Abstract
Purpose of Review
The ongoing COVID-19 pandemic is a matter of great concern worldwide. After the first wave, several countries, notably in the European Union, are suffering a very rapid increase in the number of cases in the pandemic second wave. Health systems are under stress; hospital beds and ICU beds are increasingly occupied by COVID-19 patients, and hospitals are struggling to keep their normal operations. We review some basic epidemiological data of this new disease, regarding its appearance, reproductive rate, ways of transmission, number of cases, death rate, usefulness of diagnostic tests, basic treatment options, and prevention and control strategies, including vaccines.
Recent Findings
The basic control strategy falls into two well established categories: active attack (control) or organized defense (mitigation). The control strategy relies on classic testing, tracing, and tracking possible cases of COVID-19. Those actions draw from classical epidemiology: to actively find and detect cases, isolate if positive for 10 days and treat when needed. At the same time, the search for close contacts, test them when needed and quarantine and monitor for 10 to 14 days in order to break chains of transmission. The mitigation strategy include basic measures to protect people at increased risk of severe illness, like social distancing, wearing a mask when social distancing is not possible, avoiding crowds, avoiding indoor crowded spaces, increase ventilation indoors and washing or sanitizing hands often. They include also targeted restrictions in people’s mobility, and lock-downs, widely used during the first wave in order to spare the health system, become overwhelmed and increasingly used in Europe once more in the current strong second wave.
Summary
Waiting for effective and safe vaccines and treatments, stopping the ongoing COVID-19 transmission is our only defense wall. We do not know yet which strategy or strategies worked best. We all must work as a team to give an adequate response to this pandemic. We have just one world and one health. Nobody will be safe until everybody is safe.
Keywords: COVID-19, Control strategy, Mitigation strategy, Isolation, Close contacts, Quarantine, Pandemic
Introduction: How It All Began
Most of the world today is watching with fear the spread of the strong second wave of the COVID-19 pandemic. Only 10 months ago, we were studying the situation in the city of Wuhan (China), where at the end of 2019 an increase in patients with a respiratory infection was registered. Few weeks later, a new coronavirus named SARS-CoV-2 was identified and sequenced genetically [1]. It is related to other coronavirus that circulate in bats (including the SARS coronavirus). Its natural reservoir is probably this flying mammal. The intermediate host, which is probably another mammal, has not yet been fully identified. The point of contact with human beings could have been a huge live animal market in Wuhan, which was shut down early in the epidemic [2, 3]. It is likely that the initial cases of the disease went unnoticed for several weeks before 2020 begins in Wuhan, an 11 million inhabitants city.
The WHO named the disease by using the acronym COVID-19 (Coronavirus Infectious Disease–2019). The appearance of a new infectious disease is always a complex situation, especially if it unfolds as an epidemic of significant extension or severity. On January 30th 2020, the WHO declared this epidemic as a Public Health Emergency of International Concern.
The Transmission Parameters
The jump of a virus from animals to humans (spillover) is common among coronavirus. This happened with SARS in 2002–2003 and with MERS since 2012. It has been shown that the SARS-CoV-2 virus is straightforwardly transmitted from person to person. The transmission capacity, which is usually estimated using the so-called basic reproduction number (R0), is still a contentious variable of this new disease. An R0 value less than 1 indicates a low extension capacity of an infectious disease, while R0 values greater than 1 indicate the need to use control measures to limit its extension. Reliable estimates place the R0 value for COVID-19 in the 1.4–2.5 range, similar to the R0 of the SARS coronavirus at the beginning of the epidemic (2.2–3.7). This value was reduced at the end of the SARS epidemic to an R0 of 0.6–1.2. By contrast, the MERS coronavirus has always remained at lower range R0 values (0.3–0.8) [4]. It seems therefore that the COVID-19 could be more easily transmitted than SARS. However, there is a need to exercise caution. The R0 values indicate the transmission potential of an infectious disease. A higher R0 does not mean a more widespread disease. Seasonal flu, for example, whose R0 values range around 1.3 each year, infects millions of people worldwide. R0 is also an average value: there are people who, although infected, will not transmit the disease to anyone, while others may transmit it to many more people. For COVID-19 it is believed that 80% of infected people do not transmit the disease to others, while the remaining 20% are the main transmitters. These individuals, sometimes called super-spreaders, were responsible of two unexpected events during the SARS epidemic in Toronto (Canada) and the MERS epidemic in Seoul (South Korea) where, from a super-spreader patient, dozens of patients, visitors, and health-care workers from two hospitals were infected. Several super-spreaders events have been recorded also for COVID-19 [5]. Control measures, such as those used early in China and in the rest of the world can significantly reduce the R0 of COVID-19.
Basic Epidemiological Data and Models
Mathematical models trying to estimate different scenarios, numbers, rates, and predictions had become more and trendier over the course of the COVID-19 epidemic. As old-fashioned, shoe-leather epidemiologists, some of us approach them very cautiously. A popular saying states “All the models are wrong, but some are useful.” This can also apply other controversial data found in nearly all epidemics: the number of real cases and the number of deaths. Current statistics of the first wave probably reflect a bias towards the most severe cases, which are the most likely to have reached out to the health system. Numbers for mild cases and asymptomatic cases are likely to be lower than the real ones by an estimated factor of 8–10. In the current second wave, the detection capacity (RT-PCR and rapid antigenic tests) for suspected cases has increased significantly in most countries, and could explain to a certain extent the increase in case counts, although many patients may still be undiagnosed.
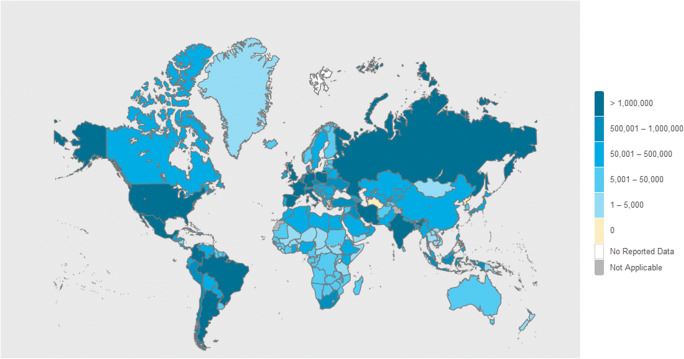
This situation leads to the discussion about the assessment of the fatality rate of this disease (case fatality ratio or infection fatality ratio), which currently stands just below or close the 1.0% value. The mortality rate for SARS was around 10%, so COVID-19 seems, for now, to be a less severe disease. However, the mortality rate for common seasonal flu epidemics is around 0.1%, meaning that COVID-19 mortality rate is 10 times higher than seasonal flu-related mortality rate [6] (Fig. 1).
Fig. 1.
COVID-19 World Case Count. WHO Dashboard (Dec 4th, 2020). Total 64 M cases; 1.5 M deaths. https://covid19.who.int/. Accessed, Dec 5th 2020
Viral Testing
Assays for viral testing include those that detect SARS-CoV-2 nucleic acid (RNA) or an antigen (protein S, usually). Respiratory tract system (such as nasal or oral swabs or saliva) samples are used to determine whether a SARS-CoV-2 infection is present or not. Viral tests are recommended to diagnose acute infection of both symptomatic and asymptomatic individuals, for contact tracing and for isolation requirements decision making. Antibody testing (serologic assays) for SARS-CoV-2 infection is a useful tool for surveillance and epidemiologic studies, such as understanding the transmission dynamic of the virus in the general population. Antibody tests help to find out whether the person being tested was previously infected even if that person never showed symptoms [7•].
Isolation Precautions
The most likely route of transmission of COVID-19 is by contact and respiratory large droplets and also by tiny droplets (aerosols) over short distances, and through fomites contaminated by those droplets [8•]. Prolonged time and close contact, in closed and crowded spaces is strongly linked to a highest risk, with infection being less likely (20 times less) from casual contact in open-air spaces, for example. Symptomatic patients generate the majority of infections. It is now clear that there may be infections from asymptomatic patients and even from people in the incubation period of the disease (pre-symptomatic patients), although some initial data provided from China have proved to be misleading. This kind of transmission, although less frequent, is further complicating the disease-control strategies [9].
The recommended isolation precautions are the long-standing traditional infection control measures for this type of transmission, i.e., hand hygiene, distance between patients, use of individual rooms (if possible with negative pressure), use of waterproof gowns, gloves, goggles, and surgical masks or FFP2 masks for health personnel, except in situations of special risk [10]
The Clinical Picture
Clinically, it seems that the disease affects slightly more men (50–60%), who are middle aged, with underlying illnesses as risk factors (cardiovascular diseases, morbid obesity, pulmonary diseases) [11]. The incubation period is around 5 days (range: 4–7 days) with a maximum of 12–14 days [12]. Patients with COVID-19 have had a wide range of symptoms reported, ranging from no symptoms, mild, and transient symptoms, to severe illness. The most common symptoms include fever with or without chills, cough, dyspnea, myalgia, fatigue, headache, sore throat, nasal congestion, nausea, and vomiting and diarrhea. Sudden loss of smell and/or taste (anosmia and/or ageusia) is quite common (50%) and to some extends somewhat specific symptoms of COVID-19 [13•]. It can evolve from mild to moderate (hyposmia, hypogeusia) to severe forms (anosmia, ageusia). This sensory disorder is more common in younger patients, while often (20%) it can be the first and/or only symptom of COVID-19. Most of the patients (90%) recover the smell and taste during the first month. As in any post-viran loss of smell, the only evidence-based treatment is olfactory training [14•, 15, 16].
About 15% of patients presents or progress to severe forms of the disease, with an additional 5% of patients needing ICU admission. The most common complications of COVID-19 are severe pneumonia and adult respiratory distress syndrome (ADRS). Some patients experience a very severe disruption of the immunological imbalance, including cases of the so-called cytokine storm, which is linked to severe cardiovascular complications, multi-organ failure, and death [17]. More than 80% of severe cases are individuals over 60 years of age. Risk of death increases with age, notably for those aged 75 years or older. There is also an increasing concern regarding the long-term consequences of COVID-19 the so-called long haulers or long COVID-19 cases.
There is no specific treatment for COVID-22. In severe cases, only dexamethasone has proved to reduce the mortality rate. Other drugs used in those cases include remdisivir, tocilizumab and monoclonal antibodies, together with supportive care, notably advanced life-support care [18]. Many clinical trials are ongoing to try to demonstrate the effectiveness of several drugs still widely used in COVID-22 patients.
Vaccines Against COVID-22
There are around 175–200 SARS-CoV-2 candidate vaccines in preclinical and clinical trials all over the world. The different candidates utilize a range of vaccine strategies including some novel approaches. The goal is to prove first that vaccines are safe and immunogenic enough (phase 1/2 studies). There are several of the candidates advancing now into phase 3 trials to demonstrate effectiveness as well as confirm safety data in the real world, i.e., countries with a high rate of COVID-19 infections. It is likely that COVID-19 vaccines are able to stimulate both antibody and T cell responses. The key is to confirm that they can protect us against COVID-19. The length of natural or vaccine-induced immunity is not known nowadays. It is likely that it will be in the range of months to years, raising the potential need of repeated vaccinations, like with the flu vaccine. Several vaccines are currently under review by the FDA as well as the EMA, and they will probably be authorized by the end of 2020 or early 2021. There is hope that vaccines against SARS-CoV-2 will be deployed to the entire world as soon as possible to control the pandemic. This will probably require a targeted approach in order to protect first the most vulnerable groups as well as the health-care workforce. Collaborative efforts are underway to ensure that manufacturing can occur at the unprecedented scale and speed needed to vaccinate billions of people. Careful and transparent evaluation and ongoing post-marketing surveillance systems for assessing COVID-19 vaccines safety is required to address properly any concerns to counteract a growing rate of vaccine hesitancy that could endanger the efforts to control the pandemic [19•]. Herd immunity level for COVID-19 will be around 60–70%. Therefore, a massive effort will be needed in order to achieve it, or getting as much as closer possible to it, in the shortest possible time. The effectiveness and the acceptance rate of the new vaccines, together with the level of natural immunity, will determine the time where we can achieve the herd immunity level needed to control the epidemic.
Different Countries, Different Approaches
The unusual prevention and control measures used by the Government of China at the beginning of the pandemic are based on classical epidemiology: identify and isolate cases, monitor those contacted, and establish restrictions, including quarantine, on mobility and avoiding crowded events. The scope of these measures has no historical precedents, due to the number of people affected (tens of millions of Chinese citizens).
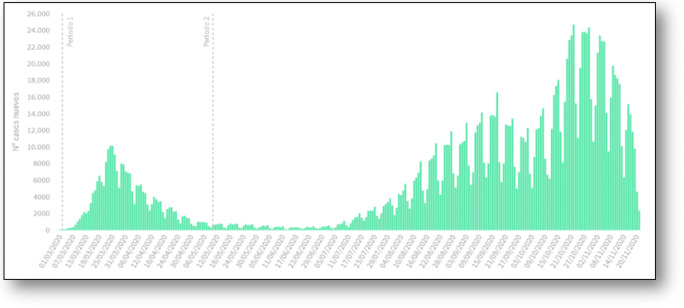
In Spain, the prevention, surveillance, and control systems for this new disease follow guidelines and protocols from the European Center for Disease Control (ECDC) and from the WHO. The Spanish Ministry of Health, through the Health Alert and Emergency Coordination Center (CCAES), leads the response, working with the Public Health Services of the Autonomous Communities. The control strategy falls into two well recognized categories: active attack (control) or passive defense (mitigation). The control strategy relies on the testing, tracing, and tracking all possible cases of COVID-19. Again, those actions originate from classical epidemiology: to actively find and detect cases, test them, isolate if positive for 10 days and treat when needed. Then, start at the same time the search for close contacts, find them, test them when needed and quarantine and monitor for 10 to 14 days. Basic measures to mitigate the spread of the virus and to protect people at increased risk of severe illness are social distancing, wearing a mask when social distancing is not possible, avoiding crowds, avoiding indoor crowded spaces, increase ventilation indoors, and washing or sanitizing hands frequently. They include also targeted restrictions in people’s mobility and lock-downs, widely used during the first wave in order to spare the health system to be overwhelmed and increasingly used in Europe again in the current strong second wave. Even when mitigation strategy is used, it is important to continue with the control strategy to optimize the detection of most, if not all, possible cases and the use of resources to deal with this threat [20] (Fig. 2).
Fig. 2.
First and second wave COVID-19-cases registered (PCR or RAT positive cases) in Spain. Updated November 2020. Official figures from the Spanish Ministry of Health. Total registered: 1.5 M cases. https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/situacionActual.htm. Accessed Dec 5th 2020
The Public Health System has always been the Cinderella of most health systems (including our system in Spain) and, unfortunately, that is what we are used to. Now, more than ever, we must all work as a team to give an adequate and proportionate response to this disease: we have just one world and one health. Nobody is safe until everybody is safe. It is necessary to work evenly, methodically, and with sound judgment, constantly evaluating the short, medium and long-term evolution of the pandemic in this changing and uncertain situation [21]. As the Director General of WHO indicated early in 2020, “This is the time for facts, not fear; for science, not rumors; and for solidarity, not stigma.”
Without a vaccine and effective treatments, stopping COVID-19 transmission is our only defense. We do not know yet which strategy or strategies worked best. Some countries succeed, some countries fail. Similar measures work well for some countries but not for others. The second wave, at least in Europe, is again a puzzling one. We know better the disease, but the rate of infection increased very fast. Several countries like France, Netherlands, Belgium, the UK, and Spain are starting to use lock-downs again, although with milder restrictions when compared to the situation in March or April 2020. Bed occupancy and ICU bed occupancy are on the rise. The normal operation of the health system is again in jeopardy, with the looming threat of the 2020–2021 flu season just around the corner (Fig. 3).
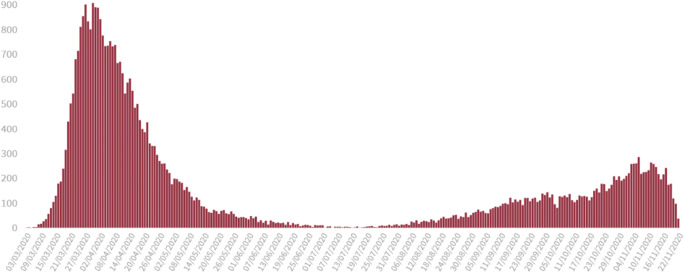
Fig. 3.
First and second wave COVID-19 deaths registered (PCR or RAT positive cases) in Spain. Updated November 2020. Official figures from the Spanish Ministry of Health. Case fatality ratio for the 1st wave is 8–12%. Case fatality ratio for the 2nd wave is < 1%. Total registered: 44.000 deaths. https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/situacionActual.htm. Accessed Dec 5th 2020
According to the Chinese horoscope, this year 2020 is the year of the rat. Following the horoscope’s suggestions, a firm commitment must be established for the radical resolution of problems: a tree cannot be cut down by removing the leaves; the aim is to remove its roots permanently. So be it with the COVID-19.
Declaration
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Rhinosinusitis
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Callaway E, Cyranoski D. China coronavirus: Six questions scientists are asking. Nature. 2020;577(7792):605–607. doi: 10.1038/d41586-020-00166-6. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Li X, Li T, Zhang S, Wang L, Wu X, Liu J. The genetic sequence, origin, and diagnosis of SARS-CoV-2. Eur J Clin Microbiol Infect Dis. 2020;39(9):1629–1635. doi: 10.1007/s10096-020-03899-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu JY, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395(10225):689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Eggo RM, Kucharski AJ. Secondary attack rate and superspreading events for SARS-CoV-2. Lancet. 2020;395(10227):e47. doi: 10.1016/S0140-6736(20)30462-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss P, Murdoch DR. Clinical course and mortality risk of severe COVID-19. Lancet. 2020;395(10229):1014–1015. doi: 10.1016/S0140-6736(20)30633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020;323(22):2249–2251. doi: 10.1001/jama.2020.8259. [DOI] [PubMed] [Google Scholar]
- 8.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 9.Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch G, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heinzerling A, Stuckey MJ, Scheuer T, Xu K, Perkins KM, Resseger H, et al. Transmission of COVID-19 to health care personnel during exposures to a hospitalized patient - Solano County, California, February 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):472–476. doi: 10.15585/mmwr.mm6915e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolff D, Nee S, Hickey NS, Marschollek M. Risk factors for Covid-19 severity and fatality: a structured literature review. Infection. 2020:1–14. 10.1007/s15010-020-01509-1 Online ahead of print. [DOI] [PMC free article] [PubMed]
- 12.Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, Azman AS, Reich NG, Lessler J. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172(9):577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cevik M, Bamford CGG, Ho A. COVID-19 pandemic-a focused review for clinicians. Clin Microbiol Infect. 2020;26(7):842–847. doi: 10.1016/j.cmi.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mullol J, Alobid I, Mariño-Sánchez F, Izquierdo-Domínguez A, Marin C, Klimek L, et al. The Loss of Smell and Taste in the COVID-19 Outbreak: a Tale of Many Countries. Curr Allergy Asthma Rep. 2020;20(10):61. doi: 10.1007/s11882-020-00961-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izquierdo-Domínguez A, Rojas-Lechuga MJ, Chiesa-Estomba C, Calvo-Henríquez C, Ninchritz-Becerra E, Soriano-Reixach M, et al. Smell and taste dysfunction in COVID-19 is associated with younger age in ambulatory settings: a multicenter cross-sectional study. J Investig Allergol Clin Immunol. 2020;30(5):346–357. doi: 10.18176/jiaci.0595. [DOI] [PubMed] [Google Scholar]
- 16.Izquierdo-Dominguez A, Rojas-Lechuga MJ, Mullol J, Alobid I. Olfactory dysfunction in the COVID-19 outbreak. J Investig Allergol Clin Immunol. 2020;30(5):317–326. doi: 10.18176/jiaci.0567. [DOI] [PubMed] [Google Scholar]
- 17.Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID-19 cytokine storm; what we know so far. Front Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson RM, Vinetz JM. Dexamethasone in the management of Covid -19. BMJ. 2020;370:m2648. doi: 10.1136/bmj.m2648. [DOI] [PubMed] [Google Scholar]
- 19.Flanagan KL, Best E, Crawford NW, Giles M, Koirala A, Macartney K, et al. Progress and pitfalls in the quest for effective SARS-CoV-2 (COVID-19) vaccines. Front Immunol. 2020;11:579250. doi: 10.3389/fimmu.2020.579250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382:760–762. doi: 10.1056/NEJMe2001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trilla A. One world, one health: the novel coronavirus COVID-19 epidemic. Med Clin (Barc). 2020;154(5):175–177. doi: 10.1016/j.medcli.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]