Abstract
Background
Remote rural riverine villages account for most of the reported malaria cases in the Peruvian Amazon. As transmission decreases due to intensive standard control efforts, malaria strategies in these villages will need to be more focused and adapted to local epidemiology.
Methods
By integrating parasitological, entomological, and environmental observations between January 2016 and June 2017, we provided an in-depth characterization of malaria transmission dynamics in 4 riverine villages of the Mazan district, Loreto department.
Results
Despite variation across villages, malaria prevalence by polymerase chain reaction in March 2016 was high (>25% in 3 villages), caused by Plasmodium vivax mainly and composed of mostly submicroscopic infections. Housing without complete walls was the main malaria risk factor, while households close to forest edges were more commonly identified as spatial clusters of malaria prevalence. Villages in the basin of the Mazan River had a higher density of adult Anopheles darlingi mosquitoes, and retained higher prevalence and incidence rates compared to villages in the basin of the Napo River despite test-and-treat interventions.
Conclusions
High heterogeneity in malaria transmission was found across and within riverine villages, resulting from interactions between the microgeographic landscape driving diverse conditions for vector development, housing structure, and human behavior.
Keywords: malaria, transmission, heterogeneity, Amazon, incidence, prevalence, human biting rate, entomological inoculation rate, Peru
The history of malaria in Peru shows that important reductions in the malaria burden can be obtained with intensive and comprehensive standard control measures, but also that this progress can quickly be lost if there is not a long-term country plan to consolidate the achievements and prevent malaria resurgence [1]. For instance, the Global Fund-sponsored PAMAFRO project supported the strengthening of microscopic diagnosis, the implementation of active test-and-treat interventions, and the distribution of long-lasting insecticidal mosquito nets (LLINs), with a community-based intercultural approach, between 2005 and 2010 in the Peruvian Amazon. During this period, malaria declined drastically in the most affected department of Loreto from 54 291 reported cases (25% due to Plasmodium falciparum) in 2005 to 10 504 cases (20% due to P. falciparum) in 2010 [1, 2]. Regrettably, the marked reduction of international donors’ support and the unusually heavy rains that caused floods in riverine villages starting in 2012, led to a 5-fold increase in cases between 2010 and 2015 (60 302 cases) [1, 3].
The political and financial commitment from the Peruvian government was crucial not only to respond to this malaria resurgence, but also to establish a long-term initiative called Plan Malaria Cero started in 2017, with the ambitious aim of eliminating malaria in the country by 2036 [4]. Intensive malaria control interventions based on the PAMAFRO experience in high-risk malaria villages contributed to a substantial reduction in the malaria incidence in Loreto, reporting 22 037 cases in 2019 [5]. As transmission decreases, malaria strategies need to be more focalized and adapted to the local malaria epidemiology determined by the complex interactions among Plasmodium parasites, human behavior, and highly variable environments driving changes in mosquito vector behavior and habitat suitability [1, 6, 7].
Rural riverine villages, characterized by poverty and limited access to health facilities, concentrate an important proportion of reported malaria cases in Loreto [8]; however, transmission dynamics in these villages remain understudied [9, 10]. By accounting for the ecological heterogeneity of 2 distinctive river basins, we identified 4 riverine villages (2 on each river) in the endemic district of Mazan in Loreto department and calculated the prevalence of malaria infections by quantitative real-time PCR (qPCR) in March and September 2016, and March 2017. These parasitological measurements were then related to incidence rates of reported malaria episodes between January 2016 and June 2017, and to available entomological and environmental observations in the same period [11, 12], to better characterize malaria transmission in these villages.
METHODS
Study Area
The district of Mazan is home to approximately 13 900 inhabitants, with one-third of them living in Mazan town near the confluence of the Napo River and its tributary the Mazan River about 50 km from Iquitos city (capital of Loreto). Mazan was among the districts in Loreto with the highest annual parasite index in 2017 (annual parasite incidence, 96.4 cases per 1000 inhabitants; P. vivax, 1066 cases; P. falciparum, 374 cases) [5, 13]. Most cases (>80%) occurred in approximately 20 villages located in the Mazan and Napo River basins. The exophilic, exophagic, and anthropophagic Anopheles darlingi is the main malaria vector [12]. The primary interventions prior to this study were routine passive case detection, seasonally indoor residual spraying, and the delivery of LLINs in the first months of 2016.
Design and Selected Villages
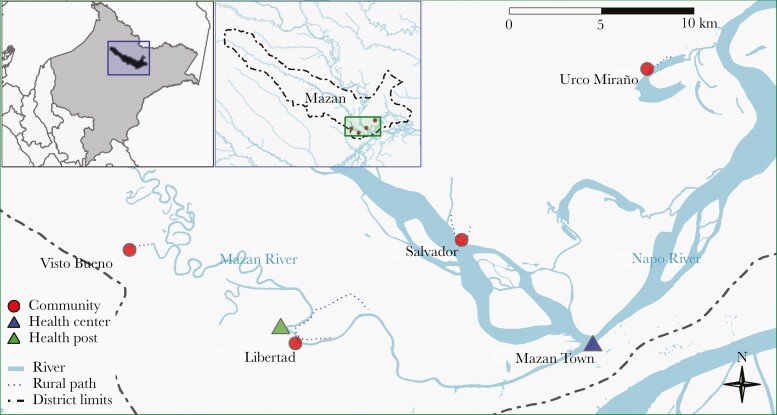
This study integrated available sociodemographic, parasitological, entomological and environmental data from Libertad (LIB) (3.496°S, 73.234°W) and Visto Bueno (VIB) (3.449°S, 73.317°W) in the Mazan basin, and Salvador (SAL) (3.445°S, 73.154°W) and Urco Miraño (URC) (3.361°S, 73.064°W) in the Napo basin (Figure 1 and Supplementary Table 1).
Figure 1.
Study area, 4 riverine villages in Mazan district, Loreto department.
The Direction of Loreto (477–2016), Universidad Peruana Cayetano Heredia (UPCH, 64746), and WHO Ethics Review Committee (0002669) approved entomological collections, while UPCH (66235) approved sociodemographic and parasitological surveys.
Baseline Data
Households were censused, georeferenced, and updated during surveys in March and September 2016 and March 2017. Mobile devices programmed with Open Data Kit allowed for the collection of sociodemographic and epidemiological data, including questions about LLIN use, and the time to go to sleep and wake up.
Prevalence Data
Malaria prevalence by both microscopy and qPCR were estimated from datasets of a pilot study that assessed the effects and costs of population-wide malaria test-and-treat interventions (in addition to passive case detection) before the high malaria transmission season in the Peruvian Amazon [13, 14]. Visits to all censused households in VIB and URC were made in March and September 2016 and March 2017. Available household members in March 2016 and March 2017 had axillary temperature taken and history of fever or any other malaria-compatible symptom documented. Finger-prick blood samples were collected for immediate microscopy in the field and dried blood spots for later analysis by qPCR. Health workers from the Ministry of Health accompanied personnel conducting field research activities, and treated microscopically confirmed infections regardless of symptoms according to national guidelines [15]. The same procedures were carried out in September 2016, but microscopic diagnosis was not performed by the pilot study; individuals with any malaria-compatible symptom were referred to health facilities for diagnosis and treatment.
Additional household visits in LIB and SAL were made in April 2016 and April 2017 (30 days after visits in March). A supplementary intervention in these villages consisted of making qPCR results available within 3 days of the sample collection in March and April of 2016 and 2017, allowing for the treatment of qPCR-positive individuals who were negative in an initial microscopic test. These qPCR tests were processed at the local research laboratory in Iquitos (Supplementary Material).
Incidence Data
Passive case-detection data of microscopically confirmed malaria episodes between January 2016 and June 2017 were obtained from the Ministry of Health surveillance systems [5] and verified with data registered at health facilities. Monthly incidence rates were calculated as the number of registered episodes (× 100) divided by the number of individuals censused during the baseline survey. In cases of recurrent registered episodes, intervals ≥ 60 days for P. vivax and ≥ 30 days for P. falciparum were used to identify independent episodes [16].
Entomological and River Level Data
Published data of indoor and outdoor 12-hour mosquito collections by human landing catch were used to estimate human biting rates (HBRs), the hourly biting behavior of An. darlingi, and entomological inoculation rates (EIRs) in March, June, September, and November 2016, and March 2017 [12]. Available vector and human behavior data were used to provide a rough estimate of the proportion of human exposure to An. darlingi occurring indoors, in scenarios in which people are or are not protected by LLIN, as described by Monroe et al [17]. Larval sampling data from water bodies located by ground inspection within 1 km of each village (September and November 2016, and March 2017) identified An. darlingi breeding sites [11]. Daily water levels of the Napo River measured by a hydrological station near the confluence of the Napo and Mazan rivers were averaged monthly [18].
Data Analysis
Baseline characteristics between villages were compared using the χ2 test. Trend charts related monthly incidence rates, malaria prevalence, HBRs, EIRs, and river levels. Uni- and multivariate mixed-effects logistic regression models determined risk factors for malaria infection by qPCR in March 2016. The following potential risk factors were assessed as fixed effects: age, sex, occupation, malaria antecedent, LLIN ownership, LLIN use, and housing structure. Random effects accounted for individuals nested within households, and households nested within villages. Factors with P values < .2 for the Wald test in the univariate analysis were included in the full multivariate model. Manual backward elimination guided by the minimization of the Akaike information criterion reduced of the number of factors in final models. Likelihood ratio tests compared nested models.
Households were mapped and their age-standardized malaria prevalence estimated considering the population’s age structure. Global Moran index (I) assessed the spatial autocorrelation of malaria prevalence in villages. Local Moran I with correction by false discovery rate assessed the variation in spatial autocorrelation over the village area, allowing for the classification of each household into 5 categories: high-high (a high prevalent household surrounded by high prevalent households), low-low (a low prevalent household surrounded by low prevalent households), outliers high-low and low-high, and not significant. A maximum neighborhood distance criterion of 550 meters was chosen for LIB, URC, and SAL [10], whereas it was set at 200 meters for VIB given the smaller distance between households. Malaria prevalence was weighted according to the inverse of the distance to the neighboring house. All analysis and maps were done using R version 2.15 software (R Development Core Team, R Foundation for Statistical Computing).
RESULTS
A total of 205 households and 1015 residents were censused and distributed as follows: LIB (60 households, 297 individuals), VIB (15 households, 60 individuals), URC (53 households, 284 individuals), and SAL (77 households, 374 individuals) (Supplementary Table 1). The overall ratio of female to male was 0.9, with most individuals aged younger than 20 years (median, 16.2). Secondary or higher education level was higher in URC (28.7%) than in other villages (approximately 10%) (P < .001). Forest-related economic activities predominated (31.2%–41.7%) with no differences between villages. Compared to LIB and SAL, residents in URC and VIB more frequently reported malaria antecedent in the past year as well as appropriate LLIN household coverage (P < .01). LLIN use the previous night ranged between 75.3% and 81.4%, with no differences across villages. Housing structures without complete wooden walls were common (63.2%), especially in LIB (73.7%) (P < .001). In contrast to VB and LIB, in URC and SAL, tin frequently replaced palm leaves as ceiling material and more participants reported having electricity at home (P < .001) (Supplementary Table 2 and Supplementary Table 3). This latter finding was negatively associated with the proportion of individuals sleeping at 20:00 hours in URC (75%) and SAL (70%) in comparison with those in VB (88%) and LIB (96%) (P < .001).
Samples for qPCR analysis were available for 823 individuals in March 2016, 843 in September 2016, and 800 in March 2017. Malaria prevalence was the highest in March 2016 for all villages (LIB, 40.9% [95% confidence interval, CI, 34.5%–47.5%]; VIB, 27.5% [95% CI, 16.3%–42.0%]; SAL, 9.2% [95% CI, 6.4%–13.0%]; and URC, 30.7% [95% CI, 24.7%–37.4%]), with P. vivax to P. falciparum ratios (LIB, 5:1; VIB, 1.3:1; SAL, 28:1; and URC, 21:1) and submicroscopic to microscopic infection ratios (LIB, 93:1; VIB, 13:1; SAL, 29:1; and URC, 16:1) varying widely across villages (Table 1, Figure 2A, Figure 3A, Figure 4A, and Figure 5A). Submicroscopic mixed infections were found in LIB (6 infections) and SAL (1 infection). The prevalence in September 2016 decreased for all villages (LIB, 11.8% [95% CI, 8.1%–16.7%]; VIB, 23.6% [95% CI, 13.7%–37.3%]; SAL, 3.8% [95% CI, 2.0%–6.7%]; and URC, 13.4% [95% CI, 9.4%–18.7%]), but the P. vivax to P. falciparum ratio continued to fluctuate across villages (LIB, 6:1; VIB, 3:1; SAL, 2:1; and URC, 28:1). The lowest malaria prevalence, composed of mainly submicroscopic infections, were found in March 2017 (LIB, 8.1% [95% CI, 4.9%–12.8%]; VIB, 6.1% [95% CI, 1.6%–17.9%]; SAL, 4.4% [95% CI, 2.5%–7.4%]; and URC, 1.8% [95% CI, 0.6%–4.9%]) with P. vivax to P. falciparum infection ratios ranging between 1:2 and 2:1.
Table 1.
Prevalence of Malaria by qPCR in March 2016, September 2016, and March 2017
| March 2016 | September 2016a | March 2017 | ||||
|---|---|---|---|---|---|---|
| Infection Detected | n (%) | 95% CI | n (%) | 95% CI | n (%) | 95% CI |
| Libertad | ||||||
| Total samples (n) | 230 | 238 | 211 | |||
| Overall | 94 (40.9) | 34.5–47.5 | 28 (11.8) | 8.1–16.7 | 17 (8.1) | 4.9–12.8 |
| P. vivax | 73 (31.7) | 25.9–38.2 | 24 (10.1) | 6.7–14.8 | 11 (5.2) | 2.8–9.4 |
| Submicroscopic | 72 (31.3) | 25.5–37.8 | … | … | 7 (3.3) | 1.5–7.0 |
| Microscopic | 1 (0.4) | .0–2.8 | … | … | 4 (1.9) | .6–5.1 |
| P. falciparum | 15 (6.5) | 3.8–10.7 | 4 (1.7) | .5–4.5 | 6 (2.8) | 1.2–6.4 |
| Submicroscopic | 15 (6.5) | 3.8–10.7 | … | … | 6 (2.8) | 1.2–6.4 |
| Microscopic | 0 (0.0) | … | … | 0 (0.0) | ||
| Mixed | 6 (2.6) | 1.1–5.9 | 0 (0.0) | 0 (0.0) | ||
| Visto Bueno | ||||||
| Total samples (n) | 51 | 55 | 49 | |||
| Overall | 14 (27.5) | 16.3–42.0 | 13 (23.6) | 13.7–37.3 | 3 (6.1) | 1.6–17.9 |
| P. vivax | 8 (15.7) | 7.5–29.1 | 9 (16.4) | 8.2–29.3 | 2 (4.1) | .7–15.1 |
| Submicroscopic | 8 (15.7) | 7.5–29.1 | … | … | 2 (4.1) | .7–15.1 |
| Microscopic | 0 (0.0) | 0 (0.0) | ||||
| P. falciparum | 6 (11.8) | 4.9–24.6 | 3 (5.5) | 1.4–16.1 | 1 (2.0) | .1–12.2 |
| Submicroscopic | 5 (9.8) | 3.7–22.2 | … | … | 1 (2.0) | .1–12.2 |
| Microscopic | 1 (2.0) | .1–11.8 | … | … | 0 (0.0) | |
| Mixed | 0 (0.0) | 1 (1.8) | .1–11.0 | 0 (0.0) | .2–9.1 | |
| Salvador | ||||||
| Total samples (n) | 327 | 319 | 318 | |||
| Overall | 30 (9.2) | 6.4–13.0 | 12 (3.8) | 2.0–6.7 | 14 (4.4) | 2.5–7.4 |
| P. vivax | 28 (8.6) | 5.9–12.3 | 7 (2.2) | 1.0–4.7 | 9 (2.8) | 1.4–5.5 |
| Submicroscopic | 27 (8.3) | 5.6–11.9 | … | … | 7 (2.2) | 1.0–4.7 |
| Microscopic | 1 (0.3) | .0–2.0 | … | … | 2 (0.6) | .1–2.5 |
| P. falciparum | 1 (0.3) | .0–2.0 | 4 (1.3) | .4–3.4 | 5 (1.6) | .6–3.8 |
| Submicroscopic | 1 (0.3) | .0–2.0 | … | … | 5 (1.6) | .6–3.8 |
| Microscopic | 0 (0.0) | … | … | 0 (0.0) | ||
| Mixed | 1 (0.3) | .0–2.0 | 1 (0.3) | .0–2.0 | 0 (0.0) | |
| Urco Miraño | ||||||
| Total samples (n) | 215 | 231 | 222 | |||
| Overall | 66 (30.7) | 24.7–37.4 | 31 (13.4) | 9.4–18.7 | 4 (1.8) | .6–4.9 |
| P. vivax | 63 (29.3) | 23.4–35.9 | 28 (12.1) | 8.3–17.2 | 1 (0.5) | .0–2.9 |
| Submicroscopic | 59 (27.4) | 21.7–34.0 | … | … | 1 (0.5) | .0–2.9 |
| Microscopic | 4 (1.9) | .6–5.0 | … | … | 0 (0.0) | |
| P. falciparum | 3 (1.4) | .4–4.4 | 1 (0.4) | .0–2.8 | 2 (0.9) | .2–3.6 |
| Submicroscopic | 3 (1.4) | .4–4.4 | … | … | 2 (0.9) | .2–3.6 |
| Microscopic | 0 (0.0) | … | … | 0 (0.0) | ||
| Mixed | 0 (0.0) | 2 (0.9) | .2–3.4 | 1 (0.5) | .0–2.9 | |
Abbreviations: CI, confidence interval; P. falciparum, Plasmodium falciparum; P. vivax, Plasmodium vivax; qPCR, quantitative polymerase chain reaction.
aMicroscopic diagnosis not performed.
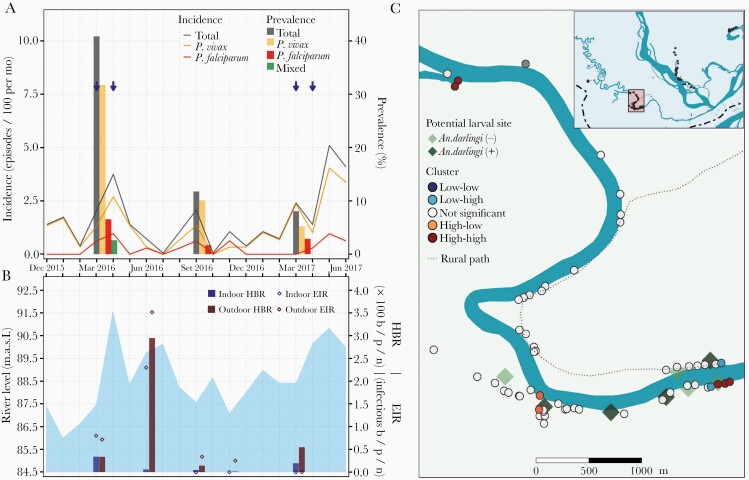
Figure 2.
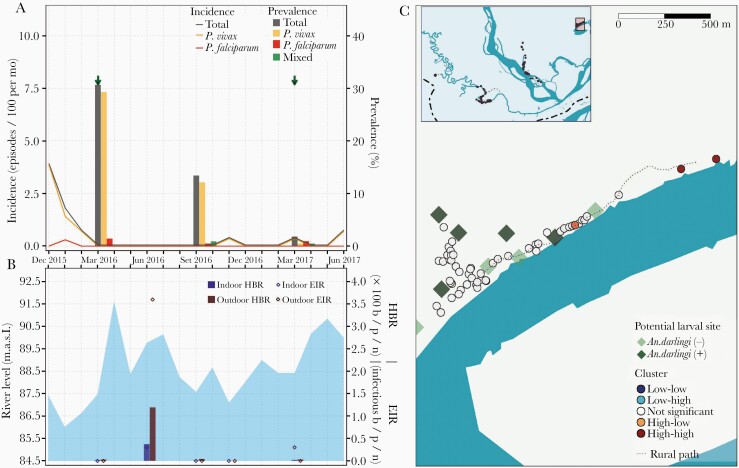
Parasitological, entomological, and river-level observations between January 2016 and June 2017, and spatial clusters of malaria prevalence in March 2016 in Libertad village. A, Evolution of malaria prevalence and incidence rates: Arrows indicate months when test-and-treat interventions were conducted with polymerase chain reaction (PCR) result available for malaria treatment within 3 days of the sample collection. B, Bars represent human biting rates (HBR) and points entomological inoculation rates (EIR) estimated in March, June, September and November 2016 and in March 2017. Shaded area represents monthly water levels of the Napo River. C, Spatial distribution of households classified according to local Moran I as high-high, high-low, not significant, low-high, and low-low clusters of malaria prevalence in March 2016. Abbreviations: b/p/n, bites/person/night; MASL, meters above sea level.
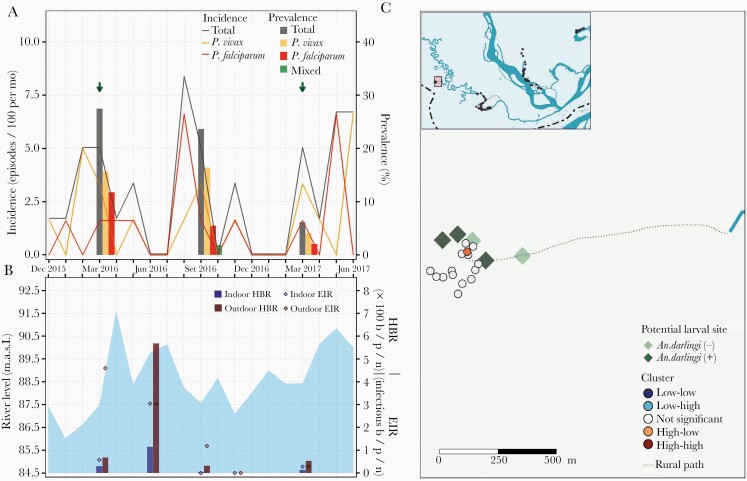
Figure 3.
Parasitological, entomological, and river level observations between January 2016 and June 2017, and spatial clusters of malaria prevalence in March 2016 in Visto Bueno village. A, Evolution of malaria prevalence and incidence rates: Arrows indicate months when test-and-treat interventions were conducted using microscopy. B, Bars represent human biting rates (HBR) and points entomological inoculation rates (EIR) estimated in March, June, September, and November 2016 and in March 2017. Shaded area represents monthly water levels of the Napo River. C, Spatial distribution of households classified according to local Moran I as high-high, high-low, not significant, low-high, and low-low clusters of malaria prevalence in March 2016. Abbreviations: b/p/n, bites/person/night; MASL, meters above sea level.
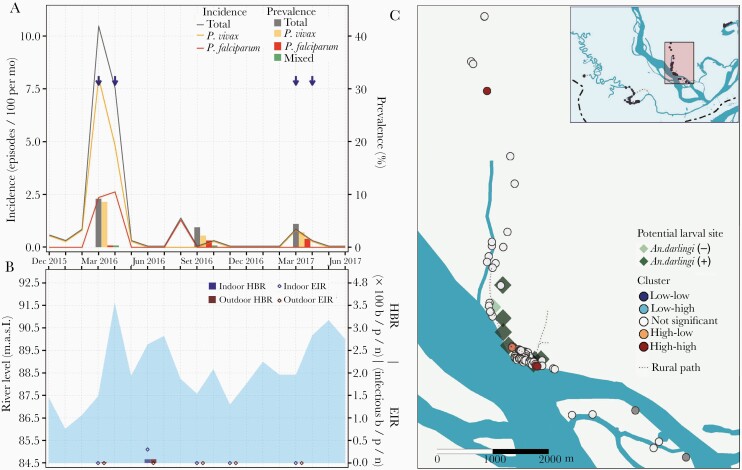
Figure 4.
Parasitological, entomological, and river level observations between January 2016 and June 2017, and spatial clusters of malaria prevalence in March 2016 in Salvador village. A, Evolution of malaria prevalence and incidence rates: Arrows indicate months when test-and-treat interventions were conducted with polymerase chain reaction (PCR) result available for malaria treatment within 3 days of the sample collection. B, Bars represent human biting rates (HBR) and points entomological inoculation rates (EIR) estimated in March, June, September, and November 2016 and in March 2017. Shaded area represents monthly water levels of the Napo River. C, Spatial distribution of households classified according to local Moran I as high-high, high-low, not significant, low-high, and low-low clusters of malaria prevalence in March 2016. Abbreviations: b/p/n, bites/person/night; MASL, meters above sea level.
Figure 5.
Parasitological, entomological, and river level observations between January 2016 and June 2017, and spatial clusters of malaria prevalence in March 2016 in Urco Miraño village. A, Evolution of malaria prevalence and incidence rates: Arrows indicate months when test-and-treat interventions were conducted using microscopy. B, Bars represent human biting rates (HBR) and points entomological inoculation rates (EIR) estimated in March, June, September, and November 2016 and in March 2017. Shaded area represents monthly water levels of the Napo River. C, Spatial distribution of households classified according to local Moran I as high-high, high-low, not significant, low-high, and low-low clusters of malaria prevalence in March 2016. Abbreviations: b/p/n, bites/person/night; MASL, meters above sea level.
In all villages, HBRs reached the highest levels 2 months after the peak of the Napo River (June 2016) (Figure 2B, Figure 3B, Figure 4B, and Figure 5B), and were followed by an increase in incidence in August–September 2016 (second peak) of predominantly P. vivax episodes in LIB and P. falciparum episodes in VIB and SAL. Only in VIB, the highest EIR was found in March 2016, not coinciding with the highest HBR. In comparison with villages in the Napo basin (SAL and URC), villages in the Mazan basin (LIB and VIB) had higher HBR and EIR levels, and maintained higher prevalence and incidence rates despite test-and-treat interventions. In contrast to the highest HBR levels outside houses in most surveys, differences between outdoor and indoor EIR values were less obvious. About 90% of human exposure to An. darlingi bites between 18:00 and 6:00 hours occurred indoors where LLINs were not available and/or used, according to the matched analysis of mosquito and human behavior data. The current LLIN usage in villages would prevent approximately 60% of human exposure (Supplementary Table 4).
The multivariate model for malaria infection by qPCR in March 2016 confirmed the influence of clustered data at household and village level, and the increased risk for individuals living in houses with incomplete walls (adjusted OR [aOR], 1.7; 95% CI, 1.1–2.6) (Table 2). Malaria prevalence was also slightly higher in individuals between 10 and 29 years old (aOR, 1.5; 95% CI, 1.0–2.3; P = .073) and men (aOR, 1.4; 95%CI, 1.0–2.0; P = .067) compared respectively with younger children and women, but not significantly.
Table 2.
Uni- and Multivariate Risk Factor Analysis for Malaria Infection in March 2016
| Risk Factor | n/N % | OR | OR 95% CI | aOR | aOR 95% CI |
|---|---|---|---|---|---|
| Age group, y | |||||
| <10 | 60/ 290 (20.7) | Ref | |||
| 10–29.9 | 70/ 243 (28.8) | 1.5** | .9–2.2 | 1.5** | 1.0–2.3 |
| ≥30 | 74/ 290 (25.5) | 1.2 | .8–1.8 | 1.2 | 0.8–1.8 |
| Sex | |||||
| Female | 89/ 399 (22.3) | Ref | |||
| Male | 115/ 424 (27.1) | 1.3 | .9–1.9 | 1.4** | 1.0–2.0 |
| Occupation | |||||
| Not forest related | 128/ 515 (24.9) | Ref | |||
| Forest related | 76/ 308 (24.7) | 1.0 | .7–1.4 | ||
| Malaria in the past year | |||||
| No | 90/ 395 (22.8) | Ref | |||
| Yes | 111/ 413 (26.9) | 1.3 | .9–1.9 | ||
| Sleep under a LLIN the previous night | |||||
| Yes | 154/ 640 (24.1) | Ref | |||
| No | 48/ 176 (27.3) | 1.2 | .8–1.9 | ||
| 1 LLIN for 2 people | |||||
| Yes | 89/ 390 (22.8) | Ref | |||
| No | 115/ 433 (26.6) | 1.3 | .9–1.9 | ||
| Indoor residual spray in past year | |||||
| Yes | 154/ 484 (31.8) | Ref | |||
| No | 49/ 336 (14.6) | 0.9 | .5–1.5 | ||
| Number of external walls in house | |||||
| Complete, 4 | 52/ 288 (18.1) | Ref | |||
| None/incomplete, 0–3 | 152/ 535 (28.4) | 1.6* | 1.1–2.5 | 1.7* | 1.1–2.6 |
| Ceiling material | |||||
| Tin, calamina | 63/ 283 (22.3) | Ref | |||
| Palm leaves | 141/ 540 (26.1) | 1.0 | .6–1.6 | ||
| Electricity available | |||||
| Yes | 74/ 345 (21.4) | Ref | |||
| No | 130/ 478 (27.2) | 0.8 | .5–1.3 | ||
| Distance to Anopheles darlingi larval site | |||||
| ≥ 200 meters | 140/ 485 (28.9) | Ref | |||
| < 200 meters | 63/ 314 (20.1) | 0.8 | .5–1.2 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; n, number of malaria infected people; N, total number of people; OR, odds ratio; Ref, reference.
* P < .05, **P < .01
Global Moran I confirmed spatial autocorrelation of age-standardized household prevalence in March 2016 in 2 villages: LIB (I = 0.13, P = .04) and URC (I = 0.09, P = .04). Local Moran I identified high-high households (high-risk clusters) in LIB, URC, and SAL, but not in VIB (Figure 2C, Figure 3C, Figure 4C, and Figure 5C). High-risk clusters in LIB and URC were, respectively, 5 and 2 of the outermost households in the villages, while in SAL this included 2 households in the most populated area and 1 outermost household near a narrow river gorge that joins the Napo River. In addition, high-low outliers (high prevalent households surrounded by low prevalent households) were identified in all villages, located mainly in the most populated areas. High-risk clusters were not closer to An. darlingi larval sites when compared with other households (P > .05).
DISCUSSION
The combined analysis of parasitological, entomological, and environmental observations between January 2016 and June 2017 allowed for an in-depth characterization of malaria transmission dynamics in riverine villages in Mazan, an Amazonian district where poverty, limited accessibility to health care, and village-specific ecology create challenges for malaria control. Despite some degree of heterogeneity across villages, malaria prevalence in the survey in March 2016 was high (> 25% in 3 villages), comprising mostly P. vivax submicroscopic infections. Whereas the housing without complete walls was the main risk factor for malaria, the proximity to An. darlingi breeding sites was not associated with malaria. Interestingly, villages in the Mazan basin had the highest entomological indicators and maintained the highest prevalence and incidence rates among study villages despite test-and-treat interventions.
The earlier high malaria incidence rates in LIB, VIB, and SAL in March–April 2016 in comparison to historically seasonal patterns of malaria incidence in the Mazan district (with peaks usually in June–July) are mainly explained by increased test-and-treat efforts in those months as part of a concurrent intervention pilot study. These efforts may have affected the temporal dynamics of malaria transmission, bringing a second peak of malaria incidence in these villages 2–3 months after their highest HBR levels in June 2016. The submicroscopic parasite carriers may have contributed to a silent infectious reservoir able to maintain mild-moderate transmission, despite population-wide malaria testing and treatment of confirmed infections in March–April of 2016 and 2017, and the drop in vector-human contact rates in the dry season of 2016. Indeed, several cross-sectional surveys in the Peruvian Amazon have consistently observed that a majority of P. vivax and P. falciparum infections could only be detected by sensitive molecular tests, suggesting the important role of these submicroscopic infections in sustaining malaria transmission [19–21]. For instance, a recent cohort study that screened full populations for malaria in riverine endemic villages showed that most low-density infections escaped standard surveillance using microscopy, even if residents were consecutively screened at intervals of 10 days over a month [14].
The persistently higher prevalence and incidence rates in the Mazan basin compared to those in the Napo basin (regardless of the type of test-and-treat intervention implemented) were in agreement with higher HBRs and EIRs in Mazan villages compared with the Napo ones. Contrary to what was expected, An. darlingi breeding sites in the Mazan basin were not the most numerous and densely inhabited by larvae among the study villages. Indeed, our group recently showed that SAL (Napo basin) was the village with the greatest number of An. darlingi larvae and anopheline larvae in general during the same study period, despite having the lowest density of adult mosquitoes [11, 12]. Notably, the presence and density of mosquito larvae in aquatic habitats and consequently the number of adults capable of malaria transmission are regulated by a variety of ecosystem processes operating and interacting at several organizational levels and spatial and temporal scales [22]. Understanding the patterns in the productivity of larval habitats requires knowledge about the local interactions between abiotic (eg, hydrology, temperature, shade, pH, salinity, and nutrients) and biotic (eg, predation and competition) factors [23–25]. A recent study of larval habitats in Mazan and Napo river watersheds found a correlation between An. darlingi larval sites and low forest coverage, low sunlight, and emergent vegetation [11]. Biotic factors, however, such as larval competition remain understudied [26]; it would be interesting to know, for instance, whether the proportion of An. darlingi larvae among total anopheline larvae (higher in water bodies in LIB and VIB than that in SAL) is a better determinant of the abundance of adult mosquitoes than larval densities (higher in SAL than in LIB and VIB).
The microgeographic landscape composition may also be an important determinant of the productivity of larval breeding sites, the abundance of adult mosquitoes, and ultimately malaria transmission in villages [26]. A recent study by our group, with high-resolution imagery captured by drones, showed remarkably heterogeneous land cover composition in study villages, suggesting a high diversity of locations at a microgeographical scale where An. darlingi can breed [27]. Published orthomosaics from this imagery [27] together with additional field visits confirmed that all outermost households classified as spatial clusters of high malaria prevalence (high-high) in LIB, SAL, and URC were located near forest edges. Habitats in marsh and forest edges (especially in early stages of settlement) are able to promote malaria transmission by both increasing entomological risk and increasing human–vector contact as a result of human behavioral patterns associated with economical activities [28]. In an urban area in Senegal, for instance, An. arabiensis densities and EIR estimates showed a negative correlation with the distance to the marsh edges [29], while in rural villages in south-eastern Tanzania, high densities of malaria vectors were observed to be clustered in peripheral houses [30]. Numerous studies in the Brazilian Amazon also found a relationship between malaria and proximity to forest, finding high densities of larval and adult An. darlingi in forest fringes, as well as increased malaria infections in populations living or working near forest edges [31, 32].
With the main malaria vector An. darlingi biting predominantly outdoors, it was not surprising to find a trend of the association of men and individuals aged 10–29 years with increased malaria infection. Similar relationships have been detected in many cross-sectional surveys in the Amazon, explained by the increased outdoor-, social-, and forest-related activities of men, adolescents, and young adults, usually when mosquitoes are more active [19, 20, 33]. However, housing without complete walls as the main risk factor for malaria (observed in other studies in the Amazon) [34, 35] and indoor EIRs as high as outdoor EIRs in some villages (at different times) suggests that intradomiciliary transmission is common in some settings. Indeed, accounting for human behavior in villages [17], we estimated that approximately 90% of human exposure to An. darlingi at dusk and dawn would occur indoors where LLINs were not available and/or used. Several studies in Africa reported that main vectors are not predominantly endophagic, but they overwhelmingly preferred feeding at times when most humans are indoors [36–39]. This feeding pattern driven by the mosquito preference for humans in the indoor environment has also been observed in coendemic settings for P. vivax and P. falciparum in Salomon Islands [40] and Colombia [41], which are dominated by vectors that feed predominantly outdoors.
The successful application of different discipline methodologies to understand malaria transmission dynamics in endemic and remote riverine villages in Mazan together with the comprehensive integration of parasitological, vector, and environmental data prospectively collected during 18 months can be underscored as the main strengths of our study. Nevertheless, difficult access to villages together with budget constraints limited the number of collections of An. darlingi mosquitoes during the study period, preventing the statistical assessment of the relationship between parasitological and entomological indicators. Similarly, water level data of the Mazan River were not available to relate to those indicators in LIB and VIB; however, the fact that the highest HBR in these villages occurred 2 months after the peak level of Napo River as in SAL and URC, suggests that water levels of the Mazan River follow the same trends as those of the Napo. Moreover, there are logistical limitations to ground exploration for the identification of all potential breeding sites, and the assessment of proximity to them as a malaria risk factor. Finally, we only performed the risk factor analysis and the spatial analysis with prevalence data of March 2016, because the prevalence of other surveys was likely affected by the deployment of test-and-treat interventions.
Our study confirmed higher prevalence and incidence rates associated with higher density of adult An. darlingi in the Mazan basin compared with the Napo basin. High microheterogeneity in malaria transmission was observed within the same villages, as a result of interactions between the microgeographic landscape driving diverse conditions for malaria vector development, the housing structure, and human behavior. Considering the evidence for indoor malaria transmission in the study villages, we recommend the delivery and opportune replacement of LLINs to increase coverage and prevention levels to An. darlingi exposure, and education interventions to insure their appropriate use and conservation. Local initiatives to improve housing conditions should be encouraged because they are not only able to reduce malaria, but also they improve health and well-being in general. New vector strategies such as spatial repellents [42] and attractive toxic sugar baits [43], capable of altering mosquito feeding behavior and reducing mosquito densities, both indoor and outdoor, can complement the use of LLINs to further reduce malaria transmission. Similarly, community environment-based management, aimed at removing and/or reducing temporary water collections near houses, and larval source management of permanent and large breeding sites, have been found to be effective vector measures in some endemic settings [44, 45]. The feasibility, operability, and effectiveness of new vector control strategies need to be assessed prior to implementation in the heterogeneous Amazon basin landscape.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank all residents and local authorities from riverine villages in Mazan for their enthusiastic participation in the study, as well as all field workers for their dedication during the study. This publication has been possible thanks to the authorization and permits N.0424-2012-AG-DGFFS-DGEFFS from Dirección de Gestión Forestal y de Fauna Silvestre and Dirección General Forestal y de Fauna Silvestre del Ministerio de Agricultura de la República del Perú.
Author contributions. A. R. A., M. M., N. S., and D. G. conceived and designed the study. D. M. G., M. M., M. S., M. G., J. B., F. A., K. A., G. C. E., and K. P. supervised the fieldwork. M. S., J. C. M., and K. P. supervised the laboratory assays. A. R. A., K. A., and G. C. E. contributed to the data management and the consolidation of the fieldwork and laboratory data. A. R. A., D. M. G., and N. S. conducted the analysis. A. R. A. prepared the figures and tables and wrote the first draft of the paper. M. M., D. M. G., N. S., J. E. C., J. V., A. L. C., and D. G. contributed to the result interpretation and writing of the paper. All authors read and approved the final manuscript.
Financial support. This work was supported by the Consejo Nacional de Ciencia, Tecnología e Innovación Tecnológica (grant number 008-2014-FONDECYT); the Académie de Recherche et d’Enseignement Supérieur-Commission de la Coopération au Développement of Belgium (grant number ARES-CCD, PRD-Peru 2014–2019 to N. S., A. L. C., and A. R. A.); World Health Organization Special Programme for Research and Training in Tropical Diseases (grant number 201460655 to D. G.); and National Institutes of Health, National Institute of Allergy and Infectious Diseases (grant numbers U19AI089681 to J. M. V. and R01AI110112 to J. E. C.). A. R. A. is a Postdoctoral Researcher of the Fonds de la Recherche Scientifique (FNRS, Belgium).
Supplement sponsorship. The supplement is sponsored by TDR, the Special Programme for Research and Training in Tropical Diseases, based at the World Health Organization.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Rosas-Aguirre A, Gamboa D, Manrique P, et al. Epidemiology of Plasmodium vivax malaria in Peru. Am J Trop Med Hyg 2016; 95:133–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soto-Calle V, Rosas-Aguirre A, Llanos-Cuentas A, et al. Spatio-temporal analysis of malaria incidence in the Peruvian Amazon region between 2002 and 2013. Sci Rep 2017; 7:40350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ministerio de Salud del Perú. Tendencia y situación de las enfermedades sujetas a vigilancia epidemiológica: malaria. Bol Epidemiol 2015; 24:975–86. [Google Scholar]
- 4.Ministerio de Salud del Peru (MINSA). Plan malaria cero periodo 2017–2021. resolucion ministerial No 244–2017. Lima, Peru: MINSA, 2017. [Google Scholar]
- 5.Ministerio de Salud del Perú. Boletin epidemiologico del Peru. Bol Epidemiol 2019; 28:1346–451. [Google Scholar]
- 6.Coura JR, Suárez-Mutis M, Ladeia-Andrade S. A new challenge for malaria control in Brazil: asymptomatic Plasmodium infection—a review. Mem Inst Oswaldo Cruz 2006; 101:229–37. [DOI] [PubMed] [Google Scholar]
- 7.Cotter C, Sturrock HJ, Hsiang MS, et al. The changing epidemiology of malaria elimination: new strategies for new challenges. Lancet 2013; 382:900–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solano-Villarreal E, Valdivia W, Pearcy M, et al. Malaria risk assessment and mapping using satellite imagery and boosted regression trees in the Peruvian Amazon. Sci Rep 2019; 9:15173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker BS, Paredes Olortegui M, Peñataro Yori P, et al. Hyperendemic malaria transmission in areas of occupation-related travel in the Peruvian Amazon. Malar J 2013; 12:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrasco-Escobar G, Gamboa D, Castro MC, et al. Micro-epidemiology and spatial heterogeneity of P. vivax parasitaemia in riverine communities of the Peruvian Amazon: A multilevel analysis. Sci Rep 2017; 7:8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prussing C, Saavedra MP, Bickersmith SA, et al. Malaria vector species in Amazonian Peru co-occur in larval habitats but have distinct larval microbial communities. PLoS Negl Trop Dis 2019; 13:e0007412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saavedra MP, Conn JE, Alava F, et al. Higher risk of malaria transmission outdoors than indoors by Nyssorhynchus darlingi in riverine communities in the Peruvian Amazon. Parasit Vectors 2019; 12:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreno-Gutierrez D, Rosas-Aguirre A, Llanos-Cuentas A, et al. Economic costs analysis of uncomplicated malaria case management in the Peruvian Amazon. Malar J 2020; 19:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreno-Gutierrez D, Llanos-Cuentas A, Luis Barboza J, et al. Effectiveness of a malaria surveillance strategy based on active case detection during high transmission season in the Peruvian amazon. Int J Environ Res Public Health 2018; 15:2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ministerio de Salud del Perú (MINSA). Norma técnica para la atención de la malaria y malaria severa en el Perú. NTS Nro. 054-MINSA/DGSP-V.01, modificada en Febrero 2015. Lima, Peru: MINSA, 2015. [Google Scholar]
- 16.Bautista C, Chan A, Ryan J, et al. Epidemiology and spatial analysis of malaria in the northern Peruvian Amazon. Am J Trop Med Hyg 2007; 75:1216–22. [PubMed] [Google Scholar]
- 17.Monroe A, Moore S, Okumu F, et al. Methods and indicators for measuring patterns of human exposure to malaria vectors. Malar J 2020; 19:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Servicio de hidrografia y navegación de la Amazonía. Avisos a los navegantes fluviales. Marina de Guerra del Peru. 2016. https://www.dhn.mil.pe/shnaNEW/boletines/Avilona/Avisos/12-12-2016.pdf. Accessed 18 August 2020.
- 19.Rosas-Aguirre A, Speybroeck N, Llanos-Cuentas A, et al. Hotspots of malaria transmission in the Peruvian Amazon: rapid assessment through a parasitological and serological survey. PLoS One 2015; 10:e0137458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosas-Aguirre A, Guzman-Guzman M, Gamboa D, et al. Micro-heterogeneity of malaria transmission in the Peruvian Amazon: a baseline assessment underlying a population-based cohort study. Malar J 2017; 16:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrasco-Escobar G, Miranda-Alban J, Fernandez-Miñope C, et al. High prevalence of very-low Plasmodium falciparum and Plasmodium vivax parasitaemia carriers in the Peruvian Amazon: insights into local and occupational mobility-related transmission. Malar J 2017; 16:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rejmankova E, Grieco J, Achee N, et al. Freshwater community interactions and malaria. In: Collinge SK and Ray C, eds. Disease ecology . Oxford, UK: Oxford University Press, 2007:90–104. [Google Scholar]
- 23.Washburn JO. Regulatory factors affecting larval mosquito populations in container and pool habitats: implications for biological control. J Am Mosq Control Assoc 1995; 11:279–83. [PubMed] [Google Scholar]
- 24.Knight TM, Chase JM, Goss CW, Knight JJ. Effects of interspecific competition, predation, and their interaction on survival and development time of immature Anopheles quadrimaculatus. J Vector Ecol 2004; 29:277–84. [PubMed] [Google Scholar]
- 25.Stresman GH. Beyond temperature and precipitation: ecological risk factors that modify malaria transmission. Acta Trop 2010; 116:167–72. [DOI] [PubMed] [Google Scholar]
- 26.Manguin S. Anopheles mosquitoes—new insights into malaria vectors,2013. https://www.intechopen.com/books/anopheles-mosquitoes-new-insights-into-malaria-vectors. Accessed 5 April 2020.
- 27.Carrasco-Escobar G, Manrique E, Ruiz-Cabrejos J, et al. High-accuracy detection of malaria vector larval habitats using drone-based multispectral imagery. PLoS Negl Trop Dis 2019; 13:e0007105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacDonald AJ, Mordecai EA. Amazon deforestation drives malaria transmission, and malaria burden reduces forest clearing. Proc Natl Acad Sci U S A 2019; 116:22212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trape JF, Lefebvre-Zante E, Legros F, et al. Vector density gradients and the epidemiology of urban malaria in Dakar, Senegal. Am J Trop Med Hyg 1992; 47:181–9. [DOI] [PubMed] [Google Scholar]
- 30.Russell TL, Lwetoijera DW, Knols BG, Takken W, Killeen GF, Kelly-Hope LA. Geographic coincidence of increased malaria transmission hazard and vulnerability occurring at the periphery of two Tanzanian villages. Malar J 2013; 12:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barros FSM, Honório NA. Deforestation and malaria on the amazon frontier: larval clustering of Anopheles darlingi (Diptera: Culicidae) determines focal distribution of malaria. Am J Trop Med Hyg 2015; 93:939–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barros FS, Arruda ME, Gurgel HC, Honório NA. Spatial clustering and longitudinal variation of Anopheles darlingi (Diptera: Culicidae) larvae in a river of the Amazon: the importance of the forest fringe and of obstructions to flow in frontier malaria. Bull Entomol Res 2011; 101:643–58. [DOI] [PubMed] [Google Scholar]
- 33.Chuquiyauri R, Paredes M, Peñataro P, et al. Socio-demographics and the development of malaria elimination strategies in the low transmission setting. Acta Trop 2012; 121:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leandro-Reguillo P, Thomson-Luque R, Monteiro WM, de Lacerda MV. Urban and architectural risk factors for malaria in indigenous Amazonian settlements in Brazil: a typological analysis. Malar J 2015; 14:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corder RM, Paula GA, Pincelli A, Ferreira MU. Statistical modeling of surveillance data to identify correlates of urban malaria risk: a population-based study in the Amazon Basin. PLoS One 2019; 14:e0220980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huho B, Briët O, Seyoum A, et al. Consistently high estimates for the proportion of human exposure to malaria vector populations occurring indoors in rural Africa. Int J Epidemiol 2013; 42:235–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Killeen GF. A revival of epidemiological entomology in Senegal. Am J Trop Med Hyg 2018; 98:1216–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Killeen GF. Characterizing, controlling and eliminating residual malaria transmission. Malar J 2014; 13:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seyoum A, Sikaala CH, Chanda J, et al. Human exposure to anopheline mosquitoes occurs primarily indoors, even for users of insecticide-treated nets in Luangwa Valley, South-east Zambia. Parasit Vectors 2012; 5:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russell TL, Beebe NW, Bugoro H, et al. Frequent blood feeding enables insecticide-treated nets to reduce transmission by mosquitoes that bite predominately outdoors. Malar J 2016; 15:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elliott R. Studies on man vector contact in some malarious areas in Colombia. Bull World Health Organ 1968; 38:239–53. [PMC free article] [PubMed] [Google Scholar]
- 42.Bohbot JD, Dickens JC. Insect repellents: modulators of mosquito odorant receptor activity. PLoS One 2010; 5:e12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conn JE, Norris DE, Donnelly MJ, et al. Entomological monitoring and evaluation: diverse transmission settings of ICEMR projects will require local and regional malaria elimination strategies. Am J Trop Med Hyg 2015; 93:28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tusting LS, Thwing J, Sinclair D, et al. Mosquito larval source management for controlling malaria. Cochrane Database Syst Rev 2013; ( 8):CD008923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castro MC. Malaria transmission and prospects for malaria eradication: the role of the environment. Cold Spring Harb Perspect Med 2017; 7:a025601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.