Abstract
The physical properties of a biomaterial play an essential role in regulating immune and reparative activities within the host tissue. This study aimed to evaluate the immunological impact of material stiffness of a glycol-chitosan hydrogel designed for vocal fold tissue engineering. Hydrogel stiffness was varied via the concentration of glyoxal cross-linker applied. Hydrogel mechanical properties were characterized through atomic force microscopy and shear plate rheometry. Using a transwell setup, macrophages were co-cultured with human vocal fold fibroblasts that were embedded within the hydrogel. Macrophage viability and cytokine secretion were evaluated at 3, 24, and 72 hr of culture. Flow cytometry was applied to evaluate macrophage cell surface markers after 72 hr of cell culture. Results indicated that increasing hydrogel stiffness was associated with increased anti-inflammatory activity compared to relevant controls. In addition, increased anti-inflammatory activity was observed in hydrogel co-cultures. This study highlighted the importance of hydrogel stiffness from an immunological viewpoint when designing novel vocal fold hydrogels.
Keywords: fibroblast, hydrogel, immunomodulation, macrophage, stiffness
1 |. INTRODUCTION
Hydrogels are water-swollen polymer networks that have been engineered for biomaterial therapeutics of defective tissues and organs.1–4 There is growing interest in tuning the biomechanical properties of hydrogels to facilitate favorable tissue regeneration outcomes.5–7 For example, hydrogel stiffness can affect focal adhesion maturation and the levels of tyrosine phosphorylation of FAK and paxillin expression.8,9 In addition, porosity can significantly affect the onset and severity of chronic inflammation.10
Various methods have been reported for tuning hydrogel properties for desired biological outcomes. For instance, the degradation rate of PEG-based hydrogels can be modified by introducing α-hydroxy acids or enzymatically cleavable peptides to the PEG macromer backbone.11,12 Furthermore, hydrogel stiffness can be modified by varying cross-linking density present via, for example, altering the concentration of cross-linker applied.13,14
The immunomodulatory properties of vocal fold hydrogels have been explored using vocal fold fibroblasts (VFFs), macrophages (Mφ), mesenchymal stem cells, and adipose-derived stem cells.15–21 Specifically, VFFs represent the most abundant cell type found within the lamina propria where they serve to maintain and repair the vocal fold mucosa.22–24 VFFs have been recognized for their mechanosensitive and immunomodulatory properties including their ability to suppress pro-inflammatory cytokine production.25–28 Fibroblast-like cells have been suggested to have some behavioral capacities similar to innate immune cells and also play a role in inflammatory resolution.29 Furthermore, VFFs influence the local tissue environment through the provision of glycosaminoglycans (GAGs), proteogylcans, elastin, and collagen that help dictate the migration, growth, and differentiation of surrounding cells.22,25,30
Hydrogel physical properties have been demonstrated to influence VFF activity in vitro. For example, decreased cross-linking density of PEGDA hydrogels was shown to induce increased GAG synthesis and decreased elastin synthesis from VFFs.31 HA-gelatin composite microgels demonstrated that other physical properties, such as a controlled degradation rate and increased surface area, can enhance VFF behavior including their adhesion capacity and motility.32
In contrast, Mφ are antigen-presenting immune cells that are one of the early cellular responders to an implanted hydrogel. Critically, Mφ dysregulation can induce excessive fibrosis of the local tissue.33 Hydrogel physical properties have been associated with alterations in Mφ phenotype alongside changes in chemokine, cytokine, and matrix metalloproteinase production.34–36 For example, the pore size of poly (2-hydroxyethyl methacrylate-methacrylic acid) hydrogels was associated with a shift in Mφ phenotype and associated inflammatory activity.37 Pore sizes >20 μm induced a shift to an M2 phenotype whereas nonporous hydrogels were related to an increased M1 population.
Co-cultures of VFFs and Mφ have been applied to quantify immunomodulatory properties of vocal fold hydrogels.18 Evidence of crosstalk between VFFs and Mφ through paracrine signaling has been demonstrated.38 For instance, Mφ secrete IL-10 following a shift from an “inflammatory” to a “tissue repair” phenotype.34,35,39 IL-10 presence has been associated with promoting healthy, nonfibrotic VFFs in PEGDA hydrogels.19 In addition, fibroblasts stimulated by IL-10 have been shown to synthesize ECM components essential to reestablishing normal tissue structure and function.40 These findings further support the idea that the immunomodulatory properties of vocal fold hydrogels can be exploited to promote inflammatory resolution and ECM remodeling for effective tissue repair.
Stiffness is a notable physical design parameter of vocal fold hydrogels because of the unique elastic and stiffness properties human vocal fold tissue requires for phonation. Depending upon age and gender, human vocal folds typically vibrate at frequencies in the range of 100–300 Hz during phonation.41–44 The Young’s moduli, or stiffness, of human vocal folds ranges from 2.45 to 29.4 kPa.45 If vocal fold hydrogel stiffness is not tuned to match physiological standards, phonatory function will not be properly restored. More importantly, in addition to the importance of vocal fold hydrogel stiffness for replicating the native tissue environment, stiffness offers an opportunity to modulate the innate immune response.
Cells sense and respond to the surrounding local matrix through mechanotransduction.46,47 Interactions between a biomaterial and the local tissue environment have been shown to regulate cellular behavior including contractibility, differentiation, motility, and spreading.48–51 In particular, local cells can sense the material stiffness and trigger specific intracellular signaling events. Material stiffness has been demonstrated to influence focal adhesion formation and maturation.8,9 Soft hydrogels (0.1–1 kPa) can limit focal adhesion maturation and increase their turnover rate. Stiffer hydrogels (10–100 kPa) can produce extended focal adhesions with increased levels of tyrosine phosphorylation of FAK and paxillin expression. Cytoskeleton assembly can also be modulated through material stiffness. For example, fibroblasts cultured on soft polyacrylamide gels (~0.2 kPa) displayed a spherical appearance with no stress fibers, whereas on stiffer gels (~3.6 kPa) fibroblasts were oblong with a visible cytoskeleton.52
A mechanically tunable glycol-chitosan hydrogel has been proposed for vocal fold tissue engineering.14 The concentration of glyoxal, a dialdehyde cross-linker, was used to modify the stiffness of chitosan-based hydrogels.53 Higher glyoxal concentrations produce stiffness values more closely aligned with human vocal folds that have been reported to have an average Young’s modulus of 12.66 kPa.45 Unfortunately, increased glyoxal content within glycol-chitosan hydrogels has previously been associated with reduced VFF viability.14 As such, an appropriate glyoxal concentration must be selected that provides the hydrogel with mechanical integrity and relevant stiffness while minimizing cytotoxic effects upon cells.54,55
However, VFF hydrogel monocultures provide only limited information on the nature of the innate inflammatory response associated with glycol-chitosan hydrogels. Further investigation is necessary to quantify inflammatory effects related to varying the glyoxal concentration of the hydrogels. Given the significance of Mφ in biomaterial-related inflammatory activity, Mφ inclusion in a subsequent in vitro model will enable the inflammatory response to be quantified more extensively.56
The primary purpose of this study was to investigate the response of human Mφ to stiffness variations of a glycol-chitosan hydrogel in the presence of human VFFs. Mechanical properties of the hydrogel were characterized with atomic force microscopy (AFM) and oscillatory shear rheometry. Mφ inflammatory activity was characterized over a period of 72 hr via assessments of cytokine production, cell viability, and cell phenotyping. The hypothesis was that stiffer hydrogels would be associated with increased Mφ pro-inflammatory activity over a 72 hr period due to the cytotoxicity of the glyoxal cross-linker.
2 |. MATERIALS AND METHODS
2.1 |. Glycol-chitosan hydrogel preparation
A stock solution of glycol-chitosan hydrogel was prepared by mixing 4 g of glycol-chitosan powder (Sigma-Aldrich, Lot #SLBV0662) with 80 ml of 1 × phosphate buffered saline (PBS) (Wisent Bioproducts, QC, Canada) at room temperature to form a glycol-chitosan solution with a concentration of 4.76% (wt/wt %). The solution was rotated (MX-RDPro, Montreal Biotech Inc.) at 10 rpm for 24 hr. Following rotation, the solution was sterilized by autoclaving (SV-120, Steris Amsco Century) for 30 min at 121°C before being stored at 4°C until use.
2.2 |. Glyoxal cross-linker preparation
A stock solution of 10% glyoxal cross-linker solution was prepared in a fume hood at room temperature by mixing 5 ml of 40% glyoxal solution (Sigma-Aldrich, Lot #MKBV9169V) with 15 ml 1 × PBS. The 10% glyoxal solution was then sterilized by autoclaving and stored at 4°C. Prior to each experiment, 10% glyoxal solution was mixed with sterile PBS in a biosafety cabinet to produce three glyoxal solutions with concentration 0.005, 0.01, and 0.02% respectively.
2.3 |. Mechanical characterization–oscillatory shear rheometry
A TA Instrument Rheometer-AR2000 (New Castle, DE) was used to determine the storage and loss moduli of the cross-linked glycol-chitosan hydrogel. Parallel plates with a diameter of 20 mm and gap length 500 μm were used for testing and the plate temperature was set to 37°C. In a test tube, 400 μl of the glycol-chitosan hydrogel was mixed with 500 μl of defined glyoxal cross-linker (0.005, 0.01, or 0.02%). 300 μl of the resultant mix was applied to the bottom rheometer plate. 200 μl of baby oil (Johnson & Johnson) was applied around the plate edge to prevent sample dehydration. Soak time was set to 2 hr to allow hydrogel gelation prior to testing. Following soak time, a controlled stress frequency sweep test was performed between the angular frequency range 0.1–100 rad/s.
2.4 |. Mechanical characterization–AFM
AFM was used to measure substrate mechanical properties of hydrogels on cellular scale.4 Cross-linked hydrogel samples were prepared in 35 mm glass bottomed petri dishes (MatTek Corporation, USA) by mixing 400 μl of the glycol-chitosan hydrogel with 500 μl of defined glyoxal cross-linker (0.005, 0.01, or 0.02%). The hydrogel was distributed using a cell scraper to form a thin film across the dish surface. Each hydrogel sample was placed in an incubator (37°C, 5% CO2) for 2 hr to facilitate gelation. Following gelation, PBS was added atop the hydrogel to prevent dehydration. AFM (JPK Nano-wizard 3, Bio-Science, Berlin, Germany) was then applied to each hydrogel to determine the Young’s modulus of each respective cross-linked hydrogel. A rectangular silicon cantilever (0.6 N/m) with an attached 25 μm polystyrene spherical particle (Novascan Technologies, Inc., Boone, IA) was applied for all testing. 20 unique points on each hydrogel surface were tested and results were averaged to obtain the mean Young’s modulus of each hydrogel.
2.5 |. THP-1 monocyte culture
The human monocyte cell line THP-1 (ATCC TIB202, Manassas, VA) was maintained in suspension in Roswell Park Memorial Institute medium (RPMI) 1640 (Wisent Bioproducts, QC, Canada) supplemented with 10% (vol/vol) fetal bovine serum (FBS) (Wisent Bioproducts, QC, Canada) and 1% (vol/vol) penicillin streptomycin (Wisent Bioproducts, QC, Canada). Cell cultures were stored in a humidified incubator (37°C, 5% CO2) for amplification.
2.6 |. Induced differentiation of THP-1 monocytes
To obtain THP-1 Mφ, 5 ml of THP-1 monocytes at a concentration of approximately 1 × 106 cells/ml were transferred to a tissue culture treated cell culture dish (100 × 20 mm, Eppendorf).57 Phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich) was added to the cells at a concentration of 20 ng/ml. Cells were incubated for 24 hr to allow differentiation. A light microscope (Axiovert 40C, Zeiss) was used to confirm differentiation visualized by cell adherence to the petri dish surface and morphological changes. Phenotypical changes were verified with flow cytometry as described further below.58
2.7 |. VFF culture
Immortalized human VFFs donated by the Thibeault laboratory (University of Wisconsin-Madison)59 were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Wisent Bioproducts, QC, Canada) supplemented with 10% (vol/vol) FBS, 1% (vol/vol) penicillin streptomycin and 1% (vol/vol) nonessential amino acid (Sigma-Aldrich). VFF cultures were stored in a humidified incubator (37°C, 5% CO2). At approximately 90% confluency, media was removed and cells were washed with PBS before TrypLE (Gibco) was added for 5 min to facilitate cell-surface detachment. A light microscope was used to confirm cell detachment. Supplemented DMEM was added to stop dissociation. Cells were counted using trypan blue (Invitrogen) and a hemocytometer (Fisher Scientific, Cat #0267110) before being centrifuged at 900 rpm for 5 min. Media was removed and cells were resuspended in supplemented phenol red-free DMEM at a working volume of approximately 1 × 106 cells/ml. VFFs with a passage number of 6–10 were used throughout this study.
2.8 |. Transwell cell culture
Four testing groups were prepared for transwell culture (Table 1).
TABLE 1.
Testing groups applied for transwell culture
| Culture | Group | Label | Setup |
|---|---|---|---|
| Hydrogel co-culture of THP-1 Mφ and VFFs | Experimental | (Mφ + VFF + hydrogel) | Mφ seeded on basolateral transwell membrane, VFFs embedded in hydrogel in upper well |
| Hydrogel monoculture of THP-1 Mφ | Control | (Mφ + hydrogel) | Mφ seeded on basolateral transwell membrane, acellular hydrogel in upper well |
| Nonhydrogel co-culture of THP-1 Mφ and VFFs | Control | (Mφ + VFF) | Mφ seeded on basolateral transwell membrane, VFFs seeded on apical transwell membrane |
| Nonhydrogel monoculture of THP-1 Mφ | Control | (Mφ) | Mφ seeded on basolateral transwell membrane |
Abbreviations: Mφ, macrophages; VFFs, vocal fold fibroblasts.
To prepare PMA-induced Mφ for experiments, media and non-adherent cells were aspirated from the petri dish. Mφ were then washed with 5 ml 1 × PBS before 2 ml of TrypLE (Fisher Scientific, Ottawa, ON, Canada) was applied. The dish was placed in an incubator (37°C, 5% CO2) for 5 min to facilitate dissociation. Cells were checked under a light microscope for nonadherence before 5 ml of supplemented phenol-red free RMPI (Wisent Bioproducts, QC, Canada) was added to stop dissociation. Cells were transferred to a 50 ml polypropylene centrifuge tube and the dish surface was washed with an additional 5 ml of RPMI to collect remaining cells. Cells were stained with trypan blue and counted using a cell hemocytometer before being centrifuged at 900 rpm for 5 min. Media was aspirated and the cells resuspended in supplemented phenol red-free RPMI at a working volume of approximately 1.5 × 106 cells/ml.
Tissue culture treated 6-well transwell plates (polycarbonate membrane, 0.4 μm pore, Corning, Cat #3412) were used for all cell cultures (Figure 1). Transwell inserts enabled the physical separation of VFFs and Mφ while permitting cytokine transport between cell types, mimicking paracrine signaling.60 500 μl of Mφ were seeded on the basolateral underside of a tissue culture-treated transwell and placed in an incubator (37°C, 5% CO2) top-side down for 3 hr. After incubation, transwells plates were gently turned over (top-side up) and 1 ml of supplemented phenol-red free RPMI media was added to lower wells. In the upper well of (Mφ + VFF + hydrogel) cultures, 400 μl of glycol-chitosan hydrogel was mixed with 500 μl of glyoxal cross-linker (0.005, 0.01, or 0.02%) and 200 μl of HVFF cells. For (Mφ + hydrogel) cultures, 200 μl of DMEM media was applied instead of VFFs. Finally, (Mφ + VFF) and (Mφ) cultures contained no hydrogel in the upper well and instead applied only 200 μl of VFFs ([Mφ + VFF]) or DMEM media ([Mφ]) in the upper well. Transwell plates were stored in an incubator for 2 hr to permit hydrogel gelation. After 2 hr, plates 750 μl of supplemented DMEM was added to all transwell upper wells to prevent hydrogel dehydration and provide VFFs with additional nutrients. Plates were then incubated for 3, 24, or 72 hr.
FIGURE 1.
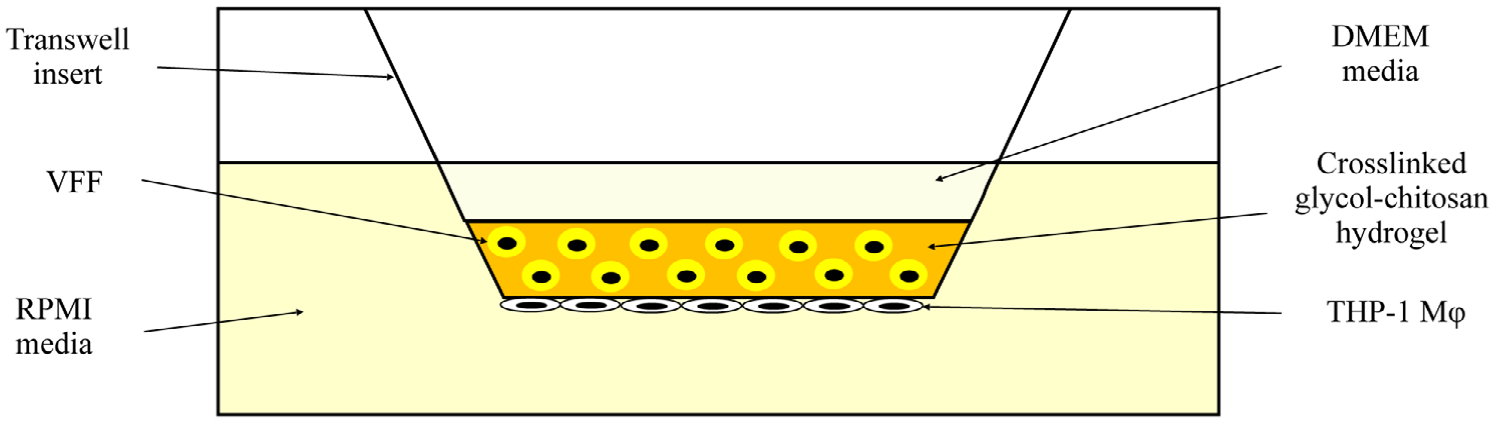
Transwell setup for the ([Mφ + VFF + hydrogel]) culture. Mφ were seeded on the basolateral transwell membrane and VFFs were embedded in the hydrogel. For (Mφ + hydrogel), VFFs were excluded. For (Mφ + VFF), the hydrogel was excluded. For (Mφ), VFFs, and the hydrogel were excluded. DMEM, Dulbecco’s modified Eagle’s medium; Mφ, macrophages; VFFs, vocal fold fibroblasts
Supernatant samples from the lower well, representative of the 3, 24, and 72 hr Mφ response, were collected and stored at −80°C prior to analysis. Immediately following supernatant collection, Mφ on the basolateral transwell membrane was stained for viability analysis. Separate culture plates were used for each time-point to ensure supernatants collected were cumulative over the defined time period for cytokine protein analyses. For Mφ phenotyping via flow cytometry, only 72 hr culture samples were examined. All experiments were performed in at least triplicate.
2.9 |. Secreted pro- and anti-inflammatory cytokine levels
Enzyme-linked immunosorbent assays (ELISAs) were used to measure the concentration of pro- and anti-inflammatory cytokines in supernatant samples. Human tumor necrosis factor alpha (TNF-α) (Cat. #KHC3011, Invitrogen, Vienna, Austria) and human interleukin 10 (IL-10) (Cat. #HS100C, R&D systems, MN) were assessed to determine the respective pro- and anti-inflammatory response. Standard curves were applied to measure the quantity of analyte present in each sample. After thawing samples on ice, vials were briefly centrifuged (1,200 rpm) to remove debris prior to loading into the respective ELISA plates. Total protein concentration was quantified using the Bradford Protein Assay (Cat. #5000002, Bio-Rad Laboratories, Canada) and used to normalize ELISA data. All assays were performed according to the manufacturer’s requirements.
2.10 |. Cell viability
The Mφ seeded on the basolateral underside of the transwell membrane were washed with 1 × PBS before being stained using a LIVE/DEAD viability/cytotoxicity assay (Invitrogen; Eugene, OR, Lot #1932445) in accordance with the manufacturer’s instructions. The plate was incubated for 30 min in darkness at room temperature before subsequently being washed twice more with PBS. The transwell insert was then placed into 35 mm glass bottom culture dishes (MatTek Corporation) containing 250 μl of fresh PBS. An inverted confocal fluorescence microscope (LSM710, Zeiss, Germany) was used to image the stained samples. Images were acquired using a 10× objective with channels of 488 nm (LIVE, green channel) and 514 nm (DEAD, red channel).
Image acquisition and analysis were performed using Zen System (Zeiss, Germany) and Imaris version 7.5.6 (Bitplane, South Windsor, CT) software. Mφ viability (%) was determined by dividing the number of live Mφ by the total Mφ count (live + dead) and multiplying the result by 100. A colocalization channel was applied to account for cells that fluoresced both green and red; such cells were counted as dead for viability calculations.
2.11 |. Cell phenotyping
Four-color flow cytometry was conducted to assess how the glycol-chitosan hydrogel influenced Mφ phenotype. To harvest Mφ for flow cytometry, RPMI media was aspirated from each lower well and 1.2 ml of PBS was added for washing. PBS was then aspirated and 1 ml of TrypLE was added before the plate was placed in an incubator (37°C, 5% CO2) for 5 min to facilitate dissociation. Following incubation the plate was tapped to promote further cell detachment before 1.2 ml of supplemented RPMI was added to each well to stop dissociation. Cells were then collected and transferred to a 50 ml tube containing 5 ml of supplemented RPMI. The basolateral membrane of the transwell was washed with a further 1 ml of RPMI to collect any remaining Mφ that were subsequently added to the 50 mL tube. Cells were centrifuged at 900 rpm before being resuspended in 10 ml of PBS and centrifuged again at 900 rpm for washing. Finally, cells were resuspended at a working volume of 1 × 10^6 cells/ml in sodium azide- and protein-free 1 × DPBS. 150 μl of cell solution was transferred to each labeled flow cytometry tube (BD Biosciences) and 5 μl of Human BD Fc Block (BD #564219) was added per tube followed by 10 min incubation at room temperature. A preprepared antibody cocktail was added to each cell aliquot suspension. The cocktail comprised 50 μl of Brilliant Stain Buffer (BSB) (BD #563794) and 5 μl of each of the four markers. The four-marker panel constructed for this experiment consisted of CD11b (#562721), CD33 (#564588), CD80 (#560925), and CD206 (#564063) (BD Life Sciences, San Jose, CA) (Table 1). CD11b and CD33 identified nonpolarized, basal Mφ58 and confirmed monocyte to Mφ differentiation. CD80 identified pro-inflammatory Mφ61. CD206 identified anti-inflammatory Mφ.61 (Mφ + VFF + hydrogel) cultures were compared to (Mφ + hydrogel) cultures, and (Mφ) cultures.
Samples were prepared as unstained controls (50 μl BSB only), single stained controls (50 μl BSB + 5 μl of one antibody), fluorescence minus one (FMO) controls (50 μl BSB + 5 μl of three of the four antibodies), or multi-stained samples (50 μl BSB + 5 μl of all antibodies). Following staining, cells were incubated on ice for 30 min (4°C), protected from light. Cells were washed by adding 2 ml of DPBS per tube and centrifuging cells at 300 g for 5 min followed by supernatant aspiration. Cells were resuspended in 400 μl of PBS for flow cytometry analysis utilizing the BD LSR Fortessa Cell Analyzer (without UV).
FlowJo Version 10 (TreeStar, Ashland, OR) was used for data analysis. Relevant compensation matrices were constructed for each sample and applied to the data. Singlets were gated out initially by comparing SSC-W versus SSC-A for each sample. Next, multistain samples were compared to relevant FMO controls via quadrant gates to separate populations of cells and identify an upregulation of a specific marker as denoted in Table 2. Single-stained and unstained controls were used for further confirmation of gate borders. In this study, CD33+/CD80+ (pro-inflammatory) and CD33+/CD206+ (anti-inflammatory) populations were of principal interest for evaluating Mφ polarization.
TABLE 2.
Basal, pro-inflammatory, and anti-inflammatory Mφ markers for flow cytometry
| Marker | ||||
|---|---|---|---|---|
| Cell phenotype | CD11b | CD33 | CD80 | CD206 |
| Basal Mφ | + | + | − | − |
| Pro-inflammatory Mφ | + | + | + | − |
| Anti-inflammatory Mφ | + | + | − | + |
Abbreviation: Mφ, macrophages.
2.12 |. Statistical analysis
A three-way ANOVA (with type III sums of squares) was used to compare the effect of three between-group factors, namely of hydrogel presence, culture type (VFF presence), and time (3, 24, 72 hr) upon cytokine production (TNF-α, IL-10) and Mφ viability respectively.
Separately, a further three-way ANOVA (with type III sums of squares) was used to compare the effect of three between-group factors, namely of cross-linker concentration (.005%, .01%, .02%), culture type (VFF presence), and time (3, 24 , 72 hr) upon cytokine production (TNF-α, IL-10) and Mφ viability respectively.
The normality of the distribution of cytokine production and viability were tested and a Box Cox transformation was applied on IL-10, TNF-α and viability variables in order to increase their normality for statistical testing.62 The residuals of the statistical models have been assessed ensuring that they were normally distributed and presented a homogenous variance. Post hoc comparisons using the Tukey HSD test were performed for further evaluations of significant differences. The criteria for significance were *p < .05, **p < .01, ***p < .001, and ****p < .0001. Analyses were carried out in R using the package “car” (Version 3.0–10).63
3 |. RESULTS
3.1 |. Mechanical characterization
Twenty indentation tests were performed on each of the three-hydrogel samples. Data points were averaged to produce the mean Young’s modulus and SD of each hydrogel (Table 3). Results demonstrated that Young’s modulus increased with glyoxal cross-linker concentration. As the intended clinical use for this hydrogel would be in the vocal folds, for context, the mean Young’s modulus of in vivo human vocal folds at rest has previously been reported as approximately 12.66 kPa (2.45–29.4 kPa range).45
TABLE 3.
The Young’s modulus of three glycol-chitosan hydrogels measured using atomic force microscopy indicated as the mean modulus across 20 measurements ± SD
| Glyoxal concentration (%) | Young’s modulus (kPa) |
|---|---|
| 0.005 | 1.11 ± 0.344 |
| 0.01 | 3.35 ± 0.790 |
| 0.02 | 7.86 ± 0.999 |
Shear plate rheometry was used to determine the storage modulus (G’) and loss modulus (G”) of the materials. All glycol-chitosan hydrogel samples tested demonstrated G’ values that remained larger than G” through the testing period (Figure 2). Results also indicated that the hydrogels tested had the capacity to elastically store energy and return, to an extent, to their initial configuration prior to mechanical force application. The stiffest hydrogel (0.02% glyoxal cross-linker) possessed the greatest elastic capabilities due to an increased level of cross-linking.
FIGURE 2.
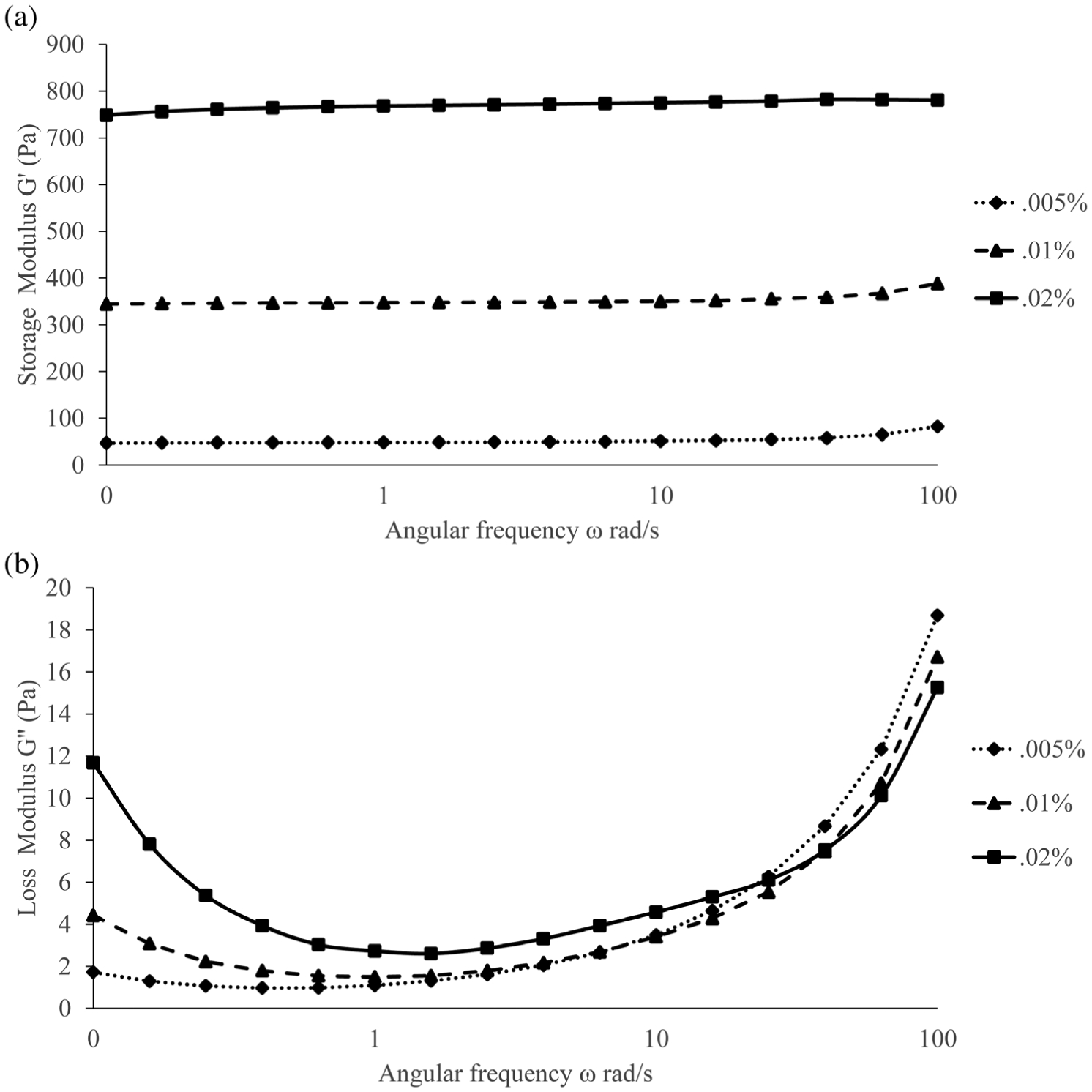
The (a) storage modulus and (b) loss modulus of the three glyoxal cross-linked glycol-chitosan hydrogels assessed using shear plate rheometry. Glyoxal cross-linker concentrations used were 0.005, 0.01, and 0.02%
3.2 |. TNF-α cytokine secretion
3.2.1 |. All cultures: ANOVA for time by hydrogel presence by culture type
From the TNF-α ELISA results, a significant interaction effect was found (Table 4) between time and culture type (F [2, 58] = 4.66, p < .05) (Figure 3a). Further analyses indicated significant main effects: (1) time (F [2, 58] = 6.94, p < .01) and (2) hydrogel presence (F [1, 58] = 40.70, p < .0001) (Figure 3b). All other fixed effects and interactions were nonsignificant.
TABLE 4.
Results of the three-way ANOVA assessing the effect of time, hydrogel presence, and culture type on TNF-α detected. Bold indicates significant results
| Source | Sum of squares | Df | Mean squares | F value | p |
|---|---|---|---|---|---|
| Time (T) | 48.9 | 2 | 24.5 | 6.94 | .002 |
| Hydrogel presence (HP) | 143.3 | 1 | 143.3 | 40.70 | .000 |
| Culture type (CT) | 11.8 | 1 | 11.8 | 3.34 | .073 |
| T × HP | 18.2 | 2 | 9.1 | 2.58 | .084 |
| T × CT | 32.8 | 2 | 16.4 | 4.66 | .013 |
| HP × CT | 7.8 | 1 | 7.8 | 2.21 | .142 |
| T × HP × CT | 3.3 | 2 | 1.7 | 0.46 | .631 |
| Residuals | 204.3 | 58 | 3.5 |
FIGURE 3.
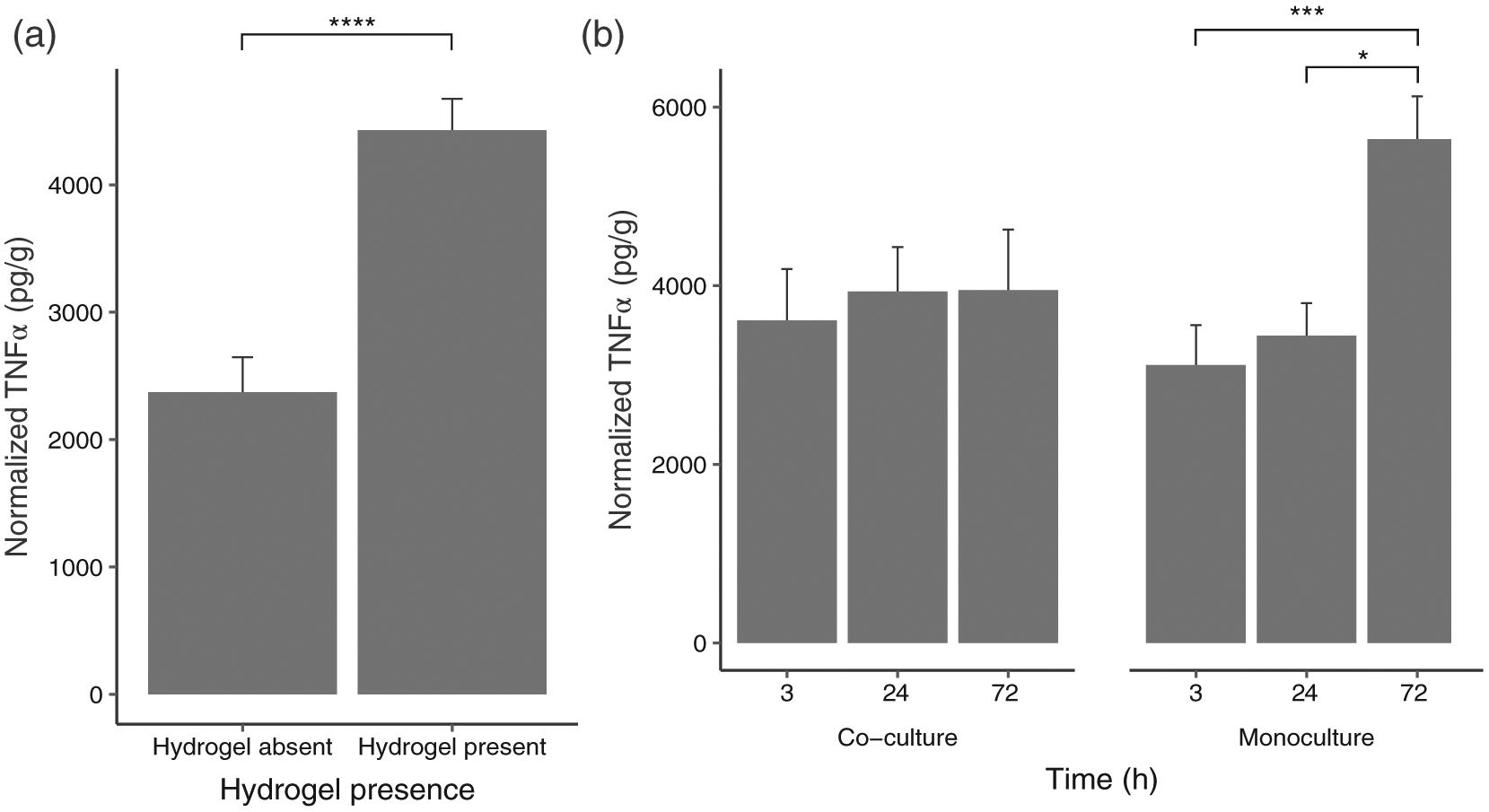
(a) Interaction between culture type and Time on TNF-α detected (co-culture: N = 36; monoculture N = 36); (b) Main effect of hydrogel presence on TNF-α concentrations (hydrogel absent control group: N = 18; hydrogel present groups: N = 54). Data are graphed as concentration normalized to total protein levels (pg/g protein). The bars and error bars represent the mean and standard error of the data respectively. *p < .05, ***p < .001, ****p < .0001
Results from Tukey’s HDS indicated that overall TNF-α levels were higher for hydrogel cultures than for nonhydrogel cultures (p < .0001). While there was no significant difference between different time points for co-culture, TNF-α levels at 72 hr were significantly different from those at 3 hr (p < .001) and 24 hr (p < .05) for monoculture.
3.2.2 |. Hydrogel cultures: ANOVA for time by culture type by cross-linker concentration
Given the significant effect of hydrogel presence, a further ANOVA was performed for hydrogel cultures with cross-linker concentration included as a variable. Significant interaction effects (Table 5) were found for time by culture types by cross-linker concentration (F [4, 35] = 4.98, p < .01) (Figure 4), (2) time by culture type (F [2, 35] = 5.68, p < .01), (3) time by cross-linker concentration interaction (F [4, 35] = 16.55, p < .0001). Further analyses indicated significant main effects of: (1) time (F [2, 35] = 11.83, p < .0001), (2) cross-linker concentration (F [2, 35] = 14.88, p < .0001). All other fixed effects and interactions were nonsignificant. The simple effects from Tukey’s HDS tests indicated that overall TNF-α levels increased with time (3 ≃ 24 < 72 hr, p < .001) and cross-linker concentration (0.005 < 0.01 < 0.02%, p < .05).
TABLE 5.
Results of the three-way ANOVA assessing the effect of time, culture type, and cross-linker concentration on TNF-α detected. Bold indicates significant results
| Source | Sum of squares | Df | Mean squares | F value | p |
|---|---|---|---|---|---|
| Time (T) | 30.3 | 2 | 15.2 | 11.83 | .000 |
| Culture type (CT) | 0.2 | 1 | 0.2 | 0.12 | .727 |
| Cross-linker concentration (CC) | 38.1 | 2 | 19.05 | 14.88 | .000 |
| T × CT | 14.5 | 2 | 7.25 | 5.68 | .007 |
| T × CC | 84.7 | 4 | 21.18 | 16.55 | .000 |
| CT × CC | 0.9 | 2 | 0.45 | 0.36 | .070 |
| T × CT × CC | 25.5 | 4 | 6.4 | 4.98 | .003 |
| Residuals | 44.8 | 35 | 1.3 |
FIGURE 4.
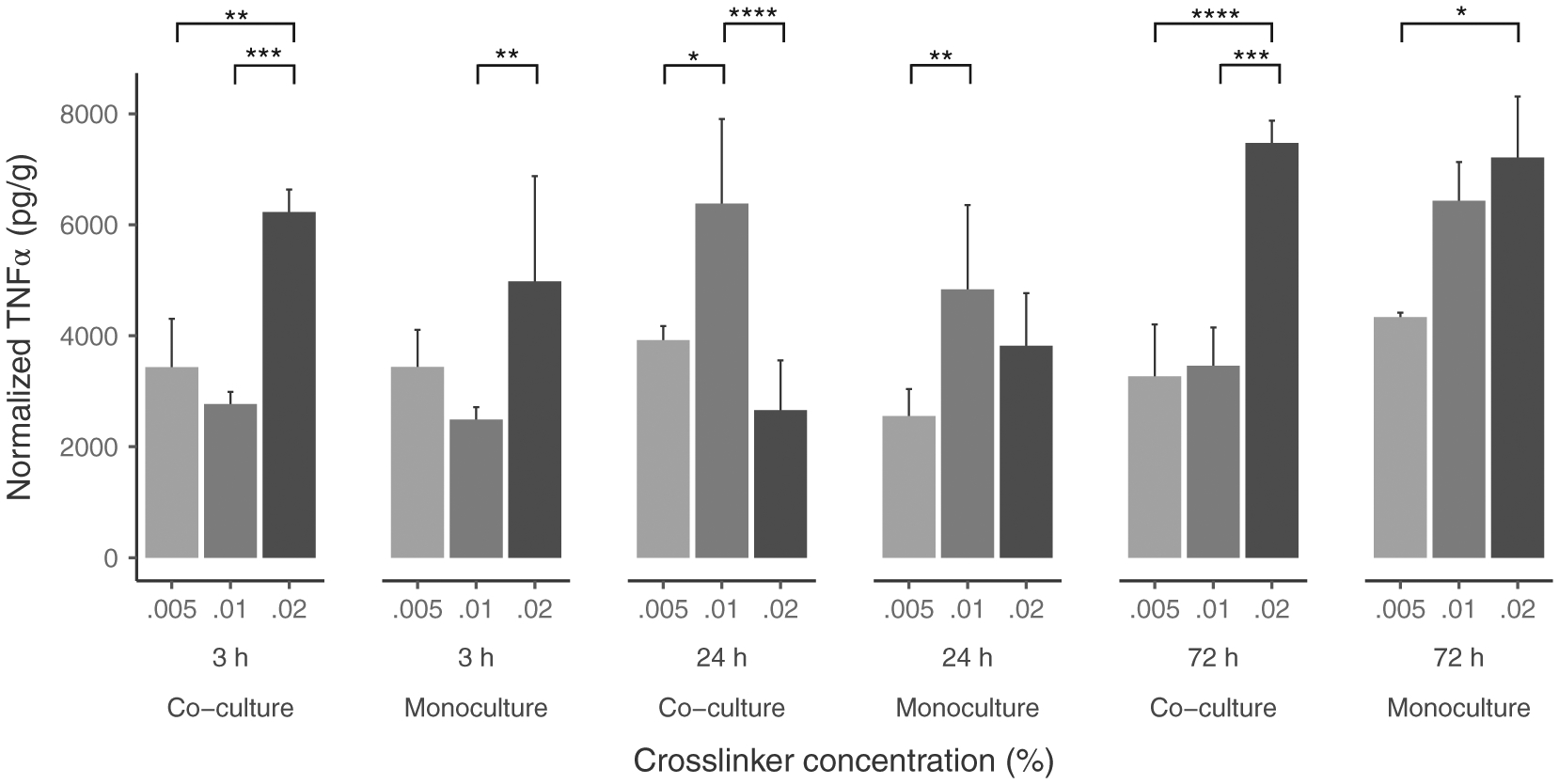
Three-way interaction between cross-linker concentration, Time and culture type on TNF-α detected in hydrogel cultures. Data is graphed as concentration normalized to total protein levels (pg/g protein). The bars and error bars represent the mean and standard error of the data respectively (N = 9 per co/monoculture group). *p < .05, **p < .01, ***p < .001, ****p < .0001
Further Tukey’s HDS tests indicated that the cross-linker concentration effect depended on both time and culture type (Table 6).
TABLE 6.
Post hoc comparisons summary for secreted TNF-α in hydrogel cultures. Bold indicates significant results
| Cross-linker concentration | ||||
|---|---|---|---|---|
| Time | Culture type | 0.005–0.01% | 0.01–0.02% | 0.005–0.02% |
| 3 hr | (Mφ + VFF + hydrogel) | 1.00 (n.s.) | −4.30 (p < .001) | −3.30 (p < .01) |
| 3 hr | (Mφ + hydrogel) | 1.57 (n.s.) | −3.40 (p < .01) | −1.83 (n.s.) |
| 24 hr | (Mφ + VFF + hydrogel) | −2.61 (p < .05) | 4.69 (p < .0001) | 2.08 (n.s.) |
| 24 hr | (Mφ + hydrogel) | −3.25 (p < .01) | 1.24 (n.s.) | 2.01 (n.s.) |
| 72 hr | (Mφ + VFF + hydrogel) | −0.31 (n.s.) | −4.37 (p < .001) | −4.68 (p < .0001) |
| 72 hr | (Mφ + hydrogel) | −2.16 (n.s.) | −0.67 (n.s.) | −2.83 (p < .05) |
Abbreviations: Mφ, macrophages; VFFs, vocal fold fibroblasts.
At 3 hr, the general trend was similar for monoculture and co-culture: no significant difference between 0.005% and 0.01% concentrations and a significant difference between 0.01 and 0.02% concentrations for monoculture (p < .01) and co-culture (p < .001). TNF-α levels for 0.02% concentration were significantly higher than 0.005% concentration only for co-culture (p < .01).
At 24 hr, TNF-α levels were significantly higher for 0.01% than for 0.005% concentrations both for monoculture (p < .01) and co-culture (p < .05). However, while TNF-α levels were significantly lower for 0.02% concentrations than at 0.01% concentration for co-culture (p < .0001), there was no significant difference for monoculture between 0.01 and 0.02% concentrations.
At 72 hr, no significant difference was found between 0.005 and 0.01% concentration levels for either culture type. However, for 0.02% concentration, TNF-α levels in co-culture were significantly higher than for 0.01% concentration (p < .001). Levels for 0.02% were higher than0.005% both for co-culture (p < .0001) and monoculture (p < .05).
3.3 |. Il-10
3.3.1 |. All cultures: ANOVA for time by hydrogel presence by culture type
From the IL-10 ELISA results, significant interaction effects (Table 7) were found for: (1) time by hydrogel presence interaction (F [2, 58] = 11.45, p < .0001) (Figure 5(a)), and (2) time by culture type interaction (F [2, 58] = 3.30, p < .05) (Figure 5(b)). All main effects were found to be significant, namely: (1) time (F [2, 58] = 14.85, p < .0001), (2) hydrogel presence (F [1, 58] = 15.27, p < .0001), and (3) culture type (F [1, 58] = 7.16, p < .01). All other fixed effects and interactions were non-significant.
TABLE 7.
Results of the three-way ANOVA assessing the effect of time, hydrogel presence, and culture type on IL-10 detected. Bold indicates significant results
| Source | Sum of squares | Df | Mean squares | F value | p |
|---|---|---|---|---|---|
| Time (T) | 3,024.7 | 2 | 1,512.35 | 14.85 | .000 |
| Hydrogel presence (HP) | 1,555.3 | 1 | 1,555.3 | 15.27 | .000 |
| Culture type (CT) | 729.3 | 1 | 729.3 | 7.16 | .009 |
| T × HP | 2,333.0 | 2 | 1,166.5 | 11.45 | .000 |
| T × CT | 673.2 | 2 | 336.6 | 3.30 | .044 |
| HP × CT | 299.6 | 1 | 299.6 | 2.94 | .092 |
| T × HP × CT | 633.5 | 2 | 316.75 | 3.11 | .052 |
| Residuals | 5,907.8 | 58 | 101.86 |
FIGURE 5.
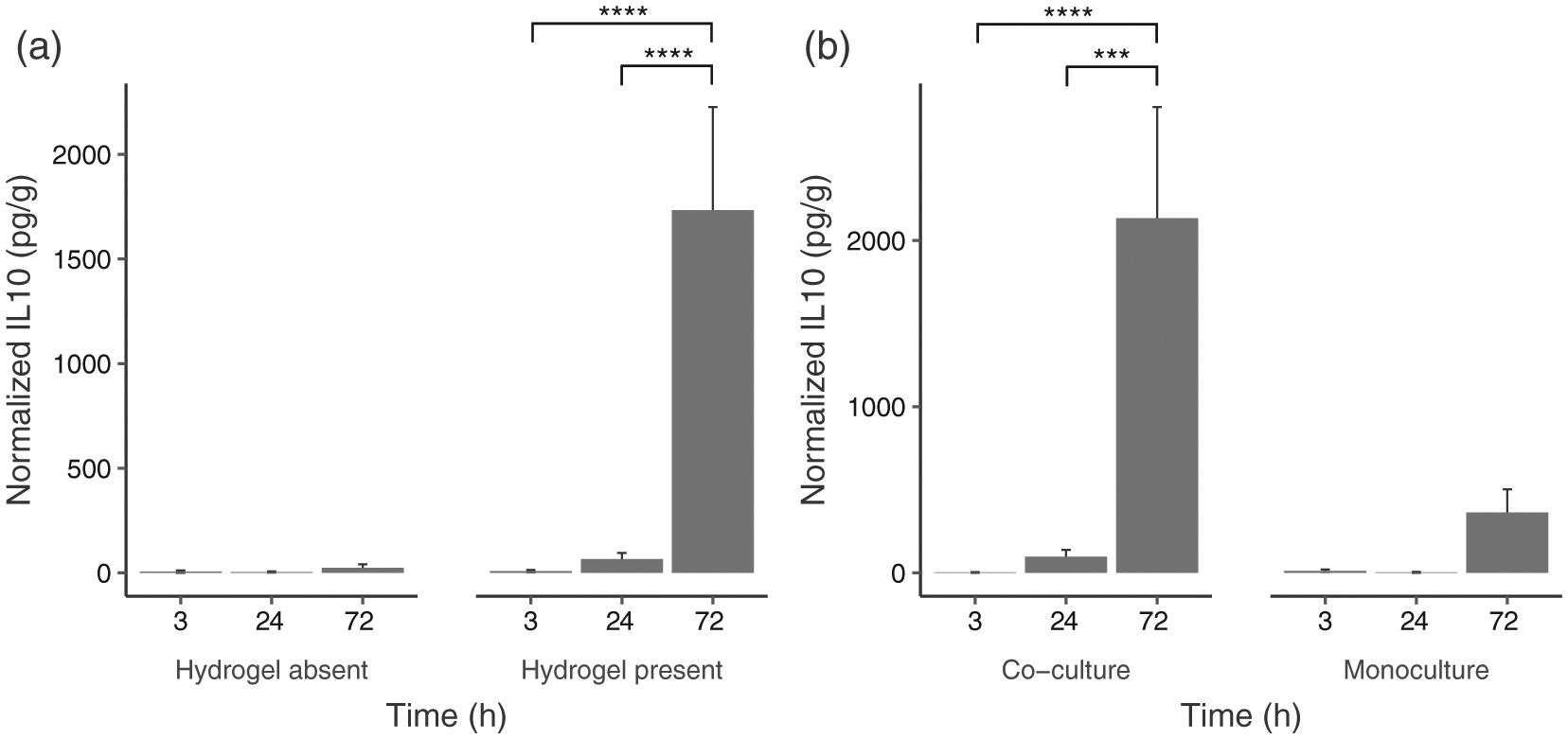
(a) Interaction between hydrogel presence and time on IL-10 detected (Absent: N = 18; Present: N = 54); (b) Interaction between culture type and time on IL-10 detected (co-culture: N = 36; monoculture = 36). Data are graphed as concentration normalized to total protein levels (pg/g protein). The bars and error bars represent the mean and standard error of the data respectively. ***p < .001, ****p < .0001
Results from Tukey’s HDS indicated that overall IL-10 levels were not significantly different across time for nonhydrogel cultures. For hydrogel culture, IL-10 levels at 72 hr were significantly different than levels observed at 3 hr (p < .0001) and 24 hr (p < .0001). While there was no significant difference between different time points for monoculture, IL-10 levels at 72 hr were significantly different from those at 3 hr (p < .0001) and 24 hr (p < .001) for co-culture.
3.3.2 |. Hydrogel cultures: ANOVA for time by culture type by cross-linker concentration
Given the significant effect of hydrogel presence, a further ANOVA was performed for hydrogel cultures with cross-linker concentration included as a variable. Significant interaction effects (Table 8) were found: (1) time by culture type by cross-linker concentration (F [4, 35] = 3.60, p < .05) (Figure 6), (2) time by culture type (F [2, 35] = 21.99, p < .0001), (3) time by cross-linker concentration (F [4, 35] = 5.72, p < .01). Further analyses revealed main effects: (1) time (F [2, 35] = 80.65, p < .0001), (2) culture type (F [1, 35] = 33.45, p < .0001), (3) cross-linker concentration (F [2, 35] = 6.68, p < .01). All other fixed effects and interactions were nonsignificant.
TABLE 8.
Results of the three-way ANOVA assessing the effect of time, culture type, and cross-linker concentration on IL-10 detected. Bold indicates significant results
| Source | Sum of squares | Df | Mean squares | F value | p |
|---|---|---|---|---|---|
| Time (T) | 10,314.0 | 2 | 5,157.0 | 80.65 | .000 |
| Culture type (CT) | 2,138.0 | 1 | 2,139.0 | 33.45 | .000 |
| Cross-linker concentration (CC) | 855.0 | 2 | 427.0 | 6.68 | .003 |
| T × CT | 2,813.0 | 2 | 1,406.0 | 21.99 | .000 |
| T × CC | 1,464.0 | 4 | 366.0 | 5.72 | .001 |
| CT × CC | 180.0 | 1 | 180.0 | 1.40 | .259 |
| T × CT × CC | 920.0 | 4 | 230.0 | 3.60 | .015 |
| Residuals | 2,238.0 | 35 | 64.0 |
FIGURE 6.
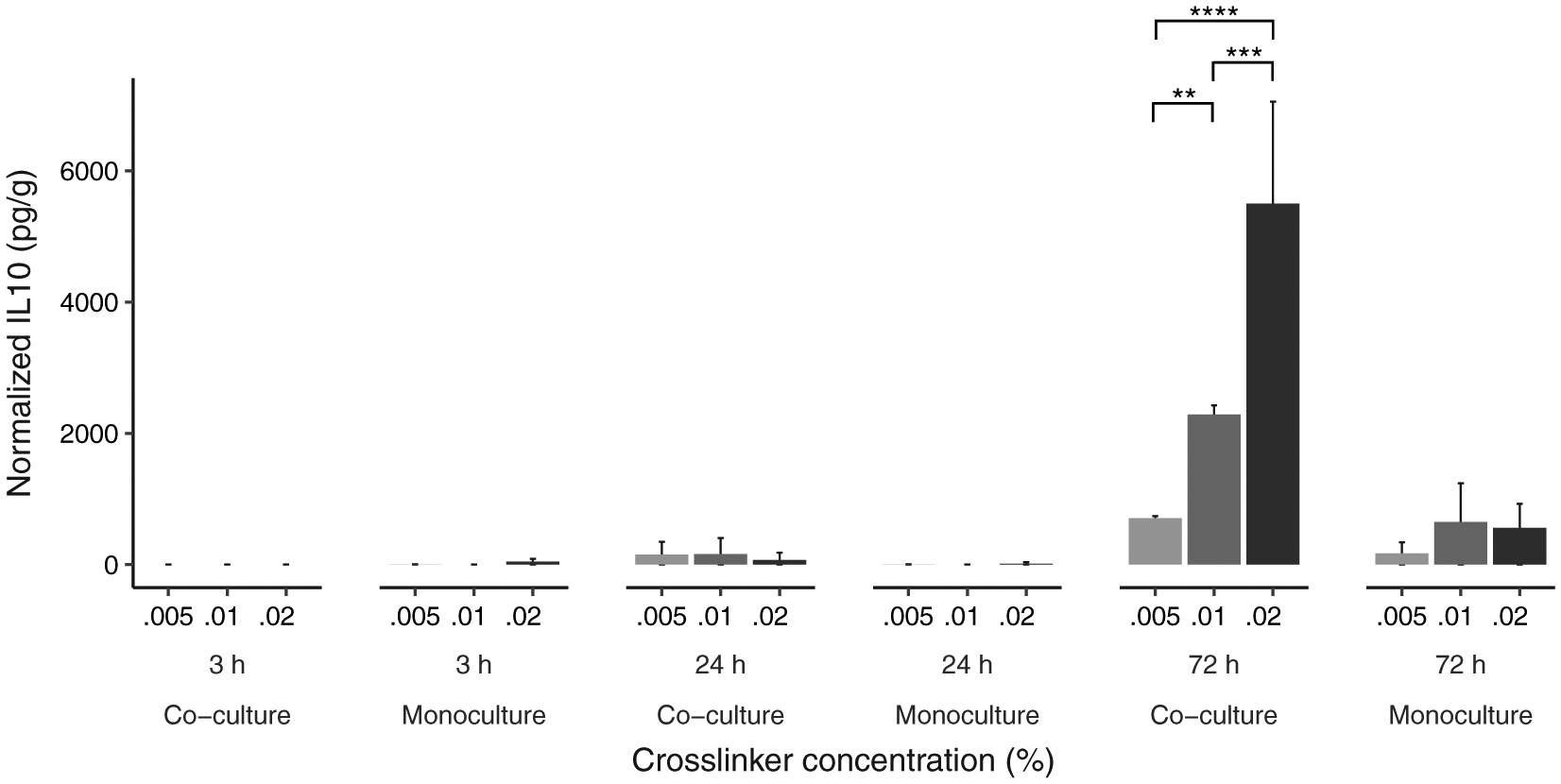
Three-way interaction between cross-linker concentration, time and culture type on IL-10 detected in hydrogel cultures. Data is graphed as concentration normalized to total protein levels (pg/g protein). The bars and error bars represent the mean and standard error of the data respectively (N = 9 per co/monoculture group). **p < .01, ***p < .001, ****p < .0001
Simple effects from Tukey’s HDS tests indicated that IL-10 levels increased with time (3 ≃ 24 hr and 24 < 72 hr, p < .001), cross-linker concentration (0.005% < 0.02%, p < .01), and were overall higher for co-culture ([Mφ + VFF + hydrogel] > [Mφ + hydrogel], p < .01). These simple effects were qualified by the interaction between culture type, time, and cross-linker concentration.
Finally, Tukey’s HDS tests revealed no significant difference between different levels of cross-linker concentration for both culture types at 3 hr and at 24 hr, nor at 72 hr for monocultures (Table 9). A significant difference between 0.005% and 0.01% cross-linker concentration (p < .01) and between 0.01% and 0.02% cross-linker concentration (p < .01) was found only at 72 hr for co-culture.
TABLE 9.
Post hoc comparisons summary for secreted IL-10 in hydrogel cultures. Bold indicates significant results
| Cross-linker concentration | ||||
|---|---|---|---|---|
| Time | Culture type | 0.005–0.01% | 0.01–0.02% | 0.005–0.02% |
| 3 hr | (Mφ + VFF + hydrogel) | 0.00 (n.s.) | 0.00 (n.s.) | 0.00 (n.s.) |
| 3 hr | (Mφ + hydrogel) | 0.71 (n.s.) | −5.36 (n.s.) | −4.65 (n.s.) |
| 24 hr | (Mφ + VFF + hydrogel) | 0.05 (n.s.) | 3.52 (n.s.) | 3.57 (n.s.) |
| 24 hr | (Mφ + hydrogel) | 0.65 (n.s.) | −2.20 (n.s.) | −1.54 (n.s.) |
| 72 hr | (Mφ + VFF + hydrogel) | −21.21 (p < .01) | −25.79 (p < .001) | −46.97 (p < .0001) |
| 72 hr | (Mφ + hydrogel) | −11.37 (n.s.) | 1.29 (n.s.) | −10.09 (n.s.) |
Abbreviations: Mφ, macrophages; VFFs, vocal fold fibroblasts.
3.4 |. Macrophage viability
Confocal images of Mφ stained with the viability assay are shown in Figure 7. Live/dead cell counts were statistically analyzed to determine differences across all cultures. As one data point ([Mφ + VFF + hydrogel], 0.005% cross-linker, 3 hr) was more than three standard deviations away from the mean it was considered an outlier and removed from the group analysis.
FIGURE 7.
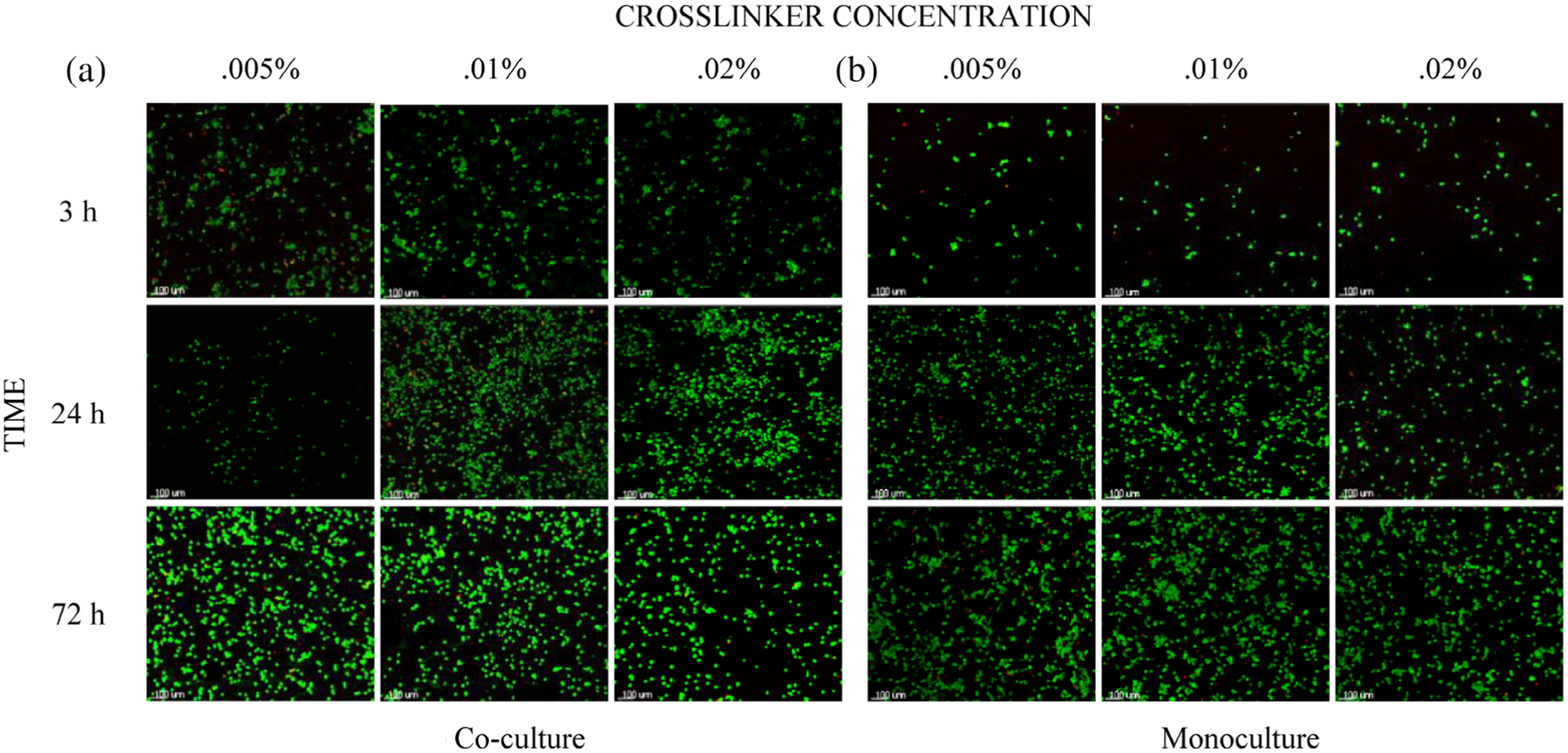
Live/dead confocal images of Mφ on the underside of the basal membrane following culture with glyoxal cross-linked glycol-chitosan hydrogels. Panel A displays Mφ from (Mφ + VFF + hydrogel). Panel B displays Mφ from (Mφ + hydrogel). Live cells fluoresce green, dead cells fluoresce red. Cell density and distribution varied to an extent across the membrane surface and between samples. All images were taken at ×100 original magnification (scale bar = 100 μm). Mφ, macrophages; VFFs, vocal fold fibroblasts
3.4.1 |. All cultures: ANOVA for time by hydrogel presence by culture type
A significant interaction effect (Table 10) was found between hydrogel presence and culture type (F [1, 60] = 5.06, p < .05) (Figure 8). A main effect of hydrogel presence (F [1, 60] = 13.20, p < .0001) was indicated. All other fixed effects and interactions were nonsignificant. Results from Tukey’s HDS indicated that non-hydrogel cultures had higher viability than hydrogel cultures (p < .001). While there was no significant effect of culture type for nonhydrogel cultures, viability of co-culture was significantly higher than for monoculture for hydrogel cultures (p < .01).
TABLE 10.
Results of the three-way ANOVA assessing the effect of time, hydrogel presence, and culture type on Mφ viability. Bold indicates significant results
| Source | Sum of squares | Df | Mean squares | F value | p |
|---|---|---|---|---|---|
| Time (T) | 84.0 | 2 | 42.0 | 0.53 | .590 |
| Hydrogel presence (HP) | 1,046.0 | 1 | 1,046.0 | 13.20 | .000 |
| Culture type (CT) | 9.0 | 1 | 9.0 | 0.11 | .741 |
| T × HP | 284.0 | 2 | 142.0 | 1.80 | .175 |
| T × CT | 400.0 | 2 | 200.0 | 2.52 | .089 |
| HP × CT | 401.0 | 1 | 401.0 | 5.06 | .020 |
| T × HP × CT | 9.0 | 2 | 4.5 | 0.06 | .944 |
| Residuals | 4,752.0 | 60 | 79.2 |
FIGURE 8.
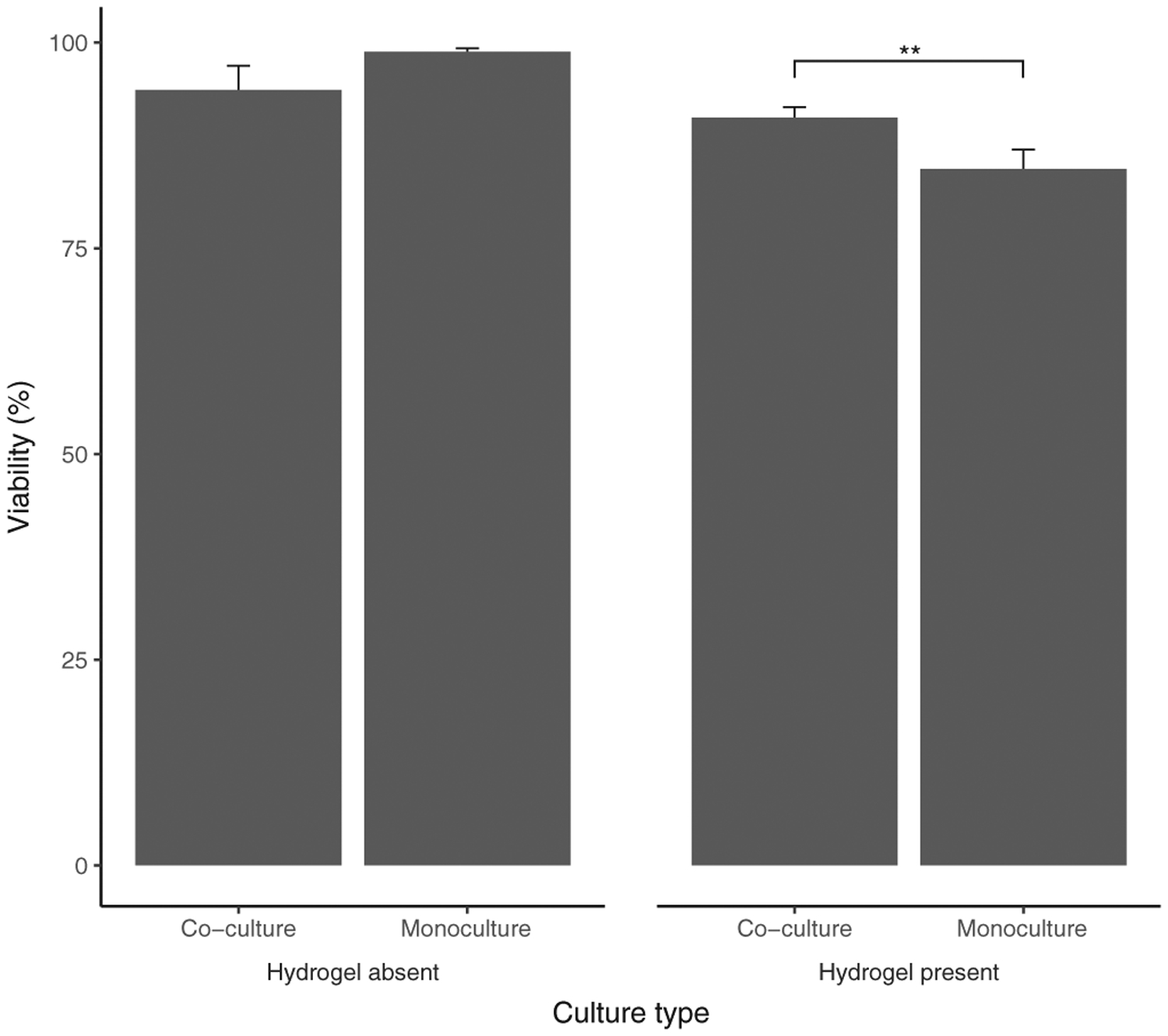
Interaction between culture type and hydrogel presence for Mφ viability (%). The bars and error bars represent the mean Mφ viability and standard error of the data respectively (Absent: N = 18; Present: N = 54). **p < .01. Mφ, macrophages
A subsequent ANOVA for hydrogel cultures revealed no significant main effect or interaction (Supplementary Table 3). This result indicated that cross-linker concentration did not significantly influence viability.
3.5 |. Macrophage phenotyping
Flow cytometry was used to identify Mφ subpopulations in this study. The total percentage (%) of cells that displayed CD33+/CD80+ (pro-inflammatory) and CD33+/CD206+ (anti-inflammatory) expression are indicated in Table 11. The quadrant method applied to obtain populations is described in Figure 9. Overall, the population of CD33 +/206+ cells appeared more dominant than CD33+/80+ across all samples. The mean CD33+/206+ population for (Mφ + VFF + hydrogel) was consistently higher than (Mφ + hydrogel) across all hydrogel stiffness variations tested. The CD33+/80+ population remained relatively consistent across all hydrogel cultures regardless of hydrogel stiffness or VFF presence.
TABLE 11.
Mean % ± SD of CD33 +/CD80+ and CD33+/CD206+ cells in each culture group. Minimum number of events recorded per sample was 10,000
| Culture | Cross-linker concentration | CD33+/CD80+ population (%) | CD33+/CD206+ population (%) |
|---|---|---|---|
| (Mφ + VFF + hydrogel) | 0.005% | 6.2 ± 1.1 | 90.2 ± 0.1 |
| 0.01% | 8.2 ± 6.1 | 87.0 ± 4.2 | |
| 0.02% | 6.8 ± 1.5 | 81.7 ± 4.4 | |
| (Mφ + hydrogel) | 0.005% | 7.0 ± 0.7 | 68.0 ± 1.6 |
| 0.01% | 8.9 ± 2.9 | 61.0 ± 1.6 | |
| 0.02% | 10.1 ± 6.7 | 77.9 ± 5.7 | |
| (Mφ) | N/A | 4.7 ± 1.3 | 77.7 ± 13.8 |
Abbreviations: Mφ, macrophages; VFFs, vocal fold fibroblasts.
FIGURE 9.
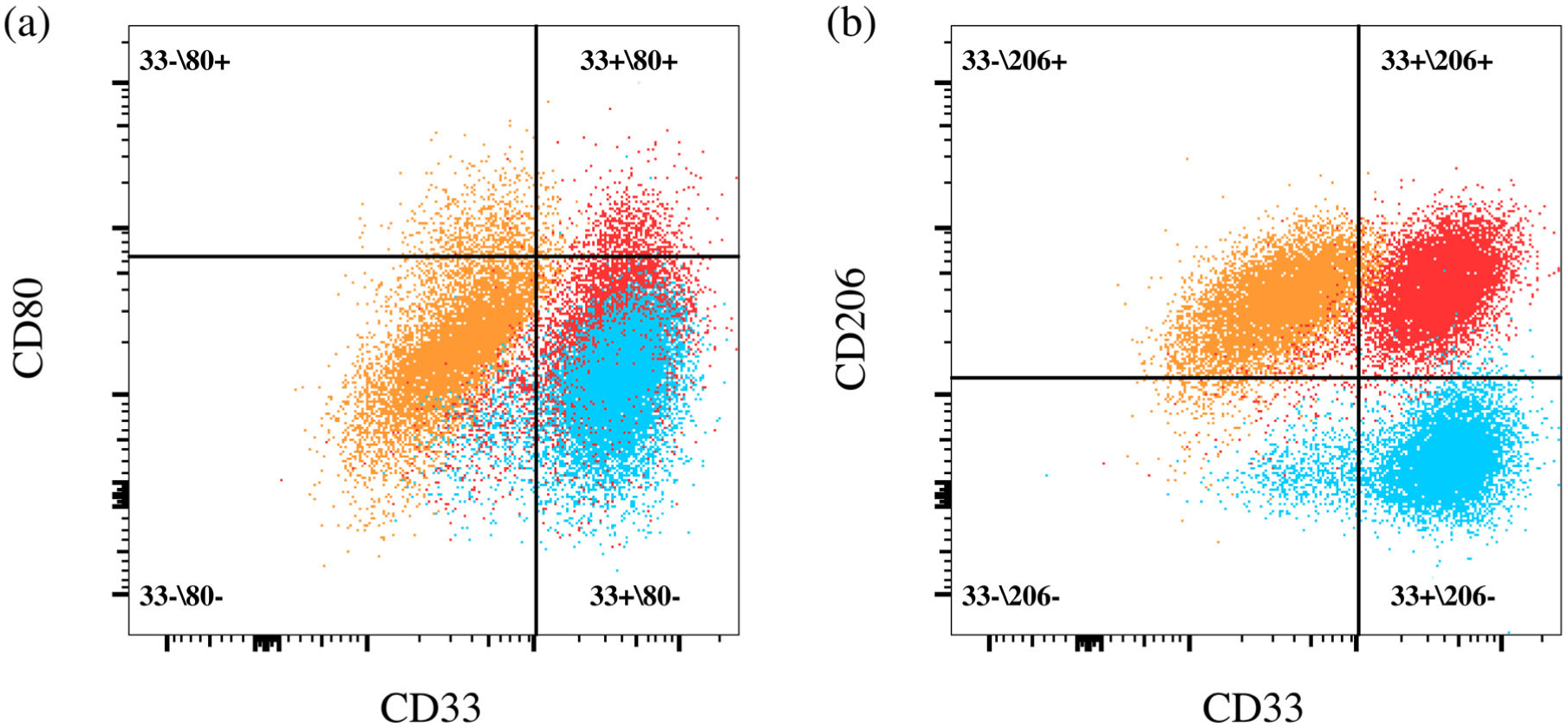
Representative examples of the quadrant gates drawn to determine upregulations of (a) CD80 or (b) CD206 in comparison to relevant FMO controls. The example shown is for a hydrogel co-culture (0.005% glyoxal) sample. Quadrant names refer to whether that given population expressed none, one, or both markers. Numbers refer to the percentage of cells from the multistained samples only that were found in a given quadrant population. Contour plots (5% levels) in FlowJo were examined prior to gate drawing to assist with defining upregulated populations. Color key: Red = Multistain (A, B); Blue = FMO (80) (A) FMO (206) (B); Orange = FMO (33) (A, B). FMO, fluorescence minus one
4 |. DISCUSSION
The use of hydrogels to treat vocal fold scarring has the potential to improve patient therapeutic outcomes compared to current clinical practices.1,64 When developing vocal fold hydrogels, it is necessary to establish the material composition that minimizes undesirable inflammation, maintains optimum mechanical integrity, and promotes beneficial immunomodulatory effects.48,55 Hydrogel stiffness is a tunable material property that can be harnessed to modulate the immune response.4,46,65,66 Hydrogel stiffness can be modified via the concentration of cross-linking agents applied.14,53 However, chemical cross-linkers, such as glyoxal, are cytotoxic and thus defining an optimum concentration that provides mutual mechanical and immunomodulatory benefits is critical for regenerative biomaterial design.67
In the current study, the concentration of glyoxal cross-linker applied to a glycol-chitosan hydrogel was investigated for its effect upon the inflammatory activity of Mφ. As increased hydrogel stiffness was acquired via increased glyoxal concentration, it was hypothesized that stiffer hydrogels would induce increased pro-inflammatory Mφ activity.14 Further, the presence of VFFs was hypothesized to promote anti-inflammatory Mφ activity via crosstalk between the cell types.38 Results in this study indicated that hydrogel presence, hydrogel stiffness, and VFF presence all induced significant changes in Mφ inflammatory activity.
First, the ELISA results showed that hydrogel presence in cell cultures produced a significantly increased cytokine concentration of pro-inflammatory TNF-α (Figure 3) and anti-inflammatory IL-10 (Figure 4). The effect of hydrogel presence was anticipated because Mφ, as early responding immune cells, respond to foreign materials as part of the nonspecific inflammatory response that represents a major component of the innate immune response.68 The chemical and physical properties of biomaterials strongly influence immune cell recruitment.69–71 In the present study, the hydrogel components likely had a direct impact on the inflammatory activity that occurred. For example, the glyoxal cross-linker may have induced elevated pro-inflammatory activity given that it is a known cytotoxic material and has previously been associated with decreased VFF viability when used in high concentrations.14,67 Cell death triggers an inflammatory response in the local environment that may have caused increased Mφ pro-inflammatory activity via the secretion of danger signals from dead cells that can stimulate inflammation.72 In fact, Mφ and VFFs have been identified as a major cell source of high-mobility group protein 1 (HMGB1), a typical danger signal cytokine, within the vocal fold mucosa.28,36 An alternative source of increased pro-inflammatory activity could be the hydrogel’s chitosan polymer backbone. As a natural polymer, chitosan can include impurities within its polymer network.48 Depending upon the nature of these impurities, they could significantly increase the immunogenicity of the hydrogel, thus increasing associated inflammatory activity.73
Mφ viability was found to decrease with hydrogel presence (Figure 8). A possible explanation is that apoptosis typically occurs following inflammation resolution.74 Hydrogel presence was responsible for inducing Mφ activation and inflammation. However, ultimately inflammation was resolved as suggested by the increase in IL-10 levels and large anti-inflammatory Mφ population detected via flow cytometry. Hence, greater Mφ apoptosis likely occurred following this resolution, leading to the significant difference found for Mφ viability between hydrogel presence and absence.
Second, hydrogel stiffness was associated with alterations in inflammatory activity in this study. Hydrogel stiffness has been demonstrated to influence cellular activity and can potentially be harnessed to direct the inflammatory response toward resolution.6,50 The stiffness of the material cells that contact is important in facilitating their adhesion, contractility, and migration.75 For the glycol-chitosan hydrogel, the stiffness ranged from 1.11 to 7.86 kPa (Table 3). Increased stiffness may lead to significantly increased levels of the pro-inflammatory cytokine, TNF-α (Figure 4). Interestingly, increased stiffness was also associated with significantly higher concentrations of the anti-inflammatory cytokine IL-10. Elevated IL-10 levels can promote healthy, nonfibrotic VFFs and thus modulate Mφ toward inflammatory resolution.19,38 The inherent plasticity of Mφ enables them to switch between pro- and anti-inflammatory phenotypes when polarized by external stimuli.76 Hence local Mφ populations can include coexistent pro- or anti-inflammatory Mφ subpopulations. Flow cytometry results, collected at the 72 hr time-point, indicated the presence of such coexistent Mφ subpopulations. Notably, the presence of both pro- and anti-inflammatory Mφ for the stiffest hydrogel could explain its association with peak levels for both the pro- and anti-inflammatory cytokines assessed.
Stiffer hydrogels have also been associated with modulating fibroblast activities including extended focal adhesions, spreading, and a well-defined cytoskeleton.8,9,52 In contrast, soft matrices were shown to induce cells to display increased TNF receptor clustering associated with NF-κB pathway activation.77 The NF-κB pathway is related to the production of excessive pro-inflammatory cytokines, such as TNF-α that induces fibroblasts to overproduce ECM proteins associated with fibrosis.78,79 Fibroblasts may have the capacity to tune their internal stiffness to that of the surrounding substrate as a potential mechanism for directing localized inflammatory activities.80 Such fibroblast activity may be another possible cause of the increased cytokine concentrations detected for the stiffest hydrogels in this study.
Third, the presence of VFFs in the model was associated with increased anti-inflammatory activity compared to controls. Throughout the inflammatory response to a foreign material, such as the hydrogel presented, Mφ and fibroblasts communicate constantly via autocrine and paracrine signaling.60 The release of pro-inflammatory factors from Mφ, including TNF-α, influences the growth, migration, and activity of fibroblasts and ultimately reinforces the local inflammatory response.35 TNF-α also has involvement in the stimulation of MMP secretion from Mφ and VFFs in vocal fold tissue.64,81,82 Results from the ELISA assessment of TNF-α indicated that the presence of VFFs was associated with lower TNF-α levels compared to monoculture controls as time increased (Figure 4). This finding agreed with literature elsewhere that VFF may suppress Mφ pro-inflammatory cytokine production and regulate inflammatory activity via toll-like receptor 4 (TLR4).38,83
IL-10 typically peaks after the initial pro-inflammatory response and is associated with inflammatory resolution.5 In this study, IL-10 production was delayed compared to pro-inflammatory TNF-α for all cultures assessed, with minimal IL-10 detected prior 72 hr. However, at 72 hr a significant increase in IL-10 was detected for (Mφ + VFF + hydrogel) compared to (Mφ + hydrogel) (Figure 6), potentially driven by the VFFs modulating Mφ inflammatory activity.38 Flow cytometry findings also suggested that VFF presence produced a greater proportion of anti-inflammatory (CD33+/CD206+) Mφ compared to respective monoculture controls.
4.1 |. Limitations and future directions
Mφ and fibroblasts, as two principal effector cell types of the initial inflammatory response to biomaterials, were included in this study. The incorporation of other immune cells, such as neutrophils or T-cells, should be considered for further hydrogel evaluation. As an alternative to primary cells, Mφ and VFF cell lines were used in this study to ensure a sufficient supply of cells for the multiple time-points and measurements included in the evaluation. However, despite the advantages of immortalized cell lines, such as ease of manipulation and expansion, significant drawbacks are attached to their use. For example, THP-1 Mφ have an inability to mimic the full spectrum of human Mφ phenotypes and are restricted to simplified models investigating polarization and functional implications.61,84 Future work with the glycol-chitosan hydrogel should attempt to replicate inflammatory trends observed in this study while using primary cells or small-animal in vivo models.
In this study, a transwell system was applied to study the paracrine signaling in co-cultures of Mφ and VFFs in response to the glycol-chitosan hydrogel. Mφ were seeded on the basolateral transwell membrane while VFFs were cultured within the hydrogel of the upper well. However, the membrane of the transwell insert presented notable technical challenges in studying the interaction of Mφ with the hydrogel surface and characterizing VFFs with confocal microscopy. Future in vitro investigations with more advanced 3D culture models, such as organoids, may help circumvent aforesaid issues related to the transwell co-culture system.
Last, cross-linker concentration not only varies hydrogel stiffness but also other material properties such as pore size and degradation profile. As these materials properties are often dependent, decoupling them is experimentally challenging. Work is ongoing to develop a computational model of biomaterials to decipher how individual and combined material properties influence cell activities and long-term tissue growth.
5 |. CONCLUSION
Increased hydrogel stiffness modulated Mφ behavior toward inflammatory resolution as shown by the heightened levels of IL-10. The presence of VFFs within hydrogel cultures further suppressed pro-inflammatory activity as shown by significant changes in cytokine concentrations and the dominant anti-inflammatory macrophage population detected. An in vitro evaluation of material-related inflammation that considers hydrogel physical properties and crosstalk between Mφ and VFFs is recommended for use during the determination of optimum vocal fold hydrogel characteristics from the immunological perspective.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported the National Sciences and Engineering Research Council of Canada (RGPIN-2018-03843), Canada Research Chair research stipend (950-231265) and the National Institutes of Health (R01 DC-005788). Microscopy images and image processing/analysis for this manuscript were performed in the McGill University Life Sciences Complex Advanced BioImaging Facility (ABIF). The flow cytometry work was performed in the Flow Cytometry Core Facility of the Life Science Complex supported by funding from the Canadian Foundation for Innovation. We would also like to acknowledge the donation of HVFF by Prof. Susan Thibeault’s laboratory (University of Wisconsin-Madison) and the access to the AFM and the rheometer facilities in Prof. Luc Mongeau’s laboratory (McGill University).
Footnotes
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in a collection in figshare at https://doi.org/10.6084/m9.figshare.c.5178068.v1
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Li L, Stiadle JM, Lau HK, et al. Tissue engineering-based therapeutic strategies for vocal fold repair and regeneration. Biomaterials. 2016; 108:91–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burdick JA. Injectable gels for tissue/organ repair. Biomed Mater. 2012;7:20201. [DOI] [PubMed] [Google Scholar]
- 3.Burdick JA, Prestwich GD. Hyaluronic acid hydrogels for biomedical applications. Adv Mater. 2011;23:41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caliari SR, Burdick JA. A practical guide to hydrogels for cell culture. Nat Methods. 2016;13:405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vishwakarma A, Bhise NS, Evangelista MB, et al. Engineering immunomodulatory biomaterials to tune the inflammatory response. Trends Biotechnol. 2016;34:470–482. [DOI] [PubMed] [Google Scholar]
- 6.Tsou YH, Khoneisser J, Huang PC, Xu X. Hydrogel as a bioactive material to regulate stem cell fate. Bioact Mater. 2016;1:39–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao X, Peng R, Ding J. Cell-material interactions revealed via material techniques of surface patterning. Adv Mater. 2013;25:5257–5286. [DOI] [PubMed] [Google Scholar]
- 8.Peyton SR, Raub CB, Keschrumrus VP, Putnam AJ. The use of poly(ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cells. Biomaterials. 2006; 27:4881–4893. [DOI] [PubMed] [Google Scholar]
- 9.Mason BN, Califano JP, Reinhart-King CA. Matrix stiffness: a regulator of cellular behavior and tissue formation. In: Bhatia S, ed. Engineering Biomaterials for Regenerative Medicine. New York: Springer; 2012. [Google Scholar]
- 10.Young JL, Tuler J, Braden R, et al. In vivo response to dynamic hyaluronic acid hydrogels. Acta Biomater. 2013;9:7151–7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawhney AS, Pathak CP, Hubbell JA. Bioerodible hydrogels based on photopolymerized poly(ethylene glycol)-co-poly(alpha-hydroxy acid) diacrylate macromers. Macromolecules. 1993;26:581–587. [Google Scholar]
- 12.West JL, Hubbell JA. Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules. 1999;32: 241–244. [Google Scholar]
- 13.Luo Y, Kobler JB, Heaton JT, Jia X, Zeitels SM, Langer R. Injectable hyaluronic acid-dextran hydrogels and effects of implantation in ferret vocal fold. J. Biomed. Mater. Res.-Part B Appl. Biomater 2010;93B: 386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heris HK, Latifi N, Vali H, Li N, Mongeau L. Investigation of chitosanglycol/glyoxal as an injectable biomaterial for vocal fold tissue engineering. Procedia Eng. 2015;110:143–150. [Google Scholar]
- 15.Chen X, Thibeault SL. Cell–cell interaction between vocal fold fibroblasts and bone marrow mesenchymal stromal cells in three-dimensional hyaluronan hydrogel. J Tissue Eng Regen Med. 2016;10: 437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King SN, Hanson SE, Chen X, Kim J, Hematti P, Thibeault SL. In vitro characterization of macrophage interaction with mesenchymal stromal cell-hyaluronan hydrogel constructs. J Biomed Mater Res Part A. 2014;102:890–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanson SE, King SN, Kim J, Chen X, Thibeault SL, Hematti P. The effect of mesenchymal stromal cell-hyaluronic acid hydrogel constructs on immunophenotype of macrophages. Tissue Eng Part A. 2011;17:2463–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erndt-Marino JD, Jimenez-Vergara AC, Diaz-Rodriguez P, et al. In vitro evaluation of a basic fibroblast growth factor-containing hydrogel toward vocal fold lamina propria scar treatment. J Biomed Mater Res-Part B Appl Biomater. 2018;106:1258–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H, Erndt-Marino J, Diaz-Rodriguez P, et al. In vitro evaluation of anti-fibrotic effects of select cytokines for vocal fold scar treatment. J Biomed Mater Res Part B Appl Biomater. 2019;107:1056–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L, Mahara A, Tong Z, et al. Recombinant resilin-based bioelastomers for regenerative medicine applications. Adv Healthc Mater. 2016;5:266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park H, Karajanagi S, Wolak K, et al. Three-dimensional hydrogel model using adipose-derived stem cells for vocal fold augmentation. Tissue Eng - Part A. 2010;16:535–543. [DOI] [PubMed] [Google Scholar]
- 22.Chen X, Thibeault SL. Characteristics of age-related changes in cultured human vocal fold fibroblasts. Laryngoscope. 2008;118:1700–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jetté ME, Hayer SD, Thibeault SL. Characterization of human vocal fold fibroblasts derived from chronic scar. Laryngoscope. 2013;123: 738–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ling C, Yamashita M, Waselchuk EA, Raasch JL, Bless DM, Welham NV. Alteration in cellular morphology, density and distribution in rat vocal fold mucosa following injury. Wound Repair Regen. 2010;18:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catten M, Gray SD, Hammond TH, Zhou R, Hammond E. Analysis of cellular location and concentration in vocal fold lamina propria. Otolaryngol - Head Neck Surg. 1998;118:663–667. [DOI] [PubMed] [Google Scholar]
- 26.Bartlett RS, Gaston JD, Ye S, Kendziorski C, Thibeault SL. Mechanotransduction of vocal fold fibroblasts and mesenchymal stromal cells in the context of the vocal fold mechanome. J Biomech. 2019;83:227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garg A, Yuen S, Seekhao N, et al. Towards a physiological scale of vocal fold agent-based models of surgical injury and repair: sensitivity analysis, calibration and verification. Appl Sci 2019;9:2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li-Jessen NYK, Powell M, Choi AJ, Lee BJ, Thibeault SL. Cellular source and proinflammatory roles of high-mobility group box 1 in surgically injured rat vocal folds. Laryngoscope. 2017;127: 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392–402. [DOI] [PubMed] [Google Scholar]
- 30.Gray SD. Cellular physiology of the vocal folds. Otolaryngol Clin North Am. 2000;33:679–697. [DOI] [PubMed] [Google Scholar]
- 31.Liao H, Munoz-Pinto D, Qu X, Hou Y, Grunlan MA, Hahn MS. Influence of hydrogel mechanical properties and mesh size on vocal fold fibroblast extracellular matrix production and phenotype. Acta Biomater. 2008;4:1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heris HK, Daoud J, Sheibani S, Vali H, Tabrizian M, Mongeau L. Investigation of the viability, adhesion, and migration of human fibroblasts in a hyaluronic acid/gelatin microgel-reinforced composite hydrogel for vocal fold tissue regeneration. Adv Healthc Mater. 2016;5: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wynn TA, Vannella KM. Review macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44:450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mescher AL. Macrophages and fibroblasts during inflammation and tissue repair in models of organ regeneration. Regeneration. 2017;4: 39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li NYK, Lee BJ, Thibeault SL. Temporal and spatial expression of high-mobility group box 1 in surgically injured rat vocal folds. Laryngoscope. 2012;122:364–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madden LR, Mortisen DJ, Sussman EM, et al. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc Natl Acad Sci. 2010;107:15211–15216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.King SN, Chen F, Jetté ME, Thibeault SL. Vocal fold fibroblasts immunoregulate activated macrophage phenotype. Cytokine. 2013; 61:228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karin M, Clevers H. Reparative inflammation takes charge of tissue regeneration. Nature. 2017;529:307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown WS Jr, Morris RJ, Hollien H, Howell E. Speaking fundamental frequency characteristics as a function of age and professional singing. J Voice. 1991;5:310–315. [Google Scholar]
- 42.Titze IR. On the relation between subglottal pressure and fundamental frequency in phonation. J Acoust Soc Am. 1989;85:901–906. [DOI] [PubMed] [Google Scholar]
- 43.Titze IR, Hitchcock RW, Broadhead K, et al. Design and validation of a bioreactor for engineering vocal fold tissues under combined tensile and vibrational stresses. J Biomech. 2004;37:1521–1529. [DOI] [PubMed] [Google Scholar]
- 44.Kutty JK, Webb K. Tissue engineering therapies for the vocal fold lamina propria. Tissue Eng Part B. 2009;15(3):249–262. [DOI] [PubMed] [Google Scholar]
- 45.Tran QT, Gerratt BR, Berke GS, Kreiman J. Measurement of young’s modulus in the in vivo human vocal folds. Ann Otol Rhinol Laryngol. 1993;102:584–591. [DOI] [PubMed] [Google Scholar]
- 46.Guvendiren M, Burdick JA. Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nat Commun. 2012;3:792–800. [DOI] [PubMed] [Google Scholar]
- 47.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. [DOI] [PubMed] [Google Scholar]
- 48.Mariani E, Lisignoli G, Borzì RM, Pulsatelli L. Biomaterials: foreign bodies or tuners for the immune response? Int J Mol Sci. 2019;20: 636–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Discher DE, Janmey P, Wang Y. Tissue cells feel and respond to the stiffness of their substrate. Mater Biol. 2005;310:1139–1143. [DOI] [PubMed] [Google Scholar]
- 50.Blakney AK, Swartzlander MD, Bryant SJ. The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly(ethylene glycol)-based hydrogels. J Biomed Mater Res - Part A. 2012;100 A:1375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sadtler K, Wolf MT, Ganguly S, et al. Divergent immune responses to synthetic and biological scaffolds. Biomaterials. 2019;192:405–415. [DOI] [PubMed] [Google Scholar]
- 52.Yeung T, Georges PC, Flanagan LA, et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60:24–34. [DOI] [PubMed] [Google Scholar]
- 53.Berger J, Reist M, Mayer JM, Felt O, Peppas NA, Gurny R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur J Pharm Biopharm. 2004; 57:19–34. [DOI] [PubMed] [Google Scholar]
- 54.Zhu J, Marchant RE. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev Med Devices. 2011;8:607–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guyot C, Lerouge S. Can we achieve the perfect injectable scaffold for cell therapy? Futur Sci OA. 2018;4:FSO284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grotenhuis N, Bayon Y, Lange JF, Van Osch GJVM, Bastiaansen-Jenniskens YM. A culture model to analyze the acute biomaterial-dependent reaction of human primary macrophages. Biochem Biophys Res Commun. 2013;433:115–120. [DOI] [PubMed] [Google Scholar]
- 57.Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer. 1980;26:171–176. [DOI] [PubMed] [Google Scholar]
- 58.Mittar D, Paramban R, Catherine M. Flow cytometry and high-content imaging to identify markers of monocyte-macrophage differentiation. BD Biosci Appl Note. 2011:1–20. [Google Scholar]
- 59.Chen X, Thibeault SL. Novel isolation and biochemical characterization of immortalized fibroblasts for tissue engineering vocal fold lamina Propria. Tissue Eng Part C. 2009;15:201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holt DJ, Chamberlain LM, Grainger DW. Cell-cell signaling in co-cultures of macrophages and fibroblasts. Biomaterials. 2011;31:9382–9394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shiratori H, Feinweber C, Luckhardt S, et al. THP-1 and human peripheral blood mononuclear cell-derived macrophages differ in their capacity to polarize in vitro. Mol Immunol. 2017;88:58–68. [DOI] [PubMed] [Google Scholar]
- 62.Box GEP, Cox DR. An analysis of transformations. J. Royal Stat. Soc.: Series B (Methodological). 1964;26:211–243. [Google Scholar]
- 63.John F, Sanford W, Brad P, et al. R Package “car”. (2020).
- 64.Bartlett RS, Thibeault SL, Prestwich GD. Therapeutic potential of gel-based injectables for vocal fold regeneration. Biomed Mater. 2012;7: 024103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McWhorter FY, Davis CT, Liu WF. Physical and mechanical regulation of macrophage phenotype and function. Cell Mol Life Sci. 2015;72: 1303–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Irwin EF, Saha K, Rosenbluth M, Gamble LJ, Castner DG, Healy KE. Modulus-dependent macrophage adhesion and behavior. J Biomater Sci Polym Ed. 2008;19:1363–1382. [DOI] [PubMed] [Google Scholar]
- 67.Kielhorn J, Pohlenz-Michel C, Schmidt S, Mangelsdorf I. Concise international chemical assessment document 57: GLYOXAL. World Heal. Organ 2004;1–41. [Google Scholar]
- 68.Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ. Mediation of biomaterial-cell interactions by adsorbed proteins: a review. Tissue Eng. 2005;11:1–18. [DOI] [PubMed] [Google Scholar]
- 69.Vitte J, Benoliel AM, Pierres A, Bongrand P. Is there a predictable relationship between surface physical-chemical properties and cell behaviour at the interface? Eur Cells Mater. 2004;7:52–63. [DOI] [PubMed] [Google Scholar]
- 70.Zhang L, Cao Z, Bai T, et al. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat Biotechnol. 2013;31: 553–556. [DOI] [PubMed] [Google Scholar]
- 71.Milleret V, Buzzi S, Gehrig P, et al. Protein adsorption steers blood contact activation on engineered cobalt chromium alloy oxide layers. Acta Biomater. 2015;24:343–351. [DOI] [PubMed] [Google Scholar]
- 72.Rock KL, Kono H. The inflammatory response to cell death. Annu Rev Pathol. 2008;3:99–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singh SK. Impact of product-related factors on immunogenicity of biotherapeutics. J Pharm Sci. 2011;100:354–387. [DOI] [PubMed] [Google Scholar]
- 74.Elmore S Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wong SW, Lenzini S, Cooper MH, Mooney DJ, Shin J. Soft extracellular matrix enhances inflammatory activation of mesenchymal stromal cells to induce monocyte production and trafficking. Sci Adv. 2020;6:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sullivan DE, Ferris M, Nguyen H, Abboud E, Brody AR. TNF-alpha induces TGF-beta-1 expression in lung fibroblasts at the transcriptional level via AP-1 activation. J Cell Mol Med. 2009;13: 1866–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Luedde T, Schwabe RF. NF-κB in the liver - linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011;8: 108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Solon J, Levental I, Sengupta K, Georges PC, Janmey PA. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys J. 2007;93:4453–4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gaston J, Thibeault SL. Hyaluronic acid hydrogels for vocal fold wound healing. Biomatter. 2013;3:e23799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brown BN, Ratner BD, Goodman SB, Amar S, Badylak SF. Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials. 2012;33: 3792–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang J, Hori K, Ding J, et al. Toll-like receptors expressed by dermal fibroblasts contribute to hypertrophic scarring. J Cell Physiol. 2011; 226:1265–1273. [DOI] [PubMed] [Google Scholar]
- 84.Tedesco S, De Majo F, Kim J, et al. Convenience versus biological significance: are PMA-differentiated THP-1 cells a reliable substitute for blood-derived macrophages when studying in vitro polarization? Front Pharmacol. 2018;9:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


