Abstract
Residual malaria transmission is the actual maintained inoculation of Plasmodium, in spite of a well-designed and implemented vector control programs, and is of great concern for malaria elimination. Residual malaria transmission occurs under several possible circumstances, among which the presence of exophilic vector species, such as Anopheles dirus, or indoor- and outdoor-biting vectors, such as Anopheles nili, or specific behavior, such as feeding on humans indoors, then resting or leaving the house the same night (such as Anopheles moucheti) or also changes in behavior induced by insecticides applied inside houses, such as the well-known deterrent effect of permethrin-treated nets or the irritant effect of DDT. The use of insecticides may change the composition of local Anopheles populations, such as A. arabiensis taking up the place of A. gambiae in Senegal, A. aquasalis replacing A. darlingi in Guyana, or A. harrisoni superseding A. minimus in Vietnam. The change in behavior, such as biting activity earlier than usually reported—for example, Anopheles funestus after a large-scale distribution of long-lasting insecticidal nets—or insecticide resistance, in particular the current spread of pyrethroid resistance, could hamper the efficacy of classic pyrethroid-treated long-lasting insecticidal nets and maintained transmission. These issues must be well documented in every situation to elaborate, implement, monitor, and evaluate tailored vector control programs, keeping in mind that they must be conceived as integrated programs with several well and appropriately coordinated approaches, combining entomological but also parasitological, clinical, and social methods and analyses. A successful integrated vector control program must then be designed to reduce transmission and incidence rates of malaria morbidity and overall mortality.
Keywords: Residual malaria transmission, Anopheles vectors, outdoor biting behavior, vector control methods, vector behavior changes
After a successful period of malaria burden reduction with scaling up of long-lasting insecticidal net (LLIN) distribution, indoor residual spraying (IRS), and artemisin combined therapy, along with intermittent presumptive treatment, rapid detection tests, and possible other approaches [1], malaria remained a worrying public health problem, and its control is at a crossroads [2].
Residual malaria transmission (RMT), which corresponds to Plasmodium transmission, could be considered according to 3 main biological components: (1) entomological, such as remaining transmission even after well-done vector control programs; (2) parasitological, corresponding to remaining Plasmodium parasites in the blood (or elsewhere in the body, such as in the liver), even after well done and adapted drug administration; and (3) clinical, with remaining clinical illness even after appropriately conducted treatment with correct drugs and dosage. The remaining vectors and parasites could be due to resistance (to insecticide or drugs respectively) and several other biological and socioeconomic factors; in the present article we consider only residual Plasmodium transmission, main factors involved, and issues for vector control.
Despite high coverage of insecticide-treated nets (ITN) or IRS, the main vector control methods currently implemented, outdoor and evening or early morning malaria transmission occurs in many malaria-endemic regions and is considered RMT [3]. It represents a key challenge across all malaria-endemic countries. RMT is defined as the transmission of Plasmodium that remains once universal coverage of LLINs (>80%) and/or maximal coverage of IRS has been achieved using insecticides to which the local vectors are susceptible [4].
Malaria transmission still occurs in many endemic countries and this could be due to various factors, including insecticide resistance spread, vector behavior and environment changes, and the role of secondary vectors, to cite a few. Actually, vector control methods implemented indoors have the greatest impact on mosquitoes entering, biting, resting, or/and living inside these treated houses, such as endophilic, endophagic, and anthropophilic mosquitoes. Indoor malaria transmission and morbidity rates have been greatly reduced in the last decades with the scaling up of LLINs and IRS [1].
Inversely exophilic, exophagic, zoophilic mosquitoes are not in contact with insecticide-treated surfaces and could maintain some levels of malaria transmission outdoor even in properly treated areas. There is great variability in the behavior (biting and resting) of Anopheles between and within species and populations, and outdoor biting could be a natural or an induced behavior due to the insecticide used inside the house.
NATURAL VARIABILITY OF ANOPHELES POPULATIONS
In Africa, South of Sahara, the main vectors, Anopheles gambiae and Anopheles funestus are essentially anthropophilic, endophilic, endophagic, biting mainly during the second part of the night [5, 6], while Anopheles arabiensis, also belonging to the A. gambiae complex, has a more plastic behavior, being often quite zoophilic, exophilic, and exophagic. Variability in biting and resting behaviors were noticed with different species [5] in natural conditions without any vector control intervention.
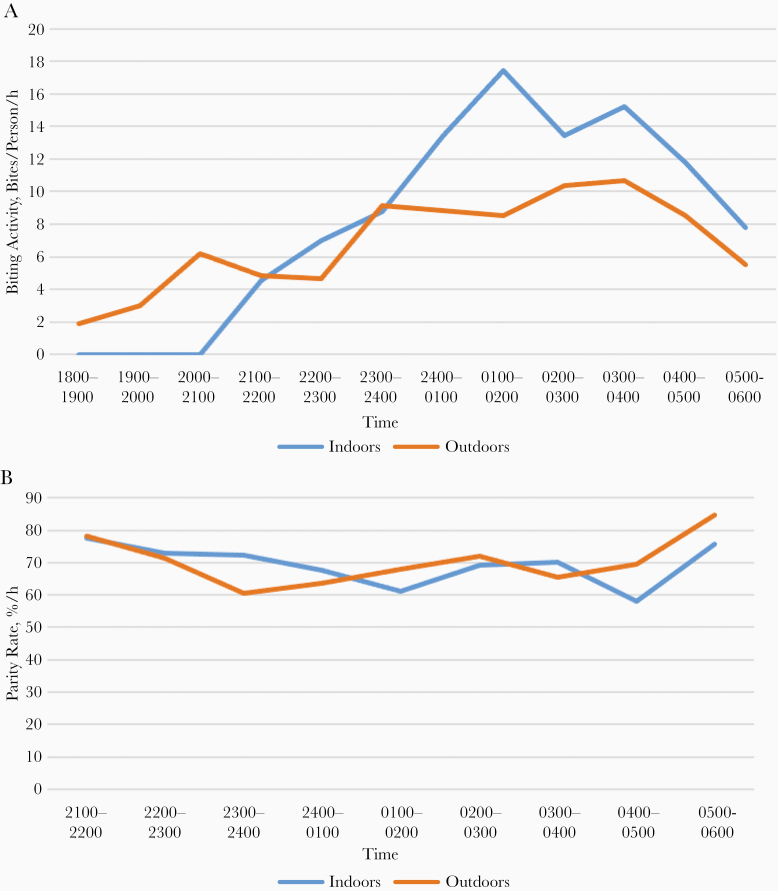
For instance, in Africa south of the Sahara, in rural forested area of Congo (Brazzaville), near permanent rivers such as Lououlou and Louholo, night catches on humans showed that Anopheles nili bites outdoors as well as indoors [7] with 3 successive steps: bites began outdoors while people stay outside for usual social activities, then bites indoors and outdoors were similar, while people progressively entered the house for sleeping, an then bites indoors increased at a largely higher rate than outdoors, while most people of the village are sleeping inside their houses (Figure 1A). It clearly showed that the behavior of A. nili adapted to human behavior. However, malaria transmission could also occur outside, which was reported when an infected bite was noticed at 2200 outdoors. The Anopheles populations biting indoors and outdoors seemed the same, as the parity rate was similar all night (Figure 1B) with an average of 64.8% (n = 895) inside and 67.4% (n = 420) outside.
Figure 1.
Biting activity and parity rate of Anopheles nili for indoor (blue) and outdoor (orange) human habitations. A, Biting activity as hourly number of bites in humans. B, Parity rate per hour.
In Burkina Faso, 12% of the well-known anthropophilic vector species, A. gambiae, were attracted to cattle odor traps, and only 40% of specimens caught inside houses had blood fed on humans [8]. In Sao Tome, A. gambiae appeared exophagic and fed on dogs [9], and in Bioko Island (Equatorial Guinea), the same species was reported as partially exophagic and biting early at night [10] (after initiation of some vector control programs).
In Eritrea, 36% of infective bites occurred outdoors [11]; in Northeastern Tanzania, 12% of malaria transmission occurred before sleeping time [12]; and in Uganda, up to 36% of indoor and 49% of outdoor transmission occurred before sleeping time [13]. In Latin America, the main malaria vector is Anopheles darlingi, greatly anthropophilic, biting during sleeping hours or early in the morning [14], but the main biting peak was also noticed in the evening [15], and outdoor biting was observed in French Guiana [16]. In Nicaragua, in some areas with mainly Plasmodium vivax, 50% of infected bites occurred before sleeping time [17].
In Southeast Asia, the level of behavioral heterogeneity of Anopheles species and populations according to ecological situations is even higher than on the other continents [18]. Anopheles dirus is mostly anthropophilic, exophagic, and exophilic (Table 1), but in Lao People’s Democratic Republic some populations appeared having endophagic and endophilic trends [19]. In western Thailand, populations showed a more zoophilic behavior than anthropophilic with an early biting peak [20]. Other A. dirus populations can also blood feed during the daytime in dark forested areas [21]. Anopheles minimus can be observed with anthropophilic or zoophilic tendencies, as well as endophilic and endophagic behavior, according to region [18]; the biting behavior of Anopheles maculatus (biting earlier than A. dirus or A. minimus) changes according to locality [3].
Table 1.
Mosquito Taxa and Biting Rate by Setting and Collection Methoda
| Setting | Collection Method |
Anopheles Taxon | Biting Rate, Bites/Person/Night |
|---|---|---|---|
| Village | Cow bait | A. maculatus | 0.5 |
| OHLC | A. maculatus | 0.1 | |
| Farm hut | IHLC | A. dirus | 4.3 |
| A. maculatus | 0.1 | ||
| OHLC | A. dirus | 5.7 | |
| A. maculatus | 0.5 | ||
| Forest | OHLC | A. dirus | 4.5 |
| A. maculatus | 0.2 |
Abbreviations: IHLC, indoor human landing collection; OHLC, outdoor human landing collection.
aData from Hung et al [81].
An in-depth study on the adult ecology of A. minimus and A. dirus was undertaken in the Khanh Hoa Province of Vietnam [22] with a trial on the effects of bed nets impregnated with permethrin. The observation showed that the distribution of A. dirus is restricted to forests and areas where the forest has been replaced mainly by plantation of jackfruit, citrus, and rubber (Table 1). Its density was very low during the dry season, with breeding sites restricted to deep forests. A. minimus is breeding in streams in the vicinity of the village (Figure 2B) and it appeared strongly endophagic in all seasons, but the highest densities were noticed during the dry season, because during the rainy season the speed of water flow in the larval habitats and small streams, and along river banks, is too fast and larvae are flushed out (Figure 2B). The biting activities of both species occurred indoors and outdoors throughout the night, but inside houses the biting peaks were not the same; A. dirus had an earlier peak than A. minimus, and A. dirus had higher densities outdoors than indoors.
Figure 2.
Breeding sites of Anopheles vectors in Vietnam. A, Anopheles dirus from Khan Hoa Province (photograph provided by P. C.). B, Anopheles minimus from Hoa Binh Province (photograph provided by S. M.).
The results of this study showed that the number of specimens collected during biting and resting collections decrease during the second part of the night (after 0200) and that fully fed females were found outdoors on wall surfaces, suggesting that A. dirus leaves the houses the same night and fulfills the rest on the gonotrophic cycle outside. Considering that people usually stay outside the nets until 2100, it was estimated that 40% of A. dirus and 17% of A. minimus bite before 2200 showing thus the potential of RMT even if permethrin-treated nets (PTNs) are implemented in villages. In the same study done in Vietnam, the A. dirus biting peak was much higher outdoors than indoors, while for A. minimus the human-landing density was 10-fold higher indoors than outdoors. Implementation of PTNs (0.2 g active ingredient/m2) induced a reduction of 94% of the indoor biting pressure of these main vectors, even for unprotected people living in the same house who benefited from the mass effect of the nets [22]; this reduction of human-vector contact was most likely due to the deterrent or excito-repellent effect of PTNs.
In another study by Garros et al [23], also done in Khanh Phu, Vietnam, the wide use of PTNs coincided with the significant reduction of the prevalence of A. minimus from 100% to 2% between 1999 and 2002, replaced by its sibling species A. harrisoni, which prevalence increased from 3% in 1997 to 88% in 2002. Marchand et al [22] concluded that “normal use of treated nets during sleeping hours may be expected to prevent about 80% of the number of bites from A. minimus, but due to its early biting habits, at most 60% of those from A. dirus, since the seasons of both species alternate, it may be predicted that full use of impregnated nets will, at least initially, have less impact during the rainy season, when A. dirus is most important. This effect may be quite marked since, in addition, this species is also more active biting outdoors.” This change in Anopheles species composition may have important consequences on RMT and the role of the so-called secondary vectors should not be neglected [24].
INDUCED CHANGES
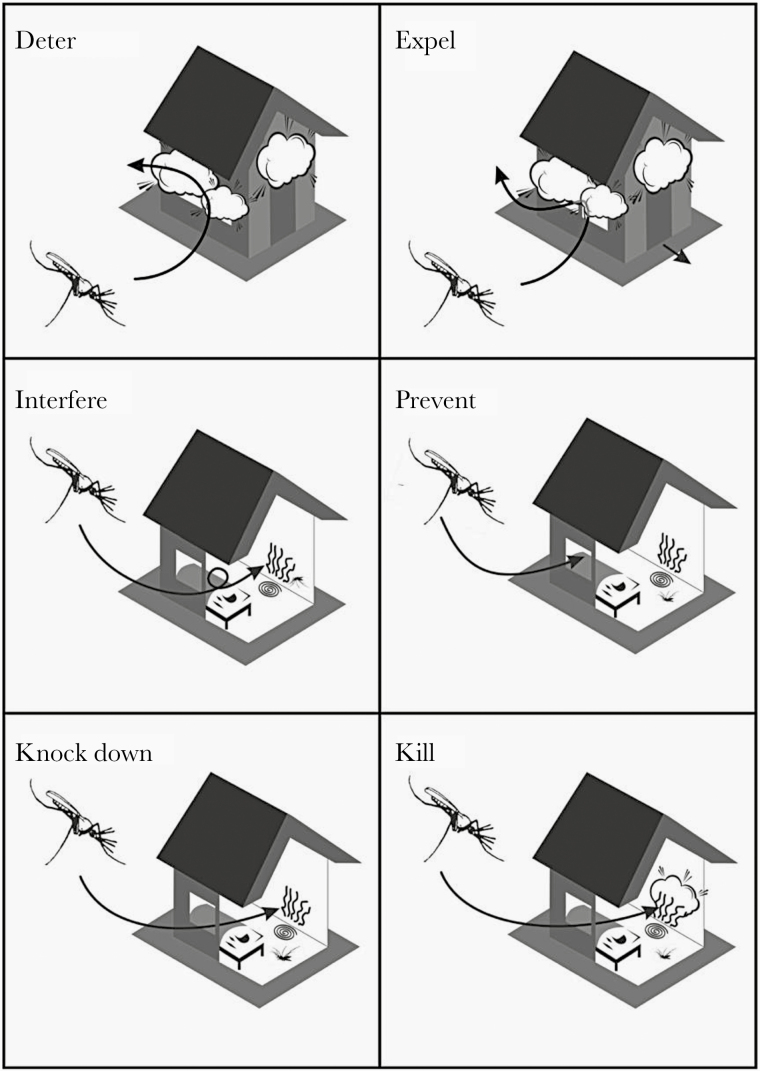
Implementation of some insecticides inside a house can have different impact on the behavior of mosquitoes (Figure 3), such as (1) a deterrent effect (avoidance or reduction of mosquitoes to enter inside a treated house); (2) an expellent or repellent effect (mosquito enters the house but, owing to insecticide irritancy, leaves the treated house quickly, before having received enough dose of insecticide); (3) an interference effect on normal flight and biting behavior; (4) a preventive effect (keeping mosquitoes from entering the house); (5) a knock-down effect for mosquitoes coming near the impregnated surface to bite the host; and (6) a killing or insecticide effect of the chemical uses.
Figure 3.
Main behaviors induced by chemicals implemented in houses for vector control (source: Guillaume Carnevale).
For Durnez and Coosemans [3], insecticides could induce 3 main behavioral shifts. The first is behavioral plasticity, which corresponds to mosquitoes having a high degree of irritability or repellency even at the first exposure to insecticide [25], able to detect its presence and then avoid it [26]. This is also considered “protective avoidance” and could be observed in the case of large-scale LLIN or IRS implementation. Endophilic mosquitoes can change to outdoor behavior after enough contact with the insecticide; they can survive, and malaria transmission still occurs. The second behavioral shift is protective behavior, such as exophagy, exophily, zoophily, and early biting, which also leads to a short (if any) contact with the insecticide inside the house. Mosquito species, populations, or subpopulations may present a high or low repellency response [27]. The third shift is behavioral resistance, in which insecticide induces a selection of mutations favoring the mosquito’s survival, such as insecticide resistance. This happens after and with implementation of indoor vector controls.
The impact of indoor vector control operations could lead to some RMT in several ways. These include (1) a shift in biting or resting behavior, from endophagic, endophilic anthropophily to exophagic, exophilic zoophily; (2) a change in species composition within the same complex or group, such as A. arabiensis in place of A. gambiae, Anopheles rivulorum in place of A. funestus, or A. harrisoni in place of A. minimus [23]; and (3) increasing importance of local or secondary vectors, such as Anopheles aquasalis after elimination of A. darlingi in Guyana [28] or exophagic Anopheles barbirostris in Thailand [29].
Shifts in Biting and Resting Behavior
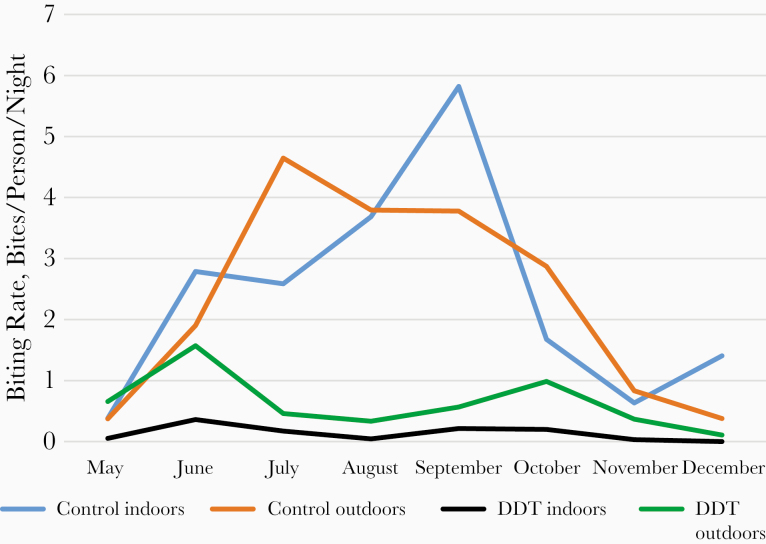
The irritant effect of DDT inducing and increasing the exophilic behavior of A. gambiae was well observed in DDT-treated houses of the Pilot Zone of Bobo-Dioulasso (Burkina Faso) [30], where it showed the important following results (Figure 4). In nontreated areas, the biting rates of A. gambiae were similar inside and outside (P = .93). In DDT-treated areas, the biting rate of A. gambiae was significantly higher outside than inside (P = .003), clearly showing the irritant effect of the product increasing the exit of mosquitoes; this phenomenon was observed during the 8 months of the trial (Figure 4). In dieldrin-treated villages, the biting rates were similar inside and outside (P = .93). Compared with control areas, DDT significantly reduced the biting pressure both inside (P < .001) and outside (P = .04). The reduction of the biting rate inside DDT-treated houses was of great epidemiological importance for malaria control, while the significant increase in the biting rate outside versus inside DDT-treated houses is of great concern for the pursuit of malaria control, despite the efficient insecticide use of IRS.
Figure 4.
Monthly human biting rate of Anopheles gambiae in control and DDT-treated houses of the pilot zone of Bobo-Dioulasso, Burkina Faso [28].
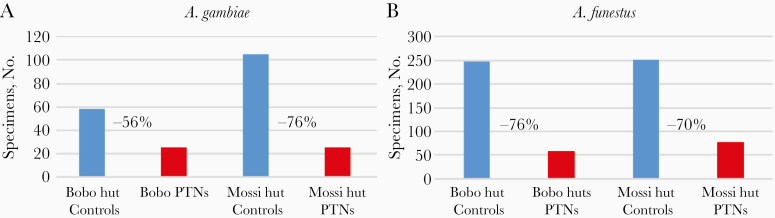
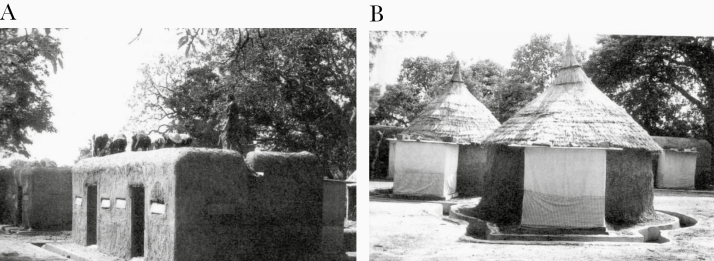
The deterrent effect of permethrin was well noticed during the first trial of ITN in the experimental huts of Soumousso, Burkina Faso [31], where samples of A. gambiae caught indoors with PTN was reduced by 56% in Bobo huts and 76% in Mossi huts (Figures 5A and 6), compared with huts with untreated nets. For A. funestus, the sample size was reduced by 76% in Bobo huts and 70% in Mossi-type huts (Figures 5B and 6).
Figure 5.
Number of specimens of Anopheles vectors caught in controls and permethrin-treated nets (PTNs) in Soumousso experimental huts. A, Anopheles gambiae. B, Anopheles funestus.
Figure 6.
Experimental huts in the World Health Organization (WHO) collaborative field station in Soumousso, Burkina Faso. A, Bobo hut design. B, Mossi hut design. (Photographs provided by Frédéric Darriet) [29].
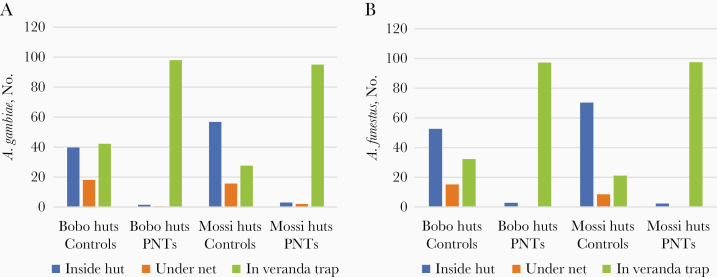
In addition, locations where specimens were collected during morning hour (eg, inside the hut, the net, and the veranda trap) clearly showed the expel effect of PTNs. The results in Figure 7 clearly showed that most specimens (>95%) were collected inside veranda traps in huts equipped with PTNs for both species, A. gambiae (Figure 7A) and A. funestus (Figure 7B), which confirmed the expel effect of PTNs, which increases the exit behavior (exophily) of both species.
Figure 7.
Number of Anopheles collected in 3 locations inside the experimental huts, inside the hut (blue), under nets (orange), or in veranda traps (green) in Bobo and Mossi huts with either untreated nets (Controls) or permethrin-treated nets (PTNs). A, Anopheles gambiae. B, Anopheles funestus.
This increase in exophilic behavior (fewer mosquitoes entering treated huts and more specimens leaving quickly the treated huts) is epidemiologically important, as it clearly shows that PNT confers great personal/familial protection. However, if more mosquitoes are active outside, more will bite and transmit malaria parasites to unprotected people. Therefore, for proper public health management, high (>80%) or universal coverage is absolutely required.
Outdoor RMT has been consistently reported in many areas where interventions such as LLIN or IRS in houses were implemented without much impact [4, 32], while the question of the need for outdoor vector control for malaria elimination was raised [33]. Variations in the ecology of the local vectors, such as shift in biting time from late to early biting [3], increased outdoor biting behavior [34], and changes in species composition [23] have been shown to contribute to maintain transmission [35]. The change in behavior was well noticed in The Gambia [35], as Anopheles coluzzii, well known to be anthropophilic, endophilic, endophagic, and a late-night biter [36], and A. arabiensis, known to be more zoophilic, exophilic, exophagic, and an early-night biter [3], had similar biting patterns after large-scale distribution of LLINs and IRS.
In Benin, decreased proportions of endophily and endophagy in A. gambiae sensu lato (s.l.) populations were observed after IRS and ITN interventions [36]. In the IRS arm, the endophilic rate went from 67.1% before to 4.5% after vector control intervention, whereas in the control arm, the endophilic rate stayed stable at 51.7% (P > .05). In the LLIN arm, endophilic rates also decreased after vector control intervention. For the endophagic rate, proportions of mosquitoes biting indoors in the IRS arm decreased from 67.1% before intervention to 42.9% after intervention, while for the LLIN arm, the reduction in endophagy was less pronounced but still significant, going from 71.3% to 57.5% [37].
Similar observations were reported from Tanzania, Benin, Bioko Island (Equatorial Guinea), and Nigeria, where the increasing use of ITN or IRS raised the proportions of outdoor feeding (exophagy) malaria vector populations such as A. gambiae and A. funestus [32, 38–40]. No shift to increased exophagy of A. gambiae after vector control implementation was reported from Tanzania [32, 41], Burkina Faso [42], or The Gambia [43].
Shift in Biting Time
In Kenya, a shift was observed to an early evening biting time of A. gambiae s.l. after implementation of PTN [44]; the same was also reported from Tanzania with A. gambiae and A. funestus [45, 46]. In Benin, a shift to early morning biting activity was noticed after implementation of a large-scale coverage of LLINs [38].
In Dielmo, Senegal, A. funestus showed a behavioral change in biting activity after 2 massive deployments of LLINs, in July 2008 and July 2011. Anthropophilic and endophilic behavior remained, while diurnal feeding was adopted, essentially on humans [47]. Classically, the biting cycle of A. funestus presented an indoor peak at 0100–0300 and an outdoor peak at 0200–0500 [48], while in Dielmo increasing aggressiveness was observed between 0700 and 1100, corresponding to the time when people are not under LLINs but are instead involved in early household and farming activities [47].
It is clear that in such circumstances, malaria transmission is still occurring despite LLINs inside houses or indoor IRS, and this situation induces RMT of great concern, because the ways to eliminate outdoor transmission are not quite available. However, shifts in biting behavior have not systematically been noticed with ITN or IRS implementation. In Djoumouna village (Congo), the temporary presence inside a house of a bed net impregnated with deltamethrin (12.5 or 25 mg active ingredient/m2) has not induced any shift in the biting cycle of A. gambiae [49]. This was also reported from Bioko Island (Equatorial Guinea) [10], Kenya [50] with PTNs, and The Gambia [43] and for A. funestus in Kenya [50].
Shift in Species Composition of the Anopheles Population
In several studies, a shift was noticed from indoor to outdoor feeding behavior, which could be due to a shift in species composition, for example, from A. gambiae to A. arabiensis, which is more zoophilic and exophilic than A. gambiae [32, 34, 51, 52]. In Vietnam, a shift from A. minimus to A. harrisoni, which is more exophagic and exophilic, was reported after the wide use of permethrin-treated bed nets [23].
In Dielmo, Senegal, a shift in species composition in the A. gambiae complex after implementation of LLINs nets was described with a drastic decrease in the proportions of A. coluzzii and A. gambiae after the introduction of LLINs, which was concomitant with an increase in the proportion of A. arabiensis [53]. In Kenya and Tanzania, where ITNs were implemented at large scale, the proportion of A. arabiensis increased while the densities of A. gambiae and A. funestus decreased [34, 51].
This phenomenon was also noticed after IRS implementation in Kenya, where A. funestus disappeared and was replaced by the more exophagic A. rivulorum [54] or Anopheles parensis [55], which took over the breeding sites of the declining species. However, the same vector species were noticed after ITN implementation in Bioko Island, Equatorial Guinea [10], or Kenya [50].
Understanding the contribution of outdoor resting Anopheles to RMT is important in scaling up vector control toward malaria elimination in South Africa [56]. In KwaZulu-Natal Province (South Africa), the main vectors were A. funestus and A. arabiensis [57]; the former, highly anthropophilic and endophagic, was well controlled by repeated IRS campaigns done for several years and nearly disappeared [58]. For A. arabiensis, which bites and rests outdoors, its control is less amenable to being controlled by indoor vector control operations and is greatly involved in RMT [59].
Other species are also involved in RMT, such as Anopheles merus (A. gambiae complex) in Mozambique [60] and Mpumalanga Province, South Africa [61]; Anopheles vandeeni, an outdoor resting mosquito and member of the A. funestus group [62], considered a secondary vector [63]; and A. parensis, mainly zoophilic and resting indoors or outdoors, which was found to be positive to Plasmodium falciparum circumsporozoite (CSP) [56] and could therefore be involved in RMT in the northern KwaZulu Natal Province of South Africa.
Secondary vectors, such as A. rivulorum, Anopheles leesoni, and A. parensis were also incriminated in Tanzania [64–66], as well as A. rivulorum and Anopheles longipalpis in Kenya [67, 68], all of which may contribute to RMT. However, the global lack of studies on secondary vectors prevent any precise information on their specific role in malaria transmission [24].
Shift in Biting Preference
Large-scale use of ITN in Kenya induced a shift of A. gambiae and A. funestus from humans (protected) to animals, mainly cattle [69]; the same shift from human, not readily accessible, to locally available animals was also noticed in Burkina Faso [8]. However, a large number of studies found no particular trophic deviation induced by ITN or IRS on A. gambiae [39, 41, 42, 70], A. funestus [41], or A. arabiensis [71].
Shift in Resting Place
In French Guiana A. darlingi disappeared from inside houses after IRS campaigns, while staying outside in the peridomestic environment [16]. Shifts to exophily were observed in species from all geographic regions, including Afrotropical (A. arabiensis), Australasian (Anopheles farauti), Oriental (A. dirus), and Neotropical (A. darlingi) [3].
VECTOR CONTROL METHODS FOR OUTDOOR-BITING MOSQUITOES
Among the available vector control methods currently used, most of them target indoor-biting (endophagic) and indoor-resting (endophilic) mosquitoes. Very few approaches are efficiently designed to control exophagic or exophilic mosquitoes, although new approaches have recently been developed. This is a review on some currently available methods to control outdoor-biting Anopheles vectors.
LLIN Implementation
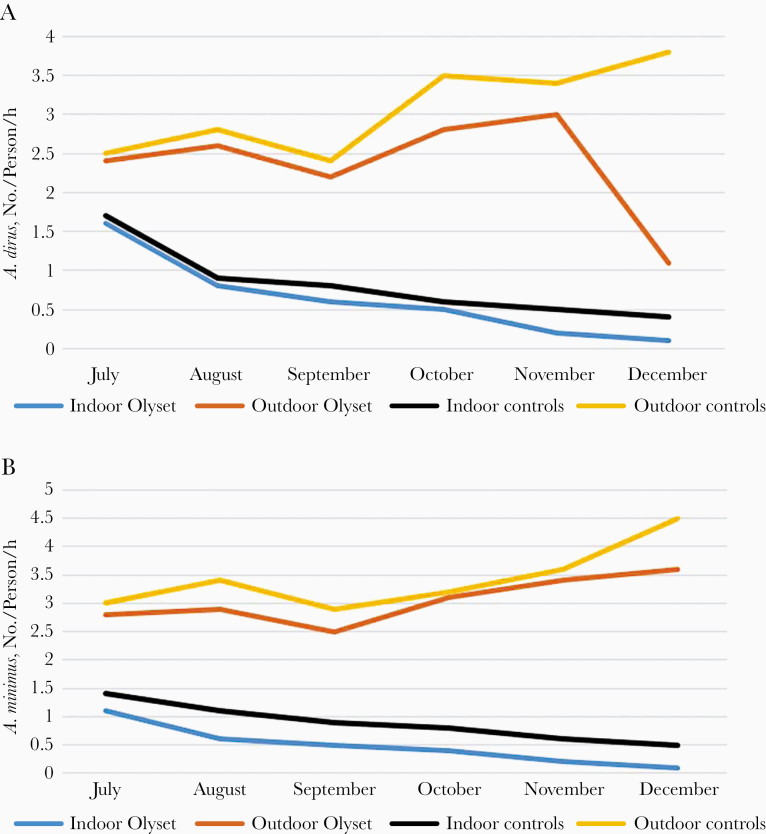
In the Rattanakiri Province of Cambodia, a trial of Olyset LLINs (Permethrin) was implemented against A. dirus and A. minimus (Yeng Chhelang and Lek Sandy, unpublished report). For A. dirus, the average densities of bites per person per hour were 0.6 and 0.8 indoors in Olyset and control villages, respectively, and 2.4 versus 3.1 outdoors, owing to the exophagic behavior of this species (Figure 8A). The bite densities (bites per person per hour) were significantly higher outdoors than indoors in control (P = .002), as well as in Olyset villages (P = .04), confirming the common exophagic behavior of this species (Figure 8A).
Figure 8.
Monthly evolution of the biting density (bites per person per hour) of Anopheles vectors indoors and outdoors in controls and Olyset net villages. A, Anopheles dirus; B, Anopheles minimus.
When comparing Olyset villages and controls, densities were similar inside (P = .43), as well as outside (P = .13). According to Chhelang and Sandy (unpublished report), the total indoor biting density, per person per hour, was reduced by 71.4% in Olyset areas and by 55.6% in control areas. Outdoors, the reduction rate was only 14.3% in Olyset areas, and the density increased by 16.1% in control villages, showing that even against an exophagic vector such as A. dirus, implementation of PTN could still be worthwhile in reducing human-biting pressure.
For A. minimus, a reduction rate of 72.2% in indoor density was reported, compared with 30.8% in control areas, while there was a reduction rate of 3.2% in outdoor density in Olyset areas and an increase of 11.8% in control areas (Figure 8B). Trends of the monthly evolution of bite density (bites per person per hour) were remarkably similar. Hourly bite densities were significantly higher outdoors than indoors in both Olyset (P = .002) and control areas (P = .002), and similar inside (P = .80) and outside (P = .25) treated and control areas (Figure 8B).
The entomological data recorded after the implementation of Olyset nets not only clearly showed a reduction in indoor biting densities, but it was also very efficient in decreasing the whole population of exophilic mosquitoes by 71.4% for A. dirus and 72.2% for A. minimus (Figure 8). Another important result to underline is that during the 7-month period, the number of malaria cases was reduced by 91.1% and deaths by 100% (Chhelang and Sandy, unpublished report).
Larval Source Management
Fish-Based Biological Control
In Djibouti, when the main malaria vector was A. arabiensis, a large-scale larval control program was implemented in the capital based on the autochthonous larvivorous fish Aphanius dispar (Figure 9A), along with the use of locally Bacillus thuringiensis or Bacillus sphaericus [72]. First, all A. arabiensis breeding sites were localized, and 800 wells appeared as actual or potential sites. Besides, the local larvivorous fish A. dispar was collected and reared. Then, each well received 5–10 fish, which were checked for their presence regularly and restocked if needed. Local or temporary ponds with stagnant rain water were also treated first with temephos (0.1–0.5 ppm) and then with B. thuringiensis (0.1–0.2 g/m2) or B. sphaericus (1–5 g/m2), the latter product lasting longer than B. thuringiensis (3 weeks). This large larval control program induced a 73% reduction of positive breeding sites, and 90% of expected clinical malaria cases were avoided [72].
Figure 9.
Fish used in biological control against mosquito larvae. A, Aphanius díspar. B, Poecilia reticulata (guppy). C, Gambusia affinis.
A. dispar was also successfully used in Assab, Ethiopia, against Anopheles culicifacies adanensis [73]. Cisterns, wells, and barrels found positive for larvae received this fish with monthly restocking, which was enough to maintain actual control. It thus appeared that 34% (range of 18%–60%) of unstocked site were positive, while 1.6% only of the fish-treated breeding sites still harbored some larvae. It is important to underline that stocking of larvivorous fish in wells and household water storage containers was well accepted by the participants, and, based on the results of this study, larvivorous fishes were introduced on an operational scale for the control of malaria transmission in Assab, with voluntary participation of the population.
A study on A. dispar in India [74] showed that the mean (standard deviation) daily consumption of larvae in laboratory was as follows: Anopheles stephensi, 128 (0.2) to 204 (6); Culex quinquefasciatus 24 (4) to 58 (10); and Aedes aegypti, 43 (5) to 68 (2). In water tanks, the fish A. dispar reduced larval counts by 93% by day 7 and by 98% by day 21 (P < .01), showing high larval propensity (Figure 9A).
According to these studies from Djibouti and India, and considering that A. stephensi is now present in Djibouti [75], larval control based on A. dispar should be implemented to control this efficient urban malaria vector. It was recently reported that A. stephensi invaded Djibouti and Ethiopia, potentially spreading to other areas of Africa [76]. This invasion of A. stephensi increases the major threat of urban malaria transmission in East Africa and requires urgent control measures to prevent malaria epidemics in cities, which would cause a public health disaster [77].
In Thailand, other species of fish, including guppy and Gambusia (Figures 9B, 9C, and 10) were used for the control of vectors with community participation [78, 79], in addition to IRS with deltamethrin and ITN. Environmental modifications to reduce the larval habitats were also implemented, targeting A. minimus and A. maculatus, but it seemed unfeasible for A. dirus, despite the fact that it is the most potent vector.
Figure 10.
Releasing larvivorous fish, Chanthaburi, Thailand (photograph by Tawat Kantasri, courtesy of Frédérick Gay) [78].
Larviciding, Environmental Management, and Outdoor Residual Spraying
In KwaZulu Natal Province (South Africa), A. funestus almost disappeared after repeated IRS campaigns, leaving room for A. arabiensis, which became the main vector, primarily responsible for the bulk of RMT in South Africa. A. parensis, while being known as strongly zoophilic, was also found to be positive for P. falciparum CSP [56]. As Burke et al [54] mentioned, the 2 P. falciparum–positive specimens of A. parensis were caught resting outdoors, which highlights the importance of addressing RMT in South Africa by targeting both indoor- and outdoor-resting vectors. Therefore, besides continuing IRS programs, intensive larval source management programs, including winter larviciding and community outreach programs designed to educate on personal protection measures and treatment seeking, are under development to address this issue.
In Indonesia, interesting intersectoral collaborations were developed, for instance, one with the Ministry of Agriculture that provided a legal base regarding regulations on irrigation and planting schedules, which proved critical to malaria vector management success in Java. The Ministry of Public Works has strived to reduce the sources of larval habitats in endemic areas, and vector control was done with environmental management (Figure 11A), larviciding spray (Figure 11B) and community participation, as well as classic IRS [80].
Figure 11.
Mosquito larval source reduction in Indonesia. A, Elimination of manmade breeding places carried out by the Department of Public Works. B, Larviciding by health department personnel (photographs by Bangkit Hutajulu, courtesy of Frédérick Gay) [80].
To control an outbreak in Central Vietnam, larval source reduction and outdoor residual spraying were implemented (Figure 12). The outdoor residual spraying control method deserves further study and evaluation of its impact in reducing RMT [81].
Figure 12.
Outdoor residual spraying at a malaria epidemic focus in Dak Lak Province, Vietnam (photograph by Huu Thuy, courtesy of Frédérick Gay); [81].
Long-Lasting Insecticide-Treated Hammocks
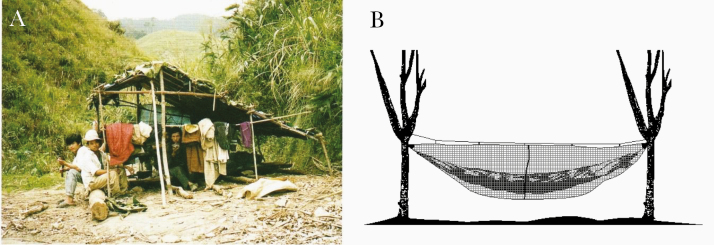
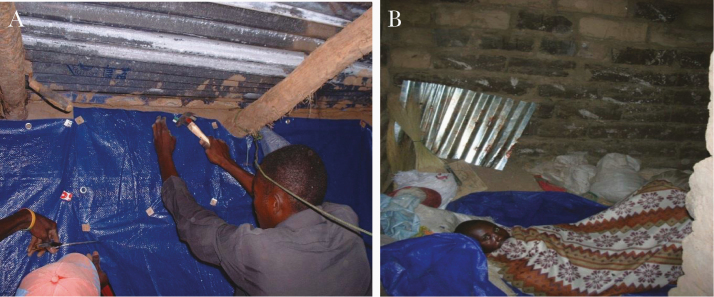
A main issue in malaria control concern migrants farmers, woodcutters who are living in temporary shelters without any protection (Figure 13A) [82]. One solution could be to provide them with LLINs or/and long-lasting insecticide-treated hammocks (LLIHs) (Figure 13B) when they go to work in forested areas prone to A. dirus [83–86]. Another way to prevent them from malaria transmission would be to use topical repellent or/and impregnated clothes to reduce the biting pressure.
Figure 13.
Field conditions and protection against outdoor-biting mosquitoes. A, Woodcutters in their temporary shelter in Vietnam (photograph by Vo Hinh, courtesy of Frédérick Gay) [82]. B, Long-lasting insecticide-treated hammock (source: Guillaume Carnevale).
A trial using LLIHs with the classic LLIN Olyset Net sewn at the backside of the hammock was implemented in Western Cambodia in Pang Rolim village, A. minimus being the main vector, and Dey Krahorn Leu village, with A. dirus predominant [83]. Implementation of treated hammocks reduced by 45% A. minimus bites in both villages, while for A. dirus a reduction of 46% was observed only during the second survey (P = .005). These results mean that the personal protective effect of LLIHs against exophagic vectors and nuisance due to mosquitoes was variable according to species, villages, and surveys. Nearly half of the A. minimus bites could be avoided by using LLIHs. A similar result was obtained against A. dirus but only during the second survey (end of the rainy season), and no evidence of protection was found in the middle of the rainy season [77].
In central Vietnam, an interesting biological and social study was recently implemented in Khanh Hoa Province considering vectors and common use of impregnated nets (ITNs or LLINs) according to place of residence (village) and/or temporary work (farm, forest) [87]. ITN/LLIN coverage in the village was reported to be >90%. The vast majority (>90%) of individuals owned a forest farm plot; 72% sometimes slept in the farm huts overnight, and 33.2% sometimes stayed overnight in the forest. Only 12.1% of forest workers regularly used a net overnight in the forest, and 1.1% sometimes did so. Mosquitoes were collected on a single cow bait tent trap outside, as well as human-landing catch indoors and outdoors. The results confirmed the absence of A. dirus in villages with universal coverage of treated nets, and the importance of this coverage inside and outside farm huts and in forests [81].
The normal sleeping time in the community in farm huts was at 2100, while 45% of the biting peak of A. dirus s.l. and 100% of A. maculatus s.l. occurred before 2100. For Anopheles mosquitoes outdoor in the forest and inside farm huts, the peak biting activity was highest at 2000–2100. Three A. dirus s.l. were found to be sporozoite positive, 2 with P. falciparum, both collected outdoors, 1 near the farm hut and 1 in the forest, and 1 with P. vivax, also outdoors at farm huts.
This study in Vietnam showed entomological inoculation rates for P. falciparum of 17.8 infectious bites per person per year in the outdoor farm hut site and 25.3 per person per year in the forest, specifically from A. dirus s.l. The entomological inoculation rate for P. vivax from A. dirus s.l. in the forest site was also 25.3 infectious bites per person per year [87]. These observations are well in line with some others done in Central Vietnam, showing that human outdoor activities, especially at night, favor exposure to malaria vectors that cannot be prevented by sleeping under LLINs and some risk factors relating to evening outdoor exposure may have been missed in previous studies [88].
Insecticide-Treated Plastic Sheeting
Considering that biting rates in farm huts were comparable to those seen in the forest, because farm huts offered little to no protection due to poor structure, freely allowing entry of mosquitoes through the walls and floors, it is interesting to try here the recently developed insecticide-treated plastic sheeting (ITPS). ITPS can be pinned on walls, inside or outside huts, by people themselves, and on the floor, serving several purposes (Figure 14).
Figure 14.
Use of ZeroFly insecticide-treated plastic sheeting (ITPS) in villages in Angola. A, Hanging ITPS on the wall. B, Sleeping on ITPS spread on the floor. (Photographs provided by P. C.)
ITPS was successfully used in refugee camps in Sierra Leone [89] and Afghanistan [90], and ZeroFly ITPS was also implemented in India [91, 92] against A. stephensi and A. culicifacies. Entomological and parasitological data showed the high efficacy of ZeroFly sheeting in controlling malaria. It is worth noting that, for Sharma et al [92], the introduction of ZeroFly plastic sheeting in a community-based intervention program was an operationally feasible way to contain malaria, especially in high-transmission areas.
A village-scale control program around Balombo town (Benguela Province, Angola) was implemented in 2007 using deltamethrin-treated ITPS as a wall lining or ZeroFly [93], used either alone or combined with deltamethrin-treated LLINs or after λ-cyhalothrin IRS. The results showed an overall reduction of 90% of the entomological inoculation rate by A. funestus and A. gambiae and 80% of the Anopheles density. In addition, 70% reduction in P. falciparum prevalence, 65% reduction in the gametocyte index, and 55% reduction in the number of cases in children <9 years old were also recorded [93].
One main concern was the withdrawal of ITPS by the population in some villages (Figure 15), often discarded outside, underscoring the need for information education communication campaigns. Therefore, ITPS deserves special attention as it can also be used on the outside walls of huts and temporary shelters of farmer migrants in Southeast Asia to protect them against outdoor Anopheles vectors when staying near their houses for usual social events before sleeping.
Figure 15.
Example of a wall lining withdrawn from houses and laid on the fence in Angola (photograph provided by P. C.).
It should also be possible, if and where feasible, to place ITPS between well-known breeding sites, such as streams for A. minimus (Figure 2) or pools for A. dirus, and villages to form a barrier to protect people. Hand-made fences around villages to protect against wild animals could also be used to support ITPS, which would provide a protection to villagers against outdoor-biting mosquitoes (Figure 15).
Zooprophylaxis
A recent analysis of 34 articles dealing with blood feeding and resting habits of A. arabiensis considered 4 types of zooprophylaxis: passive, active, combination, or insecticide zooprophylaxis [94], defined as follows. Passive zooprophylaxis is the natural prophylactic effect of cattle seen when cattle density within a community is increased. Active zooprophylaxis refers to the deliberate introduction of domestic animals to divert mosquitoes away from human settlements toward other nontransmitting hosts. Combination zooprophylaxis is the use of ITNs and IRS integrated with livestock placed in a separate shed, to induce a push-pull effect, thereby reducing disease incidence. Finally, insecticide zooprophylaxis is the treatment of cattle by sponging or dipping the cattle with insecticides to pass on a lethal dose to blood-feeding mosquitoes. For Asale et al [94], the studies examined showed that zooprophylaxis can have positive, negative, or no effects on malaria vector control.
Two studies analyzed the push-pull combined actions with the deliberate introduction of LLINs and IRS used as the pushing factor, while zoophilic and opportunistic mosquito species such as A. arabiensis are attracted by domestic animals (ie, the pulling factor) [95, 96]. Other studies used the additive role of LLINs in zooprophylaxis, which demonstrated by modeling that scaling up mass coverage of LLINs to 80% in the community and ensuring 80% coverage of livestock treatment with pyrethroids could lead to a global reduction and elimination of the disease [97, 98].
According to a recent review of literature, zooprophylaxis could be part of an effective strategy of integrated vector management to reduce malaria transmission under specific ecological and geographic conditions [99]. However, the current scientific evidence base is inconclusive on the role of socioeconomic factors, the optimal distance between livestock and human sleeping quarters, and the effect of animal species and densities on zooprophylaxis, because 2 opposite phenomena could occur, zooprophylaxis or zoopotentiation. The first, zooprophylaxis, corresponds to the reduced malaria risk in areas where predominant mosquito species do not prefer human hosts, where livestock are kept at a distance from human sleeping quarters at night, and where mosquito nets or other protective measures are used. While the second, zoopotentiation, refers to the fact livestock may actually draw mosquitoes to humans, increasing malaria transmission, which occurs where livestock are housed within or near human sleeping quarters at night and where mosquito species prefer human hosts [99]. In fact, various parameters interfere starting with the Anopheles species being targeted, and each situation has to be carefully studied before embarking in a large-scale zooprophylaxis program, but the possibility has to still be kept in mind [100].
Reproduction and Swarming
Another innovative approach is targeting male mosquito mating behavior for malaria control [101], based on sound knowledge of the reproduction of the concerned Anopheles species, such as A. arabiensis [102, 103], a vector mainly involved in RMT following the effect of LLINs in decreasing A. gambiae and A. funestus. For a long time, a large amount of work was devoted to the sterile insect technique preferentially targeting males [104–106], and it is gaining new emphasis with RMT not yet controlled by currently available techniques such as IRS and LLINs [107–109]. Considering the well-known risk of reinvasion by mosquitoes, the mass release of sterile males can be done only in remote villages or islands, as developed for the control of Aedes albopictus in La Réunion Island [110].
Personal Protection With Skin Repellents or Permethrin-Impregnated Clothes
A number of studies has been devoted to skin repellents [102–106, 111–115], with several recommendations of use, such as the those provided by the former French organization Afssaps [116]. Special attention should be given to the use of skin repellents for newborns, babies, and small children. The French Pediatric Society [106] stated that before infants are old enough to walk on their own, skin repellents must be avoided unless there is a high risk of vector-borne diseases, such as an outbreak of dengue or other arboviruses, but infants must be protected by treated mosquito nets; for those aged 6–12 months, recommendations include once-daily para-menthane-3,8-diol or PMD (Citriodiol) 20%–30%, N,N-diethyl-m-toluamide (DEET) 10%–30%, or IR 35/35 20% [106].
Other recommendations and considerations include conditions and limits of use, duration, mosquito species of the area, perspiration, use of sunscreens [117], time to spend in at-risk areas and during at-risk periods [118]. Skin repellents confer protection against outdoor-biting vectors and deserve further attention for their promotion as a public health tool.
Soap containing 20% DEET and permethrin (either 0.5 or 1.0%) were tested on Penang Island, Malaysia against Anopheles lesteri and other outdoor mosquito species [119]. An interesting efficacy was noticed, with 80%–100% reduction in mosquito landing and biting rates according to the species tested and residual effects were registered up to 4 hours. In high mosquito densities, small percentages of A. lesteri landed on treated skin. The use of the soap repellent formulations could be envisaged for personal or familial protection, while considering cost-effectiveness, safety, sustainability, and acceptability. Such soap could also be used to “wash and treat” clothes; this could be tested in terms of efficacy and efficiency against outdoor mosquitoes to protect day and night workers exposed to biting pressure.
Permethrin-impregnated clothes were largely tested and used against several species of mosquitoes in various situations [120–126]. Clothes could be treated by dipping, as with nets, the method used in several parts of Vietnam, by spraying [127], or already industrially treated [128]. Treated clothes can keep their efficacy for 2 months and resist to 6–8 washes (with cold water) when normally used. Resistance could be increased to 33 washes (cold water) with an increased dose of permethrin; the efficacy is quickly reduced when the clothing is washed in hot water (60°C) with detergent. Clothes could also be treated with repellents such as DEET [127] but with short residual activity.
Permethrin-treated clothes could also be used with skin repellents on “unprotected” area of the body [129], and such combinations showed great efficacy against exceptionally aggressive Culiseta impatiens (2400 bites per hour). Permethrin on clothes gave a 93.4% protection, DEET on skin gave 99.7% protection, and using both conferred 99.9% protection [130].
A field evaluation of personal protection methods against outdoor-biting mosquitoes was conducted in 2 study sites in Xieng-Ngeun district, Luang Prabang Province, northern Lao People’s Democratic Republic, where the more abundant species were Culex vishnui s.l. and A. albopictus, and the putative malaria vectors were Anopheles barbumbrosus s.l., A. minimus s.l., A. barbirostris s.l., A. dirus s.l., A. maculatus s.l., Anopheles epiroticus, and Anopheles umbrosus [126].
The study showed that: (1) mosquito coils in a metal casing worn on a belt provided 92.3% protection against all mosquito species during the afternoon (1200–1800) and 68.8% during the evening (1700–2300); (2) the combination of permethrin-treated clothing, plus PMD or para-menthane-3,8-diol (menthoglycol or Citriodiol) resulted in 68.2% protection in the afternoon and 52.3% in the evening; (3) the combination of untreated overalls with PMD resulted in 55% protective efficacy, and only 25.2% in the evening; and (4) long permethrin-treated clothing resulted in 61.1% protection during the afternoon and 43% in the evening.
The conclusion of this study was that portable mosquito coils were highly protective against outdoor-biting mosquitoes, although there are safety concerns related to their use. The combination of permethrin-treated clothing and PMD repellent represent an alternative treatment for protection against outdoor-biting mosquitoes.
The use of synthetic repellents against mosquitoes (Table 2), particularly DEET, has raised some issues on safety and health risks to humans and the environment. Therefore, plants-based repellents should be increasingly studied to serve as safer alternatives to synthetic repellents. In addition the raw material to produce plant-based mosquito repellents can be readily accessible, locally accepted, and even quite popular in some communities, and affordable to low-income communities, while quite efficient in preventing mosquito bites. At present, the development of natural product-based repellents with more effective and long-lasting protection is under study. Many active ingredients have been studied, most of them being essential oils [131–134]. Studies have shown that 2 plant-based compounds such as β-caryophyllene oxide [135] and vetiver oil [136] have great potentials as natural insect repellents against A. minimus, A. dirus, A. albopictus, and A. aegypti. These plant-based repellents were shown to be environmentally friendly and safe for public use [135, 137].
Table 2.
Active Ingredients of Skin Repellents and Concentrations by Age Group and for Pregnant Womana
| Age Group | Active Ingredients | Concentration, % |
|---|---|---|
| 30 mo to 12 y | PMD (Citriodiol)b | 20–50 |
| IR 35/35 | 20–35 | |
| DEETb | 20–35 | |
| KBR 3023c | 20–30 | |
| >12 y | PMD (Citriodiol)b | 20–50 |
| IR 35/35 | 20–35 | |
| DEETb | 20–50 | |
| KBR 3023c | 20–30 | |
| Pregnant women | IR 35/35 | 20–35 |
Abbreviations: IR, insect repellent; DEET, N,N-diethyl-m-toluamide; KBR 3023, icaridine or picaridine.
aData from Sorge et al [106].
bNot used in patients with a history of seizure.
cNot used for >1 month.
Attractive Toxic Sugar Bait
The attractive toxic sugar bait method is based on the “lure and kill” strategy, in which the instinct of mosquitoes to search and feed on sugar sources is exploited [138, 139]. The attractive toxic sugar bait is coformulated with low-risk toxic substances, such as boric acid, and can be deployed in bait stations or sprayed on plants, providing an interesting way to control outdoor mosquitoes [140–146].
Nanosynthetized Insecticides
A paint, containing 2 organophosphates, chlorpyrifos and diazinon, and insect growth regulator, pyriproxyfen, was tested under laboratory conditions for 12 months after World Health Organization pesticide evaluation scheme (WHOPES) phase I procedures [147]. After treatment, delayed mosquito mortality rates were high (87%–100%), even in organophosphate-resistant A. gambiae females on all surfaces except cement treated at 1 kg/12 m2. One year after treatment, delayed mortality rates were 93%–100% in organophosphate-resistant females on nonporous surfaces at both doses. On cement, death rates were low 12 months after treatment regardless of the dose and the resistance status. Fecundity, fertility and adult emergence were reduced after treatment, even at the lower dose (P < .01). A reduction in fecundity was still observed 9 months after treatment at both doses (P < .01), and adult emergence was reduced at the higher dose (P < .01) [148]. Owing to its spatial mortality pattern (mortality at distance without contact with insecticide treated surface) [149], insecticide paint for an outdoor residual spraying could be an interesting way to control outdoor mosquitoes when surfaces of the huts or shelters allow its use.
Conclusions
The burden of malaria dropped strikingly in the last decade with the large-scale implementation of vector control methods essentially based on LLIN and IRS, and with availability of artemisin combined therapy [1]. However, control programs have to deal with some key problems, such as insecticide resistance [150] and RMT, transmission that is maintained even after well-done vector control operations against susceptible vectors. RMT is due to several factors, such as potential shifts in vector behavior from endophagy to exophagy, from endophily to exophily, and from anthropophily to zoophily. RMT is also occurring owing to the selection of outdoor-biting, resting, or zoophilic behavior of Anopheles vector populations (A. arabiensis, A. minimus) or outdoor-biter species, such as A. dirus, the jungle mosquito present in remote forested areas of Southeast Asia, where it presents a serious challenge to malaria control [151]. Change in vector composition is also another factor affecting RMT, with some species taking over breeding sites and replacing the eliminated ones, such as A. rivulorum [54], A. parensis [55] or A. harrisoni [23].
These situations could be quite different according to local conditions and natural or induced changes, with a dynamic process, such as environmental management or modifications, plantations (eg, rice and rubber), deforestation for agricultural projects, social and cultural habits, economical situations, migrants (to and from forested areas), resettled populations, climatic changes, Anopheles genetic background, and issues with species identification in complexes or groups such as A. gambiae, A. funestus, A. minimus, A. maculatus, A. dirus, to cite a few, in which sibling species cannot be morphologically differentiated [152, 153].
One species can have different behavior and vector capacity according to the region or country: for example Anopheles aconitus is a swamp breeder in Indonesia [154], where it is one of the common species. It rests outdoors in bushes, predominantly zoophilic but bites humans readily [155], can enter houses and cattle sheds, and starts biting as early as 1800 until 0100. It can also rest inside houses or cattle sheds, and it is a secondary vector in Orissa (India) but reported as an important vector in Java and Sumatra (Indonesia), even with low vector competence [156, 157].
Therefore, there is no single solution, no magic bullet applicable everywhere, but rather adapted and tailored measures, which can be implemented only after sound knowledge of every situation with comprehensive understanding on biological, social, and economic factors involved in RMT, needing multisectorial coordination to elaborate and implement relevant plan of action, ranging from community-based involvement to international cooperation to prevent reintroduction of malaria at international borders.
Several tools are now available for vector control of outdoor Anopheles mosquitoes, and each has its advantages and issues. These tools must be used in an integrated vector management led by field-oriented pluridisciplinary teams targeting the reduction of malaria transmission, morbidity, and mortality rates and the elimination of the disease in the foreseeable future [158].
Notes
Financial support. This work study was supported by the French National Research Institute for Sustainable Development (IRD).
Supplement sponsorship . The supplement is sponsored by TDR, the Special Programme for Research and Training in Tropical Diseases, based at the World Health Organization.
Potential conflicts of interest. Both authors: No reported conflicts. Both authors have submitted the ICMJS Form for Disclosure of Potential of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. World malaria report. Geneva, Switzerland: World Health Organization, 2015. [Google Scholar]
- 2. World Health Organization. World malaria report. Geneva, Switzerland: World Health Organization, 2017. [Google Scholar]
- 3. Durnez L, Coosemans M. Residual transmission of malaria: an old issue for new approaches. In: Manguin S, ed. Anopheles mosquitoes—new insights into malaria vectors. Rijeka, Croatia: IntechOpen, 2013:671–704. [Google Scholar]
- 4. Killeen GF. Characterizing, controlling and eliminating residual malaria transmission. Malar J 2014;13:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gillies MT, De Meillon B. The Anophelinae of Africa South of the Sahara: Ethiopian zoogeographical region. South Afr Inst Med Res 1968;54:1–343. [Google Scholar]
- 6. Sinka ME, Bangs MJ, Manguin S, et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic précis. Parasit Vectors 2010; 3:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carnevale P, Zoulani A. Agressivité d’Anopheles nili (Theobald), 1904 à l’intérieur et à l’extérieur des maisons. Cah ORSTOM, sér Ent Méd Parasitol. 1975; 13:69–73. [Google Scholar]
- 8. Lefèvre T, Couagna L, Dabira K, et al. Beyond nature and nurture: phenotypic plasticity in blood-feeding behaviour of Anopheles gambiae s.s. when human are not readily accessible. Am J Trop Med Hyg 2009; 81:122–5. [DOI] [PubMed] [Google Scholar]
- 9. Sousa CA, Pinto J, Almeida AP, Ferreira C, do Rosário VE, Charlwood JD. Dogs as a favored host choice of Anopheles gambiae sensu stricto (Diptera: Culicidae) of São Tomé West Africa. J Med Entomol 2001; 38:122–5. [DOI] [PubMed] [Google Scholar]
- 10. Reddy M, Overgaard H, Abaga S, et al. Outdoor host feeding behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island, Equatorial Guinea. Malar J 2011; 10:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shililu J, Ghebremeskel T, Seulu F, et al. Seasonal abundance, vector behaviour, and malaria transmission in Erithrea. J Amer Mosq Cont Assoc 2004; 20:155–64. [PubMed] [Google Scholar]
- 12. Maxwell CA, Wakibara J, Tho S, Curtis CF. Malaria-infective biting at different hours of the night. Med Vet Entomol 1998; 12:325–7. [DOI] [PubMed] [Google Scholar]
- 13. Okello PE, Van Bortel W, Byaruhanga AM, et al. Variation in malaria transmission intensity in seven sites throughout Uganda. Am J Trop Med Hyg 2006; 75:219–25. [PubMed] [Google Scholar]
- 14. Hiwat H, Issaly J, Gaborit P, et al. Behavioral heterogeneity of Anopheles darlingi (Diptera: Culicidae) and malaria transmission dynamics along the Maroni River, Suriname, French Guiana. Trans R Soc Trop Med Hyg 2010; 104:207–13. [DOI] [PubMed] [Google Scholar]
- 15. Moutinho PR, Gil LH, Cruz RB, Ribolla PE. Population dynamics, structure and behavior of Anopheles darlingi in a rural settlement in the Amazon rainforest of Acre, Brazil. Malar J 2011; 10:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Girod R, Gaborit P, Carinci R, Issaly J, Fouque F. Anopheles darlingi bionomics and transmission of Plasmodium falciparum, Plasmodium vivax and Plasmodium malariae in Amerindian villages of the Upper-Maroni Amazonian forest, French Guiana. Mem Inst Oswaldo Cruz 2008; 103:702–10. [DOI] [PubMed] [Google Scholar]
- 17. Kroeger A, González M, Ordóñez-González J. Insecticide-treated materials for malaria control in Latin America: to use or not to use? Trans R Soc Trop Med Hyg 1999; 93:565–70. [DOI] [PubMed] [Google Scholar]
- 18. Trung HD, Bortel WV, Sochantha T, Keokenchanh K, Briët OJ, Coosemans M. Behavioural heterogeneity of Anopheles species in ecologically different localities in Southeast Asia: a challenge for vector control. Trop Med Int Health 2005; 10:251–62. [DOI] [PubMed] [Google Scholar]
- 19. Vythilingam I, Sidavong B, Chan ST, et al. Epidemiology of malaria in Attapeu Province, Lao PDR in relation to entomological parameters. Trans R Soc Trop Med Hyg 2005; 99:833–9. [DOI] [PubMed] [Google Scholar]
- 20. Tananchai C, Tisgratog R, Juntarajumnong W, et al. Species diversity and biting activity of Anopheles dirus and Anopheles baimaii (Diptera: Culicidae) in a malaria prone area of western Thailand. Parasit Vectors 2012; 5:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Obsomer V, Defourny P, Coosemans M. The Anopheles dirus complex: spatial distribution and environmental drivers. Malar J 2007; 6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marchand RP, Tuyen Quang N, Quang Hoanh N, Tho Vien N.. The Khanh Phu malaria research project. Hanoi, Vietnam: Medical Publication House; 1997. [Google Scholar]
- 23. Garros C, Marchand RP, Quang NT, Hai NS, Manguin S. First record of Anopheles minimus C and significant decrease of An. minimus A in central Vietnam. J Am Mosq Control Assoc 2005; 21:139–43. [DOI] [PubMed] [Google Scholar]
- 24. Hamon J, Mouchet J. Secondary vectors of human malaria in Africa [in French]. Med Trop (Mars) 1961; 21(special):643–60. [PubMed] [Google Scholar]
- 25. Muirhead-Thomson R. The significance of irritability, behaviourstic avoidance and allied phenomena in malaria eradication. Bull WHO. 1960; 22:721–34. [PMC free article] [PubMed] [Google Scholar]
- 26. Badyaev A. Stress-induced variation in evolution from behavioural plasticity to genetic assimilation. Proc Bio Sci/Roy Soc. 2005; 272:877–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pothikasikorn J, Chareonviriyaphap T, Bangs M, Prabaripai A. Behavioural responses to DDT and pyrethroids between Anopheles minimus species A and C, malaria vectors in Thailand. Amer J Trop Med Hyg 2005; 73:343–9. [PubMed] [Google Scholar]
- 28. Giglioli G. Ecological change as a factor in renewed malaria transmission in an eradicated area: a localized outbreak of A. aquasalis-transmitted malaria on the Demerara river estuary, British Guiina, in the fifteenth year of A. darlingi and malaria eradication. Bull WHO 1963; 29:1131–45. [PMC free article] [PubMed] [Google Scholar]
- 29. Limrat D, Rojruthai B, Apiwathnasorn C, Samung Y, Prommongkol S. Anopheles barbirostris/campestris as a probable vector of malaria in Aranyaprathet, Sa Kaeo Province. Southeast Asian J Trop Med Public Health 2001; 32:739–44. [PubMed] [Google Scholar]
- 30. Hamon J, Choumara R, Adam J, Bailly H, Ricossé J. Le paludisme dans la zone pilote de Bobo Dioulasso Haute-Volta. Cahiers de l’ORSTOM. 1959; 1:125. [Google Scholar]
- 31. Darriet F, Robert V, Tho Vien N, Carnevale P. Evaluation de l’efficacité sur les vecteurs de paludisme de la permethrine en imprégnation de moustiquaires intactes et trouées. WHO/VBC/84899 and WHO/MAL/841008, Geneva, Switzerland: World Health Organization; 1984.
- 32. Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, Killeen GF. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J 2011; 10:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhu L, Müller GC, Marshall JM, et al. Is outdoor vector control needed for malaria elimination? an individual-based modelling study. Malar J 2017; 16:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lindblade KA, Gimnig JE, Kamau L, et al. Impact of sustained use of insecticide-treated bednets on malaria vector species distribution and culicine mosquitoes. J Med Entomol 2006; 43:428–32. [DOI] [PubMed] [Google Scholar]
- 35. Mwesigwa J, Achan J, Di Tanna GL, et al. Residual malaria transmission dynamics varies across The Gambia despite high coverage of control interventions. PLoS One 2017; 12:e0187059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Caputo B, Nwakanma D, Jawara M, et al. Anopheles gambiae complex along The Gambia river, with particular reference to the molecular forms of An. gambiae s.s. Malar J 2008; 7:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Padonou GG, Gbedjissi G, Yadouleton A, et al. Decreased proportions of indoor feeding and endophily in Anopheles gambiae s.l. populations following the indoor residual spraying and insecticide-treated net interventions in Benin (West Africa). Parasit Vectors 2012; 5:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moiroux N, Gomez MB, Pennetier C, et al. Changes in Anopheles funestus biting behavior following universal coverage of long-lasting insecticidal nets in Benin. J Infect Dis 2012; 206:1622–9. [DOI] [PubMed] [Google Scholar]
- 39. Molineaux L, Gramiccia G.. The Garki project: Research on the epidemiology and control of malaria in the Sudan savanna of West Africa. Geneva, Switzerland: World Health Organization; 1980. [Google Scholar]
- 40. Molineaux L, Shidrawi GR, Clarke JL, Boulzaguet JR, Ashkar TS. Assessment of insecticidal impact on the malaria mosquito’s vectorial capacity, from data on the man-biting rate and age-composition. Bull World Health Organ 1979; 57:265–74. [PMC free article] [PubMed] [Google Scholar]
- 41. Magesa SM, Wilkes TJ, Mnzava AE, et al. Trial of pyrethroid impregnated bednets in an area of Tanzania holoendemic for malaria. Part 2. Effects on the malaria vector population. Acta Trop 1991; 49:97–108. [DOI] [PubMed] [Google Scholar]
- 42. Ilboudo-Sanogo E, Cuzin-Ouattara N, Diallo DA, et al. Insecticide-treated materials, mosquito adaptation and mass effect: entomological observations after five years of vector control in Burkina Faso. Trans R Soc Trop Med Hyg 2001; 95:353–60. [DOI] [PubMed] [Google Scholar]
- 43. Quiñones ML, Lines JD, Thomson MC, Jawara M, Morris J, Greenwood BM. Anopheles gambiae gonotrophic cycle duration, biting and exiting behaviour unaffected by permethrin-impregnated bednets in The Gambia. Med Vet Entomol 1997; 11:71–8. [DOI] [PubMed] [Google Scholar]
- 44. Mbogo CN, Baya NM, Ofulla AV, Githure JI, Snow RW. The impact of permethrin-impregnated bednets on malaria vectors of the Kenyan coast. Med Vet Entomol 1996; 10:251–9. [DOI] [PubMed] [Google Scholar]
- 45. Njau R, Mosha F, Nguma J. Field trials of pyrethroid impregnated bednets in northern Tanzania. I. Effects on malaria transmission. Insect Science and its application 1993;5:575–84. [Google Scholar]
- 46. Braimah N, Drakeley CJ, Kweka E, et al. Tests of bednet traps (Mbita traps) for monitoring mosquito populations and time of biting in Tanzania and possible impact of prolonged insecticide treated net use. Int J Trop Insect Sci 2005; 25:208–13. [Google Scholar]
- 47. Sougoufara S, Diédhiou SM, Doucouré S, et al. Biting by Anopheles funestus in broad daylight after use of long-lasting insecticidal nets: a new challenge to malaria elimination. Malar J 2014; 13:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fontenille D, Lepers JP, Campbell GH, Coluzzi M, Rakotoarivony I, Coulanges P. Malaria transmission and vector biology in Manarintsoa, high plateaux of Madagascar. Am J Trop Med Hyg 1990; 43:107–15. [DOI] [PubMed] [Google Scholar]
- 49. Zoulani A, Carnevale P, Penchenier L. Influence des moustiquaires imprégnées de deltaméthrine sur le cycle d’agressivité d’Anopheles gambiae à Djoumouna (Congo). Ann Soc Belg Med Trop 1994; 74:83–91. [PubMed] [Google Scholar]
- 50. Mathenge EM, Gimnig JE, Kolczak M, Ombok M, Irungu LW, Hawley WA. Effect of permethrin-impregnated nets on exiting behavior, blood feeding success, and time of feeding of malaria mosquitoes (Diptera: Culicidae) in western Kenya. J Med Entomol 2001; 38:531–6. [DOI] [PubMed] [Google Scholar]
- 51. Bayoh MN, Mathias DK, Odiere MR, et al. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malar J 2010; 9:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mutuku FM, King CH, Mungai P, et al. Impact of insecticide-treated bed nets on malaria transmission indices on the south coast of Kenya. Malar J 2011; 10:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sougoufara S, Harry M, Doucouré S, Sembène PM, Sokhna C. Shift in species composition in the Anopheles gambiae complex after implementation of long-lasting insecticidal nets in Dielmo, Senegal. Med Vet Entomol 2016; 30:365–8. [DOI] [PubMed] [Google Scholar]
- 54. Gillies M, Smith A. The effect of a residual house spraying campaign in East Africa on species balance in the Anopheles funestus group: the replacement of A. funestus Giles by A. rivulorum Leeson. Bull Ent Res. 1960; 51:243–52. [Google Scholar]
- 55. Gillies M, Furlong M. An investigation into the behaviour of Anopheles parensis Gilles at Malindi on the Kenya coast. Bull Ent Res. 1963; 55:1–16. [Google Scholar]
- 56. Burke A, Dahan-Moss Y, Duncan F, et al. Anopheles parensis contributes to residual malaria transmission in South Africa. Malar J 2019; 18:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Brooke B, Koekemoer L, Kruger P, Urbach J, Misiani E, Coetzee M. Malaria vector control in South Africa. S Afr Med J 2013; 103:784–8. [DOI] [PubMed] [Google Scholar]
- 58. Coetzee M, Hunt RH, Wilkerson R, Della Torre A, Coulibaly MB, Besansky NJ. Anopheles coluzzii and Anopheles amharicus, new members of the Anopheles gambiae complex. Zootaxa 2013; 3619:246–74. [PubMed] [Google Scholar]
- 59. Dandalo LC, Brooke BD, Munhenga G, et al. Population dynamics and Plasmodium falciparum (Haemosporida: Plasmodiidae) infectivity rates for the malaria vector Anopheles arabiensis (Diptera: Culicidae) at Mamfene, KwaZulu-Natal, South Africa. J Med Entomol 2017; 54:1758–66. [DOI] [PubMed] [Google Scholar]
- 60. Cuamba N, Mendis C. The role of Anopheles merus in malaria transmission in an area of southern Mozambique. J Vector Borne Dis 2009; 46:157–9. [PubMed] [Google Scholar]
- 61. Mbokazi F, Coetzee M, Brooke B, et al. Changing distribution and abundance of the malaria vector Anopheles merus in Mpumalanga province, South Africa. Public Health Action 2018; 8:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Coetzee M, Koekemoer LL. Molecular systematics and insecticide resistance in the major African malaria vector Anopheles funestus. Annu Rev Entomol 2013; 58:393–412. [DOI] [PubMed] [Google Scholar]
- 63. Burke A, Dandalo L, Munhenga G, et al. A new malaria vector mosquito in South Africa. Sci Rep 2017; 7:43779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wilkes TJ, Matola YG, Charlwood JD. Anopheles rivulorum, a vector of human malaria in Africa. Med Vet Entomol 1996; 10:108–10. [DOI] [PubMed] [Google Scholar]
- 65. Temu EA, Minjas JN, Tuno N, Kawada H, Takagi M. Identification of four members of the Anopheles funestus (Diptera: Culicidae) group and their role in Plasmodium falciparum transmission in Bagamoyo coastal Tanzania. Acta Trop 2007; 102:119–25. [DOI] [PubMed] [Google Scholar]
- 66. Kyalo D, Amratia P, Mundia CW, Mbogo CM, Coetzee M, Snow RW. A geo-coded inventory of anophelines in the Afrotropical Region south of the Sahara: 1898-2016. Wellcome Open Res 2017; 2:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kawada H, Dida GO, Sonye G, Njenga SM, Mwandawiro C, Minakawa N. Reconsideration of Anopheles rivulorum as a vector of Plasmodium falciparum in western Kenya: some evidence from biting time, blood preference, sporozoite positive rate, and pyrethroid resistance. Parasit Vectors 2012; 5:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ogola EO, Fillinger U, Ondiba IM, et al. Insights into malaria transmission among Anopheles funestus mosquitoes, Kenya. Parasit Vectors 2018; 11:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Boch C, OPedersen E, Mukoko D, Ouma J. Permethrin-impregnated bednets effects on resting and feeding behaviour of lymphatic filariasis vector mosquitoes in Kenya. Med Vet Entomol. 1998; 12:52–9. [DOI] [PubMed] [Google Scholar]
- 70. Lindsay SW, Alonso PL, Armstrong Schellenberg JR, et al. A malaria control trial using insecticide-treated bed nets and targeted chemoprophylaxis in a rural area of The Gambia, west Africa. 7. Impact of permethrin-impregnated bed nets on malaria vectors. Trans R Soc Trop Med Hyg. 1993; 87(suppl 2):45–51. [DOI] [PubMed] [Google Scholar]
- 71. Fornadel C, Norris L, Glass G, Norris D. Analysis of Anopheles arabiensis blood feeding behaviour in southern Zambia during two years after introduction of insecticide-treated bed nets. Amer J Trop Med Hyg. 2010; 83:848–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Louis JP, Albert JP. Malaria in the Republic of Djibouti. Strategy for control using a biological antilarval campaign: indigenous larvivorous fishes (Aphanius dispar) and bacterial toxins [in French]. Med Trop (Mars). 1988;48:127–31. [PubMed] [Google Scholar]
- 73. Fletcher M, Teklehaimanot A, Yemane G. Control of mosquito larvae in the port city of Assab by an indigenous larvivorous fish, Aphanius dispar. Acta Trop 1992; 52:155–66. [DOI] [PubMed] [Google Scholar]
- 74. Haq S, Yadav RS. Geographical distribution and evaluation of mosquito larvivorous potential of Aphanius dispar (Rüppell), a native fish of Gujarat, India. J Vector Borne Dis 2011; 48:236–40. [PubMed] [Google Scholar]
- 75. Faulde MK, Rueda LM, Khaireh BA. First record of the Asian malaria vector Anopheles stephensi and its possible role in the resurgence of malaria in Djibouti, Horn of Africa. Acta Trop 2014; 139:39–43. [DOI] [PubMed] [Google Scholar]
- 76. Sinka ME, Pironon S, Massey NC, et al. A new malaria vector in Africa: Predicting the expansion range of Anopheles stephensi and identifying the urban populations at risk. Proc Natl Acad Sci U S A 2020; 117:24900–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Takken W, Lindsay S. Increased threat of urban malaria from Anopheles stephensi mosquitoes, Africa. Emerg Infect Dis 2019; 25:1431–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Faragalla IA. The usefulness of the MMFO course and the extent to which it added to my real job situation. Mekong Malaria Forum 1999; 4: 30–1.
- 79. Thimasarn K. Current malaria control in Thailand. Mekong Malaria Forum 2000; 5: 10–3.
- 80. Laihad FJ. Gebrak Malaria: Indonesia’s Roll Back Malaria initiative. Mekong Malaria Forum 1999; 4: 6–9.
- 81. Hung LX, Cong LD, Hung NM. Comparison of the effectiveness of residual spraying and bed net impregnation for malaria prevention in central Vietnam. Mekong Malaria Forum 1999; 4: 16–20.
- 82. Hinh NV, Dinh LV. Malaria outbreak risk and preventive measures in A Luoi district, Thua Thien Hu province, Vietnam 1997–1998. Mekong Malaria Forum 1999; 4: 21–7.
- 83. Sochantha T, Van Bortel W, Savonnaroth S, Marcotty T, Speybroeck N, Coosemans M. Personal protection by long-lasting insecticidal hammocks against the bites of forest malaria vectors. Trop Med Int Health 2010; 15:336–41. [DOI] [PubMed] [Google Scholar]
- 84. Morel CM, Thang ND, Erhart A, et al. Cost-effectiveness of long-lasting insecticide-treated hammocks in preventing malaria in South-central Vietnam. PLoS One 2013; 8:e58205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Magris M, Rubio-Palis Y, Alexander N, et al. Community-randomized trial of lambdacyhalothrin-treated hammock nets for malaria control in Yanomami communities in the Amazon region of Venezuela. Trop Med Inter Hlth. 2007; 12:392–403. [DOI] [PubMed] [Google Scholar]
- 86. Thang ND, Erhart A, Speybroeck N, et al. Long-lasting insecticidal hammocks for controlling forest malaria: a community-based trial in a rural area of central Vietnam. PLoS One 2009; 4:e7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Edwards HM, Chinh VD, Le Duy B, et al. Characterising residual malaria transmission in forested areas with low coverage of core vector control in central Viet Nam. Parasit Vectors 2019; 12:454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bannister-Tyrrell M, Xa NX, Kattenberg JH, et al. Micro-epidemiology of malaria in an elimination setting in Central Vietnam. Malar J 2018; 17:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Burns M, Rowland M, N’Guessan R, et al. Insecticide-treated plastic sheeting for emergency malaria prevention and shelter among displaced populations: an observational cohort study in a refugee setting in Sierra Leone. Am J Trop Med Hyg 2012; 87:242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Graham K, Mohammad N, Rehman H, et al. Insecticide-treated plastic tarpaulins for control of malaria vectors in refugee camps. Med Vet Entomol 2002; 16:404–8. [DOI] [PubMed] [Google Scholar]
- 91. Mittal PK, Sreehari U, Razdan RK, Dash AP. Evaluation of the impact of ZeroFly®, an insecticide incorporated plastic sheeting on malaria incidence in two temporary labour shelters in India. J Vector Borne Dis 2011; 48:138–43. [PubMed] [Google Scholar]
- 92. Sharma SK, Upadhyay AK, Haque MA, et al. Field evaluation of ZeroFly—an insecticide incorporated plastic sheeting against malaria vectors & its impact on malaria transmission in tribal area of northern Orissa. Indian J Med Res 2009; 130:458–66. [PubMed] [Google Scholar]
- 93. Brosseau L, Drame PM, Besnard P, et al. Human antibody response to Anopheles saliva for comparing the efficacy of three malaria vector control methods in Balombo, Angola. PLoS One 2012; 7:e44189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Asale A, Duchateau L, Devleesschauwer B, Huisman G, Yewhalaw D. Zooprophylaxis as a control strategy for malaria caused by the vector Anopheles arabiensis (Diptera: Culicidae): a systematic review. Infect Dis Poverty 2017; 6:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Iwashita H, Dida GO, Sonye GO, et al. Push by a net, pull by a cow: can zooprophylaxis enhance the impact of insecticide treated bed nets on malaria control? Parasit Vectors 2014; 7:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kaburi J, Githuto J, Muthami L, Ngure P, Mueke J, Mwandawiro C. Effects of long-lasting insecticidal nets and zooprophylaxis on mosquito feeding behavior and density in Mwea, central Kenya. J Vector Dis 2009; 46:184–90. [PubMed] [Google Scholar]
- 97. Levens W. Mathematical modelling of co-application of long lasting insecticidal nets and insecticides zooprophylaxis against the resilience of Anopheles arabiensis for effective malaria prevention [thesis ]. University of Dar es Salaam Tanzania, 2013. [Google Scholar]
- 98. Killeen GF, Smith TA. Exploring the contributions of bed nets, cattle, insecticides and excitorepellency to malaria control: a deterministic model of mosquito host-seeking behaviour and mortality. Trans R Soc Trop Med Hyg 2007; 101:867–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Donnelly B, Berrang-Ford L, Ross NA, Michel P. A systematic, realist review of zooprophylaxis for malaria control. Malar J 2015; 14:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bettini S, Romi R. Zooprophylaxis: old and new problems [in Italian]. Parassitologia 1998; 40:423–30. [PubMed] [Google Scholar]
- 101. Diabate A, Tripet F. Targeting male mosquito mating behaviour for malaria control. Parasit Vectors 2015; 8:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Combemale P. The prescription of repellents [in French]. Med Trop (Mars) 2001; 61:99–103. [PubMed] [Google Scholar]
- 103. Debboun M, Frances SP, Strickman D.. Insect repellents: principles, methods and uses. Boca Raton, FL: CRC Press; 2007. [Google Scholar]
- 104. Peterson C, Coats J. Insect repellents—past, present and future. Pesticide Outlook 2001; 12:154–8. [Google Scholar]
- 105. Sanghong R, Junkum A, Chaithong U, et al. Remarkable repellency of Ligusticum sinense (Umbelliferae), a herbal alternative against laboratory populations of Anopheles minimus and Aedes aegypti (Diptera: Culicidae). Malar J 2015; 14:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sorge F, Imbert P, Laurent C, et al. ; Groupe de Pédiatrie Tropicale; Société Française de Pédiatrie . Children arthropod bites protective measures: insecticides and repellents [in French]. Arch Pediatr 2007; 14:1442–50. [DOI] [PubMed] [Google Scholar]
- 107. Benedict MQ, Robinson AS. The first releases of transgenic mosquitoes: an argument for the sterile insect technique. Trends Parasitol 2003; 19:349–55. [DOI] [PubMed] [Google Scholar]
- 108. Helinski ME, Hassan MM, El-Motasim WM, Malcolm CA, Knols BG, El-Sayed B. Towards a sterile insect technique field release of Anopheles arabiensis mosquitoes in Sudan: irradiation, transportation, and field cage experimentation. Malar J 2008; 7:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Helinski ME, Knols BG. Mating competitiveness of male Anopheles arabiensis mosquitoes irradiated with a partially or fully sterilizing dose in small and large laboratory cages. J Med Entomol 2008; 45:698–705. [DOI] [PubMed] [Google Scholar]
- 110. Le Goff G, Damiens D, Ruttee AH, et al. Field evaluation of seasonal trends in relative population sizes and dispersal pattern of Aedes albopictus males in support of the design of a sterile male release strategy. Parasit Vectors 2019; 12:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Barnard DR. Repellents and toxicants for personal protection. Geneva, Switzerland: World Health Organization, 2000. [Google Scholar]
- 112. Brown M, Hebert AA. Insect repellents: an overview. J Am Acad Dermatol 1997; 36:243–9. [DOI] [PubMed] [Google Scholar]
- 113. Maia MF, Kliner M, Richardson M, Lengeler C, Moore SJ. Mosquito repellents for malaria prevention. Cochrane Database Syst Rev 2018; 2:CD011595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Rowland M, Downey G, Rab A, et al. DEET mosquito repellent provides personal protection against malaria: a household randomized trial in an Afghan refugee camp in Pakistan. Trop Med Int Health 2004; 9:335–42. [DOI] [PubMed] [Google Scholar]
- 115. Wilson AL, Chen-Hussey V, Logan JG, Lindsay SW. Are topical insect repellents effective against malaria in endemic populations? a systematic review and meta-analysis. Malar J 2014; 13:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Carnevale P, Robert V.. Les anophèles: biologie, transmission du Plasmodium et lutte antivectorielle. Marseille, France: IRD Ed, Collections Didactiques, 2009. [Google Scholar]
- 117. Buka RL. Sunscreens and insect repellents. Curr Opin Pediatr 2004; 16:378–84. [DOI] [PubMed] [Google Scholar]
- 118. Rozendaal J. Vector control. Methods for use by individuals and communities. Geneva, Switzerland: World Health Organization Library, World Health Organization, 1997. [Google Scholar]
- 119. Yap HH. Effectiveness of soap formulations containing DEET and permethrin as personal protection against outdoor mosquitoes in Malaysia. J Am Mosq Control Assoc 1986; 2:63–7. [PubMed] [Google Scholar]
- 120. Eamsila C, Frances SP, Strickman D. Evaluation of permethrin-treated military uniforms for personal protection against malaria in northeastern Thailand. J Am Mosq Control Assoc 1994; 10:515–21. [PubMed] [Google Scholar]
- 121. Kimani EW, Vulule JM, Kuria IW, Mugisha F. Use of insecticide-treated clothes for personal protection against malaria: a community trial. Malar J 2006; 5:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Romi R, Peragallo M, Sarnicola G, Dommarco R. Impregnation of uniforms with permethrin as a mean of protection of working personnel exposed to contact with hematophagous arthropods [in Italian]. Ann Ig 1997; 9:313–9. [PubMed] [Google Scholar]
- 123. Rowland M, Durrani N, Hewitt S, et al. Permethrin-treated chaddars and top-sheets: appropriate technology for protection against malaria in Afghanistan and other complex emergencies. Trans R Soc Trop Med Hyg. 1999; 93:465–72. [DOI] [PubMed] [Google Scholar]
- 124. Schreck CE, Posey K, Smith D. Durability of permethrin as a potential clothing treatment to protect against blood-feeding arthropods. J Econ Entomol 1978; 71:397–400. [DOI] [PubMed] [Google Scholar]
- 125. Soto J, Medina F, Dember N, Berman J. Efficacy of permethrin-impregnated uniforms in the prevention of malaria and leishmaniasis in Colombian soldiers. Clin Infect Dis 1995; 21:599–602. [DOI] [PubMed] [Google Scholar]
- 126. Tangena JA, Thammavong P, Chonephetsarath S, Logan JG, Brey PT, Lindsay SW. Field evaluation of personal protection methods against outdoor-biting mosquitoes in Lao PDR. Parasit Vectors 2018; 11:661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Schreck CE, Snoddy EL, Spielman A. Pressurized sprays of permethrin or DEET on military clothing for personal protection against Ixodes dammini (Acari: Ixodidae). J Med Entomol 1986; 23:396–9. [DOI] [PubMed] [Google Scholar]
- 128. Deparis X, Frere B, Lamizana M, et al. Efficacy of permethrin-treated uniforms in combination with DEET topical repellent for protection of French military troops in Côte d’Ivoire. J Med Entomol 2004; 41:914–21. [DOI] [PubMed] [Google Scholar]
- 129. Schreck C, Haile D, Kline D. The effectiveness of permethrin and DEET alone or in combination for protection against Aedes taeniorhynchus. Amer J Trop Med Hyg 1984;33:725–30. [DOI] [PubMed] [Google Scholar]
- 130. Lillie TH, Schreck CE, Rahe AJ. Effectiveness of personal protection against mosquitoes in Alaska. J Med Entomol 1988; 25:475–8. [DOI] [PubMed] [Google Scholar]
- 131. Gerber EJ, Novak RJ. Considerations on the use of botanically-derived repellent product. In: Debboun M, Frances SP, Strickman D, eds. Insect repellents: principles, methods and uses. Boca Raton, FL: CRC Press; 2007:305–9. [Google Scholar]
- 132. Moore SJ, Lenglet A, Hill N. Plant-based insect repellents. In: Debboun M, Frances SP, Strickman D, eds. Insect repellents: principles, methods and uses. Boca Raton, FL: CRC Press; 2007:275–303. [Google Scholar]
- 133. Sathantriphop S, Achee NL, Sanguanpong U, Chareonviriyaphap T. The effects of plant essential oils on escape response and mortality rate of Aedes aegypti and Anopheles minimus. J Vector Ecol 2015; 40:318–26. [DOI] [PubMed] [Google Scholar]
- 134. Tisgratog R, Sanguanpong U, Grieco JP, Ngoen-Kluan R, Chareonviriyaphap T. Plants traditionally used as mosquito repellents and the implication for their use in vector control. Acta Trop 2016; 157:136–44. [DOI] [PubMed] [Google Scholar]
- 135. Nararak J, Sathantriphop S, Kongmee M, et al. Excito-repellent activity of β-caryophyllene oxide against Aedes aegypti and Anopheles minimus. Acta Trop 2019; 197:105030. [DOI] [PubMed] [Google Scholar]
- 136. Tisgratog R, Sukkanon C, Grieco JP, et al. Evaluation of the constituents of vetiver oil against Anopheles minimus (Diptera: Culicidae), a malaria vector in Thailand. J Med Entomol 2018; 55:193–9. [DOI] [PubMed] [Google Scholar]
- 137. Nararak J, Giorgio CD, Sukkanon C, et al. Excito-repellency and biological safety of β-caryophyllene oxide against Aedes albopictus and Anopheles dirus (Diptera: Culicidae). Acta Trop 2020; 210:105556. [DOI] [PubMed] [Google Scholar]
- 138. Beier JC, Wilke ABB, Benelli G. Newer approaches for malaria vector control and challenges of outdoor transmission. In: Manguin S, Dev V, eds. Towards malaria elimination—a leap forward. London, UK: IntechOpen; 2018:387–402. [Google Scholar]
- 139. Fiorenzano JM, Koehler PG, Xue RD. Attractive toxic sugar bait (ATSB) for control of mosquitoes and its impact on non-target organisms: a review. Int J Environ Res Public Health 2017; 14:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Beier JC, Müller GC, Gu W, Arheart KL, Schlein Y. Attractive toxic sugar bait (ATSB) methods decimate populations of Anopheles malaria vectors in arid environments regardless of the local availability of favoured sugar-source blossoms. Malar J 2012; 11:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Muller GC, Beier JC, Traore SF, et al. Successful field trial of attractive toxic sugar bait (ATSB) plant-spraying methods against malaria vectors in the Anopheles gambiae complex in Mali, West Africa. Malar J 2010; 9:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Muller GC, Kravchenko VD, Schlein Y. Decline of Anopheles sergentii and Aedes caspius populations following presentation of attractive toxic (spinosad) sugar bait stations in an oasis. J Am Mosq Control Assoc 2008; 24:147–9. [DOI] [PubMed] [Google Scholar]
- 143. Xue RD, Ali A, Kline DL, Barnard DR. Field evaluation of boric acid- and fipronil-based bait stations against adult mosquitoes. J Am Mosq Control Assoc 2008; 24:415–8. [DOI] [PubMed] [Google Scholar]
- 144. Xue RD, Barnard DR. Boric acid bait kills adult mosquitoes (Diptera: Culicidae). J Econ Entomol 2003; 96:1559–62. [DOI] [PubMed] [Google Scholar]
- 145. Xue RD, Kline DL, Ali A, Barnard DR. Application of boric acid baits to plant foliage for adult mosquito control. J Am Mosq Control Assoc 2006; 22:497–500. [DOI] [PubMed] [Google Scholar]
- 146. Xue RD, Muller GC, Kline DL, Barnard DR. Effect of application rate and persistence of boric acid sugar baits applied to plants for control of Aedes albopictus. J Am Mosq Control Assoc 2011; 27:56–60. [DOI] [PubMed] [Google Scholar]
- 147. Mosqueira B, Duchon S, Chandre F, Hougard JM, Carnevale P, Mas-Coma S. Efficacy of an insecticide paint against insecticide-susceptible and resistant mosquitoes. Part 1: laboratory evaluation. Malar J 2010; 9:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Mosqueira B, Chabi J, Chandre F, et al. Efficacy of an insecticide paint against malaria vectors and nuisance in West Africa—part 2: field evaluation. Malar J 2010; 9:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Mosqueira B, Chabi J, Chandre F, et al. Proposed use of spatial mortality assessments as part of the pesticide evaluation scheme for vector control. Malar J 2013; 12:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. World Health Organization. Global report on insecticide resistance in malaria vectors. 2010–2016. Geneva, Switzerland: World Health Organization, 2018. [Google Scholar]
- 151. Kondrashin A, Jung R, Akiyama J. Ecological aspects of forest malaria in Southeast Asia. In: Sharma VP, Kondrashin AV, eds. Forest malaria in Southeast Asia. New Delhi, India: Proceedings of an Informal Consultative meeting, WHO/Malaria Research Center; 1991:1–28. [Google Scholar]
- 152. Manguin S. Anopheles mosquitoes—new insights into malaria vectors. Rijeka, Croatia: IntechOpen, 2013:813. [Google Scholar]
- 153. Manguin S, Garros C, Dusfour I, Harbach RE, Coosemans M. Bionomics, taxonomy, and distribution of themajor malaria vector taxa of Anopheles subgenus Cellia in Southeast Asia: an updated review. Infect Genet Evol 2008; 8:489–503. [DOI] [PubMed] [Google Scholar]
- 154. Nair C. Habits and adult anopheline mosquitoes of Malaya. Malayan Med J. 1947; 1:166–72. [Google Scholar]
- 155. Wharton R. The habits of adult mosquitoes in Malaya. I. Observations on anophelines in window trap huts and cattlesheds. Ann trop Med Parasit 1951; 45:141–54. [DOI] [PubMed] [Google Scholar]
- 156. Sharma V, Prasittisuk C, Kondrashin A. Magnitude of forest-related malaria in the WHO Southeast Asia Region. In: Sharma VP, Kondrashin AV, eds. Forest malaria in Southeast Asia. New Delhi, India: Proceedings of an Informal Consultative meeting, WHO/Malaria Research Center; 1991:29–53. [Google Scholar]
- 157. Dev V. Vector biology and control: an update for malaria elimination initiative in India. New Delhi, India: The National Academy of Sciences, 2020 . [Google Scholar]
- 158. World Health Organization. World malaria report. Geneva, Switzerland: World Health Organization, 2018. [Google Scholar]