Abstract
Background
Cancer outcomes in sub-Saharan Africa (SSA) remain suboptimal, in part due to poor patient retention. Many patients travel long distances to receive care, and transportation costs are often prohibitively expensive. These are well-known and established causes of delayed treatment and care abandonment in Malawi and across SSA.
Methods
We sent visit reminder texts and offered upfront money to cover transportation costs through a mobile money transfer (MMT) platform to lymphoma patients enrolled in a prospective cohort in Malawi. The primary aim was to test the feasibility of upfront MMTs.
Results
We sent 1034 visit reminder texts to 189 participating patients. Of these texts, 614 (59%) were successfully delivered, with 536 (52%) responses. 320/536 (60%) MMTs were sent to interested patients and 312/320 (98%) came to their appointment on time. Of 189 total patients, 120 (63%) were reached via text and 84 (44%) received MMTs a median of three times (IQR 2–5). Median age of reachable patients was 41 (IQR 30–50), 75 (63%) were male, 62 (52%) were HIV+ and 79 (66%) resided outside of Lilongwe.
Conclusion
MMTs were a feasible way to cover upfront transportation costs for patients reachable via text, however many of our patients were unreachable. Future studies exploring barriers to care, particularly among unreachable patients, may help improve the efficacy of MMT initiatives and guide retention strategies throughout SSA.
Keywords: global health ethics, global oncology, patient retention, research reimbursement and compensation, sub-Saharan Africa
Introduction
The cancer burden is increasing in sub-Saharan Africa (SSA), with an estimated 1 million new cases and 683 000 deaths in 2018.1,2 Age-standardized incidence across the region is projected to increase by 10–20% between 2015 and 2020.1,2 Recent efforts to address this growing burden include focused programs to train local healthcare providers as well as setting-specific initiatives to build oncology research and clinical care capacity.3 The advancement of oncologic care across the region has been supported in part by bilateral partnerships with academic cancer centers in high-income countries and engagement of the World Health Organization and pharmaceutical companies for improved access to medications.3,4
The successes of these advancements are threatened by pervasive barriers patients encounter related to the accessibility of healthcare, and cancer care in particular.5–8 Oncology services, including chemotherapy administration and supportive care resources, are often only available in hospitals located in large urban areas.9 Many patients travel long distances to receive care, and transportation costs are often prohibitively expensive.10 Patient retention remains a major challenge across the region in both research and clinical care, and contributes to suboptimal patient outcomes.6,8,11–14
Kamuzu Central Hospital (KCH) in Lilongwe serves a catchment area of approximately 9 million people. It is one of two public referral hospitals providing cancer treatment in Malawi. We have repeatedly demonstrated that complex chemotherapy can be administered safely with good outcomes even in a resource-limited healthcare setting.15–22 However, our efforts to maximize efficacy and minimize treatment-related toxicity require frequent visits and intensive treatment. Since beginning the KCH Lymphoma Cohort Study in 2013, which is a prospective study that provides standard of care treatment, we have facilitated clinic attendance by reimbursing the transportation costs associated with scheduled patient visits—a practice that is mandated by the Malawi National Health Sciences Research Committee (NHSRC).23 Despite receiving reimbursement at the completion of their clinic visits, many patients still come late to scheduled visits or miss them entirely, and visit timeliness is likely worse outside of a study situation where these reimbursements are not available.
Previous qualitative research by our group identified both long travel distances and transportation costs to reach the central hospital for cancer care as major barriers to care for patients.10 This was expected due to limited district-level healthcare infrastructure, and are both well-reported causes of care abandonment throughout the region.7,13,24 In response to these findings, we built a comprehensive retention plan. As part of this plan, we sent appointment reminders and offered patients transportation reimbursements via mobile phone money transfer (MMT) technology to lessen the upfront costs associated with receiving care. We report our experience using MMT to facilitate timely patient visits and better support patients throughout long-term, multi-visit care in Malawi.
Methods
Participants and setting
From October 2018 to January 2020, we implemented MMT among adult lymphoma patients enrolled in an ongoing prospective KCH Lymphoma Cohort Study that provides standard of care treatment at KCH in Lilongwe, Malawi. MMTs are a common and accessible way for patients to receive money through local cellular networks, even in remote settings like those in rural Malawi.
MMT program design and methods
The primary outcome of this pilot study was to test the feasibility of upfront MMT to participants receiving treatment, and the secondary outcome was to explore if the MMT intervention decreased treatment delays. We ensured all patients receiving care had a mobile phone, provided basic mobile phones (7955 Malawi Kwacha (MK), US$11) as needed to patients who signed an agreement to return the phone upon treatment completion and collected between one and three active phone numbers per patient. Additionally, we provided an active Airtel SIM card (300 MK, US$0.40), which has the best network coverage in Malawi and allows MMT between different network carriers. Our research associate underwent registration and training to become an Airtel agent so we could help patients set up mobile phones/mobile money accounts in clinic. Appointment reminders for all scheduled clinic visits were sent via text message, as well as a request to receive upfront transport reimbursement via MMT. To receive an MMT, participants had to respond ‘yes’ via text, call or a free ‘call me back’ message to not use their own airtime. If the patient had low literacy, we either sent a text message to a guardian/caregiver with documented literacy or directly called the patient. Patients who declined MMT were not contacted for future visits, unless they specified that they would like to continue to be offered the MMT option for future scheduled clinic visits in addition to the visit reminder text. One mobile phone was used in clinic to send texts, call and send MMTs to participants.
As an alternative to transportation reimbursement at the completion of patient visits, we offered upfront transportation funds through the MMT platform for upcoming scheduled clinic visits. MMTs were sent to interested patients the Friday preceding their scheduled clinic visit, which usually fell on Monday or Tuesday of the following week. The upfront transfers and post-visit reimbursements were standardized at 3500 MK (US$4.8), per the fixed amount for research participation mandated by the NHSRC for studies initiated prior to 2017. Exceptions were made on a case-by-case basis for patients whose transportation cost was much greater than the predetermined amount and could be verified via travel receipts. To demonstrate the distance our participants traveled to receive care at KCH, we mapped the home district of origin for patients contacted during the MMT intervention. A small number of patients temporarily relocate to Lilongwe district during treatment, but this data was not captured.
Historically (pre-MMT), we collected active numbers for patients at study enrollment, as well as numbers for guardians/caregivers, to maintain communication throughout treatment. If a participant did not have a phone, we would collect phone numbers from a family member, friend and/or neighbor. We did not provide phones or SIM cards, and did not send appointment reminders. We provided the standardized transport reimbursement of 3500 MK (US$4.8) at the completion of the study visit in person in clinic.
Participant interviews
We attempted to contact all unreachable patients to explore barriers and guide future improvements to the initiative. The interview guide was adapted from our previous qualitative study that explored abandonment of care.6 Interviews were conducted by phone in Chichewa by a trained research assistant. A 14-question semi-structured interview guide included location of home district, travel time to clinic, barriers to receiving care, barriers to consistent mobile phone access, sociodemographic characteristics and general understanding of cancer treatment.
Analysis
For the purposes of our analysis, patients were considered reached or unreached. Patients were considered reached if they had an active phone number, received the text message we sent and responded to the text message. Patients were considered not reached if they had an inactive phone number, the text message did not deliver or they did not respond to the delivered text message.
We conducted an exploratory analysis to compare treatment delays during the 17-month MMT intervention, October 2018 through January 2020, to 17-month pre-intervention historical controls seen January 2017 through September 2018. Patients were considered on time if they came to the clinic within +/- seven days of their scheduled return visit. Patients who arrived more than seven days early to their appointment were excluded from the analysis, as these visits were often unscheduled due to sickness. We further analyzed the demographic characteristics of patients who arrived on time versus those who did not and stratified by pre/post MMT initiation.
Cohort characteristics were summarized using simple descriptive statistics, and statistical differences between groups were assessed using an alpha-level of 0.05 and a one-sided t-test for roughly normally distributed data, Wilcoxon Rank Sum test for nonparametric data and Chi-squared for categorical data. Analyses were conducted using R 3.5.2 (New York, New York).
Ethical approval
All participants gave written informed consent. This study was approved by the University of North Carolina Institutional Review Board and Malawi NHSRC.
Results
Between October 2018 and January 2020, we attempted patient contact 1034 times via text/phone call to a total of 189 participating patients (Figure 1). We gave 23 phones to patients who did not have a mobile phone. Of 1034 appointment reminder texts sent, 696 (67%) reached an active phone number, and 614 (59%) texts were successfully delivered (Figure 1). Among 614 delivered texts, 536 (87%) responded to the text; 216/536 (40%) declined MMTs, and 320/536 (60%) MMTs were sent (Figure 1). Nearly all MMTs (n = 312/320, 98%) resulted in on-time visits. Of the eight (2%) that did not come to their appointment on time, two (25%) died, two (25%) came seven to 21 days late, three (37%) came more than 21 days late and one (13%) abandoned care. Of the 23 borrowed phones, 20 (87%) were returned at treatment completion and three (13%) were not returned because the participants died at home.
Figure 1.
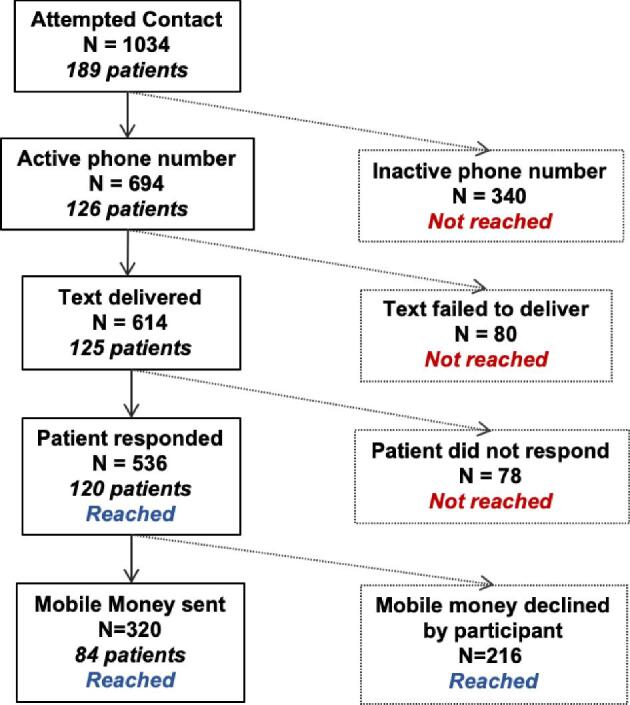
Flow chart of lymphoma patient contact during mobile money transfers (MMTs) intervention in Malawi.
Most patients (117/189, 62%) we contacted during the MMT intervention resided outside of Lilongwe district (Figure 2). There were no significant differences in the demographic characteristics of the patients who were reachable/unreachable via text (Table 1). We reached 120 (61%) patients via text (Table 1). Of those reached, the median age was 41 years (Interquartile range (IQR) 30–50), 75 (63%) were male and 62 (52%) were HIV+ (Table 1). The most common diagnoses were: 49 (41%) diffuse large B-cell lymphoma, 15 (13%) Hodgkin lymphoma, 15 (11%) low grade non-Hodgkin lymphoma and 11 (9%) multicentric Castleman disease. Most reachable patients (84/120, 70%) received MMTs a median of three times (IQR 2–5). The median MMT amount was 3700 MK, approximately US$5, with a range of 3700 MK to 23 000 MK (US$5 to US$30).
Figure 2.
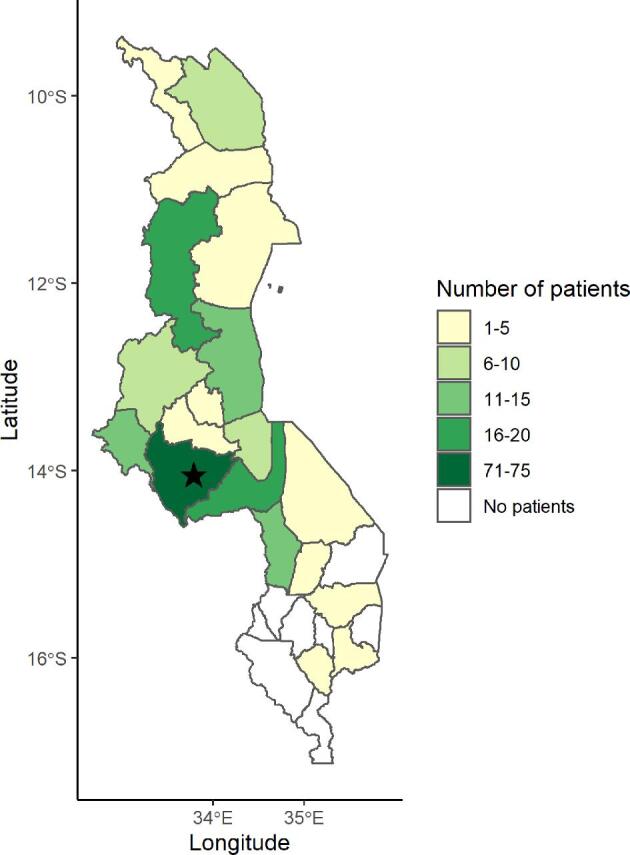
District of origin of patients receiving care at Kamuzu Central Hospital in Lilongwe, Malawi who were contacted during the mobile money transfers (MMT) intervention (n = 189). The black star represents the location of Kamuzu Central Hospital in Lilongwe, Malawi.
Table 1.
Baseline characteristics of patients seen during the pre-mobile money transfer (MMT) period compared to those contacted during the MMT initiative. Patients were considered reached if they had an active phone number, received the text message we sent and responded
| Pre-MMT | Post-MMT initiation | Pre vs. post | ||
|---|---|---|---|---|
| All | Reached | Not reached | p-value | |
| Variable | n = 213 | n = 120 | n = 69 | |
| Male sex, n (%) | 129 (61) | 75 (63) | 44 (64) | 0.71 |
| Age in years, median (IQR) | 41 (30–54) | 41 (30–50) | 41 (32–52) | 0.33 |
| Resides outside of Lilongwe, n (%) | 133 (62)* | 79 (66) | 38 (55) | 1.00 |
| HIV-positive, n (%) | 115 (54)** | 62 (52) | 40 (58) | 0.96 |
| Diagnosis, n (%) | 0.98 | |||
| Diffuse large B-cell | 88 (41) | 49 (41) | 29 (42) | |
| Hodgkin | 19 (9) | 15 (13) | 9 (13) | |
| Low grade non-Hodgkin lymphoma | 20 (9) | 15 (13) | 4 (6) | |
| Multicentric Castleman disease | 18 (8) | 11 (9) | 5 (7) | |
| Burkitt lymphoma | 18 (8) | 9 (7) | 7 (10) | |
| Plasmablastic | 14 (7) | 5 (4) | 7 (10) | |
| Acute lymphoblastic leukemia | 9 (4) | 5 (3) | 2 (3) | |
| Other lymphoma | 27 (13) | 11 (9) | 3 (4) | |
*Missing 7.
**Missing 1.
We conducted an exploratory analysis of treatment delays in our longitudinal cohort, comparing the 17 months after initiating MMTs to the 17 months prior to initiation. There were 213 patients seen during the pre-intervention period, and their demographic characteristics were similar to the 189 seen post-MMT initiation (Table 1); 114 patients were seen in both analysis periods, and this overlap was expected. During the pre-intervention period, there were 1622 scheduled clinic visits, of which 1433 (88%) patients arrived on time (Table 2). On average, patients returned to the clinic within a median 1.0 day (IQR 0–1) of their scheduled return visit. Following the initiation of MMTs, there were 1122 scheduled clinic visits. We attempted contact for 1034 (92%) of these visits, and 1000 (89%) patients arrived on time (Table 2). Patients returned to the clinic within a median 1.0 day (IQR 0–1). The proportion of patients who returned on time, the mean treatment delay and median treatment delay did not significantly change with the MMT initiative (p = 0.57; p = 0.31 and p = 0.61, respectively). Among on-time visits, mean days between scheduled clinic visit and actual return was 0.83 (SD 1.33) for the pre-MMT visits and 0.95 (SD 1.59) for the post-MMT visits (p = 0.05; Table 3). In addition, a larger proportion of patients seen in post-MMT visits resided outside of Lilongwe (Pre: 62%, Post: 67%, p = 0.008). Median treatment delay among those who did not arrive on time (> seven days late) did not differ between pre-MMT visits 15 days (IQR 9–39) and 15 days (IQR 10–30) for post-MMT visits (p = 0.67) (Table 3). There were no significant differences in the demographic characteristics of patients who arrived on time versus those who did not arrive on time (> seven days late) (Table 3).
Table 2.
Clinic visit volume stratified by pre- and post-initiation of mobile money transfers (MMTs) for lymphoma patients in Malawi
| Pre-MMT | Post-MMT | p-value | |
|---|---|---|---|
| Number of patient visits | 1622 | 1122 | |
| Average treatment delay in days, mean (SD) | 8.0 (52.5) | 6.1 (35.5) | 0.31 |
| Average treatment delay in days, median (IQR) | 1.0 (0–1.0) | 1.0 (0–1.0) | 0.61 |
| On-time visits, n (%) | 1433 (88) | 1000 (89) | 0.57 |
Table 3.
Characteristics of participants who arrived on time (+/- seven days) vs. not on time (> seven days) to clinical visits pre and post mobile money transfer (MMT) initiation for lymphoma patients in Malawi
| On-time visits (+/- seven days) | Not on-time visits (> seven days) | |||||
|---|---|---|---|---|---|---|
| Pre-MMT | Post-MMT | p-value | Pre-MMT | Post-MMT | p-value | |
| Number of patient visits | 1433 | 1000 | 189 | 122 | ||
| Average treatment delay in days, mean (SD) | 0.83 (1.33) | 0.95 (1.59) | 0.05 | 62.1 (143) | 48.6 (98) | 0.36 |
| Average treatment delay in days, median (IQR) | 1.0 (0–1.0) | 1.0 (0–1.0) | 0.88 | 15.0 (9.0–39.0) | 15.0 (10.0–30.0) | 0.67 |
| Male sex, n (%) | 898 (63) | 557 (56) | 0.001 | 107 (57) | 71 (58) | 0.92 |
| Age in years, median (IQR) | 40 (27–53) | 40 (29–49) | 0.48 | 38 (27–51) | 40 (30–46) | 0.90 |
| Resides outside of Lilongwe, n (%) | 891 (62) | 666 (67) | 0.008 | 126 (67) | 79 (65) | 0.90 |
| HIV-positive, n (%) | 713 (50) | 459 (46) | 0.09 | 93 (49) | 71 (58) | 0.13 |
We conducted semi-structured phone interviews with patients who were unreachable through the MMT initiative to further explore barriers to care; we attempted to contact 58 patients and reached seven (8.3%). The majority (6, 86%) reported regular access to a mobile phone during the initiative, but few (2, 29%) were able to charge their phone at least once per week. Only one (14%) could reliably purchase airtime. All patients (7, 100%) expressed understanding the goals of their chemotherapy treatment as well as what could happen if their chemotherapy cycles were delayed or missed. The main barriers to receiving care were transportation issues (4, 57%) and childcare responsibilities (3, 43%). Respondents reported traveling a median 1.5 hours (IQR 1.5–5.0) to KCH, which included a median of 40 minutes (IQR 12.5–158) by foot from their homes to the closest minibus depot. Most lived in homes with iron sheet roofing (5, 71%) versus a grass-thatched roof (2, 29%), cement (4, 57%) versus dirt flooring (3, 43%), with traditional latrine pit toilets (7, 100%) and a tap (4, 57%) as their primary water source versus borehole (3, 43%).
Discussion
We report the first attempt to integrate MMTs into long-term, multi-visit oncology care in Malawi and, to our knowledge, throughout SSA. We transferred upfront transportation reimbursement, as opposed to reimbursement at the completion of a patient's visit, using a mobile phone platform in an attempt to reduce the financial burden associated with long travel distances to care and expensive transportation. Although one-third of contact attempts were unsuccessful, the initiative was well-used among reachable and interested patients. Nearly all (98%) patients who received MMTs came to their clinical visits on time. In light of ongoing attempts to increase patient retention and ultimately improve disease outcomes, the integration of MMTs may be a valuable strategy for which our findings provide important initial insight.
Health ethicists often cite compensating patient engagement as directly jeopardizing patient autonomy.23 Nyangulu et al. reviewed the ethics of research compensation in Malawi and argue that the NHSRC mandated amount of US$5 to US$10 places undue influence on research participants.23 We agree that the ethics of reimbursing transportation costs contingent on research participation are challenging, particularly among patient populations where the costs associated with receiving care are not insignificant.23 However, we offered MMT to patients enrolled in our KCH Lymphoma Cohort Study, which is a prospective single-arm study where participants receive standard of care treatment, including curative-intent chemotherapy and supportive care at all visits. Participants are not asked to come for research-only related visits, it is not a clinical trial and no investigational drugs are used, resulting in minimal participant risk. The primary goal is to improve outcomes among cancer patients in Malawi. Our research is embedded into standard clinical care, which allows us to provide optimized care and treatment in a setting where drug availability is sporadic and the healthcare system is extremely understaffed. Further, the majority of our patients (62%) reside outside of Lilongwe, with many clustered in the Northern region, and oncology services are not available in their home districts. Although public sector healthcare in Malawi is provided at no cost to patients, the financial strain associated with missed work and transportation costs to access cancer care cannot be understated. Many of our patients are subsistence farmers, and most Malawians do not make more than US$2 per day. Given these economic constraints, upfront reimbursement is perhaps more equitable than reimbursing after the completion of a clinical visit.
Among visits where an upfront MMT was sent, nearly all patients arrived on time (98%). There were initial reservations that patients might misuse upfront transportation reimbursements and not come to their clinical visits or not return borrowed phones at treatment completion; however, we are happy to report that this rarely occurred. Based on anecdotal feedback from our research assistant, those who declined to receive MMT and preferred to receive the reimbursement at the completion of their clinic visits (our historical pre-MMT method) often did so because they did not want to misuse the funds prior to their visit. In contrast to our hypothesis, following the initiation of MMTs, we did not observe a significant improvement in on-time clinical visits in our exploratory analysis. The majority of our patients arrived to scheduled clinic visits on time both pre MMT (88%) and post MMT (89%; p = 0.57). One explanation suggested by these data is that the MMT initiative did not reach the subset of patients who faced the most significant barriers to care.
Approximately half (48%) of our contact attempts were unsuccessful—340 reached an inactive phone number, 80 texts failed to deliver and 78 delivered texts did not get a response. Possible reasons for being unreachable include: inactive phone number due to mobile network de-activating service; text message not delivered, which occurs if the phone is turned off, not charged, out of network range or the SIM card is changed to a different carrier (which is common as there are two mobile networks in Malawi); or participant is unable to respond to a delivered text message, often a result of no pre-paid airtime loaded on their phone. These challenges are also present in other mobile health efforts in low-income settings; a review of these efforts reported that healthcare workers found communicating with patients via a mobile health platform overall beneficial, but experienced challenges related to poor network connection, access to electricity and the cost of recharging phones.25
In our MMT intervention, receiving and withdrawing the MMT does not require airtime, but the participant must have some amount of airtime loaded on their phone to respond to our visit reminder text and offer to send upfront MMT. If interested, our clinical MMT coordinator then initiates the MMT and the patient withdraws the transfer at a local mobile money booth. This process is relatively affordable—for each upfront reimbursement of 3500MK (US$4.8) via MMT, associated network fees were 210 MK (US$0.28) when sending to Airtel customers and 525 MK (US$0.71) when sending to out-of-network participants. We gave active Airtel SIM cards to all participants in order to encourage in-network MMT transactions and decrease overall costs.
Other mobile phone contact initiatives in SSA have largely focused on communicating broadcast messages to patients focused on improving adherence to ante- and post-natal care, childhood vaccine adherence, HIV prevention and care, as well as communicating lab results to patients.26–33 This approach can be successful without soliciting a response from the patient, via text or call.26–33 Regional studies have also encountered challenges when sending participants text messages, and report that only 20–25% of participants received and/or responded to their messages.29,33 An intervention in rural Uganda successfully used text messaging to communicate abnormal laboratory results that required clinical follow-up and offered patients who returned within seven days post-visit transportation reimbursement, but did not report whether patients responded directly via text.28 In contrast to these interventions, sending MMTs is a multi-step process and requires patient response to be successful. One group sent automatic mobile cash transfers to mothers who vaccinated their children on time once the clinic had confirmed adherence.31 Most (90%) participants reported receiving the SMS and 83% reported receiving an MMT; however, this approach did not require a response.31 There are no reports from SSA on using upfront MMT to promote treatment adherence and retention in cancer care.
We specifically contacted patients who were unreachable to identify alternative ways we could support them during cancer care; most cited transportation cost, distance and childcare responsibilities as continued challenges. They also reiterated that having reliable access to a phone charger and funds to purchase airtime were particularly challenging. We attempted to set up a toll-free clinic number for patients to call as part of the MMT intervention, which would mitigate insufficient airtime funds, but the cost was not sustainable as the mobile phone company charges 100 000 MK (US$135) monthly to maintain a toll-free line. Given our experience, future interventions include providing solar chargers and/or battery packs for phone charging and sending airtime to participants to facilitate a response. One participant suggested having a hostel in Lilongwe where patients could stay while receiving treatment, especially for those who travel long distances for care and are not able to re-locate to Lilongwe during treatment. Patient hostels reduce both accommodation and transportation barriers and have been successfully implemented in several other sites across SSA.34–36 We are working with KCH leadership to develop a plan for establishing and sustaining a hostel for cancer patients. There is no cancer treatment facility in the Northern region, which contributes to extremely long travel times with high associated costs. We urge the Ministry of Health to establish a cancer treatment facility in the Northern region, which will likely alleviate barriers related to travel distance.
Study limitations include the large number of unreachable patients, potential existing differences between the pre- and post-intervention time periods as well as overlap between the patient groups, the small sample size for the phone interviews to assess barriers and inadequate sample size to determine the effect of the MMT intervention on treatment delays.
Our experience suggests we need better strategies to reach patients who were unreachable via the MMT intervention (69/189). To further understand how we can best support this cohort of patients, future qualitative studies are needed to identify specific barriers to cancer care and explore ways to overcome these barriers. Furthermore, a larger clinical trial is needed to evaluate if upfront MMT results in decreased treatment delays. We encourage key stakeholders and the Malawi Ministry of Health to partner with local mobile phone network providers to establish toll-free health lines without monthly charges to improve remote access to healthcare, particularly for patients receiving long-term, multi-visit care.
Conclusions
MMTs were a feasible and effective way to cover upfront transportation costs for patients who were reachable via text and interested. Nearly all (98%) patients who received MMTs came to their appointment on time. However, it appears the MMT intervention failed to reach those patients who faced the most significant barriers to care.
Acknowledgements
The authors would like to thank the patients and their families for agreeing to participate in the project. We would also like to thank Airtel Malawi for their services to set up mobile money transfers for our patients. We are also grateful to the leadership of Kamuzu Central Hospital, Malawi Ministry of Health, UNC Project-Malawi, and UNC Lineberger Comprehensive Cancer Center for support of this study.
Contributor Information
Grace K Ellis, UNC Project-Malawi, Lilongwe, Malawi.
Agness Manda, UNC Project-Malawi, Lilongwe, Malawi.
Hillary Topazian, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Christopher C Stanley, UNC Project-Malawi, Lilongwe, Malawi.
Ryan Seguin, UNC Project-Malawi, Lilongwe, Malawi.
Caroline E Minnick, UNC Project-Malawi, Lilongwe, Malawi.
Blessings Tewete, UNC Project-Malawi, Lilongwe, Malawi.
Asekanadziwa Mtangwanika, UNC Project-Malawi, Lilongwe, Malawi.
Mena Chawinga, UNC Project-Malawi, Lilongwe, Malawi.
Sara Chiyoyola, UNC Project-Malawi, Lilongwe, Malawi.
Maria Chikasema, UNC Project-Malawi, Lilongwe, Malawi.
Ande Salima, UNC Project-Malawi, Lilongwe, Malawi.
Stephen Kimani, UNC Project-Malawi, Lilongwe, Malawi; University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Edwards Kasonkanji, UNC Project-Malawi, Lilongwe, Malawi.
Victor Mithi, UNC Project-Malawi, Lilongwe, Malawi.
Bongani Kaimila, UNC Project-Malawi, Lilongwe, Malawi.
Matthew S Painschab, UNC Project-Malawi, Lilongwe, Malawi; University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Satish Gopal, National Cancer Institute, Center for Global Health, Rockville, MD, USA.
Katherine D Westmoreland, UNC Project-Malawi, Lilongwe, Malawi; University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Authors’ contributions
KDW designed the study and provided oversight and mentorship throughout the project. GKE analyzed and interpreted the data with assistance from KDW, HT and CS. GKE, AM, RS and CEM wrote the paper with revisions from KDW, MSP, SK, SG, RS, HT and CS. AM was the project manager who sent the weekly text reminders and mobile money transfers to patients. AM and AS collected the clinical data. BT, AM, MC, SC, MC, SK, EK, VM, BK, MSP, SG and KDW provided clinical care.
Funding
This work was supported by the Burkitt Lymphoma Fund for Africa (BLFA) [KDW]; the National Institute of Health (NIH) (K01TW011191 [KDW], K01TW01147 [MSP], K01TW009488 [SG] and U2GPS001965); the NIH National Cancer Institute (NCI) (U54CA190152 [SG], P30CA016086, UM1CA121947 [KDW, MSP, SK, BK], and L30CA233709 [MSP]); by an NIH National Institute of General Medical Sciences Award (T32GM086330 [KDW]); and by an NIH Research Training Grant (D43TW009340 [KDW, MSP, SK]) funded by the NIH Fogarty International Center, National Institute of Neurological Disorders and Stroke, National Institute of Mental Health, National Heart, Lung, and Blood Institute and National Institute of Environmental Health Sciences. The funding agencies had no role in study design, data collection, data analysis, data interpretation, writing of the report or the decision to submit it for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Competing interests
The authors declare no competing financial interests.
Data available
None.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2. Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study global burden of disease cancer collaboration. JAMA Oncol. 2017;3(4):524–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gopal S, Loehrer PJ. Global oncology. JAMA - J Am Med Assoc. 2019;322(5):397–398. [DOI] [PubMed] [Google Scholar]
- 4. Robertson J, Barr R, Shulman LN, Forte GB, Magrini N. Essential medicines for cancer: WHO recommendations and national priorities. Bull World Health Organ. 2016;94(10):735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goh XTW, Tan YB, Thirumoorthy T, Kwan YH. A systematic review of factors that influence treatment adherence in paediatric oncology patients. J Clin Pharm Ther. 2016;42(1):1–7. [DOI] [PubMed] [Google Scholar]
- 6. Stanley CC, van der Gronde T, Westmoreland KD, et al. Risk factors and reasons for treatment abandonment among children with lymphoma in Malawi. Support Care Cancer. 2018;26(3):967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Varela C, Young S, Mkandawire N, Groen RS, Banza L, Viste A. Transportation barriers to access healthcare for surgical conditions in Malawi: a cross-sectional nationwide household survey. BMC Public Health. 2019;19(1):264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Geoffroy E, Harries AD, Bissell K, et al. Bringing care to the community: expanding access to health care in rural Malawi through mobile health clinics. Public Heal action. 2014;4(4):252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. The Lancet Oncology . Cancer control in Africa: infrastructure, not philanthropy. Lancet Oncol. 2017;18(11):1423. [DOI] [PubMed] [Google Scholar]
- 10. Stanley CC, van der Gronde T, Westmoreland KD, et al. Risk factors and reasons for treatment abandonment among children with lymphoma in Malawi. Support Care Cancer. 2018;26(3):967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Makaula P, Bloch P, Banda HT, et al. Primary health care in rural Malawi – a qualitative assessment exploring the relevance of the community-directed interventions approach. BMC Health Serv Res. 2012;12:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stanley CC, Westmoreland KD, Itimu S, et al. Quantifying bias in survival estimates resulting from loss to follow-up among children with lymphoma in Malawi. Pediatr Blood Cancer. 2017;64(6): e26370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Slone JS, Chunda-Liyoka C, Perez M, et al. Pediatric malignancies, treatment outcomes and abandonment of pediatric cancer treatment in Zambia. PLoS One. 2014;9(2):e89102–e89102. doi:10.1371/journal.pone.0089102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Freeman E, Semeere A, Wenger M, et al. Pitfalls of practicing cancer epidemiology in resource-limited settings: the case of survival and loss to follow-up after a diagnosis of Kaposi's sarcoma in five countries across sub-Saharan Africa. BMC Cancer. 2016;16(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Masamba L. Guest Editorial: The state of oncology in Malawi in 2015. Malawi Med J. 2015;27(3):77. [PMC free article] [PubMed] [Google Scholar]
- 16. Gopal S, Fedoriw Y, Kaimila B, et al. CHOP chemotherapy for aggressive non-Hodgkin Lymphoma with and without HIV in the antiretroviral therapy era in Malawi. Galardy PJ, ed. PLoS One. 2016;11(3):e0150445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Painschab MS, Westmoreland KD, Kasonkanji E, et al. Prospective study of Burkitt lymphoma treatment in adolescents and adults in Malawi. Blood Adv. 2019;3(4):612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zuze T, Painschab MS, Seguin R, et al. Plasmablastic lymphoma in Malawi. Infect Agent Cancer. 2018;13(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zuze T, Ellis GK, Kasonkanji E, et al. Modified EPOCH for high-risk non-Hodgkin lymphoma in sub-Saharan Africa. Cancer Med. 2020;9(1):77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Youngblood VM, Nyirenda R, Nyasosela R, et al. Outcomes and prognostic factors for women with breast cancer in Malawi. Cancer Causes Control. 2020;31(4):393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stanley CC, Westmoreland KD, Heimlich BJ, et al. Outcomes for paediatric Burkitt lymphoma treated with anthracycline-based therapy in Malawi. Br J Haematol. 2016;173(5):705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Westmoreland KD, Stanley CC, Montgomery ND, et al. Hodgkin lymphoma, HIV, and Epstein–Barr virus in Malawi: longitudinal results from the Kamuzu Central Hospital lymphoma study. Pediatr Blood Cancer. 2017;64(5):e26302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nyangulu W, Mungwira R, Nampota N, et al. Compensation of subjects for participation in biomedical research in resource-limited settings: a discussion of practices in Malawi. BMC Med Ethics. 2019;20(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Njuguna F, Mostert S, Slot A, et al. Abandonment of childhood cancer treatment in Western Kenya. Arch Dis Child Educ Pract Ed. 2014;99(7):609–614. [DOI] [PubMed] [Google Scholar]
- 25. Odendaal WA, Anstey Watkins J, Leon N, et al. Health workers’ perceptions and experiences of using mHealth technologies to deliver primary healthcare services: a qualitative evidence synthesis. Cochrane database Syst Rev. 2020;3:CD011942. doi:10.1002/14651858.CD011942.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Linde DS, Andersen MS, Mwaiselage JD, Manongi R, Kjaer SK, Rasch V. Text messages to increase attendance to follow-up cervical cancer screening appointments among HPV-positive Tanzanian women (Connected2Care): study protocol for a randomised controlled trial. Trials. 2017;18(1):555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Siedner MJ, Haberer JE, Bwana MB, Ware NC, Bangsberg DR. High acceptability for cell phone text messages to improve communication of laboratory results with HIV-infected patients in rural Uganda: a cross-sectional survey study. BMC Med Inform Decis Mak. 2012;12(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Siedner MJ, Santorino D, Lankowski AJ, et al. A combination SMS and transportation reimbursement intervention to improve HIV care following abnormal CD4 test results in rural Uganda: a prospective observational cohort study. BMC Med. 2015;13(1):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chib A, Wilkin H, Ling LX, Hoefman B, Van Biejma H. You have an important message! Evaluating the effectiveness of a text message HIV/AIDS campaign in Northwest Uganda. J Health Commun. 2012;17(Suppl. 1):146–157.22548607 [Google Scholar]
- 30. Forrest JI, Wiens M, Kanters S, Nsanzimana S, Lester RT, Mills EJ. Mobile health applications for HIV prevention and care in Africa. Curr Opin HIV AIDS. 2015;10(6):464–471. [DOI] [PubMed] [Google Scholar]
- 31. Wakadha H, Chandir S, Were EV, et al. The feasibility of using mobile-phone based SMS reminders and conditional cash transfers to improve timely immunization in rural Kenya. Vaccine. 2013;31(6):987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gibson DG, Ochieng B, Kagucia EW, et al. Mobile phone-delivered reminders and incentives to improve childhood immunisation coverage and timeliness in Kenya (M-SIMU): a cluster randomised controlled trial. Lancet Glob Heal. 2017;5(4):e428–e438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lefevre AE, Mohan D, Hutchful D, et al. Mobile technology for community health in Ghana: what happens when technical functionality threatens the effectiveness of digital health programs? BMC Med Inform Decis Mak. 2017;17(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Safina KS. Bridging cancer care gaps, providing homes away from home- relevancy of cancer patients hostels in Uganda. 4th Edition of International Cancer Conference. 2020. Available from: https://magnusconferences.com/cancer-oncology/program/scientific-program/bridging-cancer-care-gaps-providing-homes-away-from-home-relevancy-of-cancer-patients-hostels-in-uganda [Accessed 7th May 2020]. [Google Scholar]
- 35. Kersten E, Scanlan P, Dubois SG, Matthay KK. Current treatment and outcome for childhood acute leukemia in Tanzania. Pediatr Blood Cancer. 2013;60(12):2047–2053. [DOI] [PubMed] [Google Scholar]
- 36. Shad A, Challinor J, Cohen ML. Paediatric oncology in Ethiopia: an INCTR-USA and Georgetown University Hospital twinning initiative with Tikur Anbessa Specialized Hospital. Cancer Control. 2013:108–112. http://cancercontrol.info/wp-content/uploads/2014/08/cc2013_108-112-Ethiopia-PEDIATRIC-ONCOLOGY-NEW_2012.pdf. [Google Scholar]


