ABSTRACT
Macroautophagy is a catabolic process critical for the degradation of intracellular material, but its physiological functions in vertebrates are not fully understood. Here, we discuss our recent finding that macroautophagy plays a role in lamellar body maturation. The lamellar body is a lysosome-related organelle and stores phospholipid-containing surfactant complexes that reduce the surface tension of the air–water interface in order to inflate the airspace in lungs and swim bladders. In the epithelial cells of these organs, autophagosomes fuse with immature lamellar bodies to increase their size and lipid contents. This function is essential for respiration after birth in mice and for maintaining buoyancy in zebrafish. These findings unveil a novel function of macroautophagy in the maturation of surfactant-containing lamellar bodies.
KEYWORDS: Autophagy, lamellar body, lung, lysosome-related organelle, swim bladder, zebrafish
The lung and the swim bladder are homologous air-filled organs. To expand their air spaces, the action of a surfactant, which reduces the surface tension on the air–water interface, is crucial. The surfactant is a lipid–protein complex composed mostly of phospholipids and is produced by the lamellar body in epithelial cells in both the lung (type II pneumocytes) and swim bladder. The lamellar body is a lysosome-related organelle that originates from late endosomes/multivesicular bodies and has an acidic pH and degradative activities. The maturation of lamellar bodies is accompanied by an increase in size and the incorporation of a large amount of surfactant phospholipids, which are synthesized in the endoplasmic reticulum. SFTPB (surfactant protein B) and ABCA3 (ATP binding cassette subfamily A member 3) are essential for the proper load of phospholipids into lamellar bodies. Additionally, previous in vitro studies using transformed mink alveolar epithelial cells have suggested a possible role for macroautophagy (hereafter autophagy) in the incorporation of surfactant lipid into lamellar bodies. However, in these studies, the role of autophagy was assessed using 3-methyladenine, a class III PtdIns3K inhibitor that suppresses not only autophagy but also multivesicular body formation. Thus, it was not directly demonstrated whether autophagy was indeed required for lamellar body formation, particularly in vivo.
We performed an unbiased phenotypic screening by using zebrafish lacking representative autophagy-related (atg) genes and revealed that autophagy plays a critical role in the maturation of lamellar bodies in the swim bladder [1]. We also found that this function is conserved in the lung of mice. Loss of autophagy in zebrafish and mice results in defective maturation of lamellar bodies in swim bladder epithelial cells and type II pneumocytes. In wild-type animals, LC3-positive autophagosomes frequently fused with lamellar bodies. The fraction of LC3-positive lamellar bodies increases after inhibition of V-ATPase in an ex vivo lung culture system, suggesting that the internal contents are actively degraded after autophagosome-lamellar body fusion. Being unable to properly inflate the airspace, atg-deficient zebrafish cannot maintain their position in the water and type-II-pneumocyte-specific Rb1cc1/Fip200-deficient mice show neonatal lethality with respiratory failure. These findings demonstrated that autophagy is indeed important for lamellar body maturation and inflation of airspaces in vivo.
What is the function of autophagy in lamellar body maturation? Autophagy deficiency leads to a reduction in the size and lipid contents of lamellar bodies without affecting the cellular level of surfactant phospholipids (e.g., dipalmitoylphosphatidylcholine), maturation of SFTPB, and overall development of the swim bladder and lung. Thus, autophagy may be important for the remobilization of lipids to lamellar bodies rather than lipid synthesis or cellular differentiation. This function might be mediated by at least two mechanisms. First, autophagosomes might supply the sources of surfactant lipids by providing the partially degraded membranes of engulfed organelles (e.g., the inner autophagosomal membranes and endomembranes). Second, the outer autophagosomal membranes fuse with the limiting membrane of immature lamellar bodies or late endosomes, thereby contributing to lamellar body expansion. However, given that autophagy is also important for intracellular quality control and glycogen metabolism in the lung, the actual roles of autophagy in lamellar body maturation will need to be further investigated in the future.
Our zebrafish screens and mice experiment also revealed that upstream ATG proteins, but not ATG conjugation system components (e.g., ATG5), are required for lamellar body maturation. In addition, zebrafish lacking upstream Atgs die earlier compared with those lacking Atg conjugation systems; this is similar to the case of mice lacking upstream ATG proteins, who die in utero, whereas those lacking ATG conjugation systems die within 1 day after birth. These phenotypic differences could be explained by the formation of autophagosomes even without the ATG conjugation systems (although at a reduced rate). Alternatively, these differences may be attributed to autophagy-independent functions of ATG proteins. However, as we observed the defective inflation of the swim bladder in zebrafish lacking all classes of upstream Atg proteins (Rb1cc1, Atg13, Atg101, Atg14, Atg9a and Atg9b, Atg2a and Atg2b, Vmp1), it is unlikely that the observed phenotype is caused by the non-autophagic functions of these upstream factors. Further comprehensive investigation using all classes of ATG proteins is expected to delineate the precise causes of these phenotypic differences.
In summary, our study revealed that autophagosomes fuse with immature lamellar bodies (derived from late endosomes) to promote their maturation, which is crucial for the inflation of the swim bladder in zebrafish and the lung in mice (Figure 1). This process resembles the formation of amphisomes, which are generated by the fusion of autophagosomes with late endosomes. The presence of multilamellar structures in endolysosomes has been reported in several other cell types under physiological and pathological conditions (e.g., viral infections, cancer, and lysosome storage diseases). Thus, autophagy may be more broadly involved in the formation of multilamellar structures in cells where lipids are actively loaded to endolysosomes, degraded, stored, and/or secreted.
Figure 1.
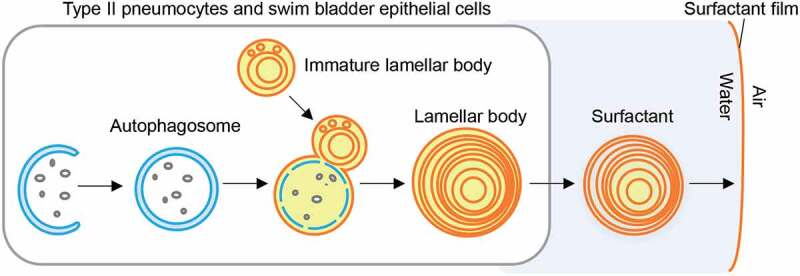
Model of the function of autophagy in lamellar body maturation in the swim bladder and lung. In the epithelial cells of these organs, autophagosomes are formed and fuse with immature lamellar bodies to increase their size and expand surfactant lipid storage. After the fusion of lamellar bodies with the plasma membrane, the surfactant is secreted and spread on the air-liquid interface as a lipid monolayer in order to lower the surface tension. Autophagy increases the size and lipid contents of lamellar bodies by remobilizing the surfactant lipids and membranes
Funding Statement
This work was supported by Exploratory Research for Advanced Technology [ERATO; grant JPMJER1702 to N.M.] from the Japan Science and Technology Agency (JST), a Grant-in-Aid for Scientific Research on Innovative Areas [Grant 25111005 to N.M.], and a Grant-in-Aid for Young Scientists [Grant 18K14694 to H.M.] from the Japan Society for the Promotion of Science.
Disclosure statement
No potential conflict of interest was reported by the authors.
Reference
- [1].Morishita H, Kanda Y, Kaizuka T, et al. Autophagy is required for maturation of surfactant-containing lamellar bodies in the lung and swim bladder. Cell Rep. 2020. December 8;33(10):108477. [DOI] [PMC free article] [PubMed] [Google Scholar]


