Abstract
Microorganisms that inhabits human digestive tract affect global health and enteric disorders. Previous studies have documented the effectiveness and mode of action of probiotics and classified as human-friendly biota and a competitor to enteric pathogens. Statistical studies reported more than 1.5 billion cases of gastrointestinal infections caused by enteric pathogens and their long-term exposure can lead to mental retardation, temporary or permanent physical weakness, and leaving the patient susceptible for opportunistic pathogens, which can cause fatality. We reviewed previous literature providing evidence about therapeutic approaches regarding probiotics to cure enteric infections efficiently by producing inhibitory substances, immune system modulation, improved barrier function. The therapeutic effects of probiotics have shown success against many foodborne pathogens and their therapeutic effectiveness has been exponentially increased using genetically engineered probiotics. The bioengineered probiotic strains are expected to provide a better and alternative approach than traditional antibiotic therapy against enteric pathogens, but the novelty of these strains also raise doubts about the possible untapped side effects, for which there is a need for further studies to eliminate the concerns relating to the use and safety of probiotics. Many such developments and optimization of the classical techniques will revolutionize the treatments for enteric infections.
Keywords: Probiotics, Bacterial strains, Enteric infections, Regulation of gut barrier, Therapeutic approach
Introduction
Every year, 1.5 billion cases occur of infections related to enteric system mostly diarrheal in children and they are also ranked at the fifth spot in causing deaths in people of almost all ages that accounts for approximately 2.2 billion on whole (Siddiqui et al. 2021). Young children that are more likely to be under 5 years are more prone to such infections and chances or these infections are greatest in developing countries (Wong et al. 2015). Disabled physical growth, mental retardation, lack of apprehension, and perception can be the results of early age infections in children (Sujlana et al. 2017). In most of the cases, these infections are caused by contaminated foods and it constitutes 70% of all diarrheal infections. There are many sources of enteric infections; it can be bacterial, viral or parasitic by nature they hinder activities of intestines with or without causing diarrhoea (Martins et al. 2009) Diseases caused by food possess critical harm to public health as given in Table 1. With the inception of Sustainable development goals or SDGs, these issues have reached significant attention. SDG 6, which is based on ensuring accessibility and sustainable water and sanitation management for all, represents the increased emphasis on water and sanitation problems on the globalist stage. The resources of water have covered all aspects of development in SDG 6 discussions, such as health, food security, pathogenic diseases, and in preserving a healthy ecosystem (Bartram et al. 2018).
Table 1.
Diseases caused by foodborne pathogens (Bintsis 2017)
| Diseases | Causative pathogens | References |
|---|---|---|
| Vomiting, diarrhea | Isospora; Taenia, Cronobacter, Salmonella, Bacillus, Shigella, Vibrio, Noro virus, Staphylococcus, Rota virus, Entamoeba, Cryptosporidium, Cyclospora, Giardia | Bintis et al. (2017) |
| Arthritis | Campylobacter, Yersinia, Shigella, Salmonella | Wei and Zhao (2020) |
| Hemorrhagic uremic syndrome (HUS) kidney disease | Shigella spp., Shiga-toxin-producing E. coli (STEC) | Bintis et al. (2017) |
| Guillain Barre syndrome | Campylobacter | Lai et al. (2020), Chen et al. (2020a) |
| Miscarriage, neonatal infection | Listeria, Toxoplasma | Bintis et al. (2017) |
| Paralysis | Clostridium botulinum, Campylobacter, Sea food toxin | Corr et al. (2009) |
| Malignancies and auto-immune diseases | Mycotoxin | Lai et al. (2020), Chen et al (2020a) |
| Allergic reactions | Seafood toxin | Qin et al. (2005) |
Diarrhoea is the second major cause of death in children below 5 years of age and kills nearly 3 million children per year. Diarrhoea can persist for many days, and it can keep body in dehydration and deprivation of salts state, optimal amounts of those are essential for survival. In the recent reports for most cases, serious dehydration and water loss were the major triggers of diarrheal deaths (Florez 2020). Nearly, 1.6 million people died globally in 2017 because of diarrhoea, and in sub-Saharan African and South Asia countries 90% deaths occurred due to diarrhoea. In Africa 2015, Centres for diseases control and prevention surveyed that approximately 330,000 deaths were reported in the previous year (Keto et al. 2020).
There are many ways by which such infections can propagate more than 200 mediums are known through which infections caused by food can be caused such as microbes, physical or chemical agents (Vieco-Saiz et al. 2019). It is evaluated by the CDC that 44% of all the foodborne infections including hospitalization and deaths are the result of 31 known causing agents (Amalaradjou and Bhunia 2012). Concerning current critical public health situations, many advances are made in this field to prevent, detect, and control the pathogens causing food-related diseases. It is calculated that a straight reduction of 10% in foodborne infections can lead to the protection of 5 million people overall (Amalaradjou and Bhunia 2013). To control the prevalence of foodborne diseases probiotics are an effective way. It is, therefore, more proficient and feasible substitute as it is safe and healthy for consumers, so the use of probiotics can be proven as a beneficial strategy. Out of all causing agents accountable for enteric infections about 22 pathogens are kept under strict examination because of their high extent of viability, death rate, and morbidity. These incorporate Brucella species, Campylobacter species., Enteroaggregative Escherichia coli (EAggEC), Enteropathogenic Escherichia coli (EPEC), Enterotoxigenic Escherichia coli (ETEC), Shiga-poison delivering Escherichia coli (STEC), Helicobacter pylori, Hepatitis A infection, Hepatitis E infection, Listeria monocytogenes, Mycobacterium Bovis, Vibrio cholera O1/O139, Non-cholera Vibrio spp, Norovirus, Rotavirus, Prions, Salmonella spp. (non-typhoidal), Salmonella enterica serovar Typhi, Shigella species., and Yersinia species, and poisons from Staphylococcus aureus, Clostridium perfringens and Bacillus cereus (Amalaradjou and Bhunia 2013).
For improvement of growth and rate of production of meat from animals, many steps are taken to control food-related pathogens in either humans or animals. Antibiotics are being in Zhang et al. 2020). On the other hand, there is also a huge risk of antibiotic resistance that has forced scientists to use antimicrobials that are more natural. These antimicrobials are used as alternatives to antibiotics and include essential oils, bacteriocins, plant extract (Dwivedi et al. 2011), probiotics (Amalaradjou and Bhunia 2012) and bacteriophages (Hagens and Loessner 2010). Acids play a vital role in increasing the effect of antimicrobials such as organic acids are used to wash and clean animal’s old rotten bones, fruits, and animals (Sirsat et al. 2009). Acids can be used collectively to show synergistic effects in antimicrobials. If we use acids with oxidizing agents, for example hydrogen peroxide, it can give us enhanced results. Some other processes can also be useful for enhancement of the effect such as thermal and non-thermal techniques (Luksiene and Arturas 2009).
Live microbial feed association with health has a long history that goes back to thousands of years. (Nazir et al. 2018). The use of the term 'probiotic' was first adopted in 1974, but Parker described it as species and entities that have a beneficial impact on the host animal and have contributed to its gut microbial equilibrium, the definition of probiotics has been improved many times (Nazir et al. 2018). Metchnikof and his colleagues reported on their first documented study of probiotics, which discussed the positive effects of fermented milk on the health and longevity of people and their opinion about the lower intestinal flora. Hence, in 1908, he was awarded the medicine Nobel Prize for his cellular (phago) immunity theory and his proposition to transform the "toxic" forum into a host friendly colony for the Bacillus bulgaricus inspired generations of scientists and food product developers (Özdemir 2013). Since then, detailed studies have been carried out on human beneficial impact of probiotics and their connection to the prevention and treatment of gut-related diseases such as bacterial and antibiotic-associated diarrhoea, lactose intolerance, irritable bowel syndrome, indigestion and stomach bloating (Ritchie and Romanuk 2012). Because of the huge and remarkable impact of probiotic in improving intestinal health and improvement of overall human being, the interest for probiotic substances has elevated colossally. In 2007, the worldwide retail for probiotic production for example food-based products and bioengineered probiotics worth was about $14.9 billion which is expanded up to US$16 billion out of 2008. Moreover, the probiotic market is foreseen to extend from $37.7 in 2016 to $71.9 billion by 2025, at a CAGR of 7.49% (Granato et al. 2010).
In addition to enhancing the intestinal overall health, probiotics have been recorded to have beneficial effects on chronic disease, for example, cancer-like malignant growth (Xu et al. 2012), high serum cholesterol, and allergy (Prescott and Björkstén 2007), and easing back the illness and indications of the HIV-tainted individual, for example, bacterial translocations just as vulvovaginal candidiasis in ladies (Nwosu et al. 2014). The inhibitory and remedial impact of probiotics on malignancy (cancer) has been set up through many components including the balance of gut microbiota, improvement of gut hindrance capacities, debasement of potential cancer-causing agents, and improved immune system (Nazir et al. 2018). For example, a study found that the probiotic Bacillus-Polyfermenticus exerts an anti-cancer impact on colon cancer growth cells of human stimulating (IgG) creation and tweaks the quantity of CD8þ, NK cells or CD4þ (Shi et al. 2016). In another study, including 54 women found that, an everyday probiotic utilization for a half year upgraded the removal of human papillomavirus (HPV) which is a known cause of cervical cancer (Van Puyenbroeck et al. 2012). Besides, past in vivo examinations, it is demonstrated that the administration of probiotics is effective in developing the profiles of lipids along with the decrease in plasma or serum cholesterol level and triglycerides, or addition of (HDL) cholesterol and LDL-cholesterol (Ettinger et al. 2014). For instance, the Lactobacillus-reuteri probiotic (NCIMB-30242) or a couple of different strain of Bifidobacterium and Lactobacillus has demonstrated that they contain some potential in decreasing serum, the level of cholesterol and particularly decreasing the cholesterol level of LDL which can build up one of the significant antecedents of numerous constant sicknesses including illnesses of cardiovascular, hyperlipidemia, hypertension, high blood pressure and development of atherosclerotic plaque in the veins. Probiotics have been utilized to re-establish the ecosystem of the enteric microbial biological system and manage the pathogenic contaminations, which is described as “live microorganisms which confer a health benefit to the host when properly administered (Ceapa et al. 2013). Their administration helps deter and combat foodborne diseases by various mechanism including, but not limited to competitive exclusion of GIT pathogenes, modulation of host immune systems and enhanced intestinal barrier management (Wohlgemuth et al. 2009). Evaluation of enteric pathogens has proven effective, but there are limitations. It is generic in nature and often does not hinder the attachment of specific pathogens at specific infection sites and causes low immune response levels (Zuo et al. 2020). An extensive understanding of the confinements of traditional probiotics, pathogens, and the components through which it causes infection gives prospects to structure new strains of probiotic with required characteristics and functions. Using genetic manipulation, the novel bioengineered probiotic strains of probiotics have been created. Working with customary in the case of novel strain probiotics can be reinforced that has impact basic strides in pathogenesis. These strains can also be utilized to administrate the medications or antibodies, target on a particular pathogen or toxin, the surface of receptors, and upgrade an invulnerable reaction or defence system inside the host (Amalaradjou and Bhunia 2013).
Dysbiosis results as consequences of disruption in microbiome homeostasis, making host susceptible to pathogen introduction and virulence induction leading to disease onset. Various clinical trials has provided conclusive evidence about the correlation of dysbiosis with the occurrence of inflammatory bowel disease and infectious colitis. Not only these but it is also regarded as one of the major and important contributory factors related to other diseases including obesity, necrotizing enterocolitis, type I and type II diabetes, irritable bowel syndrome, and colon cancer (Liu et al. 2016). In fact, the adverse effects of antibiotics are well documented because of the protracted disturbance of the residing GIT microbiota. Probiotics (predominantly Lactobacillus and Bifidobacterium), which have been used widely in humans to reduce cholesterol level, increase intolerance to lactose, mitigate diarrhoea and protect them from enteric infections, modulate gut microbiota positively, and improve gut homeostasis. Even though probiotic strains are naturally commensal bacteria and are widely accepted as healthy and safe, there have been some safety concerns with their use. Lactobacillus, for example, researchers have linked it to the infections in immunocompromised patients, and some cases of probiotic bacteraemia have been identified in these patients. In this correlation, the abrupt suspension of the consumption of probiotics can potentially damage this homeostasis of host GIT microbiota, particularly in immunocompromised groups, which are more vulnerable to environmental disturbance (Liu et al. 2016).
Gut dysbiosis probably precedes food allergy, and the timing is critical factor. Gut microbiota may be distinct from each food allergy. Murine models support the role of gut microbiota in shaping immune maturation and tolerance. Gut microbiota can affect susceptibility to food allergies by modulating immunity from type 2 and influence immunisation and tolerance, regulate populations of basophils and promote the function of intestinal barrier (Zhao et al. 2019). Interventional investigations of probiotics, prebiotics, symbiotic, and faecal microbiota relocate are in progress or intended to additional proselyte our insight into the gut microbiome of food hypersensitivity to clinical practice. Future investigations in this field will incorporate stretching out flow research foci to incorporate the virile, mycobiome, and communications between the microbiome, host, and environment, just as growing momentum research foci to incorporate the virile, mycobiome, and collaborations between the microbiome, host, and climate. Future examinations will be encouraged by thorough and lucid investigation plans, multidimensional profiling, and frameworks science draws near (Zhao et al. 2019).
Probiotics
Probiotics are living microbes that, when administered properly, impart health benefits to the host and keeps a person healthy in accordance with the coined definition by World Health Organization and Food and Agriculture Organization United State "WHO and FAO" with small textual differences (Hill et al. 2014; Reid et al. 2019). However, dead bacteria and their constituents may exhibit the properties of probiotics as well. The bacteria, frequently reported to exhibit the properties of probiotic, are the strains of lactic acid bacteria and Bifidobacterium, and are used in numerous healthy foods and dietary supplements. Preferably, a real probiotic should be of safe human origin, healthy, and free from vectors capable of transmitting resistance to antibiotic and factors of pathogenicity or toxicity (Plaza-Diaz et al. 2019). Identification of substances produced by one microorganism that stimulates growth factors of another have revamped the beneficial impact of symbiotic bacteria on animals relating to their enteric flora level (Bortoluzzi et al. 2020). Various studies have reported different possible hypothesis regarding classification of probiotics in terms of evolution. Advancements in genomic-tools provided us greater accuracy in classification of different species of probiotics and mechanism of action (Reid 2016). Several lactic acid bacteria (LAB) is considered as probiotics, because of these bacteria have the ability to release lactose when fermented with sugar-rich substances (Plaza-Diaz et al. 2019). According to their phenotypic and morphologic characteristic, initially lactic acid bacteria were divided into Microbacterium, Betacoccus, Streptobacterium, Tetracoccus, Termobacterium, and Betabacterium. Now, just Streptococcus is retained, while rest of the bacteria were renamed into Enterococcus species, Lactobacillus species, and Bifidobacterium (Mohania et al. 2008).
Lactobacillus genus morphologically belongs to the Firmicutes phylum, class Bacilli, order Lactobacillales, and family Lactobacillaceae is made up of over 170 species Gram-positive, anaerobic, facultative, catalase-negative, non-spore-forming rod-shaped bacteria. It is used for the production of fermented food which is derived from both plants (cereal and vegetables) and animals (meat and milk) (Zhang et al. 2018). Bifidobacterium are generally Gram-positive, non-motile anaerobic, pleomorphism, non-sporting bacteria that produce acetic acids, formic acids, and lactic acids as the outcome of fermenting carbohydrates (Vlkova et al. 2002). In contrast to Lactobacillus, the cultivation of Bifidobacterium is very complex because of their obligate-anaerobes qualities and frequently requires more caring when it is (Abou-Kassem et al. 2020) produced in dairy items, for example, yogurt and it is also used in the formation of probiotic products.
Today, the concern in the probiotic analysis and manufacturing is on promoting an association of several probiotic spp. This is because it revealed that it has a great effect on an individual's health as compared to the one probiotic use. Such as there were eight distinct compounds of VSL, #3 probiotics present. That were shown to be efficient in the treatment of different illnesses such as strengthening the immune system, enhancing the resistance against hepatic insulin in diabetes patient, diarrhea, bowel disorder, and ulcerative colitis (Dong et al. 2016; Schlee et al. 2008). Besides, the organizations of Bifidobacterium strains with (LA) &&Lactobacillus acidophilus has shown to be efficient in decreasing the occurrence of NEC (necrotizing-enter colitis) as well as NEC-related death in severe disorder neonates (Nair and Soraisham 2013). The ability of probiotic items will decrease when mutual interruption occurs between the probiotic consortia. Hence, it is necessary to assure that probiotic consortia will not mutually interact between themselves. For example, the basic study of probiotic items having 15 bacteria revealed an efficient development in the recovery of the diseases, that is why a single strain is not enough, whereas a mixture of strains can be more useful (Nair and Soraisham 2013).
On the other hand, several bacteria considered as a probiotic, but some bacteria do not contain its desirable features. According to researchers (Mitropoulou et al. 2013), many perspectives should be kept in mind before bacteria is considered as a probiotic. The probiotic bacteria should be nonpathogenic to assure the protection of probiotic items and should be generally identified as safe (GRAS) for individual acceptance via the FDA Drug Administration and US Food.
Apart from the beneficial bacteria residing human gut, there are living microbes present in gastrointestinal system, Clostridium difficile and H. pylori, being most common some others are included as well, possess certain threats to health. Additionally, probiotic characteristics analysis checklist should be carried out in vivo assays as well as in vitro and the effects should reflect in controlled human study. The probiotic bacteria should be capable of remaining alive in the severe situation of the gastrointestinal region and gut to assure its effectiveness (Amin et al. 2020). It is claimed that probiotic bacteria can endure the bile salt and gastric juice. This is because several bacteria known as probiotic microbes that cannot sustain the level of acidity in stomach and bile salt. This situation raises several discussions on probiotic between the researchers, entrepreneurs, and observers. Hence, it revealed that non-viable probiotic devour the beneficial impacts as well on health system (Lahtinen 2012; Ouwehand and Salminen 1998; Akter et al. 2020). The reason is that neither the complete system nor the clinical aspects of probiotic bacteria accompanying DNA segments or cell walls can impact useful or beneficial effects on individuals (Kechagia et al. 2013). It was revealed that viable and non-viable LB show a related beneficial impact towards lactic acid sensitivity by lactase-inadequate subjects. Furthermore, in the therapy of gastrointestinal tract, several probiotic bacteria exhibited clinical ability in reducing the period of loose stools in both forms viable and non-viable. Although, several probiotic bacteria, for example, Saccharomyces boulardii, have a useful impact on an individual treatment that should in a viable form, varying from several Lactobacillus species exhibited effectiveness in viable and non-viable form (Sen et al. 2020). Therefore, researchers need to further evaluate probiotic capability and if treatment is effective or not, depending on the disease and probiotic bacteria. Hence, researchers recommended that when probiotic bacteria are present in viable form then it will show the best curative result on an individual (Hill et al. 2014). The reason is that a viable form of probiotics can colonize as well as attach on the surface of the gastrointestinal region, giving aggressive elimination of microbes that is why they keep the enteric flora stable. Researchers revealed that non-viable or dead probiotic bacteria have not been able to give such type of process and hence their advantageous effects are limited. Besides, the present term of probiotic is stressed on the requirements of a viable form of probiotics as explained in earlier parts.
Studies that are more effective must be done to observe the effectiveness of probiotics to lower the gastric juices and help the bile salt activity, which also affects processing, and production food-based probiotic products when made commercially. The reason behind this is that the processing of probiotic bacteria sometimes decreased as the food production, accumulation, and distribution. Several reviews have explained huge changes and poor processing of bacteria particularly Bifidobacterium, in diary items for example, in preparations of yogurt (Ananya et al. 2020). The severity of Bifidobacterium to lowering pH and H2O2 together with low processing in food items during accumulation remains a big issue in several probiotic items. Hence, techniques for example encapsulation and immobilization are applied to assure and stable the features and viability of probiotic items used. Generally, encapsulation and immobilization of probiotics give stability and defence mechanism of cells against physical and chemical changes for example densities of high cells, greater productivity, more active fermentation, bile salts and maturation measurement, effectiveness, cell loads, temperature and pH, utilization substrate improved (Mitropoulou et al. 2013). Therefore, this procedure is away from the focus of this article, hence researchers have not talked about this paper extensively. Besides, protection, the adequate dosage of probiotics taken is a different key element to assure the beneficial impact on the gastrointestinal system and on the overall person’s health using that specific probiotics.
However, the knowledge is limited about its viability and still considered as uncertain. Usually, it is acceptable that the concentration of probiotic items should remain minimum of approximately 106 (CFU per mL) or in grams and at least 108–109 probiotic bacteria should be used regularly, which will give the beneficial effects to the humans and it will keep the immune system healthy, but for most people, probiotics appear to be safe. If you want to try them, and you have a healthy immune system, they should not cause any unpleasant side effects And there is likely to be a huge difference between the pharmaceutical-grade probiotics that show promise in clinical trials and the yoghurts and supplements sold in shops (Sanders 2008).
Probiotic mechanism of action against enteric infections
Use of probiotics can avoid or lessen the development of different pathogens in the gastrointestinal tract either by contest for supplements or adhesion to gastrointestinal area (Fuller 1991; Ohashi and Ushida 2009). Pathogens in any condition expect supplements to replicate and either cause or increase the level of infections. The gastrointestinal tract for its plenitude in nutrients is notable. For the establishment of bacterial colonization, it creates an appropriate environment. The capability of probiotics to win over the microbes for these supplements favours their development as compared to the pathogens (Khaneghah et al. 2020).
Probiotics can produce certain metabolites during the contest for supplements, for example, unstable unsaturated fats disturbing the pH of the gastrointestinal tract. The pH reduction of the gastrointestinal tract makes an unfavourable situation for microbes and will restraint the growth of pathogens, as per the fact that the most of pathogens cannot grow at low pH (Biswasroy et al. 2020). Space adhesion competition describes the situation where probiotics prevent pathogens from colonizing favoured sites like intestinal villi, goblet cells and colonial crypts (Chichlowski et al. 2007). The key factor of pathogenicity of intestinal microbes is the attachment to the surface of enteric epithelial cells (Cai et al. 2020). Simultaneously, the process called Colonization Resistant, by which the surface of enteric mucosal is prevented from the attachment and reproduction of pathogens, also have crucial importance (Corr et al. 2009). These probiotics tie to the enteric cells through electrostatic associations, steric powers, or surface of explicit proteins. Therefore, they can bind to these cells in large numbers on their surfaces (Sarkar et al. 2020). Therefore, they physically block the binding sites which results in no spaces left for the microbes to cling and in this way providing no chance to cause disease (Khorshidian et al. 2020). Probiotic lactic acid bacteria have a more prominent ability to stick on the epithelial tissues than microbes (Lee et al. 2003). Bifidobacteria and Lactobacilli are related to carbohydrates binding abilities with some enteric pathogenic microbes (Neeser et al. 2000). L. reuteri and B. bifdum can bind to the glycolipid’s sides to the surface of host tissue to inhibit some specific microbes that can also bind to the surface of glycolipids (Wohlgemuth et al. 2010). Researchers described that they observed that in the presence of Bacillus subtilis the attachment between the Salmonella enteritidis on the surface of enteric epithelial tissues decreases to a significant number. Therefore, this is how the microbial growth is inhibited in the gastrointestinal tract, which is the hotspot for most of such infections. This was verified with low pathogen survival due to their growth by insufficient nutrition. And proliferation of adherent space in the gastrointestinal tract and limited availability (Mathipa and Thantsha 2017).
Regulation of gut barrier by probiotic metabolites
Secondary metabolites such as bacteriocins, extracellular vesicles, short fatty acids chain, indole, and extracellular proteins (secreted proteins) are formed by probiotics. It can prevent the gut epithelial barrier while integrating with certain antimicrobial peptides secretion, instantly enhancing the mucus secretion via goblet cells and promoting the TJs protein expression (Liu et al. 2020). Table 2 enlists some of the secondary metabolites of probiotic bacteria and their health benefits.
Table 2.
Secondary metabolites of probiotic bacteria and their health benefits (Mikkili et al. 2019)
| Secondary metabolites of probiotic bacteria and their health benefits | ||
|---|---|---|
| Compounds | Health benefits | Probiotic bacterium |
| Bacteriocins | Acts antagonistically for gut pathogens and act as a signaling molecule, Enhance probiotic bacterium's' ability to | Lactococcus lactis |
| Acetic acid |
Acetate is metabolized in muscle and used to produce adenosine5′-triphosphate (ATP). Defense-related activities in host epithelial cells |
Bifdobacterium sps |
| Enterocins | Antimicrobial activity against Pseudomonas aeruginosa | Enterococcus casselifavus MI001 |
| Exopolysaccharides | Antioxidant activity | B. coagulants RK-02 |
| Lactic Acid | Used as substrate for glucose, cholesterol, and lipids metabolism Lowers pH in vaginal environment | Lactobacillus sps |
| Amino acid metabolites | Essential nutrients supports the growth | Enterobacteriaceae |
| Enzymes | Hydrolysis of starch and β-galactosides | Lactobacillus sp G3_4_1TO2 and P. freudenreichii |
Probiotic-secreted protein
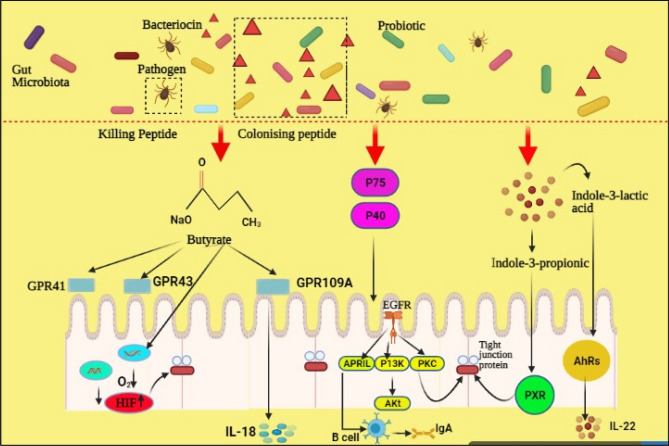
It is confirmed that the probiotic-secreted proteins are involved in the host and symbiotic bacterial relationship. The L. Plantarum BMCM12 secretes extracellular proteins and considerably reduces microbes' adhesion or strengthens the gut barrier. It is shown that 2 proteins secreted by p40, p75, and LGG improve IEC homeostasis. In addition, these 2 proteins p40 and p75 protect the H2O2-induced TJs protein, which is disrupted by the mechanism of protein kinase-C (PKC) as shown in the figure no. 01. Similarly, it is reported that LGG protein (HM0539) prevent the gut integrity via modulating the expression of TJs Liu et al. 2020).
Indole
Generally, tryptophan’s-containing bacteria produce Indole###. It is a particular barrier signal for gut symbiotic bacteria. It is stated that indole can be produced via symbiotic E. coli. A study reported that the chemotaxis of pathogenic could be inhibited by indole. As well as, it inhibits the bacterial attachment with epithelium lining while promoting gene expression, which is involved in the function of the gut epithelium (Liu et al. 2020).
Extracellular vesicles
Extracellular vesicles are the structure of lipid bilayer produced by gut microflora. It is composed of lipopolysaccharides, lipids, proteins, and nucleic acids. Extracellular vesicles are involved in a host and bacterial symbiotic relationship and in the stability of intestinal homeostasis. It has been stated that the oral use of A. muciniphila obtained from extracellular vesicles can reduce colitis caused by dextran sulphate sodium while improving allergic cell infiltration of the large intestinal wall and improvements in intestinal length (Liu et al. 2020).
Short fatty acids chains
Short fatty acids chain primarily contain propionate, acetate, and butyrate that are metabolites produced by the gut microbiota through indigestible fermented proteins and carbohydrates. Butyrate is a preferable energy source for intestinal epithelial cells between short fatty acids chain. The study revealed that the lower concentration (≤ 2 mM) of butyrate showed a beneficial impact on the single line barrier of Caco-2 cells like TER increased and permeability of inulin decreased. Besides, microbial-based butyrate improves the TJs protein activity and in vivo study indicated that it suppresses the paracellular permeability as well as stimulates the secretion of mucin by goblet cells, mainly MUC2, that protects the destruction of enterocytes by pathogenic bacteria. Butyrate is a histone deacetylase inhibitor. It is reported that it can bind with G-coupled protein receptors, including GPR43, GPR41, and GPR109A. It has been confirmed that in the epithelium cells, GPR109A plays an essential role in the formation of IL-18 and stability of intestinal homeostasis. The initiation of AMP-activated protein kinase is one of the processes by which the butyrate enhances the function of intestinal epithelial barrier. Butyrate can enhance the histones H3 and H4 acetylation and H3 methylation on the MUC2 receptor at the same time, thus protecting the mucosal barrier. Moreover, butyrate acts as an inhibitor for the expression of TJs protein penetrability claudin-2 by the mechanism of IL-10RA. The antimicrobial cathelicidin production, LL-37 directly related to butyrate. Butyrate can stimulate the intestinal epithelium's O2 intake to the level of HIF stable as well as it promotes the expression of HIF target genes protective barrier (Liu et al. 2020).
Bacteriocins
Bacteriocins are a class of antimicrobial peptides synthesized by ribosomes. Bacteriocins are classified into two distinct classes: (class I) lanthionine-containing bacteria or bacteriocins and (class II) non-lanthionine-containing bacteria. Class 1 of bacteriocins is consist of antibiotics from the polypeptide chain and single peptide chain. These bacteria including lacticin 3147, nisin, and lacticin 481 that are synthesized ribosomal antimicrobial peptides formed by Gram + ve bacteria. While class II of bacteriocins is composed of three subclasses, subclass-I, subclass-II, subclass-III and subclass-IV, respectively.
It has been reported that bacteriocins can serve as colonizing peptides of many gut microbes, which is promoting them to gain a competitive edge over the other strains and occupying existing environments in the gut. Research findings analyse that EcN releases 2 antimicrobial peptides microcin-M and microcin-H47 with lower molecular weight. It is stated that it can be detected by the receptors of catecholate siderophore thus, increase EcN's compatibility with the other microbes. The microbiota in the stool of mice is visibly affected by bacteriocin formed by the strains of Enterococcus faecium KH24. As well as lowering the quantity of E.coli, then bacteriocin substantially enhances the concentration of Lactobacillus. Bacteriocins also serve as destroying peptides, because they can interact with the production of microbes particularly Gram-negative bacteria while crossing the inner membrane or distracting the synthesis of the cell wall. L. reuteri is called reuterin, can be secreted secondary metabolites with the wide-spectrum activity of ant microbes, which inhibits directly from bacteria or pathogens. Also, nisin, produced by Lactobacillus lactis and Streptococcus lactis, is capable of restricting the reproduction and growth of most Gram-positive bacteria and their spores, specifically against Streptococcus haemolytic and S. aureus. The effect of metabolites of probiotics is shown in Fig. 1.
Fig. 1.
Effects of metabolites of probiotics on gut. Indole 3-propionic acid has the ability to bind to PXR thus upregulating the expression of tight junction protein. The indole-3-lactic acid activates AhRs of the gut epithelium and promotes the expression of IL-22. Isolated from LGG, the soluble proteins P40 and P75 can activate EGFR and subsequently control the expression of APRIL into the epithel and, therefore, stimulate cell secretion of lgA via B-cells. Besides, P40 and p75 maintain gut homeostasis by activating EGFR–PIK3–Akt signaling pathway to. Furthermore, these two proteins prevent tight junctional disruption of the pathways that rely on protein kinase-C. Butyrate can bind to the GPCR, like GPR41, GPR109A, and GPR43, and induce colonic epithel to produce IL-18. Butyrate also motivates gut epithelia O2 intakes to preserve HIF stability and to enhance the expression of HIF target genes that prevent barrier protective disease. Moreover, probiotic-based bacteriocins serve as colonizing peptides to enable producers to gain a competitive edge over other strains and to occupy niches in the intestines. Alternatively, bacteriocins has the ability to act as a killer peptide, which specifically prevents pathogen adhesion of the mucus layer and strengthens the first intestinal barrier (Liu et al. 2020)
Production of inhibitory substances
Microbes to obtain the benefit when fighting for supplements and space release the antimicrobial substances. Antimicrobials have an immediate restraint on specific pathogens (Volzing et al. 2013). Probiotics are associated closely with each other with context to the mechanisms used to inhibit pathogenic microorganisms. As mentioned earlier, repression of pathogenic growth happens because of the ability of probiotics to emit natural organic acids, for example, lactic acid and acetic acids (Alakomi et al. 2000). The pH of the surrounding environment decreases because of the production of some natural acids that are making the microbial environment too acidic, thus, accordingly barring microbes that can’t endure acidic environment (Wohlgemuth et al. 2010). The organic acids have also affected the metabolism of pathogens as well as their ability to produce some toxins which creates resistance against disease making it difficult to cure with conventional medicines.
The probiotic antimicrobial activity is dependent on a couple of factors particularly environmental factors (Servin 2004). Metabolites with antibacterial characteristics for example H2O2 and bacteriocins can be produced in addition to the acids mentioned above, which are called as non-lactic acidic molecules (Dobson et al. 2012; Oscáriz et al. 1999). In a characteristic biological environment, for microbial competition, the tiny antipathogenic peptides of bacteriocins are produced (Volzing et al. 2013). They can serve as colonizing peptides by allowing probiotics to enter a previously inhabited niche on the intestinal epithelial cells. This competitive advantage enables increased density on the surface of the host intestines of probiotic bacteria (Dobson et al. 2012). As well as they can act as killing peptides, by straightforwardly influencing the microbes. A study was designed to evaluate the antimicrobial efficacy of the bacteriocins: leucocin, lacticin, and pediocin against the gastric pathogen H. pylori (Kim et al. 2003).
These bacteriocins showed results with significant inhibition of the proliferation of H. pylori, and lacticin was found showing the most inhibitory effect against this gastric bug. L. acidophilus produce metabolites which includes acidophilin, lactocidin and acidolin (Oscáriz et al. 1999), while bifidobacteria yields some substances like bacteriocins. Due to the synergistic action of lactic acid and secreted non-lactic acid molecules, the antibacterial effects of probiotic Lactobacillus that inhibited the growth and resulted in pathogenic death were triggered. The expression of host cell antimicrobial peptides can also be induced by other probiotic strains. The host’s intestines can produce defensins that can prevent pathogens from functioning to help protect the intestinal barrier (Dobson et al. 2012).
Immune system modulation
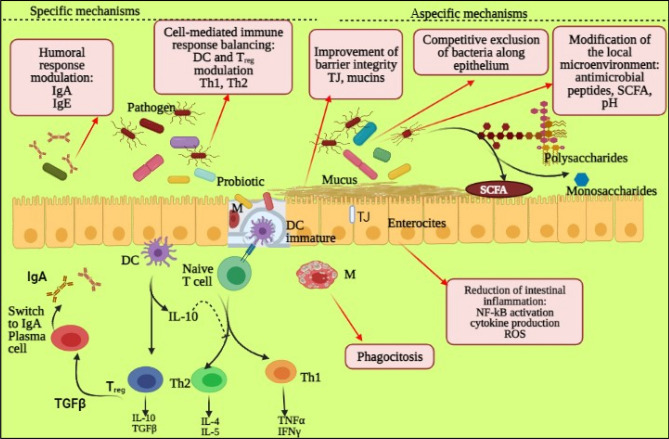
By the incitement of host resistance, the probiotics can dislodge the microbes. The concept of probiotic displacement of pathogens within the GIT by inducing specific and nonspecific immunity for bacterial pathogens causing intestinal infections is supported by substantial evidence. (Fang et al. 2000). By activating lymphocytes and producing antibodies, they activate the immune system of the host against pathogenic antigens (Ng et al. 2009). The effects of various cells inherent in innate and adaptive immunity, like macrophages, dendritic cells, T-cells and B-cells, which increase gut pathogens’ phagocytosis can also be stimulated. (Viaşu-Bolocan et al. 2013). The gut-associated lymphoid tissue adheres to probiotic strains like &&Lactobacillus rhamnosus and Lactobacillus plantarum, which boost both systemic and mucosal immunity; (Behnsen et al. 2013). These probiotics increase immunity, which disrupts the adhesion of pathogens to the intestinal epithelial cells through modulating the production of gut mucin (MUC2 and MUC3). This prevents pathogen translocation and the whole and Fig. 2 shows the whole process of how probiotic cause immune system modulation.
Fig. 2.
Immune system modulation using probiotics: Probiotics’ immunomodulatory acts. Specific pathways: probiotics’ role in the humoral immune system and cell-mediated responses. Relevant mechanisms: improvement of the role of the epithelial barrier, competitive removal of epithelial bacteria, specific microenvironment modifications, and elimination of the intestines inflammation (Tan et al. 2015)
Moreover, inducing epithelial cells to produce and secrete epithelial TGFβ and interleukins (IL-10 and IL-6). The probiotics can be perceived through the defence mechanism by identifying substances, for example, T like receptors can recognize the immune system. In 2000, a clinical trial was performed with thirty normal people were divided into 3 groups, with every treatment option comprising for a.
Span of 7 days and it was observed that Lactococcus lactis inactive drug (ethylcellulose), and Lactobacillus GG (Fang et al. 2000). The vaccine of Salmonella typhi Ty21a was given to all treatment subjects. The outcomes demonstrated an increase in the humoral immune response in the group of people who received probiotics rather than the control group. Probiotics can invigorate the making of antibodies in the enteric lumen, explicitly in antibody A. Immunoglobulin IgA leads the primary line defence mechanisms against the disease and creates resistance against the pathogenic microorganism as well as can repress the attachment of the receptors of adhesive cells on the surface of pathogenic cells and induce microbial agglutination. It is demonstrated that the oral administration of L. casei upgraded the convergence of IgA in newborn children experiencing looseness of the bowels, in this way reducing the time duration of these symptoms. (Ng et al. 2009). Administration of L. rhamnosus increased nonspecific humoral response in children with acute gastroenteritis was reflected in an increase in circulating IgG, IgA and IgM levels. In addition to all the above, probiotics may induce an anti-inflammatory response that can be used to help lower gastroenteritis, enterocolitis and irritable bowel syndrome inflammation. (Behnsen et al. 2013). Anti-inflammatory reaction is activated when strains induce the dendritic cells activation that naturally produce interleukin 10 (IL-10) which is a cytokine that plays an important role in lowering inflammation. The level of pro-inflammatory cytokines also decreases in inflammation (Viaşu-Bolocan et al. 2013).
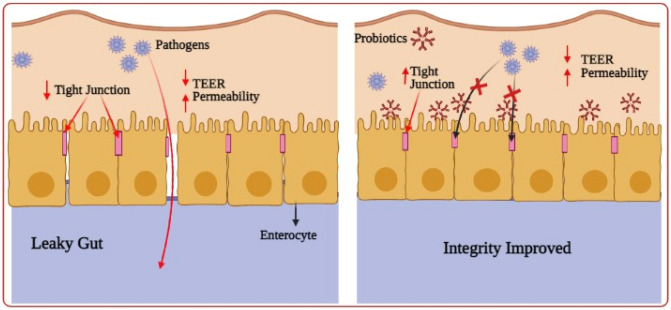
Improved barrier function
To prevent pathogens to get entry into the intestinal cells and resulting in local and systemic infections, the integrity of the intestinal barrier must be maintained. Gut microbial pathogens have the potential to destabilize the barrier whenever there is a disproportion in the microbial intestinal ecosystem. (Culligan et al. 2009). Probiotics reduce paracellular permeability, protect against pathogens and improve the physical protective potency of the mucosal membrane (Ohland et al. 2010). Probiotics reduce the epithelial penetrability defence system, giving inborn immunity against microbes and upgrading the physical barrier of the mucosal membrane (Boirivant and Strober 2007). They can also restore the barrier after damage triggered by intestinal pathogens. Probiotics will induce mucosal portion, chloride and the portion of water and bind submucosal cells through strong connective proteins to rebuild the intestinal barrier (Wohlgemuth et al. 2010).
Consequently, the normalization of colonic physiological function has resulted in an improvement in histological disorders and barrier integrity (Madsen et al. 2001). Tight junction creates constant intracellular barrier among the epithelial cells, which is needed to regulate selective solute movement across the epithelium and to separate tissue spaces. Various proteins are expressed on the TJ and their expression interruption leads to an unstable epithelial barrier (Madsen et al. 2001). TJs are generally divided into four protein groups, namely regulatory proteins, adaptor proteins, trans-membrane proteins, post-transcriptional and transcriptional regulators (Lodemann, 2010). These proteins function to help preserve intestinal integrity in a coordinated manner that can be well represented via indicators such as permeability of paracellular and (TEER) trans-epithelial electrical resistance permeability of monolayer cell (Wan et al. 2016).
The bar molded shape mucins are expressed by Goblet cells which are either secreted into the lumen to form the mucous layer or localized to the cell membrane (McCool et al. 1994). Eighteen types of mucin glycoproteins are expressed in humans (Culligan et al. 2009). In the enteric cell lines of humans, Lactobacillus spp, improve mucin articulation (MUC3 and MUC2 by HT29; MUC2 by Caco-2 cells), hence blocking cell binding and attack by infectious Escherichia coli ( Kim et al. 2008). The defective gene of mice IL-10 demonstrates the processing way with a VSL#3 probiotic such as Bacillus infantis, bulgaricus, thermophilus, Bifidobacterium longum, delbrueckii subspecies, Lactobacillus casei, S. salivarius subspecies, Bacillus breve.
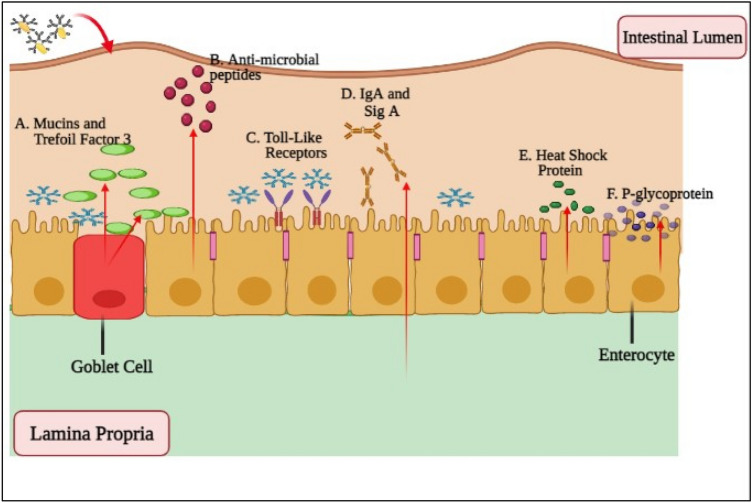
Extensive studies on the probiotic ability to affect immune system revealed that probiotic bacteria may affect both adaptive and innate components of the human immune system. Certain probiotic function in the gut lumen by producing innate immune molecules, which include mucins from goblet cell-derived, trefoil factors, defenses formed via intestinal Paneth cells while some attenuate the creation of TLRs, secreted immunoglobulin A, P-glycoproteins, and heat shock protein as shown in Fig. 3.
Fig. 3.
Regulation of Intestinal barrier mechanism using probiotics (Wan et al. 2016)
It is revealed the L. acidophilus enhances the effect of occludin, which is a significant part of tight junction in the enteric mucosal membrane of animals with ligation of inter-sphinteric fistula tract and aperture, which leads to reduce pathogenic translocation (Anderson and Van Itallie 2009). It is revealed that microbial probiotics, explicitly L. acidophilus and Streptococcus thermophilus, anticipated the decrease in the entero-invasive Escherichia coli-initiated phosphorylation of the proteins of the occludin and zonula occludens 1, subsequently gives the information about Tight Junction structure (Qin et al. 2005). In addition to innate defense mechanisms, probiotic bacteria tend to engage in the regulation of immune response mediated through intestinal epithelial cell, including cytokine production from enterocyte and M cell-mediated (GALT) gut-associated lymphoid tissue immune response as shown in Figs. 4 and 5, respectively. Besides, it is indicated that the reconstruction of the Tight Junction protein of ZO-1 is inhibited by Lactobacillus casei, which is away from the cell–cell contact, by a disease with enteropathogenic Escherichia coli. (Resta-Lenert and Barrett, 2003). In Table 3, information regarding some commercially available probiotics and their mode of action is give.
Fig. 4.
Regulation of innate defence mechanism using probiotics (Wan et al. 2016)
Fig. 5.
Regulation of Adaptive immune system using probiotics (Wan et al. 2016)
Table 3.
| Mechanism of action of probiotic | Pathogen | Probiotic bacterial Strain | Function | References |
|---|---|---|---|---|
| Competitive exclusion |
Enteropathogenic E. coli L. monocytogenes, Yersinia pseudotuberculosis S.enterica serovar Typhimurium |
L. johnsonii L. acidophilus |
Inhibited adhesion of Caco-2 cells to pathogenic organisms | Otte et al. (2004) |
|
Klebsiella pneumonia Enterotoxigenic E. coli EPEC |
L. casei | Inhibited adhesion of Caco-2 cells to pathogenic organisms | Corr et al. (2009) | |
| E. coli O157:H7 |
L. acidophilus L. rhamnosus |
Pathogen adhesion inhibited to T-84 epithelial cell | Corr et al. (2009) | |
| Production of inhibitory substances | H. pylori | L. acidophilus | Production of lacticins A164 and BH5 | O'Hara and Shanahan (2007) |
|
E. coli Clostridium difficile |
B. longum | Bacteriocin Production | O'Hara and Shanahan (2007) | |
| L. monocytogenes | L. salivarius | bacteriocin Abp118 production | Corr et al. (2009) | |
| C. difficile | L. lactis | lacticin 3147 production | Corr et al. (2009) | |
| Modulation of Immune System | Salmonella spp. | L. reuteri | Enhance the production IgM (anti-Salmonella) | Corr et al. (2009) |
| E. coli O157:H7 | L. rhamnosus |
Increase anti-E. coli IgA (Intestinal) response Leukocyte phagocytic activity |
O'Hara and Shanahan (2007) | |
| Enhanced barrier function | E. coli | L. acidophilus | Regulated against rearrangement to F-actin | Qin et al. (2005) |
| Enteroinvasive E. coli |
L. acidophilus S. thermophilus |
Enhanced trans-epithelial resistance, development and maintenance of cytoskeletal as well as tight junctional phosphorylation of its protein |
Qin et al. (2005) Resta-Lenert and Barrett (2003) |
Clinical outcomes
The researcher evaluated the effectiveness and protection of three combination probiotic strains, in hospitalized infants, L. rhamnosus HN001, Bifidobacterium lactis Bi-07 and, L. acidophilus NCFM, as an alternative to rehydration therapy for acute watery diarrhea (Chen et al. 2020a). One of the principle therapies for acute diarrhea associated with dehydration is oral rehydration (Guarino et al. 2014). Nonetheless, the duration or frequency of diarrhea could not be significantly reduced by the electrolytes or fluid replenishment (Vandenplas et al. 2007). Previous studies demonstrated that L.rhamnosus GG, Lactobacillus reuteri, and S. boulardii, and other such microbes are effective against acute diarrhea (Guarino et al. 2015; Guarner et al. 2012). However, this study reports that a mixture of probiotic L. rhanmosus HN001, B. lactis Bi-07, and L.acidophilus NCFM reduces diarrhea in children with a mean duration of 23 h as compared to those who are subjected to the rehydration treatment alone. These results are in accordance with another study that included 63 independent studies and 8,014 participants, which stated that diarrhea duration is reduced with a mean 24 h difference through probiotic intervention.
Analogously, Chen et al. (2020b) observed the 26-h shorter mean period of diarrhea in children complemented by the combination of Enterococcus faecalis, Clostridium butyricum, and Bacillus mesentericus, relative to placebo or inactive medicine provision to children. Not important enough, but a 24-h duration decrease of diarrhea was observed in a child consuming a probiotic combination of Bifidobacterium longum, L. Rhamnosus, L. acidophilus, and S. Boulardii compared to a child undergoing oral rehydration treatment (Chen et al. 2020a; Grandy et al. 2010). In 2007, Canani et al. observed a reduction of around 45 h in diarrhea duration in children receiving a mixture therapy as compared to those who received the oral treatment only (Canani et al. 2007). The mixture included strains of L. delbrueckii (bulgaricus), L. acidophilus, S. thermophilus, and B. bibidum. On the contrary, Saavedra and Tschernia (2002) opposed all these results and demonstrated no reduction in infants’ diarrheal duration with a mixture utilizing S. thermophilus and B. bifidum. These results were expected to be due to the lower dose of treatment. On average, the mean hospital stay duration of children treated with probiotics is approximately 29 h shorter as compared to the control group (Chen et al. 2020a).
Probiotics and enteric pathogens
Probiotics against foodborne pathogens
As antibiotics are widely used and misused as therapeutic agents, bacterial antibiotic resistance and normal microflora imbalance and the presence of pharmaceutical residues in food commodities have been increased. (Fayol-Messaoudi et al. 2005). This indicated that the treatment of pathogenic bacteria required a new procedure that led to an increase in the field of research of beneficial bacteria, i.e. probiotics. Treatment and prevention of infectious diseases by various pathogenic products is one of the main reasons for the extensive study of probiotics (De LeBlanc et al. 2010). The intestinal complexity of intestine, where a number of interactions between GIT, epithelial and nutrient associated microorganisms exists, is important to be considered when examining the prevention and treatment of pathogens (Hooper and Gordon 2001). In modulating the immune function, the epithelial and immune cells play a role and are the primary defense against bacterial pathogens. The resident microflora can alter the composition and activity of the intestinal microbiota (De LeBlanc et al. 2010).
Probiotics against Helicobacter pylori
The variable portion of host Gastrointestinal Tract is affected by various pathogenic bacteria, for instance, H. pylori affects the mucosal layer of the duodenum and cause gastric problem, as well as some colonial and ileum problems, are created by Clostridium difficle and Salmonella species, while Shigella species lean towards the colonial mucosa (Dupont 1997). Past examinations have indicated that the probiotics have an impact when devoured as a major aspect of the day by day diet, they can keep up the defense mechanism in a functioning state as well as can cure the distinctive enteric issue (De LeBlanc et al. 2010). It was revealed that the specific probiotics of lactic acid bacteria repress apoptosis of macrophages affected with Salmonella anticipating salmonellosis (Carlos Valdez et al. 2001). Cano and Perdigón examined the protection proportion of Lactobacillus casei CRL-431 against Salmonella serovar Typhimurium.
In the mice models after fourteen days of the nutrition, It is revealed that controlling probiotics forestalled Salmonella serovar Typhimurium 100% related to contamination, which stop them from causing infection (Cano and Perdigón 2003).Findings of their trials were affirmed by an alternate report (De LeBlanc et al. 2010), in a mice model the preventative and persistent administration of probiotic Lactobacillus casei CRL-431 against Salmonella serovar Typhimurium was studied. The study group reported less serious infection than control group who did not receive probiotics before the introduction of the pathogen for the first 7 days and the post-infection. They have also recorded that 7 days of post-infection treatment of probiotics led to better immunity against Salmonella infection. They deduced that probiotic consistent administration decreased the number and spread of bacterial infections in the intestine and outside of the organs. Further experiments on the effectiveness of probiotic strains have been performed on different pathogens. H. Pylori is a pathogen that has an important role in pathogenesis of both adult and children's chronic active gastritis and peptic ulcer. It is a type of bacterium which mostly affects youngsters and infants (Elitsur and Yahav 2005) with increasing concrete evidence that it is an important contributor in establishment of gastric cancer (Uemura et al. 2001). H. pylori have been connected to cancer, therefore, yet there is no vaccine available that can cure patients from this infection (Ruggiero 2014). The distinctive restorative methodologies are available which can be utilized to treat H. pylori, as well as not constrained to the generally utilized triple treatment with proton pump inhibitors, amoxicillin and either metronidazole or clarithromycin or double treatment high dose amoxicillin.
After research, It is reported that a few patients despite of above mentioned treatment stay infected even after the completion of this medication for two runs of 14-day intake (Leung and Graham 2002). It is especially important to prescribe alternative drugs that may improve treatment effectiveness and/or reduce the side effects (Ruggiero 2014). There is significant evidence from various studies that emphasize the effectiveness of probiotics in the management of H. Pylori disease targeting various signs and symptoms (Lionetti et al. 2011). Scientists examined whether readily market accessible formulation containing Lactobacillus casei represses the development of H. pylori in vitro (Cats et al. 2003).
It was observed that, in in vitro environment, Lactobacillus casei restrains the development of H. pylori; with a condition that, the probiotic cells must be feasible and useable. In an alternate report, it was revealed that probiotics, for example, L. rhamnosus GG or Lactobacillus johnsonii use bactericidal or bacteriostatic mechanisms against a wide range of pathogenic microbes, as well as H. pylori (Bernet-Camard et al. 1997). A clinical trial was performed to see if the constant administration of a dietary product having L. johnsonii La1 or L. paracasei ST11 would interfere with colonization H. pylori in the gastric epithelium of children (Cruchet et al. 2003). They found out that frequent intake of the nutritional item including Lactobacillus johnsonii La1 may serve as an intriguing option against H. pylori to adjust its colonies in youngsters who are infected with this pathogenic bacteria. Researchers showed that a Ten-day fourfold treatment of helicobacter treatment with (RBC) ranitidine bismuth citrate in addition to (PPI) proton siphon inhibitors, tinidazole and amoxicillin gets a huge eradication of H. pylori, whereas when supplemented with L. casei have been shown to significantly increase the eradication rate of H. pylori infection (Tursi et al. 2004). Furthermore, it is estimated that it indicates a slightly better approach of H. pylori eradication by the augmentation of treatment with the administration of probiotics. Consequently, probiotics can be utilized as the first course of anti H. pylori therapy as well as utilized with the first line treatment approaches.
Probiotics against Shigella
Shigella is a bacteria which can resist against antibiotics (Pazhani et al. 2008). According to statistics in developing areas approximately, three to five million children are reported to die annually due to gastrointestinal infections (Sivapalasingam et al. 2006). Multiple drug resistance to Shigella is a threat in developing countries to cost-effective antimicrobials and this bacterium 's resistance pattern is now becoming a clinical problem worldwide (Mirnejad et al. 2013). The need for alternative therapies was therefore considered necessary because of increased prevalence of its antibiotic resistance (Zhang et al. 2011). The antipathogenic activity of probiotic paracasei M5-L, Lactobacillus casei Q8-L, Lactobacillus paracasei subspecies, and Lactobacillus rhamnosus J10-L against S. sonnei were tested. It is revealed that the approved Lactobacillus strains demonstrated a solid antibacterial action against Shigella sonnei. The trial to find out antibacterial activity of probiotics against Shigella sonnei showed that, Lactobacillus johnsonii F0421 showed huge repressive activity and barred, replaced Shigella sonnei clung to HT-29 cells (Zhang et al. 2012a, b). The antimicrobial activity of nisin, was studied in another study by scientists produced by Strains of lactis, against S. aureus, L. monocytogenes, Typhimurium Salmonella and Boydia shigella (Mirnejad et al. 2013). The study revealed that pathogen populations declined, due to alteration of fatty acid levels, cell viability, permeability of membrane, and nisin depolarization effect. A different trial examined the antibacterial effect of nisin, a bacteriocin produced by the lactis strains of Lactobacillus, against S. boydii, S. aureus, Lactobacillus monocytogenes, S. boydii. In addition, S. Typhimurium.
Probiotics against Lactobacillus monocytogenes
L. monocytogenes belongs to a class of bacteria that causes foodborne diseases (Zou et al. 2013). that contributes to severe impacts in the human host that cause premature delivery and stillbirth conditions for perinatal conditions by causing septicemia and meningitis in adults (Vázquez-Boland et al. 2001) There are various probiotics being used against the food-borne pathogenic microbes that cause food poisoning. Furthermore, in research, it is showed that bifidobacterial isolates can deliver antimicrobial elements (Touré et al. 2003). Scientists published six infant strains having more of antibacterial action capacity against Lactobacillus monocytogenes produced from bifidobacteria. The isolates inhibited L. monocytogenes actively by producing a heat-stable protein-substance. Their research showed that the use of bifidobacterial strains is suitable for interacting in probiotics with bacterial pathogens would enhance gut microbial ecology and offers an alternative effective strategy to prevent gastrointestinal pathogens. In 2007, studies were carried out for the pretreatment with strains of Bifidobacterium and Lactobacillus of C2Bbe1 cells, which is clone of the Caco-2 human adenocarcinoma cell line in order to show that these significantly can hinder the subsequent chances of reinfection by L. monocytogenes (Corr et al. 2007). They revealed that the earlier treatment of enteric epithelial cells besides probiotic microorganisms preceding disease with Lactobacillus monocytogenes EGDe brought about a noteworthy lessening in listerial attack about 60–90%. In a different trial where Lactobacillus strains were used against E. coli and L. monocytogenes to see its antagonistic effects, it was observed that L. plantarum WS4174 showed a much effective inhibitory effect against the Gram-positive L. monocytogenes LMO26, probably because of the lactic acid accumulation and L. monocytogenes being sensitive to lower pH (Aguilar et al. 2011).
Enteric viral infections
Viral diseases are controlled through probiotics. About 20–25% of diarrheal infection in the world is caused by rotavirus. The levels of IFNy and IL-4 in serum enhanced by L. acidophilus and Lactobacillus reuteri and it eliminates rotavirus disease (Wen et al. 2009). Norovirus, which causes 58% of foodborne diseases, probiotics proved to be an effective treatment against these as well (Mattison 2011). L. casei Shirota strain was found to be effective in controlling gastroenteritis caused by norovirus in a healthy individual, which is available as Probiotic fermented milk. A randomized double-blind trial using probiotic formulations (VSL#3) has been shown to substantially reduce the bowel frequency and oral rehydration is needed in infants (Dubey et al. 2008).
Bacterial enteric infection
Among enteric pathogens that induce the diarrheal infection, Campylobacter jejuni is responsible for around 400 million cases per year in both undeveloped and developed countries. Several trials of probiotics were tested for their efficacy in managing the infection of Campylobacter. Both lactobacilli and bifidobacteria have been shown to increase resistance to colonization in mice infected with C. jejuni or Salmonella. Probiotics have boosted lymphocyte proliferation and reversed pathogen-induced immunosuppression activity against Salmonella antigens (Ruiz-Palacios 2007). Probiotics likewise have expanded the expansion of lymphocytes against Salmonella antigens and inverted microbes-instigated immunosuppressive action (Wagner et al. 2009). Campylobacter jejuni load in poultry feces substantially reduced by symbiotic composed of prebiotic galacto-oligosaccharide and probiotic bifidobacterium longum (Baffoni et al. 2012). Acute dehydrating watery diarrhea is caused by V. cholera with 1.8 million cases and an estimated mortality rate of 27,000 per year (Girard et al. 2006). Furthermore study indicated that the administration of L. acidophilus BKM B-2020 orally in mouse as well as suckling rabbit’s disease inhibited cholera infection. Cultured cell lines are effectively attached to Probiotic Lactobacillus plantarum AS1 and decrease Vibrio parahemolyticus fixation through excluding it competitively (Satish Kumar et al. 2011). Probiotics are beneficial for treating diarrhea infection caused by E. coli. for example ETEC and STEC. A bacterium named Enterococcus faecium decreases a large number of Escherichia coli. Lactobacillus plantarum, Enterococcus faecium, Lactobacillus fermentum, Lactobacillus plantarum, Lactobacillus casei and L. acidophilus altogether diminished E. coli O157: H7 exfoliating through sheep (Lema et al. 2001). Bifidobacteria in mice decreases the production of Shiga toxin by STEC and provides a defense mechanism against Escherichia coli. STEC growth stops by Nissle and Shiga are not produced as well (Reissbrodt et al. 2009). Dendritic cells are regulated by exposure to Lactobacillus paracasei, helper T cells are also activated and develop antibodies, there is increase in enteric integrity and low regulation of pro-inflammatory cytokines and the infections which entered are protected from them (Tsai et al. 2010). Studies have proven the effectiveness of probiotics used against Salmonella enterica by preventing the spread of infection and colonization in the gut (Higgins et al. 2008).
An industrial probiotic alcoholic drink essentially diminished the Salmonella which includes in the ceca and tonsils of hens and poults (Zhang et al. 2012a, b). Salmonella infection in the tonsils and ceca chickens has been found to be lowered considerably by a commercial probiotic named as cocktail (de Moreno et al. 2010). In vivo examination utilizing a mice model exhibited that constant administration of Lactobacillus casei CRL reduces the Salmonella numbers includes in the digestive tract and extra enteric dispersal (Asahara et al. 2011). The strain of Lactobacillus, L. casei Shirota, likewise ensured protection to mice against deadly contamination with multi-resistant Salmonella Typhimurium DT104 strain (Varma et al. 2010). Probiotics have been additionally viable to other intestinal microbes, for example, S. aureus, P. mirabilis, and P. aeruginosa, E. faecalis and S. sonnei (Amalaradjou and Bhunia 2013). The Bacteriocin "Microcin S" delivering probioticE. coli G3-10 likewise smothered EPEC constancy and microgenesis (Malin et al. 1996).
Probiotics administration for infectious diarrhea in children
A few ongoing clinical investigations endeavored to build up the estimation of probiotics in the prophylaxis as well as the cure of loose watery stool infection in children (Kaila et al. 1992). Rotavirus is main reason for watery bowels in children all over the world. Lactobacillus rhamnosus GG showed the most vital advantages in sufferers with intense gastroenteritis brought about through rotavirus, which is yet the most significant pathogenic microbe for pediatric stool sickness around the world. It revealed that very much directed and acted in a few nations through a few agents with scholarly and modern help (Kaila et al. 1995). During an examination, the information for Lactobacillus reuteri and Bifidobacterium lactis have mostly founded on a couple of clinical investigations (Rautanen et al. 1998). Many different double-blinded treatments were controlled in which Finnish babies and little youngsters with rotaviral loose bowels were included. It revealed that indicated a decreased span of scenes of loose bowels among small kids who got Lactobacillus reuteri (span/1.7 days versus 2.9 days for control sufferers) (Shornikova et al. 1997a, b). It likewise revealed a shorter span of looseness of the bowels and a diminishing in rotavirus shedding with Lactobacillus rhamnosus GG, affirms the discoveries of earlier clinical examinations (Guarino et al. 1997). Other than the curative impacts, these discoveries showed that extensive outgrowths in regards of diminishing the pace of nosocomial rotaviral contamination in newborn children with a high hazard for gastrointestinal infections (Saavedra 2000). Multicenter European organization concluded that use of hypotonic oral dehydration solution and LLG at the same time can be of great benefit to children as it can reduce the time of illness and duration of hospital stay (Guandalini et al. 2000). But on the other hand, there was no improvement in the loose bowels sickness was observed when an experiment was carried out in Canada in 1972. A probiotic that was commercially available (108 lyophilized bacteria comprised of 50–60% Streptococcus thermophilus, 35–45% L. acidophilus, and 5% L. bulgaricus) was applied on 94 children who were suffering from acute diarrhea (Pearce and Hamilton 1974). In developed countries, many experiments were carried out showed that if we start the treatment earlier with simultaneous use or ORT and LGG it can be of great use and can reduce the time of suffering and also work for treatment of acidosis, but it cannot work on people with bloody diarrheal stools (Shornikova et al. 1997a, b). Whereas addition of heat-killed L.acidophilus bacteria’s to ORT has proved to be a beneficial treatment in the children having acute gastroenteritis and it can also lead to the reduction of sickness span (Simakachorn et al. 2000).
Another study was held in Paris in which 287 sound youngsters were included (mean age, 18 months and a half; extend, 7–32 months) the children were chosen from 12 different daycares (Pedone et al. 1999). Kids took one of three dairy items: milk aged by yogurt standard yogurt and Lactobacillus GG casei, or jellied milk as a treatment. There was no distinction found in the frequency of loose bowels ailment between all groups, yet the span of illness of looseness of the bowels was shorter between sufferers in the Lactobacillus casei bunch than it was between sufferers in the jellied milk society (4.3 days versus 8.0 days, individually; P = 0.009).
Probiotics for diarrheal disease in adults
There are some scarcely controlled preliminaries led in grown-ups with irresistible loose bowels. A random trial was held in the adults and they were divided into two groups, (an experimental group and a control group) who had chronic diarrhea which showed that the adults who had Enterococcus SF 68 strain in their medication had less severe diarrhea and also shorter time of illness in probiotic group as compared to control group (Buydens and Debeuckelaere 1996). In a case, an investigation was led with 23 sound volunteers who got an item that contained Lactobacillus bulgaricus and Lactobacillus acidophilus though there was no change in attack rate, incubation period, or time length of sickness was evident when they tested with Enterotoxigenic E. coli (Clements et al. 1981). A few investigations revealed that there is some preventive microscopic organism’s impact of lactic acid in sufferers with traveler’s diarrhea shows incompatible outcomes, and detailed advantages are unassuming. According to another research that have been exhibited between 282 British soldiers who ventured out to Belize and got a probiotic item that contained Lactobacillus acidophilus or KLD strain of Lactobacillus fermentum (DuPont and Ericsson 1993), and then again between 820 Finnish travelers who got Lactobacillus rhamnosus GG before making a trip to Turkey (Oksanen et al. 1990). It was shown that a decrease in the frequency of looseness of the bowels between 245 American explorers who took Lactobacillus rhamnosus GG for 1–3 weeks, contrasted and control sufferers who got fake treatment (occurrence of the span, 3.9% every day for people who got Lactobacillus rhamnosus GG versus 7.4% every day for control cases P = 0.05) (Hilton et al. 1997).
Antibiotic-associated diarrhea and probiotics
Looseness of the bowels is the most widely recognized gastroenteric symptom of anti-infection treatment regularly connected with Clostridium difficile contaminations in grown-ups and youngsters. Studies revealed that it included Saccharomyces boulardii strains (McFarland et al. 1995), Lactobacillus spp., and Bifidobacterium spp. (Colombel et al. 1987) have revealed valuable impacts in the therapy and avoidance of antimicrobial related looseness of the bowels, (Gorbach et al. 1987) revealed as an effective therapy of Clostridium difficile backsliding looseness of the bowels, without any symptoms, in five grown-ups and four kids who got Lactobacillus rhamnosus GG (Biller et al. 1995). A little report that included 16 youthful grown-ups who got erythromycin for 7 days demonstrated a considerable constructive outcome between the individuals who got in advanced yogurt Lactobacillus rhamnosus GG, contrasted with those individuals who got treatment by yogurt. The span of the looseness bowels was just about 2 days between sufferers in the revealed bunch versus 8 days between sufferers in the placebo treatment bunch. That means those who received LGG-enriched yogurt, compared with those who received placebo yogurt. The duration of diarrhea was only 2 days among patients in the study group versus 8 days among patients in the placebo group (Siitonen et al. 1990). Recently, a useful impact of Lactobacillus rhamnosus GG in the counteraction of looseness of the bowels was studied between Finnish (Arvola et al. 1999) as well as American kids with respiratory disorders. It showed reduction in loose bowel episodes and an increase in bowel consistency by ongoing antibiotic treatment when both L. bulgaricus and L. acidophilus were given then, different investigations had likewise shown no advantage. Many antibiotics were tried and different organism were also being tested but there also some limitations such as not adequate amount of control groups and sufferers so it has not allowed to imply some clinical trials (Tankanow et al. 1990).
Probiotics as vaccines
Low immune response levels may be caused by probiotics. Probiotics can, therefore, be bioengineered to provide the gastric mucosa region with immunogenic compounds to boost the immune response of host. Recombinant probiotics can mimic the effect of a vaccine to treat intestinal pathogens in the host immune system (Gardlik et al. 2012). The researchers have cloned and expressed ETEC adhesives K99 to the probiotic Lactobacillus casei in order to use a safe and highly effective vaccine to prevent K99 infections of ETEC. They observed an elevated levels in the effectiveness of the recombinant probiotic and that those over 80 per cent of the vaccinated mice had been protected against a fatal dosage of standard strains (Wen et al. 2012). Non-bactericidal illnesses can be administrated by using vaccine delivery systems with bio-engineered probiotics. The most frequent source of childhood diarrhea is rotavirus. It affects cells in the intestine (enterocytes) and then tends to cause gastroenteritis in the effected host. The viral proteins of the pathogen may interfere with water reabsorption inside the human gastrointestinal tract and cause lactose digestion inefficiency, which could contribute to milk intolerance for children. Symptoms of infection include nausea, diarrhea, vomiting, fatigue and fever (Thirabunyanon 2011). Researchers used the bioengineered Lactobacillus lactis to express infection spike protein VP8, that prompted IgA antibodies in mice and hostile to VP8 antibodies (Snydman 2008). This induction developed inside the mouse digestive tract, systemically and locally, providing 100 percent protection against rotavirus. Since oral vaccination preferred over other forms of vaccination, it is possible to use probiotics since these have the ability to resist gastrointestinal conditions as an alternate vaccination process. There are also other benefits of delivering vaccines with recombinant probiotics, such as simple administration by users, reduced risk of transmission of foodborne infections and activation of both innate and adaptive immune responses (Mathipa and Thantsha, 2017).
Probiotic against parasitic infection
Giardia lamblia is known to be one of the most widespread intestinal parasites to infect humans. It is the causal factor in giardiasis, a significant cause of diarrheal disease. Present recommended medication is generally done by a few medications. However, due to parasitic resistance alternative solutions are being pursued. At present, interest in potential biotherapeutic techniques involving natural approaches such as probiotic Lactobacillii with antiprotozoal effects is on the front. The current study on 57 albino mice of Swiss origin retrieved from the Theodor Bilharz Research Institute's animal house determined the effect of L. casei on Giardia lamblia as compared to the routinely available metronidazole in intentionally infected mice (Mazroue 2020).
Research published in 1995 involving human patients demonstrated the positive effect of probiotic yeast, S. boulardii (Reflos), not specifically to avoid giardiasis but to assist with the healing of irritable bowel syndrome resulting after infection, a problem that occurs in some patients infected with parasites (Travers et al. 2011b). This analysis was endorsed by a subsequent survey claiming S. Boulardii lowered the quantity of parasitic cysts in patients' feces treated with a blend of metronidazole and S. Boulardii compared to metronidazole-treated patients (Besirbellioglu et al. 2006).
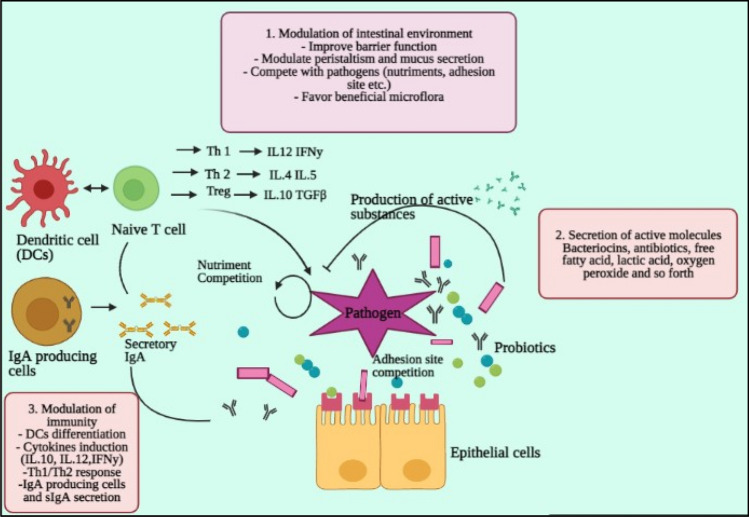
Parasites of the intestine like C. parvum, G. lamblia, B. hominis, E. histolytica, or T. spiralis (a nematode) provide Irritable Bowel Syndrome-like signs and symptoms. It also plays a possible role in the etiology of IBS (Robertson et al. 2010). There has been little assessment of the prospects of probiotics to control IBS-like signs associated with parasitic infections. Probiotics, especially, L. paracasei NCC2461, B. longum NCC2705, L. Johnsonii NCC533, and B. lactis NCC362 positively modulated the parasite expulsion. Although medical data of efficacy has begun to arise, the actual effect of probiotics on PI-IBS therapies remains extremely contentious. It is primarily owing to the complexity of comparing the research projects due to experiment design differences, the dose of probiotic, strain utilized, and the active agent (Quigley, 2007; Travers et al. 2011b). Figure 6 explains the different possible ways of pathogenic control.
Fig. 6.
Schematic diagram of the different pathways for probiotic-mediated pathogen control. At (1), probiotics may attenuate their physical and chemical surroundings (epithelial cell receptors, mucus, nutrients, pH, peristaltism, and tight junctions). At (2), probiotic supplements may yield biological molecules like antibiotics, bacteriocins, or peroxide-containing antimicrobial characteristics. At (3), probiotics can cause immune regulation, either by interfering with dendritic cells, which can, in effect, alter the segregation of naive T lymphocytes to Th1, 2, or Treg cells, corresponding to unique inductions of cytokines or by humoral response via IgA-based cells and their secretive IgAs (Travers et al. 2011b)
A probiotics' list tested on numerous eukaryotic infectious organisms. The first column lists the pathogens investigated; the second shows the probiotics examined (and, if identified, a reference to their strain; n.s. not defined) and the accompanying sources. The third section provides specifics of the study level: therapeutic in patients, in vivo in normal or laboratory models (mouse, pigs calves, rats, gerbil, chicken) or at the most basic cellular stage (cyst segregation and survival, invasion capacity, and trophozoites development). The fourth column provides the probiotic administration conditions and is denoted by A. a, being the first three days, b – next four days, c—7th – 15th days, d – increasing than fifteen days, e – specifying the after infection, and lastly, f – concomitant infection and administration. The fifth column is for the study results, which are specified in terms of ( +) or (-) impacts, depicting the parasitic load reduction in administered subjects versus the controls. – (Minus) stands for adverse probiotic effect, 0 indicates no effect, + stands for around 50% load reduction, + + means about 75% reduction of parasitic load, and + + + for about 100% reduction. The star (*) indicates a clinical case and n.d. in the results indicates reduction not determined (Travers et al. 2011b).
Limitations of conventional probiotics
Even though probiotics give various advantages to the host, they have several restrictions too. Certain examinations revealed that the strains of probiotic might be useless or ineffective because of explicit intestinal microbes. Probiotics can discharge a wide range of antimicrobial substances notwithstanding, reports recommended that there are some restrictions in the accomplishment of probiotics focusing on explicit pathogenic microbes. Consequently, the probiotic strains of different cocktail can be created to improve the impacts countering various pathogenic microbes inside the intestine (Touré et al. 2003). A brief data about the previous trials of probiotics against certain pathogens is shown in Table 4.
Table 4.
Documented trials of probiotics against specific pathogens (Travers et al. 2011a)
| Pathogen | Probiotic tested | Host | A | R |
|---|---|---|---|---|
| Ascaris suum | B. lactis (pig isolate) | Pig | d | n.d |
| Babesia microti | L. casei ATCC7469 | Mouse | a | + + + |
| b | + | |||
| Cryptosporidium parvum | L. reuteri 4000, 4020 | Mouse | c | + + + |
| L. reuteri 4000, 4020 or L. acidophilus NCFM | Mouse | c | + + | |
| L. reuteri 4000, 4020 | Mouse | c | + + | |
| L. rhamnosus GG + L. casei shirota | Human | e | ∗ | |
| VSL#3 or Actimel | Neonatal rat | a | 0 | |
| B. brevis, E. faecium, P. alcaligenes | Calf | f | 0 | |
| L. reuteri ATCC23272 or L. acidophilus NCFM | Cell culture | + | ||
| B. breve ATCC15698 or B. longum ATCC15707 | Cell culture | + + + | ||
| B. brevis, E. faecium and P. alcaligenes | Cell culture | + + + | ||
| Eimeriatenella/acervulina | Primalac | Chicken | d | + + |
| Mitomax | Chicken | d | + | |
| Mitogrow | Chicken | d | + | |
| L. acidophilus Lb33ac, L. salivarius Lb14c7 Lb16c6 | Cell culture | + + | ||
| Giardia lamblia | L. johnsonii LA1 | Cell culture | + + + | |
| L. johnsonii LA1 | Gerbil | b | + + | |
| L. casei MTCC1423 | Mouse | b | + + + | |
| E. faecium SF68 |
In contrast to prior reports probiotics displayed inhibition impact against L. monocytogenes (Kailasapathy and Chin 2000), Koo et al. revealed that probiotics have a constrained accomplishment in case of preventing L.monocytogenes to attach to monolayers of enteric region (Koo et al. 2012). They implemented three experimenting methodologies of serious prohibition of adhesion or displacement of pathogens, and competitive exclusion to decide if by using lactobacilli, that it would diminish the bond of L.monocytogenes to Caco-2 cells, they demonstrated that the rates of Lactobacillus monocytogenes attachment to cells in the actively present and in nonappearance of probiotics were almost similar. Lactobacilli and other LAB were not able to essentially diminish attachment or colonization on the layer of epithelial cells, despite being at larger quantities. Moreover, an increase in dose of the strain of probiotic providing high concentration of it was also not able to dislodge the connected Lactobacillus monocytogenes. The information from the trial demonstrated that the previously used conventional strain of lactic acid bacteria could not inhibit the growth of these pathogenic microbes. In addition, it was reported that probiotics could also stimulate low immune system response in the organism as well as low anti-inflammatory response rates (McCarthy et al. 2003). Both Bifidobacterium infantis and Lactobacillus salivarius were orally given to mice experiencing colitis. Results showed that the levels of TGF-β in mice who were given or not given the probiotics remained same as before. TGF-β is a cytokine that can reduce the inflammation, and the degrees of this cytokine were not essentially elevated yet its levels were maintained with Lactobacillus salivarius, but these are not keeping up by the use of Bifidobacterium infantis. Most probiotics are delivered as capsules or foods, so technical, digestive, and enteric stress factors must be addressed. In addition, an obstacle to its effectiveness is the large mode of action of probiotics and the variations between probiotics. The beneficial characteristics of a strain or a strain mixture may not be repeatable and may vary from individual to individual (Karimi and Peña, 2008). Furthermore, the probiotic strain, dosage, course of treatment and composition of probiotic preparation can also influence their effectiveness (Koo et al. 2012). Considering these findings, it is clear that probiotics still are nondiscriminatory and nonspecific or inefficient in some hosts (Bomba et al. 2002). The above limits imply the need for new, innovative measures for use of probiotics to prevent and treat foodborne pathogens. Previous studies reported that the use of probiotic therapy was continued for much longer period to produce the host's mucosal barrier with prophylactic and therapeutic molecules( Richter et al. 2009). But a detailed understanding of the pathogen’s behavior and the mechanisms of their diseases is needed to be successfully achieved (Amara and Shibl 2015). All these understanding can be helpful to enhance the effectiveness of probiotics and then using a particular probiotic for particular pathogens or toxins. Therefore, novel probiotic strains can be produced with improved or even targeted probiotic operation. Biotechnology provides the opportunity to produce these recombinant probiotics.
Recombinant bioengineered probiotics
Through using bioengineering, the efficiency of known probiotic strains can be boosted. Bioengineering involves the modification of the probiotic strain genes in order to improve toleration of the ecological or host environmental stress, which is not limited but also include high temperatures, acidification and oxygen, food processing and/or probiotic survival at the GIT in order to provide maximum benefit to host when administrated (Guidone et al. 2014). There are different techniques, which can be utilized in structuring, and developing of new probiotic harbouring genes of intrigue got from the pathogenic microbes. It enables the transformation of proteins, which were not in the microorganism at first. Pathogenic virulence factors can be cloned, characterized and expressed in the probiotic strain and following the delivery of the resulting bioengineered probiotic strain will prevent the infection, resulting in no clinical symptoms. Moreover, recombinant probiotics can also be used for medications, vaccinations and to enhance immuno-response and cell-bound receptors for particular pathogens or toxins (Berg and Mertz 2010). Most human receptors that are identified by or are well distinguished by enteric pathogens. Furthermore, the production of vaccine or treatment of resistance is considered unlikely by targeting a particular pathogen. Bioengineering is not a completely new field, several trials have shown the effectiveness of these probiotics against diseases (Culligan et al. 2009).
Numerous studies in recent years have focused on the feasibility of using GM probiotics as a bio-therapeutic (Sola-Oladokun et al. 2017). The basic principles of bioengineered probiotics have been knowing the certain mechanisms behind the positive and beneficial effects of wild probiotic products and pathogenicity in infectious gastrointestinal diseases (Sleator and Hill 2007). The enhancement of probiotic stress tolerance has contributed to the development of stronger recombinant strains able to cope with the production process challenges and then with the innate protection of the intestinal tract (Sheehan et al. 2006; Watson et al. 2008). Improved specificity of probiotic and its advancement as molecular delivery vehicles could significantly enhance its biotherapy potential in the areas of infection, chronic inflammations of gastrointestinal tract, psychological diseases and even cancer (Sleator 2010).
Most of the bioengineered and modified probiotics known till now focused on well-known bacterial species, for example L. lactis, E. coli, and Lactobacilli, for these advanced tools of genetic modification to increase their potential have been reported by scientists. Advances in Genetic engineering have extended the range to include the prevalent intestinal microbiota flora for modification., let us say, Bacteroides thetaiotaomicron (Mimee et al. 2015). A suite of promoters, inducible expression systems and ribosome-binding sites (RBS using recombinases and CRISPR interference was developed for B. thetaiotaomicron. The study suggests these advancements so that the GIT can be monitored and monitored in vivo, the impacts of various pathways and genes on colonizing potential and biotherapeutic utilization are focused and controlled. Integration of expert gut colonizers (and opportunist pathogens) is vital to the strict biological confinement and/or suicide processes. The risk for displacement of natural B. thetaiotaomicron populations (which is responsible for 30% of anaerobic bacteria which is culturable in the host intestine along with their bioengineered adjuvants would be much elevated compared to, for example, L. lactis or E. coli (Salyers 1984). Nonetheless, the use of similar strategies to other genus will add to the bioengineering catalog of species available in the coming years.
Scientists also developed an Escherichia coli strain that could be detected, recorded and identified during the tetracycline passage through the moray gastrointestinal system by means of a genetic processing switch. The study proposed that cell reporters should be established further and used to react to chemical biological markers that are indicator of inflammation, skin toxins, cancer and infection, or to generate diagnostics and therapeutics or antibiotic compound as a real-time response to environmental external stimuli (Kotula et al. 2014). In a study, researcher showed that P. aeruginosa planktonic biofilms and cells can be destroyed using a modular bioengineering approach for E. coli. Production of a bacteriocin (microcin S) was involved in the three-pronged approach and a nuclease (DNase I) was produced in response to P. aeruginosa produced quorum-sensing molecules, As a reaction to the same quorum-sensing molecule, the bioengineered strain moved actively into the target by reprogramming CheZ chemotaxis (N-acyl homoserin lactone (AHL), etc.) (Hwang et al. 2014).
The contribution of synthetic biology in designing and synthesizing natural products is addressed in an excellent review of Smanski and its colleagues. Significant advances in the production of new naturally produced products such as antivirals, antibiotics, anti-cancer and immunomodulatory compounds, and inhibitors are combined with constant cost reductions in DNA sequencing and synthesis technologies. An incredible number of such compound products remain unfindable; 150,000 natural compounds of the genus Streptomyces alone have been described to date, less than 5 percent of them (Smanski et al. 2016; Watve et al. 2001). These unexploited resources will without doubt produce new antibiotics, which are needed urgently to fight the increased rates of drug resistance between bacteria (Editors 2016).
In future, synthetic biology will be playing an important role in the functional enhancement of the probiotic engineering (Alivisatos et al. 2015). The development of the first cell with a synthetic genome (Gibson et al. 2010)and in recent studies, the development of a minimal bacterial genome (Hutchison et al. 2016)has provided a foundation in order to design and create completely new bacterial cells, tailored with a specific set of genes and a specific purpose. In just 20 years, the field has gone from the design, synthesis and assembly of a synthetic genome and associated biomolecules to the sequencing of the first bacterial genome (Sleator 2013). Further developments, including the automated design of genetic circuits, complete computing modelling, Big Data storage mediated by DNA and the growing genetic alphabet, will eventually lead to the next generation of true probiotics (O' Driscoll and Sleator 2013).
Conclusion
Probiotics are microbial supplements, administered to improve human gut health since a time now. Recent studies have expanded the definition of probiotics, because ability of imparting beneficial health effects has been shown to be equally present in genetically modified and non-viable microbes. However, the rationale for probiotic therapy is the standardization of the characteristics of unbalanced indigenous microflora by specific strains of healthy intestinal microflora. Probiotics also induce and contribute to eradicate nonspecific host resistance against microbial pathogens. Probiotics are currently used majorly to reduce the risk of infections linked with intestinal barrier impairment. This paper has analysed the role of probiotics in prevention and treatment of enteric infections. The probiotic efficiency of the strains varies; different bacteria or modified strains have defined adherence sites, immunological effects, and varied effects in the healthy versus inflamed mucosal environment. This paper has been discussed the bioengineered probiotic characteristics that may be exploited in the future for the development of specific prophylactic and therapeutic interventions for diseases.
Over the years, many probiotics against enteropathogens have demonstrated effectiveness and their exact mechanism of action has been established. A better correlation was established of the host pathogen interaction has also allowed the development of bio-engineered probiotics for targeted pathogens elimination. The use of engineered probiotics leads the stability of many other therapeutic alternatives. To avoid the uninhibited spread, however, the modified organism must be contained. Furthermore, their biosecurity and ability to cause allergies due to long consumption must be taken into consideration. Although several challenges lie in developing a novel probiotic against enteric infection, advancing technologies and research will continue to provide novel biotherapy in rich and economically challenged countries to deal with and prevent enteric infections.
References
- Abou-Kassem DE, Elsadek MF, Abdel-Moneim AE, Mahgoub SA, Elaraby GM, Taha AE, Ashour EA. Growth, carcass characteristics, meat quality, and microbial aspects of growing quail fed diets enriched with two different types of probiotics (Bacillus toyonensis and Bifidobacterium bifidum) Poul Sci. 2021;100(1):84–93. doi: 10.1016/j.psj.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar C, Vanegas C, Klotz B. Antagonistic effect of Lactobacillus strains against Escherichia coli and Listeria monocytogenes in milk. J Dairy Res. 2011;78(2):136. doi: 10.1017/S0022029910000877. [DOI] [PubMed] [Google Scholar]
- Akter S, Park J-H, Jung HK. Potential health-promoting benefits of paraprobiotics, inactivated probiotic cells. J Microbiol Biotechnol. 2020 doi: 10.4014/jmb.1911.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alakomi H-L, Skyttä E, Saarela M, Mattila-Sandholm T, Latva-Kala K, Helander IJAEM. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl Environ Microbiol. 2000;66(5):2001–2005. doi: 10.1128/aem.66.5.2001-2005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alivisatos AP, Blaser MJ, Brodie EL, Chun M, Dangl JL, Donohue TJ, Taha SA. A unified initiative to harness Earth's microbiomes. Science. 2015;350(6260):507–508. doi: 10.1126/science.aac8480. [DOI] [PubMed] [Google Scholar]
- Amalaradjou MA, Bhunia AK. Modern approaches in probiotics research to control foodborne pathogens. Adv Food Nutr Res. 2012;67:185–239. doi: 10.1016/b978-0-12-394598-3.00005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amalaradjou MAR, Bhunia AK. Bioengineered probiotics, a strategic approach to control enteric infections. Bioengineered. 2013;4(6):379–387. doi: 10.4161/bioe.23574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara AA, Shibl A. Role of Probiotics in health improvement, infection control and disease treatment and management. Saudi Pharmaceut J. 2015;23(2):107–114. doi: 10.1016/j.jsps.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin M, Adams MB, Burke CM, Bolch CJJAR. Isolation and screening of lactic acid bacteria associated with the gastrointestinal tracts of abalone at various life stages for probiotic candidates. Aquac Rep. 2020;17:100378. [Google Scholar]
- Ananya B, Rani SL, Brundha MP (2020) Knowledge and attitude of probiotics among outpatients visiting dental operatory. Drug Invent Today 14(2)
- Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harbor Perspect Biol. 2009;1(2):a002584. doi: 10.1101/cshperspect.a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvola T, Laiho K, Torkkeli S, Mykkänen H, Salminen S, Maunula L, Isolauri EJP. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: a randomized study. Pediatrics. 1999;104(5):e64–e64. doi: 10.1542/peds.104.5.e64. [DOI] [PubMed] [Google Scholar]
- Asahara T, Shimizu K, Takada T, Kado S, Yuki N, Morotomi M, Nomoto K. Protective effect of Lactobacillus casei strain Shirota against lethal infection with multi-drug resistant Salmonella enterica serovar Typhimurium DT104 in mice. J Appl MIcrobiol. 2011;110(1):163–173. doi: 10.1111/j.1365-2672.2010.04884.x. [DOI] [PubMed] [Google Scholar]
- Baffoni L, Gaggìa F, Di Gioia D, Santini C, Mogna L, Biavati B. A Bifidobacterium-based synbiotic product to reduce the transmission of C jejuni along the poultry food chain. Int J Food Microbiol. 2012;157(2):156–161. doi: 10.1016/j.ijfoodmicro.2012.04.024. [DOI] [PubMed] [Google Scholar]
- Bartram J, Brocklehurst C, Bradley D, Muller M, Evans B (2018) Policy review of the means of implementation targets and indicators for the sustainable development goal for water and sanitation. NPJ Clean Water 1(1):1–5
- Behnsen J, Deriu E, Sassone-Corsi M, Raffatellu M. Probiotics: properties, examples, and specific applications. Cold Spring Harbor Perspect Med. 2013;3(3):a010074. doi: 10.1101/cshperspect.a010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg P, Mertz JEJG. Personal reflections on the origins and emergence of recombinant. DNA Technol. 2010;184(1):9–17. doi: 10.1534/genetics.109.112144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernet-Camard MF, Liévin V, Brassart D, Neeser JR, Servin AL, Hudault S. The human Lactobacillus acidophilus strain LA1 secretes a nonbacteriocin antibacterial substance (s) active in vitro and in vivo. Appl Environ Microbiol. 1997;63(7):2747–2753. doi: 10.1128/aem.63.7.2747-2753.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besirbellioglu BA, Ulcay A, Can M, Erdem H, Tanyuksel M, Avci IY, Araz E, Pahsa A. Saccharomyces boulardii and infection due to Giardia lamblia. Scand J Infect Dis. 2006;38(6–7):479–481. doi: 10.1080/00365540600561769. [DOI] [PubMed] [Google Scholar]
- Biller JA, Katz AJ, Flores AF, Buie TM, Gorbach SL. Treatment of recurrent Clostridium difficile colitis with Lactobacillus GG. J Pediatr Gastroenterol Nutrit. 1995;21(2):224–226. doi: 10.1097/00005176-199508000-00016. [DOI] [PubMed] [Google Scholar]
- Bintsis TJ. Foodborne pathogens. AIMS Miccrobiol. 2017;3(3):529. doi: 10.3934/microbiol.2017.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswasroy P, Pradhan D, Sahu DK, Sahu A, Ghosh G, Rath G. Recent advances in clinical utility of probiotics in gastrointestinal tract disorders. CPB. 2020 doi: 10.2174/1389201021666201029154239. [DOI] [PubMed] [Google Scholar]
- Boirivant M, Strober WJC. The mechanism of action of probiotics. CPB. 2007;23(6):679–692. doi: 10.1097/MOG.0b013e3282f0cffc. [DOI] [PubMed] [Google Scholar]
- Bomba A, Nemcová R, Mudroňová D, Guba P. The possibilities of potentiating the efficacy of probiotics. Trends Food Sci Technol. 2002;13(4):121–126. [Google Scholar]
- Bortoluzzi C, Fernandes J, Doranalli K, Applegate A. Effects of dietary amino acids in ameliorating intestinal function during enteric challenges in broiler chickens. Anim Feed Sci Technol. 2020;262:114383. [Google Scholar]
- Buydens P, Debeuckelaere S. Efficacy of SF 68 in the treatment of acute diarrhea a placebo-controlled trial. Scand J Gastroenterol. 1996;31(9):887–891. doi: 10.3109/00365529609051997. [DOI] [PubMed] [Google Scholar]
- Cai R, Cheng C, Chen J, Xu X, Ding C, Gu BJG. Interactions of commensal and pathogenic microorganisms with the mucus layer in the colon. Gut Microb. 2020;11(4):680–690. doi: 10.1080/19490976.2020.1735606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canani RB, Cirillo P, Terrin G, Cesarano L, Spagnuolo MI, De Vincenzo A, Manguso FJB. Probiotics for treatment of acute diarrhoea in children: randomised clinical trial of five different preparations. BMJ. 2007;335(7615):340. doi: 10.1136/bmj.39272.581736.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano PG, Perdigón G. Probiotics induce resistance to enteropathogens in a re-nourished mouse model. J Dairy Res. 2003;70(4):433. doi: 10.1017/s0022029903006472. [DOI] [PubMed] [Google Scholar]
- Carlos Valdez J, Rachid M, Gobbato N, Perdigon G. Lactic acid bacteria induce apoptosis inhibition in Salmonella typhimurium infected macrophages. Food Agric Immunol. 2001;13(3):189–197. [Google Scholar]
- Cats A, Kuipers EJ, Bosschaert MA, Pot RG, Vandenbroucke-Grauls CM, Kusters JG. Effect of frequent consumption of a Lactobacillus casei-containing milk drink in Helicobacter pylori-colonized subjects. Aliment Pharmacol Therap. 2003;17(3):429–435. doi: 10.1046/j.1365-2036.2003.01452.x. [DOI] [PubMed] [Google Scholar]
- Ceapa C, Wopereis H, Rezaïki L, Kleerebezem M, Knol J, Oozeer R. Influence of fermented milk products, prebiotics and probiotics on microbiota composition and health. Best Pract Res Clin Gastroenterol. 2013;27(1):139–155. doi: 10.1016/j.bpg.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Chen K, Xin J, Zhang G, Xie H, Luo L, Yuan S, Bu Y, Yang X, Ge Y, Liu C. A combination of three probiotic strains for treatment of acute diarrhoea in hospitalised children: an open label, randomised controlled trial. Benef Microbes. 2020;11(4):339–346. doi: 10.3920/BM2020.0046. [DOI] [PubMed] [Google Scholar]
- Chen W, Peng X, Yu J, Chen X, Yuan M, Xiang R, He L, Yu D, Kang H, Pan Y, Xu Z (2020b) FengLiao affects gut microbiota and the expression levels of Na+/H+ exchangers, aquaporins and acute phase proteins in mice with castor oil-induced diarrhea. Plos one 15(7):e0236511 [DOI] [PMC free article] [PubMed]
- Chichlowski M, Croom J, McBride B, Havenstein G, Koci MJIJPS. Metabolic and physiological impact of probiotics or direct-fed-microbials on poultry: a brief review of current knowledge. Int L Poult Sci. 2007;6(10):694–704. [Google Scholar]
- Clements ML, Levine MM, Black RE, Robins-Browne RM, Cisneros LA, Drusano GL, Lanata CF, Saah AJ. Lactobacillus prophylaxis for diarrhea due to enterotoxigenic Escherichia coli. Antimicrob Agents Chemother. 1981;20(1):104–108. doi: 10.1128/aac.20.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombel J, Cortot A, Neut C, Romond C. (1987) Yoghurt with Bifidobacterium longum reduces erythromycin-induced gastrointestinal effects. Lancet [DOI] [PubMed]
- Corr SC, Gahan CG, Hill CJFI, Microbiology M. Impact of selected lactobacillus and Bifidobacterium species on Listeria monocytogenes infection and the mucosal immune response. FEMS Immunol Med Microbiol. 2007;50(3):380–388. doi: 10.1111/j.1574-695X.2007.00264.x. [DOI] [PubMed] [Google Scholar]
- Corr SC, Hill C, Gahan CG. Understanding the mechanisms by which probiotics inhibit gastrointestinal pathogens. Adv Food Nutr Res. 2009;1(56):1–5. doi: 10.1016/S1043-4526(08)00601-3. [DOI] [PubMed] [Google Scholar]
- Cruchet S, Obregon MC, Salazar G, Diaz E, Gotteland M. Effect of the ingestion of a dietary product containing Lactobacillus johnsonii La1 on Helicobacter pylori colonization in children. Nutrition. 2003;19(9):716–721. doi: 10.1016/s0899-9007(03)00109-6. [DOI] [PubMed] [Google Scholar]
- Culligan EP, Hill C, Sleator RD. Probiotics and gastrointestinal disease: successes, problems and future prospects. Gut Pathogens. 2009;1(1):1–2. doi: 10.1186/1757-4749-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De LeBlanc ADM, Castillo NA, Perdigon G. Anti-infective mechanisms induced by a probiotic Lactobacillus strain against Salmonella enterica serovar Typhimurium infection. Int J Food Microbiol. 2010;138(3):223–231. doi: 10.1016/j.ijfoodmicro.2010.01.020. [DOI] [PubMed] [Google Scholar]
- de Moreno A, Castillo NA, Perdigon G. Anti-infective mechanisms induced by a probiotic Lactobacillus strain against Salmonella enterica serovar Typhimurium infection. Int J Food Microbiol. 2010;138(3):223–231. doi: 10.1016/j.ijfoodmicro.2010.01.020&&. [DOI] [PubMed] [Google Scholar]
- Florez ID, Niño-Serna LF, Beltrán-Arroyave CP (2020) Acute infectious diarrhea and gastroenteritis in children. Curr Infect Dis Rep 22(2):1–12 [DOI] [PubMed]
- Dobson A, Cotter PD, Ross RP, Hill C. Bacteriocin production: a probiotic trait? Appl Environ Microbiol. 2012;78(1):1–6. doi: 10.1128/AEM.05576-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Teng G, Wei T, Gao W, Wang HJP. Methodological quality assessment of meta-analyses and systematic reviews of probiotics in inflammatory bowel disease and pouchitis. PLoS ONE. 2016;11(12):e0168785. doi: 10.1371/journal.pone.0168785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey AP, Rajeshwari K, Chakravarty A, Famularo G. Use of VSL# 3 in the treatment of rotavirus diarrhea in children: preliminary results. J Clin Gastroenterol. 2008;42:S126–S129. doi: 10.1097/MCG.0b013e31816fc2f6. [DOI] [PubMed] [Google Scholar]
- Dupont HL. Lactobacillus GG in prevention of traveler's diarrhea: an encouraging first step. Oxford: Oxford University Press; 1997. [DOI] [PubMed] [Google Scholar]
- DuPont HL, Ericsson CD. Prevention and treatment of traveler's diarrhea. New Engl J Med. 1993;328(25):1821–1827. doi: 10.1056/NEJM199306243282507. [DOI] [PubMed] [Google Scholar]
- Dwivedi P, Juneja V, Yan X. Novel natural food antimicrobials. Annu Rev Food Sci Technol. 2011 doi: 10.1146/annurev-food-022811-101241. [DOI] [PubMed] [Google Scholar]
- Editors PM. Antimicrobial resistance: is the world unprepared? PLoS Med. 2016;13(9):1002130–1002130. doi: 10.1371/journal.pmed.1002130&&. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elitsur Y, Yahav JJH. Helicobacter pylori infection in pediatrics. Helicobacter. 2005;10:47–53. doi: 10.1111/j.1523-5378.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- Ettinger G, MacDonald K, Reid G, Burton JP. The influence of the human microbiome and probiotics on cardiovascular health. Gut Microbes. 2014;5(6):719–728. doi: 10.4161/19490976.2014.983775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H, Elina T, Heikki A, Seppo SJFI, Microbiology M. Modulation of humoral immune response through probiotic intake. FEMS Immunol Med Microbiol. 2000;29(1):47–52. doi: 10.1111/j.1574-695X.2000.tb01504.x. [DOI] [PubMed] [Google Scholar]
- Fayol-Messaoudi D, Berger CN, Coconnier-Polter MH, Lievin-Le Moal V, Servin AL. pH-, Lactic acid-, and non-lactic acid-dependent activities of probiotic Lactobacilli against Salmonella enterica Serovar Typhimurium. Appl Environ Microbiol. 2005;71(10):6008–6013. doi: 10.1128/AEM.71.10.6008-6013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller RJG. Probiotics in human medicine. Gut. 1991;32(4):439. doi: 10.1136/gut.32.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardlik R, Palffy R, Celec P. Recombinant probiotic therapy in experimental colitis in mice. Folia Biol. 2012;58(6):238. [PubMed] [Google Scholar]
- Gibson DG, Glass JI, Lartigue C, Noskov VN, Chuang RY, Algire MA, Venter JC. Creation of a bacterial cell controlled by a chemically synthesized genome. Science. 2010;329(5987):52–56. doi: 10.1126/science.1190719. [DOI] [PubMed] [Google Scholar]
- Girard MP, Steele D, Chaignat C-L, Kieny MPJV. A review of vaccine research and development: human enteric infections. Vaccine. 2006;24(15):2732–2750. doi: 10.1016/j.vaccine.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Gorbach S, Chang TW, Goldin B (1987) Successful treatment of relapsing Clostridium difficile colitis with Lactobacillus GG. The Lancet 330(8574):1519 [DOI] [PubMed]
- Granato D, Branco GF, Cruz AG, Faria J, Shah NP. Probiotic Dairy products as functional foods. Compr Rev Food Sci Food Saf. 2010;9(5):455–470. doi: 10.1111/j.1541-4337.2010.00120.x. [DOI] [PubMed] [Google Scholar]
- Grandy G, Medina M, Soria R, Terán CG, Araya MJB. Probiotics in the treatment of acute rotavirus diarrhoea A randomized, double-blind, controlled trial using two different probiotic preparations in Bolivian children. BMC Infect Dis. 2010;10(1):253. doi: 10.1186/1471-2334-10-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guandalini S. Probiotics for prevention and treatment of diarrhea. J Clin Gastroenterol. 2011;1(45):S149–S153. doi: 10.1097/MCG.0b013e3182257e98. [DOI] [PubMed] [Google Scholar]
- Guandalini S, Pensabene L, Zikri MA, Dias JA, Casali LG, Hoekstra H, Kolacek S, Massar K, Micetic-Turk D, Papadopoulou A, de Sousa JS. Lactobacillus GG administered in oral rehydration solution to children with acute diarrhea: a multicenter European trial. J Pediatr Gastroenterol Nutr. 2000;30(1):54–60. doi: 10.1097/00005176-200001000-00018. [DOI] [PubMed] [Google Scholar]
- Guarino A, Canani RB, Spagnuolo MI, Albano F, Di Benedetto L. Oral bacterial therapy reduces the duration of symptoms and of viral excretion in children with mild diarrhea. J Pediatr Gastroenterol Nutr. 1997;25(5):516–519. doi: 10.1097/00005176-199711000-00005. [DOI] [PubMed] [Google Scholar]
- Guarino A, Ashkenazi S, Gendrel D, Vecchio AL, Shamir R, Szajewska H. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: update 2014. J Pediatr Gastroenterol Nutr. 2014;59(1):132–152. doi: 10.1097/MPG.0000000000000375. [DOI] [PubMed] [Google Scholar]
- Guarner F, Khan AG, Garisch J, Eliakim R, Gangl A, Thomson A, De Paula JA. World gastroenterology organisation global guidelines: probiotics and prebiotics october 2011. J Clin Gastroenterol. 2012;46(6):468–481. doi: 10.1097/MCG.0b013e3182549092. [DOI] [PubMed] [Google Scholar]
- Guidone A, Zotta T, Ross RP, Stanton C, Rea MC, Parente E, Ricciardi A (2014) Functional properties of Lactobacillus plantarum strains: a multivariate screening study. LWT-Food Sci Technol 56(1):69–76
- Hagens S, Loessner MJ. Bacteriophage for biocontrol of foodborne pathogens: calculations and considerations. Curr Pharm Biotechnol. 2010;11(1):58–68. doi: 10.2174/138920110790725429. [DOI] [PubMed] [Google Scholar]
- Higgins SE, Higgins JP, Wolfenden AD, Henderson SN, Torres-Rodriguez A, Tellez G, Hargis B. Evaluation of a Lactobacillus-based probiotic culture for the reduction of Salmonella enteritidis in neonatal broiler chicks. Poult Sci. 2008;87(1):27–31. doi: 10.3382/ps.2007-00210. [DOI] [PubMed] [Google Scholar]
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- Hilton E, Kolakowski P, Singer C, Smith M. Efficacy of lactobacillus GG as a diarrheal preventive in travelers. J Travel Med. 1997;4(1):41–43. doi: 10.1111/j.1708-8305.1997.tb00772.x. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Gordon JIJS. Commensal host-bacterial relationships in the gut. Science. 2001;292(5519):1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- Hutchison CA, Chuang RY, Noskov VN, Assad-Garcia N, Deerinck TJ, Ellisman MH, Gill J, Kannan K, Karas BJ, Ma L, Pelletier JF. Design and synthesis of a minimal bacterial genome. Science. 2016 doi: 10.1126/science.aad6253. [DOI] [PubMed] [Google Scholar]
- Hwang IY, Tan MH, Koh E, Ho CL, Poh CL, Chang MW. Reprogramming microbes to be pathogen-seeking killers. ACS Synth Biol. 2014;3(4):228–237. doi: 10.1021/sb400077j. [DOI] [PubMed] [Google Scholar]
- Kaila M, Isolauri E, Soppi E, Virtanen E, Laine S, Arvilommi H. Enhancement of the circulating antibody secreting cell response in human diarrhea by a human Lactobacillus strain. Pediatr Res. 1992;32(2):141–144. doi: 10.1203/00006450-199208000-00002. [DOI] [PubMed] [Google Scholar]
- Kaila M, Isolauri E, Saxelin M, Arvilommi H, Vesikari TJA. Viable versus inactivated lactobacillus strain GG in acute rotavirus diarrhoea. Arch Dis Childh. 1995;72(1):51–53. doi: 10.1136/adc.72.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kailasapathy K, Chin J. Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol Cell Biol. 2000;78(1):80–88. doi: 10.1046/j.1440-1711.2000.00886.x. [DOI] [PubMed] [Google Scholar]
- Karimi O, Peña AS. Indications and challenges of probiotics, prebiotics, and synbiotics in the management of arthralgias and spondyloarthropathies in inflammatory bowel disease. J Clin Gastroenterol. 2008;42:S136–S141. doi: 10.1097/MCG.0b013e3181662455. [DOI] [PubMed] [Google Scholar]
- Kechagia M, Basoulis D, Konstantopoulou S, Dimitriadi D, Gyftopoulou K, Skarmoutsou N, Fakiri EM (2013) Health benefits of probiotics: a review. Int Sch Res Not [DOI] [PMC free article] [PubMed]
- Keto T, Alemu Y, Mamo A. Mothers’ perception and management preference of acute diarrheal disease. Int J Public Health. 2020;9(4):338–346. [Google Scholar]
- Khaneghah AM, Abhari K, Eş I, Soares MB, Oliveira RB, Hosseini H. Interactions between probiotics and pathogenic microorganisms in hosts and foods: a review. Technology. 2020;95:205–218. [Google Scholar]
- Khorshidian N, Yousefi M, Shadnoush M, Siadat SD, Mohammadi M, Mortazavian AM. Using probiotics for mitigation of acrylamide in food products: a mini review. Curr Opin Food Sci. 2020;32:67–75. [Google Scholar]
- Kim TS, Hur JW, Yu MA, Cheigh CI, Kim KN, Hwang JK, Pyun YR. Antagonism of Helicobacter pylori by bacteriocins of lactic acid bacteria. J Food Prot. 2003;66(1):3–12. doi: 10.4315/0362-028x-66.1.3. [DOI] [PubMed] [Google Scholar]
- Kim YH, Kim SH, Whang KY, Kim YJ, Oh SJ. Inhibition of Escherichia coli O157: H7 attachment by interactions between lactic acid bacteria and intestinal epithelial cells. J Microbiol Biotechnol. 2008;18(7):1278–1285. [PubMed] [Google Scholar]
- Koo OK, Amalaradjou MA, Bhunia AK. Recombinant probiotic expressing Listeria adhesion protein attenuates Listeria monocytogenes virulence in vitro. PLoS ONE. 2012;7(1):e29277. doi: 10.1371/journal.pone.0029277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotula JW, Kerns SJ, Shaket LA, Siraj L, Collins JJ, Way JC, Silver PA. Programmable bacteria detect and record an environmental signal in the mammalian gut. Proc Natl Acad Sci USA. 2014;111(13):4838–4843. doi: 10.1073/pnas.1321321111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahtinen SJ. Probiotic viability: does it matter? Microb Ecol Health Dis. 2012;23(1):18567. doi: 10.3402/mehd.v23i0.18567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YH, Chung YA, Wu YC, Fang CT, Chen PJ. Disease burden from foodborne illnesses in Taiwan, 2012–2015. J Formos Med Assoc. 2020;119(9):1372–1381. doi: 10.1016/j.jfma.2020.03.013. [DOI] [PubMed] [Google Scholar]
- Lee YK, Puong KY, Ouwehand AC, Salminen S. Displacement of bacterial pathogens from mucus and Caco-2 cell surface by lactobacilli. J Med Microbiol. 2003;52(10):925–930. doi: 10.1099/jmm.0.05009-0. [DOI] [PubMed] [Google Scholar]
- Lema M, Williams L, Rao DR. Reduction of fecal shedding of enterohemorrhagic Escherichia coli O157: H7 in lambs by feeding microbial feed supplement. Small Rumin Res. 2001;39(1):31–39. doi: 10.1016/s0921-4488(00)00168-1. [DOI] [PubMed] [Google Scholar]
- Leung WK, Graham DY. Rescue therapy for Helicobacter pylori. Curr Treat Option Gastroenterol. 2002;5(2):133–138. doi: 10.1007/s11938-002-0060-8. [DOI] [PubMed] [Google Scholar]
- Lionetti E, Principi M, Scaccianoce G, Maurogiovanni G, La Rosa M, Ierardi E, Fontana C, Sardaro R, Cavallo L, Francavilla R. Probiotics and Helicobacter pylori. Eur Gastroenterol Hepatol Rev. 2011;7(2):121–128. [Google Scholar]
- Liu Z, Liu W, Ran C, Hu J, Zhou Z. Abrupt suspension of probiotics administration may increase host pathogen susceptibility by inducing gut dysbiosis. Sci Rep. 2016;6(1):1–2. doi: 10.1038/srep23214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Yu Z, Tian F, Zhao J, Zhang H, Zhai Q, Chen W. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microb Cell Fact. 2020;19(1):23. doi: 10.1186/s12934-020-1289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodemann U. Bioactive foods in promoting health. Amsterdam: Elsevier; 2010. Effects of probiotics on intestinal transport and epithelial barrier function; pp. 303–333. [Google Scholar]
- Luksiene Z, Arturas Z. Prospects of photosensitization in control of pathogenic and harmful micro-organisms. J Appl Microbiol. 2009;107:1415–1424. doi: 10.1111/j.1365-2672.2009.04341.x. [DOI] [PubMed] [Google Scholar]
- Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, De Simone C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121(3):580–591. doi: 10.1053/gast.2001.27224. [DOI] [PubMed] [Google Scholar]
- Malin M, Suomalainen H, Saxelin M, Isolauri E. Promotion of IgA immune response in patients with Crohn's disease by oral bacteriotherapy with Lactobacillus GG. Ann Nutr Metab. 1996;40(3):137–145. doi: 10.1159/000177907. [DOI] [PubMed] [Google Scholar]
- Martins FS, Silva AA, Vieira AT, Barbosa FH, Arantes RM, Teixeira MM, Nicoli JR. Comparative study of Bifidobacterium animalis, Escherichia coli, Lactobacillus casei and Saccharomyces boulardii probiotic properties. Arch Microbiol. 2009;191(8):623–630. doi: 10.1007/s00203-009-0491-x. [DOI] [PubMed] [Google Scholar]
- Mathipa MG, Thantsha MS. Probiotic engineering: towards development of robust probiotic strains with enhanced functional properties and for targeted control of enteric pathogens. Gut Pathog. 2017;9:28–28. doi: 10.1186/s13099-017-0178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison K. Advances in food and nutrition research. Amsterdam: Elsevier; 2011. Norovirus as a foodborne disease hazard; pp. 1–39. [DOI] [PubMed] [Google Scholar]
- Mazroue AA (2020) Assessment of the Therapeutic Effect of Probiotic Lactobacilli Alone and in Combination with Metronidazole in Murine Giardiasis. Al-Azhar Univ J Virus Res Stud 2(1):1–18
- McCarthy J, O'Mahony L, O'Callaghan L, Sheil B, Vaughan EE, Fitzsimons N, Shanahan F. Double blind, placebo controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokine balance. Gut. 2003;52(7):975–980. doi: 10.1136/gut.52.7.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool DJ, Forstner JF, Forstner GG. Synthesis and secretion of mucin by the human colonic tumour cell line LS180. Biochem J. 1994;302(1):111–118. doi: 10.1042/bj3020111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland LV, Surawicz CM, Greenberg RN, Elmer GW, Moyer KA, Melcher SA, Bowen KE, Cox JL. Prevention of b-lactam-associated diarrhea by Saccharomyces boulardii compared with placebo. Am J Gastroenterol. 1995;90(3):439–448. [PubMed] [Google Scholar]
- Mikkili I, Vekateswarulu TC, Peele A, Bobby M, Krupanidhi S. Bioactive molecules of probiotic bacteria and their mechanism of action: a review. Biotech. 2019 doi: 10.1007/s13205-019-1841-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimee M, Tucker AC, Voigt CA, Lu TK. Programming a human commensal bacterium, Bacteroides thetaiotaomicron, to sense and respond to stimuli in the murine gut microbiota. Cell Syst. 2015;1(1):62–71. doi: 10.1016/j.cels.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirnejad R, Vahdati AR, Rashidiani J, Erfani M, Piranfar V. The antimicrobial effect of lactobacillus casei culture supernatant against multiple drug resistant clinical isolates of Shigella sonnei and Shigella flexneri in vitro. Iran Red Crescent Med J. 2013;15(2):122. doi: 10.5812/ircmj.7454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitropoulou G, Nedovic V, Goyal A, Kourkoutas Y (2013) Immobilization technologies in probiotic food production. J Nutr Metab 2013(1):1–15 [DOI] [PMC free article] [PubMed]
- Mohania D, Nagpal R, Kumar M, Bhardwaj A, Yadav M, Jain S, Marotta F, Singh V, Parkash OM, Yadav H. Molecular approaches for identification and characterization of lactic acid bacteria. J Dig Dis. 2008;9(4):190–198. doi: 10.1111/j.1751-2980.2008.00345.x. [DOI] [PubMed] [Google Scholar]
- Nair V, Soraisham AS (2013) Probiotics and prebiotics: role in prevention of nosocomial sepsis in preterm infants. Int J Pediatr 2013(2):1–8 [DOI] [PMC free article] [PubMed]
- Nazir Y, Hussain SA, Abdul Hamid A, Song Y. Probiotics and their potential preventive and therapeutic role for cancer, high serum cholesterol, and allergic and HIV diseases. Biomed Res Int. 2018 doi: 10.1155/2018/3428437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeser J-R, Granato D, Rouvet M, Servin A, Teneberg S, Karlsson K-A. Lactobacillus johnsonii La1 shares carbohydrate-binding specificities with several enteropathogenic bacteria. Glycobiology. 2000;10(11):1193–1199. doi: 10.1093/glycob/10.11.1193. [DOI] [PubMed] [Google Scholar]
- Ng S, Hart A, Kamm M, Stagg A, Knight SCJI. Mechanisms of action of probiotics: recent advances. Inflam Bowel Dis. 2009;15(2):300–310. doi: 10.1002/ibd.20602. [DOI] [PubMed] [Google Scholar]
- Nwosu FC, Avershina E, Wilson R, Rudi K. Gut microbiota in HIV infection: implication for disease progression and management. Gastroenterol Res Pract. 2014;2014:803185. doi: 10.1155/2014/803185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Driscoll A, Sleator R. Synthetic DNA: the next generation of big data storage. Bioengineered. 2013 doi: 10.4161/bioe.24296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara AM, Shanahan FJT. Mechanisms of action of probiotics in intestinal diseases. Sci World J. 2007;7:31–46. doi: 10.1100/tsw.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi Y, Ushida KJASJ. Health-beneficial effects of probiotics: Its mode of action. Anim Sci J. 2009;80(4):361–371. doi: 10.1111/j.1740-0929.2009.00645.x. [DOI] [PubMed] [Google Scholar]
- Ohland CL, Macnaughton WK. Probiotic bacteria and intestinal epithelial barrier function. Am J Physiol-Gastroint Liver Physiol. 2010;298(6):807–819. doi: 10.1152/ajpgi.00243.2009. [DOI] [PubMed] [Google Scholar]
- Oksanen PJ, Salminen S, Saxelin M, Hämäläinen P, Ihantola-Vormisto A, Muurasniemi-Isoviita L, Salminen E. Prevention of travellers diarrhoea by lactobacillus GG. Ann Med. 1990;22(1):53–56. doi: 10.3109/07853899009147242. [DOI] [PubMed] [Google Scholar]
- Oscáriz JC, Lasa I, Pisabarro AG. Detection and characterization of cerein 7, a new bacteriocin produced by Bacillus cereus with a broad spectrum of activity. FEMS Microbiol Lett. 1999;178(2):337–341. doi: 10.1111/j.1574-6968.1999.tb08696.x. [DOI] [PubMed] [Google Scholar]
- Otte JM, Podolsky DK (2004) Functional modulation of enterocytes by gram-positive and gram-negative microorganisms. Am J Physiol Gastro Liver Physiol 286(4):G613–G626 [DOI] [PubMed]
- Ouwehand AC, Salminen SJJIDJ. The health effects of cultured milk products with viable and non-viable bacteria. Int Dairy J. 1998;8(9):749–758. [Google Scholar]
- Özdemir Ö. Retracted: preventative and therapeutic role of probiotics in various allergic and autoimmune disorders: an up-to-date literature review of essential experimental and clinical data. J Evid Based Complement Altern Med. 2013;18(2):121–151. doi: 10.1177/2156587212461279. [DOI] [PubMed] [Google Scholar]
- Pazhani GP, Niyogi SK, Singh AK, Sen B, Taneja N, Kundu M, Ramamurthy TJJ. Molecular characterization of multidrug-resistant Shigella species isolated from epidemic and endemic cases of shigellosis in India. J Med Microb. 2008;57(7):856–863. doi: 10.1099/jmm.0.2008/000521-0. [DOI] [PubMed] [Google Scholar]
- Pearce J, Hamilton JR. Controlled trial of orally administered lactobacilli in acute infantile diarrhea. J Pediat. 1974;84(2):261–262. doi: 10.1016/s0022-3476(74)80618-9. [DOI] [PubMed] [Google Scholar]
- Pedone C, Bernabeu A, Postaire E, Bouley C, Reinert PJI. The effect of supplementation with milk fermented by Lactobacillus casei (strain DN-114 001) on acute diarrhoea in children attending day care centres. Int J Clin Pract. 1999;53(3):179. [PubMed] [Google Scholar]
- Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A. Mechanisms of action of probiotics. Adv Nutr. 2019;10(1):S49–S66. doi: 10.1093/advances/nmy063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott SL, Björkstén B. Probiotics for the prevention or treatment of allergic diseases. J Allergy Clin Immunol. 2007;120(2):255–262. doi: 10.1016/j.jaci.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Qin H-L, Shen T-Y, Gao Z-G, Fan X-B, Hang X-M, Jiang Y-Q, Zhang H-ZJ. Effect of lactobacillus on the gut microflora and barrier function of the rats with abdominal infection. WJG. 2005;11(17):2591. doi: 10.3748/wjg.v11.i17.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley EMJJ. Bacterial flora in irritable bowel syndrome: role in pathophysiology, implications for management. J Dig Dis. 2007;8(1):2–7. doi: 10.1111/j.1443-9573.2007.00277.x. [DOI] [PubMed] [Google Scholar]
- Rautanen T, Isolauri E, Salo E, Vesikari TJ. Management of acute diarrhoea with low osmolarity oral rehydration solutions and lactobacillusstrain GG. Arch Dis Child. 1998;79(2):157–160. doi: 10.1136/adc.79.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid GJ. Probiotics: definition, scope and mechanisms of action. Best Pract Res Clin Gastroenterol. 2016;30(1):17–25. doi: 10.1016/j.bpg.2015.12.001. [DOI] [PubMed] [Google Scholar]
- Reid G, Gadir AA, Dhir RJF. Probiotics: reiterating what they are and what they are not. Front Microbiol. 2019;10:424. doi: 10.3389/fmicb.2019.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissbrodt R, Hammes WP, Dal Bello F, Prager R, Fruth A, Hantke K, Williams PHJF. Inhibition of growth of Shiga toxin-producing Escherichia coli by nonpathogenic Escherichia coli. FEMS Microbiol Lett. 2009;290(1):62–69. doi: 10.1111/j.1574-6968.2008.01405.x. [DOI] [PubMed] [Google Scholar]
- Resta-Lenert S, Barrett KJG. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC) Gut. 2003;52(7):988–997. doi: 10.1136/gut.52.7.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter JF, Gitter AH, Günzel D, Weiss S, Mohamed W, Chakraborty T. Listeriolysin O affects barrier function and induces chloride secretion in HT-29/B6 colon epithelial cells. Am J Physiol. 2009;296(6):1350–1359. doi: 10.1152/ajpgi.00040.2009. [DOI] [PubMed] [Google Scholar]
- Ritchie ML, Romanuk TN. A meta-analysis of probiotic efficacy for gastrointestinal diseases. PLoS ONE. 2012;7(4):e34938. doi: 10.1371/journal.pone.0034938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson LJ, Hanevik K, Escobedo AA, Mørch K, Langeland NJT. Giardiasis–why do the symptoms sometimes never stop? Trends Parasitol. 2010;26(2):75–82. doi: 10.1016/j.pt.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Ruggiero PJW. Use of probiotics in the fight against Helicobacter pylori. World J Gastroint Pathophysiol. 2014;5(4):384. doi: 10.4291/wjgp.v5.i4.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Palacios GM (2007) The health burden of Campylobacter infection and the impact of antimicrobial resistance: playing chicken. Clin Infect Dis 44(5):701–703. 10.1086/509936 [DOI] [PubMed]
- Saavedra JJTA. Probiotics and infectious diarrhea. Am J Gastroenterol. 2000;95(1):S16–S18. doi: 10.1016/s0002-9270(99)00811-4. [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Tschernia A (2002) Human studies with probiotics and prebiotics: clinical implications. British J Nutrit 87(S2):S241–S246 [DOI] [PubMed]
- Salyers AA. Bacteroides of the human lower intestinal tract. Annu Rev Microbiol. 1984;38:293–313. doi: 10.1146/annurev.mi.38.100184.001453. [DOI] [PubMed] [Google Scholar]
- Sanders ME. Probiotics: definition, sources, selection, and uses. Clin Infect Dis. 2008;46(2):S58–S61. doi: 10.1086/523341. [DOI] [PubMed] [Google Scholar]
- Sarkar SL, Hossain MI, Monika SA, Sanyal SK, Roy PC, Hossain MA. Probiotic potential of Pediococcus acidilactici and Enterococcus faecium isolated from indigenous yogurt and raw goat milk. Microbiol Biotechnol Lett. 2020;48(3):276–286. [Google Scholar]
- Satish Kumar R, Kanmani P, Yuvaraj N, Paari K, Pattukumar V, Arul V. Lactobacillus plantarum AS1 binds to cultured human intestinal cell line HT-29 and inhibits cell attachment by enterovirulent bacterium Vibrio parahaemolyticus. Lett Appl Microbiol. 2011;53(4):481–487. doi: 10.1111/j.1472-765X.2011.03136.x. [DOI] [PubMed] [Google Scholar]
- Schlee M, Harder J, Köten B, Stange E, Wehkamp J, Fellermann K. Probiotic lactobacilli and VSL# 3 induce enterocyte β-defensin 2. J Immunology. 2008;151(3):528–535. doi: 10.1111/j.1365-2249.2007.03587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S, Mansell TJ. Yeasts as probiotics: mechanisms, outcomes, and future potential. Fungal Genet Biol. 2020;137:103333. doi: 10.1016/j.fgb.2020.103333. [DOI] [PubMed] [Google Scholar]
- Servin ALJ. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev. 2004;28(4):405–440. doi: 10.1016/j.femsre.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Sheehan VM, Sleator RD, Fitzgerald GF, Hill C. Heterologous expression of BetL, a betaine uptake system, enhances the stress tolerance of Lactobacillus salivarius UCC118. Appl Environ Microbiol. 2006;72(3):2170–2177. doi: 10.1128/aem.72.3.2170-2177.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi LH, Balakrishnan K, Thiagarajah K, Mohd Ismail NI, Yin OS. Beneficial properties of probiotics. Trop Life Sci Res. 2016;27(2):73–90. doi: 10.21315/tlsr2016.27.2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shornikova A-V, Casas IA, Isolauri E, Mykkänen H, Vesikari TJ. Lactobacillus reuteri as a therapeutic agent in acute diarrhea in young children. J Pediatr Gastroenterol Nutr. 1997;24(4):399–404. doi: 10.1097/00005176-199704000-00008. [DOI] [PubMed] [Google Scholar]
- Shornikova AV, Isolauri E, Burkanova L, Lukovnikova S, Vesikari TJAP. A trial in the Karelian Republic of oral rehydration and Lactobacillus GG for treatment of acute diarrhoea. Acta Paediatri. 1997;86(5):460–465. doi: 10.1111/j.1651-2227.1997.tb08913.x. [DOI] [PubMed] [Google Scholar]
- Siddiqui FJ, Belayneh G, Bhutta ZA. Nutrition and infectious diseases. Cham: Humana; 2021. Nutrition and diarrheal disease and enteric pathogens; pp. 219–241. [Google Scholar]
- Siitonen S, Vapaatalo H, Salminen S, Gordin A, Saxelin M, Wikberg R, Kirkkola A-L. Effect of Lactobacillus GG yoghurt in prevention of antibiotic associated diarrhoea. Ann Med. 1990;22(1):57–59. doi: 10.3109/07853899009147243. [DOI] [PubMed] [Google Scholar]
- Simakachorn N, Pichaipat V, Rithipornpaisarn P, Kongkaew C, Tongpradit P, Varavithya W. Clinical evaluation of the addition of lyophilized, heat-killed Lactobacillus acidophilus LB to oral rehydration therapy in the treatment of acute diarrhea in children. J Pediatr Gastroenterol Nutr. 2000;30(1):68–72. doi: 10.1097/00005176-200001000-00020. [DOI] [PubMed] [Google Scholar]
- Sirsat S, Muthaiyan A, Ricke S. Antimicrobials for pathogen reduction in organic and natural poultry production. J Appl Poultry Res. 2009;18:379–388. doi: 10.3382/japr.2008-00140. [DOI] [Google Scholar]
- Sivapalasingam S, Nelson JM, Joyce K, Hoekstra M, Angulo FJ, Mintz ED. High prevalence of antimicrobial resistance among Shigella isolates in the United States tested by the National Antimicrobial Resistance Monitoring System from 1999 to 2002. Antimicrob Agents Chemother. 2006;50(1):49–54. doi: 10.1128/AAC.50.1.49-54.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleator RD. Probiotics: a viable therapeutic alternative for enteric infections especially in the developing world. Discov Med. 2010;10(51):119–124. [PubMed] [Google Scholar]
- Sleator RD. Synthetic ribosomes: making molecules that make molecules. Bioengineered. 2013;4(2):63–64. doi: 10.4161/bioe.23640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleator R, Hill CJSP. Patho-biotechnology; using bad bugs to make good bugs better. Sci Prog. 2007;90(1):1–14. doi: 10.3184/003685007780440530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smanski MJ, Zhou H, Claesen J, Shen B, Fischbach MA, Voigt CA. Synthetic biology to access and expand nature's chemical diversity. Nat Rev Microbiol. 2016;14(3):135–149. doi: 10.1038/nrmicro.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snydman DR. The safety of probiotics. Clin Infect Dis. 2008;46(2):S104–S111. doi: 10.1086/523331. [DOI] [PubMed] [Google Scholar]
- Sola-Oladokun B, Culligan EP, Sleator RD. Engineered probiotics: applications and biological containment. Annu Rev Food Sci Technol. 2017;8(1):353–370. doi: 10.1146/annurev-food-030216-030256. [DOI] [PubMed] [Google Scholar]
- Sujlana A, Goyal R, Pannu P, Opal S, Bansal P. Visual pedagogy and probiotics for hearing impaired children: a pilot study. J Indian Soc Pedod Prev Dent. 2017;35(4):353. doi: 10.4103/JISPPD.JISPPD_146_17. [DOI] [PubMed] [Google Scholar]
- Tan P, Eor J, Chun T, Kim S. Beneficial microorganisms in medical and health applications. Berlin: Springer; 2015. Immune modulation by probiotics; pp. 101–130. [Google Scholar]
- Tankanow RM, Ross MB, Ertel IJ, Dickinson DG, McCormick LS, Garfinkel JFJD. A double-blind, placebo-controlled study of the efficacy of Lactinex in the prophylaxis of amoxicillin-induced diarrhea. DICP. 1990;24(4):382–384. doi: 10.1177/106002809002400408. [DOI] [PubMed] [Google Scholar]
- Thirabunyanon M. Biotherapy for and protection against gastrointestinal pathogenic infections via action of probiotic bacteria. Maejo Int J Sci Technol. 2011;5(1):108. [Google Scholar]
- Touré R, Kheadr E, Lacroix C, Moroni O, Fliss I. Production of antibacterial substances by bifidobacterial isolates from infant stool active against Listeria monocytogenes. J Appl Microbiol. 2003;95(5):1058–1069. doi: 10.1046/j.1365-2672.2003.02085.x. [DOI] [PubMed] [Google Scholar]
- Travers M-A, Florent I, Kohl L, Grellier P. Probiotics for the control of parasites: an overview. J Parasitol Res. 2011 doi: 10.1155/2011/610769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai Y-T, Cheng P-C, Pan T-M. Immunomodulating activity of Lactobacillus paracasei subsp paracasei NTU 101 in enterohemorrhagic Escherichia coli O157H7-infected mice. J Agric Food Chem. 2010;58(21):11265–11272. doi: 10.1021/jf103011z. [DOI] [PubMed] [Google Scholar]
- Tursi A, Brandimarte G, Giorgetti GM, Modeo M. Effect of Lactobacillus casei supplementation on the effectiveness and tolerability of a new second-line 10-day quadruple therapy after failure of a first attempt to cure Helicobacter pylori infection. Med Sci Monit. 2004;10(12):662–666. [PubMed] [Google Scholar]
- Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Schlemper R. Helicobacter pylori infection and the development of gastric cancer. New Engl J Med. 2001;345(11):784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- Van Puyenbroeck K, Hens N, Coenen S, Michiels B, Beunckens C, Molenberghs G, Verhoeven V. Efficacy of daily intake of Lactobacillus casei Shirota on respiratory symptoms and influenza vaccination immune response: a randomized, double-blind, placebo-controlled trial in healthy elderly nursing home residents. Am J Clin Nutr. 2012;95(5):1165–1171. doi: 10.3945/ajcn.111.026831. [DOI] [PubMed] [Google Scholar]
- Vandenplas Y, Salvatore S, Viera M, Devreker T, Hauser BJE. Probiotics in infectious diarrhoea in children: are they indicated? Eur J Pediatr. 2007;166(12):1211–1218. doi: 10.1007/s00431-007-0497-9. [DOI] [PubMed] [Google Scholar]
- Varma P, Dinesh KR, Menon KK, Biswas R. Lactobacillus fermentum isolated from human colonic mucosal biopsy inhibits the growth and adhesion of enteric and foodborne pathogens. J Food Sci. 2010;75(9):M546–M551. doi: 10.1111/j.1750-3841.2010.01818.x. [DOI] [PubMed] [Google Scholar]
- Vázquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Domínguez-Bernal G, Goebel W, Kreft J. Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev. 2001;14(3):584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaşu-Bolocan L, Popescu F, Bica CJCHSJ. Probiotics and their immunomodulatory potential. Curr Health Sci. 2013;39:204–209. [Google Scholar]
- Vieco-Saiz N, Belguesmia Y, Raspoet R, Auclair E, Gancel F, Kempf I, Drider D. Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Front Microbiol. 2019;10:57–57. doi: 10.3389/fmicb.2019.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlkova E, Medkova J, Rada V. Comparison of four methods for identification of bifidobacteria to the genus level. Czech J Food Sci. 2002;20(5):171–174. [Google Scholar]
- Volzing K, Borrero J, Sadowsky MJ, Kaznessis YN. Antimicrobial peptides targeting Gram-negative pathogens, produced and delivered by lactic acid bacteria. ACS Synth Biol. 2013;2(11):643–650. doi: 10.1021/sb4000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner RD, Johnson SJ, Kurniasih Rubi D. Probiotic bacteria are antagonistic to Salmonella enterica and Campylobacter jejuni and influence host lymphocyte responses in human microbiota-associated immunodeficient and immunocompetent mice. Mol Nutr Food Res. 2009;53(3):377–388. doi: 10.1002/mnfr.200800101. [DOI] [PubMed] [Google Scholar]
- Wan LY, Chen ZJ, Shah NP, El-Nezami H. Modulation of Intestinal Epithelial Defense Responses by Probiotic Bacteria. Critic Rev Food Sci Nutr. 2016;56(16):2628–2641. doi: 10.1080/10408398.2014.905450. [DOI] [PubMed] [Google Scholar]
- Watson D, Sleator RD, Hill C, Gahan CG. Enhancing bile tolerance improves survival and persistence of Bifidobacterium and Lactococcus in the murine gastrointestinal tract. BMC Microbiol. 2008;8:176. doi: 10.1186/1471-2180-8-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watve MG, Tickoo R, Jog MM, Bhole BD. How many antibiotics are produced by the genus Streptomyces? Arch Microbiol. 2001;176(5):386–390. doi: 10.1007/s002030100345. [DOI] [PubMed] [Google Scholar]
- Wen K, Azevedo MS, Gonzalez A, Zhang W, Saif LJ, Li G. Toll-like receptor and innate cytokine responses induced by lactobacilli colonization and human rotavirus infection in gnotobiotic pigs. Immunopathology. 2009;127(3–4):304–315. doi: 10.1016/j.vetimm.2008.10.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L-J, Hou X-L, Wang G-H, Yu L-Y, Wei X-M, Liu J-K, Wei C-HJV. Immunization with recombinant Lactobacillus casei strains producing K99, K88 fimbrial protein protects mice against enterotoxigenic Escherichia coli. Vaccine. 2012;30(22):3339–3349. doi: 10.1016/j.vaccine.2011.08.036. [DOI] [PubMed] [Google Scholar]
- Wohlgemuth S, Loh G, Blaut M. Recent developments and perspectives in the investigation of probiotic effects. Int J Med Microbiol. 2009;300:3–10. doi: 10.1016/j.ijmm.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Wohlgemuth S, Loh G, Blaut MJIJ. Recent developments and perspectives in the investigation of probiotic effects. Int J Med Microbiol. 2010;300(1):3–10. doi: 10.1016/j.ijmm.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Wong S, Jamous A, O'Driscoll J, Sekhar R, Saif M, O'Driscoll S, Hirani SP. Effectiveness of probiotic in preventing and treating antibiotic-associated diarrhoea and/or Clostridium difficile-associated diarrhoea in patients with spinal cord injury: a protocol of systematic review of randomised controlled trials. Syst Rev. 2015;4:170. doi: 10.1186/s13643-015-0159-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R-Y, Wan Y-P, Fang Q-Y, Lu W, Cai W. Supplementation with probiotics modifies gut flora and attenuates liver fat accumulation in rat nonalcoholic fatty liver disease model. J Clin Biochem Nutr. 2012;50(1):72–77. doi: 10.3164/jcbn.11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang L, Du M, Yi H, Guo C, Tuo Y, Yang L. Antimicrobial activity against Shigella sonnei and probiotic properties of wild lactobacilli from fermented food. Microbiol Res. 2011;167(1):27–31. doi: 10.1016/j.micres.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Zhang D, Li R, Li J. Lactobacillus reuteri ATCC 55730 and L22 display probiotic potential in vitro and protect against Salmonella-induced pullorum disease in a chick model of infection. Res Vet Sci. 2012;93(1):366–373. doi: 10.1016/j.rvsc.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Zhang Y-C, Zhang L-W, Ma W, Yi H-X, Yang X, Du M, Zhang L-L. Screening of probiotic lactobacilli for inhibition of Shigella sonnei and the macromolecules involved in inhibition. Anaerobe. 2012;18(5):498–503. doi: 10.1016/j.anaerobe.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Lv J, Pan L, Zhang Y. Roles and applications of probiotic Lactobacillus strains. Appl Microbiol Biotechnol. 2018;102(19):8135–8143. doi: 10.1007/s00253-018-9217-9. [DOI] [PubMed] [Google Scholar]
- Zhang J, Cameron D, Quak S, Kadim M, Mohan N, Ryoo E, Hoekstra HJB. Rates and determinants of antibiotics and probiotics prescription to children in Asia-Pacific countries. Benef Microbes. 2020;11(4):329–338. doi: 10.3920/BM2019.0203. [DOI] [PubMed] [Google Scholar]
- Zhao W, Ho H-E, Bunyavanich S. The gut microbiome in food allergy. Ann Allergy Asthma Immunol. 2019;122(3):276–282. doi: 10.1016/j.anai.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Jung LS, Lee SH, Kim S, Cho Y, Ahn JJI. Enhanced antimicrobial activity of nisin in combination with allyl isothiocyanate against Listeria monocytogenes Staphylococcus aureus, Salmonella typhimurium and Shigella boydii. J Food Sci Technol. 2013;48(2):324–333. [Google Scholar]
- Zuo F, Chen S, Marcotte H. Engineer probiotic bifidobacteria for food and biomedical applications: current status and future prospective. Biotechnol Adv. 2020;45:107654. doi: 10.1016/j.biotechadv.2020.107654. [DOI] [PubMed] [Google Scholar]