ABSTRACT
The burden of chronic liver disease (CLD) in India is high, particularly among middle-aged men, with nearly 220,000 deaths due to cirrhosis in 2017. CLD increases the risk of infection, severe disease (e.g. hepatitis A virus or HAV superinfection, acute-on-chronic liver failure, fulminant hepatic failure), and mortality. Hence, various countries recommend HAV vaccination for CLD patients. While historic Indian studies showed high seroprevalences of protective HAV antibodies among Indian adults with CLD, the most recent ones found that nearly 7% of CLD patients were susceptible to HAV infection. Studies in healthy individuals have shown that HAV infection in childhood is decreasing in India, resulting in an increasing population of adults susceptible to HAV infection. As patients with CLD are at increased risk of severe HAV infection, now may be the time to recommend HAV vaccination among people with CLD in India.
KEYWORDS: Hepatitis A, chronic liver disease, cirrhosis, India, vaccination, endemicity
Chronic liver disease (CLD)
Hepatitis A, B, C, D, and E virus (HAV, HBV, HCV, HDV, and HEV) infections and alcohol consumption can cause liver damage, as can obesity, which can result in nonalcoholic fatty liver disease (NAFLD). CLD (disease that has lasted for ≥6 months) is a progressive deterioration in liver function, which can lead to fibrosis and cirrhosis.1 Cirrhosis often starts asymptomatically (“compensated cirrhosis”), but can ultimately progress to “decompensated cirrhosis”, during which complications of liver dysfunction and portal hypertension manifest (e.g. ascites, jaundice, variceal bleeding).2 Once a patient has decompensated cirrhosis, their survival will likely only be around 3–5 years.3 In 2017, there were an estimated 1.5 billion cases of cirrhosis and other CLDs globally.4 Liver cirrhosis was the 11th and 26th leading cause of disability-adjusted life years in men and women, respectively;5 the 13th leading cause of life years lost;6 and, along with other CLDs, resulted in over 1.3 million deaths in 2017.6
Early treatment of patients with CLD is important. The goals of treatment are to stop disease progression (generally by managing the underlying cause, e.g. antivirals, alcohol abstinence) and to manage complications (e.g. portal hypertension, hepatorenal syndrome, bleeding esophageal varices, hepatic encephalopathy, and hepatocellular carcinoma).1 Ultimately, patients may require a liver transplant, which is the second commonest major organ transplantation.7
Increased risk of severe infection
Patients with cirrhosis have a compromised immune system and are known to be at increased risk of bacterial infection,8–12 and those who become infected have a nearly 4-fold higher risk of death compared with uninfected people with cirrhosis.2 Given the effect of cirrhosis on the immune response, such patients may also be at increased risk of HAV infection. Although we could not find any confirmation of this, patients with CLD certainly appear to be at increased risk of developing more severe HAV disease if they have superimposed HAV disease.13–15 For example, in an outbreak of >300,000 HAV cases in China, mortality was 5.6-fold higher among those with HAV infection superimposed on underlying HBV infection than in those with HAV but without HBV.15 Acute HAV infection in patients with CLD can also result in acute-on-chronic liver failure (ACLF), which is associated with high rates of mortality.16
Patients with CLD are also at increased risk of developing fulminant hepatitis,13,17 also known as fulminant hepatic failure (FHF). In an Italian study, 595 adults (29.1 ± 9.8 years) with chronic HBV or HCV (without HAV antibodies) were enrolled during 1990–1997.18 Of these, 27 (4.5%) acquired HAV superinfection (10/163 of those with chronic HBV and 17/432 of those with chronic HCV). FHF developed in 0/10 chronic HBV patients and 7/17 chronic HCV patients, 6/7 of whom died. None of 191 controls (without CLD) who presented with acute HAV developed FHF.18 While this study implies that patients with chronic HBV are not at risk of FHF after HAV superinfection, results from a small Canadian study show that those with chronic HBV can have FHF after HAV superinfection. In the Canadian study, 4/60 cases of FHF during 1991–1997 were due to HAV.19 Three of these patients had CLD (2 chronic HBV infection; 1 alcoholic cirrhosis), and all 3 died (13–35 days after admission); the patient without CLD survived.19
HAV vaccination recommendations in patients with CLD
The United States (US) Advisory Committee on Immunization Practices recommends a 2-dose series of HAV or a 3-dose series of HAV+HBV vaccinations for all patients with CLD, including those with HBV, HCV, cirrhosis, NAFLD, alcoholic liver disease, autoimmune hepatitis, or alanine aminotransferase or aspartate aminotransferase level >2 the upper limit of normal.20 Similarly, in the United Kingdom (UK), patients with various chronic liver conditions are recommended to receive HAV vaccination.21
Two types of HAV vaccine are available22 – live attenuated and inactivated – of which only the latter is appropriate for immunocompromised patients such as those with CLD. The World Health Organization (WHO) has endorsed that inactivated HAV vaccines are well tolerated by patients with mild-to-moderate CLD.17 It is recommended that HAV vaccination should be given as early as possible after CLD diagnosis for maximum efficacy and safety.13,14
Situation in India
Burden and changing etiology of CLD
In a multicenter prospective study conducted in different parts of India, 1.3% of nearly 21 million patients who attended 11 hospitals during February 2010 to January 2013 had liver disease.23 One quarter of these patients had a new diagnosis of liver disease, of whom 19.8% had CLD.23 Among these 13,014 patients with newly diagnosed CLD (of whom 4413 [33.9%] had decompensated cirrhosis), mean age was 42.8 ± 14.4 years and the majority (73.0%) were male.23 The main etiologies were related to hepatitis viruses (54.9%), alcohol (17.3%), and NAFLD (12.8%).23 However, etiology varied widely by region, with HCV being the most common in the North (44.9%), HBV in the East (47.9%) and South (40.5%), alcohol in the North-East (31.9%), and NAFLD in the Central region (43.6%) and the West (39.6%).23 CLD etiologies reported in other studies have varied widely,24–34 likely due to variations by region, population, and over time. The latter has been shown in a study in a tertiary care referral hospital in Eastern India, where etiologies of CLD changed substantially from 2003 to 2011, with alcohol increasing from 22.5% to 42.0% (p = .01), cryptogenic (i.e. unknown cause) decreasing from 44.9% to 25.0% (p = .001), but no significant changes in HBV (mean 22.3%) or HCV (mean 10.9%).35
More recent studies have indicated that NAFLD could be becoming a major cause of CLD in India, with huge numbers of people potentially affected. For example, a study published in 2016 found that 30.7% of adults aged ≥35 years in a rural community in North India had NAFLD on ultrasonography,36 while one published in 2019 reported that 528 (53.5%) of male blood donors (mean age 31 ± 8 years for males and 45 ± 8 for females) in an urban community in North India had NAFLD on ultrasonography.37
Recent meta-analyses have estimated seroprevalences of HBV surface antigen (HBsAg) and anti-HCV to be 1.46%38 and 0.44–0.88%,39 respectively in India. Based on a population of approximately 1.38 billion,40 this would equate to approximately 20 million people in India having chronic HBV infection and around 6–12 million having chronic HCV infection, meaning that large numbers of people are potentially at risk for FHF, which has a very high mortality rate among patients with CLD.18,19 However, these numbers are dwarfed by the potential number of people with NAFLD, which, based on the two above-mentioned studies36,37 and the adult Indian population,40 could equate to hundreds of millions of adults with NAFLD in India. While we were unable to find data on the prevalence of alcoholic liver disease in India, given that 18% of liver-related deaths in India were due to alcohol,41 there are likely also many millions of people with alcoholic liver disease in India.
Mortality
In India, deaths due to cirrhosis nearly doubled – from 110,091 to 217,896 – between 1990 and 2017 (although there was little change in the age-standardized mortality rate).42 In 2017, 16.5% of global cirrhosis deaths (217,896 of 1,322,868) were in India.42
Mortality rates vary among patients with CLD. For example, those with alcoholic cirrhosis had higher 1-month mortality than those with nonalcoholic cirrhosis (9.8% vs. 3.2%) in a single-center study from North East India.43 In that study, patients with alcoholic versus nonalcoholic cirrhosis were more often male (97% vs. 64%) and had more advanced disease (based on various parameters).43 The rates of death or orthotopic liver transplantation within 1 year are even higher among those with a first episode of decompensation (most common presentations among 110 Indian patients with cirrhosis: overt ascites [57.3%], ultrasound-detected ascites [22.7%], and hepatic encephalopathy [13.6%]), occurring in 22.2%, 28.0%, and 20.0% of these patients, respectively.33 In an Indian retrospective study of 392 patients (median [range] age 50 [14–87] years; 80% male) who had died of liver-related causes (except liver metastasis from non-hepatic cancers), the most common causes of liver-related death were alcohol (30.1%), nonalcoholic steatohepatitis/cryptogenic (23.2%), hepatotropic viruses (18.6%), and bacterial/other infections (11.5%).34 Most patients (85.5%) had CLD, and among those with CLD, most (70.7%) had presented with cirrhosis complications (e.g. end-stage liver disease, portal hypertension, sepsis), while 29.3% presented with ACLF.34 Based on data from the WHO, approximately 54% of liver-related deaths in India are due to HBV, 18% due to alcohol, 10% due to HAV or HEV, and 10% due to HCV.41 However, acute hepatitis-related deaths are largely due to HBV (54%) or HEV (37%), with 6% due to HAV and 2% due to HCV.41
ACLF
ACLF (acute decompensation in a patient with CLD16) occurred in 121/3220 (3.8%) patients with cirrhosis and acute HAV or HEV admitted to a hospital in Lucknow during 2000–2006.32 The mean age of those with ACLF was 36.3 ± 18.0 years and 70.2% were male.32 Clinical features included jaundice (100%), ascites (78.5%), coagulopathy (77.7%), encephalopathy (55.4%), hyponatremia (41.3%), renal failure (35.5%), and sepsis (33.9%).32 ACLF was due to HEV in 66.1%, HAV in 27.3%, or both in 6.6%; the underlying CLD was mainly cryptogenic (36.4%), HBV (30.6%), or alcohol (10.7%).32 Three-month mortality among these patients with ACLF was high (44.6%).32 In another retrospective study, which included 1049 consecutive patients with ACLF (mean age 44.7 ± 12.2 years; 81.3% male) conducted in 10 tertiary centers from across India during 2011–2014, the most common precipitants of ACLF were alcohol consumption (35.7%), viral superinfection/flare (HAV, HBV, or HEV) (21.4%), and sepsis (16.6%).29 The underlying CLD was mainly alcohol (56.7%), cryptogenic (19.4%), or HBV/HCV (15.9%). During a median (range) hospital stay of 8 (4–14) days, 42.6% of patients died.29 In a single-center study in Eastern India (2012–2014), the most common precipitants of acute decompensation among 123 patients with ACLF (mean age 45.8 ± 12.1 years; 88.6% male) were recent alcohol intake (42.3%) and bacterial infection (36.6%).44 Three-month mortality was very high (71.3%), more so in alcoholics than nonalcoholics (81.1% vs. 55.9%; p = .01).44 Lastly, among 64 patients (median age 44 years; 82.8% male) with ACLF in a hospital in Hyderabad, 2015–2016, the main precipitants were infection (43.8%) and alcoholism (37.5%). Twenty-eight day mortality was high (43.8%).45
Susceptibility of Indian CLD patients to HAV
Nine out of ten old studies from India (carried out up to 2007)25–28,30,31,46–48 found that nearly all patients with CLD/cirrhosis (93.2–99.0%) had evidence of past infection with HAV (as shown by HAV-immunoglobulin G or HAV-IgG49 or anti-HAV antibodies), as did most healthy controls (71.2–100%) (Table 1). The study by Khanna et al.,50 however, reported a much lower rate of HAV-IgG among patients with cirrhosis (60.6%), possibly because they only included patients from the upper middle or upper socioeconomic classes. All of their seronegative CLD patients were vaccinated against HAV.50 The authors of most of the other studies suggested that CLD patients did not routinely require HAV vaccination (as most were already immune),25,27,28,30,31,46–48 while opinions on testing for HAV antibodies before potential vaccination were mixed (see Table 1).25,31,48
Table 1.
Seroprevalence studies showing evidence of previous HAV infection (HAV-IgG or anti-HAV) in patients in India with CLD (or specifically with cirrhosis), listed chronologically
| Reference | Study years | Population | HAV seroprevalence (%) | HAV vaccination recommendations for CLD patients | Other observations |
|---|---|---|---|---|---|
| Dhotial et al. 200246 | 2000–2001 | 42 cirrhosis | 97.6 (anti-HAV) | May not be needed in their population | Etiologies: alcohol (61.9%), HBV (16.7%), HCV (7.1%), other (14.3%) |
| Acharya et al. 200230 | 1997–2001 | 254 CLD | 97.6 (anti-HAV) | Not required | Etiologies: chronic hepatitis due to HCV (33.1%) or HBV (29.8%), cirrhosis due to HBV (18.5%) or HCV (18.5%) |
| Xavier and Anish 200331 | ≤2002a | 52 cirrhosis vs. 50 controls | 98.1 vs. 100 (anti-HAV) | Routine vaccination not required; nor routine anti-HAV screening | Main etiologies: alcoholic cirrhosis (25.0%), HBV (13.5%); high/low socioeconomic class (51.9%/48.1%) |
| Ramachandran et al. 200427 | 2001–2002 | 100 CLD vs. 79 controlsb | 99.0 vs. 100 (HAV-IgG) | Not needed | Main etiologies: cryptogenic (32.0%), alcohol (25.0%), HBV (25.0%) |
| Anand et al. 200428 | 2002 | 187 CLD vs. 89 controls | 95.7 vs. 94.6 (HAV-IgG) | Not routinely required | Main etiologies: HBV (48.7%), HCV (33.2%), cryptogenic (12.8%) |
| Duseja et al. 200447 | 1999–2000 | 55 cirrhosis | 98.2 (anti-HAV) | Routine vaccination cannot be recommended | Etiologies: alcohol (45.5%), HBV (23.6%), HCV (9.1%), Budd–Chiari Syndrome (3.6%), cryptogenic (20.0%) |
| Hussain et al. 200626 | 1999–2003 | 300 CLDc vs. 500 controls | 98.0 vs. 71.2 (HAV-IgG) | Selective vaccination of high-risk population would be a rational and cost-effective approach | Etiologies: HBV (56.3%), HCV (24.3%), alcohol (16.0%), HBV+HCV (3.3%) |
| Khanna et al. 200650 | 1999–2004 | 127 cirrhosis | 60.6% (HAV-IgG) | All seronegative cirrhotic patients were vaccinated | All patients were upper/upper middle socioeconomic class |
| Joshi et al. 200725 | 2004–2005 | 133 cirrhosis vs. 75 controlsb | 93.2 vs. 94.6 (anti-HAV) | Not routinely required screening for HAV antibodies may be more cost effective than vaccination | Main etiologies: HBV (29.3%), cryptogenic (23.3%), alcohol (21.8%), HCV (14.3%) |
| John et al. 200948 | ≤2007a | 300 CLD | 93.3 (HAV-IgG) | Routine vaccination without testing for HAV antibodies not recommended | Etiologies: alcohol (62%), HBV/HCV (12%), cryptogenic (24%), Wilson’s (2%) |
aThese studies did not report study dates, so these dates are based on “received” and “accepted” publication dates.
bAge- and sex-matched.
cAnd HBV and/or HCV infection or alcoholism; CLD patients with unclassified etiology were excluded.
CLD, chronic liver disease; HAV, hepatitis A virus; HBV, hepatitis B virus; HCV, hepatitis C virus; IgG, immunoglobulin G; NR, not reported.
However, most of these studies are old, only including patients until 2007 at the latest and, as will be discussed further below, the epidemiology of HAV is changing in India, with declining HAV infection during childhood and subsequent increasing susceptibility in adulthood. It should also be noted that, in the two latest studies in Table 1,25,48 which included patients during the mid 2000s, nearly 7% of CLD patients did not have anti-HAV/anti-IgG antibodies and were therefore susceptible to HAV infection. As suggested by Radha Krishna et al. in 200932 – based on their study that included 21 adults with ACLF due to HAV – this advice may now be outdated. In a more recent study (2011–2014), 21.4% of ACLF cases were due to HAV, HBV, or HEV superinfection, but unfortunately, the authors did not report results separately for HAV.29
Changing HAV endemicity in India
If HAV is encountered during early childhood, the resultant hepatitis is generally mild, causing no symptoms or nonspecific symptoms (e.g. fever, malaise, diarrhea)51 and providing long-term immunity against HAV.52 However, if HAV is encountered for the first time in adulthood, most people will have symptoms (e.g. jaundice, pain), and it is associated with a mortality rate of around 1%.51 HAV is also significantly more likely to result in more severe disease with increasing age.53 Historically, many people in India were exposed to HAV during childhood, resulting in life-long protection.52 However, with improved sanitation and hygiene, children are becoming less likely to be exposed to HAV, resulting in increasing number of adolescents and adults who are at risk of infection, and a paradoxical increase in morbidity and mortality due to HAV.51,52,54,55 Thus, and taking into account the high heterogeneity across the Indian continent, India is now thought to be shifting from high to intermediate HAV endemicity.51,54 This situation is particularly challenging, as in high HAV endemicity areas, most children are exposed, resulting in mild disease and lifelong immunity, while in low endemicity areas, the chance of exposure in adulthood is low.54 However, with intermediate HAV endemicity, the chance of childhood exposure is reduced, leaving more adults at risk of more severe disease.54
Declining HAV immunity in India
Various serological studies have reported that the proportion of healthy Indian people with seroprotective anti-HAV antibodies (i.e. previous HAV infection) has fallen over time.26,56–59 For example, Arankalle et al.56 reported that anti-HAV positivity decreased significantly from 1982 to 1998 among children from urban high socioeconomic populations (age 6–10 years: ~86% to 30.9%; age 11–15 years: ~94% to 46.9%; combined age p < .00001), but not in adults or urban lower middle socioeconomic populations. Das et al.57 reported that HAV-IgG seropositivity fell from 98.0% in 198260 to 54.1% in 1998 among those aged 15–24 years and from 98.6%60 to 58.7% among those aged 25–34 years (both p < .05). Hussain et al.26 reported that 71.2% of healthy subjects were positive for HAV-IgG in 1999–2003, much lower than the 94.8% reported in subjects in 1982 in an earlier study.60 Gadgil et al.58 found that HAV seropositivity among adult blood donors fell from 96.5% in 2002 to 92.1% in 2004–2005 (p < .01). Recently, Arankalle et al.59 reported that, while HAV seropositivity decreased from 1998 to 2017 among low/middle socioeconomic children and younger adults, it increased during the same time period among high socioeconomic children and adults (Figure 1A).59 This was likely due to HAV vaccination in the high socioeconomic population, although the vaccination status of the participants was not available. Figure 1A also shows that, in 1998, low/middle socioeconomic populations had considerably higher seropositivity (i.e. were much more likely to have had previous HAV infection) than high socioeconomic populations of the same age group, but by 2017, there was very little difference in seropositivity between the two populations.59 Deoshatwar et al.61 have also reported results from the same region (for select age groups) for 2017 and compared them with the same 1998 data as Arankalle et al.59 Figure 1B shows that the changes in seropositivity were less pronounced in the latter study.
Figure 1.
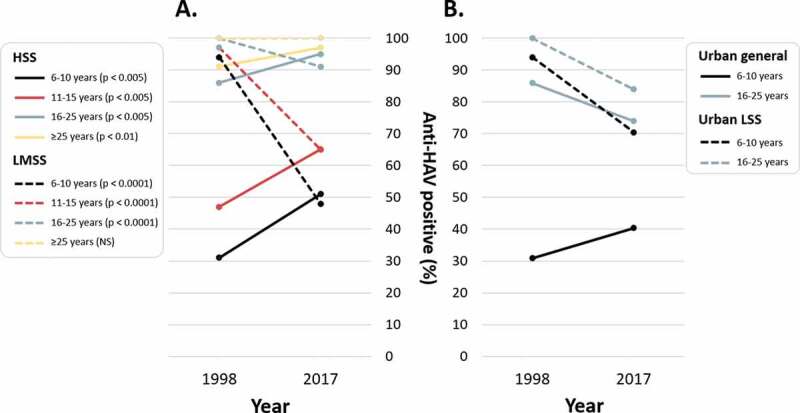
Opposing, but converging, trends in HAV susceptibility among (A) higher and lower middle socioeconomic status populations (created based on data from Arankalle et al.59) and (B) urban general and lower socioeconomic status populations (created based on data from Deoshatwar et al.61)
HAV, hepatitis A virus; HSS, high socioeconomic status; LMSS, lower middle socioeconomic status; LSS, lower socioeconomic status; NS, not significant; p, p value.
Arankalle et al.59 also reported that 90–95% of 3-month-old infants in both 1995 and 2017 were seropositive for HAV, likely due to maternal antibodies. In 1995, seropositivity fell to 13.6% by age 9 months and then increased to 41.0% by age 15 months, which must have been due to natural infection as none were vaccinated. However, in 2017, seropositivity fell to only 2.2% among unvaccinated infants at age 15 months.59 These studies all support a decrease in natural HAV infection during childhood, resulting in an increase in the number of susceptible adults.
Increasing HAV infection in adulthood
In line with declining HAV seroprotection, some studies have shown an increase in the proportion of acute viral hepatitis cases that are due to HAV over time.26,50 Hussain et al.26 studied 1932 patients with acute viral hepatitis at a tertiary care center in Northern India, of whom 11.4% overall were HAV-IgM positive (indicating current infection). This increased from 3.4% in 1999 to 12.3% in 2003 among adults (Hussain line in Figure 2; p < .004); and from 10.6% to 22.0% in children (p < .003). At another tertiary care center in Northern India, Khanna et al.50 reported increasing proportions of acute hepatitis due to HAV from 1999 to 2004 among patients with acute hepatitis aged 13–20 years (27.2% to 61.5%; p = .008), 21–30 years (13.3% to 39.5%; Khanna line in Figure 2; p = .031), and >30 years (0% to 17.3%; p = .06).
Figure 2.
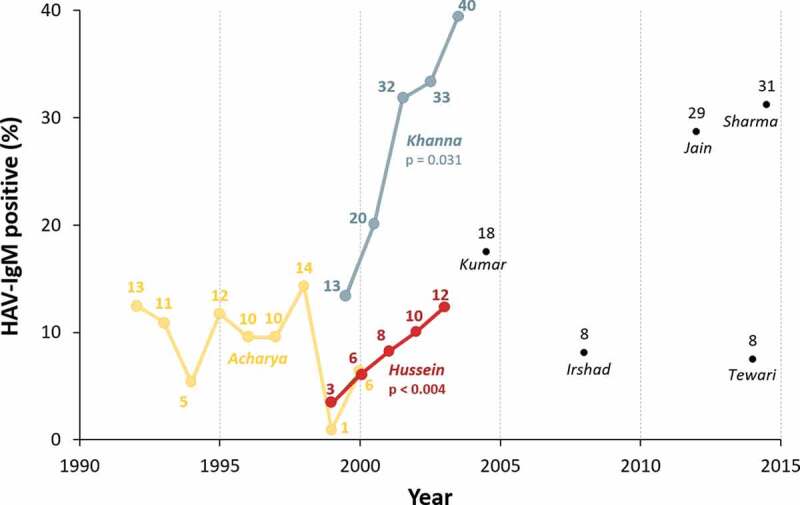
Increasing acute HAV infection among adults with acute viral hepatitis, created based on data from Acharya et al. 2003,62 Khanna et al. 2006 (middle adult age group used),50 Hussain et al. 2006,26 Kumar et al. 2007 (mainly adults),63 Irshad et al. 2010,64 Jain et al. 2013,65 Tewari et al. 2016,66 and Sharma et al. 2016 (suspected viral hepatitis).67 Further information on these studies can be found in Table 2
HAV, hepatitis A virus; IgM, immunoglobulin M.
Various other Indian studies have reported on the seroprevalence of HAV-IgM antibodies among those with acute viral hepatitis, but have reported no change over time (Acharya line in Figure 2),62 or have not studied their evolution over time63-69 (see Table 2 and single points in Figure 2). While comparisons between studies (particularly single-center studies) should be undertaken with caution, as seropositivity varies widely by socioeconomic status, age, HAV vaccination rates, region, setting, and local outbreaks, there appears to be a slight upward trend in the proportion of adults with acute viral hepatitis who have acute HAV infection (Figure 2).
Table 2.
Seroprevalence studies showing evidence of current HAV infection in patients in India with (suspected) acute viral hepatitis, listed chronologically
| Reference | Study years | Population | Age | Seroprevalence (HAV-IgM) (%) | Other observations |
|---|---|---|---|---|---|
| Poddar et al. 200268 | 1997–2000 | 172 AVH | Children | 72.7 (64.5 HAV alone; 8.1 with HCV and/or HEV) | Other etiologies: HEV 16.3%, HBV 7.6% |
| Acharya et al. 200362 | 1992–2001 | 998 AVH vs. 492 FHF | Adults | 7.7 vs 5.9 | No significant change in the proportion of AVH or FHF due to HAV from 1992 to 2001 |
| Hussain et al. 200626 | 1999–2003 | 1932 AVH | 751 children, 1181 adults | 16.2 (children), 8.4 (adults) | See line in Figure 2 for evolution over time |
| Khanna et al. 200650 | 1999–2004 | 500 AVH | 90 children, 410 adults | 72.2 (children), 28.0 (adults) | All middle/upper socioeconomic status; see line in Figure 2 for evolution over time |
| Kumar et al. 200763 | 2002–2006 | 685 AVH vs. 70 FHF vs. 53 CLD vs. 11 ATT-induced jaundice vs. 24 pregnant | 10–70 years | 17.5 vs. 4.3 vs. 0 vs. 0 vs. 0 | – |
| Irshad et al. 201064 | 2006–2009 | 76 AVH vs. 54 FHF vs. 102 CVH vs. 96 cirrhosis vs 42 HCC | Adults | 8.1 vs. 0 vs. 0 vs. 0 vs. 0 | – |
| Jain et al. 201365 | 2011–2012 | 205 AVH vs. 62 FHF | AVH: 97 children, 108 adults; FHF: 46 children, 16 adults | AVH: 34.0 (children), 28.7 (adults); FHF: 13.0 (children), 12.5 (adults) | Other etiologies (AVH and FHF combined): HEV 18.0%, HBV 16.1%, HCV 12.0% |
| Sharma et al. 201667 | 2012–2015 | 285 suspected viral hepatitis | Adults | 36.8 (31.2 HAV alone; 5.6 with HBV, HCV, or HEV) a | Other etiologies: HBV 1.8%, HCV 1.4%, HEV 1.4% |
| Tewari et al. 201666 | 2014 | 89 AVH | 36 children, 53 adults | 72.2 (children), 7.5 (adults) | – |
| Mittal et al. 201669 | 2015 | 1654 AVH | Children and adults | 7.7 | Most seropositive cases were aged 11–20 years (45.6%), 0–10 years (29.1%), 21–30 years (18.1%) |
AVH, acute viral hepatitis; ATT, antituberculosis treatment; CVH, chronic viral hepatitis; CLD, chronic liver disease; FHF, fulminant hepatic failure; HAV, hepatitis A virus; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HEV, hepatitis E virus; IgM, immunoglobulin M.
The seroprevalence among children varied widely by study, from 16.2%26 to 72.2%,50,66 with little correlation over time. This may relate to the socioeconomic status of the studied populations (which, as shown in Figure 1, used to have a large impact on seroprevalence, but nowadays, has much less impact), but most studies did not describe this parameter.
Indian HAV immunization recommendations
HAV vaccination is not included in the routine childhood immunization schedule in India.70 However, the Advisory Committee on Vaccines & Immunization Practices (ACVIP) of the Indian Academy of Pediatrics (IAP) recommends HAV vaccination for all infants, as a single dose of live attenuated vaccine at 12 months or 2 doses of inactivated vaccine at 12 and 18 months of age,51 which can be administered in a private setting paid for by the parents.22 The IAP particularly recommends HAV vaccination for various risk groups, including children with CLD and those who are carriers of HBV and HCV.51
Although the recommendation to vaccinate patients with CLD against HAV has been endorsed by the WHO,17 the Indian National Centre for Disease Control (NCDC) does not currently recommend HAV vaccination for adults with CLD in India, as “most adults have already been exposed to and are thus protected”.71 This recommendation is supported by 9/10 old studies from India (carried out up to 2007)25–28,30,31,46–48 (Table 1). However, in the current context of changing endemicity it is very unlikely to hold true and therefore we feel that this should now be reexamined.
Indian associations and scientific society recommendations relating to HAV vaccination are detailed in Table 3.72–74 The Association of Physicians of India (API) and the Indian Society of Nephrology (ISN) both indicate that patients with CLD who are not immune to HAV, those with other hepatitis virus infections, and patients awaiting or having received a liver transplant are at risk of HAV infection, but do not specifically recommend vaccination.72,73 The ISN, however, says that HAV vaccination “is indicated for all transplant candidates with CLD or those patients of end‑stage renal disease who have chronic hepatitis B or C” due to an increased risk of FHF.73 The Indian Medical Association (IMA) does not mention CLD or other hepatitis infection, but does recommend HAV for adults or children undergoing liver transplantation.74
Table 3.
Adult HAV vaccination guidelines from various associations and scientific societies in India
| API 200972 | ISN 201673 | IMA 201874 | |
|---|---|---|---|
| All adults | No | No | Yes |
| CLD and not immune to HAV | Uncleara | Uncleara | NM |
| Other hepatitis viruses | Uncleara | Uncleara | NM |
| Liver transplantation | Uncleara | Uncleara | Yes |
| Transplant candidates with CLD | NM | Yes | NM |
| ESRD and chronic HBV or HCV | NM | Yes | NM |
| Other at-risk peopleb | Uncleara | Uncleara | Yes |
aGuideline specifies that these people are at high risk for acquiring HAV, and are most likely to benefit from HAV vaccination, but it is not clear whether vaccination is recommended.
bDefinitions vary by guideline.
API, Association of Physicians of India; CLD, chronic liver disease; ESRD, end-stage renal disease; HAV, hepatitis A virus; HBV, hepatitis B virus; HCV, hepatitis C virus; IMA, Indian Medical Association; ISN, Indian Society of Nephrology; NM, not mentioned.
Authors’ recommendations
Based on the currently available evidence of shifting endemicity and increasing HAV susceptibility in adulthood in India, now may be the time to revisit the existing NCDC recommendation that HAV vaccination is not necessary for those with CLD in India.71 Instead, we propose that a recommendation for HAV vaccination of adults with CLD should be considered in India, as is already the case in the US,20 the UK,21 and Sri Lanka75 (a near neighbor of India), and also for children with CLD in India.51 While some Indian medical association/society guidelines recognize that seronegative patients with CLD are at increased risk of HAV infection, they do not clearly recommend HAV vaccination.72,73
Up-to-date serological studies among Indian patients with CLD would be beneficial to confirm whether seroprotective HAV antibodies have decreased over time in these patients, in line with what has been shown in healthy people.26,56–59,61 However, awaiting the results of such studies should not be a prerequisite for recommending HAV vaccination among patients with CLD in India.
A more targeted approach, with serological testing prior to HAV vaccination, could be a more cost-effective option than universal HAV vaccination of patients with CLD.25 However, given the limited facilities for serological testing, the associated cost, and the potential for missed opportunity for vaccination if patients do not return after serological testing, this should also not be a prerequisite.
Limitations
This was not a systematic review, so although we included all relevant manuscripts that we could find on PubMed and Embase, there may have been some manuscripts (e.g. those published in Indian journals that are not listed on PubMed or Embase) that we did not manage to capture. Also, India is a large country with high socioeconomic status heterogeneity. As seropositivity rates vary considerably with socioeconomic status, age, HAV vaccination rates, region, setting, and local outbreaks, comparisons between studies from different time periods should be undertaken with caution. Futher, as already discussed, the studies that have assessed the HAV susceptibility of CLD patients are old and, while it is likely that susceptibility among these patients has increased as it has among general adults, this should be confirmed.
Summary and conclusions
The burden of CLD in India is high, resulting in high morbidity and mortality.42 Patients with CLD are at increased risk of severe HAV disease13-15,17 and ACLF, which has a very high mortality rate.16,29,32,44,45 Hence, such patients are recommended to receive various vaccinations, including HAV vaccination, in the US,20 the UK,21 and Sri Lanka.75 Old studies from India showed a high seroprevalence of protective HAV antibodies among Indian adults with CLD,25–28,30,31,46–48 although the most recent ones (≤2007) found that nearly 7% of CLD patients did not have protective HAV antibodies and were therefore susceptible to HAV infection. Studies in healthy individuals have shown that HAV infection in childhood is decreasing in India,56–59,76 resulting in an increasing population of adults without protective antibodies, and a higher risk of HAV infection in adulthood.26,50 This is likely also the case among patients with CLD.
Based on seroprovalence data,38,39 millions of people in India likely have chronic HBV or HCV infection. Even more adults could have NAFLD36,37 and, along with an increasing amount of alcoholic liver disease in India,35 this equates to a huge population of people with chronic hepatitis infection and/or CLD. Such people are at higher risk of severe disease13,17 (HAV superinfection, ACLF, FHF) and increased mortality.15,18,19
It may, therefore, now be time to reexamine the existing Indian recommendations.71–74 Patients with CLD who do not have HAV antibodies should receive HAV vaccination. This approach could reduce morbidity, mortality, and healthcare costs of HAV infection among patients with CLD.77 In situations where antibody testing is not available or practical, CLD patients should not be excluded from HAV vaccination.
Figure 3 elaborates on the findings in a form that could be shared with patients by healthcare professionals.
Figure 3.

Plain language summary
Acknowledgments
The authors thank Business & Decision Life Sciences for editorial assistance and manuscript coordination, on behalf of GSK. Benjamin Lemaire coordinated the manuscript development and editorial support. Jenny Lloyd (Compass Medical Communications Ltd.) provided writing support.
Funding Statement
GlaxoSmithKline Biologicals S.A. took in charge all costs associated with the development and publication of this manuscript.
Author contributions
AA developed the search strategy and searched the databases. All authors participated in the design or implementation or analysis, and the development of the manuscript. All authors had full access to the data and gave final approval before submission.
Disclosure of potential conflicts of interest
Anar Andani, Shafi Kolhapure, and Ashish Agrawal are employees of the GSK group of companies. Anar Andani and Shafi Kolhapure hold shares as part of their employee remuneration. Bhaskar Raju declares no financial conflicts of interest. All authors declare no non-financial conflicts of interest.
References
- 1.Sharma A, Nagalli S.. 2020. Chronic liver disease. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Available at https://www.ncbi.nlm.nih.gov/books/NBK554597/, accessed April 21, 2020. [Google Scholar]
- 2.Arvaniti V, D’Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK.. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246–56. 1256 e1241-1245. doi: 10.1053/j.gastro.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 3.Hernaez R, Sola E, Moreau R, Gines P. Acute-on-chronic liver failure: an update. Gut. 2017;66:541–53. doi: 10.1136/gutjnl-2016-312670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GBD Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.GBD . DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2017;2018(392):1859–922. doi: 10.1016/S0140-6736(18)32335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.GBD . Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2017;2018(392):1736–88. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manzarbeitia C Liver transplantation. Available at https://emedicine.medscape.com/article/431783-print, accessed April 21, 2020.
- 8.Ghassemi S, Garcia-Tsao G. Prevention and treatment of infections in patients with cirrhosis. Best Pract Res Clin Gastroenterol. 2007;21:77–93. doi: 10.1016/j.bpg.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis. 2008;28:26–42. doi: 10.1055/s-2008-1040319. [DOI] [PubMed] [Google Scholar]
- 10.Fiuza C, Salcedo M, Clemente G, Tellado JM. In vivo neutrophil dysfunction in cirrhotic patients with advanced liver disease. J Infect Dis. 2000;182:526–33. doi: 10.1086/315742. [DOI] [PubMed] [Google Scholar]
- 11.Christou L, Pappas G, Falagas ME. Bacterial infection-related morbidity and mortality in cirrhosis. Am J Gastroenterol. 2007;102:1510–17. doi: 10.1111/j.1572-0241.2007.01286.x. [DOI] [PubMed] [Google Scholar]
- 12.Irvine KM, Ratnasekera I, Powell EE, Hume DA. Causes and consequences of innate immune dysfunction in cirrhosis. Front Immunol. 2019;10:293. doi: 10.3389/fimmu.2019.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reiss G, Keeffe EB. Review article: hepatitis vaccination in patients with chronic liver disease. Aliment Pharmacol Ther. 2004;19:715–27. doi: 10.1111/j.1365-2036.2004.01906.x. [DOI] [PubMed] [Google Scholar]
- 14.Keeffe EB. Hepatitis A and B superimposed on chronic liver disease: vaccine-preventable diseases. Trans Am Clin Climatol Assoc. 2006;117:227–37. discussion 237-228. [PMC free article] [PubMed] [Google Scholar]
- 15.Cooksley WG. What did we learn from the Shanghai hepatitis A epidemic? J Viral Hepat. 2000;7(Suppl 1):1–3. doi: 10.1046/j.1365-2893.2000.00021.x. [DOI] [PubMed] [Google Scholar]
- 16.Sarin SK, Choudhury A, Sharma MK, Maiwall R, Al Mahtab M, Rahman S, Saigal S, Saraf N, Soin AS, Devarbhavi H, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13:353–90. doi: 10.1007/s12072-019-09946-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization . WHO position paper on hepatitis A vaccines – june 2012. Wkly Epidemiol Rec 2012;87:261–76.22905367 [Google Scholar]
- 18.Vento S, Garofano T, Renzini C, Cainelli F, Casali F, Ghironzi G, Ferraro T, Concia E. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med. 1998;338:286–90. doi: 10.1056/NEJM199801293380503. [DOI] [PubMed] [Google Scholar]
- 19.Lefilliatre P, Villeneuve JP. Fulminant hepatitis A in patients with chronic liver disease. Can J Public Health. 2000;91:168–70. doi: 10.1007/BF03404264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC) . Recommended adult immunization schedule for ages 19 years or older, United States, 2020. Available at https://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html, accessed April21, 2020.
- 21.National Health Service (NHS) . NHS vaccinations and when to have them: extra vaccines for at-risk people. Available at https://www.nhs.uk/conditions/vaccinations/nhs-vaccinations-and-when-to-have-them/, accessed June 9, 2020.
- 22.Verma R, Hepatitis KP. A vaccine should receive priority in National Immunization Schedule in India. Hum Vaccin Immunother. 2012;8:1132–34. doi: 10.4161/hv.20475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukherjee PS, Vishnubhatla S, Amarapurkar DN, Das K, Sood A, Chawla YK, Eapen CE, Boddu P, Thomas V, Varshney S, et al. Etiology and mode of presentation of chronic liver diseases in India: a multi centric study. PLoS One. 2017;12:e0187033. doi: 10.1371/journal.pone.0187033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choudhuri G, Chaudhari S, Pawar D, Roy DS. Etiological patterns, liver fibrosis stages and prescribing patterns of hepato-protective agents in Indian patients with chronic liver disease. J Assoc Physicians India. 2018;66:58–63. [PubMed] [Google Scholar]
- 25.Joshi N, Rao S, Kumar A, Patil S, Hepatitis RS. A vaccination in chronic liver disease: is it really required in a tropical country like India? Indian J Med Microbiol. 2007;25:137–39. doi: 10.4103/0255-0857.32720. [DOI] [PubMed] [Google Scholar]
- 26.Hussain Z, Das BC, Husain SA, Murthy NS, Kar P. Increasing trend of acute hepatitis A in north India: need for identification of high-risk population for vaccination. J Gastroenterol Hepatol. 2006;21:689–93. doi: 10.1111/j.1440-1746.2006.04232.x. [DOI] [PubMed] [Google Scholar]
- 27.Ramachandran J, Eapen CE, Kang G, Abraham P, Hubert DD, Kurian G, Hephzibah J, Mukhopadhya A, Chandy GM. Hepatitis E superinfection produces severe decompensation in patients with chronic liver disease. J Gastroenterol Hepatol. 2004;19:134–38. doi: 10.1111/j.1440-1746.2004.03188.x. [DOI] [PubMed] [Google Scholar]
- 28.Anand AC, Nagpal AK, Seth AK, Dhot PS. Should one vaccinate patients with chronic liver disease for hepatitis A virus in India? J Assoc Physicians India. 2004;52:785–87. [PubMed] [Google Scholar]
- 29.Shalimar SV, Singh SP, Duseja A, Shukla A, Eapen CE, Kumar D, Pandey G, Venkataraman J, Puri P, Narayanswami K, et al. Acute-on-chronic liver failure in India: the Indian national association for study of the liver consortium experience. J Gastroenterol Hepatol. 2016;31(10):1742–49. doi: 10.1111/jgh.13340. [DOI] [PubMed] [Google Scholar]
- 30.Acharya SK, Batra Y, Saraya A, Hazari S, Dixit R, Kaur K, Bhatkal B, Ojha B, Panda SK. Vaccination for hepatitis A virus is not required for patients with chronic liver disease in India. Natl Med J India. 2002;15:267–68. [PubMed] [Google Scholar]
- 31.Xavier S, Anish K. Is hepatitis A vaccination necessary in Indian patients with cirrhosis of liver? Indian J Gastroenterol. 2003;22:54–55. [PubMed] [Google Scholar]
- 32.Radha Krishna Y, Saraswat VA, Das K, Himanshu G, Yachha SK, Aggarwal R, Choudhuri G. Clinical features and predictors of outcome in acute hepatitis A and hepatitis E virus hepatitis on cirrhosis. Liver Int. 2009;29:392–98. doi: 10.1111/j.1478-3231.2008.01887.x. [DOI] [PubMed] [Google Scholar]
- 33.Shah AS, Amarapurkar DN. Natural history of cirrhosis of liver after first decompensation: a prospective study in India. J Clin Exp Hepatol. 2018;8:50–57. doi: 10.1016/j.jceh.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar A, Gupta V, Arora A. Most liver related deaths in India are caused by alcohol: an audit of liver mortality from tertiary care center in North India [abstract]. Clin Gastroenterol Hepatol. 2017;15:e37. doi: 10.1016/j.cgh.2016.09.092. [DOI] [Google Scholar]
- 35.Ray G. Trends of chronic liver disease in a tertiary care referral hospital in Eastern India. Indian J Public Health. 2014;58:186–94. doi: 10.4103/0019-557X.138630. [DOI] [PubMed] [Google Scholar]
- 36.Majumdar A, Misra P, Sharma S, Kant S, Krishnan A, Pandav CS. Prevalence of nonalcoholic fatty liver disease in an adult population in a rural community of Haryana, India. Indian J Public Health. 2016;60:26–33. doi: 10.4103/0019-557X.177295. [DOI] [PubMed] [Google Scholar]
- 37.Duseja A, Najmy S, Sachdev S, Pal A, Sharma RR, Marwah N, Chawla Y. High prevalence of non-alcoholic fatty liver disease among healthy male blood donors of urban India. JGH Open. 2019;3:133–39. doi: 10.1002/jgh3.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 2015; 386:1546–55. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 39.Goel A, Seguy N, Aggarwal R. Burden of hepatitis C virus infection in India: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2019;34:321–29. doi: 10.1111/jgh.14466. [DOI] [PubMed] [Google Scholar]
- 40.Worldometer . India population. Available at https://www.worldometers.info/world-population/india-population/, accessed June 9, 2020.
- 41.Sarin SK, Kumar M, Eslam M, George J, Al Mahtab M, Akbar SMF, Jia J, Tian Q, Aggarwal R, Muljono DH, et al. Liver diseases in the Asia-pacific region: a Lancet gastroenterology & hepatology commission. Lancet Gastroenterol Hepatol. 2020;5:167–228. doi: 10.1016/S2468-1253(19)30342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.GBD . Cirrhosis collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. 2017;2020(5):245–66. doi: 10.1016/S2468-1253(19)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhattacharyya M, Barman NN, Goswami B. Survey of alcohol-related cirrhosis at a tertiary care center in North East India. Indian J Gastroenterol. 2016;35:167–72. doi: 10.1007/s12664-016-0651-2. [DOI] [PubMed] [Google Scholar]
- 44.Pati GK, Singh A, Misra B, Misra D, Das HS, Panda C, Singh SP. Acute-on-chronic liver failure (ACLF) in coastal Eastern India: “a single-center experience”. J Clin Exp Hepatol. 2016;6:26–32. doi: 10.1016/j.jceh.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kulkarni S, Sharma M, Rao PN, Gupta R, Reddy DN. Acute on chronic liver failure-in-hospital predictors of mortality in ICU. J Clin Exp Hepatol. 2018;8:144–55. doi: 10.1016/j.jceh.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dhotial A, Kapoor D, Kumar N. Seroprevalence of hepatitis A virus antibody in patients with chronic liver disease - experience from a tertiary care hospital in north India. Trop Gastroenterol. 2002;23:170–71. [PubMed] [Google Scholar]
- 47.Duseja A, Sharma S, Das K, Dhiman RK, Chawla YK. Is vaccination against hepatitis A virus required in patients with cirrhosis of the liver? Trop Gastroenterol. 2004;25:162–63. [PubMed] [Google Scholar]
- 48.John A, Chatni S, Narayanan VA, Balakrishnan V, Nair P. Seroprevalence of hepatitis A virus in patients with chronic liver disease from Kerala: impact on vaccination policy. J Indian Med Assoc. 2009;107:859–61. [PubMed] [Google Scholar]
- 49.Stoll BJ, Hansen NI, Sánchez PJ, Faix RG, Poindexter BB, Van Meurs KP, Bizzarro MJ, Goldberg RN, Frantz ID 3rd, Hale EC, et al. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics. 2011;127:817–26. doi: 10.1542/peds.2010-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khanna S, Vohra P, Jyoti R, Vij JC, Kumar A, Singal D, Tandon R. Changing epidemiology of acute hepatitis in a tertiary care hospital in Northern India. Indian J Gastroenterol. 2006;25:101–02. [PubMed] [Google Scholar]
- 51.Advisory Committee on Vaccines & Immunization Practices (ACVIP) . IAP guidebook on immunization 2018-2019. Balasubramanian S, Shastri DD, Shah AK, Chatterjee P, Pemde HK, Shivananda S, Guduru VK. eds. New Delhi (India): Jaypee Brothers Medical Publishers (P) Ltd.; 2020. [Google Scholar]
- 52.Agrawal A, Singh S, Kolhapure S, Hoet B, Arankalle V, Mitra M. Increasing burden of hepatitis A in adolescents and adults and the need for long-term protection: a review from the Indian subcontinent. Infect Dis Ther. 2019;8:483–97. doi: 10.1007/s40121-019-00270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee HW, Chang DY, Moon HJ, Chang HY, Shin EC, Lee JS, Kim KA, Kim HJ. Clinical factors and viral load influencing severity of acute hepatitis A. PLoS One. 2015;10:e0130728. doi: 10.1371/journal.pone.0130728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poddar U, Ravindranath A. Is time ripe for hepatitis A mass vaccination? Indian Pediatr. 2019;56:731–32. doi: 10.1007/s13312-019-1630-3. [DOI] [PubMed] [Google Scholar]
- 55.Aggarwal R, Goel A. Hepatitis A: epidemiology in resource-poor countries. Curr Opin Infect Dis. 2015;28:488–96. doi: 10.1097/QCO.0000000000000188. [DOI] [PubMed] [Google Scholar]
- 56.Arankalle VA, Chadha MS, Chitambar SD, Walimbe AM, Chobe LP, Gandhe SS. Changing epidemiology of hepatitis A and hepatitis E in urban and rural India (1982-98). J Viral Hepat. 2001;8:293–303. doi: 10.1046/j.1365-2893.2001.00279.x. [DOI] [PubMed] [Google Scholar]
- 57.Das K, Jain A, Gupta S, Kapoor S, Gupta RK, Chakravorty A, Kar P. The changing epidemiological pattern of hepatitis A in an urban population of India: emergence of a trend similar to the European countries. Eur J Epidemiol. 2000;16:507–10. doi: 10.1023/a:1007628021661. [DOI] [PubMed] [Google Scholar]
- 58.Gadgil PS, Fadnis RS, Joshi MS, Rao PS, Chitambar SD. Seroepidemiology of hepatitis A in voluntary blood donors from Pune, Western India (2002 and 2004-2005). Epidemiol Infect. 2008;136:406–09. doi: 10.1017/S0950268807008643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arankalle V, Tiraki D, Kulkarni R, Palkar S, Malshe N, Lalwani S, Mishra A. Age-stratified anti-HAV positivity in Pune, India after two decades: has voluntary vaccination impacted overall exposure to HAV? J Viral Hepat. 2019;26:757–60. doi: 10.1111/jvh.13074. [DOI] [PubMed] [Google Scholar]
- 60.Arankalle VA, Tsarev SA, Chadha MS, Alling DW, Emerson SU, Banerjee K, Purcell RH. Age-specific prevalence of antibodies to hepatitis A and E viruses in Pune, India, 1982 and 1992. J Infect Dis. 1995;171:447–50. doi: 10.1093/infdis/171.2.447. [DOI] [PubMed] [Google Scholar]
- 61.Deoshatwar AR, Gurav YK, Lole KS. Declining trends in Hepatitis A seroprevalence over the past two decades, 1998-2017, in Pune, Western India. Epidemiol Infect. 2020;148:e121. doi: 10.1017/S0950268820000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Acharya SK, Batra Y, Bhatkal B, Ojha B, Kaur K, Hazari S, Saraya A, Panda SK. Seroepidemiology of hepatitis A virus infection among school children in Delhi and north Indian patients with chronic liver disease: implications for HAV vaccination. J Gastroenterol Hepatol. 2003;18:822–27. doi: 10.1046/j.1440-1746.2003.03051.x. [DOI] [PubMed] [Google Scholar]
- 63.Kumar S, Ratho RK, Chawla YK, Chakraborti A. The incidence of sporadic viral hepatitis in North India: a preliminary study. Hepatobiliary Pancreat Dis Int. 2007;6:596–99. [PubMed] [Google Scholar]
- 64.Irshad M, Singh S, Joshi YK. Viral hepatitis in India: a report from Delhi. Glob J Health Sci 2010; 2:96–103. [Google Scholar]
- 65.Jain P, Prakash S, Gupta S, Singh KP, Shrivastava S, Singh DD, Singh J, Jain A. Prevalence of hepatitis A virus, hepatitis B virus, hepatitis C virus, hepatitis D virus and hepatitis E virus as causes of acute viral hepatitis in North India: a hospital based study. Indian J Med Microbiol. 2013;31:261–65. doi: 10.4103/0255-0857.115631. [DOI] [PubMed] [Google Scholar]
- 66.Tewari R, Makeeja V, Dudeja M. Prevalence of hepatitis A in southern part of Delhi, India. Int J Med Sci Public Health. 2016;5:2067–70. doi: 10.5455/ijmsph.2016.20112015426. [DOI] [Google Scholar]
- 67.Sharma AK, Dutta U, Sinha SK, Kochhar R. Upsurge in vaccine preventable hepatitis A virus infection in adult patients from a tertiary care hospital of North India. Poster presentated at the 17th International Congress on Infectious Diseases. Int J Infect Dis. abstract 43.233]. Available at https://www.researchgate.net/publication/299473755_Upsurge_in_vaccine_preventable_hepatitis_A_virus_infection_in_adult_patients_from_a_tertiary_care_hospital_of_North_India, accessed. 2016;45S:457. April23, 2020. [Google Scholar]
- 68.Poddar U, Thapa BR, Prasad A, Singh K. Changing spectrum of sporadic acute viral hepatitis in Indian children. J Trop Pediatr. 2002;48:210–13. doi: 10.1093/tropej/48.4.210. [DOI] [PubMed] [Google Scholar]
- 69.Mittal A, Bithu R, Vyas N, Maheshwari RK. Prevalence of hepatitis A virus and hepatitis E virus in the patients presenting with acute viral hepatitis at a tertiary care hospital Jaipur Rajasthan. N Niger J Clin Res. 2016;5:47–50. doi: 10.4103/2250-9658.197436. [DOI] [Google Scholar]
- 70.National Health Portal (NHP) India . Universal immunisation programme. Available at https://www.nhp.gov.in/universal-immunisation-programme_pg, accessed June 5, 2020.
- 71.National Centre for Disease Control . Viral hepatitis: the silent disease facts and treatment guidelines. Available at https://ncdc.gov.in/linkimages/guideline_hep20158117187417.pdf, accessed April 30, 2020. [Google Scholar]
- 72.Expert Group of the Association of Physicians of India on Adult Immunization in India . Executive summary: the association of physicians of India evidence-based clinical practice guidelines on adult immunization. J Assoc Physicians India 2009;57:345–56. [PubMed] [Google Scholar]
- 73.Indian Society of Nephrology . Indian Society of Nephrology guidelines for vaccination in chronic kidney disease. Indian J Nephrol. 2016;26(Suppl1):S1–S30. [Google Scholar]
- 74.Indian Medical Association . Life course immunization guidebook. A quick reference guide. Available at http://www.ima-india.org/ima/pdfdata/IMA_LifeCourse_Immunization_Guide_2018_DEC21.pdf, accessed April 5, 2019.
- 75.Sri Lanka Medical Association (SLMA) . SLMA guidelines and information on vaccines. Available at https://slma.lk/wp-content/uploads/2017/12/GSK-SLMA-Guidelines-Information-on-Vaccines.pdf, accessed June 9, 2020.
- 76.Chitambar SD, Chadha MS, Joshi MS, Arankalle VA. Prevalence of hepatitis A antibodies in western Indian population: changing pattern. Southeast Asian J Trop Med Public Health. 1999;30:273–76. [PubMed] [Google Scholar]
- 77.Waghray A, Waghray N, Khallafi H, Menon KV. Vaccinating adult patients with cirrhosis: trends over a decade in the United States. Gastroenterol Res Pract. 2016;2016:5795712. doi: 10.1155/2016/5795712. [DOI] [PMC free article] [PubMed] [Google Scholar]


