Highlights
-
•
The occurrence of rectal annular constriction due to infiltration by bladder cancer is relatively rare, and difficult diagnosis by colonoscopy.
-
•
The images including computed tomography, colonoscopy, and magnetic resonance imaging was fully recorded in our report.
-
•
The poor prognosis was noted from literature review with no record for survival >2 years after diagnosis of a rectal lesion.
Abbreviations: LDH, lactate dehydrogenase; AFP, alpha-fetoprotein; CA19-9, carbohydrate antigen 19-9; CT, computed tomography; MRI, magnetic resonance imaging; CK, cytokeratin
Keywords: Invasive urothelial carcinoma, Colonoscopy, Proctitis, Case report
Abstract
Introduction
Bladder urothelial carcinoma rarely spreads to the gastrointestinal tract, and its presentation in the rectum varies. We report a case of a patient who presented with an annular constriction of the rectum.
Presentation of case
A 60-year-old man was referred to our hospital with chief complaints of anal stricture and partial obstruction for about 1 month. Computed tomography and magnetic resonance imaging revealed diffuse wall thickening of the rectum, possible high cellularity in the lower portion of urinary bladder, and lesions in the visible pelvic bony structure. A colonoscopy showed a contiguous annular constriction from 5 to 15 cm above the anal verge. Carcinoembryonic antigen and carbohydrate antigen 19-9 levels were 39.75 ng/mL and 139.2 U/mL, respectively. A transurethral bladder biopsy revealed high-grade urothelial cell carcinoma, and anal biopsy showed a poorly differentiated carcinoma arranged in a small nested pattern within the subepithelial area of the anorectal tissue. A colostomy was performed, and the patient was transferred to another hospital for further treatment after series of survey with lung metastasis.
Discussion
Invasive bladder cancers rarely infiltrates into the rectum and is known with the difficulty diagnosis by colonoscopy. Furthermore, the secondary rectum tumor due to bladder cancer had poor record for survival in the literature review.
Conclusion
This case of bladder urothelial carcinoma penetrating to the rectum was interesting because it mimicked proctitis with diffuse annular swelling observed in the colonoscopy.
1. Introduction
Bladder urothelial carcinoma rarely spreads to the gastrointestinal tract and usually metastasizes to the lymph nodes, liver, lung, and bone. Moreover, it is common to note aggressive local invasion into adjacent organ. We report a case of a patient who presented with a bladder urothelial cancer that resulted in rectal infiltration and partial obstruction mimicking proctitis observed during a colonoscopy. This work has been reported in line with the SCARE criteria [1].
2. Presentation of case
A 65-year-old man was referred to our hospital because of anal stricture with partial obstruction for about 1 month without obvious abnormal findings from a colonoscopy and a biopsy. His history was remarkable for bladder urothelial carcinoma cTisN0M0, high grade status post-transurethral resection of a bladder tumor and two-times instillation chemotherapy with intravesical 81 mg Bacillus Calmette-Guerin once a week 2 years earlier. Physical examination revealed normal vital signs, a soft, non-tender, tympanic abdomen, and no palpable abdominal mass. A digital rectal examination revealed a narrowed rectal lumen with an intact rectal mucosa without red blood on the examining finger. The laboratory exam showed the following results: C-reactive protein, 1.17 mg/dl; normal white blood cell count, 7530/μl; negativity for cytomegalovirus, herpes simplex virus, and treponema pallidum infection; lactate dehydrogenase (LDH), 310 U/L; alpha-fetoprotein (AFP), 3.31 ng/mL; carcinoembryonic antigen, 38.75 ng/mL; carbohydrate antigen 19-9 (CA19-9), 139.2 U/mL; and prostate specific antigen, 0.861 ng/dl. Urinalysis revealed hematuria with red blood cells >100 per high-power field. Urine cytology showed abundant atypical urothelial cells with hyperchromatism.
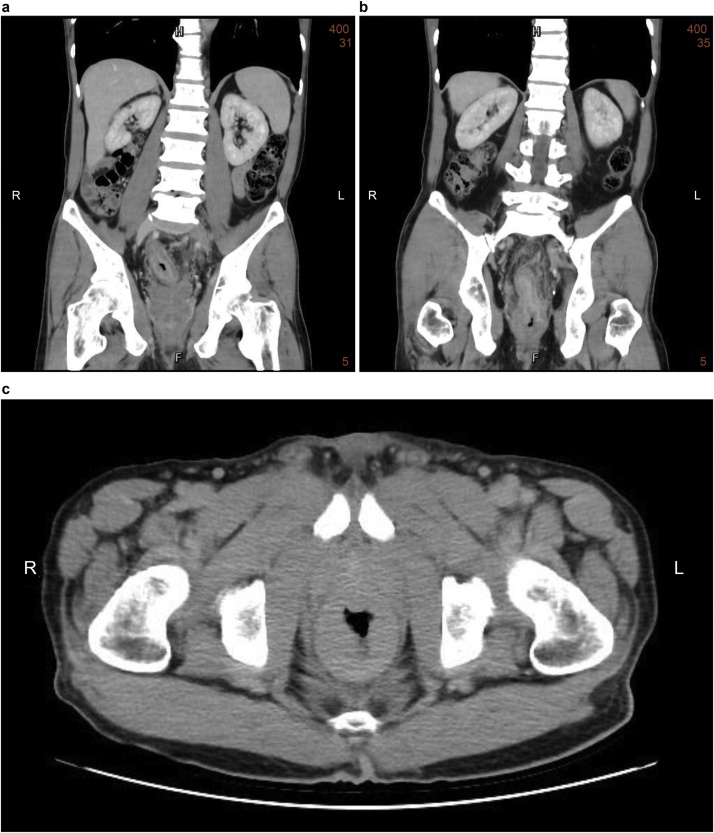
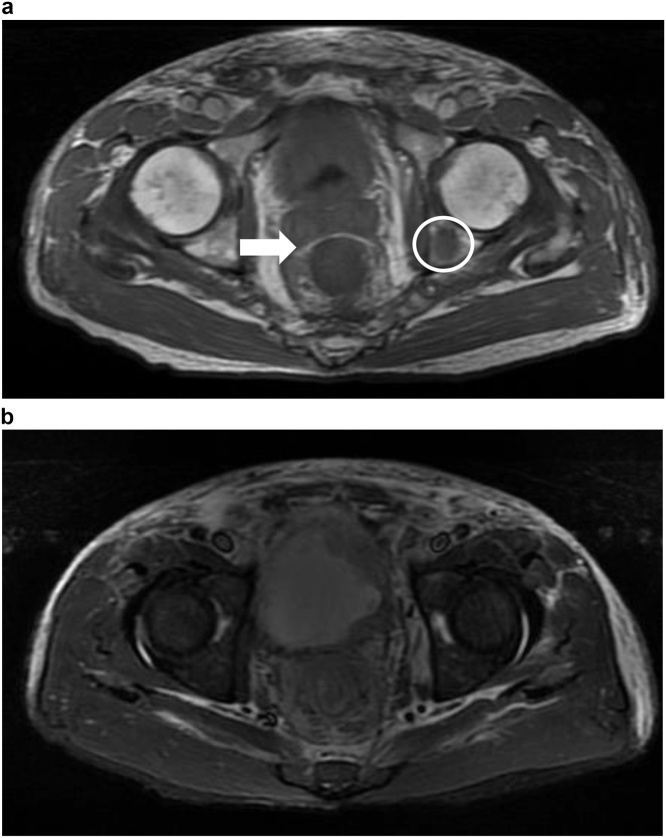
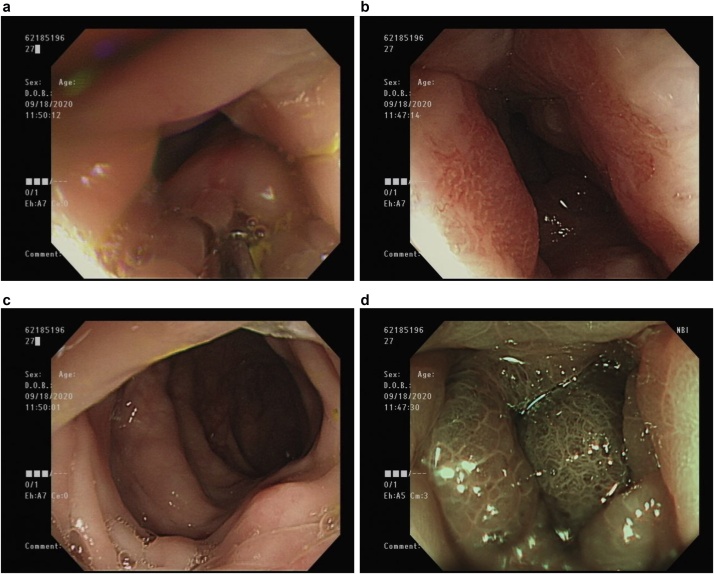
Subsequently, computed tomography (CT) (Fig. 1) and magnetic resonance imaging (MRI) (Fig. 2) revealed diffuse rectal wall thickening, diffuse lower urinary bladder wall thickening with possible high cellularity, and at least three lesions in the visible pelvic bony structure with the largest one (about 1.7 cm) in the left ischium bone. Subsequently, repeated colonoscopy was arranged after bowel preparation and showed contiguous annular constriction from 5 to 15 cm above the anal verge (Fig. 3). A biopsy revealed an inflamed colonic mucosa with a few abnormal small glands within the submucosa.
Fig. 1.
Computed tomographic image showing contiguous symmetrical rectal wall thickening.
Fig. 2.
Magnetic resonance image showing (a) intact Denonvilliers’ fascia (arrow) and bone metastasis (circle) and (b) irregular thickening of the bladder wall.
Fig. 3.
Colonoscopy.
(a) 5 cm from the anal verge, (b) 10 cm from the anal verge, (c) 15 cm from the anal verge, (d) narrow-band image at 5 cm from the anal verge.
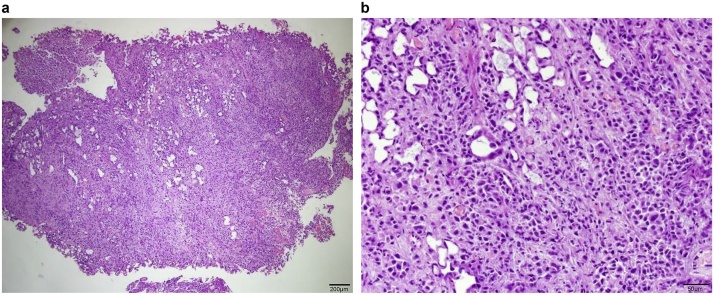
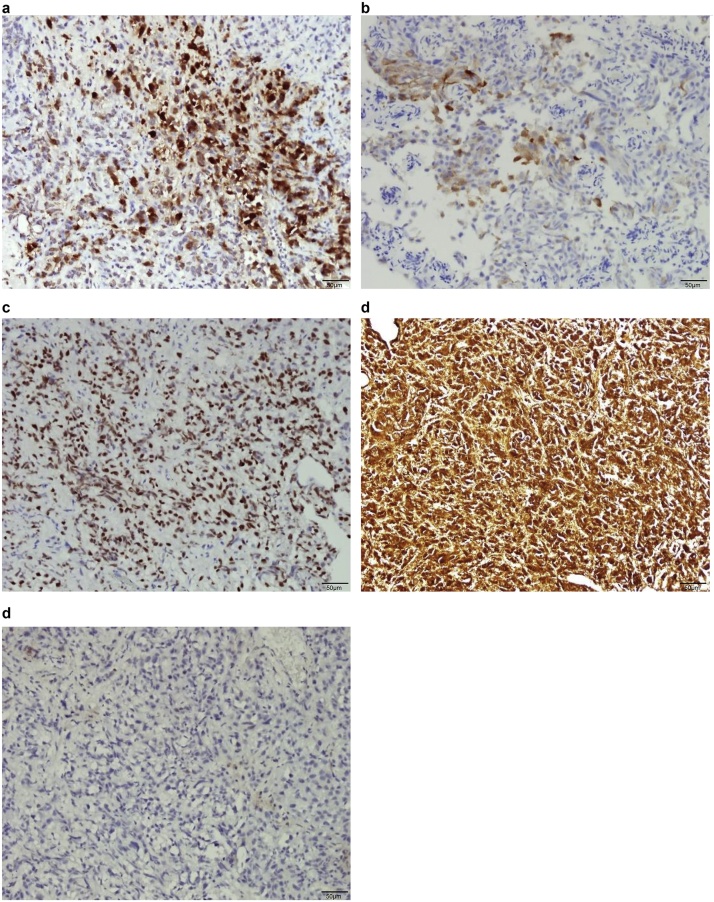
After discussion with the patient, we agreed to perform a colostomy for nutrition establishment, cystoscopy, and anal biopsy. The cystoscopy showed a diffuse wide-base bladder tumor over the bladder neck and left lateral wall. The tumor cells stained strongly positive for CK7 and were focally positive for CK20, positive for GATA-3, diffusely positive for CD138, and negative for CDX-2. Therefore, primary adenocarcinoma of the colon was less likely and tumor growth of urothelial origin was suspected (Fig. 4). An anal biopsy showed poorly differentiated carcinoma arranged in a small nested pattern within the subepithelial area of the anorectal tissue (Fig. 5) and further positive immunohistochemistry staining for GATA-3 was reported. From the pathology above, the mas caused annular constriction was consistent with direct invasion from the previous urinary bladder cancer. Unfortunately, the patient was diagnosed with lung and bone metastasis after transferring to another hospital for further evaluation and chemotherapy for invasive urothelial carcinoma cT4N1M1, high grade, plasmacytoid variant because of personal reasons after a T-loop colostomy.
Fig. 4.
Immunohistochemistry of bladder tumor cells (a) strongly positive for CK7, (b) focally positive for CK20, (c) positive for GATA-3, (d) diffusely positive for CD138, and (e) negative for CDX-2; scale bar represents 100 μM.
Fig. 5.
Histopathological examination revealed inflamed colonic mucosa with a few abnormal small glands within the submucosa with crushed artefacts suspicious of adenocarcinoma. (a) 40×, scale bar represents 200 μM; (b) 200×, scale bar represents 50 μM.
As far as we are concerned, we could only trach his out-patient department follow-up record about five months later.
3. Discussion
Invasive bladder cancers frequently show lymphatic or hematogenous metastasis. Aggressive local invasion into adjacent structures is also commonly observed. However, the occurrence of rectal annular constriction due to infiltration by bladder cancer is relatively rare. Previously, it was believed that the rectal mucosa could remain intact because it was thought that metastatic spread would not penetrate the muscular layers of the bowel [2]. However, Aneese et al. [3] reported a case of bladder urothelial carcinoma extension, via the prostate, into the rectum that formed a multinodular, oval, ulcerated, friable mass. All of the previous reports have revealed the vagaries of the presentation of invasive bladder (prostate) cancer in the rectum and the difficult diagnosis by colonoscopy.
Although the mechanism of urological tumor-related annular rectal stricture remains unclear, several studies have been reported. Langenstroer et al. [4] reported a case involving a similar clinical presentation that was noted about three years after the operation of cystoprostatectomy; they suggested that this presentation may have been caused by surgical deposition of cancer cells. Stillwell et al. [5] presented two cases of annular constrictions of the rectum and hypothesized that a locally aggressive bladder cancer may spread through the Denonvilliers’ fascia causing the problem. Kobayashil et al. [6] hypothesized that this kind of cancer cells may easily progress along the lateral pedicles to the posterior part of rectal wall and even break through the rectal wall afterward. Their hypothesis was based on the many CT results of their three cases that showed enlarge lateral pedicles with relative intact of their fat layer. From the studies mentioned above, it is currently hypothesized that the routes of invasive bladder (prostate) tumor to the rectum are (1) previous iatrogenic exposure-caused deposition; (2) direct invasion, breaking though the bladder wall via Denonvilliers’ fascia to the rectum; and (3) metastasis via the lateral pedicles of the bladder to the posterior rectal wall followed by wall infiltration.
In our case, we concluded that a secondary rectum tumor due to bladder cancer could be mimicking proctitis with continuous annular constriction caused by swelling and an intact mucosa. We had performed a tumor marker survey and a laboratory evaluation of possible infections. Details of the colonoscopy were recorded, and CT and MRI were also performed. However, we did not know the etiology of the severe swelling of the rectal mucosa until we obtained the pathology report results.
Bladder urothelial carcinoma rarely extends or metastasizes to the gastrointestinal tract, and few cases with rectum involvement have been reported [7]. Three main characteristics of secondary rectal tumors originating from the bladder or prostate were noted. First, all of the cases, including ours, involved men. A possible explanation of a sex difference is that there is a reproductive organ that acts as a barrier between urologic organs and the rectum in women. Second, high grades of the original cancers were noted, which may be related to the third feature: poor outcome. There is no record for survival >2 years after diagnosis of a rectal lesion.
4. Conclusion
In summary, we presented a case of a bladder urothelial carcinoma that had penetrated to the rectum and mimicked proctitis with diffuse annular swelling observed by colonoscopy, which may lead to a poor outcome.
Declaration of Competing Interest
The authors report no declarations of interest.
Funding
This study has not received funding from any person or institution.
Ethical approval
Our protocol adhered to the tenets of the Declaration of Helsinki and received approval from the TSGH Institutional Review Board.
Consent
Informed written consent was obtained from the patient’s legally authorized representatives for publication of this case.
Author contribution
Yu-Hong Liu contributed to the design of the study, was responsible for the management and retrieval of data, contributed to initial data analysis and interpretation, and drafted the initial manuscript. Yu-Hong Liu and Ta-Wei Pu decided upon the data collection methods and were also responsible for the data analysis decisions. Ta-Wei Pu conceptualized and designed the study, supervised all aspects of the study, critically reviewed and revised the manuscript, and approved the final manuscript for submission.
Registration of research studies
Not applicable.
Guarantor
Ta-Wei Pu, M.D.
Division of Colon and Rectal Surgery, Department of Surgery, Tri-Service General Hospital, Songshan Branch; and School of Medicine, National Defense Medical Center, Taipei, Taiwan, Republic of China.
No. 131, Jiankang Rd., Songshan District, Taipei City 10581, Taiwan (R.O.C.).
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgment
The authors are grateful to Enago for technical editing, language editing, and proofreading.
Contributor Information
Yu-Hong Liu, Email: lovewithoutreasons@gmail.com.
Ta-Wei Pu, Email: tawei0131@gmail.com.
References
- 1.Agha R.A., Franchi T., Sohrabi C., Mathew G., for the SCARE Group The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 2.Gengler L., Baer J., Finby N. Rectal and sigmoid involvement secondary to carcinoma of the prostate. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1975;125:910–917. doi: 10.2214/ajr.125.4.910. [DOI] [PubMed] [Google Scholar]
- 3.Aneese A.M., Manuballa V., Amin M., Cappell M.S. Bladder urothelial carcinoma extending to rectal mucosa and presenting with rectal bleeding. World J. Gastrointest. Endosc. 2017;9:282–295. doi: 10.4253/wjge.v9.i6.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langenstroer P., Zacharias A., Almagro U., Dewire D. Annular constriction of the rectum secondary to transitional cell carcinoma of the bladder. Urology. 1996;47:442. doi: 10.1016/S0090-4295(99)80471-3. [DOI] [PubMed] [Google Scholar]
- 5.Stillwell T.J., Rife C.C., Lieber M.M. Bladder carcinoma presenting with rectal obstruction. Urology. 1989;34:238–240. doi: 10.1016/0090-4295(89)90315-4. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi S., Kato H., Iijima K., Kinebuchi Y., Igawa Y., Nishizawa O. Annular rectal constriction due to infiltration by bladder cancer. Hinyokika Kiyo. 2006;52:569. [PubMed] [Google Scholar]
- 7.Kumar N., Raju M., Fass R. Bladder cancer presenting as lower-GI bleeding. Dig. Dis. Sci. 2009;54:2047–2048. doi: 10.1007/s10620-008-0571-9. [DOI] [PubMed] [Google Scholar]