose polyp/ 18 exp nose disease/ or exp nose/ 19 exp polyp/ 20 18 and 19 21 ((nose or nasal or rhino* or rhinitis or sinus* or sinonasal) adj3 (papilloma* or polyp*)).tw. 22 (rhinopolyp* or CRSwNP).tw. 23 16 or 17 or 20 or 21 or 22 24 solution and solubility/ 25 hypertonic solution/ 26 exp sodium chloride/ 27 exp hypotonic solution/ 28 exp mineral water/ 29 exp isotonic solution/ 30 exp sea water/ 31 (saline or "sodium chloride" or saltwater or hypertonic* or hypotonic* or isotonic* or hypersaline or "sea water" or seawater or ((salt* or thermal or mineral or sulfur* or bromic or iodic* or bromide or iodine or bromine) and (water* or solution*))).ab,ti. 32 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 33 lavage/ 34 exp nasal lavage/ 35 exp inhalational drug administration/ 36 exp intranasal drug administration/ 37 exp nose spray/ 38 aerosol/ 39 (douch* or spray* or lavag* or wash* or rinse* or rinsing or irrigat* or pulsed or nebulise* or aerosol* or buffer* or atomis* or atomiz* or (squeeze and bottle)).ab,ti. 40 (intranasal or inhalation* or irrigator).ab,ti. 41 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 42 ((nasal or intranasal or sinus or nose or sinonasal) adj3 (irrigation* or rinsing or rinse* or wash* or lavage or douch* or hygiene)).ab,ti. 43 (sterimar or NeilMed or nasaline or navage or marimer or physiomer or Emcur or "simply saline" or "nasal mist" or ayr or salex or "otrovin saline" or ISCS or Prorhinel or SSBI).ab,ti. 44 exp mineral water/ad, ih, th, tp [Drug Administration, Inhalational Drug Administration, Therapy, Topical Drug Administration] 45 32 and 41 46 42 or 43 or 44 or 45 47 23 and 46 |
ClinicalTrials.gov Condition: rhinitis OR sinusitis OR rhinosinusitis OR (nose AND polyp*) OR (nasal AND polyp*) OR CRSsNP OR CRSwNP OR CRS ICTRP Title: rhinitis OR sinusitis OR rhinosinusitis OR CRSsNP OR CRSwNP OR CR OR All: (nose AND polyp*) OR (nasal AND polyp*) NB These searches were run from 1 March 2015 to 11 August 2015, when these terms were last searched to populate the Cochrane ENT trials register in CRS |
Appendix 2. Data extraction form
| REF ID: | Study title: |
| Date of extraction: | Extracted by: |
| General comments/notes (internal for discussion): |
| Flow chart of trial | ||
| Group A (Intervention) | Group B (Comparison) | |
| No. of people screened | ||
| No. of participants randomised ‐ all | ||
| No. randomised to each group | ||
| No. receiving treatment as allocated | ||
| No. not receiving treatment as allocated ‐ Reason 1 ‐ Reason 2 |
||
| No. dropped out (no follow‐up data for any outcome available) |
||
| No. excluded from analysis1 (for all outcomes) ‐ Reason 1 ‐ Reason 2 |
||
| 1This should be the people who received the treatment and were therefore not considered 'drop‐outs' but were excluded from all analyses (e.g. because the data could not be interpreted or the outcome was not recorded for some reason). | ||
| Information to go into 'Characteristics of included studies' table | |
| Methods | X arm, double/single/non‐blinded, [multicentre] parallel‐group/cross‐over/cluster‐RCT, with x duration of treatment and x duration of follow‐up |
| Participants |
Location: country, no of sites etc. Setting of recruitment and treatment: Sample size:
Participant (baseline) characteristics:
Inclusion criteria:[state diagnostic criteria used for CRS, polyps score if available] Exclusion criteria: |
| Interventions |
Intervention (n = x): drug name, method of administration, dose per day/frequency of administration, duration of treatment Comparator group (n = y): Use of additional interventions (common to both treatment arms): |
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes:
Other outcomes reported by the study:
|
| Funding sources | 'No information provided'/'None declared'/State source of funding |
| Declarations of interest | 'No information provided'/'None declared'/State conflict |
| Notes | |
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Quote: "…" Comment: |
|
| Allocation concealment (selection bias) | Quote: "…" Comment: |
|
| Blinding of participants and personnel (performance bias) | Quote: "…" Comment: |
|
| Blinding of outcome assessment (detection bias) | Quote: "…" Comment: |
|
| Incomplete outcome data (attrition bias) | Quote: "…" Comment: |
|
| Selective reporting (reporting bias) | Quote: "…" Comment: |
|
| Other bias (see section 8.15) Insensitive/non‐validated instrument? |
Quote: "…" Comment: |
|
| Other bias (see section 8.15) | Quote: "…" Comment: |
| Findings of study: continuous outcomes | |||||||
| Results (continuous data table) | |||||||
| Outcome | Group A | Group B | Other summary stats/Notes | ||||
| Mean | SD | N | Mean | SD | N | Mean difference (95% CI), P values etc. | |
| Disease‐specific HRQL (instrument name/range) Time point: |
|||||||
| Generic HRQL (instrument name/range) Time point: |
|||||||
| Symptom score (overall) (instrument name/range) Time point: |
|||||||
|
Added total ‐ if scores reported separately for each symptom (range) Time point: |
|||||||
| Nasal blockage/obstruction/congestion (instrument name/range) |
|||||||
| Nasal discharge (instrument name/range) |
|||||||
| Facial pain/pressure (instrument name/range) |
|||||||
| Smell (reduction) (instrument name/range) |
|||||||
| Headache (instrument name/range) |
|||||||
| Cough (in children) (instrument name/range) |
|||||||
| Polyp size (instrument name/range) |
|||||||
| CT score (instrument name/range) |
|||||||
| Comments: | |||||||
| Results (dichotomous data table) | ||||||
| Outcome | Applicable review/intervention | Group A | Group B | Other summary stats/notes | ||
| No. of people with events | No. of people analysed | No. of people with events | No. of people analysed | P values, RR (95% CI), OR (95% CI) | ||
| Epistaxis/nose bleed | INCS Saline irrigation |
|||||
| Local irritation (sore throat, oral thrush, discomfort) | INCS Saline irrigation |
|||||
| Osteoporosis (minimum 6 months) | INCS | |||||
| Stunted growth (children, minimum 6 months) | INCS | Can also be measured as average height | ||||
| Mood disturbances | OCS | |||||
| Gastrointestinal disturbances (diarrhoea, nausea, vomiting, stomach irritation) |
OCS Antibiotics |
|||||
| Insomnia | OCS | |||||
| Osteoporosis (minimum 6 months) | INCS OCS |
|||||
| Discomfort | Saline irrigation | |||||
| Skin irritation | Antibiotics | |||||
| Anaphylaxis or other serious allergic reactions such as Stevens‐Johnson | Antibiotics | |||||
| Comments: | ||||||
Data and analyses
Comparison 1. Nasal saline (hypertonic, 2%, large‐volume) versus usual treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Disease‐specific HRQL ‐ measured using RSDI (range 0 to 100) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 3 months follow‐up | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 6.3 [0.89, 11.71] |
| 1.2 6 months follow‐up | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 13.5 [9.63, 17.37] |
| 2 Disease severity ‐ using single‐item score (range not known) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 3 months follow‐up | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.45, ‐0.35] |
| 2.2 6 months follow‐up | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐2.15, ‐1.04] |
| 3 Generic HRQL ‐ measured using SF‐12 (range 0 to 100) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 3 months follow‐up | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 5.30 [‐4.38, 14.98] |
| 3.2 6 months follow‐up | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 10.5 [0.66, 20.34] |
| 4 Percentage of 2‐week blocks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Sinus headache | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Frontal pain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Frontal pressure | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Nasal congestion | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Nasal discharge | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Intranasal steroids versus nasal saline (nebulised, small‐volume).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Disease severity ‐ overall score (range not known) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 3 months follow‐up (end of treatment) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐13.50 [‐14.44, ‐12.56] |
| 1.2 6 months follow‐up (3 months post end of treatment) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐7.71 [‐8.72, ‐6.70] |
| 2 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Epistaxis | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.20, 20.33] |
| 2.2 Local irritation | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.52] |
| 3 Endoscopy score ‐ measured using modified Lund‐Mackay (range unknown) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 3 months follow‐up (end of treatment) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐14.57 [‐15.15, ‐13.99] |
| 3.2 6 months follow‐up (3 months post end of treatment) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐7.37 [‐8.22, ‐6.52] |
2.1. Analysis.
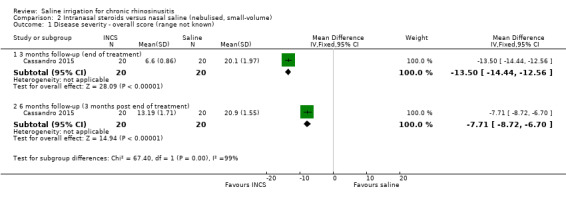
Comparison 2 Intranasal steroids versus nasal saline (nebulised, small‐volume), Outcome 1 Disease severity ‐ overall score (range not known).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cassandro 2015.
| Methods | 4‐arm, non‐blinded, single‐centre, parallel‐group RCT, with 3 months of treatment and a total of 6 months follow‐up | |
| Participants |
Location: Italy, single site, between September 2011 and April 2012 Setting of recruitment and treatment: Department of Otorhinolaryngology of the University Hospital 'San Giovanni di Dio e Ruggi d'Aragona' in Salerno Sample size:
Participant (baseline) characteristics:
Inclusion criteria:
Exclusion criteria: pregnant women |
|
| Interventions |
Intranasal corticosteroid (n = 20): mometasone furoate nasal spray (MFNS) 200 µg twice a day Nasal saline (n = 20): nebulised saline administered as aerosol therapy (NEBULA®, Air Liquide Medical Systems Italy) with 5 ml of saline twice a day Use of additional interventions (common to both treatment arms): nasal decongestants and local anaesthesia were not used |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes: 1. Disease severity symptom score using a validated 10 cm VAS for nasal obstruction, nasal discharge, postnasal drip, sneezing, cough, olfactory disturbance, facial pain, snoring and nasal dryness was recorded by the patient and guardian 2. Significant adverse effect: epistaxis Secondary outcomes: 3. Endoscopy, reported as "mean endoscopic score" ‐ scored by 2 otorhinolaryngologists using modified postoperative criteria for endoscopic appearance originally described by Lund et al 4. CT scan ‐ not fully reported 5. Adverse events: local irritation Other outcomes reported by the study:
|
|
| Funding sources | "Editorial assistance was provided by Raelene Simpson on behalf of in Science Communications, Springer Healthcare. This assistance was sponsored by IBSA". IBSA is the manufacturer of nebulised sodium hyaluronate, included in treatment arms not considered for this review | |
| Declarations of interest | No information provided | |
| Notes | There are 2 other intervention groups in this trial:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned" Comment: no further description |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomly assigned" Comment: no further description |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "…drug was administered on an open‐label basis" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "…drug was administered on an open‐label basis." Comment: subjective outcomes in a non‐blinded study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: there was no mention of drop‐outs or exclusions |
| Selective reporting (reporting bias) | Unclear risk | Comment: outcomes seem to be reported as stated in the methods section, except for CT scan score where it was stated that all groups showed improvement compared to the saline group. There was no description in the methods about how adverse events were to be collected. |
| Other bias | Unclear risk | Quote: "The 10‐cm VAS we used consisted of a statistically validated questionnaire that the patient filled out, answering the question 'how troublesome are your symptoms of rhinosinusitis?' is used. The answers range from 0 (not troublesome) to 10 (worst thinkable troublesome)" Comment: they did not fully report how scores were added up and analysed |
Rabago 2002.
| Methods | 2‐arm, unblinded, single‐centre, parallel‐group RCT, with a 6‐month duration of treatment and simultaneous follow‐up | |
| Participants |
Location: Wisconsin, USA, single site Setting of recruitment and treatment: University of Wisconsin primary care and ear, nose and throat (ENT) practices. About 80% of participants were from primary care, the others were from an ENT clinic. Sample size:
Participant (baseline) characteristics:
Inclusion criteria: patients 18 to 65 years old with 2 episodes of acute sinusitis or 1 episode of chronic sinusitis per year for 2 consecutive years were contacted. Of these, patients indicating "moderate to severe" impact of sinus symptoms on their quality of life on a Likert scale of 1 to 7 were invited to participate. Exclusion criteria: pregnancy and significant comorbidity precluding travel to a meeting or use of saline irrigation |
|
| Interventions |
Intervention (n = 52): 2.0% saline buffered with baking soda (1 heaping teaspoon of canning salt, one‐half teaspoon of baking soda and 1 pint of tap water), 150 ml through each nostril daily for 6 months administered with the SinuCleanse nasal cup Solution was mixed fresh every 1 to 2 days. Intervention duration was 6 months. Participants saw a brief demonstration film, witnessed nasal irrigation by the facilitator and demonstrated proficiency with the nasal irrigation technique before departure. Comparator group (n = 24): continued treatment of sinus disease in their usual manner Use of additional interventions (common to both treatment arms):
|
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes: 1. Health‐related quality of life, disease‐specific using RSDI at 1.5, 3 and 6 months (emotional and functional domains), range of 0 to 100 2. Disease severity symptom score: single‐item symptom severity assessment (SIA): "Please evaluate the overall severity of your sinus symptoms since you enrolled in the study". Likert scale. 3. Significant adverse effect: epistaxis Secondary outcomes: 4. Health‐related quality of life, generic, using SF‐12, range of 0 to 100 5. Adverse events: local irritation Other outcomes reported by the study:
|
|
| Funding sources | Small Grant Program from the Department of Family Medicine, University of Wisconsin, Madison | |
| Declarations of interest | No information provided | |
| Notes | Experimental participants reported using nasal irrigation on 87% of days during the study; 31 participants reported using nasal irrigation on 91% or more days, 13 participants on 76% to 90% of days, and 5 participants on 51% to 75% of days. Only 3 participants used nasal irrigation on 50% of days or less. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization scheme was prepared by the Investigational Drug Services of the University of Wisconsin Hospital and Clinics. Subjects were stratified by smoking status and then randomized by using an approximate 2:1 block design, with 10 subjects per block" Comment: randomisation schedule should have been appropriately generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "One of us (D.R., R.M., or A.Z.) facilitated each informational meeting of 1 to 6 persons. Sealed envelopes containing the patient’s randomized group assignment were distributed to subjects in the order they entered the room. The group assignment was unknown to the investigator. Subjects broke the seal and learned their assignment" Comment: envelopes given by order of arrival at the meeting. Unclear whether the usage of randomisation blocks could have affected concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Subjects broke the seal and learned their assignment. Thereafter, investigators were not blind to subjects' group assignment." Comment: neither participants nor assessors were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Persons managing and analysing data also saw unblinded data but had no contact with subjects". "… at the 6‐month assessment, subjects were shown their baseline answers for comparison because they had told us they needed to recall answers to past questions. They believed they knew whether they felt better or worse and wanted their later answers to reflect this change". Comment: there was no blinding at all. Most of outcomes are subjective responses from patients; showing patients their baseline response could put this at a higher risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "As dictated by the intention‐to‐treat model, the few missing values were imputed with multiple regression". "Dropouts tended to have slightly better baseline RSDI scores than nondropouts, 66.8 vs 58.1 points, but this difference was not significant (P = .15)." Comment: drop‐outs not high and clearly documented (12% in saline, 4% in control), but the proportion is higher in the intervention group. The baseline RSDI is also about 10 points higher in the drop‐out group ‐ these are the patients who were less unwell at baseline. |
| Selective reporting (reporting bias) | Unclear risk | All key outcomes fully reported. No reason to suspect deviation from planned analysis. However, adverse events seemed to be collected only in the intervention group and were reported as a total number of people with events. |
| Other bias | Unclear risk | Quote: "By chance all subjects from ENT clinics (n = 6) and a disproportionate percentage of subjects with chronic sinusitis were randomized to the experimental group. Neither variable was statistically significant." Comment: baseline risks appear balanced but all the ENT clinic participants ended up in the intervention group. Validated scales, RSDI and SF‐12 were used for quality of life. However, the discriminant validity of SF‐12 in CRS is still not proven. There were minor modifications in the RSDI, which should not affect its validity. There is less clarity on the validity and discriminant validity of the Single Item Assessment "Likert scale". |
CRS: chronic rhinosinusitis CRSwNP: chronic rhinosinusitis with nasal polyps CT: computerised tomography F: female INCS: intranasal corticosteroids M: male MFNS: mometasone furoate nasal spray NS: nasal saline RCT: randomised controlled trial RSDI: Rhinosinusitis Disability Index SD: standard deviation VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12615000154505 | INTERVENTION: xylitol versus saline; xylitol was not considered to be a standard treatment for CRS |
| Bachmann 2000 | INTERVENTION: Ems salt solution (1.1%) versus sodium hydrochloride solution (0.9%) |
| Cho 2010 | POPULATION: acute, subacute and chronic sinusitis INTERVENTION: nasal irrigation using benzalkonium chloride, which has other chemical properties (surfactant and antibacterial) ‐ not a saline irrigation solution |
| Cho 2015 | INTERVENTION: low concentration hypochlorous acid versus saline irrigation; hypochlorous irrigation is not considered to be a standard treatment for CRS |
| Cordray 2005 | POPULATION: seasonal allergic rhinitis |
| Culig 2010 | DURATION: treatment and follow‐up only 15 days |
| Desrosiers 2001 | INTERVENTION: antifungal irrigation versus saline irrigation |
| Friedman 2006 | INTERVENTION: hypertonic dead sea salt irrigation versus hypertonic saline irrigation; not an included comparison |
| Friedman 2012 | INTERVENTION: hypertonic dead sea salt irrigation versus hypertonic saline irrigation; not an included comparison |
| Garavello 2003 | POPULATION: seasonal allergic rhinitis |
| Garavello 2005 | POPULATION: seasonal allergic rhinoconjunctivitis |
| Heatley 2000 | INTERVENTION: hypertonic saline irrigation with a bulb syringe versus hypertonic nasal irrigation with a nasal irrigation pot versus reflexology on "established sinus contact points" DURATION: follow‐up only 2 weeks |
| Hunninghake 2012 | INTERVENTION: homeopathic agent containing wild indigo versus nasal saline |
| Jiang 2014 | POPULATION: all patients had surgery 1 month prior to starting trial |
| NCT00924404 | INTERVENTION: xylitol versus saline solution |
| NCT01700725 | INTERVENTION: xylitol versus saline solution |
| NCT02097576 | INTERVENTION: saline mixed with Manuka honey versus saline only (note: the study was withdrawn prior to enrolment) |
| Ottaviano 2011 | INTERVENTION: sulphurous, salty, bromic, iodic (SSBI) thermal water versus isotonic sodium chloride solution (ISCS); SSBI not considered to be a standard CRS treatment |
| Passali 2007 | INTERVENTION: intranasal glucan spray versus intranasal saline spray; glucan spray is not considered to be a standard treatment |
| Passali 2008 | INTERVENTION: treatment with thermal waters, which are not considered to be a standard CRS treatment |
| Passali 2008a | INTERVENTION: treatment with thermal waters, which are not considered to be a standard CRS treatment |
| Pynnonen 2007 | INTERVENTION: isotonic dead sea salt nasal spray (low‐volume) versus isotonic saline rinse irrigation (large‐volume) DURATION: follow‐up only 8 weeks |
| Rogkakou 2005 | POPULATION: persistent allergic rhinitis |
| Salami 2000 | INTERVENTION: thermal waters versus sodium chloride 0.9%. Treatment with thermal waters, which are not considered to be a standard CRS treatment. |
| Shoseyov 1998 | DURATION: follow‐up only 2 months. Study was conducted in paediatric patients with chronic maxillary sinusitis. |
| Taccariello 1999 | INTERVENTION – seawater spray versus alkaline saline irrigation |
| Ural 2009 | DESIGN: intervention and follow‐up only 10 days. A study of mucociliary clearance after using hypertonic and isotonic nasal saline in patients with CRS, acute sinusitis, allergic rhinitis and normal participants. |
| Wendeler 1997 | INTERVENTION: isotonic Emser (Epsom) salt (magnesium sulphate) solution versus tap water |
CRS: chronic rhinosinusitis
Characteristics of ongoing studies [ordered by study ID]
ISRCTN88204146.
| Trial name or title | 'Steam inhalation and nasal irrigation for recurrent sinusitis' |
| Methods | Pragmatic randomised controlled 2 x 2 factorial trial |
| Participants | Patients (both males and females) aged 18 to 65 years with recurrent or chronic sinusitis |
| Interventions | Daily nasal irrigation versus daily steam inhalation versus combined treatment group |
| Outcomes |
Primary outcome: severity of symptoms assessed by the Rhinosinusitis Disability Index (RSDI) Secondary outcomes: 1. Quality of life assessed by the EQ‐5D 2. Severity of sinus symptoms assessed by a Single Item Sinus Symptom Severity Assessment (SIA) 3. Severity of upper respiratory symptoms (coryza, sore throat, cough, earache, feeling unwell, fever) 4. Belief in the importance of antibiotics and seeing the doctor for sinusitis using validated Likert scales 5. Side effects of treatment (and also reported side effects for previous 3 months) 6. Compliance with irrigation/inhalation 7. Use of over the counter treatments (e.g. analgesics, decongestants) 8. Number of prescriptions for antibiotics for sinus‐related symptoms 9. Number of prescriptions for antibiotics in total 10. Number of GP visits regarding sinus‐related symptoms and for other respiratory symptoms |
| Starting date | 2008 |
| Contact information | Prof Paul Little (p.little@soton.ac.uk) |
| Notes | We contacted the study author. The trial is in the process of being written up for publication. |
NCT00335309.
| Trial name or title | 'Effectiveness of maxillary sinus saline irrigation in conjunction with systemic antibiotic therapy versus systemic antibiotic therapy alone in the management of chronic rhinosinusitis, a prospective randomized controlled trial' |
| Methods | Randomised, parallel assignment, open‐label controlled trial |
| Participants | Adults with chronic (over 3 months) maxillary and ethmoidal rhinosinusitis (verified by a CT scan) |
| Interventions | Normal saline 0.9% versus no saline irrigation Both arms have intravenous antibiotics of Augmentin 1 g 3 times a day for 4 days, and then per os (PO) Augmentin 875 mg twice a day for another 10 days |
| Outcomes |
Primary outcome: CT scoring Secondary outcomes: nasal endoscopy score, quality of life questionnaire |
| Starting date | October 2005 |
| Contact information | Ohad Ronen, Carmel Medical Center |
| Notes | We contacted the study author. The trial is in the process of being written up for publication. |
NCT02582099.
| Trial name or title | 'The efficacy and complication of gentamicin nasal irrigation in chronic rhinosinusitis and recurrent sinusitis' |
| Methods | Randomised, parallel assignment, double‐blind controlled trial |
| Participants | Children (7 to 18 years) with chronic rhinosinusitis and/or recurrent acute/subacute sinusitis |
| Interventions | 1. Gentamicin nasal irrigation in chronic rhinosinusitis amount 20 ml each per nostril 2. Normal saline nasal irrigation in chronic rhinosinusitis amount 20 ml each per nostril |
| Outcomes |
Primary outcome: frequency of sinusitis Secondary outcome: none listed |
| Starting date | 2015 |
| Contact information | Prof Nualanong Visitsunthorn (nualanongv@yahoo.com) |
| Notes | Estimated date of completion is December 2016 |
TCTR20140323002.
| Trial name or title | 'The effect of warm saline irrigation on mucociliary function in patients with chronic rhinosinusitis' |
| Methods | Randomised, placebo‐controlled, parallel assignment, single‐blind controlled trial |
| Participants | Adults with chronic rhinosinusitis |
| Interventions | Warm saline nasal irrigation versus "placebo" (unclear comparator) |
| Outcomes |
Primary outcome: saccharine transit time Secondary outcome: obstructive symptom score, comfort symptom score, peak nasal inspiratory flow, rhinomanometry, acoustic rhinometry |
| Starting date | 2014 |
| Contact information | Saran Ruxrungtham (saran.rux@gmail.com) |
| Notes | We made attempts to contact the study author to find out further information but could not obtain this |
CT: computed tomography GP: general practitioner PO: oral
Differences between protocol and review
As part of the discussions about the use of a total symptoms score we noted that many papers within the suite of reviews did not present information for all four elements of the EPOS criteria for defining chronic rhinosinusitis (EPOS 2012). In particular, many studies that only included patients with nasal polyps did not present information on facial pressure or pain. We made the decision that where individual symptoms were recorded, they should be presented within the outcome of disease severity symptom score within the paper as this information would be useful for the reader.
Contributions of authors
Lee Yee Chong: scoped, designed and wrote the protocol (Chong 2015). Abstract screening, full paper review, data extraction, data analysis, drafting and writing the report.
Karen Head: reviewed and edited the protocol. Abstract screening, full paper review, data extraction, data analysis and editing the report.
Claire Hopkins: clinical guidance at all stages of project scoping and protocol development. Clinical input into data analysis, reviewing and editing the report.
Carl Philpott: clinical guidance at all stages of project scoping and protocol development. Clinical input into data analysis, reviewing and editing the report.
Simon Glew: abstract screening, data extraction and clinical input into data analysis.
Glenis Scadding: abstract screening and clinical input into data analysis.
Anne GM Schilder: clinical guidance at all stages of project scoping and protocol development. Clinical input into data analysis, reviewing and editing the report.
Martin Burton: clinical input into data analysis, reviewing and editing the report.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
Funding to complete a suite of reviews on medical interventions for chronic rhinosinusitis in 2015/2016 (award reference 14/174/03), in addition to infrastructure funding for Cochrane ENT
Declarations of interest
Lee Yee Chong: none known.
Karen Head: none known.
Claire Hopkins: I have received financial support from several companies involved in producing instruments for sinus surgery: Acclarent, Sinusys, Cryolife and Medtronic.
Carl Philpott: I have previously received consultancy fees from the companies Acclarent, Navigant, Aerin Medical and Entellus.
Simon Glew: none known.
Glenis Scadding: research grants from GSK and ALK. Honoraria for articles, lectures/chairing, advisory boards and consultancy: Astra Zeneca, Brittania Pharmaceuticals, Capnia, Church & Dwight, Circassia, GSK, Groupo Uriach, Meda, Merck, MSD, Ono Pharmaceuticals, Oxford Therapeutics, Sanofi‐Aventis, Shionogi and UCB. Travel funding from ALK, Bayer, GSK and Meda.
Martin Burton: Professor Martin Burton is joint Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review.
Anne GM Schilder: Professor Anne Schilder is joint Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review. Her evidENT team at UCL is supported by her NIHR Research Professorship award with the remit to develop a UK infrastructure and programme of clinical research in ENT, Hearing and Balance. Her institution has received a grant from GSK for a study on the microbiology of acute tympanostomy tube otorrhoea.
New
References
References to studies included in this review
Cassandro 2015 {published data only}
- Cassandro E, Chiarella G, Cavaliere M, Sequino G, Cassandro C, Prasad SC, et al. Hyaluronan in the treatment of chronic rhinosinusitis with nasal polyposis. Indian Journal of Otolaryngology and Head and Neck Surgery 2015;67(3):299‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rabago 2002 {published data only}
- Rabago D, Pasic T, Zgierska A, Mundt M, Barrett B, Maberry R. The efficacy of hypertonic saline nasal irrigation for chronic sinonasal symptoms. Otolaryngology ‐ Head and Neck Surgery 2005;133(1):3‐8. [DOI] [PubMed] [Google Scholar]
- Rabago D, Zgierska A, Mundt M, Barrett B, Bobula J, Maberry R. Efficacy of daily hypertonic saline nasal irrigation among patients with sinusitis: a randomized controlled trial. Journal of Family Practice 2002;51(12):1049‐55. [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12615000154505 {published data only}
- ACTRN12615000154505. The effect of xylitol and saline nasal irrigations on the sinus microbiome in patients with chronic rhinosinusitis. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=367895 (accessed 17 February 2016).
Bachmann 2000 {published data only}
- Bachmann G, Hommel G, Michel O. Effect of irrigation of the nose with isotonic salt solution on adult patients with chronic paranasal sinus disease. European Archives of Oto‐Rhino‐Laryngology 2000;257(10):537‐41. [DOI] [PubMed] [Google Scholar]
Cho 2010 {published data only}
- Cho YS, Kim MS, Chun YH, Kim HH, Kim JT, Lee JS. A prospective randomized open trial of nasal irrigation and nasal decongestant for sinusitis in children. Pediatric Allergy and Respiratory Disease 2010;20(4):232‐7. [Google Scholar]
Cho 2015 {published data only}
Cordray 2005 {published data only}
- Cordray S, Harjo JB, Miner L. Comparison of intranasal hypertonic dead sea saline spray and intranasal aqueous triamcinolone spray in seasonal allergic rhinitis. Ear, Nose, & Throat Journal 2005;84(7):426‐30. [PubMed] [Google Scholar]
Culig 2010 {published data only}
- Culig J, Leppee M, Vceva A, Djanic D. Efficiency of hypertonic and isotonic seawater solutions in chronic rhinosinusitis. Medicinski Glasnik 2010;7(2):116‐23. [PubMed] [Google Scholar]
Desrosiers 2001 {published data only}
- Desrosiers MY, Salas‐Prato M. Treatment of chronic rhinosinusitis refractory to other treatments with topical antibiotic therapy delivered by means of a large‐particle nebulizer: results of a controlled trial. Otolaryngology ‐ Head and Neck Surgery 2001;125(3):265‐9. [DOI] [PubMed] [Google Scholar]
Friedman 2006 {published data only}
- Friedman M, Vidyasagar R, Joseph N. A randomized, prospective, double‐blind study on the efficacy of dead sea salt nasal irrigations. Laryngoscope 2006;116(6):878‐82. [DOI] [PubMed] [Google Scholar]
Friedman 2012 {published data only}
- Friedman M, Hamilton C, Samuelson CG, Maley A, Wilson MN, Venkatesan TK, et al. Dead Sea salt irrigations vs saline irrigations with nasal steroids for symptomatic treatment of chronic rhinosinusitis: a randomized, prospective double‐blind study. International Forum of Allergy and Rhinology 2012;2(3):252‐7. [DOI] [PubMed] [Google Scholar]
Garavello 2003 {published data only}
- Garavello W, Romagnoli M, Sordo L, Gaini RM, Berardino C, Angrisano A. Hypersaline nasal irrigation in children with symptomatic seasonal allergic rhinitis: a randomized study. Pediatric Allergy and Immunology 2003;14(2):140‐3. [DOI] [PubMed] [Google Scholar]
Garavello 2005 {published data only}
- Garavello W, Berardino F, Romagnoli M, Sambataro G, Gaini RM. Nasal rinsing with hypertonic solution: an adjunctive treatment for pediatric seasonal allergic rhinoconjunctivitis. International Archives of Allergy and Immunology 2005;137(4):310‐4. [DOI] [PubMed] [Google Scholar]
Heatley 2000 {published data only}
- Heatley D, McConnell KE, Kilie T, Leverson GA. Nasal irrigation for the alleviation of sinonasal symptoms. Otolaryngology ‐ Head and Neck Surgery 2000;123(2):78‐9. [DOI] [PubMed] [Google Scholar]
- Heatley DG, McConnell KE, Kille TL, Leverson GE. Nasal irrigation for the alleviation of sinonasal symptoms. Otolaryngology ‐ Head and Neck Surgery 2001;125(1):44‐8. [DOI] [PubMed] [Google Scholar]
Hunninghake 2012 {published data only}
- Hunninghake R, Davis D. A comparison of two nasal irrigation solutions with no treatment in chronic sinusitis sufferers. Townsend Letter 2012;2012(353):66‐73. [Google Scholar]
Jiang 2014 {published data only}
- Jiang RS, Liang KL, Wu SH, Su MC, Chen WK, Lu FJ. Electrolyzed acid water nasal irrigation after functional endoscopic sinus surgery. American Journal of Rhinology & Allergy 2014;28(2):176‐81. [DOI] [PubMed] [Google Scholar]
NCT00924404 {published data only}
- NCT00924404. Randomized controlled trial of xylitol versus saline rinse for chronic sinusitis. clinicaltrials.gov/show/NCT00924404 (accessed 17 February 2016).
NCT01700725 {published data only}
- Hayer SD, Rabago DP, Amaza IP, Kille T, Coe CL, Zgierska A, et al. Effectiveness of nasal irrigation for chronic rhinosinusitis and fatigue in patients with Gulf War illness: protocol for a randomized controlled trial. Contemporary Clinical Trials 2015;41:219‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01700725. Nasal irrigation for chronic rhinosinusitis and fatigue in patients with gulf war illness. clinicaltrials.gov/show/NCT01700725 (accessed 17 February 2016).
NCT02097576 {published data only}
- NCT02097576. Effectiveness of manuka honey/saline nasal rinses as an adjunct to standard medical therapy for chronic rhinosinusitis: a prospective clinical trial. clinicaltrials.gov/show/NCT02097576 (accessed 17 February 2016).
Ottaviano 2011 {published data only}
- Ottaviano G, Marioni G, Staffieri C, Giacomelli L, Marchese‐Ragona R, Bertolin A, et al. Effects of sulfurous, salty, bromic, iodic thermal water nasal irrigations in nonallergic chronic rhinosinusitis: a prospective, randomized, double‐blind, clinical, and cytological study. American Journal of Otolaryngology 2011;32(3):235‐9. [DOI] [PubMed] [Google Scholar]
Passali 2007 {published data only}
- Passali D, Fiorella R, Camaioni A, Villari G, Mora E, Passali GC, et al. Glucan solution nasal spray vs saline in the treatment of chronic rhinosinusitis: a multi‐centric double blind randomised clinical trial. Clinica Terapeutica 2007;158(2):139‐45. [PubMed] [Google Scholar]
Passali 2008 {published data only}
- Passali D, Lauriello M, Passali GC, Passali FM, Cassano M, Cassano P, et al. Clinical evaluation of the efficacy of Salsomaggiore (Italy) thermal water in the treatment of rhinosinusal pathologies. Clinica Terapeutica 2008;159(3):181‐8. [PubMed] [Google Scholar]
Passali 2008a {published data only}
- Passali FM, Crisanti A, Passali GC, Cianfrone F, Bocchi M, Messineo G, et al. Efficacy of inhalation therapy with water of Salsomaggiore (Italy) in chronic and recurrent nasosinusal inflammation treatment. Clinica Terapeutica 2008;159(3):175‐80. [PubMed] [Google Scholar]
Pynnonen 2007 {published data only}
- Pynnonen MA, Mukerji SS, Kim HM, Adams ME, Terrell JE. Nasal saline for chronic sinonasal symptoms: a randomized controlled trial. Archives of Otolaryngology‐‐Head & Neck Surgery 2007;133(11):1115‐20. [DOI] [PubMed] [Google Scholar]
Rogkakou 2005 {published data only}
- Rogkakou A, Guerra L, Massacane P, Baiardini I, Baena‐Cagnani R, Zanella C, et al. Effects on symptoms and quality of life of hypertonic saline nasal spray added to antihistamine in persistent allergic rhinitis ‐ a randomized controlled study. Allergie et Immunologie 2005;37(9):353‐6. [PubMed] [Google Scholar]
Salami 2000 {published data only}
- Salami A, Dellepiane M, Strinati F, Guastini L, Mora R. Sulphurous thermal water inhalations in the treatment of chronic rhinosinusitis. Rhinology 2010;48(1):71‐6. [DOI] [PubMed] [Google Scholar]
Shoseyov 1998 {published data only}
- Shoseyov D, Bibi H, Shai P, Shoseyov N, Shazberg G, Hurvitz H. Treatment with hypertonic saline versus normal saline nasal wash of pediatric chronic sinusitis. Journal of Allergy and Clinical Immunology 1998;101(5):602‐5. [DOI] [PubMed] [Google Scholar]
- Slawson D. Is hypertonic saline wash effective in the treatment of pediatric chronic sinusitis?. Evidence‐Based Practice 1998;1(8):6. [Google Scholar]
Taccariello 1999 {published data only}
- Taccariello M, Parikh A, Darby Y, Scadding G. Nasal douching as a valuable adjunct in the management of chronic rhinosinusitis. Rhinology 1999;37(1):29‐32. [PubMed] [Google Scholar]
Ural 2009 {published data only}
- Ural A, Oktemer TK, Kizil Y, Ileri F, Uslu S. Impact of isotonic and hypertonic saline solutions on mucociliary activity in various nasal pathologies: clinical study. Journal of Laryngology & Otology 2009;123(5):517‐21. [DOI] [PubMed] [Google Scholar]
Wendeler 1997 {published data only}
- Wendeler HM, Muller J, Dieler R, Helms J. Nasal irrigation using isotonic Emser salt solution in patients with chronic rhinosinusitis. Oto‐Rhino‐Laryngol Nova 1997;7(5‐6):254‐8. [Google Scholar]
References to ongoing studies
ISRCTN88204146 {published data only}
- ISRCTN88204146. A primary care randomised controlled trial of nasal irrigation and steam inhalation for recurrent sinusitis. www.isrctn.com/ISRCTN88204146 (accessed 17 February 2016).
NCT00335309 {published data only}
- NCT00335309. Maxillary sinus irrigation in the management of chronic rhinosinusitis. clinicaltrials.gov/show/NCT00335309 (accessed 3 March 2016).
NCT02582099 {published data only}
- NCT02582099. Comparison of the efficacy and complication of gentamicin and normal saline nasal irrigation in chronic rhinosinusitis and recurrent sinusitis: a double blind randomized control trial. clinicaltrials.gov/show/NCT02582099 (accessed 7 February 2016).
TCTR20140323002 {published data only}
- TCTR20140323002. The effect of warm saline irrigation on mucociliary function in patients with chronic rhinosinusitis. www.clinicaltrials.in.th (accessed 17 February 2016).
Additional references
Balk 2012
- Balk EM, Earley A, Patel K, Trikalinos TA, Dahabreh IJ. Empirical assessment of within‐arm correlation imputation in trials of continuous outcomes [Internet]. Report No.: 12(13)‐EHC141‐EF. AHRQ Methods for Effective Health Care. Rockville (MD): Agency for Healthcare Research and Quality, 2012. [PubMed] [Google Scholar]
Brown 2004
- Brown CL, Graham SM. Nasal irrigations: good or bad?. Current Opinion in Otolaryngology & Head and Neck Surgery 2004;12(1):9‐13. [PUBMED: 14712112] [DOI] [PubMed] [Google Scholar]
Cho 2012
- Cho SH, Hong SJ, Han B, Lee SH, Suh L, Norton J, et al. Age‐related differences in the pathogenesis of chronic rhinosinusitis. Journal of Allergy and Clinical Immunology 2012;129(3):858‐60.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chong 2016a
- Chong LY, Head K, Hopkins C, Philpott C, Schilder AGM, Burton MJ. Intranasal steroids versus placebo or no intervention for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011996.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chong 2016b
- Chong LY, Head K, Hopkins C, Philpott C, Burton MJ, Schilder AGM. Different types of intranasal steroids for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011993.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 1998
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd Edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc, 1988. [Google Scholar]
DeMarcantonio 2011
- DeMarcantonio MA, Han JK. Nasal polyps: pathogenesis and treatment implications. Otolaryngologic Clinics of North America 2011;44(3):685‐95, ix. [DOI] [PubMed] [Google Scholar]
Ebbens 2010
- Ebbens FA, Toppila‐Salmi SK, Renkonen JA, Renkonen RL, Mullol J, Drunen CM, et al. Endothelial L‐selectin ligand expression in nasal polyps. Allergy 2010;65(1):95‐102. [DOI] [PubMed] [Google Scholar]
Ebbens 2011
- Ebbens FA, Toppila‐Salmi S, Groot EJ, Renkonen J, Renkonen R, Drunen CM, et al. Predictors of post‐operative response to treatment: a double blind placebo controlled study in chronic rhinosinusitis patients. Rhinology 2011;49(4):413‐9. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical research ed.) 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
EPOS 2007
- Fokkens W, Lund V, Mullol J. European position paper on rhinosinusitis and nasal polyps 2007. Rhinology 2007;45(Suppl 20):1‐139. [PubMed] [Google Scholar]
EPOS 2012
- Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinology. Supplement 2012;50 Suppl 23:1‐298. [PubMed] [Google Scholar]
Gliklich 1995
- Gliklich RE, Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngology ‐ Head and Neck Surgery 1995;113(1):104‐9. [DOI] [PubMed] [Google Scholar]
Handbook 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Harvey 2007
- Harvey R, Hannan SA, Badia L, Scadding G. Nasal saline irrigations for the symptoms of chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD006394.pub2] [DOI] [PubMed] [Google Scholar]
Hastan 2011
- Hastan D, Fokkens WJ, Bachert C, Newson RB, Bislimovska J, Bockelbrink A, et al. Chronic rhinosinusitis in Europe ‐ an underestimated disease. A GA2LEN study. Allergy 2011;66(9):1216‐23. [DOI] [PubMed] [Google Scholar]
Head 2016a
- Head K, Chong LY, Hopkins C, Philpott C, Burton MJ, Schilder AGM. Short‐course oral steroids alone for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011991.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Head 2016b
- Head K, Chong LY, Hopkins C, Philpott C, Schilder AGM, Burton MJ. Short‐course oral steroids as an adjunct therapy for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011992.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Head 2016c
- Head K, Chong LY, Piromchai P, Hopkins C, Philpott C, Schilder AGM, et al. Systemic and topical antibiotics for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011994.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jaeschke 1989
- Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Controlled Clinical Trials 1989;10(4):407‐15. [PUBMED: 2691207] [DOI] [PubMed] [Google Scholar]
Kern 2008
- Kern RC, Conley DB, Walsh W, Chandra R, Kato A, Tripathi‐Peters A, et al. Perspectives on the etiology of chronic rhinosinusitis: an immune barrier hypothesis. American Journal of Rhinology 2008;22(6):549‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keswani 2012
- Keswani A, Chustz RT, Suh L, Carter R, Peters AT, Tan BK, et al. Differential expression of interleukin‐32 in chronic rhinosinusitis with and without nasal polyps. Allergy 2012;67(1):25‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirtsreesakul 2012
- Kirtsreesakul V, Wongsritrang K, Ruttanaphol S. Does oral prednisolone increase the efficacy of subsequent nasal steroids in treating nasal polyposis?. American Journal of Rhinology & Allergy 2012;26(6):455‐62. [DOI: 10.2500/ajra.2012.26.3820] [DOI] [PMC free article] [PubMed] [Google Scholar]
Larsen 2004
- Larsen P, Tos M. Origin of nasal polyps: an endoscopic autopsy study. Laryngoscope 2004;114(4):710‐9. [DOI] [PubMed] [Google Scholar]
Rabago 2006
- Rabago D, Barrett B, Marchand L, Maberry R, Mundt M. Qualitative aspects of nasal irrigation use by patients with chronic sinus disease in a multimethod study. Annals of Family Medicine 2006;4(4):295‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ragab 2004
- Ragab SM, Lund VJ, Scadding G. Evaluation of the medical and surgical treatment of chronic rhinosinusitis: a prospective, randomised, controlled trial. Laryngoscope 2004;114(5):923‐30. [DOI] [PubMed] [Google Scholar]
Ragab 2010
- Ragab SM, Lund VJ, Scadding G, Saleh HA, Khalifa MA. Impact of chronic rhinosinusitis therapy on quality of life: a prospective randomized controlled trial. Rhinology 2010;48(3):305‐11. [DOI] [PubMed] [Google Scholar]
Revicki 2008
- Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient‐reported outcomes. Journal of Clinical Epidemiology 2008;61(2):102‐9. [PUBMED: 18177782] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Talbot 1997
- Talbot AR, Herr TM, Parsons DS. Mucociliary clearance and buffered hypertonic saline solution. Laryngoscope 1997;107(4):500‐3. [DOI] [PubMed] [Google Scholar]
Tan 2011
- Tan BK, Li QZ, Suh L, Kato A, Conley DB, Chandra RK, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. Journal of Allergy and Clinical Immunology 2011;128(6):1198‐206.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tomassen 2011
- Tomassen P, Zele T, Zhang N, Perez‐ Novo C, Bruaene N, Gevaert P, et al. Pathophysiology of chronic rhinosinusitis. Proceedings of the American Thoracic Society 2011;8(1):115‐20. [DOI] [PubMed] [Google Scholar]
van Drunen 2009
- Drunen CM, Reinartz SM, Wigman J, Fokkens W. Inflammation in chronic rhinosinusitis and nasal polyposis. Immunology and Allergy Clinics of North America 2009;29(4):621‐9. [DOI] [PubMed] [Google Scholar]
Vlckova 2009
- Vlckova I, Navrátil P, Kana R, Pavlicek P, Chrbolka P, Djupesland PG. Effective treatment of mild‐to‐moderate nasal polyposis with fluticasone delivered by a novel device. Rhinology 2009;47(4):419‐26. [DOI] [PubMed] [Google Scholar]
Zhang 2008
- Zhang N, Zele T, Perez‐Novo C, Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T‐effector cells orchestrate mucosal inflammation in chronic sinus disease. Journal of Allergy and Clinical Immunology 2008;122(5):961‐8. [DOI] [PubMed] [Google Scholar]
Zhang 2009
- Zhang XH, Lu X, Long XB, You XJ, Gao QX, Cui YH, et al. Chronic rhinosinusitis with and without nasal polyps is associated with decreased expression of glucocorticoid‐induced leucine zipper. Clinical and Experimental Allergy 2009;39(5):647‐54. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Chong 2015
- Chong LY, Head K, Hopkins C, Philpott C, Schilder AGM. Saline irrigation for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD011995] [DOI] [PMC free article] [PubMed] [Google Scholar]


