Abstract
Phosphorus-solubilizing microorganisms is a microbial fertilizer with broad application potential. In this study, 7 endophytic phosphate solubilizing bacteria were screened out from Chinese fir, and were characterized for plant growth-promoting traits. Based on morphological and 16S rRNA sequence analysis, the endophytes were distributed into 5 genera of which belong to Pseudomonas, Burkholderia, Paraburkholderia, Novosphingobium, and Ochrobactrum. HRP2, SSP2 and JRP22 were selected based on their plant growth-promoting traits for evaluation of Chinese fir growth enhancement. The growth parameters of Chinese fir seedlings after inoculation were significantly greater than those of the uninoculated control group. The results showed that PSBs HRP2, SSP2 and JRP22 increased plant height (up to 1.26 times), stem diameter (up to 40.69%) and the biomass of roots, stems and leaves (up to 21.28%, 29.09% and 20.78%) compared to the control. Total N (TN), total P (TP), total K (TK), Mg and Fe contents in leaf were positively affected by PSBs while showed a significant relationship with strain and dilution ratio. The content of TN, TP, TK, available phosphorus (AP) and available potassium (AK) in the soil increased by 0.23–1.12 mg g−1, 0.14–0.26 mg g−1, 0.33–1.92 mg g−1, 5.31–20.56 mg kg−1, 15.37–54.68 mg kg−1, respectively. Treatment with both HRP2, SSP2 and JRP22 increased leaf and root biomass as well as their N, P, K uptake by affecting soil urease and acid phosphatase activities, and the content of available nutrients in soil. In conclusion, PSB could be used as biological agents instead of chemical fertilizers for agroforestry production to reduce environmental pollution and increase the yield of Chinese fir.
Subject terms: Ecology, Microbiology, Ecology
Introduction
Phosphorus is one of the important nutrients that affect plant growth and metabolism1, and it is also a limited natural resource, which is gradually being recognized as a new challenge for global sustainable development2, 3. Because available phosphorus is not enough to meet the needs of plant growth4. Especially the subtropical forest soil is severely deficient in phosphorus5, 6, which greatly limits the development and productivity of plants. About 52.3 billion tons of phosphorus fertilizer are used annually to maintain soil fertility. However, The recovery of P fertilizer in the soil is quite low (15–20%), and the residual P is fixed in soils7. Therefore, improving the absorption and use of P by crops is of great significance from both the ecological and economical perspective8.
Phosphate solubilizing bacteria (PSB) are a group of microorganisms that are capable of solubilizing insoluble phosphate, fixing nitrogen and secreting auxin, thereby promoting plant growth. In recent years, working on PSB mainly focused on plant growth9, 10. It has shown that seed or soil inoculated with phosphate-solubilizing bacteria (PSB) is known to improve the solubilization of fixed soil phosphorus and applied phosphates, resulting in higher performance of plants11, 12. For instance, Hansen et al.13 inoculated Bacillus simplex into winter wheat plants, which significantly increased the nutrients of plant, such as P concentration in root biomass and concentrations of Mg, Mn and S in shoot biomass. Tahir et al.14 found that inoculation with PSB significantly increased wheat growth rate, phosphorus uptake, and wheat yield increased by 10–12%. Ahmad et al.15 isolated the PSB PSB1, PSB2, and PSB7 from the rhizosphere of Zea mays and these were shown to promote dry matter yield as compared to the control. High-efficiency PSB have the potential for making a great contribution to the decrease of environmental pollution and promoting ecological balance by replacing chemical fertilizers16.
Due to the influence of indigenous microorganisms, soil environment and other factors, the phosphorus-solubilizing microorganisms in the soil are difficult to colonize successfully, resulting in unstable application effects17, 18. As an important part of the entire micro-ecosystem of plant plants, endophytes have multiple functions such as nitrogen fixation, enhancement of plant resistance, resistance to pests and diseases, and promotion of plant growth18; at the same time, most plant endophytes are facultative endophytes, and they not only exist in plants, but are also common in rhizosphere soil and other environments19. Chinese fir (Cunninghamia lanceolata) is the timber tree species with the largest afforestation area in southern China20. In recent years, due to the intensive management of fir and the shortened rotation period, the productivity of fir forests has declined21. In addition, the high iron and aluminum content of the soil in southern areas and strong leaching can easily cause the fixation and inactivation of phosphorus in the soil22, resulting in a very low utilization rate of soil phosphorus, which seriously limits the sustainable development of Chinese fir plantation. Therefore, it is of great significance to study the effect of phosphorus-solubilizing bacteria on the growth of Chinese fir for the future application of PSB on forest trees.
In this study, phosphorus-solubilizing bacteria were isolated and screened from the roots, stems, and leaves of Chinese fir, through research on the growth-promoting properties of nitrogenase activity, 1-Aminocyclopropane-1-Carboxylate (ACC) deaminase activity, Indole-3-Acetic Acid (IAA) production and siderophore. Here, we aimed to taking Chinese fir seedling as the material and investigating the effects of inoculation with PSB on the plant growth, nutrient content soil enzyme activity. The results of this study will provide basic data and practical guidance for the development and applications of PSB as biofertilizers in forestry.
Materials and methods
Plant materials
In April 2019, 2-year-old Chinese fir seedlings were collected from the Yalin Center of the Chinese Academy of Forestry in good condition and free from pests and diseases. (The use of Chinese fir seedlings in the experiment complies with national regulations).
Medium
Pikovskava (PVK) solid medium: glucose 10 g, Ca3(PO4) 25 g, CaCO35g, (NH4)2SO40.5 g, NaCl 0.2 g, MgSO4 7H2O 0.1 g, KCl 0.1 g, MnSO4 0.002 g, FeSO4 7H2O 0.002 g, agar 18 g, Distilled water 1000 mL, pH 7.0.
PVK liquid medium: PVK solid medium without agar.
Luria-Bertan (LB) medium: tryptone 10 g, yeast extract powder 5 g, NaCl 10 g, agar 18 g, distilled water 1000 mL, pH7.0.
LB liquid medium:LB solid medium without agar.
Isolation and purification of endophytes from Chinese fir seedlings
The roots, stems, and leaves of the selected Chinese fir seedlings were washed away with running water to remove the surface soil, and then washed with running water for 24 h to 36 h, and the surface moisture was absorbed with sterile filter paper. Weigh 1 g of roots, stems and leaves in a petri dish, and then carry out surface disinfection in a sterile operating table with 75% alcohol (C2H5OH) for 30 s, 5% sodium hypochlorite (NaClO) 10 min, wash the sterile water 7 times, and use sterile filter paper to absorb the water. The material after surface sterilization is cut into 2 mm × 2 mm with sterile surgical scissors, placed in a sterilized mortar and grated with a small amount of sterile quartz sand and ground into a homogenate, then diluted with sterile water to 10–1, 10–2 and 10–3, pipette to draw 150 µL of sample grinding fluid, spread on the medium, set the sterile water of the last rinse of Chinese fir tissue as a blank control, incubate with other plates under the same conditions, and verify whether the surface disinfection. Cultivate in a 28 ℃ incubator according to the characteristics of the colony phenotype, and use the streak separation method to further purify and isolate the strains until the isolation of the colony morphology is uniform for each isolate.
Note: 75% C2H5OH: 75 mL absolute ethanol + 25 mL sterile deionized water; 5% NaClO: sodium hypochlorite solution with 10% available chlorine: sterile deionized water = 1:1.
Characterization of PGP traits
Determination of phosphorus solubilizing ability
The strain was inoculated into PVK liquid medium and cultured at 28 ℃ for 180 days/min on a reciprocating shaker for 7 days, and then the pH value of the medium was measured. The culture solution was centrifuged at 8000 rpm for 15 min to remove bacterial cells. Take the supernatant and use the molybdenum antimony scandium colorimetric method23 to determine the soluble phosphorus content in the culture broth.
Determination of nitrogenase activity
An aliquot of 200 µL fresh culture was inoculated to 20 mL of nutrient broth and incubated overnight at 30℃. Bacterial growth was collected by centrifugation and was washed twice using sterile water, and resuspended by liquid limited nitrogen culture medium (OD600 = 0.2). The 3 mL suspension was transferred to a 25 mL sterilized serum vial and 2.4 mL acetylene gas (99.9999%) was driven into the serum bottle, and then incubated at 30 °C for 12 h. The ethylene content and the protein of bacterial suspension were determined as You et al.24.
1-Aminocyclopropane-1-carboxylate (ACC) deaminase activity determination
ACC deaminase activity was determined by the method of Glick et al.25 using N-free medium (Nfb)26 for bacteria and minimal medium (MM)27 for actinomycetes containing 0.3 m mol L−1 ACC (Sigma, USA) as a sole nitrogen source. MM with 0.1% (w/v) NH4(SO4)2 was used as a positive control and cultivation without ACC was used as a negative control. After incubation at 28 ℃ for 7 days for non-actinomycete bacteria and 14 days for actinomycetes, colony growth on Nfb or MM with addition of ACC indicated ACC deaminase activity.
Indole-3-acetic acid (IAA) production
IAA production was measured by colorimetric assay27. Bacterial isolates were cultured for 3 days in TY broth (without L-tryptophan or supplemented with 500 μg/mL of l-tryptophan) in the dark at 28 °C. Cells were removed from the culture medium by centrifugation at 13,000×g for 10 min; then, 1 mL of the supernatant was mixed vigorously with 2 mL of Salkowski’s reagent (4.5 g of FeCl3 per L in 10.8 M H2SO4). Samples were incubated at room temperature for 30 min and the IAA production was estimated from the optical density at 600 nm (OD600) by comparison with a standard curve prepared from known concentrations of IAA.
Siderophore production
Siderophore production was examined by using chrome azurol S (CAS) agar28. Isolate was inoculated onto CAS agar, cultured at 28 °C for 2 days, and the positive strain was indicated by an orange halo around the bacterial colony. Determine the ratio (D/d) of orange aperture diameter (D) to colony diameter (d) to determine the iron-producing carrier capacity of the strain.
Physiological and biochemical tests of phosphate-solubilizing bacteria
The conventional physiological and biochemical identification of PSB is carried out according to the methods in the “Common Bacterial System Identification Manual”, which mainly includes Gram stain, glucose hydrolysis test, lactose hydrolysis test, methyl red test, Voges-Proskauer (VP) test, hydrogen sulfide production test, gelatin liquefaction Test, citrate utilization test, malonate utilization test, denitrification test.
16 SrRNA gene sequencing
Taking the screened multifunctional PSB as the object, the bacterial genomic DNA extraction kit of Beijing Bomed Biotechnology Co., Ltd. was used to extract the DNA of the strain, using the DNA as a template, and using the bacterial universal primer 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′) PCR amplification, the amplification system is as follows: DNA template 1 uL, primer 27F 0.5 µL, 1492R 0.5 µL, 2 × TaqMix 12.5 µL, ddH2O10.5 µL. The PCR procedure is as follows: 93℃ for 3 min, 93℃ for 30 s, 56 ℃ for 30 s, 72℃ for 2 min, 32 cycles; 72 ℃ for 7 min. The amplified products were sequenced bidirectionally by BGI. After splicing the measured 16SrDNA sequences in ContigExpress, search in GenBank, EzTaxon, BIGSdb databases respectively, select the model strains with high homology. The phylogenetic tree was constructed by the neighbor-joining method using MEGA version 7.0 with the Kimura 2-parameter model29, the robustness of the tree was evaluated by performing bootstrap analyses based on 1000 replications30.
Evaluation of plant growth promotion by individual inoculation
Preparation of inoculum
The single colonies were picked out and incubated in LB broth at 180 rpm at 28 ℃ for 12 h. The above solution was inserted into 200 mL of LB broth at 1% inoculation, incubated at 180 rpm at 28℃ for 48 h and the cell pellet was resuspended in sterile distilled water and made up to a final concentration of 3 × 108 CFU/mL.
Experimental seedlings and soil
Chinese fir seedlings were provided by the Experimental Center of Subtropical Forestry, Chinese Academy of Forestry (117° 67′ E, 27° 82′ N), Jiangxi Province, China. The use of Chinese fir seedlings in the experiment complies with national regulations. Five months old seedlings with vigorous and apparently disease and pest free were used. The height and root collar diameters of seedlings were 8.7 cm and 1.36 mm, respectively. The seedling container was made of non-woven fabric, the specification was 4.5 cm × 8.0 cm (Diameter × Height). The soil was consisted of nursery medium and loess at a ratio of 9:1, was thoroughly mixed and homogenized with 3 kg slow-release fertilizer per cubic. The slow-release fertilizer is produced by American Abbes (180 g kg−1 total N, 80 g kg−1 available P, and 80 g kg−1 total K, the fertilizer effect period is 9 months). The soil exhibited the following properties: 6.34 g kg−1 total N, 0.80 g kg−1 total P, 2.50 g kg−1 total K, and a pH-value of 6.00.
Test design
The SSP2, JRP22 and HRP2, which were confirmed to have the characteristics of promoting plant growth, so pot experiments were conducted. To determine the effectiveness of phosphorus-solubilizing bacteria in plant growth of Chinese fir, a pot culture experiment was conducted between August and November 2019 in an open-sided greenhouse in Experimental Center of Subtropical Forestry, Chinese Academy of Forestry, Jiangxi Province, China. The experiment was carried out in three-factor orthogonal design with five replications for each treatment. The orthogonal experimental design of experiment is provided in Table 1, and strain, dilution ratio, inoculation method contained 3 levels. The pots with water was used as control (CK). For the irrigation of the rhizosphere (IR) treatments, 30 mL of diluted inoculum was added to the soil in the vicinity of the roots of Chinese fir. For the foliar spray (FS) treatments, 30 mL diluted bacterial cell suspension was inoculated in the leaves of Chinese fir by using a syringe. For the rhizosphere + foliar spray (IS) treatments, 15 mL of diluted inoculum was inoculated in the rhizosphere of seedlings, 15 mL was added to the leaves of seedlings by foliar spray. Each treatment contained sixteen seedlings for a total of nine treatments. A total of 3 inoculations were given in the middle of each month. The plant height and stem diameter were recorded before the first inoculation. The plants were harvested after 90 days (16 plantlets/replicate/treatment, i.e., a total of 80 plantlets per treatment) and the root biomass, stem biomass, leaf biomass, plant height and stem diameter were measured.
Table 1.
L9(34) Orthogonal design of experiment.
| Treatment | Strain | Dilution ratio | Inoculation method |
|---|---|---|---|
| T1 | SSP2 | 1:30 | IR |
| T2 | SSP2 | 1:60 | FS |
| T3 | SSP2 | 1:90 | IS |
| T4 | JRP22 | 1:30 | FS |
| T5 | JRP22 | 1:60 | IS |
| T6 | JRP22 | 1:90 | IR |
| T7 | HRP2 | 1:30 | IS |
| T8 | HRP2 | 1:60 | IR |
| T9 | HRP2 | 1:90 | FS |
| CK | – | – | IR |
IR irrigation of the rhizosphere, FS foliar spray, IS irrigation of the rhizosphere + foliar spray.
Determination of growth indicators
During the test, before each inoculation, the height of the seedlings was measured with a ruler and the ground diameter of the seedlings was measured with a vernier caliper. After the experiment, 10 plants of Chinese fir seedlings in average growth were randomly selected from each treatment, a total of 30 plants were washed with clean water to remove surface impurities, the filter paper was dried and the roots, stems, and leaves were put into paper bags respectively at 105 °C. After being degraded for 0.5 h, dried at 70 °C to a constant weight, weighed and recorded the biomass of each part.
Determination of leaf and soil nutrient content
Total N content of leaf and soil was measured by a 2300 Kjeltec Analyzer Unit (FOSS, Höganäs, Sweden). Total P and total K, total Mg and total Fe of leaf, soil TP, TK, AP and AK were extracted according to literature31 and were determined by ICP (Kleve, Germany).
Determination of soil enzyme activities
Activities of the soil urease, cellulase, sucrase, dehydrogenase and acid phosphatase were determined by spectrophotometry. Firstly, 0.05 g of soil was added to 450 mL of phosphate buffer solution (PBS, 0.1 mol L−1, pH 7.4). Then the solution was mixed by shaking, and centrifuged at 2000 rpm at 4 ℃ for 10 min and supernatant was collected with a new centrifugal tube. The supernatant and reagents were added according to the kit instructions (Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China). Absorbance at 450 nm was measured on a SpectraMax Paradigm Multi-Mode detection platform (Molecular devices, San Jose, CA, USA).
Data processing and analysis
All statistical analyses were performed using SPSS24. Data are presented in terms of means (± SE; standard error). Statistical differences were tested by one-factor ANOVA to evaluate the differences in the nutrient content of soil and plant growth status. In MEGA7.0, the Neighbor-Joining method was used to construct the phylogenetic tree, and the Bootstrap value was 1000.
Results
Colony characteristics of phosphate-dissolving bacteria
Seven strains of putative phosphate-solubilizing bacteria were isolated and purified from Chinese fir seedlings, five were from the root system of Chinese fir plants, and one was isolated from the stems of Chinese fir plants and one was from leaves. Morphological characterization of bacterial colonies generally includes the observation of shape, color, surface texture, opacity, etc. These morphological characteristics of the seven isolated strains of putative phosphorolytic bacteria were observed and recorded (Table 2). It can be seen from Table 2 that most of the bacterial colonies were white or light yellow, round, and with a wet surface, while a few were transparent.
Table 2.
Colony characteristics of phosphate solubilizing bacteria grown in PVK medium.
| Strain no | Isolated part | Shape | Color | Surface state |
|---|---|---|---|---|
| HRP2 | Stem | Round | Light yellow | Moist |
| JLP2 | Leaf | Round | White | Dry |
| SSP2 | Root | Round | White | Dry |
| JRP13 | Root | Round | White | Moist |
| JRP22 | Root | Round | Light yellow | Moist |
| JRP23 | Root | Round | Light yellow | Dry |
| JRP24 | Root | Round | White | Moist |
Physiological and biochemical tests
The physiological and biochemical characteristics of the selected PSBs are shown in Table 3. The screened strains were Gram negative and could all hydrolyze glucose. On the contrary, only JRP23 could hydrolyze lactose and produce hydrogen sulfide; however, it was also the only strain to score negative in the citrate test. Only JLP2 was methyl-red positive, and SSP2, JRP22, and JRP23 were methyl-red negative. Only JLP2, SSP2, and JRP22 showed a positive Voges–Proskauer (VP) test result. Only JRP23 and JRP24 failed to hydrolyze gelatin. Only JRP22 scored positive in the malonate test. HRP2, JLP2, JRP23, and JRP24 could not perform denitrification. Among these strains, JLP2, SSP2, and JRP22 exhibited similar physiological and biochemical characteristics; they could all use glucose and lactose as energy sources and scored negative in the methyl-red test and positive in the VP test. However, we could not classify strains according to their physiological and biochemical features; therefore, we carried out molecular identification to determine their respective genus and species.
Table 3.
Physiological and biochemical features of phosphate-solubilizing bacteria.
| Test | HRP2 | JLP2 | SSP2 | JRP13 | JRP22 | JRP23 | JRP24 |
|---|---|---|---|---|---|---|---|
| Gram staining | − | − | − | − | − | − | − |
| Glucose hydrolysis | + | + | + | + | + | + | + |
| Lactose hydrolysis | + | + | + | + | + | − | − |
| Methyl red | + | − | − | + | − | − | + |
| V-P test | − | + | + | - | + | − | − |
| Hydrogen sulfide production | − | − | − | − | − | + | − |
| Gelatin liqueaction | + | + | + | + | + | − | − |
| Citrate | + | + | + | + | + | − | − |
| Malanate | − | − | − | − | + | − | − |
| Denitrification | − | − | + | + | + | − | − |
16S rRNA gene sequences
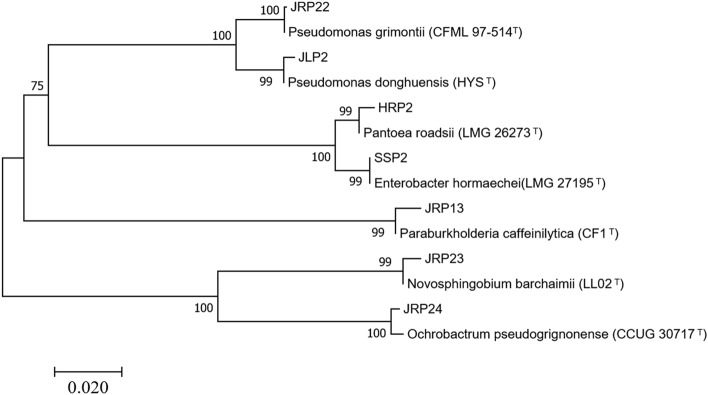
The nucleotide sequences of the 16S rRNA genes of the seven selected strains, approximately 1350 bp long, were amplified by PCR and sequenced. These sequences were compared by BLAST with those in the GenBank, EzTaxon, and BIGSdb databases, and the results are shown in Table 4. These results showed that the seven strains of growth-promoting bacteria belonged to six genera. In particular, two strains (JLP2 and JRP22) displayed high sequence similarity with Pseudomonas (98.75–100%). Moreover, the similarity between the 16S rRNA gene sequence of strain HRP2 and that of the genus Pantoea was 98.84%. The remaining four strains (SSP2, JRP13, JRP23, and JRP24) showed high sequence similarity with the genera Enterobacter, Paraburkholderia, Novosphingobium, and Ochrobactrum, respectively. In addition, the 16S rRNA gene sequence of strain JRP22 was identical to that of Pseudomonas grimontii (CFML97-514). Therefore, strain HRP2, displaying the strongest phosphate solubilization ability among these bacteria, belongs to the genus Pantoea, while the next most efficient phosphate-solubilizing bacteria, SSP2 and JRP22, belong to the genus Enterobacter and Pseudomonas. Using MEGA7.0 software, Neighbor-Joining was used to construct a phylogenetic tree of phosphate solubilizing bacteria (Fig. 1) to determine the type of each strain. The phylogenetic tree in the figure only shows values with a self-extension value greater than 70.
Table 4.
Identification of PSB based on their 16S rRNA gene sequences.
| Strain no. | Type strain | Identity (%) | Accession number |
|---|---|---|---|
| JLP2 | Pseudomonas donghuensis(HYS) | 99.73 | NR136501.2 |
| HRP2 | Pantoea roadsii(LMG26273) | 98.84 | NR114154.1 |
| SSP2 | Enterobacter hormaechei(LMG 27195) | 99.86 | NR126208.1 |
| JRP13 | Paraburkholderia caffeinilytica(CF1) | 99.31 | NR152088.1 |
| JRP22 | Pseudomonas grimontii(CFML97-514) | 100.00 | NR025102.1 |
| JRP23 | Novosphingobium barchaimii(LL02) | 99.69 | NR118314.1 |
| JRP24 | Ochrobactrum pseudogrignonense(CCUG30717) | 100.00 | NR042599.1 |
Figure 1.
Phylogenetic evolutionary tree based on 16S rRNA sequences.
Growth-promoting traits of phosphate-solubilizing bacteria
To identify possible growth-promoting traits of the isolated bacteria, we screened them for several PGP-associated enzymatic activities or for the production of PGP compounds (Table 5). The results showed that three strains of PSB displayed nitrogenase activity, accounting for 40% of the tested strains, which led to C2H2 reduction rates ranging from 73.19 to 83.06 nmol mL−1 h−1. Among these, the HRP2 strain showed the highest nitrogenase activity, with a C2H2 reduction rate of 83.06 nmol mL−1 h−1. Overall, the nitrogenase activities of these three strains of PSBdid not differ much (p > 0.05). Next, we tested the seven strains for their ACC deaminase activity. As shown in Table 5, three strains exhibited ACC deaminase activity ranging from 0.22 to 0.78 µmol mg−1 h−1. Among these, the ACC deaminase activity of strain HRP2 was the highest, at 0.78 µmol mg−1 h−1. Strains SSP2 and JRP22 exhibited similar ACC deaminase activities, at 0.22 µmol mg−1 h−1 and 0.50 µmol mg−1 h−1, respectively. Overall, the ACC deaminase activities of these three strains differed significantly (p < 0.05). Moreover, among the tested strains, five strains had the ability to produce IAA, at a concentration between 6.27 and 14.44 µg mL−1. Among these, strain JRP22 displayed the highest IAA production, followed by JLP2 (15.64 µg mL−1); the strain producing the lowest amount of IAA was SSP2 (6.27 µg mL−1). Most of the isolated PSBshowed the ability to produce IAA, which differed significantly among strains (p < 0.05). Finally, the siderophore production ability of the PSBwas tested by growth on Chrome azurol S (CAS) plates. The results showed that five strains could secrete iron carriers. Among these, JRP22 and HRP2 displayed ratios between the diameter of the siderophore-containing area and the colony diameter of 1.81, 0.91, which were significantly higher than those of the other strains (p < 0.05).
Table 5.
Determination of the growth-promotion ability of phosphate-solubilizing bacteria.
| Strain no | Soluble phosphorus content (mg L−1) | Nitrogenase activity (C2H2 nmol mL−1 h−1) | ACC deaminase (µmol mg−1 h−1) | IAA (µg mL−1) | Siderophore D/d |
|---|---|---|---|---|---|
| HRP2 | 123.73 ± 7.17a | 83.06 ± 2.50a | 0.78 ± 0.03a | 7.64 ± 0.61c | 0.91 ± 0.03b |
| JLP2 | 44.51 ± 2.2.52f | – | – | 12.42 ± 0.67b | – |
| SSP2 | 116.51 ± 1.96b | 76.09 ± 2.79b | 0.22 ± 0.03c | 6.27 ± 0.75c | 0.88 ± 0.06b |
| JRP13 | 67.76 ± 2.26e | – | – | 11.51 ± 0.82b | – |
| JRP22 | 79.36 ± 1.54c | 73.19 ± 2.24b | 0.50 ± 0.02b | 14.44 ± 1.10a | 1.81 ± 0.08a |
| JRP23 | 74.74 ± 1.67d | – | – | – | 0.72 ± 0.03cf |
| JRP24 | 67.21 ± 1.67e | – | – | – | 0.87 ± 0.05b |
ACC 1-aminocyclopropane-1-carboxylate deaminase, IAA indole-3-acetic acid.
Significant differences among the seven bacterial strains for each growth promotion indicator were determined using one-way analysis of variance at p < 0.05. The data are shown as mean ± SD (n = 3).
Different lowercase letters represent significant differences among strains isolated from Chinese fir at p < 0.05.
Effect of PSBs on Chinese fir growth
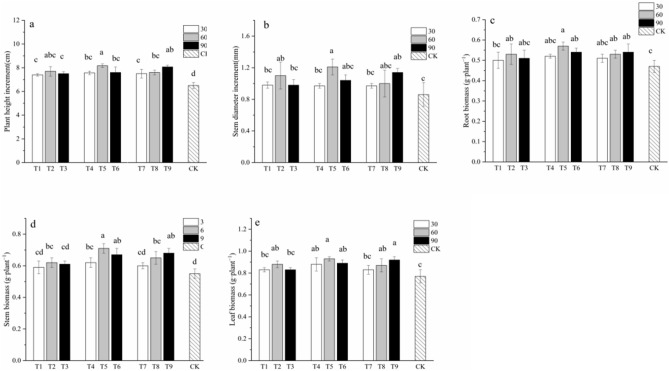
The three most promising phosphate-solubilizing bacterial strains, SP13, RP2, and RP22, were further examined for their growth-promoting activity for Chinese fir seedlings. Chinese fir seedlings were inoculated with increasingly diluted cultures (OD600 = 1.0, diluted by a factor of 30, 60, or 90) of strain SSP2 (seedlings T1, T2, and T3), strain JRP22 (seedlings T4, T5, and T6), and strain HRP2 (seedlings T7, T8, and T9). Compared to the CK, the inoculation with Enterobacter hormaechei SSP2, Pseudomonas grimontii JRP22, and Pantoea roadsii HRP2 significantly promoting Chinese fir seedlings growth (Fig. 2). Chinese fir seedlings inoculated with different strains exhibited significantly different increases in plant height (p < 0.05), which was, in all cases, significantly higher than that of control seedlings (p < 0.05). Among inoculated seedlings, T5 seedlings exhibited the highest increase in plant height (8.2 cm), which was about 1.26 times that of the control. T9 and T2 seedlings followed, with 1.24-fold and 1.18-fold increases plant height, respectively, as compared to that of control seedlings. Moreover, different treatments led to different growth-promoting effects on the ground diameter of Chinese fir seedlings (p < 0.05). Indeed, T5 seedlings showed the highest increase in ground diameter (1.21 mm), with an increase of 40.69% compared to that of the control, followed by T9 seedlings with an increase of 32.56% compared to that of the control. However, there was no significant difference in ground diameter growth of seedlings treated with the other dilutions of inocula compared with that of the control. Furthermore, compared to that of the control, the root dry weights of T5 and T9 seedlings both increased significantly (p < 0.05). In particular, the root dry weight of T5 seedlings was the highest, with an increase of 21.28% with respect to that of the control. In addition, the stem weights of T5, T6, and T9 seedlings were significantly higher than that of the control (p < 0.05), with increases of 29.09%, 21.82%, and 23.64%, respectively. Additionally, T5 and T9 seedlings displayed a significant increase in the dry weight of leaves (p < 0.05), which increased by 20.78% and 19.48%, respectively, compared with that of the control. In general, the quality of seedlings treated with strains JRP22 and HRP2 was higher than that of seedlings treated with strain SSP2. In addition, for inoculation with a single strain, dilution by a factor of 60 or 90 led to more significant increases in biomass (p < 0.05).
Figure 2.
Growth response of Chinese fir seedlings inoculated with PSBs. (a) Plant height increment. (b) Stem diameter increment. (c) Root biomass. (d) Stem biomass. (e) Leaf biomass. Different lowercase letters represent significant differences at p < 0.05.
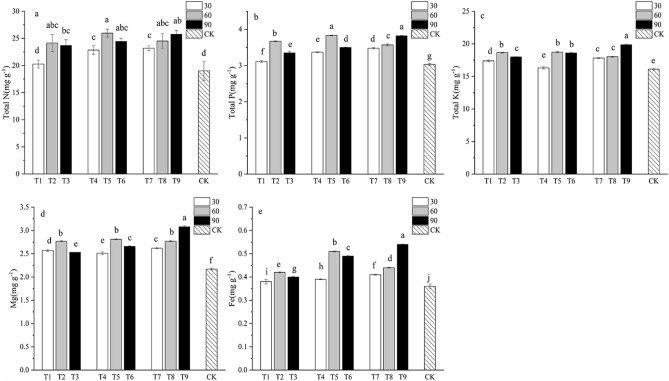
Effect of PSBs on the nutrient content of Chinese fir
PSBs had a significant impact on the concentration of TN, TP, TK, Mg and Fe (Fig. 3). The T9 treatment significantly increased leaf TK, Mg and Fe content compared to the others treatments. The TN, TP, TK, Mg and Fe contents were significantly higher for T5 and T9 than for CK. The strain, dilution ratio and inoculation method had significant impact on the TN, TP concentration (Fig. 3a–c). All treatments, apart from inoculation with Enterobacter hormaechei SSP2 (T1) resulted in significantly increased TN content of Chinese fir compared to the control (CK). Compared to the treatment with dilution ratio of 1:30, the treatment with dilution ratio of 1:60 and 1:90 resulted in higher content of TN, TP, TK, Mg and Fe (Fig. 3a–c). The concentration of Mg and Fe significantly increased compared to CK (Fig. 3d–e).
Figure 3.
Leaf nutrient content of Chinese fir at 90 days after inoculation of PSBs. (a) Leaf total N content. (b) Leaf total P content. (c) Leaf total K content. (d) Leaf Mg content. (e) Leaf Fe content. Different lowercase letters represent significant differences at p < 0.05.
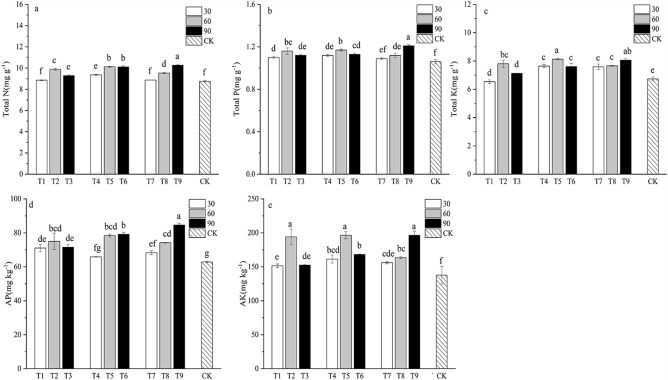
Nutrient content of soil
The PSBs significantly increased the nutrient content of soil compared with the control (Fig. 4). Similar to nutrient content of Chinese fir, inoculation with PSBs at the dilution ratio of 1:60 and 1:90 showed higher NPK content. Inoculation with HRP2 at the dilution ratio of 1:90 (T9) showed higher TN, TP, TK, AP and AK in soils than SSP2 and JRP22.
Figure 4.
Soil nutrient content of Chinese fir at 90 days after inoculation of PSBs. (a) Soil total N content. (b) Soil total P content. (c) Soil total K content. (d) Soil AP content. (e) Soil AK content. Different lowercase letters represent significant differences at p < 0.05.
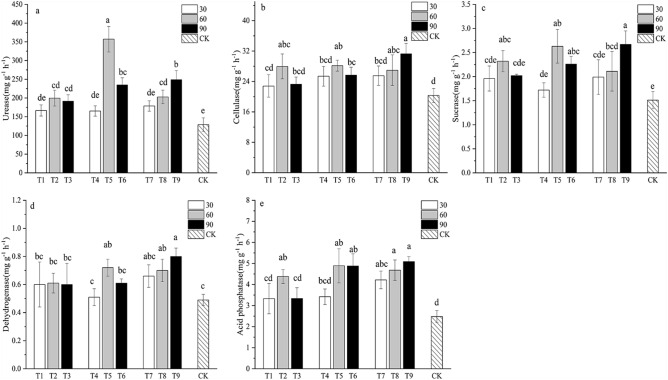
Soil enzyme activities
In this study, we found that soil urease, cellulase, sucrase, dehydrogenase and acid phosphatase activities all were influenced significantly by strain and dilution ratio (Fig. 5). Overall, inoculation of PSBs significantly increased the five enzyme activities. Strain and dilution ratio significantly influenced soil urease and acid phosphatase activity, whereas inoculation method had not-significant effects on the six enzyme activities. The urease activity was highest in T5 inoculated with JRP22 at the dilution ratio of 1:60 (Fig. 5a). The other five enzyme activities were highest in T9.
Figure 5.
Soil enzyme activity at 90 days after inoculation of PSBs. (a) Soil total urease activity. (b) Soil cellulase activity. (c) Soil sucrase activity. (d) Soil dehydrogenase activity. (e) Soil acid phosphatase activity. Different lowercase letters represent significant differences at p < 0.05.
Discussion
Among the PSBs isolated in this study, the number of Pseudomonas strains was the highest. Currently known phosphorolytic bacteria mainly include species of the genera Burkholderia, Bacillus, Pseudomonas, Erwinia, and Klebsiella, among which Pseudomonas, Burkholderia, and Bacillus have been extensively studied32, 33. The phosphate solubilization abilities of the strains isolated and screened in this study ranged from 44.29 to 195.61 mg L−1. These were significantly higher than the phosphate solubilization abilities of bacteria isolated from the roots of Moroccan wheat and grown in liquid medium, which ranged from 59.1 to 90.2 µg mL−134. On the contrary, Qiao et al.35 found that phosphorus-solubilizing bacteria from maize rhizosphere soil could dissolve phosphorus up to 487.67 mg L−1 and proposed that the ability of each strain was related to its physiological and metabolic characteristics. Studies have shown that fungi are more efficient than bacteria at solubilizing phosphate. Indeed, the phosphate solubilization ability of most bacteria ranges from 26.9 to 43.3 mg L−1, while phosphate solubilization ability of fungi is generally 3.2 to 5.4 times higher than that of bacteria (59.6 to 145.4 mg L−1)36, 37. Interestingly, the HRP2, SSP2, and JRP22 bacterial strains isolated in this study displayed a phosphate solubilization ability in the range of those of fungi and 2.9–7.2 times higher than the average ability of bacteria. This result indicates that these three strains are very good at solubilizing phosphate, with the HRP2 strain reaching an activity of up to 195.61 mg L−1. Moreover, in this study we found that the seven isolated strains possessed ACC deaminase activity, ranging from 0.2 to 0.74 µmol mg−1 h−1, consistent with the results reported by Han et al.38. Among them, strain HRP2 (Burkholderia ubonensis) exhibited the highest ACC deaminase activity (0.74 µmol mg−1 h−1), which was higher than that of the B. ubonensis strain CBPB (0.21 µmol mg−1 h−1) isolated from the rhizosphere of rice previously reported by Poonguzhali et al.39. Such a difference may be related to the soil type and the type of host plant from which strains were isolated. In fact, different habitats can greatly influence the physiological and metabolic processes of a bacterial strain40. IAA is an important plant hormone that participates in many important physiological processes in plants, including cell growth and division, and tissue differentiation, thereby promoting the growth of plant roots and shoots41, 42. Among the strains tested in this study, strain HRP2 displayed the strongest IAA production capacity (18.00 µg mL−1), although this was quite lower than that of B. ubonensis CBPB-HIM (28.02 µg mL−1) previously reported by Poonguzhali et al.39 but similar to those of the isolates characterized by Abderrazak et al.32, which exhibited IAA production capacities of 2.9–21.2 µg mL−1. Siderophores are small organic compounds produced by microorganisms under iron deficiency, which gather insoluble iron compounds from the environment and convert them into a form that can be absorbed and utilized by plants43. Therefore, siderophores promote plant growth by enhancing iron absorption by plants44. Among the strains tested in this study, strain JRP22 (P. grimontii) showed the highest production of siderophores, with an iron carrier circle diameter to colony diameter ratio of 1.80. On the contrary, Chen et al.45 found seven Pseudomonas isolates producing iron carriers with an iron carrier circle diameter to colony diameter ratio of 1.50–12.00. These findings indicate that Pseudomonas strains possess a highly variable siderophore production ability. Some studies have pointed out that the ability of bacterial strains to produce siderophores is mainly related to their genetic characteristics, different strains secrete different types of siderophores depending on the degree of iron chelation in the soil43.
Studying the influence of PSB on the morphological and physiological characteristics of Chinese fir seedlings and thereby unraveling the growth-promoting properties of these bacteria can prove useful for the application of PSB to forest trees. Plant height, ground diameter, biomass, and other morphological growth indicators are a direct manifestation of the efficiency of seedling growth. The results of this study showed that phosphorus-solubilizing bacteria promote the growth of Chinese fir seedlings in terms of plant height, ground diameter, and root, stem, and leaf biomass to varying degrees. This might be due to the PSB strains dissolving the insoluble phosphate in the soil and enhancing the available P content by producing organic acid and extracellular phosphatases46, 47. Another possibility might be related to the metabolism of PSB, producing a variety of plant hormones, acids, and vitamins48.This is consistent with the findings of Cui et al.49 and Xue et al.50, who demonstrated that phosphorus-solubilizing bacteria can promote plant growth and increase rhizome and leaf biomass. The observed increases in plant height, ground diameter, and biomass are likely due to the ability of PSB to dissolve phosphorus and nitrogen, which promote efficient absorption and use of nutrients to synthesize organic matter and increase plant biomass. Similarly, Pereira et al.51 found that the inoculation of PSB significantly promoted plant height, ground diameter, and biomass, which can be due to the organic acids production (such as gluconic, formic, and citric acids) by these strains. In this study, the plant height growth and biomass of seedlings treated with bacteria were significantly higher than those of control seedlings. This may be due to the fact that the seedlings used in this study were sown in the same year, and most of the nutrients in the seedlings thus flowed to the roots and leaves52. The results of this study showed that the growth-promoting effects of the strains JRP22 and HRP2 were stronger than that of strain SSP2; this phenomenon may depend on differences in growth-promoting characteristics and environmental adaptability among these strains. When plants were treated with the same strain, higher dilutions (by a factor of 60 or 90) triggered a more obvious growth promotion effect. Indeed, the plant height, ground diameter, and stem and leaf dry weight of seedlings treated with low bacterial concentrations were significantly higher than those of plants treated with high bacterial concentrations. This finding is consistent with the results of Xu et al.53 and of Luan et al.54 in red bean. Altogether, these results indicate that phosphorus-solubilizing bacteria can promote plant growth within a certain range of concentrations. It turns out that the mechanism of promoting growth is a complex and comprehensive effect.
Soil nitrogen, phosphorus and potassium content is considered to be an important indicator of soil fertility, which reflects the storage and supply of nutrients in the soil55. PSBs increase the content of nitrogen and phosphorus in soil by dissolving insoluble phosphate in the soil and fixing nitrogen56, 57. PSB not only solubilize and mineralize P from insoluble compounds but also release other nutrients58, 59. This study found that the effect of PSBs on soil nutrient content was significantly different. Compared to the control, the content of TN, TP, TK, AP and AK increased significantly, demonstrating that PSB elevated the amounts of available N, P, and K in the soil and subsequently provided better nutrition for plant growth60, 61, which is consistent with the results of Matsumura et al.62 and Wang et al.63 found that inoculation with PSBs significantly increased the accumulation of available phosphorus and nitrogen in the soil, and effectively promoted the growth of Willow seedlings.
Soil enzymes are catalysts for biochemical reactions in soil and it affects microbial activity in the soil and the characteristics of biochemical processes64, 65. The results of this study indicated that PSBs significantly increase the enzyme activity of soil. This may be because the organic acids secreted by the strains during the growth and metabolism process lead to the decrease of soil pH, which is more conducive to the increase of soil enzyme activity66. Khati et al.67 studied the response mechanism of corn to phosphate-dissolving bacteria and found that soil enzyme activity increased by 2 to 3 times. Soil cellulase and soil dehydrogenase are closely related to microbial biomass56, 68, 69. This study showed that the cellulase and dehydrogenase activity of T9 was the highest, indicating that the treatment had a large number of substrate microorganisms. Shahzad70 reported that PSBs significantly increased the activity of soil dehydrogenase, beta-glucosidase, and urease.
Conclusion
Seven strains of phosphorus-solubilizing bacteria cloud be isolated from the roots, stems, and leaves of Chinese fir. The isolated strains were characterized and identified by analysis of colony characteristics, physiological and biochemical testing, and phylogenetic analysis based on 16S rRNA gene sequencing. Found that these seven phosphate solubilizing bacteria belong to the genus Pseudomonas, Pantoea, Enterobacter, Paraburkholderia, Novosphingobium, Ochrobactrum. Next, their growth-promoting characteristics, such as their ability to fix nitrogen, produce IAA, and secrete siderophores, were studied. Interestingly, the phosphate solubilization ability of the bacterial strain HRP2 reached the level of the phosphate solubilization ability of fungi. The strain showing the highest nitrogenase activity was SSP2, while the best iron carrier producer was JRP22. In the growth promotion test, compared with the control, PSBs significantly improved the growth of Chinese fir seedlings, including plant height, stem diameter, biomass, and nutrient content of Chinese fir. At the same time, soil nutrient content and enzyme activity were significantly increased, such as total N, total P, total K, AP, AK, soil urease, cellulase, sucrase, dehydrogenase, nitrate reductase and acid phosphatase. Increased enzyme activity was significantly associated with increased nutrient content. In the future, these isolated and identified strains can be tested for the presence of virulence factors to understand the influence of the tested strains on the composition and richness of endophytic microbial communities to fully evaluate the application potential of these strains as microbial fertilizers in agroforestry.
Acknowledgements
This work is supported by The National Key Research and Development Program of China (2016YFD0600300), the Special Fund for Basic Scientific Research Business of Central Public Research Institutes (CAFYBB2018SY001).
Author contributions
Conceptualization, J.C. and R.J.; Methodology, J.C. and R.J.; Software, J.C. and Y.W.; Validation, J.C., Y.W., G.Z., Y.D., L.H. and R.J.; Formal Analysis: J.C., Y.W. and G.Z.; Investigation, J.C., Y.D. and R.J.; Resources, J.C. and R.J.; Data Curation, J.C.; Writing—Original Draft Preparation, J.C.; Writing—Review and Editing: J.C., Y.W., G.Z. and R.J.; Visualization, J.C.; Supervision, R.J.; Project Administration, J.C.; Funding Acquisition: R.J.
Funding
This research was funded by State Key Research Development Program of China (Grant Number 2016YFD0600300), this study is a part of the project entitled “Study on efficient cultivation technology of Chinese fir plantation”. And Special Fund for Basic Scientific Research Business of Central Public Research Institutes (CAFYBB2018SY001). This study is a part of the project entitled “Screening and research of microbial germplasm resources for forest soil function”.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bononi L, Chiaramonte JB, Pansa CC, et al. Phosphorus-solubilizing Trichoderma spp. from Amazon soils improve soybean plant growth. Sci. Rep. 2020;10:2858. doi: 10.1038/s41598-020-59793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Syers JK, Johnston AE, Curtin D. Efficiency of soil and fertilizer phosphorus use. In: Curtin D, Syers K, Johnston AE, Curtin D, editors. Reconciling Changing Concepts of Soil Phosphorus Behavior with Agronomic Information. FAO; 2008. [Google Scholar]
- 3.Sattari SZ, Bouwman AF, Giller KE, van Ittersum MK. Residual soil phosphorus as the missing piece in the global phosphorus crisis puzzle. Proc. Natl. Acad. Sci. U.S.A. 2012;109:6348–6353. doi: 10.1073/pnas.1113675109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hedley MJ, White RE, Nye PH. Plant-induced changes in the rhizosphere of rape (Brassica napus var. emerald) seedlings. III. Changes in l value, soil phosphate fractions and phosphatase activity. New Phytol. 1982;91:45–56. doi: 10.1111/j.1469-8137.1982.tb03291.x. [DOI] [Google Scholar]
- 5.Kochian LV. Plant nutrition: Rooting for more phosphorus. Nature. 2012;488:466–467. doi: 10.1038/488466a. [DOI] [PubMed] [Google Scholar]
- 6.Zeng YL, Fang X, Xiang WH, et al. Stoichiometric and nutrient resorption characteristics of dominant tree species in subtropical Chinese forests. Ecol. Evol. 2017;7:11033–11043. doi: 10.1002/ece3.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weil RR, Brady NC. Soil phosphorus and potassium. In: Brady N, Weil RR, editors. The Nature and Properties of Soils. 15. Pearson; 2017. pp. 6433–6695. [Google Scholar]
- 8.Abbas M, Shah JA, Irfan M, et al. Remobilization phosphorus in wheat cultivars under induced phosphorus deficiency. J. Plant Nutr. 2018;41:1–12. doi: 10.1080/01904167.2018.1458871. [DOI] [Google Scholar]
- 9.Bakhshandeh E, Pirdashti H, Lendeh KS. Phosphate and potassium-solubilizing bacteria effect on the growth of rice. Ecol. Eng. 2017;103:164–169. doi: 10.1016/j.ecoleng.2017.03.008. [DOI] [Google Scholar]
- 10.Nacoon S, Jogloy S, Riddech N, et al. Interaction between phosphate solubilizing bacteria and arbuscular mycorrhizal fungi on growth promotion and tuber inulin content of Helianthus tuberosus L. Sci. Rep. 2020;10:4916. doi: 10.1038/s41598-020-61846-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ekin Z. P-solubilizing bacteria and phosphorus fertilizer applications to sunflower improve seed set, seed filling efficiency and concentration of macro-and micro-nutrients of seeds. Turk. J. Field Crop. 2011;16(2):183–189. [Google Scholar]
- 12.Richardson AE. Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Funct. Plant Biol. 2001;28(9):897–906. doi: 10.1071/PP01093. [DOI] [Google Scholar]
- 13.Hansen V, et al. Seed inoculation with Penicillium bilaiae and Bacillus simplex affects the nutrient status of winter wheat. Biol. Fertil. Soils. 2019;56:97–109. doi: 10.1007/s00374-019-01401-7. [DOI] [Google Scholar]
- 14.Tahir M, et al. Inoculation of pqqE gene inhabiting Pantoea and Pseudomonas strains improves the growth and grain yield of wheat with a reduced amount of chemical fertilizer. J. Appl. Microbiol. 2020 doi: 10.1111/jam.14630. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad DA, Bhat AK. Genotypic characterization and efficacy of phosphate solubilising bacteria in improving the crop yield of Zea mays. Forests. 2019;14:34–39. [Google Scholar]
- 16.Zak D, Goldhammer T, Cabezas A, et al. Top soil removal reduces water pollution from phosphorus and dissolved organic matter and lowers methane emissions from rewetted peatlands. J. Appl. Ecol. 2018;55:311–320. doi: 10.1111/1365-2664.12931. [DOI] [Google Scholar]
- 17.Reyes I, Valery A, Valduz Z. Phosphate-solubilizing microorganisms isolated from rhizospheric and bulk soils of colonizer plants at an abandoned rock phosphate mine. Plant Soil. 2006;287:69–75. doi: 10.1007/s11104-006-9061-z. [DOI] [Google Scholar]
- 18.Huang J, Sheng XF, He LY. Biodiversity of phosphate-dissolving and plant growth promoting endophytic bacteria of two crops. Acta Microbiol. Sin. 2010;50(6):710–716. [PubMed] [Google Scholar]
- 19.Lodewyckx C, Vangronsveld J, Porteous F, et al. Endophytic bacteria and their potential applications. Crc. Crit. Rev. Plant Sci. 2002;21:583–606. doi: 10.1080/0735-260291044377. [DOI] [Google Scholar]
- 20.Wu J, Lin H, Guo L, et al. Biomass and nutrients variation of Chinese fir rooted cuttings under conventional and exponential fertilization regimes of nitrogen. Forests. 2019 doi: 10.3390/f10080615. [DOI] [Google Scholar]
- 21.Zhao JR. The Effect of Chinese Fir Forest Ecosystem Conversion on Soil Phosphorus Forms and Its Mechanism. Fujian Agriculture and Forestry University; 2012. [Google Scholar]
- 22.Zhao QG, Hang GQ, Ma YQ. The problems and countermeasures of the red soil ecosystem in southern China. Acta Ecol. Sin. 2013;33(24):7615–7622. [Google Scholar]
- 23.Zhang XS. Study on the determination method of effective phosphorus content in fermentation broth. J. Huzhou Voc. Technol. Coll. 2008;06(3):1–3. [Google Scholar]
- 24.You M, et al. Expression of the nifH gene of a Herbaspirillum endophyte in wild rice species: Daily rhythm during the light-dark cycle. Appl. Environ. Microbiol. 2005;71:8183–8190. doi: 10.1128/AEM.71.12.8183-8190.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glick BR, Karaturovıc D, Newell P. A novel procedure for rapid isolation of plant growth-promoting rhizobacteria. Can. J. Microbiol. 1995;41:533–536. doi: 10.1139/m95-070. [DOI] [Google Scholar]
- 26.Dobereiner J, Day JM. Associative symbiosis in tropical grasses: characterization of microorganisms and dinitrogen fixing sites. In: Newton W, Nyman C, editors. Proceeding of the first International Symposium on Nitrogen Fixation. Washington State University Press; 1976. pp. 518–538. [Google Scholar]
- 27.Patten CL, Glick BR. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl. Environ. Microb. 2002;68(8):3795–3801. doi: 10.1128/AEM.68.8.3795-3801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 29.Kumar S, Stecher G, Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Felsenstein J. Confdence limits on phylogenies: An approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 31.Wang C, Xue L, Dong Y, et al. Unravelling the functional diversity of the soil microbial community of Chinese fir plantations of different densities. Forests. 2018;9:532. doi: 10.3390/f9090532. [DOI] [Google Scholar]
- 32.Abderrazak R, Omar Z, Fatima ZA, et al. Isolation, selection and characterization of root-associated rock phosphate solubilizing bacteria in Moroccan wheat (Triticum aestivum L.) Geomicrobiol. J. 2020;2020:230–241. [Google Scholar]
- 33.Xu YT. Study on the influence of microorganisms in the sediments of West Lake on the cycle of organic phosphorus. Zhejiang University; 2005. [Google Scholar]
- 34.Du L, Wang SP, Chen G, et al. Screening and identification of a high-efficiency phosphate-solving bacterium and its ability to dissolve phosphorus. Soil Fertil. Sci. China. 2017;3:136–141. [Google Scholar]
- 35.Qiao CC, Wang TT, Wang RF, et al. Screening of high-efficiency phosphate-solubilizing bacteria and its growth promoting effect. J. Nanjing Agric. Univ. 2017;40(4):664–670. [Google Scholar]
- 36.Qin LJ, Yang YZ, Yang XY. Research progress of phosphorus solubilizing and phosphorus solubilizing mechanism of soil phosphorus-solubilizing microorganisms. Life Sci. Res. 2019;23(1):59–64. [Google Scholar]
- 37.Lin QM, Zhao XR, Sun YX, et al. Community characters of soil phosphobacteria in four ecosystems. Soil Environ. 2000;9(1):34–37. [Google Scholar]
- 38.Han K, Tian ZY, Liu K, et al. Effect of endophytic bacteria on the salt tolerance of wheat with ACC deaminase activity on the seaside mallow (Kosteletzkya pentacarpos) Plant Physiol. J. 2015;51(2):212–220. [Google Scholar]
- 39.Poonguzhali S, Madhaiyan M, Sa T. Quorum-sensing signals produced by plant-growth promoting Burkholderia strains under in vitro and in planta conditions. Res. Microbiol. 2007;158(3):287–294. doi: 10.1016/j.resmic.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 40.Chi YB, Cui T, Cui QL, et al. Effect of high pressure on the physiological activity of bacteria in milk. Chin. Sci. Bull. 1997;42(5):526–529. doi: 10.1360/csb1997-42-5-526. [DOI] [Google Scholar]
- 41.Karcz W, Burdach Z. Regulation of growth, development and whole organism physiology. A comparison of the effects of IAA and 4-Cl-IAA on growth, proton secretion and membrane potential in maize coleoptile segments. J. Exp. Bot. 2002;53(10):1089–1098. doi: 10.1093/jexbot/53.371.1089. [DOI] [PubMed] [Google Scholar]
- 42.He LJ, Liu WH, Qi J, et al. Effects of IAA on the physiology and growth of the seedlings of sagegrass. Grassl. Lawn. 2019;2:32–38. [Google Scholar]
- 43.Shilev S, Babrikova I, Babrikov T. Consortium of plant growth-promoting bacteria improves spinach (Spinacea oleracea L.) growth under heavy metal stress conditions. J. Chem. Technol. Biotechnol. 2020;95(4):932–939. [Google Scholar]
- 44.Saha M, Sarkar S, Sarkar B, et al. Microbial siderophores and their potential applications: A review. Environ. Sci. Pollut. Res. 2016;23(5):3984–3999. doi: 10.1007/s11356-015-4294-0. [DOI] [PubMed] [Google Scholar]
- 45.Chen, S. X. Pseudomonas SPF-1 iron-producing carrier conditions and iron carrier properties. Wuhan University (2005).
- 46.Parekh LJ, Gyaneshwar P, Kumar GN, et al. Involvement of a phosphate starvation inducible glucose dehydrogenase in soil phosphate solubilization by Enterobacter asburiae. FEMS Microbiol. Lett. 1999;171:223–229. doi: 10.1111/j.1574-6968.1999.tb13436.x. [DOI] [Google Scholar]
- 47.Bashan Y, Kamnev AA, de Bashan LE. A proposal for isolating and testing phosphate-solubilizingbacteria that enhance plant growth. Biol. Fertil. Soils. 2013;49:1–2. doi: 10.1007/s00374-012-0756-4. [DOI] [Google Scholar]
- 48.Yu X, Liu X, Zhu TH, et al. Isolation and characterization of phosphate-solubilizing bacteriafrom walnut and their effect on growth and phosphorus mobilization. Biol. Fertil. Soils. 2011;47:437–446. doi: 10.1007/s00374-011-0548-2. [DOI] [Google Scholar]
- 49.Cui WG, Yin YS, Zhang FB, et al. Analysis of potato bacterial diversity and screening of high-yield IAA strains. Soil Fertil. China. 2020;01:223–231. [Google Scholar]
- 50.Xue YY, Ye W, Yang S, et al. Isolation and identification of a phosphate-solubilizing bacteria and its growth-promoting effect. Agric. Res. Arid Areas. 2019;37(4):253–262. [Google Scholar]
- 51.Pereira SA, Castro PML. Phosphate-solubilizing rhizobacteria enhance Zea mays growth in agricultural P-deficient soils. Ecol. Eng. 2014;73:526–535. doi: 10.1016/j.ecoleng.2014.09.060. [DOI] [Google Scholar]
- 52.Sarikhani MR, Aliasgharzad N, Khoshru BP. Solubilizing potential of some plant growth promoting bacteria used as ingredient in phosphatic biofertilizers with emphasis on growth promotion of Zea mays L. Geomicrobiol. J. 2019;37(4):327–335. doi: 10.1080/01490451.2019.1700323. [DOI] [Google Scholar]
- 53.Xu YP, Zang RC, Chen WL, et al. Growth-promoting effect of Enterobacter cloacae B8 fermentation broth on plants and IAA analysis. J. Zhejiang Univ. (Agric. Life Sci.) 2011;27(3):282–284. [Google Scholar]
- 54.Luan, H. H. The effect of growth-promoting bacteria combined with nitrogen and phosphorus on the growth and development of adzuki beans. Shanxi Normal University (2018).
- 55.Guan F, Xia M, Tang X, et al. Spatial variability of soil nitrogen, phosphorus and potassium contents in Moso bamboo forests in Yong'an City, China. CATENA. 2017;150:161–172. doi: 10.1016/j.catena.2016.11.017. [DOI] [Google Scholar]
- 56.Li H, Qiu Y, Yao T, et al. Effects of PGPR microbial inoculants on the growth and soil properties of Avena sativa, Medicago sativa, and Cucumis sativus seedlings. Soil Till. Res. 2020;199:104577. doi: 10.1016/j.still.2020.104577. [DOI] [Google Scholar]
- 57.Rezakhani L, Motesharezadeh B, Tehrani MM, Etesami H, Hosseini HM. Effect of silicon and phosphate-solubilizing bacteria on improved phosphorus (P) uptake is not specific to insoluble P-fertilized sorghum (Sorghum bicolor L.) Plants J. Plant Growth Regul. 2020;39:239–253. doi: 10.1007/s00344-019-09978-x. [DOI] [Google Scholar]
- 58.Oteino N, Lally RD, Kiwanuka S, et al. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015;6:745. doi: 10.3389/fmicb.2015.00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prodhan MA, Finnegan PM, Lambers H. How does evolution in phosphorus-impoverished landscapes impact plant nitrogen and sulfur assimilation? Trends Plant Sci. 2019;24:69–82. doi: 10.1016/j.tplants.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 60.Kerkhoff AJ, Fagan WF, Elser JJ, et al. Phylogenetic and growth form variation in the scaling of nitrogen and phosphorus in the seed plants. Am. Nat. 2006;168:E103–E122. doi: 10.1086/507879. [DOI] [PubMed] [Google Scholar]
- 61.Diao CP, Wang ZH, Li SS, et al. Differences in grain nitrogen contents of high-yielding wheat cultivars and relation to NPK uptake and utilization in drylands. J. Plant Nutr. Fertil. 2018;24:285–295. [Google Scholar]
- 62.Matsumura A, Daimon H. An evaluation of phosphorus uptake in Sesbania cannabina when ferric phosphate is applied in the presence of phosphate-solubilizing rhizobia. Legume Res. 2018;41:311–315. doi: 10.18805/lr-357. [DOI] [Google Scholar]
- 63.Wang Z, Chen Z, Xu Z, Fu X. Effects of phosphate-solubilizing bacteria and N-2-fixing bacteria on nutrient uptake, plant growth, and bioactive compound accumulation in Cyclocarya paliurus (Batal.) Iljinskaja. Forests. 2019 doi: 10.3390/f10090772. [DOI] [Google Scholar]
- 64.Zhang Y, Zhou G, Wu N, et al. Soil enzyme activity changes in different-aged spruce forests of the eastern Qinghai-Tibetan Plateau. Pedosphere. 2004;14(3):305–312. [Google Scholar]
- 65.Piotrowska-D L, Ugosz A, Charzynski P. The impact of the soil sealing degree on microbial biomass, enzymatic activity, and physicochemical properties in the Ekranic Technosols of Toruń (Poland) J. Soils Sediments. 2015;15(1):47–59. doi: 10.1007/s11368-014-0963-8. [DOI] [Google Scholar]
- 66.Chen YF, Ye JR, Kong QQ. Potassium-solubilizing activity of bacillus aryabhattai SK1-7 and its growth-promoting effect on Populus alba L. Forests. 2020;11:1348. doi: 10.3390/f11121348. [DOI] [Google Scholar]
- 67.Khati P, et al. Nanochitosan supports growth of Zea mays and also maintains soil health following growth. 3Biotech. 2017;7:2. doi: 10.1007/s13205-017-0668-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mou L, Wu HG, Gu GJ, et al. Characteristics of soil microbes and enzyme activities of four vegetation types in karst rift basins of Yunnan. Chin. J. Appl. Environ. Biol. 2020;26(06):1–10. [Google Scholar]
- 69.Wu ZH, Liu JH, Fu L, et al. Effects of phosphate solubilizing bacteria on soybean rhizosphere soil enzyme activities and microbial community. J. China Agric. Univ. 2017;22(11):58–67. [Google Scholar]
- 70.Shahzad SM, et al. Interaction of compost additives with phosphate solubilizing rhizobacteria improved maize production and soil biochemical properties under dryland agriculture. Soil Till. Res. 2017;174:70–80. doi: 10.1016/j.still.2017.06.004. [DOI] [Google Scholar]