Abstract
The pleiotropic cytokine interleukin-6 (IL-6) has been implicated in the pathogenesis of COVID-19, but uncertainty remains about the potential benefits and harms of targeting IL-6 signalling in patients with the disease. The efficacy and safety of tocilizumab and sarilumab, which block the binding of IL-6 to its receptor, have been tested in adults with COVID-19-related acute respiratory illness in randomised trials, with important differences in trial design, characteristics of included patients, use of co-interventions, and outcome measurement scales. In this Series paper, we review the clinical and methodological heterogeneity of studies of IL-6 receptor antagonists, and consider how this heterogeneity might have influenced reported treatment effects. Timing from clinical presentation to treatment, severity of illness, and concomitant use of corticosteroids are among the factors that might have contributed to apparently inconsistent results. With an understanding of the sources of variability in these trials, available evidence could be applied to guide clinical decision making and to inform the enrichment of future studies.
This is the third in a Series of four papers about COVID-19
Introduction
Interleukin-6 (IL-6) is a cytokine that has diverse and pivotal roles in the inflammatory and immune responses to infection, and as an important downstream regulator of the coagulation cascade.1 Dysregulation of IL-6 signalling pathways has been linked to inflammatory-mediated conditions such as rheumatoid arthritis, juvenile idiopathic arthritis, and the cytokine release syndrome that can sometimes follow chimeric antigen receptor T-cell therapy.2, 3, 4 The characterisation of IL-6 as a pleiotropic cytokine implicated in different diseases has led to the search for therapeutic interventions that target the blockade of IL-6 and its downstream signalling pathways.5 Tocilizumab and sarilumab are monoclonal antibodies that target the IL-6 receptor (IL-6R) and are approved for the treatment of the aforementioned IL-6-mediated conditions.5
The COVID-19 pandemic continues to exert unprecedented pressure on health-care systems worldwide, with a large number of patients requiring critical care and organ support, including invasive mechanical ventilation.6, 7, 8 The pathogenesis of COVID-19-associated acute hypoxaemic respiratory failure shares many features with that of acute respiratory distress syndrome (ARDS) due to other causes,9 but perhaps with more profound coagulation abnormalities and an accentuated inflammatory response.10, 11, 12, 13 The early observation that IL-6 concentrations were higher in patients who subsequently developed severe forms of COVID-19 respiratory failure led to the hypothesis that IL-6R blockade could be clinically beneficial in this population.14, 15, 16, 17 Several observational cohort studies and randomised controlled trials (RCTs) have reported the use of tocilizumab and sarilumab in patients with COVID-19-related respiratory failure.18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 Important differences in the design of these studies—and therefore, perhaps unsurprisingly, in the results reported30, 31—need to be considered in drawing conclusions about their implications for clinical practice.
In view of the fact that clinical and methodological heterogeneity between individual studies might limit the extent to which pooled effect estimates can yield relevant conclusions,32 we aimed to summarise the rationale and evidence for the use of IL-6R antagonists in patients with COVID-19-related acute respiratory illness. In this Series paper, we review the studies reported so far, discuss the clinical and methodological sources of heterogeneity in treatment effects, and consider how available and emerging evidence could be applied in clinical practice and in the enrichment of ongoing and future clinical trials.33
Key messages.
-
•
Tocilizumab and sarilumab block the binding of interleukin-6 to its receptor (IL-6R), reducing downstream effects on inflammation and the innate immune response
-
•
Several randomised controlled trials have examined the effects of tocilizumab or sarilumab in patients with COVID-19, with inconsistent results
-
•
Patients with severe COVID-19, especially those requiring high-flow nasal oxygen, non-invasive ventilation, or invasive mechanical ventilation, are likely to benefit from treatment with tocilizumab
-
•
Time from clinical deterioration, rather than time from symptom onset, might be a better marker to define optimum treatment timing
-
•
Patients with non-severe COVID-19, those with irreversible organ dysfunction, and patients with concurrent bacterial or fungal infections are less likely to benefit from IL-6R blockers, and their use might be associated with harm
-
•
An ongoing need exists to better identify patients with COVID-19 who are likely to benefit from tocilizumab (and other anti-inflammatory treatments); future studies will need to establish the optimum time for treatment initiation and whether beneficial effects of IL-6R blockade are maintained beyond the initial 28 days
Studies of IL-6R antagonists
Up to March 5, 2021, we identified ten RCTs of tocilizumab or sarilumab (including 7050 patients) that had been completed and reported: nine published after peer review20, 21, 22, 23, 24, 25, 26, 28, 29 and one available as a preprint.27 Additionally, we identified several reports of observational studies.18, 19 The table provides a summary of the main characteristics and findings of the available RCTs. The included studies enrolled patients with COVID-19 of varying degrees of severity (both in terms of clinical signs and the level of respiratory support required) and a median time from symptom onset to randomisation (and drug initiation) that ranged from 6 to 12 days. Nine RCTs tested tocilizumab (3375 patients),20, 21, 22, 23, 24, 25, 26, 27, 28 one of which tested sarilumab in a subset of 48 patients,26 and one trial randomised patients to different doses of sarilumab (334 patients) or placebo.29 Tocilizumab was generally administered at a dose of 8 mg/kg intravenously, with at least half of the studies allowing a repeat dose within the first 24 h. Sarilumab was administered intravenously at doses ranging from 200 mg to 400 mg.
The primary outcome of these studies varied (table ), with most trials using a combination of invasive mechanical ventilation or all-cause death, and at least half of the studies using a variation of an ordinal scale to summarise patients' clinical status or requirement for organ support—for example, ranging from 1 (discharged or ready for discharge) to 7 (death).22, 23, 24, 25, 28, 29 Three of the studies reported statistically significant improvements in their prespecified primary endpoints in patients randomised to receive tocilizumab. Specifically, the EMPACTA trial24 reported a reduction in the composite outcome of need for invasive mechanical ventilation or death at day 28 with tocilizumab versus placebo (hazard ratio 0·56, 95% CI 0·33–0·97). The recently reported REMAP-CAP trial26 showed an improvement in organ support-free days or death at day 21 with the use of tocilizumab compared with standard care (odds ratio 1·64; 95% credible interval 1·25–2·14). Finally, the RECOVERY trial27 showed a benefit of tocilizumab in terms of all-cause mortality at 28 days (risk ratio 0·86, 95% CI 0·77–0·96) and several prespecified secondary outcomes, including the composite of invasive mechanical ventilation or death (0·85, 0·78–0·93). None of the other six trials demonstrated benefit,20, 21, 22, 23, 25, 28 and one study was stopped early because of a signal for increased mortality associated with the use of tocilizumab.23
Table.
Main characteristics of selected trials of tocilizumab and sarilumab in patients with COVID-19
| Design | Main intervention | Inclusion and exclusion criteria | Number of participants* | Time to enrolment | Primary outcome | Other outcomes | Main results | Comments† | |
|---|---|---|---|---|---|---|---|---|---|
| CORIMUNO-19,20 published October, 2020 (NCT04331808) | Open-label RCT; 1:1 ratio | Tocilizumab 8 mg/kg versus usual care; option for second dose at 72 h | Adults with COVID-19-related moderate, severe, or critical pneumonia requiring O2 ≥3 L/min; patients on non-invasive or invasive mechanical ventilation excluded | 131 (64 tocilizumab, 67 usual care) | Mean of 10 days from symptom onset; median of 1 day from hospitalisation | Score >5 on WHO 10-point Clinical Progression Scale at day 4; survival free from mechanical ventilation at day 14 | Clinical status; overall survival; time to discharge; time to oxygen supply dependency | Indeterminate for primary outcome (median HR 0·58, 90% CrI 0·33–1·00); indeterminate for secondary outcomes | 17% of patients on steroids; none on remdesivir; not critically ill |
| RCT-TCZ-COVID-19,21 published October, 2020 (NCT04346355) | Open-label RCT; 1:1 ratio | Tocilizumab 8 mg/kg versus standard of care; second dose at 12 h | Patients aged ≥18 years with COVID-19 pneumonia, PaO2/FiO2 200–300, and inflammatory phenotype, without mechanical ventilation at baseline; patients admitted to ICU, of advanced age, or with high burden of comorbidities excluded | 126 (60 tocilizumab, 66 standard of care) | Median of 8 days from symptom onset; median of 2 days from hospitalisation | Clinical worsening at 14 days, including ICU admission, death, or PaO2/FiO2 <150 | ICU admission; death at 14 and 30 days | Indeterminate for primary outcome (RR 1·05, 0·59–1·86); indeterminate for secondary outcomes | <5% of patients on steroids; none on remdesivir; not critically ill |
| BACC Bay,22 published December, 2020 (NCT04356937) | Double-blind RCT; 2:1 ratio | Tocilizumab 8 mg/kg versus placebo; single dose | Patients aged 19–85 years with confirmed COVID-19, hyperinflammatory state, and pulmonary infiltrates, fever, or need for supplemental O2; patients with higher risk of infection or O2 >10 L/min excluded | 243 (161 tocilizumab, 82 placebo) | Median of 9 days from symptom onset; within 72 h of worsening | Mechanical ventilation or death | Clinical worsening (ordinal scale) | Indeterminate for primary outcome (HR 0·83, 0·38–1·81); indeterminate for secondary outcome | 10% of patients on steroids; 32% on remdesivir; 4% on HFNO |
| TOCIBRAS,23 published January, 2021 (NCT04403685) | Open-label RCT; 1:1 ratio | Tocilizumab 8 mg/kg versus standard of care; single dose | Adults hospitalised with severe COVID-19 receiving supplemental O2 or mechanical ventilation, with high inflammatory markers; patients with uncontrolled infection, liver disease, or renal disease excluded | 129 (65 tocilizumab, 64 standard of care) | Mean of 10 days from symptom onset | Clinical status (ordinal scale) at day 15 | Death at 28 days | Indeterminate for primary outcome (OR 1·54, 0·66–3·66); indeterminate for secondary outcome | 71% pf patients on steroids; none on remdesivir; 32% on non-invasive ventilation or HFNO; 16% on invasive mechanical ventilation |
| EMPACTA,24 published January, 2021 (NCT04372186) | Double-blind RCT; 2:1 ratio | Tocilizumab 8 mg/kg versus placebo; option for second dose at 8–24 h | Patients aged ≥18 years with COVID-19 pneumonia receiving supplemental O2; patients on non-invasive or invasive mechanical ventilation, with active infection, or at risk of imminent death excluded | 388 (259 tocilizumab, 129 placebo) | Median of 8 days from symptoms; median of 1 day from diagnosis | Mechanical ventilation or death by day 28 | Median time to hospital discharge; improvement in clinical status (ordinal scale) | Positive for primary outcome (HR 0·56, 0·33–0·97); indeterminate for secondary outcomes | >80% of patients on steroids; >70% on antivirals; 27% critically ill or on HFNO |
| COVACTA,25 published February, 2021 (NCT04320615) | Double-blind RCT; 2:1 ratio | Tocilizumab 8 mg/kg versus placebo; option for second dose at 8–24 h | Patients aged ≥18 years with COVID-19 pneumonia, and SpO2 ≤93% or PaO2/FiO2 <300; patients with active infection or at risk of imminent death excluded | 452 (301 tocilizumab, 151 placebo) | Mean of 12 days from symptom onset; median of 5 days from mechanical ventilation | Clinical status (7-category ordinal scale) at day 28 | Death at 28 days; ventilator-free days during 28 days | Indeterminate for primary outcome (between-group difference in median clinical status −1·00, −2·50 to 0·00); indeterminate for secondary outcomes | 22% of patients on steroids (more in the placebo group); 25% on antivirals; 37% on invasive mechanical ventilation |
| REMAP-CAP,26 published February, 2021 (NCT02735707) | Open-label RCT (adaptive platform trial); balanced assignment (ratio dependent on number of interventions available at each site) | Tocilizumab 8 mg/kg or sarilumab 400 mg versus standard of care; option for second dose of tocilizumab at 12–24 h | Critically ill patients aged ≥18 years receiving respiratory or cardiovascular organ support, enrolled within 24 h of ICU admission; patients at risk of imminent death excluded | 865 (353 tocilizumab, 48 sarilumab, 402 standard of care) | Median of 1 day from hospital admission to randomisation; median of 14 h from ICU admission | Organ support-free days or death up to 21 days | In hospital or death at 90 days; time to ICU discharge; time to hospital discharge | Positive for primary outcome (tocilizumab OR 1·64, 95% Crl 1·25–2·14; sarilumab 1.76, 1·17–2·91); positive for secondary outcomes | >80% of patients on steroids; 33% on remdesivir; 29% on HFNO; 42% on non-invasive ventilation; 29% on invasive mechanical ventilation |
| RECOVERY,27 preprint published February, 2021 (NCT04381936) | Open-label RCT (platform trial); 1:1 ratio | Tocilizumab 8 mg/kg versus usual care; option for second dose at 12–24 h | Patients aged ≥18 years with severe COVID-19, with SpO2 <92% on air or requiring O2 therapy, and C-reactive protein ≥75 mg/L; patients with active infection excluded | 4116 (2022 tocilizumab, 2094 usual care) | Median of 10 days from symptom onset; median of 2 days from hospitalisation | All-cause death at 28 days | Time to hospital discharge; invasive mechanical ventilation or death at 28 days | Positive for primary outcome (RR 0·86, 0·77–0·96); positive for secondary outcomes | >80% of patients on steroids; 22% on remdesivir; 41% on non-invasive ventilation or HFNO; 14% on invasive mechanical ventilation |
| COVINTOC,28 published March, 2021 (CTRI/2020/05/025369) | Open-label RCT; 1:1 ratio | Tocilizumab 6 mg/kg versus standard of care; option for second dose at 12 h to 7 days | Patients aged ≥18 years admitted to hospital with moderate (respiratory rate 15–30 per min, SpO2 90–94%) to severe (respiratory rate ≥30 per min, SpO2 <90%, or ARDS or septic shock) COVID-19; patients with active infection or at risk of imminent death excluded | 180 (90 tocilizumab, 90 standard of care) | Not reported | Progression of COVID-19 from moderate to severe or from severe to death up to day 14 | Time to clinical improvement; proportion of patients with improvement in ASTCT CRS grade | Indeterminate for primary outcome (mean difference −3·7, −18·2 to 11·2); indeterminate for secondary outcomes | 91% of patients on steroids; 42% on remdesivir; 27% on non-invasive ventilation; 5% on invasive mechanical ventilation |
| Lescure et al,29 published March, 2021 (NCT04327388) | Double-blind RCT (adaptive trial); 2:2:1 ratio | Sarilumab 400 mg or sarilumab 200 mg versus placebo; option for second dose at 24–48 h | Patients aged ≥18 years admitted to hospital with severe COVID-19 pneumonia or with critical disease requiring supplemental O2 or ICU admission; patients with active infection, dysfunction of ≥2 organ systems, on renal replacement therapy or extracorporeal support, or at risk of imminent death excluded | 420 (173 sarilumab 400 mg, 161 sarilumab 200 mg, 86 placebo) | Median of 5 days from dyspnoea onset; median of 3 days from hospitalisation | Time to clinical improvement (2 or more points on 7-point ordinal scale) | Death at 28 days | Indeterminate for primary outcome sarilumab 400 mg HR 1·14, 0·84–1·54; sarilumab 200 mg 1·03, 0·75–1·40; indeterminate for secondary outcomes | 20% of patients on steroids; <1% on remdesivir; 6% on HFNO; 2% on non-invasive ventilation; 12% on invasive mechanical ventilation |
All measures of effect are shown alongside 95% CI (frequentist analysis) unless otherwise specified. ARDS=acute respiratory distress syndrome. ASTCT=American Society for Transplantation and Cellular Therapy. Crl=credible interval. CRS=cytokine release syndrome. HFNO=high-flow nasal oxygen. HR=hazard ratio. ICU=intensive care unit. OR=odds ratio. PaO2/FiO2=ratio of partial pressure of arterial oxygen to fraction of inspired oxygen. RR=risk ratio. SpO2=oxygen saturation.
Total numbers might differ in the modified intention-to-treat analysis in individual trials.
Steroid and remdesivir use reflect pre-randomisation data where available, as a proportion of the entire sample enrolled (ie, all arms in the study).
Clinical sources of heterogeneity
What are the drivers of these inconsistent findings, despite similar tocilizumab dosing protocols across studies? Several characteristics of these trials might explain, at least partly, the discordant results observed, including the following: the severity of disease; the timing of IL-6R blockade in relation to the course of clinical deterioration; the concurrent use of other drugs such as corticosteroids; and the presence of elevated inflammatory markers. A better understanding of these sources of variability might enhance the application of knowledge at the bedside and inform the rationale for future RCTs.
Severity of disease
Although all RCTs included hospitalised patients who required supplemental oxygen, important variability exists in the initial severity of disease used for the inclusion or exclusion of participants (table). Indeed, whereas certain trials excluded patients who required invasive or non-invasive mechanical ventilation (eg, CORIMUNO-19,20 RCT-TCZ-COVID-19,21 BACC Bay,22 and EMPACTA24), others considered the need for intensive care unit (ICU) admission and the use of non-invasive or invasive ventilation as inclusion criteria (eg, REMAP-CAP26).
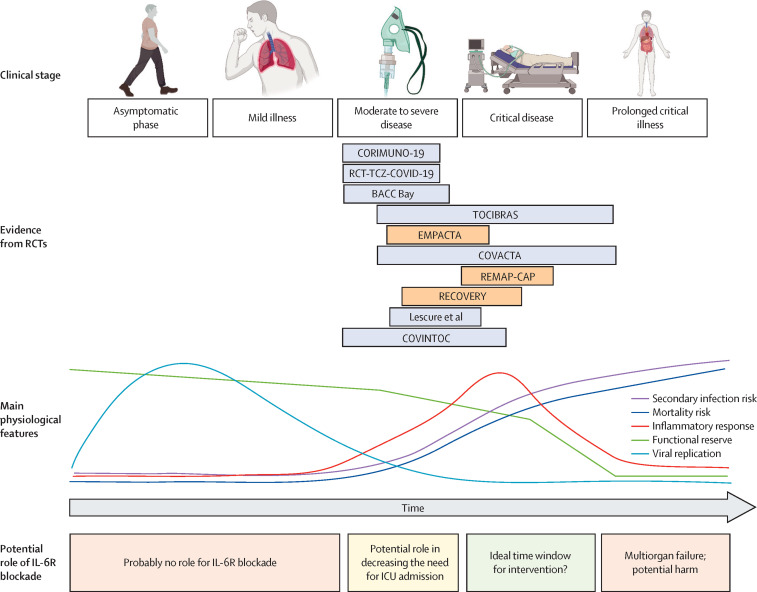
There are two essential mechanisms by which the varying severity of illness might explain the conflicting results of these trials. First, different degrees of clinical severity might be associated with different magnitudes of inflammation, with those at the more severe end of the spectrum, with the highest levels of inflammatory cascade activation, expected to benefit most from IL-6R blockade. This hypothesis is supported by the beneficial effect observed in studies that enrolled patients early in their critical care trajectory or in those with established but new-onset organ failure.26, 27 For example, REMAP-CAP, the study that demonstrated one of the largest estimates of benefit with IL-6R blockade, included only patients within 24 h of requiring non-invasive oxygenation (ie, high-flow nasal oxygen or non-invasive ventilation) or invasive mechanical ventilatory support. However, this relationship might not hold true for patients with the most severe disease (especially if a long time has elapsed from symptom onset or clinical deterioration), because proliferation of the inflammatory cascade might be too advanced for benefits to be seen with targeted IL-6R blockade (figure ). Even if the relative effect of IL-6R antagonists was consistent across disease severities, studies that included patients with less severe disease, and therefore a lower absolute risk of mortality—eg, RCT-TCZ-COVID-1921 had an overall 30-day mortality of less than 3%—would probably be underpowered to observe meaningful mortality differences between groups. Such reduced power would not allow definitive advice to be given on when and in whom to start therapy based only on the magnitude of inflammation.
Figure.
Proposed use of IL-6R blockade in patients with COVID-19-associated hypoxaemic respiratory failure
A conceptual model of the time-course of infection from the asymptomatic phase to more severe phases in patients who develop critical illness. The model includes completed RCTs and the main physiological features of disease in each phase. We propose a potential time window in which IL-6R blockade might be more effective. The length of each RCT box is proportional to the time from symptom onset or hospitalisation to randomisation in each trial population, and the location corresponds to the severity, on average, of enrolled patients. For many patients, the progression from the asymptomatic phase to critical illness occurs in a short period of time, and the current representation might not be applicable in the case of rapid progression. Trials that appear in light orange are those that were positive for the primary outcome. ICU=intensive care unit. IL-6R=interleukin-6 receptor. RCT=randomised controlled trial. Figure originally created using BioRender.
Timing of randomisation
Clinicians caring for hospitalised patients with severe COVID-19-related hypoxaemic respiratory failure often look retrospectively at two important time intervals: first, the time from onset of symptoms; and second, the time since clinical deterioration.34, 35 Although viral replication probably peaks earlier, the peak of the inflammatory response to SARS-CoV-2 often coincides with or shortly precedes clinical deterioration.36 Once the inflammatory cascade achieves a state of hyperactivation, it might be too late to intervene, and it has therefore been hypothesised that a time window exists within which therapies targeting the inflammatory response (eg, IL-6 inhibitors) will be most beneficial.37 This window might correlate with a time just before or just after clinical deterioration, perhaps when organ dysfunction is developing and potentially at its most reversible.38 Although several trials have included subgroup analyses based on the interval between symptom onset and randomisation, the time of randomisation relative to clinical deterioration might be more important in defining the period of maximum efficacy of IL-6R blockade.
The REMAP-CAP trial26 used a 24-h window for randomisation after the initiation of organ support (mainly invasive or non-invasive oxygenation strategies) irrespective of the time of symptom onset. The fact that this study showed benefit with the use of IL-6R blockade, coupled with the likely detrimental effects observed in a trial23 that intervened at a later stage, seems to suggest that IL-6R antagonists need to be administered specifically around the time of clinical deterioration. However, the RECOVERY trial27 did not show an important interaction between the use of tocilizumab and the time from symptom onset (≤7 days vs >7 days). This finding is not inconsistent with the hypothesis that IL-6R blockade might be more effective when given closer to the time of clinical deterioration, and a subgroup analysis considering this time interval might be more valuable. Unfortunately, on the basis of the available data, it is not possible to confidently answer the question of whether time from symptom onset or clinical deterioration influences the efficacy of administered IL-6R antagonists (figure). However, given that the salient time point of clinical deterioration seems, in principle, to be a better marker for the initiation of anti-inflammatory therapy, subgroup analysis or an individual patient data meta-analysis evaluating this hypothesis might be a useful starting point.
Concomitant use of corticosteroids
The use of co-interventions in the trials we identified was highly variable, probably owing to differences not only in time of trial conduct (eg, before or after the widespread use of corticosteroids),39 but also in local clinical practice. The table summarises the proportion of patients that received corticosteroids or the antiviral agent remdesivir40 in each RCT. How the differential use of these treatments might have modified the effect of tocilizumab remains an important potential source of heterogeneity between trials. Of note, RECOVERY27 found an interaction between the use of corticosteroids and tocilizumab: the subgroup of patients who received both treatments appeared to benefit the most. A benefit associated with a higher intensity of immunosuppression when both treatments are combined might partly explain the differential estimated effects among trials with different proportions of patients receiving corticosteroids. This possibility is especially relevant given the current widespread use of steroids in patients with COVID-19.26 Furthermore, it raises the question of whether the same beneficial immunomodulatory effect could be achieved more easily and with less associated cost by using a higher dose of corticosteroids. Finally, it should be noted that the differential use of co-interventions across trials (rather than within trials) should not have influenced the validity of the estimated effects within studies (ie, due to random allocation and blinding of study treatments). Adaptive platform trials have the potential advantage of being able to study multiple interventions simultaneously, and more adequately assess treatment-by-treatment interactions.41, 42
Other differences in standard of care or usual care that varied across trials and settings, including other drugs or methods of delivery of supportive therapy, might also have contributed to the observed differences in treatment effect.43 Decisions about the features of the standard-of-care arm might limit the applicability of study findings in different settings (and be a source of heterogeneity between different trials), in the presence of effect modification or practice misalignment.43
Baseline inflammatory status
Of note, several of the IL-6 inhibitor RCTs (RCT-TCZ-COVID-19,21 BACC Bay,22 TOCIBRAS,23 RECOVERY;27 table) used serological markers to enrich the study population, including patients with elevated inflammatory markers (eg, C-reactive protein, D-dimer, or ferritin). Intuitively, this strategy should select patients with an increased inflammatory response, who might benefit most from IL-6R blockade. However, of these trials, only RECOVERY27 reported a beneficial effect with the use of tocilizumab, and TOCIBRAS23 showed potential harm. Moreover, the benefit of tocilizumab treatment was evident across all C-reactive protein subgroups in the REMAP-CAP trial.26
These observations do not necessarily prove that enriching the population of COVID-19 patients for RCTs of IL-6R blockade will not be beneficial. Rather, they might indicate that commonly used inflammatory markers are not sensitive enough to adequately select patients who are likely to benefit, since their biological association with IL-6 might not be linear or temporally simultaneous. Additional enrichment strategies could include the use of specific cytokine profiles or the combination of serological markers with observed clinical phenotypes (eg, acute illness severity scores).44 The extent to which the concentrations of circulating serum cytokines, and in particular IL-6, reflect or affect the response to tocilizumab in patients with COVID-19 is also uncertain. Several studies have shown that increased IL-6 concentrations are associated with subsequent clinical worsening and need for escalating respiratory support measures in patients with COVID-19.45, 46, 47 However, a systematic review and meta-analysis found that IL-6 concentrations in critically ill patients with COVID-19 were not significantly higher than those in patients with less severe disease.10 Nevertheless, it should be noted that if IL-6 is indeed part of the causal pathway that leads to clinical worsening and severe disease, this might occur even at lower serological concentrations. Moreover, the biological effects of IL-6 might depend not only on the concentrations of the soluble cytokine.1, 48 Hence, in our view, these findings do not necessarily argue against the efficacy of blockers of IL-6 signalling in patients with COVID-19, but instead suggest that the timing of serum IL-6 measurement or relative rather than absolute changes in IL-6 concentrations might be key to identifying which patients will benefit from treatment. Whether IL-6 acts as a predictor or an effector (or both) of clinical deterioration is a fundamental question that needs to be answered.
Finally, among the RCTs that showed no difference with tocilizumab, patients randomised to receive tocilizumab had higher concentrations of IL-6, IL-10, and interferon-γ after its administration.23 Moreover, observational reports have suggested that an increase in alternative, non-IL-6 cytokine expression might represent a surrogate of non-response to tocilizumab.49 This rebound might reflect a feedback mechanism triggered by receptor blockade or by diverting inflammation through collateral pathways, and could be detrimental depending on the half-life of the initial treatment. A longer-lasting or more widespread cytokine blockade might be needed to counter the overactivated inflammatory cascade.
Methodological sources of heterogeneity
Observational studies
Several observational studies have been published that examined the use of tocilizumab in patients with COVID-19.18, 19 These studies were generally well done, and most attempted to account for confounding, which is commonly a concern with observational data and limits the ability to estimate causal effects.50 The results of observational studies have been largely consistent regarding the beneficial effects of IL-6R blockade on clinical outcomes in patients with COVID-19-related hypoxaemic respiratory failure.30 This consistency—in contrast to the discordant findings of RCTs—could have a causal or a non-causal explanation. For example, observational studies have included mostly patients with higher levels of disease severity, who are thus expected to have a more marked inflammatory state, potentially increasing statistical power. Furthermore, observational studies are subject to residual confounding that could bias the results in both directions (ie, either away or towards the null). For example, it is possible that physicians were more likely to use tocilizumab or sarilumab in patients who were more likely to survive or, by contrast, in those who were sicker as a last-resort salvage attempt. Indeed, assessment of the efficacy of IL-6R blockade in patients with COVID-19 should be based primarily on the available RCTs, given the benefits of randomisation in balancing known and unknown prognostic factors. Notwithstanding their limitations, large observational studies might have an important role: first, in shedding light on the subgroups of patients with COVID-19 who might benefit the most from IL-6R blockade; second, in providing crucial information on side-effects and secondary infections that occur at low rates and are therefore challenging to study in the context of an RCT; and third, in describing the effects of IL-6R blockade among people who are usually excluded from RCTs, such as immunosuppressed patients and pregnant women.
Absence of blinding
Several of the RCTs that evaluated the effects of IL-6R blockade in patients with COVID-19 have been conducted as open-label studies. Moreover, blinded RCTs could have been unmasked by changes in inflammatory markers such as C-reactive protein in the tocilizumab arm.51 An absence of blinding could have affected the internal validity of individual trials through co-intervention and differential decision-making regarding intubation and ICU admission, which were, in several studies, part of the scales used to define either primary or secondary outcomes.
Outcome selection and analytical strategy
The primary endpoints of the RCTs reported so far have varied (table). The most frequently selected endpoints have been ordinal scales assessing clinical improvement or worsening, or the composite of death and invasive mechanical ventilation. Together with differences in the baseline severity of enrolled patients, the use of different outcomes might have contributed to inconsistencies in the reported results. Such inconsistencies might reflect differences in statistical power (due to the chosen endpoint) and the likelihood of tocilizumab to influence some (if not all) outcomes under consideration.
The use of early stopping rules might also explain inconsistent results across trials, with studies that were stopped early more likely to reflect a false positive (type I error) or a false negative (type II error) result.52 The analytical strategy—ie, the use of a Bayesian design, as in REMAP-CAP,26 versus frequentist approaches—might be another source of observed discrepancies.53 For example, the use of adaptive platforms within a Bayesian framework could maximise the finding and interpretation of a beneficial effect. Furthermore, the choice of priors and stopping rules affect the decision to stop enrolment (or not) and will thus change the final estimated effect of a given trial. Importantly, it has been shown that the conclusions from frequentist and Bayesian analysis of the same evidence are not always consistent.53
Challenges and future directions
Knowledge of the available evidence, alongside a deeper understanding of the differences among trials examining IL-6R antagonists, might help to inform the application of this evidence to clinical practice and the enrichment of ongoing and future RCTs. Current evidence points to a beneficial effect of tocilizumab—in terms of reduced need for invasive mechanical ventilation, reduced all-cause death, and improved clinical status—in patients with more severe COVID-19, whereas patients with non-severe disease probably do not benefit. It is also likely that the benefit of IL-6R blockade is closely linked to the time of maximum inflammation manifested as clinical deterioration.
Several relevant research questions remain unanswered (panel ). First and foremost, exploration of the timing of tocilizumab administration and enhanced patient selection to maximise benefit and reduce harm is warranted. Second, given that most RCTs excluded patients with ongoing or suspected active bacterial or fungal infections, the role and safety profile of IL-6R blockade in this setting remains unknown. Although most RCTs did not identify safety concerns with tocilizumab, surveillance for potentially detrimental effects, such as increased risk of infection, once the drug is used more widely should be heightened. Third, the extent to which the benefit observed with tocilizumab can be extended to sarilumab remains unknown. The relatively small number of patients randomised to sarilumab precludes further depiction of the benefits observed overall as a class effect for all IL-6R antagonists. Fourth, given the relatively short time-frame of outcome assessment in the EMPACTA,24 REMAP-CAP,26 and RECOVERY27 trials, an additional unanswered question is whether the beneficial effects observed in these trials will be maintained at longer follow-up periods, such as 60 or 90 days—something that will probably be monitored by regulatory agencies. Longer-term follow-up is important, especially given the long hospitalisation period of some patients with COVID-19-associated critical illness.54
Panel. Key research questions.
-
•
What is the optimum timing, from clinical deterioration in the course of COVID-19, for the use of monoclonal antibodies that target the interleukin-6 receptor (IL-6R)?
-
•
What are the baseline characteristics that define the patients who are more likely to benefit from IL-6R blockade?
-
•
With the increasing use of tocilizumab for patients with COVID-19, what is the efficacy and safety profile in patients with or at risk of active bacterial or fungal infections?
-
•
Is the benefit of IL-6R blockade among patients with severe COVID-19 a class effect (or isolated to tocilizumab)?
-
•
Do the efficacy results of trials extrapolate to longer follow-up periods, such as 60 or 90 days?
-
•
What is the cost-effectiveness of tocilizumab in patients with COVID-19?
Notwithstanding the ongoing need for further evidence, significant additional challenges remain. First, assuming efficacy, the cost-effectiveness of tocilizumab is unknown. This is especially relevant in the context of widespread infection and extreme pressure on health systems. Second, it is worth mentioning the role and weight of individual trial results released as preprints when evaluating the evidence for a specific intervention. The reports on the use of tocilizumab serve as a relevant case study in which, for a moderate amount of time, therapeutic decision making was based on preprints (on this occasion, these were very similar to the fully published reports).26 Ongoing discussions are needed to assess the benefits of rapid access to trial results that are perceived to be practice-changing, to weigh these benefits against the risks of dissemination before peer-review, and to consider the need for quality control. The use of preprints is an aspect of current research and clinical practice that is likely to have long-lasting consequences, far beyond the current COVID-19 pandemic.55
Conclusions
Should all severely ill patients with COVID-19-associated acute hypoxaemic respiratory failure receive tocilizumab? A one-size-fits-all approach is unlikely to be the answer. Given the inconsistency in the data and the challenges in accurately quantifying and identifying potential explanations for such heterogeneity, strong recommendations for unselected populations are probably not possible. However, we argue that tocilizumab is probably beneficial among patients who require respiratory support, soon after the time of clinical deterioration. Future studies of immunomodulatory therapy for patients with COVID-19 should integrate the knowledge gained in order to maximise sample enrichment, carefully monitor for harm such as secondary infections, and evaluate benefits for longer follow-up periods. Furthermore, tools to predict the response to tocilizumab (and other anti-inflammatory treatments) in terms of serological markers or clinical patterns might prove to be helpful in guiding optimal tailored therapy at the bedside.
Search strategy and selection criteria
We searched PubMed and the medRxiv preprint server for articles published in or translated into the English language between Jan 1, 2020, and March 5, 2021, using combinations of the terms “COVID-19”, “tocilizumab”, “sarilumab”, “randomized clinical trial”, and “randomized controlled trial”. FA and BLF established relevance on the basis of content, focusing on observational cohorts and randomised controlled trials reporting the effects of tocilizumab or sarilumab on clinically relevant outcomes for adult patients with COVID-19. We also manually retrieved articles from the reference lists of selected papers. The final reference list was generated on the basis of relevance to this Series paper, with the aim of advancing understanding of the studies of tocilizumab, and the implications of their findings, in patients with COVID-19.
Declaration of interests
EF reports personal fees from ALung Technologies, Baxter, Getinge, and MC3 Cardiopulmonary, outside of the submitted work. NDF reports personal fees from Xenios and Baxter, outside of the submitted work. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
FA and BLF are each partially supported by a Vanier Canada Graduate Scholarship from the Canadian Institutes of Health Research. The authors' funding sources had no role in the preparation of this manuscript.
Contributors
FA and BLF contributed to conception and design of the work, literature search and data acquisition, data interpretation, and drafting and revision of the manuscript. LB, EF, NDF, SH, SHK, EL, LM, and SR contributed to data interpretation, and drafting and revision of the manuscript. OGVU contributed to conception and design of the work, literature search and data acquisition, and revision of the manuscript. BR and LDS contributed to conception and design of the work, data interpretation, and drafting and revision of the manuscript, and were responsible for overall supervision of the work. All authors approved the final version of the manuscript.
References
- 1.Garbers C, Heink S, Korn T, Rose-John S. Interleukin-6: designing specific therapeutics for a complex cytokine. Nat Rev Drug Discov. 2018;17:395–412. doi: 10.1038/nrd.2018.45. [DOI] [PubMed] [Google Scholar]
- 2.Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med. 2020;383:2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388:2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 4.Schoels MM, Van Der Heijde D, Breedveld FC, et al. Blocking the effects of interleukin-6 in rheumatoid arthritis and other inflammatory rheumatic diseases: systematic literature review and meta-analysis informing a consensus statement. Ann Rheum Dis. 2013;72:583–589. doi: 10.1136/annrheumdis-2012-202470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choy EH, De Benedetti F, Takeuchi T, Hashizume M, John MR, Kishimoto T. Translating IL-6 biology into effective treatments. Nat Rev Rheumatol. 2020;16:335–345. doi: 10.1038/s41584-020-0419-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piroth L, Cottenet J, Mariet AS, et al. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respir Med. 2021;9:251–259. doi: 10.1016/S2213-2600(20)30527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim ZJ, Subramaniam A, Reddy MP, et al. Case fatality rates for patients with COVID-19 requiring invasive mechanical ventilation. Am J Respir Crit Care Med. 2021;203:54–66. doi: 10.1164/rccm.202006-2405OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan E, Beitler JR, Brochard L, et al. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir Med. 2020;8:816–821. doi: 10.1016/S2213-2600(20)30304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leisman DE, Ronner L, Pinotti R, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;8:1233–1244. doi: 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 12.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campochiaro C, Dagna L. The conundrum of interleukin-6 blockade in COVID-19. Lancet Rheumatol. 2020;2:e579–e580. doi: 10.1016/S2665-9913(20)30287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Price CC, Altice FL, Shyr Y, et al. Tocilizumab treatment for cytokine release syndrome in hospitalized patients with coronavirus disease 2019: survival and clinical outcomes. Chest. 2020;158:1397–1408. doi: 10.1016/j.chest.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guirao JJ, Cabrera CM, Jiménez N, Rincón L, Urra JM. High serum IL-6 values increase the risk of mortality and the severity of pneumonia in patients diagnosed with COVID-19. Mol Immunol. 2020;128:64–68. doi: 10.1016/j.molimm.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu J, Pang J, Ji P, et al. Elevated interleukin-6 is associated with severity of COVID-19: a meta-analysis. J Med Virol. 2021;93:35–37. doi: 10.1002/jmv.26085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta S, Wang W, Hayek SS, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2021;181:41–51. doi: 10.1001/jamainternmed.2020.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biran N, Ip A, Ahn J, et al. Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study. Lancet Rheumatol. 2020;2:e603–e612. doi: 10.1016/S2665-9913(20)30277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermine O, Mariette X, Tharaux PL, Resche-Rigon M, Porcher R, Ravaud P. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:32–40. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salvarani C, Dolci G, Massari M, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:24–31. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383:2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veiga VC, Prats JAGG, Farias DLC, et al. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. BMJ. 2021;372:n84. doi: 10.1136/bmj.n84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;384:20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384:1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The REMAP-CAP Investigators Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horby PW, Pessoa-Amorim G, Peto L, et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial. medRxiv. 2021 doi: 10.1101/2021.02.11.21249258. published online Feb 11. (preprint). [DOI] [Google Scholar]
- 28.Soin AS, Kumar K, Choudhary NS, et al. Tocilizumab plus standard care versus standard care in patients in India with moderate to severe COVID-19-associated cytokine release syndrome (COVINTOC): an open-label, multicentre, randomised, controlled, phase 3 trial. Lancet Respir Med. 2021 doi: 10.1016/S2213-2600(21)00081-3. published online March 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lescure F-X, Honda H, Fowler RA, et al. Sarilumab treatment of hospitalised patients with severe or critical COVID-19: a multinational, randomised, adaptive, phase 3, double-blind, placebo-controlled trial. Lancet Respir Med. 2021 doi: 10.1016/S2213-2600(21)00099-0. published online March 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parr JB. Time to reassess tocilizumab's role in COVID-19 pneumonia. JAMA Intern Med. 2021;181:12–15. doi: 10.1001/jamainternmed.2020.6557. [DOI] [PubMed] [Google Scholar]
- 31.Murthy S, Lee TC. IL-6 blockade for COVID-19: a global scientific call to arms. Lancet Respir Med. 2021 doi: 10.1016/S2213-2600(21)00127-2. published online March 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imrey PB. Limitations of meta-analyses of studies with high heterogeneity. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2019.19325. [DOI] [PubMed] [Google Scholar]
- 33.Bhatt DL, Mehta C. Adaptive designs for clinical trials. N Engl J Med. 2016;375:65–74. doi: 10.1056/NEJMra1510061. [DOI] [PubMed] [Google Scholar]
- 34.Chen SL, Feng HY, Xu H, et al. Patterns of deterioration in moderate patients with COVID-19 from Jan 2020 to Mar 2020: a multi-center, retrospective cohort study in China. Front Med (Lausanne) 2020;7 doi: 10.3389/fmed.2020.567296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manson JJ, Crooks C, Naja M, et al. COVID-19-associated hyperinflammation and escalation of patient care: a retrospective longitudinal cohort study. Lancet Rheumatol. 2020;2:e594–e602. doi: 10.1016/S2665-9913(20)30275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castelnovo L, Tamburello A, Lurati A, et al. Anti-IL6 treatment of serious COVID-19 disease: a monocentric retrospective experience. Medicine (Baltimore) 2021;100 doi: 10.1097/MD.0000000000023582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gandhi RT, Lynch JB, del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020;383:1757–1766. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 39.The RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 — final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilkinson E. RECOVERY trial: The UK covid-19 study resetting expectations for clinical trials. BMJ. 2020;369 doi: 10.1136/bmj.m1626. [DOI] [PubMed] [Google Scholar]
- 42.Angus DC, Berry S, Lewis RJ, et al. The REMAP-CAP (Randomized Embedded Multifactorial Adaptive Platform for Community-acquired Pneumonia) study. Rationale and design. Ann Am Thorac Soc. 2020;17:879–891. doi: 10.1513/AnnalsATS.202003-192SD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Angriman F, Masse MH, Adhikari NKJ. Defining standard of practice: pros and cons of the usual care arm. Curr Opin Crit Care. 2019;25:498–504. doi: 10.1097/MCC.0000000000000642. [DOI] [PubMed] [Google Scholar]
- 44.Knight SR, Ho A, Pius R, et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: development and validation of the 4C Mortality Score. BMJ. 2020;370 doi: 10.1136/bmj.m3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Del Valle DM, Kim-Schulze S, Huang HH, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26:1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laing AG, Lorenc A, del Molino del Barrio I, et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat Med. 2020;26:1623–1635. doi: 10.1038/s41591-020-1038-6. [DOI] [PubMed] [Google Scholar]
- 47.Herold T, Jurinovic V, Arnreich C, et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146:128. doi: 10.1016/j.jaci.2020.05.008. 36.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McElvaney OJ, Curley GF, Rose-John S, McElvaney NG. Interleukin-6: obstacles to targeting a complex cytokine in critical illness. Lancet Respir Med. 2021 doi: 10.1016/S2213-2600(21)00103-X. published online April 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guillén L, Padilla S, Fernández M, et al. Preemptive interleukin-6 blockade in patients with COVID-19. Sci Rep. 2020;10 doi: 10.1038/s41598-020-74001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hernán MA, Sauer BC, Hernández-Díaz S, Platt R, Shrier I. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J Clin Epidemiol. 2016;79:70–75. doi: 10.1016/j.jclinepi.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Cáceres C, Martínez R, Bachiller P, Marín L, García JM. The effect of tocilizumab on cytokine release syndrome in COVID-19 patients. Pharmacol Rep. 2020;72:1529–1537. doi: 10.1007/s43440-020-00186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walter SD, Guyatt GH, Bassler D, Briel M, Ramsay T, Han HD. Randomised trials with provision for early stopping for benefit (or harm): the impact on the estimated treatment effect. Stat Med. 2019;38:2524–2543. doi: 10.1002/sim.8142. [DOI] [PubMed] [Google Scholar]
- 53.Yarnell CJ, Abrams D, Baldwin MR, et al. Clinical trials in critical care: can a Bayesian approach enhance clinical and scientific decision making? Lancet Respir Med. 2021;9:207–216. doi: 10.1016/S2213-2600(20)30471-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rees EM, Nightingale ES, Jafari Y, et al. COVID-19 length of hospital stay: a systematic review and data synthesis. BMC Med. 2020;18:270. doi: 10.1186/s12916-020-01726-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carneiro CFD, Queiroz VGS, Moulin TC, et al. Comparing quality of reporting between preprints and peer-reviewed articles in the biomedical literature. Res Integr Peer Rev. 2020;5:16. doi: 10.1186/s41073-020-00101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]