ABSTRACT
An increase in invasive meningococcal disease (IMD) incidence was observed in Tuscany in 2015/2016, mainly due to hypervirulent clonal complex (cc) 11 strains. In a post-hoc analysis, we assessed bactericidal activity of antibodies in sera from children primed with MenACWY-CRM or MenC-CRM conjugate vaccines and receiving a MenACWY-CRM booster dose against 5 meningococcal C (MenC) strains isolated from IMD cases. Sera collected from 90 infants/toddlers who participated in a phase III, open-label study (NCT00667602) and its extension (NCT01345721) were tested by serum bactericidal activity assay with human complement (hSBA). Children were primed with either MenACWY-CRM at 6–8 and 12 months of age (group 2_MenACWY; N = 30), MenACWY-CRM (group 1_MenACWY; N = 30), or MenC-CRM at 12 months of age (group 1_MenC; N = 30); all received MenACWY-CRM booster dose at 22–45 months of age. Four tested strains (FI001–FI004) were C:P1.5–1,10-8:F3-6:ST-11 (cc11) and 1 (FI005) was C:P1.7–4,14-6:F3-9:ST-1031 (cc334). Overall, immune responses tended to be higher against Fl002–FI004 than Fl001 and Fl005. Geometric mean titers were high in group 2_MenACWY (range: 94.8 [FI005]–588.1 [FI004]) and very high post-boosting with MenACWY-CRM in all groups (176.9 [FI005]–3911.0 [FI004]). Seroresponse rates tended to be higher in group 1_MenC (33.3% [FI005]–93.3% [FI004]) than in group 1_MenACWY (16.7% [FI005]–73.3% [FI004]). Irrespective of strains tested or the identity/number of priming doses, ≥96.7% of children had hSBA titers ≥1:8 post-MenACWY-CRM booster dose. MenACWY-CRM and MenC-CRM elicited bactericidal antibodies and immunological memory against hypervirulent cc11 and cc334 MenC strains responsible for IMD outbreaks.
KEYWORDS: Hypervirulent MenC strains, cc11/cc334 clonal complexes, outbreak, MenACWY-CRM conjugate vaccine, MenC-CRM conjugate vaccine
Introduction
An increase of Neisseria meningitidis serogroup C (MenC) invasive meningococcal disease (IMD) was reported during January 2015–February 2016 in Tuscany, a region located in the center of Italy.1 Within this period of slightly more than 1 year, 43 confirmed MenC IMD cases, of which 10 fatal, were reported, accounting for approximately 38% of all confirmed MenC IMD cases recorded since 2000 in the region (overall, 111 MenC IMD cases from January 2000 to February 2016). The vast majority (87.5%) of MenC strains isolated during this outbreak belonged to the clonal complex (cc) 11, with cc334 isolated in fewer individuals.1
This post-hoc analysis aimed to evaluate the magnitude of immune responses against hypervirulent cc11 and cc334 strains in sera collected from infants/toddlers after priming with a monovalent MenC conjugate vaccine (MenC-CRM; Menjugate, GSK) or a quadrivalent MenA, MenC, MenW, MenY conjugate vaccine (MenACWY-CRM; Menveo, GSK) and a booster dose of MenACWY-CRM.
Methods
Post-hoc analysis methodology
Two completed studies, part of the clinical development program of MenACWY-CRM, were selected for this analysis, based on the population evaluated (infants/toddlers, at high risk of IMD) and the vaccine schedules (priming with different vaccines and doses, and assessment of booster responses). The first one was a phase III, open label, randomized, multicenter study (NCT00667602) performed in Germany and Australia in 2011. This study was conducted to evaluate immune responses and safety after priming of infants/toddlers with 1 or 2 doses of MenACWY-CRM, as compared to priming with a single dose of MenC-CRM. The second selected study was its extension, a phase IIIb trial (NCT01345721) performed in Germany to assess the immunogenicity and safety of a booster dose of MenACWY-CRM vaccine, 10–33 months following the last priming dose in the parent study. The vaccines’ composition has been previously described.2,3
The selection of these two studies was facilitated by the availability of remaining aliquots of sera for retest; sera were selected from those obtained by individuals who participated in both studies and had evaluable samples at all timepoints of interest: Visits 3 (pre-second dose for group 2_MenACWY and pre-first dose for groups 1_MenACWY and 1_MenC) and 4 (1 month post-Visit 3) of the parent study and Visits 7 (pre-booster dose) and 8 (1 month post-booster dose) of its extension (Figure 1). The only sera that were a priori excluded from this post-hoc analysis, irrespective of their availability for retesting, were those obtained from children enrolled in one of the sites from Germany for which noncompliance with Good Clinical Practices was documented during the conduction of the original phase III study. Retest of samples was allowed by the original informed consent obtained from the children’s parents.
Figure 1.
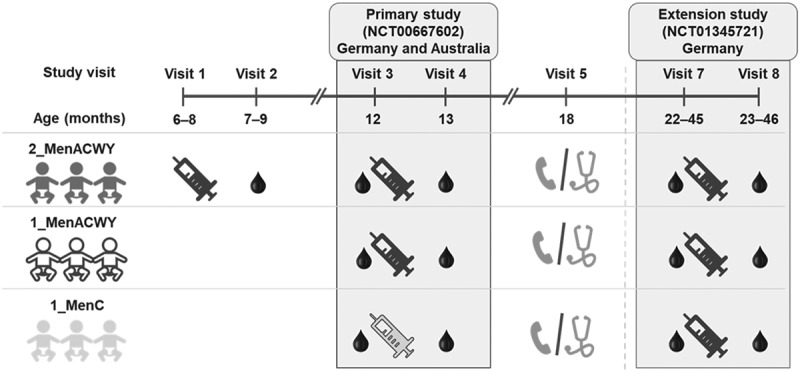
Study design for parent and extension studies
Group 2_MenACWY, MenACWY-CRM vaccination at 6–8 and 12 months of age; Group 1_MenACWY, MenACWY-CRM vaccination at 12 months of age; Group 1_MenC, MenC-CRM vaccination at 12 months of age. All children received a booster dose of MenACWY-CRM at 22–45 months of age.Note: Visits enclosed in gray boxes correspond to timepoints considered in the analysis. Syringes indicate vaccine administration (black for MenACWY-CRM and gray for MenC-CRM).Visit 3 was pre-second dose for group 2_MenACWY and pre-first dose for groups 1_MenACWY and 1_MenC; Visit 4 was post-primary vaccination, Visit 7, pre-booster vaccination, Visit 8, post-booster vaccination. Visit 6 (blood sample collection to evaluate persistence of immune response at 6–18 months post-primary vaccination) applied only to children enrolled at the Australian sites.
Overall, sera from a randomly selected subset of 90 children, who took part in both the parent and the extension study and from whom ≥300 μL of sera were available at each timepoint, were analyzed for antibody responses: sera from 30 children from each of the parent study groups (group 2_MenACWY: priming with 2 MenACWY-CRM doses [at 6–8 and 12 months of age], group 1_MenACWY: priming with 1 MenACWY-CRM dose [at 12 months of age], group 1_MenC: priming with 1 dose of MenC-CRM [at 12 months of age]), who also received a booster dose at 22–45 months of age in the extension study (Figure 1).
Tested strains
Among the IMD cases observed in Tuscany, 5 hypervirulent MenC strains, isolated by the Laboratory of Immunology and Immunopathology at the Meyer Hospital of Florence during the years 2015 and 2016, were made available for serum bactericidal assay testing.
A genotypic analysis of the ferric enterobactin transport protein A (fetA) and porin A (PorA) variable regions was performed.
The phylogenetic network, based on PubMLST4 core genome distances, was determined. The distance matrix between isolates was calculated using the BIGSdb Genome Comparator tool,5 based on the number of loci carrying different alleles for each pair of strains. The scheme used was N. meningitidis cgMLST v1.0, a core genome scheme that includes 1605 loci found to be present in ≥95% N. meningitidis isolates.6 Neighbor-net diagrams were generated using SplitsTree5.7
Immunogenicity assessment
Antibody titers against the hypervirulent MenC strains were tested using a non-validated serum bactericidal activity (SBA) assay with human complement (hSBA), developed by the Preclinical Evidence Generation Serology Department (GSK, Siena, Italy) and performed, with minor modifications, as previously described.8 During the development of the assay, critical parameters for target strains, incubation times, complement sources and test acceptance criteria were defined and reproducibility was demonstrated. The lower limit of quantitation for the hSBA assay was determined as a titer of 1:4.
Statistical analysis
The analyses were purely descriptive in nature and were not statistically powered to detect differences between groups.
A one-way analysis of variance (ANOVA) model with vaccine group as a factor was used to calculate the hSBA geometric mean titers (GMTs) and geometric mean ratios (GMRs; Visit 4/Visit 3 and Visit 8/Visit 7). The GMTs, GMRs and 2-sided 95% confidence intervals (CIs) were computed by exponentiating (base 10) the least square means and their 95% CIs of the logarithmically transformed (base 10) hSBA titers. Titers below the detection limit (<1:4) were set to half that limit.
GMR was not calculated for group 2_MenACWY as the blood draw at Visit 3 for this group did not correspond to pre-vaccination levels (Visit 3 occurred 4–6 months post-first vaccine dose).
Seroresponse was defined for children with Visit 3 hSBA titer <1:4 as an hSBA titer ≥1:8 at Visit 4, and for children with Visit 3 hSBA titer ≥1:4 as a ≥ 4-fold rise in hSBA titers at Visit 4 (1 month after the first meningococcal vaccine dose for children in group 1_MenACWY and group 1_MenC of the parent study). The associated 2-sided 95% Clopper-Pearson CIs were calculated.
The percentages of children with hSBA titers ≥1:4 and ≥1:8 against the hypervirulent MenC strains and the associated 2-sided 95% Clopper-Pearson CIs were calculated at all timepoints (Visits 3, 4, 7 and 8).
After having randomly selected the 90 children based on sera availability for retesting, a post-hoc analysis of the same immunogenicity endpoints against the MenC reference strain used in the 2 clinical studies was performed using the original datasets restricted to the subset of selected children. Bactericidal activity against the reference MenC strain was tested (only in the original trials) with a validated manual hSBA assay9,10 at the GSK Clinical Sciences Laboratory (Marburg, Germany).
Results
Baseline characteristics for study population
The demographic and other baseline characteristics of the 90 children whose sera were available for retesting are presented in Appendix Table A1 and were similar across groups.
Overall, the mean age (± standard deviation) at time of study enrollment in the parent study was 6.6 ± 0.6 months. Slightly more males than females were included in the groups 2_MenACWY and 1_MenACWY, compared to the group 1_MenC. Across groups, most children (93–100%) were Caucasian.
MenC strains
Genetic characterization of the 5 hypervirulent MenC strains showed that 4 of them (FI-001–FI-004) belonged to the C:P1.5–1,10-8:F3-6:ST-11 (cc11) clonal complex, while FI-005 belonged to the C:P1.7–4,14-6:F3-9:ST-1031 (cc334) clonal complex. Four strains (FI-001–FI-004) were almost identical, and distinct from FI-005 (cc334), isolated from sporadic IMD cases (Figure 2). Isolate FI-005 had an average distance of 1430 loci from the other 4 isolates.
Figure 2.
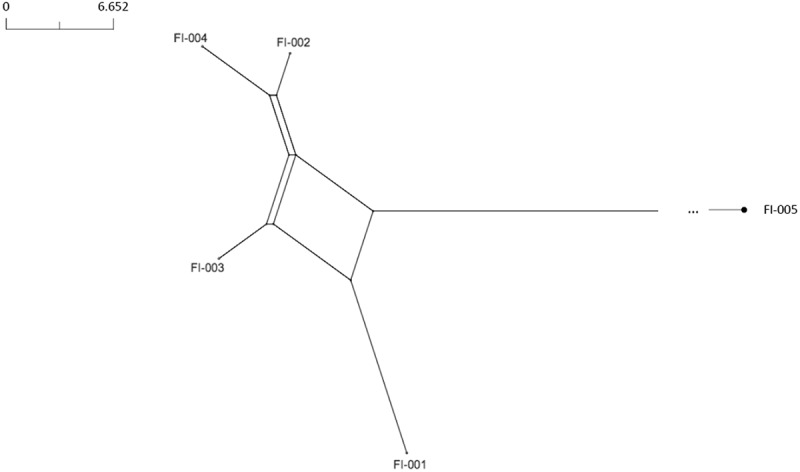
Core genome analysis of the hypervirulent MenC strains
Immunogenicity
Geometric mean titers and geometric mean ratios
Approximately 1 month after completion of the primary series, hSBA GMTs tended to be lower against the cc334 strain (FI-005, range 3.6–94.8, across groups) than against the other cc11 strains (FI-001–FI-004, range 5.3–588.1, across groups and strains), with the highest titers following 2-dose and the lowest after 1-dose of MenACWY-CRM (Figure 3a). Visit 4/Visit 3 GMRs ranged from 1.7 (FI-005, group 1_MenACWY) to 23.7 (FI-004, group 1_MenC) (Figure 3b).
Figure 3.
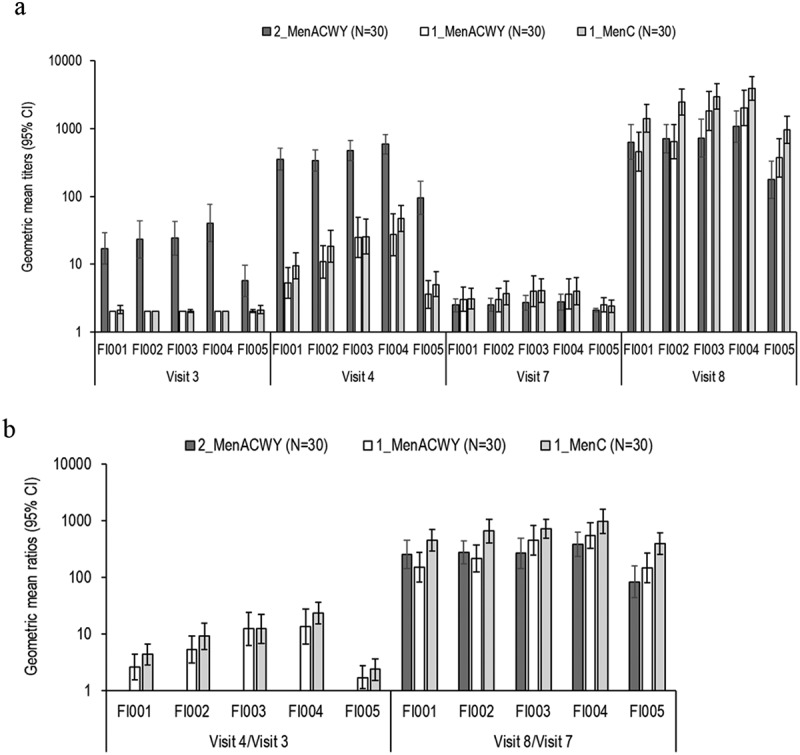
hSBA geometric mean titers (A) and geometric mean ratios (B) for hypervirulent MenC strains
hSBA, serum bactericidal activity assay with human complement; 95% CI, 95% confidence interval; N, number of children; Group 2_MenACWY, MenACWY-CRM vaccination at 6–8 and 12 months of age; Group 1_MenACWY, MenACWY-CRM vaccination at 12 months of age; Group 1_MenC, MenC-CRM vaccination at 12 months of age.
Before the MenACWY-CRM booster dose (10–33 months post-priming), hSBA GMTs ranged from 2.1 (FI-005, group 2_MenACWY) to 4.1 (FI-003, group 1_MenC) (Figure 3a).
One month post-booster dose, high hSBA GMTs were observed against all five hypervirulent MenC strains in all 3 groups, and especially in the group of children primed with MenC-CRM. hSBA GMTs ranged from 176.9 (group 2_MenACWY) to 955.4 (group 1_MenC) for FI-005 and from 456.1 (FI-001, group 1_MenACWY) to 3911.0 (FI-004, group 1_MenC) for strains belonging to cc11 (Figure 3a). Visit 8/Visit 7 GMRs ranged from 84.4 (group 2_MenACWY) to 397.1 (group 1_MenC) for FI-005, and from 150.5 (FI-001, group 1_MenACWY, FI-001) to 977.8 (FI-004, group 1_MenC) for cc11 strains (Figure 3b).
When tested against the vaccine reference strain (Appendix Table A2), hSBA GMTs were higher in the 2_MenACWY group (279.9), compared to the other 2 (23.9 and 35.6, groups 1_MenACWY and 1_MenC, respectively). Following the MenACWY-CRM booster dose, GMTs were 554.1, 956.0 and 1935.4 in groups 2_MenACWY, 1_MenACWY and 1_MenC, respectively. GMRs ranged from 156.6 (group 1_MenACWY) to 449.2 (group 1_MenC).
Seroresponse rates
Seroresponse rates were lower for the FI-005 strain (16.7% and 33.3%, groups 1_MenACWY and 1_MenC, respectively) and higher for the FI004 strain (73.3% and 93.3%, groups 1_MenACWY and 1_MenC, respectively) (Figure 4). When analyzing immune responses against the MenC vaccine reference strain 1 month post-primary series, ≥82.8% of children in groups 1_MenACWY and 1_MenC achieved seroresponse following priming (Appendix Table A2).
Figure 4.
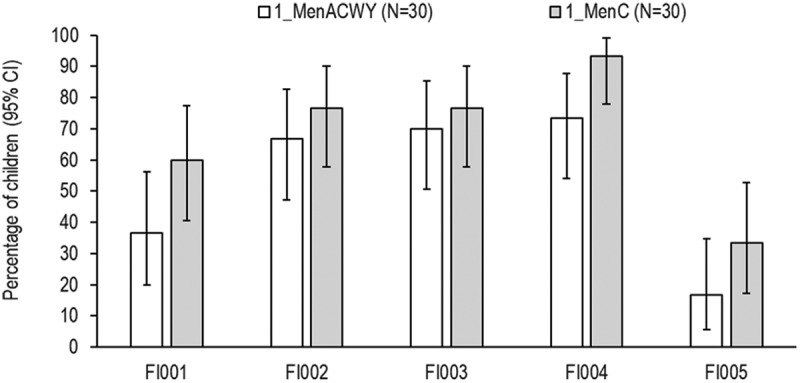
Percentages of children with seroresponse against hypervirulent MenC strains
95% CI, 95% confidence interval; N, number of children; Group 1_MenACWY, MenACWY-CRM vaccination at 12 months of age; Group 1_MenC, MenC-CRM vaccination at 12 months of age. Seroresponse was defined for children with Visit 3 hSBA titer <1:4 as an hSBA titer ≥1:8 at Visit 4, and for children with Visit 3 hSBA titer ≥1:4 as a ≥ 4-fold rise in hSBA titers at Visit 4.
Percentages of children with hSBA titers ≥1:4 and ≥1:8
One month after completion of the primary series, percentages of children with hSBA titers ≥1:4 ranged from 90.0% (FI005) to 100% (FI001–FI004) in group 2_MenACWY, from 26.7% (FI005) to 80.0% (FI004) in group 1_MenACWY, and from 53.3% (FI005) to 100% (FI004) in group 1_MenC (Table 1). Against the reference MenC strain, similar percentages of children had hSBA titers ≥1:4 across groups, ranging from 90.0% (group 1_MenACWY) to 100% (group 2_MenACWY) (Appendix Figure A1).
Table 1.
Percentages of children with hSBA antibody titers ≥1:4 and ≥1:8 against hypervirulent MenC strains (all time points). hSBA, serum bactericidal activity assay with human complement; %, percentages of children with hSBA titers equal to or above the specified threshold; 95% CI, 95% confidence interval; N, number of children in each group; Group 2_MenACWY, MenACWY-CRM vaccination at 6–8 and 12 months of age; Group 1_MenACWY, MenACWY-CRM vaccination at 12 months of age; Group 1_MenC, MenC-CRM vaccination at 12 months of age; All children were vaccinated with booster dose of MenACWY-CRM at 22–45 months of age. Visit 3 was pre-second dose for group 2_MenACWY and pre-first dose for groups 1_MenACWY and 1_MenC; Visit 4 was post-primary vaccination, Visit 7, pre-booster vaccination, Visit 8, post-booster vaccination
| % (95% CI) |
|||||||
|---|---|---|---|---|---|---|---|
| 2_MenACWY (N=30) |
1_MenACWY (N=30) |
1_MenC (N=30) |
|||||
| hSBA titers ≥1:4 | hSBA titers ≥1:8 | hSBA titers ≥1:4 | hSBA titers ≥1:8 | hSBA titers ≥1:4 | hSBA titers ≥1:8 | ||
| FI001 | Visit 3 | 86.7 (69.3; 96.2) | 76.7 (57.7; 90.1) | 0 (0; 11.6) | 0 (0; 11.6) | 3.3 (0.1; 17.2) | 3.3 (0.1; 17.2) |
| Visit 4 | 100 (88.4; 100) | 100 (88.4; 100) | 50.0 (31.3; 68.7) | 36.7 (19.9; 56.1) | 80.0 (61.4; 92.3) | 63.3 (43.9; 80.1) | |
| Visit 7 | 16.7 (5.6; 34.7) | 6.7 (0.8; 22.1) | 13.3 (3.8; 30.7) | 13.3 (3.8; 30.7) | 23.3 (9.9; 42.3) | 20.0 (7.7; 38.6) | |
| Visit 8 | 96.7 (82.8; 99.9) | 96.7 (82.8; 99.9) | 96.7 (82.8; 99.9) | 96.7 (82.8; 99.9) | 100 (88.4; 100) | 100 (88.4; 100) | |
| FI002 | Visit 3 | 86.7 (69.3; 96.2) | 76.7 (57.7; 90.1) | 0 (0; 11.6) | 0 (0; 11.6) | 0 (0; 11.6) | 0 (0; 11.6) |
| Visit 4 | 100 (88.4; 100) | 100 (88.4; 100) | 70.0 (50.6; 85.3) | 66.7 (47.2; 82.7) | 86.7 (69.3; 96.3) | 76.7 (57.7; 90.1) | |
| Visit 7 | 16.7 (5.6; 34.7) | 10.0 (2.1; 26.5) | 13.3 (3.8; 30.7) | 13.3 (3.8; 30.7) | 33.3 (17.3; 52.8) | 23.3 (9.9; 42.3) | |
| Visit 8 | 100 (88.4; 100) | 100 (88.4; 100) | 100 (88.4; 100) | 96.7 (82.8; 99.9) | 100 (88.4; 100) | 100 (88.4; 100) | |
| FI003 | Visit 3 | 90.0 (73.5; 97.9) | 76.7 (57.7; 90.1) | 0 (0; 11.6) | 0 (0; 11.6) | 3.3 (0.1; 17.2) | 0 (0; 11.6) |
| Visit 4 | 100 (88.4; 100) | 100 (88.4; 100) | 76.7 (57.7; 90.1) | 70.0 (50.6; 85.3) | 86.7 (69.3; 96.2) | 76.7 (57.7; 90.1) | |
| Visit 7 | 23.3 (9.9; 42.3) | 10.0 (2.1; 26.5) | 30.0 (14.7; 49.4) | 20.0 (7.7; 38.6) | 43.3 (25.5; 62.6) | 26.7 (12.3; 45.9) | |
| Visit 8 | 100 (88.4; 100) | 96.7 (82.8; 99.9) | 96.7 (82.8; 99.9) | 96.7 (82.8; 99.9) | 100 (88.4; 100) | 100 (88.4; 100) | |
| FI004 | Visit 3 | 93.3 (77.9; 99.2) | 90.0 (73.5; 97.9) | 0 (0; 11.6) | 0 (0; 11.6) | 0 (0; 11.6) | 0 (0; 11.6) |
| Visit 4 | 100 (88.4; 100) | 100 (88.4; 100) | 80.0 (61.4; 92.3) | 73.3 (54.1; 87.7) | 100 (88.4; 100) | 93.3 (77.9; 99.2) | |
| Visit 7 | 20.0 (7.7; 38.6) | 16.7 (5.6; 34.7) | 23.3 (9.9; 42.3) | 20.0 (7.7; 38.6) | 30.0 (14.7; 49.4) | 26.7 (12.3; 45.9) | |
| Visit 8 | 100 (88.4; 100) | 100 (88.4; 100) | 100 (88.4; 100) | 100 (88.4; 100) | 100 (88.4; 100) | 100 (88.4; 100) | |
| FI005 | Visit 3 | 40.0 (22.7; 59.4) | 40.0 (22.7; 59.4) | 3.3 (0.1; 17.2) | 0 (0; 11.6) | 3.3 (0.1; 17.2) | 3.3 (0.1; 17.2) |
| Visit 4 | 90.0 (73.5; 97.9) | 90.0 (73.5; 97.9) | 26.7 (12.3; 45.9) | 20.0 (7.7; 38.6) | 53.3 (34.3; 71.66) | 36.7 (19.9; 56.1) | |
| Visit 7 | 6.7 (0.8; 22.1) | 0 (0; 11.6) | 13.3 (3.8; 30.7) | 10.0 (2.1; 26.5) | 13.3 (3.8; 30.7) | 6.7 (0.8; 22.1) | |
| Visit 8 | 96.7 (82.8 99.9) | 96.7 (82.8; 99.9) | 96.7 (82.8; 99.9) | 96.7 (82.8; 99.9) | 100 (88.4; 100) | 100 (88.4; 100) | |
Irrespective of the strain tested and the priming schedule used, ≥96.7% of children had hSBA titers ≥1:4 after the MenACWY-CRM booster dose (Table 1). All children showed titers ≥1:4 against the MenC vaccine reference strain.
One month post-primary series, the percentages of children with hSBA titers ≥1:8 were in the range of 90.0% (strain FI005) and 100% (all other hypervirulent strains) in group 2_MenACWY, of 20.0% (strain FI005) and 73.3% (strain FI004) in group 1_MenACWY, and of 36.7% (strain FI005) and 93.3% (strain FI004) in group 1_MenC (Table 1). After the primary series, similar percentages of children with hSBA titers ≥1:8 against the reference MenC strain were observed, ranging from 83.3% (group 1_MenACWY) to 100% (group 2_MenACWY) (Appendix Figure A1).
At 10–33 months following the last priming dose, the percentages of children with hSBA titers ≥1:8 were lower than those observed post-vaccination, ranging from 0% to 26.7% before booster administration, and raising to ≥96.7% 1 month post-booster dose against all strains (Table 1). All children had hSBA titers ≥1:8 against the MenC vaccine reference strain after booster administration (Appendix Figure A1).
Discussion
To our knowledge, this study is among the first to analyze vaccine performance against MenC strains responsible for outbreaks of IMD and the first to assess MenC-CRM/MenACWY-induced bactericidal activity against hypervirulent MenC strains circulating in Italy.
Immune responses against five hypervirulent MenC isolated during an IMD outbreak in Tuscany were evaluated using sera from children previously immunized against MenC. The sera were collected during studies conducted as part of the clinical development program of MenACWY-CRM, which were selected for this post-hoc analysis based on several considerations. The first was the interest in exploring the responses in infants and toddlers, who are among the age groups at higher risk for IMD. The second was the potential to evaluate different MenACWY-CRM/MenC-CRM priming schedules and boosting with MenACWY-CRM, in terms of bactericidal activity against hypervirulent MenC strains.
The trigger for this post-hoc analysis was the unexpectedly higher incidence of MenC IMD cases observed in 2015–2016 in Tuscany: 0.83 per 100,000 inhabitants in 2015 and 1.98 in the first 2 months of 2016, compared to previous years (an average incidence rate for 2012–2014 of 0.08 per 100,000 inhabitants, and a range from 0.05 in 2014 to 0.11 in 2012).1,11 Among the 43 MenC IMD cases reported between January 2015 and February 2016, 5 occurred in individuals who had been vaccinated with a MenC conjugate vaccine. Apart from 1 case in an individual (62 years old) vaccinated on the same day of the symptom onset, in the remaining 4 cases (9–22 years old), the vaccine was administered 2–10 years before the symptom onset. Of the overall 10 cases with fatal outcome, 9 were among those unvaccinated. In 35 out of the 40 samples analyzed, the MenC strain was confirmed as C:P1.5–1,10-8:F3-6:ST-11 (cc11).1 A recently published paper reported that the estimated effectiveness of MenC-vaccines was still high (77%; 95% CI: 36–92) during the outbreak, and similar to that calculated for 2006–2016, over the entire period since the introduction of MenC vaccination in Tuscany (80%; 95% CI: 54–92).11
In response to this outbreak, an immunization campaign was launched in the region, initially with a single dose of meningococcal tetravalent (MenACWY) conjugate vaccine actively offered to individuals 11–19 years old, even if already vaccinated with MenC conjugate vaccines in childhood. The immunization campaign was then continuously adapted to the evolving epidemiology, for instance extended to older individuals (20–44 years) residing in the area of the local health units with at least 1 reported MenC IMD case, using MenC conjugate vaccines as an alternative option to the tetravalent vaccine (actively offered only to 11–20 years age group).1
This post-hoc analysis provides a descriptive assessment of antibody responses induced by vaccination with MenC or MenACWY-CRM conjugate vaccines against hypervirulent cc11 or cc334 strains, isolated from MenC IMD cases reported in Tuscany in 2015–2016. Sera were tested with a SBA assay, which is the “gold standard” for measuring the ability of vaccine-induced antibodies to kill N. meningitidis.12,13 SBA measures complement-mediated killing via antibody and the assay generally uses exogenously added human (hSBA) or rabbit complement (rSBA; an easier source of large volumes of complement without the need for human donors) to mediate killing. For meningococcal vaccines, a generally-accepted correlate of protection is a level of bactericidal antibodies ≥1:8, or even ≥1:4, when using hSBA14–16 and ≥1:8 when using rSBA.17 In this post-hoc analysis, the hSBA assay was used consistently with the test method used in the original study.
Bactericidal antibody titers varied according to the hypervirulent strain tested and overall resulted in higher titers against the 4 cc11 strains than against the cc334 one, an observation apparently not explained by differences in the capsule composition, as the sialic acid quantitation did not yield significantly different results among the five strains (data not shown). The difference in bactericidal activity may thus rather be related to a different susceptibility to complement-mediated bacterial killing.
When testing sera taken approximately 1 month after priming, immune responses were more robust following 2 doses of MenACWY-CRM, administered 4–6 months apart, than after 1 dose of MenC-CRM or MenACWY-CRM given at 12 months of age, as shown by the percentages of children with putative protective hSBA titers.
Seroresponse rates could not be assessed for group 2_MenACWY, as no blood draw was taken before first MenACWY-CRM dose. For the other two groups, a better performance against the hypervirulent MenC strains was shown when children were primed with MenC-CRM compared to MenACWY-CRM. Similar findings were observed against the MenC vaccine reference strain in the same subset of 90 children, an outcome which could be predicted considering that: i) for group 2_MenACWY, a second dose of the quadrivalent conjugate vaccine was administered 4–6 months apart, maximizing the possibility to mount robust primary responses following a “prime-prime” schedule, compared to those elicited by a single dose of the vaccine, and ii) for group 1_MenC, the MenC antigen content administered per dose was twice as much (10 μg) as MenACWY-CRM (5 μg) and the vaccine contained aluminum hydroxide adjuvant.2,3
When analyzing the bactericidal activity after a MenACWY-CRM booster dose, priming with MenC-CRM elicited higher titers compared to either 1- or 2-dose MenACWY-CRM priming. The better performance in terms of anamnestic response following MenC-CRM priming can be explained by the amount of antigen and the presence of the adjuvant in its formulation. Of note, 1 dose of MenACWY-CRM at 12 months tended to result in a better priming than 2 doses of the same vaccine administered at 6–8 and 12 months. However, this should be interpreted with caution, as it may be limited by the selection of the analyzed subset, which was mainly based on availability of sufficient quantities of samples for retesting, in a small group of participants from the clinical trials. Nevertheless, this finding was also observed when restricting the analysis to the MenC vaccine reference strain, an observation that might be biased by the potential effect of the different time interval between the booster dose and the last priming dose, which could vary between 13 and 33 months.
Overall, irrespective of the priming series and the different hypervirulent MenC strain tested, bactericidal titers ≥1:4 and ≥1:8 were detected in sera from almost all children following the booster dose.
This analysis has several limitations, the main being that it was planned and performed post-hoc, it is descriptive in nature and based on sera from a limited number of children (N = 90). Another limitation is the design of the original clinical trials, which were the source of the samples for retesting, with a wide chronological range allowed between primary and booster vaccination. In addition, testing against the hypervirulent strains was performed using a non-validated hSBA assay, while the results of the original analysis against the MenC vaccine reference strain were obtained with a validated hSBA assay, thus further preventing any formal comparison.
Notwithstanding these potential limitations, this study brings important information on the performance of meningococcal vaccination against hypervirulent MenC strains. As meningococcal strain circulation and the burden of disease in the field vary across different geographical areas and over time and can hardly be predicted, this analysis may provide insights on possible vaccination strategies in case of hypervirulent MenC outbreaks.
In conclusion, the conjugate vaccines MenACWY-CRM and MenC-CRM were able to elicit immune responses to hypervirulent MenC strains to different extents, depending on the priming series and the strain tested. While 2 MenACWY-CRM doses, administered 4–8 months apart, seemed to provide better priming to infants/toddlers, 1 dose of MenC-CRM at 12 months of age resulted in higher serum bactericidal activity following MenACWY-CRM booster dose administered approximately 1–2 years after. Irrespective of the strain tested and the priming vaccine and schedule adopted, putative protective levels of bacterial antibodies (hSBA titers ≥1:8) were detected in almost all sera collected after the MenACWY-CRM booster dose.
Acknowledgments
The authors thank the children from whom sera were collected and their parents, as well as investigators and nurses involved in the original clinical trials. The authors are grateful to Frans Corthals (GSK, science writing), Lucia Fontana & Daniela Proietti (GSK, pre-clinical team) for their contribution to this post-hoc analysis. The authors thank the Modis and Business & Decision Life Sciences platforms for editorial assistance, manuscript coordination and writing support, on behalf of GSK. Petronela M. Petrar (Modis, on behalf of GSK) provided medical writing assistance and Bruno Dumont (Business & Decision Life Sciences, on behalf of GSK) coordinated manuscript development and editorial support.
Appendix.
Table A1.
Demographic characteristics of patients at the time of enrollment in the parent study
| 2_MenACWY (N = 30) | 1_MenACWY (N = 30) | 1_MenC (N = 30) | |
|---|---|---|---|
| Mean age ± SD, months | 6.6 ± 0.6 | 6.5 ± 0.4 | 6.8 ± 0.8 |
| Female sex, n (%) | 13 (43) | 13 (43) | 16 (53) |
| Ethnic origin, n (%) | |||
| Caucasian | 28 (93) | 28 (93) | 30 (100) |
| Asian | 1 (3) | 1 (3) | 0 (0) |
| Other | 1 (3) | 1 (3) | |
| Mean weight ± SD, kg | 7.8 ± 1.2 | 7.9 ± 0.8 | 8 ± 0.9 |
| Mean height ± SD, cm | 68.1 ± 3.0 | 68.6 ± 2.9 | 68.2 ± 3.0 |
N, number of children; SD, standard deviation; n (%), number (percentage) of children in the specified category. Group 2_MenACWY, MenACWY-CRM vaccination at 6–8 and 12 months of age; Group 1_MenACWY, MenACWY-CRM vaccination at 12 months of age; Group 1_MenC, MenC-CRM vaccination at 12 months of age.
Table A2.
hSBA GMTs, GMRs and seroresponse rates against the MenC vaccine reference strain (all time points)
| Value (95% CI) |
|||
|---|---|---|---|
| 2_MenACWY (N = 30) | 1_MenACWY (N = 30) | 1_MenC (N = 30) | |
| Visit 3 GMT | 19.7 (12.0; 32.5) | 2.1 (1.9; 2.4) | 2.6 (1.9; 3.5) |
| Visit 4 GMT | 279.9 (204.0; 384.1) | 23.9 (14.7; 39.0) | 35.6 (23.5; 53.9) |
| Visit 4/Visit 3 GMR | - | 11.3 (7.0; 18.4) | 13.8 (8.2; 23.2) |
| Visit 7 GMT | 3.5 (2.7; 4.7) | 3.9 (2.4; 6.2) | 4.3 (2.8; 6.6) |
| Visit 8 GMT | 554.1 (352.3; 871.5) | 956.0 (580.1; 1575.6) | 1935.4 (1321.3; 2834.8) |
| Visit 8/Visit 7 GMR | 156.6 (99.1; 247.6) | 247.6 (171.4; 357.6) | 449.2 (291.2; 692.8) |
| Seroresponse rate | - | 83.3% (65.3; 94.4) | 82.8% (64.2; 94.2) |
hSBA, serum bactericidal activity assay with human complement; 95% CI, 95% confidence interval; GMT, geometric mean titer, GMR, geometric mean ratio; N, number of children; Group 2_MenACWY, MenACWY-CRM vaccination at 6–8 and 12 months of age; Group 1_MenACWY, MenACWY-CRM vaccination at 12 months of age; Group 1_MenC, MenC-CRM vaccination at 12 months of age.
Note: Seroresponse was defined for children with Visit 3 hSBA titer <1:4 as an hSBA titer ≥1:8 at Visit 4, and for children with Visit 3 hSBA titer ≥1:4 as a ≥ 4-fold raise in hSBA titers at Visit 4.
For group 1_MenC, N = 29 for the seroresponse rate.
Figure A1.
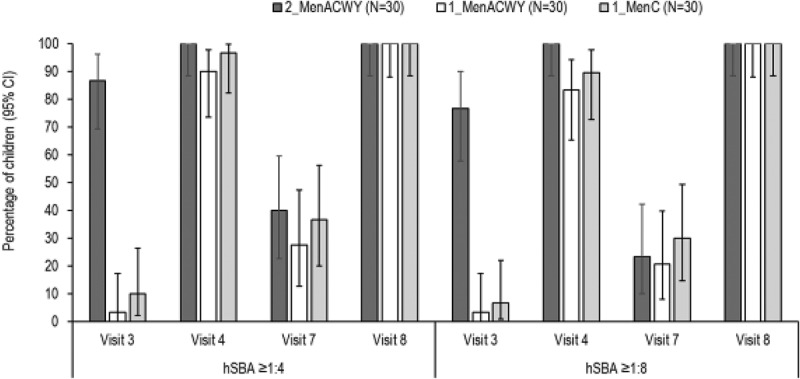
Percentages of children with hSBA titers ≥1:4 or ≥1:8 against the MenC vaccine reference strain (all time points)
95% CI, 95% confidence interval; N, number of children; Group 2_MenACWY, MenACWY-CRM vaccination at 6–8 and12 months of age; Group 1_MenACWY, MenACWY-CRM vaccination at 12 months of age; Group 1_MenC, MenC-CRM vaccination at 12 months of age.Note: For group 1_MenC, N = 29 for Visit 4.
Funding Statement
GlaxoSmithKline Biologicals SA funded this research and was involved in all stages of study conduct, including analysis of the data. GlaxoSmithKline Biologicals SA also took in charge all costs associated with the development and publication of this manuscript.
Author’s contribution
All authors participated in the design or implementation or analysis and interpretation of the study; and the development of this manuscript. All authors had full access to the data and gave final approval before submission.
Disclosure of potential conflicts of interest
MMG, AB, PK, ST, LS, EM, AB, MB, TM, MC, and MP are employees of the GSK group of companies. MMG, PK and MP also hold shares in the GSK group of companies. At the time the analysis was conducted, MB was an employee of Randstad Italia SPA, working as a contractor for GSK. MM, FN, SR, and CA declare no financial conflicts of interest. All authors declare no non-financial conflict of interest.
Data availability statement
The product that is studied in this clinical study, together with the rights to the data and results generated, have been transferred to GSK by Novartis. The results summary for these studies (NCT00667602 and its extension NCT01345721) are available on the Novartis Clinical Trials Results website and can be accessed at https://www.novctrd.com/. For interventional studies that evaluate GSK medicines, anonymized patient-level data are made available to independent researchers, subject to review by an independent panel, at www.clinicalstudydatarequest.com within six months of publication. To protect the privacy of patients and individuals involved in our studies, GSK does not publicly disclose patient level data. Patient-level data for this study will be made available on www.clinicalstudydatarequest.com upon request, subject to any pre-existing rights and obligations and/or consents required under the relevant agreements governing or related to these studies.
Trademark statement
Menveo and Menjugate are trademarks owned or licensed by the GSK group of companies.
References
- 1.Stefanelli P, Miglietta A, Pezzotti P, Fazio C, Neri A, Vacca P, Voller F, D’Ancona FP, Guerra R, Iannazzo S, et al. Increased incidence of invasive meningococcal disease of serogroup C/clonal complex 11, Tuscany, Italy, 2015 to 2016. Euro Surveill. 2016;21(12). doi: 10.2807/1560-7917.ES.2016.21.12.30176. [DOI] [PubMed] [Google Scholar]
- 2.Summary of product characteristics . Menjugate. [accessed 2020 February10]. http://gskpro.com/content/dam/global/hcpportal/en_MT/PDF/Homepage/Products/productlisting/menjugate/Menjugate%20spc.pdf.
- 3.European Medicines Agency . Summary of product characteristics. Menveo. [accessed 2020 February10]. http://www.ema.europa.eu/en/documents/product-information/menveo-epar-product-information_en.pdf.
- 4.Jolley KA, Bray JE, Maiden MCJ.. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018;3:124. doi: 10.12688/wellcomeopenres.14826.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jolley KA, Maiden MC.. BIGSdb: scalable analysis of bacterial genome variation at the population level. BMC Bioinform. 2010;11:595. doi: 10.1186/1471-2105-11-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bratcher HB, Corton C, Jolley KA, Parkhill J, Maiden MC. A gene-by-gene population genomics platform: de novo assembly, annotation and genealogical analysis of 108 representative Neisseria meningitidis genomes. BMC Genomics. 2014;15(1):1138. doi: 10.1186/1471-2164-15-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huson DH, Bryant D. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol. 2006;23(2):254–67. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- 8.Borrow R, Aaberge IS, Santos GF, Eudey TL, Oster P, Glennie A, Findlow J, Hoiby EA, Rosenqvist E, Balmer P, et al. Interlaboratory standardization of the measurement of serum bactericidal activity by using human complement against meningococcal serogroup B, strain 44/76-SL, before and after vaccination with the Norwegian MenBvac outer membrane vesicle vaccine. Clin Diagn Lab Immunol. 2005;12(8):970–76. doi: 10.1128/CDLI.12.8.970-976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snape MD, Perrett KP, Ford KJ, John TM, Pace D, Yu LM, Langley JM, McNeil S, Dull PM, Ceddia F, et al. Immunogenicity of a tetravalent meningococcal glycoconjugate vaccine in infants: a randomized controlled trial. JAMA. 2008;299(2):173–84. doi: 10.1001/jama.2007.29-c. [DOI] [PubMed] [Google Scholar]
- 10.Vu DM, Welsch JA, Zuno-Mitchell P, Dela Cruz JV, Granoff DM. Antibody persistence 3 years after immunization of adolescents with quadrivalent meningococcal conjugate vaccine. J Infect Dis. 2006;193(6):821–28. doi: 10.1086/500512. [DOI] [PubMed] [Google Scholar]
- 11.Pezzotti P, Miglietta A, Neri A, Fazio C, Vacca P, Voller F, Rezza G, Stefanelli P. Meningococcal C conjugate vaccine effectiveness before and during an outbreak of invasive meningococcal disease due to Neisseria meningitidis serogroup C/cc11, Tuscany, Italy. Vaccine. 2018;36(29):4222–27. doi: 10.1016/j.vaccine.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129(6):1307–26. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McIntosh ED, Broker M, Wassil J, Welsch JA, Borrow R. Serum bactericidal antibody assays - The role of complement in infection and immunity. Vaccine. 2015;33(36):4414–21. doi: 10.1016/j.vaccine.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 14.Borrow R, Balmer P, Miller E. Meningococcal surrogates of protection-Serum bactericidal antibody activity. Vaccine. 2005;23(17–18):2222–27. doi: 10.1016/j.vaccine.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 15.Frasch CE, Borrow R, Donnelly J. Bactericidal antibody is the immunologic surrogate of protection against meningococcal disease. Vaccine. 2009;27(Suppl 2):B112–116. doi: 10.1016/j.vaccine.2009.04.065. [DOI] [PubMed] [Google Scholar]
- 16.Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17(7):1055–65. doi: 10.1128/cvi.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Findlow H, Plikaytis BD, Aase A, Bash MC, Chadha H, Elie C, Laher G, Martinez J, Herstad T, Newton E, et al. Investigation of different group A immunoassays following one dose of meningococcal group A conjugate vaccine or A/C polysaccharide vaccine in adults. Clin Vaccine Immunol. 2009;16(7):969–77. doi: 10.1128/cvi.00068-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The product that is studied in this clinical study, together with the rights to the data and results generated, have been transferred to GSK by Novartis. The results summary for these studies (NCT00667602 and its extension NCT01345721) are available on the Novartis Clinical Trials Results website and can be accessed at https://www.novctrd.com/. For interventional studies that evaluate GSK medicines, anonymized patient-level data are made available to independent researchers, subject to review by an independent panel, at www.clinicalstudydatarequest.com within six months of publication. To protect the privacy of patients and individuals involved in our studies, GSK does not publicly disclose patient level data. Patient-level data for this study will be made available on www.clinicalstudydatarequest.com upon request, subject to any pre-existing rights and obligations and/or consents required under the relevant agreements governing or related to these studies.


