Abstract
Background:
Although the classic Reed-Sternberg (RS) cell is considered a diagnostic of Hodgkin's disease, RS-like cells have been noted in various benign and malignant conditions. The presence of these cells can be a diagnostic challenge.
Aims:
Our aim was to see if cells morphologically resembling or identical to RS cells occur in conditions other than Hodgkin lymphoma (HL) and what proportion of cases show binucleate cells in various benign and malignant conditions. We also want to discuss the diagnostic utility of cytomorphological features in predicting HL.
Material and Methods:
This study is a retrospective analysis of 2086 cytology cases. The cytosmears were screened for the presence of binucleate cells. A detailed cytomorphological analysis of cytosmears with binucleate cells was performed, and the diagnostic utility of cytomorphological criteria in delineating HL was examined.
Results:
Out of 2086 smears, 55 (2.6%) cytosmears showed binucleated RS/RS-like cells. Out of these 55 cases, 6 were HL, 3 cases were non-HL (NHL), 3 were sarcoma, 32 were carcinoma, and 11 were benign/inflammatory lesions. The presence of prominent eosinophilic nucleoli, granulomas, and eosinophils had good sensitivity and specificity in predicting the diagnosis of HL.
Conclusion:
A detailed cytomorphological analysis is of limited value in categorizing the RS and RS-like cells. However, few cytomorphological features such as prominent eosinophilic nucleoli, eosinophils, granulomas, and lack of clustering of atypical cells can help us in predicting HL.
Keywords: Hodgkin's disease, lymphoma, Reed-Sternberg cells
INTRODUCTION
A binucleate cell is an uncommon finding on cytosmears. The Reed-Sternberg (RS) cell is a diagnostic of Hodgkin's lymphoma (HL). Large binucleate cells resembling RS cells can be seen in a variety of benign and malignant conditions such as infectious mononucleosis, non-Hodgkin lymphoma (NHL), melanoma, poorly differentiated carcinoma, undifferentiated nasopharyngeal carcinoma, solid tumors, and sarcomas.[1,2,3,4,5] Although these conditions are relatively easy to identify by histopathological study, their presence in fine-needle aspiration (FNA) cytology smears may be more difficult to evaluate.
In this study, we wanted to see if cells morphologically resembling or identical to RS cells occur in conditions other than HL and what proportion of cases show binucleate cells in various benign and malignant categories. We also wanted to study the diagnostic utility of cytomorphologic criteria in the correct classification of binucleate cells as RS or RS-like cells.
MATERIALS AND METHODS
This retrospective study was carried out in our Department of Pathology. All the aspiration cytosmears/imprint smears performed between January 2016 and December 2019 were retrieved, and the slides were reviewed for the presence of binucleate RS and RS-like cells, irrespective of age, sex, and site. The cytological diagnosis was noted and correlated with the histopathological diagnosis. Both air-dried (MGG) and alcohol-fixed cytosmears (H&E) were studied. The proportion of cases showing binucleated cells in various benign and malignant categories was calculated.
Only cases with histopathological correlation were included for further analysis [Table 1] to ascertain the nature of the binucleate cells. The cytomorphologic characteristics of cases showing binucleation were analyzed in detail by two pathologists. The cases with binucleate cells were grouped into various categories based on their respective histopathological diagnosis, and the cytomorphological characteristics were compared [Tables 1 and 2]. For assessing the cell size, the mature lymphocyte and RBCs in the background were used for comparison. The cell size was classified as <50 μ, 50–70 μ, 71–90 μ, and >90 μ. The cytoplasmic color was classified as acidophilic, basophilic, or amphophilic. The presence/absence of cytoplasmic granules was also scored. The nuclear features including its shape, nuclear membrane, and chromatin pattern were studied in detail. When nucleoli were detected, they were screened for size, outline, and color. The smear pattern, architectural features, and other characteristics of the cytosmears were also analyzed [Table 2]. The eosinophils were graded as absent (0/10 HPF), occasional (1–5/10 HPF), few (6–10/10 HPF), and numerous (>10/10 HPF).
Table 1.
Comparison of individual cellular features of Binucleated cells in various categories
| Variables | Benign/Inflam n (%) | Carcinoma n (%) | HL n (%) | NHL n (%) | Sarcoma n (%) | P |
|---|---|---|---|---|---|---|
| Size of cell (µ) | ||||||
| <50 | 0 | 0 | 2 (33.3) | 0 | 1 (33.3) | |
| 50-70 | 2 (50.0) | 5 (23.8) | 3 (50.0) | 1 (33.3) | 1 (33.3) | 0.111 |
| 71-90 | 2 (50.0) | 9 (42.9) | 0 | 1 (33.3) | 1 (33.3) | |
| >90 | 0 | 7 (33.3) | 1 (16.7) | 1 (33.3) | 0 | |
| Cytoplasm | ||||||
| Acidophilic | 2 (50.0) | 8 (38.1) | 5 (83.3) | 1 (33.3) | 1 (33.3) | |
| Basophilic | 0 | 11 (52.4) | 0 | 1 (33.3) | 1 (33.3) | 0.072 |
| Amphophilic | 2 (50.0) | 2 (9.5) | 1 (16.7) | 1 (33.3) | 1 (33.3) | |
| Granularity | ||||||
| Present | 1 (25.0) | 4 (19.0) | 0 | 0 | 0 | 0.844 |
| Absent | 3 (75.0) | 17 (81.0) | 6 (100.0) | 3 (100.0) | 3 (100.0) | |
| Nucleus shape | ||||||
| Round | 2 (50.0) | 9 (42.9) | 2 (33.3) | 2 (66.7) | 2 (66.7) | |
| Round to Oval | 0 | 12 (57.1) | 4 (66.7) | 1 (33.3) | 1 (33.3) | 0.080 |
| Oval | 2 (50.0) | 0 | 0 | 0 | 0 | |
| Nuclear Membrane | ||||||
| Regular | 3 (75.0) | 16 (76.2) | 4 (66.7) | 2 (66.7) | 2 (66.7) | 0.966 |
| Irregular | 1 (25.0) | 5 (23.8) | 2 (33.3) | 1 (33.3) | 1 (33.3) | |
| Chromatin | ||||||
| Hyperchromatic | 0 | 2 (9.5) | 0 | 0 | 0 | |
| Fine | 1 (25.0) | 5 (23.8) | 2 (33.3) | 0 | 2 (66.7) | 0.171 |
| Coarse | 1 (25.0) | 14 (66.7) | 3 (50.0) | 3 (100.0) | 1 (33.3) | |
| Vesicular | 2 (50.0) | 0 | 1 (16.7) | 0 | 0 | |
| Chromatin clumping | ||||||
| Present | 1 (25.0) | 13 (61.9) | 1 (16.7) | 3 (100.0) | 1 (33.3) | 0.075 |
| Absent | 3 (75.00) | 8 (38.1) | 5 (83.3) | 0 | 2 (66.7) | |
| Nucleoli | ||||||
| Present | 3 (75.0) | 15 (71.4) | 5 (83.3) | 2 (66.7) | 1 (33.3) | 0.669 |
| Absent | 1 (25.0) | 6 (28.6) | 1 (16.7) | 1 (33.3) | 2 (66.7) | |
| Nucleoli size | ||||||
| Absent | 1 (25.0) | 6 (28.6) | 1 (16.7) | 1 (33.3) | 2 (66.7) | |
| Small | 2 (50.0) | 7 (33.3) | 0 | 1 (33.3) | 0 | 0.359 |
| Inclusion | 1 (25.0) | 8 (38.1) | 5 (88.3) | 1 (33.3) | 1 (33.3) | |
| Nucleoli color | ||||||
| Absent | 1 (25.0) | 6 (28.6) | 1 (16.7) | 1 (33.3) | 2 (66.7) | |
| Acidophilic | 1 (25.0) | 3 (14.3) | 5 (83.3) | 2 (66.7) | 0 | 0.011 |
| Basophilic | 2 (50.0) | 12 (57.1) | 0 | 0 | 1 (33.3) | |
| Nucleolar membrane | ||||||
| Absent | 1 (25.0) | 6 (28.6) | 1 (16.7) | 1 (33.3) | 2 (66.7) | |
| Regular | 2 (50.0) | 12 (57.1) | 3 (50.0) | 1 (33.3) | 1 (33.3) | 0.864 |
| Irregular | 1 (25.0) | 3 (14.3) | 2 (33.3) | 1 (33.3) | 0 |
Table 2.
Comparison of smear pattern, architectural features, and other characteristics in various categories
| Variables | Benign/Inflam n (%) | Carcinoma n (%) | HL n (%) | NHL n (%) | Sarcoma n (%) | P |
|---|---|---|---|---|---|---|
| Cellularity | ||||||
| High | 0 | 15 (71.4) | 1 (16.7) | 3 (100.0) | 1 (33.3) | |
| Medium | 3 (75.0) | 5 (23.8) | 5 (83.3) | 0 | 1 (33.3) | 0.005 |
| Low | 1 (25.0) | 1 (4.8) | 0 | 0 | 1 (33.3) | |
| Atypical cell clusters | ||||||
| Present | 2 (50.0) | 21 (100.0) | 0 | 1 (33.3) | 0 | <0.001 |
| Absent | 2 (50.0) | 0 | 6 (100.0) | 2 (66.7) | 3 (100.0) | |
| Multinucleate cells | ||||||
| Present | 0 | 8 (38.1) | 5 (83.3) | 2 (66.7) | 2 (66.7) | 0.058 |
| Absent | 4 (100.0) | 13 (61.9) | 1 (16.7) | 1 (33.3) | 1 (33.3) | |
| Eosinophils | ||||||
| Absent | 1 (25.0) | 18 (85.7) | 0 | 2 (66.7) | 3 (100.0) | |
| Occasional | 2 (50.0) | 3 (14.3) | 0 | 1 (33.3) | 0 | <0.001 |
| Few | 1 (25.0) | 0 | 2 (33.3) | 0 | 0 | |
| Numerous | 0 | 0 | 4 (66.7) | 0 | 0 | |
| Stromal fragments | ||||||
| Present | 0 | 3 (14.3) | 0 | 0 | 1 (33.3) | 0.632 |
| Absent | 4 (100.0) | 18 (85.7) | 6 (100.0) | 3 (100.0) | 2 (66.7) | |
| Granuloma | ||||||
| Present | 0 | 0 | 5 (83.3) | 0 | 0 | <0.001 |
| Absent | 4 (100.0) | 21 (100.0) | 1 (16.7) | 3 (100.0) | 3 (100.0) | |
| Necrosis | ||||||
| Present | 1 (25.0) | 6 (28.6) | 1 (16.7) | 2 (66.7) | 0 | 0.513 |
| Absent | 3 (75.0) | 15 (71.4) | 5 (83.3) | 1 (33.3) | 3 (100.0) |
Fisher's exact test was used to determine the statistical significance of the observed difference in cytomorphological criteria in various categories. All reported P values were two-sided, and a P value of less than 0.05 was considered significant for statistical tests.
The statistical analysis was performed by using SPSS for Windows (version 20.0. Armonk, New York: IBM Corporation).
The study was approved by the institutional ethics committee, and a waiver of informed consent was granted.
RESULTS
In this study, we have included a total of 2086 cytology cases. Out of these 2086 cases, 6 were HL, 10 were NHL, 3 were sarcoma, 248 were carcinoma, 1424 were benign/inflammatory lesions, and 395 were nondiagnostic/inconclusive cases. Among these 2086 cases, 55 cytosmears showed binucleated RS/RS-like cells. Out of these 55 cases, 6 were HL, 3 cases were NHL, 3 were sarcoma, 32 were carcinoma, and 11 were benign/inflammatory lesions. The proportion of cases showing binucleate RS-like cells in various categories were 100% in HL, 30% in NHL, 12.9% in carcinoma, 100% in sarcoma, and 0.7% in benign/inflammatory conditions. Among these 55 cases, only 37 cases had a corresponding histopathological diagnosis. Among these 37 cases, 15 cases were from lymph nodes, 10 from the breast, 4 from soft tissues, 3 from the lung, 2 from the thyroid, and 1 each from bone, liver, and bladder lesions. The age of the patients ranged from 7 to 70 years, with 21 males and 16 females. On the basis of the histopathological diagnosis, these 37 cases were grouped into 5 broad categories of which 6 were HL, 3 were NHL, 21 were carcinoma, 3 cases were sarcoma, and 4 were benign/inflammatory lesions. RS cells in HL and RS-like cells in other malignant conditions are shown in Figures 1 and 2, respectively.
Figure 1.
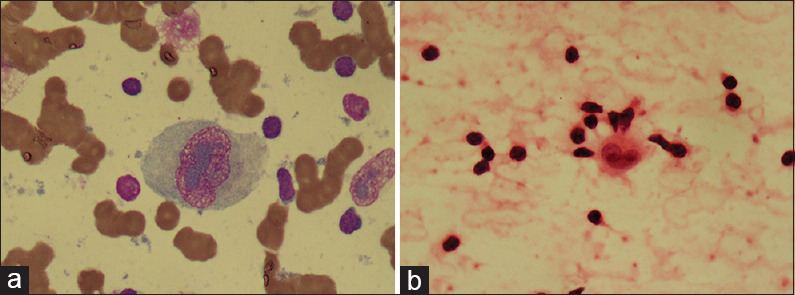
(a) RS cell in Hodgkin's lymphoma showing pale-greyish magenta–colored inclusion-like nucleoli (MGG, ×100) (b) RS cell in Hodgkin's lymphoma showing eosinophilic inclusion-like nucleoli (H and E, ×100)
Figure 2.
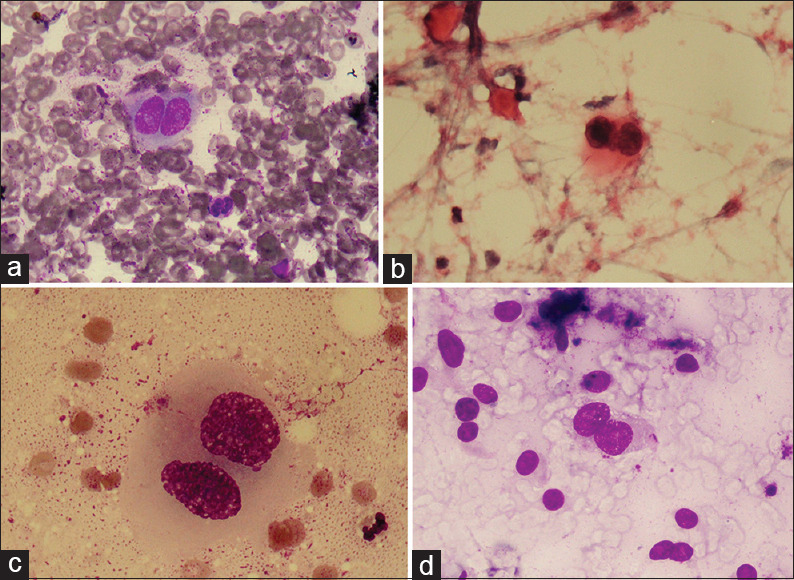
RS-like cell in other malignancies (a) non-Hodgkin's lynmphoma (MGG x100) (b) RS- like cell in non-Hodgkin's lymphoma (H and E, ×100) (c) RS-like cells in carcinoma (MGG, x100) (d) RS-like cell in sarcoma (MGG, ×40)
The detailed cytomorphological analysis of cytosmears of the 37 cases is displayed in Tables 1 and 2. The nuclear and cytoplasmic features [Table 1] are of limited use in distinguishing RS and RS-like cells. Only the nucleoli color varied significantly. All HL cases except one showed binucleated cells with prominent nucleoli. The majority of the carcinomas showed basophilic nucleoli, whereas HL and NHL showed acidophilic nucleoli. The cytosmears of one of the cases with binucleated cells along with irregular nuclear membrane, chromatin clumping, and inclusion-like nucleoli turned out to be a benign lesion. It was reported on biopsy as a benign myofibroblastic tumor. The binucleate cells observed in the cytosmears were probably binucleate myofibroblasts [Figure 3a and b].
Figure 3.
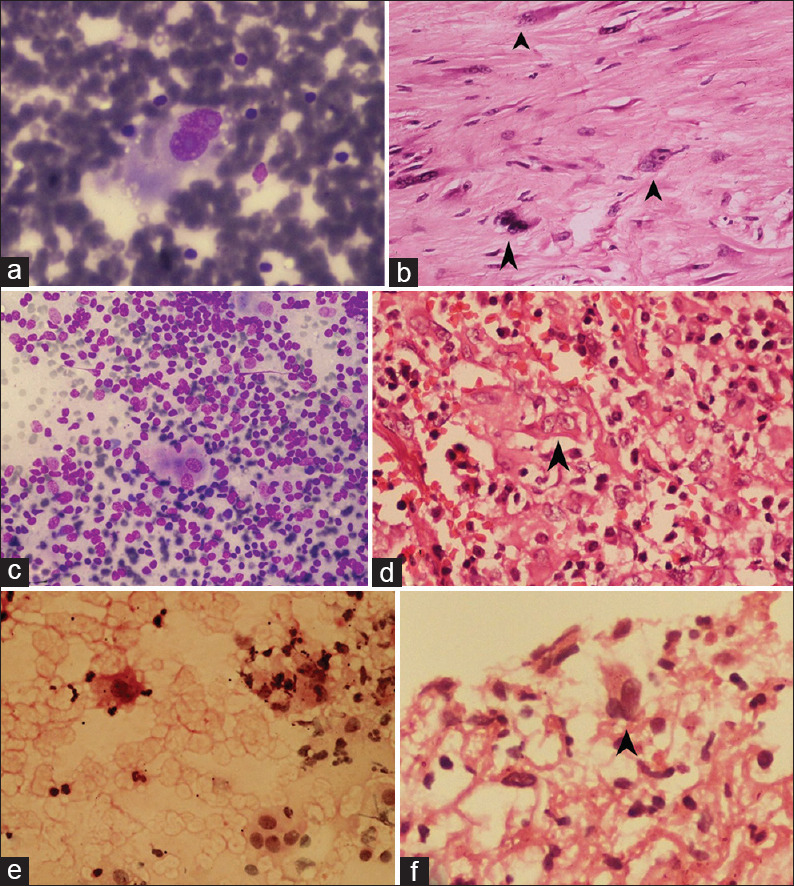
(a) Cytosmears showing RS-like cells in benign myofibroblastic tumor b) Corresponding histology of the same case showing binucleated myofibroblast (c) Cytosmears showing RS-like cells in angiolymphoid hyperplasia with eosinophilia (d) Corresponding histology of the same case (e) Imprint smear of a lung biopsy showing binucleated cells (f) Corresponding biopsy revealing features of pneumonia with binucleated cells (a, c, e – MGG ×40, b, d, f - H and E, ×40, arrowhead shows RS-like cells)
The majority of the carcinoma and NHL cases presented with high cellularity [Table 2]. All 21 cases of carcinoma had clusters of atypical cells (P < 0.001). Numerous eosinophils were noted in HL. Few/occasional eosinophils were seen in 2 benign cases, 3 carcinomas, and 1 NHL as well. The difference in the grades of eosinophils in various categories was statistically significant (P < 0.001). A benign lymph node aspirate showed binucleate RS-like cells and few eosinophils in the background mimicking HL, but in the biopsy, it turned out to be angio-lymphoid hyperplasia with eosinophilia. Reactive, plump, binucleate endothelial cells mimicked RS cells in the cytosmears [Figure 3c and d]. Similarly, the presence of granuloma was also statistically significant (P < 0.001). Out of the six cases of HL, five cases showed granuloma. The sensitivity, specificity, positive and negative predictive values of eosinophilic nucleoli, and the presence of granuloma and eosinophils in predicting the diagnosis of HL were calculated. The eosinophilic nucleoli had 83.3% of sensitivity, 80.5% of specificity, 45.5% of positive predictive value, and 96.2% of negative predictive value. The sensitivity, specificity, and positive and negative predictive values of the presence of granuloma were 83.3%, 100%, and 100% and 96.9%, respectively. The presence of a few or more eosinophils (≥6/10 HPF) had 100.0% of sensitivity, 96.8% of specificity, 85.7% of positive predictive value, and 100.0% of negative predictive value. The presence of numerous eosinophils (≥10/10 HPF) had 66.7% of sensitivity, 100.0% of specificity, and 100.0% and 93.95% of positive and negative predictive values, respectively. All these cytomorphologic criteria possess good sensitivity and specificity in predicting the diagnosis of HL.
DISCUSSION
FNA has become an acceptable tool in the primary diagnosis because of our better understanding of cytomorphology. Overlapping morphologic features is often a dilemma in diagnostic cytopathology.[6]
Binucleate cells in cytosmears often pose a diagnostic challenge due to the diverse cytomorphological characteristics of these cells. The presence of RS cells in aspirates or imprints, as well as in tissue sections of involved organs, suggests the diagnosis of Hodgkin's disease. The RS cell is usually large and pleomorphic. It has single or multiple nuclei. If there is one nucleus, it may be large and bean-shaped, or it may be multilobulated. The nucleus is characterized by heavy clumps of chromatin and by distinct round or ovoid eosinophilic inclusion-like nucleoli in H and E staining. The nucleoli appear pale-greyish to magenta color in May-Grunwald Giemsa (MGG) staining. The cytoplasm is acidophilic to basophilic. In tissue sections stained with hematoxylin and eosin, the cytoplasm is acidophilic. A homogeneous light blue is the characteristic color in the smears of bone marrow and imprints of tissues stained with Romanowsky dyes. Classical type RS cells have two bean-shaped nuclei, which are found to be mirror images of each other and with their hilar indentations apposed.[7]
The presence of notorious RS-like binucleate cells has been reported in cytosmears in various other conditions such as B-cell and T-cell NHL-like diffuse large B-cell lymphoma (DLBCL), anaplastic large cell lymphoma (ALCL), peripheral T-cell lymphoma (PTCL), polymorphic mantle cell lymphoma, etc. These RS-like cells also raise the suspicion of melanoma, poorly differentiated carcinoma, undifferentiated nasopharyngeal carcinomas, solid tumors, and sarcomas in appropriate clinical settings.[8,9,10]
In the present study, 2.3% of the cytosmears showed RS-like binucleate cells. Donahue et al. reported that these large atypical mononuclear or binucleate cells with RS-like features were present in about 1% of lymph node aspirates. These cells were thought to represent immunoblasts or reactive histiocytes that were often difficult to discern from true RS cells based on morphology alone.[10] Our study also reveals that it is difficult to distinguish RS from RS-like cells based on nuclear and cytoplasmic characteristics alone.
The cells of metastatic carcinoma and sarcoma, including amelanotic malignant melanoma, are sometimes large and manifest striking nucleoli. However, these cells are usually cohesive and arranged in sheets or clumps.[7] In the present study, it was found that smear patterns and clustering of cells provide some clue to the nature of the binucleate cells. The presence of eosinophils and granulomas in cytosmears also helps in the diagnosis of HL.
RS-like cells are often seen in NHL. Morphologic and immunophenotypic features of these cells, along with careful scrutiny of the inflammatory background are essential for the appropriate classification.[4] The presence of eosinophils in the background gives a clue to diagnose HL. In the present study, the presence of eosinophils in the background was significantly associated with HL. Two-thirds of HL cases showed numerous eosinophils, and the rest showed a few eosinophils.
Peripheral T-cell lymphoma (PTCL) can be misdiagnosed as HL due to the morphologic overlap between these conditions. Mathur et al. reported that a careful analysis of cytomorphologic features could be useful in at least suggesting a diagnosis of PTCL.[5] PTCL, not otherwise specified (NOS), characteristically display small, intermediate, and large atypical lymphoid cells with the percentage of large cells ranging from 20 to 90. In contrast, HL reveals mature lymphocytes and neoplastic RS cells with eosinophilic macronucleoli. The lymphoid cells in HL do not reveal nuclear cleaving/indentation as in PTCL.
The hallmark cells in ALCL are known to show varied cytomorphology and can be a diagnostic challenge. RS-like cells, lymphocytes admixed with eosinophils, presence of spindle cells, and absence of lymphoglandular bodies can be seen in ALCL also. The combination of hallmark and wreath-like multinucleate giant cells is helpful to predict anaplastic large cell lymphoma (ALCL) on cytology. The nucleoli in RS-like cells of ALCL are basophilic and irregular and not eosinophilic as seen in HL.[11]
In one case of HL, nucleoli were not detected in our study. On reviewing the cytosmears, it was noticed that the smears were not optimally fixed, and some amount of drying effect was obvious. To detect eosinophilic nucleoli, optimally alcohol fixed, Pap, and H & E smears are essential. Sahay et al. also mentioned that problems caused by fixation delay could result in loss of chromatin, halo, fading, or complete disappearance of nuclei.[12] Ahmed et al. observed that degenerative changes due to delayed fixation can lead to misdiagnosis in urine cytology.[13]
Benign conditions such as reactive lymphoid hyperplasia, as well as post-transplant lymphoproliferative disorders could also display these large atypical mononuclear or binucleate cells with RS-like features.[10]
In our study, RS-like cells were observed in angiolymphoid hyperplasia with eosinophilia. Murthy et al. also experienced diagnostic difficulties in differentiating Kimura's disease (KD) from HL due to the presence of numerous eosinophils, lymphocytes, and the occasional presence of atypical mononuclear RS-–like cells in cytosmears. But histopathological examination confirmed the diagnosis. KD and angiolymphoid hyperplasia with eosinophilia (ALHE) are close mimickers of HL.[14] Strum et al. noted the presence of RS–like cells in nine cases of malignancy and four benign conditions (biopsies) such as proliferative myositis, rubeola, infectious mononucleosis, and thymoma.[15] We also noted RS-like cells in the cytosmears of some cases, which later proved to be benign lesions [Figure 3a-f].
Granulomatous response in HL can be a confounding factor, which might distract the observer from the underlying pathology.[8] Kumari et al. observed well-formed granuloma in 5/18 cases of HL.[8] In our study, five out of six cases had granulomatous reaction along with binucleate cells (83.3%). Thus, a meticulous search of atypical cells in a reactive background is helpful.
Finally, we conclude that RS cells are characteristic of HL. However, RS-like cells can be seen uncommonly in cytosmears of other benign and malignant conditions also. The pathologist must be aware of various diagnostic pitfalls. Our study shows that even a detailed analysis of the various cellular characteristic of binucleate cells in cytosmears is of limited value in distinguishing RS from RS-like cells. However, few cytomorphological features such as prominent eosinophilic nucleoli, eosinophils, granulomas, and lack of clustering of atypical cells can help us to predict HL. Also, to detect eosinophilic nucleoli optimal fixation is essential.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Miliasuskas J. Lymphnode. In: Orell SR, Sterrett GF, editors. Orell and Sterrett's Fine Needle Aspiration Cytology. 5th ed. Philadelphia: Elsevier Publishing; 2012. pp. 77–117. [Google Scholar]
- 2.Arber DA. Lymhnodes. In: Goldblum JR, Lamps LW, Mckenny JK, Myers JL, editors. Rosai and Ackerman's Surgical Pathology. 11th ed. Elsevier Publishing; 2017. pp. 1530–631. [Google Scholar]
- 3.Kumar V, Abbas AK, Aster JK. Disease of White blood cells, Lymphnode, Spleen and Thymus.Robbins and Cotran Pathologic basis of Diseases. South Asia edition. Elsevier Publishing; 2014. pp. 579–628. [Google Scholar]
- 4.Gomez-Gelvez JC, Smith LB. Reed-sternberg–like cells in non-hodgkin lymphomas. Arch Pathol Lab Med. 2015;139:1205–10. doi: 10.5858/arpa.2015-0197-RAI. [DOI] [PubMed] [Google Scholar]
- 5.Mathur S, Verma K. Peripheral T-cell lymphoma not otherwise specified vs. Hodgkin's lymphoma on fine needle aspiration cytology. Acta Cytol. 2005;49:373–7. doi: 10.1159/000326168. [DOI] [PubMed] [Google Scholar]
- 6.Mourad WA, Nazer AI, Tulbah A. Cytomorphologic differentiation of Hodgkin's lymphoma and Ki-1+anaplastic large cell lymphoma in fine needle aspirates. Acta Cytol. 2003;47:744–8. doi: 10.1159/000326599. [DOI] [PubMed] [Google Scholar]
- 7.Yang YH, Palmer SD. The morphology of Reed-Sternberg cells in bone marrow. Am J Clin Pathol. 1963;39:115–20. doi: 10.1093/ajcp/39.2.115. [DOI] [PubMed] [Google Scholar]
- 8.Rashmikumari TR, Rajalakshmi T. Fine needle aspiration cytology in the diagnosis of Hodgkin lymphoma: Hits and misses. J Cytol. 2008;25:10–12. [Google Scholar]
- 9.Siddaraju N, Yaranal PJ, Mishra MM, Soundararaghavan J. Fine needle aspiration cytology in recurrent amelanotic melanoma. Acta Cytol. 2007;51:829–32. doi: 10.1159/000325851. [DOI] [PubMed] [Google Scholar]
- 10.Iacobuzio-Donahue CA, Clark DP, Ali SZ. Reed-Sternberg-like cells in lymph node aspirates in the absence of Hodgkin's disease: Pathologic significance and differential diagnosis. Diagn Cytopathol. 2002;27:335–9. doi: 10.1002/dc.10195. [DOI] [PubMed] [Google Scholar]
- 11.Agnihothri MA, Kothari KS, Naik LP, Patil S. Anaplastic large cell: A great mimic on cytology. J Cytol. 2017;34:165–7. doi: 10.4103/0970-9371.208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahay K, Mehendiratta M, Rehani S, Kumra M, Sharma R, Kardam P. Cytological artifacts masquerading interpretation. J Cytol. 2013;30:241–6. doi: 10.4103/0970-9371.126649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed HG, Tom MA. The consequence of delayed fixation on subsequent preservation of urine cells. Oman Med J. 2011;26:14–8. doi: 10.5001/omj.2011.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murthy SV, Geethamala K, Rao SM. Kimura's Disease: A cytodiagnostic dilemma with brief review of literature. Int Med J Sifa Univ. 2015;2:62–5. [Google Scholar]
- 15.Strum SB, Park JK, Rappaport H. Observation of cells resembling Sternberg-Reed cells in conditions other than Hodgkin's disease. Cancer. 1970;26:176–90. doi: 10.1002/1097-0142(197007)26:1<176::aid-cncr2820260123>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]


