Abstract
Background
Determinants of COVID-19 vaccine acceptance among healthcare workers (HCW) remains poorly understood. We assessed HCWs’ willingness to be vaccinated and reasons underlying hesitancy.
Methods
Cross-sectional survey across 17 healthcare institutions. HCWs eligible for vaccination (Pfizer-BioNTech mRNA) in December 2020 were invited to receive immunization. Multivariate logistic regression was performed to identify predictors of acceptance. Reasons for refusal among those who never intended to be vaccinated (ie, firm refusers) and those who preferred delaying vaccination (ie, vaccine hesitants) were assessed.
Results
Among 2,761 respondents (72% female, average age, 44), 2,233 (80.9%) accepted the vaccine. Physicians, environmental services workers and healthcare managers were more likely to accept vaccination compared to nurses. Male sex, age over 50, rehabilitation center workers, and occupational COVID-19 exposure were independently associated with vaccine acceptance by multivariate analysis. Factors for refusal included vaccine novelty, wanting others to receive it first, and insufficient time for decision-making. Among those who declined, 74% reported they may accept future vaccination. Vaccine firm refusers were more likely than vaccine hesitants to distrust pharmaceutical companies and to prefer developing a natural immunity by getting COVID-19.
Conclusions
Vaccine hesitancy exists among HCWs. Our findings provide useful information to plan future interventions and improve acceptance.
Key Words: SARS-CoV-2, Healthcare personnel, Vaccination campaigns, Attitudes, Vaccine safety, Vaccine hesitancy
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing Coronavirus Disease 2019 (COVID-19) emerged in late 2019 and reached the level of pandemic in March 2020.1 As of March 2021, 120 million cumulative cases and more than 2.6 million deaths have occurred worldwide.2 International collaborative efforts have led to the rapid development of vaccines against COVID-19 and on December 9, 2020, a first COVID-19 vaccine (Pfizer-BioNTech mRNA COVID-19 vaccine) was authorized for use in Canada.3 Due to limited supply, healthcare workers (HCW) and residents of long-term care facilities were prioritized to be immunized first.4 A group of public healthcare institutions in Montreal were notified that they would act as one of the first pilot sites for COVID-19 vaccination in Canada and would be administering the vaccination to HCWs from December 14 to December 28, 2020.
The success of this immunization campaign ultimately relies on individuals’ acceptance of novel vaccines, but the actual level of acceptance among HCWs remains poorly understood. Vaccine hesitancy (ie, the delay in acceptance or refusal of vaccination despite availability of vaccination services)5 remains a pervasive issue in the general population as well as among HCWs across the globe.6, 7, 8, 9, 10 Most of the studies investigating COVID-19 vaccine acceptance so far have assessed individuals’ intention to receive the vaccine, rather than their explicit acceptance of the vaccine once it was available. It is well recognized that intention does not always correlate with actual behavior, including for vaccination.11 , 12 Furthermore, vaccine hesitancy varies across time, context and for different vaccines. Determinants of acceptance of other vaccines (for example, the influenza vaccine) among HCWs may thus not be directly applicable to the new COVID-19 vaccines.13
Given the paucity of data regarding vaccine acceptance among HCWs, we conducted a survey across multiple healthcare institutions to measure their willingness to accept and schedule receiving the first dose of a COVID-19 vaccine, as well as to understand the reasons underlying vaccine hesitancy or refusal.
Methods
Study population and settings
We conducted a multicenter cross-sectional study to assess HCWs’ acceptance of the COVID-19 vaccine at the Centre Intégré Universitaire de Santé et de Services Sociaux Centre-Ouest-de-Montréal (CIUSSS COMTL), Canada. The CIUSSS COMTL is a large public organization employing 12,000 HCWs that provides a broad range of healthcare services. It includes over 3000 care beds spread across 20 healthcare institutions, including an academic acute care center, 6 long-term care facilities, 2 rehabilitation centers, 2 day centers, 6 centers for local community services (CLSC), and 3 specialized hospitals. These institutions were notified on December 4, 2020, that they would act as one of the pilot sites for COVID-19 vaccination in Canada following authorization for the use of a first COVID-19 vaccine. The 2,000 allocated doses of vaccine were expected to be administered within 14 days according to allocation and prioritization plans.
Given the lack of information regarding HCW acceptance of this new vaccine, a rapid-response strategy was developed that combined invitations for vaccination, screening and registration into a unified process to which information regarding vaccine acceptance and reasons for refusal were integrated. First, an email invitation was sent to all eligible HCWs, informing them that they could receive the COVID-19 vaccine. Eligible individuals included all HCWs who provide direct patient care, those who were in contact with potentially infectious material, and essential workers who did not provide direct patient care but whose job could not be performed via telework.14 An introduction letter was included to outline the survey objectives and inform respondents that participation was voluntary. It contained an internet link to allow them to either accept or decline vaccination. HCWs who declared that they accepted the vaccine were pre-registered for an appointment for vaccine administration at a dedicated HCW vaccination clinic. Hence, this survey measured actual acceptance or refusal of the COVID-19 vaccine, rather than mere intention. The final vaccination appointment was obtained after taking into account the HCW's rank in the prioritization sequence determined by the Quebec Health Ministry, which was based on health sectors and settings (eg. those working in long-term care facilities were given priority over those working in acute care facilities).14 , 15 In those who refused the vaccine, additional questions were asked to collect motives for refusal and assess intention to be vaccinated in the future.15
Survey questionnaire
As this study involved different directorates across multiple institutions, 3 different questionnaires were created to reach all HCWs. These questionnaires shared the same set of 20 core questions regarding vaccine acceptance and refusal, but varied in the types of socio-demographic questions that were collected. For example, the questionnaire sent to nurses contained information regarding the institution where they worked, whether they were reassigned to a COVID-19 unit, and included their age and gender. In contrast, questionnaires sent to physicians, residents and medical students (hereafter collectively referred to as “physicians”) contained information regarding department and service, but did not contain questions about gender, age or assignment to a COVID-19 unit. These variations were meant to accommodate the specific needs of the different directorates to plan the vaccination schedule. All questionnaires were available in English and French, taking approximately 5 minutes to complete (Appendix 1). Questions were created based on input from co-authors and experts in qualitative methods in infectious disease and vaccine hesitation.16 , 17 Responses were either in multiple choice or free-text form. The survey instrument was composed of 2 parts. The first section contained basic socio-demographic and employment information. In the second section, participants responded to questions about whether they were presently interested in receiving the vaccine. When respondents refused vaccination, they were asked to indicate how important a series of 15 factors were in their decision to decline the vaccine, by choosing 1 of 4 options: “Not important,” “somewhat important,” “very important,” or “I don't know.” In addition, respondents who declined vaccination were asked if they would be interested in receiving the vaccine at a later date, and if so, they were asked to choose among the following options: “In a few days,” “in a few weeks,” “in a few months” or “next year.”
Ethical considerations
The study was conducted in accordance with Article 2.5 of the 2018 Tri-Council Policy Statement for the Ethical Conduct for Research Involving Humans (studies used for assessment management and improvement purposes).18 The Institutional Review Board approved the analysis and publication of these results (protocol # 2021-2806). Respondents were considered to be consenting to participate in the study through the act of answering and submitting the questionnaire.
Statistical analyses
Variables included in this analysis included sex, age, occupational exposure to patients with COVID-19, institution of employment and profession. Discrete variables were reported as numbers and proportions in each category. The variable age was categorized to simplify interpretation.19 Given the high number of employment positions in the CIUSSS, job titles were regrouped into the following 6 categories: nurses and orderlies, physicians, health care managers, environmental services workers, administrative workers, and other HCWs (which includes, among others, pharmacists, radiology technicians, physiotherapists and respiratory therapists). Variables associated with vaccine acceptance were assessed through univariate logistic regression providing crude odds ratio (OR) and 95% confidence intervals (CI). In a subsequent step, we performed a forced-entry multivariate logistic regression procedure introducing all covariates with a p-value < 0.05 to identify covariates independently associated with acceptance of the COVID-19 vaccine. When any of the covariates included in the model had missing values, the data related to a respondent were excluded. Notably, physicians were excluded from this multivariate analysis as information regarding their age and gender was not available.
Respondents who declined vaccination were further grouped into 2 categories based on their interest, or lack thereof, in receiving the vaccine at a later date. These 2 groups included: those who may eventually accept vaccination (ie, vaccine hesitants), and those who never intend to receive the vaccine (ie, firm refusers). A chi-square test was used to compare reasons for refusal among vaccine hesitants and firm refusers. All tests were 2-sided and a P value <.05 was considered to indicate statistical significance. All analyses were performed using SAS version 9.4 (SAS Institute Cary NC).
Results
Basic demographics
Overall, 2,761 respondents answered the survey between December 15 and December 28, 2020. Of these, 2,233 (80.9%) accepted to receive the vaccine and 528 (19.1%) declined. Socio-demographic information can be found in Table 1 . Most respondents (n = 1,234, 72%) were female and average age was 44. Respondents worked in a wide range of healthcare settings including acute care hospitals (n = 1,478), rehabilitation centers (n = 261), long-term care facilities (n = 137), local community health centers and primary care clinics (n = 338). Their occupation spanned the entire spectrum of care, including nurses and orderlies (n = 638), physicians (n = 520), environmental services workers (n = 122), healthcare managers (n = 69) and administrative workers (n = 616). Approximately one quarter (n = 781, 28.3%) had occupational exposure to patients infected with COVID-19.
Table 1.
Predictors of COVID-19 mRNA Vaccine acceptance among 2761 healthcare workers in Montreal, Canada, December 15 to 28, 2020 (Univariate analysis)
| Variables | Respondents (%) | COVID-19 Vaccine acceptance |
|||
|---|---|---|---|---|---|
| (%) | OR | 95% C.I. | P value | ||
| Sex (n = 1,709) | |||||
| Female | 1,234 (72.2) | 77.6 | 1 | ||
| Male | 373 (21.8) | 84.5 | 1.56 | 1.15-2.13 | .005 |
| Unknown | 102 (6.0) | ||||
| Age group (n = 1,709) | |||||
| < 30 y.o. | 244 (14.3) | 75.4 | 1 | ||
| 30-39 y.o. | 413 (24.2) | 74.8 | 0.97 | 0.67-1.40 | .87 |
| 40-49 y.o. | 406 (23.8) | 77.1 | 1.10 | 0.76-1.59 | .62 |
| 50-59 y.o. | 393 (23.0) | 84.0 | 1.71 | 1.15-2.54 | .008 |
| ≥ 60 y.o. | 151 (8.8) | 90.7 | 3.19 | 1.71-5.95 | <.001 |
| Unknown | 102 (6.0) | ||||
| Occupational exposure to patients with COVID-19 (n = 2,761) | |||||
| No | 1,980 (71.7) | 77.1 | 1 | ||
| Yes | 781 (28.3) | 90.5 | 2.84 | 2.19-3.69 | <.001 |
| Type of institution (n = 2,761) | |||||
| Acute care hospitals | 1,478 (53.5) | 81.0 | 1 | ||
| Long-term care facility | 137 (5.0) | 82.5 | 1.11 | 0.70-1.75 | .67 |
| Primary care clinics | 338 (12.2) | 80.8 | 0.99 | 0.73-1.33 | .93 |
| Rehabilitation centers | 261 (9.5) | 83.5 | 1.19 | 0.84-1.69 | .33 |
| Multi-Sites, others, unknown | 547 (19.8) | 79.0 | 0.88 | 0.69-1.13 | .31 |
| Profession (n = 2,761) | |||||
| Nurses and orderlies | 638 (23.1) | 73.7 | 1 | ||
| Physicians* | 520 (18.8) | 95.6 | 7.72 | 4.91-12.16 | <.001 |
| Healthcare managers | 69 (2.5) | 87.0 | 2.38 | 1.16-4.91 | .02 |
| Environmental services workers | 122 (4.4) | 82.8 | 1.72 | 1.04-2.84 | .03 |
| Other healthcare workers† | 687 (24.9) | 78.8 | 1.33 | 1.03-1.71 | .03 |
| Administrative workers | 616 (22.31) | 76.8 | 1.18 | 0.91-1.53 | .20 |
| Unknown | 109 (4.0) | ||||
CI, confidence Interval; OR, odds ratio; y.o., year old.
category including physicians, residents and medical students.
other healthcare workers includes, but is not limited to, pharmacists, radiology technicians, physiotherapists and respiratory therapists.
Variables associated with acceptance of COVID-19 vaccine
By univariate analysis (Table 1), the following factors were significantly associated with acceptance of the COVID-19 vaccine (P < .05): male sex, age over 50 years old, and occupational exposure to patients infected with COVID-19. Also, physicians, healthcare managers, environmental services workers, and other HCWs were more likely to accept the vaccine compared with nurses and orderlies. The variables associated with near universal (>90%) acceptance of the vaccine were age over 60 (91% acceptance; OR, 3.2; 95% CI, 1.7-5.9; P < .001); physicians (96% acceptance; OR, 7.7; 95% CI, 4.9-12.2; P < .001) and occupational exposure to COVID-19 infected patients (91% acceptance; OR, 2.8; 95% CI, 2.2-3.7; P < .001). By contrast, there was no association between the type of workplace and acceptance of the vaccine.
By multivariate analysis (Table 2 ), male sex, age over 50 years old, occupational exposure to patients infected with COVID-19, and work in rehabilitation centers were independently associated with acceptance of the COVID-19 vaccine.
Table 2.
Predictors of COVID-19 mRNA vaccine acceptance (multivariate analysis)
| Variables | COVID-19 vaccine acceptance (n = 1709) |
P value | ||
|---|---|---|---|---|
| aOR | 95% C.I. | |||
| Sex* | ||||
| Male | 1.62 | 1.16 | 2.26 | .004 |
| Age group | ||||
| <30 y.o. | 1 | |||
| 30-39 y.o. | 0.98 | 0.68 | 1.43 | .93 |
| 40-49 y.o. | 1.04 | 0.71 | 1.52 | .86 |
| 50-59 y.o. | 1.62 | 1.07 | 2.44 | .02 |
| ≥ 60 y.o. | 3.28 | 1.74 | 6.18 | <.001 |
| Occupational exposure to patients with COVID-19 | ||||
| Yes | 3.88 | 2.29 | 6.58 | <.001 |
| Type of Institution | ||||
| Acute care hospitals | 1 | |||
| Rehabilitation centers | 1.76 | 1.17 | 2.66 | .007 |
| Long-term care facilities | 1.47 | 0.85 | 2.55 | .17 |
| Primary care clinics | 1.27 | 0.87 | 1.86 | .22 |
| Multi-Sites, others, unknown | 1.20 | 0.86 | 1.66 | .28 |
| Profession† | ||||
| Nurses and orderlies | 1 | |||
| Other healthcare workers | 1.06 | 0.71 | 1.57 | .78 |
| Administrative workers | 0.91 | 0.61 | 1.34 | .62 |
| Healthcare managers | 1.82 | 0.83 | 4.00 | .14 |
| Environmental services workers | 0.93 | 0.50 | 1.74 | .83 |
aOR, adjusted odds ratio; CI, Confidence Interval; y.o., year old.
Reference group female.
Physicians were excluded from this multivariate analysis due to missing data.
Reasons for refusal of COVID-19 vaccine
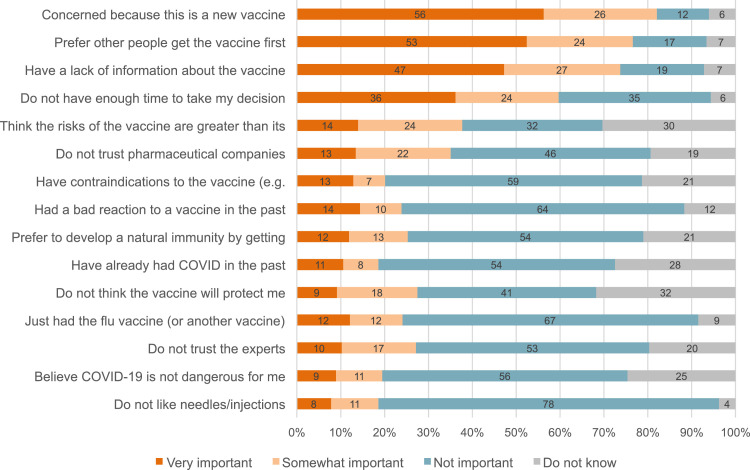
The reasons provided for refusing the vaccine are presented in Figure 1 . Of the 15 reasons provided to vaccine refusers so as to assess potential reasons underlying their decision, 4 (27%) were identified as “important” or “very important” by more than half of those who refused the vaccine. These included: the concern that this vaccine is new (82% agreement); the preference to let other people receive the vaccine first (77% agreement); the lack of available information about the vaccine (74% agreement); and the lack of time to make their decision (60% agreement). Notably, a lack of trust in pharmaceutical companies and in experts was mentioned by 35% and 27% of vaccine refusers, respectively. A quarter of respondents believed that they would prefer to develop immunity by contracting the COVID-19 virus rather than through vaccination.
Fig 1.
Distribution of reasons for refusal by level of importance among healthcare workers who refused to be vaccinated. Reasons are presented as percentages in decreasing frequency of “very important” answers.
Vaccine hesitants vs firm refusal among current refusers
Of the 528 respondents who declined vaccination, most (391 respondents; 74.1%) indicated that they may accept receiving it in the future (ie, vaccine hesitants). Of these, approximately half (53.2%) declared that they would be open to receive it in a few months; and one third (31.9%) reported that they would be open to receive it next year. Only a quarter (137 respondents, 25.9%) mentioned they do not intend to ever receive the vaccine (ie, firm refusers).
Globally, firm refusers provided more reasons to decline vaccination than hesitants (mean number of “somewhat important” or “very important” reasons: 6.6 vs 5.4, respectively, P value <.001). Vaccine hesitants and firm refusers also differed markedly in their motives for currently refusing the vaccine (Table 3 ). Hesitants were significantly more likely to mention the desire to let other people get the vaccine first, the lack of information, and the lack of time to make their decision as “somewhat important” or “very important” reasons to decline vaccination, compared to firm refusers. In contrast, firm refusers were more likely than hesitants to mention a lack of trust in experts and in pharmaceutical companies, to report a perception that the risks of the COVID-19 vaccine are greater than its benefits, to a hold the belief that COVID-19 is not dangerous or that the vaccine will not protect them, to declare that they prefer to develop a natural immunity by getting COVID-19, to report a history of adverse reactions to vaccines or to report having a contraindication to the vaccine. Firm refusers were also more likely to report an aversion to needles and injections. No association was found between hesitants and firm refusers in terms of status and socioprofessional characteristics (sex, age, profession, type of establishment).
Table 3.
Comparison of reasons for non-vaccination considered important between firm refusers and vaccine hesitants
| Reason | If you refuse now, would you be interested in receivingthe vaccine later? |
||
|---|---|---|---|
| NoFirm RefusersN (%)*N = 137 | YesHesitantsN (%)*N = 391 | P value | |
| Have already had COVID in the past | 31 (31.0) | 67 (23.7) | .3386 |
| Just had the flu vaccine (or another vaccine) | 20 (16.5) | 107 (29.6) | .005 |
| Prefer other people get the vaccine first | 81 (70.4) | 323 (85.5) | <.0001 |
| Have a lack of information about the vaccine | 86 (69.9) | 303 (82.6) | .004 |
| Do not like needles/injections | 39 (30.7) | 59 (15.5) | <.0001 |
| Did not have enough time to take my decision | 67 (53.2) | 248 (66.7) | .01 |
| Do not trust the experts | 64 (59.3) | 79 (25.0) | <.0001 |
| Do not trust big pharma companies | 68 (60.7) | 117 (37.3) | <.0001 |
| Think the risks of the vaccine are greater than its benefits | 74 (74.0) | 125 (46.6) | <.0001 |
| Do not think the vaccine will protect me | 61 (65.6) | 84 (31.5) | <.0001 |
| Be concerned because this is a new vaccine | 107 (85.6) | 326 (87.8) | .6142 |
| Prefer to develop a natural immunity by getting COVID-19 | 67 (64.4) | 67 (21.4) | <.0001 |
| Believe COVID-19 is not dangerous for me | 48 (52.8) | 55 (17.9) | <.0001 |
| Had a bad reaction to a vaccine in the past | 52 (46.0) | 74 (21.0) | <.0001 |
| Have contraindications to the vaccine (eg. allergy) | 36 (37.5) | 70 (21.9) | .0002 |
Proportion of Somewhat important / Very Important after exclusion of "I don't know" category.
Discussion
Vaccine hesitancy may be a significant impediment to controlling the COVID-19 pandemic and the barriers to COVID-19 vaccine uptake among HCWs are not completely understood. In our study, most HCWs accepted to receive the COVID-19 vaccine, but approximately one fifth of respondents refused vaccination. However, most of those who refused were open to vaccination at a later time, and only a small proportion (less than 5% of all respondents) had no intention to ever receive the COVID-19 vaccine. Taken as a whole, these data suggest that the COVID-19 vaccine acceptance among HCWs appears to be higher compared to other adult-administered vaccines. On average, less than 50% of Canadian HCWs receive the influenza vaccine each year.20, 21, 22 While it is still unclear, some speculate that vaccine hesitancy may be context or disease specific and several models have been developed to try and understand the motivations behind vaccine hesitancy.23
To date, few studies have investigated acceptance of the COVID-19 vaccines specifically among HCWs, and most published studies assessed intention rather than actual vaccine uptake.17 , 24, 25, 26 A recent study addressing willingness to accept a future COVID-19 vaccine among physicians and nurses in France, Belgium and Quebec, revealed that 48.6% of participants reported high acceptance, whereas 23% reported moderate acceptance, and 28.4% reported hesitancy or reluctance.17 The most important factors independently associated with hesitancy or reluctance included concerns about the safety of the vaccine developed in an emergency state.17 The factors associated with increased intention included male gender, older age, physician profession and contact with suspected or confirmed COVID-19 cases, findings that were also identified in our study.27 Fewer data are available regarding actual HCWs’ COVID-19 vaccine uptake.28 Vaccine uptake in the early months after vaccine rollout were reported to be 33% in Saudi Arabia, 64% in the U.K. and 70% in Pakistan.28, 29, 30 In a multicenter study of 1398 HCWs in 20 emergency departments in the United States, 94% reported having been offered the vaccine and 86% reported having received it.26 The main reason to decline vaccination was a concern about vaccine safety.26 Vaccine acceptance may, however, vary over time as additional information about risks and safety become more widely available. For example, in the spring of 2020, a survey conducted in Hong Kong revealed that 37% of nurses were hesitant to receive the COVID-19 vaccine.24 In comparison, a study conducted later in time, between March and July 2020 observed a higher vaccine acceptance (76.9%) among 2047 HCWs in France.25
Given the novelty of the vaccine and based on the theory of Diffusion of Innovation, our team suspected that many current vaccine refusers might accept vaccination at a later time.31 Our study identified the desire to let other individuals receive the vaccine first as an important reason to decline vaccination. It also identified 2 subgroups among those who refuse the vaccine: vaccine hesitants and firm refusers, of which the former was more frequent than the latter.16 This suggests that many who hesitate or refuse vaccination initially, could accept vaccination in the future, providing that their reasons for hesitancy are alleviated.16 Based on our findings, the promotion of data reinforcing vaccine safety and the use of positive reinforcement signals such as “I am vaccinated” buttons, could potentially prove useful in encouraging vaccine hesitants to eventually be vaccinated. Additional avenues to pursue in future studies include examining reasons that motivate HCWs to accept vaccination, such as self protection, societal risk, or public acknowledgement of vaccination through social media, as these may prove to be valuable targets in ongoing vaccination campaigns.
To our knowledge, our study is among the first to investigate actual COVID-19 vaccine acceptance among HCWs. In addition, our study provides insight on the reasons for refusing COVID-19 vaccines among HCWs who are hesitant (and who could be motivated toward acceptance), as well as among the minority who are unlikely to change their decision. HCWs are not only among the first to be vaccinated in most jurisdictions, but they are also role models for the general public, therefore their acceptance and recommendation may influence hesitant members of the general population to eventually accept vaccination.32 It is thus crucial that we address barriers to vaccine acceptance in this group. Our findings suggest that providing more information on the safety and efficacy of the new vaccines and promoting positive peer influence could be key in addressing the major concerns of the HCWs who hesitate to be vaccinated.
Our study has limitations, including having been conducted in the early days of the vaccination campaign. Respondents’ beliefs at the time of the survey in December 2020 may not be reflective of current beliefs. Some variables were not available for all respondents, which prevented their inclusion in the multivariate analysis. Futhermore, our study is specific to a certain socio-cultural context. Important sociodemographic and health-related information such as race, education and comorbidity were not collected.33 Finally, response rate in our study is unknown, and a bias may exist in which those who were not willing to accept the vaccine may have been less likely to answer the survey. However, the proportion of respondents who accepted vaccination in our study is similar to other studies performed in Quebec.34 Still, our data could be useful as a “baseline acceptance level” against which future studies could be compared to assess the impact of vaccination promotion strategies.
Conclusion
In conclusion, our study suggests that early on in a vaccination campaign, most HCWs are willing to be vaccinated with the novel mRNA COVID-19 vaccine, whether in the present or in the future, and also identifies several reasons underlying vaccine hesitancy. These findings could be used in the future to tailor communications and promotion campaigns to increase vaccine uptake.
Footnotes
Conflicts of interest: Dr Longtin reported receiving research grants from Merck, Becton Dickinson and Gojo, outside of submitted work. Dr Longtin is a scholar researcher funded by the Fonds de recherche en santé du Québec. All other authors declare having no potential conflicts of interest.
Funding: Centre intégré universitaire de santé et services sociaux centre-ouest-de-l'ile-de-Montréal (CIUSSS) and Institut national de santé publique du Québec (INSPQ). The sponsors had no role in the collection, analysis and interpretation of data, writing of the report, or in the decision to submit the article for publication.
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.ajic.2021.04.079.
Appendix. SUPPLEMENTARY MATERIALS
References
- 1.World Health Organization . World Health Organization; Geneva: 2020. WHO Director-General's Opening Remarks at the Media Briefing on COVID-19 - 11 March 2020.https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11-march-2020 Available at: Accessed March 13, 2021. [Google Scholar]
- 2.World Health Organization . World Health Organization; Geneva: 2021. WHO Coronavirus Disease (COVID-19) Dashboard.https://covid19.who.int/ Available at: Accessed March 13, 2021. [Google Scholar]
- 3.National Advisory Committee on Immunization (NACI) Government of Canada; Ottawa: 2021. Recommendations on the Use of COVID-19 Vaccines. 2021.https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines.html Available at: Accessed March 13, 2021. [Google Scholar]
- 4.National Advisory Committee on Immunization (NACI) Government of Canada; Ottawa: 2020. Guidance on the Prioritization of Key Populations for COVID-19 Immunization. [Google Scholar]
- 5.Macdonald NE. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33:4161–4164. doi: 10.1016/j.vaccine.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 6.Karafillakis E, Dinca I, Apfel F, Cecconi S, Wurz A, Takacs J. Vaccine hesitancy among healthcare workers in Europe: a qualitative study. Vaccine. 2016;34:5013–5020. doi: 10.1016/j.vaccine.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 7.Verger P, Fressard L, Collange F. vaccine hesitancy among general practitioners and its determinants during controversies: a national cross-sectional survey in France. EBioMedicine. 2015;2:891–897. doi: 10.1016/j.ebiom.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kun E, Benedek A, Meszner Z. Vaccine hesitancy among primary healthcare professionals in Hungary. Orv Hetil. 2019;160:1904–1914. doi: 10.1556/650.2019.31538. [DOI] [PubMed] [Google Scholar]
- 9.AlMarzooqi LM, AlMajidi AA, AlHammadi AA, AlAli N, Khansaheb HH. Knowledge, attitude, and practice of influenza vaccine immunization among primary healthcare providers in Dubai health authority, 2016-2017. Hum Vaccin Immunother. 2018;14:2999–3004. doi: 10.1080/21645515.2018.1507667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tankwanchi AS, Jaca A, Larson HJ, Wiysonge CS, Vermund SH. Taking stock of vaccine hesitancy among migrants: a scoping review protocol. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-035225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Godin G, Belanger-Gravel A, Eccles M, Grimshaw J. Healthcare professionals' intentions and behaviours: a systematic review of studies based on social cognitive theories. Implement Sci. 2008;3:36. doi: 10.1186/1748-5908-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brewer NT, Chapman GB, Rothman AJ, Leask J, Kempe A. Increasing vaccination: putting psychological science into action. Psychol Sci Public Interest. 2017;18:149–207. doi: 10.1177/1529100618760521. [DOI] [PubMed] [Google Scholar]
- 13.Roller-Wirnsberger R, Lindner S, Kolosovski L. The role of health determinants in the influenza vaccination uptake among older adults (65+): a scope review. Aging Clin Exp Res. 2021;33:2123–2132. doi: 10.1007/s40520-021-01793-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comité sur l'immunisation du Québec . Institut National de Santé Publique du Québec; Québec: 2020. Avis Préliminaire sur les Groupes Prioritaires Pour la Vaccination Contre la COVID-19 au Québec.https://www.inspq.qc.ca/publications/3085-groupes-prioritaires-vaccination-covid Available at: Accessed March 11, 2021. [Google Scholar]
- 15.Government of Canada Planning Guidance for Immunization Clinics for COVID-19 Vaccines. Ottawa. 2020 https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/guidance-documents/planning-immunization-clinics-covid-19-vaccines.html Available at: Accessed March 10, 2021. [Google Scholar]
- 16.Dube E, Laberge C, Guay M, Bramadat P, Roy R, Bettinger J. Vaccine hesitancy: an overview. Hum Vaccin Immunother. 2013;9:1763–1773. doi: 10.4161/hv.24657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verger P, Scronias D, Dauby N. Attitudes of healthcare workers towards COVID-19 vaccination: a survey in France and French-speaking parts of Belgium and Canada, 2020. Euro Surveill. 2021;26:2002047. doi: 10.2807/1560-7917.ES.2021.26.3.2002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.2018. Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, and Social Sciences and Humanities Research Council, Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans. [Google Scholar]
- 19.Statistics Canada . Government of Canada; Ottawa: 2004. Policy on Standards. [Google Scholar]
- 20.Gruben V, Siemieniuk RA, McGeer A. Health care workers, mandatory influenza vaccination policies and the law. CMAJ. 2014;186:1076–1080. doi: 10.1503/cmaj.140035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ontario Respiratory Virus Bulletin . Public Health Ontario; Toronto (ON): 2014. [Google Scholar]
- 22.Bish A, Yardley L, Nicoll A, Michie S. Factors associated with uptake of vaccination against pandemic influenza: a systematic review. Vaccine. 2011;29:6472–6484. doi: 10.1016/j.vaccine.2011.06.107. [DOI] [PubMed] [Google Scholar]
- 23.Betsch C, Schmid P, Heinemeier D, Korn L, Holtmann C, Böhm R. Beyond confidence: development of a measure assessing the 5C psychological antecedents of vaccination. PLOS ONE. 2018;13 doi: 10.1371/journal.pone.0208601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwok KO, Li KK, Wei WI, Tang A, Wong SYS, Lee SS. Editor's Choice: influenza vaccine uptake, COVID-19 vaccination intention and vaccine hesitancy among nurses: a survey. Int J Nurs Stud. 2021;114 doi: 10.1016/j.ijnurstu.2020.103854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gagneux-Brunon A, Detoc M, Bruel S. Intention to get vaccinations against COVID-19 in French healthcare workers during the first pandemic wave: a cross-sectional survey. J Hosp Infect. 2021;108:168–173. doi: 10.1016/j.jhin.2020.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schrading WA, Trent SA, Paxton JH. Academic Emergency Medicine; 2021. Vaccination Rates and Acceptance of SARS-CoV-2 Vaccination Among US Emergency Department Health Care Personnel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galanis PA, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Intention of health care workers to accept COVID-19 vaccination and related factors: a systematic review and meta-analysis. medRxiv. 2020 2020.12.08.20246041. [Google Scholar]
- 28.Martin CA, Marshall C, Patel P. Association of demographic and occupational factors with SARS-CoV-2 vaccine uptake in a multi-ethnic UK healthcare workforce: a rapid real-world analysis. medRxiv. 2021 doi: 10.1371/journal.pmed.1003823. 2021.02.11.21251548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malik A, Malik J, Ishaq U. Acceptance of COVID-19 vaccine in Pakistan among health care workers. medRxiv. 2021 doi: 10.1371/journal.pone.0257237. 2021.02.23.21252271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barry M, Temsah M-H, Aljamaan F. COVID-19 vaccine uptake among healthcare workers in the fourth country to authorize BNT162b2 during the first month of rollout. medRxiv. 2021 doi: 10.1016/j.vaccine.2021.08.083. 2021.01.29.21250749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers EM. 3rd ed. The Free Press; New York, NY: 1983. Diffusion of Innovations. [Google Scholar]
- 32.Yaqub O, Castle-Clarke S, Sevdalis N, Chataway J. Attitudes to vaccination: a critical review. Soc Sci Med. 2014;112:1–11. doi: 10.1016/j.socscimed.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 33.Alley SJ, Stanton R, Browne M. As the pandemic progresses, how does willingness to vaccinate against COVID-19 evolve? Int J Environ Res Public Health. 2021;18:797. doi: 10.3390/ijerph18020797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dionne M, Dubé E, Hamel D, Rochette L, Tessier M. Institut national de santé publique du Québec: Gouvernement du Québec; 2021. COVID-19 - Pandémie et vaccination; p. 7.https://www.inspq.qc.ca/covid-19/sondages-attitudes-comportements-quebecois/vaccination/fevrier-2021 Available at: Accessed April 10, 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.