Abstract
Background
Nutritional risk index (NRI) has been shown to better predict survival than body mass index (BMI) or albumin after several cardiovascular interventions. Under assessment herein is whether NRI can have higher predictive value than conventional parameters for short-term survival after transcatheter aortic valve replacement (TAVR).
Methods
A prospective cohort study was performed. In-hospital, 1-month and 3-month survival was evaluated. Since most patients undergoing TAVR are over 65, the NRI definition for a geriatric population (GNRI) was used. The impact of baseline BMI, albumin levels, and GNRI on in-hospital and short-term survival was assessed.
Results
One hundred fifty two patients aged 82 ± 5.4 were included. In-hospital, 1-month, and 3-month mortality was 5.3%, 5.9%, and 9.2%, respectively. Mean GNRI was 112.7 ± 11.9, and was significantly lower in patients who died in-hospital (101.0 ± 8.8 vs. 113.3 ± 11.7), at 30 days (103.4 ± 10.9 vs. 113.3 ± 11.7), and at 90 days (104.0 ± 9.6 vs. 113.6 ± 11.8) than in survivors (all, p < 0.05). Three-month mortality in patients with no nutritional risk was 6.8% (9/132) vs. 25% (5/20) in patients with malnutrition (p = 0.022). In univariate analysis, GNRI predicted in-hospital, 30-day, and 90-day mortality (all, p < 0.05). Predictive value remained significant after adjusting for age, EuroSCORE II, and STS-Score (p < 0.05). Based on receiver operating curves, GNRI (AUC: 0.73) showed a better discrimination for 3-month mortality than albumin (0.69), weight (0.67) or BMI (0.62). The optimal cut-off value was 109.8.
Conclusions
The geriatric nutritional risk index predicts short-term mortality after TAVR and has a higher discriminating ability than other commonly used nutritional variables. It is a simple parameter that identifies those patients who could benefit from pre-procedural nutritional therapy.
Keywords: aortic valve stenosis, body mass index, transcatheter aortic valve replacement, hypoalbuminemia
Introduction
Malnutrition is frequent in elderly patients and has been shown to affect survival in several cardiovascular diseases, such as chronic heart failure [1] or coronary artery disease [2]. Transcatheter aortic valve replacement (TAVR) is mainly performed in high-risk patients, the vast majority of which are geriatric patients. In such patients, nutritional status could be a useful prognostic factor to be considered before any planned TAVR. Nutritional status in patients undergoing TAVR has been evaluated in several ways, including body mass index (BMI) and laboratory parameters such as albumin levels. Higher BMI and higher albumin levels have been previously associated with more favorable outcomes after TAVR [3]. Regarding albumin, low baseline levels have been shown to predict in-hospital, 30-day and long-term mortality [3, 4]. Furthermore, a meta-analysis has shown BMI, as a continuous variable, to be associated with a better early prognosis after TAVR [5]. The nutritional risk index (NRI), originally described by Buzby et al. [6], is a simple tool that combines both clinical and laboratory parameters. Since its introduction, it has been applied in several medical specialties, mainly in the field of oncology [7, 8]. NRI has been recently shown to have a better prognostic value than both BMI and albumin in several cardiovascular diseases and procedures, including acute or chronic heart failure [1, 9], heart transplants [10], coronary artery disease [2] or percutaneous coronary interventions [11]. NRI is not only an easy tool to assess nutritional status, but it does not require any complex or additional test to those performed routinely on admission. The geriatric nutritional risk index (GNRI) is a version of the NRI adapted for elderly patients; thus, it could be particularly useful for the population usually undergoing TAVR. The present study sought to elucidate the impact of nutritional status, measured with both GNRI and conventional parameters, on clinical outcomes and particularly short-term survival after TAVR.
Methods
Study population
A prospective, observational, cohort study was performed in patients undergoing TAVR with a new generation valve prosthesis using a transfemoral access from July 2016 to September 2017 in the documented center. Consecutive patients with symptomatic severe aortic stenosis having a prohibiting risk for surgical aortic valve replacement were included, as assessed by a multidisciplinary Heart Team. Patients with an isolated or combined severe aortic regurgitation and patients requiring a valve-in-valve procedure were also included.
Procedures
Pre-procedural baseline demographic, clinical and laboratory characteristics were assessed and baseline nutritional data, including serum albumin and BMI, were obtained. New York Health Association (NYHA) class was assessed, and EuroSCORE II and STS scores were documented.
After a Heart Team decision, TAVR procedures were carried out according to standard techniques. The choice of prosthesis was left to operator discretion. Use of local anesthesia and conscious sedation was the aim for all patients. Procedural details were also recorded.
In-hospital survival was evaluated and at follow-up to assess vital status, which was performed at 1 and 3 months through outpatient visits and/or with telephone interviews by a physician.
Nutritional assessment based on GNRI
Since most patients undergoing TAVR are older than 65, the NRI definition adapted to an old population was used, as described by Bouillanne et al. [12]: Geriatric (G) NRI = (1.489) × Albumin (g/L) + [(41.7 × (present weight/ideal weight)].
In order to be consistent with GNRI use, patients under 65 years were excluded from the analysis. Ideal weight (WLo) was calculated according to Lorentz equations [12]:
Based on this definition, patients were divided into four grades of nutrition-related risk, as suggested in the literature [12]: GNRI > 98 (no risk), GNRI 92 to ≤ 98 (low risk), GNRI 82 to < 92 (moderate risk), and GNRI < 82 (major risk). Due to the low number of malnourished individuals, for inferential categorical analyses, all patients with some degree of malnutrition were combined into one category (GNRI ≤ 98) and those without malnutrition into another one (GNRI > 98).
Outcomes
The primary endpoint of this study was overall mortality at 3 months. Secondary endpoints included in-hospital and 1-month mortality. Exploratory variables were length of stay in hospital, and NYHA improvement at 3 months after TAVR.
Statistical analysis
Categorical variables were described with frequencies and percentages, and continuous variables were reported with mean ± standard deviation (SD) if normally distributed or median (range) if not normally distributed. The Fisher test or χ2 was used to compare categorical variables. The Student t-test was used to compare means and the Mann-Whitney U test was used to compare medians. Primary and secondary endpoints were assessed hierarchically in the following pre-specified order: 3-month, 1-month, and in-hospital mortality. All other endpoints were considered exploratory, and no adjustments were made for multiplicity of tests. Survival prediction was evaluated by means of a logistic regression (adjusted by potential confounding factors). Statistical significance was based on a p-value < 0.05. Receiver operating curves (ROC) were created to assess sensitivity and specificity of the GNRI in predicting survival, as well as those for individual components of the index. The best cut-off value was decided using the highest value of the Youden index. SPSS statistical software package version 24.0 was used for all analyses.
All patients gave signed and informed consent prior to intervention and the study was performed under the protocol, which was approved by the local ethics committee (296/16).
Results
Study population
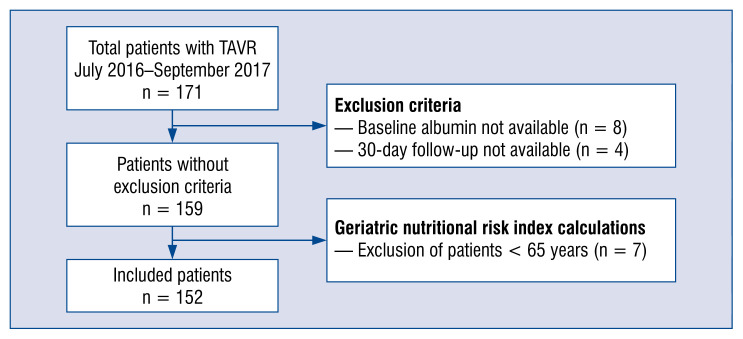
Out of 171 patients who underwent TAVR between July 2016 and September 2017, 8 patients were excluded from the analysis due to unavailable baseline albumin levels and 4 patients were excluded due to missing follow-up data. In order to be consistent with GNRI use, 7 patients under 65 years were excluded from the analysis. A flow-chart of patient exclusion in the present study population is shown in Figure 1.
Figure 1.
Flowchart of the study population; TAVR — transcatheter aortic valve replacement.
Baseline and procedural characteristics
Transcatheter aortic valve replacement was performed in 152 patients using various new generation prostheses including Portico valve (St. Jude Medical) (n = 91), Sapien 3 valve (Edwards Lifesciences) (n = 20), Evolut R valve (Medtronic) (n = 20), and Symetis valve (Boston Scientific) (n = 21).
Overall mean ± SD age was 82 ± 5.4 years, and 41.4% of patients were female. EuroSCORE II and STS score were 5.3 ± 6 and 4.0 ± 2.8, respectively.
Baseline and procedural characteristics of the whole population and in patients with and without malnutrition are shown in Table 1. Most patients had hypertension (93%), and other common comorbidities were coronary artery disease (58%), diabetes (35%), and most patients had some degree of chronic renal failure. No significant differences were shown between groups except regarding nutritional parameters, including weight, albumin and GNRI. Both EuroSCORE II and STS scores differed significantly between groups as expected.
Table 1.
Baseline and procedural characteristics of the population according to geriatric nutritional risk index (GNRI).
| Overall population (n = 152) | Patients with no nutritional risk (GNRI > 98) (n = 132) | Patients with nutritional risk (GNRI ≤ 98) (n = 20) | P | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age [years] | 82 ± 5.4 | 81.6 ± 5.4 | 84.4 ± 4.9 | 0.032 |
| Sex (female) | 41.4% (n = 63) | 41.7% (n = 55) | 40% (n = 8) | 0.888 |
| Weight [kg] | 77.0 ± 14.0 | 79.0 ± 13.6 | 63.9 ± 8.9 | 0.0001 |
| Ideal weight [kg] | 62.2 ± 7.4 | 62.0 ± 7.3 | 63.4 ± 8.3 | 0.442 |
| Height [cm] | 167.1 ± 9.6 | 166.9 ± 9.5 | 168.7 ± 10.7 | 0.427 |
| BMI [kg/m2] | 26.9 (16.4–41.7) | 27.5 (19.5–41.7) | 21.9 (16.4–31.1) | 0.0001 |
| Albumin [g/dL] | 4.2 (2.5–5) | 4.2 (2.6–5) | 3.5 (2.5–4.4) | 0.0001 |
| GNRI | 112.7 ± 11.9 | 115.6 ± 9.8 | 93.5 ± 3.6 | 0.0001 |
| Frailty | 68.4% (n = 104) | 67.4% (n = 89) | 75% (n = 15) | 0.611 |
| Chronic renal failure | 96.7% (n = 147) | 96.2% (n = 127) | 100% (n = 20) | 0.999 |
| Carotid occlusive disease | 18.4% (n = 28) | 17.4% (n = 23) | 25% (n = 5) | 0.535 |
| Peripheral artery disease | 15.1% (n = 23) | 15.9% (n = 21) | 10% (n = 2) | 0.740 |
| Previous cardiac surgery | 13.2% (n = 20) | 11.4% (n = 15) | 25% (n = 5) | 0.146 |
| Previous MI | 10.5% (n = 16) | 9.1% (n = 12) | 20% (n = 4) | 0.230 |
| Previous stroke | 13.8% (n = 21) | 13.6 (n = 18) | 15% (n = 3) | 0.999 |
| Previous TIA | 2% (n = 3) | 2.3% (n = 3) | 0% (n = 0) | 0.999 |
| Coronary artery disease | 57.9% (n = 88) | 56.8% (n = 75) | 65% (n = 13) | |
| Porcelain aorta | 17.1% (n = 26) | 18.9% (n = 25) | 5% (n = 1) | 0.200 |
| COPD | 15.8% (n = 24) | 15.2% (n = 20) | 20% (n = 4) | 0.525 |
| Diabetes | 34.9% (n = 53) | 34.1% (n = 45) | 40% (n = 8) | 0.621 |
| Hypertension | 92.8% (n = 141) | 92.4% (n = 122) | 95% (n = 19) | 0.999 |
| EuroSCORE II | 5.4 ± 6.1 | 4.8 ± 4.8 | 9.4 ± 10.5 | 0.002 |
| STS score | 4.1 ± 2.8 | 3.6 ± 1.8 | 7.2 ± 5.1 | 0.0001 |
| Procedural characteristics | ||||
| Type of valve: | ||||
| Portico | 59.9% (n = 91) | 59.1% (n = 78) | 65% (n = 13) | |
| Evolut | 13.2% (n = 20) | 12.9% (n = 17) | 15% (n = 3) | |
| Symetis | 13.8% (n = 21) | 13.6% (n = 18) | 15% (n = 3) | |
| Sapien 3 | 13.2% (n = 20) | 14.4% (n = 19) | 5% (n = 1) | |
| Contrast dye [mL] (n = 150) | 140 (10–550) | 150 (10–550) | 125 (50–240) | 0.249 |
| Fluoroscopy time [min] (n = 149) | 18.4 (7.9–230) | 18.4 (7.9–230) | 18.4 (8.0–47.0) | 0.802 |
| Simultaneous PCI (n = 150) | 2.7% (n = 4) | 3.1% (n = 4) | 0% (n = 0) | 0.999 |
Bold figures show significant differences; BMI — body mass index; COPD — chronic obstructive pulmonary disease; MI — myocardial infarction; PCI — percutaneous coronary intervention; TIA — transient ischemic attack
Nutritional results
Overall baseline mean GNRI value was 112.7 ± 11.9, median BMI was 26.9 (16.4–41.7) kg/m2, and median albumin level was 4.2 (2.5–5) g/dL. Based on GNRI values, 86.8% of patients had no nutritional risk (GNRI > 98), 9.9% had low risk (GNRI 92 to ≤ 98), 3.3% had moderate risk (GNRI 82 to < 92), and no patients were at major risk (GNRI < 82) prior to intervention, with median GNRI values being 115.6 ± 9.8, 95.3 ± 1.8, and 88.1 ± 1.8, respectively. Mean BMI and albumin values varied within categories but did not show a clear tendency.
Mean age was 81.6 ± 5.4 years in no risk patients, 83.7 ± 4.1 years in low risk patients, and 86.2 ± 6.9 years in patients at moderate risk. Lower GNRI values (thus, more severe malnutrition) were associated with older age; however, this did not reach statistical significance.
Clinical outcomes and survival
Overall mortality was 5.3% in-hospital, 5.9% at 1 month, and 9.2% at 3 month follow up. Causes of 3-month mortality were the following: cardiovascular (3 refractory cardiogenic shock, and 1 electromechanical dissociation), non-cardiovascular (4 life-threatening bleeding, 1 life-threatening cerebrovascular accident, 1 critical limb ischemia, 1 acute kidney failure, 2 multi-organ failure syndrome), and 1 unknown cause.
Three-month mortality in patients with no nutritional risk was 6.8% (9/132) vs. 25% (5/20) in patients with some degree of malnutrition according to GNRI (p = 0.022, the Fisher test). Mortality at 1-month and in-hospital also showed a similar trend: 4.5% (6/132) in well-nourished patients vs. 15% (3/20) in malnourished patients at 1 month, and 3.8% (5/132) in well-nourished patients vs. 15% (3/20) in malnourished patients in-hospital, with differences not reaching statistical significance.
Mean GNRI values were significantly lower in patients who died in-hospital (101.0 ± 8.8 vs. 113.3 ± 11.7), at 30 days (103.4 ± 10.9 vs. 113.3 ± 11.7), and at 90 days (104.0 ± 9.6 vs. 113.6 ± 11.8) than in those who survived (two-sample Student t-test, all, p < 0.05). Results were also significant for baseline albumin levels when comparing patients who died within 3 months after the intervention vs. those who survived: 3.7 (2.5–4.8) vs. 4.2 (2.7–5) (p = 0.018, Mann-Whitney U Test), respectively. BMI showed a numerical difference but did not reach statistical significance. Further details are shown in Table 2.
Table 2.
Baseline characteristics of the population according to short-term survival.
| Patients with survival at 3-months (n = 138) | Patients who died at 3-months (n = 14) | P | |
|---|---|---|---|
| Baseline characteristics | |||
| Age [years] | 82 ± 5.3 | 81.8 ± 6.9 | 0.919 |
| Sex (female) | 39.9% (n = 55) | 57.1% (n = 8) | 0.259 |
| Weight [kg] | 77.8 ± 13.9 | 69.14 ± 13.5 | 0.027 |
| Ideal weight [kg] | 62.4 ± 7.3 | 60.1 ± 8.3 | 0.282 |
| Height [cm] | 167.4 ± 9.5 | 164.5 ± 10.6 | 0.291 |
| BMI [kg/m2] | 27.2 (18.1–41.7) | 25.1 (16.4–37.4) | 0.133 |
| Albumin [g/dL] | 4.2 (2.7–5) | 3.7 (2.5–4.8) | 0.018 |
| GNRI | 113.6 ± 11.8 | 104 ± 9.6 | 0.004 |
| Frailty | 66.7% (n = 92) | 85.7% (n = 12) | 0.227 |
| Chronic renal failure | 96.4% (n = 133) | 100% (n = 14) | 0.999 |
| Carotid occlusive disease | 18.1% (n = 25) | 21.4% (n = 3) | 0.723 |
| Peripheral artery disease | 15.9% (n = 22) | 7.1% (n = 1) | 0.696 |
| Previous cardiac surgery | 14.5% (n = 20) | 0% (n = 0) | 0.217 |
| Previous MI | 10.1% (n = 14) | 14.3% (n = 2) | 0.644 |
| Previous stroke | 13.8% (n = 19) | 14.3% (n = 2) | 0.999 |
| Previous TIA | 1.4% (n = 2) | 7.1% (n = 1) | 0.253 |
| Coronary artery disease | 60% (n = 80) | 57.1% (n = 8) | |
| Porcelain aorta | 18.1% (n = 25) | 7.1% (n = 1) | 0.466 |
| COPD | 15.2% (n = 21) | 21.4% (n = 3) | 0.465 |
| Diabetes | 35.5% (n = 49) | 28.6% (n = 4) | 0.772 |
| Hypertension | 93.5% (n = 129) | 85.7% (n = 12) | 0.268 |
| EuroSCORE II | 5.5 ± 6.2 | 4.3 ± 4.5 | 0.482 |
| STS score | 4.0 ± 2.7 | 4.6 ± 3.1 | 0.486 |
| Procedural characteristics | |||
| Type of valve: | |||
| Portico | 71.4% | 84.6% | |
| Evolut | 12.5% | 7.7% | |
| Symetis | 0% | 7.7% | |
| Sapien | 3 | 16.1% | 0% |
| Contrast dye [mL] (n = 150) | 140 (10–550) | 185 (110–270) | 0.096 |
| Fluoroscopy time [min] (n = 149) | 18.1 (7.9–230) | 20.6 (12.7–47) | 0.138 |
| Simultaneous PCI (n = 150) | 2.2% (n = 3) | 7.7% (n = 1) | 0.327 |
Bold figures show significant differences; BMI — body mass index; COPD — chronic obstructive pulmonary disease; MI — myocardial infarction; PCI — percutaneous coronary intervention; TIA — transient ischemic attack
In univariate analysis, GNRI significantly predicted in-hospital, 30-day and 90-day mortality (all, p < 0.05). Predictive capacity of GNRI remained significant in multivariate analysis after adjusting for potential confounders including age, and pre-interventional risk-scores (EuroSCORE II and STS-Score) (p < 0.05, logistic regression). No other baseline characteristics were significant independent predictors in univariate analysis. Albumin level was also significantly predictive, and BMI was numerically higher in patients who survived.
In order to investigate if the predictive value of GNRI was mainly driven by results in patients with high vs. low general clinical risk, some post hoc exploratory analyses in subgroups of patients were performed as defined by EuroSCORE/STS risk level. The overall trend was confirmed in all subgroups. In patients with an intermediate/high EuroSCORE II risk (n = 55), mortality rates were 2.44% in patients with no nutritional risk vs. 21.43% in patients with some degree of nutritional risk (p < 0.05). In patients with a low EuroSCORE II risk (n = 97), mortality rates were 8.80% vs. 33.30%, respectively (p = 0.11). In patients with an intermediate/high STS risk (n = 54), mortality rates were 8.80% in patients with no nutritional risk vs. 33.30% in patients with some degree of nutritional risk (p = 0.34). In patients with a low STS risk (n = 98), mortality rates were 9.76% vs. 23.08%, respectively (p = 0.08). In regression analyses the GNRI predictive capacity reached significance in the STS high/intermediate group (p < 0.05) and the EuroSCORE low-risk group (p = 0.01).
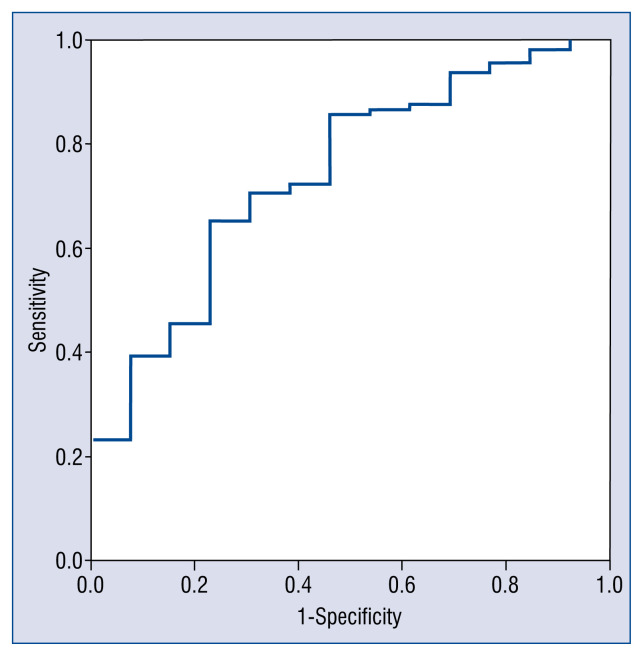
According to ROC, GNRI showed a better discrimination for 3-month mortality than its individual components (3-month: area under curve [AUC] GNRI: 0.73 vs. AUC albumin: 0.69 vs. AUC weight: 0.67) or BMI (AUC BMI: 0.62). Similar results were found for in-hospital and 1-month mortality. ROC for GNRI and 3-month mortality is shown in Figure 2. The optimal GNRI cut-off in the present series was 109.8.
Figure 2.
Receiver operating curve for geriatric nutrition risk index and 3-month mortality; area under the curve: 0.74; 95% confidence interval: 0.60–0.88; p < 0.005.
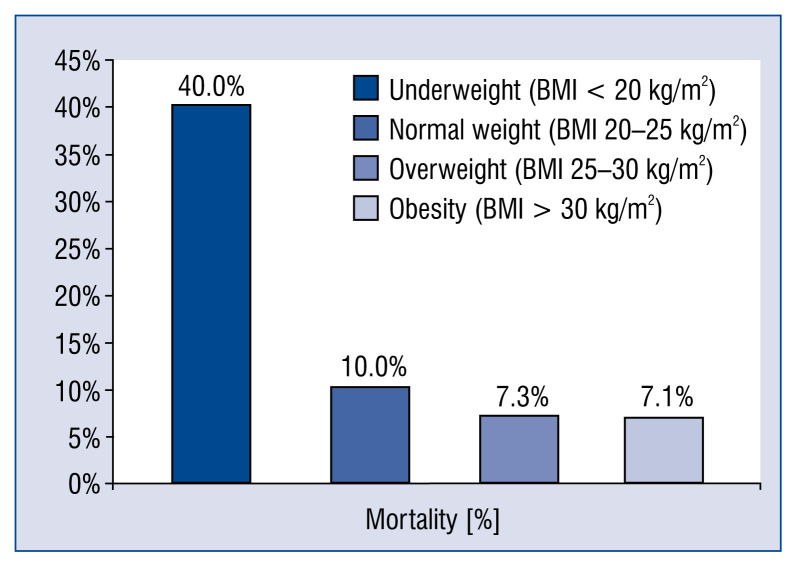
In a subgroup analysis based on the traditional BMI classification, patients with underweight (BMI < 20 kg/m2) showed a numerically higher mortality than normal weight, overweight, and obese patients, with the difference not reaching statistical significance. Detailed mortality percentages are shown in Figure 3.
Figure 3.
Relationship between 3-month mortality and body mass index (BMI) classification.
The NYHA class change at 3 months after TAVR could be assessed in 118 patients. An improvement of at least one level was shown in most of them (84.7%). Such an improvement was observed in 86.6% of patients with no nutrition risk vs. 69.2% of patients with some degree of nutritional risk (p = NS).
No significant differences in median length of stay in hospital were observed between different nutritional status groups (9 days in patients with no degree of malnutrition vs. 10 days in patients with some degree of malnutrition).
Discussion
Overall outcomes in the current TAVR population are in line with those previously described in the literature, with short-term mortality and inhospital complications according to Valve Academic Research Consortium-2 criteria being similar to those reported for all new generation valves [13–15].
According to available research, this is the first prospective cohort study on the predictive value of GNRI in TAVR patients in a European population, in which an improved predictive value of GNRI as compared to commonly used nutritional parameters is shown and a practical clinical threshold is estimated. Differences between patients who died and survivors at 90 days were significant regarding GNRI, weight and albumin, but not regarding BMI. The overall GNRI predictive value is supported by the uniform trend observed in exploratory analyses in all risk level subgroups defined by EuroSCORE and STS scores. Specifically, the significant predictive value of GNRI in some subgroups suggests a potential added value of GNRI to predict futility of TAVR.
Geriatric nutritional risk index showed a higher discrimination in prediction of short-term mortality than its individual parameters or BMI, as shown by ROC-curves. A preliminary GNRI cut-off value of 109.8 is suggested; further studies in larger populations are warranted to confirm its clinical value. The trend to a less common NYHA improvement in patients with some degree of nutritional risk is consistent with the overall negative impact of poor nutrition on clinical outcomes.
Data analysis has recently appeared from a Japanese registry which has also suggested that GNRI has a prognostic value in TAVR [16]. Patient details were based on registry records and information on deaths were obtained from the treating hospital or by calling family members. Although no comparison of its predictive value with other nutritional markers were reported, a significantly increased mortality rate was also found in patients with lower GNRI values.
In the present cohort, no patients with a very high-risk malnutrition were identified, but several showed some extent of malnutrition. A possible explanation is that patients with severe malnutrition or who are frail may have been excluded for TAVR screening due to the presumed futility of the intervention.
The present results are in line with previously published studies showing a good predictive value of pre-operative GNRI in other cardiovascular therapies such as heart failure [17], heart transplant [10] or more recently percutaneous coronary intervention [11]. Other reports have shown low GNRI to delay rehabilitation after cardiac surgery in elderly patients [18], which remains to be studied after TAVR.
Several studies have shown that low levels of pre-procedural albumin are associated with short-term and mid-term mortality [3, 4, 19]. These results have been confirmed in the present study. However, the GNRI (combining both albumin and other body mass parameters) showed an even better discrimination capacity in predicting short-term mortality after TAVR than pre-procedural albumin.
Body mass index as a continuous variable has previously been shown to be associated with a better short-term prognosis after TAVR [5]. Continuous BMI data in the current study did not significantly predict mortality, probably due to the low number of events. However, median BMI was lower in patients not surviving at 3 months. When categorizing patients according to BMI values, underweight patients (BMI < 20 kg/m2) showed a numerically higher mortality (40%) than all groups with a higher BMI (7.1–10%), with the difference not reaching statistical significance. However, this association has been significant in other studies with a long-term follow-up [20].
The interpretation of BMI as a risk factor suggesting malnutrition in patients undergoing TAVR is complicated by the so-called “obesity paradox” resulting in a better survival in several cardiovascular interventions including TAVR [21, 22]. Previous studies have shown that overweight and obese patients undergoing TAVR show better outcomes than those with a low BMI [23]. A recent meta-analysis showed better short- and long-term survival in obese patients (BMI > 30 kg/m2) compared to patients of normal weight [20]. The finding of GNRI being better than BMI and albumin in predicting in-hospital/short-term survival in TAVR, even after adjusting for potential confounders, could reflect an immediate negative effect of malnutrition rather than a favorable effect of overweight/obesity.
Several nutritional tools have been used in TAVR to assess nutritional status such as grip strength, gait speed, bioimpedance analysis, or nutritional questionnaires (e.g. Mini Nutritional Assessment [MNA]) [24, 25]. The main limitation of GNRI is that it is mainly based on albumin, a biochemical marker that can be affected by other co-morbidities, such as hepatic cirrhosis; moreover, inflammatory disorders are known to result in a catabolic state and a reduced liver synthesis of albumin. The major strength of GNRI is that it is practical, since it only involves one calculation including the routinely measured BMI and albumin levels on admission, and no extra equipment or measuring devices are required. The need for a formula to estimate GNRI could certainly be a practical drawback. However, an online calculator is available at http://touchcalc.com/calculators/gnri. Routine recording of pre-interventional GNRI is not only easy to perform, but it provides a useful nutritional assessment tool to identify those patients at risk of malnutrition. GNRI is suggested to be helpful to classify patients regarding their short-term mortality risk. This might help to decide which patients could benefit from a nutritional intervention prior to TAVR.
Malnutrition is frequent in elderly patients undergoing TAVR and it should not be overlooked when stratifying patients. Therefore, measuring baseline GNRI values and assessing the improvement of such index prior to TAVR could be useful in protecting this vulnerable group of patients. As already proven in other heart diseases (e.g. heart failure) [26], GNRI is a modifiable factor, both in terms of pre-interventional albumin levels and pre-interventional BMI (i.e. weight), and a strategy to improve nutritional status before an intervention such as TAVR should be considered. Further randomized trials are warranted to test this hypothesis, and to assess the practicality and time needed to improve nutritional status in such patients.
Previous studies in TAVR have shown that some parameters besides the conventional risk scores (EuroSCORE II and STS score) offer prognostic information; that is diabetes mellitus, mobility and nutritional status measured with questionnaires [25]. Other studies have suggested adding baseline albumin levels to risk stratification factors before TAVR [4, 27]. If the present results are confirmed by further studies, GNRI could be considered in risk scores, for it has a stronger prognostic discriminating ability than the nutritional parameters already included in such scores and other specific measurements such as albumin.
Being a single-center investigation with a limited sample size are limitations in the present study; however, the results are consistent and strongly significant. Some other limitations must also be acknowledged. Firstly, as in any observational study, although an adjustment was used for the imbalance in major baseline characteristics, confounding factors due to unmeasured variables cannot be excluded. Secondly, this is the first study from a single center; further studies will be needed at a multicenter level for these findings to be extrapolated to a wider population. Thirdly, cause of death was not always available because some of the follow-up data on vital status were obtained from a family member who was not aware of the exact cause of death. Therefore, data on specific causes of death should be interpreted with caution. Fourthly, long-term survival was not analyzed in this study; however, GNRI showed a strong association with survival in the short-term.
Conclusions
Geriatric nutritional risk index predicts short-term mortality in patients undergoing TAVR and appears to have a higher discriminating ability than other commonly used nutritional variables, such as serum albumin and BMI. It is a simple and easy to calculate parameter, and its routine use could be useful in identifying those patients who could benefit from nutritional therapy prior to intervention. Further prospective, multicenter studies with a longer follow-up, as well as randomized trials using an established GNRI threshold, and GNRI improvement prior to TAVR are needed to confirm this relationship in the long-term.
Footnotes
Conflict of interest: Mariuca Vasa-Nicotera is proctor for Abbott and Medtronic, Stephan Fichtlscherer and Thomas Walther are proctors and report consultancy activities for Abbott and Edwards Lifesciences. All other authors have no conflicts of interest related to the subject of the article.
References
- 1.Sargento L, Vicente Simões A, Rodrigues J, et al. Geriatric nutritional risk index as a nutritional and survival risk assessment tool in stable outpatients with systolic heart failure. Nutr Metab Cardiovasc Dis. 2017;27(5):430–437. doi: 10.1016/j.numecd.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Kunimura A, Ishii H, Uetani T, et al. Impact of Geriatric Nutritional Risk Index on cardiovascular outcomes in patients with stable coronary artery disease. J Cardiol. 2017;69(1):383–388. doi: 10.1016/j.jjcc.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Koifman E, Magalhaes MA, Ben-Dor I, et al. Impact of pre-procedural serum albumin levels on outcome of patients undergoing transcatheter aortic valve replacement. Am J Cardiol. 2015;115(9):1260–1264. doi: 10.1016/j.amjcard.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto M, Shimura T, Kano S, et al. Prognostic Value of Hypoalbuminemia After Transcatheter Aortic Valve Implantation (from the Japanese Multicenter OCEAN-TAVI Registry) Am J Cardiol. 2017;119(5):770–777. doi: 10.1016/j.amjcard.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Takagi H, Umemoto T ALICE (All-Literature Investigation of Cardiovascular Evidence) Group. „Obesity paradox” in transcatheter aortic valve implantation. J Cardiovasc Surg (Torino) 2017;58(1):113–120. doi: 10.23736/S0021-9509.16.09233-8. [DOI] [PubMed] [Google Scholar]
- 6.Buzby GP, Williford WO, Peterson OL, et al. A randomized clinical trial of total parenteral nutrition in malnourished surgical patients: the rationale and impact of previous clinical trials and pilot study on protocol design. Am J Clin Nutr. 1988;47(2 Suppl):357–365. doi: 10.1093/ajcn/47.2.357. [DOI] [PubMed] [Google Scholar]
- 7.Bo Y, Wang K, Liu Y, et al. The geriatric nutritional risk index predicts survival in elderly esophageal squamous cell carcinoma patients with radiotherapy. PLoS One. 2016;11(5):e0155903. doi: 10.1371/journal.pone.0155903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shoji F, Matsubara T, Kozuma Y, et al. Preoperative Geriatric Nutritional Risk Index: A predictive and prognostic factor in patients with pathological stage I non-small cell lung cancer. Surg Oncol. 2017;26(4):483–488. doi: 10.1016/j.suronc.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 9.La Rovere MT, Maestri R, Olmetti F, et al. Additional predictive value of nutritional status in the prognostic assessment of heart failure patients. Nutr Metab Cardiovasc Dis. 2017;27(3):274–280. doi: 10.1016/j.numecd.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Barge-Caballero E, García-López F, Marzoa-Rivas R, et al. Prognostic value of the nutritional risk index in heart transplant recipients. Rev Esp Cardiol (Engl Ed) 2017;70(8):639–645. doi: 10.1016/j.rec.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Wada H, Dohi T, Miyauchi K, et al. Prognostic impact of the geriatric nutritional risk index on long-term outcomes in patients who underwent percutaneous coronary intervention. Am J Cardiol. 2017;119(11):1740–1745. doi: 10.1016/j.amjcard.2017.02.051. [DOI] [PubMed] [Google Scholar]
- 12.Bouillanne O, Morineau G, Dupont C, et al. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82(4):777–783. doi: 10.1093/ajcn/82.4.777. [DOI] [PubMed] [Google Scholar]
- 13.Eggebrecht H, Mehta RH. Transcatheter aortic valve implantation (TAVI) in Germany 2008–2014 on its way to standard therapy for aortic valve stenosis in the elderly? EuroIntervention. 2016;11(9):1029–1033. doi: 10.4244/EIJY15M09_11. [DOI] [PubMed] [Google Scholar]
- 14.Jakobsen L, Terkelsen CJ, Søndergaard L, et al. Short- and long-term mortality and stroke risk after transcatheter aortic valve implantation. Am J Cardiol. 2018;121(1):78–85. doi: 10.1016/j.amjcard.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Mas-Peiro S, Vasa-Nicotera M, Weiler H, et al. Thirty-day outcomes in 100 consecutive patients undergoing transfemoral aortic valve replacement with the portico valve on an all-comer basis. J Invasive Cardiol. 2017;29(12):431–436. [PubMed] [Google Scholar]
- 16.Shibata K, Yamamoto M, Kano S, et al. Importance of Geriatric Nutritional Risk Index assessment in patients undergoing transcatheter aortic valve replacement. Am Heart J. 2018;202:68–75. doi: 10.1016/j.ahj.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 17.Minamisawa M, Miura T, Motoki H, et al. Geriatric nutritional risk index predicts cardiovascular events in patients at risk for heart failure. Circ J. 2018;82(6):1614–1622. doi: 10.1253/circj.CJ-17-0255. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa M, Izawa KP, Satomi-Kobayashi S, et al. Poor preoperative nutritional status is an important predictor of the retardation of rehabilitation after cardiac surgery in elderly cardiac patients. Aging Clin Exp Res. 2017;29(2):283–290. doi: 10.1007/s40520-016-0552-3. [DOI] [PubMed] [Google Scholar]
- 19.Bogdan A, Barbash IM, Segev A, et al. Albumin correlates with all-cause mortality in elderly patients undergoing transcatheter aortic valve implantation. EuroIntervention. 2016;12(8):e1057–e1064. doi: 10.4244/EIJY15M10_09. [DOI] [PubMed] [Google Scholar]
- 20.Sannino A, Schiattarella GG, Toscano E, et al. Meta-Analysis of Effect of Body Mass Index on Outcomes After Transcatheter Aortic Valve Implantation. Am J Cardiol. 2017;119(2):308–316. doi: 10.1016/j.amjcard.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 21.Bundhun PK, Li N, Chen MH. Does an obesity paradox really exist after cardiovascular intervention?: a systematic review and meta-analysis of randomized controlled trials and observational studies. Medicine (Baltimore) 2015;94(44):e1910. doi: 10.1097/MD.0000000000001910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konigstein M, Havakuk O, Arbel Y, et al. The obesity paradox in patients undergoing transcatheter aortic valve implantation. Clin Cardiol. 2015;38(2):76–81. doi: 10.1002/clc.22355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abawi M, Rozemeijer R, Agostoni P, et al. Effect of body mass index on clinical outcome and all-cause mortality in patients undergoing transcatheter aortic valve implantation. Neth Heart J. 2017;25(9):498–509. doi: 10.1007/s12471-017-1003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimura T, Yamamoto M, Kano S, et al. Impact of frailty markers on outcomes after transcatheter aortic valve replacement: insights from a Japanese multicenter registry. Ann Cardiothorac Surg. 2017;6(5):532–537. doi: 10.21037/acs.2017.09.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eichler S, Salzwedel A, Harnath A, et al. Nutrition and mobility predict all-cause mortality in patients 12 months after transcatheter aortic valve implantation. Clin Res Cardiol. 2018;107(4):304–311. doi: 10.1007/s00392-017-1183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bourdel-Marchasson I, Emeriau JP. Nutritional strategy in the management of heart failure in adults. Am J Cardiovasc Drugs. 2001;1(5):363–373. doi: 10.2165/00129784-200101050-00006. [DOI] [PubMed] [Google Scholar]
- 27.Grossman Y, Barbash IM, Fefer P, et al. Addition of albumin to traditional risk score improved prediction of mortality in individuals undergoing transcatheter aortic valve replacement. J Am Geriatr Soc. 2017;65(11):2413–2417. doi: 10.1111/jgs.15070. [DOI] [PubMed] [Google Scholar]