Abstract
The natural infection of sand flies by Leishmania was investigated in Andean areas located between the Central and Eastern Cordilleras of northern Peru where cutaneous leishmaniasis caused by Leishmania (Viannia) peruviana is endemic. Sand flies were captured at five locations along the Utcubamba River in the Department of Amazonas, and morphologically identified under a microscope. Among 422 female sand flies dissected, the most dominant species was Pintomyia verrucarum (320 flies), followed by Pi. maranonensis (83 flies), Pi. robusta (13 flies), and Lutzomyia castanea (6 flies). Genetic analysis of sand flies from these areas together with those from other areas revealed that individuals of Pi. verrucarum were closely related regardless of morphological variation of their spermathecae. On the other hand, individuals of Pi. maranonensis collected in the study area were distant from those of other areas with genetic distances over the intraspecific level but mostly below the interspecific level, suggesting the unique characteristics of sand flies in this area. The natural infection of sand flies by flagellate parasites was detected mainly in the hindgut of each one of Pi. verrucarum and Pi. maranonensis. Both parasite species were identified as L. (V.) peruviana based on cytochrome b and mannose phosphate isomerase gene analyses. In addition, parasite species obtained from the lesion of a patient with cutaneous leishmaniasis in the study area in this period was identified as L. (V.) peruviana. These results strongly suggest that Pi. verrucarum and Pi. maranonensis are responsible for the transmission of L. (V.) peruviana in these areas. This is the first report of the natural infection of Pi. maranonensis by L. (V.) peruviana.
Author summary
Phlebotomine sand flies are tiny insects of the family Psychodidae in the order Diptera, and female sand flies suck blood for egg production. Approximately 1,020 sand fly species have been recorded in the world, of which about 550 species are in the New World. Only a part of them are associated with medically important infectious diseases such as leishmaniasis, and importantly, each vector species transmits specific species of Leishmania. Since the infecting Leishmania species is the major determinant of the clinical outcome and its endemicity is largely dependent on the prevalence of the vector species, the identification of circulating sand flies and vector species, which determine transmissible parasite species, is important to predict the risk and expansion of the disease in endemic and surrounding areas. However, the vector species involved in disease transmission remains unidentified in most endemic areas because the infection rate in sand fly populations is very low. In the present study, sand flies were investigated in the Department of Amazonas in the Eastern Andes of northern Peru, in which cutaneous leishmaniasis caused by Leishmania (Viannia) peruviana is endemic, to clarify the transmission mechanism of leishmaniasis in these areas. In addition, genetic analyses of circulating sand flies were performed to elucidate the characteristics of sand flies in these areas.
Introduction
Phlebotomine sand flies are blood-sucking insects belonging to the family Psychodidae in the order Diptera [1,2]. To date, 1,020 sand fly species have been recorded in the world, of which about 550 species are in the New World [3]. The identification of sand fly species is medically important since approximately 10 percent of them are responsible for the transmission of human pathogens such as Leishmania protozoa [2,4,5]. In addition, each vector species transmits specific Leishmania species, and the infecting species is the major determinant of the clinical outcomes, such as cutaneous, mucocutaneous, and visceral disorders [4–6]. Therefore, studies on sand fly fauna and the identification of vector species of leishmaniasis in endemic and surrounding areas are important for predictions of the risk of transmission and expansion of the disease.
Peru is one of the most highly endemic countries for cutaneous leishmaniasis (CL), distributed through the country from highlands to lowlands, whereas mucocutaneous leishmaniasis (MCL) in this country is endemic mostly in Amazonian areas [7,8]. Six Leishmania species and several hybrids have been recorded as responsible for leishmaniasis in this country [7–9]. Of these, predominant causative agents are L. (V.) peruviana, L. (V.) braziliensis, and L. (V.) guyanensis, mainly circulating in the Andean highlands, tropical rainforest, and northern to central rainforest areas, respectively [7,8,10,11]. Approximately 190 sand fly species have been recorded in Peru, and information on prevalent sand fly species is accumulating, especially in Andean areas; however, the vector species responsible for the transmission of Leishmania remains to be identified in most endemic areas [12]. The vector species of L. (V.) peruviana was identified as Lutzomyia ayacuchensis in the western valley of central Andes [13] and Lu. peruensis in northern and central Andes [14–17]. Pintomyia verrucarum, a widely distributing species in Andean highlands, was reported to have the capacity to transmit L. (V.) peruviana under experimental conditions [18], and the natural infection of Pi. verrucarum by Leishmania species was detected by PCR using pooled sand fly samples from the west Andean slope in central Peru, although the parasites were not identified at the species level [19]. In addition, Lu. tejadai was reported as a vector of a hybrid of L. (V.) braziliensis and L. (V.) peruviana in the central Andes of Peru [20].
In the present study, to further disclose circulating sand fly species and identify the vectors of Leishmania protozoa, sand flies were investigated in endemic areas along the Utcubamba River in Provinces of Chachapoyas, Luya, and Bongara, Department of Amazonas located in the Eastern Andes of northern Peru where CL caused by L. (V.) peruviana is endemic [7,8].
Materials and methods
Ethics statement
Verbal informed consent was obtained prior to the sample collection, providing information on the process of diagnosis and Leishmania species analysis, following the guidelines of the Ethics Committee of the Ministry of Health, Peru. The study was approved by the ethics committee of Jichi Medical University (approval number: 17–080) [8,9].
Sand fly collection
Sand flies were captured with a mouth aspirator on protected human bait between 18:30 and 21:00 and CDC light traps operated throughout the night from 18:00–06:00 for 11 nights in July 2019 around patients’ houses in the rural area at five localities along the Utcubamba River in the Provinces of Chachapoyas, Luya, and Bongara, Department of Amazonas (Fig 1). Female sand flies were dissected and identified at the species level mainly based on the morphology of their spermathecae [6,21]. They were also examined under light microscopy for natural flagellate infections, and samples were fixed individually in absolute ethanol. Ethanol-fixed specimens were dried up and individually lysed in 50 μL of DNA extraction buffer [150 mM NaCl, 10 mM Tris-HCl (pH 8.0), 10 mM EDTA and 0.1% sodium dodecyl sulfate (SDS)] containing proteinase K (100 μg/mL). The samples were incubated at 37°C overnight, heated at 95°C for 5 min, and then 0.5 μL of each sample was directly used as a template for PCR amplification [16,17,20,22,23].
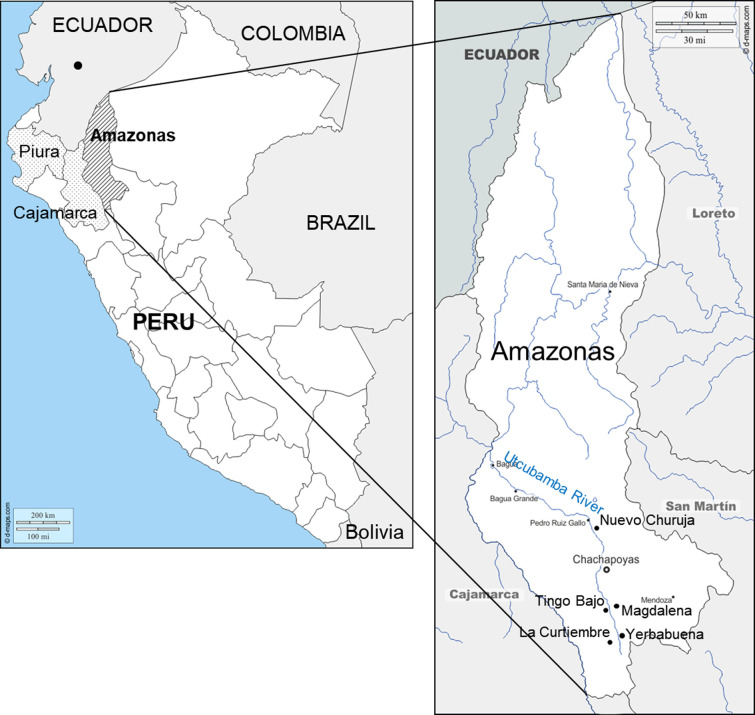
Fig 1. Maps of study areas.
Left panel: Department of Amazonas, Peru, is shown by the hatched lines, and Departments of Piura and Cajamarca, Peru, are shown by the small dots. A black spot in Ecuador shows the collection site of Pi. maranonensis in the Province of Chimborazo, Ecuador. Right panel: Sample collection sites in the Department of Amazonas: La Curtiembre, Yerbabuena, Magdalena, Tingo Bajo, and Nuevo Churuja. (Adapted from a map available at https://www.d-maps.com/carte.php?num_car=4764&lang=en and https://www.d-maps.com/carte.php?num_car=190488&lang=en).
Clinical samples
A clinical sample was collected from a patient suspected of having CL. A tissue sample was taken by scraping the margins of an active cutaneous lesion of the patient, spotting the scrapings onto an FTA Classic Card (Whatman, Newton Center, MA) and storing it at room temperature. Two-mm-diameter disks containing the sample spot were punched out from the card and washed three times with FTA Purification Reagent (Whatman) and once with Tris-EDTA buffer. The disks were air-dried and directly subjected to PCR amplification [7,8,20].
Identification of Leishmania species
Leishmania species were identified by cytochrome b (cyt b) gene sequence analysis [8,9,16]. PCR amplification with a pair of specific primers: L.cyt-S (5’-GGTGTAGGTTTTAGTYTAGG-3’) and L.cyt-R (5’-CTACAATAAACAAATCATAATATRCAATT-3’), was carried out with 30 cycles of denaturation (95°C, 1 min), annealing (55°C, 1 min), and polymerization (72°C, 1 min) using Ampdirect Plus reagent (Shimadzu Biotech, Tsukuba, Japan). PCR products were directly cloned into the plasmid using a pGEM-T Easy Vector System (Promega, Madison, WI), and the sequence of the insert was determined by the dideoxy chain termination method using a BigDye Terminator v.3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA).
Differentiation between L. (V.) braziliensis and L. (V.) peruviana
Differentiation between L. (V.) braziliensis and L. (V.) peruviana was performed by PCR-RFLP analysis of the mannose phosphate isomerase (mpi) gene, as described previously [8,9,16]. Briefly, the mpi gene fragment was amplified with a pair of primers: MPI-S (5’-GCTCTTCCTGTCGGACAGCGAGC-3’) and MPI-R (5’-TCACTCTCGAAGGGAGTTCG-3’), and digested with the restriction enzyme, VpaK11BI (Takara Bio, Shiga, Japan). The RFLP pattern was analyzed by 3% agarose gel electrophoresis.
Sequence analysis of sand fly cytochrome oxidase I (COI) gene
The sand fly COI gene fragment was amplified with universal COI primers: LCO1490 (5’-GGTCAACAAATCATAAAGATATTGG-3’) and HCO2198 (5’-TAAACTTCAGGGTGACCAAAAAATCA-3’), using a high-fidelity DNA polymerase, KOD-Plus-ver.2 (TOYOBO, Tokyo, Japan) [24–26]. The PCR products were purified using a FastGeneGel/PCR Extraction kit (NIPPON Genetics, Tokyo, Japan) to remove excessive primers, and the sequences were directly determined with a forward primer, as described above.
Phylogenetic analysis
The obtained sequences were aligned with CLUSTAL W software [27] and examined using the program MEGA (Molecular Evolutionary Genetics Analysis) version 6 [28]. Phylogenetic trees were constructed by the maximum likelihood (ML) method with the best ML model selected based on the lowest BIC score (Bayesian Information Criterion) in MEGA 6 [28]. Branch support for the ML tree was calculated using the bootstrapping method with 1,000 replicates [28]. Phylogenetic analysis of the Leishmania cyt b gene included sequences from L. (L.) infantum (GenBank accession number: AB095958), L. (L.) donovani (AB095957), L. (L.) major (AB095961), L. (L.) tropica (AB095960), L. (L.) amazonensis (AB095964), L. (L.) mexicana (AB095963), L. (V.) panamensis (AB095968), L. (V.) guyanensis (AB095969), L. (V.) braziliensis (AB095966), L. (V.) peruviana (AB433282), L. (V.) lainsoni (AB433280), L. (V.) naiffi (AB433279), and L. (V.) shawi (AB433281). Phylogenetic analysis of the sand fly COI gene included sequences of Pi. verrucarum from the Department of Amazonas [Ama10 (FJ437242), Ama11 (FJ437243), and Ama14 (FJ437244)], Piura [Piu01 (AB984460) and Piu17 (FJ437264)], Cajamarca [Caj09 (FJ437247), Yuram02 (FJ437269), and Yumpe15 (FJ437267)], and Lima [Lim01 (AB984463) and Lim07 (FJ437256)] in Peru, and sequences of Pi. maranonensis from the Department of Cajamarca in Peru [Caja01 (LC593648), Caja02 (LC593649), Caja03 (LC593650), Caja04 (LC593651), Caja05 (LC593652), Caja06: (LC593653), Caja07 (LC593654), Caja08 (LC593655), Caja09 (LC593656), Caja10 (LC593657), Caja1H (LC593671), Caja5C (LC593672), Cola-a (LC593658), Cola-b (LC593659), Cola02 (LC593660), Cola03 (LC593661)], and Province of Chimborazo in Ecuador [Huig8C (LC593670)].
Results
Detection and identification of flagellates in sand flies
In the present study, 422 female sand flies were dissected for identification at the species level, and four species were recognized. Among them, the most dominant species, Pi. verrucarum (320 flies), was captured in all localities, and the remaining three species, Pi. maranonensis (83 flies), Pi. robusta (13 flies), and Lu. castanea (6 flies), were captured only in Nuevo Churuja (Table 1). Interestingly, Pi. verrucarum showed variations in the morphology of spermathecae, regardless of the collection sites; Some were shrinking, some were round, and the others were elongated (S1 Fig).
Table 1. Identification of sand fly species and detection of flagellates within sand flies by microscopic examination.
| Locality | District | Sand fly species | No. captured | No. infected |
|---|---|---|---|---|
| La Curtiembre | Santo Tomas | Pi. verrucarum | 59 | 0 |
| Yerbabuena | La Jalca | Pi. verrucarum | 39 | 0 |
| Magdalena | Magdalena | Pi. verrucarum | 29 | 0 |
| Tingo Bajo | Tingo | Pi. verrucarum | 176 | 1 |
| Nuevo Churuja | Churuja | Pi. verrucarum | 17 | 0 |
| Pi. maranonensis | 83 | 1 | ||
| Pi. robusta | 13 | 0 | ||
| Lu. castanea | 6 | 0 |
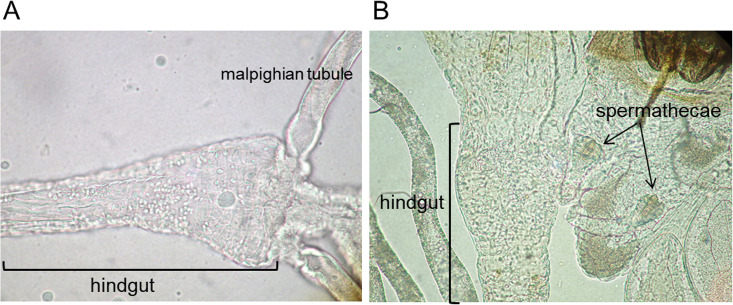
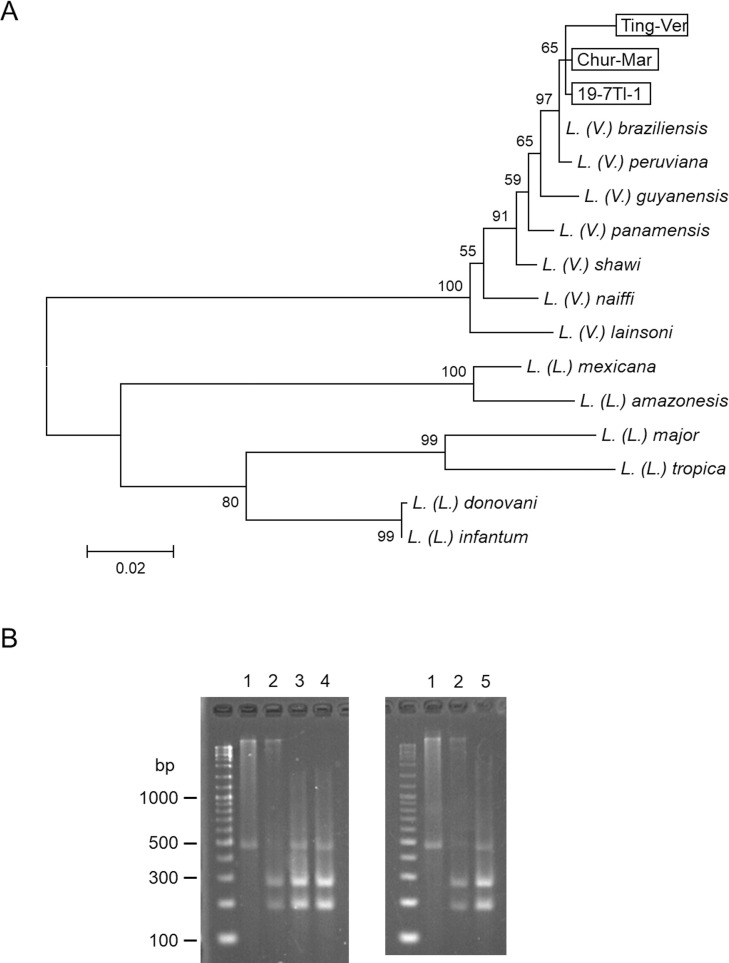
Natural infection of sand flies by flagellates was detected mainly in the hindgut of each one of Pi. verrucarum from Tingo Bajo and Pi. maranonensis from Nuevo Churuja under the microscope (Fig 2). The infection rates of Pi. maranonensis and Pi. verrucarum with Leishmania in the present study were 0.3% and 1.2%, respectively. The cyt b gene fragments were amplified from the parasites and subjected to sequence analyses. The cyt b gene sequences from Pi. verrucarum (Ting-Ver) (GenBank accession number: LC593674) and Pi. maranonensis (Chur-Mar) (LC593675) had a greater degree of homology with those of L. (V.) peruviana and L. (V.) braziliensis (98.9–99.7%) than other Leishmania species. A phylogenetic analysis showed that sequences from Pi. verrucarum (Ting-Ver) and Pi. maranonensis (Chur-Mar) could be divided into the clade of L. (V.) peruviana and L. (V.) braziliensis (Fig 3A). Parasite species from Pi. verrucarum (Ting-Ver) and Pi. maranonensis (Chur-Mar) were further differentiated by PCR-RFLP analysis of the mpi gene targeting a single-nucleotide polymorphism [7,8,16,17]. The RFLP pattern of both samples corresponded to that of L. (V.) peruviana but not L. (V.) braziliensis (Fig 3B), indicating that flagellates detected within Pi. verrucarum and Pi. maranonensis were both L. (V.) peruviana. This is the first report of the natural infection of Pi. maranonensis by L. (V.) peruviana.
Fig 2. Natural infection of sand flies by flagellates.
Natural infection of sand flies by flagellates was detected mainly in the hindgut of Pi. verrucarum from Tingo Bajo (A) and Pi. maranonensis from Nuevo Churuja (B).
Fig 3. Identification of Leishmania species by cytochrome b and mannose phosphate isomerase gene analyses.
A. A phylogenetic analysis of cytochrome b gene was performed by the maximum likelihood method together with sequences from 13 Leishmania species. The scale bar represents 0.02% divergence. Bootstrap values are shown above or below branches. Ting-Ver: Leishmania-positive Pi. verrucarum from Tingo Bajo, Chur-Mar: Leishmania-positive Pi. maranonensis from Nuevo Churuja, 19-7TI-1: a patient’s specimen from Tingo Bajo. B. PCR-RFLP analysis of mannose phosphate isomerase genes from L. (V.) braziliensis (lane 1), L. (V.) peruviana (lane 2), Leishmania-positive Pi. verrucarum from Tingo Bajo (lane 3), a patient’s specimen from Tingo Bajo (lane 4), and Leishmania-positive Pi. maranonensis from Nuevo Churuja (lane 5).
Identification of Leishmania species from a patient’s specimen
During this field research, a clinical sample was obtained from the cutaneous lesion of a patient with CL in Tingo Bajo where a flagellate-infected sand fly was detected. The cyt b gene fragment was analyzed, and the sequence (19-7TI-1) (GenBank accession number: LC593673) had a greater degree of homology with those of L. (V.) peruviana and L. (V.) braziliensis (99.5–99.7%) than others. The result was supported by a phylogenetic analysis (Fig 3A). PCR-RFLP analysis of the mpi gene showed that the parasite species was L. (V.) peruviana (Fig 3B).
COI gene analyses of Pintomyia verrucarum and Pintomyia maranonensis
Since Pi. verrucarum showed morphological variations of spermathecae regardless of their habitat, genetic analysis based on the COI gene was performed on Pi. verrucarum and Pi. maranonensis, in which Leishmania infection was detected in this study, to identify their genetic diversities in comparison with those from other areas.
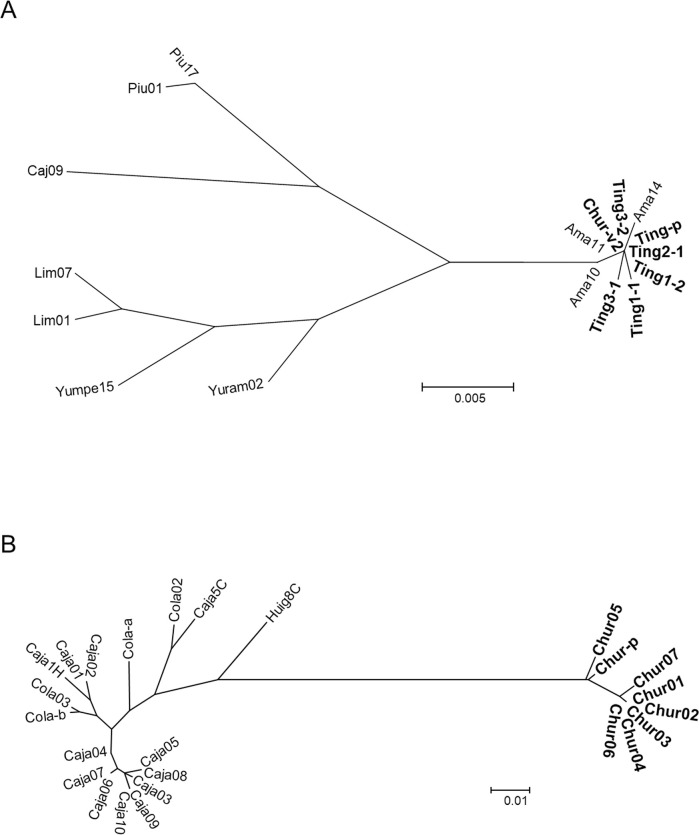
Sequences of 630-bp COI gene fragments were determined in 7 Pi. verrucarum including the Leishmania-positive sample (Ting-p) collected in this study (GenBank accession numbers: LC593641- LC593647), and a phylogenetic analysis was performed together with those from other areas registered in GenBank (Fig 1). Pintomyia verrucarum captured in Tingo Bajo (Ting1-1, Ting1-2, Ting2-1, Ting3-1, Ting3-2, and Ting-p) and Nuevo Churuja (Chur-v2) of the Department of Amazonas were closely related to each other with genetic distances of 0.0–0.2% independent of the morphological variation of spermathecae (Fig 4A). COI gene sequences of Pi. verrucarum from the Department of Amazonas registered in GenBank (Ama10, Ama11, and Ama14) were located in the same clade as our samples. On the other hand, COI gene sequences of Pi. verrucarum from Departments of Piura (Piu01 and Piu17), Cajamarca (Caj09, Yuram02, and Yumpe15), and Lima (Lim01 and Lim07) composed different clades from those of Amazonas samples (Fig 4A). Genetic distances based on Kimura 2-parameter values were 2.4–2.8% between Amazonas and Piura samples, 2.1–3.1% between Amazonas and Cajamarca samples, and 2.3–2.8% between Amazonas and Lima samples, all of which were intraspecific diversity levels (<6.0%) [26].
Fig 4. Phylogenetic analyses of cytochrome oxidase I (COI) gene sequences among Pi. verrucarum and among Pi. maranonensis.
A. A phylogenetic analysis of the COI gene of Pi. verrucarum collected in this study (Ting1-1, Ting1-2, Ting2-1, Ting3-1, Ting3-2, Ting-p, and Chur-v2) was performed together with those from Departments of Amazonas (Ama10, Ama11, and Ama14), Piura (Piu01 and Piu17), Cajamarca (Caj09, Yuram02, and Yumpe15), and Lima (Lim01 and Lim07) in Peru. The scale bar represents 0.005% divergence. B. A phylogenetic analysis of the COI gene of Pi. maranonensis collected in this study (Chur01-Chur07 and Chur-p) was performed together with those from the Department of Cajamarca in Peru (Caja01-Caja10, Caja1H, Caja5C, Cola-a, Cola-b, Cola02, and Cola03) and the Province of Chimborazo in Ecuador (Huig8C). The scale bar represents 0.01% divergence.
COI gene sequences from 8 Pi. maranonensis including Leishmania-infected sample (Chur-p) collected in this study were also determined (GenBank accession numbers: LC593662- LC593669) and analyzed together with those from other areas in Peru and Ecuador (Fig 1). Pintomyia maranonensis captured in Nuevo Churuja (Chur01-Chur07 and Chur-p) were closely related to each other with genetic distances of 0.0–1.6% (Fig 4B). However, unexpectedly, they were distant from those obtained from other areas such as the Department of Cajamarca in Peru (Caja01-Caja10, Caja1H, Caja5C, Cola-a, Cola-b, Cola02, and Cola03), and the Province of Chimborazo in Ecuador (Huig8C), all of which are relatively closely related in spite of the geographic distance (Fig 4B). Genetic distances were 0.0–3.2% among Cajamarca samples and 3.9–4.1% between Cajamarca and Ecuador samples. On the other hand, genetic distances between Nuevo Churuja samples analyzed in this study and Cajamarca samples were 7.1–8.5%, which was over the intraspecific level (<6.0%) but mostly below the interspecific level (>8.4%) [26].
Discussion
In the present study, the natural infection of sand flies by Leishmania was investigated in the Eastern Andes of northern Peru where cutaneous leishmaniasis caused by L. (V.) peruviana is endemic. Of 422 female sand flies examined, infection by flagellates was detected in Pi. verrucarum and Pi. maranonensis. Both flagellates were identified as L. (V.) peruviana, which is the causative agent of CL in these areas, strongly suggesting that Pi. verrucarum and Pi. maranonensis are responsible for the transmission of CL. This is the first report of the natural infection of Pi. maranonensis by L. (V.) peruviana. Further genetic analysis of these sand flies revealed that Pi. verrucarum was closely related to flies from other areas regardless of the morphological variation of spermathecae, whereas Pi. maranonensis was genetically unique when compared with flies from other areas.
The study area is located between the Central and Eastern Cordilleras of the Andes in northern Peru [29]. Epidemiological studies on leishmaniasis in the Peruvian Andes, including sand fly research, have been carried out mainly in endemic areas located in the North, Center, and South of the Western Andes, and occasionally in the North and Central inter-Andean valleys of the Central Andes and in the southern zone of the Eastern Andes. Lu. ayacuchensis and Lu. peruensis, which distribute in western valleys of the Central Andes and Northern and Central Andes, respectively, were identified as vectors of L. (V.) peruviana, the primary etiological agent of CL in the Peruvian Andes [13–16]. On the other hand, the vector responsible for the transmission of CL remains to be elucidated in endemic areas of the Eastern Andes. Interestingly, the distribution of both Lu. ayacuchensis and Lu. peruensis has not been reported in these areas, suggesting that some factors that may affect distributing sand fly species, such as the ecosystem, fauna, and flora, are unique in these areas.
In the study areas, L. (V.) peruviana was identified as the causative agent of CL in previous studies [7,8], and this was confirmed in the present study. The vector species responsible for the transmission of L. (V.) peruviana has not been identified. The present study detected flagellate parasites mainly in the hindguts of Pi. verrucarum and Pi. maranonensis, and both parasites were identified as L. (V.) peruviana, strongly suggesting that these sand fly species are responsible for the transmission of CL in these areas. Pi. verrucarum is widely distributed in Andean mountainous areas of the Eastern and Central Cordillera, and well-known as the primary vector of Bartonella bacilliformis, the etiologic agent of Carrion’s disease, also known as Oroya fever [12]. Pintomyia verrucarum was reported to have the capacity to transmit L. (V.) peruviana under experimental conditions [18], and the natural infection of the sand fly by unidentified Leishmania species was detected by PCR in an endemic area [19]. These findings strongly suggest that Pi. verrucarum is responsible for the transmission of L. (V.) peruviana. The present study showed that Pi. verrucarum is the natural vector of L. (V.) peruviana in endemic areas of CL for the first time. Moreover, natural infection by L. (V.) peruviana was detected unexpectedly in another sand fly species, Pi. maranonensis, suggesting that, at least, two sand fly species are associated with the transmission of L. (V.) peruviana in the study areas.
Since morphological variation of spermathecae was noted in Pi. verrucarum during the microscopic examination, genetic analysis of these flies was performed to identify the difference depending on the morphological characteristics. Interestingly, individuals of Pi. verrucarum in the study areas were closely related to each other regardless of the morphological variation, and their genetic diversities were within the intraspecific level when compared with those from other areas, suggesting that spermathecae of Pi. verrucarum are more flexible than those of other species. This finding should be taken into account on the morphological identification of sand flies, especially when unfixed specimens are used. Another Leishmania-positive sand fly species, Pi. maranonensis, was also subjected to genetic analysis, although morphological variation was not observed. Unexpectedly, Pi. maranonensis in the study area was genetically distant from flies of other areas with genetic distances between intraspecific and interspecific levels. This is the first report on the transmission of Leishmania species by Pi. maranonensis. Since Pi. maranonensis in the study area may have unique characteristics of the intestinal environment that result in acquiring the vectorial capacity, further studies associated with vector competence, such as mid- and hindgut molecules and the microbiome, would be interesting [30–32].
In the present study, the natural infection of sand flies by Leishmania was microscopically examined in the northern Peruvian Andes on the Cordillera Central where CL caused by L. (V.) peruviana is endemic. Both flagellates detected within Pi. verrucarum and Pi. maranonensis were identified as L. (V.) peruviana, strongly suggesting that Pi. verrucarum and Pi. maranonensis are responsible for the transmission of leishmaniasis in these areas. Genetic divergence of Pi. verrucarum in these areas was confirmed to be at the intraspecific level regardless of their morphological variations, whereas Pi. maranonensis was genetically characteristic regarding the genetic distance between intra- and interspecific levels when compared with flies from other areas. Since Pi. maranonensis has never reported to transmit Leishmania species, this species in the study area is expected to have unique characteristics associated with the vectorial capacity. Further studies on the microenvironment of the mid- and hindgut, in which particular parasite species can develop, will be expected to provide further insight into the vector competence of sand flies. On the other hand, more detailed morphological and molecular analyses of Pi. maranonensis in the study area will be needed since the possibility of a new species cannot be ruled out, based on the finding that genetic distances of the COI gene with those from other areas were over the intraspecific level.
Supporting information
(TIF)
Acknowledgments
We would like to thanks Norma Cruz Vilcarromero, Director of Public Health and Regional Coordinator of Indigenous Peoples, Amazonas, and members of Centro de Salud of Tingo, Yerbabuena, Churuja and Magdalena, Amazonas, for providing the facilities for the execution of the investigation in their respective jurisdictions. We also would like to thank Urbano Perez Mego (Colasay, Department of Cajamarca) and Pablo Lara de la Cruz (Salas, Department of Lambayeque) for their support in the collection of sand flies.
Data Availability
Sequence data are availavle on the Genbank (accession numbers: LC593641-LC593675).
Funding Statement
HK was funded by the Ministry of Education, Culture and Sports, Science and Technology (MEXT) of Japan (Grant Nos. 25257501 and 17H01685)https://www.mext.go.jp/en/index.htm. AGC was funded by the Universidad Nacional Mayor de San Marcos: Research Projects for Groups of 2019 (No. 03556-R-19) https://www.unmsm.edu.pe/. The funders play no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Killick-Kendrick R. The biology and control of phlebotomine sand flies. Clin Dermatol. 1999; 17:279–289. 10.1016/s0738-081x(99)00046-2 [DOI] [PubMed] [Google Scholar]
- 2.Munstermann LE. Phlebotomine sand flies, the Psychodidae. In Biology of Disease Vectors, 2nd ed.; Marquardt WC, Black WC, Freier JE, Hagedorn HH, Hemingway J, Higgs S, James AA, Kondratieff B, Moore CG, Eds.; Elsevier: San Diego, CA, USA. 2004; pp.141–151. [Google Scholar]
- 3.Galati EAB. Morfologia e terminologia de Phlebotominae (Diptera: Psychodidae). Classificação e identificação de táxons das Américas. Vol I. Apostila da Disciplina Bioecologia e Identificação de Phlebotominae do Programa de Pós-Graduação em Saúde Pública. Faculdade de Saúde Pública da Universidade de São Paulo, São Paulo. 2019. [Google Scholar]
- 4.Bates PA. Transmission of Leishmania metacyclic promastigotes by phlebotomine sand flies. Int J Parasitol. 2007; 37:1097–1106. 10.1016/j.ijpara.2007.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kato H, Gomez EA, Cáceres AG, Uezato H, Mimori T, Hashiguchi Y. Molecular epidemiology for vector research on leishmaniasis. Int J Environ Res Public Health. 2010a; 7:814–826. 10.3390/ijerph7030814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young DG, Duncan MA. Guide to the identification and geographic distribution of Lutzomyia sand flies in Mexico, the West Indies, Central and South America (Diptera: Psychodidae), Memoirs of the American Entomological Institute, vol. 54, Associated Publishers-American Entomological Institute, Gainsville, FL. 1994. [Google Scholar]
- 7.Kato H, Cáceres AG, Mimori T, Ishimaru Y, Sayed AS, Fujita M, et al. Use of FTA cards for direct sampling of patients’ lesions in the ecological study of cutaneous leishmaniasis. J Clin Microbiol. 2010b; 48:3661–3665. 10.1128/JCM.00498-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kato H, Caceres AG, Seki C, Silupu Garcia CR, Holguin Mauricci C, Castro Martinez SC, et al. Further insight into the geographic distribution of Leishmania species in Peru by cytochrome b and mannose phosphate isomerase gene analyses. PLoS Negl Trop Dis. 2019; 13:e0007496. 10.1371/journal.pntd.0007496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabbabi A, Cáceres AG, Bustamante Chauca TP, Seki C, Choochartpong Y, Mizushima D, et al. Nuclear and kinetoplast DNA analyses reveal genetically complex Leishmania strains with hybrid and mito-nuclear discordance in Peru. PLoS Negl Trop Dis. 2020; 14:e0008797. 10.1371/journal.pntd.0008797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucas CM, Franke ED, Cachay MI, Tejada A, Cruz ME, Kreutzer RD, et al. Geographic distribution and clinical description of leishmaniasis cases in Peru. Am J Trop Med Hyg. 1998; 59:312–317. 10.4269/ajtmh.1998.59.312 [DOI] [PubMed] [Google Scholar]
- 11.Arevalo J, Ramirez L, Adaui V, Zimic M, Tulliano G, Miranda-Verástegui C, et al. Influence of Leishmania (Viannia) species on the response to antimonial treatment in patients with American tegumentary leishmaniasis. J Infect Dis. 2007; 195:1846–1851. 10.1086/518041 [DOI] [PubMed] [Google Scholar]
- 12.Zorrilla V, Vásquez G, Espada L, Ramírez P. Update on tegumentary leishmaniasis and Carrion’s disease vectors in Peru. Rev Peru Med Exp Salud Publica. 2017; 34:485–496. 10.17843/rpmesp.2017.343.2398 [DOI] [PubMed] [Google Scholar]
- 13.Caceres AG, Villaseca P, Dujardin JC, Bañuls AL, Inga R, Lopez M, et al. Epidemiology of Andean cutaneous leishmaniasis: incrimination of Lutzomyia ayacuchensis (Diptera: psychodidae) as a vector of Leishmania in geographically isolated, upland valleys of Peru. Am J Trop Med Hyg. 2004; 70:607–612. [PubMed] [Google Scholar]
- 14.Perez JE, Villaseca P, Caceres A, Lopez M, Zolessi A, Campos M, et al. Leishmania (Viannia) peruviana isolated from the sandfly Lutzomyia peruensis (Diptera: Psychodidae) and a sentinel hamster in the Huayllacallan Valley, Ancash, Peru. Trans R Soc Trop Med Hyg. 1991; 85:60. 10.1016/0035-9203(91)90158-u [DOI] [PubMed] [Google Scholar]
- 15.Perez JE, Veland N, Espinosa D, Torres K, Ogusuku E, Llanos-Cuentas A, et al. Isolation and molecular identification of Leishmania (Viannia) peruviana from naturally infected Lutzomyia peruensis (Diptera: Psychodidae) in the Peruvian Andes. Mem Inst Oswaldo Cruz. 2007; 102:655–658. 10.1590/s0074-02762007005000077 [DOI] [PubMed] [Google Scholar]
- 16.Kato H, Cáceres AG, Gomez EA, Mimori T, Uezato H, Marco JD, et al. Molecular mass screening to incriminate sand fly vectors of Andean-type cutaneous leishmaniasis in Ecuador and Peru. Am J Trop Med Hyg. 2008; 79:719–721. [PubMed] [Google Scholar]
- 17.Kato H, Gomez EA, Cáceres AG, Vargas F, Mimori T, Yamamoto K, et al. Natural infections of man-biting sand flies by Leishmania and Trypanosoma species in the northern Peruvian Andes. Vector Borne Zoonotic Dis. 2011; 11:515–521. 10.1089/vbz.2010.0138 [DOI] [PubMed] [Google Scholar]
- 18.Davies CR, Fernandez M, Paz L, Roncal N, Llanos-Cuentas A. Lutzomyia verrucarum can transmit Leishmania peruviana, the aetiological agent of Andean cutaneous leishmaniasis. Trans R Soc Trop Med Hyg. 1993; 87:603–606. 10.1016/0035-9203(93)90103-w [DOI] [PubMed] [Google Scholar]
- 19.Perez JE, Ogusuku E, Inga R, Lopez M, Monje J, Paz L, et al. Natural Leishmania infection of Lutzomyia spp. in Peru. Trans R Soc Trop Med Hyg. 1994; 88:161–164. 10.1016/0035-9203(94)90276-3 [DOI] [PubMed] [Google Scholar]
- 20.Kato H, Cáceres AG, Hashiguchi Y. First evidence of a hybrid of Leishmania (Viannia) braziliensis/L. (V.) peruviana DNA detected from the phlebotomine sand fly Lutzomyia tejadai in Peru. PLoS Negl Trop Dis. 2016; 10:e0004336. 10.1371/journal.pntd.0004336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galati EAB, Cáceres AG, Le Pont F. Descricos de duas especies novas de Phlebotominae (Diptera, Psychodidae) e consideracoes sobre o subgénero Pifanomyia Ortiz & Scorza. Rev Bras Entomol. 1995; 39:431–446. [Google Scholar]
- 22.Kato H, Uezato H, Gomez EA, Terayama Y, Calvopiña M, Iwata H, et al. Establishment of a mass screening method of sand fly vectors for Leishmania infection by molecular biological methods. Am J Trop Med Hyg. 2007; 77:324–329. [PubMed] [Google Scholar]
- 23.Kato H, Calvopiña M, Criollo H, Hashiguchi Y. First human cases of Leishmania (Viannia) naiffi infection in Ecuador and identification of its suspected vector species. Acta Trop. 2013; 128:710–713. 10.1016/j.actatropica.2013.09.001 [DOI] [PubMed] [Google Scholar]
- 24.Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. 1994; 3:294–299. [PubMed] [Google Scholar]
- 25.Kato H, Cáceres AG, Gomez EA, Mimori T, Uezato H, Hashiguchi Y. Genetic divergence in populations of Lutzomyia ayacuchensis, a vector of Andean-type cutaneous leishmaniasis, in Ecuador and Peru. Acta Trop. 2015; 141:79–87. 10.1016/j.actatropica.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 26.Nzelu CO, Cáceres AG, Arrunátegui-Jiménez MJ, Lañas-Rosas MF, Yañez-Trujillano HH, Luna-Caipo DV, et al. DNA barcoding for identification of sand fly species (Diptera: Psychodidae) from leishmaniasis-endemic areas of Peru. Acta Trop. 2015; 145:45–51. 10.1016/j.actatropica.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 27.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994; 22:4673–4680. 10.1093/nar/22.22.4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013; 30:2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Velásquez MT. Andes Mountains, mountain system, South America. 2020; https://www.britannica.com/place/Andes-Mountains. [Google Scholar]
- 30.Kamhawi S, Ramalho-Ortigao M, Pham VM, Kumar S, Lawyer PG, Turco SJ, et al. A role for insect galectins in parasite survival. Cell. 2004; 119:329–341. 10.1016/j.cell.2004.10.009 [DOI] [PubMed] [Google Scholar]
- 31.Dostálová A, Volf P. Leishmania development in sand flies: parasite-vector interactions overview. Parasit Vectors. 2012; 5:276. 10.1186/1756-3305-5-276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Telleria EL, Martins-da-Silva A, Tempone AJ, Traub-Csekö YM. Leishmania, microbiota and sand fly immunity. Parasitology. 2018; 145:1336–1353. 10.1017/S0031182018001014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
Data Availability Statement
Sequence data are availavle on the Genbank (accession numbers: LC593641-LC593675).