Abstract
Chronic lymphocytic leukemia (CLL) is the second most common hematologic malignancy, and it is characterized by lymphocytic leukocytosis and secondary hematologic deficiencies. While it most commonly presents as a systemic disease, extramedullary involvement may rarely occur. The literature surrounding CLL metastatic disease to the gallbladder is particularly sparse. Interestingly, we describe a case of a 67-year-old female who presented with painless jaundice and was found to have a rapidly growing gallbladder wall mass which was determined to be CLL metastatic disease after extensive surgical resection. It is important for radiologists to recognize the possibility of CLL metastatic disease to the gallbladder when evaluating potential cases of cholecystitis due to the overlapping spectrum of imaging findings. Cognizant radiologists can potentially save patients from surgical intervention as CLL is classically treated with chemotherapy.
Keywords: Lymphoma, Gallbladder neoplasms, Cholecystitis, Adenocarcinoma, Body radiology, Emergency radiology
Introduction
Chronic lymphocytic leukemia (CLL) is a malignancy of B cells that is estimated to cause 21,000 new cases and 4,000 deaths this year in the United States [1]. Despite a slow decline in both the incidence and mortality of CLL, it remains as one of the most common leukemias [1]. The clinical presentation of CLL may vary, but it is defined as the presence of ≥5 × 109/L (5000/μL) B-lymphocytes in the peripheral blood for >3 months [2]. While CLL is typically considered indolent, it may cause extramedullary disease in various organs. The most common locations include the central nervous system and skin, although infiltration into the prostate, myocardium, thyroid, and lung have been reported [3], [4], [5], [6], [7]. We report a rare case and radiologic findings of CLL metastasis into the gallbladder presenting as a focal mass.
Case report
A 67-year-old female with a past medical history of hyperlipidemia, mild jaundice, and untreated and indolent CLL presented with elevated liver function tests (LFTs) to her primary care provider. At that time, the patient denied abdominal pain. The abnormal liver tests were attributed to her statin use, which were discontinued. 3-month follow-up laboratory workup revealed persistently elevated LFTs and physical exam was significant for jaundice - she was instructed to go to the emergency department. Evaluation in the emergency department was significant for elevated total bilirubin (4.6 mg/dl), but leukocytosis or elevated CA19-9 was not present. Ultrasound of the abdomen (Fig. 1) demonstrated a slightly echogenic liver without intrahepatic ductal dilation or common bile duct dilation. Multiple echogenic foci were noted in the markedly distended gallbladder with accompanying pericholecystic fluid and edema; the presumptive diagnosis of cholelithiasis with possible acute cholecystitis was suggested.
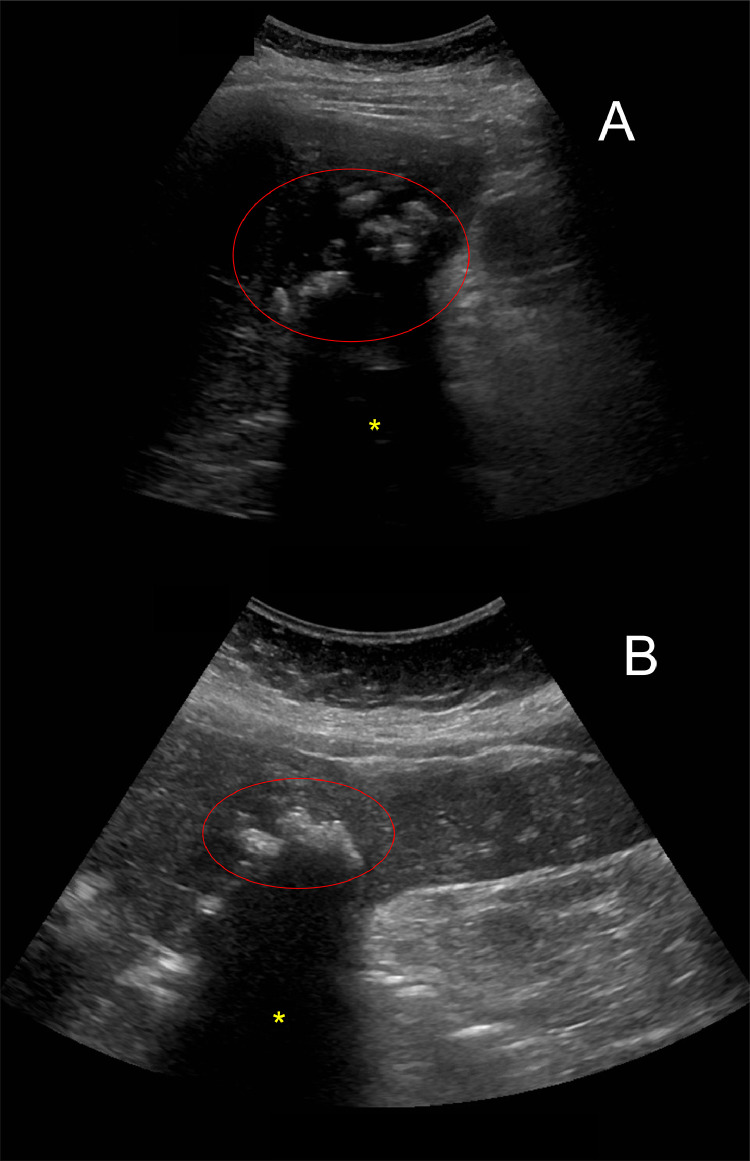
Fig. 1.
Sagittal (A) and left-lateral decubitus (B) ultrasound images of the gallbladder demonstrating echogenic material (red circle) inside the gallbladder lumen and evidence of acoustic shadowing (yellow asterisk).Color version of figure is available online.
The pancreas was not completely visualized due to overlying bowel gas.
Computed tomography (CT) of the abdomen in the emergency department revealed common bile duct (CBD) dilatation with portacaval and porta hepatis lymphadenopathy (Fig. 2 and 3). At this time, the gallbladder wall was reported to be indistinct and cholelithiasis was present. Due to the bilirubinemia and unequivocal clinical picture, further evaluation with magnetic resonance imaging (MRI) of the abdomen using cholangiopancreatography (MRCP) protocol was performed, which demonstrated cholelithiasis in the gallbladder neck, thickened gallbladder wall and CBD dilatation (Fig. 4). However, no distinct mass was identified by the radiologist and the impression reported findings consistent with possible acute cholecystitis.
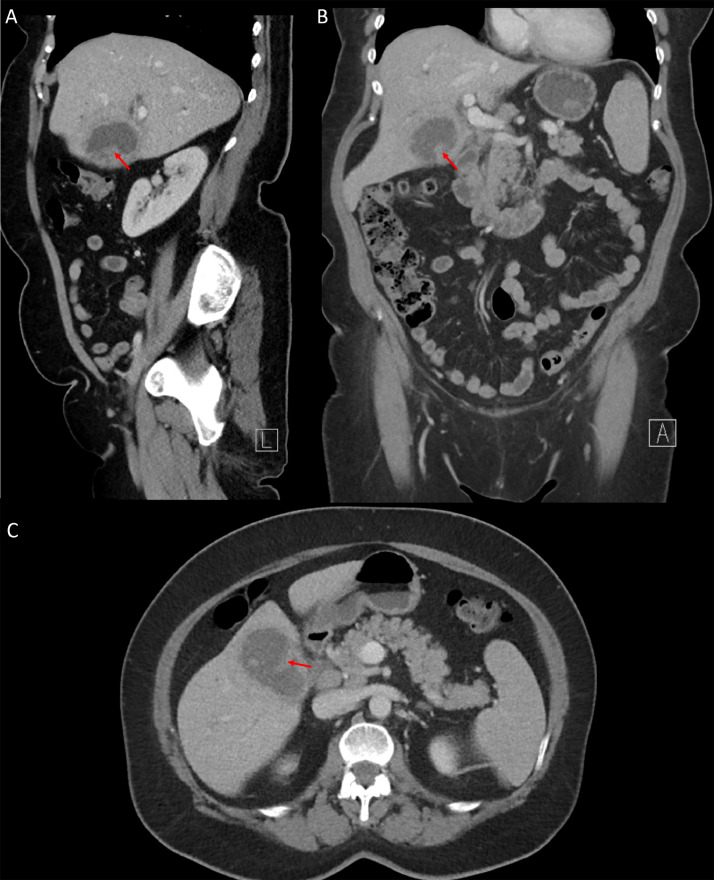
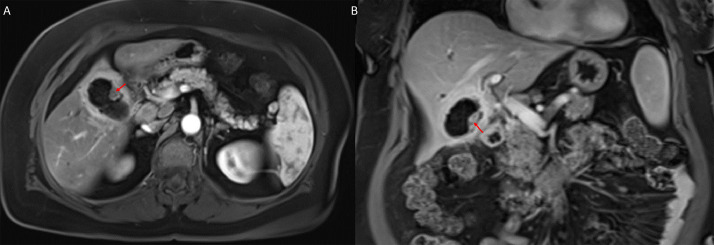
Fig. 2.
Sagittal (A), coronal (B), and axial (C) contrast-enhanced CT images of the abdomen and pelvis demonstrate an enlarged gallbladder, with indistinct walls, and the presence of cholelithiasis. Retrospective review reveals an iso-attenuating lesion in the inferior portion of the gallbladder (red arrows), which was thought to represent a gallbladder wall fold at the time of original interpretation. Color version of figure is available online.
Fig. 3.
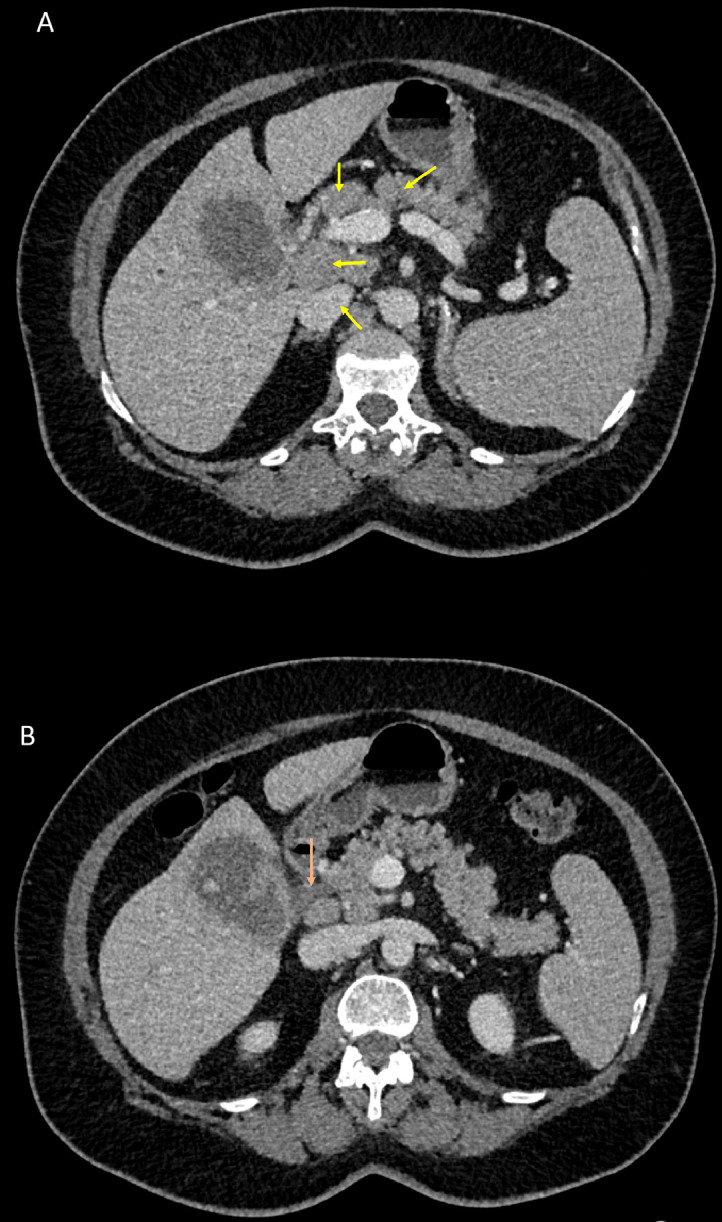
Axial intravenous contrast-enhanced CT images of the abdomen and pelvis demonstrate lymphadenopathy (A; yellow arrows) and common biliary duct dilatation (B; orange arrow). Color version of figure is available online.
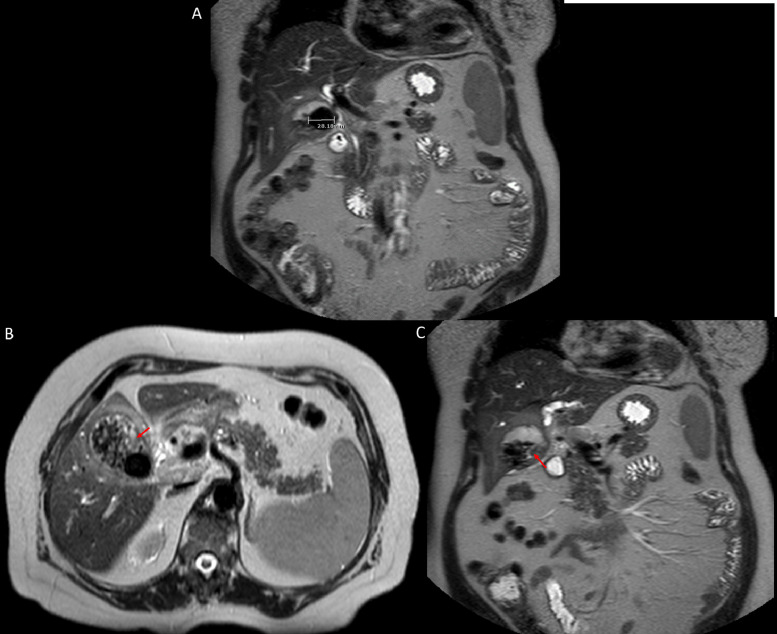
Fig. 4.
Coronal (A, C) and axial (B) MR images of the abdomen with cholangiopancreatography protocol reveal a 2.8 cm gallstone as well as surrounding sub-centimeter calculi and layering sludge (red arrow). Color version of figure is available online.
The patient's bilirubin continued to increase the following day to 12.1 mg/dl, and she was scheduled for endoscopic retrograde cholangiopancreatography (ERCP). A biliary tract obstruction was identified without presence of a mass or stone (Fig. 5). Cells for cytology were obtained from the narrowed bile duct segment, sphincterotomy with stent placement was performed, and fine needle aspiration (FNA) of the largest porta hepatis node was completed. Brushings did not reveal a neoplastic process but were significant for fibrosis and edema. FNA of the lymph node demonstrated findings consistent with the patient's known history of CLL. Following the ERCP, the patient's bilirubin decreased, and she was discharged as she was asymptomatic, with a follow-up outpatient appointment to the hepatobiliary surgery (HBS) service.
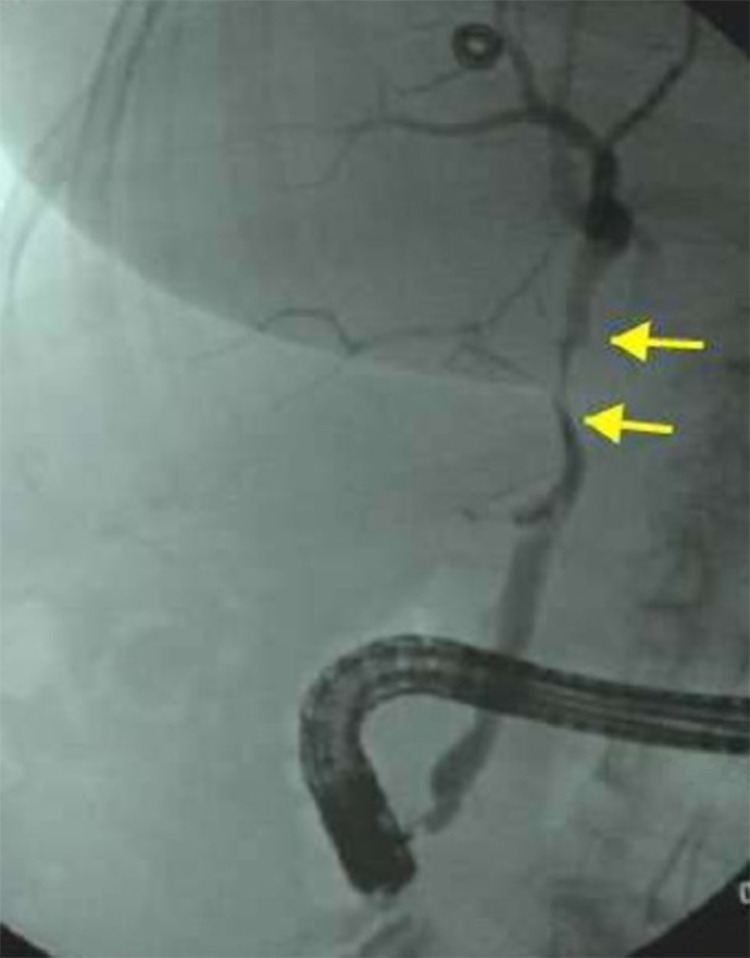
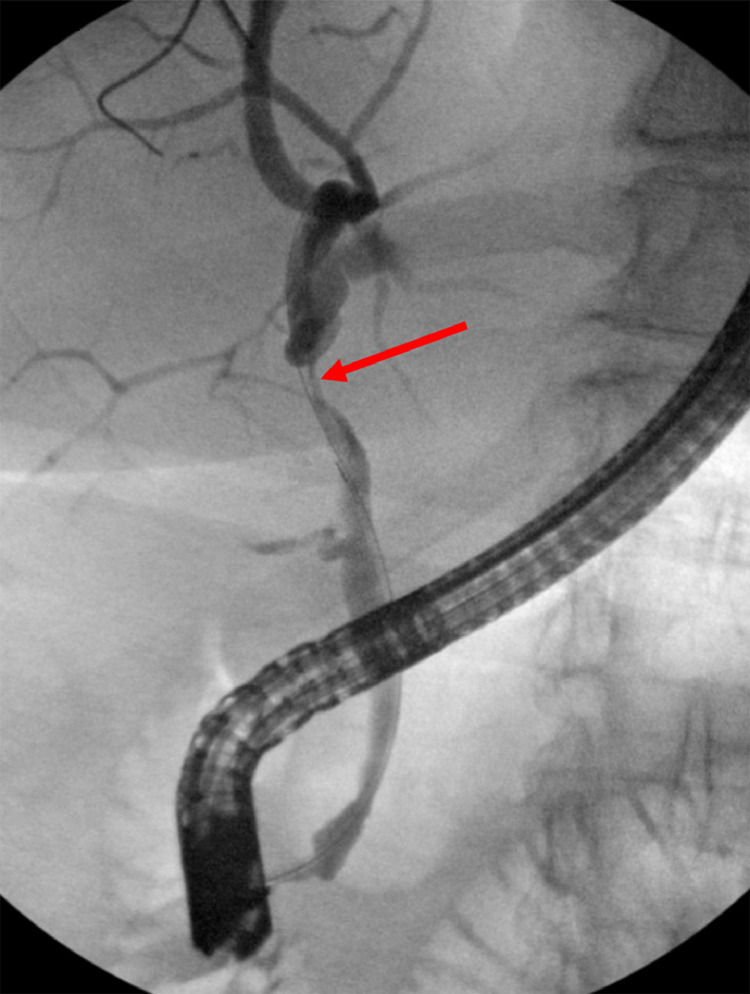
Fig. 5.
Fluoroscopic images of the abdomen during endoscopic retrograde cholangiography demonstrating strictures in the common hepatic duct (yellow arrows). Color version of figure is available online.
1 month later, another MRI of the abdomen using MRCP protocol was performed prior to her follow-up HBS appointment. The MRI revealed intrahepatic and extrahepatic ductal dilation, gallbladder wall thickening, portacaval nodes, and a 1.3 × 1.2 cm enhancing lesion arising from the gallbladder (Fig. 6), which appeared to be more conspicuous as compared to the prior MR examination. During a second ERCP, significant segmental strictures in the upper third of the main bile duct and common hepatic duct were noted (Fig. 7). Biliary duct brushings were obtained and cytopathological analysis was reported to demonstrate biliary adenocarcinoma with local extension. At this time, it was noted that the patient did not have an elevated CA19-9.
Fig. 6.
Axial (A) and coronal (B) contrast enhanced T1-weighted MR images of the abdomen with subtraction demonstrate an enhancing mass arising from the anteroinferior gallbladder wall (red arrow). Color version of figure is available online.
Fig. 7.
Fluoroscopic images of the abdomen during endoscopic retrograde cholangiography reveals a stricture in the common hepatic duct (red arrow). Color version of figure is available online.
After completing 3 cycles of gemcitabine-cisplatin, the patient elected for an extended hepatectomy with extrahepatic bile duct resection and cholecystectomy. The final surgical pathology of the resected liver, bile ducts, and gallbladder revealed small lymphocytic lymphoma without evidence of primary hepatic or biliary carcinoma (Fig. 8). The lymphoma was noted to extensively involve the liver parenchyma, hepatic and bile ducts, and gallbladder (Fig. 9). The lymphoma also involves the proximal bile duct and hepatic resection margins. Further microscopic examination of the 7 resected lymph nodes (periportal, celiac, and superior pancreatic lymph nodes) also revealed small lymphocytic lymphoma. The patient experienced multiple post-operative complications including bile leak, right phrenic artery injury, and surgical site abscess formation. After 2 years of post-surgery follow-up, the patient has not any evidence of recurrence.
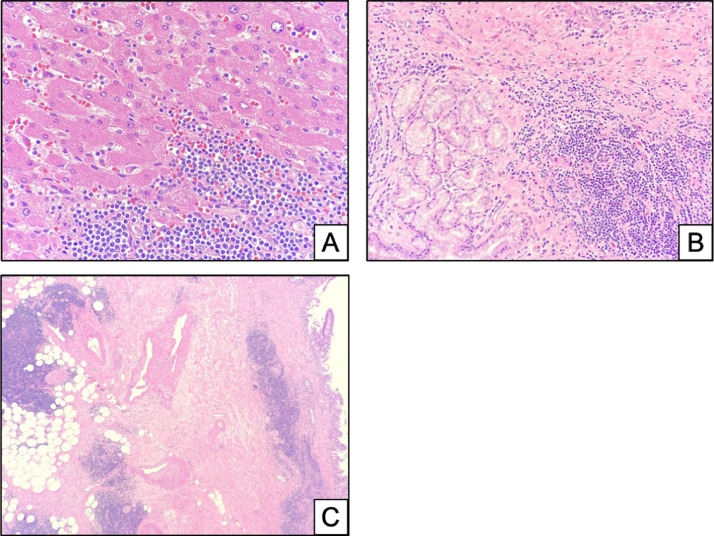
Fig. 8.
Histopathology images (H&E, 20X) demonstrate lymphocytic infiltrate surrounding submucosal glands (A, H&E 20X) consisted of both B and T cells as seen by immunohistochemistry (IHC) for CD20 (C, 20X) and CD3 (D, 20X). IHC for CD5 (B, 20X) showed aberrant staining the CD20-positive B cells and normal staining of CD3-positive T cells. The population of abnormal B cells showed lighter staining than the normal T cell population as seen in (B). The CD20/CD5 B cells were also positive for CD23 and negative for Cyclin D1 by immunohistochemistry (not shown).
Fig. 9.
Histopathology image (A, H&E 20X) showing unremarkable liver parenchyma with diffuse and nodular infiltration by small, monotonous lymphocytes with clumped chromatin. Second histopathology image (B, H&E 20X) showing nodular infiltrates of small lymphocytes appeared adjacent to submucosal glands near the common bile duct. Third histopathology images (C, H&E 5X) showing nodules and diffuse infiltrates of small, bland lymphocytes dissecting through fibrous tissue surrounding the common bile duct and into the surrounding fat.
Discussion
Chronic lymphocytic leukemia is the second most common hematologic malignancy (behind diffuse large B cell lymphoma) characterized by the accumulation of functionally incompetent monoclonal lymphocytes [1]. With a median age at diagnosis of 70 and a 5-year survival rate of 86.1%, CLL is classically considered an indolent disease [1]. However, cytogenetic studies have further stratified CLL into high- and low-risk categories largely based on the mutational status of the immunoglobulin heavy chain gene (IgVH), CD-38, and zeta-associated protein (ZAP-70) [8,9]. High- and low-risk stratification approximates that only one-third of cases of CLL are truly indolent and may not necessitate treatment. Another one-third of patients may progress several years after the initial diagnosis, while the remaining one-third of patients will need immediate treatment at the time of diagnosis [10].
Extramedullary CLL (EM-CLL), a serious and rare manifestation of CLL, occurs when CLL presents outside the blood or bone marrow with or without the presence of systemic CLL. Prognostication and survival after diagnosis of patients with EM-CLL largely depends on the organs involved, with the most commonly reported sites being skin (33%) and the central nervous system (27%) [3]. Less commonly, the remaining involvements are gastrointestinal (14%), genitourinary or gynecologic (10%), lung (5%), and ocular (5%) [3]. Patients with EM-CLL at the time of relapse tend to have a lower survival rate than those who had EM-CLL at the time of initial systemic diagnosis [3]. Extramedullary lymphoma involvement of the gallbladder specifically is rare, and consequently has been infrequently reported in literature. EM lymphoma to the gallbladder may often represent primary non-Hodgkin lymphoma from mucosa-associated lymphoid tissue [11,12].
One of the challenging aspects to this case was the determining the pathological correlate to the observed radiologic findings, in part due to the rarely reported distinguishing radiologic features of gallbladder lymphoma and its overlap with imaging findings of cholecystitis. Additionally, as with our case, the differentiation between gallbladder lymphoma and a primary gallbladder neoplasm may not be clear. Evaluation of primary gallbladder neoplasm using MRI typically reveals aT1-weighted hypointense and T2-weighted hyperintense mass within, or arising from, the gallbladder, which may also extend into the liver. Associated findings of biliary obstruction or porta hepatic lymphadenopathy may also be present. Interestingly, this patient's imaging appeared more in line with a primary gallbladder neoplasm given the findings of a contrast-enhancing nodular foci within the gallbladder lumen.
To our knowledge, only 3 case reports have been published pertaining to this topic, all of which were clinically suggestive of cholecystitis at the time of patient presentation. Radiological findings are not clearly described in the published cases, and two of the three cases do not describe imaging findings at all. The first case involves a 62-year-old woman with a history of CLL who underwent an emergency cholecystectomy for clinically consistent acute cholecystitis. Specific imaging findings were not reported in the publication with the exception that a gallbladder stone was not present. Surgical histology of the gallbladder revealed transmural infiltration with small lymphoid cells consistent with CLL metastatic disease [11]. The second case involved a 71-year-old male who underwent an elective laparoscopic cholecystectomy after an episode of gallstone pancreatitis. Again, imaging findings of the gallbladder were not described in the publication. Surgical histopathology of the gallbladder showed acute-on-chronic inflammatory infiltrate with a dense, intramural, small, and monomorphous lymphoid infiltrate. Further analysis confirmed these lymphoid cells to be of CLL phenotype. Interestingly, the patient lacked typical CLL symptoms or laboratory perturbations. 1 year following the cholecystectomy, the patient developed thrombocytopenia and a bone marrow biopsy confirmed marrow infiltration consistent with CLL [13]. The third and final case describes a 75-year-old female patient presenting with an acute cholecystitis-like picture on imaging. Both ultrasound and MRI findings demonstrate gallbladder wall thickening without focal mass or obstructing stone. The patient underwent a cholecystectomy and surgical histopathology was consistent with gallbladder wall infiltration of CLL [14].
Given that specific imaging findings of lymphoma metastatic disease to the gallbladder were not described in the known case reports and that all cases that were initially diagnosed involved the spectra of cholecystitis, it can be assumed that the radiological presentation was most consistent with imaging characteristics of cholecystitis (classically gallbladder wall thickening and pericholecystic fluid). However, unlike any of the cases described, we present a patient with a known diagnosis of CLL who was found to have a rapidly growing and distinct gallbladder mass, which was initially concerning for primary gallbladder adenocarcinoma. Extensive surgical resection revealed CLL metastatic disease with cytopathology demonstrating tumor cells positive for CD10 and CD5 and negative for CD3, CD10 and cyclin D1. To the best of our best knowledge, there has never been reported a case of CLL metastatic disease to the gallbladder presenting as a focal mass extending from the gallbladder wall.
When evaluating an elderly patient with acalculous cholecystitis with or without a known diagnosis of CLL, lymphoma metastatic disease to the gallbladder and bile ducts should be considered. Although the most common and likely diagnosis in a patient with right upper quadrant pain will be cholecystitis, the presence of lymphoma should be considered and, if radiologic findings are consistent, mentioned in the correct clinical scenario. Early recognition of lymphoma metastatic disease may save patients from extensive surgery and its associated morbidity.
Conclusion
While chronic lymphocytic leukemia is often considered an indolent disease, it may rarely infiltrate other organs. Extramedullary chronic lymphocytic leukemia carries a worse prognosis depending on the time of diagnosis and organ involvement. Gallbladder infiltration is rarely reported in the literature and infiltrative disease may present before a systemic process is identified. Early diagnosis of chronic lymphocytic leukemia metastasis to the gallbladder may allow for proper treatment with chemotherapeutic agents and avoid potentially unnecessary surgery. Radiologists must remember that gallbladder lymphoma can present as an infiltrative process, or as a distinct mass arising from the gallbladder wall, as illustrated from our case presentation.
Ethics approval
This is a retrospective case report not requiring ethics approval.
Patient Consent
Consent to participate: All patient data has been removed and no informed consent is required to participate
Consent for publication: All patient data has been removed and no informed consent is required to publish.
All attempts to remove identifying information and personal health information (PHI) have been made. No written consent is required for participation or publication.
Availability of data and materials
Not applicable
Code availability
Not applicable
Authors’ contribution
All authors contributed to writing the manuscript. All authors read and approved the final manuscript.
Footnotes
Acknowledgement: No funding was received to assist with the preparation of this manuscript.
Competing Interest: The authors declare that they have no conflict of interest.
References
- 1.Siegel R.L, Miller K.D, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. Epub 2020 Jan 8. PMID: 31912902. [DOI] [PubMed] [Google Scholar]
- 2.Hallek M, Cheson B.D, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745–2760. doi: 10.1182/blood-2017-09-806398. Epub 2018 Mar 14. PMID: 29540348. [DOI] [PubMed] [Google Scholar]
- 3.Ratterman M, Kruczek K, Sulo S, Shanafelt T.D, Kay N.E, Nabhan C. Extramedullary chronic lymphocytic leukemia: systematic analysis of cases reported between 1975 and 2012. Leuk Res. 2014;38(3):299–303. doi: 10.1016/j.leukres.2013.08.009. Epub 2013 Sep 6. PMID: 24064196. [DOI] [PubMed] [Google Scholar]
- 4.Benekli M, Büyükaşik Y, Haznedaroğlu I.C, Savaş M.C, Ozcebe O.I. Chronic lymphocytic leukemia presenting as acute urinary retention due to leukemic infiltration of the prostate. Ann Hematol. 1996;73(3):143–144. doi: 10.1007/s002770050216. PMID: 8841103. [DOI] [PubMed] [Google Scholar]
- 5.Applefeld M.M, Milner S.D, Vigorito R.D, Shamsuddin A.M. Congestive heart failure and endocardial fibroelastosis caused by chronic lymphocytic leukemia. Cancer. 1980;46(6):1479–1484. doi: 10.1002/1097-0142(19800915)46:6>1479::aid-cncr2820460631<3.0.co;2-r. PMID: 7417948. [DOI] [PubMed] [Google Scholar]
- 6.Fain J.S, Naeim F, Becker D.P, Van Herle A, Yan-Go F, Petrus L. Chronic lymphocytic leukemia presenting as a pituitary mass lesion. Can J Neurol Sci. 1992;19(2):239–242. PMID: 1623453. [PubMed] [Google Scholar]
- 7.Hyjek E, Isaacson P.G. Primary B cell lymphoma of the thyroid and its relationship to Hashimoto's thyroiditis. Hum Pathol. 1988;19(11):1315–1326. doi: 10.1016/s0046-8177(88)80287-9. PMID: 3141260. [DOI] [PubMed] [Google Scholar]
- 8.Shanafelt T.D, Geyer S.M, Kay N.E. Prognosis at diagnosis: integrating molecular biologic insights into clinical practice for patients with CLL. Blood. 2004;103(4):1202–1210. doi: 10.1182/blood-2003-07-2281. Epub 2003 Oct 23PMID: 14576043. [DOI] [PubMed] [Google Scholar]
- 9.Chiorazzi N, Rai K.R, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352(8):804–815. doi: 10.1056/NEJMra041720. PMID: 15728813. [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Wang Y.L. Prognostic and predictive molecular biomarkers in chronic lymphocytic leukemia. J Mol Diagn. 2020;22(9):1114–1125. doi: 10.1016/j.jmoldx.2020.06.004. Epub 2020 Jun 29PMID: 32615167. [DOI] [PubMed] [Google Scholar]
- 11.Chim C.S, Loong F, Chung L.P. Chronic lymphocytic leukaemia involving the gallbladder. Br J Haematol. 2001;115(4):717. doi: 10.1046/j.1365-2141.2001.03209.x. PMID: 11843800. [DOI] [PubMed] [Google Scholar]
- 12.Bickel A, Eitan A, Tsilman B, Cohen H.I. Low-grade B cell lymphoma of mucosa-associated lymphoid tissue (MALT) arising in the gallbladder. Hepatogastroenterology. 1999;46(27):1643–1646. PMID: 10430312. [PubMed] [Google Scholar]
- 13.Rao V, Watkins R, Kaleem A, Cooke J, Wedgwood K. Leukaemic infiltration of gall bladder - unusual presentation of occult chronic lymphocytic leukaemia. J Surg Case Rep. 2011;2011(1):7. doi: 10.1093/jscr/2011.1.7. PMID: 24950545; PMCID: PMC3649194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dasanu C.A, Mesologites T, Homsi S, Ichim T.E, Alexandrescu D.T. Chronic lymphocytic leukemia presenting with cholecystitis-like symptoms and gallbladder wall invasion. South Med J. 2010;103(5):482–484. doi: 10.1097/SMJ.0b013e3181d7e38f. PMID: 20375943. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable