Highlights
-
•
Quality assurance of all treatment plans (1854 patients) in the DBCG HYPO trial is presented.
-
•
More strict dose constraints for high-dose volumes of the breast in whole breast RT are suggested.
-
•
Laterality-specific dose constraints for organs at risk (OAR) in whole breast RT are presented.
Keywords: Breast cancer, Radiation therapy, Fractionation, OAR dose constraints
Abstract
Purpose
Quality assessment of the treatment plans in the Danish Breast Cancer Group (DBCG) HYPO trial was carried out based on prospectively reported dosimetric parameters and evidence-based dose constraints for whole breast radiation therapy were derived.
Materials and methods
From 2009 to 2014, 1882 patients (pts) were randomised between 50 Gy/25fractions (fr) versus 40 Gy/15fr. Doses to CTVp_breast (V95%, V107%-V110%, Dmax, and in addition for 40 Gy plans V105%-V107%), ipsilateral lung (V20Gy/V17Gy), heart (V20Gy/V17Gy, V40Gy/V35Gy), and left anterior descending coronary artery (LADCA) (Dmax) and use of respiratory gated technique were prospectively reported to the DBCG database. After end of accrual, these dosimetric parameters from all plans in the trial were compared to the pre-specified treatment constraints.
Results
In total, 1854 pts from eight radiation therapy (RT) centres in three countries were treated. No statistically significant differences were found between the results for 40 Gy and 50 Gy plans, except for CTVp_breast hot-spot volume (V107%-V110%). Of the 40 Gy pts, 90% with CTVp_breast > 600 mL and 95% with CTVp_breast ≤ 600 mL had a CTVp_breast hot-spot volume (V105%-V107%) <2%. In 95% of the 50 Gy plans, the CTVp_breast absolute hot-spot volume (V107%-V110%) was <0.5 mL and 1.7 mL for CTVp_breast ≤ 600 mL and > 600 mL, respectively. Compliance was >99% for both heart and lung constraints. Largest deviation from protocol constraints was found for the volume of CTVp_breast covered with 95% of the prescription dose or more (V95%). The CTV dose coverage (V95%) was >94.3% in 95% of the right-sided pts, whereas the figures for 95% of the left-sided pts treated with and without respiratory gating were 93.2% and 88.8%, respectively.
Conclusion
A high degree of compliance with protocol dose constraints was found for treatment plans in the DBCG HYPO trial. New constraints for dose to organs at risk and high-dose volumes in the breast are suggested for breast-only RT planning.
1. Introduction
For decades, the standard fractionation for radiation therapy (RT) after breast conserving surgery was 50 Gy/25fractions (fr), because early experience with moderately hypofractionated breast RT resulted in excess radiation-associated loco-regional morbidity [1], [2]. Since then, 3D CT-based treatment planning has emerged. Furthermore, the α/β ratio for late normal tissue changes in the breast has been reported to be around 3 Gy, supporting modest increase to fraction size above 2 Gy [3]. Such schedules were tested in randomised trials in Canada and the UK. The Canadian trial tested 42.5 Gy/16fr versus 50 Gy/25fr [4], whereas the UK START Trial B tested 40 Gy/15fr/3weeks versus 50 Gy/25fr/5weeks [5], [6]. The favourable results from these trials paved the way for more widespread use of moderate hypofractionation for breast RT.
After the initial encouraging results from the START Trial B [5], the Danish Breast Cancer Group (DBCG) RT Committee decided to reintroduce moderately hypofractionated RT through a clinically controlled randomised trial, including patients with an indication for breast-only RT. The fractionation design of the DBCG HYPO trial was similar to the START Trial B, however with an additional constraint for breast doses in the range of 105% to 107% of the prescription dose in the 40 Gy arm compared to the 50 Gy arm. The primary endpoint in the DBCG HYPO trial was 3-year breast induration grade 2–3.
A quality assessment (QA) program was set up to ensure that the technical guidelines were followed in the participating centres (4 in Denmark, 2 in Norway and 2 in Germany). For each individual treatment plan, a selection of dosimetric parameters were prospectively reported to the DBCG database. After end of accrual, analysis of these parameters evaluated the compliance of the participating centers with the trial protocol. Recently, the first results from the trial were reported with median 7.3-year follow-up. The nine-year loco-regional recurrence risks in the DBCG HYPO study were 3.3% (95% Cl, 2.0% to 5.0%) and 3.0% (95% Cl, 1.9% to 4.5%) in the 50 Gy and 40 Gy arm, respectively, and radiation-associated cardiac and lung disease was seldom [7]. This supported that the dose constraints applied for the treatment planning in this study were appropriate, and the dosimetric results from the study may be used to improve the constraints for future planning.
2. Materials and methods
Patients older than 40 years with a T1-2 N0-N1(mic) invasive adenocarcinoma or ductal carcinoma in situ (DCIS) referred to postoperative whole breast RT were eligible for inclusion in the DBCG HYPO trial. No RT of regional nodes was allowed. Stratification factors were breast size (smaller/larger than 600 mL), boost (yes/no), systemic treatment and institution. The patients were randomized to whole breast irradiation 50 Gy/25fr/5weeks versus 40 Gy/15fr/3weeks. Boost was prescribed as sequential 5–8 fr of 2 Gy according to national or institutional guidelines (supplementary material in [7]). A written informed consent according to Good Clinical Practice guidelines and local and national rules of participating institutions was obtained. The trial was approved by the Ethics Committee in Region Midt, Denmark, on behalf of all participating Danish centres, and by local Ethics Committees for the non-Danish centres (clinicaltrials.gov number NCT00909818).
2.1. Planning CT scanning
Treatment preparation was according to DBCG RT guidelines. The neck and breast region including both lungs was CT scanned with the patient positioned in the institutional standard fixation for this patient group. The only requirement was that a daily reproducibility of approx. 5 mm should be achieved during the course of treatment. The institutional guideline for respiratory gating and image verification were followed and had to be identical for the two treatment arms..
2.2. Delineation of target volumes and organs at risk
The CTVp_breast was defined according to DBCG guidelines, which was identical to the ESTRO guidelines [8], [9]. CTVp_boost was defined as a 5 mm expansion from the volume including surgical clips and a relevant part of the surgical cavity guided by pre-surgical imaging and the surgical report, and cropped inside the CTVp_breast. PTVs were constructed from the CTVs following the institutional standard CTV-PTV margins for whole breast RT, and cropped to 5 mm below the skin. Heart, ipsilateral lung and contralateral breast were defined as organs at risk (OAR) [10].
2.3. Treatment planning
The recommended treatment technique was a one-isocenter technique with two tangential fields with parallel posterior field borders. Wedges or field-in-field segments were applied to obtain a homogeneous dose distribution. However, inverse optimized IMRT planning was also an option. The institutional dose calculation algorithm was used. There was no restriction on the photon energy used except that the 95% isodose curve should cover the breast 5 mm below the surface. The CTVp_breast was to be covered with doses in the range 95%-105% (40 Gy plans) and 95%-107% (50 Gy plans) of the prescription dose and Dmax ≤ 110%. For the 40 Gy arm, a volume of up to 2% of the CTVp_breast could receive a dose between 105% and 107% of the prescription dose (V105%-V107%≤2%). In both treatment arms, an absolute volume of the CTVp_breast of up to 2 mL could receive a dose in the range 107%-110% of the prescription dose (V107%-V110%<2mL). In this way, hot spots in the breast were defined for doses >105% for 40 Gy plans and >107% for 50 Gy plans, respectively. No part of the CTVp_breast except for build-up regions should be covered with doses <95% of the prescription dose (V95%≥95%), corresponding to under-dosed volumes.
The maximum dose to <25% of the ipsilateral lung was 20 Gy (50 Gy plans) and 17 Gy (40 Gy plans) (V20Gy/V17Gy ≤ 25%). <10% and 5% of the heart should receive 20 Gy and 40 Gy, respectively, in 50 Gy plans and 17 Gy and 35 Gy, respectively, in 40 Gy plans (V20Gy/V17Gy ≤ 10% and V40Gy/V35Gy ≤ 5%). Delineation of the left anterior descending coronary artery (LADCA) and contralateral breast was optional. If LADCA was delineated, DLADCA,max was 17 Gy and 20 Gy in 40 Gy and 50 Gy plans, respectively. The dose to the contralateral breast should be as low as possible. It is noteworthy that the constraints for 50 Gy/25fr were all based on consensus (in lack of evidence) in the DBCG RT Committee dating back to the days where CT-based RT planning was introduced. The committee was worried about excess morbidity after hypofractionated RT, so the constraints were deliberately stricter for the 40 Gy plans. Highest priority was given to CTVp_boost or tumor bed if no boost, thereafter the priority was heart > ipsilateral lung > CTVp_breast > PTVp_breast > contralateral breast, however, every treatment plan was to be balanced and approved after considering information about the patient, comorbitidy, tumour characteristics and other treatment factors.
2.4. Data available for analysis
The dosimetric parameters for CTVp_breast (V95%, V107%-V110%, Dmax, and in addition for 40 Gy plans V105%-V107%), ipsilateral lung (V20Gy/V17Gy), heart (V20Gy/V17Gy, V40Gy/V35Gy) and LADCA (Dmax) were prospectively reported to the DBCG database. Prior to analysis, the data quality was investigated, and if values deviated much from expected values, the centre was asked to confirm the value. Use of respiratory gating was added to the database in 2012 [7], thus, data on gating prior to 2012 was collected retrospectively from the centres.
2.5. Data analysis
Compliance with the protocol treatment constraints and 50th (median), 90th, and 95th percentiles for dosimetric parameters were determined for both treatment arms, and for various subgroups of patients according to laterality, use of respiratory gating, and CTVp_breast volume. Student’s t-test in MS Excel version 2010 was used to test for statistical significance (p < 0.05).
3. Results
Between May 2009 and March 2014, 1854 eligible patients were treated [7]. RT characteristics from their treatment plans are presented in table 1. Table 2 shows the compliance with the predefined dose constraints. Data on modified treatment plans was reported for 5 pts (0.3%) and of these, 3 pts had a new plan during the treatment course, whereas the remaining two were replanned prior to treatment start.
Table 1.
Characteristics of the 1854 patients treated in the DBCG HYPO trial.
| Fractionation schedule | |||
|---|---|---|---|
| 50 Gy | 40 Gy | Total | |
| Number of patients (n) | 937 | 917 | 1854 |
| Age [y] mean (min–max) | 59 (42–83) | 59 (41–82) | 59 (41–83) |
| CTVp_breast [mL] mean (median, IQR) | 709 (644, 502) | 721 (635, 531) | 715 (640, 520) |
| Laterality | |||
| Right (n) | 455 | 445 | 900 |
| Left (n) | 482 | 472 | 954 |
| Gating (left-sided patients) (n) | |||
| Yes | 231 | 226 | 457 |
| No | 251 | 246 | 497 |
| Boost (n) | |||
| No | 721 | 703 | 1424 |
| 10 Gy/5 fr | 183 | 185 | 368 |
| 16 Gy/8 fr | 33 | 29 | 62 |
Table 2.
Compliance with dose constraints in the DBCG HYPO trial for volume of CTVp_breast receiving>95% of prescription dose (V95%), volume of CTVp_breast receiving 105–107% (40 Gy plans) and 107–110% of prescription dose (V105%-V107%, V107%-V110%), maximum dose to CTVp_breast (Dmax), volumes of lung and heart receiving 20 Gy (50 Gy plans) or 17 Gy (40 Gy plans) (V20Gy/V17Gy), heart receiving 40 Gy (50 Gy plans) or 35 Gy (40 Gy plans) (V40Gy/V35Gy), and maximum dose to left anterior descending coronary artery (LADCA).
| 50 Gy | 40 Gy | ||||||
|---|---|---|---|---|---|---|---|
| Protocol constraint | Compliance | Non compliance | Missing data | Compliance | Non compliance | Missing data | |
| V95% ≥ 95% | 767 (81.9%) | 166 (17.7%) | 4 (0.4%) | 730 (79.6%) | 185 (20.2%) | 2 (0.2%) | |
| V105%-V107% ≤2% | – | – | – | 846 (92.3%) | 68 (7.4%) | 3 (0.3%) | |
| V107%-V110% <2cm3 | 899 (95.9%) | 35 (3.7%) | 3 (0.3%) | 907 (98.9%) | 9 (1.0%) | 1 (0.1%) | |
| Dmax ≤ 110% | 927 (98.9%) | 7 (0.7%) | 3 (0.3%) | 911 (99.3%) | 5 (0.5%) | 1 (0.1%) | |
| Lung V20Gy/V17Gy ≤ 25% | 924 (98.6%) | 8 (0.9%) | 5 (0.5%) | 912 (99.5%) | 3 (0.3%) | 2 (0.2%) | |
| Heart V20Gy/V17Gy ≤ 10% |
Right | 431 (94.7%) | 0 (0.0%) | 24 (5.3%) | 412 (92.6%) | 0 (0.0%) | 33 (7.4%) |
| Left | 480 (99.6%) | 0 (0.0%) | 2 (0.4%) | 470 (99.6%) | 1 (0.2%) | 1 (0.2%) | |
| Heart V40Gy/V35Gy ≤ 5% | Right | 431 (94.7%) | 0 (0.0%) | 24 (5.3%) | 412 (92.6%) | 0 (0.0%) | 33 (7.4%) |
| Left | 480 (99.6%) | 0 (0.0%) | 2 (0.4%) | 470 (99.6%) | 1 (0.2%) | 1 (0.2%) | |
| LADCA Dmax ≤ 20 Gy/17 Gy | Right | 404 (88.8%) | 0 (0.0%) | 51 (11.2%) | 395 (88.8%) | 0 (0.0%) | 50 (11.2%) |
| Left | 411 (85.3%) | 29 (6.0%) | 42 (8.7%) | 382 (79.3%) | 47 (9.8%) | 43 (8.9%) | |
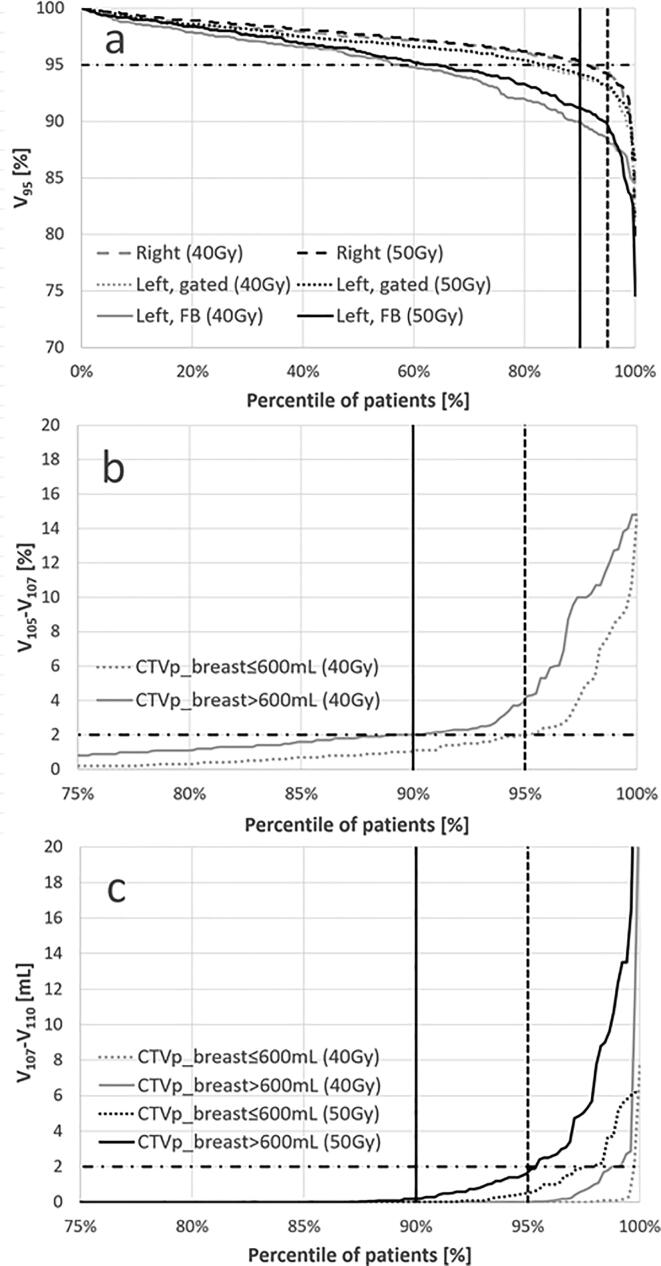
The largest number of deviations from the constraint was found for the dose coverage (V95%), however, no statistically significant difference was found between the under-dosed volumes of CTVp_breast in the 50 Gy and 40 Gy plans (Fig. 1a). The best dose coverage of CTVp_breast was reported for right-sided patients with V95%>94.3% in 95% of the plans, whilst the coverage in left-sided gated patients was significantly less with V95%>93.2% (p < 0.001), and even worse for left-sided patients treated in free breathing (FB), where V95%>88.8% (p < 0.001) (Fig. 1a). In the 40 Gy treatment arm, non-compliance to the constraint V105%-V107%≤2% was found in 68 treatment plans of which 47 and 21 patients had large (CTVp_breast > 600 mL) and small breasts (CTVp_breast ≤ 600 mL), respectively, corresponding to 90% of the large-breasted and 95% of the small-breasted patients having treatment plans with a CTVp_breast hot-spot volume (V105% -V107%) up to 2% (Fig. 1b). Larger breast hot-spot volumes were observed in 50 Gy plans compared to 40 Gy plans (p = 0.001) as 2.1% (small breasts) and 4.7% (large breasts) of the 40 Gy patients received breast doses above 107% of the prescription dose, whereas the corresponding figures for 50 Gy patients were 9.0% and 14.8% (Fig. 1c). However, in 95% of the 50 Gy plans the CTVp_breast absolute hot-spot volume (V107%-V110%) was<0.5 mL and 1.7 mL for small and large breast, respectively.
Fig. 1.
a: Volume of CTVp_breast covered with 95% of prescription dose (V95%), b: Volume of CTVp_breast with a hot spot dose between 105% and 107% of the prescription dose (V105%-V107%) for 40 Gy plans, and c: Volume of CTVp_breast with a hot spot dose between 107% and 110% of the prescription dose (V107%-V110%) for both 40 Gy and 50 Gy plans versus percentile of patients. In b and c results are shown for patients with a CTVp_breast smaller than or equal to 600 mL and CTVp_breast>600 mL. Constraints are shown with horizontal dashed-dotted lines, whereas the vertical lines show the 90th (solid line) and 95th (dashed line) percentiles. In c, two (27.0 and 48.6 mL) and one (24.3 mL) data points are outside the scale for CTVp_breast > 600 mL (50 Gy plans) and CTVp_breast > 600 mL (40 Gy plans), respectively.
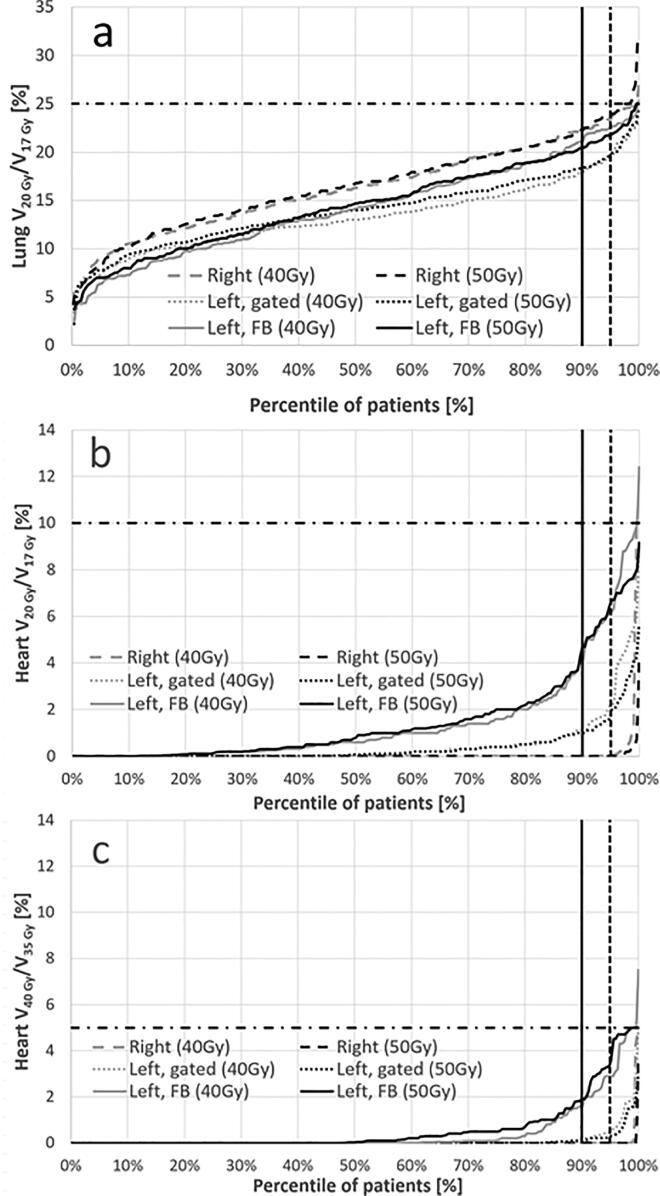
For both lung and heart constraints, compliance was > 99% (table 2). Delineation of the heart was not carried out in 57 (6%) of the right-sided patients, whereas data was missing for only three left-sided patients. Since it was optional to delineate LADCA, a higher number of missing data was expected for LADCA. For ipsilateral lung, the highest lung V20Gy/17Gy values were seen for right-sided patients, followed by FB and gated left-sided patients, respectively (Fig. 2a). No statistically significant difference was found between the lung values for 40 Gy plans and 50 Gy plans with p = 0.50 (right), p = 0.06 (left, gated), and p = 0.53 (left, FB). The heart data are shown in Fig. 2b and c. Also, for the heart no statistically significant difference was found when comparing 40 Gy plans and 50 Gy plans with p-values for V20Gy/17Gy of 0.31 (right), 0.27 (left, gated) and 0.80 (left, FB) and corresponding values for V40Gy/35Gy of 0.25, 0.38, and 0.16. Table 3 summarizes the 50th (median), 90th, and 95th percentiles for lung, heart and breast dosimetric parameters.
Fig. 2.
a. Lung V20Gy (50 Gy plans) and V17Gy (40 Gy plans) b. Heart V20Gy (50 Gy plans) and V17Gy (40 Gy plans), and c. Heart V40Gy (50 Gy plans) and V35Gy (40 Gy plans) versus percentile of patients. The constraints in the trial protocol are shown with horizontal dashed-dotted lines, whereas the vertical lines show the 90th (solid line) and 95th (dashed line) percentiles.
Table 3.
50th (median), 90th, and 95th percentile values for lung V20Gy/V17Gy, heart V20Gy/V17Gy and V40Gy/V35Gy, and volume of CTVp_breast covered with 95% of prescription dose (V95%) for right-sided and left-sided patients treated with respiratory gating and in free breathing, respectively. The same figures are given for volume of CTVp_breast with doses between 105% and 107% of prescription dose (V105%-V107%) (40 Gy) and between 107% and 110% of prescription dose (V107%-V110%) for 40 Gy plans, 50 Gy plans, and pooled.
| Percentile | Right | Left, gating | Left free breathing | |
|---|---|---|---|---|
| Lung V20Gy/V17Gy [%] | 50th | 16.6 | 13.5 | 14.3 |
| 90th | 22.3 | 18.3 | 20.9 | |
| 95th | 23.8 | 19.6 | 22.3 | |
| Heart V20Gy/V17Gy [%] | 50th | 0.0 | 0.1 | 0.7 |
| 90th | 0.0 | 1.1 | 4.5 | |
| 95th | 0.0 | 2.0 | 6.4 | |
| Heart V40Gy/V35Gy [%] | 50th | 0.0 | 0.0 | 0.0 |
| 90th | 0.0 | 0.1 | 1.9 | |
| 95th | 0.0 | 0.4 | 3.2 | |
| CTVp_breast V95% [%] | 50th | 97.6 | 97.0 | 95.5 |
| 90th | 95.2 | 94.1 | 90.4 | |
| 95th | 94.3 | 93.2 | 88.8 | |
| Percentile | 40 Gy/15fx | 50 Gy/25fx | All | |
| CTVp_breast V105% -V107% [%] | 50th | 0.0 | NA | NA |
| 90th | 1.8 | NA | NA | |
| 95th | 2.7 | NA | NA | |
| CTVp_breast V107% -V110% [mL] | 50th | 0.0 | 0.0 | 0.0 |
| 90th | 0.0 | 0.1 | 0.0 | |
| 95th | 0.0 | 1.2 | 0.3 | |
4. Discussion
This paper presents dosimetric parameters from the individual CT-based treatment plans of all patients treated in the DBCG HYPO trial. Compliance with the pre-defined dose constraints was high for all prospectively reported parameters, except the under-dosed volume of CTVp_breast (1-V95%). For 95% of the right-sided patients, CTVp_breast V95% was>94.3% whereas the corresponding values for left-sided patients were 93.2% (gated) and 88.8% (FB), showing that the heart constraints had higher priority in the protocol than CTVp_breast coverage. This reflects the focus on the risk of late cardiac morbidity in this group of early breast cancer patients [11], [12], [13] and a recent analysis of cardiac substructures in the Danish HYPO cohort showed decreasing mean heart and LADCA doses with treatment year [14]. However, the excellent clinical results obtained in this study with nine-year loco-regional recurrence risks of 3.3% (95% Cl, 2.0% to 5.0%) for the 50 Gy arm and 3.0% (95% Cl, 1.9% to 4.5%) for the 40 Gy arm indicate that this trade-off was feasible [7]. No statistically significant differences were found between the results for 40 Gy and 50 Gy plans except for CTVp_breast hot-spot volume (V107%-V110%).
The statistically significant larger proportion of 50 Gy patients with a higher hot-spot breast volume compared to 40 Gy patients was a result of the additional dose constraint of V105%-V107%≤2% for 40 Gy plans. As the patients in the two arms had similar breast sizes, it was expected, that more 50 Gy plans could have had a smaller hot-spot volume if the additional 40 Gy hot-spot constraint had been applied to the 50 Gy patients. The lower hot spot volumes for 40 Gy patients may be due to the use of a higher proportion of high energy photon beam in the treatment plan, thereby increasing the build-up zone in the breast. However, this was not reflected in the under-dosed volumes of CTVp_breast, where no statistically significant difference was observed between the 50 Gy and 40 Gy plans. Thus, the hot-spot constraint V105%-V107%≤2% used in the protocol is recommended as a hot-spot constraint for whole breast RT plans in the future.
From a clinical perspective, no patients were hospitalized with radiation pneumonitis in the DBCG HYPO trial, and only very few patients had died from heart or lung disease with no indication of an excess risk [7]. For the lung and heart, the 95th percentile values for lung and heart presented in table 3 are therefore suggested as new OAR constraints for whole-breast RT planning. These constraints are to our knowledge the first constraints for doses to OAR based on dosimetric data collected from all treatment plans in a randomized trial, and they represent constraints depending on laterality and use of respiratory gated technique.
The high degree of compliance (>99%) with the heart protocol constraints was due to the fact that in the treatment planning the heart had the highest priority after the tumour bed. Shortly after the end of recruitment in the HYPO trial an initial analysis of the heart doses from the Danish HYPO patients was presented to the DBCG RT Committee, and it was decided to change the heart constraints in the RT guidelines to V35Gy < 1% and V17Gy < 5% for whole-breast planning. These values may now be reduced even further by the results presented here. For loco-regional treatment the corresponding DBCG constraints are ≤ 5% and ≤ 10%, respectively.
In accordance with other large trials (IMPORT LOW, FAST, and FAST Forward), this trial recommended two tangential fields with field-in-field segments to obtain a homogeneous dose distribution [5], [15], [16]. However, 3D CRT forward field-in-field planning can be tedious. Therefore, several planning studies with a limited number of patients have investigated alternative field arrangements using either two inversed planned tangential IMRT fields, IMRT with more than two fields or volumetric modulated arc therapy (VMAT) [17], [18], [19]. The advantage of a tangential field technique is that irradiation of the contralateral side of the patient is minimised. Furthermore, Aznar et al. showed in a systematic review of lung doses from breast cancer RT that tangential fields spared both the ipsilateral and contralateral lung better than IMRT for whole breast RT without nodal irradiation [20]. With breathing adaption, 3D-CRT and tangential IMRT fields also had higher potential for sparing the heart compared to a partial VMAT technique [21]. These conclusions were recently validated in another study showing that the 3D-CRT in many situations is to be preferred [28].
Formation of fibrosis is dependent on several factors including hot-spot volumes in the breast [22]. This study has shown that breast hot-spot volumes also for large breasts can be minimized with tangential fields. Thus, this technique is far from an outdated method for whole breast RT.
This report was limited to the dosimetric parameters defined prior to the start of the trial and manually reported to the DBCG database. In this way it was possible to collect data from all patients treated. However, at that time the DBCG RT committee decided to report values related to CTVp_breast, whereas PTVp_breast values were not included in the list of manually reported values. All treatment plans from the Danish centres were also prospectively collected in a national storage facility of DICOM data (plan data bank), where values for analysis are not limited to a pre-defined set. Due to legislation issues at the time of recruitment, it was not possible for the Norwegian and German centres to export treatment plans to the Danish data bank. Thus, only results for the predefined and prospectively reported parameters can be presented for the total number of treatment plans in the trial.
Brink et al. demonstrated that the data quality in a treatment plan data bank was superior to manually reported data [23]. Therefore, quality assurance of the manually entered dosimetric parameters was carried out prior to the data analysis. If a dosimetric value deviated much from the expected range, the responsible physicist at the centre was asked to confirm the value. Dose coverage of the CTVp_breast (V95%) was the dosimetric parameter with most need of corrections during the data validation, confirming the findings in [23]. This was probably because the parameter to be prospectively reported was 1-V95% while the value most easily read from the treatment planning systems was V95%. Thus, for manually recording and reporting dose values it is recommended to choose parameters which are easily read from dose volume histograms.
During the trial period, the fraction of left-sided patients treated with respiratory gating increased from 7% in 2009–2010 to 85% in 2013–2014, thus in total 457 (48%) of the left-sided patients were treated with gating, whereas the remaining 497 left-sided patients were treated in FB. Results from treatment plans for 684 left-sided Danish patients in the DBCG HYPO trial submitted to the plan data bank presented by Berg et al were in harmony with other studies reporting that respiratory gated technique can improve target, lung and heart dose [24], [25], [26]. This was also observed in the complete data set from this trial. Apart from a lower heart exposure for gated patients, also lower lung V20Gy/17Gy values were obtained, potentially resulting in a decreased risk of a second lung cancer [27].
In conclusion, the prospectively collected treatment data from all treated breast cancer patients in the DBCG HYPO trial demonstrated a high degree of compliance with pre-specified constraints for treatment planning. This suggests that more strict dose constraints for organs at risk and for high dose breast volumes can be applied and are recommended for future whole breast RT planning.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank the patients who participated in this trial and the staff at participating centres and in the DBCG office. BVO was supported by the Danish Cancer Society and CIRRO (Centre for Interventional Research in Radiation Oncology) and the DCCC RT (Danish Comprehensive Cancer Centre RadioTherapy Group).
References
- 1.Overgaard M., Bentzen S.M., Juul Christensen J., Hjøllund Madsen E. The value of the NSD formula in equation of acute and late radiation complications in normal tissue following 2 and 5 fractions per week in breast cancer patients treated with postmastectomy irradiation. Radiother Oncol. 1987;9(1):1–11. doi: 10.1016/s0167-8140(87)80213-x. [DOI] [PubMed] [Google Scholar]
- 2.Friberg S., Rudén B.-I. Hypofractionation in radiotherapy: An investigation of injured Swedish women, treated for cancer of the breast. Acta Oncol. 2009;48(6):822–831. doi: 10.1080/02841860902824917. [DOI] [PubMed] [Google Scholar]
- 3.Yarnold J., Ashton A., Bliss J., Homewood J., Harper C., Hanson J. Fractionation sensitivity and dose response of late adverse effects in the breast after radiotherapy for early breast cancer: long-term results of a randomised trial. Radiother Oncol. 2005;75(1):9–17. doi: 10.1016/j.radonc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Whelan T.J., Pignol J.-P., Levine M.N., Julian J.A., MacKenzie R., Parpia S. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362(6):513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 5.Bentzen S.M., Agrawal R.K., Aird E.G.A., Barrett J.M., Barrett-Lee P.J., Bliss J.M. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet. 2008;371:1098–1107. doi: 10.1016/S0140-6736(08)60348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haviland J.S., Owen J.R., Dewar J.A., Agrawal R.K., Barrett J., Barrett-Lee P.J. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14(11):1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 7.Offersen BV, Alsner J, Nielsen HM, Jakobsen EH, Nielsen MH, Krause M et al. Hypofractionated versus standard fractionated radiotherapy of 1882 patients with early breast cancer or ductal carcinoma in situ in the randomized phase III trial: the DBCG HYPO trial. J Clin Oncol 2020;38:JCO.20.01363-3625. [DOI] [PubMed]
- 8.Nielsen M.H., Berg M., Pedersen A.N., Andersen K., Glavicic V., Jakobsen E.H. Delineation of target volumes and organs at risk in adjuvant radiotherapy of early breast cancer: national guidelines and contouring atlas by the Danish Breast Cancer Cooperative Group. Acta Oncol. 2013;52(4):703–710. doi: 10.3109/0284186X.2013.765064. [DOI] [PubMed] [Google Scholar]
- 9.Offersen B.V., Boersma L.J., Kirkove C., Hol S., Aznar M.C., Biete Sola A. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol. 2015;114(1):3–10. doi: 10.1016/j.radonc.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 10.Feng M., Moran J.M., Koelling T., Chughtai A., Chan J.L., Freedman L. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79(1):10–18. doi: 10.1016/j.ijrobp.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor C.W., Brønnum D., Darby S.C., Gagliardi G., Hall P., Jensen M.-B. Cardiac dose estimates from Danish and Swedish breast cancer radiotherapy during 1977–2001. Radiother Oncol. 2011;100(2):176–183. doi: 10.1016/j.radonc.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darby S.C., Ewertz M., McGale P., Bennet A.M., Blom-Goldman U., Brønnum D. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 13.Laugaard Lorenzen E., Christian Rehammar J., Jensen M.-B., Ewertz M., Brink C. Radiation-induced risk of ischemic heart disease following breast cancer radiotherapy in Denmark, 1977–2005. Radiother Oncol. 2020;152:103–110. doi: 10.1016/j.radonc.2020.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Finnegan R., Lorenzen E.L., Dowling J., Jensen I., Berg M., Thomsen M.S. Analysis of Cardiac Substructure Dose in a Large, Multi-Centre Danish Breast Cancer Cohort (the DBCG HYPO trial): Trends and Predictive Modelling. Radiother Oncol. 2020;153:130–138. doi: 10.1016/j.radonc.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Yarnold J.R. First results of the randomised UK FAST Trial of radiotherapy hypofractionation for treatment of early breast cancer (CRUKE/04/015) Radiother Oncol. 2011;100:93–100. doi: 10.1016/j.radonc.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 16.Brunt A.M., Haviland J.S., Wheatley D.A., Sydenham M.A., Alhasso A., Bloomfield D.J. FAST-Forward phase III multi-centre non-inferiority randomised controlled trial of 1- versus 3-week hypofractionated breast radiotherapy: 5-year results for efficacy and late normal tissue effects. Lancet. 2020;395:1613–1626. doi: 10.1016/S0140-6736(20)30932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penninkhof J., Spadola S., Breedveld S., Baaijens M., Lanconelli N., Heijmen B. Individualized Selection of Beam Angles and Treatment Isocenter in Tangential Breast Intensity Modulated Radiation Therapy. Int J Rad Oncol Biol Phys. 2017;98(2):447–453. doi: 10.1016/j.ijrobp.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Virén T., Heikkilä J., Myllyoja K., Koskela K., Lahtinen T., Seppälä J. Tangential volumetric modulated arc therapy technique for left-sided breast cancer radiotherapy. Radiat Oncol. 2015;10:79. doi: 10.1186/s13014-015-0392-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karpf D., Sakka M., Metzger M., Grabenbauer G.G. Left breast irradiation with tangential intensity modulated radiotherapy (t-IMRT) versus tangential volumetric modulated arc therapy (t-VMAT): trade-offs between secondary cancer induction risk and optimal target coverage. Radiat Oncol. 2019;14:156. doi: 10.1186/s13014-019-1363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aznar M.C., Duane F.K., Darby S.C., Wang Z., Taylor C.W. Exposure of the lungs in breast cancer radiotherapy: A systematic review of lung doses published 2010–2015. Radiother Oncol. 2018;126(1):148–154. doi: 10.1016/j.radonc.2017.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vikström J., Hjelstuen M.H., Wasbø E., Mjaaland I., Dybvik K.I. A comparison of conventional and dynamic radiotherapy planning techniques for early-stage breast cancer utilizing deep inspiration breath hold. Acta Oncol. 2018;57(10):1325–1330. doi: 10.1080/0284186X.2018.1497294. [DOI] [PubMed] [Google Scholar]
- 22.Donovan E., Bleakley N., Denholm E., Evans P., Gothard L., Hanson J. Randomised trial of standard 2D radiotherapy (RT) versus intensity modulated radiotherapy (IMRT) in patients prescribed breast radiotherapy. Radiother Oncol. 2007;82(3):254–264. doi: 10.1016/j.radonc.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Brink C., Lorenzen E.L., Krogh S.L., Westberg J., Berg M., Jensen I. DBCG hypo trial validation of radiotherapy parameters from a national databank versus manual reporting. Acta Oncol. 2018;57(1):107–112. doi: 10.1080/0284186X.2017.1406140. [DOI] [PubMed] [Google Scholar]
- 24.Berg M., Lorenzen E.L., Jensen I., Thomsen M.S., Lutz C.M., Refsgaard L. The potential benefits from respiratory gating for breast cancer patients regarding target coverage and dose to organs at risk when applying strict dose limits to the heart: results from the DBCG HYPO trial. Acta Oncol. 2018;57(1):113–119. doi: 10.1080/0284186X.2017.1406139. [DOI] [PubMed] [Google Scholar]
- 25.Vikström J., Hjelstuen M.H.B., Mjaaland I., Dybvik K.I. Cardiac and pulmonary dose reduction for tangentially irradiated breast cancer, utilizing deep inspiration breath-hold with audio-visual guidance, without compromising target coverage. Acta Oncol. 2011;50(1):42–50. doi: 10.3109/0284186X.2010.512923. [DOI] [PubMed] [Google Scholar]
- 26.Nissen H.D., Appelt A.L. Improved heart, lung and target dose with deep inspiration breath hold in a large clinical series of breast cancer patients. Radiother Oncol. 2013;106(1):28–32. doi: 10.1016/j.radonc.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 27.Grantzau T., Thomsen M.S., Væth M., Overgaard J. Risk of second primary lung cancer in women after radiotherapy for breast cancer. Radiother Oncol. 2014;111(3):366–373. doi: 10.1016/j.radonc.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Stick L.B., Lorenzen E.L., Yates E.S., Anandadas C., Andersen K., Aristei C. Selection criteria for early breast cancer patients in the DBCG proton trial - The randomised phase III trial strategy. CTRO. 2021;27:126–131. doi: 10.1016/j.ctro.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]