Abstract
Background
Elevated proinflammatory cytokines are associated with greater COVID-19 severity. We aimed to assess safety and efficacy of sarilumab, an interleukin-6 receptor inhibitor, in patients with severe (requiring supplemental oxygen by nasal cannula or face mask) or critical (requiring greater supplemental oxygen, mechanical ventilation, or extracorporeal support) COVID-19.
Methods
We did a 60-day, randomised, double-blind, placebo-controlled, multinational phase 3 trial at 45 hospitals in Argentina, Brazil, Canada, Chile, France, Germany, Israel, Italy, Japan, Russia, and Spain. We included adults (≥18 years) admitted to hospital with laboratory-confirmed SARS-CoV-2 infection and pneumonia, who required oxygen supplementation or intensive care. Patients were randomly assigned (2:2:1 with permuted blocks of five) to receive intravenous sarilumab 400 mg, sarilumab 200 mg, or placebo. Patients, care providers, outcome assessors, and investigators remained masked to assigned intervention throughout the course of the study. The primary endpoint was time to clinical improvement of two or more points (seven point scale ranging from 1 [death] to 7 [discharged from hospital]) in the modified intention-to-treat population. The key secondary endpoint was proportion of patients alive at day 29. Safety outcomes included adverse events and laboratory assessments. This study is registered with ClinicalTrials.gov, NCT04327388; EudraCT, 2020-001162-12; and WHO, U1111-1249-6021.
Findings
Between March 28 and July 3, 2020, of 431 patients who were screened, 420 patients were randomly assigned and 416 received placebo (n=84 [20%]), sarilumab 200 mg (n=159 [38%]), or sarilumab 400 mg (n=173 [42%]). At day 29, no significant differences were seen in median time to an improvement of two or more points between placebo (12·0 days [95% CI 9·0 to 15·0]) and sarilumab 200 mg (10·0 days [9·0 to 12·0]; hazard ratio [HR] 1·03 [95% CI 0·75 to 1·40]; log-rank p=0·96) or sarilumab 400 mg (10·0 days [9·0 to 13·0]; HR 1·14 [95% CI 0·84 to 1·54]; log-rank p=0·34), or in proportions of patients alive (77 [92%] of 84 patients in the placebo group; 143 [90%] of 159 patients in the sarilumab 200 mg group; difference −1·7 [−9·3 to 5·8]; p=0·63 vs placebo; and 159 [92%] of 173 patients in the sarilumab 400 mg group; difference 0·2 [−6·9 to 7·4]; p=0·85 vs placebo). At day 29, there were numerical, non-significant survival differences between sarilumab 400 mg (88%) and placebo (79%; difference +8·9% [95% CI −7·7 to 25·5]; p=0·25) for patients who had critical disease. No unexpected safety signals were seen. The rates of treatment-emergent adverse events were 65% (55 of 84) in the placebo group, 65% (103 of 159) in the sarilumab 200 mg group, and 70% (121 of 173) in the sarilumab 400 mg group, and of those leading to death 11% (nine of 84) were in the placebo group, 11% (17 of 159) were in the sarilumab 200 mg group, and 10% (18 of 173) were in the sarilumab 400 mg group.
Interpretation
This trial did not show efficacy of sarilumab in patients admitted to hospital with COVID-19 and receiving supplemental oxygen. Adequately powered trials of targeted immunomodulatory therapies assessing survival as a primary endpoint are suggested in patients with critical COVID-19.
Funding
Sanofi and Regeneron Pharmaceuticals.
Introduction
The emergence of SARS-CoV-2 in December, 2019, and the associated disease COVID-191 has resulted in more than 110 million confirmed infections and more than 2·4 million deaths worldwide as of Feb 19, 2021. COVID-19-associated pneumonia can rapidly progress to acute respiratory distress syndrome, estimated to affect up to 41% of patients with severe COVID-19.2 In some patients, COVID-19 can cause damage to additional organs, including the heart, brain, kidney, and liver.3 In the first several months of the pandemic, there were no treatments with proven efficacy for patients with severe or critical COVID-19; therefore, carefully designed randomised, controlled trials of novel or repurposed medications were, and still are, warranted.
Research in context.
Evidence before this study
We searched PubMed with no date or language restrictions up to March 18, 2020, for published clinical trials of sarilumab in patients with laboratory-confirmed COVID-19. The search terms were (“COVID-19” or “2019-nCoV” or “SARS-CoV-2”) AND “sarilumab” AND (“clinical trial” or “randomised controlled trial”). We found no published clinical trials reporting outcomes of sarilumab in patients with COVID-19.
Added value of this study
This study was the first completed, large, global, randomised, placebo-controlled clinical trial, to our knowledge, of intravenous sarilumab added to local standard of care for patients with COVID-19 done in hospitals in Asia, Europe, and North and South America. Neither the primary endpoint of time to improvement of two or more points on an ordinal seven-point clinical status scale nor the key secondary endpoint of proportion of patients alive at day 29 showed superiority of intravenous sarilumab over placebo. There were no dose-related increases in the incidence of infections, serious infections, or adverse events leading to death in the sarilumab groups compared with the placebo group. The types of adverse events were generally consistent with those observed in clinical trials of sarilumab in patients with rheumatoid arthritis and no new safety signal for sarilumab was identified in patients admitted to hospital for COVID-19.
Implications of all the available evidence
This randomised double-blind, placebo-controlled study did not show efficacy of intravenous sarilumab when added to local hospital standard of care for patients who were severely or critically ill with COVID-19. Numerical differences in survival among the critically ill, although not statistically significant in this trial, should be further evaluated in an adequately powered clinical trial in patients with COVID-19.
Literature to date supports an association of elevated proinflammatory cytokines with acute, life-threatening respiratory injury observed in patients with COVID-19.4 Among these cytokines, interleukin (IL)-6 appears to play a prominent role in the pathogenesis of COVID-19-related acute respiratory distress syndrome. Results of a meta-analysis of laboratory findings indicated that 53% of patients with COVID-19 have increased IL-6 concentrations.5 A meta-analysis of 23 clinical trials involving 3400 patients showed that patients with severe COVID-19 had higher concentrations of IL-6 than those with mild disease, and even higher concentrations were observed in patients who died.6 Two additional meta-analyses7, 8 and a large, prospective cohort study of patients admitted to hospital with COVID-199 also showed an association between elevated IL-6 and COVID-19-related mortality. Many of these findings were first published during the early months of the pandemic, suggesting that inhibition of IL-6 signalling might have value as a treatment to manage inflammatory manifestations of COVID-19 pneumonia.
Sarilumab is a human monoclonal antibody, which inhibits the binding of IL-6 to its α receptor and is approved for treatment of adults with moderate to severely active rheumatoid arthritis.10, 11 Because sarilumab inhibits both soluble and membrane-bound forms of IL-6 receptor,10, 12 potentially suppressing proinflammatory signalling by both pulmonary epithelial and immune cells,13 we hypothesised it could reduce the severity of pulmonary complications of COVID-19, including respiratory failure. Here, we report results of a 60-day, randomised, placebo-controlled trial, in which we aimed to assess the safety and efficacy of sarilumab in patients admitted to hospital with severe to critical COVID-19.
Methods
Study design and participants
This study was an adaptive, phase 3, multicentre, randomised, double-blind trial. The study was done at 45 hospitals in Argentina, Brazil, Canada, Chile, France, Germany, Israel, Italy, Japan, Russia, and Spain (appendix pp 1–3). Because of the uncertainties of assessing treatment efficacy in COVID-19 pneumonia at the time of study design, the initial protocol allowed adaptations such as modification of the provisional phase 3 endpoints, sample size re-estimation before entering phase 3, or closing a dose group while the study remained blinded. Patients were assessed daily (vital signs were recorded ≥4 times per day from day 1–3, and ≥2 times per day from day 4–29) while hospitalised until discharge, or death, with a final follow-up on day 60.
We enrolled adults (≥18 years) who had been admitted to hospital for laboratory-confirmed SARS-CoV-2 infection in any specimen within 2 weeks before random assignment and with evidence of pneumonia by chest imaging or chest auscultation and no alternative explanation for the clinical presentation. Patients also had to meet criteria for severe disease (defined as administration of supplemental oxygen by nasal cannula, simple face mask, or another similar device) or critical disease (defined as need for supplemental oxygen delivered by non-rebreather mask or high-flow nasal cannula, use of invasive or non-invasive ventilation, or treatment in an intensive care unit).
Patients were excluded from the study if they had, in the investigator's opinion, at least one of the following: a low probability of surviving 48 h or remaining at the investigational site beyond 48 h, dysfunction of two or more organ systems, need for extracorporeal life support, or renal replacement therapy at screening; absolute neutrophil count less than 2000 cells per mm3; aspartate aminotransferase or alanine aminotransferase (ALT) exceeding five-times the upper limit of normal at screening; less than 50 000 platelets per mm3 at screening; known active, incompletely treated, suspected or known extrapulmonary tuberculosis; previous or concurrent use of immunosuppressant drugs at screening, including, but not limited to, IL-6 inhibitors or Janus kinase inhibitors within 30 days of baseline; anti-CD20 agents without evidence of B-cell recovery to baseline concentrations or IL-1 receptor antagonist within 1 week of baseline; abatacept within 8 weeks of baseline; tumour necrosis factor α inhibitors within 2–8 weeks of baseline; alkylating agents, including cyclophosphamide, within 6 months of baseline; cyclosporine, azathioprine, mycophenolate mofetil, leflunomide, or methotrexate within 4 weeks of baseline; or intravenous immunoglobulin within 5 months of baseline; use of systemic chronic (eg, oral) corticosteroids for a condition not related to COVID-19 at doses higher than prednisone 10 mg/day or equivalent at screening; or suspected or known active systemic bacterial or fungal infections within 4 weeks of screening.
The protocol was approved by the institutional review boards at each participating hospital and by national ethics committees, as required by local and national regulations. The study was done in accordance with the International Conference on Harmonisation Guidelines for Good Clinical Practice and the World Medical Association's Declaration of Helsinki and its amendments. Before participating in the trial, informed consent was obtained from all patients or their legally authorised or appointed representatives, as specified by local law and in compliance with or exceeding ethics committee requirements.
Four amendments were made to the original protocol. Amended protocol 01 (March 26, 2020) implemented clarifications to the original version. Amended protocol 02 (April 8, 2020) implemented changes from phase 2 primary and key secondary endpoints to the phase 3 primary and key secondary endpoints and added an option for a second dose. Amended protocol 03 (April 29, 2020) added an interim analysis when approximately 50% of the total planned number of patients reached day 15, for review by the independent data monitoring committee and unmasked representatives of the sponsor's senior management, who were not involved in study conduct; clarified the extent to which the sponsor could adapt the study following review of an interim report; and removed the use of vasopressors as an exclusion criterion. Amended protocol 04 (June 11, 2020) closed enrolment into the 200 mg group after senior management review of interim results and subsequent confirmation by review of the independent data monitoring committee, which favoured the 400 mg dose.
Randomisation and masking
Eligible patients were randomly assigned (2:2:1) to one dose of intravenous sarilumab 400 mg, sarilumab 200 mg, or placebo according to a central randomisation scheme using permuted blocks of five and implemented through an interactive response technology. Randomisation was stratified by severity of illness (severe or critical) and use of systemic corticosteroids (yes or no). Patients, care providers, outcome assessors, and investigators remained masked to assigned intervention throughout the course of the study. An unmasked pharmacist was responsible for the preparation and dispensation of all study interventions.
Procedures
Sarilumab 400 mg, sarilumab 200 mg, or placebo were prepared according to instructions provided in the pharmacy manual. After confirming the randomisation number accessed via interactive response technology, the hospital pharmacist added the contents of prefilled syringes of sarilumab 200 mg solution for subcutaneous injection supplied by the sponsor into a specified volume of locally sourced 0·9% sodium chloride solution for intravenous infusion (two syringes for the 400 mg dose, one syringe for the 200 mg dose, and 0·9% sodium chloride solution for the placebo dose) to produce an intravenous bag containing a colourless solution to be administered by masked hospital staff as one intravenous infusion. Patients could have the intravenous infusion stopped for a safety-related issue, in which case they did not continue with dosing. An option for a second dose existed (within the assigned treatment group) within 24–48 h of the first dose, based on the investigator's benefit-risk assessment (amended protocol 02; April 8, 2020).
Efficacy assessments included a once per day assessment of clinical status until discharge, body temperature (day 1–3 four times a day; day 4–29 twice a day), oxygen administration (day 1–3 four times a day; day 4–29 results recorded as assessed), resting oxygen saturation (SpO2 day 1–3 four times a day; day 4–29 results recorded as assessed), and National Early Warning Score 2.14 Safety procedures and assessments included clinical laboratory testing (done locally at each hospital), targeted physical examination, and concomitant medication review. Vital signs were recorded daily until discharge. Surveillance testing for bacterial and fungal infection was done locally, on days 7 and 15. In addition to the positive SARS-CoV-2 result required for inclusion, nasopharyngeal (when feasible) and blood samples were collected at baseline and on days 7, 15, 21, and 29, or on the day of hospital discharge and analysed by the local laboratories and a central laboratory. Serum IL-6 and other biomarkers were analysed in a central laboratory. Blood samples were also taken for measurement of sarilumab concentration. Other than central laboratory results, all clinical data were entered by investigators at each site into an electronic clinical research form and validated remotely by the sponsor's monitoring team.
Outcomes
The primary efficacy endpoint was time from baseline to clinical improvement of two or more points on a seven-point ordinal scale, with numerical values defined as follows: (1) death; (2) admitted to hospital, on invasive mechanical ventilation or extracorporeal membrane oxygenation; (3) admitted to hospital, on non-invasive ventilation or high-flow oxygen devices; (4) admitted to hospital, requiring supplemental oxygen; (5) admitted to hospital, not requiring supplemental oxygen, requiring ongoing medical care (COVID-19-related or otherwise); (6) admitted to hospital, not requiring supplemental oxygen, no longer requiring ongoing medical care; and (7) discharged from hospital. Discharge before day 29 was considered as a two-point improvement. The key secondary efficacy endpoint was the proportion of patients alive at day 29.
The original phase 2 primary endpoint was the time to resolution of fever for at least 48 h without antipyretics or until discharge (original protocol; March 18, 2020). However, the unanticipated rapid rate of enrolment made the plan to use the phase 2 analysis to select phase 3 efficacy endpoints unfeasible. As a result, the primary and key secondary endpoints for phase 3, as described above, were adopted a priori in amended protocol 02 (April 8, 2020).
Other secondary efficacy endpoints included differences in time-to-event endpoints by treatment (eg, time to improvement of one or more points on the seven-point scale, fever resolution, or discharge from hospital), score changes at specific timepoints (eg, proportion with a one-point improvement or worsening), and event durations (eg, mechanical ventilation, hospital stay).
Safety was assessed by investigator reports of adverse events, serious adverse events, adverse events of special interest (infusion-related reactions; hypersensitivity reactions; absolute neutrophil count <500 cells per mm3 with or without concurrent invasive infection; increase in ALT of at least three-times the upper limit of normal if normal at baseline or more than three-times the upper limit of normal and at least two-times more than the baseline concentration if abnormal at baseline; invasive bacterial or fungal infections of clinical significance with confirmed diagnosis based on the investigator's assessment with appropriate diagnostic tests and consultations; symptomatic overdose),15 and clinical laboratory parameters including lymphocyte count, neutrophil count, and ALT on days 1, 4, 7, 15, 21, and 29 (if still hospitalised).
Statistical analysis
This study addressed the null hypothesis of no difference in time to an improvement of two or more points on the seven-point scale between a sarilumab dose group and placebo. In sample size determination, approximately 400 patients randomly assigned (2:2:1) were estimated to provide 90% or greater power for pairwise comparison between each sarilumab dose (approximately 160 patients each) and placebo (approximately 80 patients) using a log-rank test of superiority at a two-sided significance level of 0·05. Assumptions, based in part on the results of a placebo-controlled study of remdesivir in China,16 included accrual duration of 3 months, each patient being followed up for 29 days or more, and proportions of patients with a two-point improvement at day 15 being 45% for placebo and 70% for sarilumab.
The modified intention-to-treat (mITT) and safety populations included all randomly assigned patients given study medication. Primary analysis was planned at day 29 and final analysis at day 60. Analysis of the primary endpoint in the mITT population involved a stratified log-rank test with treatment as a fixed factor. Estimation of treatment effect was provided as a hazard ratio (HR), generated using a stratified Cox proportional hazards model with treatment as a covariate. Patients without improvement were censored at the last observation timepoint; patients who took rescue medication in the study without previous improvement were censored at rescue medication start date. Patients who died were deemed no improvement starting from death date. The proportional hazards assumption was assessed by visual inspection of the plot of log(−log[survival]) versus log(survival time) to determine whether curves were parallel among treatments. Analysis of the key secondary endpoint in the mITT population involved a Cochran-Mantel-Haenszel test, with estimation of treatment effect reported as the difference in percentage of patients alive at day 29 (sarilumab minus placebo). An administrative interim analysis was prespecified for a point in time when approximately 50% of total planned patients (approximately 200) reached day 15. Multiplicity was addressed for the primary and key secondary endpoints for the primary analysis at day 29, by means of hierarchical testing: (1) primary endpoint sarilumab 400 mg versus placebo; (2) key secondary endpoint sarilumab 400 mg versus placebo; (3) primary endpoint sarilumab 200 mg versus placebo; and (4) key secondary endpoint sarilumab 200 mg versus placebo. The trial was monitored by an external independent data monitoring committee with ongoing access to unblinded clinical data. This study is registered with ClinicalTrials.gov, NCT04327388; EudraCT, 2020-001162-12; and WHO, U1111-1249-6021.
Role of the funding source
The study sponsor designed the study and was responsible for data collection, data analysis, and data interpretation.
Results
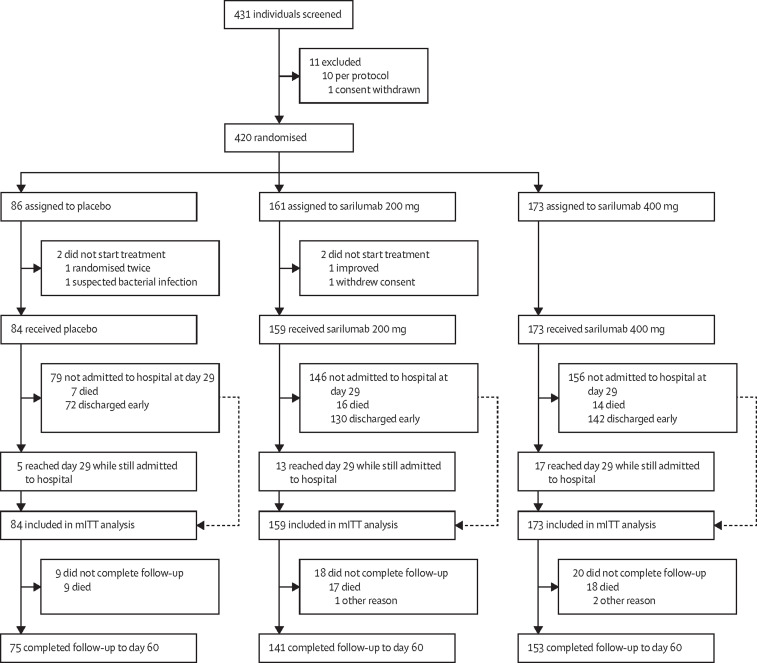
The first patient was screened on March 28, 2020, and the last patient was randomly assigned on July 3, 2020. The last patient's last visit was on Sept 2, 2020. Of 431 patients who were screened, 420 were randomly assigned and 416 received placebo (n=84 [20%]), sarilumab 200 mg (n=159 [38%]), or sarilumab 400 mg (n=173 [42%]; figure 1 ). Within each treatment group, less than 10% of patients received a second masked infusion of the assigned treatment (appendix p 4).
Figure 1.
Trial profile
mITT=modified intention-to-treat.
Baseline demographic, clinical, and laboratory characteristics were similar overall among the treatment groups, with exceptions including sex distribution, ferritin concentration, and proportions of patients with fever and obesity (table 1 ). Median age of patients was 59·0 years (IQR 50·0–68·0) and 37% of participants were women. According to investigator-reported severity, 61% had severe disease and 39% had critical disease. Two (<1%) patients randomly assigned into the severe disease stratum were recorded in the electronic clinical research form as having multisystem organ dysfunction because they required renal replacement therapy. Fever was reported in 218 (52%) patients. Median duration of hospital stay before dosing was 3·0 days (IQR 2·0–4·0). Use of systemic corticosteroids (including dexamethasone), antiviral medications, antibacterial medications (including azithromycin), and hydroxychloroquine or chloroquine before, before and during, and after first infusion of study medication did not differ substantially across treatment groups (appendix p 5).
Table 1.
Baseline patient characteristics
| All patients (N=416) | Placebo (n=84) | Sarilumab 200 mg (n=159) | Sarilumab 400 mg (n=173) | ||
|---|---|---|---|---|---|
| Age, years | 59·0 (50·0–68·0) | 60·0 (53·0–69·5) | 58·0 (51·0–67·0) | 58·0 (48·0–67·0) | |
| Sex | |||||
| Men | 261 (63%) | 54 (64%) | 108 (68%) | 99 (57%) | |
| Women | 155 (37%) | 30 (36%) | 51 (32%) | 74 (43%) | |
| Race | |||||
| Asian | 20 (5%) | 6 (7%) | 5 (3%) | 9 (5%) | |
| Black | 9 (2%) | 1 (1%) | 3 (2%) | 5 (3%) | |
| White | 321 (77%) | 67 (80%) | 126 (79%) | 128 (74%) | |
| Other* | 66 (16%) | 10 (12%) | 25 (16%) | 31 (18%) | |
| Ethnicity | |||||
| Hispanic or Latino | 150† (36%) | 31 (37%) | 53 (33%) | 66 (38%) | |
| Weight, kg | 83·0 (74·0–98·0) | 83·4 (72·0–97·4) | 83·0 (74·0–98·0) | 83·5 (74·0–98·0) | |
| Body-mass index ≥30 kg/m2 | 147/350 (42%) | 29/69 (42%) | 55/133 (41%) | 63/148 (43%) | |
| Comorbidities | |||||
| Hypertension | 177 (43%) | 39 (46%) | 68 (43%) | 70 (40%) | |
| Diabetes | 110 (26%) | 18 (21%) | 45 (28%) | 47 (27%) | |
| Obesity | 86 (21%) | 12 (14%) | 37 (23%) | 37 (21%) | |
| Neoplasm‡ | 42 (10%) | 6 (7%) | 17 (11%) | 19 (11%) | |
| Dyslipidaemia | 41 (10%) | 6 (7%) | 16 (10%) | 19 (11%) | |
| Coronary artery disease | 22 (5%) | 6 (7%) | 7 (4%) | 9 (5%) | |
| Chronic obstructive pulmonary disease | 18 (4%) | 6 (7%) | 4 (3%) | 8 (5%) | |
| Asthma | 17 (4%) | 3 (4%) | 10 (6%) | 4 (2%) | |
| Chronic kidney disease | 18 (4%) | 5 (6%) | 7 (4%) | 6 (3%) | |
| Severity of illness | |||||
| Severe§ | 252 (61%) | 55 (65%) | 92 (58%) | 105 (61%) | |
| Critical¶ | 162 (39%) | 29 (35%) | 65 (41%) | 68 (39%) | |
| Multisystem organ dysfunction | 2 (<1%) | 0 | 2 (1%) | 0 | |
| Clinical status on seven-point scale | |||||
| 2 | 50 (12%) | 9 (11%) | 17 (11%) | 24 (14%) | |
| 3 | 60 (14%) | 11 (13%) | 28 (18%) | 21 (12%) | |
| 4 | 304 (73%) | 64 (76%) | 112 (70%) | 128 (74%) | |
| 5 | 2 (<1%) | 0 | 2 (1%) | 0 | |
| Signs and symptoms | |||||
| Body temperature, °C‖ | 38·1 (0·9) | 38·0 (0·9) | 38·1 (0·9) | 38·2 (1·0) | |
| Fever** | 218 (52%) | 36 (43%) | 84 (53%) | 98 (57%) | |
| Cough | 298 (72%) | 58 (69%) | 112 (70%) | 128 (74%) | |
| Dyspnoea | 357 (86%) | 75 (89%) | 131 (82%) | 151 (87%) | |
| Time from dyspnoea onset to baseline, days | 5·0 (2·0–9·0) | 7·0 (3·0–10·0) | 5·0 (2·0–10·0) | 4·0 (2·0–9·0) | |
| Duration of hospital stay before dosing, days | 3·0 (2·0–4·0) | 4·0 (2·0–6·0) | 3·0 (1·0–4·0) | 2·0 (2·0–4·0) | |
| Admitted to ICU before dosing | 148 (36%) | 28 (33%) | 61 (38%) | 59 (34%) | |
| Duration of ICU stay before dosing, days | 2·0 (1·0–3·0) | 1·0 (1·0–3·5) | 2·0 (1·0–3·0) | 2·0 (1·0–3·0) | |
| Oxygen flow rate, L/min | 5·0 (3·0–8·0) | 5·0 (2·0–7·0) | 5·0 (3·0–9·0) | 5·0 (3·0–7·0) | |
| Percentage SpO2 | 95·0% (93·0–96·0) | 94·0% (93·0–96·0) | 95·0% (93·0–96·0) | 94·0% (93·0–96·0) | |
| Percentage FiO2 | 40·0% (32·0–55·0) | 40·0% (28·0–50·0) | 40·0% (32·0–60·0) | 40·0% (32·0–55·0) | |
| SpO2 to FiO2 ratio | 237·5 (173·6–300·0) | 240·0 (190·0–332·1) | 230·0 (165·0–296·9) | 237·5 (172·7–293·8) | |
| Type of oxygen delivery device | |||||
| Nasal cannula | 175 (42%) | 41 (49%) | 67 (42%) | 67 (39%) | |
| Simple face mask | 111 (27%) | 21 (25%) | 44 (28%) | 46 (27%) | |
| Non-rebreather face mask | 44 (11%) | 8 (10%) | 12 (8%) | 24 (14%) | |
| High-flow nasal cannula | 26 (6%) | 3 (4%) | 14 (9%) | 9 (5%) | |
| Non-invasive ventilation | 7 (2%) | 2 (2%) | 3 (2%) | 2 (1%) | |
| Invasive mechanical ventilation | 48 (12%) | 9 (11%) | 16 (10%) | 23 (13%) | |
| Other | 5 (1%) | 0 | 3 (2%) | 2 (1%) | |
| Use of extracorporeal membrane oxygenation | 0 | 0 | 0 | 0 | |
| Use of renal replacement therapy | 2 (<1%) | 0 | 2 (1%) | 0 | |
| Use of vasopressors | 12 (3%) | 1 (1%) | 5 (3%) | 6 (3%) | |
| Systemic corticosteroid use before dosing | 83 (20%) | 16 (19%) | 25 (16%) | 42 (24%) | |
| Laboratory findings | |||||
| SARS-CoV-2 virus detected†† | 391 (94%) | 80 (95%) | 147 (92%) | 164 (95%) | |
| C-reactive protein, mg/L | 94·6 (48·1–167·9) | 95·5 (55·5–184·4) | 94·1 (44·6–176·8) | 96·1 (48·1–160·6) | |
| IL-6, pg/mL | 12·3 (4·8–25·5) | 13·0 (3·6–23·5) | 11·6 (5·1–23·5) | 12·7 (5·5–26·5) | |
| Soluble IL-6 receptor, ng/mL | 42·4 (33·4–58·0) | 43·8 (32·1–61·8) | 41·2 (33·7–59·2) | 43·0 (33·7–54·4) | |
| D-dimer, mg/L | 0·50 (0·20–0·99) | 0·53 (0·17–1·14) | 0·48 (0·23–1·02) | 0·54 (0·16–0·97) | |
| Ferritin, μg/L | 765·0 (437·5–1309·0) | 979·6 (458·0–1644·0) | 694·6 (477·5–1270·5) | 737·0 (375·5–1151·0) | |
| Neutrophil to lymphocyte ratio | 5·3 (3·5–9·2) | 5·5 (3·8–8·8) | 5·1 (3·5–9·8) | 5·4 (3·4–8·5) | |
Data are median (IQR), n (%), n/N (%), or mean (SD). FiO2=fractional concentration of oxygen in inspired air. ICU=intensive care unit. IL-6=interleukin 6. SpO2=oxygen saturation.
Includes race not reported, other, or unknown.
136 (91%) of 150 Hispanic or Latino patients were in the white race category.
Includes benign, malignant, and unspecified neoplasms.
Severe disease was defined by supplemental oxygen administration by nasal cannula, simple face mask, or another similar device.
Critical disease was defined by one of the following criteria: supplemental oxygen delivered by non-rebreather mask or high-flow nasal cannula, use of invasive or non-invasive ventilation, or treatment in an ICU.
Defined as the highest temperature during the screening period.
Defined as body temperature greater than 37·4°C (axilla), greater than 38·0°C (oral), or greater than 38·4°C (rectal or tympanic).
Based on nasopharyngeal or serum PCR samples collected before first infusion.
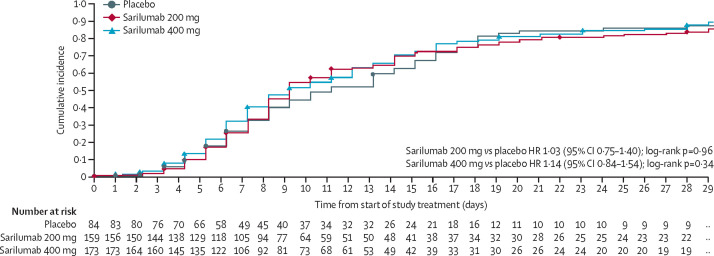
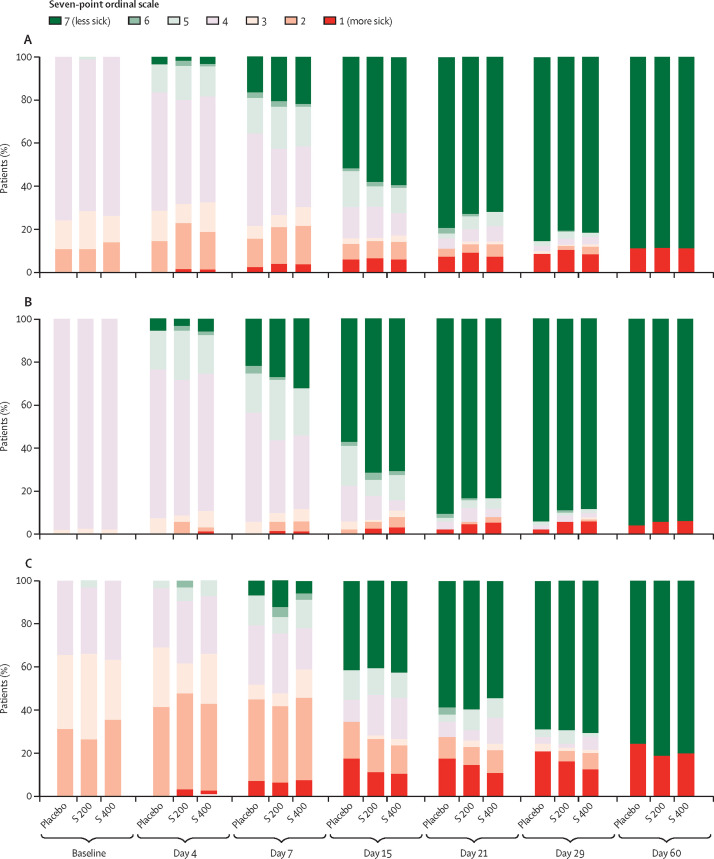
For the primary endpoint of time to improvement of two or more points on a seven-point clinical assessment scale, no significant difference was observed between sarilumab doses and placebo up to day 29 (figure 2 , appendix p 6). Differences in progression over time between patients with severe and critical disease on the clinical status scale are shown in figure 3 .
Figure 2.
Primary endpoint
Time to improvement of two or more points in clinical status from baseline on a seven-point ordinal scale (Kaplan-Maier curves; day 29 analysis). Number censored included in appendix (p 6). HR=hazard ratio.
Figure 3.
Ordinal scale point category from baseline to day 60
Findings shown for all patients (A), patients who were severely ill (B), and patients who were critically ill (C). S 200=sarilumab 200 mg. S 400=sarilumab 400 mg.
In addition, no significant differences were observed in the overall proportions of patients alive at day 29 (92% [77 of 84] in the placebo group; 90% [143 of 159] in the sarilumab 200 mg group; and 92% [159 of 173] in the sarilumab 400 mg group; table 2 ).
Table 2.
Summary of endpoints according to disease severity at day 29
|
All patients* |
Severe disease† |
Critical disease† |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo (n=84) | Sarilumab 200 mg (n=159) | Sarilumab 400 mg (n=173) | Placebo (n=55) | Sarilumab 200 mg (n=92) | Sarilumab 400 mg (n=105) | Placebo (n=29) | Sarilumab 200 mg (n=65) | Sarilumab 400 mg (n=68) | |
| Time to ≥2-point improvement on seven-point clinical status scale‡ | |||||||||
| Median Kaplan-Meier estimates, days§ | 12·0 (9·0 to 15·0) | 10·0 (9·0 to 12·0) | 10·0 (9·0 to 13·0) | 12·0 (9·0 to 14·0) | 9·0 (9·0 to 10·0) | 9·0 (8·0 to 10·0) | 15·0 (8·0 to 25·0) | 12·0 (9·0 to 19·0) | 13·0 (11·0 to 16·0) |
| p value | NA | 0·96 | 0·34 | NA | 0·59 | 0·62 | NA | 0·70 | 0·53 |
| Hazard ratio vs placebo | NA | 1·03 (0·75 to 1·40) | 1·14 (0·84 to 1·54) | NA | 1·11 (0·77 to 1·61) | 1·10 (0·77 to 1·59) | NA | 0·96 (0·53 to 1·72) | 1·13 (0·64 to 2.00) |
| Analysis of proportion of patients alive at day 29 | |||||||||
| Patients alive at day 29¶ | 77 (92%) | 143 (90%) | 159 (92%) | 54 (98%) | 87 (95%) | 99 (94%) | 23 (79%) | 55 (85%) | 60 (88%) |
| Difference vs placebo‖ | NA | −1·7 (−9·3 to 5·8) | 0·2 (−6·9 to 7·4) | NA | −3·6 (−9·4 to 2·2) | −3·9 (−9·6 to 1·8) | NA | 5·3 (−11·8 to 22·5) | 8·9 (−7·7 to 25·5) |
| p value vs placebo | NA | 0·63 | 0·85 | NA | 0·27 | 0·26 | NA | 0·60 | 0·25 |
Data are median (95% CI), hazard ratio (95% CI), n (%), or difference (95% CI), unless specified. Data are for the modified intention-to-treat population. NA=not applicable. ICU=intensive care unit.
Analyses for all patients were stratified by severity of illness (severe, critical) and use of systemic corticosteroids as entered in interactive response technology. Includes two patients in the 200 mg group stratified to the multisystem organ dysfunction category who are not included in the severe or critical disease columns.
For analyses of severe and critical disease, the category was based on severity entered by the investigator in the electronic clinical research form and the stratification factor for disease severity was removed from the model.
Patients without improvement were censored at the last observation timepoint; patients who took rescue medication in the study without previous improvement were censored at rescue medication start date; patients who died were categorised as no improvement, starting from death date.
Two-sided 95% CI was computed using the Brookmeyer and Crowley method (log-log transformation).
One death in the sarilumab 200 mg group was included in the all patients summary but not in the severe or critical categories, as the patient had multiorgan failure.
Based on asymptomatic confidence limits.
The proportions of patients discharged due to recovery by day 29 were 83% (70 of 84) in the placebo group, 79% (126 of 159) in the sarilumab 200 mg group, and 79% (137 of 173) in the sarilumab 400 mg group, and the percentages of patients alive at day 60 were 89% (75 of 84) in the placebo group, 89% (142 of 159) in the sarilumab 200 mg group, and 90% (155 of 173) in the sarilumab 400 mg group (appendix p 7). Additional secondary endpoints related to fever, oxygenation, and hospital status are shown in the appendix (p 7).
Prespecified analysis of day 29 data showed no significant difference in survival between sarilumab 400 mg (n=60 [88%]) and placebo (n=23 [79%]; difference +8·9% [95% CI −7·7 to 25·5]; p=0·25) for the patients with critical disease (table 2). Kaplan-Meier curves by disease severity for the primary endpoint, survival, and discharge due to recovery (appendix p 11) and Forest plots of selected subgroups for time to improvement of two points and percentage of patients alive at day 29 are shown in the appendix (pp 12–13).
In patients with either severe or critical disease, differences between sarilumab 400 mg and placebo on the seven-point clinical status scale were greater during the first 2 weeks of treatment than during the second 2 weeks (figure 2). The time–concentration plot of sarilumab concentration after intravenous infusion showed an initially rapid decline over the first 4 days and a slower decline from day 7 onward, with nearly complete elimination by day 21 even among patients who received two doses of 400 mg sarilumab within the first 48 h (appendix p 14).
Changes in C-reactive protein (CRP) and neutrophil counts were considered pharmacodynamic markers of systemic IL-6 signalling inhibition. The decline in mean CRP was steeper in the sarilumab groups than in the placebo group, with a rebound at day 7 in the 200 mg group and day 15 in the 400 mg group (appendix p 14). As expected with IL-6 receptor inhibition, neutrophil counts were decreased in the sarilumab groups and lower for a longer period of time with the 400 mg dose than the 200 mg dose, but again appeared to increase after day 4 in the 200 mg group and after day 15 in the 400 mg group. In the placebo group, neutrophil counts were stable up until day 7 but were higher at day 15 (appendix p 14). Neutrophil to lymphocyte ratios and D-dimer concentrations did not appear to be influenced by sarilumab concentration, and mean ALT elevation only appeared higher than placebo in the sarilumab groups at day 7 (appendix p 14). The time–concentration plots for IL-6 and soluble IL-6 receptor (appendix p 14) were consistent with what has been previously reported for sarilumab after subcutaneous injection.17 Mean soluble IL-6 receptor concentration in the placebo group remained low (<100 ng/mL) up to day 29.
The rates of treatment-emergent adverse events, infections (including serious infections), and treatment-emergent adverse events leading to death were similar among the treatment groups (table 3 ). In patients given sarilumab, the types of adverse events of special interest were generally consistent with the established safety profile of sarilumab and the intravenous route of administration and occurred more frequently than in the placebo group. Overall, 11% (44 of 416) of patients died due to a a treatment-emergent adverse event, with similar rates between treatment groups (placebo [11%]; sarilumab 200 mg [11%], sarilumab 400 mg [10%]; table 3).
Table 3.
Summary of adverse events in patients with more than one adverse event
| Placebo (n=84) | Sarilumab 200 mg (n=159) | Sarilumab 400 mg (n=173) | ||
|---|---|---|---|---|
| Any treatment-emergent adverse event | 55 (65%) | 103 (65%) | 121 (70%) | |
| Any serious treatment-emergent adverse event | 20 (24%) | 42 (26%) | 51 (29%) | |
| Any serious infection | 10 (12%) | 18 (11%) | 22 (13%) | |
| Pneumonia | 0 | 1 (1%) | 6 (3%) | |
| COVID-19 pneumonia | 2 (2%) | 11 (7%) | 4 (2%) | |
| Bacterial pneumonia | 1 (1%) | 1 (1%) | 3 (2%) | |
| Any treatment-emergent adverse event leading to death | 9 (11%) | 17 (11%) | 18 (10%) | |
| Any adverse event of special interest | 18 (21%) | 53 (33%) | 76 (44%) | |
| Alanine aminotransferase increase | 16 (19%) | 48 (30%) | 55 (32%) | |
| Invasive bacterial or fungal infection | 3 (4%) | 8 (5%) | 15 (9%) | |
| Grade ≥2 hypersensitivity reaction | 0 | 1 (1%) | 7 (4%) | |
| Grade 4 neutropenia | 0 | 3 (2%) | 6 (3%) | |
| Grade ≥2 infusion-related reaction | 0 | 1 (1%) | 6 (3%) | |
Data are n (%).
Because standards of care for patients admitted to hospital with COVID-19 evolved over the course of the trial, in a post-hoc analysis, the proportions of patients initiating or continuing selected medications were plotted by week of study conduct and treatment group (appendix p 15). Use of systemic corticosteroids appeared to wane during the first 6 weeks of study conduct, then increased to a peak usage in 70% (81 of 116) of patients 13 weeks after the first randomly assigned patient started receiving a corticosteroid (appendix p 15). This uptick in corticosteroid usage coincided with increased enrolment of patients with critical disease. Over the course of the study, initiation of systemic corticosteroids did not appear to be related to treatment group. Use of antiviral medications, hydroxychloroquine or chloroquine, and any combinations (appendix pp 15–16) declined over the course of the trial. For the medications of interest, changes in background therapy appeared balanced across the treatment groups. In subgroup analyses (data not shown), no significant interactions between use of systemic corticosteroids, antiviral medications, antibiotic medications, or hydroxychloroquine or chloroquine and time to clinical improvement of two or more points or survival at day 29 were identified. Only two patients (one in each of the sarilumab groups) were given remdesivir or convalescent plasma during the trial.
Discussion
In this multinational, randomised, placebo-controlled trial of patients with severe or critical COVID-19 who were receiving the local standard of care, there was no observed benefit of intravenous sarilumab over placebo. The treatment groups had similar rates of serious infections and adverse events leading to death, and types of adverse events were consistent with previous clinical trial data for sarilumab.10 No new safety signals for sarilumab were observed in these patients with COVID-19.
We suggest several potential reasons for why sarilumab was not effective as a treatment for COVID-19 in this clinical trial. First, IL-6 suppression alone might be insufficient to quell the inflammatory phase of the disease.18 Open-label studies in patients with COVID-19 have suggested clinical improvement with tocilizumab, another IL-6 inhibitor.17, 19, 20 However, in other published randomised trials, which also included patients admitted to hospital with COVID-19 pneumonia, tocilizumab did not reduce disease severity at day 4 or mortality at day 28,21 clinical worsening at day 14,22 or time to intubation or death.22
Second, we did not select patients on the basis of commonly available biological and clinical markers of inflammation (eg, elevated CRP) or worsening prognosis (eg, neutrophil counts or uncontrollable fever); consequently, we might not have included a sufficient number of patients for whom immunomodulatory therapy would have been appropriate. Additionally, we might not have chosen an optimal time in the disease course of COVID-19 to administer sarilumab.
Third, immunomodulation might only be beneficial for the most serious cases of COVID-19. Results of a large, open-label, controlled trial of dexamethasone (n=2104) versus usual care (n=4321) for patients admitted to hospital with COVID-19 suggested that the magnitude of survival benefit is related to intensity of respiratory support.23 In our study, a numerical difference in survival favouring sarilumab was only seen in the patients who required intensive respiratory support (oxygen by non-rebreather mask or high-flow nasal cannula, use of invasive or non-invasive ventilation), or treatment in an intensive care unit. The differences in treatment response between patients with severe disease and critical disease might be qualitatively reflected in the different evolution of clinical status over the course of the trial—ie, earlier improvement in the sarilumab groups among patients who were severely ill up to day 15, and greater proportions of patients surviving after day 15 among patients who were critically ill (figure 3). Kaplan-Meier time-to-event curves up to day 60 also suggested a faster improvement and earlier discharge due to improvement in the sarilumab groups than the placebo group among patients with severe disease; and higher probability of survival among patients given sarilumab than those given placebo in those who were critically ill (table 2, appendix p 11). EMPACTA, a randomised, placebo-controlled study of tocilizumab in 389 patients with COVID-19 not receiving mechanical ventilation, showed a reduced likelihood of progression to mechanical ventilation or death but not a robust effect on survival.24 A preliminary report of REMAP-CAP, an ongoing, multifactorial, adaptive-platform trial (n=803), which enrolled a more critically ill population than EMPACTA (patients who required oxygen by high-flow nasal cannula, non-invasive or invasive mechanical ventilation, or vasopressor cardiovascular support), suggests that sarilumab and tocilizumab increased the number of respiratory or cardiovascular organ support-free days and improved the odds of hospital survival.25
Fourth, frequent use of systemic corticosteroids might have reduced the differences between the investigational treatment and the placebo control groups. More than 60% of patients in the trial received at least one dose of systemic corticosteroids before, during, or after infusion of the study medication and the frequency of systemic corticosteroid use varied during the study.
Fifth, considering the limited amount of clinical data available to estimate the efficacy of usual care for patients admitted to hospital with COVID-19, this study might have been underpowered, as suggested by trial results disclosed after selection of the phase 3 endpoints.26, 27
Sixth, a single intravenous administration of sarilumab 400 mg might be insufficient to control the inflammatory phase of COVID-19 beyond 14 days, as suggested by the reduction in sarilumab concentration between day 7 and day 14 and subsequent rebound in CRP concentration and neutrophil counts after day 15. Alternatively, the intravenous route of administration, although theoretically advantageous, might not have resulted in a time–concentration profile suited for COVID-19.
Lastly, the efficacy endpoints chosen might have been insufficiently sensitive for the wide range of disease severity studied in this trial. Additionally, an ordinal clinical status scale based on intensity of respiratory support could be too crude to measure treatment effects in patients with an acute systemic disease involving multiple organ systems.
Despite these limitations, survival at day 29 was possibly higher by 9% in the sarilumab groups than the placebo group for patients who required non-invasive or invasive mechanical ventilation or extracorporeal membrane oxygenation at baseline, which is not inconsistent with survival reported for REMAP-CAP.25 Therefore, we think the results of this study do not exclude the possibility of a benefit from targeted immunomodulation in patients admitted to hospital with COVID-19 pneumonia with critical illness and suggest that subsequent randomised trials of targeted immunomodulatory therapies in this disease focus on patients who are critically ill and are adequately powered to assess survival as a primary endpoint.
For details see https://www.sanofi.com/en/science-and-innovation/clinical-trials-and-results/our-data-sharing-commitments
Data sharing
Qualified researchers can request access to patient-level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient-level data will be anonymised and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data sharing criteria, eligible studies, and process for requesting access can be found online.
Acknowledgments
Acknowledgments
The authors and Sanofi thank the patients for their participation in the trial, as well as the investigators. Special thanks to Meng Zhang, Christine Xu, and Evelyne Dombrecht of Sanofi for their expertise, dedication, and leadership of a remarkable team, and to the members of the independent data monitoring committee (Kevin Winthrop, Victor Ortega, Mitchell Levy, Steve Dahlberg), and the independent statistical data analysis center (Department of Biostatistics and Medical Informatics, University of Wisconsin–Madison, USA) for their diligence and equipoise under extraordinary circumstances. This study was funded by Sanofi (Paris, France) and Regeneron Pharmaceuticals (Tarrytown, USA). Medical writing assistance was provided by Richard J Hogan, and Vojislav Pejović of Eloquent Scientific Solutions, a division of Envision Pharma Group, and editorial and graphics assistance was provided by Eloquent Scientific Solutions. This support was funded by Sanofi.
Contributors
F-XL, JSL, GS, and OH take responsibility for the integrity of the data and the accuracy of the data analysis. JSL, GS, PW, and OH provided input on the trial design. F-XL, HH, and RAF were responsible for acquisition and interpretation of data. F-XL and OH drafted the manuscript. F-XL, HH, RAF, NP, and OH critically revised the manuscript. GS contributed to statistical analysis. F-XL, HH, RAF, JSL, and PW contributed to conducting the trial. All authors reviewed and approved the final version of the manuscript. All authors, including those affiliated with the study sponsor, contributed to writing the manuscript, and reviewed and approved the manuscript. The authors had full access to all data in the study and had final responsibility for the decision to submit for publication.
Declaration of interests
F-XL has received lecture fees from Merck Sharp & Dohme and Gilead Science. JSL, GS, PW, NP, and OH are employees of Sanofi and hold stock or stock options in the company. All other authors declare no competing interests.
Contributor Information
Sarilumab COVID-19 Global Study Group:
Ignacio J. Bazzalo, Marcelo M. Casas, Sebastián A. Nuñez, Yael Pere, Carlos M. Ibarrola, Marco A. Solis Aramayo, Maria C. Cuesta, Andrea E. Duarte, Pablo M. Gutierrez Fernandez, Maria A. Iannantuono, Erica A. Miyazaki, Javier P. Silvio, Dario G. Scublinsky, Alessandra Bales, Daniela Catarino, Elie Fiss, Sara Mohrbacher, Victor Sato, Antonio Baylao, Adilson Cavalcante, Francini Correa, Celso A. de Andrade, Juvencio Furtado, Nelson Ribeiro Filho, Valéria Telles, Leopoldo T. Trevelin, Ricardo Vipich, Rodrigo Boldo, Paula Borges, Suzana Lobo, Graziela Luckemeyer, Luana Machado, Maysa B. Alves, Ana C. Iglessias, Marianna M. Lago, Daniel W. Santos, Hugo Chapdelaine, Emilia L. Falcone, Rahima Jamal, Me-Linh Luong, Madeleine Durand, Stephane Doucet, François-Martin Carrier, Bryan A. Coburn, Lorenzo Del Sorbo, Sharon L. Walmsley, Sara Belga, Luke Y. Chen, Allison D. Mah, Theodore Steiner, Alissa J. Wright, J. Hajek, Neill Adhikari, Robert A. Fowler, Nick Daneman, Kosar A. Khwaja, Jason Shahin, Carolina Gonzalez, Rafael Silva, Marcelo Lindh, Gabriel Maluenda, Patricia Fernandez, Maite Oyonarte, Martin Lasso, Alexandre Boyer, Didier Bronnimann, Hoang-Nam Bui, Charles Cazanave, Helene Chaussade, Arnaud Desclaux, Mailys Ducours, Alexandre Duvignaud, Denis Malvy, Lisa Martin, Didier Neau, Duc Nguyen, Thierry Pistone, Gaetane Soubrane-Wirth, Julie Leitao, Clotilde Allavena, Charlotte Biron, Sabelline Bouchez, Benjamin Gaborit, Antoine Gregoire, Paul Le Turnier, Anne-Sophie Lecompte, Raphael Lecomte, Maeva Lefebvre, Francois Raffi, David Boutoille, Pascale H. Morineau, Romain Guéry, Emmanuel Chatelus, Nathalie Dumoussaud, Renaud Felten, Florina Luca, Bernard Goichot, Francis Schneider, Marie-Caroline Taquet, Matthieu Groh, Mathilde Roumier, Mathilde Neuville, Antoine Bachelard, Valentina Isernia, F-Xavier Lescure, Bao-Chau Phung, Anne Rachline, Aurelie Sautereau, Dorothee Vallois, Yves Bleher, Delphine Boucher, Clémentine Coudon, Jean Esnault, Thomas Guimard, Sophie Leautez-Nainville, Dominique Merrien, Marine Morrier, Pauline Motte-Vincent, Romain Gabeff, Hélène Leclerc, Céline Cozic, Romain Decours, Ronan Février, Gwenhael Colin, Sophie Abgrall, Dorothee Vignes, Raluca Sterpu, Mira Kuellmar, Melanie Meersch-Dini, Raphael Weiss, Alexander Zarbock, Christiane Antony, Marc Berger, Thorsten Brenner, Christian Taube, Frank Herbstreit, Sebastian Dolff, Margarethe Konik, Karsten Schmidt, Markus Zettler, Oliver Witzke, Boris Boell, Jorge Garcia Borrega, Philipp Koehler, Thomas Zander, Fabian Dusse, Othman Al-Sawaf, Philipp Köhler, Dennis Eichenauer, Matthias Kochanek, Alexander Shimabukuro-Vornhagen, Sibylle Mellinghoff, Annika Claßen, Jan-Michel Heger, Charlotte Meyer-Schwickerath, Paul Liedgens, Katrin Heindel, Ana Belkin, Asaf Biber, Mayan Gilboa, Itzchak Levy, Vladislav Litachevsky, Galia Rahav, Anat Finesod Wiedner, Tal Zilberman-Daniels, Yonatan Oster, Jacob Strahilevitz, Sigal Sviri, Elena M. Baldissera, Corrado Campochiaro, Giulio Cavalli, Lorenzo Dagna, Giacomo De Luca, Emanuel Della Torre, Alessandro Tomelleri, Davide Bernasconi De Luca, Amedeo F. Capetti, Massimo Coen, Maria V. Cossu, Massimo Galli, Andrea Giacomelli, Guido A. Gubertini, Stefano Rusconi, Giulia J. Burastero, Margherita Digaetano, Giovanni Guaraldi, Marianna Meschiari, Cristina Mussini, Cinzia Puzzolante, Sara Volpi, Marina Aiello, Alarico Ariani, Alfredo A. Chetta, Annalisa Frizzelli, Andrea Ticinesi, Domenico Tuttolomondo, Stefano Aliberti, Francesco B. Blasi, Marta F. Di Pasquale, Sofia Misuraca, Tommaso Pilocane, Edoardo Simonetta, Alessio M. Aghelmo, Claudio Angelini, Enrico Brunetta, Giorgio W. Canonica, Michele Ciccarelli, Sara Dal Farra, Maria De Santis, Sebastian Ferri, Marco Folci, Giacomo M. Guidelli, Enrico M. Heffler, Ferdinando Loiacono, Giacomo Malipiero, Giovanni Paoletti, Rosa Pedale, Francesca A. Puggioni, Francesca Racca, Aurora Zumbo, Morihiko Satou, Hitoshi Honda, Tatyana Lisun, Denis Protsenko, Nikolay Rubtsov, Irina Beloglazova, Daria Fomina, Mariana Lysenko, Sofia Serdotetskova, Vitali Firstov, Ivan Gordeev, Ilia Kokorin, Ksenia Komissarova, Nina Lapochkina, Elena Luchinkina, Valentin Malimon, Sevinch Mamedguseyinova, Ksenia Polubatonova, Natalia Suvorova, Jose Arribas, Alberto M. Borobia Perez, Fernando de la Calle Prieto, Juan Carlos Figueira, Rocio Motejano Sanchez, Marta Mora-Rillo, Concepcion Prados Sanchez, Javier Queiruga Parada, Francisco Fernandez Arnalich, Maria Guerro Barrientos, Alejandro Bendala Estrada, Aranzazu Caballero Marcos, Maria E. Garcia Leoni, Rita García-Martínez, Ana María Collado, Patricia Munoz Garcia, Ana Torres do Rego, María V. Villalba García, Almudena Burrillo, Maricela Valerio Minero, Paloma Gijon Vidaurreta, Sonsoles Infante Herrero, Elena Velilla, Marina Machado, Maria Olmedo, Blanca Pinilla, Benito Almirante Gragera, Maria de la Esperanza Cañas Ruano, Sofia Contreras Medina, Alejandro Cortés Herrera, Vicenç Falcó Ferrer, Ricard Ferrer Roca, Xavier Nuvials Casals, Esteve Ribera Pascuet, Paula Suanzes Diez, Pedro Rebollo Castro, Felipe Garcia Alcaide, Alejandro Soriano, Aina Oliver Caldes, Ana González Cordón, Celia Cardozo, Lorena De la Mora Cañizo, Romina Pena López, Sandra Chamorro, Clara Crespillo-Andujar, Rosa Escudero Sanchez, Jesús Fortún-Abete, Begoña Monge-Maillo, Ana Moreno Zamora, Francesca Norman, Matilde Sanchez Conde, Sergio Serrano Villar, and Pilar Vizcarra
Supplementary Material
References
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Z, Deng H, Ou C, et al. Clinical symptoms, comorbidities and complications in severe and non-severe patients with COVID-19: a systematic review and meta-analysis without cases duplication. Medicine. 2020;99 doi: 10.1097/MD.0000000000023327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robba C, Battaglini D, Pelosi P, Rocco PRM. Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Expert Rev Respir Med. 2020;14:865–868. doi: 10.1080/17476348.2020.1778470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kox M, Waalders NJB, Kooistra EJ, Gerretsen J, Pickkers P. Cytokine levels in critically ill patients with COVID-19 and other conditions. JAMA. 2020;324 doi: 10.1001/jama.2020.17052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang ZL, Hou YL, Li DT, Li FZ. Laboratory findings of COVID-19: a systematic review and meta-analysis. Scand J Clin Lab Invest. 2020;80:441–447. doi: 10.1080/00365513.2020.1768587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu J, Pang J, Ji P, et al. Elevated interleukin-6 is associated with severity of COVID-19: a meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.26085. published online May 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian W, Jiang W, Yao J, et al. Predictors of mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. J Med Virol. 2020;92:1875–1883. doi: 10.1002/jmv.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeng F, Huang Y, Guo Y, et al. Association of inflammatory markers with the severity of COVID-19: a meta-analysis. Int J Infect Dis. 2020;96:467–474. doi: 10.1016/j.ijid.2020.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.KEVZARA (sarilumab) Summary of Product Characteristics Paris, France. 2017. https://www.ema.europa.eu/en/documents/product-information/kevzara-epar-product-information_en.pdf
- 11.KEVZARA (sarilumab) Prescribing Information Bridgewater, NJ. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761037s000lbl.pdf
- 12.Raimondo MG, Biggioggero M, Crotti C, Becciolini A, Favalli EG. Profile of sarilumab and its potential in the treatment of rheumatoid arthritis. Drug Des Devel Ther. 2017;11:1593–1603. doi: 10.2147/DDDT.S100302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirano T, Murakami M. COVID-19: A new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020;52:731–733. doi: 10.1016/j.immuni.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams B. The National Early Warning Score 2 (NEWS2) in patients with hypercapnic respiratory failure. Clin Med. 2019;19:94–95. doi: 10.7861/clinmedicine.19-1-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calabrese C, Rajendram P, Sacha GL, Calabrese L. Practical aspects of targeting IL-6 in COVID-19 disease. Cleve Clin J Med. 2020 doi: 10.3949/ccjm.87a.ccc018. published online October 7. [DOI] [PubMed] [Google Scholar]
- 19.Gupta S, Wang W, Hayek SS, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2021;181:41–51. doi: 10.1001/jamainternmed.2020.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toniati P, Piva S, Cattalini M, et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020;19 doi: 10.1016/j.autrev.2020.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hermine O, Mariette X, Tharaux PL, et al. Effect of tocilizumab vs usual care in adults hospitalized with Covid-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:32–40. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salvarani C, Dolci G, Massari M, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with covid-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:24–31. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. published online July 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;384:20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19 – preliminary report. medRxiv. 2021 doi: 10.1101/2021.01.07.21249390. published online Jan 7. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers can request access to patient-level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient-level data will be anonymised and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data sharing criteria, eligible studies, and process for requesting access can be found online.