Abstract
Introduction
In order to improve our understanding of the fundamental limits of core interventions and guide efforts based on prioritization and identification of effective/novel interventions with great potentials to interrupt persistent malaria transmission in the context of high vector control coverage, the drivers of persistent disease transmission were investigated in three eco-epidemiological settings; forested areas in Cameroon, coastal area in Kenya and highland areas in Ethiopia.
Methods
Mosquitoes were sampled in three eco-epidemiological settings using different entomological sampling techniques and analysed for Plasmodium infection status and blood meal origin in blood-fed specimens. Human behavioural surveys were conducted to assess the knowledge and attitude of the population on malaria and preventive measures, their night activities, and sleeping pattern. The parasitological analysis was conducted to determine the prevalence of Plasmodium infection in the population using rapid diagnostic tests.
Results
Despite the diversity in the mosquito fauna, their biting behaviour was found to be closely associated to human behaviour in the three settings. People in Kenya and Ethiopia were found to be more exposed to mosquito bites during the early hours of the evening (18-21h) while it was in the early morning (4-6 am) in Cameroon. Malaria transmission was high in Cameroon compared to Kenya and Ethiopia with over 50% of the infected bites recorded outdoors. The non-users of LLINs were 2.5 to 3 times more likely to be exposed to the risk of acquiring malaria compared to LLINs users. Malaria prevalence was high (42%) in Cameroon, and more than half of the households visited had at least one individual infected with Plasmodium parasites.
Conclusions
The study suggests high outdoor malaria transmission occurring in the three sites with however different determinants driving residual malaria transmission in these areas.
Keywords: persistent malaria transmission, human behavior, malaria vectors, Kenya, Ethiopia, Cameroon
Despite the fact that >90% of malaria cases still occur in Africa, the burden of the disease distribution is largely heterogeneous across the continent [1]. Some countries such as Morocco are now free of malaria whereas others, including Cameroon, Nigeria, and Democratic Republic of the Congo, are classified as high-burden, high-impact countries because they have the highest contribution to malaria morbidity and mortality. Yet tremendous success has been achieved in disease control during the last decade. In many countries the disease prevalence has been reduced by half and several are heading toward the elimination phase. This success is considered to have resulted from the scale-up of key vector control interventions such as long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS) [2–4]. However, since 2014 the trend in disease reduction has stalled, with several regions reporting increased prevalence of malaria cases or no reduction at all in disease morbidity and mortality [4], suggesting that several factors may be limiting the efficacy of core interventions. Insecticide resistance is considered as a major threat undermining current core vector control interventions; several other factors are thought to be equally affecting the efficiency of these tools, but these factors have so far not been fully assessed [5–8].
Undertaking malaria vector surveillance could contribute important information regarding local vector population ecology, behavior, and dynamics after the scaling up of control interventions or could enable better appraisal of residual/persistent malaria transmission [8]. Several vector species have been reported to sustain residual/persistent malaria transmission including Anopheles arabiensis and Anopheles gambiae in Africa, Anopheles dirus in Southeast Asia, and Anopheles albimanus and Anopheles darlingi in the Americas [8]. Persistent malaria transmission is considered to be driven by outdoor biting and resting behavior of vectors, reduced or limited contact of mosquitoes with treated materials, or early exiting from houses or early-evening biting behavior [8–10]. Such vector behaviors could occur naturally or may result from insecticide-induced selective forces on malaria vectors (irritancy, repellence, and/or toxicity) [11]. Understanding factors contributing to the limited, or even attenuated performance of control interventions could enable in improving approaches for better disease prevention and control by these tools or provide knowledge for designing improved disease prevention and control tools. It is still not clear whether persistent malaria transmission is a consequence of mosquito feeding on humans in the absence of protection, including being indoors but not under nets, or being outdoors away from protected houses due to occupational, domestic, or recreational activities, or if vectors succeed to bite and transmit disease while people are still protected [5, 6].
In Africa, >20 species are considered as malaria vectors; however, a few are responsible for most of malaria transmission [12], and the predominant vectors vary from one eco-epidemiological setting to another. For instance in West Africa, vectors such as An. gambiae, Anopheles coluzzii, and An. arabiensis are the main vectors responsible for most malaria cases [13]. In Central Africa, species such as An. gambiae, An. coluzzii, Anopheles funestus, Anopheles nili, and Anopheles moucheti play a major role in malaria transmission [14–18]. In the coastal area of East Africa, the main malaria vector species include An. gambiae, An. arabiensis, and An. funestus [19–23], whereas in highland areas, species such as An. arabiensis and Anopheles coustani also play a major role in malaria transmission [23, 24]. Most species of the An. gambiae complex and An. funestus group have developed resistance to insecticides [15, 20, 25–28]. Other species such as An. nili, An. moucheti, and An. coustani are still susceptible to most insecticides [29], but they display high outdoor biting and resting behavior, which enable them to escape the current control interventions that target indoor feeding and resting behavior of mosquitoes [15, 16]. It is also not known whether persistent transmission of malaria results from the combination of the complexity of the vectorial system and poor coverage by LLINs and IRS or if this differs from one eco-epidemiological setting to another. In the present study, data collected from 3 sub-Saharan African countries with different eco-epidemiological features—forested areas of Cameroon, a coastal area of Kenya, and highland areas of Ethiopia—were assessed to better understand factors promoting persistent malaria transmission in different malaria-endemic foci in sub-Saharan Africa.
METHODS
Study Sites
The study was conducted in 3 different eco-epidemiological settings of sub-Saharan Africa: Cameroon (forested area), Kenya (coastal area), and Ethiopia (highland area) (Figure 1 and Supplementary Table 1).
Figure 1.
Map showing the study sites in the 3 sub-Saharan African malaria-endemic countries.
Cameroon
The study was conducted in Olama (3°24′N; 11°18′E) and Nyabessan (2°80′N; 10°25′E) along the rivers Nyong and Ntem, respectively. They culminate at about 300–600 m above sea level. Both villages display high and perennial malaria transmission patterns and are located within the Congo-Guinean phytogeographic zone, characterized by a typical equatorial climate with 2 rainy seasons extending from March to June and September to November. Mean annual rainfall ranges from 1600 to 1800 mm [15] with daily temperature varying from 18°C to 28°C. Agriculture and fishing are the main socioeconomic activities of residents. Over the last decade, 3 (2004, 2011, and 2015), LLIN mass distribution campaigns have been conducted [30]. In 2019, the national malaria control program through the Ministry of Public Health launched the fourth LLIN distribution campaign. Anopheles gambiae sensu lato (s.l.), An. nili, and An. moucheti are the predominant malaria vectors in Nyabessan. In Olama, the main vector is An. moucheti followed by An. gambiae s.l. The coverage and usage of LLINs in the studied villages were high (approximately 96% and 90%, respectively).
Kenya
The study was conducted in 6 villages: Chodari (3°7676′N; 39°7710′E), Ngombeni (3°7402′N; 39°7721′E), Mapawa (3°7402′N; 39°7721′E), Ziani (3°7484′N; 39°7207′E), Makata (3°7323′; 39°7965′E) and Pingilikani (3°7596′; 39°7895′E) along the coastal strip of Kilifi County in the coastal region of Kenya that is malaria endemic. Kilifi’s climate is considered tropical with precipitation mainly occurring during April through June and October through December. Mean annual precipitation ranges from 750 to 1200 mm [31]. Anopheles gambiae sensu stricto (s.s.), An. arabiensis, Anopheles merus, and An. funestus are the primary vectors of malaria and show a highly anthropophilic behavior [31]. The main socioeconomic activities are mainly fishing, farming, and tourism. The government of Kenya through the Ministry of Health has been distributing LLINs through a 3-year mass distribution cycle, and the last distribution was done in 2017. This was aimed at ensuring universal coverage in the region. LLIN coverage in 2016 in the study villages was 96.4%, and the usage rate was 78%.
Ethiopia
The study was undertaken in Bore Tika (9°02′00′′N, 38°44′48′′E) and Chewaka (8°26′02′′E, 36°19′48′′N) villages in southwestern Ethiopia. Bore Tika is a village situated 350 km southwest of Addis Ababa in the suburb of Jimma town in Seka district at an altitude ranging from 1716 to 1752 m above sea level. The mean annual rainfall of the area is 1489 mm with temperature ranging from 11°C to 27°C. It is situated around Gilgel-Gibe river, which provides most of the mosquitoe breeding sites in the area. Chewaka village is situated in Chewaka district, Buno Bedele zone, Oromia Regional state, Ethiopia. Its altitude ranges from 1247 to 1262 m above sea level. The inhabitants are resettled communities from eastern Ethiopia due to recurrent drought. The population has a habit of spending early part of the night chewing khat (Catha edulis). The main socioeconomic activities of the community are mixed farming. Maize, sorghum, teff, khat, haricot beans, sesame, and pepper are widely cultivated. Coffee is an important cash crop in the area. In the country; in addition to IRS used as a major vector control tool since 1959, LLIN use has been scaled up since 2005, resulting in >64 million nets distributed (US President’s Malaria Initiative [PMI], 2016). In 2016, LLIN coverage and utilization in the study site were around 70.4% and 67.88%, respectively.
House Selection
During the study, households where mosquito collection was conducted were randomly selected in each site and consent was obtained from the head of household. Houses were grouped according to trapping methods, that is, human landing catch (HLC; 10 houses), CDC light traps (10 houses), window exit traps (WETs; 5 houses) and backpack aspirators (10 houses). Other houses were used for pyrethrum spray catches (PSCs). Within the same sampling site, a distance of 50–100 m was maintained between houses and the number of homesteads sampled at each period of the study was dependent on the availability of traps.
Mosquito Capture/Collection
Adult mosquitoes were collected from the selected houses using HLCs, CDC light traps, PSCs, WETs, and backpack aspirators. These sampling tools were used based on their availability in each setting.
Human Landing Catches
Six to 10 houses were selected randomly in each of the selected villages per country for HLCs. In each of the 10 houses, indoor and outdoor mosquito collections were carried out from 6:00 pm to 6:00 am by 2 teams of 2 people per house using mouth aspirators. Outdoor mosquito collection was carried out about 8 m from each homestead/house selected for indoor mosquito collection by HLCs. The 2 teams (indoor/outdoor collectors) swapped positions in each homestead every hour of the night. Paper cups for mosquito collection were exchanged hourly following mosquito capture.
Light Trap Catches
Ten houses were also selected for indoor and outdoor light trap catches from 2 study villages in each country in a similar way houses were selected for HLCs. Thus, the 10 houses served as sentinel stations for indoor and outdoor mosquito collection using LTCs. Mosquitoes were collected indoor and outdoor from 6:00 pm to 6:00 am from each selected house using standard CDC light traps (Centers for Disease Control and Prevention, Atlanta, Georgia). Traps were hung from the ceiling or from roof supports at the foot end of the bed where people sleep at night and the body of the trap was suspended about 1.5 m from the floor. Traps were also hung outdoor in the veranda or other outdoor structure for outdoor mosquito collection. Traps were set by trained research team members and run from 6:00 pm to 6:00 am. Collection bags were attached to the trap by stretching the open end of the bag over the bottom rim of the trap, and a label marked with the date and the site number was placed inside the collection bag. The number of people who slept in and animals kept inside the house the previous night were also recorded. Collection bags were retrieved from traps in each house in the morning from 7:00 am to 8:00 am by the collection team.
Pyrethrum Spray Catches
Ten houses were randomly selected in each of the selected villages for spray sheet collection. Mosquitoes were collected from 6:00 am to 9:00 am. For this pyrethrum spray collection all the eaves, windows, and other exit points were closed or covered. White sheets were spread on the floor, aerosol insecticide was sprayed in the entire room, and the house was closed for 15 minutes after spraying. Prior to spraying, the heads of households were informed about the purpose and the time of spray and given clear instructions as to what they have to do before and after their houses have been sprayed. After 15 minutes, all of the knocked-down mosquitoes lying on the white sheets were collected carefully with forceps and placed in paper cups.
Mosquito Collection Using Backpack Aspirator
Ten houses were also selected in each of the 2 selected sites in each country. Mosquitoes were collected from houses with human habitation, animal shed, and mixed habitation from 6:00 am to 9:00 am using backpack aspirator by trained collectors.
Window Exit Traps
Window exit traps were performed in at least 5 different houses at least for 3 consecutive days per site per season. Traps were set at 6:00 pm and collected at 6:00 am the following day.
Mosquito Identification
All mosquitoes collected were sorted into anophelines and culicines. All of the Anopheles mosquitoes were identified using taxonomic keys [31]. The An. gambiae complex, An. funestus group, and An. moucheti complex were further identified to sibling species using species-specific polymerase chain reaction (PCR). The wings and/or legs were used for sibling species identification [33, 34]. Genomic DNA was extracted from the legs and wings of subsamples of the collected specimens using the methods of Collins et al [35, 36] and amplified using specific diagnostic primers for An. gambiae s.s., An. coluzzi, An. arabiensis, Anopheles amharicus, and An. merus for An. gambiae complex [34, 37]. Anopheles moucheti was identified according to Kengne et al [38] using specific primers of An. moucheti subsp moucheti, An. moucheti subsp nigeriensis, and An. moucheti subsp bervoetsi. The protocol developed by Koekemoer et al [39] was used for molecular identification of member of the An. funestus group using specific primers of An. leessoni, An. parensis, An. rivulorum, and An. vaneedeni.
Mosquito Processing
The cephalothoraces of all mosquitoes were tested separately for the presence of sporozoite of Plasmodium falciparum and/or Plasmodium vivax using enzyme-linked immunosorbent assay (ELISA) [40–42]. Fully blood-fed females collected by other collection methods other than HLC were also tested for blood meal sources by ELISA [43]. Blood meal sources were tested against human and bovine antibodies in all sites. In addition, goat and chicken antibodies were also used in Cameroon while only goat antibodies were added in Kenya.
Human Behavioral/Occupational Factors Associated With Malaria Transmission
A semi-structured questionnaire was administered to participants in different sites to assess whether or not they sleep under their nets regularly, when they go to sleep and when they wake up in the morning, and leave their nets. In randomly selected houses, we targeted the parents (mothers and fathers) and an individual in the household who gave consent to participate in the study. The mothers gave information on their activities and those of their children in the morning and/or in the evening. Rapid diagnostic tests SD BIOLINE Malaria Ag. P.f./Pan (Standard Diagnostics, Republic of Korea) were used for malaria diagnosis for each respondent consenting to take part in the study to assess whether people reporting going to bed earlier and using nets are less affected by malaria than those going to bed late or not using nets. The association between the risk of malaria infection and the use of nets in each group was assessed using logistic regression analysis. The study was conducted twice a year during the dry and wet seasons in Cameroon and Ethiopia.
Ethical Approval
This study was approved by the World Health Organization Ethics Review Committee (Protocol ID ERC.0002666), the Kenya Medical Research Institute Scientific Ethics Review Unit (KEMRI/SERU/CGMR-C/024/3148), the Cameroon National Ethics Committee for Research on Human Health (CNERSH) under the ethical clearance number 2016/01/685/CE/CNERSH/SP, and the Institutional Review Board of the College of Health Sciences, Jimma University (reference number HRPGG/269/2015). Written informed consent was obtained from the parents/guardians of the children during the malaria prevalence surveys, and assent was obtained for participants aged >13 years and <18 years.
Data Analysis
Mosquito density was expressed as the mean number of mosquitoes of each species per trap per night. Calculation of the entomological inoculation rate (EIR) provided point measures of malaria transmission intensity. EIR is a commonly used metric that estimates the number of bites by infectious mosquitoes per person per unit time, which is a direct measure of malaria transmission intensity in an area. Sporozoite rates between mosquito species were compared using Fisher exact test.
Biting Behavior/Cycle
To assess the behavior of malaria vectors, the indices proposed in Seyoum et al [44] were used. The mean proportion of bites on humans lacking a net that was obtained from a given vector population in the absence of any protective measure (πh,i) was calculated by weighing the mean indoor (Bi) and outdoor (Bo) biting rates for each hour of the night (t) by the proportion of humans reporting to have been indoors (I) and outdoors (1-I), respectively.
A univariate analysis was also carried out to independently determine whether any of the prospectively defined independent factors (age, sex, net usage, and sleeping behavior) was significantly associated with risk of malaria infection. To assess relative risk, odds ratios (ORs) with 95% confidence intervals (CIs) were computed using MedCalc Statistical Software version 15.8 (MedCalc Software, Ostend, Belgium; https://www.medcalc.org).
RESULTS
Mosquito Species Composition
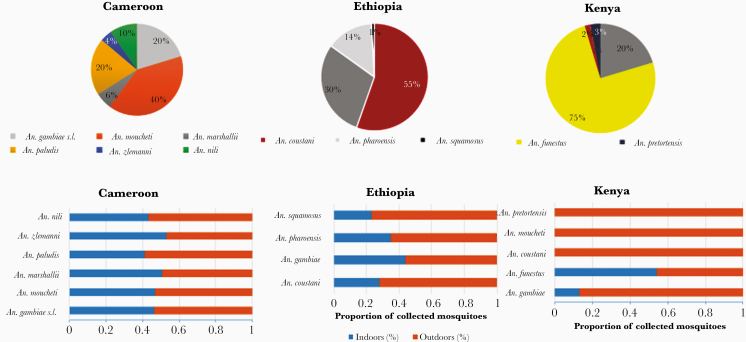
The mosquito fauna collected in the 3 ecological/epidemiological settings (countries) was diverse and country specific (Figure 2; Supplementary Table 2). A total of 17 837 anophelines were collected. Anopheline mosquitoes collected in high densities in each site were considered as major vectors.
Figure 2.
Anopheline mosquito species composition and behavior from Kenya, Ethiopia, and Cameroon.
In the forested area of Cameroon, of the 15 925 anophelines collected, An. moucheti (n = 6344 [39.84%]) was the predominant malaria vector, followed by An. gambiae s.l. (n = 3232 [20.3%]). Other species included Anopheles paludis (n = 3202 [20.11%]), Anopheles marshallii (n = 942 [6%]), An. nili (n = 1538 [10%]), and Anopheles ziemanni (n = 667 [4%]). More than one-third of the collected Anopheles mosquitoes (An. nili, An. paludis, An. ziemanni, An. coustani, An. marshallii, An. pharoensis, An. pretoriensis, and An. squamosus) were secondary malaria vectors (n = 6349 [39.9%]). Molecular identification of An. gambiae complex using PCR revealed the presence of An. gambiae s.s. (n = 319 [72.17%]) and An. coluzzi (n = 123 [27.82%]) as members of the An. gambiae complex whereas molecular identification of An. nili (n = 40) and An. moucheti (n = 192) revealed the presence of Anopheles ovengensis (n = 40 [100%]) and An. moucheti subsp moucheti (n = 192 [100%]), respectively.
In the coastal area of Kenya, 415 Anopheles mosquitoes comprising 7 species were collected, of which An. funestus group (n = 311 [75 %]) was the predominant anopheline species, followed by An. gambiae s.l. (n = 84 [20%]). Other species included An. pretoriensis (n = 12 [2.9%]), An. coustani (n = 6 [1.4%]), An. moucheti (n = 1 [0.24%]), and An. squamosus (n = 1 [0.24%]). Molecular identification of An. gambiae complex showed that 80.95% (n = 68) belonged to An. arabiensis and 19.05% (n = 16) belonged to An. gambiae s.s.; An. funestus s.s. and An. rivulorum were found as members of the An. funestus group based on molecular identification.
Of the 2023 anopheline mosquitoes collected in the highlands of Ethiopia, An. coustani group (n = 945 [47%]) was the predominant anopheline species followed by An. gambiae s.l. (n = 852 [42%]). Those remaining (n = 226 [11%]) belonged to An. pharoensis and An. squamosus. Molecular identification of subsamples of An. gambiae complex revealed the presence of only An. arabiensis.
Mosquito Behavior
Using HLC and CDC light traps, higher proportions of Anopheles mosquitoes were collected outdoors (9388/17 130; 54.80% [95% CI, 53.57%–55.92%]) than indoors (7742/17 130; 45.20% [95% CI, 44.2%–46.26%]) in all 3 countries. This outdoor host-seeking behavior is country dependent; it varies from 67% (95% CI, 62.63%–71.53%) in Ethiopia to 54% (95% CI, 53.19%–55.5%) in Kenya and Cameroon. It was also observed that higher density/proportion of An. gambiae s.l. was collected in all settings using PSC or aspirator (Prokopack). Other species such as An. moucheti were collected exiting houses in Cameroon (Figure 2; Supplementary Table 2). Of mosquitoes collected by Prokopack aspirator (Ethiopia), a high density/proportion of all anopheline mosquito species was collected from animal sheds compared with human and mixed habitation.
Blood Meal Source
Freshly fed mosquitoes collected using sampling methods other than HLC were analyzed for their blood meal origin. It was observed that a high proportion of mosquitoes collected were blood-fed (>60%) except in Cameroon where only An. gambiae s.l. had a blood feeding rate exceeding 10%. In general, An. gambiae s.l., An. coustani, and An. funestus have high blood feeding rates. In Cameroon, the human blood index was very high (99%) with mixed (human and chicken) blood meal found in Ethiopia and Kenya. We detected blood meal from animal sources in 89.49% of the mosquitoes (bovine for Ethiopia and goat and bovine in Kenya) (Table 1). Mixed blood meals were observed in all settings, confirming the opportunistic behavior of most anopheline species such as An. gambiae s.l., An. coustani, An. pharoensis, and An. funestus. The blood meal source of about half of the Ethiopian mosquitoes was of unknown origin (unidentified) whereas only 14% and 3.3% were from unknown vertebrate sources in Cameroon and Kenya, respectively.
Table 1.
Blood Meal Source of Malaria Vectors in Kenya, Ethiopia, and Cameroon
| Country | Anopheles Species | Collected | Tested | BF Rate | Human | Goat | Bovine | H + C | H + B | H + G | G + B | H + B + G | Unknown |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethiopia |
An. coustani | 168 | 116 | 69.05 | 0 (0) | NA | 60 (51.7) | NA | 4 (3.4) | NA | NA | NA | 52 (44.8) |
| An. arabiensis | 486 | 327 | 67.28 | 4 (1.2) | NA | 141 (43.1) | NA | 23 (7.0) | NA | NA | NA | 159 (48.6) | |
| An. pharoensis | 96 | 44 | 45.83 | 0 (0) | NA | 18 (40.9) | NA | 1 (2.3) | NA | NA | NA | 25 (56.8) | |
| Total | 750 | 487 | 64.93 | 4 (0.8) | NA | 219 (44.9) | NA | 28 (5.7) | NA | NA | NA | 236 (48.5) | |
| Kenya |
An. funestus s.s. | 78 | 58 | 74.36 | 8 (14.0) | 5 (9.0) | 1 (2.0) | 0 (0) | 1 (2.0) | 24 (41) | 8 (14) | 11 (19) | 0 (0) |
| An. arabiensis | 15 | 12 | 80 | 0 (0) | 2 (17) | 0 (0) | 0 (0) | 1 (8) | 4 (33) | 2 (17) | 2 (17) | 1 (8) | |
| An. pretoriensis | 2 | 0 | 0 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (33) | 1 (33) | 0 (0) | 1 (33) | |
| An. gambiae s.s. | 0 | 0 | 0 | 0 (0) | 1 (20) | 0 (0) | 0 (0) | 0 (0) | 3 (60) | 0 (0) | 1 (20) | 0 (0) | |
| Total | 95 | 62 | 66.67 | 8 (12.9) | 8 (12.9) | 1 (1.6) | 0 (0) | 1 (1.6) | 32 (51.6) | 11 (17.7) | 14 (22.6) | 2 (3.2) | |
| Cameroon |
An. gambiae s.l. | 355 | 100 | 28.17 | 77 (77) | 0 (0) | 0 (0) | 3 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 20 (20) |
| An. nili | 59 | 5 | 8.47 | 5 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| An. moucheti | 313 | 25 | 7.99 | 25 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| An. paludis | 175 | 4 | 2.29 | 4 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Total | 902 | 134 | 14.86 | 111 (82.8) | 0 (0) | 0 (0) | 3 (2.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 20 (14.9) |
Data are presented as No. (%) unless otherwise indicated. Mosquito species shown in italics.
Abbreviations: BF, blood feeding; G + B, goat plus bovine blood; H + B, human plus bovine blood; H + B + G, human plus bovine plus goat blood; H + C, human plus chicken blood; H + G, human plus goat blood; NA, not available; s.l., sensu lato; s.s., sensu stricto.
MOSQUITO BITING ACTIVITY AND SLEEPING BEHAVIOR (INDOOR AND OUTDOOR EXPOSURE TO MALARIA INFECTION)
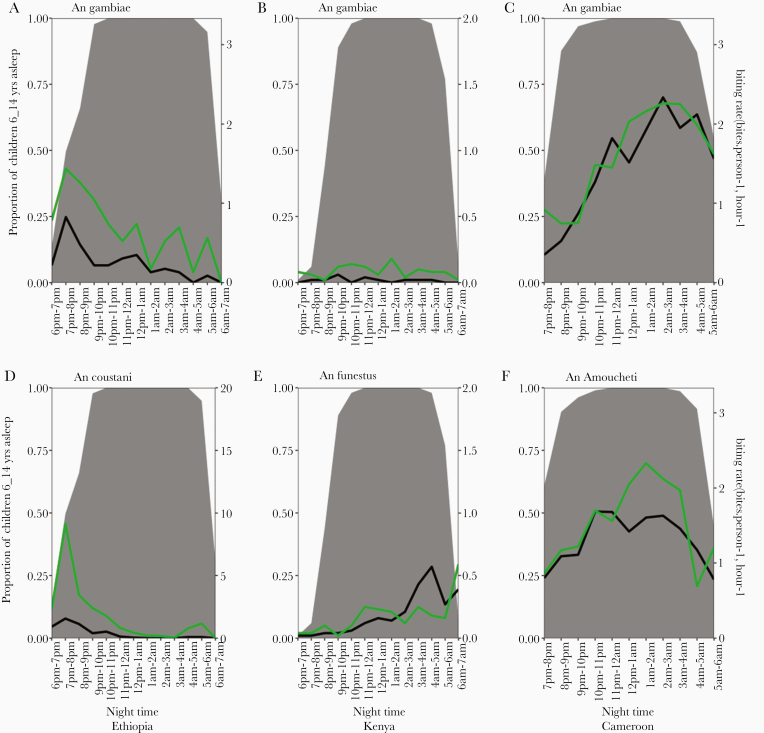
More than 70% of children aged 6–14 years were reported to be asleep between 6:00 pm and 9:00 pm; this rose monotonically over the course of the night, reaching 100% by 10:00 pm in all 3 countries (Figure 3). Similar trends were observed in children aged <5 and >15 years (Supplementary Data). However, up to 50% of adult household members (fathers and mothers) were awake after 9:00 pm with the latest sleeping at around midnight. The timing of human activity, both indoors and outdoors, may modulate vector-human contact. For example, in Ethiopia, peak biting activity for both An. coustani group and An. gambiae s.l. occurs early in the evening (6:00–8:00 pm) before children go to sleep under the protection of insecticide-treated nets (ITNs), while in Cameroon, both An. moucheti and An gambiae s.l. peak biting occurs between 10:00 pm and 2:00 am, when a majority of household members are under the protection of ITNs (Figure 3). In Kenya, a stable biting activity is observed for indoor feeding vectors; however, for outdoor feeding and resting mosquitoes, especially An. funestus group, biting activity gradually increases throughout the night with peak activity between 3:00 am and 5:00 am.
Figure 3.
Hourly biting activity of Anopheles mosquitoes both indoors (black lines) and outdoors (green lines) for the Anopheles vector and Anopheles gambiae sensu lato. Gray shading represents the proportion of children aged 6–14 years asleep at each hour of the night.
Indoor and Outdoor Exposure of Malaria Infection
Using Seyoum’s formula, the proportion of exposure occurring indoors/outdoors was estimated [45]. In general, indoor exposure (πi) was 0.82, 0.27, and 0.84, respectively, in Cameroon, Ethiopia, and Kenya. Indoor exposure of mosquito varies with human population age, mosquito species, and country. In Cameroon, πi for An. gambiae was 0.84 while exposure to An. moucheti was 0.81. In Ethiopia, most species bite outdoor with high proportion of people outdoors during the night; πi was 0.48 and 0.2 for An. gambiae s.l. and An. coustani, respectively. In Kenya, πi was 0.47 and 0.9 for An. gambiae s.l. and An. funestus, respectively.
Between 7:00 pm and 9:00 pm (early evening), 18.31%, 85.67%, and 91.90% of exposures occurred outdoors in Cameroon, Ethiopia, and Kenya, respectively. However, from 10:00 pm to 4:00 am, nearly all exposures occurred indoors, while between 4:00 am and 6:00 am, outdoor/indoor exposure was high in all settings except in Kenya (45.76% in Cameroon, 40.66% in Ethiopia, and 4.87% in Kenya).
Indoor and Outdoor Malaria Transmission Intensity
A total of 14 140 Anopheles mosquitoes (12 802 from Cameroon, 1022 from Ethiopia, and 316 from Kenya) collected by HLC were tested for Plasmodium circumsporozoite protein (CSP) using ELISA. In Cameroon, the indoor and outdoor sporozoite rate was 1.4 and 1.6%, respectively, with an overall sporozoite rate of 1.5%. Infection rates for An. gambiae s.l., An. moucheti, An. nili, An. paludis, An. zeimanni, and An. marshali were 6.0%, 0.7%, 0.9%, 0.7%, 0.3%, and 0.2%, respectively. In Ethiopian sites, the sporozoite rate for An. gambiae s.l. and An. coustani was 0.5% and 0.2%, respectively, all being from outdoor collections. The fact that no infection could be detected indoors could be linked to the limit of detection with the sample size used. None of the tested An. pharoensis and An. squamosus specimens from Ethiopia were positive for CSPs. In Kenya, the indoor and outdoor sporozoite rate was 3.7% and 2.7%, respectively, with an overall sporozoite rate of 3.0%. Anopheles gambiae s.l., An. funestus group, and An. pretoriensis contributed to infection rates in Kenyan sites (Table 2).
Table 2.
Malaria Transmission Intensity in Cameroon, Ethiopia, and Kenya
|
Anopheles Species |
Biting Locations | HBR (Bites/Person/Night) | Sporozoite Index, %a | EIR (Infective Bites/Person/Year) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cameroon | Ethiopia | Kenya | Cameroon | Ethiopia | Kenya | Cameroon | Ethiopia | Kenya | ||
|
An. gambiae s.l. |
Indoor | 5.22 | 3.13 | 0.17 | 0.05 [0.04–0.06] (1171) | 0.000 […] | 0 (11) | 95.28 | 0.00 | 0.00 |
| Outdoor | 6.35 | 8.61 | 0.85 | 0.06 [0.05–0.08] (1004) | 0.005 [0.004–0.01] | 0.018 [0.0–0.1] (55) | 139.04 | 15.87 | 5.63 | |
| Total | 5.78 | 5.87 | 0.508 | 0.06 [0.05–0.07] (2175) | 0.004 [0.002–0.005] | 0.015 [0.0–0.08] (66) | 126.69 | 7.93 | 2.815 | |
|
An. moucheti
|
Indoor | 10.72 | 0.00 | 0.00 | 0.008 [0.004–0.011] (2604) | NA | NA | 31.32 | NA | 0.00 |
| Outdoor | 12.65 | 0.00 | 0.02 | 0.006 [0.0033–0.009] (2545) | NA | 0 (1) […] | 27.71 | NA | 0.00 | |
| Total | 11.69 | 0.00 | 0.008 | 0.007 [0.005–0.009] (5149) | NA | 0 (1) […] | 29.86 | NA | 0.00 | |
|
An. funestus group |
Indoor | 0.00 | 0.00 | 1.86 | NA | NA | 0.04 [0.01–0.09] (121) | 0.00 | NA | 28.15 |
| Outdoor | 0.00 | 0.00 | 1.71 | NA | NA | 0.03 [0.01–0.09] (111) | 0.00 | NA | 16.89 | |
| Total | 0.00 | 0.00 | 1.785 | NA | NA | 0.03 [0.02–0.07] (232) | 0.00 | NA | 22.52 | |
| Other species |
Indoor | 10.64 | 6.13 | 0.00 | 0.005 [0.003–0.008] (2765) | 0.000 […] | 0 (0) | 19.42 | 0.0 | 0.00 |
| Outdoor | 13.47 | 26.57 | 0.26 | 0.007 [0.004–0.011] (2713) | 0.002 [0.0–0.01] | 0.058 [0.001–0.33] (17) | 34.42 | 15.9 | 5.63 | |
| Total | 12.06 | 16.35 | 0.13 | 0.006 [0.004–0.008] (5478) | 0.001 [0.0–0.01] | 0.058 [0.001–0.33] (17) | 26.40 | 7.9 | 2.81 | |
| All species |
Indoor | 26.59 | 9.26 | 2.03 | 0.014 [0.011–0.017] (6540) | 0.000 […] | 0.037 [0.01–0.08] (132) | 135.85 | 0.00 | 28.15 |
| Outdoor | 32.47 | 35.17 | 2.83 | 0.016 [0.013–0.019] (6262) | 0.002 [0.001–0.01] | 0.027 [0.01–0.06] (184) | 189.64 | 31.74 | 28.15 | |
| Total | 29.53 | 22.22 | 2.43 | 0.015 [0.013–0.017] (12 802) | 0.002 [0.001–0.01] | 0.03 [0.015–0.05] (316) | 161.67 | 15.87 | 28.15 |
Abbreviations: EIR, entomological inoculation rate; HBR, human biting rate; NA, not available; s.l., sensu lato.
aSporozoite Index shown as percent of plasmodium positive mosquitoes and number tested and compared by Fischers exact test.
The overall annual indoor entomological rates (EIRs) for Cameroon, Ethiopia, and Kenya were 135.85, 0, and 28.15 infective bites per person per year, respectively, whereas the annual outdoor EIRs for the 3 sites were 189.64, 31.74, and 28.15 infective bites per person per year, respectively.
CONTRIBUTION OF OUTDOOR BITING BEHAVIOR AND SECONDARY/MINOR MALARIA VECTORS IN MALARIA TRANSMISSION
In these 3 countries, An. moucheti, An. gambiae s.l., and An. funestus group are defined as major vectors while others (An. paludis, An. ovengensis, An. marshallii, An. ziemanni, An. pretoriensis, An. coustani, An. pharoensis, and An. squamosus) are defined as secondary or minor vectors. The contribution of these vectors to malaria transmission and the outdoor biting behavior was estimated from the total transmission index (EIR). In Cameroon, 58.24% of the total transmission occurs outdoors while secondary vectors contribute to 13.68% of total transmission. In Kenya, 50% of transmission occurs outdoors while secondary vectors contribute to <10% of the transmission (7.1%). Surprisingly, all transmission occurs outdoors in Ethiopia while secondary vectors contribute to 50% of the total transmission occurring in the country.
SLEEPING BEHAVIOR AND ACTIVITIES KEEPING PEOPLE OUTDOORS IN THE EVENING
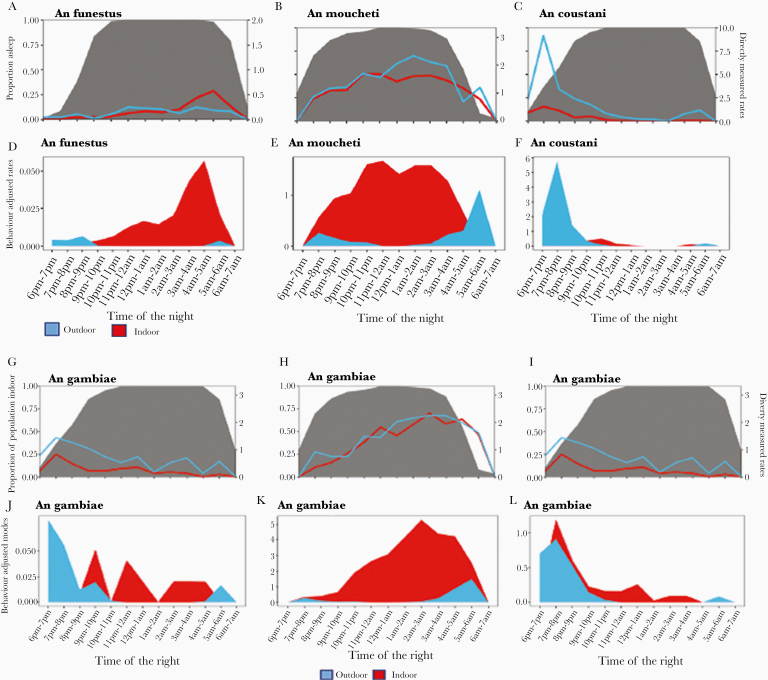
Sleeping patterns varied between ecological settings (Figure 4). The proportion of people staying outdoors was high in the evening (6:00–8:00 pm) with the majority going to bed after this period. Except in Ethiopia where the household head (father) went asleep early while children and mother went to bed late (by 9:00 pm), most fathers and school-aged children were late-night sleepers. In most cases, early morning/outdoor activities began at 4:00 am. Different activities were observed and justified to maintain these outdoor activities and the activities were sex, age, and country specific. In most cases, mothers were engaged in preparing food/coffee or taking care of babies with the help of female adolescents, while fathers and male children took care of the animals, read Quran, and studied their lessons. Also, it was observed in Cameroon and Kenya that fathers chat in the evening while drinking alcohol (palm wine or beers). In Cameroon, watching TV (football matches) or playing “songo” or selling of food and alcoholic beverages in the evening were the most common activities keeping adolescents outdoor.
Figure 4.
Exposure risk to indoor and outdoor mosquito bites with age category in Ethiopia, Cameroon, and Kenya. A1, A2, and A3 represent directely measured human biting rate and human location for Anopheles gambiae sensu lato in Ethiopia, Cameroon, and Kenya, respectively. B1, B2, and B3 represent behavior-adjusted biting rate for an individual for An. gambiae and C1, C2, and C3 for Anopheles coustani, Anopheles moucheti, and Anopheles funestus, respectively, for Ethiopia, Cameroon, and Kenya.
It was observed in Cameroon that time spent outdoors increased during popular games such as football (European champions league) or social ceremonies such as wedding, burials, funerals, parties, festivities, or toward the end of each month when people get their salary.
CHARACTERISTICS OF THE STUDY POPULATION
Sociodemographic characteristics of the study population were country dependent. House walls were constructed mainly from wood and mud in Cameroon, and with mixed materials in Ethiopia and Kenya. Iron sheeting was the main roofing material in Ethiopia and Cameroon whereas grass was the main material in Kenya. It was common to see between 1 and 5 persons per house in all the sites. Bed net ownership (percentage of houses having at least 1 net) was high in all sites with 96.8%, 70.4%, and 96.7%, respectively, in Cameroon, Ethiopia, and Kenya; utilization (proportion who slept in the net the previous night) was also high—67.88%, 94.54%, and 78% in Ethiopia, Cameroon, and Kenya, respectively (Table 3).
Table 3.
Sociodemographic and Socioeconomic Characteristics of Surveyed Populations in Kenya, Ethiopia, and Cameroon
| Characteristic | Kenya | Ethiopia | Cameroon | |||
|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | No. | (%) | |
| No. of households | 304 | … | 274 | … | 186 | … |
| LLIN ownership | 294 | (96.7) | 193 | (70.4) | 180 | (96.8) |
| LLIN usage | 294 | (78) | 186 | (67.88) | 178 | (95.7) |
| Sex of head of household | ||||||
| Male | 114 | (37. 5) | 86 | (31.4) | 105 | (56.45) |
| Female | 190 | (62.5) | 188 | (68.6) | 81 | (43.55) |
| Age of head of household, y | ||||||
| 15–34 | 103 | (36.14) | 150 | (58.37) | 78 | (47.56) |
| 35–54 | 111 | (38.95) | 82 | (31.91) | 64 | (39.02) |
| ≥55 | 71 | (24.91) | 25 | (9.73) | 28 | (17.07) |
| No. of household members | ||||||
| 1–5 | 184 | (61.33) | 172 | (63.24) | 78 | (41.9) |
| 6–10 | 113 | (37.67) | 89 | (32.72) | 74 | (39.8) |
| ≥11 | 3 | (1.00) | 11 | (4.04) | 34 | (18.3) |
| No. of household members aged <5 y | ||||||
| 1–5 | 242 | (100) | 97 | (100) | 110 | (59.14) |
| 6–10 | 0 | (0) | 0 | (0) | 7 | (3.8) |
| ≥10 | 0 | (0) | 0 | (0) | 2 | (1.07) |
| No. of rooms per household | ||||||
| 1–5 | … | … | 252 | (97.30) | 128 | (68.82) |
| 6–10 | … | … | 7 | (2.70) | 58 | (31.18) |
| ≥10 | … | … | 0 | (0) | 3 | (1.61) |
| Main wall material | ||||||
| Brick | 8 | (2.66) | 0 | (0) | 6 | (3.2) |
| Cement/paint | 0 | (0.00) | 0 | (0) | 15 | (8.1) |
| Mud plastered | 0 | (0.00) | 0 | (0) | 28 | (15.1) |
| Wood | 16 | (5.32) | … | (0) | 73 | (39.2) |
| Mixed | 267 | (88.70) | 261 | (100) | … | … |
| Mud | 0 | (0.00) | 0 | (0) | 64 | (34.4) |
| Stone | 10 | (3.32) | 0 | (0) | 0 | (0) |
| Main roof material | ||||||
| Cement | 0 | (0.00) | 0 | (0) | 1 | (0.55) |
| Grass | 220 | (77.46) | 8 | (2.99) | 4 | (2.18) |
| Iron sheet | 64 | (22.54) | 260 | (97.01) | 178 | (97.27) |
No. is the number or size of each group; % is the proportion (number in each group divided by the total).
Abbreviation: LLIN, long-lasting insecticidal net.
DETERMINANTS OF MALARIA PREVALENCE
In this part, only Cameroon collected data accurately (see Table 4).
Table 4.
Risk of Malaria Infection by House Type, Sleeping Behavior, and Population Age in Cameroon
| Characteristic | Prevalencea | OR (95% CI) | P Value |
|---|---|---|---|
| Sleeping behavior | |||
| Sleep late | (30) 69/229b | 0.57 (.41–.80)c | <.001 |
| Sleep early | (43) 255/569 | 1 | |
| Wake up early | (38) 57/150 | 0.89 (.62–1.29) | .72 |
| Wake up late | 40) 267/675 | 1 | |
| House type | |||
| Cement | (33) 61/185 | 1 | |
| Wood | (53) 188/355 | 2.29 (1.58–3.32) | <.0001 |
| Mud | (26.9) 73/271 | 0.75 (.5–1.13) | .16 |
| Age, y | |||
| [0–5] | (42) 126/300 | 1 | NA |
| [5–10] | (53) 128/240 | 1.58 (1.12–2.22) | .008 |
| [11–15] | (42) 63/151 | 0.98 (.66–1.47) | .95 |
| >16 | (12) 18/145 | 0.2 (.11–.34) | .000 |
| Use of LLIN | |||
| Yes | (35.31) 220/623 | 1 | |
| No | (54.32) 88/162 | 2.17 (1.53–3.09) | <.0001 |
The values in bold show significance (P Value).
Abbreviations: CI, confidence interval; LLIN, long-lasting insecticidal net; OR, odds ratio.
aMalaria Prevalence.
bnumber infected/number screened.
c95% CI.
Age-Specific Malaria Prevalence
A total of 836 individuals (430 in Olama and 406 in Nyabessan) were tested with a malaria rapid diagnostic test (RDT). Of this population, 351 were found to be infected with Plasmodium species, representing a prevalence of 42%. The prevalence of infections was high in the age group 5–10 years. Children aged <16 years have a high risk of malaria infection.
House Type and Malaria Prevalence
A total of 258 houses were visited for malaria detection during the study. The proportion of people detected with malaria parasites was high, at 57.36% (95% CI, 51.67%–78.25%). The prevalence of malaria infection in the inhabitants varied significantly according to house characteristics (χ 2 = 4.58; P = .032). People living in cement houses were found to have fewer malaria parasite infections compared to others living in mud or wood houses.
LLIN Utilization and Malaria Prevalence
RDT was used to detect malaria infection in people. It appears that people not using LLINs were twice more at risk of malaria infection (OR, 2.17 [95% CI, 1.53–3.09]) as compared to users.
DISCUSSION
The study objectives were to document entomological and anthropogenic behavioral factors, which could contribute to persistent malaria transmission in 3 settings with high coverage with LLINs or IRS and with different ecological features across sub-Saharan Africa: Central African equatorial forest region (Cameroon), East African coastal area (Kenya), and East African highlands (Ethiopia).
Persistent transmission of malaria was recorded in the 3 sites and was in conformity with continuous malaria transmission occurring in these areas. Yet the EIR rate was different from one country to the other and this could be due to different malaria burden in the 3 sites [2]. These differences in malaria transmission may be due to the difference observed in the level of outdoor mosquito biting across the 3 countries, which may likely result in differences in persistent/residual transmission and the effectiveness of current malaria prevention actions. Surprisingly, the high outdoor biting behavior of malaria vectors from Ethiopia was not associated with high malaria transmission and may be due to the low proportion of the major vector An. gambiae s.l. Study sites in Cameroon display the highest EIR followed by Kenya and Ethiopia. The following could suggest different protection provided by indoor-based control tools (treated nets and/or IRS) or different efficiency/competence of major vectors in the area. In Cameroon, vector populations were found to be highly anthropophilic compared to Kenya and Ethiopia. This close reliance on human blood could be a major factor increasing the risk of malaria transmission. Yet in all the sites, malaria transmission was high outdoors compared to indoors. Despite this high transmission, estimates weighted with human behavior as described in Monroe et al [46] showed high exposure risk indoors in all sites except in Ethiopia, where high proportions of mosquitoes and population are found outdoors during the night. The proportion of bites preventable by control tools such as LLINs was not estimated during this study and could constitute a limitation [46, 47].
Up to 15 species were recorded during the study period, consistent with the high diversity of the anopheline fauna across Africa [12]. Anopheles gambiae s.l. was recoorded as the major malaria vector in all settings, contributing for >90% of the total transmission. Apart from An. gambiae s.l., which was present in the 3 sites, differences in species composition and abundance were recorded between the 3 settings and were in accordance with the peculiarity of each site [12]. In Cameroon the main vectors were An. gambiae s.l. and An. moucheti. These species were responsible for >90% of malaria transmission cases in the forest region; these findings are consistent with previous studies in the area [15, 48]. Other species such as An. paludis and An. ovengensis were also recorded infected and involved in malaria transmission as reported in earlier studies [15–17, 48]. In Kenya, vectors collected included An. gambiae s.l. and An. arabiensis. These are well-known vectors in the country [23, 49–53]. In Ethiopia, 2 anopheline species, An. gambiae s.l. and An. coustani, were found to be infected with Plasmodium species. These species are known to display different biting, feeding, and resting behaviors [54–56]. Although Ethiopia is situated in a highland area, the malaria situation in the country is largely complex with both P. falciparum and P. vivax as the main parasites together with the presence of new vectors such as Anopheles stephensi in the country.
Studies conducted in the 3 sites indicated high exophagic behavior of mosquitoes. The following could suggest high influence of treated nets that could, through their excito-repellency effect, reduce the entry of mosquito indoors or could be due to a change of behavior of mosquitoes in relation with human activities or genetic changes [57].
Improving malaria control strategies also requires a good understanding of when and where people are most likely to be exposed to mosquito infective bites. In addition to entomological surveys, human population surveys were conducted to link human activities to the mosquito biting cycle. A majority of people return home by 10:00 pm and wake up by 4:00–5:00 am; this behavior makes exposure to mosquito infective bites preventable if we assume that they use LLINs at night and that anopheline bites mostly occur in the middle of the night when people are asleep. However, as reported elsewhere [5, 6, 58, 59], different social or livelihood activities that lasted throughout the night, such as feasts, burials, and commemorations, could disrupt usual sleeping patterns and reduce the use of LLINs during the night. In Kenya and Ethiopia, it appeared that high anopheline biting densities outdoors were recorded between 6:00 pm and 9:00 pm, whereas in Cameroon a high outdoor biting rate was recorded instead between 4:00 am and 6:00 am. The latter could result from the fact that many people wake up in the early hours to prepare for work or to do some domestic activities before going to work or school. Similar behavior has been reported elsewhere [6]. Although a certain number of similar activities was found to maintain people outdoors in the 3 countries (eg, cooking, fetching water, eating, and relaxing/chatting), a certain number of peculiarities was also observed. In Ethiopia, for example, herding cattle or sharing sleeping quaters with cattle was also recorded, and this could have increased the risk of being bitten by zoophilic mosquito species. Mosquitoes such as An. coustani displaying a high opportunistic behavior were recorded to be frequently infected in the area. In Kenya, where people sleep in the same house with cattle, different species were found to be infected with malaria parasites. In Cameroon, despite the high anthropophilic behavior of mosquitoes, a change in the biting activities of vectors closely associated with human behavior was recorded. Anopheles gambiae and An. moucheti, the main vectors in the region, were found to display high biting rates early in the evening or early in the morning when people were out of the house conducting a certain number of activities.
The main vector tools used by the population in the different study sites included IRS and LLINs; these tools are efficient when people are indoors or asleep in bed. People working outdoors are considered to be vulnerable to mosquito infectious bites. No additional tools to target outdoor biting mosquitoes were used by the population in either sites. These observations have similarly been reported in Tanzania where the use of repellent was not observed during outdoors activities [6, 60–63]. The absence of tools that could protect people from mosquito bites outdoors is a critical gap in vector control measures that needs to be covered to reduce the risk of malaria transmission, particularly in the context of malaria elimination.
High malaria prevalence in the human population was observed in Cameroon despite high bed net usage. The following could be due to the influence of different factors such as sleeping time, the number of people residing within a house, and the proportion of people using bed nets or the type of house design/construction. Unexpectedly, people going to bed early were twice likely to be infected than other age group. The waking up time in the morning was not associated with a risk of malaria transmission; this could be due to the limited power of analysis as not everyone responded to this question. Children aged 5–10 years were more likely to be infected than the older age groups. Nonusers of ITNs were also found to be twice more infected by P. falciparum than ITN users [15], highlighting the importance of ITN use despite the increased prevalence of insecticide resistance in vector populations, which must be preserved to improve the control of malaria. The misuse of control tools by residents may hinder the fight against malaria. There is a need for continuous sensitization of the population to improve bed net usage and malaria prevention. House characteristics also appeared to be an important risk factor, but this has so far not been deeply assessed. It is possible that stopping collection at 6:00 am might have underestimated the exposure rate to mosquito bites because a substantial proportion of exposure could be taking place after 6:00 am, as highlighted elsewhere [46, 47]. Poor house construction was associated with increased malaria transmission risk. Previous studies also established a direct relationship between house characteristics and malaria transmission [64–66].
CONCLUSIONS
Although there is proper use of LLINs in the equatorial forest, highland, and coastal regions in Africa, effective in preventing indoor malaria transmission, people still complain about the persistence of disease transmission. Malaria risk/transmission was found to be higher outdoors, during early evening (before bed time) or early morning, and during social activities. High transmission was found in the equatorial forest where a high density of An. gambiae s.l., a primary vector in Africa, was found. Secondary vectors also contribute to malaria transmission. These observations suggest that complementary tools to LLINs are still needed to protect people when outdoors if malaria elimination is still on the agenda. These findings highlight the need to search additional means to control malaria transmission mainly vectored by outdoor-seeking mosquitos and other related diseases in these foci where the population could be exposed to the risk of outbreaks due to the transmission of pathogens from primates to humans. As human behavioral activities exposing populations to malaria risk are similar to those found in other countries in Africa (Tanzania) and elsewhere (Vietnam), addressing these issues with existing tools could be difficult. New personal protection tools that do not rely on mosquito biting behavior will be needed in the arsenal of control tools. In addition, the use of an integrated vector control approach to improve the performance of LLINs and limit the expansion of insecticide resistance could be indicated. In addition, more sensitization needs to be done within endemic regions to promote the use of disease control measures and to avoid human activities that increase the risk of exposure and transmission of malaria.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. D. Y., C. A. N., and J. M. conceived and designed the study, contributed to tool and protocol development, helped manage data collection, advised on data analyses and interpretation, and edited the manuscript. B. R. managed entomological field data collection, performed epidemiological studies and laboratory analysis, analyzed results and data interpretation, wrote the first draft of the manuscript, and wrote the final manuscript. M. R. and T. D. managed entomological field data collection, analyzed results and data interpretation, and contributed to the draft of the manuscript. J. M., C. M., P. I., A. K., M. W., A. A., Z. B., K. T., E. K., P. A.-A., T. T., and F. N. were involved in data collection and participated in laboratory analysis. C. A. N., J. M., D. Y., and M. R. critically reviewed the manuscript. All authors read and approved the final manuscript.
Acknowledgments. We express our gratitude to the populations who participated in this study.
Financial support. The present study was supported by funds from the World Health Organization’s Special Programme for Research and Training in Tropical Diseases (2016/602099-0), which approved the final study design and protocols. C. A. N. was supported by a Wellcome Trust Senior Fellowship in Public Health and Tropical Medicine (202687/Z/16/Z).
Supplement sponsorship . The supplement is sponsored by TDR, the Special Programme for Research and Training in Tropical Diseases, based at the World Health Organization.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Sixth Pan African Mosquito Control Association, September 2019, Yaoundé, Cameroon.
References
- 1. Kelly-Hope LA, McKenzie FE. The multiplicity of malaria transmission: a review of entomological inoculation rate measurements and methods across sub-Saharan Africa. Malar J 2009; 8:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bhatt S, Weiss D, Cameron E, Bisanzio D, Mappin B, Dalrymple U. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015; 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. WHO recommendations for achieving universal coverage with long-lasting insecticidal nets in malaria control. Geneva, Switzerland: WHO, 2013. [Google Scholar]
- 4. World Health Organization. World malaria report 2018. Geneva, Switzerland: WHO, 2018. [Google Scholar]
- 5. Monroe A, Mihayo K, Okumu F, et al. Human behaviour and residual malaria transmission in Zanzibar: findings from in-depth interviews and direct observation of community events. Malar J 2019; 18:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Finda MF, Moshi IR, Monroe A, et al. Linking human behaviours and malaria vector biting risk in south-eastern Tanzania. PLoS One 2019; 14:e0217414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moiroux N, Damien GB, Egrot M, Djenontin A, Chandre F, Corbel V. Human exposure to early morning Anopheles funestus biting behavior and personal protection provided by long-lasting insecticidal nets. PLoS One 2014; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Killeen GF: Characterizing, controlling and eliminating residual malaria transmission. Malar J 2014; 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Msellemu D, Namango HI, Mwakalinga VM, Ntamatungiro AJ, Mlacha Y, Mtema ZJ. The epidemiology of residual Plasmodium falciparum malaria transmission and infection burden in an African city with high coverage of multiple vector control measures. Malar J 2016; 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moiroux N, Gomez M, Pennetier C, Elanga E, Djenontin A, Chandre F. Changes in Anopheles funestus biting behavior following universal coverage of long-lasting insecticidal nets in Benin. J Infect Dis 2012; 206. [DOI] [PubMed] [Google Scholar]
- 11. Molineaux L, Gramiccia G. The Garki Project. Research on the epidemiology and control of malaria in the Sudan Savanna of West Africa. Geneva, Switzerland: World Health Organization,1980. [Google Scholar]
- 12. Sinka ME, Bangs MJ, Manguin S, et al. A global map of dominant malaria vectors. Parasit Vectors 2012; 5:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dia I, Konate L, Samb B, et al. Bionomics of malaria vectors and relationship with malaria transmission and epidemiology in three physiographic zones in the Senegal River Basin. Acta Trop 2008; 105:145–53. [DOI] [PubMed] [Google Scholar]
- 14. Doumbe-Belisse P, Ngadjeu CS, Sonhafouo-Chiana N, et al. High malaria transmission sustained by Anopheles gambiae sl occurring both indoors and outdoors in the city of Yaoundé, Cameroon. Wellcome Open Res 2018; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bamou R, Mbakop LR, Kopya E, et al. Changes in malaria vector bionomics and transmission patterns in the equatorial forest region of Cameroon between 2000 and 2017. ParasitVectors 2018; 11:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Antonio-Nkondjio C, Kerah C, Simard F, Awono-Ambene H, Mouhamadou C, Tchuinkam T. Complexity of malaria vectorial system in Cameroon: contribution of secondary vectors to malaria transmission. J Med Entomol 2006; 43. [DOI] [PubMed] [Google Scholar]
- 17. Awono-Ambene P, Antonio-Nkondjio C, Toto J, et al. Epidemological importance of the Anopheles nili group of malaria vectors in equatorial villages of Cameroon, Central Africa. Sci Med Afr 2009; 1:13–20. [Google Scholar]
- 18. Ndo C, Kopya E, Donbou MA, Njiokou F, Awono-Ambene P, Wondji C. Elevated Plasmodium infection rates and high pyrethroid resistance in major malaria vectors in a forested area of Cameroon highlight challenges of malaria control. Parasit Vectors 2018; 11:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaindoa EW, Matowo NS, Ngowo HS, Mkandawile G, Mmbando A, Finda M. Interventions that effectively target Anopheles funestus mosquitoes could significantly improve control of persistent malaria transmission in south-eastern Tanzania. PLoS One 2017; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Finda MF, Limwagu AJ, Ngowo HS, et al. Dramatic decreases of malaria transmission intensities in Ifakara, south-eastern Tanzania since early 2000s. Malar J 2018; 17:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Walker M, Winskill P, Basanez MG, Mwangangi JM, Mbogo C, Beier JC. Temporal and micro-spatial heterogeneity in the distribution of Anopheles vectors of malaria along the Kenyan coast. Parasit Vectors 2013; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mwangangi JM, Midega J, Kahindi S, Njoroge L, Nzovu J, Githure J. Mosquito species abundance and diversity in Malindi, Kenya and their potential implication in pathogen transmission. Parasitol Res 2012; 110. [DOI] [PubMed] [Google Scholar]
- 23. Mwangangi J, Mbogo C, Orindi B, Muturi E, Midega J, Nzovu J. Shifts in malaria vector species composition and transmission dynamics along the Kenyan coast over the past 20 years. Malar J 2013; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kawada H, Dida GO, Sonye G, Njenga SM, Mwandawiro C, Minakawa N. Reconsideration of Anopheles rivulorum as a vector of Plasmodium falciparum in western Kenya: some evidence from biting time, blood preference, sporozoite positive rate, and pyrethroid resistance. Parasit Vectors 2012; 5:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mandeng SE, Awono-Ambene HP, Bigoga JD, et al. Spatial and temporal development of deltamethrin resistance in malaria vectors of the Anopheles gambiae complex from north Cameroon. PLoS One 2019; 14:e0212024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Menze BD, Riveron JM, Ibrahim SS, Irving H, Antonio-Nkondjio C, Awono-Ambene PH. Multiple insecticide resistance in the malaria vector Anopheles funestus from northern Cameroon is mediated by metabolic resistance alongside potential target site insensitivity mutations. PLoS One 2016; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Antonio-Nkondjio C, Tene Fossog B, Kopya E, Poumachu Y, Menze Djantio B, Ndo C. Rapid evolution of pyrethroid resistance prevalence in Anopheles gambiae populations from the cities of Douala and Yaounde (Cameroon). Malar J 2015; 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Antonio-Nkondjio C, Sonhafouo-Chiana N, Ngadjeu CS, Doumbe-Belisse P, Talipouo A, Djamouko-Djonkam L. Review of the evolution of insecticide resistance in main malaria vectors in Cameroon from 1990 to 2017. Parasit Vectors 2017; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bamou R, Kopya E, Djamouko-Djonkam L, et al. Assessment of the anophelinae blood seeking bionomic and pyrethroids resistance of local malaria vectors in the forest region of southern Cameroon. JEZS 2020; 8:1054–62. [Google Scholar]
- 30. Antonio-Nkondjio C, Sonhafouo-Chiana N, Ngadjeu CS, et al. Review of the evolution of insecticide resistance in main malaria vectors in Cameroon from 1990 to 2017. Parasit Vectors 2017; 10:472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa South of the Sahara (Afro-Tropical region). Johannesburg: South African Institute for Medical Research; 1987: 55. [Google Scholar]
- 32. Mbogo CM, Mwangangi JM, Nzovu J, et al. Spatial and temporal heterogeneity of Anopheles mosquitoes and Plasmodium falciparum transmission along the Kenyan coast. Am J Trop Med Hyg 2003; 68:734–42. [PubMed] [Google Scholar]
- 33. Koekemoer LL, Kamau L, Hunt RH, Coetzee M. A cocktail polymerase chain reaction (PCR) assay to identify the Anopheles funestus (Diptera: Culicidae) group. Am J Trop Med Hyg 2002; 6:78–83. [DOI] [PubMed] [Google Scholar]
- 34. Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg 1993; 49:520–9. [DOI] [PubMed] [Google Scholar]
- 35. Collins FH, Mehaffey PC, Rasmussen MO, Brandling-Bennett AD, Odera JS, Finnerty V. Comparison of DNA-probe and Isoenzyme methods for differentiating Anopheles gambiae and Anopheles arabiensis (Diptera: Culicidae). J Med Entomol 1988; 25:6–20. [DOI] [PubMed] [Google Scholar]
- 36. Collins FH, Petrarca V, Mpofu S, et al. Comparison of DNA probe and cytogenetic methods for identifying field collected Anopheles gambiae complex mosquitoes. Am J Trop Med Hyg 1988; 39:545–50. [DOI] [PubMed] [Google Scholar]
- 37. Paskewitz SM, Collins FH. Use of the polymerase chain reaction to identify mosquito species of the Anopheles gambiae complex. Med Vet Entomol 1990; 4:367–73. [DOI] [PubMed] [Google Scholar]
- 38. Kengne P, Antonio-Nkondjio C, Awono-Ambene H, Simard F, Awolola T, Fontenille D. Molecular differentiation of three closely related members of the mosquito species complex Anopheles moucheti, by mitochondrial and ribosomal DNA polymorphism. Med Vet Entomol 2007; 21:177–82. [DOI] [PubMed] [Google Scholar]
- 39. Koekemoer L, Kamau L, Hunt R, Coetzee M. A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am J Trop Med Hyg 2002; 66:804–11. [DOI] [PubMed] [Google Scholar]
- 40. Beier JC, Perkins PV, Wirtz RA, Whitmire RE, Mugambi M, Hockmeyer WT. Field evaluation of an enzyme-linked immunosorbent assay (ELISA) for Plasmodium falciparum sporozoite detection in anopheline mosquitoes from Kenya. Am J Trop Med Hyg 1987; 36:459–68. [DOI] [PubMed] [Google Scholar]
- 41. Wirtz RA, Burkot TR. Detection of malarial parasites in mosquitoes. Adv Dis Vect Res 1991; 8:77–106. [Google Scholar]
- 42. Wirtz RA, Zavala F, Charoenvit Y, et al. Comperative testing of Plasmodium falciparum circumsporozoite antibody. Bull World Health Organ 1987; 65:39–45. [PMC free article] [PubMed] [Google Scholar]
- 43. Beier JC, Perkins PV, Wirtz RA, et al. Bloodmeal identification by direct enzyme-linked immunosorbent assay (ELISA), tested on Anopheles (Diptera: Culicidae) in Kenya. J Med Entomol 1988; 25:9–16. [DOI] [PubMed] [Google Scholar]
- 44. Seyoum A, Sikaala CH, Chanda J, et al. Human exposure to anopheline mosquitoes occurs primarily indoors, even for users of insecticide-treated nets in Luangwa Valley, south-east Zambia. Parasit Vectors 2012; 5:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Seyoum A, Sikaala CH, Chanda J, Chinula D, Ntamatungiro AJ, Hawela M. Human exposure to anopheline mosquitoes occurs primarily indoors, even for users of insecticide-treated nets in Luangwa Valley, south-east Zambia. Parasit Vectors 2012; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Monroe A, Moore S, Okumu F, et al. Methods and indicators for measuring patterns of human exposure to malaria vectors. Malar J 2020; 19:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huho B, Briet O, Seyoum A, Sikaala C, Bayoh N, Gimnig J. Consistently high estimates for the proportion of human exposure to malaria vector populations occurring indoors in rural Africa. Int J Epidemiol 2013; 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mbakop LR, Awono-Ambene PH, Mandeng SE, et al. Malaria transmission around the Memve’ele Hydroelectric Dam in South Cameroon. A combined retrospective and prospective study, 2000–2016. Inter J Env Res Pub Health 2019; 16:1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dida GO, Anyona DN, Abuom PO, et al. Spatial distribution and habitat characterization of mosquito species during the dry season along the Mara River and its tributaries, in Kenya and Tanzania. Infect Dis Poverty 2018; 7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ogola E, Villinger J, Mabuka D, et al. Composition of Anopheles mosquitoes, their blood-meal hosts, and Plasmodium falciparum infection rates in three islands with disparate bed net coverage in Lake Victoria, Kenya. Malar J 2017; 16:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mwangangi J, Shililu J, Muturi E, et al. Dynamics of immature stages of Anopheles arabiensis and other mosquito species (Diptera: Culicidae) in relation to rice cropping in a rice agro-ecosystem in Kenya. J Vector Ecol 2006; 31:241–5. [DOI] [PubMed] [Google Scholar]
- 52. Kamau A, Mwangangi JM, Rono MK, Mogeni P, Omedo I, Midega J. Variation in the effectiveness of insecticide treated nets against malaria and outdoor biting by vectors in Kilifi, Kenya. Wellcome Open Res 2018; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mbogo CM, Mwangangi JM, Nzovu J, Gu W, Yan G, Gunter JT. Spatial and temporal heterogeneity of Anopheles mosquitoes and Plasmodium falciparum transmission along the Kenyan coast. Am J Trop Med Hyg 2003; 68. [PubMed] [Google Scholar]
- 54. Daygena TY, Massebo F, Lindtjørn B. Variation in species composition and infection rates of Anopheles mosquitoes at different altitudinal transects, and the risk of malaria in the highland of Dirashe Woreda, south Ethiopia. Parasit Vectors 2017; 10:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kenea O, Balkew M, Tekie H, Gebre-Michael T, Deressa W, Loha E. Human-biting activities of Anopheles species in south-central Ethiopia. Parasit Vectors 2016; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kenea O, Balkew M, Tekie H, et al. Comparison of two adult mosquito sampling methods with human landing catches in south-central Ethiopia. Malar J 2017; 16:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sougoufara S, Sokhna C, Diagne N, Doucouré S, Sembène PM, Harry M. The implementation of long-lasting insecticidal bed nets has differential effects on the genetic structure of the African malaria vectors in the Anopheles gambiae complex in Dielmo, Senegal. Malar J 2017; 16:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Monroe A, Asamoah O, Lam Y, Koenker H, Psychas P, Lynch M. Outdoor-sleeping and other night-time activities in northern Ghana: implications for residual transmission and malaria prevention. Malar J 2015; 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Monroe A, Moore S, Koenker H, Lynch M, Ricotta E. Measuring and characterizing night time human behaviour as it relates to residual malaria transmission in sub-Saharan Africa: a review of the published literature. Malar J 2019; 18:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mathania MM, Kimera SI, Silayo RS. Knowledge and awareness of malaria and mosquito biting behaviour in selected sites within Morogoro and Dodoma regions Tanzania. Malar J 2016; 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Moshi IR, Ngowo H, Dillip A, Msellemu D, Madumla EP, Okumu FO. Community perceptions on outdoor malaria transmission in Kilombero Valley, southern Tanzania. Malar J 2017; 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Koenker HM, Loll D, Rweyemamu D, Ali AS. A good night’s sleep and the habit of net use: perceptions of risk and reasons for bed net use in Bukoba and Zanzibar. Malar J 2013; 12:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Makungu C, Stephen S, Kumburu S, Govella NJ, Dongus S, Hildon ZJL. Informing new or improved vector control tools for reducing the malaria burden in Tanzania: a qualitative exploration of perceptions of mosquitoes and methods for their control among the residents of Dar es Salaam. Malar J 2017; 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Thomas S, Ravishankaran S, Asokan A, et al. Socio-demographic and household attributes may not necessarily influence malaria: evidence from a cross sectional study of households in an urban slum setting of Chennai, India. Malar J 2018; 17:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Krech R. Social determinants of Malaria: does equity matter? Geneva, Switzerland: World Health Organization, 2013. http://rbm.acw-server1.co.uk/files/files/about/MultisectoralApproach/Plenary-WHO-Social-Determinants.pdf. Accessed 14 August 2017. [Google Scholar]
- 66. Liu JX, Bousema T, Zelman B, Gesase S, Hashim R, Maxwell C. Is housing quality associated with malaria incidence among young children and mosquito vector numbers? Evidence from Korogwe, Tanzania. PLoS One 2014; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Noor AM, Amin AA, Akhwale WS, Snow RW. Increasing coverage and decreasing inequity in insecticide-treated bed net use among rural Kenyan children. PLoS Med 2007; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Loha E, Deressa W, Gari T, et al. Long-lasting insecticidal nets and indoor residual spraying may not be sufficient to eliminate malaria in a low malaria incidence area: results from a cluster randomized controlled trial in Ethiopia. Malar J 2019; 18:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Woyessa A, Deressa W, Ali A, Lindtjørn B. Ownership and use of long-lasting insecticidal nets for malaria prevention in Butajira area, south-central Ethiopia: complex samples data analysis. BMC Public Health 2014; 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.