Abstract
The dynamic and unpredictable nature of expressive vocabulary dropout in progressive anomia presents a challenge for language intervention. We evaluated whether eye gaze patterns during naming could predict anomia for same items in the near future. We tracked naming accuracy and gaze patterns as patients with semantic (n=7) or logopenic (n=2) variants of Primary Progressive Aphasia or amnestic Alzheimer’s Disease (n=1), named photographs of people and objects. Patients were tested three or more times spaced roughly evenly over an average duration of 19.1 months. Target words named accurately at baseline were retrospectively coded as either known (i.e., consistently named) or vulnerable (i.e., inaccurately or inconsistently named) based on naming accuracy over the study interval. We extracted gaze data corresponding to successful naming attempts and implemented logistic mixed effects models to determine whether common gaze measures could predict each word’s naming status as known or vulnerable. More visual fixations and greater visual fixation dispersion predicted later anomia. These findings suggest that eye tracking may yield a biomarker of the robustness of particular target words to future expressive vocabulary dropout. We discuss the potential utility of this finding for optimizing treatment for progressive anomia.
Keywords: Primary Progressive Aphasia, Naming, Anomia, Eyetracking, Dementia
1. Introduction
Naming impairment (i.e., anomia) is a functionally debilitating symptom of numerous neurological disorders. Progressively worsening anomia is especially prevalent in the semantic and logopenic variants of Primary Progressive Aphasia (svPPA & lvPPA) (Budd et al, 2010, Gorno-Tempini et al, 2011; Kamath, Sutherland, & Chaney, 2020, Woollams et al., 2008). Anomia and associated word finding impairments are also common within the amnestic variant of Alzheimer’s Disease (AD) (Flanagan et al., 2016; McKhann et al., 2011; Reilly et al., 2011). Neuropsychological rehabilitation has recently seen a surge of interest in treating language disorders incurred in these clinical populations, including the promise of synergistic gains from pairing behavioral interventions with non-invasive brain stimulation (Meyer et al., 2019; Tippett et al., 2015; Hung et al., 2017; Tsapkini et al., 2014).
The effectiveness of most traditional anomia treatments is gauged by measuring improvements in naming accuracy for words a patient cannot name across repeated baseline attempts (Conroy, Sage, & Lambon Ralph, 2009; Edmonds & Kiran, 2006; Best, Greenwood, Grassly, Herbert, Hickin, & Howard, 2013). The fundamental assumption of this approach is that patients can successfully build or re-establish durable pathways between word forms and meaning (Raymer et al, 2008). This assumption of a durable neural substrate for relearning is violated in progressive anomia where neurodegeneration causes what patients and caregivers often refer to as language loss (e.g., “Mary lost the name of the dog.”) or language forgetting (e.g., “Frank forgot the kids’ names”).
The question of whether words are lost or forgotten remains central to neuropsychology, and the answers have profound implications for neurorehabilitation. Patients with semantic dementia or svPPA are thought to experience naming impairment with a primary etiology in the erosion of conceptual knowledge (Gorno-Tempini et al., 2011; Macoir et al., 2015; Patterson, 2007; Snowden, Goulding, & Neary, 1989; Woollams, Cooper-Pye, Hodges, & Patterson, 2008). In contrast, anomia in post stroke aphasia is thought to predominantly reflect impaired linguistic access to otherwise grossly intact conceptual knowledge (Dell et al., 1997; Geschwind et al., 1968; Goodglass & Baker, 1976; Jefferies, 2013; Jefferies & Lambon Ralph, 2006; Levelt et al., 1998; Meyer et al., 1998; Noonan et al., 2013; Oldfield & Wingfield, 1965; Rogers et al., 2015; Thompson et al., 2015; Warrington & Shallice, 1984; Whitney et al., 2011). Progressive anomia in svPPA, lvPPA, and amnestic AD is characterized by worsening impairment in producing the names of objects and people, a phenomenon we hereafter refer to as expressive vocabulary dropout.
The unpredictable nature of expressive vocabulary dropout presents a significant obstacle for restorative language treatment in progressive anomia. The assumption of a stable or improving baseline upon which gains might be built must be abandoned. A patient with progressive anomia may successfully name the family dog at baseline only to experience persistent anomia for the same dog two months later. Treatments premised upon ad hoc identification of inaccurately named words must constantly update a patient’s inventory of known words, an untenable prospect for an adult lexicon exceeding 40,000 words (Brysbaert et al, 2016).
Maintenance is gaining momentum as an alternative approach to restorative treatments for progressive anomia (Jokel et al., 2006; Jokel & Anderson, 2012; Reilly, 2016). The logic of a maintenance intervention is that patients may experience better functional outcomes by focusing on the preservation of a small set of known words relative to the reacquisition of “lost” expressive vocabulary. One key advance for optimizing such treatment would involve identifying target words that are weakening prior to overt dropout. A sensitive index of the robustness of a particular target word could more effectively guide treatment dosage to slow or prevent dropout. In the study to follow, we examined whether eye gaze patterns during visual confrontation naming could yield such a physiological marker of future expressive vocabulary dropout in a cohort of patients with progressive anomia.
1.1. Aims and hypotheses
Many past studies have focused on phonological, lexical, and semantic factors that mediate anomia. However, the contribution(s) of visual processing to anomia remain less clear (Harnish et al, 2010). In a healthy visual system, object recognition involves a fluid division of labor between bottom-up, stimulus driven processing and top-down conceptual expectancies (Bar, 2003). As information streams forward from primary visual cortices, conceptual expectancies and predictive processing impute missing elements (e.g., edge boundaries, discontinuities) needed to perceive scenes and parse their constituent elements (Humphreys et al., 1997). Conceptual biases rapidly orient visual attention to salient features such as faces (Bar, 2003; Bar et al., 2006; Yarbus, 1967). In this respect, semantic memory speeds object recognition and allows us to make rapid inferences about the identity of people and objects. In turn, semantic impairment may offer an in vivo model of visual object recognition in the absence of top-down support (Bueno et al., 2019; Faria et al., 2018; Seckin, Mesulam, Voss, et al., 2016; Ungrady et al., 2019b).
A bottom-up visual search is characterized by more visual fixations1, higher saccade amplitude, and greater fixation dispersion (Rayner, 2009; Seckin et al, 2016). Clinically, such patterns translate to longer periods of aimless scanning with attentional capture governed by visual interest (e.g., a red sweater, a sparkly object) rather than semantic informativeness. Figure 1 illustrates a hypothetical distribution of visual attention that might be observed when naming a photograph of cat. In our prior work, healthy adult visual fixation patterns during successful picture naming are characterized by a small number of fixations (often none) densely clustered around distinctive features such as faces (Binney, Ashaie, Zuckerman & Reilly, 2018). Our aim in this study was to evaluate whether similar gaze patterns could yield a marker of later vocabulary dropout in progressive anomia. We hypothesize that lexical-semantic degradation proceeds non-uniformly across different words and that gaze metrics can provide an index of the health/integrity of a particular word prior to its dropout from a patient’s expressive vocabulary.
Figure 1.

Fixation count vs. fixation dispersion
Note: These data are fictional and for illustrative purposes only. Red circles represent discrete visual fixations. Fixation count is the total number of fixations (circles) during a given time interval. Fixation dispersion reflects the spatial distribution of the fixations. The most efficient visual search is represented by the cat in the lower left quadrant.
2. Methods
2.1. Overview
We examined visual confrontation naming in a longitudinal cohort of patients with svPPA (n=7), lvPPA (n=2), or amnestic AD (n=1) who were simultaneously undergoing a maintenance-based language treatment (Hung et al., 2017; Reilly, 2016). Due to either life circumstances rendering some patients unable to complete part of the treatment (e.g., moving into a nursing home, rapid cognitive decline, other illnesses) or non-viable eyetracking data (e.g., patient looked off the screen, calibration errors, etc.), only seven patients yielded full datasets, whereas the remaining three patients had partial datasets. Briefly, the treatment involved a combination of naming and semantic feature generation of a set of personalized target photographs arrayed within a memory book (see section 2.3 Stimulus characteristics). Treatment was co-facilitated by the patient’s primary caregiver and a visiting clinician (author M.B.U.). Upon enrollment, each patient received baseline neuropsychological testing and were subsequently retested every 4–6 months for approximately two years (see Table 1). All treatment and assessment sessions (including eyetracking) were completed in the patient’s home. During each assessment session, we evaluated eye gaze patterns as patients named personalized photographs of common objects and people. At the study’s conclusion we contrasted eye gaze patterns for target words that were consistently named accurately across all timepoints relative to items that were omitted or inaccurately named. Crucially, we focused exclusively upon items that were named accurately at baseline.
Table 1.
Patient Demographics, Testing Duration, and Total Numbers of Datapoints
| ID | Dx | Age | Edu | Years Post Disease Onset | Total Duration Enrolled (months) |
Total Assessments | Total Naming Attempts | Total Viable Trials | % Viable Trials |
|---|---|---|---|---|---|---|---|---|---|
| 1 | lv | 69 | 13 | 2 | 10 | 3 | 250 | 161 | 64.4 |
| 2 | sv | 66 | 16 | 1 | 23 | 4 | 720 | 444 | 61.7 |
| 3 | sv | 60 | 16 | 7 | 19 | 3 | 233 | 207 | 88.8 |
| 4 | lv | 65 | 14 | 3 | 24 | 5 | 725 | 560 | 77.2 |
| 5 | sv | 59 | 12 | 5 | 20 | 4 | 758 | 433 | 57.1 |
| 6 | AD | 79 | 16 | 4 | 17 | 4 | 757 | 416 | 55.0 |
| 7 | sv | 65 | 12 | 2 | 28 | 5 | 878 | 608 | 69.2 |
| 8 | sv | 65 | 15 | 3 | 23 | 5 | 956 | 471 | 49.3 |
| 9 | sv | 64 | 18 | 3 | 23 | 5 | 956 | 718 | 75.1 |
| 10 | sv | 65 | 19 | 6 | 31 | 4 | 433 | 85 | 20.0 |
Note: ‘Dx’ (diagnosis): lv=logopenic variant PPA, sv=semantic variant PPA, AD= amnestic variant Alzheimer’s Disease;; ‘Age’ denotes age in years at study onset; ‘Edu’ represents years of formal schooling; “Years Post Disease Onset’ reflects the approximate interval in years between self-reported symptom onset and initiation of the treatment study; ‘Total Duration Enrolled’ reflects the duration in months each patient took active part in the study; ‘Total Assessments’ reflects the total number of repeated administrations of the naming assessment and neuropsychological battery; ‘Total Naming Attempts’ reflects each patient’s total number of recorded naming attempts across repeated assessments; ‘Total Viable Trials’ constitutes the number of discrete naming trials with recorded accuracy and viable gaze data uncontaminated by artifacts (e.g., blinks, coughing, excessive head motion); ‘% Viable Trials’ reflects the proportion of viable trials divided by the total number of naming attempts.
Treating and assessing patients with progressive anomia using personalized picture stimuli in their own homes offers significant advantages in terms of ecological validity, reduced caregiver burden, and greater potential for context dependent learning transfer. Nevertheless, these advantages are counterbalanced by the compromise of experimental control. Testing in the home using personalized picture stimuli introduces numerous confounds including variable luminance contours, idiosyncratic distractions, and differences in the quality and visual complexity of photographs. As such, these data lack the precision of laboratory-based psycholinguistic and eyetracking studies. Despite these limitations, however, we were able to collect many repeated naming attempts in a naturalistic setting with viable gaze data spanning an extended period of time.
In the analyses to follow, we first extracted all naming attempts with viable gaze data that were named successfully at the study’s baseline. We then marked each target word as either known (consistently named) or vulnerable (named inconsistently or never again named accurately after the baseline). We then revisited the baseline production and all subsequent accurate responses for both known and vulnerable words to assess whether a particular set of gaze patterns could portend whether a word would be forgotten later during the course of the study.
This research was conducted in accord with ethical principles of medical research outlined in the Declaration of Helsinki. The study was approved by the institutional review boards of Temple University and the University of Pennsylvania. Deidentified raw data and computer scripts in R Markdown file format are available for download via the Open Science Framework (OSF) at https://osf.io/gjc7y/ .
2.2. Participants
Participants included older adults diagnosed by an experienced behavioral neurologist in accord with published criteria for svPPA (N=7) or lvPPA (N=2) (Gorno-Tempini et al., 2011). This sample also included one patient with the amnestic variant of Alzheimer’s Disease (McKhann et al., 2011). Upon enrollment, we completed baseline neuropsychological testing and eyetracking. We re-administered this battery every four to six months for up to two years. All patients were followed for at least three successive timepoints but not all patients completed the two-year testing interval. Table 1 lists demographic detail including each patient’s total duration of enrollment and the number of viable naming attempts that were entered into the statistical model. Participants averaged 4.2 repeated assessments spaced over 19.2 months.
Table 2 lists neuropsychological data characterizing the patient cohort on repeated measures of global cognition, confrontation naming ability, and semantic memory. In an effort to reduce patient fatigue and distress, we administered short forms of the Boston Naming Test (Kaplan, Goodglass, & Weintraub, 1983; Mack, Freed, Williams, & Henderson, 1992) and an unvalidated 26-item short form of the original 52-item Pyramids & Palm Trees Test (PPT) (Howard & Patterson, 1992). This abbreviated version of the PPT included all even-numbered triads from the original battery (see Cousins, Ash, Olm, & Grossman, 2018). We scaled each patient’s raw score to the original point range (1–52) followed by a z-transformation using parameters from Wierenga et al.’s (2018) study of a sex-balanced sample of older American adults from the North Florida region. These older adults (n=20, mean age=75 years) approached ceiling accuracy on the PPT with a mean of 51.05 and standard deviation of 0.49. Z-score derivations for the PPT-short form in Table 2 reflect the following computation:
Table 2.
Neuropsychological Data at Baseline and Study Endpoint
| ID | Dx | Dur | MoCA (of 30) |
BNT-short (of 15) |
PPT-s Pics (raw score, z-score) |
PPT-s Words (raw score, z-score) |
Trails A/B (seconds) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | Tend | T1 | Tend | T1 | Tend | T1 | Tend | T1 | Tend | |||
| 1 | lv | 10 | 15^ | 16^ | 11 | 8^ | 26 (1.93) | 25 (−2.14) | 26 (1.93) | 25 (−2.14) | 67^/118 | 49/155 |
| 2 | sv | 23 | 22^ | 18^ | 4^ | 4^ | 23 (−10.31) | 19 (−26.63) | 19 (−26.63) | 17 (−34.80) | 27/44 | 45/60 |
| 3 | sv | 19 | 16^ | 19^ | 4^ | 3^ | 22 (−14.39) | 21 (−18.47) | 19 (−26.63) | 22 (−14.39) | 40/118 | 40/109 |
| 4 | lv | 24 | 15^ | 10^ | 12 | 10^ | 25 (−2.14) | 23 (−10.31) | 24 (−6.22) | 24 (−6.22) | 95^/58 | NC^/NC^ |
| 5 | sv | 20 | 21^ | 18^ | 2^ | 1^ | 25 (−2.14) | 22 (−14.39) | 20 (−22.55) | NA | 19/54 | 34/74 |
| 6 | AD | 17 | 15^ | 10^ | 4^ | 4^ | 20 (−22.55) | 15 (−42.96) | 20 (−22.55) | 17 (−34.80) | 25/60 | 40/93 |
| 7 | sv | 28 | 16^ | 13^ | 4^ | 4^ | 22 (−14.39) | 20 (−22.55) | 18 (−30.71) | 18 (−30.71) | 16/45 | 19/40 |
| 8 | sv | 23 | 14^ | 7^ | 2^ | 1^ | 15 (−42.96) | 12 (−55.2) | 14 (−47.04) | 12 (−55.2) | 70^/245^ | NC^/NC^ |
| 9 | sv | 23 | 17^ | 20^ | 3^ | 2^ | 23 (−10.31) | 21 (−18.47) | 25 (−2.14) | 20 (−22.55) | 41/96 | 35/75 |
| 10 | sv | 31 | 17^ | 18^ | 1^ | 2^ | 12 (−55.2) | 15 (−42.96) | 13 (−51.10) | 12 (−55.2) | 42/101 | 52/114 |
Note: ‘Dx’ (diagnosis): lv=logopenic variant PPA, sv=semantic variant PPA, AD= amnestic variant Alzheimer’s Disease; ‘Dur’ = duration in months each patient took part in the study; ‘MoCA’ = Montreal Cognitive Assessment raw score total of 30; uncorrected MoCA scores less than 26 suggest impaired global cognition (Nasreddine et al., 2005); ‘BNT’=Boston Naming Test 15-item short-form Version 4; healthy adult mean accuracy is 13.2 items (sd=1.6) (Mack et al., 1992); ‘PPT-s Pics’ = unvalidated 26-item subset of the Pyramids and Palms Trees Test (Howard & Patterson, 1992) z-transformed to the original 52-item accuracy distribution for healthy older American adults reported by Wierenga et al 2008 (see method); ‘PPT-s Pics’ = picture version of the PPT unvalidated short form; ‘Trail Making Test-Form A’ (maximum time is 150 seconds), Trails-B=Trail Making Test-Form B (maximum time is 300 seconds), scores rounded to the nearest second (Tombaugh, 2004; Weintraub et al., 2009). ‘NC’ denotes that a participant did not complete the task within the allotted time.
indicates that the raw score exceeded worse than two standard deviations below the mean(s) reported for healthy controls. The following norms for each neuropsychological test were used: MoCA: ^ indicates a score below 26 (Nasreddine et al., 2005); Trails A/B: Weintraub et al., 2018; BNT: Mack et al., 1992
2.3. Stimulus characteristics
Stimuli included a combination of personalized and stock photographs of objects and people as detailed by Reilly (2016). At the study’s baseline, patients selected target items (N=100) (hereafter referred to as ‘trained’ items) to be treated during language intervention. Patients and their caregivers together selected trained items from fixed lists representing the following semantic categories: people, places, foods, household items, hygiene, clothes, activities. Treatment target selection proceeded with the constraint that trained items should be highly frequent, familiar, and have high functional utility in daily communication. After this training lexicon was established, we visited patients in their homes and either shot new photos or acquired older photos (e.g., patient’s friends, grandchildren) corresponding to the target words. Stock photos were used for targets that did not require personalized images (e.g., fruit). Untrained ‘control’ items (N=100) were composed of unassigned words from the original stimulus lists. A set of famous face photographs served as the untrained items for known people. Photos were sized to a uniform dimension (500 × 500 pixels), and in some cases post-processed to enhance focus on the target figure, blurring distracting backgrounds and/or labels. Stimulus lists are available for inspection and use at https://osf.io/gjc7y/.
Every patient’s training set differed with respect to the target words and visual attributes of the picture stimuli (e.g., quality, complexity, color variation). This property of the treatment study precluded comprehensive item-level psycholinguistic matching across participants. Items were roughly matched on familiarity, and patients named items sampled across the same distribution of semantic categories.
2.4. Eyetracking and Naming Procedures
Confrontation naming was facilitated by the use of an automated stimulus delivery program (Experiment Center) coupled to a remote infrared eyetracking system (SMI RED X 120 Hz) (SensoriMotoric Instruments Inc., Boston, MA). We collected monocular right eye movements at a sampling rate of 120 Hz (spatial resolution < 0.03°). Patients were seated at a distance of 65cm ± 5cm from the infrared illuminator positioned at the base of a 17” Dell Windows-based laptop computer. Each eyetracking session was initiated with a 5-point calibration and validation sequence. Target images were then presented in a randomized order using a gaze contingent technique. This gaze trigger involved a small, invisible, rectangular area of interest (AOI) surrounding an attention fixation cross positioned at the top and center of the screen. Once the AOI accrued 1000ms of cumulative dwell time (saccades and fixations), the next photograph automatically advanced. Patients were cued to verbally state the name of each item and were permitted as much time as needed to provide an answer. We scored only spontaneous responses using a binary coding scheme: accurate or inaccurate. If the participant self-corrected their spontaneous response, we counted their final response.
2.5. Item Level Coding
We implemented a coding schema designed to partition individual naming attempts into two broad categories, known vs. vulnerable. At the conclusion of the study, we isolated all naming attempts for words that were accurately named at the baseline, omitting gaze data for target words that were never accurately named. Known words included targets named accurately across all timepoints. Vulnerable words were named accurately at the baseline but were then either inconsistently named or inaccurately named across all timepoints thereafter. We omitted trials corresponding to inaccurate naming attempts and/or trials with poor eyetracking fidelity (e.g., excessive motion, blinks). Gaze analyses were windowed to approximately three seconds following the onset of the cue (2750ms ± 250ms of gaze data per trial).
2.6. Data Analyses
We employed logistic mixed effects models to evaluate item-level prediction of ‘known vs. vulnerable’ for each naming attempt by each patient at each successive timepoint. The dependent variable in these analyses was a dichotomous coding (0 or 1) of whether the target item represented a known or vulnerable word on each discrete naming attempt. We assessed discrimination of word status (known or vulnerable) from a principled set of predictors that index visual attention (Rayner, 2009). Predictors included fixation count, fixation dispersion, saccade count, saccade velocity, and saccade duration. Each of these gaze measures provides complementary information regarding visual attention and search efficiency. Table 3 represents a correlation matrix detailing bivariate relations between eyetracking metrics. Two of the predictors (i.e., fixation count, fixation dispersion) provide unique but highly correlated (R=.93) information. Fixation dispersion provides a measure of the spatial distribution of fixations during a given time interval, whereas fixation count provides a relative index of focus/depth. Due to multicollinearity between fixation count and dispersion, we evaluated these variables in two separate logistic mixed effects models using the ‘lme4’ package of R (Bates et al., 2015).
Table 3.
Correlation matrix eyetracking measures
| Fixation Duration | Fixation Dispersion | Saccade Count | Saccade Duration | Saccade Velocity | |
|---|---|---|---|---|---|
| Fixation Count | .46 | .93 | .72 | −0.04 | .20 |
| Fixation Duration | .35 | .07 | −.67 | −.04 | |
| Fixation Dispersion | .71 | .08 | .16 | ||
| Saccade Count | .32 | .46 | |||
| Saccade Duration | .22 |
Note: Spearman R-values represent bivariate correlations across all naming trials within and between-participants as employed in the mixed effects analyses.
We modeled the identical set of random effects in both models, including patient, item, and training condition. Patient ID was entered as a statistical control for idiosyncratic individual differences across participants. Item was entered to control for the variance imposed by stimulus-level attributes (e.g., visual complexity, familiarity). Training Condition was entered as a random effect to control for greater repeated exposure among treated targets relative to untreated control items. The dependent variable in both binary logistic models was a word’s status as either known or vulnerable (0 or 1) on each naming attempt. The two models differed only in terms of swapping in/out the two highly correlated variables of fixation count and dispersion
Model A (i.e., fixation count but no dispersion) was specified as follows:
Model B (i.e., fixation dispersion but no count) was specified as follows:
3. Results
Table 4 represents naming accuracy by each patient at baseline and study conclusion. Patients showed no evidence of decline in naming accuracy for trained items [paired t(7)=−0.08, p=.94] but did show a significant decline in untrained items [paired t(8)=4.58, p=.002]. Descriptive statistics for each of the eyegaze measures at each timepoint for trained and untrained items are listed in Appendix A accessible via the OSF at https://osf.io/gjc7y/.
Table 4.
Naming Accuracy for Trained and Untrained Items
| ID | Dx | Trained | Untrained | ||
|---|---|---|---|---|---|
| T1 | Tend | T1 | Tend | ||
| 1 | lv | NA | NA | .69 | .61 |
| 2 | sv | 1.00 | .92 | .56 | .22 |
| 3 | sv | .82 | .95 | NA | NA |
| 4 | lv | .88 | .89 | .70 | .68 |
| 5 | sv | .90 | .75 | .51 | .17 |
| 6 | AD | .69 | .58 | .57 | .42 |
| 7 | sv | .89 | .98 | .54 | .33 |
| 8 | sv | .58 | .62 | .45 | .20 |
| 9 | sv | .89 | .98 | .57 | .46 |
| 10 | sv | NA | NA | .24 | .14 |
Note: Accuracy values represent proportion correct.
3.1. Logistic Mixed Effects Models Predicting Known vs. Vulnerable Status
Model A revealed fixation dispersion as a significant predictor of word trajectory [Estimate=.072, standard error=.034, z=−2.60, p=.01], indicating that patients more diffusely fixated across the planes of vulnerable target items (see also Figure 2). Model B revealed fixation count as a significant predictor of vulnerability to expressive vocabulary dropout [Estimate=−0.05, standard error=.03, z=−1.99, p=.04], indicating that words that were more vulnerable to expressive vocabulary dropout elicited more fixations. None of the other predictors were statistically significant in either model. Full model specifications, statistical coding, and output are available at https://osf.io/gjc7y/.
Figure 2.

Eyetracking measures for known vs. vulnerable items
Note: In the scatterplots above, each dot represents an individual patient’s performance across both conditions.
Figure 3 illustrates gaze markers distinguishing vulnerable from known words. Vulnerable words elicited a higher mean fixation dispersion (614.62 pixels vs 589.35 pixels, Cohen d=.12) and higher mean fixation count (7.88 fixations vs. 7.70, fixations Cohen d=.67).
Figure 3.
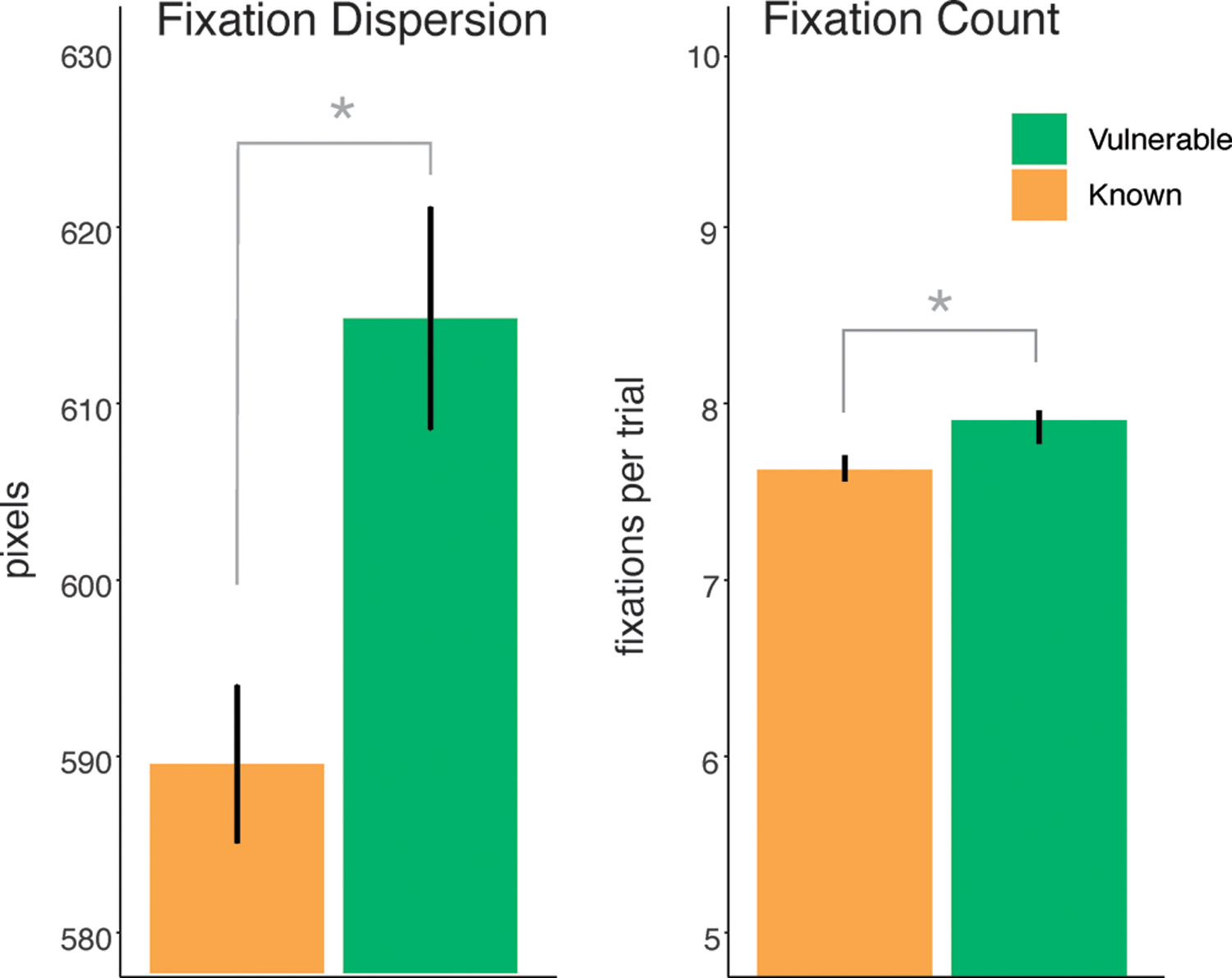
Gaze pattern differences for known vs. vulnerable words
Note: Error bars reflect standard error of the mean (SEM)
4. General Discussion
We hypothesized that eye gaze patterns could yield a sensitive index of the ‘health’ of a target word regarding its susceptibility to future dropout. We evaluated this hypothesis in a longitudinal cohort of patients experiencing progressive anomia. All of the target words were named accurately at baseline. Some words were consistently named (i.e., known words), whereas other words dropped from the patients’ expressive vocabulary (i.e., vulnerable words) over the study interval. At the conclusion of the study, we delineated target words as either known or vulnerable and contrasted their respective gaze patterns while items were accurately named (i.e., prior to dropout). This retrospective design afforded prediction of item-level dropout.
The principal finding was that more visual fixations and greater visual fixation dispersion (see Figure 1) were predictive of expressive vocabulary dropout in this patient cohort. This trend held both for treated and untreated control words. To follow, we address implications, limitations, and future applications of these findings.
4.1. Implications for neuropsychological rehabilitation
Patients with AD, lvPPA, and svPPA experience expressive vocabulary dropout, the etiology of which varies by atrophy distributions (Gorno-Tempini et al., 2011; McKhann et al., 2011; Woollams et al., 2008). Despite variability in the clinical phenotypes underlying these neurological disorders, all are characterized by progressive anomia and an evolution to semantic and/or lexical-semantic impairments as disease severity worsens (Bayles, Tomoeda, Kaszniak, & Trosset, 1991; Chen et al, 2020; Funayama et al, 2013).
We reasoned that as the lexical-semantic substrate for word meaning erodes, patients would be compelled to pursue a bottom-up visual search strategy during picture naming. Such an unsupervised visual search strategy is characterized by longer periods of undirected scanning with a more diffuse array of fixations and increased susceptibility to attentional capture by ‘pop out’ effects (Nothdurft, 2006). A semantically impaired patient might, for example, fixate on visual lures such as the red hue and white stars of a dog’s collar rather than the dog itself. The presence of such a maladaptive visual search strategy may provide a diagnostic marker of the ‘health’ of a particular target item prior to its overt dropout.2 In turn, clinicians may leverage this information to identify words that require more intensive training.
4.2. Study limitations
It would be premature to tout the promise of eye gaze as a biomarker of expressive vocabulary dropout in progressive anomia. Much remains to be learned about the validity and generalizability of these results prior to its implementation in guiding treatment. The overarching limitation of this study involved lack of precision experimental control. We tested patients in their homes using a portable remote eyetracking unit that resulted in lower fidelity gaze data than would normally be acquired in a laboratory. Another potential source of error related to weak stimulus matching and uncontrolled item-level variability (e.g., visual complexity, word length) across patients. Rayner (2009) noted that fixation count is strongly positively correlated with visual complexity of a scene array. Naming a photograph of a person posed against a white featureless background ensures a lower number of fixations than naming the same person at a crowded county fair. We attempted to control for such differences by enhancing focus on the target while blurring distractors. Nevertheless, complexity differences persisted across picture stimuli. Such variability represents an important consideration in light of the small effect size differences observed here.
Sample size and heterogeneity of the patient cohort represent another limitation. We collected many repeated observations on a small number of patients (N=10) over a relatively unprecedented duration. These data are vulnerable to small sample bias and the impact of outliers and idiosyncratic individual differences. A related issue was that inclusion was based on progressive anomia rather clinical diagnosis. As such, the sample included patients with svPPA, lvPPA, and amnestic AD. Each of these disorders has a unique phenotype, and such heterogeneity may threaten external validity.
4.3. Future directions and concluding remarks
Additional research is needed to establish the reliability of gaze metrics for optimizing treatment in progressive anomia. Clinical validation of this particular technique will require more extensive and better controlled testing. Improvements to the study’s design will elucidate how gaze metrics are mediated by differences in regional brain atrophy, disease etiology, and semantic functioning. This knowledge might be attained by assessing a larger, more balanced patient cohort characterized along a more exhaustive range of a semantic, visual, and neuroimaging measures. Future studies will also benefit from collecting higher fidelity eye gaze data in a controlled laboratory setting and validating gaze metrics against other markers such as reaction times (i.e., naming latencies) or semantic feature generation.
ACKNOWLEDGEMENT
We thank the patients, their families, and the University of Pennsylvania FTD Center for supporting this research. We are also grateful to two anonymous reviewers for their care, encouragement, and dedication to helping us create something we are truly proud of.
*This work was funded by US Public Health Service Grant R01 DC013063
Footnotes
Disclosure of Competing Interests
The authors report no conflicts of interest, financial or otherwise.
Fixations are defined as periods of focused visual attention within a particular area of interest. The threshold of what constitutes a fixation vary across eyetracking systems. The eyetracker used here (SMI Red-M 120 Hz) delineates fixation as dwell time exceeding a duration of 80ms within a spatially disbursed area subtending no more than 2 degrees of visual angle (100 pixels).
Numerous case studies have highlighted altered higher level visual processing in PPA and AD, including changes in stylistic preferences for visual art (Baddeley et al., 2001; Green & Patterson, 2009; Miller & Miller, 2013; Perry & Hodges, 1999; Vernet et al., 2014; Rizzo et al., 2000; Viskontas et al., 2011).
REFERENCES
- Baddeley AD, Baddeley HA, Bucks RS, & Wilcock GK (2001). Attentional control in Alzheimer’s disease. Brain, 124(8), 1492–1508. [DOI] [PubMed] [Google Scholar]
- Bar M (2003). A cortical mechanism for triggering top-down facilitation in visual object recognition. Journal of Cognitive Neuroscience, 15(4), 600–609. [DOI] [PubMed] [Google Scholar]
- Bar M, Kassam KS, Ghuman AS, Boshyan J, Schmid AM, Dale AM, Hämäläinen MS, Marinkovic K, Schacter DL, & Rosen BR (2006). Top-down facilitation of visual recognition. Proceedings of the National Academy of Sciences, 103(2), 449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, & Walker S (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1), 1–48. https://doi.org/doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Bayles KA, Tomoeda CK, Kaszniak AW, & Trosset MW (1991). Alzheimer’s disease effects on semantic memory: Loss of structure or impaired processing? Journal of Cognitive Neuroscience, 3(2), 166–182. [DOI] [PubMed] [Google Scholar]
- Beeson PM, King RM, Bonakdarpour B, Henry ML, Cho H, & Rapcsak SZ (2011). Positive effects of language treatment for the logopenic variant of primary progressive aphasia. Journal of Molecular Neuroscience, 45(3), 724–736. 10.1007/s12031-011-9579-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binney RJ, Ashaie SA, Zuckerman BM, Hung J, & Reilly J (2018). Frontotemporal stimulation modulates semantically-guided visual search during confrontation naming: A combined tDCS and eye tracking investigation. Brain and Language, 180, 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brysbaert M, Stevens M, Mandera P, & Keuleers E (2016). How many words do we know? Practical estimates of vocabulary size dependent on word definition, the degree of language input and the participant’s age. Language Sciences, 1116. 10.3389/fpsyg.2016.01116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd MA, Kortte K, Cloutman L, Newhart M, Gottesman RF, Davis C, Heidler-Gary J, Seay MW, & Hillis AE (2010). The nature of naming errors in primary progressive aphasia versus acute post-stroke aphasia. Neuropsychology, 24(5), 581–589. 10.1037/a0020287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno APA, Sato JR, & Hornberger M (2019). Eye tracking–The overlooked method to measure cognition in neurodegeneration? Neuropsychologia, 133, 107191 (Epub). doi: https://doi.org/doi: 10.1016/j.neuropsychologia.2019.10719 [DOI] [PubMed] [Google Scholar]
- Cappa SF, Binetti G, Pezzini A, Padovani A, Rozzini L, & Trabucchi M (1998). Object and action naming in Alzheimer’s disease and frontotemporal dementia. Neurology, 50(2), 351–355. [DOI] [PubMed] [Google Scholar]
- Chen Y, Huang L, Chen K, Ding J, Zhang Y, Yang Q, Lv Y, Han Z, & Guo Q (2020). White matter basis for the hub-and-spoke semantic representation: Evidence from semantic dementia. Brain, Epub Ahead of Print. 10.1093/brain/awaa057 [DOI] [PMC free article] [PubMed]
- Coccia M, Bartolini M, Luzzi S, Provinciali L, & Lambon Ralph MA (2004). Semantic memory is an amodal, dynamic system: Evidence from the interaction of naming and object use in semantic dementia. Cognitive Neuropsychology, 21(5), 513–527. [DOI] [PubMed] [Google Scholar]
- Conroy P, Sage K, & Lambon Ralph M (2009). Improved vocabulary production after naming therapy in aphasia: Can gains in picture naming generalise to connected speech? International Journal of Language & Communication Disorders, 44(6), 1036–1062. 10.1080/13682820802585975 [DOI] [PubMed] [Google Scholar]
- Cousins KAQ, Ash S, Olm CA, & Grossman M (2018). Longitudinal changes in semantic concreteness in semantic variant Primary Progressive Aphasia (svPPA). ENeuro, 5(6). 10.1523/ENEURO.0197-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell GS, Schwartz MF, Martin N, Saffran EM, & Gagnon DA (1997). Lexical access in aphasic and nonaphasic speakers. Psychological Review, 104(4), 801–838. [DOI] [PubMed] [Google Scholar]
- Dressel K, Huber W, Frings L, Kammerer D, Saur D, Mader I, Hall M, Weiller C, & Abel S (2010). Model-oriented naming therapy in semantic dementia: A single-case fMRI study. Aphasiology, 24(12), 1537–1558. [Google Scholar]
- Farah MJ, & McClelland JL (1991). A computational model of semantic memory impairment: Modality specificity and emergent category specificity. Journal of Experimental Psychology: General, 120(4), 339–357. [PubMed] [Google Scholar]
- Faria AV, Race D, Kim K, & Hillis AE (2018). The eyes reveal uncertainty about object distinctions in semantic variant primary progressive aphasia. Cortex, 103, 372–381. [DOI] [PubMed] [Google Scholar]
- Flanagan KJ, Copland DA, van Hees S, Byrne GJ, & Angwin AJ (2016). Semantic feature training for the treatment of anomia in Alzheimer Disease: A preliminary investigation. Cognitive & Behavioral Neurology, 29(1), 32–43. 10.1097/WNN.0000000000000088 [DOI] [PubMed] [Google Scholar]
- Funayama M, Nakagawa Y, Yamaya Y, Yoshino F, Mimura M, & Kato M (2013). Progression of logopenic variant Primary Progressive Aphasia to apraxia and semantic memory deficits. BMC Neurology, 13(1), 158. 10.1186/1471-2377-13-158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funnell E, & Hodges JR (1991). Progressive loss of access to spoken word forms in a case of Alzheimer’s disease. Proceedings of the Journal of Biological Sciences, 243(1307), 173–179. 10.1098/rspb.1991.0028 [doi] [DOI] [PubMed] [Google Scholar]
- Geschwind N, Quadfasel F, & Segarra JM (1968). Isolation of the speech area. Neuropsychologia, 6(4), 327–340. [Google Scholar]
- Goodglass H, & Baker E (1976). Semantic field, naming, and auditory comprehension in aphasia. Brain and Language, 3(3), 359–374. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, & Grossman M (2011). Classification of primary progressive aphasia and its variants. Neurology, 76(11), 1006–1014. https://doi.org/WNL.0b013e31821103e6 [pii] 10.1212/WNL.0b013e31821103e6 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green HA, & Patterson K (2009). Jigsaws-A preserved ability in semantic dementia. Neuropsychologia, 47(2), 569–576. [DOI] [PubMed] [Google Scholar]
- Harnish SM, Neils-Strunjas J, Eliassen J, Reilly J, Meinzer M, Clark JG, & Joseph J (2010). Visual discrimination predicts naming and semantic association accuracy in Alzheimer disease. Cognitive and Behavioral Neurology, 23(4), 231–239. 10.1097/WNN.0b013e3181e61cf1 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia CG, Sage K, Lambon Ralph MA, & Berthier ML (2009). Relearning and retention of verbal labels in a case of semantic dementia. Aphasiology, 23(2), 192–209. 10.1080/02687030801942999 [DOI] [Google Scholar]
- Hodges J, Patterson K, Graham N, & Dawsom K (1996). Naming and knowing in dementia of Alzheimer’s Type. Brain and Language, 325(54), 302–325. [DOI] [PubMed] [Google Scholar]
- Hoffman P, Jones RW, & Ralph MA (2012). The degraded concept representation system in semantic dementia: Damage to pan-modal hub, then visual spoke. Brain, 135(Pt 12), 3770–3780. 10.1093/brain/aws282 [DOI] [PubMed] [Google Scholar]
- Howard D, & Patterson K (1992). The pyramids and palm trees test: A test of semantic access from words and pictures. Thames Valley Test Company. [Google Scholar]
- Hubel DH, & Wiesel TN (1962). Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. Journal of Physiology, 160, 106–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys GW, Riddoch MJ, & Price CJ (1997). Top-down processes in object identification: Evidence from experimental psychology, neuropsychology and functional anatomy. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 352(1358), 1275–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung J, Bauer A, Grossman M, Hamilton RH, Coslett HB, & Reilly J (2017). Semantic feature training in combination with transcranial direct current stimulation (tDCS) for progressive anomia. Frontiers in Human Neuroscience, 11. 10.3389/fnhum.2017.00253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies E (2013). The neural basis of semantic cognition: Converging evidence from neuropsychology, neuroimaging and TMS. Cortex, 49(3), 611–625. 10.1016/j.cortex.2012.10.008 [DOI] [PubMed] [Google Scholar]
- Jefferies E, & Lambon Ralph MA (2006). Semantic impairment in stroke aphasia versus semantic dementia: A case-series comparison. Brain, 129(8), 2132–2147. [DOI] [PubMed] [Google Scholar]
- Jokel R, & Anderson ND (2012). Quest for the best: Effects of errorless and active encoding on word re-learning in semantic dementia. Neuropsychological Rehabilitation, 22(2), 187–214. 10.1080/09602011.2011.639626 [doi] [DOI] [PubMed] [Google Scholar]
- Jokel R, Rochon E, & Leonard C (2006). Treating anomia in semantic dementia: Improvement, maintenance, or both? Neuropsychological Rehabilitation, 16(3), 241–256. 10.1080/09602010500176757 [DOI] [PubMed] [Google Scholar]
- Kamath V, Sutherland ER, & Chaney G-A (2020). A meta-analysis of neuropsychological functioning in the logopenic variant of Primary Progressive Aphasia: Comparison with the semantic and non-fluent variants. Journal of the International Neuropsychological Society, 26(3), 322–330. 10.1017/S1355617719001115 [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, & Weintraub S (1983). The Boston Naming Test. Lea and Febiger. [Google Scholar]
- Klein LA, & Buchanan JA (2009). Psychometric properties of the Pyramids and Palm Trees Test. Journal of Clinical and Experimental Neuropsychology, 31(7), 803–808. 10.1080/13803390802508926 [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Graham KS, Ellis AW, & Hodges JR (1998). Naming in semantic dementia–what matters? Neuropsychologia, 36(8), 775–784. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Mcclelland JL, Patterson K, Galton CJ, & Hodges JR (2001). No right to speak? The relationship between object naming and semantic impairment: Neuropsychological evidence and a computational model. Journal of Cognitive Neuroscience, 13(3), 341–356. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Pobric G, & Jefferies E (2009). Conceptual knowledge is underpinned by the temporal pole bilaterally: Convergent evidence from rTMS. Cerebral Cortex, 19(4), 832–8. 10.1093/cercor/bhn131 [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Sage K, Jones RW, & Mayberry EJ (2010). Coherent concepts are computed in the anterior temporal lobes. Proceedings of the National Academy of Sciences USA, 107(6), 2717–2722. 10.1073/pnas.0907307107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levelt WJM, Praamstra P, Meyer AS, Helenius P, & Salmelin R (1998). An MEG study of picture naming. Journal of Cognitive Neuroscience, 10(5), 553–567. [DOI] [PubMed] [Google Scholar]
- Livingstone M, & Hubel D (1988). Segregation of form, color, movement, and depth: Anatomy, physiology, and perception. Science, 240(4853), 740–749. [DOI] [PubMed] [Google Scholar]
- Mack WJ, Freed DM, Williams BW, & Henderson VW (1992). Boston Naming Test: Shortened versions for use in Alzheimer’s disease. Journal of Gerontology, 47(3), P154–P158. [DOI] [PubMed] [Google Scholar]
- Macoir J, Laforce R Jr., Brisson M, & Wilson MA (2015). Preservation of lexical-semantic knowledge of adjectives in the semantic variant of primary progressive aphasia: Implications for theoretical models of semantic memory. Journal of Neurolinguistics, 34, 1–14. 10.1016/j.jneuroling.2014.11.003 [DOI] [Google Scholar]
- Martin N, & Saffran EM (1990). Repetition and verbal STM in transcortical sensory aphasia: A case study. Brain and Language, 39(2), 254–288. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, & Phelps CH (2011). The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s and Dementia, 7(3), 263–269. https://doi.org/S1552-5260(11)00101-4 [pii] 10.1016/j.jalz.2011.03.005 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Rogalski E, Wieneke C, Cobia D, Rademaker A, Thompson C, & Weintraub S (2009). Neurology of anomia in the semantic variant of primary progressive aphasia. Brain, 132(9), 2553–2565. https://doi.org/awp138 [pii] 10.1093/brain/awp138 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AM, Tippett DC, Turner RS, & Friedman RB (2019). Long-term maintenance of anomia treatment effects in primary progressive aphasia. Neuropsychological Rehabilitation, 29(9), 1439–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AS, Sleiderink AM, & Levelt WJ (1998). Viewing and naming objects: Eye movements during noun phrase production. Cognition, 66(2), B25–B33. [DOI] [PubMed] [Google Scholar]
- Miller ZA, & Miller BL (2013). Chapter 5—Artistic creativity and dementia. In Finger S, Zaidel DW, Boller F, & Bogousslavsky J (Eds.), Progress in Brain Research (Vol. 204, pp. 99–112). Elsevier. 10.1016/B978-0-444-63287-6.00005-1 [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Badirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, & Chertkow H (2005). The Montreal Cognitive Assessment, MoCA: A Brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, & Benson DF (1998). Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology, 51(6), 1546–1554. [DOI] [PubMed] [Google Scholar]
- Noonan KA, Jefferies E, Eshana S, Garrard P, & Lambon Ralph MA (2013). Demonstrating the qualitative differences between semantic aphasia and semantic dementia: A novel exploration of nonverbal semantic processing. Behavioural Neurology, 26(1–2), 7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothdurft H-C (2006). Salience and target selection in visual search. Visual Cognition, 14(4–8), 514–542. 10.1080/13506280500194162 [DOI] [Google Scholar]
- Oldfield RC, & Wingfield A (1965). Response latencies in naming objects. Quarterly Journal of Experimental Psychology, 17(4), 273–281. 10.1080/17470216508416445 [DOI] [PubMed] [Google Scholar]
- Patterson K (2007). The reign of typicality in semantic memory. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 362(1481), 813–821. https://doi.org/1MW426N6VV32M223 [pii] 10.1098/rstb.2007.2090 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RJ, & Hodges JR (1999). Attention and executive deficits in Alzheimer’s disease: A critical review. Brain, 122(3), 383–404. [DOI] [PubMed] [Google Scholar]
- Raymer AM, Beeson P, Holland A, Kendall D, Maher LM, Martin N, Murray L, Rose M, Thompson CK, Turkstra L, Altmann L, Boyle M, Conway T, Hula W, Kearns K, Rapp B, Simmons-Mackie N, & Gonzalez Rothi LJ (2008). Translational research in aphasia: From neuroscience to neurorehabilitation. Journal of Speech, Language, and Hearing Research, 51(1), S259–75. 10.1044/1092-4388(2008/020) [DOI] [PubMed] [Google Scholar]
- Rayner K (2009). Eye movements and attention in reading, scene perception, and visual search. The Quarterly Journal of Experimental Psychology, 62(8), 1457–1506. [DOI] [PubMed] [Google Scholar]
- Reilly J (2016). How to constrain and maintain a lexicon for the treatment of progressive semantic naming deficits: Principles of item selection for formal semantic therapy. Neuropsychological Rehabilitation, 26(1), 126–156. 10.1080/09602011.2014.1003947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly J, Martin N, & Grossman M (2005). Verbal learning in semantic dementia: Is repetition priming a useful strategy? Aphasiology, 19, 329–339. [Google Scholar]
- Reilly J, Peelle JE, Antonucci SM, & Grossman M (2011). Anomia as a marker of distinct semantic memory impairments in Alzheimer’s disease and semantic dementia. Neuropsychology, 25(4), 413–26. 10.1037/a0022738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo M, Anderson SW, Dawson J, & Nawrot M (2000). Vision and cognition in Alzheimer’s disease. Neuropsychologia, 38(8), 1157–1169. 10.1016/S0028-3932(00)00023-3 [DOI] [PubMed] [Google Scholar]
- Rogers SL, & Friedman RB (2008). The underlying mechanisms of semantic memory loss in Alzheimer’s disease and semantic dementia. Neuropsychologia, 46(1), 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TT, Ivanoiu A, Patterson K, & Hodges JR (2006). Semantic memory in Alzheimer’s disease and the frontotemporal dementias: A longitudinal study of 236 patients. Neuropsychology, 20(3), 319–335. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Patterson K, Jefferies E, & Ralph MAL (2015). Disorders of representation and control in semantic cognition: Effects of familiarity, typicality, and specificity. Neuropsychologia, 76, 220–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckin M, Mesulam M-M, Rademaker AW, Voss JL, Weintraub S, Rogalski EJ, & Hurley RS (2016). Eye movements as probes of lexico-semantic processing in a patient with primary progressive aphasia. Neurocase, 22(1), 65–75. 10.1080/13554794.2015.1045523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckin M, Mesulam M-M, Voss JL, Huang W, Rogalski EJ, & Hurley RS (2016). Am I looking at a cat or a dog? Gaze in the semantic variant of primary progressive aphasia is subject to excessive taxonomic capture. Journal of Neurolinguistics, 37, 68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T, & Jackson M (1988). Lissauer on agnosia. Cognitive Neuropsychology, 5(2), 153–156. [Google Scholar]
- Snowden JS, Goulding PJ, & Neary D (1989). Semantic dementia: A form of circumscribed cerebral atrophy. Behavioural Neurology, 2, 167–182. [Google Scholar]
- Snowden JS, Harris JM, Saxon JA, Thompson JC, Richardson AM, Jones M, & Kobylecki C (2019). Naming and conceptual understanding in frontotemporal dementia. Cortex, 120, 22–35. 10.1016/j.cortex.2019.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson HE, Robson H, Lambon Ralph MA, & Jefferies E (2015). Varieties of semantic ‘access’ deficit in Wernicke’s aphasia and semantic aphasia. Brain, 138(12), 3776–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tippett DC, Hillis AE, & Tsapkini K (2015). Treatment of primary progressive aphasia. Current Treatment Options in Neurology, 17(8), 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh T (2004). Trail Making Test A and B: Normative data stratified by age and education. Archives of Clinical Neuropsychology, 19(2), 203–214. 10.1016/S0887-6177(03)00039-8 [DOI] [PubMed] [Google Scholar]
- Tsapkini K, Frangakis C, Gomez Y, Davis C, & Hillis AE (2014). Augmentation of spelling therapy with transcranial direct current stimulation in primary progressive aphasia: Preliminary results and challenges. Aphasiology, 28(8–9), 1112–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungrady MB, Flurie M, Zuckerman BM, Mirman D, & Reilly J (2019). Naming and knowing revisited: Eyetracking correlates of anomia in progressive aphasia. Frontiers in Human Neuroscience, 13, 354. 10.3389/fnhum.2019.00354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet M, Quentin R, Chanes L, Mitsumasu A, & Valero-Cabré A (2014). Frontal eye field, where art thou? Anatomy, function, and non-invasive manipulation of frontal regions involved in eye movements and associated cognitive operations. Frontiers in Integrative Neuroscience, 8. 10.3389/fnint.2014.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villard S, & Kiran S (2017). To what extent does attention underlie language in aphasia? Aphasiology, 31(10), 1226–1245. [Google Scholar]
- Viskontas IV, Boxer AL, Fesenko J, Matlin A, Heuer HW, Mirsky J, & Miller BL (2011). Visual search patterns in semantic dementia show paradoxical facilitation of binding processes. Neuropsychologia, 49(3), 468–78. 10.1016/j.neuropsychologia.2010.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK, & Shallice T (1984). Category specific semantic impairments. Brain, 107(3), 829–854. [DOI] [PubMed] [Google Scholar]
- Weintraub S, Besser L, Dodge HH, Teylan M, Ferris S, Goldstein FC, Giordani B, et al. (2018). Version 3 of the Alzheimer disease centers’ neuropsychological test batter in the uniform data set (UDS). Alzheimer Disease and Associated Disorders, 32(1), 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, et al. (2009). The Alzheimer’s disease centers’ uniform data set (UDS): The neuropsychological test battery. Alzheimer Disease and Associated Disorders, 23(2), 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney C, Kirk M, O’Sullivan J, Lambon Ralph MA, & Jefferies E (2011). The neural organization of semantic control: TMS evidence for a distributed network in left inferior frontal and posterior middle temporal gyrus. Cerebral Cortex, 21(5), 1066–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CE, Benjamin M, Gopinath K, Perlstein WM, Leonard CM, Rothi LJ, Conway T, Cato MA, Briggs R, & Crosson B (2008). Age-related changes in word retrieval: Role of bilateral frontal and subcortical networks. Neurobiology of Aging, 29(3), 436–451. https://doi.org/S0197-4580(06)00395-2 [pii]. [DOI] [PubMed] [Google Scholar]
- Woollams AM, Cooper-Pye E, Hodges JR, & Patterson K (2008). Anomia: A doubly typical signature of semantic dementia. Neuropsychologia, 46(10), 2503–2514. [DOI] [PubMed] [Google Scholar]
- Yarbus AL (1967). Eye movements and vision. Plenum Press. [Google Scholar]


