ABSTRACT
We describe the early phases of a COVID-19 epidemic in two contiguous Italian regions, Lombardy and Veneto, which were heavily and simultaneously hit by SARS-CoV-2 in Italy but showed markedly different disease outcome in terms of case fatality rate, SARS-CoV-2-attributable mortality and hospitalization. We discuss data limitations together with similarities and differences of the regional context possibly affecting COVID-19 control in the two regions. We conclude that the better COVID-19 outcome in Veneto was due, at least in part, to the adoption of a strategy of active search of asymptomatic SARS-CoV-2 infections (Reasoned Mass Testing), instead of a strategy strictly based on the detection of symptomatic cases.
KEYWORDS: SARS-CoV-2 Mass testing, covid-19, lombardy, veneto, mortality, case Fatality Rate
Introduction
The importance of active searching for asymptomatic SARS-CoV2 infections (hereafter referred to as Reasoned Mass Testing (RMS)) for an effective control of COVID-19 epidemics in closed institutional settings (e.g. skilled nursing facilities and hospitals) has been highlighted [1,2]. It may therefore seem intuitive that, whenever feasible, RMS could provide a real, additional advantage over the classical symptom-based case detection (SBCD) strategy. Whether and to what extent the RMS strategy has helped to control SARS-CoV-2 transmission in wide regional communities remains, however, unproven. Although very instructive, the results of the two surveys made in the small Italian city of Vò Euganeo, where contact tracing and reconstruction of the transmission chains revealed the critical role of asymptomatic subjects in SARS-CoV-2 transmission, cannot be simply translated into a much wider regional context [3,4].
We believe a good case for testing whether RMS provides better epidemic control than SBCD is the comparison of COVID-19 mortality indicators in two geographically contiguous Italian regions, Lombardy and Veneto, which were simultaneously hit by SARS-CoV-2 on 21 February 2020, when the COVID-19 epidemic started in Italy, and rapidly caused rampant accumulations of critically ill patients [4,5]. The same day, both regions started active disease surveillance, attempting identification and prompt isolation of all SARS-CoV-2-infected subjects [5]. However, four days after, Lombardy adopted a stringent Symptom-Based Case Detection (SBCD) strategy, likely on the assumption that only COVID-19 fully symptomatic patients could efficiently transmit infection, while Veneto continued the initial approach and actually strengthened the search for potentially contagious, asymptomatic subjects by adopting extensive SARS-CoV-2 testing.
Here, we compare two hard outcome indicators of the epidemic’s course in the two regions along three months of COVID-19 spread in Italy, inclusive of pre- and post-national lockdown periods (which lasted 68 days) and discuss the different outcomes in the context of various regional factors that possibly affected the two regions’ COVID-19 containment strategy.
Methods
Data were extracted from official reports by the Italian Ministry of Health (available at https//www.salute.gov.it). We calculated the following outcome indicators: 1. The case fatality rate (CFR), as described by Baud et al. [6] and normalized as reported by Pachetti et al. [7]; 2. The overall COVID-19-attributable mortality in the regional population. We also measured the ratio of hospitalized to non-hospitalized subjects. The time intervals considered were from 21 February, when the epidemic started in the two regions, to 28 March, when the two different regional strategies were closely followed, and from 29 March to 31 May when, following a national lockdown and the WHO call to for enhanced diagnostic testing, Lombardy progressively loosened its SBCD strategy and markedly increased SARS-CoV-2 testing.
Finally, it is here assumed that both RMS and SBCD are coupled with a comparable efficiency of contact tracing, isolation of positive cases and quarantine of close contacts in the two regions.
Results
As shown in Figure 1 and Supplementary Table 1, at the first timepoint measurement (March, 28th), there was more than 6 times lower lethality and 7.5 lower COVID-19-attributable mortality in Veneto than in Lombardy. There was also a 2.5 lower rate of hospitalization in Veneto than in Lombardy (Table 1). Mortality difference between the two regions was even higher at the second timepoint (16 April). Thereafter, regional CFR and mortality differences were progressively reduced and at the last timepoint considered (31 May), the difference between the two regions in both of these hard COVID-19 outcomes was reduced to nearly half those of 28 March. However, these outcomes did not become equal, and Veneto had significantly lower CFR and mortality than Lombardy also at the final examined timepoint.
Figure 1..
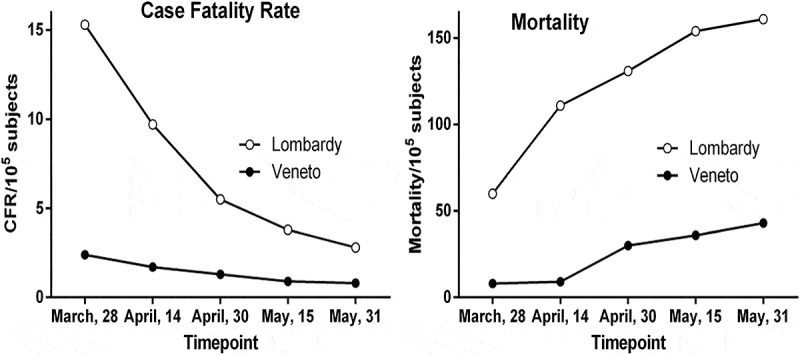
CFR (left panel) and COVID-19-attributable mortality (right panel) over the first wave of the SARS-CoV-2 pandemic in Lombardy and Veneto. CFR 95% Confidence intervals are shown in Supplementary Table 1
TABLE 1.
SARS-CoV-2 Positivity over testing and hospitalization in two Italian Regions, Lombardy (L) and Veneto (V)
| Timepoints | Positivity/Tested, %(95% CI) |
Hospitalization/Positive |
||||
|---|---|---|---|---|---|---|
| L | V | L/V | L | V | L/V | |
| March,28 | 38.0 | 4.3 | 9.02 | 0.95 | 0.42 | 2.26 |
| (37.7-38.2) | (4.2-4.4) | |||||
| April,16 | 28.6 | 3.4 | 8.41 | 0.58 | 0.17 | 3.41 |
| (28.5-28.8) | (3.3-3.45) | |||||
| April,30 | 32.9 | 4.3 | 7.65 | 0.26 | 0.16 | 1.62 |
| (32.7-38.1) | (4.2-4.3) | |||||
| May, 15 | 25.4 | 3.4 | 7.47 | 0.23 | 0.17 | 1.35 |
| (25.2-25.5) | (3.4-3.5) | |||||
| May,31 | 19.9 | 2.8 | 7.11 | 0.18 | 0.08* | 2.25* |
| (19.9-20.0) | (2.8-2.8) | |||||
L, Lombardy; V, Veneto
*strong uncertainty about the veracity of the sudden and high increase of home isolation and care of positive subjects in Veneto at this timepoint
Table 1 shows the ratios of SARS-CoV-2 positivity/testing and hospitalization/positivity between the two regions. It is noted that Lombardy increased its number of tests by more than 4 times in the time interval considered, and, at the last timepoint its ratio of positive SARS-CoV-2 to tested subjects was considerably reduced, though not reaching Veneto’s ratio. With increased testing and identification of positive subjects in Lombardy, hospitalization was also reduced, and trended more closely with that of Veneto (Table 1).
It is important to consider the contextual factors that are known to affect SARS-CoV-2 transmission and disease outcomes, which may have played a role in the different values in Lombardy and Veneto (Table 2). These regions are quite comparable in mean age, average personal income and life expectancy. They also have an almost identical proportion of elderly subjects (75–90 years of age) who are known to have a particularly poor COVID-19 prognosis [5]. Differences possibly affecting Lombardy are those related to population density, which is markedly lower in Veneto. The organization of health services appears to be mostly public and territorially spread in Veneto, while in Lombardy it is more hospital-based, with a robust private sector [8].
TABLE 2.
Similarities and differences in some regional factors between Lombardy and Veneto
| Factor | Lombardy | Veneto |
|---|---|---|
| Population (million) | 10.06 | 4,90 |
| Population density (Km.square) | 421.0 | 267.4 |
| Average personal Income (KEuro/annum) | 24.70 | 21.99 |
| Mean age (years) | 44.7 | 45.1 |
| 75-90 years old as % of the population | 10.8 | 10.9 |
| Life expectancy in years Male/female | 76.3/(83.1 | 76.9/83.7 |
| Health Servicesa | Mostly public and hospital-based | Public/private and territorial-based |
(a) See Ref. 9,11
Discussion
The largest and most remarkable differences between COVID-19 outcome indicators in Lombardy and Veneto were in the pre-lockdown period when CFR in Lombardy was disproportionately high (15.3%; Figure 1 and Supplementary Table 1) and where nearly half of all SARS-CoV-2 subjects were hospitalized (Table 1). Over the same particularly tough period of the epidemic in Italy, Veneto had substantially lower CFR (2.4%) and less than one third of infected subjects were hospitalized (Table 1). Of note, the ratio tested/positive SARS-CoV-2 subjects was nearly ten times higher in Veneto than in Lombardy, attesting to the much higher capacity of testing and identification of SARS-CoV-2 positive subjects in this region.
CFR has been discussed [6,9] for uncertainties about the real denominator (number of infected subjects), particularly in the initial epidemic period as the one discussed above. The particularly high CFR in Lombardy is likely explained by the very low denominator resulting from the SBCD strategy that can be biased toward detection of more severe clinical cases, and consequent lack of SARS-CoV-2 testing of mildly symptomatic subjects, a fact largely supported by the very high level of hospitalization in that region (Table 1). When SBCD was loosened and more intense testing for SARS-CoV-2 was implemented, leading to increased identification and isolation of milder cases and asymptomatic infections (post-lockdown period), the CFR became more realistic owing to a more accurate denominator. Hospitalization was also markedly reduced.
Notably, Veneto’s CFR remained remarkably low throughout the period examined, reaching a value (below 1%), assumed to be the ‘real world’ lethality under optimal case detection conditions, efficient contact tracing, quarantine and lack of confounding factors [9]. This interpretation is supported by the hospitalization data since a higher number of asymptomatic or minimally symptomatic subjects would not require hospitalization.
It is important to put the above differences in its regional context, and ask which of the main regional factors has favored or hindered RMS strategy. One highly relevant factor determining CFR is population age [5]. Notably, the populations of the two regions have a similar median age and an almost equal percentage of members of the highest risk age group in its population (75–90 years old), which has been found to strongly correlate with CFR [10].
There are, however, other important, COVID-19 relevant differences between the two regions which may have played a role both in the choice of control strategy and their implementation. In particular the lower population density and the greater number of hospital-integrated territorial health services [8] have certainly favored RMS implementation in Veneto while making it more difficult to apply in Lombardy, at least in the initial epidemic period when this region may have also suffered a shortage of testing capacity [8,11]. It has recently been shown that population density played an important role on the reproduction rate of SARS-CoV-2 infection in US counties, but differences in population density were there much higher (5–10 times) than that between Lombardy and Veneto (less than 0.5 times higher for Lombardy) [11]. The reduction of the mortality gap with Veneto when Lombardy increased SARS-CoV-2 testing would suggest that RMS implementation was indeed possible also in Lombardy if it had been chosen as a suitable strategy at the beginning of the epidemic, and tools for extensive SARS-CoV-2 testing had been made available. Finally, worse disease outcomes in Lombardy might also depend on a particularly high burden of unrecognized COVID-19 cases before the first official one [11], but this remains undetermined until strong comparative seroprevalence studies clarify the matter.
Data and interpretation made here have several limitations. The first is that there is some uncertainty about the real number of SARS-CoV-2 tests performed in the two regions, particularly in the first epidemic period (the first two timepoints in Figure 1 and Supplementary Table 1) when official Italian authorities reported only cumulative data, with no separation of the number of tested subjects from total tests made, and so this data includes repeated tests on the same subject. However, because the data of both regions were affected by this uncertainty, it is unlikely to significantly change the trend along the whole period examined. Another limitation is our assumption that all what follows from case identification, i.e. case isolation, contact tracing and quarantine, was done with similar efficiency in both regions, a fact for which there is currently no evidence. Although both regions were simultaneously hit by the virus, the initial high rate of infection transmission in Lombardy, as discussed above, probably reduced the contact tracing efficiency in that region. In addition, our data do not take into account possible regional specificities in sample transport and processing, laboratory systems, adherence to isolation protocols and the public response to physical containment measures and imposed lockdown. Finally, we are aware that other unknown, confounding factors might have played a role.
All the above considered, we believe the comparative data and trends reported here support our view that RMS strategy played an important role in the rather noteworthy COVID-19 outcome differences between the two regions. The better outcome in Veneto region has some resemblance to what happened in some countries that enjoyed a remarkable success in epidemic controls by massive SARS-CoV-2 testing [12], RMS application, if early implemented, can help control COVID-19 epidemics in a wide regional and national contexts, as it does in closed institutional settings or small communities [1–3]. The availability of more rapid testing tools can add to the value of mass testing, provided that these new diagnostics have acceptable performance in terms of sensitivity and specificity [13,14].
Acknowledgments
The authors would like to thank Antonella Torosantucci for drawing Figure 1, and Christo Hall for proofreading.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].MM A, Hatfield KM, Reddy SC, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382(22):2081–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gandhi S, Havlir.DY. Y.. Asymptomatic transmission, the achilles’ heel of current strategies to control COVID-19. N Eng J Med. 2020; DOI: 10.1056/NEJMe2009758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Crisanti A, Cassone A. In one Italian town, we showed mass testing could eradicate the coronavirus.TheGuardian https://www.theguardian.com/commentisfree/2020/mar/20/eradicated-coronavirus-mass-testing-COVID-19-italy-vo (2020).
- [4].Lavezzo E, Franchin E, Ciavarella. C. et al. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo’. Nature. 2020;584(7821):425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Onder G, Rezza G, Brusaferro S. Case-Fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA 2020; published online March23 DOI: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- [6].Baud D, Xiaolong Q, Nielsen-Saines K, et al. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. Published online 2020 March12 10.1016/S1473-3099(20)30195-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pachetti M, Marini B, Giudici F et al. Impact of lockdown on COVID-19 case fatality rate and virus mutations spread. Research Square preprint 2020, DOI 10.21203/rs.3.rs-32317/v1 [DOI] [PMC free article] [PubMed]
- [8].Binkin N, Michieletto F, Salmaso S, et al. Protecting our health care workers while protecting our communities during the COVID-19 pandemic: a comparison of approaches and early outcomes in two Italian Regions, 2020. medRxiv preprint doi: 10.1101/2020.04.10.20060707 [DOI]
- [9].Rajgor DD, Lee MH, Archuleta S, et al., Swee chye quek. The many estimates of the COVID-19 case fatality rate. Lancet Infect.Dis. Published online 2020 March27 10.1016/S1473-3099(20)30244-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hoffmann C, Wolf E. Older age groups and case-specific fatality rate of COVID-19 in Europe, US and Canada. Infection. 2020. DOI: 10.1007/s.15010-070-01538-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Grasselli G, Pesenti A, Cecconi M et al. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA 2020; published online March13. DOI: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- [12].SM L, Lee DH. Lessons learned from battling COVID-19: the Korean experience. Int.J.Environ.Res.Public Health. 2020;17(20):E7548. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rubin D, Huang J, Brian T, et al. Association of social distancing, population density, and temperature with the instantaneous reproduction number of SARS-CoV-2 in counties across the United States jamanetworkopen. 2020;3(7):e2016099. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Guglielmi G. The explosion of new coronavirus tests that could help to end the pandemic. Nature. 2020;583(7817):506–509. [DOI] [PubMed] [Google Scholar]


