Abstract
Background
Plasmodium vivax (P vivax) is a focus of malaria elimination. It is important because P vivax and Plasmodium falciparum infection are co‐endemic in some areas. There are asymptomatic carriers of P vivax, and the treatment for P vivax and Plasmodium ovale malaria differs from that used in other types of malaria. Rapid diagnostic tests (RDTs) will help distinguish P vivax from other malaria species to help treatment and elimination. There are RDTs available that detect P vivax parasitaemia through the detection of P vivax‐specific lactate dehydrogenase (LDH) antigens.
Objectives
To assess the diagnostic accuracy of RDTs for detecting P vivax malaria infection in people living in malaria‐endemic areas who present to ambulatory healthcare facilities with symptoms suggestive of malaria; and to identify which types and brands of commercial tests best detect P vivax malaria.
Search methods
We undertook a comprehensive search of the following databases up to 30 July 2019: Cochrane Infectious Diseases Group Specialized Register; Central Register of Controlled Trials (CENTRAL), published in the Cochrane Library; MEDLINE (PubMed); Embase (OVID); Science Citation Index Expanded (SCI‐EXPANDED) and Conference Proceedings Citation Index‐Science (CPCI‐S), both in the Web of Science.
Selection criteria
Studies comparing RDTs with a reference standard (microscopy or polymerase chain reaction (PCR)) in blood samples from patients attending ambulatory health facilities with symptoms suggestive of malaria in P vivax‐endemic areas.
Data collection and analysis
For each included study, two review authors independently extracted data using a pre‐piloted data extraction form. The methodological quality of the studies were assessed using a tailored Quality Assessment of Diagnostic Accuracy Studies‐2 (QUADAS‐2) tool. We grouped studies according to commercial brand of the RDT and performed meta‐analysis when appropriate. The results given by the index tests were based on the antibody affinity (referred to as the strength of the bond between an antibody and an antigen) and avidity (referred to as the strength of the overall bond between a multivalent antibody and multiple antigens). All analyses were stratified by the type of reference standard. The bivariate model was used to estimate the pooled sensitivity and specificity with 95% confidence intervals (CIs), this model was simplified when studies were few. We assessed the certainty of the evidence using the GRADE approach.
Main results
We included 10 studies that assessed the accuracy of six different RDT brands (CareStart Malaria Pf/Pv Combo test, Falcivax Device Rapid test, Immuno‐Rapid Malaria Pf/Pv test, SD Bioline Malaria Ag Pf/Pv test, OnSite Pf/Pv test and Test Malaria Pf/Pv rapid test) for detecting P vivax malaria. One study directly compared the accuracy of two RDT brands. Of the 10 studies, six used microscopy, one used PCR, two used both microscopy and PCR separately and one used microscopy corrected by PCR as the reference standard. Four of the studies were conducted in Ethiopia, two in India, and one each in Bangladesh, Brazil, Colombia and Sudan.
The studies often did not report how patients were selected. In the patient selection domain, we judged the risk of bias as unclear for nine studies. We judged all studies to be of unclear applicability concern. In the index test domain, we judged most studies to be at low risk of bias, but we judged nine studies to be of unclear applicability concern. There was poor reporting on lot testing, how the RDTs were stored, and background parasitaemia density (a key variable determining diagnostic accuracy of RDTs). Only half of the included studies were judged to be at low risk of bias in the reference standard domain, Studies often did not report whether the results of the reference standard could classify the target condition or whether investigators knew the results of the RDT when interpreting the results of the reference standard. All 10 studies were judged to be at low risk of bias in the flow and timing domain.
Only two brands were evaluated by more than one study. Four studies evaluated the CareStart Malaria Pf/Pv Combo test against microscopy and two studies evaluated the Falcivax Device Rapid test against microscopy. The pooled sensitivity and specificity were 99% (95% CI 94% to 100%; 251 patients, moderate‐certainty evidence) and 99% (95% CI 99% to 100%; 2147 patients, moderate‐certainty evidence) for CareStart Malaria Pf/Pv Combo test.
For a prevalence of 20%, about 206 people will have a positive CareStart Malaria Pf/Pv Combo test result and the remaining 794 people will have a negative result. Of the 206 people with positive results, eight will be incorrect (false positives), and of the 794 people with a negative result, two would be incorrect (false negative).
For the Falcivax Device Rapid test, the pooled sensitivity was 77% (95% CI: 53% to 91%, 89 patients, low‐certainty evidence) and the pooled specificity was 99% (95% CI: 98% to 100%, 621 patients, moderate‐certainty evidence), respectively. For a prevalence of 20%, about 162 people will have a positive Falcivax Device Rapid test result and the remaining 838 people will have a negative result. Of the 162 people with positive results, eight will be incorrect (false positives), and of the 838 people with a negative result, 46 would be incorrect (false negative).
Authors' conclusions
The CareStart Malaria Pf/Pv Combo test was found to be highly sensitive and specific in comparison to microscopy for detecting P vivax in ambulatory healthcare in endemic settings, with moderate‐certainty evidence. The number of studies included in this review was limited to 10 studies and we were able to estimate the accuracy of 2 out of 6 RDT brands included, the CareStart Malaria Pf/Pv Combo test and the Falcivax Device Rapid test. Thus, the differences in sensitivity and specificity between all the RDT brands could not be assessed. More high‐quality studies in endemic field settings are needed to assess and compare the accuracy of RDTs designed to detect P vivax.
Plain language summary
Rapid tests for diagnosing malaria caused by Plasmodium vivax in people living in areas where malaria is very common
What is the aim of the review?
Malaria infection is caused mainly by two species of malaria parasite: Plasmodium falciparum and Plasmodium vivax. The aim of this review was to evaluate rapid diagnostic tests (RDTs) to diagnose P vivax infection.
Why are rapid tests for P vivax malaria important?
For clinical management, knowing which parasite species is causing the malaria is important as the drug treatments differ. For P vivax infection, an additional drug is required to eliminate the infection from the liver. For public health control of malaria, we know that P falciparum is declining over the previous 15 years, and infections from P vivax have therefore increased in importance.
What was studied in this review?
RDTs provide results quickly and are often as a dipstick. We studied RDTs that specifically test for P vivax malaria. RDTs are simple to use, point‐of‐care tests. They are suitable for use in rural settings by primary healthcare workers, using drop of blood on the dipstick that causes colour change and a distinct line that indicates a positive test result. Healthcare workers in rural areas can perform RDTs for P vivax without needing a laboratory or special equipment. We wanted to find out which brands of RDTs were the most accurate for diagnosing P vivax malaria. We compared the new tests against the standard form of diagnosis with microscopy, and also more recent methods polymerase chain reaction (PCR): a molecular method to identify P vivax DNA in blood samples.
What are the main results of the review?
We included 10 studies that looked at the accuracy of six diagnostic test brands for detecting P vivax malaria in people with suspected malaria symptoms. The studies were conducted in Ethiopia (four studies), India (two studies) and Bangladesh, Brazil, Colombia, and Sudan (one study each).
Compared with microscopy, the Care Start Malaria Pf/Pv Combo test performed well with 99% sensitivity and specificity (four studies). This means that:
• for every 100 people tested who have P vivax malaria, one person will have a negative test result, and might not receive the right treatment soon enough;
• for every 100 people tested who do not have P vivax malaria, one will have a positive result, and might receive unnecessary treatment.
Compared with microscopy, the Falcivax Device Rapid test had a sensitivity of 77% and a specificity of 99% (two studies). This means that:
• For every 100 people tested who have P vivax malaria, 23 people will have a negative test result; and,
• for every 100 people tested who do not have P vivax malaria, one person will have a positive result.
We are moderately confident (certain) in the accuracy results for the Care Start Malaria Pf/Pv Combo test. The results are from a small number of studies (four), so our findings may change when results from further studies become available.
We are less confident in the accuracy results for the Falcivax Device Rapid test, because these came from only two studies. Our findings for this test will probably change when results from further studies become available.
Our results are based on a small number of studies, so we could not reliably assess all six brands of antibody test or compare their accuracy. Most studies included in this review had limitations: it was not clear how people were selected for testing, or how the study results were assessed and checked, which could have affected the results. Some rapid antibody tests were investigated by only one study. Some studies did not report clearly how common P malaria was in the area where the study was done.
How up‐to‐date is this review?
The review authors searched for studies published up to 30 July 2019.
Summary of findings
Summary of findings 1. Summary of findings table for RDTs for diagnosing P vivax malaria.
| Population: people presenting with symptoms of uncomplicated malaria | ||||||
| Prior testing: none | ||||||
| Setting: ambulatory healthcare settings in P vivax endemic areas | ||||||
| Index tests: immunochromatography‐based rapid diagnostic tests (RDTs) for P vivax malaria that meet the WHO malaria RDT performance criteria (WHO 2017b) | ||||||
| Reference standards: conventional microscopy, polymerase chain reaction (PCR) | ||||||
| Target condition:P vivax malaria | ||||||
| Importance: accurate and fast diagnosis of P vivax from other malaria species allows appropriate treatment to be provided quickly | ||||||
| Study design: retrospective or prospective cohort or cross‐sectional | ||||||
| Findings: 10 studies of six different RDT brands (CareStart Malaria Pf/Pv Combo test, Falcivax Device Rapid test, Immuno‐Rapid Malaria Pf/Pv test, SD Bioline Malaria Ag Pf/Pv test, OnSite Pf/Pv test and Test Malaria Pf/Pv rapid test) for P vivax malaria were included. Only two brands (CareStart Malaria Pf/Pv Combo test and Falcivax Device Rapid test) were evaluated against the same reference standard by more than one study. | ||||||
| Limitations: a small number of studies were included in the analyses and meta‐analyses were only possible for two RDT brands. Studies often did not report how patients were selected, the blinding of the RDT results to the reference standard and the storage conditions and lot testing of RDTs. | ||||||
| Outcome | № of studies | № of patients | Numbers in a cohort of 1000 patients tested (95% CI)a | Certainty of the evidence (GRADE)b | ||
| Prevalence of 0.5% | Prevalence of 5% | Prevalence of 20% | ||||
| Test (reference standard): CareStart Malaria Pf/Pv Combo test (microscopy), pooled sensitivity (95% CI) = 99% (94% to 100%) and pooled specificity (95% CI) = 99% (99% to 100%), positive likelihood ratio (95% CI) = 141.09 (68.18 to 292.00) and negative likelihood ratio (95% CI) = 0.01 (0.00 to 0.06) | ||||||
|
True positives (patients with P vivax malaria) |
4 | 251 | 5 (5 to 10) | 50 (47 to 50) | 198 (188 to 200) | ⊕⊕⊕⊝ MODERATE1 |
|
False negatives (patients incorrectly classified as not having P vivax malaria) |
0 (0 to 0) | 0 (0 to 3) | 2 (0 to 12) | |||
|
True negatives (patients without P vivax malaria) |
2147 | 985 (980 to 995) | 941 (941 to 950) | 792 (792 to 800) | ⊕⊕⊕⊝ MODERATE1 |
|
| False positives (patients incorrectly classified as having P vivax malaria) | 10 (0 to 10) | 9 (0 to 9) | 8 (0 to 8) | |||
| Test (reference standard): Falcivax Device Rapid test (microscopy), pooled sensitivity (95% CI) = 77% (53% to 91%) and pooled specificity (95% CI) = 99% (98% to 100%), positive likelihood ratio (95% CI) = 120.31 (43.10 to 335.87) and negative likelihood ratio (95% CI) = 0.23 (0.10 to 0.53) | ||||||
|
True positives (patients with P vivax malaria) |
2 | 89 | 4 (3 to 5) | 39 (27 to 46) | 154 (106 to 182) | ⊕⊕⊝⊝ LOW1,2 |
|
False negatives (patients incorrectly classified as not having P vivax malaria) |
1 (0 to 2) | 11 (4 to 23) | 46 (18 to 94) | |||
|
True negatives (patients without P vivax malaria) |
621 | 985 (975 to 995) | 941 (931 to 950) | 792 (784 to 800) | ⊕⊕⊕⊝ MODERATE1 |
|
| False positives (patients incorrectly classified as having P vivax malaria) | 10 (0 to 20) | 9 (0 to 19) | 8 (0 to 16) | |||
aMedian values were chosen from ranges of prevalence considered to be moderate, low, and very low transmission settings for P vivax (WHO 2017c). bMethods are lacking to assess the determinants and extent of publication bias for diagnostic studies. However, in this table, we considered publication bias ‘undetected'. 1Downgraded for risk of bias by one. 2Downgraded for imprecision by two due to wide confidence intervals.
GRADE certainty of the evidence.
High: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Background
Target condition being diagnosed
Malaria is a life‐threatening disease caused by Plasmodium species (Plasmodium spp.), transmitted by the bite of a female Anopheles mosquito. Currently, there are five established Plasmodium spp. that cause malaria in humans. The two most common are Plasmodium falciparum (P falciparum) and Plasmodium vivax (P vivax). P vivax malaria is a relapsing form, which is rarely fatal but can cause serious anaemia in children (White 2018). There has been an increased focus on P vivax, as malaria‐endemic settings that also have P falciparum have made progress in P falciparum control. In the World Health Organization (WHO) regions of the Americas, South‐East Asia and Eastern Mediterranean, P vivax is the predominant Plasmodium spp., and causes 64%, greater than 30%, and greater than 40% of all malaria cases, respectively, in these regions (WHO 2017a). People with malaria caused by P vivax can have relapses due to the dormant liver stage hypnozoites. People can carry hypnozoites ranging from a few weeks to more than 12 months before reporting symptoms again (Campo 2015). Primaquine is recommended additionally to standard malaria treatment for P vivax and Plasmodium ovale (P ovale) to clear these liver stage parasites. Due to this, it is important to have diagnostic tests that are highly sensitive and that can specifically detect P vivax from other Plasmodium spp.
Index test(s)
Rapid diagnostic tests (RDTs) (WHO 2003), detect parasite‐specific antigens in a drop of fresh blood through lateral flow immunochromatography (WHO 2006). Generally, RDTs do not require a laboratory, any special equipment, or specialized training. They are easy to use and can give results as a simple positive or negative result within 15 to 20 minutes based on the antibody affinity (referred to as the strength of the bond between an antibody and an antigen) and avidity (referred to as the strength of the overall bond between a multivalent antibody and multiple antigens) (Talman 2007; WHO 2006). Therefore, RDTs are, in general, suitable for remote areas with limited facilities and lack of laboratory expertise. They typically have a shelf life of 24 months and need to be kept dry and away from temperature extremes (greater than 40°C). They may fail to detect malaria where there are low levels of Plasmodium parasites (and antigens) in the blood and false positives are possible due to cross reactions with other disease conditions, presence of certain immunological factors, and gametocytaemia (Gatton 2018; Kakkilaya 2003).
There is strong evidence that storage conditions of the RDT affect their performance (Moonasar 2007). The parasite density of the blood sample can also affect the performance of the RDT. The WHO malaria RDT product‐testing programme report investigated the effect of parasite density by testing individual products under laboratory conditions using standardized blood samples at low and high parasite densities (200 and 2000 parasites/µL), and reported the ‘panel detection score' (WHO 2012). An existing Cochrane Review on non‐falciparum RDTs found that parasite density and storage conditions are often poorly reported in field studies (Abba 2014). Moreover, due to the lag period between when the RDT was evaluated by the WHO malaria RDT product testing programme to when the RDT is actually used in the field, manufacturers may have modified the RDT during this period.
Different types of RDT use different types of antibody or combination of antibodies to detect Plasmodium antigens. Some antibodies aim to detect a particular species while others are pan‐malarial, aiming to detect all types of Plasmodium spp. Currently, all commercial RDTs specific for P vivax use P vivax‐specific lactate dehydrogenase (LDH) antigens (WHO 2017b).
Clinical pathway
People of any age with malaria typically present to medical care with non‐specific symptoms of fever, headache, chills, or rigors. The RDTs are most commonly used at the point of presentation with these symptoms, most often in settings where quality microscopy is not available. Parasitological diagnosis is recommended prior to commencing on any treatment (WHO 2015a).
Prior test(s)
It is unlikely that patients would have had previous testing for their current infection prior to presentation to healthcare centres with symptoms of malaria. One key benefit of RDTs is the ease of use at point of care. For the purpose of this review, we did not address the sensitivity or specificity of P vivax‐specific RDTs for confirming efficacy of treatment as this is not recommended practice.
Role of index test(s)
Malaria is a common cause of fever in endemic regions. Given the non‐specific symptoms patients with malaria often present with, a parasitological test is recommended to make a formal diagnosis (WHO 2015b). Often people of any age or gender presenting to a healthcare clinic with a history of fever in a malaria‐endemic region will undergo a malaria test as part of a routine initial work‐up. As such, the population receiving the index test would be identified solely on the basis of the clinical history and physical examination. RDTs have a role in malaria diagnosis where there is no access to good quality microscopy services and in outbreak investigation or surveys of parasite prevalence. The pre‐test probability of clinical malaria is an important determinant of the RDT performance. In the absence of strong clinical suspicion of malaria, it may not be reliable to use an RDT, because the test results from this device could potentially be misleading or inaccurate. Reliable diagnosis of P vivax malaria with RDTs would not only benefit the individual by allowing treatment of the blood stage and latent hypnozoite stage, but also would have benefits at a population level by potentially reducing low‐level ongoing transmission due to relapsing disease. Widespread use of accurate RDTs can facilitate greater diagnosis and treatment rates of P vivax malaria in areas where there is inadequate access to high‐quality microscopy.
True positive results would allow effective treatment of active disease and facilitate prevention of relapse using drugs that target the liver stage hypnozoites such as primaquine or tafenoquine, thus effectively treating individuals and reducing the risk of onward transmission. True negative results facilitate accurate diagnosis by narrowing differential diagnoses of people presenting to care with fever and non‐specific symptoms. False positives would potentially lead to over treatment of individuals with primaquine, tafenoquine and either chloroquine or artemisinin combination therapies and would mean that patients are not treated for the actual cause of their symptoms. False negatives would lead to potential relapsing disease and potentially ongoing transmission at the population level.
Alternative test(s)
Microscopic examination of Giemsa‐stained thick and thin blood films remains the conventional laboratory method. Microscopic examination has good sensitivity and specificity, and it allows species and stage differentiations and quantification of parasites, all of which are important in assessing disease severity, monitoring response to treatment, and prescribing appropriate therapy. Intensive examination is more likely to reveal parasitaemia so the test is carried out with a fixed number of fields examined. Infections may be missed if slides are not examined carefully (Wongsrichanalai 2007). Very low parasitaemia may be missed even by good quality microscopy; the limit of detection of thick smear microscopy has been estimated at approximately four to 20 asexual parasites per µL, although a threshold of 50 to 100 asexual parasites per µL is more realistic under field conditions (Wongsrichanalai 2007). False positive results are also possible; if blood slides are not prepared carefully, artefacts may be formed, which can be mistaken for Plasmodium parasites (Wongsrichanalai 2007).
The polymerase chain reaction (PCR), a molecular method based on DNA amplification, is the most analytically sensitive method of detecting parasites in the blood. Compared to microscopy, PCR is less prone to observer error and more sensitive at low levels of parasitaemia (Han 2017; Snounou 1993). For PCR, the limit of detection may be as low as 0.004 asexual parasites per µL (Hänscheid 2002). This increased ability to detect low level parasitaemia is important as submicroscopic parasitaemiae may have clinical and public health significance and the prevalence of asymptomatic submicroscopic infection is high in some areas (Chen 2016). PCR is currently not widely available due to logistical constraints and the need for specially‐trained technicians and a well‐equipped laboratory. It is usually used only for research purposes.
Rationale
P vivax is becoming increasingly important, especially in regions targeting malaria elimination. In areas of co‐endemicity, P vivax malaria is increasing disproportionally compared to P falciparum malaria. Moreover, treatment for P vivax and P ovale malaria differs from treatments for other types of malaria. Therefore, it is important that the RDT correctly distinguish P vivax from other species. Geographically, P vivax has a much wider infection range compared to other Plasmodium spp. This may increase over time due to climate change (Culleton 2012). Historically, autochthonous transmission of P vivax also occurred in temperate climates, such as that of England (Dobson 1994). Autochthonous transmission is referred to as the spread of a disease from one individual and received by another individual from the same place. An existing Cochrane Review assessing RDTs for diagnosing uncomplicated non‐falciparum malaria was conducted in 2014 (Abba 2014). A subset of this review included RDTs that diagnosed P vivax. This review only assesses the diagnostic accuracy of RDTs that specifically detect P vivax with Pvivax‐specific LDH) antigens.
Objectives
To assess the diagnostic accuracy of RDTs for detecting P vivax malaria parasitaemia in people living in malaria‐endemic areas who present to ambulatory healthcare facilities with symptoms suggestive of malaria, and to identify which types and brands of commercial tests best detect P vivax malaria.
Methods
Criteria for considering studies for this review
Types of studies
We included retrospective or prospective cohort or cross‐sectional studies that assessed the accuracy of an RDT, or compared the accuracy of two or more RDTs, in the same study population (i.e. comparative accuracy studies). We excluded case‐control studies because they are known to overestimate test accuracy (Whiting 2011). Eligible studies included a consecutive series of patients, or a randomly selected series of patients. If the study did not explicitly state that the sampling was consecutive or random, the study was considered unclear but was still included. We excluded studies if they did not present sufficient data to allow us to extract or deduce the number of true positives, false positives, false negatives, and true negatives (i.e. 2 x 2 table data). We also excluded studies published in predatory journals, which is referred to as journals that accept articles for publication for a fee without providing peer‐review or quality checks for plagiarism or ethical approval.
Participants
Studies recruiting people living in P vivax‐endemic areas attending ambulatory healthcare settings with symptoms of uncomplicated malaria were eligible.
We excluded studies if participants:
had travelled from non‐malarious region to malarious regions, e.g. travellers or displaced populations;
had been previously treated for their current malaria infection or the test was performed to assess whether treatment was successful, or both;
had symptoms of severe malaria as defined by the WHO clinical definition (WHO 2014);
did not have symptoms of malaria as defined by history of fever, headache, or chills/rigors; or
were recruited through active case finding (for example, door to door surveys).
Index tests
Studies evaluating any immunochromatography‐based RDT specifically designed to detect P vivax malaria. We only included RDTs that met the WHO malaria RDT performance criteria (WHO 2017b).
Target conditions
Studies aimed at detecting P vivax malaria.
Reference standards
Studies that diagnosed P vivax malaria using at least one of the following two reference standards:
Conventional microscopy of thick blood smears and thin blood smears. Presence of asexual parasites of any density is regarded as a positive smear. Once the diagnosis is established – usually by detecting parasites in the thick smear – the laboratory technician can examine the thin smear to determine the malaria species and the parasitaemia, or the percentage of the patient’s red blood cells that are infected with malaria parasites. The thin and thick smears are able to provide all three of these vital pieces of information. Ideally, blood smears would be examined independently and in duplicate with more than 100 high‐power fields;
PCR, including quantitative PCR (qPCR), nested PCR (nPCR), and real‐time PCR (rPCR). We also included studies that used loop‐mediated isothermal amplification (LAMP). Most PCR‐based assays for P vivax are only available as laboratory‐developed tests, which means they are rarely used clinically outside of research projects where P vivax malaria is endemic. They are especially useful for diagnosing asymptomatic people as the assays have high sensitivity. Molecular diagnostics theoretically have a lower limit of detection than both RDTs and microscopy depending on the training of microscopists and quality of samples analysed. Significant variation exists between molecular diagnostics developed including type of input material (DNA, RNA, or whole blood), target gene, (number of) species detected, primer/probe composition and concentration, amplification technique (PCR or isothermal), read‐out (gel‐electrophoresis, fluorescence detection, lateral flow), and whether it is qualitative or quantitative. However, no important differences have been found in the accuracy of these tests (Roth 2016).
For studies that used both reference standards, we extracted 2 x 2 data for each reference standard and stratified the analyses by reference standard.
Search methods for identification of studies
We attempted to identify all relevant studies regardless of language or publication status (published, unpublished, in press, and in progress).
Electronic searches
We searched the following databases up to 30 July 2019 using the search terms and strategy described in Appendix 1: Cochrane Infectious Diseases Group Specialized Register; Central Register of Controlled Trials (CENTRAL), published in the Cochrane Library (issue 7, 2019); MEDLINE (PubMed, from 1966); Embase (OVID, from 1947); Science Citation Index Expanded (SCI‐EXPANDED) and Conference Proceedings Citation Index‐ Science (CPCI‐S), both in the Web of Science, from 1900; LILACS (BIREME).
We also searched the WHO International Clinical Trials Registry Platform (WHO ICTRP; www.who.int/ictrp/en/) and ClinicalTrials.gov (clinicaltrials.gov/ct2/home) for trials in progress, using "vivax malaria", "Plasmodium vivax", and "rapid diagnostic test*" or RDT* as search terms.
Searching other resources
We checked the reference lists of studies identified by the above methods.
Data collection and analysis
Selection of studies
Three review authors (RA, LC and SJ) independently assessed the study eligibility by examining the title and abstract of each article identified by the literature search and excluded obviously irrelevant studies. If a review author considered the abstract to be potentially eligible, we obtained the full‐text article. Three review authors independently assessed each full‐text article against the predefined inclusion and exclusion criteria, as stated in the ‘Criteria for considering studies for this review' section, and resolved any disagreements by discussion. All articles that were excluded after full‐text assessment are listed with reasons for exclusion in the ʽCharacteristics of excluded studies' table. We illustrated the study selection process with a PRISMA flow diagram (Figure 1).
1.
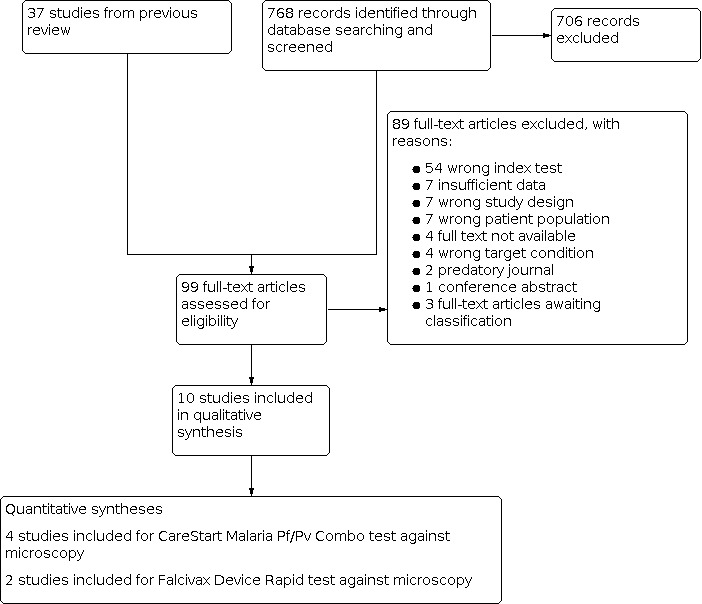
Study flow diagram.
Data extraction and management
Three review authors (RA, LC and SJ) independently extracted data using a pre designed data extraction form.
We extracted the following data.
Authors, publication year, and journal.
Study design.
Study start date.
Characteristics study participants (age, gender, co morbidities, and pregnancy).
Study inclusion/exclusion criteria.
Study setting.
Malaria species in study setting.
Malaria prevalence and endemicity in study setting.
Reference standard.
Index test (brand name, target antigen, and batch numbers).
Additional tests (and their results).
RDT and reference standard setting.
Lot testing of RDT used.
Transport and storage conditions of RDTs.
Training level of person performing index test.
Training level of person performing reference standard (and if available the WHO certified training level of the microscopist).
Number of high power fields observed in microscopy.
Parasite density of microscopy positive cases or PCR.
Observers or repeats used.
Number of indeterminate, missing or unavailable test results.
Number of true positives, false positives, false negatives, and true negatives.
Type of molecular amplification assay.
Volume of blood samples.
Limit of detection for PCR.
We resolved any discrepancies in data extraction by discussion. We contacted the authors of primary studies when we could not resolve any disagreements.
Assessment of methodological quality
We used the revised tool for the Quality Assessment of Diagnostic Accuracy Studies (QUADAS‐2) to assess the risk of bias and applicability of included studies (Whiting 2011). We tailored the tool to the context of the review as shown in Appendix 2. Three review authors (RA, LC and SJ) independently assessed methodological quality using the tailored QUADAS‐2 tool. We resolved any disagreements through consensus. We used both graphics and text to summarize the results.
Statistical analysis and data synthesis
We stratified all analyses by the type of reference standard used. We plotted estimates of sensitivity and specificity from the included studies in forest plots and in receiver operating characteristic (ROC) space using the software, Review Manager 5 (RevMan 5) (RevMan 2014). We planned to perform meta‐analysis using the bivariate model to estimate summary sensitivities and specificities (summary points) (Chu 2006; Macaskill 2010; Takwoingi 2015b). However, due to sparse data or few studies, we simplified the models to univariate random effects logistic regression models to pool sensitivity and specificity separately (Takwoingi 2015a). We performed meta‐analyses using the 'meqrlogit' command in Stata (STATA 2015). Due to the limited number of included studies we did not perform meta‐analyses to compare the accuracy of different RDT brands as planned. However, we summarized individual study estimates from head‐to‐head comparisons of brands in a table.
Investigations of heterogeneity
We intended to investigate any heterogeneity from the pooled analyses with pre‐specified factors, as stated in our secondary objective. Due to the limited number of studies, we were unable to investigate heterogeneity as planned.
Sensitivity analyses
We did not have sufficient data for sensitivity analyses.
Assessment of the certainty of the evidence
We assessed the certainty of the evidence for comparisons where there were sufficient studies enabling meta‐analyses (i.e. quality of evidence or confidence in effect estimates) using the GRADE approach and GRADEpro Guideline Development Tool software (GRADE 2013; GRADEpro GDT 2015). In the context of a systematic review, the ratings of the certainty of the evidence reflect the extent of our confidence that the estimates of test accuracy are correct. As recommended, we rated the certainty of the evidence as either high (not downgraded), moderate (downgraded by one level), low (downgraded by two levels), or very low (downgraded by more than two levels) for four domains: risk of bias, indirectness, inconsistency, and imprecision. For sensitivity and specificity, the certainty of the evidence initially started as high when there were high‐quality cross‐sectional or cohort studies that enrolled participants with diagnostic uncertainty. If we found a reason for downgrading the certainty of the evidence, we classified the reason as either serious (downgraded by one level) or very serious (downgraded by two levels).
Three review authors (RA, LC and SJ) discussed judgments and reached a consensus. We applied GRADE in the following way.
Risk of bias: we used QUADAS‐2 to assess risk of bias.
Indirectness: we considered indirectness from the perspective of test accuracy. We used QUADAS‐2 to assess applicability concerns and looked for important differences between the populations studied (for example, in the transmission intensity as defined by the WHO World Malaria Report or WHO malaria country profiles for the corresponding year), the setting, and the review question.
Inconsistency: GRADE recommends downgrading for unexplained inconsistency in sensitivity and specificity estimates.
Imprecision: we considered the width of the confidence intervals (CIs), and asked ourselves, “would we make a different decision if the lower or upper limit of the 95% confidence interval (CI) represented the truth?” In addition, we calculated absolute numbers of true positives, false negatives, false positives, and true negatives, as well as ranges for these values based on the CIs of the pooled estimates of sensitivity and specificity for various prevalences of P vivax malaria; we also made judgements on imprecision using these calculations. We also calculated positive and negative likelihood ratios with their 95% CIs.
Assessment of reporting bias
We did not assess publication bias due to the uncertainty about the determinants of publication bias for diagnostic accuracy studies, and the inadequacy of tests for detecting funnel plot asymmetry (Deeks 2005).
Results
Results of the search
We identified and screened 768 reports through the database searches conducted on 30 July 2019. We excluded 706 of these reports based on their title or abstract alone. We considered the remaining 62 articles for full‐text screening, along with the 37 studies included in the non‐falciparum malaria review by Abba 2014. Of the 109 articles, we excluded 99 for various reasons as reported in the Characteristics of excluded studies section, shown in Figure 1. We included 10 studies, of which five studies (Alam 2011; Chanie 2011; Mekonnen 2010;Singh 2010; Sharew 2009) were also included in the review by Abba 2014. The 10 studies assessed six different RDT brands (CareStart Malaria Pf/Pv Combo test, Falcivax Device Rapid test, Immuno‐Rapid Malaria Pf/Pv test, SD Bioline Malaria Ag Pf/Pv test, OnSite Pf/Pv test and Test Malaria Pf/Pv rapid test). One study directly compared the accuracy of two RDT brands (Falcivax Device Rapid test and OnSite Pf/Pv test) (Alam 2011). The six RDT brands detect P vivax as part of a mixed infection with P vivax‐specific LDH antigens. The tests have two test lines, an HRP‐2 line to detect P falciparum and an pLDH line to detect P vivax. For our analysis we only considered the presence of the pLDH line.
Of the 10 included studies, six used microscopy (Chanie 2011; Costa 2019; Hailu 2014; Mekonnen 2010; Sharew 2009; Singh 2010), one used PCR (Mussa 2019), two used both microscopy and PCR separately (Alam 2011; Saha 2017), and one used microscopy corrected by PCR (Mendoza 2013) as the reference standard. Four of the studies were conducted in Ethiopia (Chanie 2011; Hailu 2014; Mekonnen 2010; Sharew 2009), two in India (Saha 2017; Singh 2010), and one each in Bangladesh (Alam 2011), Brazil (Costa 2019), Colombia (Mendoza 2013), and Sudan (Mussa 2019).
There was a lack of detail on how the RDTs were stored and whether RDT lots were quality‐controlled prior to testing. Key study characteristics that may affect the performance of RDTs (e.g. training level of person performing the RDT, storage conditions, and parasite density of microscopy‐positive cases or PCR) are summarised in Table 2.
1. Summary of key study characteristics.
| Study | Country | Sample size | Sex | Age | RDT brand | Personnel performing RDT | Storage conditions of RDT | Reference standard | Personnel performing reference standard | Parasite density of positive cases |
| Alam 2011 | Bangladesh | 338 | 49.7% male 50.3% female |
Median (range): 14 years (18 months to 82 years) | OnSite Pf/Pv test (CTK Biotech Inc, USA) Falcivax Device Rapid test for malaria Pv/Pf (Zephyer Biomedicals, Goa) |
An experienced medical technologist | Unclear, although study stated that the instructions of the manufacturers were followed. | PCR and microscopy (separately) | Slides assessed by two independent microscopists | Of 21 P vivax positive slides, parasite count ranged from 32 to 25,120 parasites/μL of blood, with a median of 5,040 (IQR 520 to 17,160) parasites/μL blood. |
| Chanie 2011 | Ethiopia | 1092 | 51.4% male 48.6% female | Mean (SD): 22 (12.8) years | CareStart Malaria Pf/Pv Combo test (Access Bio Inc, Somerset, NJ) | Experienced malaria technicians | Kept at the local temperature of the region without any controlling system of the storage temperature during data collection | Microscopy | Experienced technicians examined the slides | Not reported |
| Costa 2019 | Brazil | 181 | 64.1% male 35.9% female | Mean (SD): 41.7 (14.4) years | Immuno‐Rapid Malaria Pf/Pv test (Wama Diagnostica, Sao Paulo, Brazil) | Hospital laboratory staff | According to manufacturer's instructions (2ºC to 30ºC until the expiration date) | Microscopy | Experienced microscopists then examined the slides | Mean parasitaemia detected by TBS for P vivax malaria was 1,206.5 parasites/mm3 blood |
| Hailu 2014 | Ethiopia | 398 | 44.2% male 55.8% female | Range: 1 to 70 years | CareStart Malaria Pf/Pv Combo test (Access Bio Inc, Somerset, NJ) | Not reported | Stored at room temperature according to manufacturer's instructions | Microscopy | Two experienced malaria technologists performed the microscopy | Not reported |
| Mekonnen 2010 | Ethiopia | 240 | 57.5% male 42.5% female |
Mean (range): 25 years (1 to 60 years) | CareStart Malaria Pf/Pv Combo test (Access Bio Inc, Somerset, NJ) | Experienced malaria technicians | According to manufacturer's instructions | Microscopy | Three experienced technicians examined the slides | Not reported |
| Mendoza 2013 | Colombia | 383 | 52.5% male 47.5% female | Range: 6 to 92 years | SD Bioline Malaria Ag Pf/Pv test (Standard Diagnostics Inc) | Conducted by a trained person | According to manufacturer’s recommendations (1ºC to 40ºC) | Microscopy corrected with PCR | Blood films were examined by two experienced readers | Parasitemia for P vivax ranged from 40 to 40,000 parasites/µL |
| Mussa 2019 | Sudan | 59 | 45.8% male 54.2% female | Not reported | Test Malaria Pf/Pv rapid test (Alltest Biotech, China) | Not reported | Unclear, although study stated that instructions of the manufacturer were followed | PCR | Not reported | Not reported |
| Saha 2017 | India | 200 | 56.0% male 44.0% female | Mean: 34.6 years 11 to 20 years: 20.5% 21 to 60 years: 68.5% <10 years: 2.5% > 61 years: 8.5% |
SD Bioline Malaria Ag Pf/Pv test (Standard Diagnostics Inc) | Microscopy, RDT and PCR done by different technicians | Unclear, although study stated that instructions of the manufacturer were followed | PCR and Microscopy (separately) | Blood films were examined by two microscopists having >15 years of experience | Not reported |
| Sharew 2009 | Ethiopia | 668 | 54.0% males 46.0% females |
Range: 6 months to 75 years | CareStart Malaria Pf/Pv Combo test (Access Bio Inc, Somerset, NJ) | Experienced malaria technicians | Stored according to manufacturer's instructions | Microscopy | Thick and thin smears determined by two experienced malaria technicians | Not reported |
| Singh 2010 | India | 372 | Not reported | Mean (SD): 15 (14.1) years | Falcivax Device Rapid test for malaria Pv/Pf (Zephyer Biomedicals, Goa) | Two research assistants | Detailed storage information provided | Microscopy | Blood films examined by an experienced microscopist | Not reported |
PCR = polymerase chain reaction; RDT = rapid diagnostic test; SD = standard deviation; TBS =thick blood smear
Methodological quality of included studies
The results of the risk of bias and applicability assessment are summarised in Figure 2. One study was judged to be at low risk of bias in all four domains of the QUADAS‐2 tool (Saha 2017). This study assessed the SD Bioline Malaria Ag Pf/Pv test.
2.
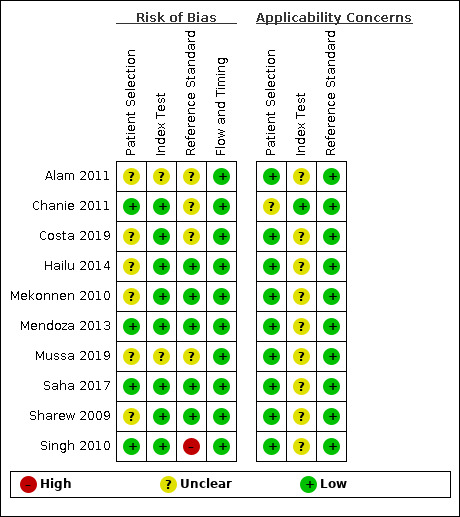
Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
Patient selection
Six (60%) studies were at unclear risk of bias in the patient selection domain because the method of participant recruitment (random or consecutive) was unclear (five studies), and/or the exclusion criteria were unclear (five studies). All studies were of low concern regarding applicability as they were all conducted in settings endemic with P vivax. However, Saha 2017 and Mussa 2019 did not report the prevalence of P vivax malaria. The remaining eight studies reported P vivax malaria or malaria in general as prevalent or endemic, but it was unclear to what degree.
Index test
We judged eight (80%) studies to be at low risk of bias in this domain because the results of the RDTs were interpreted without knowledge of the results of the reference standard. We judged the risk of bias for the remaining two studies to be unclear (Alam 2011; Mussa 2019). We judged the applicability of eight studies to be unclear, as poor reporting of the storage conditions or lot testing hampered the assessment. Singh 2010 provided thorough detail of how their RDT was stored, but it was unclear whether these conditions followed the instructions of the manufacturer. Applicability in this study was thus unclear. This study tested the temperature stability of the tests (see Table 2). Chanie 2011 evaluated the CareStart Malaria Pf/Pv Combo test. This was the only study considered to be of low applicability concern, because lot testing was reported.
Reference standard
We judged five studies to be at low risk of bias in the reference standard domain (Mendoza 2013; Saha 2017; Hailu 2014; Mekonnen 2010; Sharew 2009), while we judged one to be at high risk of bias (Singh 2010). We judged the remaining four studies to be at unclear risk of bias in this domain. It was unclear for two studies whether the results of the reference standard were interpreted without knowledge of the RDT results (Alam 2011, Mussa 2019), and it was unclear for two studies whether the results of the reference standard could classify the target condition. Costa 2019 and Chanie 2011 did not provide enough information on the reference standard to deduce if at least two microscopists independently examined the same slides from microscopy. We deemed Singh 2010 to be at high risk of bias because the second microscopist did not verify all of the reference standard results.
Flow and timing
We judged all 10 studies to be at low risk of bias in the flow and timing domain. All studies avoided partial verification, differential verification and incorporation bias, and reasons for any withdrawals were recorded. Nine studies appeared to have no uninterpretable results because the number of participants enrolled matched the number in the analysis. The remaining study reported two invalid RDT results, which were retested with the same test kits by taking fresh blood from the patients (Hailu 2014). However, it was unclear whether the same blood sample was used for the reference standard.
Test comparison
Although the QUADAS‐2 tool does not specifically address risk of bias in a test comparison, we additionally considered the potential for such bias in a study that directly compared two RDT brands (OnSite Pf/Pv test and Falcivax Device Rapid test) (Alam 2011). It was unclear whether the results of one RDT brand were interpreted without knowledge of the results of the other brand. The study used both microscopy and PCR as two separate reference standards, but it was unclear whether the conduct and interpretation of the results from these two reference standards were done independently of each other.
Findings
Verified by PCR
Three studies (Alam 2011; Mussa 2019; Saha 2017) evaluated the accuracy of four different brands of RDTs against PCR (Figure 3; Table 3). One of the studies had no cases of P vivax malaria, so sensitivity was not estimable (Mussa 2019). The sensitivities of the RDTs ranged between 77% and 86% and the specificities ranged between 93% and 100%.
3.
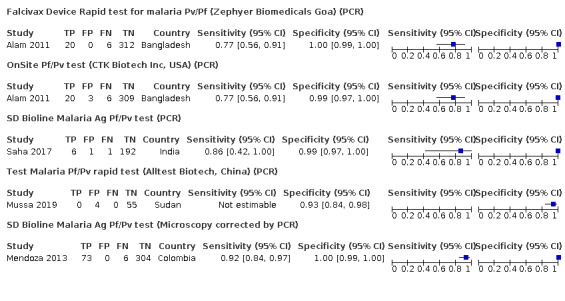
Forest plot of brands of rapid diagnostic tests verified against PCR or microscopy corrected with PCR
2. Comparison of microscopy and PCR reference standards for P vivax.
| RDT brand | Microscopy | PCR | Microscopy corrected with PCR | |||||||||
| Number of studies | Number of participants (P vivax malaria cases) | Sensitivity (95% CI) (%) | Specificity (95% CI) (%) | Number of studies | Number of participants (P vivax malaria cases) | Sensitivity (95% CI) (%) | Specificity (95% CI) (%) | Number of studies | Number of participants (P vivax malaria cases) | Sensitivity (95% CI) (%) | Specificity (95% CI) (%) | |
| CareStart Malaria Pf/Pv Combo test (Access Bio Inc, Somerset, NJ) | 4 | 2398 (251) | 99% (94% to 100%) | 99% (99% to 100%) | 0 | ‐ | ‐ | ‐ | 0 | ‐ | ‐ | ‐ |
| Falcivax Device Rapid test for malaria Pv/Pf (Zephyer Biomedicals, Goa) | 2 | 710 (89) | 77% (53% to 91%) | 99% (98% to 100%) | 1 | 338 (26) | 77% (56% to 91%) | 100% (99% to 100%) | 0 | ‐ | ‐ | ‐ |
| Immuno‐Rapid Malaria Pf/Pv test (Wama Diagnostica, Sao Paulo, Brazil) | 1 | 181 (95) | 99% (94% to 100%) | 100% (96% to 100%) | 0 | ‐ | ‐ | ‐ | 0 | ‐ | ‐ | ‐ |
| SD Bioline Malaria Ag Pf/Pv test (Standard Diagnostics Inc) | 1 | 200 (4) | 75% (19% to 99%) | 98% (95% to 99%) | 1 | 200 (7) | 86% (42% to 100%) | 99% (97% to 100%) | 1 | 383 (79) | 92% (84% to 97%) | 100% (99% to 100%) |
| OnSite Pf/Pv test (CTK Biotech Inc, USA) | 1 | 338 (21) | 90% (70% to 99%) | 99% (97% to 100%) | 1 | 338 (26) | 77% (56% to 91%) | 99% (97% to 100%) | 0 | ‐ | ‐ | ‐ |
| Test Malaria Pf/Pv rapid test (Alltest Biotech, China) | 0 | ‐ | ‐ | ‐ | 1 | 59 (0) | Not estimable | 93% (84% to 98%) | 0 | ‐ | ‐ | ‐ |
PCR = polymerase chain reaction; RDT = rapid diagnostic test.
Verified by microscopy
Eight studies conducted in four different countries evaluated the accuracy of RDTs against microscopy (Figure 4). Five different RDT brands were assessed: CareStart Malaria Pf/Pv Combo test (four studies), Falcivax Device Rapid test (two studies), Immuno‐Rapid Malaria Pf/Pv test (one study), OnSite Pf/Pv test (one study), and SD Bioline Malaria Ag Pf/Pv test (one study).
4.
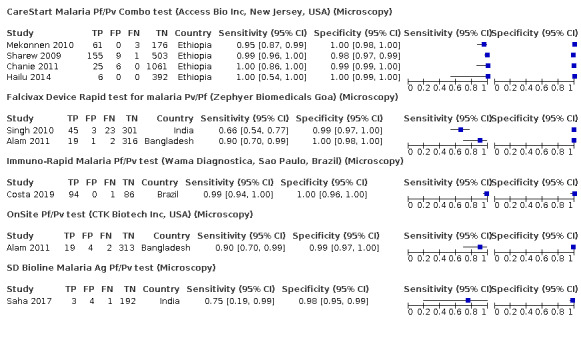
Forest plot of brands of rapid diagnostic tests verified against microscopy, within each brand sorted by sensitivity and specificity
In the four CareStart Malaria Pf/Pv Combo test studies (251 P vivax malaria cases, 2398 patients), the sensitivity ranged from 95% to 100% and specificity ranged from 98% to 100%. The pooled sensitivity (95% CI) was 99% (94% to 100%) and the pooled specificity (95% CI) was 99% (99% to 100%) (Figure 5). The positive likelihood ratio (95% CI) was 141.09 (68.18 to 292.00) and the negative likelihood ratio (95% CI) was 0.01 (0.00 to 0.06).
5.
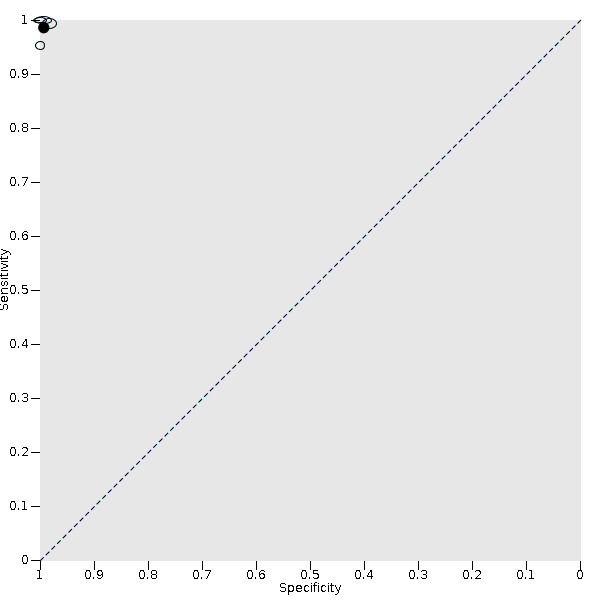
Summary ROC plot for CareStart Malaria Pf/Pv Combo test verified against microscopy. The size of each study point was scaled by the sample size of the diseased and non‐diseased groups used to estimate the study's sensitivity and specificity respectively, and reflects the precision of sensitivity and specificity in the study relative to other study points.The solid circle (summary point) represents the summary estimate of sensitivity and specificity. The summary point is not surrounded by a 95% confidence region because the bivariate model was simplified to univariate models.
The sensitivities of the Falcivax Device Rapid test from the two studies (89 P vivax malaria cases, 710 patients) were 66% (95% CI 54% to 77%) and 90% (95% CI 70% to 99%), and specificities were 99% (95% CI 97% to 100%) and 100% (95% CI 98% to 100%). The pooled sensitivity (95% CI) was 77% (53% to 91%) and the pooled specificity (95% CI) was 99% (98% to 100%). The positive likelihood ratio (95% CI) was 120.31 (43.10 to 335.87) and the negative likelihood ratio (95% CI) was 0.23 (0.10 to 0.53).
The sensitivities of the three remaining RDT brands ranged between 75% and 99% and the specificities ranged between 98% and 100% (Table 3; Figure 4).
Verified by microscopy corrected with PCR
Mendoza 2013 evaluated the accuracy of SD Bioline Malaria Ag Pf/Pv test against microscopy corrected with PCR (Figure 3). When there were discordant results between microscopy and PCR, the result of the PCR was taken, except in those in which the thick drop showed parasitic forms and the PCR was negative. The study reported a sensitivity (95% CI) of 92% (84% to 97%) and a specificity (95% CI) of 100% (99% to 100%).
Comparison between RDT brands
Alam 2011 directly compared the accuracy of Falcivax Device Rapid test and OnSite Pf/Pv test with PCR and microscopy as the reference standards. There was no evidence to suggest a difference in the sensitivity and specificity of the two brands (Table 4). Using microscopy as the reference standard, the absolute difference in sensitivity (95% CI) was 0 percentage points (‐17.8 to 17.8 percentage points) and the absolute difference in specificity (95% CI) was 0.9 percentage points (‐0.4 to 2.3 percentage points). Using PCR as the reference standard, the differences in sensitivity and specificity were similar.
3. Direct comparisons between OnSite Pf/Pv test and Falcivax Device Rapid test.
| Study | Reference standard | Sensitivity (true positives/malaria cases) (%) | Difference (95% CI) (percentage points) | P value | Specificity (true negatives/non‐cases) (%) | Difference (95% CI) (percentage points) | P value | ||
| OnSite Pf/Pv test (CTK Biotech Inc, USA) | Falcivax Device Rapid test for malaria Pv/Pf (Zephyer Biomedicals, Goa) | OnSite Pf/Pv test (CTK Biotech Inc, USA) | Falcivax Device Rapid test for malaria Pv/Pf (Zephyer Biomedicals, Goa) | ||||||
| Alam 2011 | Microscopy | 90 (19/21) | 90 (19/21) | 0 (‐17.8 to 17.8) | P = 1.00 | 99 (313/317) | 100 (316/317) | 0.9 (‐0.4 to 2.3) | P = 0.18 |
| Alam 2011 | PCR | 77 (20/26) | 77 (20/26) | 0 (‐22.9 to 22.9) | P = 1.00 | 99 (309/312) | 100 (312/312) | 1.0 (‐0.1 to 2.0) | P = 0.08 |
PCR = polymerase chain reaction.
Discussion
Summary of main results
This systematic review included 10 studies conducted in six different countries (Bangladesh, Brazil, Colombia, Ethiopia, India, and Sudan). The studies assessed six different RDT brands: CareStart Malaria Pf/Pv Combo test (four studies), Falcivax Device Rapid test for malaria Pv/Pf (three studies), Immuno‐Rapid Malaria Pf/Pv test (one study), SD Bioline Malaria Ag Pf/Pv test (three study), OnSite Pf/Pv test (two studies), and Test Malaria Pf/Pv rapid test (one study). However, only one study directly compared the accuracy of two brands.
The main findings of the review are summarised in Table 1, together with illustrations of what the findings mean. We assume median prevalences of ranges that would be classified as moderate, low, and very low transmission areas for P vivax (20%, 5%, and 0.5% respectively) in a hypothetical cohort of 1000 people suspected of having Pvivax malaria (WHO 2017c). The CareStart Malaria Pf/Pv Combo test had a pooled sensitivity (95% CI) and specificity (95% CI) of 99% (94% to 100%) and 99% (99% to 100%) when microscopy was the reference standard. For a prevalence of 20%, about 206 people will have a positive CareStart Malaria Pf/Pv Combo test result and the remaining 794 people will have a negative result. Of the 206 people with positive results, eight will be incorrect (false positives), and of the 794 people with a negative result, two would be incorrect (false negative). The potential consequence of false positive results is unnecessary initiation of treatment and over‐treatment of individuals with primaquine and either chloroquine or artemisinin combination therapies, and that patients are not treated for the actual cause of their symptoms. The consequences of false negative results are potential relapsing disease and continued risk of transmission of P vivax malaria at population level.
The Falcivax Device Rapid test had a pooled sensitivity and specificity of 77% (53% to 91%) and 99% (98% to 100%) when microscopy was the reference standard. For a prevalence of 20%, about 162 people will have a positive Falcivax Device Rapid test result and the remaining 838 people will have a negative result. Of the 162 people with positive results, eight will be incorrect (false positives), and of the 838 people with a negative result, 46 would be incorrect (false negative). A study that verified the results of the Falcivax Device Rapid test against PCR (Alam 2011), had a similar sensitivity and specificity of 77% (56% to 91%) and 100% (99% to 100%).
Strengths and weaknesses of the review
It is possible that some studies eligible for the inclusion in the review were missed by our search strategy. DTA studies are known to be poorly indexed, thus liable to be missed despite a broad literature search (Whiting 2009). However, our search was systematic, included studies published in all languages, and identified eligible studies from a previous review (Abba 2014). We also corresponded with study authors, when necessary, to obtain additional and unpublished data.
The main limitation of the review was the small number of studies included in the analyses. The meta‐analysis of the Falcivax Device Rapid test verified by microscopy included only two studies. Thus, the pooled estimate of sensitivity, and in general from analyses containing a small number of studies, should be interpreted with caution. Comparative accuracy studies are known to be typically scarce (Takwoingi 2013). Only one of the included studies compared the accuracy of two RDT brands, so we were unable to conduct comparative meta‐analyses to determine which brands were more sensitive and/or more specific. We intended to investigate any heterogeneity from the pooled analyses with pre‐specified factors, as stated in our secondary objective, but this was not possible due to the small number of studies included in the analyses.
For the diagnostic test accuracy of RDTs, there is a lack of a 'perfect reference standard'. PCR is often seen as the gold standard for malaria diagnosis, because it is less prone to observer error and more sensitive at low levels of parasitaemia (Han 2017; Snounou 1993). On the other hand, it is too analytically sensitive to be a gold standard, because it detects subclinical infections (e.g. in patients with partial immunity). Furthermore, PCR sometimes has poor sensitivity for the detection of mixed infections (Shokoples 2009). A small sample of the cases in our review are mixed infections using PCR as the reference standard (Alam 2011; Mendoza 2013), so the analysis may be flawed.
PCR is currently not widely available due to logistical constraints, namely the need for specially‐trained technicians and a well‐equipped laboratory. It is thus mostly used for research purposes and is less applicable in clinical settings. Thus, microscopy in the correct clinical setting, with well‐trained microscopists, remains the acceptable reference standard. This method is less costly than PCR, but infections can be missed if the slides are not examined carefully (Wongsrichanalai 2007). This raises the possibility that in some cases, the RDT results may in fact have been correct and the microscopy results incorrect. Alam 2011 verified RDT results against both microscopy and PCR separately, giving similar results of high specificity but lower sensitivity when verified against PCR. As mentioned previously, microscopy is more prone to observer error and is less sensitive at low levels of parasitaemia in comparison to PCR.
As reported in the Methodological quality of included studies, there was a high number of ’unclear’ evaluations of risk of bias and applicability due to poor reporting of study methods and characteristics. Nine studies (90%) did not provide enough information for us to adequately assess the selection of patients. Eight studies (80%) used an adequate reference standard, which was likely to have classified the target condition, but only four studies (40%) reported that readers of the reference standard were blinded to the results of the RDTs.
Applicability of findings to the review question
Due to the small number of studies included in this review, it is doubtful that the results obtained here can be considered to be generally applicable. Nevertheless, the findings show that the CareStart Malaria Pf/Pv Combo test verified by microscopy appeared to be both highly sensitive (missing 1% of cases) and highly specific (incorrectly classifying 1% of non‐cases as positives) in detecting P vivax alone or as part of a mixed infection. In contrast, the Falcivax Device Rapid test, verified by microscopy, appeared to be less sensitive (missing 23% of cases), but was similarly highly specific. This result should be interpreted with caution because only two studies were used to obtain the pooled estimates.
Furthermore, the RDTs are heterogeneous in terms of quality. The devices can give ambiguous test results, are prone to drying out in low‐humidity climates, resulting in lack of fluid migration. They are often not tested after they have been exposed to field conditions (Maltha 2013). In January 2020, the CareStart Malaria Pf/Pv Combo test produced by Access Bio Inc. was issued a WHO notice of concern due to their manufacturing quality assurance processes, which in turn could impact on patient safety (WHO 2020). Thus, in addition to considering results of test accuracy in published reports, end‐users must be attuned to outcomes of periodic monitoring procedures of regulatory authorities and WHO prequalification.
Comparison with previous systematic reviews
An existing Cochrane Review assessing RDTs for diagnosing uncomplicated non‐falciparum malaria was conducted in 2014 (Abba 2014). A subset of the review included RDTs that diagnosed P vivax. Our review only assessed the diagnostic accuracy of RDTs that specifically detect P vivax with Pvivax‐specific LDH antigens, however all the RDTs included in this review are combo tests that are used to detect P falciparum as well as P vivax. We included 10 studies, of which five studies (Alam 2011; Chanie 2011; Mekonnen 2010; Singh 2010; Sharew 2009) were included in the review by Abba 2014. Four studies were published following the review by Abba 2014 (Costa 2019; Hailu 2014; Mussa 2019; Saha 2017). One study (Mendoza 2013) was excluded by Abba 2014 because non‐English language studies were excluded due to resource constraints. We included studies published in all languages.
Authors' conclusions
Implications for practice.
Differentiating between Plasmodium species is particularly important in areas of co‐endemicity whereby P vivax malaria is increasing proportionally, compared to P falciparum malaria. The main analysis included in this review was CareStart Malaria Pf/Pv Combo test against microscopy as the reference standard, and this RDT was found to be both highly sensitive and specific. Owing to concerns regarding methodological quality, these findings should be interpreted with caution. Only two RDT brands were assessed by more than one study in this review, so we could not assess differences in sensitivity and specificity between RDT brands. Studies often did not report on transport, storage conditions and quality control practices for RDTs such as lot testing prior to use, therefore damage to RDTs in transit or during the study period cannot be excluded and may have negatively impacted on test results. Studies also often did not report on the background parasitaemia density. This is an important variable which influences the performance of the RDTs.
Implications for research.
More high‐quality studies are needed to assess and compare the accuracy of RDTs designed to detect P vivax. The studies should clearly report their sampling methods, if exclusion criteria were used and whether the results of index tests and reference standards were blinded from each other. Studies should also report the background parasitaemia density, if and how RDTs were quality assured prior to use, including details of transport, storage conditions, and lot testing.
In the future, the RDTs studied here may no longer be available. The quality of those that remain may be improved by the manufacturers. Thus, this review will require updating.
History
Protocol first published: Issue 2, 2019 Review first published: Issue 11, 2020
Acknowledgements
The CIDG Academic Editor of this review is Dr Jimee Hwang and the DTA Editor is Dr Karen Steingart.
We are grateful to Vittoria Lutje, Information Specialist with the Cochrane Infectious Diseases Group (CIDG), for help with the literature search strategy. We acknowledge Dr Jane Cunningham (Global Malaria Programme, World Health Organization) for her expert advice that helped shape this review.
Leslie Choi and Samuel Johnson are supported by the Research, Evidence and Development Initiative (READ‐It) project. Yemisi Takwoingi and Ridhi Agarwal were provided funding for the review through the READ‐It project. They and the CIDG editorial base are funded by UK aid from the UK government for the benefit of low‐ and middle‐income countries (project number 300342‐104). The views expressed do not necessarily reflect the UK government’s official policies.
Leslie Choi is also supported by PIIVeC, the Partnership for Increasing the Impact of Vector Control. PIIVeC is funded by the Medical Research Council of the UK (grant number MR/P027873/1) through the Global Challenges Research Fund.
Yemisi Takwoingi is supported by the UK National Institute for Health Research (NIHR) through a postdoctoral fellowship award (PDF‐2017‐10‐059) and supported by the NIHR Birmingham Biomedical Research Centre. The views expressed in this publication are those of the author(s) and not necessarily those of the National Health Service (NHS), the NIHR, or the UK Department of Health and Social Care.
Appendices
Appendix 1. Detailed search strategy
| Search set | MEDLINE (PubMed) |
| 1 | Malaria, vivax [MeSH] |
| 2 | Plasmodium vivax [MeSH] |
| 3 | “Plasmodium vivax” or “P vivax” or “vivax malaria” or “non‐falciparum Malaria” Field: Title/Abstract |
| 4 | 1 or 2 or 3 |
| 5 | Exp Reagent kits, diagnostics [MeSH] |
| 6 | "Diagnostic Tests, Routine"[Mesh] |
| 7 | rapid diagnostic test* Field: Title/Abstract |
| 8 | RDT* Field: Title/Abstract |
| 9 | Dipstick* Field: Title/Abstract |
| 10 | “Rapid diagnostic device*” Field: Title/Abstract |
| 11 | MRDD Field: Title/Abstract |
| 12 | OptiMal Field: Title/Abstract |
| 13 | “Binax NOW” or “NOW‐ICT‐Malaria” or “NOW‐Malaria‐ICT” Field: Title/Abstract |
| 14 | ParaSight or Parascreen or ParaHIT Field: Title/Abstract |
| 15 | “SD Bioline” or Carestart or Falcivax or Malascan Field: Title/Abstract |
| 16 | Immunochromatograph* or Immuno‐chromatograph* Field: Title/Abstract |
| 17 | “Antigen detection” Field: Title/Abstract |
| 18 | “Rapid malaria antigen test*” Field: Title/Abstract |
| 19 | “Combo card test*” Field: Title/Abstract |
| 20 | Immunoassay [MeSH] |
| 21 | Chromatography [MeSH] |
| 22 | Enzyme‐linked immunosorbent assay [MeSH] |
| 23 | “Rapid test*” Field: Title/Abstract |
| 24 | “Card test*” Field: Title/Abstract |
| 25 | Rapid AND (detection* or device* or test* or kit*) Field: Title/Abstract |
| 26 | 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 |
| 27 | 4 and 26 |
Web of Science
| Search set | Web of Science |
| # 6 | #5 AND #1 Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years |
| # 5 | #4 OR #3 OR #2 Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years |
| # 4 |
TOPIC: (("alere trueline" or "Rapigen biocredit" or "SD bioline" or "standard Q" or VISITECT* or PALUTOP*)) ORTOPIC: (((necviparum or "one step" or meriscreen or "onsite malaria" or paraHIt* or Quickprofile))) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years |
| # 3 |
TOPIC: (("ADVANCED QUALITY ONE STEP" or Tri‐line or BIOCREDIT or Biosynex or BioTracer or Carestart or Aspenmal)) ORTOPIC: (("combo RDT" or careUS or Coretests* or EGENS or EzDx or Falcivax or "first response" or Humasis or Karwa or KHB* or "malaria Pf (HRPII)/ PV")) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years |
| # 2 |
TOPIC: ((("rapid diagnostic test*" or RDT* or dipstick or MRDD) OR (“Binax NOW” or “NOW‐ICT‐Malaria” or “NOW‐Malaria‐ICT”))) ORTOPIC: (((ParaSight or Parascreen or ParaHIT or “SD Bioline” or Carestart or Falcivax or Malascan))) ORTOPIC: (: ((Immunochromatograph* or Immuno‐chromatograph* or "card test" or chromatography))) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years |
| # 1 |
TOPIC: (("plasmodium vivax" or "vivax malaria")) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years |
Database: Embase (OVID)
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 malaria vivax.mp. or Plasmodium vivax malaria/
2 plasmodium vivax.mp. or Plasmodium vivax/
3 ("P vivax" or "non‐falciparum Malaria").ab. or ("P vivax" or "non‐falciparum Malaria").ti.
4 1 or 2 or 3
5 diagnostic procedure/
6 "rapid diagnos$ test$ ".ab. or "rapid diagnos$ test$".ti.
7 RDT$.ab. or RDT$.ti.
8 Dipstick$.ab. or Dipstick$.ti.
9 "Rapid diagnos$ device$ ".ab. or "Rapid diagnos$ device$ ".ti.
10 MRDD.ab. or MRDD.ti.
11 ("Binax NOW" or "NOW‐ICT‐Malaria" or "NOW‐Malaria‐ICT").ab. or ("Binax NOW" or "NOW‐ICT‐Malaria" or "NOW‐Malaria‐ICT").ti.
12 (ParaSight or Parascreen or ParaHIT).ab. or (ParaSight or Parascreen or ParaHIT).ti.
13 ("SD Bioline" or Carestart or Falcivax or Malascan).ab. or ("SD Bioline" or Carestart or Falcivax or Malascan).ti.
14 ("ADVANCED QUALITY ONE STEP" or Tri‐line or BIOCREDIT or Biosynex or BioTracer or Carestart or Aspenmal).mp.
15 ("combo RDT" or careUS or Coretests* or EGENS or EzDx or Falcivax or "first response" or Humasis or Karwa or KHB* or "malaria Pf (HRPII)/ PV").mp.
16 (necviparum or "one step" or meriscreen or "onsite malaria" or paraHIt* or Quickprofile).mp.
17 ("alere trueline" or "Rapigen biocredit" or "SD bioline" or "standard Q" or VISITECT* or PALUTOP*).mp.
18 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17
19 4 and 18
Search Name: Cochrane Central Register of Controlled Trials
Issue 7 of 12, July 2019
ID Search Hits
#1 vivax malaria
#2 MeSH descriptor: [Malaria, Vivax] explode all trees
#3 MeSH descriptor: [Plasmodium vivax] explode all trees
#4 #1 or #2 or #3
#5 rapid diagnostic test*
#6 RDT*
#7 “ADVANCED QUALITY™ ONE STEP” or Tri‐line or “Aspen® Mal” or BIOCREDIT or Biosynex or BioTracer or Carestart or “combo RDT” or careUS or Coretests* or EGENS or EzDx™ or Falcivax or “first response”
#8 Humasis or Karwa or KHB* or necviparum or “one step” or meriscreen or “onsite malaria” or “paraHIt*” or Quickprofile or “alere trueline” or “Rapigen biocredit” or “SD bioline” or “standard Q” or VISITECT* or PALUTOP*
#9 “Binax NOW” or “NOW‐ICT‐Malaria” or “NOW‐Malaria‐ICT”
#10 ParaSight or Parascreen or ParaHIT
#11 “SD Bioline” or Carestart or Falcivax or Malascan
#12 Immunochromatography or Immuno‐chromatography
#13 antigen detection
#14 combo card
#15 immunoassay or chromatography
#16 Enzyme‐linked immunosorbent assay
#17 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16
#18 #17 and #4
| Database : | LILACS |
| Search on : | vivax malaria [Words] and "rapid test$" or PCR or diagnosis [Words] |
Appendix 2. QUADAS‐2 tool tailored to the context of the review
| Domain | Patient selection | Index test | Reference standard | Flow and timing |
| Description | Methods of patient selection | How index test was conducted and reported | How reference standard was conducted and reported | Describe patients that did not receive and time interval between index test or reference standard |
| Signalling questions (yes, no, or unclear) | Consecutive or random sample of patients?
|
Index test results interpreted without knowledge of the results of reference standard?
|
PCR PCR likely to correctly classify the target condition? We will answer this question as ‘yes' for all studies because PCR is an objective test with binary outcomes. Thus, there is no room for subjective interpretation of test results or poor performance of the test leading to false negatives or false positives. ‘Yes' if reference standard was PCR. Microscopy Microscopy likely to correctly classify the target condition? ‘Yes' if microscopy was performed for one sample by two independent trained microscopist examining 100 high‐power fields. ‘No' if microscopy was performed:
‘Unclear' if insufficient information was provided. |
Was there an appropriate interval between index test and reference standard?
|
| Was a case‐control design avoided? This will always be ‘yes' because case control studies will be excluded from this review. |
Pre‐specified threshold used? As the threshold is prespecified by the manufacturer in all RDTs, we will answer this question ‘yes' for all studies. |
Reference standard results interpreted without knowledge of the results of index test? We will answer this question ‘yes' for all studies using only PCR as the reference standard because PCR is an objective test with binary outcomes. Thus, there is no room for subjective interpretation of test results.
|
Did all patients receive a reference standard?
|
|
Did the study avoid inappropriate exclusions?
|
Did all patients receive the same reference standard?
|
|||
Were all patients included in the analysis?
| ||||
| Risk of bias (high, low, or unclear) | Could the selection of patients have introduced bias? | Could the conduct or interpretation of the index test have introduced bias? | Could the reference standard, its conduct, or its interpretation has introduced bias? | Could the patient flow have introduced bias? |
| Applicability concerns (high, low, or unclear) | Not applicable | Are there concerns that the index test, its conduct, or interpretation differs from the review question?
|
Are there concerns that the target condition as defined by the reference standard does not match the review question? We will answer this question ‘low' for all studies because P vivax diagnosed by light microscopy or PCR does match the review question |
Not applicable |
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
1. Test.
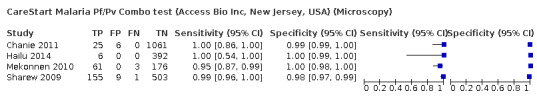
CareStart Malaria Pf/Pv Combo test (Access Bio Inc, New Jersey, USA) (Microscopy)
2. Test.

Falcivax Device Rapid test for malaria Pv/Pf (Zephyer Biomedicals Goa) (Microscopy)
3. Test.

Immuno‐Rapid Malaria Pf/Pv test (Wama Diagnostica, Sao Paulo, Brazil) (Microscopy)
4. Test.

OnSite Pf/Pv test (CTK Biotech Inc, USA) (Microscopy)
5. Test.

SD Bioline Malaria Ag Pf/Pv test (Microscopy)
6. Test.

Falcivax Device Rapid test for malaria Pv/Pf (Zephyer Biomedicals Goa) (PCR)
7. Test.

OnSite Pf/Pv test (CTK Biotech Inc, USA) (PCR)
8. Test.

SD Bioline Malaria Ag Pf/Pv test (PCR)
9. Test.

Test Malaria Pf/Pv rapid test (Alltest Biotech, China) (PCR)
10. Test.

SD Bioline Malaria Ag Pf/Pv test (Microscopy corrected by PCR)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alam 2011.
| Study characteristics | |||
| Patient Sampling |
Study design: cross‐sectional study Recruitment: did not state consecutive or random sampling Study period: May 2009 to August 2010 Population: 338 febrile patients referred for microscopy to diagnose malaria diagnosis at a health facility Inclusion and exclusion criteria: not reported |
||
| Patient characteristics and setting |
Sex: 49.7% male, 50.3% female Age: median = 14 years, range 18 months to 82 years Setting: Matiranga Upazila Health Complex (UHC), in Matiranga Upazila (sub‐district) of Khagrachari district, south‐eastern part of Bangladesh Malaria transmission: perennial transmission of malaria with 2 peaks in pre‐monsoon (March to May) and post‐monsoon (September to November) periods |
||
| Index tests |
RDT brand(s): OnSite Pf/Pv test (CTK Biotech Inc, USA) and Falcivax Device Rapid test for malaria Pv/Pf (Zephyer Biomedicals, Goa) Batch number: not reported Lot testing: not reported Storage conditions: unclear, reported manufacturer's instructions were followed for use Blinding: not reported |
||
| Target condition and reference standard(s) |
Target condition(s):P falciparum and P vivax Reference standard(s): PCR and microscopy Microscopy details:
PCR details:
Blinding: not reported |
||
| Flow and timing |
Appropriate interval between index test and reference standard: one blood sample taken from each patient. Invalid test results: None reported. |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Chanie 2011.
| Study characteristics | |||
| Patient Sampling |
Study design: cross‐sectional study Recruitment: consecutive Study period: December 2009 and January 2010 Population: 1092 febrile patients who had clinical symptoms of malaria and visited the outpatient department (OPD) of three health facilities. Inclusion and exclusion criteria: no exclusion criteria, unless the patient or the guardians of children less than 18 years old did not consent to participate. |
||
| Patient characteristics and setting |
Sex: 51.4% male, 48.6% female Age: median = 22.3 years, SD: 12.8 Setting: 75 (61.81%), 238 (21.8%) and 179 (16.4%) patients were respectively from Melkawerer Health Centre, Gewane Health Centre and Dubti Hospital, in the Afar Region, Northeast Ethiopia Malaria transmission: The study reported the transmission of malaria as unstable, with some areas of perennial transmission. Other patient characteristics: 12.5% of the patients had anti‐malarial therapy in the preceding month. |
||
| Index tests |
RDT brand(s): CareStart Malaria Pf/Pv Combo test (Access Bio Inc, Somerset, NJ) Batch number: not reported Lot testing: Lot No H38 IV and Lot No H28 IV Storage conditions: Test kits were kept at the local temperature of the region without any controlling system of the storage temperature during data collection. The quality of the package desiccant was checked before use. Blinding: Microscopy and RDT were performed independently by malaria technicians, results were recorded separately. |
||
| Target condition and reference standard(s) |
Target condition(s):P falciparum and P vivax Reference standard(s): microscopy Microscopy details:
Blinding: Microscopy and RDT was performed independently by malaria technicians, results were recorded separately. |
||
| Flow and timing |
Appropriate interval between index test and reference standard: one blood sample taken from each patient. Invalid test results: None reported. |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Costa 2019.
| Study characteristics | |||
| Patient Sampling |
Study design: prospective cross‐sectional study Recruitment: consecutive Study period: November 2016 and April 2017 Population: 181 febrile patients were recruited based on the hospital's regular admissions Inclusion and exclusion criteria: not reported |
||
| Patient characteristics and setting |
Sex: 64.1% male, 35.9% female Age: median = 41.7 years, SD: 14.4 Setting: Tertiary health unit at Fundação de Medicina Tropical Dr. Heitor Vieira Dourado in Western Brazilian Amazon Malaria transmission: study reported P vivax as "the most prevalent species in Brazil and extra‐Afican areas", but did not specify to what degree. Other patient characteristics: 93.3% of the patients had up to three previous episodes of malaria. |
||
| Index tests |
RDT brand(s): Immuno‐Rapid Malaria Pf/Pv test (Wama Diagnostica, Sao Paulo, Brazil) Batch number: not reported Lot testing: not reported Storage conditions: reported manufacturer's instructions were followed (2oC‐30oC until the expiry date) Blinding: The hospital laboratory staff who performed the RDT and thick blood smear analysis were blinded. |
||
| Target condition and reference standard(s) |
Target condition(s):P vivax and P falciparum Reference standard(s): microscopy Microscopy details:
Blinding: The hospital laboratory staff who performed the RDT and thick blood smear analysis were blinded. |
||
| Flow and timing |
Appropriate interval between index test and reference standard: RDT and microscopy were performed on admission. Invalid test results: None reported |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Hailu 2014.
| Study characteristics | |||
| Patient Sampling |
Study design: cross‐sectional study Recruitment: did not state consecutive or random sampling Study period: patients recruited in December 2011 Population: 398 febrile patients who visited the outpatient department of a health centre Inclusion and exclusion criteria: Patients with acute febrile illnesses (body temperature > 37.5 degrees C) or a history of fever during the last 2 weeks at the date of data collection were included. Patients who took antimalarials within the last 2 weeks before the data collection date or refused participation were excluded. |
||
| Patient characteristics and setting |
Sex: 44.2% male, 55.8% female Agerange: 1 and 70 years Setting: Felegeselam Health Center in Pawe Special Woredam, Northwest Ethiopia Malaria transmission: malaria transmission takes place throughout the year and that P falciparum and P vivax are co‐endemic. |
||
| Index tests |
RDT brand(s): CareStart Malaria Pf/Pv Combo test (Access Bio Inc, Somerset, NJ) Batch number: not reported Lot testing: not reported Storage conditions: RDT was stored based at room temperature and the quality of package and expiration date was checked before use. Blinding: The results of the RDT were determined earlier than microscopic results, with strict blinding to microscopic examination. |
||
| Target condition and reference standard(s) |
Target condition(s):P falciparum and P vivax Reference standard(s): microscopy Microscopy details:
Blinding: RDT with strict blinding to microscopic examination. |
||
| Flow and timing |
Appropriate interval between index test and reference standard: Blood sample was collected from each patient. Two out of 398 patients with invalid test results from RDT were retested by taking fresh blood. Unclear whether the same blood sample was used for microscopy for these patients. Invalid test results: Two patients with invalid test results. |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Mekonnen 2010.
| Study characteristics | |||
| Patient Sampling |
Study design: cross‐sectional study Recruitment: did not state consecutive or random sampling Study period: October 2007 and December 2008 Population: 240 febrile patients who were clinically suspected of malaria and visited the outpatient department of a health cent er. Inclusion and exclusion criteria: not reported. |
||
| Patient characteristics and setting |
Sex: 57.5% male, 42.5% female Age: mean = 25 years, range 1 and 60 years Setting: Serbo health cent er in Jimma zone, southern Ethiopia Malaria transmission: Study reported that P falciparum and P vivax were both prevalent. |
||
| Index tests |
RDT brand(s): CareStart Malaria Pf/Pv Combo test (Access Bio Inc, Somerset, NJ) Batch number: not reported Lot testing: not reported Storage conditions: manufacturer’s instruction was followed and the quality of package desiccant was checked before use. Blinding: The RDT and microscopy were performed by three experienced malaria technicians independently. The results of the RDT were determined before microscopic results with strict blinding to microscopic examination. |
||
| Target condition and reference standard(s) |
Target condition(s):P falciparum and P vivax Reference standard(s): microscopy Microscopy details:
Blinding: RDT with strict blinding to microscopic examination |
||
| Flow and timing |
Appropriate interval between index test and reference standard: Blood sample was collected from each patient. Invalid test results: None reported. |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Mendoza 2013.
| Study characteristics | |||
| Patient Sampling |
Study design: retrospective cross‐sectional study Recruitment: consecutive Study period: November 16 to December 2, 2010 in Córdoba and from June 14 to 25, 2011 in Chocó, Colomba Population: 383 patients who attended one of three clinics with microscopy for diagnosis of malaria. Inclusion and exclusion criteria: patient was considered as a probable case of malaria and at least 6 years of age (with consent), were included. Probable case defined as patients presenting with current or recent fever within 72 hours, who came from an endemic area in the last 15 days and who may or may not have an epidemiological relationship with diagnosed cases. The study excluded patients who did not consent to participation, lack of diligence of the clinical epidemiological record or who presented with symptoms of complicated malaria. |
||
| Patient characteristics and setting |
Sex: 52.5% male, 47.5% female Age range: 6 and 92 years Setting: 233 patients came from Córdoba, of which 121 were from Tierralta and 112 from Puerto Libertador. The remaining 150 patients were recruited in the department of Chocó, in the municipality of Quibdo. Malaria transmission: The study reported Córdoba had the highest prevalence of P vivax, unclear for Chocó. Other patient characteristics: 7.8% of the patients received treatment for malaria in the previous month of recruitment. |
||
| Index tests |
RDT brand(s): SD Bioline Malaria Ag Pf/Pv test (Standard Diagnostics Inc) Batch number: not reported Lot testing: not reported Storage conditions: reported manufacturer's instructions were followed (1ºC‐40ºC) Blinding: The results of the RDT were determined and kept separate so it does not interfere with the reference standard results. |
||
| Target condition and reference standard(s) |
Target condition(s):P falciparum and P vivax Reference standard(s): microscopy corrected by PCR Microscopy details:
PCR details:
Blinding: The results of the RDT were determined and kept seperate so it does not interfere with the reference standard results. |
||
| Flow and timing |
Appropriate interval between index test and reference standard: Multiple blood samples were taken at the same time for each patient. Invalid test results: None reported. |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Mussa 2019.
| Study characteristics | |||
| Patient Sampling |
Study design: cross‐sectional study Recruitment: did not state consecutive or random sampling Study period: Not reported Population: 59 suspected patients with P falciparum infection from different clinical centers were recruited. Inclusion and exclusion criteria: not reported |
||
| Patient characteristics and setting |
Sex: 45.8% male, 54.2% female Age: Not reported Setting: different clinics in Omdurman, Sudan Malaria transmission: Not reported |
||
| Index tests |
RDT brand(s): Test Malaria Pf/Pv rapid test cassette (Alltest Biotech, China) Batch number: not reported Lot testing: not reported Storage conditions: Unclear, reported manufacturer's instructions were followed for use Blinding: not reported |
||
| Target condition and reference standard(s) |
Target condition(s):P falciparum Reference standard(s): PCR PCR details:
Blinding: not reported |
||
| Flow and timing |
Appropriate interval between index test and reference standard: one blood sample taken from each patient. Invalid test results: None reported. |
||
| Comparative | |||
| Notes | Contacted author specifically for P vivax results. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Saha 2017.
| Study characteristics | |||
| Patient Sampling |
Study design: cross‐sectional study Recruitment: consecutive Study period: Not reported Population: 200 febrile patients in whom clinicians suspected malaria and raised the investigations. Inclusion and exclusion criteria: Patients having a fever with chills and rigor in the absence of any obvious cause such as upper respiratory tract infection. All patients diagnosed and/or treated with antimalarial drugs within the past six months were excluded. |
||
| Patient characteristics and setting |
Sex: 56.0% male, 44.0% female Age: mean = 34.6 years, <10 years: 2.5%, 11‐20 years: 20.5%, 21‐60 years: 68.5%, >61 years: 8.5% Setting: tertiary care hospital setting at the outpatient department of Kasturba Hospital, Sewagram, Wardha in Central India Malaria transmission: Not reported |
||
| Index tests |
RDT brand(s): SD Bioline Malaria Ag Pf/Pv test (Standard Diagnostics Inc) Batch number: not reported Lot testing: not reported Storage conditions: Unclear, although reported manufacturer's instructions were followed for use. Blinding: The RDT, microscopy and PCR were performed by different technicians and results of all three tests were kept blind. |
||
| Target condition and reference standard(s) |
Target condition(s):P falciparum and P vivax Reference standard(s): PCR and microscopy Microscopy details:
PCR details:
Blinding: The RDT, microscopy and PCR were performed by different technicians and results of all three tests were kept blind. |
||
| Flow and timing |
Appropriate interval between index test and reference standard: one blood sample taken from each patient. Invalid test results: None reported. |
||
| Comparative | |||
| Notes | Contacted author specifically for P vivax results. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Sharew 2009.
| Study characteristics | |||
| Patient Sampling |
Study design: cross‐sectional study Recruitment: did not state consecutive or random sampling Study period: November and December 2008 Population: 668 febrile patients who were clinically suspected of malaria and visited the outpatient department of two health centers. Inclusion and exclusion criteria: not reported. |
||
| Patient characteristics and setting |
Sex: 54.0% male, 46.0% female Age range: 6 months and 75 years Setting: Bussa and Kella health centers in Wondo Genet area, southern Ethiopa Malaria transmission: Study reported that P falciparum and P vivax were both prevalent. |
||
| Index tests |
RDT brand(s): CareStart Malaria Pf/Pv Combo test (Access Bio Inc, Somerset, NJ) Batch number: not reported Lot testing: not reported Storage conditions: manufacturer’s instruction was followed and the quality of package desiccant was checked before use. Blinding: The RDT and microscopy were performed by two experienced malaria technicians independently. The results of the RDT were determined before microscopic results with strict blinding to microscopic examination. |
||
| Target condition and reference standard(s) |
Target condition(s):P falciparum and P vivax Reference standard(s): microscopy Microscopy details:
Blinding: RDT with strict blinding to microscopic examination |
||
| Flow and timing |
Appropriate interval between index test and reference standard: Blood sample was collected from each patient. Invalid test results: None reported. |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Singh 2010.
| Study characteristics | |||
| Patient Sampling |
Study design: cross‐sectional study Recruitment: consecutive Study period: August and December 2009 Population: 372 febrile patients with clinical suspicion of malaria who visited field clinics Inclusion and exclusion criteria: excluded pregnant women and patients who took antimalarials. |
||
| Patient characteristics and setting |
Sex: Not reported Age: mean = 15 years, SD: 14.1 Setting: Bajag Primary Health Centre (PHC) of district Dindori and Satanwada PHC of district Shivpuri, India Malaria transmission: study reported that both P falciparum and P vivax as co‐endemic in the study area. |
||
| Index tests |
RDT brand(s): Falcivax Device Rapid test for malaria Pv/Pf (Zephyer Biomedicals, Goa) Batch number: not reported Lot testing: not reported Storage conditions: "For testing temperature stability of the tests, RDTs were stored at 25°C on receipt in the study sites, then allocated to separate groups for storage at 35°C & 45°C for 90 days, at 60°C for 48 hours, and at ‐10°C for 60 minutes before testing [21]. At the start of the study, the incubators were stabilized at the required temperature for three days before the RDTs to be tested were placed inside. RDTs were removed from storage to reach room temperature for 2 hours before testing and comparisons were made with control RDTs kept at 25°C until use." Blinding: Microscopy examination was conducted without reference to the results of RDTs. |
||
| Target condition and reference standard(s) |
Target condition(s):P falciparum and P vivax Reference standard(s): microscopy Microscopy details:
Blinding: microscopy conducted without reference to the results of RDTs. |
||
| Flow and timing |
Appropriate interval between index test and reference standard: Multiple samples were taken at the same time. Invalid test results: None reported. |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdelraheem 2016 | Insufficient data |
| Adams 2015 | Wrong patient population |
| Adnan 2017 | Insufficient data |
| Ageep 2013 | Wrong target condition |
| Andrade 2010 | Wrong index test |
| Arvind 2015 | Predatory journal |
| Ashton 2010 | Wrong index test |
| Ayorinde 2016 | Wrong target condition |
| Ba 2017 | Wrong index test |
| Bahk 2018 | Wrong index test |
| Barber 2013 | Wrong index test |
| Bell 2001 | Wrong index test |
| Bendezu 2010 | Wrong index test |
| Berhane 2017 | Wrong study design |
| Berzosa 2018 | Wrong index test |
| Bharti 2008 | Wrong index test |
| Bharti 2013 | Wrong study design |
| Bhide 2014 | Wrong index test |
| Birhanie 2016 | Wrong index test |
| Bisoffi 2014 | Wrong study design |
| Britton 2016 | Wrong index test |
| Chayani 2004 | Wrong index test |
| Cho 2016 | Wrong index test |
| Dahesh 2015 | Full text not available |
| Dash 2013 | Insufficient data |
| Deida 2019 | Wrong index test |
| DeKoninck 2017 | Wrong patient population |
| Dev 2004 | Wrong index test |
| Dinzouna‐Boutamba 2014 | Wrong index test |
| Dzakah 2014 | Wrong patient population |
| Ehtesham 2015 | Wrong patient population |
| Eibach 2013 | Wrong index test |
| Elahi 2013 | Wrong index test |
| Endeshaw 2012 | Wrong index test |
| Falade 2016 | Wrong target condition |
| Fernando 2004 | Wrong index test |
| Fernando 2004a | Wrong index test |
| Foster 2014 | Wrong study design |
| Fransisca 2015 | Wrong index test |
| Gabrielli 2016 | Wrong patient population |
| Ghai 2016 | Wrong index test |
| Gupta 2018 | Wrong index test |
| Harani 2006 | Wrong index test |
| Hawash 2019 | Wrong patient population |
| Jabeen 2016 | Insufficient data |
| Jahan 2019 | Wrong index test |
| Joseph 2018 | Wrong index test |
| Karimov 2013 | Full text not available |
| Kim 2013 | Wrong index test |
| Kolaczinski 2004 | Wrong index test |
| Kosack 2013 | Wrong index test |
| Kumari 2014 | Predatory journal |
| Liu 2013 | Full text not available |
| Mallepaddi 2019 | Wrong index test |
| Metzger 2011 | Wrong index test |
| Moges 2012 | Wrong index test |
| Mohon 2012 | Wrong index test |
| Olasehinde 2018 | Wrong index test |
| Pakalapati 2013 | Wrong index test |
| Pattanasin 2003 | Wrong index test |
| Puri 2013 | Wrong index test |
| Rakotonirina 2008 | Wrong index test |
| Ranjan 2016 | Insufficient data |
| Ratsimbasoa 2007 | Wrong index test |
| Ratsimbasoa 2008 | Wrong index test |
| Samane 2010 | Wrong index test |
| Selimuzzaman 2010 | Wrong index test |
| Shaikh 2013 | Insufficient data |
| Sharma 2014 | Wrong study design |
| Singh 2000a | Wrong index test |
| Singh 2003 | Wrong index test |
| Singh 2013 | Wrong study design |
| Siwal 2018 | Wrong patient population |
| Stijnberg 2013 | Conference abstract |
| Strom 2014 | Wrong target condition |
| Thongdee 2014 | Wrong index test |
| Tjitra 1999 | Wrong index test |
| Trouvay 2013 | Wrong index test |
| Valecha 2003 | Wrong index test |
| van den Broek 2006 | Wrong index test |
| Vohra 2014 | Insufficient data |
| Wang 2014 | Full text not available |
| Wongsrichanalai 2003 | Wrong index test |
| Woyessa 2013 | Wrong study design |
| Xiaodong 2013 | Wrong index test |
| Yan 2013 | Wrong index test |
Characteristics of studies awaiting classification [ordered by study ID]
Boni 2015.
| Patient Sampling | Patients were recruited from two provinces in Central Vietnam between January and August 2015. The sampling method and inclusion/exclusion criteria were not reported in the abstract. | ||
| Patient characteristics and setting | The prevalence, number of patients recruited and characteristics of patients were not reported in the abstract. | ||
| Index tests | RDT brand was not reported in abstract. No information on blinding, batch number of RDT, lot testing or storage conditions in the abstract. | ||
| Target condition and reference standard(s) | The target conditions were P falciparum and P vivax. The reference standard was microscopy examined by at least two expert microscopists. No information on number of high powered field or blinding in the abstract. | ||
| Flow and timing | Unclear whether the index test and reference standard were performed at the same time and if blood sample was taken at the same time for the tests. | ||
| Comparative | |||
| Notes | Unable to deduce the number of true positives, false positives, false negatives and true negatives for P vivax. Contacted authors for more details on methodology and results. | ||
Cheng 2013.
| Patient Sampling | This was a cross‐sectional study, patients were recruited in 2008 and in 2011. The study did not explicitly state consecutive or random sampling. Inclusion and exclusion criteria were not reported. Unclear how the febrile patients were recruited, i.e. whether they presented themselves to a health centre. | ||
| Patient characteristics and setting | 202 febrile patients (49 patients in 2008 and 153 in 2011) with fever of unknown origin in Kachine Myanmar and in Yunnan, China were recruited. 13 healthy patients were also recruited in Beijing, China, however they were not used in the analysis of the RDT. The study did not describe the characteristics of patients recruited. The study reported malaria as endemic in study area. | ||
| Index tests | CareStart Malaria HRP2/pLDH combo test (Access Bio Inc., Somerset, NJ). In the study there was no information on batch number, lot testing or storage conditions, however the study stated that the RDT was done according to manufacturer's protocol. The study did not mention blinding. | ||
| Target condition and reference standard(s) | The target conditions were P falciparum and P vivax. The reference standards were PCR and microscopy. The study reported a minimum of 100 high powered fields for microscopy. Two professional microscopists conducted the microscopic examination independently. Unclear how discrepancies for microscopy results between the two microscopists were handled, if any. With PCR, unable to deduce the number of true positives, false positives, false negatives and true negatives. | ||
| Flow and timing | The tests were performed at different times, but the blood sample was taken at the same time. All samples were frozen and stored at ‐80 oC but 2008 samples suffered freeze–and–thaw cycles. Only the 2011 samples were tested using the RDT but this was stated as 143 patients in the results rather than 153. | ||
| Comparative | |||
| Notes | Unclear whether the RDT used by the study is eligible for this review because the specific brand name was not mentioned in the main study publication, contacted authors for further information. | ||
Reda 2016.
| Patient Sampling | This was a cross‐sectional study. Patients were recruited between November and December 2014 in two health centres in Adam and Amaya, Oromia region, Ethiopia. Inclusion/ exclusion criteria or sampling method were not reported. | ||
| Patient characteristics and setting | Febrile patients with symptoms of malaria who visited the two health facilities were recruited. The study abstract did not include the prevalence of malaria or the characteristics of patients recruited. "A total of 547 febrile patients were diagnosed, of which 127 were microscopy positive for Pf (n=38) and Pv (n=85)". |
||
| Index tests | CareStart Malaria Ag Pf/Pv combo test (Access Bio Inc., Somerset, NJ) and SD BIOLINE malaria AG PF/PV test. The RDTs were performed following manufacturer's instructions. In the study abstract, there was no information on batch number or lot testing. The study abstract did not mention blinding. | ||
| Target condition and reference standard(s) | The target conditions were P falciparum and P vivax. The reference standard was microscopy. Microscopic examination was done under 100x magnifications. No information on blinding or who performed the microscopic examination in the abstract. | ||
| Flow and timing | Unclear whether the index test and reference standard were performed at the same time and if blood sample was taken at the same time for the tests. | ||
| Comparative | |||
| Notes | Unable to deduce the number of true positives, false positives, false negatives and true negatives for P vivax. Contacted authors for more details on methodology and results. | ||
Differences between protocol and review
In the protocol, the stated secondary objectives were to assess (1) the effect of transmission setting (perennial, seasonal, or epidemic) and type of malaria present in the region on the accuracy of RDTs for detecting P vivax malaria parasitaemia; (2) the effect of different generations of an RDT on test accuracy; and (3) the impact of level of training for studies that used microscopy as the reference standard. However, we were unable to conduct comparative meta‐analyses, investigations of heterogeneity, and sensitivity analyses due to the limited number of included studies.
We only assessed the certainty of the evidence using GRADE methods where there were sufficient studies for meta‐analyses.
Contributions of authors
RA, LC, and SJ screened the searches and assessed studies for inclusion, extracted data and performed methodological quality assessment. RA conducted the analyses and drafted the review under supervision by YT. LC and YT provided content expertise. YT critically revised the draft. All review authors read and approved the final draft of the review.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK
External sources
-
Foreign, Commonwealth and Development Office (FCDO), UK
Project number 300342‐104
-
National Institute for Health Research (NIHR), UK
Yemisi Takwoingi is supported by the NIHR through a postdoctoral fellowship award (PDF‐2017‐10‐059)
Declarations of interest
RA has no known conflicts of interest. LC has no known conflicts of interest. SJ has no known conflicts of interest. YT has no known conflicts of interest.
New
References
References to studies included in this review
Alam 2011 {published data only}
- Alam MS, Mohon AN, Mustafa S, Khan WA, Islam N, Karim MJ, et al. Real-time PCR assay and rapid diagnostic tests for the diagnosis of clinically suspected malaria patients in Bangladesh. Malaria Journal 2011;10:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chanie 2011 {published data only}
- Chanie M, Erko B, Animut A, Legesse M. Performance of CareStart™ Malaria Pf/Pv Combo test for the diagnosis of Plasmodium falciparum and Plasmodium vivax infections in the afar region, North East Ethiopia. Ethiopian Journal of Health Development 2011;25(3):206-11. [Google Scholar]
Costa 2019 {published data only}
- Costa MRF, Barcelos ALR, Camargo MA, Melo GC, Almeida AC, Costa AG, et al. Performance of an immuno-rapid malaria Pf/Pv rapid diagnostic test for malaria diagnosis in the Western Brazilian Amazon. Revista da Sociedade Brasileira de Medicina Tropical 2019;52:e-20170450. [DOI] [PubMed] [Google Scholar]
Hailu 2014 {published data only}
- Hailu T, Kebede T. Assessing the performance of CareStart Malaria Pf/Pv Combo test against thick blood film in the diagnosis of malaria in northwest Ethiopia. The American Journal of Tropical Medicine and Hygiene 2014;90(6):1109-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mekonnen 2010 {published data only}
- Mekonnen Z, Ali S, Belay G, Suleman S, Chatterjee S. Evaluation of the performance of CareStart™ Malaria Pf/Pv Combo rapid diagnostic test for the diagnosis of malaria in Jimma, Southwestern Ethiopia. Acta Tropica 2010;113(3):285-8. [DOI] [PubMed] [Google Scholar]
Mendoza 2013 {published data only}
- Mendoza NM, Cucunubá ZM, Aponte S, González NE, Bernal SD. Field evaluation for diagnostic accuracy of the rapid test SD Bioline Malaria Antigen Pf/Pv® in Colombia [Evaluación de campo de la precisión de la prueba de diagnóstico rápido SD Bioline Malaria Antigen Pf/Pv® en Colombia]. Biomedica 2013;33(4):587-97. [DOI] [PubMed] [Google Scholar]
Mussa 2019 {published data only}
- Mussa A, Talib M, Mohamed Z, Hajissa K. Genetic diversity of Plasmodium falciparum histidine-rich protein 2 (PfHRP2) and its effect on the performance of PfHRP2-based rapid diagnostic tests. BMC Research Notes 2019;12:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saha 2017 {published data only}
- Saha S, Narang R, Deshmukh P, Pote K, Anvikar A, Narang P. Diagnostic efficacy of microscopy, rapid diagnostic test and polymerase chain reaction for malaria using bayesian latent class analysis. Indian Journal of Medical Microbiology 2017;35(3):376-80. [DOI] [PubMed] [Google Scholar]
Sharew 2009 {published data only}
- Sharew B, Legesse M, Animut A, Jima D, Medhin G, Erko B. Evaluation of the performance of CareStart™ Malaria Pf/Pv Combo and Paracheck Pf tests for the diagnosis of malaria in Wondo Genet, Southern Ethiopia. Acta Tropica 2009;111(3):321-4. [DOI] [PubMed] [Google Scholar]
Singh 2010 {published data only}
- Singh N, Shukla MM, Shukla MK, Mehra RK, Sharma S, Bharti PK, et al. Field and laboratory comparative evaluation of rapid malaria diagnostic tests versus traditional and molecular techniques in India. Malaria Journal 2010;9:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdelraheem 2016 {published data only}
- Abdelraheem MH, Albsheer MM, Mohamed HS, Amin M, Mahdi Abdel Hamid M. Transmission of Plasmodium vivax in Duffy-negative individuals in Central Sudan. Transactions of the Royal Society of Tropical Medicine and Hygiene 2016;110(4):258-60. [DOI] [PubMed] [Google Scholar]
Adams 2015 {published data only}
- Adams M, Joshi SN, Mbambo G, Mu AZ, Roemmich SM, Shrestha B, et al. An ultrasensitive reverse transcription polymerase chain reaction assay to detect asymptomatic low-density Plasmodium falciparum and Plasmodium vivax infections in small volume blood samples. Malaria Journal 2015;14:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Adnan 2017 {published data only}
- Adnan STA, Warraich RA, Khan MA, Tabassum S. Community based evaluation of malaria rapid diagnostic test (ICT) comparing with conventional method of microscopy. Medical Forum Monthly 2017;28(7):60-63. [Google Scholar]
Ageep 2013 {published data only}
- Ageep AK. Diagnosis of malaria in Red Sea State, Sudan. Annals of Tropical Medicine and Public Health 2013;6(2):232-5. [Google Scholar]
Andrade 2010 {published data only}
- Andrade BB, Reis-Filho A, Barros AM, Souza-Neto SM, Nogueira LL, Fukatano KF, et al. Towards a precise test for malaria diagnosis in the Brazilian Amazon: Comparison among field microscopy, a rapid diagnostic test, nested PCR, and a computational expert system based on artificial neural networks. Malaria Journal 2010;9:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arvind 2015 {published data only}
- Kumar A. Evaluation of parasite LDH detection strip test (OptiMAL) for rapid diagnosis of malaria in children. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2015;6(1):557-61. [Google Scholar]
Ashton 2010 {published data only}
- Ashton RA, Kefyalew T, Tesfaye G, Counihan H, Yadeta D, Cundill B, et al. Performance of three multi-species rapid diagnostic tests for diagnosis of Plasmodium falciparum and Plasmodium vivax malaria in Oromia Regional State, Ethiopia. Malaria Journal 2010;9:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ayorinde 2016 {published data only}
- Ayorinde AF, Oyeyiga AM, Nosegbe NO, Folarin OA. A survey of malaria and some arboviral infections among suspected febrile patients visiting a health centre in Simawa, Ogun State, Nigeria. Journal of Infection and Public Health 2016;9(1):52-9. [DOI] [PubMed] [Google Scholar]
Ba 2017 {published data only}
- Ba H, Ahouidi AD, Duffy CW, Deh YB, Diedhiou C, Tandia A, et al. Evaluation of malaria rapid diagnostic test Optimal-IT® pLDH along the Plasmodium falciparum distribution limit in Mauritania [Evaluation du test de diagnostic rapide du paludisme OptiMal-IT® pLDH à la limite de la distribution de Plasmodium falciparum en Mauritanie]. Bulletin de la Societe de pathologie exotique 2017;110(1):31-7. [DOI] [PubMed] [Google Scholar]
Bahk 2018 {published data only}
- Bahk YY, Park SH, Lee W, Jin K, Ahn SK, Na BK, et al. Comparative Assessment of Diagnostic Performances of Two Commercial Rapid Diagnostic Test Kits for Detection of Plasmodium spp. in Ugandan Patients with Malaria. Korean Journal of Parasitology 2018;56(5):447-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barber 2013 {published data only}
- Barber BE, William T, Grigg MJ, Piera K, Yeo TW, Anstey NM. Evaluation of the sensitivity of a pLDH-based and an aldolase-based rapid diagnostic test for diagnosis of uncomplicated and severe malaria caused by PCR-confirmed Plasmodium knowlesi, Plasmodium falciparum, and Plasmodium vivax. Journal of Clinical Microbiology 2013;51(4):1118–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bell 2001 {published data only}
- Bell D, Go R, Miguel C, Walker J, Cacal L, Saul A. Diagnosis of malaria in a remote area of the Philippines: Comparison of techniques and their acceptance by health workers and the community. Bulletin of the World Health Organization 2001;79(10):933-41. [PMC free article] [PubMed] [Google Scholar]
Bendezu 2010 {published data only}
- Bendezu J, Rosas A, Grande T, Rodriguez H, Llanos-Cuentas A, Escobedo J, et al. Field evaluation of a rapid diagnostic test (Parascreen™) for malaria diagnosis in the Peruvian Amazon. Malaria Journal 2010;9:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berhane 2017 {published data only}
- Berhane A, Russom M, Bahta I, Hagos F, Ghirmai M, Uqubay S. Rapid diagnostic tests failing to detect Plasmodium falciparum infections in Eritrea: An investigation of reported false negative RDT results. Malaria Journal 2017;16(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berzosa 2018 {published data only}
- Berzosa P, Lucio A, Romay-Barja M, Herrador Z, González V, García L, et al. Comparison of three diagnostic methods (microscopy, RDT, and PCR) for the detection of malaria parasites in representative samples from Equatorial Guinea. Malaria Journal 2018;17(1):333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bharti 2008 {published data only}
- Bharti PK, Silawat N, Singh PP, Singh MP, Shukla M, Chand G, et al. The usefulness of a new rapid diagnostic test, the First Response Malaria Combo (pLDH/HRP2) Card Test, for malaria diagnosis in the forested belt of Central India. Malaria Journal 2008;7:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bharti 2013 {published data only}
- Bharti PK, Chand SK, Singh MP, Mishra S, Shukla MM, Singh R, et al. Emergence of a new focus of Plasmodium malariae in forest villages of District Balaghat, Central India: Implications for the diagnosis of malaria and its control. Tropical Medicine and International Health 2013;18(1):12-7. [DOI] [PubMed] [Google Scholar]
Bhide 2014 {published data only}
- Bhide M, Parekh V. Automated screening for malaria parasites on Mindray BC-6800 Hematology Analyzer: A follow-up report. International Journal of Laboratory Hematology 2014;36:80-1. [Google Scholar]
Birhanie 2016 {published data only}
- Birhanie M. Comparison of Partec Rapid Malaria Test with Conventional Light Microscopy for Diagnosis of Malaria in Northwest Ethiopia. Journal of Parasitology Research 2016;2016:3479457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bisoffi 2014 {published data only}
- Bisoffi Z, Gobbi F, Van den Ende J. Rapid diagnostic tests for malaria. BMJ 2014;348:g3846. [DOI] [PubMed] [Google Scholar]
Britton 2016 {published data only}
- Britton S, Cheng Q, Grigg MJ, Poole CB, Pasay C, William T, et al. Sensitive Detection of Plasmodium vivax Using a High-Throughput, Colourimetric Loop Mediated Isothermal Amplification (HtLAMP) Platform: A Potential Novel Tool for Malaria Elimination. PLoS Neglected Tropical Diseases 2016;10(2):e0004443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chayani 2004 {published data only}
- Chayani N, Das B, Sur M, Bajoria S. Comparison of parasite lactate dehydrogenase based immunochromatographic antigen detection assay (optimal) with microscopy for detection of malaria parasites. Indian Journal of Medical Microbiology 2004;22(2):104-6. [PubMed] [Google Scholar]
Cho 2016 {published data only}
- Cho SJ, Lee J, Lee HJ, Jo HY, Sinniah M, Kim HY, et al. A Novel Malaria Pf/Pv Ab Rapid Diagnostic Test Using a Differential Diagnostic Marker Identified by Network Biology. International Journal of Biological Sciences 2016;12(7):824-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dahesh 2015 {published data only}
- Dahesh SM, Mostafa HI. Reevaluation of malaria parasites in El-Fayoum Governorate, Egypt using Rapid Diagnostic Tests (RDTs). Journal of the Egyptian Society of Parasitology 2015;45(3):617-28. [DOI] [PubMed] [Google Scholar]
Dash 2013 {published data only}
- Dash M. Comparison of rapid immunochromatographic assays (ICT malaria P.f./P.v. test and OptiMAL test) with microscopy for detection of malaria parasites. International Journal of Pharma and Bio Sciences 2013;4(4):322-7. [Google Scholar]
Deida 2019 {published data only}
- Deida J, Tahar R, Khalef YO, Lekweiry KM, Hmeyade A, Khairy MLO, et al. Oasis Malaria, Northern Mauritania. Emerging Infectious Diseases 2019;25(2):273-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
DeKoninck 2017 {published data only}
- De Koninck AS, Cnops L, Hofmans M, Jacobs J, Van den Bossche D, Philippé J. Diagnostic performance of the loop-mediated isothermal amplification (LAMP) based illumigene® malaria assay in a non-endemic region. Malaria Journal 2017;16(1):418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dev 2004 {published data only}
- Dev V. Relative utility of dipsticks for diagnosis of malaria in mesoendemic area for Plasmodium falciparum and P. vivax in Northeastern India. Vector Borne and Zoonotic Diseases 2004;4(2):123-30. [DOI] [PubMed] [Google Scholar]
Dinzouna‐Boutamba 2014 {published data only}
- Dinzouna-Boutamba SD, Yang HW, Joo SY, Jeong S, Na BK, Inoue N, et al. The development of loop-mediated isothermal amplification targeting alpha-tubulin DNA for the rapid detection of Plasmodium vivax. Malaria Journal 2014;13:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dzakah 2014 {published data only}
- Dzakah EE, Kang K, Ni C, Tang S, Wang J, Wang J. Comparative performance of aldolase and lactate dehydrogenase rapid diagnostic tests in Plasmodium vivax detection. Malaria Journal 2014;13:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ehtesham 2015 {published data only}
- Ehtesham R, Fazaeli A, Raeisi A, Keshavarz H, Heidari A. Detection of mixed-species infections of Plasmodium falciparum and Plasmodium vivax by nested PCR and rapid diagnostic tests in Southeastern Iran. American Journal of Tropical Medicine and Hygiene 2015;93(1):181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eibach 2013 {published data only}
- Eibach D, Traore B, Bouchrik M, Coulibaly B, Coulibaly N, Siby F, et al. Evaluation of the malaria rapid diagnostic test VIKIA Malaria Ag Pf/Pan™ in endemic and non-endemic settings. Malaria Journal 2013;12:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elahi 2013 {published data only}
- Elahi R, Mohon AN, Khan WA, Haque R, Alam MS. Performance of a HRP-2/pLDH based rapid diagnostic test at the Bangladesh-India-Myanmar border area for diagnosis of clinical malaria. Malaria Journal 2013;12:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Endeshaw 2012 {published data only}
- Endeshaw T, Graves PM, Ayele B, Mosher AW, Gebre T, Ayalew F, et al. Performance of local light microscopy and the ParaScreen Pan/Pf rapid diagnostic test to detect malaria in health centers in Northwest Ethiopia. PLoS One 2012;7(4):e33014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Falade 2016 {published data only}
- Falade CO, Ajayi IO, Nsungwa-Sabiiti J, Siribie M, Diarra A, Serme L, et al. Malaria Rapid Diagnostic Tests and Malaria Microscopy for Guiding Malaria Treatment of Uncomplicated Fevers in Nigeria and Prereferral Cases in 3 African Countries. Clinical Infectious Diseases 2016;63:S290-s297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernando 2004 {published data only}
- Fernando SD, Karunaweera ND, Fernando WP, Attanayake N, Wickremasinghe AR. A cost analysis of the use of the rapid, whole-blood, immunochromatographic P.f/P.v assay for the diagnosis of Plasmodium vivax malaria in rural areas of Sri Lanka. Annals of Tropical Medicine and Parasitology 2004;98(1):5-13. [DOI] [PubMed] [Google Scholar]
Fernando 2004a {published data only}
- Fernando SD, Karunaweera ND, Fernando WP. Evaluation of a rapid whole blood immunochromatographic assay for the diagnosis of Plasmodium falciparum and Plasmodium vivax malaria. Ceylon Medical Journal 2004;49(1):7-11. [DOI] [PubMed] [Google Scholar]
Foster 2014 {published data only}
- Foster D, Cox-Singh J, Mohamad DS, Krishna S, Chin PP, Singh B. Evaluation of three rapid diagnostic tests for the detection of human infections with Plasmodium knowlesi. Malaria Journal 2014;13:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fransisca 2015 {published data only}
- Fransisca L, Kusnanto JH, Satoto TB, Sebayang B, Supriyanto, Andriyan E, et al. Comparison of rapid diagnostic test Plasmotec Malaria-3, microscopy, and quantitative real-time PCR for diagnoses of Plasmodium falciparum and Plasmodium vivax infections in Mimika Regency, Papua, Indonesia. Malaria Journal 2015;14:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gabrielli 2016 {published data only}
- Gabrielli S, Bellina L, Milardi GL, Katende BK, Totino V, Fullin V, et al. Malaria in children of Tshimbulu (Western Kasai, Democratic Republic of the Congo): Epidemiological data and accuracy of diagnostic assays applied in a limited resource setting. Malaria Journal 2016;15:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghai 2016 {published data only}
- Ghai RR, Thurber MI, El Bakry A, Chapman CA, Goldberg TL. Multi-method assessment of patients with febrile illness reveals over-diagnosis of malaria in rural Uganda. Malaria Journal 2016;15:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2018 {published data only}
- Gupta P Gupta P, Rao S, Singh N, Kalita D. Comparison between microscopy and rapid diagnostic tests in diagnosis of malaria at a tertiary care medical institution in Uttarakhand (A 3-year study). Asian Journal of Pharmaceutical and Clinical Research 2018;11(2):94-6. [Google Scholar]
Harani 2006 {published data only}
- Harani MS, Beg MA, Khaleeq L, Adil SN, Kakepoto GN, Khurshid M. Role of ICT malaria immunochromatographic test for rapid diagnosis of malaria. Journal of the Pakistan Medical Association 2006;56(4):167-71. [PubMed] [Google Scholar]
Hawash 2019 {published data only}
- Hawash Y, Ismail K, Alsharif K, Alsanie W. Malaria prevalence in a low transmission area, Jazan District of Southwestern Saudi Arabia. Korean Journal of Parasitology 2019;57(3):233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jabeen 2016 {published data only}
- Jabeen S, Farrukh U, Hameed SA, Kanwal S, Qayyum M. An investigation on the prevalence and efficiency of immunochromatographic testing in suspected malarial patients of Rawalpindi and Islamabad, Pakistan. Turkish Journal of Medical Sciences 2016;46(5):1329-34. [DOI] [PubMed] [Google Scholar]
Jahan 2019 {published data only}
- Jahan F, Khan NH, Wahid S, Ullah Z, Kausar A, Ali N. Malaria epidemiology and comparative reliability of diagnostic tools in Bannu; an endemic malaria focus in south of Khyber Pakhtunkhwa, Pakistan. Pathogens and Global health 2019;113(2):75-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Joseph 2018 {published data only}
- Joseph N, Uchila AK. Validation of malaria antigen detecting rapid diagnostic test kit: A study from highly endemic area in Coastal India. Journal of Clinical and Diagnostic Research 2018;12(9):16-20. [Google Scholar]
Karimov 2013 {published data only}
- Karimov SS, Saiburkhonov DS. Evaluation of the efficiency of rapid tests in identifying malaria patients and parasite carriers in the Republic of Tajikistan. Meditsinskaia parazitologiia i parazitarnye bolezni 2013;(1):44-5. [PubMed] [Google Scholar]
Kim 2013 {published data only}
- Kim JY, Ji SY, Goo YK, Na BK, Pyo HJ, Lee HN, et al. Comparison of rapid diagnostic tests for the detection of Plasmodium vivax malaria in South Korea. PLoS One 2013;8(5):e64353. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kolaczinski 2004 {published data only}
- Klolaczinski J, Mohammed N, Ali I, Ali M, Khan N, Ezard N, et al. Comparison of the OptiMAL rapid antigen test with field microscopy for the detection of Plasmodium vivax and P. falciparum: Considerations for the application of the rapid test in Afghanistan. Annals of Tropical Medicine and Parasitology 2004;98(1):15-20. [DOI] [PubMed] [Google Scholar]
Kosack 2013 {published data only}
- Kosack CS, Naing WT, Piriou E, Shanks L. Routine parallel diagnosis of malaria using microscopy and the malaria rapid diagnostic test SD 05FK60: The experience of Médecins Sans Frontières in Myanmar. Malaria Journal 2013;12:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumari 2014 {published data only}
- Kumari PR. A study of evaluation of rapid diagnostic techniques of Malaria in Urban Slums of Vijayawada, Krishna District, Andhra Pradesh, India. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2014;5(4):1428-49. [Google Scholar]
Liu 2013 {published data only}
- Liu H, Li XR, Li CF, Li XL, Wang HY, Nie RH. Field evaluation of SD(BIOLINE) malaria antigen Plasmodium falciparum/Plasmodium vivax rapid test kit. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2013;31(2):160-1. [PubMed] [Google Scholar]
Mallepaddi 2019 {published data only}
- Mallepaddi PC, Maity SN, Poonati R, Pyadala N, Polavarapu R, Mangamuri UK, et al. Selecting better diagnostic kits for diagnosis of malarial parasites at point of care. 3 Biotech 2019;9(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Metzger 2011 {published data only}
- Metzger WG, Vivas-Martínez S, Giron A, Vaccari E, Campos E, Rodríguez I, et al. Assessment of routine malaria diagnosis in the Venezuelan Amazon. Transactions of the Royal Society of Tropical Medicine and Hygiene 2011;105(5):262-8. [DOI] [PubMed] [Google Scholar]
Moges 2012 {published data only}
- Moges B, Amare B, Belyhun Y, Tekeste Z, Gizachew M, Workineh M, et al. Comparison of CareStart HRP2/pLDH Combo rapid malaria test with light microscopy in North-West Ethiopia. Malaria Journal 2012;11:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohon 2012 {published data only}
- Mohon AN, Elahi R, Podder MP, Mohiuddin K, Hossain MS, Khan WA, et al. Evaluation of the OnSite (Pf/Pan) rapid diagnostic test for diagnosis of clinical malaria. Malaria Journal 2012;11:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Olasehinde 2018 {published data only}
- Olasehinde GI, Oyeka UC, Oniha MI, Onile-Ere OA, Ayepola OO, Ajayi AA, et al. Data set on rapid diagnostic tests (RDTs) and microscopy for diagnosing Plasmodium falciparum and Plasmodium vivax. Data Brief 2018;20:503-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pakalapati 2013 {published data only}
- Pakalapati D, Garg S, Middha S, Kochar A, Subudhi AK, Arunachalam BP, et al. Comparative evaluation of microscopy, OptiMAL(®) and 18S rRNA gene based multiplex PCR for detection of Plasmodium falciparum & Plasmodium vivax from field isolates of Bikaner, India. Asian Pacific Journal of Tropical Medicine 2013;6(5):346-51. [DOI] [PubMed] [Google Scholar]
Pattanasin 2003 {published data only}
- Pattanasin S, Proux S, Chompasuk D, Luwiradaj K, Jacquier P, Looareesuwan S, et al. Evaluation of a new Plasmodium lactate dehydrogenase assay (OptiMAL-IT) for the detection of malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 2003;97(6):672-4. [DOI] [PubMed] [Google Scholar]
Puri 2013 {published data only}
- Puri B, Mehta P, Ingole NA, Prasad P, Mathure T. Laboratory tests for malaria: A diagnostic conundrum? South African Medical Journal 2013;103(9):625-7. [DOI] [PubMed] [Google Scholar]
Rakotonirina 2008 {published data only}
- Rakotonirina H, Barnadas C, Raherijafy R, Andrianantenaina H, Ratsimbasoa A, Randrianasolo L, et al. Accuracy and reliability of malaria diagnostic techniques for guiding febrile outpatient treatment in malaria-endemic countries. American Journal of Tropical Medicine and Hygiene 2008;78(2):217-21. [PubMed] [Google Scholar]
Ranjan 2016 {published data only}
- Ranjan P, Ghoshal U. Utility of nested polymerase chain reaction over the microscopy and immuno-chromatographic test in the detection of Plasmodium species and their clinical spectrum. Parasitology Research 2016;115(9):3375-85. [DOI] [PubMed] [Google Scholar]
Ratsimbasoa 2007 {published data only}
- Ratsimbasoa A, Randriamanantena A, Raherinjafy R, Rasoarilalao N, Ménard D. Which malaria rapid test for Madagascar? Field and laboratory evaluation of three test and expert microscopy of samples from suspected malaria patients in Madagascar. American Journal of Tropical Medicine and Hygiene 2007;76(3):481-5. [PubMed] [Google Scholar]
Ratsimbasoa 2008 {published data only}
- Ratsimbasoa A, Fanazava L, Radrianjafy R, Ramilijaona J, Rafanomezantsoa H, Ménard D. Evaluation of two new immunochromatographic assays for diagnosis of malaria. American Journal of Tropical Medicine and Hygiene 2008;79(5):670-2. [PubMed] [Google Scholar]
Samane 2010 {published data only}
- Samane AK, Nahid HZ, Saaed S, Khazen H, Ali H, Ahmad R, et al. Comparison of microscopy and RDTs techniques for laboratory detection of malaria. African Journal of Biotechnology 2010;9(10):1514-6. [Google Scholar]
Selimuzzaman 2010 {published data only}
- Selimuzzaman SM, Islam SJ, Nahar Z, Das R, Rahman MA, Rahman MA. Malariagen malaria Pf/Pv antigen rapid test: A simple and effective tool for diagnosis of malaria in the far-flung hilly areas of Bangladesh. Mymensingh Medical Journal 2010;19(1):94-9. [PubMed] [Google Scholar]
Shaikh 2013 {published data only}
- Shaikh S, Memon S, Memon H, Ahmed I. Role of rapid diagnostic tests for guiding outpatient treatment of febrile illness in Liaquat University Hospital. Pakistan Journal of Medical Sciences 2013;29(5):1167-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharma 2014 {published data only}
- Sharma J. Prevalence of malaria cases in tea garden areas of Lakhimpur District, Assam. International Journal of Pharmacy and Pharmaceutical Sciences 2014;6(8):571-3. [Google Scholar]
Singh 2000a {published data only}
- Singh N, Saxena A, Valecha N. Field evaluation of the ICT malaria P.f/P.v immunochromatographic test for diagnosis of Plasmodium falciparum and P. vivax infection in forest villages in Chhindwara, Central India. Tropical Medicine and International Health 2000;5(11):765-70. [DOI] [PubMed] [Google Scholar]
Singh 2003 {published data only}
- Singh N, Valecha N, Nagpal AC, Mishra SS, Varma HS, Subbaro SK. The hospital- and field-based performances of the OptiMAL test, for malaria diagnosis and treatment monitoring in Central India. Annals of Tropical Medicine and Parasitology 2003;97(1):5-13. [DOI] [PubMed] [Google Scholar]
Singh 2013 {published data only}
- Singh N, Bharti PK, Singh MP, Mishra S, Shukla MM, Sharma RK, et al. Comparative evaluation of bivalent malaria rapid diagnostic tests versus traditional methods in field with special reference to heat stability testing in Central India. PLoS One 2013;8(3):e58080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siwal 2018 {published data only}
- Siwal N, Singh US, Dash M, Kar S, Rani S, Rawal C, et al. Malaria diagnosis by PCR revealed differential distribution of mono and mixed species infections by Plasmodium falciparum and P. vivax in India. PLoS One 2018;13(3):e0193046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stijnberg 2013 {published data only}
- Stijnberg D, Cairo H. Rapid diagnostic test (RDT) performance of the malaria gold mining program in Suriname: Comparing the performance of two RDT's. In: 62nd Annual Meeting of the American Society of Tropical Medicine and Hygiene; 2013 13-17 November; Washington, DC. Vol. 89(5 Suppl 1). Washington, DC, USA: The American Society of Tropical Medicine and Hygiene, 2013:384.
Strom 2014 {published data only}
- Strøm GE, Moyo S, Fataki M, Langeland N, Blomberg B. PCR targeting Plasmodium mitochondrial genome of DNA extracted from dried blood on filter paper compared to whole blood. Malaria Journal 2014;13:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thongdee 2014 {published data only}
- Thongdee P, Chaijaroenkul W, Kuesap J, Na-Bangchang K. Nested-PCR and a new ELISA-based NovaLisa test kit for malaria diagnosis in an endemic area of Thailand. Korean Journal of Parasitology 2014;52(4):377-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tjitra 1999 {published data only}
- Tjitra E, Suprianto S, Dyer M, Currie BJ, Anstey NM. Field evaluation of the ICT malaria P.f/P.v immunochromatographic test in detection of Plasmodium falciparum and Plasmodium vivax in patients with a presumptive clinical diagnosis of malaria in Eastern Indonesia. Journal of Clinical Microbiology 1999;37(8):2412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trouvay 2013 {published data only}
- Trouvay M, Palazon G, Berger F, Volney B, Blanchet D, Faway E, et al. High performance of histidine-rich protein 2 based rapid diagnostic tests in French Guiana are explained by the absence of pfhrp2 gene deletion in P. falciparum. PLoS One 2013;8(9):e74269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Valecha 2003 {published data only}
- Valecha N, Singh N, Yadav RS, Dev V, Aggarwal A, Subbarao SK. Field evaluation of OptiMAL48 rapid malaria diagnostic test in India. Acta Parasitologica 2003;48(3):229-32. [Google Scholar]
van den Broek 2006 {published data only}
- den Broek I, Hill O, Gordillo F, Angarita B, Hamade P, Counihan H, et al. Evaluation of three rapid tests for diagnosis of P. falciparum and P. vivax malaria in Colombia. American Journal of Tropical Medicine and Hygiene 2006;75(6):1209-15. [PubMed] [Google Scholar]
Vohra 2014 {published data only}
- Vohra P, Mengi S, Bunger R, Pathania D, Singh VA. Comparison of peripheral blood film stained by Giemsa stain, acridine orange staining and rapid diagnostic tests for detection of P. vivax and P. falciparum in clinically suspected cases of malaria. International Journal of Pharma Medicine and Biological Sciences 2014;3(3):108-112. [Google Scholar]
Wang 2014 {published data only}
- Wang ZY, Jiang L, Zhang YG, Zhang XP, Cai L. Comparison of two rapid diagnostic tests in detection of malaria parasites. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2014;32(1):50-3. [PubMed] [Google Scholar]
Wongsrichanalai 2003 {published data only}
- Wongsrichanalai G, Arevalo I, Laoboonchai A, Yingyuen K, Miller RS, Magill AJ, et al. Rapid diagnostic devices for malaria: Field evaluation of a new prototype immunochromatographic assay for the detection of Plasmodium falciparum and non-falciparum Plasmodium. American Journal of Tropical Medicine and Hygiene 2003;69(1):26-30. [PubMed] [Google Scholar]
Woyessa 2013 {published data only}
- Woyessa A, Deressa W, Ali A, Lindtjørn B. Evaluation of CareStart™ Malaria Pf/Pv Combo test for Plasmodium falciparum and Plasmodium vivax malaria diagnosis in Butajira area, South-Central Ethiopia. Malaria Journal 2013;12:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xiaodong 2013 {published data only}
- Xiaodong S, Tambo E, Chun W, Zhibin C, Yan D, Jian W, et al. Diagnostic performance of CareStart™ malaria HRP2/pLDH (Pf/pan) Combo test versus standard microscopy on falciparum and vivax malaria between China-Myanmar endemic borders. Malaria Journal 2013;12:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yan 2013 {published data only}
- Yan J, Li N, Wei X, Li P, Zhao Z, Wang L, et al. Performance of two rapid diagnostic tests for malaria diagnosis at the China-Myanmar border area. Malaria Journal 2013;12:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Boni 2015 {published data only}
- Boni MF, Lover AA, Ngo TD, Nguyen TX, Sutamihardja A, Tran DT, et al. Potential impact of rapid diagnostic test diagnostic error on increased malaria transmission of emerging untreatable malaria. In: 64th Annual Meeting of the American Society of Tropical Medicine and Hygiene; 2015 October 25-29; Philadelphia (PA). Vol. 93(4 Suppl). Philadelphia (PA), USA: The American Society of Tropical Medicine and Hygiene, 2015:371.
Cheng 2013 {published data only}
- Cheng Z, Sun X, Yang Y, Wang H, Zheng Z. A novel, sensitive assay for high-throughput molecular detection of Plasmodia for active screening of malaria for elimination. Journal of Clinical Microbiology 2013;51(1):125-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reda 2016 {published data only}
- Reda AG. Evaluation of the performance of SD bioline malaria AG PF/PV and carestartTM malaria PF/PV combo tests for the diagnosis of malaria in two malarious areas in central Ethiopia. In: 65th Annual Meeting of the American Society of Tropical Medicine and Hygiene; 2016 November 13-17; Atlanta (GA). Vol. 95(5 Suppl). Atlanta (GA), USA: The American Society of Tropical Medicine and Hygiene, 2016:82-83.
Additional references
Abba 2014
- Abba K, Kirkham AJ, Olliaro PL, Deeks JJ, Donegan S, Garner P, et al. Rapid diagnostic tests for diagnosing uncomplicated non-falciparum or Plasmodium vivax malaria in endemic countries. Cochrane Database of Systematic Reviews 2014, Issue 12. Art. No: CD011431. [DOI: 10.1002/14651858.CD011431] [DOI] [PMC free article] [PubMed] [Google Scholar]
Campo 2015
- Campo B, Vandal O, Wesche DL, Burrows JN. Killing the hypnozoite – drug discovery approaches to prevent relapse in Plasmodium vivax. Pathogens and Global Health 2015;109(3):107-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2016
- Chen I, Clarke SE, Gosling R, Hamainza B, Killeen G, Magill A, et al. “Asymptomatic” malaria: A chronic and debilitating infection that should be treated. PLoS Medicine 2016;13(1):e1001942. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chu 2006
- Chu H, Cole SR. Bivariate meta-analysis of sensitivity and specificity with sparse data: A generalized linear mixed model approach. Journal of Clinical Epidemiology 2006;59(12):1331-2; author reply 1332-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Culleton 2012
- Culleton R, Carter R. African Plasmodium vivax: Distribution and origins. International Journal for Parasitology 2012;42(12):1091-7. [DOI] [PubMed] [Google Scholar]
Deeks 2005
- Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. Journal of Clinical Epidemiology 2005;58(9):882-93. [DOI] [PubMed] [Google Scholar]
Dobson 1994
- Dobson MJ. Malaria in England: A geographical and historical perspective. Parassitologia 1994;36(1-2):35-60. [PubMed] [Google Scholar]
Gatton 2018
- Gatton ML, Ciketic S, Barnwell JW, Cheng Q, Chiodini PL, Incardona S, et al. An assessment of false positive rates for malaria rapid diagnostic tests caused by non-Plasmodium infectious agents and immunological factors. PLoS One 2018;13(5):e0197395. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2013
- Schünemann H, Brożek J, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. Available from www.gdt.guidelinedevelopment.org/app/handbook/handbook.html (accessed 5 December 2017).
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 5 December 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Han 2017
- Han TZ, Han KT, Aye KH, Hlaing T, Thant KZ, Vythilingam I. Comparison of microscopy and PCR for the detection of human Plasmodium species and Plasmodium knowlesi in Southern Myanmar. Asian Pacific Journal of Tropical Biomedicine 2017;7(8):680-5. [Google Scholar]
Hänscheid 2002
- Hänscheid T, Grobusch MP. How useful is PCR in the diagnosis of malaria? Trends in Parasitology 2002;18(9):395-8. [DOI] [PubMed] [Google Scholar]
Kakkilaya 2003
- Kakkilaya BS. Rapid diagnosis of malaria. Laboratory Medicine 2003;34(8):602-8. [Google Scholar]
Macaskill 2010
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10: Analysing and presenting results. In: Deeks JJ, Bossuyt PM, Gatsonis C, editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0. The Cochrane Collaboration 2010. Available from: methods.cochrane.org/sdt/handbook-dta-reviews.
Maltha 2013
- Maltha J, Gillet P, Jacobs J. Malaria rapid diagnostic tests in endemic settings. Clinical Microbiology and Infection 2013;19(5):399-407. [DOI] [PubMed] [Google Scholar]
Moonasar 2007
- Moonasar D, Goga AE, Frean J, Kruger P, Chandramohan D. An exploratory study of factors that affect the performance and usage of rapid diagnostic tests for malaria in the Limpopo Province, South Africa. Malaria Journal 2007;6:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roth 2016
- Roth J, Korevaar D, Leeflang MM, Mens P. Molecular malaria diagnostics: A systematic review and meta-analysis. Critical Reviews in Clinical Laboratory Sciences 2016;53(2):87-105. [DOI] [PubMed] [Google Scholar]
Shokoples 2009
- Shokoples SE, Ndao M, Kowalewska-Grochowska K, Yanow SK. Multiplexed real-time PCR assay for discrimination of Plasmodium species with improved sensitivity for mixed infections. Journal of Clinical Microbiology 2009 Apr 1;47(4):975-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Snounou 1993
- Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Molecular and Biochemical Parasitology 1993;61(2):315-20. [DOI] [PubMed] [Google Scholar]
STATA 2015 [Computer program]
- Stata statistical software. Version 15. College Station, TX: StataCorp LLC, 2017.
Takwoingi 2013
- Takwoingi Y, Leeflang MM, Deeks JJ. Empirical evidence of the importance of comparative studies of diagnostic test accuracy. Annals of Internal Medicine 2013;158(7):544-54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Takwoingi 2015a
- Takwoingi Y, Guo B, Riley RD, Deeks JJ. Performance of methods for meta-analysis of diagnostic test accuracy with few studies or sparse data. Statistical Methods in Medical Research 2015;26(4):1896-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
Takwoingi 2015b
- Takwoingi Y, Riley DD, Deeks JJ. Meta-analysis of diagnostic accuracy studies in mental health. Evidence-Based Mental Health 2015;18(4):103-9. [DOI: 10.1136/eb-2015-102228] [DOI] [PMC free article] [PubMed] [Google Scholar]
Talman 2007
- Talman AM, Duval L, Legrand E, Hubert V, Yen S, Bell D, et al. Evaluation of the intra and inter-specific genetic variability of Plasmodium lactate dehydrogenase. Malaria Journal 2007;6:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2018
- White NJ. Anaemia and malaria. Malaria Journal 2018;17:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiting 2009
- Whiting P, Westwood M, Burke M, Sterne J, Glanville J. Systematic reviews of test accuracy should search a range of databases to identify primary studies. Journal of Clinical Epidemiology 2009;61(4):357–64. [DOI] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529-36. [DOI] [PubMed] [Google Scholar]
WHO 2003
- World Health Organization. Malaria rapid diagnosis:making it work: World Health Organization. Meeting report 20-23 January, Manila. apps.who.int/iris/bitstream/handle/10665/208030/RS_2003_GE_05_PHL_eng.pdf?sequence=1&isAllowed=y (accessed 30 Oct 2018).
WHO 2006
- World Health Organization. Towards quality testing of malaria rapid diagnostic tests: evidence and methods. Proceedings of the WHO Informal Consultation on development and methods for testing malaria rapid diagnostic tests 28 February - 2 March 2006. Available at www.portal.pmnch.org/malaria/publications/atoz/929061238X/en/ (accessed 30 Oct 2018).
WHO 2012
- World Health Organization. Malaria rapid diagnostic test performance: results of WHO product testing of malaria RDTs: Round 4 (2012). December 2012. Available at www.who.int/malaria/publications/rapid_diagnostic/en/ (accessed 30 Oct 2018).
WHO 2014
- World Health Organization, Medicines for Malaria Venture, Roll Back Malaria, and the Wellcome Trust. Severe malaria. Tropical Medicine & International Health 2014;19(s1):7-131. [Google Scholar]
WHO 2015a
- World Health Organization. Guidelines for the treatment of malaria. Third edition. April 2015. http://apps.who.int/iris/bitstream/handle/10665/162441/9789241549127_eng.pdf;jsessionid=9F90A5F3F058C3DE058158B4003EDA38?sequence=1 (accessed 26 July 2018).
WHO 2015b
- World Health Organization. The role of RDTs in malaria control. www.who.int/malaria/areas/diagnosis/rapid-diagnostic-tests/role_in_malaria_control/en/ (accessed 4 December 2017).
WHO 2017a
- World Health Organization. World Malaria Report 2017. apps.who.int/iris/bitstream/handle/10665/259492/9789241565523-eng.pdf;jsessionid=85F16FF476D140F1E6DEF84CF03783DC?sequence=1 (accessed 9 April 2018).
WHO 2017b
- World Health Organization. WHO-FIND malaria RDT evaluation programme. Last update 10 July 2017. www.who.int/malaria/areas/diagnosis/rapid-diagnostic-tests/rdt-evaluation-programme/en/ (accessed 4 December 2017).
WHO 2017c
- World Health Organization. A framework for malaria elimination. Last update March 2017. www.who.int/malaria/publications/atoz/9789241511988/en/ (accessed 29 April 2020).
WHO 2020
- World Health Organization. WHO information notice for users. Last updated January 2020. www.who.int/diagnostics_laboratory/procurement/accessbioinc_20012020_noticeforusers.pdf?ua=1 (accessed 30 July 2020).
Wongsrichanalai 2007
- Wongsrichanalai C, Barcus MJ, Muth S, Sutamihardja A, Wernsdorfer WH. A review of malaria diagnostic tools: Microscopy and rapid diagnostic test (RDT). American Journal of Tropical Medicine and Hygiene 2007;77(6 Suppl):119-27. [PMID: ] [PubMed] [Google Scholar]


