ABSTRACT
Background: Posttraumatic stress disorder (PTSD) is characterized by impairments in extinction learning and social behaviour, which are targeted by trauma-focused cognitive behavioural treatment (TF-CBT). The biological underpinnings of TF-CBT can be better understood by adding biomarkers to the clinical evaluation of interventions. Due to their involvement in social functioning and fear processing, oxytocin and arginine vasopressin might be informative biomarkers for TF-CBT, but to date, this has never been tested.
Objective: To differentiate the impact of traumatic event exposure and PTSD symptoms on blood oxytocin and vasopressin concentrations. Further, to describe courses of PTSD symptoms, oxytocin and vasopressin during an internet-based TF-CBT and explore interactions between these parameters.
Method: We compared oxytocin and vasopressin between three groups of active and former male service members of the German Armed Forces (n = 100): PTSD patients (n = 39), deployed healthy controls who experienced a deployment-related traumatic event (n = 33) and non-deployed healthy controls who never experienced a traumatic event (n = 28). PTSD patients underwent a 5-week internet-based TF-CBT. We correlated PTSD symptoms with oxytocin and vasopressin before treatment onset. Further, we analysed courses of PTSD symptoms, oxytocin and vasopressin from pre- to post-treatment and 3 months follow-up, as well as interactions between the three parameters.
Results: Oxytocin and vasopressin did not differ between the groups and were unrelated to PTSD symptoms. PTSD symptoms were highly stable over time, whereas the endocrine parameters were not, and they also did not change in mean. Oxytocin and vasopressin were not associated with PTSD symptoms longitudinally.
Conclusions: Mainly due to their insufficient intraindividual stability, single measurements of endogenous oxytocin and vasopressin concentrations are not informative biomarkers for TF-CBT. We discuss how the stability of these biomarkers might be increased and how they could be better related to the specific impairments targeted by TF-CBT.
KEYWORDS: Psychotherapy, cognitive behavioural therapy, neuropeptide, online intervention, soldiers, military
HIGHLIGHTS
To understand better why trauma-focused psychotherapy is effective, biomarkers can be added to intervention studies.
We tested whether the hormones oxytocin and vasopressin are informative biomarkers.
We found out that they are not, as they change too quickly within individuals.
Short abstract
Antecedentes: El trastorno de estrés postraumático (TEPT) se caracteriza por deficiencias en el aprendizaje de extinción y el comportamiento social, que son el objetivo del tratamiento cognitivo conductual centrado en el trauma (TF-CBT). Los fundamentos biológicos de TF-CBT se pueden entender mejor agregando biomarcadores a la evaluación clínica de las intervenciones. Debido a su participación en el funcionamiento social y el procesamiento del miedo, la oxitocina y la arginina vasopresina podrían ser biomarcadores informativos para la TF-CBT, pero hasta la fecha, esto nunca se ha probado.
Objetivo: Diferenciar el impacto de la exposición a un evento traumático y los síntomas del TEPT en las concentraciones de oxitocina y vasopresina en la sangre. Además, para describir la evolución de los síntomas del TEPT, la oxitocina y la vasopresina durante una TF-CBT basada en Internet y explorar las interacciones entre estos parámetros.
Método: Comparamos la oxitocina y la vasopresina entre tres grupos de militares activos y ex militares de las Fuerzas Armadas Alemanas (n = 100): pacientes con TEPT (n = 39), controles sanos desplegados que experimentaron un evento traumático relacionado con el despliegue (n = 33) y controles sanos no desplegados que nunca experimentaron un evento traumático (n = 28). Los pacientes con TEPT se sometieron a una TF-CBT basada en Internet durante 5 semanas. Correlacionamos los síntomas del TEPT con la oxitocina y la vasopresina antes del inicio del tratamiento. Además, analizamos la evolución de los síntomas del TEPT, la oxitocina y la vasopresina antes y después del tratamiento y el seguimiento de 3 meses, así como las interacciones entre los tres parámetros.
Resultados: La oxitocina y la vasopresina no difirieron entre los grupos y no se relacionaron con los síntomas del TEPT. Los síntomas del TEPT fueron muy estables en el tiempo, mientras que los parámetros endocrinos no lo fueron, y tampoco cambiaron en la media. La oxitocina y la vasopresina no se asociaron con los síntomas del TEPT de forma longitudinal.
Conclusiones: Principalmente debido a su estabilidad intraindividual insuficiente, las mediciones únicas de las concentraciones de oxitocina y vasopresina endógenas no son biomarcadores informativos para TF-CBT. Discutimos cómo podría aumentarse la estabilidad de estos biomarcadores y cómo podrían relacionarse mejor con las deficiencias específicas a las que se dirige TF-CBT.
Palabras clave: Psicoterapia, Terapia cognitivo-conductual, Neuropéptido, Intervención online, Soldados, Militar
Abstract
背景:创伤后应激障碍(PTSD)的特征是消退学习和社交行为受到损伤,这是聚焦创伤认知行为治疗(TF-CBT)的目标。通过将生物标志物添加到干预措施的临床评估中,可以更好地理解 TF-CBT 的生物学基础。由于催产素和精氨酸加压素可能参与社交功能和恐惧处理,因此它们可能是 TF-CBT 的信息生物标志物,但至今尚未检验。
目的:区分创伤事件暴露和 PTSD 症状对血液中催产素和加压素浓度的影响。此外,为了描述基于互联网的 TF-CBT 期间 PTSD 症状、催产素和加压素的过程,并探讨这些参数之间的相互作用。
方法:我们比较了德国武装部队三组现役和前役男性军人中的催产素和加压素(n = 100) : PTSD患者(n = 39)、经历了部署相关创伤事件的已部署健康对照者(n = 33)以及从未经历过创伤事件的未部署健康对照组(n = 28)。 PTSD 患者接受了为期5周基于互联网的 TF-CBT。在治疗开始之前,我们确定了 PTSD 症状与催产素和加压素的相关。此外,我们分析了从治疗前、后以及随访 3 个月时的 PTSD 症状、催产素和加压素的过程,以及这三个参数之间的相互作用。
结果:组间催产素和加压素没有差异,并且与 PTSD 症状无关。 PTSD 症状随时间高度稳定,而内分泌参数不稳定,其平均值也没有变化。催产素和加压素在纵向上与 PTSD 症状无关。
结论:主要由于个体内稳定性不足,对内源性催产素和加压素浓度的单次测量并不是 TF-CBT 的信息生物标志物。我们讨论了如何增加这些生物标记的稳定性,以及如何能将它们与 TF-CBT 靶向的特定损伤更好地联系起来。
关键词: 心理治疗, 认知行为疗法, 神经肽, 在线干预, 士兵,军人
1. Introduction
Posttraumatic stress disorder (PTSD) is a burdensome disease that causes severe impairments in individuals’ lives. PTSD can develop in response to a traumatic event, that is, exposure to actual or threatened death, serious injury or sexual violence, and includes symptoms such as intrusions, avoidance, negative alterations in cognition and mood, as well as marked alterations in arousal and reactivity (American Psychiatric Association et al., 2013). Globally, PTSD lifetime prevalence rates range from 1.3 to 8.9%, depending on country-specific, but also individual risk factors (Atwoli, Stein, Koenen, & McLaughlin, 2015). Among the latter, belonging to professional groups characterized by frequent and severe traumatic event exposure, such as rescue workers (Berger et al., 2012), war reporters (Feinstein, Owen, & Blair, 2002) or soldiers (Hoge et al., 2004; Wittchen et al., 2012) increases PTSD risk. Furthermore, social factors, such as exposure to an interpersonal traumatic event (Shalev et al., 2019), particularly sexual (Schumm, Briggs-Phillips, & Hobfoll, 2006; Tolin & Foa, 2006) or intimate partner violence (Forbes et al., 2014), as well as low perceived social support after traumatic event exposure (Andrews, Brewin, & Rose, 2003; Brewin, Andrews, & Valentine, 2000; Schumm, Briggs-Phillips, & Hobfoll, 2006; Ozer, Best, Lipsey, & Weiss, 2003) increase individuals’ risk to develop PTSD.
From a neurocognitive perspective, PTSD development and maintenance can be explained by deficient extinction learning (for overviews, see Parsons & Ressler, 2013; Zuj, Palmer, Lommen, & Felmingham, 2016). Fear learning represents a normal process after traumatic event exposure that can be regarded as adaptive. Learning that certain cues or contexts that co-occurred with a traumatic event might indicate danger helps individuals to avoid dangerous situations in the future. However, subsequent impaired extinction learning can be regarded as a maladaptive process after traumatic event exposure, as fear responses to trauma reminders are maintained even after repeatedly experiencing that the reminders no longer co-occur with the anticipated event (Zuj & Norrholm, 2019). Several studies reported deficient extinction learning in PTSD, as reflected in increased general and differential fear responses during extinction acquisition (Orr et al., 2000; Peri, Ben-Shakhar, Orr, & Shalev, 2000; Steiger, Nees, Wicking, Lang, & Flor, 2015; Wessa & Flor, 2007), as well as impaired extinction recall (Garfinkel et al., 2014; Milad et al., 2008, 2009).
Extinction learning is promoted in trauma-focused cognitive behavioural therapy (TF-CBT), which is one of the gold standard PTSD treatments (National Institute of Clinical Excellence, 2018). TF-CBT encompasses programmes such as cognitive processing therapy (; Monson et al., 2006; Resick & Schnicke, 1993), (prolonged) exposure therapy (Foa et al., 2005; Foa, Rothbaum, Riggs, & Murdock, 1991) or narrative exposure therapy (Hermenau et al., 2013; Neuner et al., 2004). They aim at promoting the adaptive processing of traumatic memories which is, among other techniques, achieved by psychotherapist-guided exposure. During exposure, PTSD patients repeatedly confront themselves with their traumatic memories by describing their sensory perceptions, behaviours, emotions, physiological responses and cognitions during the traumatic event, in a safe environment and supported by their psychotherapist. Over several exposure sessions, analogous to extinction learning, their initially strong, negative emotional response to the traumatic memories decreases. In recent years, technological advances made it possible to deliver TF-CBT via the internet. This helps to overcome emotional, social and practical barriers to care (Hoge et al., 2004, 2014) and thereby, to reach an increasing number of individuals who would otherwise not commit to psychotherapy. Meta-analytic research has proven that internet-based and face-to-face TF-CBTs are equally effective (Kuester, Niemeyer, & Knaevelsrud, 2016).
Given that effective treatments for PTSD exist and innovative methods to disseminate them have been developed, it is a current challenge to better understand the exact mechanisms of action underlying TF-CBT. By identifying its active ingredients, its efficacy and effectiveness can be further improved, accelerating remission and increasing response rates (Stojek, McSweeney, & Rauch, 2018). Therefore, it has been suggested to add measurements of biological correlates of behaviours, cognitions or emotions of interest to clinical evaluations of TF-CBT (Fischer & Ehlert, 2019; Yehuda et al., 2006). In this regard, two neuroanatomically closely related neuropeptides and hormones, that is, oxytocin and arginine vasopressin, seem particularly interesting. Oxytocin has previously been related to a range of positive social behaviours in healthy humans, such as caring parenting behaviours (Feldman et al., 2011, 2012), trust (Kosfeld et al., 2005; Mikolajczak et al., 2010), cooperative communication (Ditzen et al., 2009), or interpersonal closeness (Riem et al., 2019). In contrast, vasopressin has been associated with aggression (Coccaro, Kavoussi, Hauger, Cooper, & Ferris, 1998), decreased differentiation between neutral and threatening social stimuli (Thompson, Gupta, Miller, Mills, & Orr, 2004) and increased physiological responses to threatening social stimuli (Thompson, George, Walton, Orr, & Benson, 2006). Oxytocin’s and vasopressin’s involvement in social functioning already suggest their investigation as biomarkers of TF-CBT, as social factors contribute to PTSD development (Andrews, Brewin, & Rose, 2003; Brewin, Andrews, & Valentine, 2000; Schumm, Briggs-Phillips, & Hobfoll, 2006; Shalev et al., 2019; Ozer et al. 2003) and a positive therapeutic relationship is a crucial basis for successful TF-CBT (Cloitre, Stovall-McClough, Miranda, & Chemtob, 2004). Further, both neuropeptides influence fear and extinction learning, the neurocognitive processes underlying PTSD development and exposure therapy. In this regard, oxytocin has often been associated with anxiolytic effects, whereas vasopressin’s effects appear to be fear-promoting: In rats, central administration of synthetic oxytocin during fear conditioning decreased, whereas the same dose of vasopressin increased fear responses (Roozendaal et al., 1992). Further, post-conditioning central administration of oxytocin hexapeptide fragments decreased, while administration of vasopressin hexapeptide fragments increased fear recall (Stoehr, Cramer, & North, 1992). In healthy humans, intranasal administration of oxytocin decreased fear responses during extinction acquisition and recall (Acheson et al., 2013; Eckstein et al., 2015), whereas animal studies indicated that high doses of centrally administered vasopressin delayed this process (Hayes & Chambers, 2005). However, it is worth noting that oxytocin’s and vasopressin’s effects on social functioning and fear processing are not uniform, but instead strongly modulated by contextual and individual factors (Roozendaal et al., 1992; Toth, Neumann, & Slattery, 2012; Olff et al., 2013).
Regarding the investigation of these neuropeptides in research on PTSD treatments, evidence is still scarce. Some studies evaluated the effects of a single intranasal oxytocin administration on selected functions that are impaired in PTSD (e.g. Koch et al., 2019; Nawijn et al., 2016, 2017; Sack et al., 2017). One clinical trial investigated the effects of repeated intranasal oxytocin administration in individuals with recent traumatic event exposure (van Zuiden et al., 2017) and a follow-up investigation of this trial also considered the role of blood oxytocin concentrations (Engel et al., 2020). Another clinical trial evaluated the effects of intranasal oxytocin as potential TF-CBT enhancer (Flanagan, Sippel, Wahlquist, Moran-Santa Maria, & Back, 2018). A follow-up investigation of this trial also investigated the role of blood oxytocin concentrations with regard to this intervention (Sippel, King, Wahlquist, & Flanagan, 2020). The present study is the first one that included measurements of both, oxytocin and vasopressin into the clinical evaluation of TF-CBT.
1.1. Objectives
Specifically, this study aimed at answering three main scientific questions:
First, we aimed at differentiating the impact of traumatic event exposure and PTSD on endogenous oxytocin and vasopressin concentrations. Therefore, we cross-sectionally compared oxytocin and vasopressin between PTSD patients, healthy controls who were exposed to a deployment-related traumatic event and healthy controls who were never deployed. Previous evidence points towards pro-social and fear-reducing effects of oxytocin and indicates contrary effects of vasopressin. Therefore, is seems possible to expect higher oxytocin concentrations in individuals with fewer traumatic event-related strain, namely, the highest concentrations in the healthy controls who were never deployed, lower concentrations in the healthy controls who were exposed to a deployment-related traumatic event and the lowest concentrations in PTSD patients. Regarding vasopressin concentrations, the opposite pattern can be expected.
Second, we aimed at exploring the associations of oxytocin and vasopressin with the severity of PTSD symptoms. Therefore, we tested whether these parameters were correlated in PTSD patients before onset of a 5-week, internet-based TF-CBT.
Third, we aimed at describing courses and interactions between oxytocin, vasopressin and PTSD symptoms during an online psychotherapeutic intervention. Therefore, we examined courses of these three parameters from pre- to post-treatment and 3 months follow-up. We were particularly interested to explore whether oxytocin and vasopressin interacted with PTSD symptoms over time.
2. Methods and materials
2.1. Participants
Participants were active and former service members of the German Armed Forces (n = 100). We initially intended to recruit female and male service members. However, given the significantly lower proportion of women in the German Armed Forces (approximately 12%), which is even lower among deployed service members, it became evident that it was not possible to recruit a number of women that was sufficiently high for the purpose of this investigation, especially as additional factors such as menstrual cycle phase need to be considered in endocrine studies in women. Therefore, all participants in this study were male. Three groups were investigated: PTSD patients (n = 39; M = 37.74 years; SD = 9.62 years), deployed healthy controls (n = 33; M = 38.36 years; SD = 7.98 years) and non-deployed healthy controls (n = 28; M = 26.36 years; SD = 4.30 years). The group of PTSD patients consisted of service members who were treatment seeking for PTSD. Both healthy control groups did not fulfill criteria for PTSD or any other mental disorder, as confirmed by the German version of the Mini-International Neuropsychiatric Interview (Ackenheil et al., 1999; Sheehan et al., 1998). The first healthy control group consisted of service members who were deployed abroad and reported a deployment-related traumatic event according to the DSM-5 criterion (American Psychiatric Association et al., 2013), as confirmed in a telephone screening interview and by the List of the Mental Health Advisory Team (Zimmermann et al., 2014). The second healthy control group consisted of service members who were never deployed abroad and reported no traumatic event according to the DSM-5 criterion (American Psychiatric Association et al., 2013). General exclusion criteria that applied to all groups were acute psychosis, acute manic episode, current substance abuse or dependence, current suicidal ideation, neurological disorder, acute somatic disease, concurrent psychotherapeutic treatment, or unstable psychotropic medication.
Participants were recruited via advertisements in military journals, on websites and in online chat rooms for service members. Printed flyers and posters were distributed in a number of health service centres and German Armed Forces Military Hospitals. Moreover, unit commanders distributed flyers in after-deployment seminars and the study was introduced to military psychologists and psychiatrists at German Armed Forces mental health conferences.
2.2. Study design
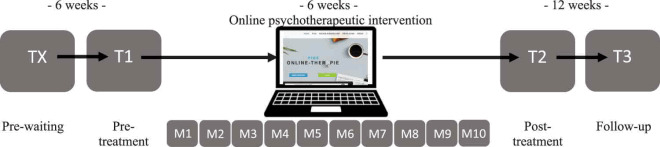
This study is part of a randomized waitlist-controlled trial investigating the feasibility, acceptability and efficacy of a 5-week internet-based TF-CBT in German Armed Forces service members (Niemeyer et al., 2020). The study design is explained and illustrated in Table 1. The intervention was based on the treatment protocols of Interapy (Lange et al., 2003) and Integrative Testimonial Therapy (Knaevelsrud, Böttche, Pietrzak, Freyberger, & Kuwert, 2017) and adapted to the military context. The model treatment protocols have been proven effective in reducing PTSD symptoms in diverse samples with large effect sizes (Hedges’s g = 0.81; CI = 0.62–1.00], Kuester, Niemeyer, & Knaevelsrud, 2016). Our protocol encompassed 10 modules of writing assignments completed by the PTSD patients for which they received written feedback by the therapists. The contact between PTSD patients and therapists was limited to the 10 texts that the patients wrote in accordance with the writing assignments and the 8 feedback letters written by the therapists (no feedback was provided after sessions 2 and 5). The therapist feedback was based on standardized templates from the treatment manual which were adapted to the patient’s specific needs. The intervention was structured in three treatment phases: biographical reconstruction, exposure and cognitive restructuring. During biographical reconstruction, patients reflected on their previous life experiences from childhood up to the traumatic event. They described positive experiences, as well as difficult ones that they had successfully handled. Then, psychoeducational texts by the therapists prepared the patients for the exposure sessions in which patients repeatedly described the worst traumatic event they had experienced. They were instructed to write in the first person and the present tense as well as to describe the most painful aspects, emotions, and sensory impressions. The subsequent treatment phase of cognitive restructuring aimed at developing a new perspective on the traumatic event. Patients were instructed to reflect on feelings such as guilt and shame, to question dysfunctional patterns in their thoughts and behaviours, to correct unrealistic assumptions, to consider possible positive consequences of the traumatic event, and to plan how they wanted to deal with such things in the future. For an even more detailed description of the treatment manual (Niemeyer et al., 2020).
Table 1.
Overview of assessments, flow of participants and available data
 | ||||||||||||||
| Assessments | ||||||||||||||
| Deployed healthy controls | - |  |
- | - | ||||||||||
| Non-deployed healthy controls | - |  |
- | - | ||||||||||
| PTSD patients, waitlist condition |  |
 |
 |
 |
||||||||||
| PTSD patients, non-waitlist condition | - |  |
 |
 |
||||||||||
| Flow of participants | ||||||||||||||
| n (deployed healthy controls) | 0 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| n (non-deployed healthy controls) | 0 | 28 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| n (PTSD patients, waitlist condition) | 19 | 17 | 17 | 16 | 16 | 13 | 13 | 13 | 13 | 13 | 13 | 13 | 13 | 12 |
| n (PTSD patients, non-waitlist condition) | 0 | 20 | 14 | 13 | 12 | 9 | 9 | 9 | 8 | 8 | 8 | 8 | 8 | 7 |
| Available data | ||||||||||||||
| CAPS-5 | ||||||||||||||
| n (deployed healthy controls) | 0 | 33 | 0 | 0 | ||||||||||
| n (non-deployed healthy controls) | 0 | 0 | 0 | 0 | ||||||||||
| n (PTSD patients, waitlist condition) | 19 | 17 | 13 | 12 | ||||||||||
| n (PTSD patients, non-waitlist condition) | 0 | 20 | 8 | 7 | ||||||||||
| Oxytocin | ||||||||||||||
| n (deployed healthy controls) | 0 | 33 | 0 | 0 | ||||||||||
| n (non-deployed healthy controls) | 0 | 261 | 0 | 0 | ||||||||||
| n (PTSD patients, waitlist condition) | 19 | 17 | 122 | 112 | ||||||||||
| n (PTSD patients, non-waitlist condition) | 0 | 182,3 | 8 | 7 | ||||||||||
| Vasopressin | ||||||||||||||
| n (deployed healthy controls) | 0 | 33 | 0 | 0 | ||||||||||
| n (non-deployed healthy controls) | 0 | 28 | 0 | 0 | ||||||||||
| n (PTSD patients, waitlist condition) | 19 | 17 | 13 | 12 | ||||||||||
| n (PTSD patients, non-waitlist condition) | 0 | 193 | 8 | 7 | ||||||||||
Note. 1 Data from two participants were defined as outliers and therefore removed. 2 Data from one participant was defined as outlier and therefore removed. 3 Data from one participant is missing as assessment was cancelled before blood sampling. M = module; CAPS-5 = Clinician-Administered PTSD Scale for DSM-5;  = Assessment was conducted in the respective group. – = Assessment was not conducted in the respective group.
= Assessment was conducted in the respective group. – = Assessment was not conducted in the respective group.
The rows representing assessments show when and how often posttraumatic stress disorder (PTSD) symptoms, oxytocin and vasopressin were assessed in the respective groups. Deployed and non-deployed healthy controls were assessed at one timepoint and did not receive the internet-based trauma-focused cognitive behavioural treatment. PTSD patients who were randomly assigned to the waitlist condition were assessed at four timepoints: once before a 6-week waiting period (TX), as well as pre-treatment (T1), post-treatment (T2) and at follow-up (T3). PTSD patients who were randomly assigned to the non-waitlist condition were assessed at three timepoints: T1, T2 and T3. The rows representing flow of participants show the number of participants assessed per group and timepoint. They also depict study and therapy dropout. The rows representing available data show the number of available data for our outcomes of interest per group and timepoint. PTSD symptoms were not assessed in non-deployed healthy controls. For cross-sectional baseline comparisons, variables of participants’ respective first assessments were compared. TX was the first assessment for PTSD patients assigned to waitlist condition and T1 was the first assessment for PTSD patients assigned to non-waitlist condition and both healthy control groups. For longitudinal analyses, PTSD patients’ T1, T2 and T3 data were used. This implies that data from PTSD patients assigned to waitlist- and non-waitlist condition were combined.
Before assignment to the trial, PTSD patients were – based on a computer-generated randomization list – assigned to the waitlist or non-waitlist condition. Patients in the waitlist condition waited for six weeks before starting treatment, patients in the non-waitlist condition started treatment immediately. Patients in the waitlist condition completed four assessments at the German Armed Forces Military Hospital Berlin, in which psychological and biological data was collected: a pre-waiting, pre-treatment, post-treatment and follow-up assessment. Patients in the non-waitlist condition completed three assessments: pre-treatment, post-treatment and follow-up. Healthy controls completed one assessment.
In order to address our first scientific question, that is, cross-sectional group comparisons, data of each group’s respective first assessment was used. For the purpose of the second and third scientific question, that is, the investigation of interactions between PTSD symptoms and endocrine parameters in relation to the internet-based TF-CBT, PTSD patients’ pre-treatment, post-treatment and follow-up data was used. Data was collected between July 2016 and July 2018.
The study was pre-registered in the Australian Clinical Trials Registry (ACTRN 12,616,000,956,404). After internal approval by the German Armed Forces, the study was approved by the Ethics committee of Freie Universität Berlin (reference number: 85/2014; addendum: 116/2016).
2.3. Psychological assessments
The German translation of the Clinician Administered PTSD Scale for DSM-5 (CAPS-5; Weathers et al. 2018) was used to assess PTSD symptoms and diagnosis. The CAPS-5 measures PTSD symptoms in the four domains: re-experiencing symptoms, avoidance symptoms, negative alterations in cognition and mood and alterations in arousal and reactivity. By means of a standardized interview with the patient, a clinician (a trained master’s level or a PhD student) rated the severity of symptoms experienced during the last month on a 5-point scale ranging from 0 = not present to 4 = extreme.
2.4. Biological assessments
2.4.1. Sampling and biochemical analyses
Endogenous oxytocin and vasopressin concentrations were measured in blood. At 8 a.m. on the first day of assessment in the case of a single-day assessment or on the second day in the case of a two-days assessment, participants were invited to the laboratory. Across all samples, there were no significant differences in oxytocin (F1,142 = 0.00, p = .96) or vasopressin concentrations (F1,147 = 0.78, p = .09) between participants with single day versus two days assessments.
Time of blood sampling was recorded and we observed high compliance to the protocol: In 83.33%, blood was drawn exactly at 8 a.m. Deviations were M = 3.00 SD = 10.00 [0.00; 75.00] minutes. Patients were instructed not to eat, drink (except for water), consume caffeine or nicotine before sampling. Compliance to these instructions was reported by the patients in 91.67% of the samples (143 samples, eat), 96.79% (151 samples, drink), 89.10% (139 samples, caffeine) and 79.49% (124 samples, nicotine), respectively. Furthermore, age, body weight, body height, and leucocytes were assessed. In 96.15% of the samples (150 samples), leucocyte values were within the normal range, 1.92% (3 samples) were considered as too low, 0.61% (1 sample) as too high and for 1.21% (2 samples), leucocyte values were unavailable.
Blood was collected in 9.00 ml serum tubes (Sarstedt, Germany). After sampling, tubes were softly shaken, then put aside for 30 minutes, protected from light, in order to allow blood to plot. Then, tubes were centrifuged at 1000xg for 10 minutes and serum was pipetted into smaller, 1.50 ml tubes (Eppendorf, Germany). Samples were stored in a freezer at −80°C. After completion of data collection, all samples were sent to the cooperating laboratory. They were extracted and analysed using a highly sensitive and selective RIA (RIAgnosis, Regensburg, Germany), as described in (Landgraf, Neumann, Holsboer, & Pittman, 1995) and in (Landgraf & Neumann, 2004). All samples were measured within the same assay, in order to avoid inter-assay variability. Intra-assay variability was < 10%. The detection limit was 0.1–0.5 pg/ml for both oxytocin and vasopressin, depending on the age of the tracers. No sample was below the detection limit. There was no significant cross-reactivity with structurally related peptides including the ring hexapeptides and the terminal tripeptides of oxytocin and vasopressin.
2.4.2. Preparation of biological data
Visual inspection and descriptive statistics revealed that oxytocin data was not normally distributed (M = 4.50 pg/ml; SD = 1.88 [1.77; 13.90]; skewness: 2.76; curtosis: 9.93). Five outliers were identified, deviating more than 3 SD from M. Outlier removal and log-transformation (LG10(1+ oxytocin)) resulted in normally distributed data that was used for analyses (M = 0.71; SD = 0.09 [0.44; 1.04]; skewness: 0.19; curtosis: 0.55). With regard to vasopressin, visual inspection and descriptive statistics revealed normally distributed data and no outliers (M = 3.50 pg/ml; SD = 0.53 [2.22; 5.01]; skewness: 0.02; curtosis: −0.72). Accordingly, complete and non-transformed data was used for analyses.
2.5. Statistical analyses
2.5.1. Group differences in endocrine parameters
For cross-sectional group comparisons, we conducted two one-way ANOVAs, using group (PTSD patients vs. deployed healthy controls vs. non-deployed healthy controls) as factor and oxytocin and vasopressin as outcomes.
2.6. Correlations before internet-based trauma-focused cognitive behavioural treatment (TF-CBT) onset
We used Pearson’s coefficient r to correlate pre-treatment oxytocin, vasopressin and PTSD symptoms (total CAPS-5 scores).
2.6.1. Courses of endocrine parameters and PTSD symptoms
In order to describe courses of oxytocin, vasopressin and PTSD symptoms, we performed three repeated-measures ANOVAs from pre-treatment over post-treatment to follow-up assessments. Time was used as within-factor, while oxytocin, vasopressin and PTSD symptoms were used as the respective outcomes. As both PTSD patient groups (waitlist and non-waitlist condition) were collapsed for longitudinal analyses, there was no between-factor.
2.6.2. Interactions of endocrine parameters with PTSD symptoms over time
In order to explore interactions between oxytocin and vasopressin with PTSD symptoms over time, we tested whether pre-treatment values of one parameter were correlated with post-treatment values of another parameter. In addition, we tested whether post-treatment values of one parameter were correlated with follow-up values of another parameter. Moreover, we created change scores, indicating an increase (positive value) or a decrease (negative value) in oxytocin, vasopressin or PTSD symptoms from one assessment to the next. We tested whether pre- and post-treatment values of one parameter were correlated with change scores from pre- to post-treatment or from post-treatment to follow-up in another parameter. Vice versa, we explored whether change scores from pre-to post-treatment and from post-treatment to follow up in one parameter were correlated with post- or follow-up values of another parameter.
Concerning the analyses in the context of the internet-based TF-CBT, all results that are presented in the manuscript are based on data of those PTSD patients with available PTSD symptoms, oxytocin and vasopressin data at pre-treatment, post-treatment and follow-up (complete cases, n = 16). This comparatively low number of cases is mainly due to the high dropout rate (as explained in Niemeyer et al., 2020). In order to test the robustness of our results, descriptive and correlational analyses were also conducted in all cases with available data in the respective parameter per assessment (n = 17–37). Results were compared and no significant differences were found. An overview of longitudinal PTSD symptoms, oxytocin and vasopressin data, as well as their longitudinal correlations and correlations with change scores is given in supplementary material 1 and 2, for all and complete cases, respectively.
Non-adjusted α was defined as .05. All analyses were performed with SPSS Statistics, version 25 (IBM).
3. Results
3.1. Flow of participants
The number of participants and available data for our outcomes of interest are illustrated in Table 1. Baseline demographic, trauma-related and psychological variables are presented in Table 2. It reveals group differences in age: Non-deployed healthy controls were younger than PTSD patients and deployed healthy controls. As deployment is a common event in most military careers, service members are non- (or not yet-)deployed only at early career stages and thus, at younger age. Except for age, no demographic group differences were detected.
Table 2.
Baseline comparisons of demographic, posttraumatic stress disorder (PTSD) symptom-related and endocrine variables
| PTSD patients (n = 39) | Deployed healthy controls (n = 33) | Non-deployed healthy controls (n = 28) | Statistics | |
|---|---|---|---|---|
| Demographic information | ||||
| Age | 37.74 (9.62)a | 38.36 (7.98) | 26.36 (4.30) | F (2, 96) = 22.09, p < .01 |
| BMI | 26.84 (3.06)a | 26.55 (3.06)a | 25.54 (2.42) | F (2, 91) = 1.77, p = .18 |
| Number of cigarettes per day | 0.94 (1.12)a | 0.42 (0.75) | 0.55 (0.96)a | F (2, 88) = 2.72, p = .07 |
| Number of deployments | 2.84 (3.05)a | 3.21 (2.76) | - | F (1, 69) = 0.28, p = .60 |
| Total number of days deployed | 357.03 (369.94)a | 415.31 (574.40)a | - | F (1, 66) = 0.27, p = .60 |
| PTSD symptoms | ||||
| PTSD diagnosis (n, %) | 25, 64.10 | 0, 0.00 | - | |
| Overall symptoms | 35.05 (14.71) | 1.88 (4.08) | - | F (1, 70) = 157.19, p < .01 |
| Re-experiencing symptoms | 9.87 (4.32) | 0.36 (1.11) | - | F (1, 70) = 150.83, p < .01 |
| Avoidance symptoms | 3.92 (2.07) | 0.18 (0.72) | - | F (1, 70) = 97.43, p < .01 |
| Negative alterations in cognition and mood | 11.13 (6.04) | 0.18 (0.53) | - | F (1, 70) = 107.61, p < .01 |
| Alterations in arousal and reactivity | 10.13 (4.34) | 1.15 (2.42) | - | F (1, 70) = 111.71, p < .01 |
| Endocrine parameters | ||||
| Oxytocin log (pg/ml) | 0.72 (0.10)a | 0.74 (0.09) | 0.68 (0.12)a | F (2, 93) = 2.32, p = .10 |
| Vasopressin pg/ml | 3.55 (0.60)a | 3.52 (0.48) | 3.54 (0.63) | F (2, 96) = 0.05, p = .95 |
Note. a Due to missing values, information was not available for all participants (see also Table 1 for main outcomes). If not indicated differently, descriptive information is presented as M (SD) and comparisons were conducted based on one-way ANOVAs (in order to compare PTSD patients, deployed healthy controls and non-deployed healthy controls) or t-tests (in order to compare PTSD patients and deployed healthy controls). All PTSD symptom-related variables are based on the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5).
Concerning deployment-related traumatic event exposure, in PTSD patients, having seen destroyed houses or villages (n = 33, 84.62%), knowing someone seriously injured or killed (n = 29, 74.36%) and having seen (parts of) dead bodies (n = 28, 71.79%) were the most frequently reported potentially traumatic events, according to the List of the Mental Health Advisory Team (Zimmermann et al., 2014). In deployed healthy controls, having seen destroyed houses or villages (n = 26, 78.79%), having experienced hostility by civilians (n = 20, 60.61%) and having seen (parts of) dead bodies (n = 19, 57.58%) were the most frequently reported potentially traumatic events. As Table 2 shows, PTSD patients and deployed healthy controls did not differ with regard to deployment-related variables, but as expected, they differed with regard to PTSD symptoms.
3.2. Group differences in endocrine parameters
The cross-sectional group comparison did not reveal any differences in endogenous oxytocin or vasopressin concentrations between PTSD patients, deployed healthy controls or non-deployed healthy controls (see Table 2 and Figure 1), although the analyses were adequately powered to detect a medium-sized effect (f = .32, α = .05; 1-β = .80).
Figure 1.
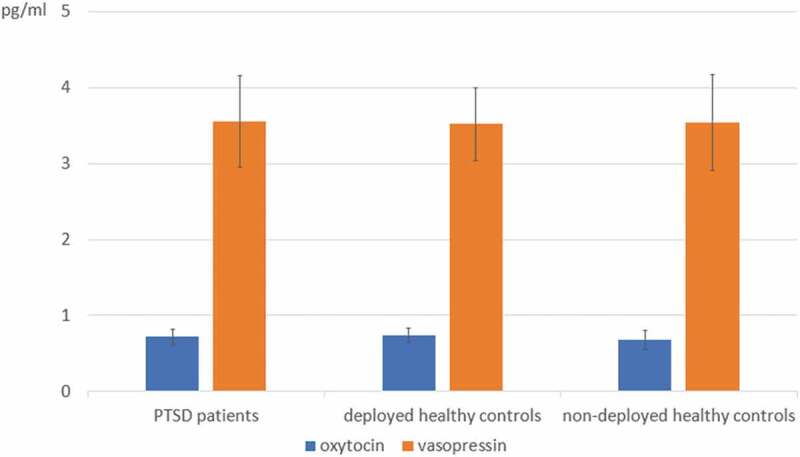
Endogenous oxytocin and vasopressin concentrations in posttraumatic stress disorder (PTSD) patients, deployed healthy controls and non-deployed healthy controls, as assessed at the participants’ first assessments. No significant group differences were detected. M and SD of log-transformed oxytocin and non-transformed vasopressin concentrations are shown
Two non-deployed healthy controls reported suffering from chronic somatic diseases that might have an impact on their endocrine states. Therefore, we repeated the cross-sectional group comparisons without these two individuals Again, groups did not significantly differ in oxytocin (F (2, 91) = 1.67, p = .19) or vasopressin concentrations (F (2, 94) = 0.06, p = .94)
3.3. Correlations before internet-based TF-CBT onset
PTSD symptoms were neither correlated with oxytocin (r = -.19, p = .47), nor with vasopressin (r = .11, p = .68) before onset of the internet-based TF-CBT. Likewise, in completers, no correlations of oxytocin and vasopressin with PTSD symptoms were detected at post-treatment or follow-up assessment (see supplementary material 2).
3.4. Courses of endocrine parameters and PTSD symptoms
Courses of PTSD symptoms, oxytocin and vasopressin are illustrated in Figure 2. In those patients who completed the TF-CBT, there was no change in mean PTSD symptoms from pre- to post-treatment and follow-up (F2, 30 = 1.00, p = .38). PTSD symptoms remained stable within individuals (pre- to post-treatment: r = .83, p < .01, post-treatment to follow-up: r = .93, p < .01). In completers, mean oxytocin did not change from pre- to post-treatment and follow-up (F2, 30 = 1.41, p = .26) and oxytocin was not stable within individuals over time (pre- to post-treatment: r = .04, p = .89, post-treatment to follow-up: r = .19, p ≤ .47). Likewise, in completers, there was no change in mean vasopressin from pre- to post-treatment and follow-up (F2, 30 = 1.24, p = .28) and it was not stable within individuals over time (pre- to post-treatment: r = .44, p = .08, post-treatment to follow-up: r = .01, p = .98).
Figure 2.
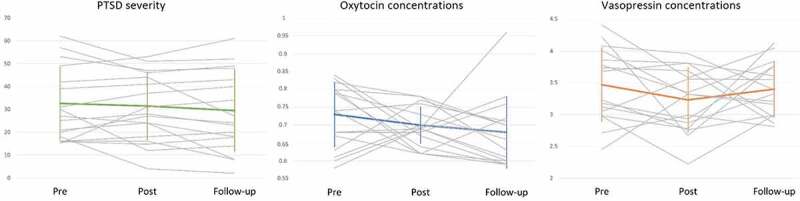
Courses of posttraumatic stress disorder (PTSD) severity (total Clinician-Administered PTSD Scale for DSM-5 scores), oxytocin (log-transformed pg/ml) and vasopressin (pg/ml) from pre-treatment to post-treatment and follow up in all PTSD patients with complete available data (n = 16). There was no significant mean change in any of the outcomes. PTSD severity remained stable within individuals, whereas oxytocin and vasopressin were not correlated within individuals over time
3.5. Interactions of endocrine parameters with PTSD symptoms over time
In completers, oxytocin and vasopressin, as assessed pre-treatment, were not correlated with PTSD symptoms post-treatment (oxytocin: r = –.25, p = .35, vasopressin: r = .27, p = .32). Likewise, pre-treatment oxytocin and vasopressin did not predict changes in PTSD symptoms from pre- to post-treatment (oxytocin: r = –.07, p = .79, vasopressin: r = .25, p = .36). Oxytocin and vasopressin post-treatment were not correlated with PTSD symptoms at follow-up (oxytocin: r = .24, p = .36, vasopressin: r = .13, p = .62), nor were they correlated with the change score of PTSD symptoms from post-treatment to follow-up (oxytocin: r = .44, p = .08, vasopressin: r = .04, p = .89).
Vice versa, in completers, PTSD symptoms pre-treatment were neither correlated with oxytocin and vasopressin, as assessed post-treatment (oxytocin: r = .12, p = .64, vasopressin: r = .22, p = .41), nor were they correlated with changes in oxytocin and vasopressin from pre- to post-treatment (oxytocin: r = .23, p = .38, vasopressin: r = .08, p = .77). Likewise, PTSD symptoms, as assessed post-treatment were not correlated with oxytocin and vasopressin, as measured at follow up (oxytocin: r = –.09, p = .73, vasopressin: r = –.18, p = .63) and they were not correlated with their change scores from post-treatment to follow-up (oxytocin: r = –.13, p = .62, vasopressin: r = –.23, p = .39).
4. Discussion
4.1. Summary of evidence
Our findings suggest that endogenous oxytocin and vasopressin concentrations are unrelated to PTSD psychopathology in patients who completed an internet-based TF-CBT. We did not detect any difference in these endocrine parameters between PTSD patients, deployed and non-deployed healthy controls. Furthermore, in patients, oxytocin and vasopressin were not correlated with PTSD symptoms when measured simultaneously. This is in line with both, previous meta-analytic evidence on cross-sectional associations between these parameters (Engel et al., 2019) as well as with the results from previous clinical trials. In the trial that evaluated repeated intranasal oxytocin administration as PTSD prevention (van Zuiden et al., 2017), blood oxytocin concentrations were neither prognostic biomarkers for PTSD symptom development, nor prescriptive biomarkers of the intervention’s effectiveness (Engel et al., 2020). Likewise, in the clinical trial that investigated the role of blood oxytocin concentrations in a prolonged exposure intervention that was augmented by intranasal oxytocin administration (Flanagan et al., 2018), these concentrations were not correlated with PTSD symptoms before intervention onset, nor did they correspond with symptom changes (Sippel et al., 2020).
Besides evaluating clinical treatment effects, we investigated oxytocin and vasopressin as possible biomarkers of internet-based TF-CBT, in order to explore treatment effects on a biological level. Our results showed that neither PTSD symptoms, nor oxytocin or vasopressin changed during internet-based TF-CBT or to follow-up in those patients who completed the TF-CBT. Notably, while PTSD symptoms were stable within individuals, the endocrine parameters were not. Our exploratory investigations revealed that oxytocin and vasopressin did not predict PTSD symptoms longitudinally and likewise, PTSD symptoms were not associated with the endocrine parameters longitudinally.
4.2. Interpretation of results
In this study, we used single measurements of oxytocin and vasopressin and yielded unsatisfactory intraindividual stability. A prerequisite to use biological measurements as biomarkers for mental disorders during psychotherapeutic or pharmacological interventions is the possibility to measure these parameters repeatedly. Only then can potential changes in biological measurements clearly be related to the intervention effect. Our study showed that this prerequisite is not given when measuring oxytocin and vasopressin only once, even though this is the most frequently applied approach for these neuropeptides in PTSD research (Engel et al., 2019).
These problems have been discussed previously. The discussions mainly focused on two critical aspects, namely, the open question to which extent endogenous oxytocin and vasopressin concentrations can reflect central processes and the diverging approaches to the biochemical analysis of oxytocin and vasopressin. Oxytocin and vasopressin are synthesized in magnocellular neurons of the supraoptic and paraventricular nuclei of the hypothalamus (Brownstein, Russell, & Gainer, 1980). Their first distribution pathway involves direct axonal projections to their central target regions. Importantly, oxytocin and vasopressin exert their psychological effects through this first distribution pathway (Brownstein, Russell, & Gainer, 1980; Landgraf & Neumann, 2004). The second distribution pathway involves paracrine oxytocin and vasopressin release into the cerebrospinal fluid (Pow & Morris, 1989). The third distribution pathway is often referred to as the hypothalamo-neurohypophysial system (Brownstein, Russell, & Gainer, 1980; Neumann, 2008): Following axonal oxytocin and vasopressin transport from the hypothalamic nuclei to the pituitary gland, these peptides are released into the peripheral circulation. Accordingly, their concentrations can be measured in peripheral tissues, such as blood. To date, it remains a matter of ongoing debate whether or how the three distribution pathways interdepend and accordingly, to which extent peripheral oxytocin and vasopressin concentrations can reflect central processes. A meta-analysis showed that under basal conditions, central and peripheral oxytocin concentrations were unrelated, but that they were positively associated when measured after stress exposure or after intranasal oxytocin administration (Valstad et al., 2017). This indicates that oxytocin’s central actions are to some extent reflected in peripheral concentrations, albeit the exact underlying mechanisms and temporal dynamics clearly need further investigation.
Concerning the biochemical analysis, the large range of reported oxytocin and vasopressin values resulting from analyses of extracted versus unextracted samples has stimulated debates (Leng & Sabatier, 2016). A meta-analysis showed that reported oxytocin concentrations were excessively higher when measured in unextracted versus in extracted blood samples (275.61 pg/ml versus 4.75 pg/ml; Engel et al., 2019). As a consequence, measurements of oxytocin concentrations in unextracted blood samples have been criticized for being invalid (Szeto et al., 2011), while other researchers pointed out that the different analysis methods might correspond with different states of oxytocin (MacLean et al., 2019). In the present study, oxytocin and vasopressin concentrations were analysed with a well-established RIA (RIAgnosis, Regensburg, Germany) that has been developed by leading researchers in the field with many years of experience (Neumann & Landgraf, 2019). Mean oxytocin concentrations of 4.50 pg/ml, as they were observed in our study, were comparable with the mean values reported in other studies that measured oxytocin concentrations in extracted blood samples (Engel et al., 2019).
Even though we relied on a valid measurement approach, the exact factors that caused the observed low intraindividual stability remain unclear. Oxytocin concentrations tend to increase in response to emotional (Barraza & Zak, 2009) and physical stimulation (Bello, White-Traut, Schwertz, Pournajafi-Nazarloo, & Carter, 2008) but interestingly, in the latter study, increases in these concentrations were also observed in the passive control condition, confirming a generally low intraindividual stability. This highlights the need to establish advanced parameters that provide stable indicators of oxytocin and vasopressin functioning. This might be achieved by increasing the number of measurements and merging them into more conclusive statistical parameters such as the area under the curve (Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003). Furthermore, confounders of oxytocin and vasopressin need to be controlled for (Engel et al., 2019). In the present study, blood sampling was highly standardized, by scheduling it at a precise time and providing precise instructions with regard to behaviours before measurements. Further, our sample was comparatively homogeneous, as it included only men with the same profession. Especially amidst the low intraindividual stability of both endocrine parameters, it remains unclear which factors actually determined the observed values. Our study’s results indicate that the values were not determined by traumatic event exposure, PTSD symptoms, sex, profession, time of day and behaviours before measurement, but it remains unresolved which factors actually influenced them.
Despite a strong theoretical rationale to investigate oxytocin and vasopressin as biomarkers of TF-CBT, which has been derived from animal studies and experimental studies in humans, the transfer to the clinical setting did not succeed. Besides their involvement in social functioning and fear processing, oxytocin and vasopressin are involved in a variety of other physiological, behavioural and psychological functions (for overviews, see Boll et al., 2018; Donaldson & Young, 2008; Gimpl & Fahrenholz, 2001; Lawson 2017; Macdonald & Feifel, 2014; Macdonald & MacDonald, 2010; Winter & Jurek, 2019; Yang et al., 2013). It appears that experimental paradigms that specifically target either social functioning or fear processing might be more appropriate to investigate oxytocin or vasopressin as potential underpinnings of psychopathological functions in a more hypothesis-driven manner. Alternatively, with regard to psychotherapy, oxytocin and vasopressin might be measured repeatedly within single sessions, such as exposure sessions. Moving from the macro- to the micro-level of psychotherapy evaluation research might be more helpful to identify the active ingredients of TF-CBT.
4.3. Limitations
Contrasting previous findings (Kuester et al., 2016), the internet-based TF-CBT evaluated in the present study was not effective in reducing PTSD symptoms (Niemeyer et al., 2020). The limited number of PTSD patients who started and completed therapy impeded a differential investigation of therapy effectiveness compared with a waiting period. However, as merging all patients into one group in order to quantify changes during the internet-based TF-CBT and follow-up period did not result in any effects, it was not necessary to compare these null-effects with a passive condition, anyway. Still, in general, an effective treatment is necessary to identify its active ingredients, and this prerequisite was not given here. The small number of patients observed limits the informative value of the observed null-findings on both, clinical and endocrine parameters. However, the results of the present study remained stable when using all values instead of those of completers only, which indicates that the findings are still plausible.
Concerning the cross-sectional group comparisons, as non-deployed healthy controls were significantly younger than the deployed healthy controls and PTSD patients, and as their younger age is a characteristic that is systematically related to their status, we cannot fully exclude the possibility that age as a confounding variable suppressed the emergence of potential group differences. However, it is worth noting that neither oxytocin (r = –.03, p = .69), nor vasopressin (r = .03, p = .70) concentrations were significantly correlated with age across all samples.
5. Conclusion
In the present investigation, oxytocin and vasopressin were not informative biomarkers of traumatic event exposure, PTSD symptoms or TF-CBT. Previous evidence from animal studies and non-clinical samples indicated that they were involved in social functioning and fear processing, two functions that are important in PTSD development and treatment. However, the informative value of single measurements of oxytocin and vasopressin was restricted, mainly due to the low intraindividual stability of these parameters. More basic research is needed in order to identify parameters of the endogenous oxytocin and vasopressin system that are more stable and less sensitive to physiological confounders. Only then can oxytocin and vasopressin successfully be implemented into the clinical evaluation of TF-CBT.
Acknowledgments
The face-to-face assessments were logistically supported by the staff of the German Armed Forces Centre of Military Mental Health, Berlin. We are grateful to Christina Kersjes, Beate Muschalla, Jan Spies and Deborah Weiss who performed diagnostic assessments.
Funding Statement
This work was supported by funding from the German Federal Ministry of Defence (Bundesverteidigungsministerium). The German Economic Foundation provided doctoral funding for SE. They had no influence on the study design, the collection, analysis and interpretation of data, the writing of the report or the decision to submit the manuscript for publication.
Disclosure statement
OFA Heinrich Rau and OTA Dr. Gerd-Dieter Willmund are employed by the German Armed Forces. Their employment influenced neither the study design nor the collection, analysis, and interpretation of the data. There are no conflicts of interest among the other authors.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, SE. The data are not publicly available, as they contain information that could compromise the privacy of research participants.
Ethical Standard
The study was approved by the Ethics committee of Freie Universität Berlin (reference number: 85/2014; addendum: 116/2016)., after internal approval by the German Armed Forces.
References
- Acheson, D. T., Feifel, D., de Wilde, S., McKinney, R., Lohr, J., & Risbrough, V. (2013). The effect of intranasal oxytocin treatment on conditioned fear extinction and recall in a healthy human sample. Psychopharmacology, 229, 199–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackenheil, M., Stotz, G., Dietz-Bauer, R., & Vossen-Wellmann, A. (1999). Deutsche Fassung des Mini-International Neuropsychiatric Interview. München: Psychiatrische Universitätsklinik München. [Google Scholar]
- American Psychiatric Association , editor. (2013). Diagnostic and statistical manual of mental disorders. DSM-5. Washington, DC: American Psychiatric Association. [Google Scholar]
- Andrews, B., Brewin, C. R., & Rose, S. (2003). Gender, social support, and PTSD in victims of violent crime. Journal of Traumatic Stress, 16, 421–427. [DOI] [PubMed] [Google Scholar]
- Atwoli, L., Stein, D. J., Koenen, K. C., & McLaughlin, K. A. (2015). Epidemiology of posttraumatic stress disorder: Prevalence, correlates and consequences. Current Opinion in Psychiatry, 28, 307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraza, J. A., & Zak, P. J. (2009). Empathy toward strangers triggers oxytocin release and subsequent generosity. Annals of the New York Academy of Sciences, 1167, 1167. [DOI] [PubMed] [Google Scholar]
- Bello, D., White-Traut, R., Schwertz, D., Pournajafi-Nazarloo, H., & Carter, C. S. (2008). An exploratory study of neurohormonal responses of healthy men to massage. Journal of Alternative and Complementary Medicine (New York, N.Y.), 14, 14. [DOI] [PubMed] [Google Scholar]
- Berger, W., Coutinho, E. S. F., Figueira, I., Marques-Portella, C., Luz, M. P., Neylan, T. C., Marmar, C. R., Mendlowicz, M. V. (2012). Rescuers at risk: A systematic review and meta-regression analysis of the worldwide current prevalence and correlates of PTSD in rescue workers. Social Psychiatry and Psychiatric Epidemiology, 47, 1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll, S., Almeida de Minas, A. C., Raftogianni, A., Herpertz, S. C., & Grinevich, V. (2018). Oxytocin and pain perception: From animal models to human research. Neuroscience, 387, 149–161. [DOI] [PubMed] [Google Scholar]
- Brewin, C. R., Andrews, B., & Valentine, J. D. (2000). Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. Journal of Consulting and Clinical Psychology, 68, 748–766. [DOI] [PubMed] [Google Scholar]
- Brownstein, M. J., Russell, J. T., & Gainer, H. (1980). Synthesis, transport, and release of posterior pituitary hormones. Science, 207, 373–378. [DOI] [PubMed] [Google Scholar]
- Cloitre, M., Stovall-McClough, K. C., Miranda, R., & Chemtob, C. M. (2004). Therapeutic alliance, negative mood regulation, and treatment outcome in child abuse-related posttraumatic stress disorder. Journal of Consulting and Clinical Psychology, 72, 411–416. [DOI] [PubMed] [Google Scholar]
- Coccaro, E. F., Kavoussi, R. J., Hauger, R. L., Cooper, T. B., & Ferris, C. F. (1998). Cerebrospinal fluid vasopressin levels: Correlates with aggression and serotonin function in personality-disordered subjects. Archives of General Psychiatry, 55, 708–714. [DOI] [PubMed] [Google Scholar]
- Ditzen, B., Schaer, M., Gabriel, B., Bodenmann, G., Ehlert, U., & Heinrichs, M. (2009). Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biological Psychiatry, 65, 728–731. [DOI] [PubMed] [Google Scholar]
- Donaldson, Z. R., & Young, L. J. (2008). Oxytocin, vasopressin, and the neurogenetics of sociality. Science (New York, N.Y.), 322, 900–904. [DOI] [PubMed] [Google Scholar]
- Eckstein, M., Becker, B., Scheele, D., Scholz, C., Preckel, K., Schlaepfer, T. E. and Hurlemann, R. (2015). Oxytocin facilitates the extinction of conditioned fear in humans. Biological Psychiatry, 78, 194–202. [DOI] [PubMed] [Google Scholar]
- Engel, S., Klusmann, H., Laufer, S., Pfeifer, A.-C., Ditzen, B., van Zuiden, M., Knaevelsrud, C., & Schumacher, S. (2019). Trauma exposure, posttraumatic stress disorder and oxytocin: A meta-analytic investigation of endogenous concentrations and receptor genotype. Neuroscience and Biobehavioral Reviews, 107, 560–601 [DOI] [PubMed] [Google Scholar]
- Engel, S., Laufer, S., Miller, R., Niemeyer, H., Knaevelsrud, C., & Schumacher, S. (2019). Demographic, sampling- and assay-related confounders of endogenous oxytocin concentrations: A systematic review and meta-analysis. Frontiers in Neuroendocrinology, 54, 100775. [DOI] [PubMed] [Google Scholar]
- Engel, S., van Zuiden, M., Frijling, J. L., Koch, S. B. J., Nawijn, L., Yildiz, R. L. W., … Olff, M. (2020). Early posttraumatic autonomic and endocrine markers to predict posttraumatic stress symptoms after a preventive intervention with oxytocin. European Journal of Psychotraumatology, 11, 1761622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein, A., Owen, J., & Blair, N. (2002). A hazardous profession: War, journalists, and psychopathology. American Journal of Psychiatry, 159, 1570–1575. [DOI] [PubMed] [Google Scholar]
- Feldman, R., Gordon, I., & Zagoory-Sharon, O. (2011). Maternal and paternal plasma, salivary, and urinary oxytocin and parent-infant synchrony: Considering stress and affiliation components of human bonding. Developmental Science, 14, 752–761. [DOI] [PubMed] [Google Scholar]
- Feldman, R., Zagoory-Sharon, O., Weisman, O., Schneiderman, I., Gordon, I., Maoz, R., Shalev, I., Ebstein, R. P. (2012). Sensitive parenting is associated with plasma oxytocin and polymorphisms in the OXTR and CD38 genes. Biological Psychiatry, 72, 175–181. [DOI] [PubMed] [Google Scholar]
- Fischer, S., & Ehlert, U. (2019). Psychoneuroendocrinology and clinical psychology. Clinical Psychology in Europe, 1(2), 1–13. [Google Scholar]
- Flanagan, J. C., Sippel, L. M., Wahlquist, A., Moran-Santa Maria, M. M., & Back, S. E. (2018). Augmenting prolonged exposure therapy for PTSD with intranasal oxytocin: A randomized, placebo-controlled pilot trial. Journal of Psychiatric Research, 98, 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa, E. B., Hembree, E. A., Cahill, S. P., Rauch, S. A. M., Riggs, D. S., Feeny, N. C., & Yadin, E. (2005). Randomized trial of prolonged exposure for posttraumatic stress disorder with and without cognitive restructuring: Outcome at academic and community clinics. Journal of Consulting and Clinical Psychology, 73, 953–964. [DOI] [PubMed] [Google Scholar]
- Foa, E. B., Rothbaum, B. O., Riggs, D. S., & Murdock, T. B. (1991). Treatment of posttraumatic stress disorder in rape victims: A comparison between cognitive-behavioral procedures and counseling. Journal of Consulting and Clinical Psychology, 59, 715–723. [DOI] [PubMed] [Google Scholar]
- Forbes, D., Lockwood, E., Phelps, A., Wade, D., Creamer, M., Bryant, R. A. and O’Donnell, M. (2014). Trauma at the hands of another: Distinguishing PTSD patterns following intimate and nonintimate interpersonal and noninterpersonal trauma in a nationally representative sample. The Journal of Clinical Psychiatry, 75, 147–153. [DOI] [PubMed] [Google Scholar]
- Garfinkel, S. N., Abelson, J. L., King, A. P., Sripada, R. K., Wang, X., Gaines, L. M., & Liberzon, I. (2014). Impaired contextual modulation of memories in PTSD: An fMRI and psychophysiological study of extinction retention and fear renewal. Journal of Neuroscience, 34, 13435–13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpl, G., & Fahrenholz, F. (2001). The oxytocin receptor system: Structure, function, and regulation. Physiological reviews, 81(2), 629–683. [DOI] [PubMed] [Google Scholar]
- Hayes, U. L., & Chambers, K. C. (2005). High doses of vasopressin delay the onset of extinction and strengthen acquisition of LiCl-induced conditioned taste avoidance. Physiology & Behavior, 84, 625–633. [DOI] [PubMed] [Google Scholar]
- Hermenau, K., Hecker, T., Schaal, S., Maedl, A., & Elbert, T. (2013). Addressing post-traumatic stress and aggression by means of narrative exposure: A randomized controlled trial with ex-combatants in the Eastern DRC. Journal of Aggression, Maltreatment & Trauma, 22, 916–934. [Google Scholar]
- Hoge, C. W., Castro, C. A., Messer, S. C., McGurk, D., Cotting, D. I., & Koffman, R. L. (2004). Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. New England Journal of Medicine, 351, 13–22. [DOI] [PubMed] [Google Scholar]
- Hoge, C. W., Grossman, S. H., Auchterlonie, J. L., Riviere, L. A., Milliken, C. S., & Wilk, J. E. (2014). PTSD treatment for soldiers after combat deployment: Low utilization of mental health care and reasons for dropout. Psychiatric Services, 65, 997–1004. [DOI] [PubMed] [Google Scholar]
- Knaevelsrud, C., Böttche, M., Pietrzak, R. H., Freyberger, H. J., & Kuwert, P. (2017). Efficacy and feasibility of a therapist-guided internet-based intervention for older persons with childhood traumatization: A randomized controlled trial. The American Journal of Geriatric Psychiatry, 25, 878–888. [DOI] [PubMed] [Google Scholar]
- Koch, S. B. J., van Zuiden, M., Nawijn, L., Frijling, J. L., Veltman, D. J., & Olff, M. (2019). Effects of intranasal oxytocin on distraction as emotion regulation strategy in patients with post-traumatic stress disorder. European Neuropsychopharmacology, 29, 266–277. [DOI] [PubMed] [Google Scholar]
- Kosfeld, M., Heinrichs, M., Zak, P. J., Fischbacher, U., & Fehr, E. (2005). Oxytocin increases trust in humans. Nature, 435, 673–676. [DOI] [PubMed] [Google Scholar]
- Kuester, A., Niemeyer, H., & Knaevelsrud, C. (2016). Internet-based interventions for posttraumatic stress: A meta-analysis of randomized controlled trials. Clinical Psychology Review, 43, 1–16. [DOI] [PubMed] [Google Scholar]
- Landgraf, R., & Neumann, I. D. (2004). Vasopressin and oxytocin release within the brain: A dynamic concept of multiple and variable modes of neuropeptide communication. Frontiers in Neuroendocrinology, 25, 150–176. [DOI] [PubMed] [Google Scholar]
- Landgraf, R., Neumann, I. D., Holsboer, F., & Pittman, Q. J. (1995). Interleukin-1β stimulates both central and peripheral release of vasopressin and oxytocin in the rat. European Journal of Neuroscience, 7, 592–598. [DOI] [PubMed] [Google Scholar]
- Lange, A., Rietdijk, D., Hudcovicova, M., van de Ven, J.-P., Schrieken, B., & Emmelkamp, P. M. G. (2003). Interapy: A controlled randomized trial of the standardized treatment of posttraumatic stress through the internet. Journal of Consulting and Clinical Psychology, 71, 901–909. [DOI] [PubMed] [Google Scholar]
- Lawson, E. A. (2017). The effects of oxytocin on eating behaviour and metabolism in humans. Nature Reviews Endocrinology, 13, 700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng, G., & Sabatier, N. (2016). Measuring oxytocin and vasopressin: Bioassays, immunoassays and random numbers. Journal of neuroendocrinology, 28(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald, K., & Feifel, D. (2014). Oxytocin׳s role in anxiety: A critical appraisal. Brain Research, 1580, 22–56. [DOI] [PubMed] [Google Scholar]
- Macdonald, K., & MacDonald, T. M. (2010). The peptide that binds: A systematic review of oxytocin and its prosocial effects in humans. Harvard Review of Psychiatry, 18, 1–21. [DOI] [PubMed] [Google Scholar]
- MacLean, E. L., Wilson, S. R., Martin, W. L., Davis, J. M., Nazarloo, H. P., & Carter, C. S. (2019). Challenges for measuring oxytocin: The blind men and the elephant? Psychoneuroendocrinology, 107, 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolajczak, M., Pinon, N., Lane, A., de Timary, P., & Luminet, O. (2010). Oxytocin not only increases trust when money is at stake, but also when confidential information is in the balance. Biological Psychology, 85, 182–184. [DOI] [PubMed] [Google Scholar]
- Milad, M. R., Orr, S. P., Lasko, N. B., Chang, Y., Rauch, S. L., & Pitman, R. K. (2008). Presence and acquired origin of reduced recall for fear extinction in PTSD: Results of a twin study. Journal of Psychiatric Research, 42, 515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad, M. R., Pitman, R. K., Ellis, C. B., Gold, A. L., Shin, L. M., Lasko, N. B., & Rauch, S. L. (2009). Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry, 66, 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson, C. M., Schnurr, P. P., Resick, P. A., Friedman, M. J., Young-Xu, Y., & Stevens, S. P. (2006). Cognitive processing therapy for veterans with military-related posttraumatic stress disorder. Journal of Consulting and Clinical Psychology, 74, 898–907. [DOI] [PubMed] [Google Scholar]
- National Institute of Clinical Excellence . (2018). Post-traumatic stress disorder. www.nice.org.uk/guidance/ng 116. [PubMed]
- Nawijn, L., van Zuiden, M., Koch, S. B. J., Frijling, J. L., Veltman, D. J., & Olff, M. (2016). Intranasal oxytocin enhances neural processing of monetary reward and loss in post-traumatic stress disorder and traumatized controls. Psychoneuroendocrinology, 66, 228–237. [DOI] [PubMed] [Google Scholar]
- Nawijn, L., van Zuiden, M., Koch, S. B. J., Frijling, J. L., Veltman, D. J., & Olff, M. (2017). Intranasal oxytocin increases neural responses to social reward in post-traumatic stress disorder. Social Cognitive and Affective Neuroscience, 12, 212–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann, I. D. (2008). Brain oxytocin: A key regulator of emotional and social behaviours in both females and males. Journal of Neuroendocrinology, 20, 858–865. [DOI] [PubMed] [Google Scholar]
- Neumann, I. D., & Landgraf, R. (2019). Tracking oxytocin functions in the rodent brain during the last 30 years: From push-pull perfusion to chemogenetic silencing. Journal of Neuroendocrinology, 31, e12695. [DOI] [PubMed] [Google Scholar]
- Neuner, F., Schauer, M., Klaschik, C., Karunakara, U., & Elbert, T. (2004). A comparison of narrative exposure therapy, supportive counseling, and psychoeducation for treating posttraumatic stress disorder in an african refugee settlement. Journal of Consulting and Clinical Psychology, 72, 579–587. [DOI] [PubMed] [Google Scholar]
- Niemeyer, H., Knaevelsrud, C., Schumacher, S., Engel, S., Kuester, A., Burchert, S., & Willmund, G.-D. (2020). Evaluation of an internet-based intervention for service members of the German armed forces with deployment-related posttraumatic stress symptoms. BMC Psychiatry, 20, 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olff, M., Frijling, J. L., Kubzansky, L. D., Bradley, B., Ellenbogen, M. A., Cardoso, C. and van Zuiden, M. (2013). The role of oxytocin in social bonding, stress regulation and mental health: An update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology, 38, 1883–1894. [DOI] [PubMed] [Google Scholar]
- Orr, S. P., Metzger, L. J., Lasko, N. B., Macklin, M. L., Peri, T., & Pitman, R. K. (2000). De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. Journal of Abnormal Psychology, 109, 290–298. [PubMed] [Google Scholar]
- Ozer, E. J., Best, S. R., Lipsey, T. L., & Weiss, D. S. (2003). Predictors of posttraumatic stress disorder and symptoms in adults: A meta-analysis. Psychological Bulletin, 129, 52–73. [DOI] [PubMed] [Google Scholar]
- Parsons, R. G., & Ressler, K. J. (2013). Implications of memory modulation for post-traumatic stress and fear disorders. Nature Neuroscience, 16, 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peri, T., Ben-Shakhar, G., Orr, S. P., & Shalev, A. Y. (2000). Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biological Psychiatry, 47, 512–519. [DOI] [PubMed] [Google Scholar]
- Pow, D. V., & Morris, J. F. (1989). Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience, 32, 435–439. [DOI] [PubMed] [Google Scholar]
- Pruessner, J. C., Kirschbaum, C., Meinlschmid, G., & Hellhammer, D. H. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28, 916–931. [DOI] [PubMed] [Google Scholar]
- Resick, P. A., & Schnicke, M. (1993). Cognitive processing therapy for rape victims: A treatment manual. Newbury Park, London, New Delhi: Sage. [Google Scholar]
- Riem, M. M. E., Kunst, L. E., Steenbakkers, F. D. F., Kir, M., Sluijtman, A., Karreman, A., & Bekker, M. H. J. (2019). Oxytocin reduces interpersonal distance: Examining moderating effects of childrearing experiences and interpersonal context in virtual reality. Psychoneuroendocrinology, 108, 102–109. [DOI] [PubMed] [Google Scholar]
- Roozendaal, B., Schoorlemmer, G. H. M., Wiersma, A., Sluyter, S., Driscoll, P., Koolhaas, J. M., & Bohus, B. (1992). Opposite effects of central amygdaloid vasopressin and oxytocin on the regulation of conditioned stress responses in male rats. Annals of the New York Academy of Sciences, 652, 460–461. [DOI] [PubMed] [Google Scholar]
- Sack, M., Spieler, D., Wizelman, L., Epple, G., Stich, J., Zaba, M., & Schmidt, U. (2017). Intranasal oxytocin reduces provoked symptoms in female patients with posttraumatic stress disorder despite exerting sympathomimetic and positive chronotropic effects in a randomized controlled trial. BMC Medicine, 15, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumm, J. A., Briggs-Phillips, M., & Hobfoll, S. E. (2006). Cumulative interpersonal traumas and social support as risk and resiliency factors in predicting PTSD and depression among inner-city women. Journal of Traumatic Stress, 19, 825–836. [DOI] [PubMed] [Google Scholar]
- Shalev, A. Y., Gevonden, M., Ratanatharathorn, A., Laska, E., van der Mei, W. F., Qi, W. and van Zuiden, M. (2019). Estimating the risk of PTSD in recent trauma survivors: Results of the international consortium to predict PTSD (ICPP). World Psychiatry, 18, 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan, D. V., Lecrubier, Y., Sheehan, K. H., Amorim, P., Janavs, J., Weiller, E. Hergueta, T., Baker, R., & Dunbar, G. C. (1998). The mini-international neuropsychiatric interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry, quiz 34–57, 59(Suppl 20), p. 22–33 [PubMed] [Google Scholar]
- Sippel, L. M., King, C. E., Wahlquist, A. E., & Flanagan, J. C. (2020). A preliminary examination of endogenous peripheral oxytocin in a pilot randomized clinical trial of oxytocin-enhanced psychotherapy for posttraumatic stress disorder. Journal of Clinical Psychopharmacology, 40, 401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger, F., Nees, F., Wicking, M., Lang, S., & Flor, H. (2015). Behavioral and central correlates of contextual fear learning and contextual modulation of cued fear in posttraumatic stress disorder. International Journal of Psychophysiology, 98, 584–593. [DOI] [PubMed] [Google Scholar]
- Stoehr, J., Cramer, C., & North, W. (1992). Oxytocin and vasopressin hexapeptide fragments have opposing influences on conditioned freezing behavior. Psychoneuroendocrinology, 17, 267–271. [DOI] [PubMed] [Google Scholar]
- Stojek, M. M., McSweeney, L. B., & Rauch, S. A. M. (2018). Neuroscience informed prolonged exposure practice: Increasing efficiency and efficacy through mechanisms. Frontiers in Behavioral Neuroscience, 12, 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto, A., McCabe, P. M., Nation, D. A., Tabak, B. A., Rossetti, M. A., McCullough, M. E., & Mendez, A. J. (2011). Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosomatic Medicine, 73, 393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, R., George, K., Walton, J. C., Orr, S. P., & Benson, J. (2006). Sex-specific influences of vasopressin on human social communication. Proceedings of the National Academy of Sciences, 103, 7889–7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, R., Gupta, S., Miller, K., Mills, S., & Orr, S. (2004). The effects of vasopressin on human facial responses related to social communication. Psychoneuroendocrinology, 29, 35–48. [DOI] [PubMed] [Google Scholar]
- Tolin, D. F., & Foa, E. B. (2006). Sex differences in trauma and posttraumatic stress disorder: A quantitative review of 25 years of research. Psychological Bulletin, 132, 959–992. [DOI] [PubMed] [Google Scholar]
- Toth, I., Neumann, I. D. and Slattery, D. A. (2012). Central administration of oxytocin receptor ligands affects cued fear extinction in rats and mice in a timepoint-dependent manner. Psychopharmacology, 223, 149–158. [DOI] [PubMed] [Google Scholar]
- Valstad, M., Alvares, G. A., Egknud, M., Matziorinis, A. M., Andreassen, O. A., Westlye, L. T., & Quintana, D. S. (2017). The correlation between central and peripheral oxytocin concentrations: A systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews, 78, 117–124. [DOI] [PubMed] [Google Scholar]
- van Zuiden, M., Frijling, J. L., Nawijn, L., Koch, S. B. J., Goslings, J. C., Luitse, J. S., … Olff, M. (2017). Intranasal oxytocin to prevent posttraumatic stress disorder symptoms: A randomized controlled trial in emergency department patients. Biological Psychiatry, 81, 1030–1040. [DOI] [PubMed] [Google Scholar]
- Weathers, F. W., Bovin, M. J., Lee, D. J., Sloan, D. M., Schnurr, P. P., Kaloupek, D. G., & Marx, B. P. (2018). The clinician-administered PTSD scale for DSM-5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychological Assessment, 30, 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessa, M., & Flor, H. (2007). Failure of extinction of fear responses in posttraumatic stress disorder: Evidence from second-order conditioning. American Journal of Psychiatry, 164, 1684–1692. [DOI] [PubMed] [Google Scholar]
- Winter, J., & Jurek, B. (2019). The interplay between oxytocin and the CRF system: Regulation of the stress response. Cell and Tissue Research, 375, 85–91. [DOI] [PubMed] [Google Scholar]
- Wittchen, H.-U., Schonfeld, S., Kirschbaum, C., Thurau, C., Trautmann, S., Steudte, S. and Zimmermann, P. (2012). Traumatic experiences and posttraumatic stress disorder in soldiers following deployment abroad: How big is the hidden problem? Deutsches Ärzteblatt International, 109, 559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H.-P., Wang, L., Han, L., & Wang, S. C. (2013). Nonsocial functions of hypothalamic oxytocin. ISRN Neuroscience, 2013, 179272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda, R., Flory, J. D., Southwick, S., & Charney, D. S. (2006). Developing an agenda for translational studies of resilience and vulnerability following trauma exposure. Annals of the New York Academy of Sciences, 1071, 379–396. [DOI] [PubMed] [Google Scholar]
- Zimmermann, P., Höfler, M., Schönfeld, S., Trautmann, S., Hauffa, R., Kowalski, J., & Wittchen, H. U. (2014). Einsatzerlebnisse und einsatzbedingte psychische Erkrankungen deutscher Soldaten – Empirische Struktur und prädiktive Wertigkeit traumatischer Stressoren (Deployment stressors and psychiatric disorders in German soldiers – Empirical structure and predictive value). Zeitschrift Für Klinische Psychologie Und Psychotherapie, 43, 180–191. [Google Scholar]
- Zuj, D. V., & Norrholm, S. D. (2019). The clinical applications and practical relevance of human conditioning paradigms for posttraumatic stress disorder. Progress in Neuro-psychopharmacology & Biological Psychiatry, 88, 339–351. [DOI] [PubMed] [Google Scholar]
- Zuj, D. V., Palmer, M. A., Lommen, M. J. J., & Felmingham, K. L. (2016). The centrality of fear extinction in linking risk factors to PTSD: A narrative review. Neuroscience and Biobehavioral Reviews, 69, 15–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, SE. The data are not publicly available, as they contain information that could compromise the privacy of research participants.


