Abstract
The COVID-19-related death rate varies between countries and is affected by various risk factors. This multicenter registry study was designed to evaluate the mortality rate and the related risk factors in Turkey. We retrospectively evaluated 1500 adults with COVID-19 from 26 centers who were hospitalized between March 11 and July 31, 2020. In the study group, 1041 and 459 cases were diagnosed as definite and highly probable cases, respectively. There were 993 PCR-positive cases (66.2%). Among all cases, 1144 (76.3%) were diagnosed with non-severe pneumonia, whereas 212 (14.1%) had severe pneumonia. Death occurred in 67 patients, corresponding to a mortality rate of 4.5% (95% CI:3.5–5.6). The univariate analysis demonstrated that various factors, including male sex, age ≥65 years and the presence of dyspnea or confusion, malignity, chronic obstructive lung disease, interstitial lung disease, immunosuppressive conditions, severe pneumonia, multiorgan dysfunction, and sepsis, were positively associated with mortality. Favipiravir, hydroxychloroquine and azithromycin were not associated with survival. Following multivariate analysis, male sex, severe pneumonia, multiorgan dysfunction, malignancy, sepsis and interstitial lung diseases were found to be independent risk factors for mortality. Among the biomarkers, procalcitonin levels on the 3rd-5th days of admission showed the strongest associations with mortality (OR: 6.18; 1.6–23.93). This study demonstrated that the mortality rate in hospitalized patients in the early phase of the COVID-19 pandemic was a serious threat and that those patients with male sex, severe pneumonia, multiorgan dysfunction, malignancy, sepsis and interstitial lung diseases were at increased risk of mortality; therefore, such patients should be closely monitored.
Keywords: COVID-19 deaths, In-hospital mortality, Risk factors
1. Introduction
The new coronavirus disease, COVID-19, caused by SARS-CoV-2, has become an unprecedented threat worldwide. As of January 20, 2021, 94,124,612 cases had been reported, resulting in 2,034,527 deaths worldwide [1]. Turkey announced its first case of COVID-19 on March 10, 2020, on the same date as the World Health Organization (WHO) declared the situation a pandemic.
By the date of this report (January 20, 2021), a total of 2,399,781 confirmed cases had been diagnosed in Turkey, resulting in 24,328 deaths, for a case-fatality rate of 1% in Turkey, according to the Turkish Ministry of Health data [2]. The case fatality rates for other countries are 1.7% for the United States of America (USA), 2.6% for the United Kingdom (UK) and 2.23% for the overall world. Mexico shows the highest case-fatality rate in the world (8.6%), followed by Italy, Indonesia, and South Africa [3,4]. The prevalence and death rates vary depending on the geographical area, the transmission rate of the virus, the percentage of the population vulnerable to the infection, the power of the community preventive measures, and the strength of the health care system, i.e., the size of the healthcare workforce, the preparedness of the healthcare system, the state of function of the primary healthcare network, and the numbers of hospital and intensive care unit (ICU) beds, ventilators and health care workers [[5], [6], [7]]. Countries with lower death rates have broad access to testing, utilize comprehensive mitigation measures and have powerful health care resources [6,7].
One additional explanation for the reported variation in death rates is the differences in the definition and reporting of the COVID-19 cases and the related deaths [8]. Currently, the cases presented on the WHO dashboard include only real-time polymerase chain reaction (RT-PCR)-positive cases, which may result in an underestimation of the real problem [1]. However, in clinical practice, the health system has been witnessing a substantial number of PCR-negative cases, who have clinical and radiographic features consistent with COVID-19 and are treated as such [[8], [9], [10]]. The estimated rates of false-negative PCR tests vary between 2-29% [10]. The accurate burden of the global pandemic would be described better by evaluating the excess mortality rate. Between March and August, US data showed a 20% increase in all-cause mortality compared with the number of expected deaths [11].
Although several papers have reported death rates and risk factors worldwide, we still do not have detailed information on the characteristics of proven or highly suspected COVID-19 cases in Turkey or on the predictors of mortality. TTD-TURCOVID 19 is a registry that contains data from 26 centers in 16 different provinces and may thus be considered representative of Turkey.
2. Material and methods
2.1. Study design
This multicenter, multidisciplinary registry study was approved by the Institutional Review Board (IRB) of Gazi University Faculty of Medicine (356/22.05.2020). The study is supported by the Turkish Thoracic Society (TTS).
The study analyzed retrospectively collected data from the hospital records to evaluate the clinical outcomes of hospitalized patients. No informed consent was required, because of the retrospective design of the study. All patients were managed according to the guidelines of the Ministry of Health of Turkey [12]. The presenting symptoms and clinical findings, comorbidities, main radiological findings, routine laboratory findings, drugs used in the treatment and clinical outcomes were recorded.
2.2. Sample size determination
The minimum required sample size for each center was calculated as 35 based on an expected mortality rate of 2.31%, with a precision of 5% and 95% confidence [13].
2.3. Case definition
The case definition was based on the WHO COVID-19 case definition sheet. Accordingly, a proven case was defined as the presence of a positive nucleic acid amplification test or a positive rapid antigen detection test together with clinical and radiographic findings that were strongly suggestive of COVID-19 [14]. Highly probable cases presented with similar clinical and radiographic findings but could not be confirmed with an RT-PCR test.
2.4. Recordings and coding
The symptoms and vital signs were coded according to the attending doctors' evaluation. Information about comorbidities was obtained from the patients’ self-declaration. Computerized tomography was coded as compatible with COVID-19, noncompatible with COVID-19 and uncertain according to the British Society of Thoracic Imaging [15].
All clinical, laboratory and radiographic findings registered at baseline, i.e. at the time of admission to the emergency department or to the ward, were used for analysis.
2.5. Final disease spectrum and mortality
The final diagnoses were made according to previously published guidelines [14,[16], [17], [18], [19]]: 1) Asymptomatic cases: PCR-positive cases with no symptoms. These cases were identified during contact tracing, mostly among health care workers. 2) Acute respiratory disease: Patients with mild acute respiratory symptoms without any signs of pneumonia. 3) Pneumonia: Patients with clinical signs of pneumonia with no signs of severe pneumonia. 4) Severe pneumonia: Pneumonia fitting any one of the following conditions: respiratory rate ≥30 breaths/min; SpO2 ≤ 90%; and PaO2/FiO2 ≤ 300 mmHg. 5) ARDS: Clinical findings meeting the Berlin 2012 ARDS diagnostic criteria [17]. 6) Multiple organ dysfunction syndrome: The presence of altered function involving at least two or more organ systems in an acutely ill patient such that homeostasis cannot be maintained without intervention [18]. 7) Sepsis: acute life-threatening organ dysfunction caused by a dysregulated host response to suspected or proven infection [16]. 8) Septic Shock: sepsis with persistent hypotension despite volume resuscitation, requiring vasopressors to maintain a mean arterial pressure (MAP) ≥65 mmHg and a serum lactate level >2 mmol/L [16]. 9) Macrophage activation syndrome (MAS): defined based on (1) worsening respiratory status defined as increased oxygen supplementation required to maintain SpO2 >93% and (2) elevation of inflammatory parameters adopted by the definition of rheumatic conditions [19].
More than one of these conditions could be registered in the database. The vital status at the end of the hospitalization period was recorded to determine the in-hospital mortality.
2.6. Laboratory findings
Four centers did not give consent to the collection and use of the biochemistry data. Detailed data on microbiology and radiology were not within the scope of this study and were not analyzed.
Hemogram, routine biochemistry, ferritin, D-dimer, C-reactive protein (CRP) and procalcitonin results were recorded.
2.7. Study period and the site distribution
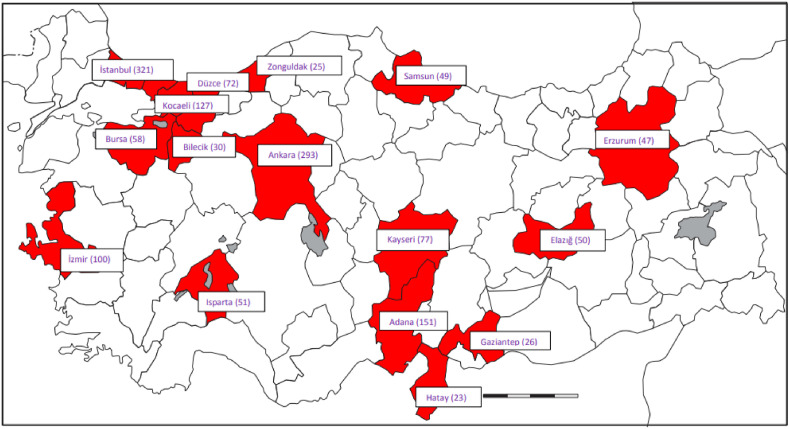
Patients admitted to the hospital between March 11 and July 18, 2020 were included in the study. There were 26 centers (17 university hospitals, 2 large tertiary hospitals, 2 secondary care hospitals and 5 private hospitals) located in 16 different provinces contributing a total of 1500 cases (Fig. 1 ). The differences in the numbers of included patients from different provinces mainly reflect the differences in the populations of those provinces.
Fig. 1.
Distribution of the 26 participating centers.
2.8. Data management
The data were recorded in an internet-based database by the physicians and were rechecked for correctness with the source documents prior to the statistical analysis.
2.9. Statistical analysis
The normality of the numerical variables was evaluated by the Shapiro-Wilk test. The Mann-Whitney U test was performed to compare survivors and non-survivors for nonnormal numerical variables. Cohen's ″d″ effect size was calculated for numerical variables. Cohen suggested that d = 0.2 be considered a ‘small’ effect size, 0.5 represents a ‘medium’ effect size and 0.8 a ‘large’ effect size [20]. Univariate and binary logistic regression analyses were performed to estimate crude and adjusted odds ratios (aORs) and 95% confidence intervals (CIs). In the multivariate analysis for biochemical, laboratory and clinical parameters, variables were selected based on significance in the univariate analysis and LASSO (least absolute shrinkage and selection operator) regression. VIF (variance inflation factor) values were calculated to evaluate multicollinearity. LASSO regression was performed by the glmnet library in R package version 4.03, and for all other statistical analyses, SPSS for Windows version 22 was used. A p value < 0.05 was considered statistically significant.
3. Results
Among the 1500 patients, 57% were male, and the mean age was 51.89 ± 17.65 years. A total of 15% were active smokers, and 120 (8%) were asymptomatic. Among the asymptomatic patients, 62 were hospitalized for the purpose of isolation and observation, as many centers did at the beginning of the pandemic. The data on the first treatment setting were recorded in 1336 patients. The majority of the cases (n = 1176, 88%) were hospitalized in the wards, 113 (8.5%) of the patients were treated in the community, and 47 (3.5%) were admitted to the ICU. Sixty-seven patients (4.5%) died due to COVID-19. A total of 1041 patients were categorized as proven cases. Among these, 993 were PCR-positive (66.2%). Repetitive positive serology was detected in 48 clinically positive patients despite negative RT-PCR results, and those were labeled proven cases according to the WHO guidelines [21]. The remaining 454 patients (30.3%) presented with clinical and HRCT findings highly suggestive of COVID-19 and were thus labeled highly probable cases.
Chest X-ray was obtained in 1364 (90.9%) patients and was abnormal in 490 (35.9%). HRCT scans were performed in 1495 patients, and 1232 (82.4%) were reported to be abnormal.
Table 1 shows the demographic and clinical findings of the cases according to their vital status. The mortality rate was similar between the proven and highly probable cases. The univariate analysis showed that the following characteristics were associated with higher mortality; male sex, age ≥65 years, active smoking, critical condition and admission to the ICU (Table 1).
Table 1.
Univariate analysis of demographic characteristics at admission.
| Table 1. | Total N (%) |
Survivors N (%) |
Non-survivors N (%) |
OR [95%CI] | P value |
|---|---|---|---|---|---|
| Proven cases/highly probable cases n (%) | 1500 1041/459 |
993 (69.3)/440(30.7) | 48 (71.6)/19(28.4) | 1.19 [0.65–1.93] | 0.684 |
| Sex n (%) | |||||
| Male | 850 (57.0) | 802 (56.4) | 48 (71.6) | 1.96 [1.14–3.36] | 0.015a |
| Female | 640 (43.0) | 621 (43.6) | 19 (28.4) | 1 (reference) | |
| Mean age (years) | 1496 | 50.98 ± 17.24 | 71.3 ± 15.06 | 1.08 [1.06–1.10] | 0.001a |
| Age groups | |||||
| <65 | 1117 (74.7) | 1095 (76.6) | 22 (32.8) | 1 (reference) | |
| ≥65 | 379 (25.3) | 334 (23.4) | 45 (67.2) | 6.71 [3.97–11.33] | 0.001a |
| BMI (kg/m2) | |||||
| <30 | 643 (78.6) | 625 (78.2) | 18 (94.7) | 5.01 [0.66–37.80] | 0.118 |
| ≥30 | 175 (21.4) | 174 (21.8) | 1 (5.3) | 1 (reference) | |
| Mean BMI (kg/m2) | 818 | 26.98 ± 4.56 | 24.41 ± 3.87 | 0.86 [0.76–0.97] | 0.014a |
| Occupation | |||||
| Health care workers | 182 (17.0) | 181 (17.4) | 1 (2.9) | 0.11 [0.01–0.83] | 0.032a |
| Factory workers | 114 (10.6) | 112 (10.8) | 2 (5.7) | 0.36 [0.08–1.54] | 0.168 |
| House wives | 315 (29.4) | 305 (29.4) | 10 (28.6) | 0.66 [0.31–1.4] | 0.278 |
| Others | 462 (43.1) | 440 (42.4) | 22 (62.9) | 1 (reference) | |
| Smoking history | |||||
| Active smokers | 204 (15.0) | 200 (15.3) | 4 (8) | 0.76 [0.26–2.23] | 0.622 |
| Ex-smokers | 256 (18.8) | 233 (17.8) | 23 (46) | 3.77 [2.08–6.84] | 0.001a |
| Never smokers | 901 (66.2) | 878 (67) | 23 (46) | 1 (reference) | |
| Ever smokers | 460 (33.8) | 433 (33) | 27 (54) | 2.38 [1.35–4.20] | 0.003a |
| First treatment setting | |||||
| Community | 113 (8.5) | 113 (8.8) | 0 (0.0) | NC | |
| Non-ICU Hospitalization | 1176 (88.0) | 1130 (88.5) | 46 (78) | 1 (reference) | |
| ICU | 47 (3.5) | 34 (2.7) | 13 (22) | 9.39 [4.65–18.99] | 0.001a |
| Final spectrum of the diseaseb | |||||
| Asymptomatic | 120 (8) | 120 (8.4) | 0 (0) | NC | 0.996 |
| Acute lower respiratory Disease |
30 (2.4) | 30 (2.5) | 0 (0) | NC | 0.998 |
| Pneumonia | 1144 (76.3) | 1082 (75.5) | 62 (92.5) | 4.02 [1.61–10.09] | 0.003a |
| Severe pneumonia | 212 (14.1) | 166 (11.6) | 46 (68.7) | 16.72 [9.73–28.72] | 0.001a |
| ARDS | 34 (2.3) | 21 (1.5) | 13 (19.4) | 16.19 [7.69–34.03] | 0.001a |
| MODs | 18 (1.2) | 4 (0.3) | 14 (20.9) | 94.37 [30.04–296.40] | 0.001a |
| Sepsis | 33 (2.2) | 21 (1.5) | 12 (17.9) | 14.67 [6.87–31.33] | 0.001a |
| Septic shock | 9 (0.6) | 2 (0.1) | 7 (10.4) | 83.47 [16.98–410.39] | 0.001a |
| MAS | 21 (1.4) | 19 (1.3) | 2 (3) | 2.29 [0.52–10.04] | 0.272 |
| Others | 3 (0.2) | 3 (0.3) | 0 (0) | NC | 0.999 |
Significant at the 0.05 level; OR: odds ratio, CI: confidence interval. NC: not calculable. BMI: body mass index. ICU: intensive care unit. ARDS: adult respiratory distress syndrome. MAS: macrophage activation syndrome. MODs: multiorgan dysfunction syndrome.
The authors could choose more than one diagnosis.
The symptoms and signs are displayed in Table 2 . The presence of dyspnea was associated with an increased risk of mortality (OR [95% CI]: 6.53 [3.64–11.71]; p = 0.001) in the univariate analysis. Similarly, increases in body temperature (p = 0.003) and respiratory rate (p = 0.001) were also associated with increased mortality. Initial SaO2 levels were noted in 480 patients, and there was not any difference between the survivor and non-survivor groups. Oxygen therapy was administered to 433 patients. The mean flow rate of the nasal oxygen was 3.41 ± 2.55 L in 255 oxygen users in whom the supplemented oxygen level was recorded. PaO2/FiO2 ratio, which was recorded in a relatively smaller number of patients (n = 266) was found to be associated with mortality (Table 3 ). A total of 23 and 37 patients were treated with high flow oxygen therapy and non-invasive mechanical ventilation (NIMV), respectively. Sixty-nine patients underwent invasive mechanical ventilation, of whom 37 (58.7%) died.
Table 2.
Univariate analysis of symptoms and signs at admission.
| Symptoms and Signs | Total N (%) |
Survivors N (%) |
Non-survivors N (%) |
OR [95%CI] | P value |
|---|---|---|---|---|---|
| Dyspnea | 549 (36.6) | 497 (34.7) | 52 (77.6) | 6.53 [3.64–11.71] | 0.001* |
| Cough | 833 (55.5) | 796 (55.5) | 37 (55.2) | 0.99 [0.6–1.62] | 0.958 |
| Fatigue | 601 (40.1) | 575 (40.1) | 26 (38.8) | 0.95 [0.57–1.56] | 0.829 |
| Muscle aches | 332 (22.1) | 319 (22.3) | 13 (19.4) | 0.84 [0.45–1.56] | 0.582 |
| Headache | 201 (13.4) | 196 (13.7) | 5 (7.5) | 0.51 [0.2–1.28] | 0.152 |
| Nausea-vomiting | 106 (7.1) | 101 (7) | 5 (7.5) | 1.06 [0.42–2.7] | 0.897 |
| Fever | 675 (45) | 638 (44.5) | 37 (55.2) | 1.54 [0.94–2.52] | 0.087 |
| Sputum | 127 (8.5) | 120 (8.4) | 7 (10.4) | 1.28 [0.57–2.85] | 0.552 |
| Diarrhea | 88 (5.9) | 86 (6) | 2 (3) | 0.48 [0.12–2] | 0.315 |
| Throat ache | 201 (13.4) | 197 (13.7) | 4 (6) | 0.4 [0.14–1.11] | 0.077 |
| Child | 96 (6.4) | 93 (6.5) | 3 (4.5) | 0.68 [0.21–2.19] | 0.513 |
| Running nose | 27 (1.8) | 27 (1.9) | 0 (0) | NC | 0.998 |
| Anosmia | 50 (3.3) | 50 (3.5) | 0 (0) | NC | 0.997 |
| Ageusia | 68 (4.5) | 68 (4.7) | 0 (0) | NC | 0.997 |
| Confusion | 20 (1.3) | 13 (0.9) | 7 (10.4) | 12.74 [4.91–33.09] | 0.001* |
| Chest tightness | 63 (4.2) | 63 (4.4) | 0 (0) | NC | 0.997 |
| Others | 174 (11.6) | 165 (11.5) | 9 (13.4) | 0.84 [0.41–1.72] | 0.632 |
| Median (IQR) body temperature (n = 1456) | 36.9 (36.5–37.8) | 36.8 (36.5–37.8) | 37.2 (36.7–38.2) | 1.5 [1.2–1.9] | 0.003* |
| Mean ± SD systolic pressure (n = 1245) | 121.9 ± 17.4 | 122.2 ± 17.2 | 115.1 ± 20.1 | 0.97 [0.95–0.99] | 0.003* |
| Mean ± SD diastolic pressure (n = 1244) | 74.6 ± 11.8 | 75 ± 11.6 | 65.7 ± 11.5 | 0.93 [0.91–0.95] | 0.001* |
| Median (IQR) respiratory rate (n = 1296) | 20 (18–22) | 20 (18–22) | 24 (22–28) | 1.19 [1.14–1.25] | 0.001* |
| Median (IQR) SaO2 (at r.t) (n = 480) | 87.7 (59.5–95.0) | 88.0 (58.3–95.0) | 84.45 (76–93) | 1.01 [0.99–1.02] | 0.247 |
Table 3.
Laboratory parameters at admission that showed a Cohen's size effect over 1.
| Laboratory parameters | Total (n = 1262) | Survivors (n = 1211) | Non-survivors (n = 51) | Cohen's Effect Size |
P value |
|---|---|---|---|---|---|
| LDH (U/l) | 235 (188–308.5) | 233 (186–301) | 403 (274–538) | 1.04 | 0.001a |
| Albumin (g/dl) | 4.03 (3.63–4.34) | 4.08 (3.69–4.35) | 3.29 (2.68–3.6) | 1.54 | 0.001a |
| Neutrophil count/mm3 | 4180 (2955–6005) | 4100 (2930–5810) | 7111 (3880–10650) | 1.10 | 0.001a |
| CRP (mg/l) | 11 (2.7–52) | 10.22 (2.6–44.76) | 108 (54.45–157.5) | 1.31 | 0.001a |
| BUN (mg/dl) | 17 (11–27) | 16 (11–26.95) | 25.9 (19–53) | 1.15 | 0.001a |
| Total protein (g/dl) | 7 (6.57–7.4) | 7 (6.6–7.4) | 6.37 (5.7–6.9) | 1.03 | 0.001a |
| CK (U/l) | 89 (57.5–147.5) | 89 (57–143) | 129 (59–310) | 1.26 | 0.023a |
| Ferritin (ng/ml) | 151 (63.53–353) | 144 (62.5–330) | 625 (204.8–1204) | 1.64 | 0.001a |
| Arterial Blood Gases (n = 416) | |||||
| pH | 7.4 (7.37–7.45) | 7.4 (7.37–7.44) | 7.41 (7.35–7.47) | 0.34 | 0.491 |
| HCO3 (mmol/l) | 24.5 (22.6–26.2) | 24.65 (23–26.4) | 23 (19–24.1) | 0.95 | 0.001a |
| Lactate (mg/dl) | 1.8 (1.3–4.9) | 1.8 (1.3–6.5) | 2 (1.5–3.5) | 0.31 | 0.688 |
| PaO2 (mmHg) | 46 (31–66) | 45.9 (30.45–66.5) | 48.45 (39–61.9) | 0.062 | 0.504 |
| PaCO2 (mmHg) | 39.15 (34–44.9) | 40 (35–45) | 32.05 (28.15–35) | 0.63 | 0.001a |
| PaO2/FiO2 n = 266 | 333.16 (250–400) | 350 (266–400) | 213.5 (119.5–251.25) | 1.82 | 0.001a |
Significant at the 0.05 level; Mann-Whitney U test. Effect size: Cohen's d. LDH: lactate dehydrogenase, CRP: C-reactive protein, BUN: blood urea nitrogen, CK: creatine kinase. HCO3: Bicarbonate, PaO2: partial oxygen pressure PaCO2: partial carbon dioxide pressure, FiO2: Fraction of inspired oxygen fraction.
The median durations of non-ICU hospital stay and ICU stay were 7 days (12–19 days) and 9 days (16–35 days), respectively. Table 4 shows the comorbidities of the patients. Comorbidities associated with the highest rates of in-hospital mortality were malignancies, immunosuppressive conditions, COPD, heart failure and interstitial lung disease. Lung carcinoma was not different from the other malignancies with respect to mortality risk (OR [95% CI]: 1.40 [0.37–5.33]) (p = 0.622) in the univariate analysis.
Table 4.
Univariate analysis of comorbidities at admission.
|
Table 3. Comorbidities |
Total N (%) |
Survivors n (%) | Non-survivors N (%) |
OR [95%CI] | P value |
|---|---|---|---|---|---|
| Hypertension | 402 (27.4) | 367 (26.2) | 35 (52.2) | 3.08 [1.88–5.05] | 0.001a |
| Asthma | 111 (7.7) | 108 (7.9) | 3 (4.6) | 0.57 [0.17–1.84] | 0.345 |
| Diabetes | 236 (16.3) | 223 (16.1) | 13 (19.7) | 1.28 [0.69–2.39] | 0.439 |
| Atherosclerosis | 145 (10) | 126 (9.1) | 19 (29.2) | 4.12 [2.34–7.26] | 0.001a |
| COPD | 90 (6.2) | 75 (5.4) | 15 (23.1) | 5.24 [2.81–9.76] | 0.001a |
| Chronic hepatic disease | 11 (0.8) | 10 (0.7) | 1 (1.6) | 2.16 [0.27–17.15] | 0.466 |
| Bronchiectasis | 12 (0.8) | 11 (0.8) | 1 (1.5) | 1.93 [0.25–15.21] | 0.531 |
| Heart failure | 64 (4.4) | 53 (3.9) | 11 (16.9) | 5.08 [2.51–10.27] | 0.001a |
| Malignancy | 76 (5.3) | 56 (4.1) | 20 (30.8) | 10.49 [5.81–18.94] | 0.001a |
| Connective tissue disorders | 25 (1.7) | 21 (1.5) | 4 (6.2) | 4.21 [1.4–12.66] | 0.001a |
| Chronic kidney disease | 51 (3.5) | 43 (3.1) | 8 (12.3) | 4.35 [1.96–9.68] | 0.001a |
| Cerebrovascular disease | 49 (3.4) | 45 (3.3) | 4 (6.3) | 1.97 [0.69–5.65] | 0.209 |
| Immunosuppressive conditions | 25 (1.7) | 20 (1.5) | 5 (7.8) | 5.73 [2.08–15.78] | 0.001a |
| Interstitial lung disease | 22 (1.5) | 18 (1.3) | 4 (6.3) | 4.99 [1.64–15.21] | 0.005a |
| Others | 277 (21.4) | 261 (21.1) | 16 (27.6) | 1.42 [0.79–2.57] | 0.243 |
Significant at the 0.05 level; OR: odds ratio, CI: confidence interval. COPD: chronic obstructive pulmonary disease.
The laboratory parameters that met the Cohen's effect size of >1 at admission are shown in Table 3, and the parameters that met the Cohen's size effect of ≥1.5 on days 3–5 of admission are shown in Table 5 . Generally, laboratory findings on days 3–5 appeared to be more strongly related to mortality than their levels at admission. Accordingly, C-reactive protein (CRP), ferritin, and albumin levels at admission and procalcitonin, ferritin, lactate dehydrogenase (LDH), and CRP levels on days 3–5 were highly predictive of mortality in univariate analysis.
Table 5.
Laboratory parameters on the 3rd-5th days after admission that showed a Cohen's size effect ≥1.5.
| Laboratory parameters on 3–5th days of admission | Total (n = 1262) | Survivors (n = 1211) | Non-survivors (n = 51) | Cohen's Effect size |
P value |
|---|---|---|---|---|---|
| Neutrophils (%) | 60.75 (52.7–70) | 60.1 (52–69) | 85 (77.7–90.3) | 1.67 | 0.001a |
| Lymphocytes (%) | 26.8 (18–34.3) | 27 (18.7–34.53) | 7.2 (4.3–12.4) | 1.50 | 0.001a |
| LDH (U/l) | 230 (180–311) | 227 (178–303) | 407.5 (328.5–575) | 1.87 | 0.001a |
| CRP (mg/l) | 11 (2.78–48.5) | 10 (2.6–43.65) | 112.5 (53–228.5) | 1.99 | 0.001a |
| Procalcitonin (ng/ml) | 0.06 (0.03–0.12) | 0.05 (0.03–0.12) | 0.78 (0.35–4.68) | 2.31 | 0.001a |
| D-dimer (mcg/ml) | 0.45 (0.28–0.94) | 0.44 (0.27–0.87) | 2.33 (0.91–4.82) | 1.52 | 0.001a |
| CK (U/l) | 66 (43–105) | 65 (43–100) | 178 (27.3–483) | 1.57 | 0.012a |
| Ferritin (ng/ml) | 189 (80.28–419) | 185.05 (79–387.05) | 677 (439–1596) | 1.92 | 0.001a |
Significant at 0.05 level; Mann-Whitney U test. Effect size: Cohen's d. LDH: lactate dehydrogenase, CRP: C-reactive protein, BUN: blood urea nitrogen, CK: creatine kinase.
After adjusting for age, sex, body mass index (BMI), disease severity, and the 5 most frequent comorbidities, in addition to procalcitonin for the antibiotic data, none of the drugs, including hydroxychloroquine, favipiravir, azithromycin or low-molecular-weight heparin (LMWH), were found to be related to survival (Table 6 ).
Table 6.
Adjusted data on drugs in relation with mortality.
| Total n (%) | Survivors n (%) | Non-survivors n (%) | OR [95%CI] | P adjusted | |
|---|---|---|---|---|---|
| Anti-virals | |||||
| Oseltamivir | 761 (54.9) | 721 (54.6) | 40 (61.) | 0,96 [0.32–2.85] | 0.946 |
| Lopinavir | 55 (4.4) | 48 (4) | 7 (14) | 2.3 [0.37–14.51] | 0.375 |
| Favipravir | 328 (25.1) | 289 (23.2) | 39 (62.9) | 1.88 [0.59–6] | 0.286 |
| Antibiotics | |||||
| Beta-lactams | 176 (14.2) | 169 (14.2) | 7 (14.3) | 0.68 [0.12–4] | 0.67 |
| Beta-lactam + beta-lactamase inhibitors | 67 (5.4) | 63 (5.3) | 4 (8.2) | 7.65 [0.6–97.76] | 0.118 |
| Doxycycline | 14 (1.1) | 14 (1.2) | 0 (0) | 0 [0 -0] | 1 |
| Clarithromycin | 35 (2.8) | 30 (2.5) | 5 (9.8) | ||
| Fluoroquinolones | 360 (27.6) | 336 (26.9) | 24 (46.2) | 0.4 [0.08–1.95] | 0.26 |
| Beta-lactams | 139 (11.1) | 112 (9.3) | 27 (47.4) | 1.73 [0.37–8.12] | 0.489 |
| Carbapenems | 96 (7.7) | 69 (5.8) | 27 (50) | 1.3 [0.2–8.46] | 0.785 |
| Immunomodulators | |||||
| Hydroxychloroquine | 1382 (93.6) | 1320 (93.6) | 62 (93.9) | 1.04 [0.1–10.82] | 0.971 |
| Azitromycine | 738 (54.7) | 704 (54.6) | 34 (57.6) | 1.54 [0.48–4.98] | 0.472 |
| Systemic steroids | 68 (5.6) | 58 (5) | 10 (20) | 0.31 [0.03–2.75] | 0.291 |
| Tocilizumab | 56 (4.6) | 50 (4.3) | 6 (12) | 0.24 [0.03–2.11] | 0.198 |
| Anticoagulants | |||||
| Low-molecular weight heparin | 911 (63.3) | 854 (62.1) | 57 (89.1) | 0.6 [0.14–2.64] | 0.497 |
| New oral anticoagulants | 31 (2.5) | 29 (2.5) | 2 (4.1) | 0.98 [0.15–6.22] | 0.983 |
3.1. Multivariate binary logistic regression analysis for mortality
A multivariate model with 1228 cases was tested, including clinical parameters, disease spectrum and comorbidities. Septic shock was excluded because of interference with sepsis. The laboratory parameters at admission and in the follow-up (the 3–5 days of admission) were tested in the models with 457 and 323 patients, respectively. Table 7 shows the tested parameters. Due to the large size of missing data, ferritin and PaO2/FiO2 ratio were not included in the model.
Table 7.
Clinical and laboratory parameters included in the logistic regression analysis.
| Clinical parameters | Adjusted OR [95% CI] | P |
|---|---|---|
| Age | 1.09 [1.06–1.12] | 0.001a |
| Sex (male vs female) | 2.47 [1.05–5.82] | 0.038a |
| Severe pneumonia | 7.56 [3.29–17.34] | 0.001a |
| Multiple organ dysfunction syndrome | 22.3 [4.05–122.94] | 0.001a |
| Interstitial lung disease | 5.27 [1.17–23.8] | 0.031a |
| Malignancy | 19.99 [8.14–49.1] | 0.001a |
| Sepsis | 8.13 [2.2–30.1] | 0.002a |
| Dyspnea | 1.77 [0.76–4.11] | 0.183 |
| Respiratory rate/min | 1.02 [0.94–1.1] | 0.634 |
| Laboratory parameters at admission | OR [95% CI] | P |
| Lymphocyte count | 1 [1–1] | 0.502 |
| D-dimer | 1.2 [0.93–1.54] | 0.162 |
| Procalcitonin | 1.01 [0.99–1.03] | 0.390 |
| BUN | 1.02 [1.01–1.03] | 0.008a |
| Albumin | 0.15 [0.07–0.33] | 0.001a |
| Laboratory parameters on days 3–5 of admission | OR [95% CI] | P |
| Lymphocyte count | 1 [1–1] | 0.449 |
| D-dimer | 1.54 [1.15–2.05] | 0.004a |
| Procalcitonin | 6.18 [1.6–23.93] | 0.008a |
| BUN | 1.01 [0.99–1.02] | 0.492 |
| Albumin | 0.37 [0.13–1.08] | 0.069 |
Significant at the 0.05 level; OR: odds ratio, CI: confidence interval. BUN: blood urea nitrogen.
3.2. Detailed evaluation of the procalcitonin-mortality relationship
Antibiotics were used in 693/1500 (46%) of the patients. Eighty-eight percent of the non-survivors and 44% of the survivors used antibiotics, respectively. In the crude analysis, antibiotic use was associated with a 9.29-fold increase in the mortality rate. Procalcitonin levels were measured twice (at admission and on the 3rd-5th day). At admission, the mean procalcitonin levels were 1.0 ± 9.50 ng/ml in patients, who were treated with antibiotics and 0.19 ± 1.32 ng/ml in patients, who were not (p = 0.001), respectively. The mean follow-up procalcitonin levels were 0.5 ± 2.27 ng/ml and 0.11 ± 0.20 ng/ml, respectively (p = 0.005). When adjusted for antibiotic use, the relationship between baseline procalcitonin levels and mortality was no longer significant (p = 0.327, OR: 1.011); however, the association between the follow-up procalcitonin levels and mortality remained significant (p = 0.001, OR: 6.18 [1,6–23,93]).
4. Discussion
The main finding of this study was that the in-hospital mortality rate was 4.5% in Turkey. The independent predictors of mortality were older age, male sex, concomitant malignancy, interstitial lung disease, severe disease, sepsis, MODs, increased BUN and decreased albumin levels at admission and increased D-dimer and procalcitonin levels during follow-up. The follow-up biomarker levels showed a larger effect size, indicating a stronger relationship with mortality. The antivirals, hydroxychloroquine and azithromycin were not associated with survival.
Previous observational studies in Turkey reported mortality rates that varied between 8.5% and 50% according to the healthcare setting, age of the study population, and disease severity [[22], [23], [24], [25], [26]]. These studies were mostly conducted with a limited number of patients in a single-center setting. One large study, which used the Ministry of Health records and included 16,942 hospitalized elderly individuals only, reported mortality rates varying between 17.9-32.2% depending on the time periods with and without public restrictions [26]. However, the study provided no clinical details of the cases. Data from the rest of the world show that the mortality rates vary between 2.3-49% depending on age and health resource utilization, very similar to the Turkish data [13,[27], [28], [29], [30], [31], [32]]. In our study, which included data from 26 centers, the population was not restricted to severe patients but consisted of a wide spectrum of patients, mostly including mild to moderate pneumonia. This may explain the relatively low mortality rate compared to previous reports from Turkey [22,26] and those from other countries [28,31,33]. To the best of our knowledge, this is the first multicenter nationwide onsite dataset from Turkey for the first wave of the pandemic.
Several studies have investigated risk factors for mortality [13,22,23,[25], [26], [27], [28],[31], [32], [33]]. Older age is the best-known predictor of mortality, and countries with large elderly populations have been shown to have higher case-fatality rates [13,27,[30], [31], [32], [33]]. In Turkey, the in-hospital mortality rate for patients aged 80 years and older was reported to be 32.8% [26]. Similarly, the mean ages of the non-survivors and survivors in our study were 71.3 and 50.9 years, respectively, indicating that age was an independent variable related to mortality. Several age-related conditions, including frailty, comorbidities and immunosenescence, possibly have roles in the increased risk.
Male sex is another frequently reported predictor of mortality. Two reports from China showed that men with COVID-19 were at greater risk for worse outcomes and death, independent of age [28,34]. Our data complement these findings, indicating that male sex is an independent risk factor associated with higher mortality.
Other clinical parameters, including symptoms and signs of the initial presentation, were also tested. Using machine learning technology, Yadaw et al. [31] reported findings from a large cohort of patients, including inpatients and outpatients, and minimum oxygen saturation, maximum body temperature and hydroxychloroquine use and admission to the hospital were found to be predictors of mortality. Cough, dyspnea and fever (35–40%) were the main symptoms of hospitalized patients with moderate to severe disease in several cohorts [22,23,25], whereas fever was the most frequent symptom (84–88.5%) in the early Chinese series [28,32]. In our study, the most frequent symptoms were cough, history of fever (45%), fatigue and dyspnea (36.6%). The frequencies of fever history and high body temperature were not as high as the Chinese data. The findings of this study differed in that none of these parameters were found to be associated with mortality in the multivariate analysis.
Comorbidities, BMI and smoking status have been widely investigated in previous studies. In the Turkish Ministry of Health database on elderly patients, diabetes mellitus, heart failure, chronic kidney disease, coronary artery disease, dementia and cancer were found to be related to mortality [26]. Our study, which included patients who were all adults, showed that malignancy and interstitial lung disease were associated with mortality. Although interstitial lung disease was diagnosed in very few patients, it remained a significant risk factor. However, there is a need for more observations in patients with interstitial lung disease.
The smoking effect on disease severity and mortality has been another important question during the pandemic. Karanasos et al. [35] showed inconclusive results in the smoking-mortality relationship using retrospective studies in their meta-analysis. They commented that diabetes and age could be confounders of the smoking effect. In Turkey, two studies have been published mainly investigating the effects of smoking [36,37]. Caliskan et al. [36] found that the active smoking rate in 565 COVID-19 patients was 20.9%, and it was associated with an increased mortality risk. However, in another study conducted in 114 hospitalized adults, the active smoking rate was 15.9%, and no relationship was observed between smoking and disease outcomes [37]. In our study, the active smoking rate was lower than that in the general population (15% vs 22.9%). Although active smoking status was not related to mortality, ex-smoking and ever smoking were found to be related to mortality.
Laboratory parameters have been investigated in relation to prognosis. In a comprehensive meta-analysis using 109 published articles, of which 42 studied mortality, Chidambaram et al. [38] analyzed clinical and laboratory parameters in relation to mortality. Leukocytosis (>10.0 × 109/L), lymphopenia (<1.1 × 109/L), and elevated C-reactive protein (>100 mg/L), LDH (>250 U/L) and D-dimer (>1 mg/L) had higher odds of severe disease and a greater risk of mortality. In a meta-analysis with a total of 5350 patients pooled from 25 studies, Huang et al. [39] showed that elevated serum CRP, PCT, D-dimer, and ferritin were associated with a poor outcome in COVID-19. Most of these previous studies were performed using admission data. However, in the current study, BUN and albumin levels at admission and D-dimer and procalcitonin levels at follow-up were found to be significantly associated with mortality. In particular, follow-up procalcitonin levels showed the highest odds ratio for mortality. In accordance with this finding, a meta-analysis of 207 studies showed that procalcitonin had the highest odds for predicting mortality [40]. High procalcitonin levels may be due to bacterial coinfections and lung injury due to cytokine release [41]. Hence, a recent study revealed that blood procalcitonin levels appeared to be disease severity-dependent in the COVID-19 pandemic. Notably, coinfection rates were only 20% and 50% in severe and critically ill patients, whereas high blood procalcitonin levels were 50% and 80%, respectively [42,43].
None of the treatments given to the patients were associated with any survival benefit. The current study reflects the treatment regimens that were used during the first wave of the pandemic. A significant proportion of the patients were treated with hydroxychloroquine. Favipiravir was a second-line agent reserved for more severe patients according to the national guideline. Although systemic corticosteroid therapy is now the mainstay of treatment of patients with respiratory failure, it was not regularly used at the early phase when the evidence was not yet available [44,45]. Anticoagulant therapy was widely used in hospitalized patients in Turkey during the first wave of the pandemic following the publication of early studies showing the presence of coagulopathy in COVID-19 and the decreased risk of mortality with the use of anticoagulants [46]. Antiplatelet therapy, on the other hand, was not part of the treatment protocols during the first wave as it was later shown to be beneficial in improving ventilation/perfusion ratio [47]. Tocilizumab was used in a small number of patients in this study and was not associated with any improvement in survival. Two meta-analyses of studies examining the effectiveness of tocilizumab in COVID-19 have recently been published, with contradictory results (5,6), possibly indicating that patient selection is of utmost importance. For optimal results, cytokine storm needs to be correctly defined using validated criteria (7).
There are some limitations of this study. First, it was retrospectively designed. Therefore, some of the values, particularly BMI, follow-up body temperatures, SpO2 and biochemical parameters, were missing, which prevented more in-depth analyses. Second, the study was conducted at the beginning of the pandemic, and due to policies in effect at that time, the majority of the patients were hospitalized; thus, severe patients may not have been represented well.
In conclusion, in this nationwide retrospective large cohort in Turkey, we have shown that several demographic, clinical and laboratory parameters were associated with mortality and that clinicians should take these into account in the management of COVID-19 patients. It may be useful to construct algorithms on the basis of these findings following their validation in prospective studies.
Author’s contributions
NK, HB, OI, CB, FOE, AS, ECE, BD, and AFK designed the study; NK, CB, PDC, SAB, OK, PA, II, AAY, YS, OBT, EA, SM, CC, AD, BK, BBK, HSO, GO, ZTY, BE, VAO, OK, ME, TUC, OA, ENT, OA, AA, DCB, YTG, FF, FD, NK, MMT, GG, SA, TT, TT, OO, OD, PYG, IB, HB, IKO, SB, BG, FB, OE, IH, HKO, GS, OU, and MA collected the data; SK and SAN analyzed the data; NK, CB, SK, SAN searched the literature and wrote the manuscript; NK, SAB, PDC, BG, OG, EM, MMT, GA and SAB edited and revised manuscript according to journal's instructions; NK, FOE, AS, MMT, OE, BG, YH, ASC and HB edited and controlled the final version of the manuscript. All of the authors approved the final version of the manuscript.
Funding
The study partially funded by The Turkish Thoracic Society.
Disclaimers
The views expressed in this article do not communicate an official position of the Turkish Thoracic Society.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank Turkish Thoracic Society for serving as a base for the setup of the network bringing the investigators of this study together.
References
- 1.WHO . 2021. WHO Coronavirus Disease (COVID-19) Dashboard [Internet]https://covid19.who.int/ [accessed 2021 Jan 20]. Available from: [Google Scholar]
- 2.Ministry of Health Republic of Turkey. COVID-19 web page of the Republic of Turkey. https://covid19.saglik.gov.tr Ministry of Health [Internet]. 2021 [accessed 2021 Jan 20]
- 3.Worldometer Countries where covid-19 has spread [Internet] https://www.worldometers.info/coronavirus/countries-where-coronavirus-has-spread/ [accessed 2021 Jan 20.
- 4.John Hopkins University of Medicine Coronavirus resource center. Mortality analysis. [Internet] https://coronavirus.jhu.edu/data/mortality [accessed 2021 Jan 20.
- 5.Cakir B. COVID-19 in Turkey: lessons learned. J. Epidemiol. Glob. Health. 2020;10:115–117. doi: 10.2991/jegh.k.200520.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matta S., et al. Morbidity and mortality trends of Covid 19 in top 10 countries. Indian J. Tubercul. 2020;67:167–172. doi: 10.1016/j.ijtb.2020.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J., et al. Hospitals' responsibility in response to the threat of infectious disease outbreak in the context of the coronavirus disease 2019 (COVID-19) pandemic: implications for low- and middle-income countries. Glob. Health J. 2020;4:113–117. doi: 10.1016/j.glohj.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bayram H., et al. Interference in scientific research on COVID-19 in Turkey. Lancet. 2020;396:463–464. doi: 10.1016/S0140-6736(20)31691-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Köktürk N., et al. COVID-19 pandemic and the global perspective of Turkish thoracic society. Turk. Thorac. J. 2020;21:419–432. doi: 10.5152/TurkThoracJ.2020.20174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watson J., et al. Interpreting a COVID-19 test result. BMJ. 2020;369 doi: 10.1136/bmj.m1808. [DOI] [PubMed] [Google Scholar]
- 11.Bauchner H., et al. Excess deaths and the great pandemic of 2020. J. Am. Med. Assoc. 2020;324:1504–1505. doi: 10.1001/jama.2020.20016. [DOI] [PubMed] [Google Scholar]
- 12.Ministry of Health Republic of Turkey covid-19 guideline. https://toraks.org.tr/site/community/news/5808 [T.C. Sağlık Bakanlığı COVID-19 Rehberi]. 2 Nisan 2020. [accessed 2020 Dec 27]
- 13.Onder G., et al. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. J. Am. Med. Assoc. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Covid-19 Case Definition. WHO/2019-nCoV/Surveillance_Case_Definition/2020.2 [accessed 2020 Dec 27].
- 15.BSTI Covid-19 guidance for the reporting radiologist https://www.bsti.org.uk/media/resources/files/BSTI_COVID19_Radiology_Guidance version_2_16.03.20.pdf Updated version 2, [accessed 2020 Dec 27]
- 16.World Health Organization. Clinical Management of Covid-19. WHO/2019-nCoV/clinical/2020.5 [accessed 2020 Dec 27].
- 17.Ranieri V.M., et al. Acute respiratory distress syndrome: the Berlin Definition. J. Am. Med. Assoc. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 18.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit. Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 19.Ravelli A., et al. Classification criteria for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a European league against rheumatism/American college of rheumatology/Paediatric rheumatology international trials organisation collaborative initiative. Ann. Rheum. Dis. 2016;75:481–489. doi: 10.1136/annrheumdis-2015-208982. [DOI] [PubMed] [Google Scholar]
- 20.Thompson B. Effect sizes, confidence intervals, and confidence intervals for effect sizes. Psychol. Sch. 2007;44:423–432. [Google Scholar]
- 21.WHO Interim Guideline https://www.who.int/publications/i/item/diagnostic-testing-for-sars-cov-2 Diagnostic testing for SARS-CoV-2. 2020 [accessed 2020 Aug 1]
- 22.Varol Y., et al. The impact of charlson comorbidity index on mortality from SARS-CoV-2 virus infection and a novel COVID-19 mortality index: CoLACD. Int. J. Clin. Pract. 2020 doi: 10.1111/ijcp.13858.e13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altunok E.S., et al. Clinical characteristics of adult patients hospitalized with laboratory-confirmed COVID-19 pneumonia. J. Infect. Chemother. 2021;27:306–311. doi: 10.1016/j.jiac.2020.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kocayigit H., et al. Characteristics and outcomes of critically Ill patients with Covid-19 in Sakarya, Turkey: a single center cohort study. Turk. J. Med. Sci. 2020 doi: 10.3906/sag-2005-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aksel G., et al. Early predictors of mortality for moderate to severely ill patients with Covid-19. Am. J. Emerg. Med. 2020 doi: 10.1016/j.ajem.2020.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esme M., et al. Older adults with coronavirus disease 2019; a nationwide study in Turkey. J. Gerontol. A. Biol. Sci. Med. Sci. 2020 doi: 10.1093/gerona/glaa219.glaa219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bahl A., et al. Early predictors of in-hospital mortality in patients with COVID-19 in a large American cohort. Intern. Emerg. Med. 2020;15:1485–1499. doi: 10.1007/s11739-020-02509-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li L.Q., et al. COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J. Med. Virol. 2020;92:577–583. doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du R.H., et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur. Respir. J. 2020;55:2000524. doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Z., et al. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. J. Am. Med. Assoc. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 31.Yadaw A.S., et al. 2020. Clinical Predictors of COVID-19 Mortality. medRxiv. [DOI] [Google Scholar]
- 32.Sun Y., et al. Characteristics and prognostic factors of disease severity in patients with COVID-19: the Beijing experience. J. Autoimmun. 2020;112:102473. doi: 10.1016/j.jaut.2020.102473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson S., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. J. Am. Med. Assoc. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin J.-M., et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front. Public. Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karanasos A., et al. Impact of smoking status on disease severity and mortality of hospitalized patients with COVID-19 infection: a systematic review and meta-analysis. Nicotine Tob. Res. 2020;22:1657–1659. doi: 10.1093/ntr/ntaa107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caliskan T., et al. Smoking and comorbidities are associated with COVID-19 severity and mortality in 565 patients treated in Turkey: a retrospective observational study. Rev. Assoc. Med. Bras. 2020;66:1679–1684. doi: 10.1590/1806-9282.66.12.1679. [DOI] [PubMed] [Google Scholar]
- 37.Chousein E.G.U., et al. Is there any effect of smoking status on severity and mortality of hospitalized patients with COVID-19 pneumonia? Tuberk. Toraks. 2020;68:371–378. doi: 10.5578/tt.70352. [DOI] [PubMed] [Google Scholar]
- 38.Chidambaram V., et al. Factors associated with disease severity and mortality among patients with COVID-19: a systematic review and meta-analysis. PloS One. 2020;15 doi: 10.1371/journal.pone.0241541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang I., et al. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther. Adv. Respir. Dis. 2020;14 doi: 10.1177/1753466620937175. 1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Izcovich A., et al. Prognostic factors for severity and mortality in patients infected with COVID-19: a systematic review. PloS One. 2020;15 doi: 10.1371/journal.pone.0241955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martins-Filho P.R., et al. Factors associated with mortality in patients with COVID-19. A quantitative evidence synthesis of clinical and laboratory data. Eur. J. Intern. Med. 2020;76:97–99. doi: 10.1016/j.ejim.2020.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu R., et al. Procalcitonin levels in COVID-19 patients. Int. J. Antimicrob. Agents. 2020;56:106051. doi: 10.1016/j.ijantimicag.2020.106051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garrido P., et al. Clinical value of procalcitonin in critically ill patients infected by SARS-CoV-2. Am. J. Emerg. Med. 2020 doi: 10.1016/j.ajem.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salton F., et al. Prolonged low-dose methylprednisolone in patients with severe COVID-19 pneumonia. Open Forum Infect. Dis. 2020;10 doi: 10.1093/ofid/ofaa421. ofaa421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Recovery Collaborative Group. Horby P., et al. Dexamethasone in hospitalized patients with COVID-19 - preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2021436. https://www.ncbi.nlm.nih.gov/pubmed/32678530 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang N., et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemostasis. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Viecca M., et al. Enhanced platelet inhibition treatment improves hypoxemia in patients with severe Covid-19 and hypercoagulability. A case control, proof of concept study. Pharmacol. Res. 2020;158:104950. doi: 10.1016/j.phrs.2020.104950. [DOI] [PMC free article] [PubMed] [Google Scholar]