Abstract
OBJECTIVE:
Women with symptoms and/or signs of myocardial ischemia but no obstructive coronary artery disease (INOCA) often have coronary vascular dysfunction and elevated risk for adverse cardiovascular events. We hypothesized that ultra-high sensitivity cardiac troponin I (u-hscTnI), a sensitive indicator of ischemic cardiomyocyte injury, is associated with coronary vascular dysfunction in women with INOCA.
APPROACH and RESULTS:
Women (N=263) with INOCA enrolled in the Women’s Ischemic Syndrome Evaluation -Coronary Vascular Dysfunction (WISE-CVD) study underwent invasive coronary vascular function testing and u-hscTnI measurements (Simoa HD-1 Analyzer, Quanterix Corporation, Lexington, MA). Logistic regression models, adjusted for traditional cardiovascular risk factors, were used to evaluate associations between u-hscTnI and coronary vascular function. Women with coronary vascular dysfunction (microvascular constriction and limited coronary epicardial dilation) had higher plasma u-hscTnI levels (both p=0.001). u-hscTnI levels were associated with microvascular constriction (OR=1.38 per doubling of u-hscTnI, 95%CI 1.03-1.84; p=0.033) and limited coronary epicardial dilation (OR=1.37 per doubling of u-hscTnI, 95%CI 1.04-1.81; p=0.026). u-hscTnI levels were not associated with microvascular dilation or coronary epicardial constriction.
CONCLUSIONS:
Our findings indicate that higher u-hscTnI is associated with coronary vascular dysfunction in women with INOCA. This suggests that ischemic cardiomyocyte injury in the setting of coronary vascular dysfunction has the potential to contribute to adverse cardiovascular outcomes observed in these women. Additional studies are needed to confirm and investigate mechanisms underlying these findings in INOCA.
Keywords: myocardial injury, INOCA, CFR, acetylcholine, vascular function, microvascular function
Graphic Abstract:
Majority of women with INOCA have coronary vascular dysfunction which is associated with cardiomyocyte injury.
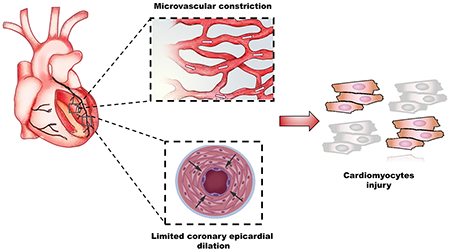
Background
The number of women with symptoms and/or signs of ischemia but no obstructive coronary artery disease (INOCA) are increasing 1. These women often have coronary vascular dysfunction, including limited coronary artery dilation and/or constriction in response to vasoactive agents 2–5. We and others have previously observed that these women are at elevated risk of major adverse cardiovascular outcomes 3, 6–9. Reduced coronary microvascular dilation assessed by coronary flow reserve (CFR) in response to intra-coronary adenosine (IC-ADO) and coronary epicardial and/or microvascular constriction in response to intra-coronary acetylcholine (IC-ACH) are associated with higher risk of adverse cardiovascular outcome, versus those with normal responses 2, 4, 5. Further, over a 9.7 years (median follow-up), the Women’s Ischemia Syndrome Evaluation [WISE] observed that limited coronary microvascular dilation, assessed invasively using vasoactive agents, predicted higher rates of death and first indexed non-fatal myocardial infarction, non-fatal stroke and non-fatal heart failure 3. Furthermore, we found that epicardial coronary constriction in response to IC-ACH was associated with increased risk of hospitalization due to angina. The mechanisms linking these findings to the development of adverse cardiovascular outcomes remain unknown.
High sensitivity cardiac troponin (hscTnI) assays are increasingly used and recently achieved regulatory approval in the United States 10. These assays enable detection of low concentrations of circulating cardiac troponins 11 and higher levels are associated with higher risk of adverse cardiovascular outcomes in the general population; patients with and without obstructive coronary artery disease (CAD) 12–17. The latest generation of cardiac troponin assays with improved analytical sensitivity (i.e. ultra-high sensitivity cardiac troponin [u-hscTnI]) have been introduced recently. These assays are capable of detecting even lower concentrations of troponin and these levels appear to represent cardiomyocyte injury, but not necessarily necrosis 11, 18.
Accordingly, we hypothesized that u-hscTnI, an indicator of cardiomyocyte injury, is associated with coronary vascular dysfunction in women with INOCA.
Methods
Women with INOCA, enrolled in the NHLBI-funded Women’s Ischemia Syndrome Evaluation - Coronary Vascular Dysfunction (WISE-CVD) study (NCT00832702), were studied. Briefly, this is prospective cohort study at Cedars-Sinai Medical Center, Los Angeles and the University of Florida, Gainesville, as previously published 19, 20. INOCA is defined as symptoms (usually angina and/or dyspnea) with signs suggesting ischemia (ECG changes during exercise, wall motion or perfusion abnormalities during echocardiography or nuclear imaging) 21 and absence of obstructive CAD by core laboratory (epicardial coronary artery diameter stenosis <50%). A subgroup of these women (N=263), underwent coronary vascular function testing and had u-hscTnI assessment.
Inclusion, exclusion criteria, demographic and clinical data were obtained from questionnaires and medical records and included age, body-mass index (BMI), history of smoking, diabetes mellitus (fasting blood glucose level >126 mg/dL or treatment with insulin or oral anti-diabetic medications), hypertension (systolic blood pressure >140 mmHg, diastolic blood pressure >90 mmHg on at least 3 occasions or treatment with antihypertensive medications), dyslipidemia (total cholesterol ≥200 mg/dL, low-density lipoprotein >130 mg/dL, or treatment with lipid-lowering medications), and use of hormonal replacement therapy, as previously published 19, 20. The protocol was approved by institutional review boards at each site and all subjects provide written informed consent.
Coronary Vascular Function Testing
Women were asked to hold long-acting nitrates, calcium-channel blockers, β-blockers, and angiotensin-converting enzyme inhibitors and angiotensin-II-receptor blockers 48 hours before the procedure 22. Women were also instructed to stop using sublingual nitroglycerin, refrain from smoking, and drinking caffeinated beverages for at least 12 hours before testing. The invasive testing protocol was previously described 2, 3, 5, 22, and performed in an outpatient setting via percutaneous femoral approach. After diagnostic angiography confirmed absence of obstructive CAD in any vessel, a Doppler-tipped guidewire (0.014-inch FloWire, Volcano Corporation, San Diego, California) was advanced through the diagnostic catheter to assess blood flow velocity and positioned in the left anterior descending coronary artery (or left circumflex coronary artery if anatomic issues prohibited safe access to the left anterior descending coronary artery). Recordings were made once a stable Doppler signal in the proximal or mid-vessel was obtained. Intra-coronary adenosine bolus injections (18–36 mcg) were used to assess coronary microvascular dilation. Average peak velocity at baseline and after adenosine injection were obtained and coronary flow reserve (CFR) was calculated using ≥2.32 as the sex-specific normal threshold as previously published 2. A graded IC-ACH infusion was infused (0.182 mcg/ml and 18.2 mcg/ml over 3 minute) to assess coronary epicardial and microvascular constriction. Coronary microvascular constriction was defined as limited increase by <50% in coronary blood flow (CBF) from baseline in response to IC-ACH, while, coronary epicardial constriction was defined as any decrease in epicardial coronary artery diameter in response to IC-ACH. 23. Finally, coronary epicardial dilation was assessed using IC-NTG (200 mcg), with normal response defined as ≥20% diameter increase 3, 22, 24.
Sample collection and analysis
Fasting blood samples were collected from the femoral arterial sheath before the use of any vasoactive medication, centrifuged and stored at −80 °C. The u-hscTnI assay was carried out on Simoa HD-1 Analyzer (Quanterix Corporation, Lexington, MA). The cardiac troponin I is sandwiched between an enzyme-labeled detection antibody and a capture antibody conjugate to magnetic beads. Beads are subsequently dispersed into femtoliter-scale wells, which are scanned on an individual basis for fluorescence 18. The assay has the limit of detection of 0.01 pg/mL, the limit of quantification of 0.079 pg/mL and upper dynamic range of 1200 pg/mL, and total precision of 10.2%, 6.0%, and 6.2% at 2.0, 5.7, and 54.4 pg/mL with a 99th percentile value of 4.89 pg/mL (90% confidence intervals [CI] 3.71- 6.25) in healthy female population 25. Under local laboratory conditions, the inter-assay coefficient of variation is <10% at 0.2871 pg/mL. The u-hscTnI 99th percentiles were also calculated as upper limits considering a robust estimation against outliers with 90% CIs.
Statistical analysis
Baseline subjects’ characteristics were summarized with means, medians, standard deviations and ranges. For non-normally distributed variables such as u-hscTnI levels, Mann-Whitney U tests were used to compare groups in unadjusted analyses. Relationships between coronary vascular function and other clinical variables (including age, BMI, history of hypertension, dyslipidemia, diabetes, use of statins and hormonal replacement therapy) were examined using logistic regression models. For multivariable analyses, u-hscTnI levels were examined as continuous variables after log-transformation (base 2) for better interpretations. Logistic regression models were also used to examine the relationship between coronary vascular function (CFR, CBF, coronary epicardial diameter change in response to IC-ACH and IC-NTG) and u-hscTnI levels after adjustment for aforementioned clinical variables. All analyses were performed using R (version 3.5.1, http://www.R-project.org). Two-sided P-value < 0.05 were considered statistically significant.
Results
Pertinent baseline characteristics of the women are summarized in Table 1. Two hundred forty-nine women (95%) underwent CFR evaluation, 229 (87%) underwent coronary epicardial diameter change evaluation in response to IC-ACH, 195 (74%) underwent CBF assessment in response to IC-ACH, and 225 (86%) had coronary epicardial diameter change evaluation in response to IC-NTG (Figure 1). CBF correlated with the change in the coronary epicardial artery diameter in response to IC-ACH and IC-NTG (r=0.5 and r=0.3; respectively, both p<0.0001). CFR did not correlate with any of the other coronary vascular function measures.
Table 1:
Pertinent Baseline Characteristics of the Women (N =263)
| Age, years (mean±SD) | 54±11 |
| Body mass index, kg/m2 (mean±SD) | 29±7 |
| History of: | |
| - Diabetes, n (%) | 27 (10%) |
| - Hypertension, n (%) | 115 (44%) |
| - Dyslipidemia, n (%) | 63 (24%) |
| - Smoking, n (%) | 91 (35%) |
| - Family history of CAD, n (%) | 108 (41%) |
| - Pregnancy, n (%) | 206 (78%) |
| Current use of hormonal replacement therapy, n (%) | 39 (15%) |
| - u-hscTnI (ng/L), median (IQR) | 0.81 (0.44-1.52) |
| Medications | |
| - Beta blockers, n (%) | 63 (24%) |
| - ACEI/ARB, n (%) | 59 (22%) |
| - CCB, n (%) | 41 (16%) |
| - Statins, n (%) | 85 (32%) |
| Coronary vascular function measures | |
| - Coronary flow reserve, median (IQR) | 2.7 (2.3-3.0) |
| - Limited coronary flow reserve, N (%) | 74 (30%) |
| - CBF in response to IC-ACH (%), median (IQR) | 38.6 (6.5-100.4) |
| - Limited CBF, N (%) | 107 (55%) |
| - Coronary diameter dilation in response to IC-ACH (%), median (IQR) | 1.02 (−7.1-9.2) |
| - Limited coronary epicardial constriction in response to IC-ACH, N (%) | 105 (46%) |
| - Coronary diameter dilation in response to IC-NTG (%), median (IQR) | 15 (6.0-24.1) |
| - Limited coronary epicardial dilation in response to IC-NTG, N (%) | 144 (64) |
SD: standard deviation, u-hscTnI: ultra-high sensitivity troponin, IQR: interquartile range, ACEI: angiotensin converting enzyme inhibitor, ARB: angiotensin-receptor blocker, CBF: coronary blood flow, IC-ACH: intra-coronary acetylcholine, IC-NTG: intra-coronary nitroglycerin. Normal coronary flow reserve ≥2.32, Normal coronary blood flow (CBF) ≥50%, normal coronary epicardial diameter change in response to IC-ACH ≥0% and normal coronary epicardial diameter change in response to IC-NTG ≥20%.
Figure 1: Distribution of Selected Coronary Vascular Function Testing in 263 Women with Evidence of Ischemia and no Obstructive Coronary Artery Disease.
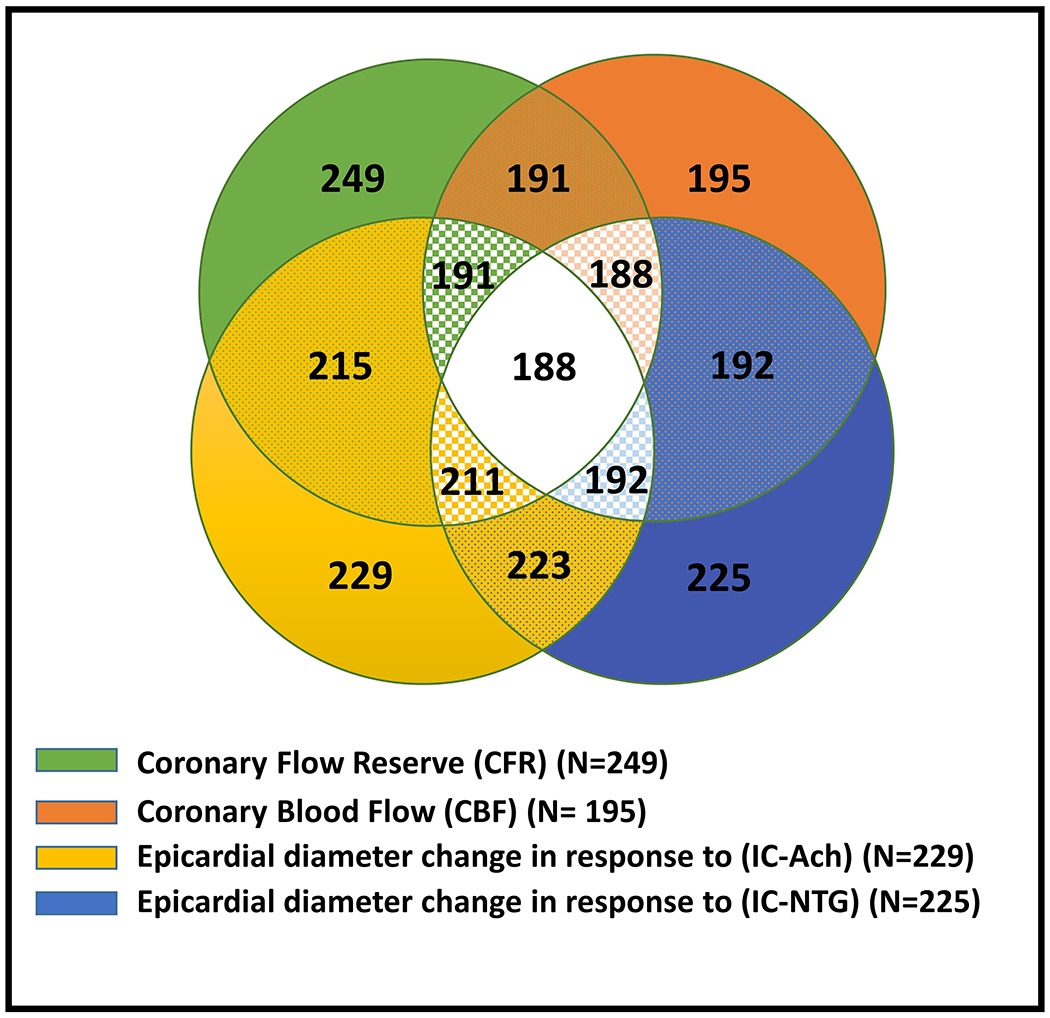
Most women underwent evaluation of >1 coronary vascular function pathway. Green represents women who underwent evaluation of coronary microvascular dilation using coronary flow reserve (CFR); orange represents women who underwent evaluation of coronary microvascular constriction using coronary blood flow (CBF); yellow represents women who underwent evaluation of coronary epicardial constriction using change in coronary artery diameter in response to intracoronary acetylcholine (IC-Ach); blue represents women who underwent evaluation of coronary epicardial dilation using change in in response to intracoronary nitroglycerin (IC-NTG).
Relations between u-hscTnI and Coronary Vascular Function
Compared to women with normal CBF, those with coronary vascular dysfunction had higher levels of plasma u-hscTnI (median: 0.9 pg/mL; interquartile range IQR [0.52-2.4] versus 0.6 pg/mL IQR [0.33-1.05], p=0.001) (Figure 3A). Women with limited epicardial coronary artery dilation to IC-NTG, had higher levels of plasma u-hscTnI versus women with normal function (median: 0.8 pg/mL IQR [0.46-1.86] versus 0.6 pg/mL IQR [0.43-1.06], p=0.001) (Figure 3D). u-hscTnI levels were not significantly different between women with normal versus limited CFR and epicardial coronary artery dilation in response to IC-ACH (Figure 3B and C).
Adjusted logistic regression models indicated that higher u-hscTnI levels were independently associated with limitations in both CBF and coronary epicardial artery dilation in response to IC-NTG (Table 2). There were no significant associations between u-hscTnI levels and CFR or coronary epicardial artery diameter change in response to IC-ACH.
Table 2:
Determinants of coronary vascular function using multivariable logistic regression models
| Odds ratio | 95% confidence interval | p value | |
|---|---|---|---|
| Coronary blood flow in response to acetylcholine <50% | |||
| Age, year | 1.02 | 0.98-1.06 | 0.38 |
| BMI, kg/m2 | 1.02 | 0.97-1.07 | 0.48 |
| Hypertension | 0.77 | 0.36-1.63 | 0.49 |
| Diabetes | 0.53 | 0.15-1.86 | 0.32 |
| Smoking history | 0.89 | 0.44-1.80 | 0.89 |
| Hyperlipidemia | 0.61 | 0.23-1.63 | 0.33 |
| Use of statin therapy | 0.61 | 1.01-6.47 | 0.047 |
| Current use of HRT | 1.41 | 0.56-3.57 | 0.47 |
| u-hscTnI, pg/mL (per 100% increase) | 1.38 | 1.03-1.84 | 0.033 |
| Coronary epicardial constriction in response to acetylcholine | |||
| Age, year | 1.02 | 0.99-1.06 | 0.19 |
| BMI, kg/m2 | 1.06 | 1.01-1.11 | 0.019 |
| Hypertension | 0.97 | 0.48-1.95 | 0.93 |
| Diabetes | 2.82 | 0.92-8.65 | 0.07 |
| Smoking history | 0.73 | 0.38-1.40 | 0.35 |
| Hyperlipidemia | 0.94 | 0.39-2.24 | 0.88 |
| Use of statins therapy | 2.15 | 0.96-4.82 | 0.06 |
| Current use of HRT | 1.67 | 0.70-3.96 | 0.25 |
| u-hscTnI, pg/mL (per 100% increase) | 1.22 | 0.95-1.57 | 0.11 |
| Coronary flow reserve in response to adenosine <2.32 | |||
| Age, year | 1.00 | 0.97-1.03 | 0.84 |
| BMI, kg/m2 | 1.01 | 0.97-1.06 | 0.62 |
| Hypertension | 1.09 | 0.57-2.09 | 0.79 |
| Diabetes | 1.12 | 0.39-3.25 | 0.83 |
| Smoking history | 2.19 | 1.16-4.13 | 0.016 |
| Hyperlipidemia | 0.89 | 0.38-2.10 | 0.80 |
| Use of statins therapy | 1.35 | 0.62-2.95 | 0.45 |
| Current use of HRT | 0.81 | 0.35-1.87 | 0.61 |
| u-hscTnI, pg/mL (per 100% increase) | 0.98 | 0.79-1.22 | 0.88 |
| Coronary epicardial dilation in response to nitroglycerin <20% | |||
| Age, year | 1.02 | 0.98-1.05 | 0.30 |
| BMI, kg/m2 | 0.97 | 0.92-1.01 | 0.16 |
| Hypertension | 0.84 | 0.41-1.71 | 0.63 |
| Diabetes | 1.44 | 0.46-4.44 | 0.53 |
| Smoking history | 1.64 | 0.84-3.19 | 0.15 |
| Hyperlipidemia | 0.63 | 0.25-1.57 | 0.33 |
| Use of statins therapy | 0.93 | 0.40-2.15 | 0.86 |
| Current use of HRT | 2.18 | 0.92-5.20 | 0.079 |
| u-hscTnI, pg/mL (per 100% increase) | 1.37 | 1.04-1.81 | 0.026 |
BMI: body mass index, HRT: hormonal replacement therapy, u-hscTnI: ultra-high sensitivity troponin I
Discussion
Among women with INOCA, higher plasma u-hscTnI levels are associated with limitations in both CBF and coronary epicardial diameter change in response IC-NTG, indicative of dysfunctional coronary vascular dilation. Further, this association appeared independent of traditional cardiovascular risk factors. Our findings suggest that in clinically stable women with INOCA, the presence of cardiomyocyte injury might be the underlying mechanistic pathway leading to adverse cardiovascular events.
Women with evidence of INOCA are increasingly prevalent. Estimates from the ACC-NCDR and the Women's Ischemia Syndrome Evaluation (WISE) databases suggest that at least 3-4 million women in the United States alone with no obstructive CAD at angiography incur health-care costs similar to obstructive CAD 26, 27. The majority of these women have coronary vascular dysfunction 2–5, which may be comprehensively evaluated with invasive physiological assessments using vasoactive agents 1, 3. Currently invasive coronary vascular function testing available in relatively few centers in the United States, despite wide-spread use of fractional flow reserve testing for obstructive CAD.
Introduction of high-sensitivity troponin immunoassays created a revolution in identification of patients at higher risk of developing adverse cardiovascular events despite having troponin levels below the 99th percentile of the upper limit in healthy individuals, but above the limit of detection 11, 18. hsTnI and u-hscTnI assays allow measurement of troponin at very low concentrations, therefore enabling detection of cardiomyocytes injury. In a population based cohort28, it was observed that hsTnT levels were associated with structural heart disease and subclinical ischemia. More importantly, patients with higher levels were at increased risk of cardiovascular death versus those with undetected levels. In large registry cohort 17, that included patients with myocardial infarction and no obstructive CAD (MINOCA), hsTnT levels were strong, independent predictors of adverse outcomes. Although the underlying etiology of MINOCA in these patients was not investigated, the majority of women with MINOCA have underlying coronary vascular dysfunction (i.e. limited dilation or constriction) 1. Our results extend these findings by providing, for the first time, new information about relationships between coronary vascular dysfunction and u-hscTnI troponin.
Cardiac myocytes are sensitive to oxygen and demands for work as oxygen sensors 29. A limitation in adequate myocardial oxygenation due to supply/demand mismatch triggers a complex and integrated events resulting in release of vasoactive metabolites from cardiomyocytes, which act on vascular endothelial cells attempting to increase CBF 30, 31. In the presence of endothelial dysfunction, failure of appropriate epicardial and/or microvascular dilation limits the increase in CBF and subsequently leads to myocardial ischemia, cardiomyocyte injury and release of cardiac troponin 32–34 (Graphic Abstract). This may explain, at least in part, our novel findings of elevated u-hscTnI levels in women with limited CBF. Others 35 suggested that in patients without obstructive CAD, there was no correlation between coronary microvascular dilation assessed by CFR and levels of hscTnI, using only a high sensitivity assay. We extended these findings and found that u-hscTnI levels were higher in women with microvascular constriction, assessed by CBF.
Previous studies suggested that the dilator response to nitroglycerin decreased with increasing age, perhaps secondary to decreased sensitivity of aging vascular smooth muscle cells or presence of mild atherosclerosis, preventing appropriate dilation 36, 37. However, when adjusting for other clinical variables, including age, we found independent associations between higher levels of u-hscTnI levels and limited coronary dilation in response to IC-NTG. This underscores the relationship between cardiomyocytes injury and coronary epicardial dilation. These results need to be further investigated in larger cohort.
Strengths and Limitations
The strengths of our study include comprehensive invasive physiological assessments of coronary vascular function and use, for the first time, of the u-hscTnI assay. This assay is very sensitive, detect cardiac troponin levels 100-fold lower versus commercially available hscTnI assays (Abbott ARCHITECT analyzer). Other strengths that helped to limit heterogeneity in this study include the fact that our selected cohort of women was sufficiently symptomatic to warrant referral for invasive coronary angiography and comprehensive testing excluded other etiologies of cardiomyocyte injury, secondary to either increase oxygen demand or decreased supply (such as anemia, arrhythmias and left ventricular hypertrophy). Limitations include that our results, from two centers with long experience in this area, may not be applicable to other centers and populations. Additionally, the extent of atherosclerosis was not assessed in this study, however a prior WISE IVUS sub-study found that in a sample of similar women, the majority have an atherosclerosis burden that is concealed by positive remodeling 38, which could potentially be related to higher u-hscTnI due to distal micro-emboli formation. Finally, the u-hscTnI assay has the ability to detect very low cardiac troponin levels. Therefore, despite carefully selected cohort and comprehensive adjustments, other etiologies of cardiomyocyte injury cannot be fully excluded. Furthermore, u-hscTnI levels were associated with coronary microvascular constriction, and coronary epicardial dilation; a mixed pattern that needs further exploration in subsequent studies.
Conclusions
In clinically stable women with INOCA, we present for the first-time evidence that higher u-hscTnI is independently associated with coronary vascular dysfunction. Our findings suggest that cardiomyocyte injury in the setting of coronary vascular dysfunction may contribute to the adverse cardiovascular outcomes observed in these women. Additional studies to confirm and investigate these findings with regard to mechanisms and prognosis in INOCA are needed.
Supplementary Material
Figure 2:
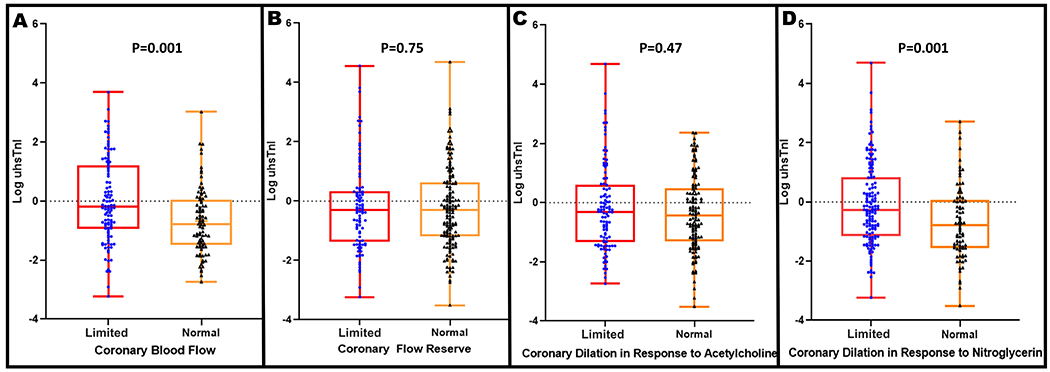
Box Plots demonstrating the relationship between the ultra-high sensitivity troponin I (u hscTnI) levels (log transformed) and (A) Normal and limited coronary blood flow response to intra-coronary acetylcholine (normal response defined as an increase in coronary blood flow in response to acetylcholine by ≥50%), (B) Normal and limited microvascular dilation to intra-coronary adenosine (normal coronary flow reserve ≥2.32), (C) Normal and limited coronary dilation in response to intra-coronary acetylcholine (normal response defined as an increase in coronary artery diameter in response to acetylcholine by ≥0%), (D) Normal and limited coronary artery dilation in response to intra-coronary nitroglycerin (normal response defined as an increase in coronary artery diameter in response to nitroglycerin by ≥20%).
Highlights.
Women with symptoms and/or signs of ischemia but no obstructive coronary artery disease (INOCA) are increasing.
Higher u-hscTnI is independently associated with coronary vascular dysfunction.
Cardiomyocyte injury in the setting of coronary vascular dysfunction may contribute to the adverse cardiovascular outcomes observed in these women.
Acknowledgments:
Sources of Funding: Ahmed AlBadri is supported by American Heart Association Postdoctoral Fellowship Award Grant (18POST34080330). This work was also supported by contracts from the National Heart, Lung, and Blood Institutes nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, grants U0164829, U01 HL649141, U01 HL649241, K23HL105787, T32HL69751, R01 HL090957, 1R03AG032631 from the National Institute on Aging, GCRC grant M01-RR00425 from the National Center for Research Resources, the National Center for Advancing Translational Sciences Grant UL1TR000124 and UL1TR001427, and the Edythe L. Broad and the Constance Austin Women’s Heart Research Fellowships, Cedars-Sinai Medical Center, Los Angeles, California, the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles, The Linda Joy Pollin Women’s Heart Health Program, and the Erika Glazer Women’s Heart Health Project, Cedars-Sinai Medical Center, Los Angeles, California. This work is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or National Institutes of Health.
Abbreviations and Acronyms
- INOCA
Ischemia but no obstructive coronary artery disease
- u-hscTnI
Ultra-high sensitivity cardiac troponin
- CFR
Coronary flow reserve
- IC-ADO
Intra-coronary adenosine
- IC-ACH
Intra-coronary acetylcholine
- IC-NTG
Intra-coronary nitroglycerin
- CBF
Coronary blood flow
- CAD
Coronary artery disease
- CI
Confidence interval
Footnotes
Disclosures: All authors report no conflict of interest related to the contents of this manuscript.
References
- 1.Merz CNB, Pepine CJ, Walsh MN, Fleg JL, Camici PG, Chilian WM, Clayton JA, Cooper LS, Crea F, Carli MD, Douglas PS, Galis ZS, Gurbel P, Handberg EM, Hasan A, Hill JA, Hochman JS, Iturriaga E, Kirby R, Levine GN, Libby P, Lima J, Mehta P, Desvigne-Nickens P, Olive M, Pearson GD, Quyyumi AA, Reynolds H, Robinson B, Sopko G, Taqueti V, Wei J, Wenger N. Ischemia and no obstructive coronary artery disease (inoca). Circulation. 2017;135:1075–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G, Bairey Merz CN. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the national heart, lung and blood institute wise (women's ischemia syndrome evaluation) study. Journal of the American College of Cardiology. 2010;55:2825–2832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.AlBadri A, Bairey Merz CN, Johnson BD, Wei J, Mehta PK, Cook-Wiens G, Reis SE, Kelsey SF, Bittner V, Sopko G, Shaw LJ, Pepine CJ, Ahmed B. Impact of abnormal coronary reactivity on long-term clinical outcomes in women. J Am Coll Cardiol. 2019;73:684–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reis SE, Holubkov R, Conrad Smith AJ, Kelsey SF, Sharaf BL, Reichek N, Rogers WJ, Merz CN, Sopko G, Pepine CJ. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: Results from the nhlbi wise study. American heart journal. 2001;141:735–741 [DOI] [PubMed] [Google Scholar]
- 5.von Mering GO, Arant CB, Wessel TR, McGorray SP, Bairey Merz CN, Sharaf BL, Smith KM, Olson MB, Johnson BD, Sopko G, Handberg E, Pepine CJ, Kerensky RA. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: Results from the national heart, lung, and blood institute-sponsored women's ischemia syndrome evaluation (wise). Circulation. 2004;109:722–725 [DOI] [PubMed] [Google Scholar]
- 6.Taqueti VR, Hachamovitch R, Murthy VL, Naya M, Foster CR, Hainer J, Dorbala S, Blankstein R, Di Carli MF. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation. 2015;131:19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR Jr., Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954 [DOI] [PubMed] [Google Scholar]
- 8.Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, Dorbala S, Blankstein R, Rimoldi O, Camici PG, Di Carli MF. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129:2518–2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, Dorbala S, Blankstein R, Di Carli MF. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126:1858–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anand A, Shah ASV, Beshiri A, Jaffe AS, Mills NL. Global adoption of high-sensitivity cardiac troponins and the universal definition of myocardial infarction. Clinical chemistry. 2019;65:484–489 [DOI] [PubMed] [Google Scholar]
- 11.Lippi G, Sanchis-Gomar F. "Ultra-sensitive" cardiac troponins: Requirements for effective implementation in clinical practice. Biochemia medica. 2018;28:030501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samman Tahhan A, Sandesara P, Hayek SS, Hammadah M, Alkhoder A, Kelli HM, Topel M, O'Neal WT, Ghasemzadeh N, Ko YA, Gafeer MM, Abdelhadi N, Choudhary F, Patel K, Beshiri A, Murtagh G, Kim J, Wilson P, Shaw L, Vaccarino V, Epstein SE, Sperling L, Quyyumi AA. High-sensitivity troponin i levels and coronary artery disease severity, progression, and long-term outcomes. Journal of the American Heart Association. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Januzzi JL Jr., Suchindran S, Hoffmann U, Patel MR, Ferencik M, Coles A, Tardif JC, Ginsburg GS, Douglas PS, Investigators P. Single-molecule hstni and short-term risk in stable patients with chest pain. Journal of the American College of Cardiology. 2019;73:251–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinning C, Keller T, Zeller T, Ojeda F, Schluter M, Schnabel R, Lubos E, Bickel C, Lackner KJ, Diemert P, Munzel T, Blankenberg S, Wild PS, Gutenberg Health S. Association of high-sensitivity assayed troponin i with cardiovascular phenotypes in the general population: The population-based gutenberg health study. Clinical research in cardiology : official journal of the German Cardiac Society. 2014;103:211–222 [DOI] [PubMed] [Google Scholar]
- 15.Eggers KM, Venge P, Lindahl B, Lind L. Cardiac troponin i levels measured with a high-sensitive assay increase over time and are strong predictors of mortality in an elderly population. Journal of the American College of Cardiology. 2013;61:1906–1913 [DOI] [PubMed] [Google Scholar]
- 16.Schulz O, Reinicke M, Berghoefer GH, Bensch R, Kraemer J, Schimke I, Jaffe AS. High-sensitive cardiac troponin i (hs-ctni) values in patients with stable cardiovascular disease: An initial foray. Clinica chimica acta; international journal of clinical chemistry. 2010;411:812–817 [DOI] [PubMed] [Google Scholar]
- 17.Hjort M, Lindahl B, Baron T, Jernberg T, Tornvall P, Eggers KM. Prognosis in relation to high-sensitivity cardiac troponin t levels in patients with myocardial infarction and non-obstructive coronary arteries. American heart journal. 2018;200:60–66 [DOI] [PubMed] [Google Scholar]
- 18.Soetkamp D, Raedschelders K, Mastali M, Sobhani K, Bairey Merz CN, Van Eyk J. The continuing evolution of cardiac troponin i biomarker analysis: From protein to proteoform. Expert review of proteomics. 2017;14:973–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomson LE, Wei J, Agarwal M, Haft-Baradaran A, Shufelt C, Mehta PK, Gill EB, Johnson BD, Kenkre T, Handberg EM, Li D, Sharif B, Berman DS, Petersen JW, Pepine CJ, Bairey Merz CN. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A national heart, lung, and blood institute-sponsored study from the women’s ischemia syndrome evaluation. Circulation. Cardiovascular imaging. 2015;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quesada O, AlBadri A, Wei J, Shufelt C, Mehta PK, Maughan J, Suppogu N, Aldiwani H, Cook-Wiens G, Nelson MD, Sharif B, Handberg EM, Anderson RD, Petersen J, Berman DS, Thomson LEJ, Pepine CJ, Merz CNB. Design, methodology and baseline characteristics of the women’s ischemia syndrome evaluation-coronary vascular dysfunction (wise-cvd). American heart journal. 2019;220:224–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Chavey WE, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS. Acc/aha 2007 guidelines for the management of patients with unstable angina/non–st-elevation myocardial infarction: Executive summary. Circulation. 2007;116:803–877 [Google Scholar]
- 22.Wei J, Mehta PK, Johnson BD, Samuels B, Kar S, Anderson RD, Azarbal B, Petersen J, Sharaf B, Handberg E, Shufelt C, Kothawade K, Sopko G, Lerman A, Shaw L, Kelsey SF, Pepine CJ, Merz CN. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: Results from the nhlbi-sponsored wise (women's ischemia syndrome evaluation) study. JACC Cardiovasc Interv. 2012;5:646–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.AlBadri A, Bairey Merz CN, Johnson BD, Wei J, Mehta PK, Cook-Wiens G, Reis SE, Kelsey SF, Bittner V, Sopko G, Shaw LJ, Pepine CJ, Ahmed B. Impact of abnormal coronary reactivity on long-term clinical outcomes in women. Journal of the American College of Cardiology. 2019;73:684–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC. Cardiovascular interventions. 2015;8:1445–1453 [DOI] [PubMed] [Google Scholar]
- 25.Mastali M, Fu Q, Sobhani K, Merz NB, Eyk JV. Abstract 19167: Ultra-high sensitive cardiac troponin i baseline levels are affected by age and sex. Circulation. 2017;136:A19167–A19167 [Google Scholar]
- 26.Shaw LJ, Merz CN, Pepine CJ, Reis SE, Bittner V, Kip KE, Kelsey SF, Olson M, Johnson BD, Mankad S, Sharaf BL, Rogers WJ, Pohost GM, Sopko G, Women's Ischemia Syndrome Evaluation I. The economic burden of angina in women with suspected ischemic heart disease: Results from the national institutes of health--national heart, lung, and blood institute--sponsored women's ischemia syndrome evaluation. Circulation. 2006;114:894–904 [DOI] [PubMed] [Google Scholar]
- 27.Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Pepine CJ, Mankad S, Sharaf BL, Rogers WJ, Pohost GM, Lerman A, Quyyumi AA, Sopko G, Investigators W. Insights from the nhlbi-sponsored women's ischemia syndrome evaluation (wise) study: Part ii: Gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. Journal of the American College of Cardiology. 2006;47:S21–29 [DOI] [PubMed] [Google Scholar]
- 28.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, McGuire DK. Association of troponin t detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winegrad S, Henrion D, Rappaport L, Samuel JL. Self-protection by cardiac myocytes against hypoxia and hyperoxia. Circulation research. 1999;85:690–698 [DOI] [PubMed] [Google Scholar]
- 30.Tirziu D, Giordano FJ, Simons M. Cell communications in the heart. Circulation. 2010;122:928–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brutsaert DL. Cardiac endothelial-myocardial signaling: Its role in cardiac growth, contractile performance, and rhythmicity. Physiological reviews. 2003;83:59–115 [DOI] [PubMed] [Google Scholar]
- 32.Summers MR, Lerman A, Lennon RJ, Rihal CS, Prasad A. Myocardial ischaemia in patients with coronary endothelial dysfunction: Insights from body surface ecg mapping and implications for invasive evaluation of chronic chest pain. European Heart Journal. 2011;32:2758–2765 [DOI] [PubMed] [Google Scholar]
- 33.Hasdai D, Gibbons RJ, Holmes DR Jr., Higano ST, Lerman A. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation. 1997;96:3390–3395 [DOI] [PubMed] [Google Scholar]
- 34.Zeiher AM, Krause T, Schachinger V, Minners J, Moser E. Impaired endothelium-dependent vasodilation of coronary resistance vessels is associated with exercise-induced myocardial ischemia. Circulation. 1995;91:2345–2352 [DOI] [PubMed] [Google Scholar]
- 35.Park K, Kim M, Cho YR, Park JS, Park TH, Kim MH, Kim YD. Association between cardiac troponin level and coronary flow reserve in patients without coronary artery disease: Insight from a thermodilution technique using an intracoronary pressure wire. Korean circulation journal. 2014;44:141–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Egashira K, Inou T, Hirooka Y, Kai H, Sugimachi M, Suzuki S, Kuga T, Urabe Y, Takeshita A. Effects of age on endothelium-dependent vasodilation of resistance coronary artery by acetylcholine in humans. Circulation. 1993;88:77–81 [DOI] [PubMed] [Google Scholar]
- 37.Zeiher AM, Drexler H, Saurbier B, Just H. Endothelium-mediated coronary blood flow modulation in humans. Effects of age, atherosclerosis, hypercholesterolemia, and hypertension. The Journal of clinical investigation. 1993;92:652–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khuddus MA, Pepine CJ, Handberg EM, Bairey Merz CN, Sopko G, Bavry AA, Denardo SJ, McGorray SP, Smith KM, Sharaf BL, Nicholls SJ, Nissen SE, Anderson RD. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: A substudy from the national heart, lung and blood institute-sponsored women’s ischemia syndrome evaluation (wise). Journal of interventional cardiology. 2010;23:511–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


