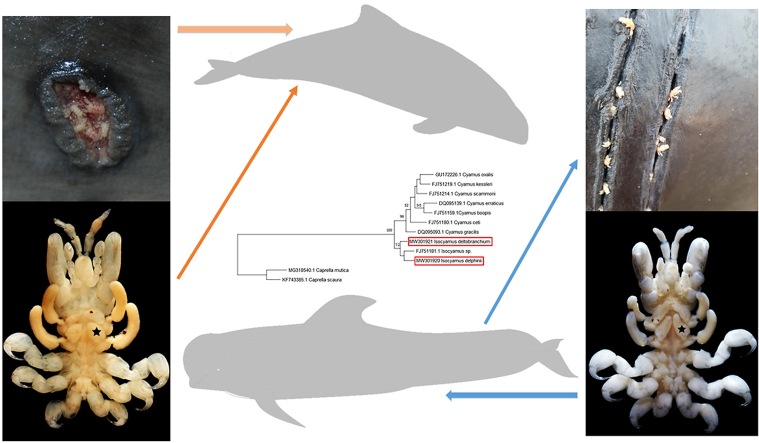
Abstract
Whale lice (Cyamidae; Amphipoda) are ectoparasitic crustaceans adapted to the marine environment with cetaceans as their host. There are few reports of cyamids occurring in odontocetes from the North Sea, and long-term studies are lacking. Marine mammal health was monitored along the German and Dutch coasts in the past decades, with extensive post mortem investigations conducted. The aim of this study was to analyse archived ectoparasite samples from stranded cetaceans from the North Sea (2010–2019), to determine species, prevalence and impact of ectoparasite infection. Ectoparasites were found on two cetacean species – harbour porpoises (Phocoena phocoena), as the most abundant cetacean species in the North Sea, and on a pilot whale (Globicephala melas), as a rare species here. Prevalence of ectoparasitic crustaceans in cetaceans was low: 7.6% in porpoises stranded in the Netherlands (n = 608) and 1.6% in porpoises stranded in Germany (n = 122). All whale lice infections were found on hosts with skin lesions characterised by ulcerations. Morphological investigations revealed characteristic differences between the cyamid species Isocyamus (I.) delphinii and I. deltobranchium identified. Isocyamus deltobranchium was determined in all infected harbour porpoises. I. delphinii was identified on only the pilot whale. Molecular analyses showed 88% similarity of mDNA COI sequences of I. delphinii with I. deltobranchium supporting them as separate species. Phylogenetic analyses of additional gene loci are required to fully assess the diversity and exchange of whale lice species between geographical regions as well as host specificity. Differing whale lice prevalences in porpoises stranded in the Netherlands and Germany could indicate a difference in severity of skin lesions between these areas. It should be further investigated if more inter- or intraspecific contact, e.g., due to a higher density of porpoises or contact with other cetaceans, or a poorer health status of porpoises in the southern North Sea could explain these differences.
Keywords: Amphipoda, Odontocetes, Ectoparasitic crustacean, Host specificity, Ecology, Pathology
Graphical abstract
Highlights
-
•
First long term study of ectoparasitic crustaceans on North Sea odontocetes.
-
•
Differences in prevalence in harbour porpoises between German and Dutch waters.
-
•
Two cyamid species identified using morphological and molecular traits.
-
•
associated to skin lesions in all investigated harbour porpoises and pilot whale.
-
•
I. deltobranchium and I. delphinii closely related.
1. Introduction
Harbour porpoises (Phocoena phocoena) are one of the reproducing marine mammal species in the North Sea (Hammond et al., 2002) while pilot whales (Globicephala melas) are mostly visitors or travelling through on their way to the Bay of Biscay or Norwegian deep (IJsseldijk et al., 2015). Both species are at the top of the food chain and can be considered as indicators for ecosystem health (Bossart, 2006) because their distribution, health status and infectious diseases can reflect changes in their habitat (Hilty and Merenlender, 2000). Little is known about their ectoparasites, although marine mammal parasites are increasingly used as biomarkers for habitat use, health monitoring, migrations of marine mammal hosts in various geographical areas and can help to better understand species interactions and their ecology (e.g. Aznar et al., 1994; MacKenzie, 2004; Vidal-Martínez et al., 2010).
Whale lice (Cyamidae; Amphipoda) are ectoparasitic crustaceans specific for cetaceans that have conquered the abandoned niche of insect ectoparasites in the marine realm. Whale lice have adapted to their marine environment in developing small, dorso-ventrally compressed body sizes with five pairs of legs ending in claw-like appendices and spines that help them to cling to their cetacean host (Rowntree, 1996). Whale lice spend their entire life on their host, without swimming stages (Roussel de Vauzème, 1834; Leger et al., 2018) and therefore rely on physical contact during reproduction, nursing or aggressive behaviour in males for transmission (Balbuena and Raga, 1991; Leger et al., 2018). This trait sets them apart from other (endo)parasites that often rely on indirect life cycles and trophic interactions involving prey organisms (Poulin and Leung, 2011). The care-giving (epimeletic) behaviour exhibited by whales and inter-specific social behavior may explain how whale lice are able to spread between host species (Boxshall et al., 2005). While slow-moving baleen whales exhibit grooves and callosities for whale lice to attach to, they tend to aggregate in the genital slit, corners of the mouth or the blowhole (Rowntree, 1996) and in lesions with thickened edges in faster swimming hydrodynamic odontocetes. Whale lice ingest small pieces of skin and scar tissue (Schell et al., 2000), but may also feed on plankton (Rowntree, 1996). Whale lice on mysticetes are known to be host-specific (Kaliszewska et al., 2005), while those on odontocetes are more generalist, especially Isocyamus species (Sedlak-Weinstein, 1992a; Martin and Heyning, 1999).
The genus Isocyamus first included only Isocyamus delphinii (Leung, 1967) which has been found on multiple toothed whales worldwide (Wardle et al., 2000; Pfeiffer, 2002; Haney et al., 2004) by now. Later I. kogiae Sedlak-Weinstein, 1992, found in pygmy sperm whale (Kogia breviceps) from Australia and Southern California (Sedlak-Weinstein, 1992a; Martin and Heyning, 1999), and I. deltobranchium, described from pilot whales (G. macrorhynchus and G. melas) in Japanese and Tasmanian waters were added (Sedlak-Weinstein, 1992b). Also, I. antarcticensis was reported from orcas (Orcinus orca) in Antarctica (Berzin and Vlasova, 1982). Recently, I. indopacetus was described as a new species parasitizing Longman's beaked whale (Indopacetus pacificus) from the South Pacific (Iwasa-Arai et al., 2017a), underlining the global distribution of Isocyamus and scarce knowledge about these generalist ectoparasites. Isocyamus delphinii (Cyamidae, Amphipoda) were reported on harbour porpoises, pilot whales, and white-beaked dolphins (Lagenorhynchus albirostris) from the North Atlantic (Balbuena and Raga, 1991; Stock 1973a, b; 1977) and have been used as indicators for social structure and behavior of their hosts (Balbuena and Raga, 1991; Balbuena et al., 1995) because they can reveal information about species interactions or migration of their host (Fraija‐Fernández et al., 2017, Lehnert et al., 2007). In porpoises, they were mostly found associated with skin lesions (Stock, 1973a; Lehnert et al., 2007) but although there are case reports describing the presence of whale lice on cetaceans in the North Sea (Stock, 1973b, 1977; Fransen and Smeenk 1991; Lehnert et al., 2007), systematic and long-term surveys regarding prevalence and intensity of infections as well as impacts on host health are lacking. Additionally, there is some uncertainty about the taxonomy and species classification of Isocyamus spp. in previous studies (Martínez et al., 2008). Therefore, the aim of this study was to report prevalence of whale lice on stranded harbour porpoises from the Dutch and German North Sea coastlines, identify the species, their microhabitat and assess the associated pathology.
This study is the first systematic survey monitoring the occurrence of ectoparasitic crustaceans specific to odontocetes, including morphological and molecular data, as well as information about microhabitat selection and pathological lesions in Dutch and German waters.
2. Material and methods
2.1. Post-mortem examinations
In the German Federal State of Schleswig-Holstein (S–H), stranded marine mammal carcasses are examined within a coordinated stranding network since 1990. Fresh or frozen cetacean carcasses are then transported to the Institute for Terrestrial and Aquatic Wildlife Research (ITAW) in Buesum, where necropsies and tissue sampling are performed following established protocols (Lehnert et al., 2005; Siebert et al., 2007; IJsseldijk, Brownlow, Mazzariol, 2019) and state of preservation as well as nutritional status are recorded (Siebert et al., 2001). In the Netherlands, stranded and bycaught cetaceans are necropsied since 2008 by the Division of Pathology of the Faculty of Veterinary Medicine of Utrecht University. Necropsies and tissue sampling are conducted following internationally standardized guidelines, including the assessment of the state of decomposition and nutritional status (IJsseldijk, Brownlow, Mazzariol, 2019).
For all cases, sex and age class were determined, with the latter based on total length (in cm, measured in a straight line alongside the body from the tip of the rostrum to the fluke notch) and assessment of reproductive organs (Table S1). According to their length (Siebert et al., 2001, 2006) or the tooth growth layer group counts (Lockyer, 1995), animals were classified as immatures (≤100 cm in length/≤0.5 years = neonates or calves; 101–130 cm in length/>0.5–4 years = juveniles) or adults (>130 cm in length/>4 years). For the animals which were not aged, gross assessment of reproductive organs was used to differentiate between immatures and matures, especially for those in the range of 120–140 cm total length. Additionally, each case was appointed a decomposition condition code (DCC), on a five-point scale with DCC1 representing very fresh carcasses and DCC5 the skeletal remains of animals. Carcasses of marine mammals are screened routinely for ecto- and endo-parasites. The level of parasitic infection is determined macroscopically and semi-quantitatively during necropsy (Siebert et al., 2001; Lehnert et al., 2005) and associated lesions recorded and submitted for histopathological investigations. In the Netherlands, prior to 2016 representative whale lice specimens were collected from most animals while in previous years only presence/absence was recorded. From 2016, all whale lice specimens were collected in 70% ethanol. Between 1st of January 2010 and 31st of May 2019 a total of 122 porpoises in DCC1-3 found stranded along the North Sea coast of Schleswig-Holstein, Germany and a total of 608 porpoises in DCC1-3 on the coast of the Netherlands were selected for this study. Proportionally, there are more neonates found on German coast whilst on the Dutch coast strandings are mainly juveniles, however for adult porpoises, proportions are comparable (IJsseldijk et al., 2020).
Necropsy reports and pictures taken during the post-mortem examinations were retrospectively analysed to assess whale lice prevalence and associated pathology. In addition, one pilot whale with whale lice infection was necropsied in the Netherlands in 2018, and information added to this study. For histopathology, samples of skin lesions were collected, fixed in 10% neutral buffered formalin and routinely embedded in paraffin wax. Tissue sections of 3–4 μm were cut and subsequently stained with haematoxylin and eosin (HE).
Whale lice from the Netherlands from sample containers were counted and a semi-quantitative level of infection was determined: mild = 5 specimens or less, moderate = 6–19, severe = 20 specimens or more. The sex ratio of the whale lice was determined individually for all hosts infected by ≤ 20 whale lice specimens by calculating the ratio of the total number of male cyamids to the total number of female cyamids.
2.2. Morphological and molecular identification
Whale lice were identified based on their morphological characteristics in accordance with the descriptions by Leung (1965), Martínez et al. (2008) and Fransen and Smeenk (1991). Juveniles, mature males, mature females with brood pouch and eggs or juveniles, and females without a brood pouch were distinguished. Males were distinguished by the genitalia located on pereon segment 7. Females were identified by their genital valve located on the pereon segment 5 and their brood pouch. All cyamids with no obvious genitalia and smaller than the smallest identifiable male were considered juveniles. To differentiate species, the shape of the accessory gills in adult males was compared (Sedlak-Weinstein, 1991; Martìnez et al., 2008).
From a subset of the infected harbour porpoises (n = 36) stranded in the Netherlands between 2010 and 2019, 699 whale lice were collected, and 29 whale lice from the single pilot whale (Table S1). The length of whale lice were measured from the anterior border of the head (excluding antennae) to the posterior border of the last segment of the pereon including the pleon using CellSens Entry software with a Stereomicroscope (Olympus CX 41) with 100x magnification and attached camera (Olympus SC30). The width was measured at pereon segment 5 for males, females, and juveniles. For documenting specimens at the Center of Natural History of the Zoological Museum Hamburg (CeNak), adults and juveniles were photographed using the BK Plus Lab System (Dun, Inc.) with 5x and 10x LD Mitutoyo objectives and integrated Canon (Canon 7D Mark II (20 Megapixels) Micro camera and the Canon 5DS (50 Megapixels) Macro camera). The images were captured and stacked with the Zerene Stacker software version 1.04.
To achieve parasite identification using gene sequence data, DNA was isolated from 6 whale lice specimens of four harbour porpoise hosts (three from Dutch and one from German waters) and four whale lice specimen from one pilot whale host using a QIAamp Tissue Kit (Qiagen, Hilden, Germany). DNA concentrations and purity were determined using a Nanodrop 2000c (Thermo Scientific) spectrophotometer. The mitochondrial gene encoding cytochrome c oxidase subunit 1 (COI, cox1) was amplified from these 10 whale lice. Approximately 740 bp of the COI gene was amplified using oligonucleotide primers Jercy (5′ TAC CAA CAT TTA TTC TGR TTT TTY GG 3′) and Patcy (5′ACT AGC ACA TTT ATC TGT CAC ATT A 3′) (Kaliszewska et al., 2005). Polymerase chain reactions (PCR) were performed in a total volume of 50 μl, comprising 25 μl of MyTaq Red Mix, 2x (Bioline GmbH, Germany), 1 μl each of primers at 20 μM, 1 μl of DNA template and 22 μl of DEPC-H2O in a TGradient cycler (Biometra, Germany). Cycling conditions were denaturation at 95 °C for 1 min, followed by 35 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 15 s and extension at 72 °C for 10 s. PCR products were visualised on a 1.5% agarose gel using SYBRSafe DNA Gel stain on a UVP Gelsolo gel documentation system (Analytik-Jena, Germany). PCR products were sent alongside primers in separate tubes to Microsynth Seqlab (Göttingen, Germany) for Sanger Sequencing. The closest match to the sequence was determined using blastn on GenBank. Sequences were aligned using Clustal W in DNAstar (version 5.07/5.52). A maximum likelihood phylogeny tree with 1000 bootstrap replicates was constructed with the sequences obtained from this study and cyamid sequences available on GenBank using the MEGA software (version X). The GTR+I+G model was chosen as the best model as indicated by ModelTest-NG (version 0.1.5) (Miller et al., 2010). Voucher specimens were deposited at the CeNak, Zoological Museum - University of Hamburg, Germany (accession nos. ZMH K-60669, ZMH K-60670, ZMH K-60671). COI sequences obtained from I. delphinii (Accession No. MW301920) and I. deltobranchium (Accession No. MW301921) were submitted to NCBI GenBank.
An extensive literature review and comparison of old I. delphinii drawings and descriptions was conducted to reconstruct historical records of I. delphinii from German and Dutch waters. Specimens collected from a pilot whale off the Faroese Islands by Balbuena and Raga (1991) were used as reference material.
2.3. Statistics
Statistical analyses were performed using R (version 3.6.2). Regression analyses were performed to show the relationship between number of juvenile whale lice and total number of whale lice found on hosts, and also length of whale lice and the total number of whale lice. Additionally, a Mann-Whitney-U Test was used to analyse whale lice infection levels within harbour porpoise host age classes (adults and juveniles) and within porpoise sexes (females and males).
3. Results
3.1. Prevalence of whale lice infections on harbour porpoises between 2010 and 2019
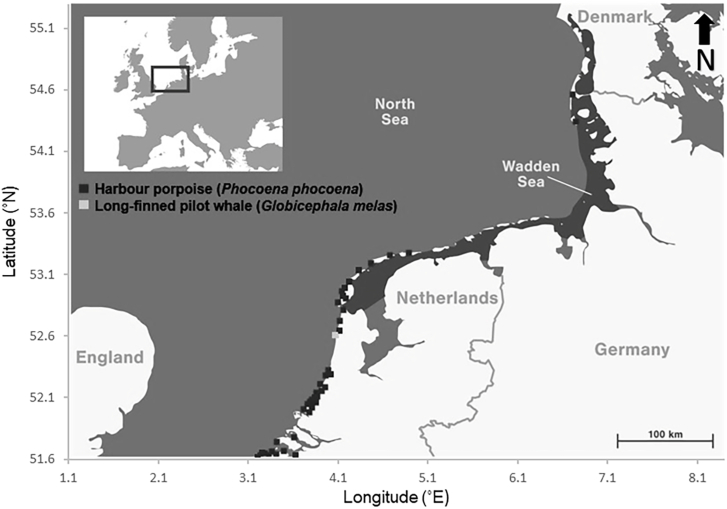
In 7.6% (n = 46) of the Dutch harbour porpoises stranded between 1st of January 2010 and 31st of May 2019 (n = 608) whale lice were found (Fig. 1). The prevalence of whale lice in adult harbour porpoises (12.6%) was twice as high as the prevalence in juvenile harbour porpoises (6.7%) between 2010 and 2019. No neonates were infected. In the freshest carcasses (DCC1), the prevalence of whale lice was the highest (14%), and it steeply declined with the carcasses becoming more putrefied (DCC2 = 10%, DCC3 = 1%). The prevalence of whale lice in emaciated porpoises (14%) was four times as high as in well-nourished porpoises (4%). Although 2014 was a year without any whale lice infected harbour porpoises, the number increased in 2015 to 10.0%, and to 12.3% in 2016. Of the infected harbour porpoise hosts with whale lice samples available for further analysis (n = 36), 55.6% (n = 20) were male and 44.4% (n = 16) were females. 50% (n = 18) were juvenile harbour porpoises and 50% (n = 18) adults. 13 male juveniles, 5 female juveniles, 7 male adults and 11 female adults were infected.
Fig. 1.
Map of German and Dutch coast with sampling locations of harbour porpoises and pilot whale.
In harbour porpoises stranded in Germany, two adult porpoises (1.6%) were found infected with whale lice (2/122), one male and one female animal, both in poor nutritional status and with skin lesions.
3.2. Morphology and demographics
Ectoparasitic crustaceans consisting of 598 mature and 93 juvenile specimens, and 8 badly damaged specimens were collected on the harbour porpoises. Among the 598 specimens, 315 male and 283 female mature individuals were counted.
All cyamids (n = 699) from the Dutch harbour porpoises (n = 36) were identified as Isocyamus deltobranchium, based on their triangular accessory gills (Fig. 2A). The specimens of I. deltobranchium presented a yellow-whitish body, thin and elongate, not ovate, with a smooth cuticle and flattened (Fig. 2B). Many of the cyamids had debris between their forearms: diatom or skin. The females with a visible brood pouch (n = 235) measured between 1.98 and 5.17 mm in length, 0.9–2.24 mm in width, the females with no brood pouch (n = 51) between 1.52 and 3.82 mm in length, 0.73–1.77 mm in width, the males (n = 315) between 1.31 and 6.04 mm in length, 0.58–2.4 mm in width. The juvenile (n = 90) specimens were 0.79–2.63 mm in length and 0.22–1.36 mm in width (Table 1).
Fig. 2.
A: Isocyamus deltobranchium adult male ventral view showing genitalia and accessory gills; B: Isocyamus deltobranchium adult female dorsal view; C: Isocyamus deltobranchium adult female ventral view showing genitalia and broodpouch; D: adult female ventral view with juveniles in brood pouch, all sampled from a stranded harbour porpoise (Phocoena phocoena) on the Dutch coast.
Table 1.
Measurements (mm) of the whale louse Isocyamus deltobranchium (n = 691) found on stranded harbour porpoises (Phocoena phocoena) (n = 36) stranded on the Dutch coast between 2010 and 2019.
| LENGTH (mm) |
WIDTH (mm) |
|||||
|---|---|---|---|---|---|---|
| min | mean | max | min | mean | max | |
| Female (n = 235) | 1.98 | 3.60 | 5.17 | 0.90 | 1.72 | 2.24 |
| Female no brood pouch (n = 51) | 1.52 | 2.68 | 3.82 | 0.73 | 1.27 | 1.77 |
| Male (n = 315) | 1.31 | 3.47 | 6.04 | 0.58 | 1.58 | 2.40 |
| Juvenile (n = 90) | 0.79 | 1.90 | 2.63 | 0.22 | 0.84 | 1.36 |
In mature female specimens of I. deltobranchium, an absence of visible ventral spines (with an optic microscope) at the base of the genital valves on pereon 5 and much-reduced accessory gills compared to those of mature male cyamids was observed. Three pairs of ventral spines were found on pereon 6 and 7 and an empty marsupium pouch formed by four segments on pereon 3 and 4 (Fig. 2C). Gravid females carry their young in a brood pouch until juveniles are released (Fig. 2D).
Six male, fifteen female and eight juvenile whale lice were recovered from the stranded adult male pilot whale. All of them (n = 29) were identified as I. delphinii, based on their elongated cylindrical accessory gills and three pairs of ventral spines on pereon segments 5 to 7. I. delphinii females with visible brood pouch (n = 10) measured between 3.66 and 5.37 mm in length, 1.61–2.4 mm in width, females with no brood pouch (n = 5) between 2.95 and 3.54 mm in length, 1.41–2.5 mm in width, males (n = 6) between 3.69 and 4.9 mm in length, 1.55–2.5 mm in width. The juvenile (n = 8) specimens were 1.7–2.08 mm in length and 0.68–0.96 mm in width (Table 2).
Table 2.
Measurements (mm) of the whale louse Isocyamus delphinii (n = 29) found on the stranded pilot whale (Globicephala melas) on the Dutch coast (n = 1) in November 2018.
| LENGTH (mm) |
WIDTH (mm) |
|||||
|---|---|---|---|---|---|---|
| min | mean | max | min | mean | max | |
| Female (n = 10) | 3.66 | 4.38 | 5.37 | 1.61 | 1.96 | 2.40 |
| Female no brood pouch (n = 5) | 2.95 | 3.21 | 3.54 | 1.41 | 2.04 | 2.50 |
| Male (n = 6) | 3.69 | 4.43 | 4.90 | 1.55 | 2.04 | 2.50 |
| Juvenile (n = 8) | 1.70 | 1.88 | 2.08 | 0.68 | 0.83 | 0.96 |
Typical characteristics to differentiate I. deltobranchium from I. delphinii are the accessory gills of males and juveniles on the ventral sides of pereons 3 and 4, triangular in form, slightly flattened and half the length of the primary gills in I. deltobranchium (Fig. 3A, asteriks). In contrast the accessory gills of I. delphinii show a cylindrical form similar in shape and length to the primary gills (Fig. 3B). In both I. delphinii as well as in I. deltobranchium a difference between males and females was observed: in females the ventral spines on pereon 5 were found to get progressively smaller as they matured and in males ventral spines were always visible on pereon 5 regardless of maturation stage. In all specimens of I. deltobranchium and I delphinii ventral spines were observed on pereon 6 and 7.
Fig. 3.
(A) Isocyamus deltobranchium and (B) Isocyamus delphinii showing the characteristic accessory gills (asterisk).
Of the 21 infected harbour porpoises stranded between 2016 and 2019 in the Netherlands, from which all whale lice present were sampled, and intensity data was thus available, 47.6% (n = 10) were infected mildly, 14.3% (n = 3) moderately and 38.1% (n = 8) were severely infected with cyamids (Table 3).
Table 3.
Levels of infection with whale lice in age classes of harbour porpoise hosts from the Netherlands between 2016 and 2019 (n = 21).
| Harbour porpoise | Levels of whale lice infection |
||
|---|---|---|---|
| Severe (n ≥ 20) | Moderate (6–19) | Mild (n ≤ 5) | |
| Adult Females (n = 4) | 0 | 1 (25%) | 3 (75%) |
| Adult Males (n = 6) | 2 (33%) | 1 (17%) | 3 (50%) |
| Juvenile Females (n = 3) | 1 (33%) | 0 | 2 (67%) |
| Juvenile Males (n = 8) | 5 (63%) | 1 (13%) | 2 (25%) |
A male-based sex ratio was found for the cyamids infecting harbour porpoises from the Netherlands. Female to male sex ratio in whale lice populations with 10 or more individuals was 1:1.29, and with 20 or more individuals found to be 1:1.32, reflecting a male-based sex ratio in the investigated porpoises. The number of juvenile cyamids seemed to increase with the total number of lice found in stranded harbour porpoises (linear regression with a positive coefficient of a = 0.2709 and R2 = 0.4382). The average length of mature whale lice on a host seemed to decrease in relation to the number of total whale lice encountered (moving average with an interval of 6, with linear regression with a negative leading coefficient (y = −0.003 + 3.3759) and R2 = 0.1902). However, neither age class nor sex of porpoise hosts had an effect on whale lice numbers on their hosts (p > 0.05) between 2016 and 2019.
3.3. Genetics
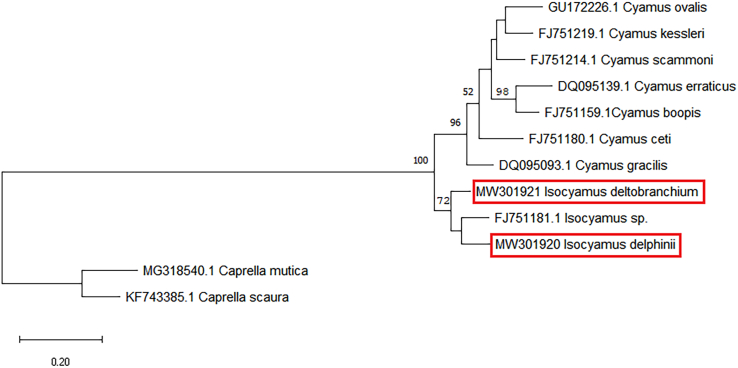
The PCR yielded a product of ~726bp. The sequences derived from I. deltobranchium of harbour porpoises were 100% identical. No differences were observed between the sequences of harbour porpoises from the Netherlands and those from German waters. Sequences of I. delphinii specimens from the pilot whale were 99% identical and differed on loci 72, 212, 344, 377 and 527. The consensus sequences of the specimens on pilot whale and harbour porpoise differed by 11.6%. The closest matches when blasted in GenBank was an Isocyamus sp. (Sequence ID: FJ751181.1) and Cyamus kessleri (Sequence ID: FJ751219.1) with 88% and 81% identity respectively.
Phylogenetic analyses with cyamid sequences available in GenBank revealed that although I. delphinii and I. deltobranchium are closely related, they are not each other's closest relatives (Fig. 4).
Fig. 4.
Maximum likelihood phylogeny tree produced with MEGA (version X), sequences in red box from this study. Bootstrap values (n = 1000 replicates) above 50% are shown above the branches.
3.4. Pathology
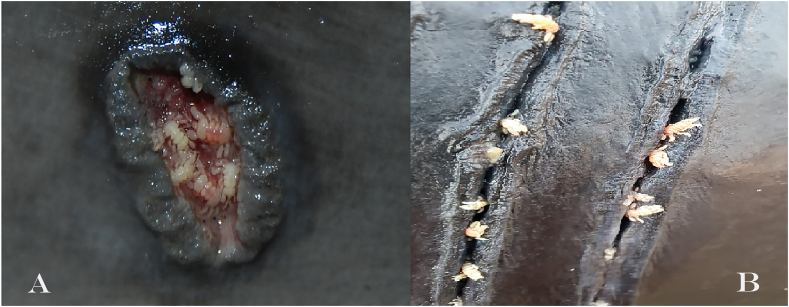
All whale lice-infected Dutch harbour porpoises (n = 46) had skin lesions, usually macroscopically characterized by round to oval shaped, well demarcated lesions with a dark rim, thickened edges and open centers (Fig. 5A), with whales lice found in the skin lesions (42/46, 91.3%) at the time of the necropsy. Of these 42 individuals, 32 had whale lice solely infecting the skin lesions, whilst in the other ten, whale lice were additionally located elsewhere: in the genital slit and loose on the body in two porpoises (2/42, 4.8%), in the blowhole in two other porpoises (2/42, 4.8%) and loose on the body in six others (6/42, 14.3%). The four harbour porpoises in which the whale lice were not found in skin lesions had whale lice loose on the body. Skin lesions with histologically visible intralesional crustaceans were available from 8 harbour porpoises from the Netherlands. The skin was ulcerated with the superficial dermis infiltrated by large numbers of degenerated neutrophils, admixed with fibrin and bacteria. Adjacent epithelium showed hyperplasia, with some vacuolization. Ulceration of the epidermis was present in all cases. Lesions severity and chronicity varied on a case-to-case basis between focal to multifocal and subacute to chronic. Some of the more extensive lesions presented with inflammation and hemorrhage affecting the entire (epi)dermis and the underlying subcutis. Skin lesions in German porpoises consisted of ulcerations with mild hyperplasia of the adjacent epidermis and granulation tissue in the superficial dermis. In one localization an intralesional lice was observed (Fig. 6). The pilot whale had rake marks (Fig. 5B) and skin lesions with intralesional whale lice, with open center and thickened edges with focal necrosis and hemorrhage.
Fig. 5.
A: Ulcerative lesion with whale lice in harbour porpoise (Phocoena phocoena); B: rake marks with whale lice on pilot whale (Globicephala melas).
Fig. 6.
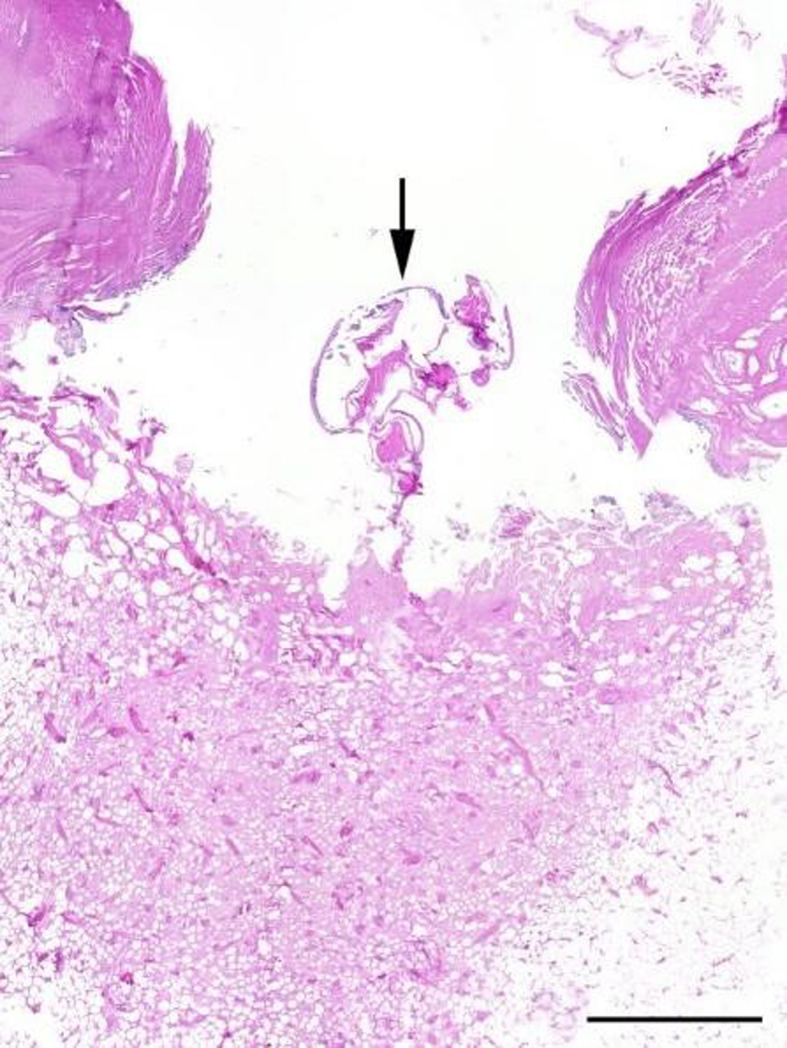
Intralesional whale lice (arrow) in lesion with mild hyperplasia of the adjacent epidermis and granulation tissue in the superficial dermis (scale bar 2 mm).
4. Discussion
This is the first systematic study on whale lice on cetaceans from the North Sea, comparing prevalence among different sexes, age classes as well as between Dutch and German waters. Data on ectoparasitic crustaceans on odontocetes in this geographical area is rare and often dated. So far, mostly opportunistic findings were reported, describing cyamid infections on harbour porpoises and a white-beaked dolphin from the North Sea (Stock, 1973a; Fransen and Smeenk 1991; Lehnert et al., 2007), but also from more rarely observed visitors like a Northern bottlenose whale (Hyperoodon ampullatus) and a sperm whale (Physeter macrocephalus) (Stock, 1973b). Few systematic surveys on whale lice have been performed worldwide, e.g., for odontocetes on I. delphinii on pilot whales off the Faroe Islands (Balbuena andRaga, 1991), and Syncyamus aequus on striped dolphins in the Mediterranean (Fraija-Fernández et al. 2017) and for baleen whales on Cyamus ceti on bowhead whales (Baleana mysticetus) (Von Duyke et al., 2016), on C. boopis on humpback whales (Megaptera novaeangliae) (Iwasa-Arai et al., 2018) and on C. ceti, C. kessleri and C. scammoni on gray whales (Callahan, 2008). Whale lice prevalence in the present study was low compared to other commonly occurring parasite species in harbour porpoises in the North Sea, like gastric and lung nematodes (30–90%), or hepatic trematodes (50%, Lehnert et al., 2005). For odontocetes, whale lice prevalence of 27% on striped dolphins in the Mediterranean (Fraija‐Fernández et al., 2017), and 30% on Faroe Island pilot whales (Balbuena and Raga, 1991) have been reported and for baleen whales a prevalence of 20% was for bowhead whales from the Bering, Chukchi, and Beaufort Seas (Von Duyke et al., 2016). A striking difference in whale lice prevalence was found between the harbour porpoises stranded in the Netherlands and those stranded on the German coastline, with a lower prevalence observed in Germany (1.6%) compared to the Netherlands (7.6%) in this study. This may be caused by the fact that porpoises inhabiting the southern North Sea have more interspecific contacts with cetacean species due to their locations closer to the channel. It has been hypothesized that whale lice are introduced to North Sea porpoises by visitors from more temperate and oceanic waters enabling interspecific transmissions (Rappé, 1990). In the southern North Sea, and since 2005 in particular on the Dutch coast, an increasing number of stranded harbour porpoises has been found (IJsseldijk et al., 2020). This had led to concerns regarding the health status of the animals inhabiting the southern North Sea. The higher prevalence of whale lice could reflect a higher prevalence of skin lesions due to a reduced health status in the Southern North Sea, and further investigations into this are needful. It remains speculative if poor nutritional status observed in severely infected porpoises in this study increases the susceptibility of porpoises to whale lice infections, e.g. by slower swimming speeds.
The prevalence of cyamids in German and Dutch waters is likely underreported, due to post-mortem emigration from dead host individuals or due to losses caused by decomposition and carcass manipulation during drift, stranding process and transport. This is reflected by decreasing prevalence of cyamids with increasing decomposition of the hosts, as observed in this study. Photographic documentation of stranded animals before handling and continued recording of whale lice in the future is recommended to record presence/absence of whale lice more reliably and to allow robust temporal analyses in due time. Most of the porpoises investigated in this study harboured 5 or less individual whale lice. Of course, intensity of infection in whale lice is biased in stranded animals, so there is little systematic information from fresh cetaceans available. In general, lower intensities on odontocetes (Fraija‐Fernández et al., 2017) were observed compared to mysticetes, where high loads of whale lice are reported (Leung, 1965; Rice and Wolman, 1971; Takeda and Ogino, 2005). Low intensities on smooth-surfaced bowhead whales (Von Duyke et al., 2016) and high intensities on right whales (Eubalaena glacialis) with lesions (Kaliszewska et al., 2005) were found, reflecting the necessity for these ectoparasites to find suitable sheltered microhabitats on their hosts.
The ectoparasitic crustaceans were identified as cyamids using morphological and molecular characteristics and revealed that different cyamid species were infecting harbour porpoises and the pilot whale investigated. The cyamid Isocyamus deltobranchium was found on the infected porpoises and is a new geographical record for the North Sea. Because first records of whale lice specimens on porpoise and dolphin hosts in the North Sea in the 1970s were reported as I. delphinii and depicted with triangular gills (Stock, 1973b, Fig.11), subsequent records may have misidentified the lice (Fransen and Smeenk, 1991). The whale lice specimens from the pilot whale were identified as I. delphinii and revealed small but significant morphological differences to I. deltobranchium regarding the shape of the accessory gills, which are triangular in I. deltobranchium and cylindrical in I. delphinii as described and depicted by Leung (1967, Fig. 2c) for I. delphinii and Sedlak-Weinstein (1992a, Pl.3, Fig. 10) for I. deltobranchium. Isocyamus deltobranchium was first described from short- and long-finned pilot whales (G. macrorhynchus & G. melas) in Japanese and Tasmanian waters (Sedlak-Weinstein, 1992b). More recently, new host records were reported for I. deltobranchium from cetaceans off the Galician coast (Martínez et al., 2008), including True's beaked whale (Mesoplodon mirus), a common dolphin (Delphinus delphis), and a harbour porpoise stranded in Spain, including SEM photographs depicting the characteristic triangular gills (Martínez et al., 2008; Fig. 1). The findings from this study are the second report from the Northern hemisphere, extending the range of I. deltobranchium northwards and highlighting the significance of porpoises as definite hosts. It remains puzzling that the investigated pilot whale was infected by I. delphinii, bearing in mind that records of I. deltobranchium in the Southern Hemisphere came from conspecifics. However, pilot whales from the Faroe Islands were infected with I. delphinii (Balbuena and Raga, 1991), indicating that the stranded pilot whale in this study may have been a visitor from the North, as suggested for other stranded pilot whales in the Netherlands (IJsseldijk et al., 2015) and highlighting the use of whale lice as indicators for habitat use in pilot whales. Both Isocyamus species encountered here had overlapping ranges in length and width, reflecting the variability in size of whale lice, depending on population size and host species.
Molecular analyses of the mDNA sequences supported that I. delphinii and I. deltobranchium are separate species but morphological and genetic differences are relatively small. All sequences of I. deltobranchium regardless of the origin of the porpoise host (Dutch or German waters) were 100% identical supporting that they are conspecific. It could also reflect a less rapid evolution of COI in I. deltobranchium or effective dispersion between hosts in the North Sea (Trobajo et al., 2010; Kaliszewska et al., 2005), however, I. deltobranchium sequences from other parts of the North Atlantic are required to support this. The intra-specific differences observed in sequences of I. delphinii specimens from the pilot whale could be a support for a faster evolution rate in the parasite than the host (Kaliszewska et al., 2005; Iwasa-Arai et al., 2017b). Phylogenetic comparison with other cyamid species COI sequences available in GenBank revealed two distinct clades, the Isocyamus and Cyamus clades. The two Isocyamus species obtained from this study were most closely related with an Isocyamus sp. sequence (Accession FJ751181.1) from GenBank, which was sampled from a short-finned pilot whale stranded in Humboldt County, California and putatively identified as I. globicipitis (Christopher Callahan, pers. comm.). According to the phylogenetic analysis, Isocyamus delphinii from this study is interpreted as sister species of the Isocyamus sp. sequence by Callahan (2008) from the US West coast, however, the genetic differences in the COI with about 12% are substantial, and similar to the molecular difference between I. delphinii and I. deltobranchium, indicating reproductive isolation for some time.
Cyamus globicipitis has been listed as a synonym of I. delphinii (Guérin-Méneville, 1836) by Leung (1967), but was retained in the genus Isocyamus by Margolis et al. (2000), who did not include I. deltobranchium in his comprehensive work, but instead combined features of I. delphinii (after Leung, 1967) and putatively I. deltobranchium (described as I. delphinii in Stock, 1973b) in his drawings (Margolis et al. 2000, Fig. 20). From the depiction of I. globicipitis in Margolis et al. (2000, ex Chevreux and Fage, 1925) the species seems similar to I. delphinii, but the sequence analysis in this study suggests that a different Isocyamus species or genetic variation between North Atlantic and North Pacific may account for the observed differences. Further sampling of whale lice specimens and investigations on additional gene loci are needed to fully understand the phylogenetic relationship of the genus Isocyamus and will help provide more information on host-parasite relationships of whale lice.
Isocyamus deltobranchium were associated with skin lesions in all infected harbour porpoises, characterised as ulcerative lesions of unknown origin in which the lice were found. Previous studies found I. deltobranchium in fresh and healing wounds of a pygmy sperm whale (Kogia breviceps) (Sedlak-Weinstein, 1992a) and pilot whales off Australia and Tasmania, with some wounds described as rounded and supposedly from cookie cutter sharks or lampreys, others like scrape marks probably from inter- or intraspecific aggression (Sedlak-Weinstein, 1992b). Cyamids have been reported to show site specificity, especially in mixed infections on baleen whales (Rowntree, 1996; Rice and Wolman, 1971). It remains speculative if I. deltobranchium is dependent on scar tissue for nutrition or needs lesions on stream-lined porpoises to hold on to its host. Whale lice reported as I. delphinii from harbour porpoises in Dutch and German waters were also found associated with skin lesions (Stock, 1973b; Lehnert et al., 2007). The male pilot whale from this study also had wounds in which the whale lice were located. In pilot whales preferred sites for attachment of I. delphinii were natural crevices like blowhole and genital slit (Balbuena and Raga, 1991), apart from wounds in adult males, where heavy infections were observed. Lesions on pilot whales with severe whale lice infections were probably caused by male aggressive behaviour (Balbuena and Raga, 1991). The extent of current pathological findings in Germany supports preceding conclusions (Lehnert et al. 2007) that whale lice commonly cause minor changes when not present in extensive numbers. Furthermore, they did not contribute to the cause of illness or death in the presented cases. It remains speculative to what extent whale lice may maintain the lesions they inhabit and impede the healing process in porpoises, however it is assumed that they are not the causative agents. The origin of lesions and their potentially more frequent occurrence in porpoises from the Netherlands need to be investigated further. It is recommended to incorporate in further research the prevalence, severity and etiology of skin lesions on all stranded individuals, since this will shed light on the relationship between whale lice and skin lesions.
5. Conclusion
This study reports the occurrence of whale lice infecting odontocetes from the Dutch and German North Sea waters. Two cyamid species were identified according to morphological and molecular characteristics. Both methods revealed small but significant differences supporting that two different Isocyamus species infected the porpoises and pilot whale under study and highlighted that more knowledge about Isocyamus taxonomy and phylogeny are important to better understand their global distribution, habitat requirements and life history. Differences in prevalence of whale lice infections in harbour porpoises between geographic regions and information about associated lesions that may be a prerogative for infections in harbour porpoises are reported. The cyamid species encountered reflect that odontocetes are highly vagile and gregarious animals. More knowledge about the ecology of ectoparasitic crustaceans is needed to better understand their use as bio or health indicators for host ecology.
Declaration of competing interest
Ethical standards: Ethical review and approval was not required for the animal study because all animals in our study were found dead, died naturally or were euthanized based on welfare grounds and none of the animals were killed for the purpose of this study. This publication was supported by Deutsche Forschungsgemeinschaft and University of Veterinary Medicine Hannover, Foundation within the funding programme Open Access Publishing.
Acknowledgements
The authors wish to thank all individuals who report and collect marine mammals in Germany and the Netherlands, in particular the S–H national park rangers and seal hunters in Germany and all volunteers and organisation associated with the Dutch stranding network, as well as all ITAW and UU colleagues and assistants for their support during necropsies. The authors wish to thank Nadine Dupérré at CeNak, Hamburg for access to photo equipment. Furthermore, we would like to thank Juan Antonio Balbuena for providing reference specimens of I. delphini from Faroese pilot whales.
The post-mortem investigations in Germany were partly funded by the Ministry of Energy, Agriculture, the Environment, Nature and Digitalisation in Schleswig-Holstein, Germany, and the Schleswig–Holstein National Park Service. The post-mortem investigations in the Netherlands are commissioned by the Dutch Ministry of Agriculture, Nature and Food Quality (2008–2015 under project reference number 140000353; 2016–2019 under project reference number WOT04-009-045), but no specific funding was acquired for this study. This publication was supported by Deutsche Forschungsgemeinschaft and the University of Veterinary Medicine Hannover, Foundation within the funding programme Open Access Publishing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2021.02.015.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Aznar F.J., Balbuena J.A., Raga J.A. Are epizoites biological indicators of a western Mediterranean striped dolphin die-off? Dis. Aquat. Org. 1994;18:159–163. [Google Scholar]
- Balbuena J.A., Raga J.A. Ecology and host relationships of the whale-louse Isocyamus delphini (Amphipoda: cyamidae) parasitizing long-finned pilot whales (Globicephala melas) off the Faroe Islands (Northeast Atlantic) Can. J. Zool. 1991;69(1):141–145. [Google Scholar]
- Balbuena J.A., Aznar F.J., Fernández M., Raga J.A. vol. 4. Elsevier Science; 1995. Parasites as indicators of social structure and stock identity of marine mammals; pp. 133–139. (Developments in Marine Biology). [Google Scholar]
- Berzin A.A., Vlasova L.P. Fauna of the cetacea cyamidae (Amphipoda) of the world ocean. Investig. Cetacea. 1982;13:149–164. [Google Scholar]
- Bossart G.D. Marine mammals as sentinel species for oceans and human health. Oceanography. 2006;19(2):134–137. doi: 10.1177/0300985810388525. [DOI] [PubMed] [Google Scholar]
- Boxshall G., Lester R., Grygier M.J., Hoeg J.T., Glenner H., Shields J.D., Lützen J. 2005. Crustacean Parasites. Rohde K. [Google Scholar]
- Callahan C.M. Humboldt State University; Arcata (CA): 2008. Molecular Systematics and Population Genetics of Whale Lice (Amphipoda: Cyamidae) Living on Gray Whale Islands. [master thesis] 54 pp. [Google Scholar]
- Fraija‐Fernández N., Fernández M., Gozalbes P., Revuelta O., Raga J.A., Aznar F.J. Living in a harsh habitat: epidemiology of the whale louse, Syncyamus aequus (Cyamidae), infecting striped dolphins in the Western Mediterranean. J. Zool. 2017;303(3):199–206. [Google Scholar]
- Fransen C.H.J.M., Smeenk C. Whale-lice (Amphipoda: cyamidae) recorded from The Netherlands. Zoologische mededelingen. 1991;65(29):393–405. [Google Scholar]
- Guérin-Méneville F.E. 1829-1844. Iconographiedu règne animal de G. Cuvier, 3 (Crustacés): 1-48. pls. 1-38. (Paris, J.-B. Bailliere). [Pl. 28, showing “Cyamus Delphinii” was published in 1836] [Google Scholar]
- Hammond P.S., Berggren P., Benke H., Borchers D.L., Collet A., Heide‐Jørgensen M.P., Heimlich S., Hiby A.R., Leopold M.F., Øien N. Abundance of harbour porpoise and other cetaceans in the North Sea and adjacent waters. J. Appl. Ecol. 2002;39:361–376. [Google Scholar]
- Haney T.A., De Almeida A.O., Reis M.S.S. A new species of cyamid (Crustacea: Amphipoda) from a stranded cetacean in Southern Bahia, Brazil. Bull. Mar. Sci. 2004;75(3):409–421. [Google Scholar]
- Hilty J., Merenlender A. Faunal indicator taxa selection for monitoring ecosystem health. Biol. Conserv. 2000;92(2):185–197. [Google Scholar]
- IJsseldijk L.L., Leopold M.F., Bravo Rebolledo E.L., Deaville R., Haelters J., IJzer J., Jepson P.D., Gröne A. Fatal asphyxiation in two long-finned pilot whales (Globicephala melas) caused by common soles (Solea solea) PloS One. 2015;10(11) doi: 10.1371/journal.pone.0141951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IJsseldijk L.L., Brownlow A.C., Mazzariol S. Joint ACCOBAMS and ASCOBANS Document. OSFPREPRINTS; 2019. Best practice on cetacean post mortem investigation and tissue sampling. [Google Scholar]
- IJsseldijk L.L., ten Doeschate M.T.I., Brownlow A., Davison N.J., Deaville R., Galatius A., Gilles A., Haelters J., Jepson P.D., Keijl G.O., Kinze C.C., Olsen M.T., Siebert U., Bie Thøstesen C., van den Broek J., Gröne A., Heesterbeek H. Spatiotemporal mortality and demographic trends in a small cetacean: strandings to inform conservation management. Biol. Conserv. 2020;249:108733. [Google Scholar]
- Iwasa-Arai T., Serejo C.S., Siciliano S., Ott P.H., Freire A.S., Elwen S., Crespo E.A., Colosio A.C., Carvalho V.L., Rodríguez-Rey G.T. The host-specific whale louse (Cyamus boopis) as a potential tool for interpreting humpback whale (Megaptera novaeangliae) migratory routes. J. Exp. Mar. Biol. Ecol. 2018;505:45–51. [Google Scholar]
- Iwasa-Arai T., Carvalho V.L., Serejo C.S. Updates on cyamidae (Crustacea: Amphipoda): redescriptions of Cyamus monodontis lütken, 1870 and Cyamus nodosus lütken, 1861, a new species of Isocyamus, and new host records for Syncyamus ilheusensis Haney, de almeida and reis, 2004. J. Nat. Hist. 2017;51(37–38):2225–2245. [Google Scholar]
- Iwasa-Arai T., Siciliano S., Serejo C.S., Rodríguez-Rey G.T. Life history told by a whale-louse: a possible interaction of a southern right whale Eubalaena australis calf with humpback whales Megaptera novaeangliae. Helgol. Mar. Res. 2017;71(1):1–6. [Google Scholar]
- Kaliszewska Z.A., Seger J., Rowntree V.J., Barco S.G., Benegas R., Best P.B., Brown M.W., Brownell R.L., Jr., Carribero A., Harcourt R., Knowlton A.R. Population histories of right whales (Cetacea: Eubalaena) inferred from mitochondrial sequence diversities and divergences of their whale lice (Amphipoda: Cyamus) Mol. Ecol. 2005;14(11):3439–3456. doi: 10.1111/j.1365-294X.2005.02664.x. [DOI] [PubMed] [Google Scholar]
- Leger J.S., Raverty S., Mena A. Pathology of Wildlife and Zoo Animals. Academic Press; 2018. Cetacea; pp. 533–568. [Google Scholar]
- Lehnert K., Raga J.A., Siebert U. Macroparasites in stranded and bycaught harbour porpoises from German and Norwegian waters. Dis. Aquat. Org. 2005;64(3):265–269. doi: 10.3354/dao064265. [DOI] [PubMed] [Google Scholar]
- Lehnert K., Fonfara S., Wohlsein P., Siebert U. Whale lice (Isocyamus delphinii) on a harbour porpoise (Phocoena phocoena) from German waters. Vet. Rec. 2007;161:526–528. doi: 10.1136/vr.161.15.526. [DOI] [PubMed] [Google Scholar]
- Leung Y.M. An illustrated key to the species of whale-lice (Amphipoda: cyamidae), ectoparasites of Cetacea, with a guide to the literature. Crustaceana. 1967;12(3):279–291. [Google Scholar]
- Leung Y.M. A collection of whale-lice (Cyamidae: Amphipoda) Bull. South Calif. Acad. Sci. 1965;64:132–143. [Google Scholar]
- Lockyer C. A review of factors involved in zonation in odontocete teeth, and an investigation of the likely impact of environmental factors and major life events on harbour porpoise tooth structure. In: Bjørge A., Donovan G.P., editors. The Biology of the Phocoenids. Rep. Int. Whal. Commn. Special Issue 16, Cambridge, UK. 1995. pp. 511–529. [Google Scholar]
- MacKenzie K. Parasites as biological tags in population studies of marine organisms: an update. Parasitology. 2004;124:S153–S163. doi: 10.1017/s0031182002001518. [DOI] [PubMed] [Google Scholar]
- Margolis L., McDonald T.E., Bousfield E.L. The whale-lice (Amphipoda: cyamidae) of the northeastern Pacific region. AMPHIPACIFICA. 2000;2(4):63–117. [Google Scholar]
- Martin J.W., Heyning J.E. First record of Isocyamus kogiae Sedlak-Weinstein, 1992 (Crustacea, Amphipoda, Cyamidae) from the eastern Pacific, with comments on morphological characters, a key to the genera of the Cyamidae, and a checklist of cyamids and their hosts. Bull. South Calif. Acad. Sci. 1999;98:26–38. [Google Scholar]
- Martínez R., Segade P., Martínez-Cedeira J.A., Arias C., García-Estévez J.M., Iglesias R. Occurrence of the ectoparasite Isocyamus deltobranchium (Amphipoda: cyamidae) on cetaceans from Atlantic waters. J. Parasitol. 2008;94(6):1239–1242. doi: 10.1645/GE-1518.1. [DOI] [PubMed] [Google Scholar]
- Miller M.A., Pfeiffer W., Schwartz T. 2010. Creating the CIPRES Science Gateway for Inference of Large Phylogenetic Trees. In 2010 Gateway Computing Environments Workshop (GCE) pp. 1–8. [Google Scholar]
- Pfeiffer C.J. Whale lice. In: Perrin W.F., Würsig B., Thewissen J.G.M., editors. Encyclopedia of Marine Mammals. Academic Press; San Diego, California: 2002. pp. 1302–1305. [Google Scholar]
- Poulin R., Leung T.L.F. Body size, trophic level, and the use of fish as transmission routes by parasites. Oecologia. 2011;166(3):731–738. doi: 10.1007/s00442-011-1906-3. [DOI] [PubMed] [Google Scholar]
- Rappé G. Isocyamus delphinii (Crustacea, Amphipoda, Cyamidae), a possible biological indicator in the North Sea. In: Evans P.G.H., Aguilar A., Smeenk C., editors. European Research on Cetaceans. Proceedings of the Fourth International Conference of the European Cetacean Society, Palma de Mallorca, Spain, March 2 to 4, 1990. 1990. pp. 121–122. [Google Scholar]
- Rice W.R., Wolman A.A. American Society of Mammalogists, Special Publication; 1971. The Life History and Ecology of the Gray Whale (Eschrichtius Robustus) No. 3. [Google Scholar]
- Roussel de Vauzème M. Mémoire sur le Cyamus ceti (Latr.) de la classe des Crustacés. Ann. Sci. Nat. Zoo. 1834;1:239–265. 2e Série. [Google Scholar]
- Rowntree V.J. Feeding, distribution, and reproductive behavior of cyamids (Crustacea: Amphipoda) living on humpback and right whales. Can. J. Zool. 1996;74:103–109. [Google Scholar]
- Schell D.M., Rowntree V.J., Pfeiffer C.J. Stable-isotope and electron-microscopic evidence that cyamids (Crustacea: Amphipoda) feed on whale skin. Can. J. Zool. 2000;78(5):721–727. [Google Scholar]
- Sedlak-Weinstein E. Three new records of cyamids (Amphipoda) from Australian cetaceans. Crustaceana. 1991;60(1):90–104. [Google Scholar]
- Sedlak-Weinstein E. A new species of Isocyamus (Amphipoda: cyamidae) from kogia breviceps (de blainville, 1838) in Australian waters. Syst. Parasitol. 1992;23(1):1–6. [Google Scholar]
- Sedlak-Weinstein E. The occurrence of a new species of Isocyamus (Crustacea, Amphipoda) from Australian and Japanese pilot whales, with a key to species of Isocyamus. J. Nat. Hist. 1992;26(5):937–946. [Google Scholar]
- Siebert U., Wünschmann A., Weiss R., Frank H., Benke H., Frese K. Post-mortem findings in harbour porpoises (phocoena phocoena) from the German North and baltic Seas. J. Comp. Pathol. 2001;124:102–114. doi: 10.1053/jcpa.2000.0436. [DOI] [PubMed] [Google Scholar]
- Siebert U., Gilles A., Lucke K., Ludwig M., Benke H., Kock K.-H., Scheidat M. A decade of harbour porpoise occurrence in German waters - analyses of aerial surveys, incidental sightings and strandings. J. Sea Res. 2006;56:65–80. [Google Scholar]
- Siebert U., Wohlsein P., Lehnert K., Baumgärtner W. Pathological findings in harbour seals (Phoca vitulina): 1996–2005. J. Comp. Pathol. 2007;137(1):47–58. doi: 10.1016/j.jcpa.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Stock J.H. Een bruinvis met luizen. Levende Nat. 1973;76(5):107–109. [Google Scholar]
- Stock J.H. Whale-lice (Amphipoda: cyamidae) in Dutch waters. Bulletin of the Zoological Museum of the University of Amsterdam. 1973;3:73–77. [Google Scholar]
- Stock J.H. Whale-lice (Amphipoda, cyamidae) on Lagenorhynchus albirostris in Dutch waters. Crustaceana. 1977;32(2):206–207. [Google Scholar]
- Takeda M., Ogino M. Record of a whale louse, Cyamus scammoni Dall (Crustacea: Amphipoda: cyamidae), from the gray whale strayed into Tokyo Bay, the Pacific coast of Japan. Bull. Natl. Sci. Mus. Ser. A Zool. 2005;31:151–156. [Google Scholar]
- Trobajo R., Mann D.G., Clavero E., Evans K.M., Vanormelingen P., McGregor R.C. The use of partial cox 1, rbc L and LSU rDNA sequences for phylogenetics and species identification within the Nitzschia palea species complex (Bacillariophyceae) Eur. J. Phycol. 2010;45(4):413–425. [Google Scholar]
- Vidal-Martinez V.M., Pech D., Sures B., Purucker S.T., Poulin R. Can parasites really reveal environmental impact? Trends Parasitol. 2010;26(1):44–51. doi: 10.1016/j.pt.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Von Duyke A.L., Stimmelmayr R., Sheffield G., Sformo T., Suydam R., Givens G.H., George J.C. Prevalence and abundance of cyamid" whale lice"(cyamus ceti) on subsistence harvested bowhead whales (balaena mysticetus) Arctic. 2016:331–340. [Google Scholar]
- Wardle W.J., Haney T.A., Worthy G.A.J. New host record for the whale louse Isocyamus delphinii (Amphipoda, Cyamidae) Crustaceana. 2000;73:639–641. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.