Abstract
Background
Stunting and severe wasting can co-occur in under-fives, predisposing them to increased risks for morbidity and mortality. The Community Management of Acute Malnutrition (CMAM) programme, which provides outpatient malnutrition care for severely wasted children, has been successful at managing severe wasting, but there are limited data on stunting among entrants into these programmes.
Methods
We performed secondary analysis of data collected from attendees of two CMAM centres in north-western Nigeria. Using WHO reference standards, we determined the prevalence of concurrent stunting (height/length-for-age <-2 SD) among severely wasted children (weight-for-height z-scores <-3 SD). We identified individual and household-level risk factors for concurrent stunting using multivariable logistic regression analysis.
Results
Our cohort comprised 472 severely wasted children and the majority (82.8%) were stunted. Age groups of 12–23 mo (adjusted OR [AOR]=2.38, 95% CI 1.26 to 4.48) and 24–35 mo (AOR=7.81, 95% CI 1.99 to 30.67), male gender (AOR=2.51, 95% CI 1.43 to 4.39) and attending the rural malnutrition clinic (AOR=3.08, 95% CI 1.64 to 5.79) were associated with a significantly increased probability of stunting.
Conclusions
Stunting prevalence is high among severely wasted children attending CMAM programmes in north-western Nigeria. Policymakers need to adapt these treatment programmes to also cater for stunting, taking into account practical programmatic realities such as available expertise and scarce resource allocation.
Keywords: community management of acute malnutrition, malnutrition, north-western Nigeria, risk factor, stunting
Introduction
Stunting describes poor linear growth and it affects more than 149 million children globally.1 The condition causes considerable morbidity in affected children and is associated with reduced cognition, poor academic performance, lower productivity in adulthood and an increased incidence of adult-onset non-communicable diseases.2,3 Stunting can also coexist with other forms of malnutrition such as wasting, predisposing those affected to as high as a 12-fold increase in mortality.4 Over the past 20 y, the global under-five stunting burden has declined in all regions of the world, except in Africa, where it has increased.1 Currently, one in every three stunted children resides in Africa and the continent is unlikely to meet the WHO target of reducing global stunting by 40% in 2025.1,5
Severe wasting, defined as weight-for-height z-scores <3 SD, is viewed as a form of acute malnutrition, and was previously thought to be distinct from stunting, which was seen as chronic malnutrition.6 It has now been observed that both entities frequently occur within the same individuals and might have similar causal pathways, necessitating recent calls for integration of malnutrition prevention, treatment and control programmes.7 The biological relationship between stunting and wasting is complex and not fully understood. Leptin, a hormone produced by fat tissue, has been identified as a plausible link for co-occurring wasting and stunting.7 This is explained by significantly reduced leptin levels occurring among severely wasted children because of decreased body fat, and a proposed biological role for the hormone in influencing catch-up growth among stunted children.8 Recent epidemiological evidence analysing growth in rural Gambian children over 4 decades also suggests that stunting might be a biological response to repeated episodes of wasting.9
In Nigeria, the average national prevalence of under-five stunting has remained static at 32% and the north-western part of the country has a higher than average prevalence of 50.4%.10 The National Nutrition and Healthy Survey estimates that about 0.5% of all Nigerian preschool children experience concomitant stunting and severe wasting.10 Locally, severe wasting has received significantly more prominence in malnutrition treatment programmes than stunting. Children who are severely wasted are treated at malnutrition outpatient clinics within the Community Management of Acute Malnutrition (CMAM) programme, which has demonstrated considerable success at treating severe wasting.11,12 The CMAM model decentralises care for severely wasted children and adopts a community-based approach, bringing acute malnutrition treatment and care closer to local communities.13 In these settings, children are frequently managed on an outpatient basis, while inpatient care is reserved for those with complications or oedematous malnutrition. Currently, no attention is given to stunting within these programmes.
Existing studies examining the relationship between stunting and wasting have largely been at population level using demographic health surveillance systems or nutrition surveys and have described sociodemographic risk factors for this, such as male gender and age <3 y.14–16 Other available studies have focused on describing stunting as a complication postdischarge from acute treatment programmes,17,18 but it is possible a large proportion of children might have been stunted prior to entrance into these programmes. There is currently emerging evidence that the prevalence of concurrent stunting might be high within these programmes and is associated with lower recovery rates among entrants into these treatment programmes.19,20
In this study, we determined the magnitude of, and examined the relationships between stunting and some of its established risk factors in a group of severely wasted preschool-aged children. These children were attending CMAM outpatient therapeutic centres in a high burden of stunting region of Nigeria. We hypothesise that the burden of concurrent stunting in these treatment programmes is high. This will have important programmatic implications in the management of severely wasted children, as policymakers may need to adapt these programmes to also cater for stunting. We hope that the findings from our study provide evidence for integrating malnutrition treatment programmes locally, and also provide a context for investigating concurrent severe wasting and stunting within other CMAM programmes, to enable a better understanding of contextual differences and thus inform global malnutrition treatment policy.
Materials and methods
Study design and setting
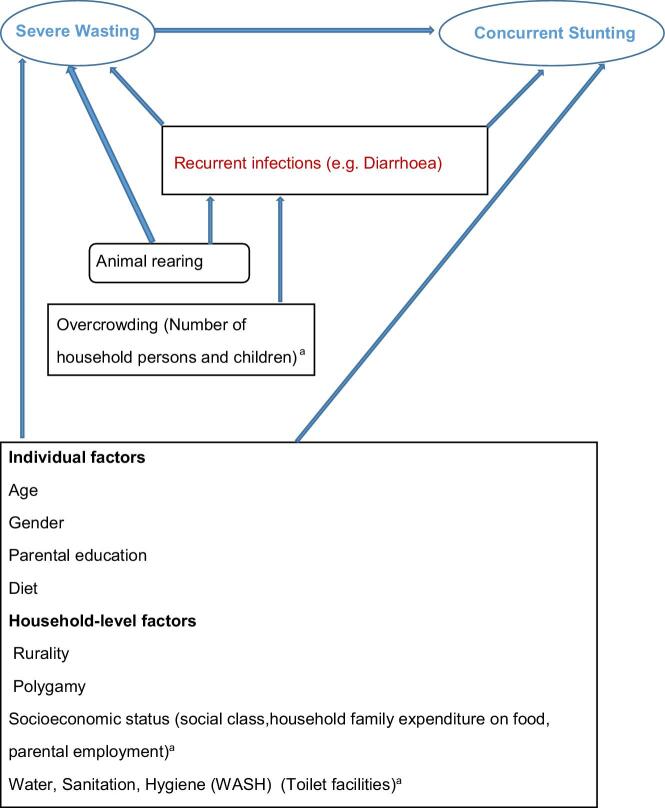
This was a secondary analysis of data collected from a previous cross-sectional study. The previous study compared helminthic prevalence and intensity of infection between severely wasted new entrants into CMAM programmes and healthy children, and determined the risk factors for this.21 We developed the conceptual framework for our secondary analysis using the variable relationship shown in Figure 1.
Figure 1.
Direct acyclic graph demonstrating conceptual framework and variable relationships with references in-situ for each association (when a variable is associated with the exposure and outcome, it is a potential confounder). Blue text: exposure variable (severe wasting), outcome variable (concurrent stunting). Red text: mediator. Black text: study variables, blue arrows represent associations. aStudy variables placed in parenthesis are proxy measures for the preceding variable.
Our study was conducted within the Nigerian CMAM programme, which is run as a collaboration between the Nigerian Federal Ministry of Health and UNICEF with the objective to improve local coverage for acute malnutrition treatment and control.22 The programme is currently run in 11 northern Nigerian states, including Kano. Kano is located in the north-western part of Nigeria, which has the highest prevalence of severe wasting in the country; it is estimated that 2% of all preschool children in this region are severely wasted.10 The state has a population of >1.8 million preschool-aged children23 and 30 CMAM outpatient therapeutic programmes (OTPs). These OTPS are located across the state in 6 out of 44 local government areas (LGAs). We conducted our study in two of these OTPs located in one rural and one urban LGA. These were Ali Akilu Memorial Hospital, a primary healthcare facility located in Madobi (a rural LGA), and Murtala Muhammed Specialist Hospital, a secondary level health facility located in Kano Municipal (an urban LGA). Within the CMAM programme, community health extension workers frequently identify severely wasted children residing in surrounding communities using simple tapes for mid-upper arm circumference and these children are referred to CMAM clinics. In some other cases, parents of children with severe wasting self-refer.
Study population
The study population were children with severe wasting who were admitted into the CMAM programme at our study sites following screening. The CMAM OTPs only attend to children with severe acute malnutrition who have severe wasting, while those with nutritional oedema are usually admitted to stabilisation centres for inpatient treatment.22 We defined severe wasting using weight-for-height z-scores <-3 SD based on WHO growth reference standards.24
Data collection
In our previous study, conducted from November 2016 to May 2017, we administered our study questionnaires to consecutive new entrants (aged 6–59 mo) into the CMAM programme in Kano state, north-western Nigeria (Figure 2).21 These children were either self-referred or identified in communities by CMAM programme case finding. During enrolment clinics, trained community health extension workers, who were part of our study team and independent of the CMAM programme, performed anthropometric measurements (length/height and weight) using standardised procedures.25 Weight was measured to the nearest 0.1 kg using a digital weighing scale (Seca 874 U manufactured by Seca, Hamburg, Germany) while length was measured to the nearest 0.1cm using a UNICEF baby/child length-height portable measuring board. Prior to study commencement, our field workers underwent a 2wk training course on anthropometric measurements and administration of our study questionnaire. We completed data collection in the urban OTP before we commenced recruitment at the rural OTP. The same research team collected data at both sites.
Figure 2.
Map of Nigeria showing Kano highlighted in the north-western region.
Study variables
Exposure variable- severe wasting
Outcome variable - concurrent stunting
Confounding variables - These were identified to be associated with both exposure and outcome variables in literature. These are listed below and are followed by references for their associations with both severe wasting and stunting:
Individual factors
Recurrent infections from overcrowding (Number of household persons and children)26
Household-level factors
Animal rearing27
Socio-economic status (social class,household family expenditure on food, parental employment)28,34 Water, Sanitation, Hygiene (WASH) (Toilet facilities)29,35
The relationships between our study variables are described using the direct acyclic graph in Figure 1.
Data analysis
For our outcome of interest (stunted or not), we plotted anthropometry on height-for-age and length-for-age WHO growth charts to determine z-score values.24 Participants whose height-for-age or length-for-age were <-3 SD were classified as having severe stunting while those with from ≥-3 SD to <-2t SD were classified as being moderately stunted.
All presented data were directly extracted from the study questionnaire except socioeconomic status (measured as social class), which was indirectly derived using a method described by Oyedeji.36 This method entails using a scoring system, based on parental occupation and education status, to determine the social class of a family, classifying them into quintiles (class I to V).36 We further categorised participants into five age groups: 6–11, 12–23, 24–35, 36–47 and 48–59 mo, to allow for comparison with other studies conducted within the CMAM programme.19
Summary statistics were derived using means and SD for normally distributed data and medians with IQR for non-normally distributed data. Comparison of baseline participant characteristics between children with severe wasting who were stunted and those without stunting was carried out using a χ2 test to compare proportions and a Mann–Whitney U test to compare medians. To adjust for possible confounding, multivariable regression analysis was used to determine ORs and their corresponding 95% CIs. All statistical analyses were performed using STATA version 13 (StataCorp LP, College Station, TX, USA).
Results
Sociodemographic features of study participants
The primary study approached 1114 parents and caregivers of study participants, of whom 1011 participants (90.8%) had complete records and anthropometric measurements.21 The cohort comprised 539 healthy children and 472 children with severe wasting. All 472 participants with severe wasting were included in our analysis. This comprised 243 males (51.5%) and 229 females (48.5%). The median age (±IQR) of the cohort was 13 (±9) mo. The majority of the study participants (82.8%) were stunted, with 64.2% having severe stunting and 18.6% being moderately stunted. Table 1 depicts the sociodemographic characteristics of both stunted and non-stunted study participants.
Table 1.
Sociodemographic characteristics of study participants and their parents.
| Variable | Stunted (%), n=391 | Not stunted (%), n=81 | p | Total (%), n=472 |
|---|---|---|---|---|
| Age group (mo) | ||||
| 6–11 | 133 (34.0) | 37 (45.7) | <0.001 | 170 (36.0) |
| 12–23 | 205 (52.4) | 34 (42.0) | 239 (50.6) | |
| 24–35 | 48 (12.3) | 3 (3.7) | 51 (10.8) | |
| 36–47 | 4 (1.0) | 1 (1.2) | 5 (1.1) | |
| 48–59 | 1 (0.3) | 6 (7.4) | 7 (1.5) | |
| Gender | ||||
| Male | 214 (54.7) | 29 (35.8) | 0.002 | 243 (51.5) |
| Female | 177 (45.3) | 52 (64.2) | 229 (48.5) | |
| Child's current main diet | ||||
| Breast milk | 75 (19.2) | 10 (12.4) | NS | 85 (18.0) |
| Semisolids | 115 (29.4) | 23 (28.4) | 138 (29.2) | |
| Household diet | 201 (51.4) | 48 (59.3) | 249 (52.8) | |
| Father's educational level | ||||
| Not formally educated | 193 (49.4) | 30 (37.0) | 0.043 | 223 (47.3) |
| Educated | 198 (50.6) | 51 (63.0) | 249 (52.8) | |
| Mother's educational level | ||||
| Not formally educated | 272 (69.6) | 51 (63.0) | NS | 323 (68.4) |
| Educated | 119 (30.4) | 30 (37.0) | 149 (31.6) | |
| Mother's employment status | ||||
| Unemployed | 105 (26.9) | 30 (37.0) | NS | 135 (28.6) |
| Employed | 286 (73.2) | 51 (63.0) | 337 (71.4) | |
| Father's employment status | ||||
| Unemployed | 3 (0.8) | 2 (2.5) | NS | 5 (1.1) |
| Employed | 388 (99.2) | 79 (97.5) | 467 (98.9) | |
| Social class | ||||
| Upper class | 10 (2.6) | 6 (7.4) | 0.03 | 16 (3.4) |
| Lower class | 381 (97.4) | 75 (92.6) | 456 (96.6) | |
| OTP location | ||||
| Rural | 243 (62.2) | 25 (30.9) | < 0.001 | 268 (56.8) |
| Urban | 148 (37.8) | 56 (69.1) | 204 (43.2) | |
NS, not significant; OTP, outpatient therapeutic programme.
Concurrently wasted and stunted children were predominately aged 12–23 mo (52.4%). In the non-stunted group, however, the largest proportion were aged 6–12 mo (45.7%). Age distribution varied significantly between both groups (p<0.001) and males were more likely to experience concurrent stunting compared with females (p=0.002). Current diet did not differ significantly between the groups.
Sixty-three percent of fathers of non-stunted children had formal education, while only 50.6% in the stunted group had any formal education and this difference was statistically significant (p=0.043). The proportions of employed parents were similar between both groups. A significantly higher proportion of children who were stunted came from a lower socioeconomic class (p=0.03) and also attended the rural OTP (p<0.001) compared with those who were not stunted.
Participant family and household characteristics
Table 2 shows the family and household characteristics of the study participants. Stunted and non-stunted child households did not differ significantly in terms of family structure or the number of people in each household. However, they differed significantly in terms of household toilet type distribution (p<0.001). Additionally, a significantly larger proportion of families with children who were concurrently stunted reared animals compared with the non-stunted group (p=0.001). The groups did not differ significantly regarding weekly family expenditure on perishable household foods.
Table 2.
Participant family and household characteristics.
| Variable | Stunted (%), n=391 | Not stunted (%), n=81 | p | Total (%), n=472 |
|---|---|---|---|---|
| Family structure | ||||
| Monogamous household | 144 (36.8) | 28 (34.6) | NS | 172 (36.4) |
| Polygamous household | 247 (63.2) | 53 (65.4) | 300 (63.6) | |
| Household toilet type | ||||
| Pit latrine | 325 (83.1) | 52 (64.2) | <0.001 | 377 (79.9) |
| Water closet | 61 (15.6) | 29 (35.8) | 90 (19.1) | |
| No household toilet | 5 (1.3) | 0 (0.0) | 5 (1.0) | |
| Household animal rearing | ||||
| Yes | 294 (75.2) | 46 (56.8) | 0.001 | 340 (72.0) |
| No | 97 (24.8) | 35 (43.2) | 132 (28.0) | |
| Number of people per household (median, IQR) | 9 (6) | 8 (6) | NS | 9 (6) |
| Number of children per household (median, IQR) | 6 (5) | 5 (5) | NS | 6 (5) |
| Weekly family expenditure on household perishable foods per person (Nigerian Naira) (median, IQR)a | 200 (272.2) | 250 (323.8) | NS | 209.2 (278.2) |
NS, not significant.
Perishable household foods were defined as money spent on animal protein and household vegetables. 1 USD = 367 Nigerian Naira (20 March 2020).
Analysis of factors associated with stunting
On multivariable analysis, age groups of 12–23 mo (Table 3; AOR=2.38, 95% CI 1.26 to 4.48) and 24–35 mo (Table 3; AOR=7.81, 95% CI 1.99 to 30.67) were associated with significantly increased odds for stunting compared with those aged <12 mo. Male children had a 2.5-fold greater probability of being stunted compared with female children (Table 3; AOR=2.51, 95% CI 1.43 to 4.39). Participants who attended the rural OTP had a threefold greater probability of stunting compared with those attending the urban OTP (Table 3; AOR=3.08, 95% CI 1.64 to 5.79). Diet, parental education and employment, family structure, number of people per household and social class were not significantly associated with increased odds of stunting (Table 3).
Table 3.
Crude ORs, AORs and 95% CIs of risk factors for stunting in the recruited cohort.
| Variable | Crude OR and 95% CI | AOR and 95% CIa |
|---|---|---|
| Age (mo) | ||
| 12–23 | 1.68 (1.00 to 2.80) | 2.38 (1.26 to 4.48) |
| 24–35 | 4.45 (1.31 to 15.11) | 7.81 (1.99 to 30.67) |
| 36–47 | 1.11 (0.12 to 10.26) | 1.72 (0.16 to 17.97) |
| 48–59 | 0.05 (0.01 to 0.40) | 0.18 (0.02 to 1.78) |
| 6–11 | 1 | 1 |
| Gender | ||
| Male | 2.17 (1.32 to 3.56) | 2.51 (1.43 to 4.39) |
| Female | 1 | 1 |
| Child's current main diet | ||
| Semisolids | 0.67 (0.30 to 1.48) | 0.96 (0.40 to 2.29) |
| Household diet | 0.56 (0.27 to 1.16) | 0.56 (0.23 to 1.34) |
| Breastfeeding | 1 | 1 |
| Paternal educational level | ||
| Any level of formal education | 0.60 (0.37 to 0.99) | 0.70 (0.40 to 1.24) |
| Not formally educated | 1 | 1 |
| Maternal educational level | ||
| Any level of formal education | 0.74 (0.45 to 1.23) | 1.43 (0.76 to 2.70) |
| Not formally educated | 1 | 1 |
| Maternal employment status | ||
| Employed | 1.60 (0.97 to 2.65) | 1.53 (0.85 to 2.75) |
| Unemployed | 1 | 1 |
| Paternal employment status | ||
| Employed | 3.27 (0.54 to 19.92) | 2.10 (0.22 to 19.93) |
| Unemployed | 1 | 1 |
| Family structure | ||
| Polygamous household | 0.91 (0.55 to 1.50) | 1.04 (0.54 to 2.01) |
| Monogamous household | 1 | 1 |
| Number of persons per household | ||
| ˃5 | 1.21 (0.70 to 2.08) | 0.99 (0.49 to 2.02) |
| ≤5 | 1 | 1 |
| Social classb | ||
| Lower class | 3.05 (1.08 to 8.64) | 2.42 (0.62 to 9.40) |
| Upper class | 1 | 1 |
| OTP type | ||
| Rural | 3.68 (2.20 to 6.15) | 3.08 (1.64 to 5.79) |
| Urban | 1 | 1 |
| Household animal rearing | ||
| Yes | 2.31 (1.40 to 3.79) | 1.04 (0.55 to 1.98) |
| No | 1 | 1 |
| Household toilet type | ||
| Modern toilet facility | 0.33 (0.20 to 0.56) | 0.59 (0.30 to 1.17) |
| Traditional sewage disposal methodc | 1 | 1 |
Each reported OR is adjusted for all other variables within the model.
Social class variable dichotomized—social class I, II and III—classified as upper class while IV and V were classified as lower class.
Traditional sewage disposal describes pit latrine and open bush disposal methods.
Discussion
Our study determined the prevalence of stunting among severely wasted children who were attending two acute malnutrition treatment clinics in north-western Nigeria. We also investigated associations for some previously established risk factors for stunting in this group. We found stunting to be highly prevalent among these children, as 82.8% of them were stunted. Being aged 12–35 mo, of male gender and attending the rural malnutrition clinic were associated with increased odds of stunting in this group.
In north-western Nigeria, approximately 50% of under-five children are stunted.10 In this study, 8 of 10 children with severe wasting were stunted, while 6 of 10 had severe stunting. These findings are similar to those from a recent study by Isanaka et al.20 in Niger, where the authors described a stunting prevalence of 79% among severely wasted under-fives attending OTP malnutrition clinics. By contrast, a recent Ugandan study described a much lower prevalence of 48.7%.19 It is, however, possible that the latter study might have underestimated the burden of stunting in these treatment programmes, as this was a retrospective cohort study of routinely collected programme data with almost 50% of eligible participants excluded on account of missing data.
In our study, >80% of our cohort who had concurrent severe wasting and stunting were aged 6–23 mo. Current evidence suggests that the stunting process is most responsive to interventions in the first 2 y of life.26 Other researchers have suggested that the critical window for reversal might extend far beyond the age of 2 y.37 Our findings thus have important implications for local malnutrition treatment programmes, which appear to have missed opportunities for early management of stunting. These programmes focus on clinical cures of severely wasted children using improvements in weight and mid-upper arm circumference and currently do not consider stunting.22 There is currently some evidence that prevalence of stunting among severely wasted children might worsen after discharge from outpatient malnutrition clinics.20 Additionally, recent evidence also suggests that declining linear growth following discharge from nutritional recovery programmes is associated with an increase of relapse.38 CMAM programmes, where severely wasted children are managed, already contain elements which can readily be adapted to also identify and control stunting in settings like ours with a high co-occurrence of both conditions.39 First, case identification for CMAM entrants should also include components such as determining height-for-age that seek to identify concurrent stunting in these children. Practically, this might work as a second tier for the screening process, where children already identified by less skilled workers in the community are further triaged by more skilled workers in the clinic setting. This would limit the opportunity cost of diverting clinic staff. Second, increasing the length of time spent within these programmes for concurrently stunted children while monitoring height-for-age might be beneficial. There is some evidence in the literature that suggests an improvement in height parameters may occur after certain thresholds in weight gain.40 Although in theory managing stunting would require a multi-interventional approach including nutrition counselling, social safety nets, growth monitoring and promotion and immunisation,41 CMAM offers a structured setting where gains can be monitored. Practically, however, further cost-benefit analysis research would be needed to evaluate this. In addition, ready-to-use therapeutic foods, a key element of CMAM, can be reformulated to treat stunting and previous randomized controlled trials have shown promising effects of some diets at reversing stunting.42–44 Other core elements of the CMAM programme, such as community engagement and acute malnutrition screening, could also be expanded to include stunting.39 These integrations would require additional expertise and resources and the key to success might reside in innovative methods that take into account operational realities in settings such as ours.
Additionally, we also found that both age group and gender are important risk factors for stunting among severely wasted new entrants into the local CMAM programme. These findings are similar to other studies among healthy under-fives.20,28,45 In our study, although severe wasting was more common in the younger age groups, on multivariable regression those aged 12–35 mo were at a greater risk of concurrent stunting compared with infants. Similar to our findings, El Taguri et al.28 in Libya observed that those aged 12–35 mo were at an increased risk of stunting. Our findings might be explained by children outside infancy having increased energy requirements that would require appropriate complementary feeding, which, if not met, play important roles in both stunting and wasting causation.26 Additionally, boys with severe wasting were more likely to be stunted compared with girls. Similar findings of a high likelihood for stunting have been observed among healthy male under-fives in other studies.10,20,46 A recent study of concurrent wasting and stunting in acute treatment programmes for malnutrition has also described significant risk factors for concurrent stunting among male children in age groups of <30 mo.14 Our findings also suggest that male gender is an important risk factor for stunting among severely wasted under-five preschool-aged children. A possible explanation might be related to differential physical activity across genders. Male children are on average more physically active than female children,47 thus it is plausible that they have a higher propensity to manifest features of a negative energy balance.
Our study also documented that children with severe wasting who attended the rural outpatient therapeutic malnutrition clinics were more likely to be stunted than those who attended the urban centre. Other studies have also documented that rural-based children are at an increased risk of stunting. One earlier study among under-five Nigerian children without wasting demonstrated an increased risk for stunting among rural-based children.18 This finding has also been observed in other low and middle income countries.45 While we acknowledge that our number of sites limits the generalisability of this finding, local CMAM sites are organised in such a way that entrants to these sites are from multiple surrounding communities, as such findings from one site generally reflect the occurrences of multiple surrounding communities. We believe this finding might be related to rural–urban inequities in access to healthcare and basic social amenities such as pipe-borne water. Stunting is strongly related to water, sanitation and hygiene (WASH) practices and health.35 Rural areas lack many of these facilities and, as such, rurality in our study might be a proxy for access to WASH. In contrast to our findings, Mushtaq et al.,32 in addition to documenting a higher prevalence of stunting in rural areas, also documented an increased risk of stunting in some urban areas. These urban areas were low socioeconomic areas, further lending credence to our assertion that an association of stunting with rurality might be the result of access to basic amenities.
Strengths and limitations
Our study is one of a few studies to describe the burden of concurrent stunting and severe wasting among new entrants to malnutrition outpatient clinics and we have documented a high burden in some north-western Nigeria clinics. This suggests a need to adapt local malnutrition treatment programmes to also cater for stunting.
Because our study was a secondary analysis of previously published cross-sectional data, we could not include follow-up data on stunting outcomes as provided by previously published studies.20 We have, however, broadened our investigation of potential risk factors for concomitant stunting by considering household-level risk factors and have described an association with rurality that would need confirmation by larger multi-site studies comparing a larger number of rural and urban malnutrition clinics. The sequential nature of our data collection by the same research team, that is, data collection which was completed in the urban area before the rural collection began, may have ensured that clinic level variations minimally affected our results.
Conclusions
The majority of children attending our studied acute malnutrition OTPs had concurrent stunting and presented at ages when stunting was potentially reversible. Local CMAM programmes might thus represent missed opportunities at managing stunting. This strongly suggests the need for more integrated malnutrition treatment services catering for both severe wasting and stunting. As a first step, these programmes need to incorporate identification of concurrent stunting into CMAM community case finding. However, further research is needed to determine the most effective ways of managing stunting within these programmes, and this will need to take into consideration practical programmatic realities such as available expertise and scarce resource allocation.
Acknowledgements
The authors would like to acknowledge all staff of the Community Management of Acute Malnutrition outpatient therapeutic programmes at Ali-Akilu Memorial Hospital, Madobi and Murtala Muhammed Specialist Hospital, Kano.
Contributor Information
Abdulazeez Imam, Department of Vaccines and Immunity, Medical Research Council Unit The Gambia at London School of Hygiene and Tropical Medicine, Atlantic Boulevard, P.O. Box 452, Fajara, Gambia.
Fatimah Hassan-Hanga, Department of Paediatrics, Bayero University Kano, Department of Paediatrics, Aminu Kano Teaching Hospital, P.M.B 3452, Kano, Nigeria.
Azeezat Sallahdeen, Department of Vaccines and Immunity, Medical Research Council Unit The Gambia at London School of Hygiene and Tropical Medicine, Atlantic Boulevard, P.O. Box 452, Fajara, Gambia.
Zubaida L Farouk, Department of Paediatrics, Bayero University Kano, Department of Paediatrics, Aminu Kano Teaching Hospital, P.M.B 3452, Kano, Nigeria.
Authors’ contributions
AI, FH and ZLF conceived and designed the study; AI, FH, AS and ZLF contributed to clinical data collection; AI analysed the data; all authors contributed to all aspects of manuscript writing including drafting the first draft of the manuscript, final manuscript revisions and approval of the final version; AI is the guarantor of the paper.
Funding
This work was not supported by any funding.
Competing interests
The authors declare no conflicts of interests.
Ethical approval
The study was approved by the Institutional Review Board of Aminu Kano Teaching Hospital (reference number: NHREC/21/08/2008/AKTH/EC/1280) and informed consent was provided by the participating caregivers prior to study enrolment.
References
- 1. WHO . UNICEF-WHO-The World Bank: Joint child malnutrition estimates - Levels and trends. WHO. http://www.who.int/nutgrowthdb/estimates/en/. Accessed 22 January 2020. [Google Scholar]
- 2. de Onis M, Branca F. Childhood stunting: a global perspective. Matern Child Nutr. 2016;12(S1):12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Emerson E, Savage A, Llewellyn G. Prevalence of underweight, wasting and stunting among young children with a significant cognitive delay in 47 low-income and middle-income countries. J Intellect Disabil Res. 2020;64(2):93–102. [DOI] [PubMed] [Google Scholar]
- 4. McDonald CM, Olofin I, Flaxman Set al.. The effect of multiple anthropometric deficits on child mortality: meta-analysis of individual data in 10 prospective studies from developing countries. Am J Clin Nutr. 2013;97(4):896–901. [DOI] [PubMed] [Google Scholar]
- 5. De Onis M, Dewey KG, Borghi Eet al.. The World Health Organization's global target for reducing childhood stunting by 2025: rationale and proposed actions. Matern Child Nutr. 2013;9:6–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grover Z, Ee LC. Protein energy malnutrition. Pediatr Clin North Am. 2009;56(5):1055–68. [DOI] [PubMed] [Google Scholar]
- 7. Briend A, Khara T, Dolan C. Wasting and stunting—similarities and differences: policy and programmatic implications. Food Nutr Bull. 2015;36(suppl 1):S15–23. [DOI] [PubMed] [Google Scholar]
- 8. Büyükgebiz B, Öztürk Y, Yilmaz S, et al. Serum leptin concentrations in children with mild protein-energy malnutrition and catch-up growth. Pediatr Int. 2004;46(5):534–8. [DOI] [PubMed] [Google Scholar]
- 9. Schoenbuchner SM, Dolan C, Mwangome Met al.. The relationship between wasting and stunting: a retrospective cohort analysis of longitudinal data in Gambian children from 1976 to 2016. Am J Clin Nutr. 2019;110(2):498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Bureau of Statistics . National nutrition and health survey (NNHS) 2018. https://www.unicef.org/nigeria/media/2181/file/Nigeria-NNHS-2018.pdf. Accessed 31 July 2019.
- 11. Collins S. Changing the way we address severe malnutrition during famine. Lancet. 2001;358(9280):498–501. [DOI] [PubMed] [Google Scholar]
- 12. Chitekwe S, Biadgilign S, Tolla Aet al.. Mid-upper-arm circumference based case-detection, admission, and discharging of under five children in a large-scale community-based management of acute malnutrition program in Nigeria. Arch Public Health. 2018;76(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Collins S, Dent N, Binns Pet al.. Management of severe acute malnutrition in children. Lancet. 2006;368(9551):1992–2000. [DOI] [PubMed] [Google Scholar]
- 14. Garenne M, Myatt M, Khara Tet al.. Concurrent wasting and stunting among under-five children in Niakhar, Senegal. Matern Child Nutr. 2019;15(2):e12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Odei Obeng-Amoako GA, Myatt M, Conkle Jet al.. Concurrently wasted and stunted children 6–59 months in Karamoja, Uganda: prevalence and case detection. Matern Child Nutr. 2020;e13000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saaka M, Galaa SZ. Relationships between wasting and stunting and their concurrent occurrence in Ghanaian preschool children. J Nutr Metab. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lelijveld N, Seal A, Wells JCet al.. Chronic disease outcomes after severe acute malnutrition in Malawian children (ChroSAM): a cohort study. Lancet Glob Health. 2016;4(9):e654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kerac M, Bunn J, Chagaluka Get al.. Follow-up of post-discharge growth and mortality after treatment for severe acute malnutrition (FuSAM study): a prospective cohort study. PloS One. 2014;9(6):e96030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Odei Obeng-Amoako GA, Wamani H, Conkle Jet al.. Concurrently wasted and stunted 6–59 months children admitted to the outpatient therapeutic feeding programme in Karamoja, Uganda: Prevalence, characteristics, treatment outcomes and response. PloS One. 2020;15(3):e0230480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Isanaka S, Hitchings MDT, Berthé Fet al.. Linear growth faltering and the role of weight attainment: prospective analysis of young children recovering from severe wasting in Niger. Matern Child Nutr. 2019;15(4):e12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Imam A, Farouk ZL, Hassan-Hanga Fet al.. A comparative cross-sectional study of prevalence and intensity of soil-transmitted helminthic infection between healthy and severe acutely malnourished pre-school aged children in Kano, Northern Nigeria. BMC Infect Dis. 2019;19(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. National Guideline for Community Management of Acute Malnutrition . Federal Ministry of Health, Family Health Department, Nutrition Division; 2011. 1–55.
- 23. Knoema. Kano – Nigeria. Data and Statistics. http://nigeria.opendataforafrica.org//apps/atlas/Kano. Accessed 31 July 2019.
- 24. WHO, UNICEF . WHO child growth standards and the identification of severe acute malnutrition in infants and children: a joint statement by the World Health Organization and the United Nations Children's Fund. Geneva: WHO; 2009.http://apps.who.int/iris/handle/10665/44129. Accessed 7 May 2017. [PubMed] [Google Scholar]
- 25. Rosalind SG. Nutritional Assessment. A Laboratory Manual. New York, NY, USA: Oxford University Press; 1993. [Google Scholar]
- 26. Prendergast AJ, Humphrey JH. The stunting syndrome in developing countries. Paediatr Int Child Health. 2014;34(4):250–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Imam A, Hassan-Hanga F, Sallahdeen Aet al.. Socio-demographic and household-level risk factors for severe acute malnutrition in pre-school children in north-western Nigeria. J Trop Pediatr. 2020May 17 [cited 2020 Jun 1] Available from: 10.1093/tropej/fmaa018 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 28. El Taguri A, Betilmal I, Mahmud SMet al.. Risk factors for stunting among under-fives in Libya. Public Health Nutr. 2009;12(8):1141–9. [DOI] [PubMed] [Google Scholar]
- 29. Miyazaki A, Matsui M, Tung Ret al.. Determinants of growth measurements in rural Cambodian infants: a cross-sectional study. Int Health. 2020May 7 [Accessed 2020 Jun 1] Available from: 10.1093/inthealth/ihaa018 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hossain A, Niroula B, Duwal Set al.. Maternal profiles and social determinants of severe acute malnutrition among children under-five years of age: a case-control study in Nepal. Heliyon. 2020;6(5):e03849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Senbanjo IO, Olayiwola IO, Afolabi WAet al.. Maternal and child under-nutrition in rural and urban communities of Lagos state, Nigeria: the relationship and risk factors. BMC Res Notes. 2013;6(1):286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mushtaq MU, Gull S, Khurshid Uet al.. Prevalence and socio-demographic correlates of stunting and thinness among Pakistani primary school children. BMC Public Health. 2011;11(1):790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Senbanjo IO, Oshikoya KA, Odusanya OOet al.. Prevalence of and risk factors for stunting among school children and adolescents in Abeokuta, southwest Nigeria. J Health Popul Nutr. 2011;29(4):364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Amsalu S, Tigabu Z. Risk factors for ever acute malnutrition inchildren under the age of five: a case-control study. Ethiop J Health Dev. 2008;22(1):21–5. [Google Scholar]
- 35. Fenn B, Bulti AT, Nduna Tet al.. An evaluation of an operations research project to reduce childhood stunting in a food-insecure area in Ethiopia. Public Health Nutr. 2012;15(9):1746–54. [DOI] [PubMed] [Google Scholar]
- 36. Oyedeji GA. Socio-economic and cultural background of hospitalized children in Ilesa. Niger J Paediatr. 1985;12:111–7. [Google Scholar]
- 37. Prentice AM, Ward KA, Goldberg GRet al.. Critical windows for nutritional interventions against stunting. Am J Clin Nutr. 2013;97(5):911–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stobaugh HC, Rogers BL, Rosenberg IHet al.. Children with poor linear growth are at risk for repeated relapse to wasting after recovery from moderate acute malnutrition. J Nutr. 2018;148(6):974–9. [DOI] [PubMed] [Google Scholar]
- 39. Bergeron G, Castleman T. Program responses to acute and chronic malnutrition: divergences and convergences. Adv Nutr. 2012;3(2):242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Walker SP, Golden MH. Growth in length of children recovering from severe malnutrition. Eur J Clin Nutr. 1988;42(5):395–404. [PubMed] [Google Scholar]
- 41. Hossain M, Choudhury N, Abdullah KABet al.. Evidence-based approaches to childhood stunting in low and middle income countries: a systematic review. Arch Dis Child. 2017;102(10):903–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Phuka JC, Maleta K, Thakwalakwa Cet al.. Complementary feeding with fortified spread and incidence of severe stunting in 6-to 18-month-old rural Malawians. Arch Pediatr Adolesc Med. 2008;162(7):619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Adu-Afarwuah S, Lartey A, Brown KHet al.. Randomized comparison of 3 types of micronutrient supplements for home fortification of complementary foods in Ghana: effects on growth and motor development. Am J Clin Nutr. 2007;86(2):412–20. [DOI] [PubMed] [Google Scholar]
- 44. Iannotti LL, Dulience SJ, Green Jet al.. Linear growth increased in young children in an urban slum of Haiti: a randomized controlled trial of a lipid-based nutrient supplement. Am J Clin Nutr. 2014;99(1):198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Paciorek CJ, Stevens GA, Finucane MMet al.. Children's height and weight in rural and urban populations in low-income and middle-income countries: a systematic analysis of population-representative data. Lancet Glob Health. 2013;1(5):e300–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Akombi BJ, Agho KE, Hall JJet al.. Stunting and severe stunting among children under-5 years in Nigeria: a multilevel analysis. BMC pediatrics. 2017;17(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Finn K, Johannsen N, Specker B. Factors associated with physical activity in preschool children. J Pediatr. 2002;140(1):81–5. [DOI] [PubMed] [Google Scholar]