Graphical abstract
Keywords: Mesenchymal stem cells, Extracellular vesicles, COVID-19, SARS-CoV-2, Acute respiratory distress syndrome, Immunomodulation
Abbreviations: ACE2, Angiotensin-Converting Enzyme 2; AD-MSC, Adipocyte-Derived MSC; AFC, Alveolar Fluid Clearance; ALI, Acute Lung Injury; ALT, Alanine Aminotransferase; Ang-1, Angiopoietin-1; ARDS, Acute Respiratory Distress Syndrome; AST, Aspartate Aminotransferase; AT II, Type II Alveolar Epithelial; BDNF, Brain-Derived Neutrophilic Factor; BM-MSC, Bone Marrow MSC; COPD, Chronic Obstructive Pulmonary Disease; CRP, C-Reactive Protein; CT, Computerized Tomography; DC, Dendritic Cell; DIC, Disseminated Intravascular Coagulation; EV, Extracellular Vesicle; GVHD, Graft-Versus-Host Disease; hESC, Human Embryonic Stem Cell; HGF, Hepatocyte Growth Factor; HIF1-α, Hypoxia-Induced Factor 1-Α; IFN, Interferon; IMRC, Immunity and Matrix-Regulatory Cell; IPF, Idiopathic Pulmonary Fibrosis; ISG, Interferon-Stimulated Genes; IV, Intravenous; KGF, Keratinocyte Growth Factor; LDH, Lactate Dehydrogenase; LIF, Leukemia Inhibitory Factor; LPS, Lipopolysaccharide; MERS-CoV, The Middle East Respiratory Syndrome Coronavirus; MIP-2, Macrophage Inflammatory Protein-2; MOD, Multiple Organ Dysfunction; MSC, Mesenchymal Stromal/Stem Cell; MVB, Multi Vesicle Body; NGF, Nerve Growth Factor; NK cell, Natural Killer Cell; PDGF, Platelet-Derived Growth Factor; PGE2, Prostaglandin E2; ROS, Reactive Oxygen Species; SARS-CoV, Severe Acute Respiratory Syndrome Coronavirus; SPA, Surfactant Protein A; SPC, Surfactant Protein C; TGF-β, Transforming Growth Factor-Β; Th, T Helper; TMPRSS2, Transmembrane Protease Serine 2; TNF- α, Tumor Necrosis Factor- Α; UC-MSC, Umbilical Cord MSC; VEGF, Vascular Endothelial Growth Factor; WBC, White Blood Cell
Abstract
In late 2019, a novel coronavirus (SARS-CoV-2) emerged in Wuhan city, Hubei province, China. Rapidly escalated into a worldwide pandemic, it has caused an unprecedented and devastating situation on the global public health and society economy. The severity of recent coronavirus disease, abbreviated to COVID-19, seems to be mostly associated with the patients’ immune response. In this vein, mesenchymal stromal/stem cells (MSCs) have been suggested as a worth-considering option against COVID-19 as their therapeutic properties are mainly displayed in immunomodulation and anti-inflammatory effects. Indeed, administration of MSCs can attenuate cytokine storm and enhance alveolar fluid clearance, endothelial recovery, and anti-fibrotic regeneration. Despite advantages attributed to MSCs application in lung injuries, there are still several issues __foremost probability of malignant transformation and incidence of MSCs-related coagulopathy__ which should be resolved for the successful application of MSC therapy in COVID-19. In the present study, we review the historical evidence of successful use of MSCs and MSC-derived extracellular vesicles (EVs) in the treatment of acute respiratory distress syndrome (ARDS). We also take a look at MSCs mechanisms of action in the treatment of viral infections, and then through studying both the dark and bright sides of this approach, we provide a thorough discussion if MSC therapy might be a promising therapeutic approach in COVID-19 patients.
1. Introduction
At the end of 2019, the novel coronavirus infection (COVID-19) pandemic was originated in Wuhan, Hubei province, China, and it was confirmed to be caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). As the rate of mortality has been growing dramatically each day, with no dedicated therapeutic agent, COVID-19 has become to be a global public health issue that demands new strategies from all around the world. SARS-CoV-2 is genetically similar to two other beta groups of coronaviruses, named severe acute respiratory coronavirus (SARS-CoV) and the Middle East respiratory syndrome coronavirus (MERS-CoV) with a mortality rate of 10% and 37%, respectively [1], [2]. Despite the lower mortality rate of the novel coronavirus infection than the SARS and MERS epidemics [3], [4], the longer incubation period made the SARS-CoV-2 more contagious with a much higher total number of deaths [5], [6]. The clinical manifestations of COVID-19 vary from asymptomatic or mild sickness with fever, cough, or lack of breath to severe respiratory failure which requires mechanical ventilation support. Patients with severe COVID-19 are highly at the risk of acute respiratory distress syndrome, secondary infection, coagulopathy, septic shock, and also multiple organ dysfunction (MOD) [5], [7], [8]. Para-clinical findings in patients with COVID-19 demonstrate hyper-activation of the immune system, which in turn results in inflammatory hyper-response, cytokine-storm, and subsequently, damage of the pulmonary tissue, dysfunction of air exchange, and ARDS [9], [10].
Due to immunomodulatory, anti-inflammatory, and other immune properties, mesenchymal stem cells (MSCs) __which according to 2019 ISCT suggestion should be expressed as mesenchymal stromal cells [11] __ can play a key role in avoiding or alleviating the cytokine storm. Presently, MSCs are largely used in cell-based therapies and their safety and efficacy, especially for the treatment of immune-mediated inflammatory diseases, have been investigated in numerous clinical trials [12], [13], [14], [15]. A large number of clinical trials introduced MSCs as the promising therapeutic approach toward improving the adverse effects of the SARS-CoV-2 infection via immunomodulatory effects, attenuating the cytokine storm, and exerting anti-inflammatory effects [16]. In this study, we review the historical evidence of successful use of MSCs for lung disease and highlight the therapeutic role of MSCs and their EVs such as immunomodulation, alveolar fluid clearance (AFC), endothelial recovery, and anti-fibrotic regeneration in the treatment of viral infections. Also, through discussing the advantage and disadvantage of this approach we deliberate if MSC therapy might be a promising therapeutic approach in COVID-19 patients.
2. An overview of MSCs in viral infections; historical evidence
In 2004, the first inclusive report on MSCs clinical implementation was published, in which allogeneic bone marrow MSCs (BM-MSCs) were injected for pediatric graft-versus-host disease (GVHD), leading to 1-year-survival of the patient [15]. Afterward, MSCs have been widely examined as a valuable treatment option for a wide spectrum of tissue/organ damage especially for immune-mediated inflammatory diseases such as GVHD, inflammatory bowel disease (IBD), Crohn's disease, rheumatoid arthritis, and ARDS [17], [18]. This is attributed to their ease of isolation and large ex vivo expansion capacity, as well as the ability to interact with other immune cells via secretion of paracrine factors and exerting their immunomodulatory effects [14]. As the lungs are in direct contact with the external environment, they are prone to different infections including viral diseases. Over the past decade, a considerable number of studies shed light on the therapeutic effects of MSCs for lung diseases, such as ARDS [19], [20], [21], [22], [23], chronic obstructive pulmonary disease (COPD) [24], [25], [26], [27], [28], Asthma [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], and idiopathic pulmonary fibrosis (IPF) [43], [44], [45], [46], [47], [48].
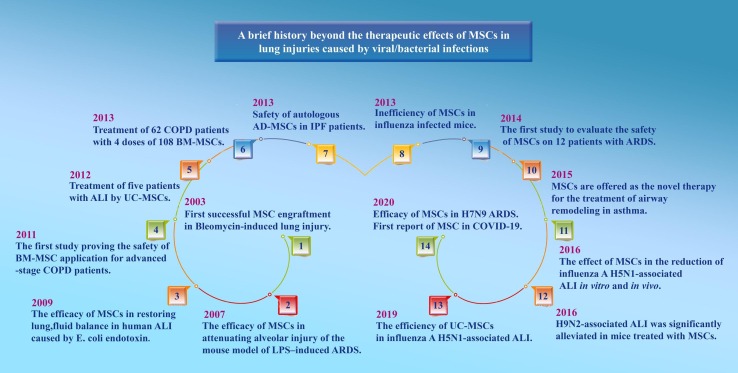
Despite the limited data from clinical studies, numerous reports suggest the therapeutic function of MSCs in preclinical models of respiratory virus-induced lung infections. A report by Chan et al. showed that the MSCs remarkably diminished the impairment of alveolar fluid clearance caused by the avian influenza virus (H5N1) in vitro and reduced A/H5N1-associated acute lung injury (ALI) in vivo [49]. Similarly, another study indicated that umbilical cord MSCs (UC-MSCs) may effectively restore alveolar fluid clearance in A/H5N1-associated ALI [50]. Furthermore, a study in a pig model provided interesting evidence showing that extracellular vesicles from MSCs may attenuate influenza virus-induced ALI [51]. Based on the results of a recent study showing that MSCs could improve the survival rate of H7N9-induced ARDS in both preclinical and clinical studies, the authors suggested that the MSC-based therapy could also be an ideal option for treatment of COVID-19 as they share similar complications such as ARDS and ALI [52]; however, in the other two in vivo studies in mice infected with A/H1N1, no improvement in inflammation or survival was seen [53], [54]. Besides, MSCs are themselves at the risk of being infected with the influenza virus [55], [56]. Future studies are needed to clarify the safety and efficacy of MSC's therapeutic role in influenza virus infections. The history beyond the therapeutic effects of MSCs in lung injuries caused by viral infections is summarized in Fig. 1 .
Fig. 1.
A glance at the history beyond the application of MSCs in lung injuries. MSC: Mesenchymal stem cell; LPS: Lipopolysaccharide; ARDS: Acute respiratory distress syndrome; ALI: Acute lung injury; BM: Bone marrow; COPD: Chronic obstructive pulmonary disease; UC: Umbilical cord; AD-MSC: Adipocyte-derived MSC; IPF: Idiopathic pulmonary fibrosis.
3. Plausible mechanisms of MSCs in viral infections
3.1. MSCs and modulation of the immune system
The principle mechanism by which MSCs exert their therapeutic functions is interaction with immune cells either via direct cell–cell contact or with the secretion of soluble immune factors (cytokines, chemokines, growth factors, angiogenic factors, and EVs). Indeed, MSC-regulated immunomodulation affects both innate and adaptive immunity by regulating the activity of neutrophils, monocytes, macrophages, dendritic cells (DC), T cells, B cells, and natural killer (NK) cells. MSCs modulate the T cell functions including inhibition of T helper (Th17) response, generation of regulatory T cells, shifting from Th1 to Th2 subset, and finally decreasing inflammation. Not only do MSCs restrain the proliferation of CD4+ and CD8+ T cell subsets [57], [58] they can also significantly influence DC development through prostaglandin E2 (PGE2) production, which eventually leads to the production of anti-inflammatory Th2 subset via differentiation of DCs to the plasmacytoid subset [59]. Apart from the modulation of the T cell responses, MSCs inhibit the proliferation of B cells and prevent their differentiation into plasma cells resulting in a reduction of immunoglobulin secretion [60], [61]. MSCs can also hamper oxidative burst in neutrophils, diminish the extracellular release of elastase and myeloperoxidase, and eventually weaken neutrophil infiltration and damage [62]. Concerning the immunomodulatory effects of these cells on innate immunity, Dayan et al. reported that MSCs could also decrease M1-type macrophages that possess a significant pro-inflammatory effect, while they increase alternatively activated M2-type macrophages via down-regulation of IL-1β and IL-6 levels [57]. Indeed, the anti-inflammatory properties of MSCs are mainly mediated by the paracrine pathways. They decrease the secretion of the pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), IL-12, and IFN-γ while elevating the level of the anti-inflammatory IL-10 mediator [63]. Taken together, all these findings reveal that MSCs could hamper the inflammatory responses, at least partly, through polarization of immune cells to the subsets which possess prominent anti-inflammatory characteristics.
3.2. MSCs and alveolar fluid clearance (AFC)
In the case of ARDS, MSCs play a role in clearing alveolar fluid which is disturbed due to cytokine storm in the lungs [64]. It has been reported that human UC-MSCs could improve the AFC impairment by producing a large amount of Angiopoietin-1 (Ang-1) [65] and hepatocyte growth factor (HGF) [50]. Ang-1 serves as a ligand for the Tie2 receptor and plays a key role in vascular stabilization due to its effects on endothelial permeability by inhibiting leukocyte-endothelium interactions and upregulating a tight-junction [66]. HGF, on the other hand, inhibits epithelial apoptosis and also regulates motility, cell growth, and morphogenesis via interaction with the c-Met receptor. Ang-1 and HGF both are considered significant mediators in the restorative properties of UC-MSCs on AFC in A (H5N1)-induced ARDS [50]. Accordingly, it has been reported that HGF could restore lung permeability via inhibition of endothelial apoptosis as well as the protection of tight junctions in a bacterial ALI model [67].
3.3. MSCs and regeneration
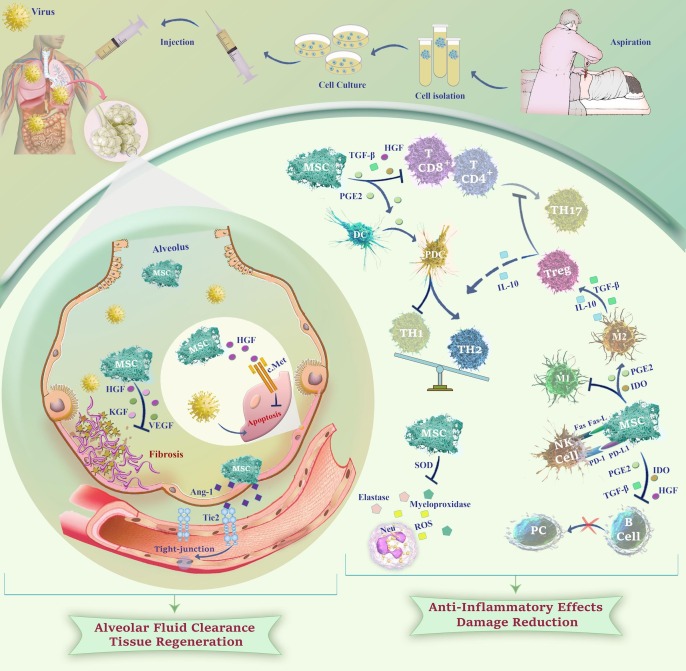
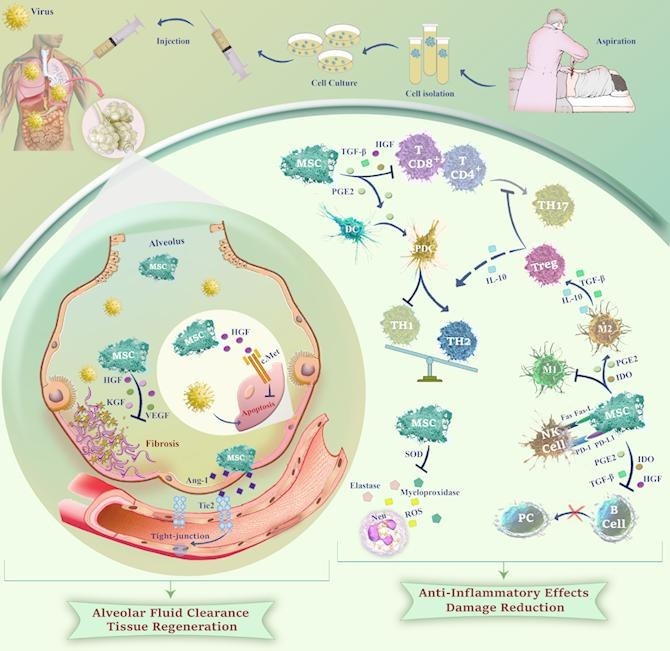
Given the fact that MSCs can secret a variety of growth factors including HGF, keratinocyte growth factor (KGF), and vascular endothelial growth factor (VEGF), they have significant ability to play essential roles in tissue regeneration in lung injuries via diminishing collagen accumulation and fibrosis [68]. HGF and VEGF can also restore pulmonary capillary permeability and stabilize the pulmonary endothelial barrier [69]. In a study by Aguilar et al., administration of MSCs expressing KGF resulted in the reduction of bleomycin-induced fibrosis, as well as decreasing collagen buildup and maintaining normal alveolar structure [70]. It has been also reported that HGF and KGF from MSCs stabilize B cell lymphoma 2 (Bcl-2), suppress hypoxia-induced factor 1-Α (HIF1-α) protein expression, and diminish reactive oxygen species (ROS) production; resulting in an anti-apoptotic effect on the alveolar epithelial cells [71]. To provide a well-conceptualized prospect, we summarized the plausible mechanisms of MSCs in viral infections in Fig. 2 .
Fig. 2.
A schematic overview of the potential mechanisms of action of MSCs against viral infections. MSCs can promote tissue regeneration and enhance alveolar fluid clearance through secretion of HGFs, KGFs, VEGFs, and Ang-1 which reduce fibrosis and facilitate injured endothelium repair. Also, MSCs may attenuate cytokine storm by regulating the immune cells in the inflammatory environment through secretion of anti-inflammatory factors leading to Treg induction, as well as TH2, PDC, and M2 macrophage polarization. Soluble factors from MSCs can prevent oxidative burst in neutrophils and reduce tissue damage via inhibition of extracellular release of elastase and myeloperoxidase.Ang-1: Angiopoietin-1; HGF: Hepatocyte growth factor; KGF: Keratinocyte growth factor; VEGF: Vascular endothelial growth factor; PGE2: Prostaglandin E2; TGF- β: Transforming growth factor-B; TH: T Helper; Treg: Regulatory T cell; IDO: Indoleamine 2,3-dioxygenase; NK cell: Natural killer cell; PD-1-PD-L1: Programmed death receptor and ligand; DC: Dendritic cell; PDC: Plasmacytoid DC; PC: Plasma cell; ROS: Reactive oxygen species; SOD: Superoxide dismutase; Neu: Neutrophil.
3.4. MSCs and production of extracellular vesicles
Extracellular vesicles (EVs) are membrane-bound vesicles about 40–1000 nm in size, produced by a variety of human cells. Exosome, microvesicles, and apoptotic bodies are three kinds of EVs. Microvesicles are separated directly by budding from the cell membrane, while exosomes are formed within the endosomal network of multi-vesicular bodies (MVBs) and their fusion with the plasma membrane [72]. MSC-derived EVs mimic many of the beneficial impacts of MSCs and transport a wide range of bioactive factors such as proteins and lipids, as well as transcription factors, mRNA, microRNA, long non-coding RNA, DNA, and signal transduction molecules [73]. Released EVs are the integral component of the cell-to-cell communication network, and upon transfer to the recipient cells, they can modulate downstream signaling. Compared with their parents, EVs administration is safer because they cannot replicate and form a tumor, they are non-immunogenic, and finally, they do not lead to emboli formation. Also, EVs are more stable and viable at − 80 °C for a longer time [74].
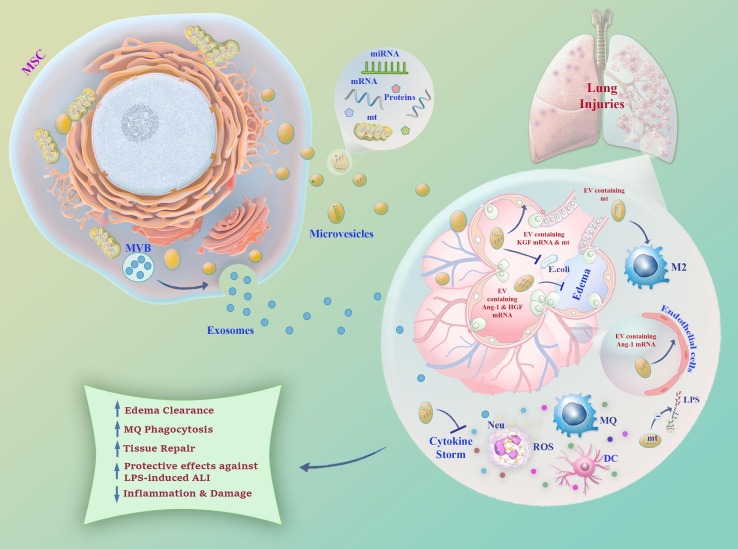
Termed as cell-free therapy, EVs have attracted special interest for their clinical application. MSC-derived EVs have been investigated in ARDS due to their ameliorating function in ALI by reducing inflammation, improving edema clearance, enhancing macrophages phagocytosis, and attenuating the severity of cytokine storm, and also tissue repair through the direct mitochondrial transfer of host cells [23], [75], [76], [77], [78]. In the study by Zhu et al., intratracheal administration of MSC-derived microvesicles was effective for pulmonary edema in the treatment of endotoxin-induced injury in mice by reducing extravascular lung water, restoring lung protein permeability, and reducing the influx of neutrophils and macrophage inflammatory protein-2 (MIP-2) levels through the expression of KGF mRNA in the injured alveolus [23]. In another study, Intratracheal administration of MSC EVs showed the same results by alleviating lung inflammation and restoring alveolar-capillary permeability in LPS-induced ALI mice. Notably, the effect was partly mediated by Ang1 mRNA, which is considered an important vascular-stabilizing factor [79]. Microvesicles derived from human MSCs could also improve the survival of mice injured with bacterial pneumonia by secreting KGF and decreasing the influx of inflammatory cells, cytokines, protein, and increasing the macrophage phagocytosis [76]. Mitochondrial transfer from BM-MSC carrying microvesicles to pulmonary alveoli ensued protective effects against LPS-induced ALI in mice [78], [80]. Moreover, the transfer of functional mitochondria from EVs was responsible for the elevation of macrophage phagocytosis through the promotion of oxidative phosphorylation [80]. Apart from ARDS, MSC-derived EVs have been also studied as a potential therapy in the management of other lung injuries including idiopathic pulmonary fibrosis, bronchopulmonary dysplasia, pulmonary arterial hypertension, asthma, and silicosis [81], [82], [83], [84]. All in all, MSC-derived EVs due to their advantages over their parents could be considered as an ideal candidate for the clinical application in a wide variety of viral infections, including COVID-19. Fig. 3 provides a schematic representation of the plausible mechanisms of MSC-derived EVs in lung injuries.
Fig. 3.
A Schematic representation of the role of MSC-derived EVs in lung injuries. Microvesicles and exosomes transfer miRNA, mRNA, protein, and mitochondria from their parents to the injured alveolus. EVs containing mitochondria and the mRNAs of KGF, HGF, and Ang-1 can decrease lung edema and enhance the tissue repair process. Mitochondrial transfer not only may hamper LPS-lung injury also inhibit cytokine storm by its modulatory effect on a variety of immune cells. EV: Extracellular vesicle; MVB: Multi-vesicular Bodies Mt: Mitochondria; Ang-1: Angiopoietin-1; HGF: Hepatocyte growth factor; KGF: Keratinocyte growth factor; LPS: Lipopolysaccharide; DC: Dendritic cell; Neu: Neutrophil; MQ: Macrophage.
4. MSCs as the potential therapy for COVID-19
SARS-CoV-2 viruses are enveloped with positive-stranded RNA of about 26 to 32 kb in length [85]. After entering the lungs, as the first organ exposed to the virus due to its way of transmission, the virus attaches to its functional receptor angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2) through the specific recognition of its surface spike (S) protein. A very similar structure also exists in the SARS virus, however, with a lower affinity binding (10–20 times) compared to the S protein of SARS-CoV-2. Notably, this binding capacity indicated the high transmission power of SARS-CoV-2 [86]. RNA is then released into the host cell and uses the replicate machinery of the infected cells [87]. The ACE2 receptor is abundantly distributed not only in alveolar epithelial cells also in the kidneys, liver, heart, and digestive organs; the fact explains why beyond ARDS, patients might go through organ damage, shock, and death from MOD [9]. Inside the host cell, the virus can trigger a “cytokine storm”. Indeed, deleterious unrestrained widespread inflammatory response arising from an excessive secretion of pro-inflammatory cytokines finally leads to deadly clinical manifestations of ARDS. The pathophysiology of SARS-CoV-2 has been largely discussed in several recent studies [88], [89], [90]. Previous studies indicated that MSCs could be at the risk of various viral infections such as influenza virus [56], hepatitis B virus [91], and human herpesvirus [92]. On the other hand, not only the lack of ACE2 receptors on MSCs may probably make their recognition by SARS-CoV-2 impossible [63] but the expression of interferon-stimulated genes (ISGs) could also induce resistance in MSCs [93]. Inline, RNA-sequence analysis for infused MSCs showed these cells do not express both ACE2 and TMPRSS2, which means MSCs cannot carry risks for cross-contamination of SARS-CoV-2. Infused MSCs also express high levels of type II alveolar epithelial (ATII) specific surfactant protein A (SPA) and surfactant protein C (SPC), indicating the potency of these cells for generating ATII cell [94]. Apart from these interesting characteristics, anti-inflammatory factors such as TGF-β, HGF, leukemia inhibitory factor (LIF), FGF, VEGF, and nerve growth factor (NGF) were highly expressed in MSCs that make these cells applicable candidates in COVID-19 treatment [63].
4.1. Therapeutic roles of MSCs and MSCs-derived EVs in COVID-19
4.1.1. Bone marrow MSCs (BM-MSCs) and umbilical cord MSCs (UC-MSCs)
To date, there have been several clinical studies publishing data regarding the use of MSCs as the novel treatment for SARS-CoV-2 cases. In the first reported study, a total of ten patients was enrolled. The intravenous (IV) administration of clinical-grade BM-MSCs was done for seven patients, while the remaining three patients served as the control group. They were closely followed for 14 days, no adverse effects were observed after MSC injection in seven cases, and MSC therapy could nearly improve all the patients' symptoms. The results of this interesting study revealed that BM-MSCs inhibit the over-activation of the immune system and prevent cytokine storm. Besides, an increase in the regulatory DC population has been observed [63].
Liang et al. have shown the safety and efficacy of treatment with UC-MSC in the modulation of the immune response and recovery of the injured tissue of a 65-years-old female severely ill COVID-19 patient. In this reported case study, a patient with a fever, fatigue, cough, and shortness of breath was treated with glucocorticoid and antiviral drugs which were ineffective. After diagnosis as a critically ill-type COVID-19 with ARDS, she was treated with triple IV infusion of 5 × 107 UC-MSCs. Following the second infusion, besides clinical improvement, white blood cell (WBC) counts and neutrophils decreased to a normal level, and lymphocytes elevated to normal range [95]. The results published by Shu et al. support that human UC-MSCs can be considered as a priority treatment option for severe COVID-19 cases. In the indicated study, a total of 41 patients were divided into 2 groups: 29 patients assigned to the placebo group and 12 assigned to the UC-MSC group. The patients were followed for 2 weeks after hUC-MSC injection. In the hUC-MSC treatment group no invasive ventilation occurred, no mortality was observed, and the patients' clinical symptoms, including shortness of breath, chest tightness, and fatigue were remarkably improved [96]. Meng et al. also conducted a clinical trial for evaluation of the safety of human UC-MSCs in the treatment of moderate and severe cases of COVID-19 in which 9 patients received 3 cycles of UC-MSCs on days 0, 3, and 6. After treatment, the PaO2/FiO2 ratio was improved, and computerized tomography (CT) scans reported improvement of lung injuries. The lung lesions were controlled for six days and completely disappeared within 2 weeks with no serious UC-MSCs infusion-associated adverse events [97]. In a case series, 8 critically ill patients on mechanical ventilation received PLX-PAD, a placenta-derived mesenchymal-like cell therapy. Despite the little sample size and the uncontrolled study design, the clinical status of patients including respiratory variables, kidney function, and positive end-expiratory pressure were improved [98]; further supporting the therapeutic efficacy of UC-MSCs in COVID-19 patients especially those with the critically ill status.
4.1.2. MSCs from other sources
Wu et al. reported IV delivery of a cell population derived from human embryonic stem cells (hESCs), termed immunity and matrix-regulatory cells (IMRCs), could hamper both fibrosis and pulmonary inflammation in a mouse model of lung tissue damage. With regards to the crisis of SARS-CoV-2 disease, IMRCs were administrated to 2 critically ill COVID-19 patients. Within 14 days following IV injection, both patients significantly recovered from pneumonia and tested negative for SARS-CoV-2. Notably, IMRCs may probably be superior to primary UC-MSCs due to stronger immunomodulatory effects, higher consistency in quality, and anti-fibrotic function [99]. Sanchez-Guijo et al. has tested the safety and efficiency of allogenic AD-MSCs for 13 patients with COVID-19 being under mechanical ventilation and treated with anti-inflammatory and anti-viral therapies. Ten out of 13 patients received two doses, 2 patients were treated with a single dose and 1 patient received 3 doses of AD-MSCs (0.98 × 106 cell/kg). Clinical improvement was seen in 69.2% of the patients and 7 patients were extubated and discharged. No adverse effect related to cell therapy was observed and the treatment resulted in the reduction of inflammatory factors including CRP, lactate dehydrogenase (LDH), ferritin, IL-6 as well as an increase in B and T lymphocytes [100]. In another clinical study by Chen et al., menstrual blood-derived MSCs were given to 25 patients with the median age of 70 years in a single, double, and triple infusion of 1 × 106 cells/kg. After treatment, abnormal signs of CT scan were improved in 16 cases, both PO2 and SO2 were increased while the levels of IL-6 and CRP were decreased. While no patients died during hospitalization, three cases underwent treatment-associated side effects, specifically heart failure, liver dysfunction, and allergic rash [101]. To provide a well-conceptualized overview, we summarized the results of previous studies that investigated the therapeutic roles of MSCs in COVID-19 in Table 1 .
Table 1.
A summary of the studies that investigated the therapeutic roles of MSCs in COVID-19.
| Severity | Dose and route | No. | Aim and outcome | Ref | ||
|---|---|---|---|---|---|---|
| BM-MSCs | ||||||
| ARDS | A single dose of 1 × 106 cells/kg; IV | 20 | Assessment of the PaO2/FiO2 ratio, oxygen saturation, sequential organ failure assessment, duration of ICU stay and hospitalization, Oxygen therapy-free days, and alterations of inflammatory parameters. | [103] | ||
| Moderate/severe | A single dose of 10 × 106 cells/kg; IV | 60 | Resulted in a fewer number of organ failure-free days, a higher oxygenation index, and a greater decrease of plasma Ang-2. | [104] | ||
| Moderate/severe | Single doses of 1 × 106 cells/kg; IV | 10 | Improved pulmonary function, decreased level of CRP, TNF-α, cytokine storm, and increased regulatory DC population. | [63] | ||
| UC-MSCs | ||||||
| ARDS | Three doses of 5 × 107 cells; IV | 1 | WBC count, neutrophils, and lymphocytes changed to normal, and inflammation symptoms relieved. | [95] | ||
| Moderate/severe | Three doses of 3 × 107 cells; IV | 18 | Decreased the level of IL-6, improved Pao2/Fio2, and ameliorated lung CT scan. | [97] | ||
| Severe/critical | A single dose of 5 × 106 cells; IV | 41 | Improved clinical symptoms, including fatigue, oxygen saturation, and shortness of breath, reduced CRP and IL-6 level, WBC count returned to the normal range, and lung inflammation improved. | [96] | ||
| ARDS |
Two doses of 0.7 × 106 cells/kg; IV A single dose of 0.3 × 106 cells/kg; IT |
1 | Improved hypoxemia and electrolyte, reduced level of CRP, and improved chest X-ray. | [105] | ||
| ARDS | Two doses of 100 ± 20 × 106; IV | 24 | Inflammatory cytokines were significantly decreased, higher survival rate with shorter time of recovery | [106] | ||
| ARDS | A single dose of 400 × 106 cells; IV | 60 | The incidence of serious adverse events, oxygenation Index, PaO2/FiO2 ratio, and sequential organ failure, length of ICU and hospital stay, and mortality rate were assessed. | [107] | ||
| Severe/critical ARDS |
A single dose of 1 × 106 cells/kg; IV Three doses of 200 × 106 cells, IV |
1 11 |
Increased SpO2 to the normal range, improved lung CT scan, neutrophils and lymphocytes returned to normal range, and decreased levels of ALT, AST, and CRP. Reduced serum levels of TNF-α, CRP, IL-6, and INF-γ, and resulted in remarkable signs of recovery in lung CT scan. |
[108], [109] | ||
| Placenta-derived MSCs | ||||||
| ARDS | One to two doses of 3 × 108 cells; IV | 8 | Decreased CRP level, and improved positive end-expiratory pressure and PaO2/FiO2 ratio. | [98] | ||
| IMRCs | ||||||
| ARDS | Single-dose; IV | 2 | Recovery from pneumonia, a decrease of pro-inflammatory cytokines (TNF-α, TGF-β), improvement of survival rate in a dose-dependent manner, and reduction of inflammation and lung fibrosis. | [99] | ||
| AD-MSCs | ||||||
| Severe/critical | Three doses of 0.98 × 106 cells/kg; IV | 13 | Reduced CRP, IL-6, ferritin, LDH, and D-dimer levels, and increased lymphocyte count. | [100] | ||
| Menstrual blood-derived MSCs | ||||||
| Severe/critical | Three doses; IV | 2 | Increased CD4+ T cells, decreased IL-6 and CRP levels, and improved partial pressure and O2 saturation. | [110] | ||
| Severe/critical | Three doses of 0.3 × 107cells, IV | 44 | Decreased rate of mortality, and significantly improved dyspnea. | [111] | ||
BM: Bone marrow; MSC: Mesenchymal stem cell; ARDS: Acute respiratory distress syndrome; IV: Intravenous; IT: Intratracheal; PaO2/FiO2: arterial oxygen partial pressure to fractional inspired oxygen; Ang-2: Angiopoietin 2; UC: Umbilical cord; WBC: White blood cell; CT: Computerized tomography; INF: Interferon; IMRCs: Immunity and matrix-regulatory cell; CRP: C-reactive protein; Spo2: Peripheral oxygen saturation; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; TNF-α: Tumor necrosis factor-Α; TGF-β: Transforming growth factor- Β; LDH: Lactate dehydrogenase; DC: Dendritic cell.
4.1.3. MSC-derived EVs
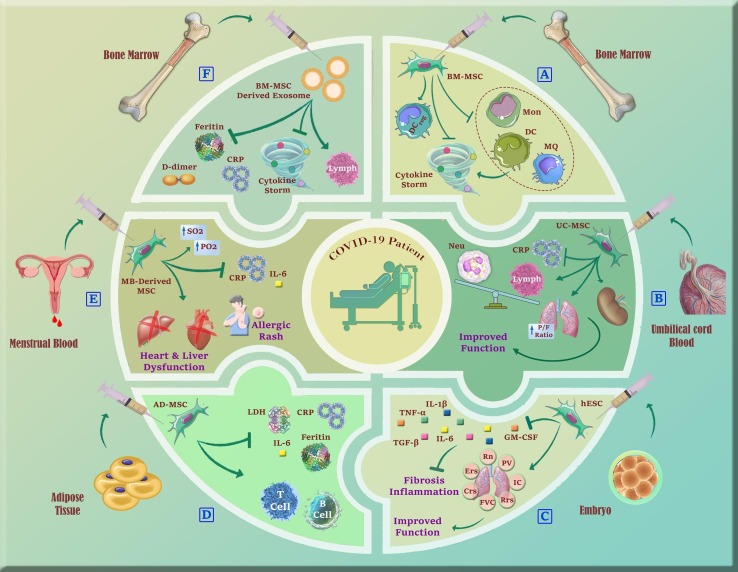
In an open-label randomized cohort study conducted by Sengupta et al., the efficiency and safety of allogenic BM-MSC-derived exosomes (ExoFloTM) have been investigated for the first time for patients with COVID-19. A single dose of ExoFlo (15 ml via the IV route) was infused into 24 critical patients with COVID-19. They showed that MSC-derived exosomes can downregulate cytokine storm, restore oxygenation, and reconstitute immunity in hospitalized patients with severe conditions. No adverse effects were attributed to the treatment and significant reduction of CRP, ferritin, and D-dimer were seen. Also, the absolute neutrophil/lymphocyte count and oxygenation were improved [102]. Up to now, 8 clinical trials using MSC-derived EVs to treat COVID-19 are registered in clinicaltrials.gov, among which 4 are still in progress (Table 2 ). Fig. 4 provides a summary of the therapeutic roles of MSCs and MSCs-derived EVs in COVID-19.
Table 2.
Ongoing clinical trials using MSC-derived extracellular vesicles for treatment of COVID-19.
| Dose | Route | No. | Phase | Status | Location | Primary Outcome Measures | Identifier |
|---|---|---|---|---|---|---|---|
| 20 doses 0.5–2 × 1010 of exosomes | Inhalation | 90 | 2 | Enrolling by invitation | Russia | 1. Safety assessments such as adverse events will be registered and monitored during all trials. 2. Safety assessments such as adverse events during the inhalation procedures. |
NCT04602442 |
| 5 doses of 2 × 108 nanovesicles | inhalation | 24 | 1 | Completed | China | 1. Adverse events assessment within 28 days. 2. Efficiency evaluation within 28 days, including the time to clinical improvement. |
NCT04276987 |
| 20 doses of 0.5–2 × 1010 exosomes |
Inhalation | 30 | 1|2 | Completed | Russian | 1. Safety assessments such as adverse events will be registered and monitored during all trials. 2. Safety assessments such as adverse events during the inhalation. |
NCT04491240 |
| 1 dose of 2–20 × 108 nanovesicles | inhalation | 27 | 1 | recruiting | China | Safety evaluation within 7 days after the first treatment, including frequency of adverse reactions. | NCT04313647 |
| 2 doses of 1 × 108 MSCs + 2 doses of EV |
IV | 60 | 2|3 | Recruiting | Iran | 1. Adverse events assessment within 28 days. 2. Blood oxygen saturation. |
NCT04366063 |
| NA | IV | 60 | 2 | Not yet recruiting | USA | 1. All-cause mortality. 2. Median days to recovery. |
NCT04493242 |
| 3 doses of 0.5–1 cc secretome | IM | 48 | 2 | Recruiting | Indonesia | NA | NCT04753476 |
| 4 doses of 2–8 × 1010 exosomes | IV | 55 | 1|2 | Not yet recruiting | USA | 1. Adverse events assessment within 90 days. 2. Quality efficacy of exosome therapy within 90 days. |
NCT04798716 |
MSCs: Mesenchymal stem cells; AT-MSCs: Adipocyte Tissue-MSCs; IV: Intravenous; EV: Extracellular vesicle; IM: Intramuscular; NA: Not available.
Fig. 4.
A schematic summary of clinical application of MSCs and their EVs from different sources in COVID-19 patients. A) BM-MSCs could modulate inflammation via downregulation of cytokine storm induced by the overactivation of pro-inflammatory immune cells. B) Administration of UC-MSCs could return immune cells to the normal range, decrease CRP level, and improve lung and kidney function. C) Human embryo-derived stem cells not only could improve lung function also prevent fibrosis and pulmonary inflammation. D) AD-MSC therapy resulted in a decreased level of inflammatory factors and increased B and T cell populations. E) Following menstrual blood-derived MSCs injection, the inflammatory factors were decreased, however, the patients underwent several treatment-associated side effects. F) Administration of BM-MSC-derived exosomes could improve the outcome of the disease through their significant attenuating effect on D-dimer, inflammation, and cytokine storm.Mon: Monocyte; DC: Dendritic Cell; MQ: Macrophage; UC-MSC: Umbilical cord MSC; CRP: C- reactive protein; Neu: Neutrophil; P/F ratio: Arterial oxygen partial pressure to fractional inspired oxygen; hESC: Human embryonic stem cell; GM-CSF: Granulocyte-macrophage colony-stimulating factor; TNF- α: Tumor necrosis factor-Α; TGF- β: Transforming growth factor-Β; AD-MSC: Adipocyte-derived MSC; LDH: Lactate dehydrogenase.
4.2. Optimizing the MSC therapy regimes
Several key points in MSC therapy should be considered to improve the capacity of MSCs in treating COVID-19, including administration route, the period window of administration, optimal dose, source of administration, and last but not least, dose frequency. IV infusion seems to be the most favorable mode of MSC delivery in ARDS and COVID-19 in the majority of clinical trials. It is the least invasive in comparison to intra-arterial or tissue injection. More importantly, due to the trapping property of MSCs, most of the cells injected via the IV route remain in the lungs that is the main organ affected mostly in ARDS and COVID-19. Also, IV administrated MSCs might be beneficial for the recovery of other organs as severe cases might suffer from MOD syndrome. However, the safety issues should be considered during IV delivery of MSCs in COVID-19 patients because of high pro-coagulant tissue factor (TF/CD142) expression in MSC products. Anticoagulation issues might have less significance in other routes of delivery such as intramuscular leading the cells into the extravascular space directly [112].
The most frequent doses for transfusion of human MSCs range from one-two million cells/kg and never exceed a dose of twelve million cells/kg. The median dose is 1 × 108 cells/patient in the IV route, however, the efficiency dose–response results have shown a narrower minimal effective dose between 100 and 150 million, and less efficacy is attributed to either higher or lower doses [13], [113]. The timing of MSC administration is also significant as the life-threatening cytokine storm, ARDS, and organ failure occur quickly. It was suggested that when symptoms are progressively getting worse, it is the optimum time for MSCs transplantation [63].
There is a preference for allogeneic transplantation of MSCs over the autologous one in the case of COVID-19 due to the time limitation for treatment, especially in severe cases [114]. On the other hand, MSC capacities are affected by age of the donor. It has been reported that MSCs received from older donors have shown a lower proliferation rate, poor homing, reduced immunomodulatory properties, and a higher percentage of apoptotic cells [115]. This means that autologous alternative is questionable in the elderly due to their liability to suffer severe COVID-19. MSCs from different sources also vary in their biological properties. According to a systematic review study by McIntyre et al., BM-MSCs and UC-MSCs are more effective in the treatment of acute lung disease [116]. However, because of the relatively low count of MSCs from BM, it might not be sufficient for the systematic disease caused by the SARS-CoV-2 virus, and among other available sources, MSCs obtained from umbilical cord __which has a higher concentration of stem cells and also obtained by a non-invasive method __ seems the most desirable. Moreover, UC-MSCs have a fast doubling time and they could be expanded faster in culture, providing enough cells for multi-organ damage in COVID-19 cases. UC-MSCs have a gene expression profile similar to embryonic MSCs; they have a higher proliferation speed, more plasticity, and unlike ESCs, possess no tumorigenic effects [117], [118], [119]. Taken together, adjusting the safe production of MSCs, in addition to settling an agreement on clinical trials concerning MSCs source and dose, administrative time, and mode of delivery might be pivotal for an effective and safe MSC-based therapy in COVID-19. Notably, pre-clinical animal data recommend the augmented MSCs either by the cytokine pre-activation, genetic engineering, or reprogramming to boost their threats potency [120], and future investigations should focus to develop modified MSCs that could safely and efficiently exert therapeutic effects.
4.3. Ongoing clinical trials of MSC therapy in COVID-19
Several clinical and pre-clinical studies have investigated the role of MSCs and their extracellular vesicles in the treatment of SARS-CoV-19 disease. Although the results of preliminary reports are promising, further trials in a larger cohort of patients are required to validate the definite safety and efficiency of MSCs and MSCs-derived EVs in mollifying symptoms associated with the COVID-19 infection. As per the time of submission of this review - April 2021, there are 74 ongoing studies related to the use of MSCs from various sources in the treatment of COVID-19 that are registered on clinicaltrial.gov. (Table 3 ).
Table 3.
Registered clinical trials on the Clinicaltrials.gov administrating MSCs to treat COVID-19.
| SC source | Dose and Route of administration | No. | Phase | Status | Location | Study design | Identifier |
|---|---|---|---|---|---|---|---|
| BM-MSCs | |||||||
| BM-MSCs | A single dose of 1–2 × 10 6 cells/kg, IV | 9 | 1 | Recruiting | Sweden | Interventional, Single Group Assignment, Open-Label Masking | NCT04447833 |
| BM-MSCs | Three times of 1.5–3 × 106 cells/kg, IV | 20 | 1|2 | Recruiting | Belgium | Single Group Assignment, Open Label | NCT04445454 |
| BM-MSCs | Two doses of 2 × 106 cells/kg, IV | 20 | 2 | Recruiting | Pakistan | Randomized, Parallel Assignment, Open Label | NCT04444271 |
| BM-MSCs | Gp 1: Three doses of 25 × 10 6 cells/kg, IV Gp 2: Three doses of 50 × 10 6 cells/kg, IV Gp 3: Three doses of 90 × 10 6 cells/kg, IV |
9 | 1 | Recruiting | Canada | Non-Randomize, Interventional, Sequential Assignment, Open Label | NCT04400032 |
| BM-MSCs | NA | 45 | 1 | Recruiting | USA | Randomized, Parallel Assignment, Quadruple Masking | NCT04397796 |
| BM-MSCs | NA | 10 | NA | Not yet recruiting | UK | Observational, Case-Only, Prospective | NCT04397471 |
| BM-MSCs | NA | 40 | 2 | Not yet recruiting | Germany | Randomized, Parallel Assignment, Open Label | NCT04377334 |
| BM-MSCs | Two doses of 2 × 106 cells/kg, IV | 223 | 3 | Active, not recruiting | USA | Randomized, Parallel Assignment, Triple Masking | NCT04371393 |
| BM-MSCs | A single dose of 1 × 106 cells/kg, IV | 20 | 1|2 | Not yet recruiting | China | Randomized, Parallel Assignment, Single Masking | NCT04346368 |
| BM-MSCs | Four doses of 1 × 108 cells/kg, IV | 30 | 1 | Not yet recruiting | USA | Single group, Parallel Assignment, Open Label | NCT04345601 |
| BM-MSCs | A single dose of 1 × 106 cells/kg | 10 | 1 | Not yet recruiting | Brazil | Single Group Assignment-Open Label | NCT04467047 |
| BM-MSCs | A single dose of 2 × 106 cells/kg | 600 | NA | Completed | Pakistan | Non-Randomized, Factorial Assignment, Open Label | NCT04492501 |
| BM-MSCs | Two doses, IV | 40 | 2 | Recruiting | USA | Randomized, Parallel Assignment, Quadruple Masking, double-blind | NCT04780685 |
| UC-MSCs | |||||||
| UC-MSCs | A single dose, IV | 40 | 1 | Completed | USA | Randomized, Single Group Assignment, Triple Masking | NCT04573270 |
| UC-MSCs | Two doses of 10 × 107 cells, IV | 60 | 1|2 | Recruiting | USA | Randomized, Parallel Assignment, Triple Masking | NCT04494386 |
| UC-MSCs | Two doses of 10 × 107 cells, IV | 21 | 1|2 | Not yet recruiting | USA | Randomized, Parallel Assignment, Double Masking | NCT04490486 |
| UC-MSCs | A single dose of 1 × 106 cells/kg, IV | 40 | 1 | Recruiting | Indonesia | Randomized, Interventional, Parallel Assignment, Triple Masking | NCT04457609 |
| UC-MSCs | Single-dose of 0.5, 1, 1.5 × 106 cells/kg, IV | 39 | 1|2 | Not yet recruiting | USA | Non-Randomized, Sequential Assignment, Open Label | NCT04452097 |
| UC-MSCs | 5 × 105 cells/kg, IV | 20 | 2 | Recruiting | Pakistan | Randomized, Parallel Assignment, Open Label | NCT04437823 |
| UC-MSCs | A single dose of 1 × 106 cells/kg, IV | 30 | 2 | Not yet recruiting | Colombia | Randomized, Parallel Assignment, Triple Masking | NCT04429763 |
| UC-MSCs | 1 × 106 cells/kg, IV | 10 | 2 | Recruiting | Mexico | Allocation: Non-Randomized, Parallel Assignment, Open Label | NCT04416139 |
| UC-MSCs | Three doses of 1 × 106 cells/kg | 30 | 1|2 | Recruiting | USA | Randomized, Single Group Assignment, Quadruple Masking | NCT04399889 |
| UC-MSCs | A single dose of 1 × 106 cells/kg | 70 | 1|2 | Not yet recruiting | USA | Randomized, Parallel Assignment, Double Masking | NCT04398303 |
| UC-MSCs | Three doses 3 × 10 6 cells, IV | 30 | 1|2 | Recruiting | Turkey | Randomized, Parallel Assignment, Quadruple Masking | NCT04392778 |
| UC-MSCs | Two doses of 50 × 106 cells, IV | 40 | 1|2 | Not yet recruiting | Colombia | Randomized, Parallel Assignment, Quadruple Masking | NCT04390152 |
| UC-MSCs | Four doses of 1 × 106 cells, IV | 60 | 1 | Active, not recruiting | China | Randomized, Parallel Assignment, Open Label | NCT04371601 |
| UC-MSCs | NA | 106 | 2 | Recruiting | Spain | Randomized, Parallel Assignment, open-label | NCT04366271 |
| UC-MSCs | Two doses of 100 × 106 cells, IV | 24 | 1|2 | Completed | USA | Randomized, Parallel Assignment, Triple Masking | NCT04355728 |
| UC-MSCs | Three doses of 4 × 107 cells, IV | 100 | 2 | Completed | China | Randomized, Parallel Assignment, Quadruple Masking | NCT04288102 |
| UC-MSCs | Four doses of 5 × 106 cells, IV | 48 | NA | Not yet recruiting | China | Randomized, Parallel Assignment, Open Label | NCT04273646 |
| UC-MSCs | Four doses of 9.9 × 107 cells, IV | 16 | 2 | Recruiting | China | Non-randomized, Single Group Assignment, Open Label | NCT04269525 |
| UC-MSCs | Three doses of 3 × 107 cells, IV | 20 | 1 | Recruiting | China | Non-Randomized, Parallel Assignment, Open Label | NCT04252118 |
| UC-MSCs | Two doses of 1 × 106 cells/kg, IV | 30 | 1|2 | Recruiting | China | Randomized, Parallel Assignment, Triple Masking | NCT04339660 |
| UC-MSCs | A single dose of 4 × 108 cells, IV | 75 | 1|2 | Recruiting | UK | Randomized, Parallel Assignment, Quadruple Masking | NCT03042143 |
| UCWJ-MSCs | Three doses of 1 × 106 cells, IV | 47 | 1|2 | Active, not recruiting | France | Randomized, Interventional, Parallel Assignment, Triple Masking | NCT04333368 |
| CB-MSCs | NA, IV | 70 | 1 | Recruiting | USA | Randomized, Parallel Assignment, Open Label | NCT04565665 |
| PLX-PAD | High and low doses, IV | 140 | 2 | Recruiting | USA | Randomized, Parallel Assignment, Quadruple Masking | NCT04389450 |
| PLX-PAD | A single dose of 3 × 108 cells, IV | 40 | 2 | Recruiting | Israel | Randomized, Parallel Assignment, Open Label | NCT04614025 |
| Placenta-MSCs | Three doses of 1 × 106 cells/kg, IV | 30 | 1|2 | Recruiting | Ukraine | Non-Randomized, Parallel Assignment, Open Label | NCT04461925 |
| Embryonic SCs | 1 × 106 cells | 9 | 1|2 | Recruiting | China | Interventional, Open Label | NCT04331613 |
| WJ-MSCs | |||||||
| WJ-MSCs | Three doses of 106 cells/kg | 30 | 2 | Not yet recruiting | Spain | Randomized, Single Group Assignment, Quadruple Masking | NCT04625738 |
| WJ-MSCs | A single dose of 10 × 107 cells, IV | 9 | 1 | Active, not recruiting | Mexico | Single Group Assignment, Open Label | NCT04456361 |
| WJ-MSCs | 106 cells, IV | 30 | 1|2 | Recruiting | Spain | Randomized, Parallel Assignment, Quadruple Masking | NCT04390139 |
| WJ-MSCs | Four doses of 1 × 106 cells/kg, IV | 5 | 1 | Recruiting | Jordan | Single Group Assignment, Open Label | NCT04313322 |
| AD-MSCs | |||||||
| AD-MSCs | A single dose of 1 × 106 cells/kg, IV | 20 | 1 | Recruiting | Mexico | Randomized, Parallel Assignment, Open Label | NCT04611256 |
| AD-MSCs | NA | 10 | 1|2 | Not yet recruiting | Korea | Single Group Assignment, Open Label | NCT04527224 |
| AD-MSCs | four times 10 × 107 cells/time | 6 | 1 | Not yet recruiting | Japan | Single Group Assignment, Open Label | NCT04522986 |
| AD-MSCs | NA, IV | 20 | 1 | Recruiting | USA | Interventional, Single Group Assignment, Open Label | NCT04486001 |
| AD-MSCs | Five doses of 1 × 108 cells | 20 | 2 | Recruiting | USA | Interventional, Single Group Assignment, Open Label | NCT04349631 |
| AD-MSCs | Three doses of 20 × 107 cells, IV | 200 | 2 | Not yet recruiting | USA | Randomized, Parallel Assignment, Double Masking | NCT04428801 |
| AD-MSCs | Five doses of 2 × 108, 1 × 108, 5 × 107 cells, IV | 100 | 2 | Enrolling by invitation | USA | Randomized, Parallel Assignment, Quadruple Masking | NCT04348435 |
| AD-MSCs | Two doses of 8 × 10 7 cells, IV | 26 | 1|2 | Active, not recruiting | Spain | Randomized, Parallel Assignment, Open Label | NCT04366323 |
| AD-MSCs | 5 × 10 7 cells/kg, IV | 20 | 1 | Not yet recruiting | USA | Non-Randomized, Sequential Assignment, Open Label | NCT04352803 |
| AD-MSCs | Four doses of 1 × 108 cells, IV | 100 | 2 | Active, not recruiting | USA | Randomized, Parallel Assignment, Quadruple Masking | NCT04362189 |
| AD-MSCs | Two doses of 1.5 × 106 cells/kg | 100 | 2 | Not yet recruiting | Spain | Randomized, Parallel Assignment-Quadruple Masking-Multi center | NCT04348461 |
| AD-MSCs | 1–1.5 × 106 cells/kg | 100 | 2 | Not yet recruiting | USA | Randomized, Parallel Assignment, Double Masking | NCT04728698 |
| AD-MSCs | Five doses, IV | 56 | 2 | Active, not recruiting | USA | Interventional, Single Group Assignment, Open Label | NCT04349631 |
| Other Sources | |||||||
| DP-MSCs | Three doses of 3 × 107, IV | 20 | 1|2 | Recruiting | China | Randomized, Interventional. Parallel Assignment, Triple Masking | NCT04336254 |
| DP-MSCs | Three doses of 1 × 106 cells, IV | 24 | 1 | Not yet recruiting | China | Interventional, Single Group Assignment, Open Label | NCT04302519 |
| LMSCs | Three doses of 1 × 108 cells, IV | 70 | 1 | Recruiting | USA | Randomized, Parallel Assignment, Double Masking | NCT04629105 |
| Cymerus MSCs | 2 million cells/kg, IV | 24 | 1|2 | Recruiting | Australia | Randomized, Parallel Assignment, Open Label | NCT04537351 |
| RNA-engineered | NA | 30 | 1|2 | Recruiting | USA | Single Group Assignment, Open Label | NCT04524962 |
| Olfactory MSCs | NA, IV | 40 | 1|2 | Enrolling by invitation | Belarus | Non-Randomized, Parallel Assignment, Open Label | NCT04382547 |
| SCE | A single dose, IV | 20 | 2 | Not yet recruiting | China | Randomized, Parallel Assignment, Single Masking | NCT04299152 |
| NHPBSCs | 1 × 106 cells, IV | 146 | 1|2 | Completed | UAE | Randomized, Open-Label | NCT04473170 |
| Progenitor | NA, IV | 400 | 2|3 | Recruiting | USA | Randomized, Parallel Assignment, Quadruple Masking, | NCT04367077 |
| Undefined-MSCs | |||||||
| MSCs | Three doses of 300 × 10, IV | 30 | 2 | Recruiting | USA | Randomized, placebo-controlled-parallel assignment, Triple Masking | NCT04466098 |
| MSCs | 250 to 750 million | 22 | 1|2 | Recruiting | USA | Randomized, Interventional, Parallel Assignment, Quadruple Masking | NCT04445220 |
| MSCs | A single dose of 1 × 10 6 cells/kg | 20 | 2 | Recruiting | Spain | Randomized, Parallel Assignment, Double Masking | NCT04615429 |
| MSCs | A single dose of 2.5,5 or 10 × 107 cells, IV | 20 | 1 | Recruiting | Brazil | Randomized, Parallel Assignment, Open Label | NCT04525378 |
| MSCs | Four doses of 2 × 10 7 cells, IV | 90 | 2 | Not yet recruiting | Brazil | Interventional, Parallel Assignment, Quadruple Masking | NCT04315987 |
| MSCs | Three doses of 1 × 10 6 cells/kg | 21 | NA | Completed | Turkey | Randomized, Parallel Assignment, Open Label | NCT04713878 |
| MSCs | A single dose of 1 × 10 6 cells/kg, IV | 24 | 2 | Recruiting | Spain | Randomized, Parallel Assignment, Triple Masking | NCT04361942 |
| DW-MSCs | Low dose gp: 5 × 107cells, IV High dose gp: 1 × 108 cells, IV |
9 | 1 | Recruiting | Indonesia | Randomized, Interventional, Parallel Assignment, Quadruple Masking | NCT04535856 |
MSC: Mesenchymal stem cell; BM-MSC: Bone marrow-MSC; UC-MSC: Umbilical cord-MSC; CM: Conditioned media; AD-MSCs: Adipocyte derived MSCs; WJ-MSCs: Warton jelly-MSC; CB-MSC: Cord blood-MSC; IMRC: immunity- and matrix-regulatory cell; DP-MSCs: Dental pulp MSCs; LMSCs: Longeveron MSCs; SCE: Stem cell educator; NHPBSC: Non-hematopoietic peripheral blood stem cells; gp: Group; IV: Intravenous; NA: Not available.
5. MSC therapy in the dark and bright side
Within the past decade, MSC-based therapies have gained interest among researchers because of their promising therapeutic roles in the treatment of inflammatory diseases. However, in regards to COVID-19, most of the clinical trials are yet ongoing and research concerning MSCs and their EVs is in its infancy. There have been only a few published results from clinical trials using MSCs for COVID-19 with none of them enrolled a large group of patients and with a detailed report of adverse effects. Therefore, despite the mentioned advantages attributed to MSCs application in lung injuries including COVID-19, there are still issues requiring to be resolved for the successful application of these cells. One of them involves the clinical-grade production of MSCs in this pandemic situation since a growing number of people are being infected with SARS-CoV-2, many of whom might be eligible for cell-based therapy meaning that we require to have a product ready-to-use on the shelf. Moreover, there are plenty of concerns regarding the stability of MSCs preparation. During ex vivo culture and passaging, MSCs go through cellular and molecular shifts [121]. The changes affect the proliferation, differentiation, homing to the site of injury, secretion of paracrine factors, the exhibition of morphological changes, and even probability of malignant transformation [122]. Previous studies reported that, in a specific vision, SCs may be compared to malignant cells as they can proliferate for a long period, suppress antitumor immunity, and escape apoptosis. Besides, pro-angiogenic factors such as platelet-derived growth factor (PDGF), VEGF, HGF, and Ang-1 released by MSCs can trigger angiogenesis [123]. This means the long-term evaluation of patients who received MSC therapy may be necessary to follow-up their tumorigenic capacity, especially in elder cases with compromised antitumor immunity.
Last but not least, it is reported that some cases of critically ill COVID-19 are highly prone to disseminated intravascular coagulation (DIC) and thromboembolism due to their pro-coagulant state. On the other hand, MSC products from various sources, especially AT-derived MSCs, express high levels of tissue factor (TF). TF is recognized as one of the crucial determinants of cell product hemocompatibility which can trigger instant blood-mediated inflammatory reaction (IBMI) following activation of the host innate immune cascade systems, including complement and coagulation by infused MSCs [124]. Being aware of the risk of lethal thrombotic complication, especially for the reactivated patients, alternative non-IV regimes, standardized hemocompatibility testing for new applied MCs, and administration of anticoagulant before application of high doses of MSCs should be seriously considered [125], [126]. All in all and taking all these concerns into account, we propose that MSCs-based therapeutic strategies should apply for COVID-19 cases only when its significant medical justifications and safety concerns are determined.
6. Conclusion and future perspective
Millions of people have been influenced with COVID-19 with no verified treatment available. Currently, supportive therapies, as well as non-specific anti-viral drugs, are mainly used for this purpose; however, MSC therapy seems to be a promising answer to this pandemic. ARDS, the major cause of respiratory failure that is characterized by injury to both the pulmonary endothelium and alveolar epithelium, is the most common complication of COVID-19 cases requiring mechanical ventilation and ICU support. IV-infused MSCs migrate directly to the lungs and secret a wide variety of factors to diminish pro-inflammatory cytokines, reduce pulmonary edema, elevate the rate of AFC, and inhibit cellular apoptosis. MSC-derived EVs has been also shown to downregulate cytokine storm, restore oxygenation, and reconstitute immunity in hospitalized COVID-19 patients with a severe condition. Notably, compared with their parents, exosomes seem to be safer because they cannot replicate and form a tumor, they are non-immunogenic, and they do not lead to emboli formation. Despite the safety and efficacy of MSCs in cell-based therapies have been shown in Phase I and Phase II of numerous clinical trials and several tens of them already made their way to Phase III, this field is in its infancy in COVID-19 and further studies regarding the ideal source and dose, optimum route of administration, and the time window of MSCs administration remains to be done.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgment
The authors would like to express their gratitude to Shahid Beheshti University of Medical Sciences (Tehran, Iran) for supporting this study.
Data availability statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.W.H. Organization, Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003, http://www. who. int/csr/sars/country/table2004_04_21/en/index. html (2003).
- 2.W.H. Organization, Middle East respiratory syndrome coronavirus (MERS-CoV), 2019.
- 3.Baud D., Qi X., Nielsen-Saines K., Musso D., Pomar L., Favre G. Real estimates of mortality following COVID-19 infection. Lancet. Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30195-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun J., He W.-T., Wang L., Lai A., Ji X., Zhai X., Li G., Suchard M.A., Tian J., Zhou J. COVID-19: epidemiology, evolution, and cross-disciplinary perspectives. Trends Mol. Med. 2020;26(5):483–495. doi: 10.1016/j.molmed.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 6.Perlman S. Another decade, another coronavirus. Mass Medical Soc. 2020 [Google Scholar]
- 7.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan. China, Jama. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang F., et al. Review of the clinical characteristics of coronavirus disease COVID-19. J. Gen. Intern. Med. 2019;2020:1–5. doi: 10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China, The lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pourbagheri-Sigaroodi A., Bashash D., Fateh F., Abolghasemi H. Laboratory findings in COVID-19 diagnosis and prognosis. Clin. Chim. Acta. 2020 doi: 10.1016/j.cca.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viswanathan S., Shi Y., Galipeau J., Krampera M., Leblanc K., Martin I., Nolta J., Phinney D., Sensebe L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy. 2019;21(10):1019–1024. doi: 10.1016/j.jcyt.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Zhao S., Wehner R., Bornhäuser M., Wassmuth R., Bachmann M., Schmitz M. Immunomodulatory properties of mesenchymal stromal cells and their therapeutic consequences for immune-mediated disorders. Stem Cells Dev. 2010;19(5):607–614. doi: 10.1089/scd.2009.0345. [DOI] [PubMed] [Google Scholar]
- 13.Galipeau J., Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22(6):824–833. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samsonraj R.M., Raghunath M., Nurcombe V., Hui J.H., van Wijnen A.J., Cool S.M. Concise review: multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem cells translational medicine. 2017;6(12):2173–2185. doi: 10.1002/sctm.17-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Blanc K., Rasmusson I., Sundberg B., Götherström C., Hassan M., Uzunel M., Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. The Lancet. 2004;363(9419):1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 16.Prockop D.J., Oh J.Y. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol. Ther. 2012;20(1):14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L.-T., Ting C.-H., Yen M.-L., Liu K.-J., Sytwu H.-K., Wu K.K., Yen B.L. Human mesenchymal stem cells (MSCs) for treatment towards immune-and inflammation-mediated diseases: review of current clinical trials. J. Biomed. Sci. 2016;23(1):1–13. doi: 10.1186/s12929-016-0289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.F. Gao, S. Chiu, D. Motan, Z. Zhang, L. Chen, H. Ji, H. Tse, Q.-L. Fu, Q. Lian, Mesenchymal stem cells and immunomodulation: current status and future prospects, Cell death & disease 7(1) (2016) e2062-e2062. [DOI] [PMC free article] [PubMed]
- 19.Wilson J.G., Liu K.D., Zhuo H., Caballero L., McMillan M., Fang X., Cosgrove K., Vojnik R., Calfee C.S., Lee J.-W. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. The Lancet Respiratory Medicine. 2015;3(1):24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.G. Bellingan, F. Jacono, J. Bannard-Smith, D. Brealey, N. Meyer, D. Thickett, D. Young, A. Bentley, B. McVerry, R. Wunderink, Primary analysis of a phase 1/2 study to assess MultiStem® cell therapy, a regenerative advanced therapy medicinal product (ATMP), in acute respiratory distress syndrome (MUST-ARDS), B14. LATE BREAKING CLINICAL TRIALS, American Thoracic Society2019, pp. A7353-A7353.
- 21.Yi X., Wei X., Lv H., An Y., Li L., Lu P., Yang Y., Zhang Q., Yi H., Chen G. Exosomes derived from microRNA-30b-3p-overexpressing mesenchymal stem cells protect against lipopolysaccharide-induced acute lung injury by inhibiting SAA3. Exp. Cell Res. 2019;383(2) doi: 10.1016/j.yexcr.2019.05.035. [DOI] [PubMed] [Google Scholar]
- 22.Chen W.-X., Zhou J., Zhou S.-S., Zhang Y.-D., Ji T.-Y., Zhang X.-L., Wang S.-M., Du T., Ding D.-G. Microvesicles derived from human Wharton’s jelly mesenchymal stem cells enhance autophagy and ameliorate acute lung injury via delivery of miR-100. Stem Cell Res. Ther. 2020;11(1):1–13. doi: 10.1186/s13287-020-01617-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Y.g. Zhu, X.m. Feng, J. Abbott, X.h. Fang, Q. Hao, A. Monsel, J.m. Qu, M.A. Matthay, J.W. Lee, Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin‐induced acute lung injury in mice, Stem cells 32(1) (2014) 116-125. [DOI] [PMC free article] [PubMed]
- 24.Shigemura N., Okumura M., Mizuno S., Imanishi Y., Nakamura T., Sawa Y. Autologous transplantation of adipose tissue-derived stromal cells ameliorates pulmonary emphysema. Am. J. Transplant. 2006;6(11):2592–2600. doi: 10.1111/j.1600-6143.2006.01522.x. [DOI] [PubMed] [Google Scholar]
- 25.Kim Y.-S., Kim J.-Y., Huh J.W., Lee S.W., Choi S.J., Oh Y.-M. The therapeutic effects of optimal dose of mesenchymal stem cells in a murine model of an elastase induced-emphysema. Tuberculosis and Respiratory Diseases. 2015;78(3):239–245. doi: 10.4046/trd.2015.78.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss D.J., Casaburi R., Flannery R., LeRoux-Williams M., Tashkin D.P. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest. 2013;143(6):1590–1598. doi: 10.1378/chest.12-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrell C.R., Miloradovic D., Sadikot R., Fellabaum C., Markovic B.S., Miloradovic D., Acovic A., Djonov V., Arsenijevic N., Volarevic V. Molecular and Cellular Mechanisms Responsible for Beneficial Effects of Mesenchymal Stem Cell-Derived Product “Exo-d-MAPPS” in Attenuation of Chronic Airway Inflammation. Anal. Cell. Pathol. 2020;2020 doi: 10.1155/2020/3153891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katsha A.M., Ohkouchi S., Xin H., Kanehira M., Sun R., Nukiwa T., Saijo Y. Paracrine factors of multipotent stromal cells ameliorate lung injury in an elastase-induced emphysema model. Mol. Ther. 2011;19(1):196–203. doi: 10.1038/mt.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.L.M. Boldrini-Leite, P.V. Michelotto Jr, S.A.B. de Moura, L.G.A. Capriglione, F.C.M. Barussi, F.Y.I. Fragoso, A.C. Senegaglia, P.R.S. Brofman, Lung Tissue Damage Associated with Allergic Asthma in BALB/c Mice Could Be Controlled with a Single Injection of Mesenchymal Stem Cells from Human Bone Marrow up to 14 d After Transplantation, Cell Transplantation 29 (2020) 0963689720913254. [DOI] [PMC free article] [PubMed]
- 30.Abreu S.C., Antunes M.A., Xisto D.G., Cruz F.F., Branco V.C., Bandeira E., Kitoko J.Z., de Araújo A.F., Dellatorre-Texeira L., Olsen P.C. Bone marrow, adipose, and lung tissue-derived murine mesenchymal stromal cells release different mediators and differentially affect airway and lung parenchyma in experimental asthma. Stem cells translational medicine. 2017;6(6):1557–1567. doi: 10.1002/sctm.16-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keyhanmanesh R., Rahbarghazi R., Ahmadi M. Systemic transplantation of mesenchymal stem cells modulates endothelial cell adhesion molecules induced by ovalbumin in rat model of asthma. Inflammation. 2018;41(6):2236–2245. doi: 10.1007/s10753-018-0866-8. [DOI] [PubMed] [Google Scholar]
- 32.Song X., Xie S., Lu K., Wang C. Mesenchymal stem cells alleviate experimental asthma by inducing polarization of alveolar macrophages. Inflammation. 2015;38(2):485–492. doi: 10.1007/s10753-014-9954-6. [DOI] [PubMed] [Google Scholar]
- 33.Royce S.G., Mao W., Lim R., Kelly K., Samuel C.S. iPSC-and mesenchymoangioblast-derived mesenchymal stem cells provide greater protection against experimental chronic allergic airways disease compared with a clinically used corticosteroid. FASEB J. 2019;33(5):6402–6411. doi: 10.1096/fj.201802307R. [DOI] [PubMed] [Google Scholar]
- 34.Malaquias M., Oyama L., Jericó P., Costa I., Padilha G., Nagashima S., Lopes-Pacheco M., Rebelatto C.L.K., Michelotto P., Xisto D. Effects of mesenchymal stromal cells play a role the oxidant/antioxidant balance in a murine model of asthma. Allergol. Immunopathol. 2018;46(2):136–143. doi: 10.1016/j.aller.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Duong K.M., Arikkatt J., Ullah M.A., Lynch J.P., Zhang V., Atkinson K., Sly P.D., Phipps S. Immunomodulation of Airway Epithelium Cell Activation by Mesenchymal Stromal Cells Ameliorates House Dust Mite-Induced Airway Inflammation in Mice. Am. J. Respir. Cell Mol. Biol. 2015;53(5):615–624. doi: 10.1165/rcmb.2014-0431OC. [DOI] [PubMed] [Google Scholar]
- 36.Zeng S.L., Wang L.H., Li P., Wang W., Yang J. Mesenchymal stem cells abrogate experimental asthma by altering dendritic cell function. Mol. Med. Rep. 2015;12(2):2511–2520. doi: 10.3892/mmr.2015.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keyhanmanesh R., Rahbarghazi R., Aslani M.R., Hassanpour M., Ahmadi M. Systemic delivery of mesenchymal stem cells condition media in repeated doses acts as magic bullets in restoring IFN-γ/IL-4 balance in asthmatic rats. Life Sci. 2018;212:30–36. doi: 10.1016/j.lfs.2018.09.049. [DOI] [PubMed] [Google Scholar]
- 38.Mirershadi F., Ahmadi M., Rezabakhsh A., Rajabi H., Rahbarghazi R., Keyhanmanesh R. Unraveling the therapeutic effects of mesenchymal stem cells in asthma. Stem Cell Res. Ther. 2020;11(1):1–12. doi: 10.1186/s13287-020-01921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Mendonca L., Felix N.S., Blanco N.G., Da Silva J.S., Ferreira T.P., Abreu S.C., Cruz F.F., Rocha N., Silva P.M., Martins V. Mesenchymal stromal cell therapy reduces lung inflammation and vascular remodeling and improves hemodynamics in experimental pulmonary arterial hypertension. Stem Cell Res. Ther. 2017;8(1):1–15. doi: 10.1186/s13287-017-0669-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Du Y.-M., Zhuansun Y.-X., Chen R., Lin L., Lin Y., Li J.-G. Mesenchymal stem cell exosomes promote immunosuppression of regulatory T cells in asthma. Exp. Cell Res. 2018;363(1):114–120. doi: 10.1016/j.yexcr.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 41.Fang S.-B., Zhang H.-Y., Wang C., He B.-X., Liu X.-Q., Meng X.-C., Peng Y.-Q., Xu Z.-B., Fan X.-L., Wu Z.-J. Small extracellular vesicles derived from human mesenchymal stromal cells prevent group 2 innate lymphoid cell-dominant allergic airway inflammation through delivery of miR-146a-5p. Journal of extracellular vesicles. 2020;9(1):1723260. doi: 10.1080/20013078.2020.1723260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonfield T.L., Koloze M., Lennon D.P., Zuchowski B., Yang S.E., Caplan A.I. Human mesenchymal stem cells suppress chronic airway inflammation in the murine ovalbumin asthma model. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2010;299(6):L760–L770. doi: 10.1152/ajplung.00182.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reddy M., Fonseca L., Gowda S., Chougule B., Hari A., Totey S. Human adipose-derived mesenchymal stem cells attenuate early stage of bleomycin induced pulmonary fibrosis: comparison with pirfenidone. Int. J. Stem Cells. 2016;9(2):192. doi: 10.15283/ijsc16041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ono M., Ohkouchi S., Kanehira M., Tode N., Kobayashi M., Ebina M., Nukiwa T., Irokawa T., Ogawa H., Akaike T. Mesenchymal stem cells correct inappropriate epithelial–mesenchyme relation in pulmonary fibrosis using stanniocalcin-1. Mol. Ther. 2015;23(3):549–560. doi: 10.1038/mt.2014.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu M., Zeng X., Wang J., Fu Z., Wang J., Liu M., Ren D., Yu B., Zheng L., Hu X. Immunomodulation by mesenchymal stem cells in treating human autoimmune disease-associated lung fibrosis. Stem Cell Res. Ther. 2016;7(1):63. doi: 10.1186/s13287-016-0319-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li F., Han F., Li H., Zhang J., Qiao X., Shi J., Yang L., Dong J., Luo M., Wei J. Human placental mesenchymal stem cells of fetal origins-alleviated inflammation and fibrosis by attenuating MyD88 signaling in bleomycin-induced pulmonary fibrosis mice. Mol. Immunol. 2017;90:11–21. doi: 10.1016/j.molimm.2017.06.032. [DOI] [PubMed] [Google Scholar]
- 47.Gad E., Salama A., El-Shafie M., Arafa H., Abdelsalam R., Khattab M. The Anti-fibrotic and Anti-inflammatory Potential of Bone Marrow-Derived Mesenchymal Stem Cells and Nintedanib in Bleomycin-Induced Lung Fibrosis in Rats. Inflammation. 2020;43(1):123–134. doi: 10.1007/s10753-019-01101-2. [DOI] [PubMed] [Google Scholar]
- 48.Mansouri N., Willis G.R., Fernandez-Gonzalez A., Reis M., Nassiri S., Mitsialis S.A., Kourembanas S. Mesenchymal stromal cell exosomes prevent and revert experimental pulmonary fibrosis through modulation of monocyte phenotypes. JCI insight. 2019;4(21) doi: 10.1172/jci.insight.128060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan M.C., Kuok D.I., Leung C.Y., Hui K.P., Valkenburg S.A., Lau E.H., Nicholls J.M., Fang X., Guan Y., Lee J.W. Human mesenchymal stromal cells reduce influenza A H5N1-associated acute lung injury in vitro and in vivo. Proc. Natl. Acad. Sci. 2016;113(13):3621–3626. doi: 10.1073/pnas.1601911113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loy H., Kuok D.I., Hui K.P., Choi M.H., Yuen W., Nicholls J.M., Peiris J.M., Chan M.C. Therapeutic Implications of Human Umbilical Cord Mesenchymal Stromal Cells in Attenuating Influenza A (H5N1) Virus-Associated Acute Lung Injury. J. Infect. Dis. 2019;219(2):186–196. doi: 10.1093/infdis/jiy478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khatri M., Richardson L.A., Meulia T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res. Ther. 2018;9(1):1–13. doi: 10.1186/s13287-018-0774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen J., Hu C., Chen L., Tang L., Zhu Y., Xu X., Chen L., Gao H., Lu X., Yu L. Clinical study of mesenchymal stem cell treating acute respiratory distress syndrome induced by epidemic Influenza A (H7N9) infection, a hint for COVID-19 treatment. Engineering. 2020 doi: 10.1016/j.eng.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Darwish I., Banner D., Mubareka S., Kim H., Besla R., Kelvin D.J., Kain K.C., Liles W.C. Mesenchymal stromal (stem) cell therapy fails to improve outcomes in experimental severe influenza. PLoS ONE. 2013;8(8) doi: 10.1371/journal.pone.0071761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gotts J.E., Abbott J., Matthay M.A. Influenza causes prolonged disruption of the alveolar-capillary barrier in mice unresponsive to mesenchymal stem cell therapy. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2014;307(5):L395–L406. doi: 10.1152/ajplung.00110.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khatri M., Saif Y.M. Influenza virus infects bone marrow mesenchymal stromal cells in vitro: implications for bone marrow transplantation. Cell Transplant. 2013;22(3):461–468. doi: 10.3727/096368912X656063. [DOI] [PubMed] [Google Scholar]
- 56.Thanunchai M., Kanrai P., Wiboon-ut S., Puthavathana P., Hongeng S., Thitithanyanont A. Tropism of avian influenza A (H5N1) virus to mesenchymal stem cells and CD34+ hematopoietic stem cells. PLoS ONE. 2013;8(12) doi: 10.1371/journal.pone.0081805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dayan V., Yannarelli G., Billia F., Filomeno P., Wang X.-H., Davies J.E., Keating A. Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction. Basic Res. Cardiol. 2011;106(6):1299–1310. doi: 10.1007/s00395-011-0221-9. [DOI] [PubMed] [Google Scholar]
- 58.Duffy M.M., Ritter T., Ceredig R., Griffin M.D. Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Res. Ther. 2011;2(4):1–9. doi: 10.1186/scrt75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yagi H., Soto-Gutierrez A., Parekkadan B., Kitagawa Y., Tompkins R.G., Kobayashi N., Yarmush M.L. Mesenchymal stem cells: mechanisms of immunomodulation and homing. Cell Transplant. 2010;19(6–7):667–679. doi: 10.3727/096368910X508762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Corcione A., Benvenuto F., Ferretti E., Giunti D., Cappiello V., Cazzanti F., Risso M., Gualandi F., Mancardi G.L., Pistoia V. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107(1):367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 61.Aggarwal S., Pittenger M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 62.Li N., Hua J. Interactions between mesenchymal stem cells and the immune system. Cell. Mol. Life Sci. 2017;74(13):2345–2360. doi: 10.1007/s00018-017-2473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q., Shan G., Meng F., Du D., Wang S. Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging disease. 2020;11(2):216. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ware L.B., Matthay M.A. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2001;163(6):1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 65.Fang X., Neyrinck A.P., Matthay M.A., Lee J.W. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J. Biol. Chem. 2010;285(34):26211–26222. doi: 10.1074/jbc.M110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saharinen P., Eklund L., Alitalo K. Therapeutic targeting of the angiopoietin–TIE pathway. Nat. Rev. Drug Discovery. 2017;16(9):635. doi: 10.1038/nrd.2016.278. [DOI] [PubMed] [Google Scholar]
- 67.Hu S., Li J., Xu X., Liu A., He H., Xu J., Chen Q., Liu S., Liu L., Qiu H. The hepatocyte growth factor-expressing character is required for mesenchymal stem cells to protect the lung injured by lipopolysaccharide in vivo. Stem Cell Res. Ther. 2016;7(1):1–13. doi: 10.1186/s13287-016-0320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li X., Yue S., Luo Z. Mesenchymal stem cells in idiopathic pulmonary fibrosis. Oncotarget. 2017;8(60) doi: 10.18632/oncotarget.18126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang Y., Chen Q.-H., Liu A.-R., Xu X.-P., Han J.-B., Qiu H.-B. Synergism of MSC-secreted HGF and VEGF in stabilising endothelial barrier function upon lipopolysaccharide stimulation via the Rac1 pathway. Stem Cell Res. Ther. 2015;6(1):1–14. doi: 10.1186/s13287-015-0257-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aguilar S., Scotton C.J., McNulty K., Nye E., Stamp G., Laurent G., Bonnet D., Janes S.M. Bone marrow stem cells expressing keratinocyte growth factor via an inducible lentivirus protects against bleomycin-induced pulmonary fibrosis. PLoS ONE. 2009;4(11) doi: 10.1371/journal.pone.0008013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bernard O., Jeny F., Uzunhan Y., Dondi E., Terfous R., Label R., Sutton A., Larghero J., Vanneaux V., Nunes H. Mesenchymal stem cells reduce hypoxia-induced apoptosis in alveolar epithelial cells by modulating HIF and ROS hypoxic signaling. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2018;314(3):L360–L371. doi: 10.1152/ajplung.00153.2017. [DOI] [PubMed] [Google Scholar]
- 72.Yáñez-Mó M., Siljander P.R.-M., Andreu Z., Bedina Zavec A., Borràs F.E., Buzas E.I., Buzas K., Casal E., Cappello F., Carvalho J. Biological properties of extracellular vesicles and their physiological functions. Journal of extracellular vesicles. 2015;4(1):27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Biancone L., Bruno S., Deregibus M.C., Tetta C., Camussi G. Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrol. Dial. Transplant. 2012;27(8):3037–3042. doi: 10.1093/ndt/gfs168. [DOI] [PubMed] [Google Scholar]
- 74.Gupta S., Krishnakumar V., Sharma Y., Dinda A.K., Mohanty S. Mesenchymal stem cell derived exosomes: a nano platform for therapeutics and drug delivery in combating COVID-19. Stem cell reviews and reports. 2020:1–11. doi: 10.1007/s12015-020-10002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee J.H., Park J., Lee J.W. Therapeutic use of mesenchymal stem cell–derived extracellular vesicles in acute lung injury. Transfusion. 2019;59(S1):876–883. doi: 10.1111/trf.14838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Monsel A., Zhu Y.-G., Gennai S., Hao Q., Hu S., Rouby J.-J., Rosenzwajg M., Matthay M.A., Lee J.W. Therapeutic effects of human mesenchymal stem cell–derived microvesicles in severe pneumonia in mice. Am. J. Respir. Crit. Care Med. 2015;192(3):324–336. doi: 10.1164/rccm.201410-1765OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hu S., Park J., Liu A., Lee J., Zhang X., Hao Q., Lee J.W. Mesenchymal stem cell microvesicles restore protein permeability across primary cultures of injured human lung microvascular endothelial cells. Stem cells translational medicine. 2018;7(8):615–624. doi: 10.1002/sctm.17-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morrison T.J., Jackson M.V., Cunningham E.K., Kissenpfennig A., McAuley D.F., O’Kane C.M., Krasnodembskaya A.D. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am. J. Respir. Crit. Care Med. 2017;196(10):1275–1286. doi: 10.1164/rccm.201701-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tang X.D., Shi L., Monsel A., Li X.Y., Zhu H.L., Zhu Y.G., Qu J.M. Mesenchymal stem cell microvesicles attenuate acute lung injury in mice partly mediated by Ang-1 mRNA. Stem cells. 2017;35(7):1849–1859. doi: 10.1002/stem.2619. [DOI] [PubMed] [Google Scholar]
- 80.Islam M.N., Das S.R., Emin M.T., Wei M., Sun L., Westphalen K., Rowlands D.J., Quadri S.K., Bhattacharya S., Bhattacharya J. Mitochondrial transfer from bone-marrow–derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 2012;18(5):759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Worthington E.N., Hagood J.S. Therapeutic use of extracellular vesicles for acute and chronic lung disease. Int. J. Mol. Sci. 2020;21(7):2318. doi: 10.3390/ijms21072318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cruz F.F., Borg Z.D., Goodwin M., Sokocevic D., Wagner D.E., Coffey A., Antunes M., Robinson K.L., Mitsialis S.A., Kourembanas S. Systemic administration of human bone marrow-derived mesenchymal stromal cell extracellular vesicles ameliorates aspergillus hyphal extract-induced allergic airway inflammation in immunocompetent mice. Stem Cells Translational Medicine. 2015;4(11):1302–1316. doi: 10.5966/sctm.2014-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee C., Mitsialis S.A., Aslam M., Vitali S.H., Vergadi E., Konstantinou G., Sdrimas K., Fernandez-Gonzalez A., Kourembanas S. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126(22):2601–2611. doi: 10.1161/CIRCULATIONAHA.112.114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Willis G.R., Fernandez-Gonzalez A., Anastas J., Vitali S.H., Liu X., Ericsson M., Kwong A., Mitsialis S.A., Kourembanas S. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am. J. Respir. Crit. Care Med. 2018;197(1):104–116. doi: 10.1164/rccm.201705-0925OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.A. Wu, Y. Peng, B. Huang, X. Ding, X. Wang, P. Niu, J. Meng, Z. Zhu, Z. Zhang, J. Wang, Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China, Cell host & microbe (2020). [DOI] [PMC free article] [PubMed]
- 86.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Seminars in immunopathology, Springer. 2017:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang X., Yu Y., Xu J., Shu H., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study, The Lancet. Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet respiratory medicine. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thanunchai M., Hongeng S., Thitithanyanont A. Mesenchymal stromal cells and viral infection. Stem Cells International. 2015;2015 doi: 10.1155/2015/860950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sundin M., Örvell C., Rasmusson I., Sundberg B., Ringden O., Le Blanc K. Mesenchymal stem cells are susceptible to human herpesviruses, but viral DNA cannot be detected in the healthy seropositive individual. Bone Marrow Transplant. 2006;37(11):1051–1059. doi: 10.1038/sj.bmt.1705368. [DOI] [PubMed] [Google Scholar]
- 93.X. Wu, V.L.D. Thi, Y. Huang, E. Billerbeck, D. Saha, H.-H. Hoffmann, Y. Wang, L.A.V. Silva, S. Sarbanes, T. Sun, Intrinsic immunity shapes viral resistance of stem cells, Cell 172(3) (2018) 423-438. e25. [DOI] [PMC free article] [PubMed]
- 94.Ma N., Gai H., Mei J., Ding F.B., Bao C.R., Nguyen D.M., Zhong H. Bone marrow mesenchymal stem cells can differentiate into type II alveolar epithelial cells in vitro. Cell Biol. Int. 2011;35(12):1261–1266. doi: 10.1042/CBI20110026. [DOI] [PubMed] [Google Scholar]
- 95.Liang B., Chen J., Li T., Wu H., Yang W., Li Y., Li J., Yu C., Nie F., Ma Z. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: A case report. Medicine. 2020;99(31) doi: 10.1097/MD.0000000000021429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shu L., Niu C., Li R., Huang T., Wang Y., Huang M., Ji N., Zheng Y., Chen X., Shi L. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 2020;11(1):1–11. doi: 10.1186/s13287-020-01875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meng F., Xu R., Wang S., Xu Z., Zhang C., Li Y., Yang T., Shi L., Fu J., Jiang T. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal transduction and targeted therapy. 2020;5(1):1–7. doi: 10.1038/s41392-020-00286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barkama R., Mayo A., Paz A., Solopov A., Mann T., Vadasz Z., Appel T., Ofir R., Shani L., Sheleg M. Placenta-Derived Cell Therapy to Treat Patients With Respiratory Failure Due to Coronavirus Disease 2019. Critical care explorations. 2020;2(9) doi: 10.1097/CCE.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.J. Wu, D. Song, Z. Li, B. Guo, Y. Xiao, W. Liu, L. Liang, C. Feng, T. Gao, Z. Wang, Immunity-and-Matrix-Regulatory Cells Derived from Human Embryonic Stem Cells Safely and Effectively Treat Mouse Lung Injury and Fibrosis, bioRxiv (2020). [DOI] [PMC free article] [PubMed]
- 100.Sánchez-Guijo F., García-Arranz M., López-Parra M., Monedero P., Mata-Martínez C., Santos A., Sagredo V., Álvarez-Avello J.-M., Guerrero J.E., Pérez-Calvo C. Adipose-derived mesenchymal stromal cells for the treatment of patients with severe SARS-CoV-2 pneumonia requiring mechanical ventilation A proof of concept study. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen X., Shan Y., Wen Y., Sun J., Du H. Mesenchymal stem cell therapy in severe COVID-19: A retrospective study of short-term treatment efficacy and side effects. The Journal of Infection. 2020 doi: 10.1016/j.jinf.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sengupta V., Sengupta S., Lazo A., Woods P., Nolan A., Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020 doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Payares-Herrera C., Martínez-Muñoz M.E., Vallhonrat I.L., de Molina R.M., Torres M.P., Trisan A., de Diego I.S., Alonso R., Zafra R., Donaire T. Double-blind, randomized, controlled, trial to assess the efficacy of allogenic mesenchymal stromal cells in patients with acute respiratory distress syndrome due to COVID-19 (COVID-AT): A structured summary of a study protocol for a randomised controlled trial. Trials. 2021;22(1):1–3. doi: 10.1186/s13063-020-04964-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Matthay M.A., Calfee C.S., Zhuo H., Thompson B.T., Wilson J.G., Levitt J.E., Rogers A.J., Gotts J.E., Wiener-Kronish J.P., Bajwa E.K. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. The Lancet Respiratory Medicine. 2019;7(2):154–162. doi: 10.1016/S2213-2600(18)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zengin R., Beyaz O., Koc E.S., Akinci I.O., Kocagoz S., Sagcan G., Ovali E., Cuhadaroglu C. Mesenchymal stem cell treatment in a critically ill COVID-19 patient: a case report. Stem cell investigation. 2020;7 doi: 10.21037/sci-2020-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.G. Lanzoni, E. Linetsky, D. Correa, S. Messinger Cayetano, R.A. Alvarez, D. Kouroupis, A. Alvarez Gil, R. Poggioli, P. Ruiz, A.C. Marttos, Umbilical cord mesenchymal stem cells for COVID‐19 acute respiratory distress syndrome: A double‐blind, phase 1/2a, randomized controlled trial, Stem cells translational medicine. [DOI] [PMC free article] [PubMed]
- 107.Gorman E., Shankar-Hari M., Hopkins P., Tunnicliffe W.S., Perkins G.D., Silversides J., McGuigan P., Jackson C., Boyle R., McFerran J. Repair of Acute Respiratory Distress Syndrome by Stromal Cell Administration in COVID-19 (REALIST-COVID-19): A structured summary of a study protocol for a randomised, controlled trial. Trials. 2020;21(1):1–3. doi: 10.1186/s13063-020-04416-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhu Y., Zhu R., Liu K., Li X., Chen D., Bai D., Luo J., Liu Y., Zhang Y., Li L. Human umbilical cord mesenchymal stem cells for adjuvant treatment of a critically ill COVID-19 patient: A case report. Infection and Drug Resistance. 2020;13:3295. doi: 10.2147/IDR.S272645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hashemian S.-M.R., Aliannejad R., Zarrabi M., Soleimani M., Vosough M., Hosseini S.-E., Hossieni H., Keshel S.H., Naderpour Z., Hajizadeh-Saffar E. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: a case series. Stem Cell Res. Ther. 2021;12(1):1–12. doi: 10.1186/s13287-021-02165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tang L., Jiang Y., Zhu M., Chen L., Zhou X., Zhou C., Ye P., Chen X., Wang B., Xu Z. Clinical study using mesenchymal stem cells for the treatment of patients with severe COVID-19. Frontiers of medicine. 2020;14(5):664–673. doi: 10.1007/s11684-020-0810-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu X., Jiang W., Chen L., Xu Z., Zhang Q., Zhu M., Ye P., Li H., Yu L., Zhou X. Evaluation of the safety and efficacy of using human menstrual blood-derived mesenchymal stromal cells in treating severe and critically ill COVID-19 patients: An exploratory clinical trial. Clinical and translational medicine. 2021;11(2) doi: 10.1002/ctm2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moll G., Drzeniek N., Kamhieh-Milz J., Geissler S., Volk H.-D., Reinke P. MSC therapies for COVID-19: importance of patient coagulopathy, thromboprophylaxis, cell product quality and mode of delivery for treatment safety and efficacy. Front. Immunol. 2020;11:1091. doi: 10.3389/fimmu.2020.01091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kabat M., Bobkov I., Kumar S., Grumet M. Trends in mesenchymal stem cell clinical trials 2004–2018: Is efficacy optimal in a narrow dose range? Stem cells translational medicine. 2020;9(1):17–27. doi: 10.1002/sctm.19-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yen B.L., Yen M.L., Wang L.T., Liu K.J., Sytwu H.K. Current status of mesenchymal stem cell therapy for immune/inflammatory lung disorders: Gleaning insights for possible use in COVID-19. Stem Cells Translational Medicine. 2020 doi: 10.1002/sctm.20-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Alt E.U., Senst C., Murthy S.N., Slakey D.P., Dupin C.L., Chaffin A.E., Kadowitz P.J., Izadpanah R. Aging alters tissue resident mesenchymal stem cell properties. Stem Cell Res. 2012;8(2):215–225. doi: 10.1016/j.scr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 116.McIntyre L.A., Moher D., Fergusson D.A., Sullivan K.J., Mei S.H., Lalu M., Marshall J., Mcleod M., Griffin G., Grimshaw J. Efficacy of mesenchymal stromal cell therapy for acute lung injury in preclinical animal models: a systematic review. PLoS ONE. 2016;11(1) doi: 10.1371/journal.pone.0147170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Arutyunyan I., Elchaninov A., Makarov A., Fatkhudinov T. Umbilical cord as prospective source for mesenchymal stem cell-based therapy. Stem cells international. 2016;2016 doi: 10.1155/2016/6901286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Weiss M.L., Troyer D.L. Stem cells in the umbilical cord. Stem cell reviews. 2006;2(2):155–162. doi: 10.1007/s12015-006-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nagamura-Inoue T., He H. Umbilical cord-derived mesenchymal stem cells: their advantages and potential clinical utility. World journal of stem cells. 2014;6(2):195. doi: 10.4252/wjsc.v6.i2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Guess A.J., Daneault B., Wang R., Bradbury H., La Perle K.M., Fitch J., Hedrick S.L., Hamelberg E., Astbury C., White P. Safety profile of good manufacturing practice manufactured interferon γ-primed mesenchymal stem/stromal cells for clinical trials. Stem cells translational medicine. 2017;6(10):1868–1879. doi: 10.1002/sctm.16-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bonab M.M., Alimoghaddam K., Talebian F., Ghaffari S.H., Ghavamzadeh A., Nikbin B. Aging of mesenchymal stem cell in vitro. BMC cell biology. 2006;7(1):14. doi: 10.1186/1471-2121-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Røsland G.V., Svendsen A., Torsvik A., Sobala E., McCormack E., Immervoll H., Mysliwietz J., Tonn J.-C., Goldbrunner R., Lønning P.E. Long-term cultures of bone marrow–derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009;69(13):5331–5339. doi: 10.1158/0008-5472.CAN-08-4630. [DOI] [PubMed] [Google Scholar]
- 123.Volarevic V., Markovic B.S., Gazdic M., Volarevic A., Jovicic N., Arsenijevic N., Armstrong L., Djonov V., Lako M., Stojkovic M. Ethical and safety issues of stem cell-based therapy. International journal of medical sciences. 2018;15(1):36. doi: 10.7150/ijms.21666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Caplan H., Olson S.D., Kumar A., George M., Prabhakara K.S., Wenzel P., Bedi S., Toledano-Furman N.E., Triolo F., Kamhieh-Milz J. Mesenchymal stromal cell therapeutic delivery: translational challenges to clinical application. Front. Immunol. 2019;10:1645. doi: 10.3389/fimmu.2019.01645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang T., Chen R., Liu C., Liang W., Guan W., Tang R., Tang C., Zhang N., Zhong N., Li S. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. The Lancet Haematology. 2020;7(5):e362–e363. doi: 10.1016/S2352-3026(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.G. Moll, J.A. Ankrum, J. Kamhieh-Milz, K. Bieback, O. Ringdén, H.-D. Volk, S. Geissler, P. Reinke, Intravascular mesenchymal stromal/stem cell therapy product diversification: time for new clinical guidelines, Trends in Molecular Medicine 25(2) (2019) 149-163. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.