Abstract
Research question
Granulosa cells (GCs) surrounding oocytes are crucial for follicular growth, oocyte development, ovulation, and luteinization under the dynamic co-stimulation of follicle stimulating hormone (FSH) and luteinizing hormone (LH). This study aimed to investigate the effect of LH levels on GCs in preovulatory follicles under gonadotropin releasing hormone antagonist-based ovarian stimulation. In vitro experiments were also conducted to study the direct effect of LH on GCs.
Methods
Twelve infertile women were divided into low (L), medium (M), and high (H) LH groups according to their serum LH levels during ovarian stimulation. RNA-sequencing (RNA-seq) was conducted to examine the transcriptome profiles of GCs obtained from the above patients during the oocyte retrieval. The activity of mitochondrial dehydrogenase was measured under the stimulation of recombinant LH (rLH) concentration gradient combined with recombinant FSH. The ultrastructures of subcellular organelles were observed.
Results
Bioinformatic analyses showed that compared with the M group, molecule and pathway changes in the L group and in the H group were similar. In cultured GCs, both insufficient and excessive rLH impaired the activity of mitochondrial dehydrogenase. With the medium rLH concentration, numerous cell connections and abundant mitochondria and liposomes were observed. Compared with the medium concentration, GCs showed smaller and rounder mitochondria, more autophagosomes, and massive organelles damages with excessive rLH, and swollen, circular, or forked mitochondria were observed with inadequate rLH.
Conclusions
RNA-seq provided a novel spectrum of transcriptome characteristics of GCs potentially affected by serum LH levels during ovarian stimulation. In vitro, rLH could directly affect GCs at the subcellular level.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-021-02066-8.
Keywords: Luteinizing hormone, Granulosa cells, Gonadotropin releasing hormone antagonist, Transcriptome, Organelle
Introduction
Granulosa cells (GCs) are somatic cells surrounding oocytes [1]. Under the coordinated stimulation of follicle stimulating hormone (FSH) and luteinizing hormone (LH), GCs proliferate and differentiate into parietal granulosa cells and cumulus cells, which are essential for follicular growth, oocyte development, ovulation, and luteinization [2]. According to the two-cell two-gonadotropin theory, LH stimulates theca cells to provide sufficient androgen as the precursor for estradiol (E2) production by GCs [3].
In human, GCs express the LHCGR (luteinizing hormone/choriogonadotropin receptor) gene throughout the follicular phase [4]. Adequate LH receptors facilitate good response of follicles to FSH [5]. In addition to promoting hormone secretion in ovarian cells, LH/FSH also affect the morphology of GCs mass [6–8]. In microscopic level, the attachment of GCs to the oocyte in the earlier stage of follicular development is essential for oocyte nuclear maturation. Then, the gradual detachment occurs in the final follicular stage, and some of the gap junctions are broken leading to GCs expansion [6]. Dispersed or clumped GCs mass pattern is associated with a higher maturation rate of oocytes [7, 8]. In vivo, LH surge in the final follicular stage regulates extracellular matrix (ECM) of GCs by stimulating the expansion and mucification of cumulus cells mass [6, 7, 9]. However, the biological function of LH in earlier follicular stage remains unclear.
The role of LH in controlled ovarian stimulation (COS) during follicular stage has received widespread attention in recent years [10–13]. It has been established to avoid premature LH surge [14] in gonadotropin releasing hormone antagonist (GnRH-ant) ovarian stimulation protocol. However, insufficient LH activity also needs attention. Recombinant LH (rLH) supplement could benefit women over 35 years and women with an unexpected low response to recombinant FSH (rFSH) monotherapy [12]. In women with hypothalamic hypogonadism, it was discovered that rLH supplement could increase rFSH sensitivity and enhance the luteinization triggered by human chorionic gonadotrophin (HCG) [13]. Our previous study reported a proportion of patients whose LH levels kept low (< 4 IU/L) without using GnRH-ant throughout COS [11]. Adding GnRH-ant caused even lower LH levels, which was associated with unfavorable clinical outcomes. Although the above clinical evidence seemed to suggest an important role of LH during follicular development, and the necessity to keep LH concentration within a moderate range [15, 16], little relevant basic research data could be found.
To explore the extensive effects of LH on GCs, we performed RNA-sequencing (RNA-seq) to compare and analyze transcriptome profiles of GCs obtained from preovulatory follicles among patients with different serum LH levels during COS. Furthermore, in vitro experiments were conducted to investigate the direct effect of rLH on GCs.
Materials and methods
Patients
This study was approved by the Ethics Committee of Beijing Chao-Yang Hospital, Capital Medical University (Beijing, China) (No. 2019-SCI-324) and conducted in accordance with the ethical standards established in the Declaration of Helsinki. Written informed consent was obtained from each patient. At the Medical Center for Human Reproduction of Beijing Chao-Yang Hospital from March 2019 to October 2020, we recruited twelve female patients who had gone through COS for oocyte retrieval. Their clinical characteristics were as follows: age 25–34 years, body mass index (BMI) 18–23 kg/m2, regular menstrual cycle with confirmed ovulation, basal FSH and LH < 10 IU/L, tubal or male factors for in vitro fertilization (IVF) treatment without polycystic ovarian syndrome (PCOS), ovarian endometrioma, systemic disease, endocrine abnormalities, or severe infections.
Ovarian stimulation protocols
Ovarian stimulation was started on days 2 or 3 of the menstrual cycle with the antrum follicles sizing 3–8 mm. Routine serum hormone tests and vaginal ultrasound examinations were performed every 1–3 days. An individualized dose of 150–300 IU rFSH (follitropin alfa; Gonal-F®, Merck Serono) were daily administered. Cetrorelix acetate (Cetrotide®; Serono, Geneva, Switzerland) was considered to be added from stimulation days 5 to 6. The 4/4 patients in group L and the 4/5 patients in group M adopted the modified flexible GnRH-ant protocol [10, 11]. The 1/5 patients in group M and 2/3 patients in group H adopted the traditional flexible GnRH-ant protocol [17]. The 1/3 patient in group H adopted the modified flexible GnRH-ant protocol. Briefly, in the traditional flexible GnRH-ant protocol, 0.25 mg cetrorelix acetate was initiated when the leading follicle reached 14 mm in diameter and/or E2 level reached > 300 pg/ml. In the modified flexible GnRH-ant protocol, cetrorelix acetate was reduced to half or zero for 1–2 days when LH level was profoundly suppressed (< 1 IU/L) with retarded follicle growth or inadequate E2 rise. Patients conducted urine LH test for home surveillance. When at least 3 follicles reached 17 mm, final oocyte maturation was triggered by HCG alfa 1500 IU (Ovitrelle®, Merck Serono) plus triptorelin 0.2 mg (Decapeptyl®, Ferring Pharmaceuticals) for 35–36 h, followed by ultrasound-guided transvaginal aspiration.
Grouping criteria
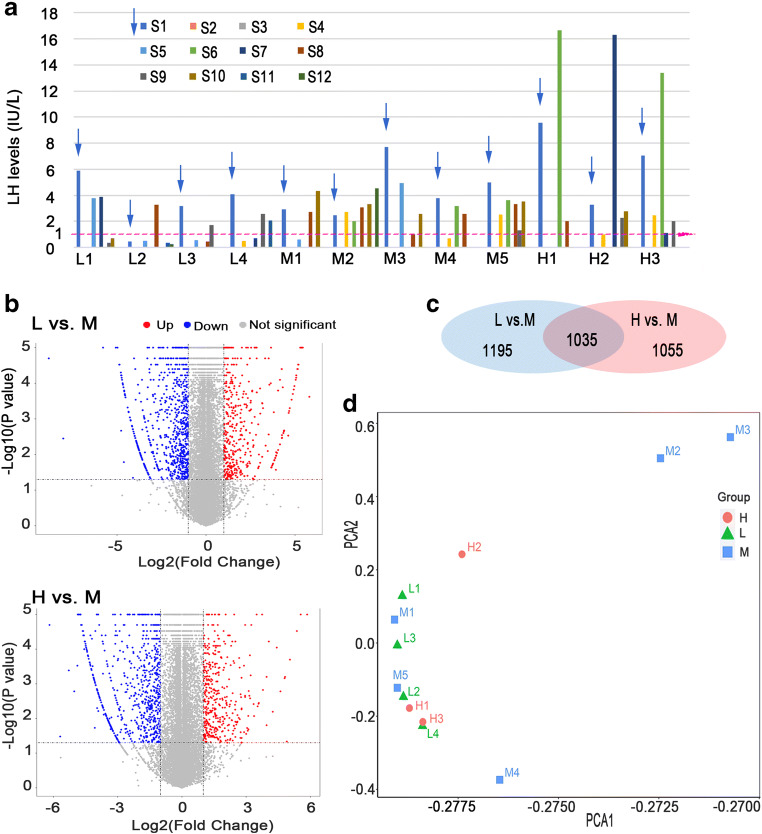
There were three groups in the RNA-seq study: 4 patients in low LH (L) group, 5 in medium LH (M) group, and 3 in high LH (H) group, respectively. The extreme cut-off values were employed to define the L group (LH < 1 IU/L) [12] and H group (LH > 10 IU/L) [18]. In L group, LH level was < 1 IU/L at least twice, with obvious inadequate E2 increment [19]. The maximal LH level in L group was 3.89 IU/L. In H group, LH level was higher than 10 IU/L once during COS before trigger day, and 0.25 mg cetrorelix acetate was given immediately. A threshold of 1–10 IU/L was primarily considered to define the M group, but the actual LH range of the M group in this study was 1.01–4.92 IU/L, without obvious E2 deficiency. Of note, the initial LH level before stimulation was not counted in all groups. The low LH level on stimulation days 4–5 was reasonable in M and H groups as a result of the negative feedback of rising E2. Detailed LH levels are illustrated in Fig. 1a.
Fig. 1.
Serum LH levels of the twelve patients during ovarian stimulation. a The X axis displayed samples, and the Y axis showed LH levels. L1–L4 were the 4 patients in group L. M1–M5 were the 5 patients in group M. H1–H3 were the 3 patients in group H. S1–S12 represented the stimulation days. The pink dotted line was the reference line of LH = 1 IU/L. b Volcano plots of differentially expressed genes (DEGs) of comparison L vs. M and H vs. M. The X axis showed the log2 of fold change (FC), and Y axis represented log10 of p values. Red dots on the right were up-regulated DEGs. Blue dots on the left were down-regulated DEGs. c The Venn diagram showed numbers of DEGs in each comparison and overlapped DEGs in both comparisons. d Principle component analysis (PCA) of the DEGs
Specimens
On the oocyte retrieval day, follicular fluid of a dominant follicle (mean diameter: 18–22 mm) without blood contamination was collected and centrifuged at 1000 rpm for 3 min. The GCs pellet was lysed in TRIzol reagent (Invitrogen, Carlsbad, CA, USA) or RIPA lysis buffer (Solarbio, Beijing, China) and immediately stored in liquid nitrogen until further use.
RNA extraction and RNA-sequencing
Total RNAs were isolated using TRIzol reagent. RNA was quantified using NanoDrop 2000 Spectrophotometers and qualified using Agilent 2100 Bioanalyzer (Thermo Fisher Scientific, MA, USA). Total RNA samples were used for subsequent experiments if met the following standards: RNA integrity number (RIN) ≥ 7.0 and a 28S:18S ≥ 1.5:1. Sequencing libraries were generated by the Beijing Genomics Institute (Shenzhen, China). The libraries were qualified by Agilent 2100 Bioanalyzer and quantified using ABI Step One Plus Real-Time PCR System. Finally, the libraries were subjected to paired-end sequencing with pair end 150 bp reading length on the BGIseq500 platform (BGI, Shenzhen, China).
Bioinformatic analyses
SOAPnuke (v1.5.2) [20] was used to filter out low quality sequencing data. Clean reads with high quality in FASTQ format were mapped to reference genome (NCBI: GCF_000001405.38_GRCh38.p12) using software HISAT2 (v2.0.4) [21]. We applied Bowtie2 (v2.2.5) [22] to align the clean reads to the reference coding gene set and then calculated the expression levels of genes by RSEM (v1.2.12) [23]. DESeq2 (v1.4.5) [24] was used to analyze differential expressed genes (DEGs) by fold change filtering (|log2(fold change)| > 1) and Student’s t-testing (p value <0.05). Gene ontology (GO) (http://www.geneontology.org/) and Kyoto Encyclopedia of Genes and Genomes (KEGG) (https://www.kegg.jp/) pathway enrichment analyses were performed by Phyper (https://en.wikipedia.org/wiki/Hypergeometric_distribution) based on a hypergeometric test. Q value with a rigorous threshold (Q value ≤ 0.05) by Bonferroni test [25] was used to correct the significant levels of terms and pathways. Gene sets in gene set enrichment analysis (GSEA) [26] were downloaded from the MSigDB database of Broad Institute (http://www.broadinstitute.org/msigdb). STRING (http://www.string-db.org/) was used in Cytoscape (v 3.7.1) [27] to construct protein-protein interaction (PPI) network.
Quantitative real-time polymerase chain reaction
Total RNAs from tissue samples were isolated using TRIzol reagent. To quantify the amount of mRNA, cDNA was synthesized using PrimeScript ™ RT reagent Kit (TaKaRa, Dalian, China). The real-time PCR analysis was performed using Power SYBR™ Green PCR Master Mix (Invitrogen, Carlsbad, CA, USA) and ABI 7500 real-time PCR system (Applied Biosystems, Foster City, CA, USA). β-actin was used as internal control. The relative expression of RNAs was calculated by 2−ΔΔCt method. All the primers were synthesized by Sangon Biotech (Shanghai, China). Primer sequences were shown in Supplementary Table S1.
Western blot analysis
The lysed cells in RIPA lysis buffer were sonicated for 5 s and centrifuged at 12,000 g for 15 min at 4 °C. The cell lysates (30 μg protein per lane) were subjected to 8% SDS-PAGE and then transferred (90 V, 1.5 h) to polyvinylidene difluoride membranes. Non-specific binding was blocked using 5% fat-free milk in Tris-buffered saline with Tween 20 for 1 h at room temperature. Membranes were incubated with appropriate amount of primary antibodies (ACTB, Proteintech, 1:2000; STAR, Proteintech, 1:1500; VIM, Cell Signaling Technology, 1:1000; HSD11B1, Proteintech, 1:800; LHCGR, Proteintech, 1:500) overnight at 4 °C. Then the membranes were incubated with HRP-conjugated anti-rabbit IgG (1:5000) for 1 h. Finally, membranes were incubated with a western blot detection reagent NcmECL Ultra Reagent A /B (NCM Biotech, Suzhou, China). Western blots were imaged with the BG-gdsAUTO710 Mini imaging system (Baygene Biotech, Beijing, China).
Granulosa cells isolation and culture
Follicular fluid harvested from 25- to 33-year-old infertile women undergoing IVF treatment with normal ovarian reserve, and normal gonadotropin response was used for GCs isolation as previously described [28, 29]. Briefly, follicular fluid was stratified by a 50% lymphocyte separation medium, and the GCs layer was collected and digested by 0.25% trypsin. GCs were cultured in DMEM/F12 medium supplemented with 10% fetal bovine serum (FBS; Gibco, NY, USA), 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco, NY, USA) in an incubator at 37 °C and 5% CO2. GCs were maintained in culture medium until day 6 to allow the optimal response to gonadotropins regardless of the COS protocols [28, 29], and then they were serum-starved overnight before stimulation by rFSH + rLH.
Mitochondrial dehydrogenase activity measurement
Isolated GCs from each of 3 patients were seeded in quintuplicate at 96-well plates and cultured for 6 days. Mitochondrial dehydrogenase activity of GCs was measured using Cell Counting Kit-8 (CCK-8) assay (Boster, Wuhan, China). Ten percent of CCK-8 substrate was added into each well. Cells were incubated at 37 °C for 2 h. Absorbance at 450 nm was tested by Varioskan Flash spectral scanning multimode reader (Thermo Fisher Scientific, Waltham, MA, USA). The initial absorbance value A1 was recorded. After starvation overnight, cells were cultured in medium without FBS serum under stimulation of increasing rLH concentrations (0, 10−3, 10−2, 10−1, 1 IU/L) combined with 1 IU/L rFSH for another 2 h. Ten percent of CCK-8 substrate was added into each well, and cells were incubated for 2 h. The after-treatment absorbance value A2 was recorded. Effect of treatment was expressed as (A2-A1)/A1.
Transmission electron microscopy
Isolated GCs from another 3 patients were each seeded into 3 culture dishes and cultured for 6 days. After starvation overnight, cells were cultured in FBS-free medium with 10−3, 10−1, and 1 IU/L rLH combined with 1 IU/L rFSH. Then, the culture medium was completely discarded, and 2.5% glutaraldehyde was added. Fixed cells were gently scraped into EP tubes. After fixation, dehydration, embedding, and curing, 50–60 nm slices were made and double stained with 2% uranium acetate and lead citrate. Finally, slices were observed under transmission electron microscope (JEM-1400, Japan).
Statistical analysis
Statistical analysis was performed using SPSS 23.0 (IBM, SPSS, Chicago, IL, USA). Figures were produced using GraphPad Prism 7.0 (GraphPad Software, Inc. La Jolla, CA, USA). Comparisons between groups were analyzed by Student’s t-test and Mann-Whitney U test as appropriate. Data were presented as mean ± s.e.m. A p value < 0.05 was considered statistically significant.
Results
Demographic data
The demographic data of the 12 patients is shown in Table 1. There were no significant differences in L vs. M and H vs. M comparisons in terms of age, antral follicle count (AFC), basal hormone levels, and rFSH consumption. Compared with M group, LH level on trigger day was significantly lower in L group. Serum E2 level on trigger day tended to be lower, and rFSH consumption tended to be higher in L group, which was consistent with previous studies that inadequate LH activity would compromise E2 release [30] and consume more exogenous FSH [31] during COS. Although the AFC among the 3 groups was similar, fewer oocytes were harvested in L group.
Table 1.
Demographic data of the twelve patients
| Parameters | Group L (low LH) | Group M (median LH) | Group H (high LH) |
|---|---|---|---|
| n = 4 | n = 5 | n = 3 | |
| Age (years) | 31.75 ± 2.18 | 29.6 ± 2.04 | 29.33 ± 2.19 |
| BMI (kg/m2) | 20.85 ± 2.35 | 21.55 ± 0.96 | 22.22 ± 2.28 |
| Menses (days) | 30.00 ± 2.31 | 32.33 ± 0.88 | 30.22 ± 1.32 |
| AFC | 14.00 ± 2.48 | 15.40 ± 0.93 | 14.00 ± 2.08 |
| Basal FSH (IU/L) | 6.08 ± 1.31 | 7.49 ± 0.70 | 7.92 ± 0.50 |
| Basal LH (IU/L) | 3.40 ± 1.12 | 4.38 ± 0.93 | 6.63 ± 1.82 |
| Basal E2 (pg/mL) | 31.77 ± 5.58 | 49.34 ± 12.23 | 31.40 ± 3.68 |
| Initiation rFSH dose (IU) | 250.00 ± 46.77 | 225.00 ± 23.72 | 225.00 ± 43.30 |
| LH on trigger day (IU/L) | 1.20 ± 0.42** | 3.50 ± 0.42 | 2.27 ± 0.24 |
| E2 on trigger day (pg/mL) | 2369.70 ± 783.70 | 4604.38 ± 646.41 | 4266.50 ± 1644.50 |
| rFSH consumption (IU) | 2637.50 ± 599.09 | 2142.50 ± 183.78 | 1841.67 ± 644.85 |
| No. of follicle > 14 mm on trigger day | 16.75 ± 4.52 | 16.20 ± 2.62 | 13.00 ± 3.46 |
| No. of oocytes retrieved | 13.20 ± 4.99 | 16.40 ± 5.60 | 15.00 ± 2.65 |
Data were presented as mean ± standard error of mean (m ± s.e.m.). BMI body mass index, AFC antrum follicle count, rFSH recombinant follicle stimulating hormone, LH luteinizing hormone, E2 estradiol. ** indicated p < 0.01 comparing group L vs. M
Differential gene expression profiles among groups
A total of 27,151 genes were detected by RNA-seq in the 12 samples of the three groups. Two thousand two hundred and thirty DEGs were identified in L group compared with M group, including 599 (26.9%) up-regulated and 1631 (73.1%) down-regulated genes. Two thousand and ninety DEGs were identified in H group compared with M group, including 593 (28.4%) up-regulated and 1497 (71.6%) down-regulated genes. DEGs are visualized by volcano plots in Fig. 1b. Venn diagram showed 1035 overlapped DEGs of the two comparisons (Fig. 1c). Principle component analysis (PCA) of the three groups is shown in Fig. 1d. The samples in L group and H group were aggregated and colocalized in adjacent area but could still be separated. Sample distribution in M group was relatively dispersed. M1 and M5 clustered with L group and H group. M2, M3, and M4 were separated from L group and H group.
The heatmap showed the top 54 DEGs annotated with MSigDB H (hallmark) description among the three groups (Fig. 2). Detailed information was presented in Supplementary Table S2. Notably, a high similarity was found between L group and H group compared with M group.
Fig. 2.
Heatmap of top differentially expressed genes (DEGs) in group expression. The X axis showed the three groups. The Y axis displayed the top 54 DEGs. In the color key, the gene expression level increased as the color changed from blue to red. On the left, genes were annotated with different colors representing MSigDB H (hallmark) terms as shown in the color list on the right
Gene ontologies clustering, classification ,and KEGG pathway analyses
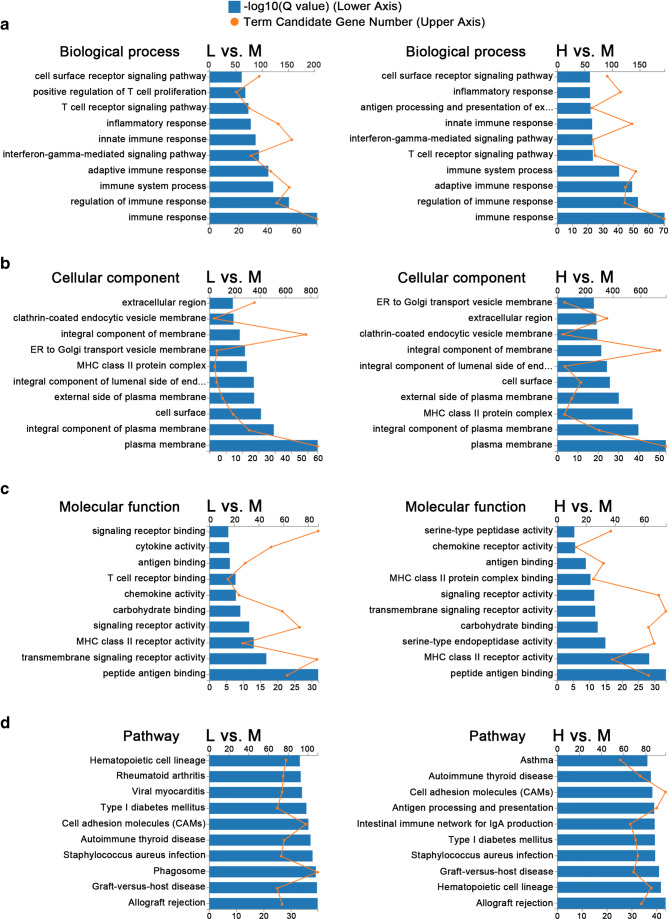
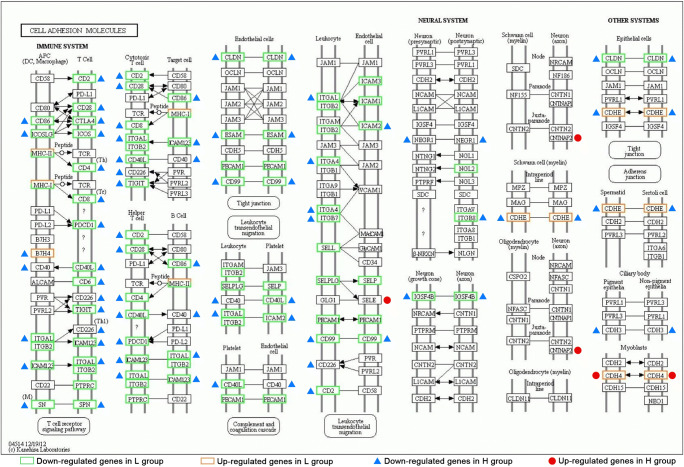
GO and KEGG analyses are illustrated in Fig. 3. Using the enrichment scores, we selected the top ten GO terms in biological processes (BP), cellular components (CC), and molecular functions (MF). Immune response, regulation of immune response, adaptive immune response, immune system process, innate immune response, inflammatory response, and cell surface receptor signaling pathway were the most significant BP in both L vs. M and H vs. M comparisons. Cell membranes and organelles such as integral component of luminal side of endoplasmic reticulum (ER), ER to Golgi transport vesicle membrane and clathrin-coated endocytic vesicle membrane, and also extracellular region were the most significant CC in both L vs. M and H vs. M comparisons. Peptide antigen binding, signal receptor activity, chemokine, and cytokine activity were the most significant MF in both L vs. M and H vs. M comparisons. According to KEGG analysis, allograft rejection, graft-versus-host disease, type I diabetes mellitus, antigen processing and presentation, cell adhesion molecules (CAMs), and autoimmune thyroid disease were significantly different in both L vs. M and H vs. M comparisons. Specifically, the enriched KEGG pathway maps of CAMs in both comparisons are integrated in Fig. 4. Most of the DEGs in CAMs pathways were down-regulated in L group and H group compared with M group and were highly overlapped.
Fig. 3.
Histograms of top 10 enriched GO and KEGG pathways of differentially expressed genes (DEGs) comparing group L vs. M and group H vs. M. The blue bars: -log10 (q value), indicated in the lower X axis. The orange nodes: gene numbers of each term, indicated in the upper X axis. a GO-BP: biological processes in GO. b GO-CC: cellular components in GO. c GO-MF: molecular functions in GO. d KEGG pathways. The left figures represented comparison L vs. M. The right figures represented comparison H vs. M
Fig. 4.
Cell adhesion molecules (CAMs) pathway map extracted from KEGG analysis integrating DEGs from both L vs. M and H vs. M comparisons. Green rectangles and blue triangles referred to down-regulation in group L and H, respectively. Orange rectangles and red dots referred to up-regulation in group L and H, respectively
Gene set enrichment analysis
Up- or down-regulated hallmarks in L group and H group compared with M group are displayed in Tables 2 and 3 with MSigDB hallmark term description. Hallmarks with a false discovery rate q value (FDR q-val) < 0.05 were considered significant. Some are illustrated in Fig. 5. Epithelial mesenchymal transition (EMT) pathway was the most significantly up-regulated hallmark in both L group and H group. The hub genes included in EMT were listed in Supplementary Table S3 and S4, representing L vs. M and H vs. M comparisons, respectively. Interferon gamma response, interferon alpha response, allograft rejection, oxidative phosphorylation, IL-6 JAK signaling, complement, inflammatory response, and KRAS signaling were down-regulated in both L group and H group. In addition, TNF-α signaling via NF-kB, IL2 STAT signaling, apoptosis, and P53 pathway were down-regulated only in L group compared with M group. MYC targets V1, DNA repair, protein secretion, adipogenesis, heme metabolism, and fatty acid metabolism were down-regulated in only H group compared with M group.
Table 2.
Up-regulated pathways in group H or L compared to group M in GSEA enrichment
| Hallmark name | Terma description | Set sizeb | NESc | FDR q value |
|---|---|---|---|---|
| Epithelial mesenchymal transition§ | Genes defining epithelial-mesenchymal transition, as in wound healing, fibrosis, and metastasis | 198 ‡ | 1.87 ‡ | < 0.002 ‡ |
| 199 † | 2.25 † | 0 † | ||
| UV response DN† | Genes downregulated in response to ultraviolet (UV) radiation | 144 | 1.55 | 0.047 |
aTerm, referred to the MSigDB H (hallmark) term
bSet size, gene set size after restricting to dataset
cNES normalized enrichment score. Group M was set as the control group. Positive NES value indicated up-regulation in groups H or L
†referred to comparison L vs. M
‡referred to comparison H vs. M
§referred to both comparisons
Table 3.
Down-regulated pathways in group H or L compared to group M in GSEA enrichment
| Hallmark name | Terma description | Set sizeb | NESc | FDR q value |
|---|---|---|---|---|
| Interferon gamma response§ | Genes up-regulated in response to IFNG | 200 | − 2.82† | 0† |
| − 2.65‡ | 0‡ | |||
| Interferon alpha response§ | Genes up-regulated in response to alpha interferon proteins | 97 | − 2.77† | 0† |
| − 2.63‡ | 0‡ | |||
| Allograft rejection§ | Genes up-regulated during transplant rejection | 196 † | − 2.52† | 0† |
| 194‡ | − 2.42‡ | 0‡ | ||
| Oxidative phosphorylation§ | Genes encoding proteins involved in oxidative phosphorylation | 200 | − 1.54† | 0.012† |
| − 2.40‡ | 0‡ | |||
| IL6 JAK STAT3 signaling § | Genes up-regulated by IL6 via STAT3, e.g., during acute phase response | 87 | − 1.84† | 0† |
| − 1.56‡ | 0.016‡ | |||
| Complement§ | Genes encoding components of the complement system, which is part of the innate immune system | 198 | − 1.82† | 0.000† |
| − 1.54‡ | 0.018‡ | |||
| Inflammatory response§ | Genes defining inflammatory response | 200 | − 2.30† | 0† |
| − 1.45‡ | 0.036‡ | |||
| KRAS signaling up § | Genes up-regulated by KRAS activation | 199 | − 1.63† | 0.004† |
| − 1.51‡ | 0.021‡ | |||
| TNFA signaling via NFKB† | Genes regulated by NF-kB in response to TNF | 200 | − 2.09 | 0 |
| IL2 STAT5 signaling † | Genes up-regulated by STAT5 in response to IL2 stimulation | 200 | − 1.94 | 0 |
| Apoptosis† | Genes mediating programmed cell death (apoptosis) by activation of caspases | 160 | − 1.71 | 0.001 |
| P53 pathway † | Genes involved in p53 pathways and networks | 200 | − 1.48 | 0.022 |
| MYC targets V1‡ | A subgroup of genes regulated by MYC version 1 (v1) | 200 | − 1.64 | 0.008 |
| DNA repair ‡ | Genes involved in DNA repair. | 150 | − 1.64 | 0.009 |
| Protein secretion‡ | Genes involved in protein secretion pathway. | 96 | − 1.66 | 0.009 |
| Adipogenesis‡ | Genes up-regulated during adipocyte differentiation (adipogenesis) | 198 | − 1.61 | 0.011 |
| Heme metabolism‡ | Genes involved in metabolism of heme (a cofactor consisting of iron and porphyrin) and erythroblast differentiation | 198 | − 1.59 | 0.012 |
| Fatty acid metabolism‡ | Genes encoding proteins involved in metabolism of fatty acids | 153 | − 1.53 | 0.017 |
aTerm, referred to the MSigDB H (hallmark) term
bSet size, gene set size after restricting to dataset
cNES normalized enrichment score. Group M was set as the control group. Negative NES value indicated down-regulation in group H or L
†referred to comparison L vs. M
‡referred to comparison H vs. M
§referred to both comparisons
Fig. 5.
Gene set enrichment plots of the differentially expressed genes (DEGs). False discovery rate q value (FDR q-val) < 0.05 was considered significant. A positive normalized enrichment score (NES) value indicated that the specific pathway was up-regulated in L or H group, compared with M group. A negative NES value indicated that the specific pathway was down-regulated in L or H group. a–i Comparing L vs. M. Only hallmarks with an FDR q-val < 0.0001 were displayed. j–n Comparing H vs. M. Only hallmarks with an FDR q-val = 0 were shown. The colored band of the X axis laid out the whole gene set involved in the specific pathway. As indicated in the color key (O), red color denoted that the gene was positively correlated with the pathway, blue color denoted that the gene was negatively correlated with the pathway, and the color density represented correlation strength. The black lines of the X axis were the DEGs encountered in run of the gene set enrichment analysis. Y axis showed the enrichment score (ES)
Protein and protein interaction network
PPI network of the DEGs related to reproduction is shown in Fig. 6a and b. Important DEGs are listed in Tables 4 and 5. Detailed information is listed in Supplementary Table SV (L vs. M) and SVI (H vs. M).
Fig. 6.
a,b The protein-protein interaction (PPI) networks of the differentially expressed genes (DEGs) related to reproduction. a PPI network of DEGs from L vs. M comparison. b PPI network of DEGs from H vs. M comparison. Pink nodes represented DEGs. Gray dotted lines represented interaction between genes. There were more DEGs in (a) than in (b), and numerous DEGs were common in both networks. c qRT-PCR validation of nine DEGs. Gene symbols were shown in the X axis; relative expression was displayed on the Y axis as mean ± s.e.m. *p < 0.05. d Western analysis of 4 proteins comparing L vs. M. LHCGR, VIM, and STAR protein levels were up-regulated in M group, and HSD11B1 protein level was up-regulated in L group
Table 4.
DEGsa associated with reproduction (L vs. M)
| Genes and descriptions | FCb | Function |
|---|---|---|
| WNT7A (Wnt family member 7A) | 0.1 | Secreted signaling protein. Essential player in female reproductive tract development and uterine function |
| GATA3 (GATA binding protein 3) | 0.2 | Transcription factor. Critical for the embryonic development and inflammatory and immune responses. Contribute to leptin inhibition of PPARc1 expression (Guan et al., 2017) |
| LEF1 (Lymphoid enhancer binding factor 1) | 0.2 | LEF1 showed lower expression levels in endometrium of patients with recurrent implantation failure compared with fertile women (Koler et al., 2009) |
| IL1B (interleukin 1 beta) | 0.2 | Pro-inflammatory cytokine. Essential player in inflammatory response, cell proliferation, differentiation, and apoptosis |
| IL10 (interleukin 10) | 0.3 | Anti-inflammatory cytokine. Present in oocytes and granulosa cells. Block NF-κB activity. Regulate JAK-STAT signaling pathway (Jatesada et al., 2013) |
| LEP (leptin) | 0.3 | Adipokine. Modulate estrogen synthesis (Chu et al., 2019) and LH secretion (Barb et al., 2004) and influence ovulation (Duggal et al., 2000) |
| PRDM1 (PR/SET domain 1) | 0.4 | Germ cell marker. Repressor of beta-interferon (β-IFN) gene expression |
| SPP1 (secreted phosphoprotein 1) | 0.4 | Extracellular structural protein. Endometrial receptivity marker |
| ETS1 (ETS proto-oncogene 1, transcription factor) | 0.4 | Transcription factor. Androgen up-regulates NR4A1 via the ETS signaling networks. ETS-NR4A1 signaling networks participate in PCOS (Song et al., 2019) |
| NOTCH1 (notch receptor 1) | 0.5 | Single-pass transmembrane receptor. Notch-1 signaling pathway activation underlined growth hormone treatment of premature ovarian failure (Liu et al., 2016) |
| ICAM1 (intercellular adhesion molecule 1) | 0.5 | Intercellular adhesion molecule. Soluble ICAM-1 in follicular fluid correlates directly with some indices of ovarian function (Viganò et al., 1998) |
| INSL3 (insulin-like 3) | 2.0 | INSL-3 initiates meiotic progression in follicle-enclosed oocytes by mediating a reduction in intra-oocyte cAMP concentration (Gambineri et al., 2007) |
| LHCGR (luteinizing hormone/choriogonadotropin receptor) | 2.1 | Transmembrane receptor. Interacts with both luteinizing hormone and chorionic gonadotropins and represents a G protein-coupled receptor |
| APOB (apolipoprotein B) | 2.1 | Apolipoprotein-B was detected in granulosa cells located at the basal layer of preovulatory follicles (Yamada et al., 1998). A positive relationship between apoB levels in follicular fluid and improved fertility parameters (Gautier et al., 2010) |
| CLU (clusterin) | 2.3 | Molecular chaperone. Responsible for aiding protein folding of secreted proteins. Known as a sensitive marker of oxidative stress |
| HAS1 (hyaluronan synthase 1) | 2.5 | Hyaluronan is a constituent of the extracellular matrix. Gonadotropin-regulated hyaluronan synthesis is involved in normal follicle growth (Takahashi et al., 2014) |
| CYP17A1 (cytochrome P450 family 17 subfamily A member 1) | 3.4 | Both 17α-hydroxylase activity and 17, 20-lyase activity. Required for the synthesis of androgenic and oestrogenic sex steroids from progestogens |
| AMH (anti-Mullerian hormone) | 8.7 | Ovarian reserve marker. Long-term usage of combined oral contraceptives significantly suppressed serum AMH level (Landersoe et al., 2020) |
aDEGs differentially expressed genes
bFC fold change
Table 5.
DEGsa associated with reproduction (H vs. M)
| Gene | FC b | Function |
|---|---|---|
| WNT7A (Wnt family member 7A) | 0.1 | Secreted signaling protein. Essential player in female reproductive tract development and uterine function |
| GATA3 (GATA binding protein 3) | 0.1 | Transcription factor. Critical for the embryonic development and inflammatory and immune responses. Contribute to leptin inhibition of PPARc1 expression (Guan et al., 2017) |
| LEF1 (lymphoid enhancer binding factor 1) | 0.3 | LEF1 showed lower expression levels in endometrium of patients with recurrent implantation failure compared with fertile women (Koler et al., 2009) |
| IL10 (interleukin 10) | 0.3 | Anti-inflammatory cytokine. Present in oocytes and granulosa cells. Block NF-κB activity. Regulate JAK-STAT signaling pathway (Jatesada et al., 2013) |
| PRDM1 (PR/SET domain 1) | 0.5 | Germ cell marker. Repressor of beta-interferon (β-IFN) gene expression |
| ETS1 (ETS proto-oncogene 1, transcription factor) | 0.5 | Transcription factor. Androgen up-regulates NR4A1 via the ETS signaling networks. ETS-NR4A1 signaling networks participate in PCOS (Song et al., 2019) |
| INSL3 (insulin-like 3) | 0.4 | INSL-3 initiates meiotic progression in follicle-enclosed oocytes by mediating a reduction in intra-oocyte cAMP concentration (Gambineri et al., 2007) |
| LHCGR (luteinizing hormone/choriogonadotropin receptor) | 2.6 | Transmembrane receptor. Interacts with both luteinizing hormone and chorionic gonadotropins and represents a G protein-coupled receptor |
| HAS1 (hyaluronan synthase 1) | 4.0 | Hyaluronan is a constituent of the extracellular matrix. Gonadotropin-regulated hyaluronan synthesis is involved in normal follicle growth |
| AMH (anti-Mullerian hormone) | 40.8 | Ovarian reserve marker. Long-term usage of combined oral contraceptives significantly suppressed serum AMH level (Landersoe et al., 2020) |
| MMP9 (matrix metallopeptidase 9) | 2.4 | Degradation of the extracellular matrix. Correlated with an increased risk for idiopathic recurrent spontaneous abortion (Pereza et al., 2012) |
| EDN1 (endothelin 1) | 4.2 | Essential player in prostaglandin F2α induced luteolysis (Doerr et al., 2008). Related with preeclampsia (Galaviz-Hernandez et al., 2016) |
| ANGPT2 (angiopoietin 2) | 0.4 | Involved in vascular permeability and inflammation. Increased in early pregnancy (Woolnough et al., 2012). Indicative of preeclampsia (Han et al., 2012) |
| FSHR (follicle stimulating hormone receptor) | 6.3 | G protein-coupled receptor. Necessary for follicular development |
aDEGs differentially expressed genes
bFC fold change
qRT-PCR validation of RNA-seq and western blot analysis
Transcripts with relatively high expression level and fold change were tested in the 3 groups by qRT-PCR (Fig. 6c). The qRT-PCR results were consistent with the sequencing data which verified the reliability of the RNA-Seq results. Four interesting proteins were analyzed using western blot methods. Changes of STAR, HSD11B1, and VIM were consistent with RNA-seq results, and LHCGR was contrast to RNA-seq result.
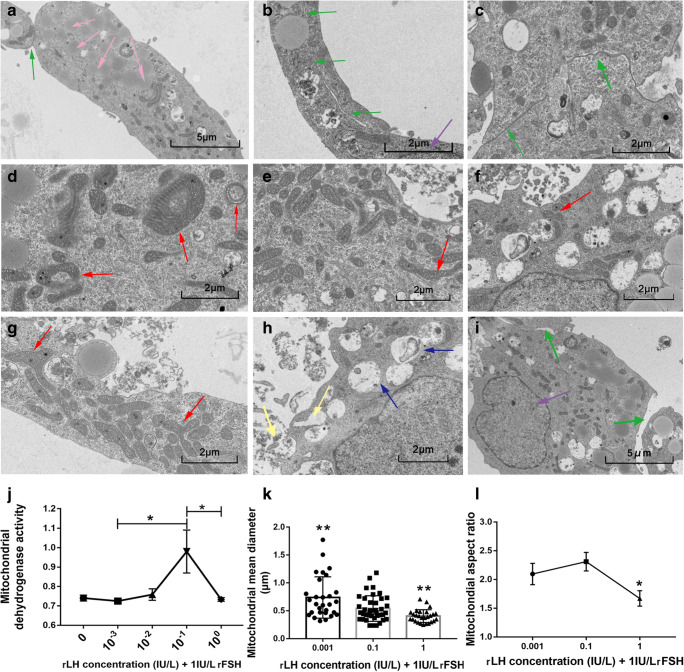
Mitochondrial dehydrogenase activity measurement
In the lower rLH concentration range, the mitochondrial dehydrogenase activity of GCs increased as rLH concentration increased, but decreased rapidly when rLH concentration continued to rise (Fig. 7j). The peak point was at the medium rLH concentration 0.1 IU/L rLH +1 IU/L rFSH.
Fig. 7.
a–i Representative images of granulosa cells (GCs) under transmission electron microscopy under co-stimulation by (0.001 IU/L, 0.1 IU/L, 1 IU/L) rLH and 1 IU/L rFSH. The magnification of the scale bar 5 μm was 2.5 thousand times, and the magnification of the scale bar 2 μm was 5 thousand times. The purple arrows referred to the cell nucleus, the red arrows referred to mitochondria, the pink arrows referred to liposomes, the blue arrows referred to autophagosomes, the green arrows referred to cell connections, and the golden arrows referred to exocytosis. a,b,c,e Images of GCs with medium concentration (0.1 IU/L rLH + 1 IU/L rFSH). a,b,c Cells were tightly connected (green arrows). a Liposomes were abundant (pink arrows). b Pseudopodia were extending. d,g Images of GCs with low concentration (0.001 IU/L rLH + 1 IU/L rFSH) stimulation. d Circular mitochondria (red arrows). g Forked mitochondria (red arrows). f,h,i Images of GCs with high concentration (1 IU/L rLH + 1 IU/L rFSH). A large number of autophagosomes (blue arrow) were visible in the cells containing undigested organelle fragments, and the endoplasmic reticulum and Golgi apparatus were significantly reduced. Cells discharged a large amount of excretion products through exocytosis (golden arrows). The gap between cells was large (green arrows). j Mitochondrial dehydrogenase activity under the co-stimulation of rLH concentration gradient (0, 0.001 IU/L, 0.01 IU/L, 0.1 IU/L, 1 IU/L) and 1 IU/L rFSH. The peak point was at 0.1 IU/L rLH + 1 IU/L rFSH. *p < 0.05. k,l Mitochondrial average diameter and aspect ratio. Compared with the medium concentration (0.1 IU/L rLH + 1 IU/L rFSH), some mitochondria with low concentration (0.001 IU/L rLH + 1 IU/L rFSH) swelled significantly. Mitochondria with high concentration (1 IU/L rLH + 1 IU/L rFSH) were significantly smaller and rounder. *p < 0.05, **p < 0.01
Transmission electron microscopic images
With the medium rLH concentration (rLH = 0.1 IU/L, rFSH = 1 IU/L), GCs showed numerous cell connections and the pseudopodia extended (Fig. 7a–c); the mitochondria were abundant and elongated (Fig. 7e and l); the liposomes were rich (Fig. 7a). Compared with medium concentration, with excessive rLH concentration (rLH = 1 IU/L, rFSH = 1 IU/L), the cell connection gap was wider (Fig. 7i); more autophagosomes, massive organelles damage, decreased ER, and Golgi apparatus were observed (Fig. 7h); mitochondria were smaller and rounder (Fig. 7f,k,l). Compared with medium concentration, with low rLH stimulation (rLH = 0.001 IU/L, rFSH = 1 IU/L), mitochondria were larger (Fig. 7k), and some mitochondria were circular (Fig. 7d) or forked (Fig. 7g); fewer liposomes, and no obvious endocytosis or exocytosis could be seen.
Discussion
LH deficiency may lead to inadequate E2 release [30, 32]. In GnRH agonist ovarian stimulation, LH levels remain low with minimal changes. In GnRH-ant ovarian stimulation, LH levels were relatively fluctuated, and E2 synthesis is susceptible to LH fluctuation [30]. To our knowledge, there is no in silico study to investigate the effect of serum LH concentration during COS using GnRH-ant protocol.
In our RNA-seq results, the heatmap showed a similar expression pattern of DEGs in L group and H group compared with M group. Clinically, once LH level exceeds 10 IU/L, 0.25 mg–0.5 mg GnRH-ant will be administered, which may overly suppress LH levels subsequently. The similar expression pattern of DEGs in L group and H group might be possibly associated with the following low LH activity after the transient high LH activity in H group.
The high overlap of changes in L group and H group compared with M group was also reflected in other bioinformatic analyses. GO analysis indicated that critical molecular functions of inflammation, and immunity were significantly affected in both L vs. M and H vs. M comparisons. In many species, such as rodent, bovine, macaque monkeys, and humans [33–36], GCs have important immunologic functions. Ovulation is comparable to immuno-inflammatory events [37, 38]. Genes related to innate immunity and immune cell function were significantly altered in cumulus cells before and after ovulation [34]. Therefore, the immunologic alterations in L and H groups might have an effect in the ovulation process. GSEA analysis showed that hallmarks such as IL-6 JAK signaling, complement, and inflammatory response were down-regulated in both L and H groups compared with M group, and NF-kB and IL2 STAT signaling were down-regulated in L group. In a previous study, IL-6 JAK signaling, complement, inflammatory response, NF-kB, and JAK/STAT signaling pathways were significantly up-regulated after ovulation trigger compared with those just before trigger [34]. Therefore, our findings strongly implied that serum LH levels during COS could possibly affect the ovulation trigger process through the pathways mentioned above. For the diseases associated with ovulation failure such as luteinized unruptured follicle syndrome [39–41], PCOS [42, 43], and empty follicle syndrome [44, 45], our findings might be helpful for studying the etiology.
As shown in Fig. 4, most of the DEGs of CAMs were down-regulated in both L and H groups compared with M group. It was reported that in cumulus cells of patients with PCOS, CAMs and extracellular matrix were down-regulated [46]. Integrin β1, a cell adhesion molecule in the granulosa layers of the bovine cystic follicle, was found significantly lower than the healthy follicles [47]. Therefore, down-regulated expression of CAMs in L and H groups might be unfavorable for follicles’ health. EMT was the most significantly up-regulated hallmark in both L and H groups compared with M group. In the EMT gene set, many DEGs encode proteins related to ECM, such as collagenase genes [48] COL3A1, COL5A1, COL1A1, COL1A2, COL4A1, COL6A3, COL4A2, and COL11A1; non-collagenous matrix protein coding genes LAMC1 and LAMA1 [49]; and other matrix relevant genes like BGN [50], MMP2 [51], MATN3 [52], and SDC1 [53]. We hypothesize that the LH during COS could affect the ECM regulation by GCs, in addition to the final LH surge as previously discussed [6–8]. Moreover, the “U shape” correlation suggested that a moderate activation of EMT was achieved by a moderate LH level during COS. Interestingly, EMT was also associated with endometriosis [54–56] and a stimulatory effect on cell migration and invasion by FSH/LH in ovarian cancer [57]. The effect of LH on EMT in our study might provide insights in the pathophysiology of endometriosis and ovarian cancer. Interestingly, the difference in cell connection observed in our in vitro study might also reflect the effect of LH function in extracellular structures.
PPI network shed light on the extensive influence of serum LH activity. Consistent with previous knowledge that peptide hormone concentration could regulate the content of its receptor [58], western blot analysis showed that LHCGR protein expression was higher in M samples than in L samples, suggesting that low LH levels during COS might reduce LHCGR protein expression for later luteal phase. Given the complexity of LH changes in H group and the low incidence of premature LH surge in clinical practice, group H was not studied in our western blot analysis. Notably, the significance of the trend of changes was subtle because the preovulatory phase was a transitional period from follicular phase to luteal phase [59].
Our in vitro study showed that rLH concentration exerted dual effects on mitochondrial dehydrogenase activity. The effects also manifested in subcellular ultrastructure changes. Cells cultured with the medium concentration of rLH showed healthier organelles. Elongated mitochondria could survive autophagic degradation and have more cristae and higher ATP synthase activity to maintain ATP production with limited nutrient [60]. With low rLH concentration, GCs showed mitochondrial morphologic changes, while other cell organelles appeared intact, indicating that low rLH might not be definitely fatal to GCs. However, the swollen and circular mitochondria reflected that cells were under mild stress [61, 62]. Forked mitochondria under low rLH stimulation and small mitochondria under excessive rLH stimulation might come from mitochondria fusion or fission, which might also reflect cellular stress [63]. Excessive rLH stimulation caused substantial autophagy in GCs. Nevertheless, this could not reflect the effect of transient high LH in COS where high LH was immediately suppressed by GnRH-ant.
In summary, this is the first in silico study combined with in vitro experiments to explore the extensive effect of LH on GCs in preovulatory follicles and GCs under culture condition. A novel spectrum of transcriptome characteristics was identified, providing multiple directions for future studies in this field. However, there are limitations in our study such as limited sample size and potential selection bias. The correlation of LH activity and LH concentrations, and cell survival conditions in vivo and in vitro were not uniform. Further studies are warranted to verify our findings and speculations.
Supplementary information
(DOCX 16 kb)
(DOCX 19 kb)
(DOCX 30 kb)
(DOCX 30 kb)
(DOCX 19 kb)
(DOCX 19 kb)
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fontana J, Martínková S, Petr J, Žalmanová T, Trnka J. Metabolic cooperation in the ovarian follicle. Physiol Res. 2020;69:33–48. doi: 10.33549/physiolres.934233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cecconi S, Ciccarelli C, Barberi M, Macchiarelli G, Canipari R. Granulosa cell-oocyte interactions. Eur J Obstet Gynecol Reprod Biol. 2004;115(Suppl 1):S19–S22. doi: 10.1016/j.ejogrb.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Fleming R, Jenkins J. The source and implications of progesterone rise during the follicular phase of assisted reproduction cycles. Reprod BioMed Online. 2010;21:446–449. doi: 10.1016/j.rbmo.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 4.Jeppesen JV, Kristensen SG, Nielsen ME, Humaidan P, Dal Canto M, Fadini R, Schmidt KT, Ernst E, Yding Andersen C. LH-receptor gene expression in human granulosa and cumulus cells from antral and preovulatory follicles. J Clin Endocrinol Metab. 2012;97:E1524–E1531. doi: 10.1210/jc.2012-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erickson GF, Wang C, Hsueh AJ. FSH induction of functional LH receptors in granulosa cells cultured in a chemically defined medium. Nature England. 1979;279:336–338. doi: 10.1038/279336a0. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Wert SE, Hendrix EM, Russell PT, Cannon M, Larsen WJ. Hyaluronic acid synthesis and gap junction endocytosis are necessary for normal expansion of the cumulus mass. Mol Reprod Dev. 1990;26:236–247. doi: 10.1002/mrd.1080260307. [DOI] [PubMed] [Google Scholar]
- 7.Thanaboonyawat I, Makemaharn O, Petyim S, Laokirkkiat P, Choavaratana R. The correlation of cumulus mucification patterns with oocyte maturation rate in vitro in FSH + LH-primed IVM cycles: a prospective study. Arch Gynecol Obstet. 2016;293:681–686. doi: 10.1007/s00404-015-3935-3. [DOI] [PubMed] [Google Scholar]
- 8.Yang S-H, Son W-Y, Yoon S-H, Ko Y, Lim J-H. Correlation between in vitro maturation and expression of LH receptor in cumulus cells of the oocytes collected from PCOS patients in HCG-primed IVM cycles. Hum Reprod. 2005;20:2097–2103. doi: 10.1093/humrep/dei045. [DOI] [PubMed] [Google Scholar]
- 9.Salustri A. Paracrine actions of oocytes in the mouse pre-ovulatory follicle. Int J Dev Biol. 2000;44:591–597. [PubMed] [Google Scholar]
- 10.Zhang D, Xia L, Xu H, Chen Q, Jin B, Zhang A, et al. Flexible low-dose GnRH antagonist protocol is effective in patients with sufficient ovarian reserve in IVF. Front Endocrinol (Lausanne) 2018;9:767. doi: 10.3389/fendo.2018.00767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu M, Liu S, Li L, Wang P, Li H, Li Y. LH levels may be used as an indicator for the time of antagonist administration in GnRH antagonist protocols-a proof-of-concept study. Front Endocrinol (Lausanne) 2019;10:67. doi: 10.3389/fendo.2019.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lisi F. To add or not to add LH: comments on recent commentaries. Reprod BioMed Online. 2006;12:415–417. doi: 10.1016/s1472-6483(10)61992-x. [DOI] [PubMed] [Google Scholar]
- 13.Recombinant human luteinizing hormone (LH) to support recombinant human follicle-stimulating hormone (FSH)-induced follicular development in LH- and FSH-deficient anovulatory women: a dose-finding study. The European Recombinant Human LH Study Group. J Clin Endocrinol Metab. 1998;83:1507–14. [DOI] [PubMed]
- 14.Hillier SG. Current concepts of the roles of follicle stimulating hormone and luteinizing hormone in folliculogenesis. Hum Reprod. 1994;9:188–191. doi: 10.1093/oxfordjournals.humrep.a138480. [DOI] [PubMed] [Google Scholar]
- 15.Häggström M. Reference ranges for estradiol, progesterone, luteinizing hormone and follicle-stimulating hormone during the menstrual cycle. Wikiversity J Med 2014;1.
- 16.Stricker R, Eberhart R, Chevailler M-C, Quinn FA, Bischof P, Stricker R. Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT analyzer. Clin Chem Lab Med. 2006;44:883–887. doi: 10.1515/CCLM.2006.160. [DOI] [PubMed] [Google Scholar]
- 17.Trenkić M, Popović J, Kopitović V, Bjelica A, Živadinović R, Pop-Trajković S. Flexible GnRH antagonist protocol vs. long GnRH agonist protocol in patients with polycystic ovary syndrome treated for IVF: comparison of clinical outcome and embryo quality. Ginekol Pol. 2016;87:265–270. doi: 10.17772/gp/62205. [DOI] [PubMed] [Google Scholar]
- 18.Regan L, Owen EJ, Jacobs HS. Hypersecretion of luteinising hormone, infertility, and miscarriage. Lancet. 1990;336:1141–1144. doi: 10.1016/0140-6736(90)92765-a. [DOI] [PubMed] [Google Scholar]
- 19.Streda R, Mardesic T, Sobotka V, Tosner J. Long GnRH agonist vs. GnRH antagonist protocol in randomized controlled trial in unselected patients--hormonal and cycle characteristics--pilot study. Ceska Gynekol. 2009;74:75–80. [PubMed] [Google Scholar]
- 20.Li R, Li Y, Kristiansen K, Wang J. SOAP: short oligonucleotide alignment program. Bioinformatics. 2008;24:713–714. doi: 10.1093/bioinformatics/btn025. [DOI] [PubMed] [Google Scholar]
- 21.Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langmead B, Salzberg SL. Fast gapped-read alignment with bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdi H. The Bonferonni and Šidák corrections for multiple comparisons. Encyclopedia of Measurement and Statistics 2007;3.
- 26.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su G, Morris JH, Demchak B, Bader GD. Biological network exploration with Cytoscape 3. Curr Protoc Bioinformatics. 2014;47:8.13.1–8.1324. doi: 10.1002/0471250953.bi0813s47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casarini L, Riccetti L, De Pascali F, Nicoli A, Tagliavini S, Trenti T, et al. Follicle-stimulating hormone potentiates the steroidogenic activity of chorionic gonadotropin and the anti-apoptotic activity of luteinizing hormone in human granulosa-lutein cells in vitro. Mol Cell Endocrinol. 2016;422:103–114. doi: 10.1016/j.mce.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Nordhoff V, Sonntag B, von Tils D, Götte M, Schüring AN, Gromoll J, et al. Effects of the FSH receptor gene polymorphism p.N680S on cAMP and steroid production in cultured primary human granulosa cells. Reprod Biomed Online. 2011;23:196–203. doi: 10.1016/j.rbmo.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 30.Kol S. Individualized treatment from theory to practice: the private case of adding LH during GnRH antagonist-based stimulation protocol. Clin Med Insights Reprod Health. 2014;8:59–64. doi: 10.4137/CMRH.S17788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alviggi C, Pettersson K, Longobardi S, Andersen CY, Conforti A, De Rosa P, et al. A common polymorphic allele of the LH beta-subunit gene is associated with higher exogenous FSH consumption during controlled ovarian stimulation for assisted reproductive technology. Reprod Biol Endocrinol. 2013;11:51. doi: 10.1186/1477-7827-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shoham Z. The clinical therapeutic window for luteinizing hormone in controlled ovarian stimulation. Fertil Steril. 2002;77:1170–1177. doi: 10.1016/s0015-0282(02)03157-6. [DOI] [PubMed] [Google Scholar]
- 33.Duffy DM. Novel contraceptive targets to inhibit ovulation: the prostaglandin E2 pathway. Hum Reprod Update. 2015;21:652–670. doi: 10.1093/humupd/dmv026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poulsen L la C, Englund ALM, Wissing MLM, Yding Andersen C, Borup R, Grøndahl ML. Human granulosa cells function as innate immune cells executing an inflammatory reaction during ovulation: a microarray analysis. Mol Cell Endocrinol. 2019;486:34–46. doi: 10.1016/j.mce.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 35.Price JC, Sheldon IM. Granulosa cells from emerged antral follicles of the bovine ovary initiate inflammation in response to bacterial pathogen-associated molecular patterns via Toll-like receptor pathways. Biol Reprod. 2013;89:119. doi: 10.1095/biolreprod.113.110965. [DOI] [PubMed] [Google Scholar]
- 36.Shimada M, Hernandez-Gonzalez I, Gonzalez-Robanya I, Richards JS. Induced expression of pattern recognition receptors in cumulus oocyte complexes: novel evidence for innate immune-like functions during ovulation. Mol Endocrinol. 2006;20:3228–3239. doi: 10.1210/me.2006-0194. [DOI] [PubMed] [Google Scholar]
- 37.Espey LL. Ovulation as an inflammatory reaction--a hypothesis. Biol Reprod. 1980;22:73–106. doi: 10.1095/biolreprod22.1.73. [DOI] [PubMed] [Google Scholar]
- 38.Espey LL. Current status of the hypothesis that mammalian ovulation is comparable to an inflammatory reaction. Biol Reprod. 1994;50:233–238. doi: 10.1095/biolreprod50.2.233. [DOI] [PubMed] [Google Scholar]
- 39.LeMaire GS. The luteinized unruptured follicle syndrome: anovulation in disguise. J Obstet Gynecol Neonatal Nurs. 1987;16:116–120. doi: 10.1111/j.1552-6909.1987.tb01446.x. [DOI] [PubMed] [Google Scholar]
- 40.Bashir ST, Baerwald AR, Gastal MO, Pierson RA, Gastal EL. Follicle growth and endocrine dynamics in women with spontaneous luteinized unruptured follicles versus ovulation. Hum Reprod. 2018;33:1130–1140. doi: 10.1093/humrep/dey082. [DOI] [PubMed] [Google Scholar]
- 41.Tomioka RB, Ferreira GRV, Aikawa NE, Maciel GAR, Serafini PC, Sallum AM, Campos LMA, Goldestein-Schainberg C, Bonfá E, Silva CA. Non-steroidal anti-inflammatory drug induces luteinized unruptured follicle syndrome in young female juvenile idiopathic arthritis patients. Clin Rheumatol. 2018;37:2869–2873. doi: 10.1007/s10067-018-4208-x. [DOI] [PubMed] [Google Scholar]
- 42.Esparza LA, Schafer D, Ho BS, Thackray VG, Kauffman AS. Hyperactive LH pulses and elevated kisspeptin and NKB gene expression in the arcuate nucleus of a PCOS mouse model. Endocrinology. 2020;161. [DOI] [PMC free article] [PubMed]
- 43.Pierre A, Peigné M, Grynberg M, Arouche N, Taieb J, Hesters L, et al. Loss of LH-induced down-regulation of anti-Müllerian hormone receptor expression may contribute to anovulation in women with polycystic ovary syndrome. Hum Reprod. 2013;28:762–769. doi: 10.1093/humrep/des460. [DOI] [PubMed] [Google Scholar]
- 44.Yuan P, He Z, Zheng L, Wang W, Li Y, Zhao H, Zhang VW, Zhang Q, Yang D. Genetic evidence of “genuine” empty follicle syndrome: a novel effective mutation in the LHCGR gene and review of the literature. Hum Reprod. 2017;32:944–953. doi: 10.1093/humrep/dex015. [DOI] [PubMed] [Google Scholar]
- 45.Lok F, Pritchard J, Lashen H. Successful treatment of empty follicle syndrome by triggering endogenous LH surge using GnRH agonist in an antagonist down-regulated IVF cycle. Hum Reprod. 2003;18:2079–2081. doi: 10.1093/humrep/deg421. [DOI] [PubMed] [Google Scholar]
- 46.Hassani F. Downregulation of extracellular matrix and cell adhesion molecules in cumulus cells of infertile polycystic ovary syndrome women with and without insulin resistance. Cell J. 2019;21:8. doi: 10.22074/cellj.2019.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohira T, Murayama C, Shimizu T, Yoshimura Y, Isobe N. Comparison of cadherin and integrin localization in bovine cystic and healthy follicles. Anim Sci J. 2013;84:303–309. doi: 10.1111/asj.12008. [DOI] [PubMed] [Google Scholar]
- 48.Thibault C, Levasseur MC. Ovulation. Hum Reprod. 1988;3:513–523. doi: 10.1093/oxfordjournals.humrep.a136737. [DOI] [PubMed] [Google Scholar]
- 49.Miner JH, Li C, Mudd JL, Go G, Sutherland AE. Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation. Development. 2004;131:2247–2256. doi: 10.1242/dev.01112. [DOI] [PubMed] [Google Scholar]
- 50.Hu L, Zang M, Wang H-X, Li J-F, Su L-P, Yan M, Li C, Yang QM, Liu BY, Zhu ZG. Biglycan stimulates VEGF expression in endothelial cells by activating the TLR signaling pathway. Mol Oncol. 2016;10:1473–1484. doi: 10.1016/j.molonc.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Imai K, Khandoker MAMY, Yonai M, Takahashi T, Sato T, Ito A, et al. Matrix metalloproteinases-2 and -9 activities in bovine follicular fluid of different-sized follicles: relationship to intra-follicular inhibin and steroid concentrations. Domest Anim Endocrinol. 2003;24:171–183. doi: 10.1016/s0739-7240(02)00235-7. [DOI] [PubMed] [Google Scholar]
- 52.Pei M, Luo J, Chen Q. Enhancing and maintaining chondrogenesis of synovial fibroblasts by cartilage extracellular matrix protein matrilins. Osteoarthr Cartil. 2008;16:1110–1117. doi: 10.1016/j.joca.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ishikawa T, Kramer RH. Sdc1 negatively modulates carcinoma cell motility and invasion. Exp Cell Res. 2010;316:951–965. doi: 10.1016/j.yexcr.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu T, Zhang X. Research progress on the role of epithelial-mesenchymal transition in pathogenesis of endometriosis. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2016;45:439–445. doi: 10.3785/j.issn.1008-9292.2016.07.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Proestling K, Birner P, Gamperl S, Nirtl N, Marton E, Yerlikaya G, Wenzl R, Streubel B, Husslein H. Enhanced epithelial to mesenchymal transition (EMT) and upregulated MYC in ectopic lesions contribute independently to endometriosis. Reprod Biol Endocrinol. 2015;13:75. doi: 10.1186/s12958-015-0063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang Y-M, Yang W-X. Epithelial-to-mesenchymal transition in the development of endometriosis. Oncotarget. 2017;8:41679–41689. doi: 10.18632/oncotarget.16472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feng D, Zhao T, Yan K, Liang H, Liang J, Zhou Y, Zhao W, Ling B. Gonadotropins promote human ovarian cancer cell migration and invasion via a cyclooxygenase 2-dependent pathway. Oncol Rep. 2017;38:1091–1098. doi: 10.3892/or.2017.5784. [DOI] [PubMed] [Google Scholar]
- 58.Catt KJ, Dufau ML. Peptide hormone receptors. Annu Rev Physiol. 1977;39:529–557. doi: 10.1146/annurev.ph.39.030177.002525. [DOI] [PubMed] [Google Scholar]
- 59.Fraser HM, Tsonis CG. Manipulation of inhibin during the luteal-follicular phase transition of the primate menstrual cycle fails to affect FSH secretion. J Endocrinol. 1994;142:181–186. doi: 10.1677/joe.0.1420181. [DOI] [PubMed] [Google Scholar]
- 60.Gomes LC, Di Benedetto G, Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol. 2011;13:589–598. doi: 10.1038/ncb2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang B, Liu Q, Bi Y. Autophagy and apoptosis are regulated by stress on Bcl2 by AMBRA1 in the endoplasmic reticulum and mitochondria. Theor Biol Med Model. 2019;16:18. doi: 10.1186/s12976-019-0113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou Y, Long Q, Wu H, Li W, Qi J, Wu Y, Xiang G, Tang H, Yang L, Chen K, Li L, Bao F, Li H, Wang Y, Li M, Liu X. Topology-dependent, bifurcated mitochondrial quality control under starvation. Autophagy. 2020;16:562–574. doi: 10.1080/15548627.2019.1634944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maycotte P, Marín-Hernández A, Goyri-Aguirre M, Anaya-Ruiz M, Reyes-Leyva J, Cortés-Hernández P. Mitochondrial dynamics and cancer. Tumour Biol. 2017;39:1010428317698391. doi: 10.1177/1010428317698391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 16 kb)
(DOCX 19 kb)
(DOCX 30 kb)
(DOCX 30 kb)
(DOCX 19 kb)
(DOCX 19 kb)