Abstract
Background
Lower limb peripheral arterial disease (PAD) is a type of cardiovascular disease where the blood vessels that carry the blood to the legs are hardened and narrowed. The most severe manifestation of PAD is critical limb ischaemia (CLI). This condition results in symptoms of intractable rest pain, non‐healing wounds and ulceration, gangrene or both. PAD affects more than 200 million people worldwide and approximately 3% to 5% of people aged over 40 have PAD, rising to 18% in people over 70 years of age. Between 5% to 10% of symptomatic PAD patients will progress to CLI over a five‐year period and the five year cumulative incidence rate for asymptomatic patients with PAD deteriorating to intermittent claudication is 7%, with 21% of these progressing to CLI. Treatment options include angioplasty, bypass or amputation of the limb, when life or limb is threatened. People with CLI have a high risk of mortality and morbidity. The mortality rates during a surgical admission are approximately 5%. Within one year of surgery, the mortality rate rises to 22%. Postoperative complications are as high as 30% and readmission rates vary between 7% to 18% in people with CLI.
Despite recent advances in surgical technology, anaesthesia and perioperative care, a proportion of surgical patients have a suboptimal recovery. Presurgery conditioning (prehabilitation) is a multimodal conditioning intervention carried out prior to surgery using a combination of exercise, with or without nutritional or psychological interventions, or both. The use of prehabilitation is gaining momentum, particularly in elderly patients undergoing surgery and patients undergoing colorectal cancer surgery, as a means of optimising fitness to improve the prognosis for people undergoing the physiological stress of surgery. People with PAD are characterised by poor mobility and physical function and have a lower level of fitness as a result of disease progression. Therefore, prehabilitation may be an opportunity to improve their recovery following surgery. However, as multimodal prehabilitation requires considerable resources, it is important to assess whether it is superior to usual care.
This review aimed to compare prehabilitation with usual care (defined as a preoperative assessment, including blood and urine tests). The key outcomes were postoperative complications, mortality and readmissions within 30 days of the surgical procedure, and one‐year survival rates.
Objectives
To assess the effectiveness of prehabilitation (preoperative exercise, either alone or in combination with nutritional or psychological interventions, or both) on postoperative outcomes in adults with PAD undergoing open lower limb surgery.
Search methods
The Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register, CENTRAL, MEDLINE, Embase, CINAHL, World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov trials register to 25 September 2019.
Selection criteria
We considered all published and unpublished randomised controlled trials (RCTs) comparing presurgery interventions and usual care. Primary outcomes were postoperative complications, mortality and readmission to hospital within 30 days of the surgical procedure.
Data collection and analysis
Two review authors independently reviewed all records identified by the searches conducted by the Cochrane Vascular Information Specialist. We planned to undertake data collection and analysis in accordance with recommendations described in the Cochrane Handbook for Systematic Reviews of Interventions.
Main results
We found no RCTs that met the inclusion criteria for this review.
Authors' conclusions
We found no RCTs conducted to determine the effects of prehabilitation on mortality or other postoperative outcomes when compared to usual care for patients with PAD. As a consequence, we were unable to provide any evidence to guide the treatment of patients with PAD undergoing surgery. To perform a randomised controlled trial of presurgery conditioning would be challenging but trials are warranted to provide solid evidence on this topic.
Plain language summary
Presurgery exercise‐based conditioning interventions (prehabilitation) in adults with peripheral arterial disease, having leg surgery
Background
Lower limb peripheral arterial disease (PAD) is a type of cardiovascular disease where the blood vessels that carry the blood to the legs are hardened and narrowed. Its most severe state (critical limb ischaemia (CLI)), results in symptoms of pain when resting, non‐healing wounds and ulceration, gangrene or both. PAD affects more than 200 million people worldwide and approximately 3% to 5% of people aged above 40 have PAD, rising to 18% in people above 70 years of age. Of those who show symptoms of PAD, between 5% and 10% will progress to CLI over a five‐year period. Of those with PAD with no symptoms, 7% will go on to develop symptoms of cramping in the legs when walking and of these patients, 21% will progress to CLI within five years. The treatment options include using a balloon or stent (a device that sits in the artery) to open it up and allow the blood to flow (angioplasty), taking a vein from the opposite leg or arm and attaching it to the artery above and below the blocked artery (bypass), or removal of the limb (amputation), when the life of the patient is at risk due to infection. People with CLI have a high risk of death and worsening health.
Despite recent advances in surgical technology, anaesthesia, and care during and after surgery, a number of surgical patients have a poor recovery. Presurgery conditioning (prehabilitation) is carried out prior to surgery and includes exercise with a nutritional or psychological intervention or both. Exercise consists of aerobic training, such as walking, running or swimming (ideally three to five times a week) and training to build muscle and strength, using weights or resistance bands (ideally two times a week). The nutritional intervention includes eating or drinking a protein supplement daily, after training or with meals, to improve the effects of any training. Psychological interventions may include breathing exercises, meditation, mindfulness, coping techniques or cognitive behavioural therapy, with an aim to reduce the anxiety of having surgery.
Presurgery conditioning has become popular in bowel surgery and for elderly patients undergoing an operation. It is used as a means of improving the condition of the patient as a way to reduce complications during and after surgery. People with PAD have difficulty walking and doing their daily living activities and as a result are often very unfit. They therefore have the potential to improve their recovery during and after surgery from presurgery conditioning. However, as presurgery conditioning requires a big commitment from both the hospital and the patient, it is important to know how presurgery conditioning compares to usual practice (preoperative assessment, including blood and urine tests and information about the operation) for reducing the number of deaths or postoperative complications, or both.
Study characteristics and key results
We performed a review of the literature (current up to 25 September 2019) to determine whether prehabilitation was associated with reduced complications and death after surgery. We identified no randomised controlled trials on this topic.
Certainty of the evidence
We found no studies undertaken to address our objectives; therefore we were not able to assess the certainty of the evidence.
Conclusion
We found no evidence to determine if presurgery conditioning is better than usual care in reducing the number of deaths or postoperative complications. Although presurgery conditioning trials are difficult to do with people with lower limb peripheral arterial disease, randomised controlled trials are needed to provide solid evidence on this topic.
Background
Description of the condition
Lower limb peripheral arterial disease (PAD) is a type of cardiovascular disease in which the blood vessels (arteries) that carry blood to the legs are hardened and narrowed, or blocked by build‐up of fatty plaques on the arterial wall (atherosclerosis) (Hiatt 2001). The most common symptom of PAD is intermittent claudication (IC). This is muscle pain, fatigue, or discomfort in the calves, thighs, or buttocks that occurs during exercise and is relieved by rest. It occurs because there is an inability to match blood flow to demand and results in a lack of oxygen supply. People with IC often have a reduction in their physical activity, walking capacity, and quality of life. Risk factors for developing PAD and contributing to a decline in health can be described as non‐modifiable (including race, gender and age) or as modifiable (including smoking, diabetes mellitus, hypertension and high cholesterol). Current treatment for PAD is focused on preventing cardiovascular events, symptom relief, restoring mobility, improving functional ability, and improving overall quality of life. Early treatment options include risk factor management using pharmacotherapy, supervised exercise, smoking cessation, weight management, and surgical procedures (Norgren 2007).
However, PAD can be a progressive disease. Approximately 10% to 20% of people with PAD will have disease progression and 5% to 10% will develop critical limb ischaemia (CLI) (NICE 2012). CLI is characterised by intractable rest pain, non‐healing wounds and ulceration, or gangrene, or both. The treatment option, when the limb is threatened or symptoms become unbearable, is in the first instance angioplasty. If this is unsuccessful or not possible due to anatomical variations or calcification of lesions, then progression may be to an open bypass procedure or amputation of the affected limb (Norgren 2007). People with IC benefit from supervised exercise programmes, to manage their claudication symptoms (Lane 2017). These supervised programmes are a crucial treatment option to manage PAD symptoms and prevent progression to a surgical procedure. However, people with CLI are often unable to manage the type of exercise offered in these programmes and so surgical intervention becomes the only option available. This difficulty with undertaking structured exercise then results in people with CLI being unable to maintain their physical fitness due to their disease progression and management of lifestyle factors, which in turn puts them at greater risk of poorer outcomes after surgery. Therefore, this Cochrane Review intended to focus only on prehabilitation conducted to optimise people prior to undergoing an open surgical intervention for PAD, and not exercise performed for symptomatic benefit.
Prevalence and impact
PAD affects more than 200 million people worldwide and is a common cause of vascular morbidity (Fowkes 2013). Total disease prevalence is approximately 3% to 5% of people aged above 40, rising to 11% to 18% in people above 70 years of age (Selvin 2004). Of those with symptomatic PAD, 5% to 10% will progress to CLI over a five‐year period (NICE 2012), and the five year cumulative incidence rate for asymptomatic patients with PAD deteriorating to intermittent claudication is 7% with 21% of these progressing to CLI (Sigvant 2016). The annual incidence of CLI is estimated at 220 to 3500 per 1 million people, with a prevalence of approximately 1% of the population (Nehler 2014; Norgren 2007). People with PAD carry a high risk for surgical intervention, which may result in poor outcomes in mortality, morbidity, and length of hospital stay. Identified risk factors of poor surgical outcome in vascular patients include age, chronic obstructive pulmonary disease, coronary artery disease, inflammatory biomarker high sensitivity C‐reactive protein, smoking, functional decline, and poor nutritional status (Ambler 2015; Hasanadka 2011; Luo 2016; Owens 2012). Surgical outcomes for vascular procedures have an in‐hospital postoperative mortality rate of 2.8% to 5% (Waton 2017), but this can be as high as 22% for bypass, below knee and above knee amputation procedures (Fortington 2013). Postoperative complication rates range from 14% in the UK, 19.9% in the USA, and up to 30% in Europe. Readmission rates vary between 7% and 18% (Fortington 2013; Kazaure 2016; Kehlet 2016; Waton 2017; Zhang 2014). A recent review that examined the surgical outcomes of bypass surgery for chronic lower limb ischaemia found that surgical outcomes did not reach statistical difference between type of procedure for mortality (P = 0.16), patency (P = 0.92), and amputation rates (P = 0.15). However, the review did find a higher risk of complications (odds ratio (OR) 1.57, 95% CI 1.09 to 2.24, P = 0.01) in patients with CLI compared to patients with IC (Antoniou 2017). Patients with CLI are also at greater risk of cardiac events due to the higher prevalence of atherosclerosis in other cardiovascular vessel beds (Farber 2016), indicating that the severity of the disease results in a poorer prognosis for patients with CLI.
Description of the intervention
Prehabilitation is a multimodal conditioning intervention carried out prior to surgery. It consists of exercise, with or without nutritional care or a psychological intervention, or both (Minnella 2017). Prehabilitation is proposed as a means to enhance functional capacity with the intention to reduce postoperative morbidity and improve postsurgical recovery.
Exercise
Exercise includes regular physical activity that is incorporated into a structured programme with the specific goal of improving fitness. The exercise programme should include aerobic and strength training, and a flexibility component to encourage improvements in both functional reserve and the musculoskeletal system. Ideally, patients should engage in aerobic exercise three to five days per week, and strength training at least two days per week for between 30 and 60 minutes as recommended by the American College of Sports Medicine (Pescatello 2014). However, prehabilitation exercise programmes vary in frequency, intensity, type and time principles. The exercise intervention should be overseen or delivered, or both, by either an exercise specialist or physiotherapist, and should commence up to 12 weeks prior to surgery, and be continued until the surgery date.
Nutritional care
Nutritional supplementation has three main functions: (i) to provide energy to enable the completion of aerobic exercise; (ii) to facilitate muscle repair; and (iii) to adapt and support the postoperative catabolic response to surgery. People who are malnourished have diminished protein and stored glycogen reserves that help to combat the metabolic stress of surgery (Gillis 2015). Carbohydrate supplementation may facilitate the completion of an exercise session via facilitation of supplementary protein. Protein supplementation helps to facilitate muscle repair and adaptation, with 1.2 g to 1.5 g protein/kg demonstrated to support the postoperative catabolic response to surgery (Braga 2009). A dietician should oversee the nutritional status of patients and can prescribe the required dose for each individual patient.
Psychological interventions
The primary aim of a psychological intervention is to reduce the anxiety and emotional burden of having surgery. A number of psychological characteristics such as mood, anxiety, health beliefs, and expectations have been suggested to influence postsurgery outcomes (Levett 2016). Psychological interventions, such as relaxation techniques, breathing exercises or meditation, as well as mindfulness, coping strategies and cognitive behavioural therapy, may all play a role in anxiety reduction (Powell 2016). An appropriately trained healthcare professional should deliver all psychological therapies.
How the intervention might work
People undergoing major surgery, who have poor cardiorespiratory reserve levels due to low levels of fitness, may be at a higher risk of morbidity and mortality due to the elevated inflammatory response invoked by surgery. This response results in an increased demand in oxygen consumption in the postoperative period, which puts the body under additional stress (Le Roy 2016). Improving the fitness of a patient prior to surgery may aid the body to withstand this trauma, and providing nutritional supplementation may help patients to overcome the metabolic stress or facilitate any exercise training effects, or both. In addition, any surgical procedure is associated with an increased anxiety state in patients about their impending operation and ability to recover. Therefore this period may provide an ideal window of opportunity to engage patients in activities to aid their recovery by reducing their emotional distress. This may be achieved through proactively engaging the patients in the preparation prior to their surgery (Carli 2010).
Exercise
Research shows that regular exercise increases aerobic capacity, antioxidant capacity, insulin sensitivity, and the ratio of lean body mass to body fat (Pierson 2001). Poor fitness scores preoperatively increase the chance of dying within 30 days postsurgery or at least result in a significantly longer stay in hospital with a greater chance of complications in cardiac and abdominal surgery (Cook 2001; Playforth 1987). A review of exercise interventions prior to abdominal and cardiac surgery found that prehabilitation resulted in fewer complications, shortened length of stay, improvements in health‐related quality of life (QoL), and a mediation in the decline of functional disability (Carli 2005). Functional capacity reflects the underlying physiological reserve and provides an insight into a person’s ability to withstand the physiological stress of major surgery (Levett 2016). Engaging in physical activity prior to surgery has the potential to enhance physiological reserve and facilitate postoperative recuperation (Carli 2015). Measures of exercise tolerance may indicate an ability to withstand the increased oxygen demand after surgery. Peak oxygen consumption of less than 15 mL.min‐1.kg‐1 and anaerobic threshold of less than 11 mL.min‐1.kg‐1 have been associated with increased postoperative complications after major thoracic and abdominal surgery (Le Roy 2016). Similarly, values of 16.6 mL.min‐1.kg‐1 and 11.8 mL.min‐1.kg‐1 have been found to be predictive of postoperative complications in abdominal aortic aneurysm repair patients (Barakat 2015).
Nutritional care
The primary goal of nutrition therapy is to optimise the nutrient stores preoperatively and to compensate for the catabolic response of surgery postoperatively. Current evidence highlights the role of whey protein in combating the inflammatory response (Yalçin 2006), and carbohydrate in avoiding insulin resistance and postoperative hyperglycaemia (Gupta 2016). The European Society for Clinical Nutrition and Metabolism recommends 1.2 g to 1.5 g protein/kg for surgical patients (Braga 2009). Whey protein is associated with an increase in protein synthesis, and plays a role in oxidative stress defence by increasing the content of intracellular stores of the antioxidant glutathione (GSH). GSH neutralizes reactive oxygen species (ROS), which aids the blunting of the inflammatory processes characteristic of the stress induced by surgery (Yalçin 2006). In a randomised controlled trial (RCT), colorectal cancer patients given a whey protein supplement of 10 g to 20 g a day, in combination with an exercise training programme for four weeks, demonstrated an improved functional performance postsurgery compared with controls (Gillis 2014). In addition, major surgical trauma results in a transient reduction of insulin sensitivity leading to an increase in glucose production, a decrease in tissue uptake of glucose, and glycogen synthesis due to hyperglycaemia, which significantly increases the risk of postoperative complications in patients undergoing cardiopulmonary bypass (Doenst 2005). In previous studies, preoperative ingestation of carbohydrates, given between 12 hours and two weeks prior to surgery, demonstrated a significant reduction in insulin resistance (Bilku 2014). This reduction of insulin resistance after cardiac surgery, has shown to be associated with a decreased risk of postoperative complications, independent of diabetic status (Sato 2010). Nutritional supplementation may therefore support a faster recovery from the metabolic stress of surgery, or improve the exercise training effect, or both.
Psychological interventions
Psychological stress induces a physiological response where the body's sympathetic nervous system is activated due to the release of hormones, adrenaline and noradrenaline. This causes an increase in heart rate, blood pressure, and respiratory rate and reduces the peripheral blood flow due to vasoconstriction. This process, if sustained for long periods, can have deleterious effects on people with PAD (Aquarius 2006), where the peripheral blood flow is already compromised and patients are at a higher risk of cardiac complications (Barakat 2015). Several studies have identified that anxiety and depression can affect postoperative outcomes, such as wound healing in cancer and general surgical procedures (Cohen 2011; Munafo 2001; Walburn 2009). Potential mechanisms for these interventions vary depending on the intervention used. Cognitive interventions aim to reduce negative emotions and thoughts related to the surgical process by either changing negative thoughts or refocusing attention elsewhere. Relaxation interventions aim to make an individual feel more relaxed, both psychologically and physiologically. Providing information about what is expected can have the potential to reduce anxiety by helping the patient to know what is normal when they undergo surgery (Powell 2016). All of these types of coping strategies can aid people's ability to tolerate or eliminate psychological stress and its potentially deleterious consequences.
Why it is important to do this review
The prevalence of PAD has risen by 23% in the decade between 2000 and 2010 and is set to increase in the future in light of an aging population and an increase in the prevalence of diabetes in Western societies (Fowkes 2013). Despite advances in surgical technology, anaesthesia, and perioperative care, which has made surgery safer and more effective, a proportion of patients undergo surgery with a suboptimal recovery (Carli 2015). Since 2000, several studies have described the role of prehabilitation exercise therapy (Arthur 2000; Carli 2010; Dronkers 2010; Hulzebos 2006; Lemanu 2013; Santa Mina 2014; Valkenet 2011), exercise and nutrition (Carli 2005), exercise and psychological interventions (Bruns 2016), and all three components (Li 2013), with varying degrees of effectiveness in elderly patients undergoing general surgery and patients undergoing colorectal cancer surgery. While there has been much work in this area in recent years, the specific interventions (modality of exercise, exercise prescription, extent of supervision of exercise, choice of nutritional supplements, where used, and duration of intervention) vary widely between studies of prehabilitation in other patient groups, making it difficult to undertake any meaningful analyses (Bolshinsky 2018; Hijazi 2017).
Currently there are no systematic reviews examining the evidence for the effectiveness of prehabilitation (including exercise with or without nutritional and psychological therapy) in patients with PAD undergoing lower limb surgery, or its effectiveness on reducing mortality and morbidity. People with PAD typically have poor mobility and physical function, which limits the diversity of exercise interventions possible. Thus, we believe a systematic review of this population is timely and appropriate. This Cochrane Review aimed to assess the evidence for prehabilitation in lower limb vascular surgery and support future directions for research. As the PAD patient pathway is complicated further by the changing status of otherwise stable PAD with presentations of acute limb ischaemia (ALI), it is essential to know if, and for whom, prehabilitation would be of benefit. Vascular surgeons could then make informed decisions about the best management for their patients, and would be equipped with a strategy to improve outcomes for those patients undergoing a surgical procedure. Additionally, a review of this kind could aim to synthesize evidence to identify relevant components of a prehabilitation programme that could be standardized to enable a meta‐analysis to be undertaken in the future.
Objectives
To assess the effectiveness of prehabilitation (preoperative exercise, either alone or in combination with nutritional or psychological interventions or both) on postoperative outcomes in adults with PAD undergoing open lower limb surgery.
Methods
Criteria for considering studies for this review
Types of studies
We intended to include all RCTs and quasi‐RCTs on prehabilitation interventions with an exercise component or in combination with a nutritional or psychological component (together or separately) compared to control (standard treatment). Any methods of randomisation were eligible and we intended to take into account any differences in methodological quality in the intended analysis.
Types of participants
We intended to include all participants aged 18 years and older, diagnosed with PAD Fontaine Classifications II, IIA, IIB, III, and IV, or Rutherford Class 2 to 6, diagnosed by a clinician using a combination of general and systemic examination and diagnostic tools (ankle brachial pressure index, toe pressure index, Doppler scan), who were undergoing lower limb surgery. We intended to include all open surgical bypass procedures including, but not limited to, supra and infrainguinal bypass and major amputations, including below, through, and above knee.
Types of interventions
We intended to include studies that compared any form of preoperative exercise alone, or in combination with nutritional or psychological interventions, or both, with standard care. Standard care was defined as a preoperative assessment and relevant preparation prior to surgery, which may have included blood and urine tests and giving information on what will happen before, during, and after the operation.
Preoperative or prehabilitation exercise included any intervention which was designed to improve fitness up to 12 weeks prior to the surgical procedure as an inpatient or outpatient. The exercise intervention was not limited to any type and could have included individual or group, facility or home‐based, or a combination thereof but was only for the purpose of improving fitness for people listed for surgery. There are likely to be variations of frequency, intensity, type and time principles used in different prehabilitation exercise programmes so we allowed for this variation when considering the inclusion of studies in this review. Exercise interventions that were designed for improving intermittent claudication (IC) symptoms only were excluded. We also excluded studies that compared the effectiveness of different types of training, such as aerobic versus strength training, unless they also had a standard care arm.
Preoperative nutritional interventions included any intervention aimed at improving the nutritional status of individuals prior to surgery. We had planned to include protein supplementation or carbohydrate loading, or both, prior to surgery; or whey protein supplementation to support exercise training.
Psychological interventions included any technique aimed at reducing the anxiety and stress of undergoing a surgical procedure. Interventions could have included inspiratory muscle training or breathing exercises, relaxation, meditation, visualisation or cognitive behavioural therapy, and smoking cessation. The smoking cessation intervention had to be part of a psychological intervention, and was only included if it was part of a prehabilitation programme to prepare the person listed for surgery.
Types of outcome measures
Primary outcomes
30‐day mortality: defined as all‐cause death occurring within 30 days of the surgical procedure
-
Postoperative complications
Non‐fatal cardiovascular events including myocardial infarction, heart failure, or stroke
Pulmonary complications: including hypoxia, atelectasis, pneumonia, and respiratory failure
Haemorrhage
Surgical site infection (SSI)
Any other complications not specified above
-
Readmission within 30 days of the surgical procedure
Major adverse limb events (MALE), including amputations or major re‐intervention
Any other cause related to surgery
Secondary outcomes
Survival at one year for all types of surgery
Amputation‐free survival in revascularisation procedures
Changes in fitness or functional capacity, or both, preintervention and postintervention (measured by VO2Peak, ventilatory anaerobic threshold (VAT), grip strength, or submaximal exercise tests)
Changes in psychological health presurgery and postsurgery (measured by validated questionnaires such as the hospital anxiety and depression scale, coping questionnaires, or psychological components in quality of life (QoL) scores)
Changes in health‐related QoL scores presurgery and postsurgery (measured by validated questionnaires such as the Short Form (SF) 36, SF12, or disease‐specific tools such as the Vascular Quality of Life (VascuQoL).
Patient adherence and acceptability of exercise, nutritional, and psychological programmes (prehabilitation programmes) as reported by the study investigators
Adverse events related to prehabilitation interventions
Length of hospital stay
For all outcomes, we intended to include the time points reported by the individual studies. Clinically relevant time points were baseline (presurgery), postintervention, and postsurgery at 30 days, three, six, and 12 months. We also planned to present outcomes that we did not include in any meta‐analyses, in narrative form.
Search methods for identification of studies
We used no restrictions on language or publication status.
Electronic searches
The Cochrane Vascular Information Specialist conducted systematic searches of the following databases for RCTs and quasi‐RCTs without language, publication year or status (published, unpublished, in press, or in progress) restriction.
Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web) (searched 30 September 2019)
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 8) in the Cochrane Register of Studies Online (CRSO)
MEDLINE (Ovid MEDLINE® Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE®) (from 1946 onwards) (searched 25 September 2019)
Embase Ovid (from 1974 onwards) (searched 25 September 2019)
CINAHL Ebsco (from 1982 onwards) (searched 25 September 2019)
The Information Specialist modelled search strategies for other databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with adaptations of the highly sensitive search strategy designed by Cochrane for identifying RCTs and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6, Lefebvre 2011). Search strategies for major databases are provided in Appendix 1.
The Information Specialist searched the following trials registries on 30 September 2019.
World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch)
ClinicalTrials.gov (clinicaltrials.gov)
Searching other resources
We planned to search the reference lists of all included studies' citations and contact authors of included studies for further information of unpublished studies. As we identified no RCTs eligible for inclusion, we sought to review the non‐randomised studies. We also did not identify any non‐randomised studies.
Data collection and analysis
Selection of studies
Two review authors (JP, SP) independently reviewed the titles and abstracts of each study to identify those that met the inclusion criteria. We retrieved the full‐text articles of the studies identified as potentially relevant by at least one of the review authors. The same two review authors independently screened the full‐text articles retrieved for inclusion or exclusion. We resolved any disagreements by discussion and therefore consultation with a third review author was unnecessary. We recorded the selection process and completed a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram.
Data extraction and management
We planned for two review authors (JP, SP) to independently extract data from the eligible studies using an adapted data extraction form provided by Cochrane Vascular. We intended to resolve any disagreements by discussion and, if necessary, consult a third review author (AH). One review author (JP) was to enter the extracted data into Review Manager 5 (RevMan 2014). A second review author (SP), was to check for accuracy and consistency against the data extraction sheets.
We planned to extract data from each included study on the following.
Lead author, date
Study participant inclusion/exclusion criteria
Country where the research was conducted
Participants' gender and age
Study design, randomisation processes, allocation concealment
Recruitment rates
Descriptions of interventions (type, length, adherence)
Intervention settings (home, hospital, gym) and resources required (expertise and numbers of staff)
Number of participants in each trial arm, withdrawals, dropouts, and loss to follow‐up
Length of follow‐up
Outcome measures and times outcomes were assessed
Funding source(s)
Assessment of risk of bias in included studies
We planned for two review authors (JP, SP) to independently assess the risk of bias in each study using the criteria below, as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Random sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective outcome reporting (reporting bias)
Other sources of bias
We intended to judge each criterion with a score of either low, high, or unclear risk of bias and to provide a statement to support each judgement. We planned to resolve any disagreements with discussion with a third review author (AH). We planned to contact any study authors should further information be required for the ‘Risk of bias' assessment and assess the likely magnitude and direction of bias and whether it was likely to impact on the findings using sensitivity analyses (see Sensitivity analysis). We intended to present ‘Risk of bias' results using a ‘Risk of bias' graph and a ‘Risk of bias' summary.
Measures of treatment effect
Dichotomous data
We planned to analyse the data based on the number of the events and the number of people assessed in the intervention and comparison groups for dichotomous outcomes. We intended to use these to calculate the risk ratio (RR) or odds ratio (OR) and associated 95% confidence intervals (CIs) to reflect uncertainty of the estimate of effect.
Continuous data
We planned to analyse data based on the mean and standard deviation (SD) and to calculate the mean difference (MD) with corresponding 95% CIs for continuous outcome measures. We intended to use standardised mean difference (SMD) with 95% CIs to combine data from trials that measure the same outcome using different scales (Higgins 2011).
Time‐to‐event data
We planned to use survival analysis to report time‐to‐event data and the intervention effect was to be expressed as a hazard ratio (HR) and associated 95% CIs guided by the methods used and described by Parmar 1998 and Tierney 2007.
Unit of analysis issues
We intended for randomised individual participants to be the unit of analysis.
Dealing with missing data
We intended to perform all analyses using an intention‐to‐treat approach, where we would have analysed all participants and their outcomes within the groups to which they were allocated, regardless of whether they received the intervention. We planned to contact study authors to request any missing data. We intended to report the levels of loss to follow‐up and assess whether this was a potential source of bias.
Assessment of heterogeneity
We planned to assess the degree of heterogeneity by visual inspection of forest plots and by using the Chi2 test for heterogeneity. We intended to assess heterogeneity of the overall results for the main outcomes using the Chi2, I2 and Tau2 statistics, according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We intended to regard statistical heterogeneity as substantial if the I2 statistic value was > 50%, but we aimed to have interpreted this value in light of the size and direction of effects and the strength of the evidence for heterogeneity based on the P value in the Chi2 test for heterogeneity, also known as Q statistic. Statistically significant heterogeneity would have been assumed if the Chi2 was greater than or exceeded the df (degrees of freedom) and the P value was < 0.05. If we had detected heterogeneity, we aimed to explore the possible reasons for it.
Assessment of reporting biases
We intended to investigate publication bias using funnel plots if 10 or more studies are included in a meta‐analysis, as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We also aimed to evaluate time lag bias, duplicate publication bias, location bias, citation bias and language bias to assess any effects on the results of the review.
Data synthesis
We planned to perform statistical analyses using Review Manager 5 (RevMan 2014). We intended to use fixed‐effect meta‐analyses for synthesising data if it was reasonable to assume that trials were estimating the same underlying treatment effect. If there was clinical heterogeneity, sufficient to expect that the underlying treatment effects differed between trials, we planned to use random‐effects meta‐analyses and produce an overall summary where the mean treatment effect was clinically meaningful. If we had identified clinical, methodological, or statistical heterogeneity across the included trials sufficient to cause concerns, we would not have reported pooled results from the meta‐analysis but would instead have used a narrative approach to the data synthesis.
Subgroup analysis and investigation of heterogeneity
We intended to perform the following subgroup analyses.
Severity of ischaemia where participants had a Fontaine score of III or IV or a Rutherford score of 4 to 6
Type of surgical procedure
Combinations of interventions used
Location of where the intervention was delivered (home, gym, or hospital)
Sensitivity analysis
We intended to repeat the analyses including high‐quality trials only. For this review, we planned to classify trials that we judged as being at low risk of bias for sequence generation and allocation concealment as high‐quality trials (Higgins 2011). We also intended to repeat these analyses with quasi‐RCTs, which would have allowed us to further investigate any effects of including data from quasi‐RCTs on the combined results.
Summary of findings and assessment of the certainty of the evidence
Based on the methods described in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we intended to present the findings of this review in a ‘Summary of findings' table. We planned to present results for the main comparisons of the review and for the following outcomes, which we considered to be of the greatest clinical relevance to both patients and health professionals.
30‐day mortality
Postoperative complications
Readmission within 30 days
Survival at one year for all types of surgery
Changes in fitness or functional capacity, or both, preintervention and postintervention
Changes in psychological health presurgery and postsurgery
Changes in health‐related quality of life (QoL) scores presurgery and postsurgery
For each assumed risk cited in the table(s), we planned to provide a source and rationale. We intended to prepare the table using the GRADE profiler (GRADEpro GDT 2015). We intended to use the GRADE approach to assess the certainty of the evidence as high, moderate, low, or very low based on the risk of bias, inconsistency, indirectness, imprecision, and publication bias (Atkins 2004; Higgins 2011). If meta‐analysis was not possible, we planned to present the results in a narrative ‘Summary of findings' table format. Please see Table 1 for an example ‘Summary of findings' table.
1. Example Summary of Findings table.
| Prehabilitation compared with standard care for peripheral arterial disease (PAD) | ||||||
|
Patient or population: adults with PAD undergoing lower limb surgery Settings: hospital Intervention: prehabilitation1 Comparison: standard care 2 | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with standard care | Risk with prehabilitation | |||||
| 30‐day mortality | Study population | RR [value] ([value] to [value]) | [value] ([value]) | [Delete as
appropriate] ⊕⊝⊝⊝ very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
||
| [value] per 1000 | [value] per 1000 ([value] to [value]) | |||||
|
Postoperative complications3 (follow‐up) |
The mean [outcome] ranged across control groups from [value][measure] | The mean [outcome] in the intervention groups was [value] [lower/higher] [(value to value lower/higher)] | [value] ([value]) | [Delete as
appropriate] ⊕⊝⊝⊝ very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
||
|
Readmission (within 30 days of surgery) |
Study population | RR [value] ([value] to [value]) | [value] ([value]) | [Delete as
appropriate] ⊕⊝⊝⊝ very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
||
| [value] per 1000 | [value] per 1000 ([value] to [value]) | |||||
| One‐year survival | Study population | RR [value] ([value] to [value]) | [value] ([value]) | [Delete as
appropriate] ⊕⊝⊝⊝ very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
||
| [value] per 1000 | [value] per 1000 ([value] to [value]) | |||||
|
Changes in fitness preintervention and postintervention (follow‐up) |
The mean [outcome] ranged across control groups from [value][measure] | The mean [outcome] in the intervention groups was [value] [lower/higher] [(value to value lower/higher)] | [value] ([value]) | [Delete as
appropriate] ⊕⊝⊝⊝ very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
||
|
Changes in psychological health presurgery and postsurgery (follow‐up) |
The mean [outcome] ranged across control groups from [value][measure] | The mean [outcome] in the intervention groups was [value] [lower/higher] [(value to value lower/higher)] | [value] ([value]) | [Delete as
appropriate] ⊕⊝⊝⊝ very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
||
|
Changes in health related QoL scores presurgery and postsurgery (follow‐up) |
The mean [outcome] ranged across control groups from [value][measure] | The mean [outcome] in the intervention groups was [value] [lower/higher] [(value to value lower/higher)] | [value] ([value]) | [Delete as
appropriate] ⊕⊝⊝⊝ very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; QoL: quality of life; PAD: peripheral arterial disease; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Prehabilitation is a multimodal conditioning intervention consisting of three components, including exercise, psychological, and nutritional care prior to having a surgical procedure. 2Standard care is a preoperative assessment and relevant preparation prior to surgery, which may include blood and urine tests, and information given on what will happen before, during, and after the operation. 3Postoperative complications will include non‐fatal cardiovascular events (myocardial infarction, heart failure, or stroke), pulmonary complications (hypoxia, atelectasis, pneumonia, respiratory failure), haemorrhage, and surgical site infection.
Results
Description of studies
Results of the search
The PRISMA flow chart in Figure 1 shows the number of studies assessed. The search strategy identified 14,597 references. After removal of duplicates, we screened 10,788 records by title and abstract. We obtained the full text for two references for further investigation. We excluded two studies (Gohil 2013; NCT02767895), and noted the reason in the Characteristics of excluded studies table.
1.
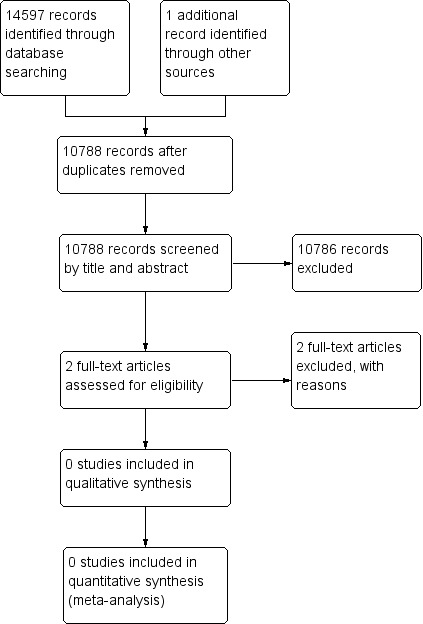
Study flow diagram.
Included studies
We identified no eligible studies for inclusion.
Excluded studies
Of the two full texts examined (Gohil 2013; NCT02767895), we excluded one study as it did not address the review question and therefore did not meet the inclusion criteria. We excluded the other study because we were unable to ascertain any details of revascularisation despite attempts to contact the principal investigator.
Risk of bias in included studies
We did not include any eligible studies in this review.
Effects of interventions
Unfortunately, we could not include any eligible studies in this review. Therefore, we were unable to assess the effects of interventions on outcomes.
Discussion
Summary of main results
We found no randomised controlled trials that evaluated the effects of prehabilitation including preoperative exercise, either alone or in combination with nutritional or psychological interventions or both, on postoperative outcomes in adults with PAD undergoing open lower limb surgery versus usual care.
Overall completeness and applicability of evidence
We identified no completed randomised controlled trials (RCTs) or cohort studies that compared the effects of prehabilitation versus usual care on postoperative outcomes in patients with PAD undergoing lower limb surgery. We did identify one study registered on the ClinicalTrials.gov registry: a pilot trial to ascertain the feasibility and acceptability of recruiting people to a healthy behaviour program, including increasing physical activity, reducing stress and promoting healthy behaviours for four weeks prior to surgery (NCT02767895). The primary outcomes were the feasibility and acceptability of participation including screening, and recruitment to the trial. Secondary outcome measures included whether there was an increase in physical activity and quality of life prior to surgery. The key inclusion criteria for participants were documented lower extremity PAD, being sedentary, and able to walk at least a block. Participants with any signs of CLI were excluded. This study recruited seven patients in just over a year and was terminated without any results due to lack of recruitment and resources. Despite attempts to contact the principal investigator, we could not ascertain any further details.
There are a number of potential reasons why recruitment to this trial was challenging. Firstly, the trial excluded all participants with any signs of CLI, therefore the pool of available patients was vastly narrowed. Typically, in the UK, revascularisation surgery is only undertaken in people with IC if symptoms are so severe that it is limiting activities of daily living; therefore the majority of patients will be presenting with CLI (NICE 2012).The study investigators possibly chose to exclude patients with CLI because the exercise component was geared towards a walking intervention and patients with CLI often find walking difficult due to wounds, pain and swelling. Therefore, future trials need to consider and include upper body exercise to enable patients with CLI to take part, thus widening the eligible patient population.
Secondly, the programme offered to participants was self‐directed with no support other than through the information contained on the study's website. It has been demonstrated that unsupervised exercise programmes result in lower physical activity engagement, and a resultant decrease in muscular endurance and cardiovascular health, compared to supervised programmes (Fennell 2016). Furthermore, in a recent qualitative review of people's experience of living with PAD, the authors found that people lacked an understanding of how to effect important behaviour change (e.g. walking exercise) to reduce their risk of PAD disease progression (Abaraogu 2018) and fewer than half the people with IC were able to discuss the benefits of regular exercise (Harwood 2017). This finding supports the assumption that prehabilitation may not be an intervention that people with PAD would consider they could manage without support. Therefore, unsupervised programmes may be futile. Programmes that offer participants support, education about the benefits of exercise, and supervision are required.
Undertaking a randomised controlled trial (RCT) to assess the benefits of prehabilitation in the PAD population presents challenges due to the natural history of the condition. In the UK, surgery is not routinely performed on people with IC unless symptoms are severely limiting lifestyle and angioplasty has failed or is unsuitable (NICE 2012). For those who have progressed to CLI, greater than 50% of these people present as medical emergencies requiring surgical treatment to save either life or limb in a time‐critical period. Therefore, this raises several key challenges for undertaking prehabilitation research trials. Firstly, it may be difficult to identify suitable patients, in a timely way, for prehabilitation to be undertaken. People are, in the first instance, scheduled for an angioplasty and if this is unsuccessful, they often proceed to a bypass procedure during the same admission. This means that people would need to be approached and recruited to prehabilitation even though they may only undergo an angioplasty. Secondly, in order to achieve adequate power, the trial will need to be multi‐centred and will require considerable resources with full engagement of other major vascular centres. Thirdly, the exercise intervention needs further development as lower body exercise may not be feasible in all patients with PAD. Arm ergometry has been shown to improve the walking capacity of patients with IC through a combination of systemic and local skeletal adaptations (Treat‐Jacobson 2009). However, programmes containing arm ergometry need further exploration to determine: the frequency, intensity, and duration of exercise to establish what people with PAD can manage; if patients adhere to the exercise regime; and if vascular centres typically have the equipment available for this type of training.
In light of the above issues raised, we recommend that future trials should begin with a feasibility trial or pilot RCT. This would enable refinement of both the exercise intervention and the recruitment pathway. It would also facilitate an understanding of the feasibility of vascular centres being able to deliver as well as engage with prehabilitation interventions. This information should help to pave the way for future trials studying the effectiveness of presurgery conditioning interventions in the PAD population.
Quality of the evidence
We are unable to assess the certainty of the evidence in the absence of eligible studies included in this review.
Potential biases in the review process
The Cochrane Information Specialist conducted a comprehensive search of the literature, and two review authors reviewed all of the references independently. We resolved any disagreement by discussion limiting any bias.
Agreements and disagreements with other studies or reviews
We found no other reviews or non‐randomised studies of presurgery conditioning programmes using exercise alone or in combination with nutrition and/or psychological interventions in PAD patients having lower limb surgery.
Authors' conclusions
Implications for practice.
We identified no randomised controlled trials (RCTs) that sought to determine the effects of presurgery conditioning programmes on mortality and other postoperative outcomes when compared to usual care for people with peripheral arterial disease (PAD). Despite the purported benefit in other clinical populations, we cannot currently recommend a specific prehabilitation programme for people with PAD undergoing a revascularisation or amputation procedure.
Implications for research.
There are no RCTs from which we can draw conclusions about this review question. An attempt has been made but was terminated due to a difficulty with recruitment and resources. Therefore we recommend recruiting people with intermittent claudication with lifestyle limiting symptoms, for whom a supervised exercise programme did not work, and people with critical limb ischaemia (CLI). Although there will be some challenges to executing a complex intervention and identifying patients within a suitable timeframe, RCTs are needed to determine if preconditioning patients prior to surgery improves patient outcomes. In light of the above issues raised, we recommend that future trials should begin with a feasibility trial or pilot RCT. This would enable the refinement of both the exercise intervention and the recruitment pathway. 'Conventional' exercise prehabilitation programmes based on walking, running or cycling may not be possible for people with CLI and therefore interventions, such as arm ergometry, need to be personalised and tailored to the individual patient's limitations. This approach will also facilitate an understanding of the feasibility of vascular centres being able to deliver as well as engage with prehabilitation interventions. It will also ensure that future trials considering the effectiveness and economic impact of prehabilitation in people with PAD can be achieved.
History
Protocol first published: Issue 9, 2019 Review first published: Issue 9, 2020
Notes
We have based parts of the Methods section of this protocol on a standard template established by Cochrane Vascular.
Acknowledgements
We gratefully thank Dr Marlene Stewart, Dr Cathryn Broderick, Ms Candida Fenton and the editorial board of Cochrane Vascular for their assistance with the preparation of this review.
The review authors and the Cochrane Vascular editorial base are grateful to the following peer reviewers for their time and comments: Mr Robert Davies, Department of Vascular Surgery, Leicester Royal Infirmary, UK; A/Prof Kerry Hitos, Westmead Clinical School, University of Sydney, Australia; Dr Simon J Howell, St James's University Hospital, Leeds, UK; Kristin Osika, USA. We are also grateful to the consumer reviewer who opted to remain anonymous.
Appendices
Appendix 1. Database searches 25 September 2019
| Source | Search strategy | Hits retrieved |
| VASCULAR REGISTER IN CRS‐Web | #1 peripheral arterial disease OR Peripheral Vascular Diseases OR PVD OR Atherosclerosis OR Arteriosclerosis OR Arteriosclerosis Obliterans OR Arterial Occlusive Diseases AND INREGISTER #2 prehabilitation OR prehab or exercise OR training OR gym OR physical activity AND INREGISTER #3 #1 AND #2 |
916 |
| CENTRAL | #1 MESH DESCRIPTOR Arteriosclerosis 953 #2 MESH DESCRIPTOR Arteriolosclerosis 0 #3 MESH DESCRIPTOR Arteriosclerosis Obliterans 83 #4 MESH DESCRIPTOR Atherosclerosis 1123 #5 MESH DESCRIPTOR Arterial Occlusive Diseases 836 #6 MESH DESCRIPTOR Intermittent Claudication 849 #7 MESH DESCRIPTOR Ischemia 1658 #8 MESH DESCRIPTOR Peripheral Vascular Diseases EXPLODE ALL TREES 2879 #9 atherosclero* or arteriosclero* or PVD or PAOD or PAD 28755 #10 (((arter* or vascular or vein* or veno* or peripher*) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*))):TI,AB,KY 13066 #11 (peripheral adj3 dis*):TI,AB,KY 6047 #12 (claudic* or IC):TI,AB,KY 5036 #13 (isch* or CLI):TI,AB,KY 40070 #14 arteriopathic:TI,AB,KY 7 #15 dysvascular*:TI,AB,KY 25 #16 (leg adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)):TI,AB,KY 151 #17 (limb adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)):TI,AB,KY 289 #18 (lower adj3 extrem* adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)):TI,AB,KY 133 #19 ((iliac or femoral or popliteal or femoro* or fempop* or crural) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)):TI,AB,KY 1903 #20 MESH DESCRIPTOR Leg 2832 #21 MESH DESCRIPTOR Iliac Artery 160 #22 MESH DESCRIPTOR Popliteal Artery 316 #23 MESH DESCRIPTOR Femoral Artery 934 #24 MESH DESCRIPTOR Tibial Arteries 40 #25 ((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)):TI,AB,KY 2183 #26 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 83002 #27 MESH DESCRIPTOR Exercise EXPLODE ALL TREES 22042 #28 MESH DESCRIPTOR Exercise Therapy EXPLODE ALL TREES 12031 #29 (Active living):TI,AB,KY 94 #30 (Active travel):TI,AB,KY 9 #31 (Circuit training):TI,AB,KY 219 #32 (Cross train*):TI,AB,KY 57 #33 Cross‐train*:TI,AB,KY 57 #34 (martial art*):TI,AB,KY 172 #35 (Motor activity):TI,AB,KY 4817 #36 (Physical activit*):TI,AB,KY 23968 #37 tennis:TI,AB,KY 923 #38 (Water exercise*):TI,AB,KY 111 #39 (Weight train*):TI,AB,KY 411 #40 Ambulation:TI,AB,KY 2613 #41 Badminton:TI,AB,KY 51 #42 Cycling:TI,AB,KY 4491 #43 Cyclist*:TI,AB,KY 1026 #44 Dance:TI,AB,KY 616 #45 Dancer:TI,AB,KY 29 #46 Dancing:TI,AB,KY 389 #47 Exercis*:TI,AB,KY 85039 #48 Golf*:TI,AB,KY 142 #49 Gymnastic*:TI,AB,KY 276 #50 gym:TI,AB,KY 395 #51 Jogging:TI,AB,KY 369 #52 Judo:TI,AB,KY 55 #53 Karate:TI,AB,KY 37 #54 Rowing:TI,AB,KY 244 #55 Run:TI,AB,KY 11653 #56 Running:TI,AB,KY 5102 #57 Swim*:TI,AB,KY 1090 #58 Treadmill:TI,AB,KY 7068 #59 Walk*:TI,AB,KY 24409 #60 Weight‐training:TI,AB,KY 382 #61 #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 127776 #62 before:TI,AB,KY 187740 #63 prior:TI,AB,KY 76957 #64 preoperative:TI,AB,KY 28758 #65 pre‐op:TI,AB,KY 404 #66 pre‐surgical:TI,AB,KY 352 #67 #62 OR #63 OR #64 OR #65 OR #66 270446 #68 #61 AND #67 30304 #69 prehabilit*:TI,AB,KY 194 #70 prehab:TI,AB,KY 37 #71 #68 OR #69 OR #70 30369 #72 #26 AND #71 2250 |
2250 |
| Clinicaltrials.gov | peripheral arterial disease OR Peripheral Vascular Diseases OR PVD OR Atherosclerosis OR Arteriosclerosis OR Arteriosclerosis Obliterans OR Arterial Occlusive Diseases | prehabilitation OR prehab or exercise OR training OR gym OR physical activity | 560 |
| ICTRP Search Portal | peripheral arterial disease OR Peripheral Vascular Diseases OR PVD OR Atherosclerosis OR Arteriosclerosis OR Arteriosclerosis Obliterans OR Arterial Occlusive Diseases | prehabilitation OR prehab or exercise OR training OR gym OR physical activity | 178 |
| Medline (Ovid MEDLINE® Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE®) 1946 to present | 1 Arteriosclerosis/ 2 Arteriolosclerosis/ 3 Arteriosclerosis Obliterans/ 4 Atherosclerosis/ 5 Arterial Occlusive Diseases/ 6 Intermittent Claudication/ 7 Ischemia/ 8 exp Peripheral Vascular Diseases/ 9 (atherosclero* or arteriosclero* or PVD or PAOD or PAD).ti,ab. 10 ((arter* or vascular or vein* or veno* or peripher*) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 11 (peripheral adj3 dis*).ti,ab. 12 (claudic* or IC).ti,ab. 13 (isch* or CLI).ti,ab. 14 arteriopathic.ti,ab. 15 dysvascular*.ti,ab. 16 (leg adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 17 (limb adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 18 (lower adj3 extrem* adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 19 ((iliac or femoral or popliteal or femoro* or fempop* or crural) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 20 Leg/ 21 Iliac Artery/ 22 Popliteal Artery/ 23 Femoral Artery/ 24 Tibial Arteries/ 25 ((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 26 or/1‐25 27 exp Exercise/ 28 exp Exercise Therapy/ 29 Active living.ti,ab. 30 Active travel.ti,ab. 31 Circuit training.ti,ab. 32 Cross train*.ti,ab. 33 Cross‐train*.ti,ab. 34 martial art*.ti,ab. 35 Motor activity.ti,ab. 36 Physical activit*.ti,ab. 37 tennis.ti,ab. 38 Water exercise*.ti,ab. 39 Weight train*.ti,ab. 40 Ambulation.ti,ab. 41 Badminton.ti,ab. 42 Cycling.ti,ab. 43 Cyclist*.ti,ab. 44 Dance.ti,ab. 45 Dancer.ti,ab. 46 Dancing.ti,ab. 47 Exercis*.ti,ab. 48 Golf*.ti,ab. 49 Gymnastic*.ti,ab. 50 gym.ti,ab. 51 Jogging.ti,ab. 52 Judo.ti,ab. 53 Karate.ti,ab. 54 Rowing.ti,ab. 55 Run.ti,ab. 56 Running.ti,ab. 57 Swim*.ti,ab. 58 Treadmill.ti,ab. 59 Walk*.ti,ab. 60 Weight‐training.ti,ab. 61 or/27‐60 62 before.ti,ab. 63 prior.ti,ab. 64 preoperative.ti,ab. 65 pre‐op.ti,ab. 66 pre‐surgical.ti,ab. 67 or/62‐66 68 61 and 67 69 prehabilit*.ti,ab. 70 prehab.ti,ab. 71 68 or 69 or 70 72 26 and 71 73 randomized controlled trial.pt. 74 controlled clinical trial.pt. 75 randomized.ab. 76 placebo.ab. 77 drug therapy.fs. 78 randomly.ab. 79 trial.ab. 80 groups.ab. 81 or/73‐80 82 exp animals/ not humans.sh. 83 81 not 82 84 72 and 83 |
2787 |
| Embase 1974 to present | 1 arteriosclerosis/ 2 arteriolosclerosis/ 3 peripheral occlusive artery disease/ 4 atherosclerosis/ 5 intermittent claudication/ 6 ischemia/ 7 exp peripheral vascular disease/ 8 (atherosclero* or arteriosclero* or PVD or PAOD or PAD).ti,ab. 9 ((arter* or vascular or vein* or veno* or peripher*) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 10 (peripheral adj3 dis*).ti,ab. 11 (claudic* or IC).ti,ab. 12 (isch* or CLI).ti,ab. 13 arteriopathic.ti,ab. 14 dysvascular*.ti,ab. 15 (leg adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 16 (limb adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 17 (lower adj3 extrem* adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 18 ((iliac or femoral or popliteal or femoro* or fempop* or crural) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 19 leg/ 20 iliac artery/ 21 popliteal artery/ 22 femoral artery/ 23 tibial artery/ 24 ((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 25 or/1‐24 26 exp exercise/ 27 exp kinesiotherapy/ 28 Active living.ti,ab. 29 Active travel.ti,ab. 30 Circuit training.ti,ab. 31 Cross train*.ti,ab. 32 Cross‐train*.ti,ab. 33 martial art*.ti,ab. 34 Motor activity.ti,ab. 35 Physical activit*.ti,ab. 36 tennis.ti,ab. 37 Water exercise*.ti,ab. 38 Weight train*.ti,ab. 39 Ambulation.ti,ab. 40 Badminton.ti,ab. 41 Cycling.ti,ab. 42 Cyclist*.ti,ab. 43 Dance.ti,ab. 44 Dancer.ti,ab. 45 Dancing.ti,ab. 46 Exercis*.ti,ab. 47 Golf*.ti,ab. 48 Gymnastic*.ti,ab. 49 gym.ti,ab. 50 Jogging.ti,ab. 51 Judo.ti,ab. 52 Karate.ti,ab. 53 Rowing.ti,ab. 54 Run.ti,ab. 55 Running.ti,ab. 56 Swim*.ti,ab. 57 Treadmill.ti,ab. 58 Walk*.ti,ab. 59 Weight‐training.ti,ab. 60 or/26‐59 61 before.ti,ab. 62 prior.ti,ab. 63 preoperative.ti,ab. 64 pre‐op.ti,ab. 65 pre‐surgical.ti,ab. 66 or/61‐65 67 60 and 66 68 prehabilit*.ti,ab. 69 prehab.ti,ab. 70 67 or 68 or 69 71 25 and 70 72 randomized controlled trial/ 73 controlled clinical trial/ 74 random$.ti,ab. 75 randomization/ 76 intermethod comparison/ 77 placebo.ti,ab. 78 (compare or compared or comparison).ti. 79 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 80 (open adj label).ti,ab. 81 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 82 double blind procedure/ 83 parallel group$1.ti,ab. 84 (crossover or cross over).ti,ab. 85 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 86 (assigned or allocated).ti,ab. 87 (controlled adj7 (study or design or trial)).ti,ab. 88 (volunteer or volunteers).ti,ab. 89 trial.ti. 90 or/72‐89 91 71 and 90 |
6745 |
| CINAHL | S86 S71 AND S85 S85 S72 OR S73 OR S74 OR S75 OR S76 OR S77 OR S78 OR S79 OR S80 OR S81 OR S82 OR S83 OR S84 S84 MH "Random Assignment" S83 MH "Triple‐Blind Studies" S82 MH "Double‐Blind Studies" S81 MH "Single‐Blind Studies" S80 MH "Crossover Design" S79 MH "Factorial Design" S78 MH "Placebos" S77 MH "Clinical Trials" S76 TX crossover OR "cross‐over" S75 AB placebo* S74 TX random* S73 TX trial* S72 TX "latin square" S71 S24 AND S70 S70 S67 OR S68 OR S69 S69 TX prehab S68 TX prehabilit* S67 S60 AND S66 S66 S61 OR S62 OR S63 OR S64 OR S65 S65 TX pre‐surgical S64 TX pre‐op S63 TX preoperative S62 TX prior S61 TX before S60 S24 AND S59 S59 S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 OR S57 OR S58 S58 TX Weight‐training S57 TX Walk* S56 TX Treadmill S55 TX Swim* S54 TX Running S53 TX Run S52 TX Rowing S51 TX Karate S50 TX Judo S49 TX Jogging S48 TX gym S47 TX Gymnastic* S46 TX Golf* S45 TX Exercis* S44 TX Dancing S43 TX Dancer S42 TX Dance S41 TX Cyclist* S40 TX Cycling S39 TX Badminton S38 TX Ambulation S37 TX Weight train* S36 TX Water exercise* S35 TX tennis S34 TX Physical activit* S33 TX Motor activity S32 TX martial art* S31 TX Cross‐train* S30 TX Cross train* S29 TX Circuit training S28 TX Active travel S27 TX Active living S26 (MH "Therapeutic Exercise+") S25 (MH "Exercise+") S24 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 S23 TX ((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) n3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)) S22 (MH "Tibial Arteries") S21 (MH "Femoral Artery") S20 (MH "Popliteal Artery") S19 (MH "Iliac Artery") S18 (MH "Leg") S17 TX ((iliac or femoral or popliteal or femoro* or fempop* or crural) n3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)) S16 TX (lower n3 extrem* n3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)) S15 TX (limb n3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)) S14 TX (leg n3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)) S13 dysvascular* S12 TX arteriopathic S11 TX isch* or CLI S10 TX claudic* or IC S9 TX peripheral n3 dis* S8 TX ((arter* or vascular or vein* or veno* or peripher*) n3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)) S7 TX atherosclero* or arteriosclero* or PVD or PAOD or PAD S6 (MH "Peripheral Vascular Diseases+") S5 (MH "Ischemia") S4 (MH "Intermittent Claudication") S3 (MH "Arterial Occlusive Diseases") S2 (MH "Atherosclerosis") S1 (MH "Arteriosclerosis") |
1161 |
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Gohil 2013 | Not a randomised controlled trial and participants did not meet the inclusion of exercise for preparation prior to surgery. |
| NCT02767895 | We were unable to ascertain any details of revascularisation despite attempts to contact the principal investigator therefore did not meet inclusion criteria of being open surgery. |
Contributions of authors
JP: conceived the review question, developed the protocol, co‐ordinated protocol development, completed the first draft of the protocol, made an intellectual contribution, edited the protocol, identified included and excluded studies, completed first draft of the review, guarantor of the review, approved the final version prior to submission. SP: developed the protocol, made an intellectual contribution, edited the protocol, identified included and excluded studies, edited the review, approved the final version prior to submission. GS: conceived the review question, developed the protocol, made an intellectual contribution, edited the protocol, edited the review, approved the final version prior to submission. AH: developed the protocol, made an intellectual contribution, edited the protocol, edited the review, approved the final version prior to submission. LI: developed the protocol, made an intellectual contribution, edited the protocol, edited the review, approved the final version prior to submission. CH: made an intellectual contribution, edited the review, approved the final version prior to submission. IC: conceived the review question, developed the protocol, made an intellectual contribution, edited the protocol, edited the review, approved the final version prior to submission.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
JP: none known. SP: none known. GS: has received speaker's fees from BSN Medical and his academic department has, in the past, received unconditional funding from Diomed/Angiodynamics. This was used to help fund a research nurse to assist with objective assessments in the context of RCTs. AH: none known. LI: none known. CH: none known. IC: none known.
New
References
References to studies excluded from this review
Gohil 2013 {published data only}
- Gohil R. Short term physiological changes secondary to exercise in intermittent claudication [MD thesis]. Hull (UK): Hull York Medical School, 2013. [Google Scholar]
NCT02767895 {published data only}
- NCT02767895. Prehabilitation for PAD revascularization patients [A pilot trial of prehabilitation among patients undergoing PAD revascularization]. clinicaltrials.gov/ct2/show/NCT02767895 (first received 10 May 2016).
Additional references
Abaraogu 2018
- Abaraogu UO, Ezenwankwo EF, Dall PM, Seenan CA. Living a burdensome and demanding life: a qualitative systematic review of patients experiences of peripheral arterial disease. PLOS ONE 2018;13(11):e0207456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ambler 2015
- Ambler GK, Brooks DE, Al Zuhir N, Ali A, Gohel MS, Hayes PD, et al. Effect of frailty on short- and mid-term outcomes in vascular surgical patients. British Journal of Surgery 2015;102(6):638-45. [DOI] [PubMed] [Google Scholar]
Antoniou 2017
- Antoniou GA, Georgiadis GS, Antoniou SA, Makar RR, Smout JD, Torella F. Bypass surgery for chronic lower limb ischaemia. Cochrane Database of Systematic Reviews 2017, Issue 4. Art. No: CD002000. [DOI: 10.1002/14651858.CD002000.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aquarius 2006
- Aquarius AE, De Vries J, Henegouwen DP, Hamming JF. Clinical indicators and psychosocial aspects in peripheral arterial disease. Archives of Surgery 2006;141(2):161-6. [DOI] [PubMed] [Google Scholar]
Arthur 2000
- Arthur HM, Daniels C, McKelvie R, Hirsh J, Rush B. Effect of a preoperative intervention on preoperative and postoperative outcomes in low-risk patients awaiting elective coronary artery bypass graft surgery. A randomized, controlled trial. Annals of Internal Medicine 2000;133(4):253-62. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barakat 2015
- Barakat HM, Shahin Y, McCollum PT, Chetter IC. Prediction of organ-specific complications following abdominal aortic aneurysm repair using cardiopulmonary exercise testing. Anaesthesia 2015;70(6):679-85. [DOI] [PubMed] [Google Scholar]
Bilku 2014
- Bilku DK, Dennison AR, Hall TC, Metcalfe MS, Garcea G. Role of preoperative carbohydrate loading: a systematic review. Annals of the Royal College of Surgeons of England 2014;96(1):15-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolshinsky 2018
- Bolshinsky V, Li MH, Ismail H, Burbury K, Riedel B, Heriot A. Multimodal prehabilitation programs as a bundle of care in gastrointestinal cancer surgery: a systematic review. Diseases of the Colon and Rectum 2018;61(1):124-38. [DOI] [PubMed] [Google Scholar]
Braga 2009
- Braga M, Ljungqvist O, Soeters P, Fearon K, Weimann A, Bozzetti F, et al. ESPEN Guidelines on Parenteral Nutrition: surgery. Clinical Nutrition (Edinburgh, Scotland) 2009;28(4):378-86. [DOI] [PubMed] [Google Scholar]
Bruns 2016
- Bruns ERJ, den Heuvel B, Buskens CJ, Duijvendijk P, Festen S, Wassenaar EB, et al. The effects of physical prehabilitation in elderly patients undergoing colorectal surgery: a systematic review. Colorectal Disease 2016;18(8):267-77. [DOI] [PubMed] [Google Scholar]
Carli 2005
- Carli F, Zavorsky GS. Optimizing functional exercise capacity in the elderly surgical population. Current Opinion in Clinical Nutrition and Metabolic Care 2005;8(1):23-32. [DOI] [PubMed] [Google Scholar]
Carli 2010
- Carli F, Charlebois P, Stein B, Feldman L, Zavorsky G, Kim DJ, et al. Randomized clinical trial of prehabilitation in colorectal surgery. British Journal of Surgery 2010;97(8):1187-97. [DOI] [PubMed] [Google Scholar]
Carli 2015
- Carli F, Scheede-Bergdahl C. Prehabilitation to enhance perioperative care. Anesthesiology Clinics 2015;33(1):17-33. [DOI] [PubMed] [Google Scholar]
Cohen 2011
- Cohen L, Parker PA, Vence L, Savary C, Kentor D, Pettaway C, et al. Presurgical stress management improves postoperative immune function in men with prostate cancer undergoing radical prostatectomy. Psychosomatic Medicine 2011;73(3):218-25. [DOI] [PubMed] [Google Scholar]
Cook 2001
- Cook JW, Pierson LM, Herbert WG, Norton HJ, Fedor JM, Kiebzak GM, et al. The influence of patient strength, aerobic capacity and body composition upon outcomes after coronary artery bypass grafting. Thoracic and Cardiovascular Surgeon 2001;49(2):89-93. [DOI] [PubMed] [Google Scholar]
Doenst 2005
- Doenst T, Wijeysundera D, Karkouti K, Zechner C, Maganti M, Rao V, et al. Hyperglycemia during cardiopulmonary bypass is an independent risk factor for mortality in patients undergoing cardiac surgery. Journal of Thoracic and Cardiovascular Surgery 2005;130(4):1144. [DOI] [PubMed] [Google Scholar]
Dronkers 2010
- Dronkers JJ, Lamberts H, Reutelingsperger IMMD, Naber RH, Dronkers-Landman CM, Veldman A, et al. Preoperative therapeutic programme for elderly patients scheduled for elective abdominal oncological surgery: a randomized controlled pilot study. Clinical Rehabilitation 2010;24(7):614-22. [DOI] [PubMed] [Google Scholar]
Farber 2016
- Farber A, Eberhardt RT. The current state of critical limb ischemia: a systematic review. JAMA Surgery 2016;151(11):1070-7. [DOI] [PubMed] [Google Scholar]
Fennell 2016
- Fennell C, Peroutky P, Glickman EL. Effects of supervised training compared to unsupervised training on physical activity, muscular endurance and cardiovascular parameters. MOJ Orthopaedics & Rheumatology 2016;5(4):00184. [Google Scholar]
Fortington 2013
- Fortington LV, Geertzen JHB, Netten JJ, Postema K, Rommers GM, Dijkstra PU. Short and long term mortality rates after a lower limb amputation. European Journal of Vascular and Endovascular Surgery 2013;46(1):124-31. [DOI] [PubMed] [Google Scholar]
Fowkes 2013
- Fowkes FGR, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 2013;382(9901):1329-40. [DOI] [PubMed] [Google Scholar]
Gillis 2014
- Gillis C, Li C, Lee L, Awasthi R, Augustin B, Gamsa A, et al. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology 2014;121(5):937-47. [DOI] [PubMed] [Google Scholar]
Gillis 2015
- Gillis C, Carli F. Promoting perioperative metabolic and nutritional care. Anesthesiology 2015;123(6):1455-72. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 28 June 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Gupta 2016
- Gupta R, Gan TJ. Preoperative nutrition and prehabilitation. Anesthesiology Clinics 2016;34(1):143-53. [DOI] [PubMed] [Google Scholar]
Harwood 2017
- Harwood AE, Broadbent E, Totty JP, Smith GE, Chetter IC. “Intermittent claudication a real pain in the calf”—Patient experience of diagnosis and treatment with a supervised exercise program. Journal of Vascular Nursing 2017;35(3):131-5. [DOI] [PubMed] [Google Scholar]
Hasanadka 2011
- Hasanadka R, McLafferty RB, Moore CJ, Hood DB, Ramsey DE, Hodgson KJ. Predictors of wound complications following major amputation for critical limb ischemia. Journal of Vascular Surgery 2011;54(5):1374-82. [DOI] [PubMed] [Google Scholar]
Hiatt 2001
- Hiatt WR. Medical treatment of peripheral arterial disease and claudication. New England Journal of Medicine 2001;344(21):1608-21. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hijazi 2017
- Hijazi Y, Gondal U, Aziz O. A systematic review of prehabilitation programs in abdominal cancer surgery. International Journal of Surgery 2017;39:156-62. [DOI] [PubMed] [Google Scholar]
Hulzebos 2006
- Hulzebos EHJ, Helders PJM, Favié NJ, De Bie RA, Brutel de la Riviere A, Van Meeteren NLU. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery: a randomized clinical trial. JAMA 2006;296(15):1851-7. [DOI] [PubMed] [Google Scholar]
Kazaure 2016
- Kazaure HS, Chandra V, Mell MW. Unplanned reoperations after vascular surgery. Journal of Vascular Surgery 2016;63(3):730-6. [DOI] [PubMed] [Google Scholar]
Kehlet 2016
- Kehlet M, Jensen LP, Schroeder TV. Risk factors for complications after peripheral vascular surgery in 3,202 patient procedures. Annals of Vascular Surgery 2016;36:13-21. [DOI] [PubMed] [Google Scholar]
Lane 2017
- Lane R, Harwood A, Watson L, Leng GC. Exercise for intermittent claudication. Cochrane Database of Systematic Reviews 2017, Issue 12. Art. No: CD000990. [DOI: 10.1002/14651858.CD000990.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Le Roy 2016
- Le Roy B, Selvy M, Slim K. The concept of prehabilitation: what the surgeon needs to know? Journal of Visceral Surgery 2016;153(2):109-12. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C , Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lemanu 2013
- Lemanu DP, Singh PP, MacCormick AD, Arroll B, Hill AG. Effect of preoperative exercise on cardiorespiratory function and recovery after surgery: a systematic review. World Journal of Surgery 2013;37(4):711-20. [DOI] [PubMed] [Google Scholar]
Levett 2016
- Levett DZH, Edwards M, Grocott M, Mythen M. Preparing the patient for surgery to improve outcomes. Best Practice and Research. Clinical Anaesthesiology 2016;30(2):145-57. [DOI] [PubMed] [Google Scholar]
Li 2013
- Li C, Carli F, Lee L, Charlebois P, Stein B, Liberman AS, et al. Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surgical Endoscopy 2013;27(4):1072-82. [DOI] [PubMed] [Google Scholar]
Luo 2016
- Luo H, Yang H, Huang B, Yuan D, Zhu J, Zhao J. Geriatric nutritional risk index (GNRI) independently predicts amputation In chronic critical limb ischemia (CLI). PLoS One 2016;11(3):e0152111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Minnella 2017
- Minnella EM, Bousquet-Dion G, Awasthi R, Scheede-Bergdahl C, Carli F. Multimodal prehabilitation improves functional capacity before and after colorectal surgery for cancer: a five-year research experience. Acta Oncologica (Stockholm, Sweden) 2017;56(2):295-300. [DOI] [PubMed] [Google Scholar]
Munafo 2001
- Munafò MR, Stevenson J. Anxiety and surgical recovery. Reinterpreting the literature. Journal of Psychosomatic Research 2001;51(4):589-96. [DOI] [PubMed] [Google Scholar]
Nehler 2014
- Nehler MR, Duval S, Diao L, Annex BH, Hiatt WR, Rogers K, et al. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. Journal of Vascular Surgery 2014;60(3):686-695.e2. [DOI] [PubMed] [Google Scholar]
NICE 2012
- National Clinical Guideline Centre. Lower limb peripheral arterial disease: Diagnosis and management 147. www.nice.org.uk/guidance/cg147/resources/peripheral-arterial-disease-diagnosis-and-management-pdf-35109575873989 (accessed 26/05/2020) 2012:1-299.
Norgren 2007
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR. Inter-society consensus for the management of peripheral arterial disease (TASC II). Journal of Vascular Surgery 2007;45(1):S5-S67. [DOI] [PubMed] [Google Scholar]
Owens 2012
- Owens CD, Kim JM, Hevelone ND, Gasper WJ, Belkin M, Creager MA, et al. An integrated biochemical prediction model of all-cause mortality in patients undergoing lower extremity bypass surgery for advanced peripheral artery disease. Journal of Vascular Surgery 2012;56(3):686-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [DOI] [PubMed] [Google Scholar]
Pescatello 2014
- Pescatello LS, American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins Health, 2014. [Google Scholar]
Pierson 2001
- Pierson LM, Herbert WG, Norton HJ, Kiebzak GM, Griffith P, Fedor JM, et al. Effects of combined aerobic and resistance training versus aerobic training alone in cardiac rehabilitation. Journal of Cardiopulmonary Rehabilitation 2001;21(2):101-10. [DOI] [PubMed] [Google Scholar]
Playforth 1987
- Playforth MJ, Smith GM, Evans M, Pollock AV. Pre-operative assessment of fitness score. British Journal of Surgery 1987;74(10):890-2. [DOI] [PubMed] [Google Scholar]
Powell 2016
- Powell R, Scott NW, Manyande A, Bruce J, Vögele C, Byrne-Davis LMT, et al. Psychological preparation and postoperative outcomes for adults undergoing surgery under general anaesthesia. Cochrane Database of Systematic Reviews 2016, Issue 5. Art. No: CD008646. [DOI: 10.1002/14651858.CD008646.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Santa Mina 2014
- Santa Mina D, Clarke H, Ritvo P, Leung YW, Matthew AG, Katz J, et al. Effect of total-body prehabilitation on postoperative outcomes: a systematic review and meta-analysis. Physiotherapy 2014;100(3):196-207. [DOI] [PubMed] [Google Scholar]
Sato 2010
- Sato H, Carvalho G, Sato T, Lattermann R, Matsukawa T, Schricker T. The association of preoperative glycemic control, intraoperative insulin sensitivity, and outcomes after cardiac surgery. Journal of Clinical Endocrinology and Metabolism 2010;95(9):4338-44. [DOI] [PubMed] [Google Scholar]
Selvin 2004
- Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation 2004;110(6):738-43. [DOI] [PubMed] [Google Scholar]
Sigvant 2016
- Sigvant B, Lundin F, Wahlberg E. The risk of disease progression in peripheral arterial disease is higher than expected: a meta-analysis of mortality and disease progression in peripheral arterial disease. European Journal of Vascular and Endovascular Surgery 2016;51(3):395-403. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Treat‐Jacobson 2009
- Treat-Jacobson D, Bronas UG, Leon AS. Efficacy of arm-ergometry versus treadmill exercise training to improve walking distance in patients with claudication. Vascular Medicine 2009;14:203-13. [DOI] [PubMed] [Google Scholar]
Valkenet 2011
- Valkenet K, de Port IGL, Dronkers JJ, Vries WR, Lindeman E, Backx FJG. The effects of preoperative exercise therapy on postoperative outcome: a systematic review. Clinical Rehabilitation 2011;25(2):99-111. [DOI] [PubMed] [Google Scholar]
Walburn 2009
- Walburn J, Vedhara K, Hankins M, Rixon L, Weinman J. Psychological stress and wound healing in humans: a systematic review and meta-analysis. Journal of Psychosomatic Research 2009;67(3):253-71. [DOI] [PubMed] [Google Scholar]
Waton 2017
- Waton S, Johal A, Heikkila K, Cromwell D, Loftus I, Boyle J. National Vascular Registry: 2017 Annual report. www.vsqip.org.uk/content/uploads/2018/05/2017-NVR-Annual-Report.pdf (accessed 9 July 2019).
Yalçin 2006
- Yalçin AS. Emerging therapeutic potential of whey proteins and peptides. Current Pharmaceutical Design 2006;12(13):1637-43. [DOI] [PubMed] [Google Scholar]
Zhang 2014
- Zhang JQ, Curran T, McCallum JC, Wang L, Wyers MC, Hamdan AD, et al. Risk factors for readmission after lower extremity bypass in the American College of Surgeons National Surgery Quality Improvement Program. Journal of Vascular Surgery 2014;59(5):1331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Palmer 2019
- Palmer J, Pymer S, Smith GE, Harwood AE, Ingle L, Huang C, Chetter IC. Presurgery conditioning interventions (prehabilitation) in adults undergoing lower limb surgery for peripheral arterial disease. Cochrane Database of Systematic Reviews 2019, Issue 9. Art. No: CD013407. [DOI: 10.1002/14651858.CD013407] [DOI] [PMC free article] [PubMed] [Google Scholar]


