ABSTRACT
Antibiotic resistance among gram-negative bacteria continues to rise globally at an alarming rate. New vaccines that prevent bacterial infections and reduce antibiotic use could provide a potential solution to these problems. This study focused on development of an investigational vaccine to prevent recurrent urinary traction infections (UTI) caused by gram-negative bacteria that use type 1 pili to adhere to, invade, and colonize human bladders. The vaccine antigen is FimH, an adhesin protein on the tip of type 1 pili with a lectin binding domain that enables attachment to glycoproteins on mammalian bladders. This was a phase 1, open-label, dose escalation study evaluating the vaccine in 67 healthy women with and without histories of recurrent UTI. The objectives of the study were to evaluate the safety, tolerability, and immunogenicity of different dosages of the antigen and adjuvant of the vaccine. All dosages were well-tolerated and a low incidence of systemic reactions occurred. No serious adverse events related to the vaccine were reported. The vaccine induced both binding and functional antibodies. The women with histories of recurrent UTI demonstrated greater than 150-fold increases in antibodies against the N-terminal region of FimH. Based on the results of this phase 1 study, this vaccine is proceeding to a double-blind, randomized, placebo-controlled phase 2 study. If this vaccine is successful in future studies, it could potentially prevent millions of recurrent UTI globally and reduce the development of antibiotic resistance.
KEYWORDS: Urinary tract infection, vaccine, FimH, TLR4 agonist, phase 1
Introduction
About 3 million people experience recurrent urinary tract infections (UTI) each year in the United States.1 Recurrent UTI, usually defined as 3 or 4 UTI during a 12-month period, has a substantial impact on day-to-day functioning due to moderate or severe symptoms and persistent recurrence at episodic intervals.2–4 Severe symptoms include pain and/or burning during urination, frequent urination, suprapubic pressure, fever, and nausea. More serious long-term outcomes include treatment failures, hospitalizations including prolonged stays and readmissions, bacteremia, severe sepsis, septic shock, in-hospital mortality, and the spread of antibiotic resistance.5–15 For more than 20 years, healthcare providers have prescribed antibiotics with refills for patients with recurrent UTIs so that these individuals can self-treat as symptoms occur, without needing to confirm that bacteria are present in urine.16 This treatment strategy empowers an individual to begin treatment earlier, so symptoms resolve faster. While such a treatment strategy that expeditiously resolves UTI is appropriate for reducing suffering and preventing sepsis, a preventive strategy would be preferable.
When cultures are obtained from midstream urine collections from symptomatic patients, Escherichia coli is the predominant species of bacteria identified, and these cultures are predictive of E. coli in the bladder.17,18 Therefore, physicians empirically treat UTI with antibiotics that target E. coli or another gram-negative species of bacteria.17 In contrast, identifying gram-positive bacteria from midstream urine collections during UTI symptoms is not predictive of their presence in the bladder.18 These gram-positive bacteria may be from periurethral contamination and not be the cause of UTI, and so it has been recommended to cautiously use this evidence to treat UTI.18,19
Since E. coli is the predominant uropathogen identified from midstream urine collections during UTI symptoms, and its detection correlates to its presence in the bladder, a vaccine for recurrent UTI must have a highly conserved antigen essential for E. coli virulence. FimH is an adhesin protein on type 1 pili of E. coli. Type 1 pili are bacterial appendages that promote attachment via branched oligomannose-containing glycoproteins on mammalian bladders. Type 1 pili and FimH are conserved among all Enterobacteriaceae including Klebsiella pneumoniae.20 There is extensive evidence indicating that FimH is essential for E. coli to colonize bladders in mice and that it enables the formation of intracellular biofilms in bladders.21–23 Preclinical studies have demonstrated that active immunization against FimH reduces bladder colonization in mice and monkeys.23–27 Therefore, FimH was selected as a vaccine antigen candidate with a proposed indication to reduce recurrent UTI in humans. The limited stability of full-length FimH requires it to be in a non-covalent complex with its natural chaperone protein FimC. This complex forms spontaneously during recombinant expression of FimC and FimH. Therefore, the antigen of the vaccine is FimCH.
Based on preclinical evidence, the treatment hypothesis of the vaccine is that anti-FimH IgG will reduce bacterial colonization of bladder surfaces in rUTI patients, thereby reducing the frequency of recurrent UTI. This requires that serum anti-FimH IgG transude to the bladder surface in humans. Anti-FimH IgG has been detected in vaginal washes of immunized monkeys, suggesting that it does transude to mucosal surfaces in mammals.24 Such transudation also has precedent in the human papillomavirus (HPV) vaccines. Human studies investigating the HPV vaccines demonstrate strong correlations between HPV-specific IgG levels in serum and in cervicovaginal secretions.28 This suggests that high serum IgG titers are indicative of the presence of IgG localized to mucosal surfaces.
The current study was conducted to evaluate the safety and immunogenicity of FimCH adjuvanted with a TLR4 agonist in healthy adults with and without a history of recurrent UTI. The primary objectives were to determine the safety and tolerability of different dosages of vaccines in the two separate populations of subjects, and the immunogenicity of different vaccine dosages. The secondary objective was to determine the duration and sustainability of the antibody responses in the two separate populations of subjects. The current indication of the FimCH vaccine is for the prevention of recurrent UTI caused by E. coli.
Materials and methods
Vaccines
The vaccine used in this study consisted of FimCH (antigen) and Phosphorylated HexaAcyl Disaccharide (PHAD®; adjuvant). Two dosages of FimCH (50 µg or 107 µg) and four dosages of PHAD® (10 µg, 20 µg, 40 µg, or 43 µg) were evaluated. Prior to administration, the vaccine was prepared on-site by adding PHAD® buffered suspension and/or sterile water to a vial of FimCH in 20 mM trisodium citrate and mixing. Intramuscular injections of 0.3 mL or 0.5 mL were administered on days 1, 30, 90, and 180 into the deltoid muscle of the non-dominant arm (Table 1).
Table 1.
Study cohorts and vaccination schedule
| History of UTI | Cohort | Number of Subjects Enrolled | Vaccinations at Days 1, 30, 90, and 180 | Volume of Injectiona |
|---|---|---|---|---|
| Nob | 1 | 5 | 107 µg FimCH | 0.5 mL |
| Nob | 2 | 8 | 50 µg FimCH 10 µg adjuvant |
0.3 mL |
| Nob | 3 & 4 | 16 | 50 µg FimCH 20 µg adjuvant |
0.3 mL |
| Nob | 5 | 8 | 107 µg FimCH 40 µg adjuvant |
0.3 mL |
| Yesc | 6 | 16 | 50 µg FimCH 40 µg adjuvant |
0.3 mL |
| Yesc | 7 | 14 | 107 µg FimCH 43 µg adjuvant |
0.5 mL |
aTwo different injection volumes were required to administer the appropriate dosages for each cohort because the vaccines were prepared from single concentrations of adjuvant drug product and FimCH drug product
bNo history of UTI for the previous 24 months prior to study enrolment.
cHistory of ≥5 UTIs in the last 24 months with at least one of these occurrences being documented as positive urine culture for E. coli at ≥103 CFU/mL prior to study enrolment.
FimCH drug substance was manufactured at the Center for Biocatalysis and Bioprocessing (Coralville, IA). FimCH drug product was manufactured at Ajinomoto Bio-Pharma Services (San Diego, CA). FimCH drug product consisted of FimCH in 20 mM trisodium citrate, pH 5.4. The adjuvant suspension drug product was manufactured at AMRI, Inc. (Burlington, MA). The adjuvant suspension drug product consisted of PHAD®, dipalmitoylphosphatidylcholine, 10 mM trisodium citrate, pH 6.0, and 0.02% of polysorbate 80. PHAD® was purchased from Avanti Polar Lipids (Alabaster, AL). All drug product was stored at Sherpa Clinical Packaging (San Diego, CA; now PCI Pharma Services).
Study design and subjects
This phase 1, open-label, antigen-controlled, dose escalation study was conducted at six clinical sites enrolling subjects from December 2013 to November 2015. The clinical sites were located in Maryland, Michigan, North Carolina, South Carolina, and Utah. Per protocol, the length of study was up to 19 months, which included 30 days before the first vaccination and 18 months after the first vaccination. Because several of the formulations included an adjuvant, the FDA requested that the subjects be followed for twelve months after the final vaccination at day 180.
Per protocol, the study included 67 females, ages 21–64, in 7 cohorts. The FDA requested a dose-escalation study because the vaccine formulations contained an adjuvant. Based on preclinical evidence, about 40 µg of adjuvant was selected as the highest adjuvant dose for this first human study. To comply with the FDA’s request, 10 µg of adjuvant was selected as the starting dose for the first cohort of subjects receiving adjuvant (cohort 2). The sample sizes of each cohort were not based on hypotheses. Each of cohorts 1 to 3 and 5 to 7 first vaccinated three dose leader subjects to evaluate the severity of local and systemic reactions prior to fully enrolling each cohort. A safety review committee allowed enrollment of subsequent cohorts after review of safety assessments. This study was not randomized. Subjects in cohorts 1 to 5 did not have a history of UTI in the previous 24 months prior to enrollment into the study. Subjects in cohorts 6 and 7 had ≥ 5 documented UTI in the last 24 months, including at least 1 UTI with a urine culture identifying E. coli. Subjects in cohorts 3 and 4 received the same dosages and schedule to increase the number of subjects in a cohort preceding the highest adjuvant dose.
Subjects were eligible if in generally good health with no clinically significant abnormal findings at screening from medical history, physical exam, 12-lead ECG, vital signs, oral temperature, and clinical laboratory evaluations. Subjects were seronegative to the N-terminus of FimH defined as less than 10 µg/mL according to a validated ELISA. Subjects had to test negative for drugs, alcohol, hepatitis, and HIV. Subjects of child-bearing potential had to practice defined contraception methods. Exclusion criteria included clinically significant disease in the opinion of the investigator or systemic bacterial infection within 2 months preceding the first vaccination, known history of neurogenic bladder, interstitial cystitis, urinary diversion, congenital urinary tract abnormality, presence of indwelling catheter, or history of kidney stones, pyelonephritis, or sepsis within the last 2 years. Exclusion criteria also included the use of an experimental agent within the past month, an experimental vaccine or antibody within the previous 12 months or plans to receive another experimental agent during this study, or administration of any licensed vaccine 30 days prior to the first dose of the study vaccine through 30 days after the last study vaccine (with the exception of a flu vaccine), or being treated with gammaglobulin or rituximab. Subjects with a history of immunosuppression, alcoholism and/or drug abuse within the last 3 years, anti-psychotic drugs for management of psychosis, hospitalization within the past 5 years prior to enrollment for psychiatric illness, history of suicide attempt, or confinement for danger to self or others, seizure disorder or taking anti-seizure medication, active malignancy or a history of any hematological malignancy, bleeding or coagulation disorder were excluded.
The study protocol, amendments to the protocol, and informed consent forms were approved by a central Institutional Review Board. The study was conducted in accordance with the Guideline for Good Clinical Practice, International Conference on Harmonization Tripartite Guideline, International Ethical Guidelines for Biomedical Research Involving Human Subjects, and the Declaration of Helsinki. All subjects provided written consent before inclusion. The study protocol is shown in the Supplemental Material.
Safety assessments
Assessments of oral temperature; local injection site symptoms including pain, tenderness, erythema, and induration; systemic abnormalities including nausea/vomiting, diarrhea, headache, fatigue, and myalgia; and other adverse events were solicited from subjects during each vaccination visit, clinical visits 2 or 3 days post-vaccination, and 7 days post-vaccination via diary cards and contact by telephone. Unsolicited assessments including any adverse events were requested from each subject via separate diary cards 8 days to 28 days post-vaccination and 90-day diary cards were collected until the end of the study. Additional unsolicited assessments including any adverse events were conducted on days 150, 270, 450, and 540 by contact via telephone. Assessments of clinical abnormalities in laboratory evaluations including serum chemistries and complete blood counts were conducted on days 1, 3, 31, 33, 60, 90, 92, 180, 182, 210, and 360. Urine analyses and cultures were conducted on days 1, 31, 60, 90, 120, 180, 210, and 360. Vital signs were assessed on days 1, 3, 31, 33, 60, 90, 92, 120, 180, 182, 210, and 360.
Immunogenicity assessments
Antibody responses specific to the N-terminal region of FimH were assessed in all subjects from sera obtained prior to first vaccination, and on days 30, 60, 90, 120, 180, 210, and 360. The ELISA was validated and performed at AI Biotech (Richmond, VA). Blinded sera samples were sent to the testing site. For the ELISA, the N-terminal domain of FimH was coated onto microtiter plates. After incubation, the plates were washed and test sera were added. After binding, the plates were washed and then a protein-L horseradish peroxidase (HRP) conjugate was added. After binding and washing, HRP substrate (3,3ʹ,5,5ʹ-tetramethylbenzidine) was added, and after 15 minutes the reaction was quenched. Optical densities were measured at 450/550. Seropositive is ELISA-dependent and defined per protocol as equivalent to 4 times greater than the limit of detection and about 1.5 times greater than the lower limit of quantitation. Each plate included a positive control of rabbit anti-FimH IgG raised against the N-terminal portion of FimH. A separate ELISA was validated using a human anti-FimH IgG standard that was produced from sera from four immunized subjects in this study (data not shown).
Definition and treatment of UTI
Recurrent UTI were characterized by one or more of the following UTI symptoms: frequency, urgency, and dysuria on urination, gross hematuria, or elicited suprapubic tenderness upon examination. When available, bacterial culture results from clean-catch midstream urine collections were reported along with UTI symptoms. Subjects who experienced UTI during the study were encouraged per protocol to seek diagnosis and treatment from a study investigator. The protocol definition of recurrent UTI was based on previously published physician treatment practices (2001) and a survey of 102 urologists in 2010.4,16 Of the 102 urologists who responded, 96 prescribe antibiotic refills to recurrent UTI patients so patients can self-treat based on symptoms without urine cultures. In addition, Lilian M. Abbo and Thomas M. Hooton state that “cultures are not recommended for most women with acute uncomplicated cystitis … ”19 Therefore, for this first phase 1 study, all data to determine how recurrent UTI are reported by patients and diagnosed by physicians were collected.
In contrast, published definitions of UTI for the last thirty years typically identify ≥102 or ≥103 CFU/mL of a species of bacteria from midstream urine collections at the time of UTI symptoms to diagnose an uncomplicated UTI.29–32 To be consistent with UTI criteria established by previous publications, the recurrent UTI disclosed in this report required all of the following 1) one or more of frequency, urgency, and dysuria on urination, gross hematuria, or elicited suprapubic tenderness upon examination, 2) diagnosis of a UTI by a physician, 3) prescribed antibiotics, and 4) a positive bacterial culture of ≥103 CFU/mL from a clean-catch midstream urine collection. These diagnosed UTI are listed in Table 2. Since evidence exists that enterococci or group B streptococci identified in midstream urine collections may not be the cause of UTI, identifying these bacteria from a clean-catch midstream urine collection during UTI symptoms does not necessarily conclude they are the cause of the recurrent UTI.18 This same report found that K. pneumoniae identified from midstream urine collections were predictive of its presence in the bladders of women.18
Table 2.
UTI diagnosed by physicians with bacteria detected in midstream urine collections during the 19-month study
| Subject No. | Study Day of Onset of UTI Symptoms |
Recorded UTI Symptoms | ≥103 CFU/mL identified from Urine Culture | If listed, bacteria identified fromurine culture was resistant to either TMP-SMX, FQ, NF, or CFTRX | Physician Prescribed Antibiotic(s) |
|---|---|---|---|---|---|
| COHORT 6 | |||||
| 101–06-004 | 2 | DFU | Enterococci | None | Macrobid® |
| 107 | DFU | E. coli | TMP-SMX, FQ | Bactrim™ DS, Ceftin® | |
| 284 | U | E. coli | None | Ceftin®, Cipro® | |
| 530 | DU | E. coli | FQ, TMP-SMX | Bactrim™, Cipro® XR | |
| 101–06-005 | 158 | DFU | E. coli | TMP-SMX | Macrobid® |
| 101–06-007 | Screen Visit | F | K. pneumoniae | NF | Cipro® |
| 101–06-008 | 47 | DFSU | Coag-neg staphylococci | FQ | Augmentin® |
| 162 | F | Coag-neg staphylococci | FQ | Bactrim™ DS | |
| 276 | DH | Group B streptococci | None | Cipro® | |
| 335 | DF | Coag-neg staphylococci | FQ | Cipro®, Macrobid® | |
| 102–06-004 | 105 | DS | E. coli | None | Macrobid®, sulfamethoxazole |
| 102–06-005 | 6 | DS | E. coli | FQ | Macrobid® |
| 27 | D | Enterococci | None | Cipro® | |
| 38 | FU | E. coli | None | Sulfamethoxazole | |
| 103–06-001 | 196 | DF | K. pneumoniae | TMP-SMX, NF, CFTRX | Macrobid® |
| 105–06-002 | 34 | DFU | K. pneumoniae | NF | Bactrim™ |
| 436 | FSU | Enterococci | None | Cipro® | |
| 106–06-001 | 251 | DFSU | E. coli | None | Cipro® |
| 106–06-002 | 38 | U | E. coli | None | Cipro® |
| 112 | DFU | E. coli | None | Cipro® | |
| 143 | FU | K. pneumoniae | NF | Cipro® | |
| 208 | DFU | K. pneumoniae | NF | Cipro® | |
| 373 | FU | E. coli | None | Cipro® | |
| 106–06-003 | 9 | DU | E. coli | FQ | Azo Gantrisin®, Macrobid® |
| 52 | DFU | E. coli | FQ, TMP-SMX | Cipro® | |
| 70 | DF | E. coli | FQ | Macrobid® | |
| 97 | FU | E. coli | FQ | Macrodantin® | |
| 109 | FU | E. coli | FQ | Macrobid® | |
| 141 | U | Enterococci | FQ | Macrobid® | |
| 226 | DFSU | E. coli | FQ, TMP-SMX | Macrobid® | |
| 243 | DFSU | E. coli | None | Cipro® | |
| 293 | DFU | E. coli | FQ, TMP-SMX | Bactrim™ | |
| 327 | DFU | E. coli | FQ, TMP-SMX | Macrobid® | |
| COHORT 7 | |||||
| 101–07-004 | Screen | DS | E. coli | FQ | Macrobid® |
| 14 | DU | S. saprophyticus | None | Macrobid® | |
| 102–07-001 | 101 | S | E. coli | TMP-SMX | Cipro® |
| 181 | DH | E. coli | TMP-SMX | Cipro® | |
| 105–07-003 | 232 | DFU | E. coli | None | Levaquin® |
| 105–07-005 | 3 | DFU | E. coli | None | Cipro® |
| 155 | DFSU | E. coli | None | Cipro® | |
| 106–07-001 | 3 | DFS | E. coli | None | Cipro® |
| 98 | U | Coag-neg staphylococci | FQ | Cipro® | |
| 215 | FU | MUF | None | Cipro® | |
| 106–07-003 | 120 | FU | E. coli | None | Macrobid® |
| 106–07-007 | 23 | DFU | E. coli | None | Cipro® |
| 57 | DFU | E. coli | None | Cipro® | |
| 110 | DFSU | E. coli | None | Bactrim™ | |
| 118 | DFSU | Citrobacter freundii | TMP/SMX | Cipro® | |
| 156 | FSU | K. pneumoniae | None | Cipro® | |
| 325 | DFSU | Enterococci | None | Cipro® | |
| 415 | DFS | Citrobacter group | None | Cipro® | |
| 107–07-003 | Screen | FU | E. coli | FQ, TMP/SMX | Cipro® |
| 29 | DFU | E. coli | FQ, TMP/SMX | Cipro® | |
| 60 | DU | E. coli | FQ, TMP/SMX | Augmentin® | |
| 86 | U | E. coli | FQ, TMP/SMX | Keflex® | |
| 122 | DH | E. coli | FQ, TMP/SMX | Cipro® | |
| 177 | FU | Enterococci | None | Cipro® | |
| 213 | FU | E. coli | FQ, TMP/SMX | Rocephin® IM | |
| 235 | DFU | K. pneumoniae | None | Zyvox® | |
| 288 | DFU | K. pneumoniae | None | Augmentin® | |
| 428 | FS | E. coli | None | Augmentin® | |
| 486 | DU | E. coli | FQ, TMP/SMX | Cipro® |
D is dysuria; F is frequency; U is urgency; H is hematuria; S is suprapubic tenderness upon examination; coag-neg is coagulase-negative; group B streptococci is Streptococcus agalactiae; MUF is mixed urogenital flora; CFTRX is ceftriaxone; FQ are fluoroquinolones; NF is nitrofurantoin; TMP-SMX is trimethoprim sulfamethoxazole; Augmentin is amoxicillin/clavulanate potassium; azo gantrisin is phenazopyridine hydrochloride and sulfamethoxazole; Bactrim DS is TMP-SMX; Cipro is ciprofloxacin; Macrobid is NF; Macrodantin is NF; Rocephin IM is ceftriaxone intramuscular injection; Zyvox is linezolid; Keflex is cephalexin.
Statistical analyses
The sample sizes of each cohort were not based on hypotheses, and all analyses were descriptive. No hypotheses were tested. No inferential statistical analyses were conducted. Adverse events are reported for all subjects who received the first vaccination. Immunogenicity analyses are reported for those subjects who received vaccinations within the study windows, whose day 210 samples were obtained within the study windows, and who had no major protocol violations. Geometric mean antibody titers with 95% confidence intervals were calculated.
Results
Subjects
Sixty-seven female subjects 18 to 64 years of age were enrolled into the study and received at least the first vaccination. Forty-two subjects were white, and 25 subjects were black (Table 3). Of these subjects, 57 received all four vaccinations (Figure 1). Prior to the fourth vaccination, one subject was lost to follow-up, eight subjects were terminated early because of inclusion or exclusion criteria of the protocol, and one subject was no longer willing to participate because of local moderate erythema and induration. After receiving the fourth and final vaccination, two subjects were lost to follow-up and three subjects withdrew consent after the day 450 phone call, one subject relocated out-of-state and withdrew consent, and one subject was terminated because of an exclusion criterion of the protocol.
Table 3.
Subject demographics
| Characteristics | Cohort 1 N = 5 |
Cohort 2 N = 8 |
Cohort 3 N = 8 |
Cohort 4 N = 8 |
Cohort 5 N = 8 |
Cohort 6 N = 16 |
Cohort 7 N = 14 |
|---|---|---|---|---|---|---|---|
| Age at vaccination, years | |||||||
| Mean | 34 | 34 | 36 | 35 | 42 | 50 | 48 |
| Min, max | 27, 40 | 23, 56 | 24, 47 | 18, 48 | 22, 62 | 25, 64 | 23, 63 |
| BMI, (kg/m2) | |||||||
| Mean | 28 | 26 | 26 | 33 | 26 | 26 | 26 |
| Min, max | 23, 35 | 19, 36 | 23, 44 | 17, 38 | 19, 39 | 24, 33 | 20, 30 |
Source: Sequoia Sciences; Data listing 16.2.4.1; Baseline Demographics Safety Population; BMI – body mass index.
Figure 1.
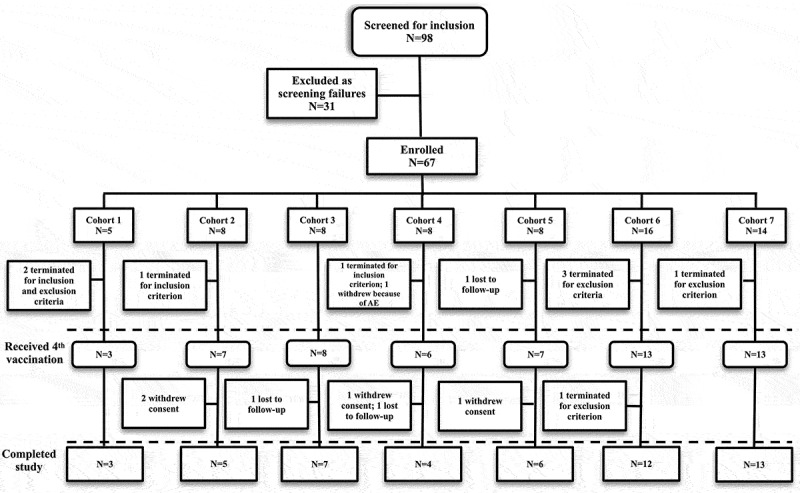
Subject disposition
Criteria for cohorts 6 and 7 required that subjects have a documented history of UTI; in these two cohorts, 30 subjects were enrolled, 26 received all four vaccinations, and 25 completed the 19-month study. These women were 25 to 64 years of age with body mass indices from 20 to 33 kg/m2. The 30 women who were enrolled presented medical documentation for 191 UTI in the previous 24 months. This documentation included 84 antibiotic susceptibility reports of E. coli identified from clean-catch midstream urine collections. These susceptibility data demonstrated that 15 women each had at least one UTI with E. coli resistant to trimethoprim-sulfamethoxazole, and 14 women each had at least one UTI with E. coli resistant to ciprofloxacin. Despite antibiotic refills being listed in these records, the use of these refills was not investigated or tabulated, so it is reasonable to assume these 30 women had additional undocumented UTI in the previous 24 months. Fourteen of the subjects had been prescribed antibiotic prophylaxis at least once in the previous 24 months.
Anti-FimH antibody responses
Table 4 lists FimH antibody responses by cohort. All 57 subjects who received 4 vaccinations were seropositive thirty days after the fourth vaccination (day 210). Subjects in cohorts 1, 2, 3/4, 5, 6, and 7 demonstrated fold increases in anti-FimH antibody responses at day 210 of 78, 72, 142, 341, 158, and 235, respectively. Of 56 subjects at day 360, 52 (93%) were seropositive. Subjects in cohorts 3/4, 5, 6, and 7 demonstrated fold increases in anti-FimH antibody responses at day 360 of 16, 51, 19, and 20, respectively.
Table 4.
Anti-FimH antibody responses for subjects in cohorts 1 to 7
| |
Cohort 1 |
Cohort 2 |
Cohorts 3 and 4 |
Cohort 5 |
Cohort 6 |
Cohort 7 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | 107 µg FimCH | 50 µg FimCH 10 µg adjuvant |
50 µg FimCH 20 µg adjuvant |
50 µg FimCH 40 µg adjuvant |
50 µg FimCH 40 µg adjuvant |
107 µg FimCH 43 µg adjuvant |
||||||||||||
| N | GMT | 95% CI | N | GMT | 95% CI | N | GMT | 95% CI | N | GMT | 95% CI | N | GMT | 95% CI | N | GMT | 95% CI | |
| 1 | 5 | - | - | 8 | - | - | 16 | - | - | 8 | - | - | 16 | - | - | 14 | 4 | 3–6 |
| 30 | 5 | 10 | 1,76 | 8 | 14 | 4,47 | 16 | 8 | 4,19 | 7 | 20 | 4,93 | 15 | 20 | 6,65 | 14 | 15 | 7,33 |
| 60 | 4 | 65 | 6,764 | 8 | 63 | 16,240 | 13 | 124 | 47,329 | 7 | 456 | 139,1498 | 15 | 125 | 48,323 | 14 | 155 | 53,453 |
| 90 | 4 | 32 | 3,400 | 8 | 40 | 13,124 | 14 | 61 | 27,137 | 7 | 223 | 62,804 | 14 | 82 | 34,197 | 13 | 89 | 29,278 |
| 120 | 4 | 149 | 2,9253 | 7 | 125 | 26,607 | 14 | 435 | 180,1051 | 7 | 426 | 121,1505 | 14 | 389 | 228,662 | 13 | 322 | 153,678 |
| 180 | 3 | 22 | 5,97 | 7 | 51 | 13,202 | 14 | 95 | 46,197 | 7 | 188 | 36,991 | 13 | 141 | 72,275 | 13 | 105 | 39,280 |
| 210 | 3 | 235 | 9,5872 | 7 | 215 | 71,651 | 14 | 427 | 175,1043 | 7 | 1024 | 263,3993 | 13 | 473 | 247,904 | 13 | 706 | 294,1691 |
| 360 | 3 | 22 | 6,82 | 7 | 23 | 6,84 | 14 | 49 | 24,102 | 6 | 154 | 47,507 | 13 | 57 | 28,116 | 13 | 61 | 24,154 |
GMT – geometric mean titer; fold increase calculated using ½ the limit of quantitation of 3 µg/ml.
For the 26 subjects in cohorts 6 and 7 that received the fourth and final vaccination, functional antibody responses evaluated at 30 days after the fourth vaccination were previously reported.33 In summary, 24 of 26 subjects demonstrated an increase in functional antibody responses.
Safety and tolerability
As shown in Table 5, the majority of local reactions reported by subjects within seven days after vaccination were mild and moderate pain and burning, tenderness and induration. The majority of severe local reactions reported by subjects within seven days after vaccination were pain and burning and tenderness. These local reactions all resolved within seven days.
Table 5.
Summary of local injection site symptom assessments reported in all subjects within 7 days of vaccination
| |
|
Cohort 1 |
Cohort 2 |
Cohorts 3 & 4 |
Cohort 5 |
Cohort 6 |
Cohort 7 |
|---|---|---|---|---|---|---|---|
| Local InjectionSite Reactions | 5 subjects (n = 17) | 8 subjects (n = 30) | 16 subjects (n = 61) | 8 subjects (n = 29) | 16 subjects (n = 58) | 14 subjects (n = 54) | |
| Pain/Burning | Mild and moderate | 6 (35%) | 11 (37%) | 21 (34%) | 15 (52%) | 26 (45%) | 36 (67%) |
| Severe | 0 | 0 | 0 | 0 | 2 (3%) | 2 (4%) | |
| Tenderness | Mild and moderate | 7 (41%) | 14 (47%) | 23 (38%) | 8 (28%) | 44 (76%) | 41 (76%) |
| Severe | 0 | 0 | 1 (2%) | 0 | 1 (2%) | 5 (9%) | |
| Erythema/Redness | Mild and moderate | 1 (6%) | 3 (10%) | 3 (5%) | 6 (21%) | 6 (10%) | 13 (24%) |
| Severe | 0 | 0 | 0 | 1 (3%) | 1 (2%) | 1 (2%) | |
| Induration/Swelling | Mild and moderate | 0 | 1 (3%) | 2 (3%) | 7 (24%) | 9 (16%) | 20 (37%) |
| Severe | 0 | 0 | 0 | 0 | 1 (2%) | 1 (2%) |
Data (n) are from 249 vaccinations among 67 subjects. A continuum of events related to a single injection is defined as a single event, graded as the most severe occurrence.
Across all of the 249 vaccinations, very few systemic reactions were reported by subjects within seven days after vaccination. These reported systemic reactions included 8 mild and 2 moderate headaches, 2 reports of mild nausea, and 4 reports of mild and moderate fatigue. No subject reported severe systemic reactions. These systemic reactions all resolved.
Unsolicited adverse events (AEs) were reported by 72% of subjects. The most frequent unsolicited AEs were UTI, nasopharyngitis, arthralgia, back pain, fatigue, and headache. No clinically significant hematological, biochemical, or urine values were reported.
Three serious adverse events were reported during the study. All were determined to be not related to the vaccine. No deaths or life-threatening AEs occurred during the study. No AEs of special interest were reported. A review of the type and frequency of reported adverse events across cohorts did not indicate any trends within or across cohorts.
Physician diagnosed UTI in the women of cohorts 6 and 7 who completed the 19-month study
Per protocol, the number of UTIs was recorded for each subject in cohorts 6 and 7 (those with a documented history of recurrent UTI prior to the study). Using a conservative definition of UTI that requires ≥103 CFU/mL detected in a midstream urine collection during symptoms (see Materials and Methods), 19 (76%) of the subjects who completed the 19-month study had a combined total of 62 episodes of UTI during the study (Table 2). Of these 62 UTI, cultures showed that 39 (63%) had E. coli present in the urine at the time of symptoms, 8 (13%) had K. pneumoniae, 6 (10%) had enterococci, 4 (6%) had coagulase-negative staphylococci, 2 (3%) had Citrobacter spp., 1 (1.6%) had Staphylococcus saprophyticus, 1 (1.6%) had group B streptococci, and 1 (1.6%) had mixed urogenital flora. Since this was not a placebo-controlled trial, the objectives of the study did not include endpoints evaluating recurrent UTI. As an exploratory analysis, we compared the UTI that occurred in the first half of the study with those in the second half of study. In the first 9 months of the study, 30 UTI had E. coli present in the urine at the time of symptoms, 7 had K. pneumoniae, and 10 had other bacteria. In the next 10 months of the study, 9 UTI had E. coli present in the urine at the time of symptoms, 1 had K. pneumoniae, and 5 had other bacteria.
Discussion
This phase 1 study demonstrated the safety and immunogenicity of the adjuvanted FimCH vaccine in women ages 21 to 64. All dosages were well-tolerated and no severe systemic reactions occurred. In all cohorts, a low incidence of systemic reactions occurred. No serious adverse events related to the vaccine were reported. No trends of reported adverse events across cohorts were identified.
All 57 subjects who received four vaccinations were seropositive thirty days after the fourth vaccination. Fifty-two of 56 (93%) subjects were seropositive at day 360. The GMT increased based on the adjuvant dosage. No apparent differences in immunogenicity were observed between subjects in cohort 5 without histories of recurrent UTI and in cohorts 6 and 7 with histories of recurrent UTI. Therefore, about 100 µg of FimCH and 40 µg of adjuvant are the dosages selected for the next human studies.
The exploratory analysis suggests that the subjects had notably fewer recurrent UTI in the second half of the study after achieving peak antibody responses compared to the first half of the study. Since plasma and B cells produce antibodies at the surfaces of bladders in women with recurrent UTI, and the antibodies induced by the vaccine in cohorts 6 and 7 reduced the binding of E. coli to bladder cells in vitro, we are optimistic that administering the vaccine in future studies will reduce recurrent UTI.33–35 However, at this stage of development no inferences can be made of the vaccine’s efficacy at reducing recurrent UTI.
To the best of our knowledge, no vaccine or other therapeutic indicated for a reduction in recurrent UTI is currently being evaluated in human studies. A vaccine against O-antigens of E. coli is currently under development for extra-intestinal pathogenic E. coli infections.36 However, it is unknown if this vaccine will seek an indication for reducing recurrent UTI. It is currently enrolling subjects 60 years of age and older, and it does not have endpoints evaluating a reduction in UTI.
Several limitations exist when investigating a preventive vaccine in patients with histories of recurrent UTI. First, since no vaccine to prevent UTI has been approved for use, no correlate of protection has been experimentally determined. Therefore, the relative quantities of functional antibodies required to sufficiently reduce the bacterial colonization of human bladders are unknown.
Second, it is unknown why some healthy women get recurrent UTI and others do not. Most females have their intestinal tracts colonized by uropathogens. Although it has been known for years that intestinal colonization leads to asymptomatic bacteriuria, this does not necessarily lead to UTIs.37 Thus, there may be unknown immune variations in certain individuals which contribute to recurrences.
Third, the virulence mechanisms of gram-negative bacteria are extensive, and many have not yet been adequately studied.38 E. coli’s and K. pneumoniae’s persistence on human skin is significant, and research suggests E. coli may be able to utilize dozens of adhesins.30,31,39–41 The impact of these virulence mechanisms and adhesins on persistence is not understood and may also contribute to potentially reducing the effectiveness of the vaccine in certain patients.
Since E. coli and other uropathogens causing recurrent UTI originate and recur from the patient’s own intestinal tract, our treatment hypothesis assumes that the infectious challenge is more frequent and of a higher burden than other viral and bacterial diseases. E. coli is routinely found via periurethral and vaginal swabs and is common in the urine.30,31,37 Furthermore, the relative abundances of different species of bacteria including E. coli in human intestinal tracts vary widely.42 In fact, the relative abundance of E. coli has been shown to vary by more than 10,000-fold among individuals.43 Therefore, certain recurrent UTI patients may require more time after achieving peak antibody titers to reduce recurrences than others simply because their microbial burden is greater. The impacts of these variables are difficult to quantify, and they warrant thoroughly investigating the efficacy of the functional antibodies induced by the vaccine.
Conclusion
Based on the favorable safety and immunogenicity results presented herein, the adjuvanted FimCH vaccine is proceeding to a double-blind, randomized, placebo-controlled phase 2 study. The highest adjuvant dose of 40 µg has been selected for the next study. The outcomes will evaluate the reduction in UTI symptoms and UTI in recurrent patients after achieving peak antibody responses. If this vaccine proves effective at preventing recurrent UTI in the next study, it would offer great hope to reduce the morbidities and mortality among this patient population.
Supplementary Material
Acknowledgments
We thank Ginger Constantine, MD for medical monitoring; Derry Ridgeway, MD and Ginger Constantine, MD for their contributions on the safety review committee; Innovative Analytics (Kalamazoo, MI) for data collection and storage and preparation of the data tables and statistical analysis; Karen Goldenthal, MD for assisting with development of the study protocol; William Egan, PhD for contributions to analytical development; Tatyana Touzova for contributions to analytical development and quality assurance; Thomas M. Hooton, MD for advice and expertise pertaining to patients with recurrent UTI; Jerry Pinkner for guidance and expertise with FimCH manufacturing; and Scott Hultgren, PhD for advice, expertise and insights pertaining to the virulence mechanisms involved with recurrent UTI.
Funding Statement
This study was funded by Sequoia Sciences. Employees of Sequoia Sciences are named as authors on this manuscript and contributed to the design and execution of the phase 1 study, and interpretation of data. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure of potential conflicts of interest
This study was funded by Sequoia Sciences. All authors were employees, contractors, consultants, or shareholders of Sequoia Sciences.
Contributors
GRE and HH conceived the phase 1 study design, development of the study protocol, and data analysis and interpretation. LR contributed with the development of the study protocol and execution of the study. AMS, ED, KGC, ASL, NS, and JP were clinical investigators and designees and contributed to subject recruitment, study procedures, and data acquisitions. LR and HH provided study medical and safety oversight. SMM provided advice and expertise. GRE and CMS wrote the manuscript.
Abbreviations
- UTI
urinary tract infections
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.National Disease and Therapeutic Index, IMS Health, Plymouth Meeting, PA. 2014. ISC# 595.0, 595.9, 597.8, 599.0.
- 2.Foxman B, Barlow R, D’Arcy H, Gillespie B, Sobel JD.. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000;10(8):509–15. doi: 10.1016/s1047-2797(00)00072-7. [DOI] [PubMed] [Google Scholar]
- 3.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Dis Mon. 2003;49(2):53–70. doi: 10.1067/mda.2003.7. [DOI] [PubMed] [Google Scholar]
- 4.Urologist UTI Survey . Sequoia Sciences, Inc. and HRA Research; 2010
- 5.van Duin D, Cober E, Richter SS, Perez F, Kalayjian RC, Salata RA, Evans S, Fowler VG, Kaye KS, Bonomo RA. Impact of therapy and strain type on outcomes in urinary tract infections caused by carbapenem-resistant Klebsiella pneumoniae. J Antimicrob Chemother. 2015. April;70(4):1203–11. doi: 10.1093/jac/dku495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fingar K, Washington R. Trends in Hospital Readmissions for Four High-Volume Conditions, 2009-2013. Healthcare Cost and Utilization Project; 2015. Statistical Brief #196. p. 1–10. [Google Scholar]
- 7.Johnston KJ, Thorpe KE, Jacob JT, Murphy DJ. The incremental cost of infections associated with multidrug-resistant organisms in the inpatient hospital setting-A national estimate. Health Serv Res. 2019;54(4):782–92. doi: 10.1111/1475-6773.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakellariou C, Gurntke S, Steinmetz I, Kohler C, Pfeifer Y, Gastmeier P, Schwab F, Kola A, Deja M, Leistner R. Sepsis Caused by Extended-Spectrum Beta-Lactamase (ESBL)-Positive K. pneumoniae and E. coli: comparison of Severity of Sepsis, Delay of Anti-Infective Therapy and ESBL Genotype. PLoS One. 2016;11(7):e0158039. doi: 10.1371/journal.pone.0158039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy MM, Gesten FC, Phillips GS, Terry KM, Seymour CW, Prescott HC, Friedrich M, Iwashyna TJ, Osborn T, Lemeshow S. Mortality Changes Associated with Mandated Public Reporting for Sepsis. The Results of the New York State Initiative. Am J Respir Crit Care Med. 2018;198(11):1406–12. doi: 10.1164/rccm.201712-2545OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatfield KM, Dantes RB, Baggs J, Sapiano MRP, Fiore AE, Jernigan JA, Epstein L. Assessing Variability in Hospital-Level Mortality Among U.S. Medicare Beneficiaries with Hospitalizations for Severe Sepsis and Septic Shock. Crit Care Med. 2018;6(11):1753–60. doi: 10.1097/CCM.0000000000003324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Critchley IA, Cotroneo N, Pucci MJ, Mendes R. The burden of antimicrobial resistance among urinary tract isolates of Escherichia coli in the United States in 2017. PLoS One. 2019;14(12):e0220265. doi: 10.1371/journal.pone.0220265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jernigan JA, Hatfield KM, Wolford H, Nelson RE, Olubajo B, Reddy SC, McCarthy N, Paul P, McDonald LC, Kallen A, et al. Multidrug-Resistant Bacterial Infections in U.S. Hospitalized Patients, 2012-2017. N Engl J Med. 2020;382(14):1309–19. doi: 10.1056/NEJMoa1914433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayakawa K, Gattu S, Marchaim D, Bhargava A, Palla M, Alshabani K, Gudur UM, Pulluru H, Bathina P, Sundaragiri PR, et al. Epidemiology and risk factors for isolation of Escherichia coli producing CTX-M-type extended-spectrum beta-lactamase in a large U.S. Medical Center. Antimicrob Agents Chemother. 2013;57(8):4010–18. doi: 10.1128/AAC.02516-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marchaim D, Chopra T, Bhargava A, Bogan C, Dhar S, Hayakawa K, Pogue JM, Bheemreddy S, Blunden C, Shango M, et al. Recent exposure to antimicrobials and carbapenem-resistant Enterobacteriaceae: the role of antimicrobial stewardship. Infect Control Hosp Epidemiol. 2012;33(8):817–30. doi: 10.1086/666642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayden MK, Lin MY, Lolans K, Weiner S, Blom D, Moore NM, Fogg L, Henry D, Lyles R, Thurlow C, et al. Prevention of colonization and infection by Klebsiella pneumoniae carbapenemase-producing enterobacteriaceae in long-term acute-care hospitals. Clin Infect Dis. 2015;60(8):1153–61. doi: 10.1093/cid/ciu1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta K, Hooton TM, Roberts PL, Stamm WE. Patient-Initiated Treatment of Uncomplicated Recurrent Urinary Tract Infections in Young Women. Ann Intern Med. 2001;135:9–16. doi: 10.7326/0003-4819-135-1-200107030-00004. [DOI] [PubMed] [Google Scholar]
- 17.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 18.Hooton TM, Roberts PL, Cox ME, Stapleton AE. Voided Midstream Urine Culture and Acute Cystitis in Premenopausal Women. N Engl J Med. 2013;369:1883–91. doi: 10.1056/NEJMoa1302186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abbo LM, Hooton TM. Antimicrobial Stewardship and Urinary Tract Infections. Antibiotics. 2014;3:174–92. doi: 10.3390/antibiotics3020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hung CS, Bouckaert J, Hung D, Pinkner J, Widberg C, DeFusco A, Auguste CG, Strouse R, Langermann S, Waksman G, et al. Structural basis of tropism of Escherichia coli to the bladder during urinary tract infection. Mol Microbiol. 2002;44(4):903–15. doi: 10.1046/j.1365-2958.2002.02915.x. [DOI] [PubMed] [Google Scholar]
- 21.Martinez JJ, Mulvey MA, Schilling JD, Pinkner JS, Hultgren SJ. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 2000;19:2803–12. doi: 10.1093/emboj/19.12.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright KJ, Seed PC, Hultgren SJ. Development of intracellular bacterial communities of uropathogenic Escherichia coli depends on type 1 pili. Cell Microbiol. 2007;9:2230–41. doi: 10.1111/j.1462-5822.2007.00952.x. [DOI] [PubMed] [Google Scholar]
- 23.Langermann S, Palaszynski S, Barnhart M, Auguste G, Pinkner JS, Burlein J. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science. 1997;276:607–11. doi: 10.1126/science.276.5312.607. [DOI] [PubMed] [Google Scholar]
- 24.Langermann S, Mollby R, Burlein JE, Palaszynski SR, Auguste CG, DeFusco A, Strouse R, Schenerman M, Hultgren S, Pinkner J, et al. Vaccination with fimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J Infect Dis. 2000;181:774–78. doi: 10.1086/315258. [DOI] [PubMed] [Google Scholar]
- 25.Thankavel K, Madison B, Ikeda T, Malaviya R, Shah AH, Arumugam PM, Abraham SN. Localization of a domain in the FimH adhesin of Escherichia coli type 1 fimbriae capable of receptor recognition and use of a domain-specific antibody to confer protection against experimental urinary tract infection. J Clin Invest. 1997;100:1123–36. doi: 10.1172/JCI119623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poggio TV, La Torre JL, Scodeller EA. Intranasal immunization with a recombinant truncated FimH adhesin adjuvanted with CpG oligodeoxynucleotides protects mice against uropathogenic Escherichia coli challenge. Can J Microbiol. 2006;52:1093–102. doi: 10.1139/w06-065. [DOI] [PubMed] [Google Scholar]
- 27.O’Brien VP, Hannan TJ, Yu L, Livny J, Roberson EDO, Schwartz DJ, Souza S, Mendelsohn CL, Colonna M, Lewis AL, et al. A mucosal imprint left by prior Escherichia coli bladder infection sensitizes to recurrent disease. Nature Microbiol. 2016;2:16196. doi: 10.1038/nmicrobiol.2016.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwarz TF, Spaczynski M, Schneider A, Wysocki J, Galaj A, Perona P, Poncelet S, Zahaf T, Hardt K, Descamps D, et al. HPV Study Group for Adult Women. Immunogenicity and tolerability of an HPV-16/18 AS04-adjuvanted prophylactic cervical cancer vaccine in women aged 15-55 years. Vaccine. 2009;27:581–87. doi: 10.1016/j.vaccine.2008.10.088. [DOI] [PubMed] [Google Scholar]
- 29.Rubin RH, Shapiro ED, Andriole VT, Davis RJ, Stamm WE. Evaluation of New Anti-Infective Drugs for the Treatment of Urinary Tract Infection. Clin Infect Dis. 1992;15(Supplement 1):S216–S227. doi: 10.1093/clind/15.Supplement_1.S216. [DOI] [PubMed] [Google Scholar]
- 30.Czaja CA, Stamm WE, Stapleton AE, Roberts PL, Hawn TR, Scholes D, Samadpour M, Hultgren SJ, Hooton TM. Prospective Cohort Study of Microbial and Inflammatory Events Immediately Preceding Escherichia coli Recurrent Urinary Tract Infection in Women. J Infect Dis. 2009;200(4):528–36. doi: 10.1086/600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hooton TM, Roberts PL, Stapleton AE. Cefpodoxime vs Ciprofloxacin for Short-Course Treatment of Acute Uncomplicated Cystitis: A Randomized Trial. JAMA. 2012;307(6):583–89. doi: 10.1001/jama.2012.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huttner A, Hatz C, van den Dobbelsteen G, Abbanat D, Hornacek A, Frölich R, Dreyer AM, Martin P, Davies T, Fae K, et al. Safety, immunogenicity, and preliminary clinical efficacy of a vaccine against extraintestinal pathogenic Escherichia coli in women with a history of recurrent urinary tract infection: a randomised, single-blind, placebo-controlled phase 1b trial. Lancet Infect Dis. 2017;17(5):528–37. doi: 10.1016/S1473-3099(17)30108-1. [DOI] [PubMed] [Google Scholar]
- 33.Starks CM, Miller MM, Broglie PM, Cubbison J, Martin SM, Eldridge GR. Optimization and qualification of an assay that demonstrates that a fimH vaccine induces functional antibody responses in women with histories of urinary tract infections. Hum Vaccin Immunother. 2020. in press. doi: 10.1080/21645515.2020.1770034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Nisco NJ, Neugent M, Mull J, Chen L, Kuprasertkul A, de Souza Santos M, Palmer KL, Zimmern P, Orth K. Direct Detection of Tissue-Resident Bacteria and Chronic Inflammation in the Bladder Wall of Postmenopausal Women with Recurrent Urinary Tract Infection. J Mol Biol. 2019;431(21):4368–79. doi: 10.1016/j.jmb.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlager TA, LeGallo R, Innes D, Hendley JO, Peters CA. B Cell Infiltration and Lymphonodular Hyperplasia in Bladder Submucosa of Patients with Persistent Bacteriuria and Recurrent Urinary Tract Infections. J Urol. 2011;186:2359–64. doi: 10.1016/j.juro.2011.07.114. [DOI] [PubMed] [Google Scholar]
- 36.Janssen Research & Development LLC . A study of three different doses of VAC52416 (ExPEC10V) in adults aged 60 to 85 years in stable health. [accessed 2020 July20]. Clinicaltrials.govAccessionNCT03819049Accession NCT03819049
- 37.Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. IDSA Guidelines for Asymptomatic Bacteriuria. Clin Infect Dis. 2005;40:643–54. doi: 10.1086/427507. [DOI] [PubMed] [Google Scholar]
- 38.Wurpel DJ, Beatson SA, Totsika M, Petty NK, Schembri MA. Chaperone-usher Fimbriae of Escherichia coli. PLoS One. 2013;8(1):e52835. doi: 10.1371/journal.pone.0052835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thurlow CJ, Prabaker K, Lin MY, Lolans K, Weinstein RA, Hayden MK. Anatomic sites of patient colonization and environmental contamination with Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae at long-term acute care hospitals. Infect Control Hosp Epidemiol. 2013;34(1):56–61. doi: 10.1086/668783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Korea CG, Badouraly R, Prevost MC, Ghigo JM, Beloin C. Escherichia coli K-12 possesses multiple cryptic but functional chaperone-usher fimbriae with distinct surface specificities. Environ Microbiol. 2010;12:1957–77. doi: 10.1111/j.1462-2920.2010.02202.x. [DOI] [PubMed] [Google Scholar]
- 41.Thänert R, Reske KA, Hink T, Wallace MA, Wang B, Schwartz DJ, Seiler S, Cass C, Burnham CA, Dubberke ER, et al. Comparative Genomics of Antibiotic-Resistant Uropathogens Implicates Three Routes for Recurrence of Urinary Tract Infections. mBio. 2019;10(4):e01977–19. doi: 10.1128/mBio.01977-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Human Microbiome Project Consortium . A framework for human microbiome research. Nature. 2012;486:215–21. 7402 doi: 10.1038/nature11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Magruder M, Sholi AN, Gong C, Zhang L, Edusei E, Huang J, Albakry S, Satlin MJ, Westblade LF, Crawford C, et al. Gut uropathogen abundance is a risk factor for development of bacteriuria and urinary tract infection. Nat Commun. 2019;10(1):5521. doi: 10.1038/s41467-019-13467-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


