Abstract
Background:
Alzheimer’s disease (AD) is a heterogeneous and multifactorial disorder with an insidious onset and slowly progressive disease course. To date, there are no effective treatments, but biomarkers for early diagnosis and monitoring of disease progression offer a promising first step in developing and testing potential interventions. Cerebral vascular imaging biomarkers to assess the contributions of vascular dysfunction to AD are strongly recommended to be integrated into the current amyloid-β (Aβ) [A], tau [T], and neurodegeneration [(N)] - the “AT(N)” biomarker system for clinical research. However, the methodology is expensive and often requires invasive procedures to document cerebral vascular dysfunction. The retina has been used as a surrogate to study cerebral vascular changes. There is growing interest in the identification of retinal microvascular changes as a safe, easily accessible, low cost, and time-efficient approach to enhancing our understanding of the vascular pathogenesis associated with AD.
Evidence Acquisition:
A systemic review of the literature was performed regarding retinal vascular changes in AD and its prodromal stages, focusing on functional and structural changes of large retinal vessels (vessels visible on fundus photos) and microvasculature (pre-capillary arterioles, capillary, and post-capillary venules) that are invisible on fundus photos.
Results:
Static and dynamic retinal microvascular alterations such as retinal arterial wall motion, blood flow rate, and microvascular network density were reported in AD, mild cognitive impairment (MCI), and even in the preclinical stages of the disease. The data are somewhat controversial and inconsistent among the articles reviewed and were obtained based on cross-sectional studies that used different patient cohorts, equipment, techniques, and analysis methods.
Conclusions:
Retinal microvascular alterations exist across the AD spectrum. Further large scale, within-subject longitudinal studies using standardized imaging and analytical methods may advance our knowledge concerning vascular contributions to the pathogenesis of AD.
Keywords: retinal blood flow, retinal vasculature, Alzheimer’s disease (AD), mild cognitive impairment (MCI), preclinical AD
Late-onset sporadic Alzheimer’s disease (AD), an insidious onset, and slowly progressing neurodegenerative disorder, is the leading cause of disability in older people worldwide. Due to the aging population, dramatically increasing global prevalence of AD poses huge epidemiological burden on society and healthcare system in all countries in the world. There are currently about 35 million AD patients worldwide and 7 million new cases yearly (1). As a major public health concern, it is critical to effectively prevent and treat AD to slow down the growth of the disease. The cerebral neuronal dysfunction usually manifests decades before the appearance of the cognitive function decline, stressing the importance of sensitive and easy accessible biomarkers for early diagnosis and monitoring of disease progression (2). However, existing biomarkers are inefficient for diagnosis, prognosis and tracking, since they are often costly, require special equipment and facilities (MRI), invasive (bloodborne markers) and often inconclusive (3,4).
AD is considered to be a heterogeneous disease with multifactorial causes (4–6). The pathogenesis of AD is unclear. Although amyloid-β (Aβ) [A], tau [T], and neurodegeneration [(N)] - the AT(N)” biomarker system (7,8) has been recommended for AD diagnosis in clinical research, no causal relationship has been established between the A and T biomarkers and clinical symptoms (4). Accumulating evidence indicates vascular abnormalities are inextricably linked to the pathogenesis of AD (9,10). Patients with AD commonly have multiple vascular risk factors, and the control of these vascular risk factors helps to improve their cognitive function (11). Evidently, cerebral microvascular alterations, especially at the capillary level (12–15), are among the major pathogenic contributors to cognitive impairment and dementia (16). The decreased cerebral perfusion is reported before the development of cognitive function decline (17), as well as in patients with AD (18–20). Hence, the incorporation of cerebral vascular dysfunction biomarkers into the “AT(N)” biomarker system is needed (4). However, it is difficult to monitor the cerebral microvascular changes in vivo (21).
As an extension of the central nervous system, retinal structural and vascular alterations reflect the cerebral AD-related pathologic changes (22). Indeed, significant thinning of retinal nerve fiber layer (RNFL) and/or the combined ganglion and plexiform layer (GCIPL) that correlate with MRI brain volume decline are reported in patients with AD (23,24), and those patients with MCI who converted to AD (25). Hence, retinal structural changes have been suggested to be the markers in staging disease progression (25). Furthermore, clinic- and population-based studies using fundus photography have demonstrated less complex retinal vasculature, retinal arteriolar narrowing, retinal venular widening, and smaller, more tortuous retinal vessels in patients with AD, compared to cognitively normal controls (CN) (26–30). The present review summarizes the multiple retinal vascular imaging methods used to evaluate the human retinal vascular function with a special focus on the microvasculature that is invisible on fundus photos in patients with AD spectrum. The correlations between retina, brain, and AT[N] system were examined.
METHODS
PubMed and Google Scholar were searched using the keywords “retinal blood flow,” “retinal vasculature,” “Alzheimer’s disease,” “mild cognitive impairment,” and “preclinical AD.” All published articles regarding functional changes of large retinal vessels (vessel visible on fundus photo) and microvasculature (pre-capillary arterioles, capillary, and post-capillary venules) that are invisible on fundus photos are reviewed and included. The studies solely based on fundus photo images and retinal structural changes were excluded.
RESULTS
Static retinal microvascular structural alterations in the AD spectrum
As cerebral capillary dysfunction is related to AD-mediated neurodegeneration (31), it is crucial to study the retinal capillary network alterations. However, the resolution of fundus photography is not high enough to visualize the microvascular network (32). The introduction of optical coherence tomography angiography (OCTA), a non-invasive imaging modality, greatly facilitates visualization of retinal microvasculature (pre-capillary arterioles, capillary, post-capillary venules) that would otherwise be invisible with fundus photography (33). Therefore, OCTA greatly simplifies the study of the retinal microvasculature in AD. Two layers of the retinal microvascular network are often defined and studied by OCTA. These microvascular layers include the superficial vascular plexus (SVP) and deep vascular plexus (DVP). SVP sits in the RNFL and GCIPL, and DVP positions in the inner nuclear (INL) and outer plexiform (OPL) layers (Figs.1 and 2) (34). A summary of OCTA findings of retinal microvascular changes across the AD spectrum is provided in Table 1.
Fig.1. OCTA for Quantitative Analysis of Microvascular Network in Intra-retinal Layer.
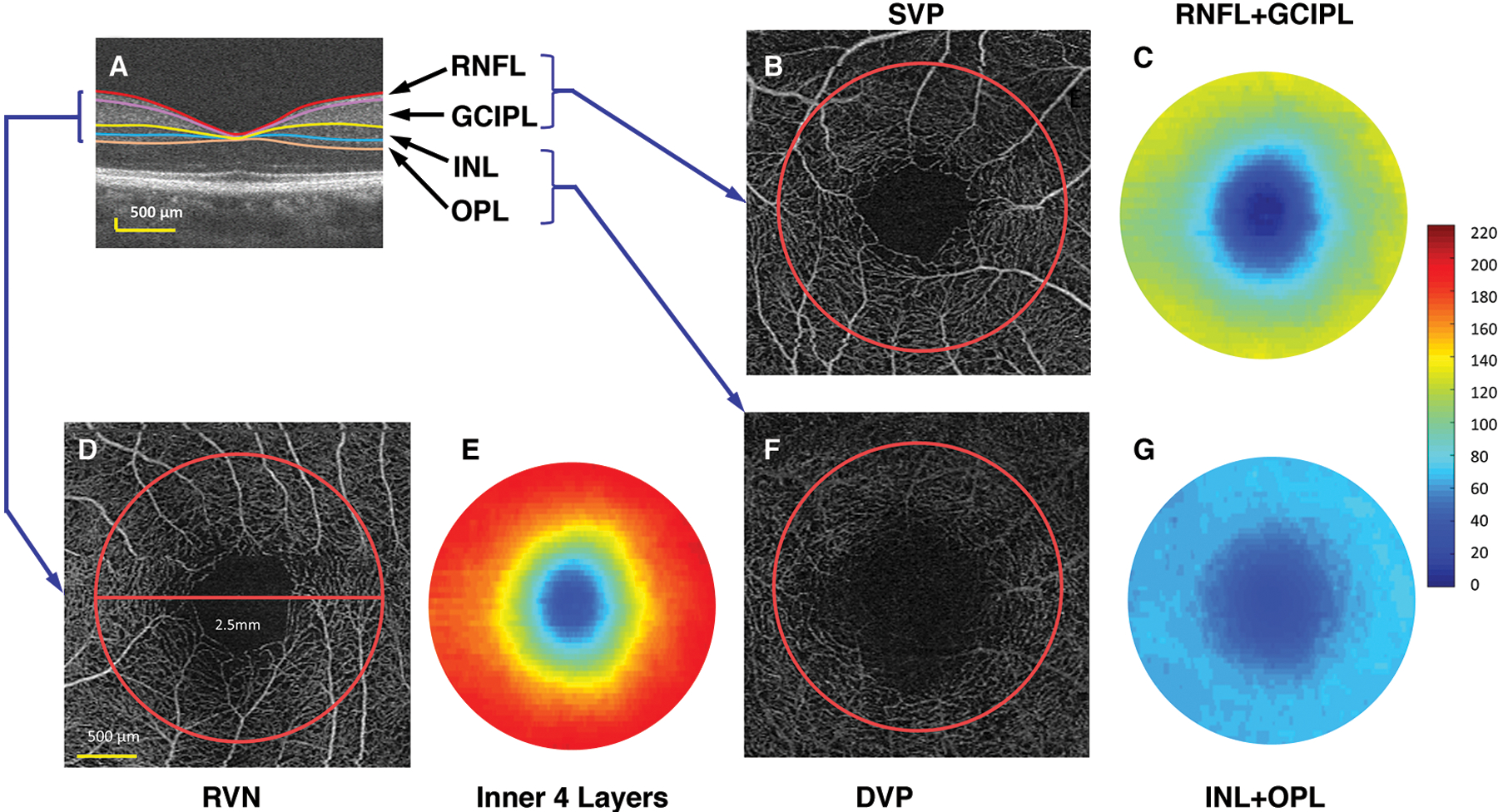
(A) Intraretinal layers were imaged using UHR-OCT and segmented using the Orion software. RNFL: retinal nerve fiber layer; GCIPL: combined ganglion cell and inner plexiform layer; INL: inner nuclear layer; OPL: outer plexiform layer. (B) The SVP (superior vascular plexus) of a scan of 3 × 3 mm imaged using OCTA with the analyzed area of a 2.5-mm disc (red circle). (C) The thickness map of the RNFL + GCIPL in a circular area (ϕ 2.5 mm). The VVD (volumetric vascular density) of the SVP (VVDs) was the vessel density of the SVP (analyzed as fractal dimension Dbox) divided by the tissue volume of the RNFL and GCIPL in the disc (ϕ 2.5 mm). (D) The RVN: retinal vascular network. (E) The thickness map of the inner retina, including RNFL, GCIPL, INL, and OPL. The VVDr was the vessel density of the RVN (analyzed as fractal dimension Dbox) divided by the tissue volume of the inner retina. (F) The DVP: deep vascular plexus (G) The thickness map of the INL and OPL in a disc (ϕ 2.5 mm). The VVDd was the vessel density of the DVP (analyzed as fractal dimension Dbox) divided by the tissue volume of the INL and OPL (cited from Lin et al.(34) under open-access license)
Fig. 2. Representative images of the retinal microvascular networks imaged using optical coherence tomography angiography (OCTA).
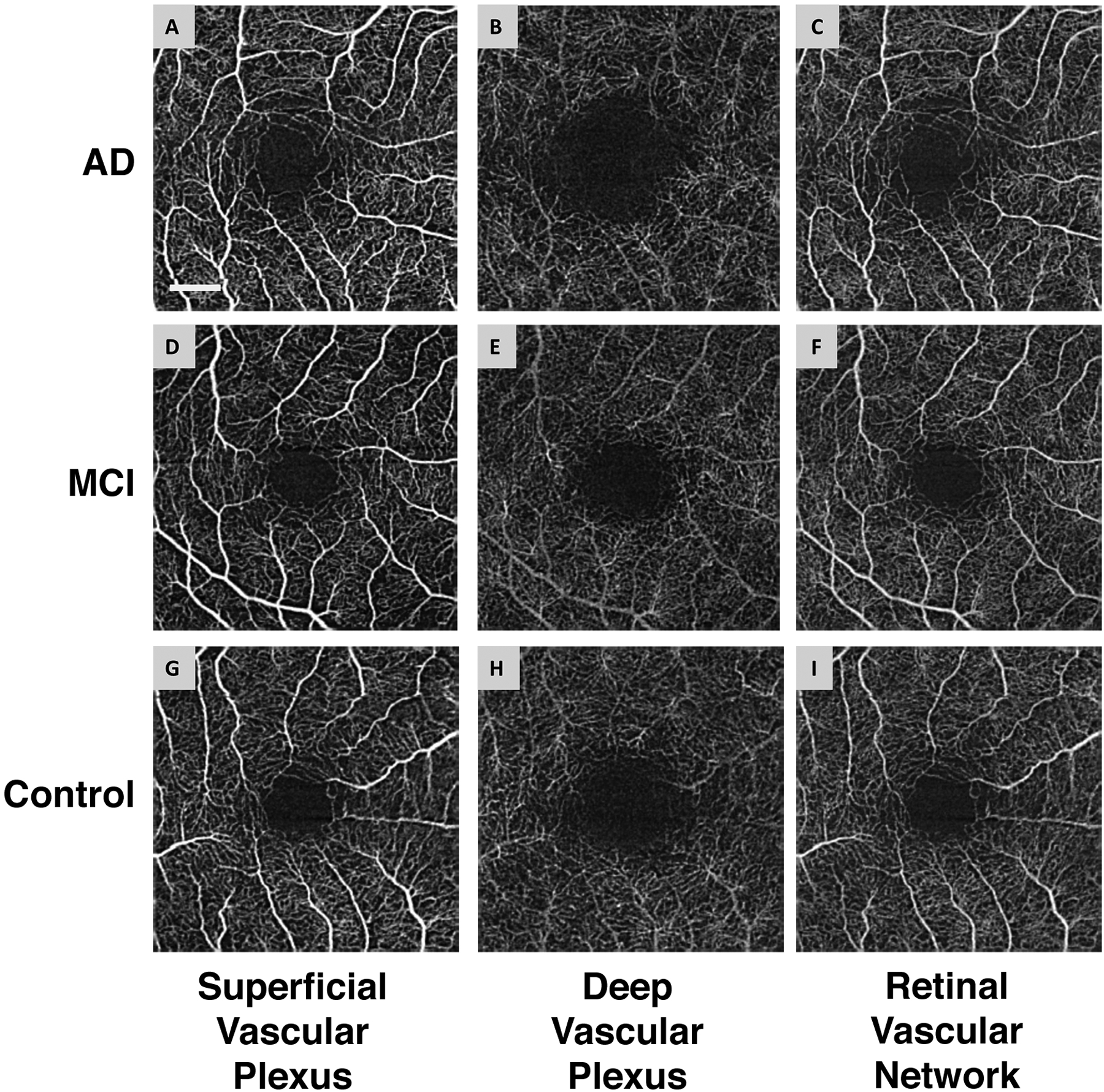
Patients with Alzheimer’s disease (AD) (A, B, and C) and mild cognitive impairment (MCI) (D, E, and F), as well as a control subject (G, H, and I), were imaged. Compared to the normal control (G and I), the large vessels in AD and MCI patients had similar densities in the superficial vascular plexus (A and D) and retinal vascular network (C and F) but showed some degrees of tortuosity. The microvessels in the deep vascular plexus in AD (B) and MCI (E) patients appeared to be less dense compared to the normal control (H). Note: the deep vascular plexus images are raw images, showing the graphic projection artifact of the large vessels. Bar = 0.5 mm. (Reproduced and used with permission obtained through RightsLink)
Table 1.
OCTA Findings of Retinal Microvascular Changes in AD spectrum
| Authors | Disease | No. of pts | ATN biomarker | OCTA findings | Correlation | OCT device |
|---|---|---|---|---|---|---|
| van de Kreeke et al.57 | pre-AD vs. CN | 13 vs. 111 | Aβ PET | VD higher in RVN in pre-AD; | VD & BPND | Zeiss (Cirrus 5000 Angioplex) |
| same FAZ size | ||||||
| O’Bryhim et al.54 | pre-AD vs. CN | 14 vs. 18 | CSF or Aβ PET | enlarged FAZ in pre-AD | inner foveal thinning | Avanti RTVue XR (Optovue) |
| Bulut et al.36 | AD vs. CN | 26 vs. 26 | none | VD lower in RVN; | MMSE | Zeiss (Cirrus 5000 Angioplex) |
| enlarged FAZ | ||||||
| Jiang et al.35 | AD vs. MCI vs. CN | 12 vs. 19 vs. 21 | none | AD vs. CN: VD lower in RVN, SVP, and DVP; | GCIPL & DVP VD | Zeiss (Cirrus 5000 Angioplex) |
| MCI vs. CN: VD lower at superonasal quant DVP; | ||||||
| Trend of VD loss from CN to MCI to AD | ||||||
| Grewal et al.40 | AD vs. CN | 1 vs. 1 (MZ) | none | VD lower in SVP; | n/a | Zeiss (Cirrus 5000 Angioplex) |
| enlarged FAZ | ||||||
| Yoon et al.37 | AD vs. MCI vs. CN | 39 vs. 37 vs. 133 | none | AD vs. MCI & AD vs. NC: VD lower in SVP; | n/a | Zeiss (Cirrus 5000 Angioplex) |
| MCI vs. NC: no difference | ||||||
| Yoon et al.38 | AD vs. MCI | 9 vs. 7 | none | AD vs MCI: VD lower in SVP | inverse correlation between ILV with VD | Zeiss (Cirrus 5000 Angioplex) |
| Zabel et al.41 | AD vs. CN | 27 vs. 27 | Aβ PET | AD vs. NC: DV lower in DVP; | n/a | Avanti RTVue XR (Optovue) |
| enlarged FAZ | ||||||
| Lahme et al.10 | AD vs. CN | 36 vs. 38 | CSF | VD lower in SVP & RPCs | Fazekas scale WMT | Avanti RTVue XR (Optovue) |
| but no CSF Aβ or tau level | ||||||
| Zhang et al.39 | aMCI/eAD vs. CN | 32 vs. 16 | none | VD lower in SVP | MoCA | Avanti RTVue XR (Optovue) |
| Querques et al.49 | AD vs. MCI vs. CN | 12 vs. 12 vs. 32 | CSF | none | n/a | Zeiss (Cirrus 5000 Angioplex) |
| den Haan et al.52 | AD vs. CN | 48 vs. 38 | CSF or Aβ PET | none | n/a | Zeiss (Cirrus 5000 Angioplex) |
AD: Alzheimer’s disease; pre-AD: preclinical AD; MCI: mild cognitive impairment; CN: cognitively normal control; aMCI/eAD: amnestic MCI/early AD; pts: patients; CSF: CSF Aβ PET and/or Tau; WMT: white matter tissue; OCTA: optical coherence tomography angiography; RVN: retinal vascular network; SVP: superior vascular plexus; DVP: deep vascular plexus; RPCs: radial peripapillary capillaries of optic nerve head; VD: vessel density; FAZ: fovea avascular zone; BPND: parametric global cortical non-displaceable binding protein (Aβ); ILV: inferolateral ventricle volume; MZ: monozygotic twins.
Allowing for the differences in patient cohorts, equipment and techniques, several groups reported significantly reduced densities of SVP in patients with late-onset and sporadic AD, compared to CN (10,35–40). The loss of retinal microvasculature is not only restricted to the macular area but also at radial peripapillary capillaries (RPC), indicating the widespread existence of this loss (10). The reduced density of DVP in AD (35,41) and MCI were also reported (35), and associated with GCIPL thinning (35). Furthermore, more profound loss of DVP in AD compared to MCI indicated that retinal vascular abnormalities might contribute to the potential conversion from MCI to AD (35). More interestingly, the SVP density was found to be highly correlated with the Fazekas scale (42) of brain white matter (10), and negatively associated with the expansion of inferolateral ventricle volume (ILV) (37). The Fazekas scale is used to quantify the amount of white matter T2 hyperintense lesions. IVL is known to strongly correlate with cognitive function decline in patients with MCI and AD (38,43). The reduction in retinal vessel density echoes the cerebral hypoperfusion measured by brain MRI in patients with both AD and MCI (18–20). The diminished retinal vascular density could be attributed to aging and vascular risk factors such as diabetes and hypertension. In addition, Aβ is also known to accumulate in small vessel walls and contributes to the degeneration of the vascular smooth muscle cells and loss of vessel wall integrity (44–46), resulting in cerebral amyloidal angiopathy (47,48).
In contrast, Querques et al. did not find a significant difference in retinal microvascular density using OCTA in patients with AD, compared to CN. Of note, type 2 diabetes mellitus (T2DM) was an exclusion criterion in this study (49), whereas T2DM is a major risk factor for AD (50,51). Similar results were reported by den Haan et al. (52), when studying a group of AD patients who were relatively young: from 57.3 to 73.5 years (mean ± standard deviation: 65.4 ± 8.1). It is known that vascular dysfunction may not contribute significantly in early-onset AD (53), which might be one of the underlying reasons that no significant difference in retinal microvascular density was found in their study.
The size of the fovea avascular zone (FAZ) is correlated to the area with no capillary perfusion, and the enlarged FAZ indicates the loss of the retinal micro-vessels (Fig.3) (54,55). The enlarged FAZ was found in preclinical AD (cognitively normal individuals with positive AD biomarkers) (56), similarly as in AD (36,40,41). The enlarged FAZ is correlated with inner foveal thinning (49,56), sustained that retinal microvascular and neuronal alterations could happen very early before the patients have any cognitive function decline. Interestingly, no difference of FAZ size, but significantly higher retinal microvessel density in both macular and optic nerve head in preclinical AD was reported by van de Kreeke et al (57). The findings were thought to be due to the inflammation and increased blood flow that related to amyloid accumulation in the early stage, mirroring the CNS changes (58).
Fig. 3. Foveal Avascular Zone (FAZ) and perfusion map.
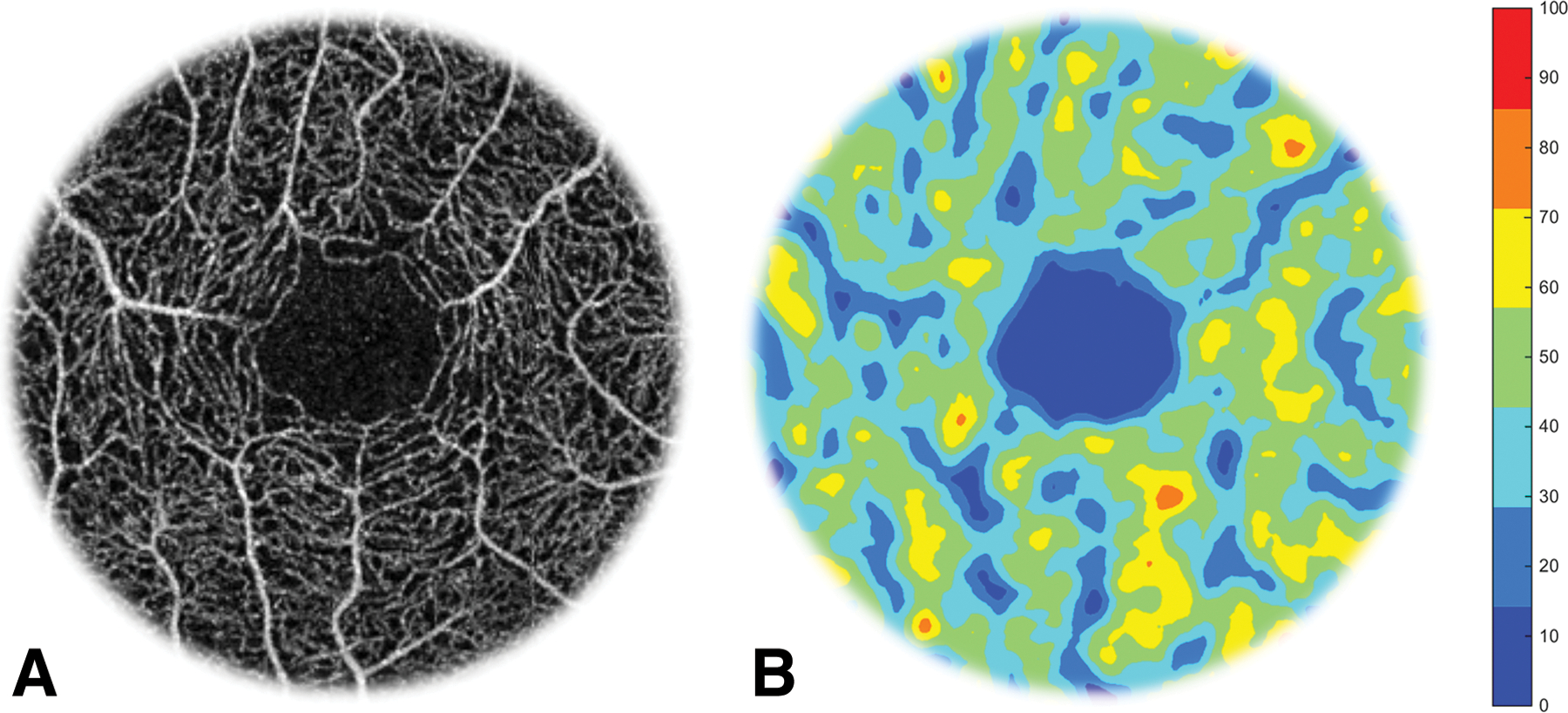
The enface angiography vessel image was obtained using optical coherence tomography (OCT)angiography (Avanti OptoVue; OptoVue). The FAZ is located in the center (the dark area) of the macula in the enface angiography of the retina (A). To visualize capillary perfusion, a Gaussian density filter (4 × 4 pixels) was used to process the skeletonized vascular images and the capillary density was color coded to create a capillary perfusion map (B), which shows the non-perfusion in the center of the fovea. The bar denotes to percent regional perfusion rate.
Given the thinning of RNFL and GCIPL evidently coexists with the vascular dysfunction through the disease course of AD (35,38,39). Simultaneous analysis of both the microvasculature and the neural structure by measuring the volumetric vascular density (Fig.1) (34,59) may reveal the more precise information of retinal vascular changes in AD spectrum and could be potentially used in the future retinal vascular study.
Functional retinal vascular alterations in the AD spectrum
In addition to the static microvascular structural alterations, impaired cerebral vasomotion (the spontaneous rhythmic modulation of arterial diameter) and related blood flow alterations are known to contribute to the pathogenesis of AD (60,61). Dysregulation of cerebral blood flow such as reduced blood flow when resting and responding to neuronal stimulation in AD is evident (62,63). To explore the retinal vascular dysfunction in AD, various types of equipment have been used to study the retinal blood flow and vessel reactions. A dynamic vessel analyzer (DVA; Imedos Systems UG, Jena, Germany) measures the retinal vessel responses to diffuse luminance flicker (64–67). The changes of retinal arterial and venous diameter visible on the fundus photos were measured at baseline, during and after a flickering-light stimulation (64–67). Significantly reduced retinal arterial dilation response to flicker light impulse was found in patients with AD and MCI, compared to NC, and these were correlated with CSF Aβ level (49). In contrast, Golzan et al. (68) reported increased arterial pulsation that was positively associated with neocortical Aβ scores in older adults with subjective memory loss and AD. Their analysis was performed after adjusting for comorbidities such as hypertension, diabetes, and smoking. Similarly, increased but delayed arterial vasodilatation in patients with AD compared to NC and MCI were found by Kotliar et al. (69), whereas decreased arterial dilation was found in MCI compared to NC (69). The reasons behind these conflicting reports may be due to different patient cohorts, dissimilar techniques, or other ophthalmologic and systemic conditions such as diabetes that are known to impact retinal vascular functions (66,67). However, the observed abnormal arterial wall movement indicated possible neurovascular coupling impairment secondary to arterial endothelial dysfunction (70) or vessel rigidity (71). Chronic hypoperfusion in the brain and thickened basal membrane of cerebral vessels are reported in patients with AD (72). Furthermore, the alteration of vascular response in subjects with MCI and subjective memory loss implies the vascular dysfunction presented early in the disease course (49,69).
Laser Doppler retinal blood flowmetry (CLBF 100, Canon, Tokyo, Japan) was used to measure retinal blood flow rate (BFR) based on the blood column diameter and the centerline blood velocity in the central retinal artery and vein (73). The measurement is not synchronized with the cardiac cycle. Significantly reduced venous BFR in patients with AD, compared to CN and MCI, and in patients with MCI compared to CN were reported (74,75). Compared to CLBF, which measure central retinal arterial and venular function, the RFI system (Optical Imaging Ltd., Rehovot, Israel) assesses the blood flow rate in pre-capillary arterioles and post-capillary venules (76,77). The fundus camera-based device uses the motion of red blood cell clusters as the intrinsic motion contrast to non-invasively measure the blood flow velocity and blood flow rate (RBF) in the pre-capillary arterioles and post-capillary venules, while synchronizing with the cardiac cycle to ensure the measurement at the same time point of the cardiac rhythm (Fig. 4) (76,77). Significantly lower RBFs in both arterioles and venules of patients with AD and MCI compared to NC were reported (78). There was no significant difference in age, gender, heart rate, blood pressure, or vascular risk factors among these three groups (78). More interestingly, the retinal tissue perfusion (RTP) measured based on the BFV and tissue volume in which the blood supply perfuse, was significantly lower in patients with AD compared to NC (79). The RTP was also found to be correlated with GCIPL thickness in these patients with AD (Fig.4) (79). These findings appeared to echo cerebral hypoperfusion (18–20), and the reduced central retinal arterial dilation obtained by the DVA test (49). A summary of retinal vascular findings as possible image biomarkers within the AD spectrum is provided in Table 2.
Fig.4. Retinal blood flow and tissue volume of the inner retina.
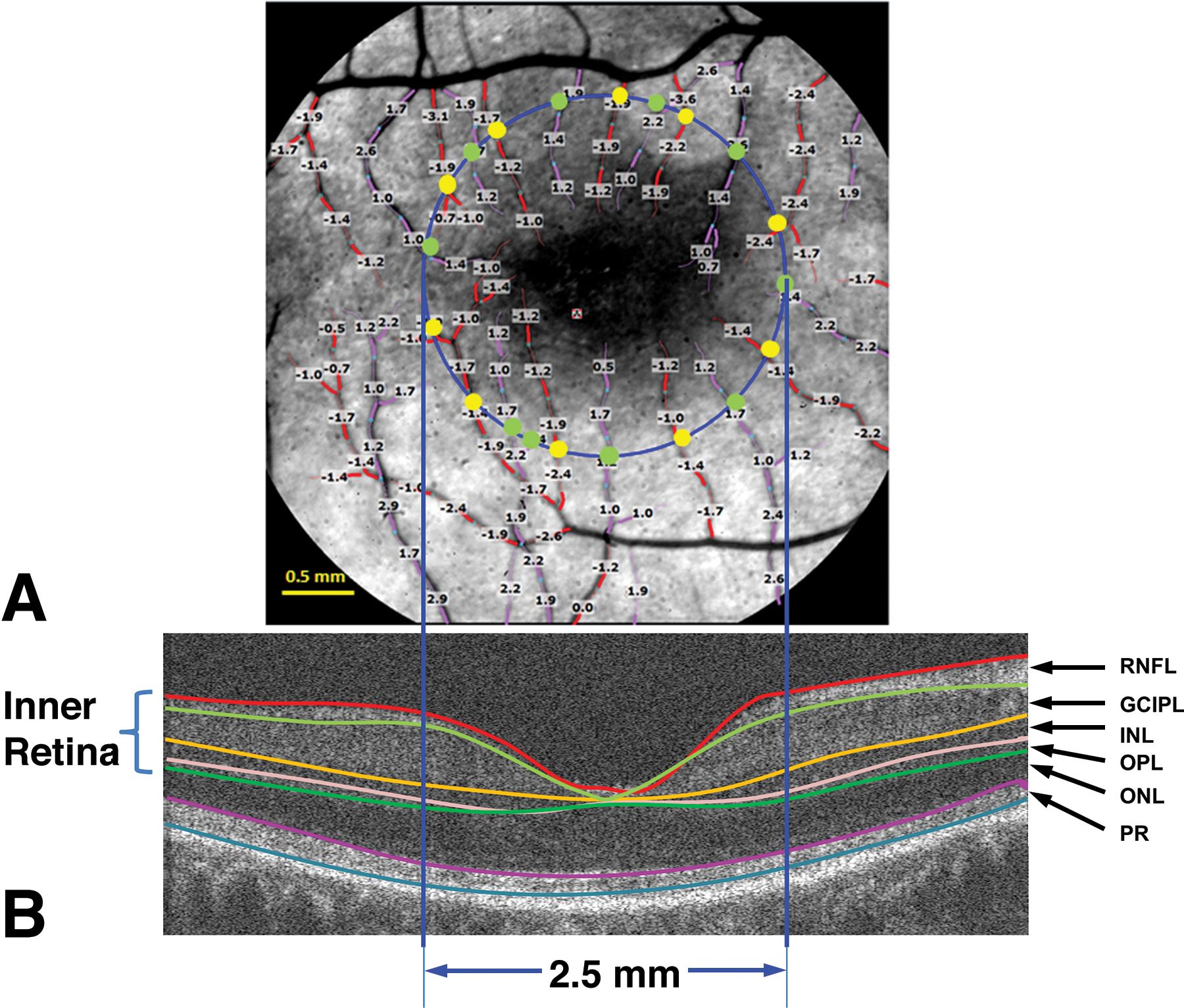
a. A representative image of the retina from an AD patient was imaged using the RFI with a FOV of 4.3 × 4.3 mm (20-degrees setting) centered on the fovea, corresponding to the dark area in the center. The blood flow velocities (mm/s) were overlaid with the vessels. The arterioles marked in red have negative velocity values, indicating that blood is flowing away from the heart (flow moving towards the fovea). The venules marked in purple have positive velocity values, indicating that blood is flowing towards the heart. To analyze the blood flow in the macula, a 2.5 mm circle (blue) centered on the fovea was outlined. Vessel diameters of the vessels crossing the circle were measured at the locations marked as yellow and green dots. The arteriolar blood flow of these arterioles was calculated using the velocity and diameter (yellow dot). The venular blood flow of these venules was also calculated using velocity and diameter (green dot). All the flow measurements in the arterioles (all yellow dots) were summed to obtain the total arteriolar flow of the macula. Similarly, all the flow measurements in the venules (all green dots) were summed to obtain the total venular flow of the macula. b. To measure the tissue volume, the same eye was imaged using a custom UHR-OCT with a raster scan of 6 × 6 mm. To calculate the tissue-dependent blood perfusion, the volumetric tissue volume of the inner retina, including RFNL, GCIPL, INL, and OPL, was measured using segmentation software (Orion, Voxeleron) in the round area with a diameter of 2.5 mm centered on the fovea. RNFL: retinal nerve fiber layer; GCIPL: ganglion cell-inner plexiform layer; INL: inner nuclear layer; OPL: outer plexiform layer; ONL: outer nuclear layer; and PR: retinal photoreceptor (cited from Gameiro et al.(79) under open-access license).
Table 2.
Retinal Vascular Findings as Possible Image Biomarkers in AD spectrum
| Retinal finding | Imaging modality | Advantage | Disadvantage |
|---|---|---|---|
| VD in SVP and DVP10,35–41,52,54 | OCTA | wide availability, no pupil dilation |
no standardized analysis, requirement of clear ocular media |
| FAZ zone size36,40,41,54,56,57 | OCTA | wide availability no pupil dilation |
no standardized analysis, requirement of clear ocular media |
| Vessel response to flicker68,69 | Dynamic vessel analyzer | dynamic analysis of vasodilatation | no wide accessibility, large vessels only |
| BFR74,75 | Laser Doppler retinal blood flowmetry | direct measurement of blood flow | relative measurement of all flow information, no synchronization of heartbeat, larger vessels only no wide accessibility |
| BFR78 | RFI | direct measurement of blood velocity and flow measurements in venules and arterioles synchronization of heat beat. |
Bright visible light, pupil dilation, requirement of clear ocular media no wide accessibility |
| RTP79 | RFI | direct measurement of tissue perfusion direct measurement of blood velocity and flow measurements in venules and arterioles synchronization of heat beat. |
Bright visible light, pupil dilation, requirement of clear ocular media no wide accessibility requirement of retinal tissue volume information |
AD: Alzheimer’s disease; VD: vessel density, SVP; SVP: superficial vascular plexus; DVP; deep vascular plexus; OCTA: optical coherence tomography angiography; FAZ: foveal avascular zone; BFR: blood flow rate; RFI: retinal function imager; RTP: retinal tissue perfusion.
CONCLUSIONS
In summary, there is a growing body of evidence showing important vascular contributions associated with AD. Both static and dynamic retinal vascular changes have been reported in patients with AD, MCI, and even in preclinical stages, supporting the strong vascular contribution to the pathogenesis of AD. The current literature on retinal vascular changes in AD, however, is inconsistent and likely due to different equipment and analysis methods as well as different patients’ characteristics with respect to age range, disease stage, comorbidities, diagnostic criteria, and differing types of neurocognitive assessments. Furthermore, almost all the studies are cross-sectional comparisons of patients with AD versus CN, although some of them involved MCI and preclinical AD patients. To understand the possible causal link between vascular dysfunction and AD, it will be important to study biomarkers associated with disease onset and progression using a large-scale prospective within-subject design, following retinal vasculature among individuals in the early preclinical stages to (subjective memory complaints) to symptomatic AD.
Adults over 50 usually have reduced vision due to presbyopia and require routine eye care with follow-up. Retinal imaging is usually necessary and convenient to perform during their regular eye exam. Non-invasive, cost-effective, and easily accessible retinal imaging, such as OCTA, can become a universally available screening tool to identify persons at high risk of AD based on retinal vascular changes. Once developed, these retinal vascular biomarkers, especially at the capillary level, may also be used to monitor disease progression and therapeutic efficacy. To better understand the link between the eye and brain vasculature and other neural structures, future studies with simultaneous imaging of the eye and the brain in patients with AD, MCI, and its preclinical stages are also warranted to further validate the applications of the retinal imaging in AD.
Acknowledgments
Grant/financial support: The work has been supported by the NIH Center Grant P30 EY014801, NIH NINDS 1R01NS111115-01 (Wang), Ed and Ethel Moore Alzheimer’s Disease Research Program (Florida Health, 20A05, to Jiang), and a grant from Research to Prevent Blindness (RPB).
Footnotes
Financial Disclosures: none
References
- 1.Dementia: Fact Sheet. World Health Organization;2019 (WHO).; 2019.
- 2.Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: the challenge of the second century. Sci Transl Med. 2011;3:77sr1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma N, Singh AN. Exploring Biomarkers for Alzheimer’s Disease. J Clin Diagn Res. 2016;10:KE01–KE06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sweeney MD, Montagne A, Sagare AP, Nation DA, Schneider LS, Chui HC, Harrington MG, Pa J, Law M, Wang DJJ, Jacobs RE, Doubal FN, Ramirez J, Black SE, Nedergaard M, Benveniste H, Dichgans M, Iadecola C, Love S, Bath PM, Markus HS, Salman RA, Allan SM, Quinn TJ, Kalaria RN, Werring DJ, Carare RO, Touyz RM, Williams SCR, Moskowitz MA, Katusic ZS, Lutz SE, Lazarov O, Minshall RD, Rehman J, Davis TP, Wellington CL, Gonzalez HM, Yuan C, Lockhart SN, Hughes TM, Chen CLH, Sachdev P, O’Brien JT, Skoog I, Pantoni L, Gustafson DR, Biessels GJ, Wallin A, Smith EE, Mok V, Wong A, Passmore P, Barkof F, Muller M, Breteler MMB, Roman GC, Hamel E, Seshadri S, Gottesman RF, van Buchem MA, Arvanitakis Z, Schneider JA, Drewes LR, Hachinski V, Finch CE, Toga AW, Wardlaw JM, Zlokovic BV. Vascular dysfunction-The disregarded partner of Alzheimer’s disease. Alzheimers Dement. 2019;15:158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nation DA, Sweeney MD, Montagne A, Sagare AP, D’Orazio LM, Pachicano M, Sepehrband F, Nelson AR, Buennagel DP, Harrington MG, Benzinger TLS, Fagan AM, Ringman JM, Schneider LS, Morris JC, Chui HC, Law M, Toga AW, Zlokovic BV. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25:270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson AR, Sweeney MD, Sagare AP, Zlokovic BV. Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer’s disease. Biochim Biophys Acta. 2016;1862:887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, Hampel H, Jagust WJ, Johnson KA, Knopman DS, Petersen RC, Scheltens P, Sperling RA, Dubois B. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87:539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santos CY, Snyder PJ, Wu WC, Zhang M, Echeverria A, Alber J. Pathophysiologic relationship between Alzheimer’s disease, cerebrovascular disease, and cardiovascular risk: A review and synthesis. Alzheimers Dement (Amst). 2017;7:69–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lahme L, Esser EL, Mihailovic N, Schubert F, Lauermann J, Johnen A, Eter N, Duning T, Alnawaiseh M. Evaluation of Ocular Perfusion in Alzheimer’s Disease Using Optical Coherence Tomography Angiography. J Alzheimers Dis. 2018;66:1745–1752. [DOI] [PubMed] [Google Scholar]
- 11.de la Torre JC. Is Alzheimer’s disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 2004;3:184–190. [DOI] [PubMed] [Google Scholar]
- 12.Hunter JM, Kwan J, Malek-Ahmadi M, Maarouf CL, Kokjohn TA, Belden C, Sabbagh MN, Beach TG, Roher AE. Morphological and pathological evolution of the brain microcirculation in aging and Alzheimer’s disease. PLoS One. 2012;7:e36893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown WR, Thore CR. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol. 2011;37:56–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhat NR. Vasculoprotection as a Convergent, Multi-Targeted Mechanism of Anti-AD Therapeutics and Interventions. J Alzheimers Dis. 2015;46:581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ostergaard L, Jespersen SN, Engedahl T, Gutierrez JE, Ashkanian M, Hansen MB, Eskildsen S, Mouridsen K. Capillary dysfunction: its detection and causative role in dementias and stroke. Curr Neurol Neurosci Rep. 2015;15:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D, Lamb BT, Montine TJ, Nedergaard M, Schaffer CB, Schneider JA, Wellington C, Wilcock DM, Zipfel GJ, Zlokovic B, Bain LJ, Bosetti F, Galis ZS, Koroshetz W, Carrillo MC. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers Dement. 2015;11:710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park KW, Yoon HJ, Kang DY, Kim BC, Kim S, Kim JW. Regional cerebral blood flow differences in patients with mild cognitive impairment between those who did and did not develop Alzheimer’s disease. Psychiatry Res. 2012;203:201–206. [DOI] [PubMed] [Google Scholar]
- 18.Hays CC, Zlatar ZZ, Wierenga CE. The Utility of Cerebral Blood Flow as a Biomarker of Preclinical Alzheimer’s Disease. Cell Mol Neurobiol. 2016;36:167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruitenberg A, Den HT, Bakker SL, van Swieten JC, Koudstaal PJ, Hofman A, Breteler MM. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol. 2005;57:789–794. [DOI] [PubMed] [Google Scholar]
- 20.Austin BP, Nair VA, Meier TB, Xu G, Rowley HA, Carlsson CM, Johnson SC, Prabhakaran V. Effects of hypoperfusion in Alzheimer’s disease. J Alzheimers Dis. 2011;26 Suppl 3:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith EE, Beaudin AE. New insights into cerebral small vessel disease and vascular cognitive impairment from MRI. Curr Opin Neurol. 2018;31:36–43. [DOI] [PubMed] [Google Scholar]
- 22.Ngolab J, Honma P, Rissman RA. Reflections on the Utility of the Retina as a Biomarker for Alzheimer’s Disease: A Literature Review. Neurol Ther. 2019;8:57–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.den HJ, Janssen SF, van de Kreeke JA, Scheltens P, Verbraak FD, Bouwman FH. Retinal thickness correlates with parietal cortical atrophy in early-onset Alzheimer’s disease and controls. Alzheimers Dement (Amst). 2018;10:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu S, Ong YT, Hilal S, Loke YM, Wong TY, Chen CL, Cheung CY, Zhou J. The Association Between Retinal Neuronal Layer and Brain Structure is Disrupted in Patients with Cognitive Impairment and Alzheimer’s Disease. J Alzheimers Dis. 2016;54:585–595. [DOI] [PubMed] [Google Scholar]
- 25.Choi SH, Park SJ, Kim NR. Macular Ganglion Cell -Inner Plexiform Layer Thickness Is Associated with Clinical Progression in Mild Cognitive Impairment and Alzheimers Disease. PLoS One. 2016;11:e0162202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheung CY, Ong YT, Ikram MK, Ong SY, Li X, Hilal S, Catindig JA, Venketasubramanian N, Yap P, Seow D, Chen CP, Wong TY. Microvascular network alterations in the retina of patients with Alzheimer’s disease. Alzheimers Dement. 2014;10:135–142. [DOI] [PubMed] [Google Scholar]
- 27.Frost S, Kanagasingam Y, Sohrabi H, Vignarajan J, Bourgeat P, Salvado O, Villemagne V, Rowe CC, Macaulay SL, Szoeke C, Ellis KA, Ames D, Masters CL, Rainey-Smith S, Martins RN, AIBL Research Group. Retinal vascular biomarkers for early detection and monitoring of Alzheimer’s disease. Transl Psychiatry. 2013;3:e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams MA, McGowan AJ, Cardwell CR, Cheung CY, Craig D, Passmore P, Silvestri G, Maxwell AP, McKay GJ. Retinal microvascular network attenuation in Alzheimer’s disease. Alzheimers Dement (Amst). 2015;1:229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheung CY, Ong YT, Ikram MK, Chen C, Wong TY. Retinal microvasculature in Alzheimer’s disease. J Alzheimers Dis. 2014;42 Suppl 4:S339–S352. [DOI] [PubMed] [Google Scholar]
- 30.Cheung CY, Chan VTT, Mok VC, Chen C, Wong TY. Potential retinal biomarkers for dementia: what is new? Curr Opin Neurol. 2019;32:82–91. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen RB, Egefjord L, Angleys H, Mouridsen K, Gejl M, Moller A, Brock B, Braendgaard H, Gottrup H, Rungby J, Eskildsen SF, Ostergaard L. Capillary dysfunction is associated with symptom severity and neurodegeneration in Alzheimer’s disease. Alzheimers Dement. 2017;13:1143–1153. [DOI] [PubMed] [Google Scholar]
- 32.Spaide RF, Klancnik JM Jr., Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015;133:45–50. [DOI] [PubMed] [Google Scholar]
- 33.Fingler J, Readhead C, Schwartz DM, Fraser SE. Phase-contrast OCT imaging of transverse flows in the mouse retina and choroid. Invest Ophthalmol Vis Sci. 2008;49:5055–5059. [DOI] [PubMed] [Google Scholar]
- 34.Lin Y, Jiang H, Liu Y, Rosa GG, Gregori G, Dong C, Rundek T, Wang J. Age-Related Alterations in Retinal Tissue Perfusion and Volumetric Vessel Density. Invest Ophthalmol Vis Sci. 2019;60:685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang H, Wei Y, Shi Y, Wright CB, Sun X, Gregori G, Zheng F, Vanner EA, Lam BL, Rundek T, Wang J. Altered Macular Microvasculature in Mild Cognitive Impairment and Alzheimer Disease. J Neuroophthalmol. 2018;38:292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bulut M, Kurtulus F, Gozkaya O, Erol MK, Cengiz A, Akidan M, Yaman A. Evaluation of optical coherence tomography angiographic findings in Alzheimer’s type dementia. Br J Ophthalmol. 2018;102:233–237. [DOI] [PubMed] [Google Scholar]
- 37.Yoon SP, Thompson AC, Polascik BW, Calixte C, Burke JR, Petrella JR, Grewal DS, Fekrat S. Correlation of OCTA and Volumetric MRI in Mild Cognitive Impairment and Alzheimer’s Disease. Ophthalmic Surg Lasers Imaging Retina. 2019;50:709–718. [DOI] [PubMed] [Google Scholar]
- 38.Yoon SP, Grewal DS, Thompson AC, Polascik BW, Dunn C, Burke JR, Fekrat S. Retinal Microvascular and Neurodegenerative Changes in Alzheimer’s Disease and Mild Cognitive Impairment Compared with Control Participants. Ophthalmol Retina. 2019;3:489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang YS, Zhou N, Knoll BM, Samra S, Ward MR, Weintraub S, Fawzi AA. Parafoveal vessel loss and correlation between peripapillary vessel density and cognitive performance in amnestic mild cognitive impairment and early Alzheimer’s Disease on optical coherence tomography angiography. PLoS One. 2019;14:e0214685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grewal DS, Polascik BW, Hoffmeyer GC, Fekrat S. Assessment of Differences in Retinal Microvasculature Using OCT Angiography in Alzheimer’s Disease: A Twin Discordance Report. Ophthalmic Surg Lasers Imaging Retina. 2018;49:440–444. [DOI] [PubMed] [Google Scholar]
- 41.Zabel P, Kaluzny JJ, Wilkosc-Debczynska M, Gebska-Toloczko M, Suwala K, Zabel K, Zaron A, Kucharski R, Araszkiewicz A. Comparison of Retinal Microvasculature in Patients With Alzheimer’s Disease and Primary Open-Angle Glaucoma by Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci. 2019;60:3447–3455. [DOI] [PubMed] [Google Scholar]
- 42.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. [DOI] [PubMed] [Google Scholar]
- 43.Jack CR Jr., Shiung MM, Gunter JL, O’Brien PC, Weigand SD, Knopman DS, Boeve BF, Ivnik RJ, Smith GE, Cha RH, Tangalos EG, Petersen RC. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004;62:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blaise R, Mateo V, Rouxel C, Zaccarini F, Glorian M, Bereziat G, Golubkov VS, Limon I. Wild-type amyloid beta 1–40 peptide induces vascular smooth muscle cell death independently from matrix metalloprotease activity. Aging Cell. 2012;11:384–393. [DOI] [PubMed] [Google Scholar]
- 45.Vinters HV, Wang ZZ, Secor DL. Brain parenchymal and microvascular amyloid in Alzheimer’s disease. Brain Pathol. 1996;6:179–195. [DOI] [PubMed] [Google Scholar]
- 46.Kalaria RN, Premkumar DR, Pax AB, Cohen DL, Lieberburg I. Production and increased detection of amyloid beta protein and amyloidogenic fragments in brain microvessels, meningeal vessels and choroid plexus in Alzheimer’s disease. Brain Res Mol Brain Res. 1996;35:58–68. [DOI] [PubMed] [Google Scholar]
- 47.Knight MJ, McCann B, Kauppinen RA, Coulthard EJ. Magnetic Resonance Imaging to Detect Early Molecular and Cellular Changes in Alzheimer’s Disease. Front Aging Neurosci. 2016;8:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weller RO, Boche D, Nicoll JA. Microvasculature changes and cerebral amyloid angiopathy in Alzheimer’s disease and their potential impact on therapy. Acta Neuropathol. 2009;118:87–102. [DOI] [PubMed] [Google Scholar]
- 49.Querques G, Borrelli E, Sacconi R, De VL, Leocani L, Santangelo R, Magnani G, Comi G, Bandello F. Functional and morphological changes of the retinal vessels in Alzheimer’s disease and mild cognitive impairment. Sci Rep. 2019;9:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carvalho C, Cardoso S. Diabetes-Alzheimer’s Disease Link: Targeting Mitochondrial Dysfunction and Redox Imbalance. Antioxid Redox Signal. 2020. [DOI] [PubMed] [Google Scholar]
- 51.Jayaraj RL, Azimullah S, Beiram R. Diabetes as a risk factor for Alzheimer’s disease in the Middle East and its shared pathological mediators. Saudi J Biol Sci. 2020;27:736–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.den Haan J, van de Kreeke JA, van Berckel BN, Barkhof Frederik, Teunissen CE, Scheltens P, Verbraak FD, Bouwman FH. Is retinal vasculature a biomarker in amyloid proven Alzheimer’s disease? Alzheimer’s & Dementia: Diagnosis, Assessment & Disesase Monitoring. 2019:383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Armstrong A Risk factors for Alzheimer’s disease. Folia Neuropathol. 2019;57:87–105. [DOI] [PubMed] [Google Scholar]
- 54.O’Bryhim BE, Apte RS, Kung N, Coble D, Van Stavern GP. Association of Preclinical Alzheimer Disease With Optical Coherence Tomographic Angiography Findings. JAMA Ophthalmol. 2018;136:1242–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bresnick GH, Condit R, Syrjala S, Palta M, Groo A, Korth K. Abnormalities of the foveal avascular zone in diabetic retinopathy. Arch Ophthalmol. 1984;102:1286–1293. [DOI] [PubMed] [Google Scholar]
- 56.O’Bryhim BE, Apte RS, Kung N, Coble D, Van Stavern GP. Association of Preclinical Alzheimer Disease With Optical Coherence Tomographic Angiography Findings. JAMA Ophthalmol. 2018;136:1242–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van de Kreeke JA, Nguyen HT, Konijnenberg E, Tomassen J, den BA, Ten KM, Yaqub M, van BB, Lammertsma AA, Boomsma DI, Tan SH, Verbraak F, Visser PJ. Optical coherence tomography angiography in preclinical Alzheimer’s disease. Br J Ophthalmol. 2020;104:157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement (N Y). 2018;4:575–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang H, Gameiro GR, Liu Y, Lin Y, Jeffrey H, Deng Y, Gregori G, Delgado S, Wang J. Visual Function and Disability are Associated with Increased Retinal Volumetric Vessel Density in Patients with Multiple Sclerosis. Am J Ophthalmol. 2020;Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Di Marco LY, Farkas E, Martin C, Venneri A, Frangi AF. Is Vasomotion in Cerebral Arteries Impaired in Alzheimer’s Disease? J Alzheimers Dis. 2015;46:35–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol (1985). 2006;100:328–335. [DOI] [PubMed] [Google Scholar]
- 63.Iadecola C Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. [DOI] [PubMed] [Google Scholar]
- 64.Corvi F, La SC, Benatti L, Querques L, Lattanzio R, Bandello F, Querques G. Impact of Intravitreal Ranibizumab on Vessel Functionality in Patients With Retinal Vein Occlusion. Am J Ophthalmol. 2015;160:45–52. [DOI] [PubMed] [Google Scholar]
- 65.Corvi F, Querques G, La SC, Lattanzio R, Bandello F. DYNAMIC AND STATIC RETINAL VESSEL ANALYSES IN PATIENTS WITH MACULAR EDEMA SECONDARY TO RETINAL VEIN OCCLUSION. Retina. 2015;35:2052–2059. [DOI] [PubMed] [Google Scholar]
- 66.La SC, Corvi F, Bandello F, Querques G. Static characteristics and dynamic functionality of retinal vessels in longer eyes with or without pathologic myopia. Graefes Arch Clin Exp Ophthalmol. 2016;254:827–834. [DOI] [PubMed] [Google Scholar]
- 67.Benatti L, Corvi F, Tomasso L, Darvizeh F, La SC, Querques L, Bandello F, Querques G. Dynamic functionality and static changes of retinal vessels in diabetic patients treated with intravitreal ranibizumab. Acta Diabetol. 2017;54:39–43. [DOI] [PubMed] [Google Scholar]
- 68.Golzan SM, Goozee K, Georgevsky D, Avolio A, Chatterjee P, Shen K, Gupta V, Chung R, Savage G, Orr CF, Martins RN, Graham SL. Retinal vascular and structural changes are associated with amyloid burden in the elderly: ophthalmic biomarkers of preclinical Alzheimer’s disease. Alzheimers Res Ther. 2017;9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kotliar K, Hauser C, Ortner M, Muggenthaler C, Diehl-Schmid J, Angermann S, Hapfelmeier A, Schmaderer C, Grimmer T. Altered neurovascular coupling as measured by optical imaging: a biomarker for Alzheimer’s disease. Sci Rep. 2017;7:12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Newman EA. Functional hyperemia and mechanisms of neurovascular coupling in the retinal vasculature. J Cereb Blood Flow Metab. 2013;33:1685–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nagel E, Vilser W. Autoregulative behavior of retinal arteries and veins during changes of perfusion pressure: a clinical study. Graefes Arch Clin Exp Ophthalmol. 2004;242:13–17. [DOI] [PubMed] [Google Scholar]
- 72.Kalaria RN. Small vessel disease and Alzheimer’s dementia: pathological considerations. Cerebrovasc Dis. 2002;13 Suppl 2:48–52. [DOI] [PubMed] [Google Scholar]
- 73.Yoshida A, Feke GT, Mori F, Nagaoka T, Fujio N, Ogasawara H, Konno S, Mcmeel JW. Reproducibility and clinical application of a newly developed stabilized retinal laser Doppler instrument. Am J Ophthalmol. 2003;135:356–361. [DOI] [PubMed] [Google Scholar]
- 74.Feke GT, Hyman BT, Stern RA, Pasquale LR. Retinal blood flow in mild cognitive impairment and Alzheimer’s disease. Alzheimers Dement (Amst). 2015;1:144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Berisha F, Feke GT, Trempe CL, McMeel JW, Schepens CL. Retinal abnormalities in early Alzheimer’s disease. Invest Ophthalmol Vis Sci. 2007;48:2285–2289. [DOI] [PubMed] [Google Scholar]
- 76.Landa G, Jangi AA, Garcia PM, Rosen RB. Initial report of quantification of retinal blood flow velocity in normal human subjects using the Retinal Functional Imager (RFI). Int Ophthalmol. 2012;32:211–215. [DOI] [PubMed] [Google Scholar]
- 77.Lopes de Faria JM, Andreazzi DD, Larico Chavez RF, Arthur AM, Arthur R, Iano Y. Reliability and validity of digital assessment of perifoveal capillary network measurement using high-resolution imaging. Br J Ophthalmol. 2014;98:726–729. [DOI] [PubMed] [Google Scholar]
- 78.Jiang H, Liu Y, Wei Y, Shi Y, Wright CB, Sun X, Rundek T, Baumel BS, Landman J, Wang J. Impaired retinal microcirculation in patients with Alzheimer’s disease. PLoS One. 2018;13:e0192154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gameiro GR, Jiang H, Liu Y, Deng Y, Sun X, Nascentes B, Baumel B, Rundek T, Wang J. Retinal tissue hypoperfusion in patients with clinical Alzheimer’s disease. Eye and Vision. 2018;5:21. [DOI] [PMC free article] [PubMed] [Google Scholar]


