Abstract
Background
Reinfection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been documented, raising public health concerns. SARS-CoV-2 reinfections were assessed in a cohort of antibody-positive persons in Qatar.
Methods
All SARS-CoV-2 antibody-positive persons from April 16 to December 31, 2020 with a PCR-positive swab ≥14 days after the first-positive antibody test were investigated for evidence of reinfection. Viral genome sequencing was conducted for paired viral specimens to confirm reinfection. Incidence of reinfection was compared to incidence of infection in the complement cohort of those who were antibody-negative.
Findings
Among 43,044 antibody-positive persons who were followed for a median of 16.3 weeks (range: 0–34.6), 314 individuals (0.7%) had at least one PCR positive swab ≥14 days after the first-positive antibody test. Of these individuals, 129 (41.1%) had supporting epidemiological evidence for reinfection. Reinfection was next investigated using viral genome sequencing. Applying the viral-genome-sequencing confirmation rate, the incidence rate of reinfection was estimated at 0.66 per 10,000 person-weeks (95% CI: 0.56–0.78). Incidence rate of reinfection versus month of follow-up did not show any evidence of waning of immunity for over seven months of follow-up. Meanwhile, in the complement cohort of 149,923 antibody-negative persons followed for a median of 17.0 weeks (range: 0–45.6), incidence rate of infection was estimated at 13.69 per 10,000 person-weeks (95% CI: 13.22–14.14). Efficacy of natural infection against reinfection was estimated at 95.2% (95% CI: 94.1–96.0%). Reinfections were less severe than primary infections. Only one reinfection was severe, two were moderate, and none were critical or fatal. Most reinfections (66.7%) were diagnosed incidentally through random or routine testing, or through contact tracing.
Interpretation
Reinfection is rare in the young and international population of Qatar. Natural infection appears to elicit strong protection against reinfection with an efficacy ~95% for at least seven months.
Funding
Biomedical Research Program, the Biostatistics, Epidemiology, and Biomathematics Research Core, and the Genomics Core, all at Weill Cornell Medicine-Qatar, the Ministry of Public Health, Hamad Medical Corporation, and the Qatar Genome Programme.
Keywords: SARS-CoV-2, Epidemiology, Reinfection, Immunity, Genetics
Research in context.
Evidence before this study
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has spread worldwide, causing disease and mortality, as well as social disruption and economic loss. In addition to the risk of first infection, reinfection during this prolonged pandemic has raised additional public health concerns. PubMed and medRxiv preprint searches were updated on March 24, 2021 using the search criteria ("SARS-CoV-2″ OR "COVID-19″) AND “reinfection.” These searches identified eight cohort studies providing estimates for the efficacy of natural infection against reinfection. These studies were conducted in Austria, Denmark, Qatar, Switzerland, the UK, and the USA, and reported an efficacy against reinfection ranging between 80 and 95%.
Added value of this study
The study assessed cumulative risk of SARS-CoV-2 reinfection and incidence rate of reinfection in a nationwide cohort of 43,044 antibody-positive individuals who were followed for up to 35 weeks. The total cohort follow-up time exceeded 600,000 person-weeks, which is comparable to or greater than the follow-up time of COVID-19 vaccine trials. The study demonstrated and confirmed through viral genome sequencing that SARS-CoV-2 reinfection does occur, but only rarely, with the cumulative risk of reinfection being ~2 per 1000 persons after 35 weeks of follow-up, and the incidence rate of reinfection estimated at <1 per 10,000 person-weeks. Meanwhile, in the complement cohort of 149,923 antibody-negative persons, the cumulative risk of infection was much higher, estimated at ~31 per 1000 persons after 46 weeks of follow-up, and the incidence rate of infection was estimated at ~14 per 10,000 person-weeks. Efficacy of natural infection against reinfection was estimated at 95%. The study showed that there was no evidence for waning of protective immunity against reinfection in this cohort for over 7 months.
Implications of all the available evidence
There is concrete evidence that reinfection can occur in individuals with detectable antibodies for SARS-CoV-2 infection, even in some with high antibody titers. However, the occurrence of reinfection is rare for over 7 months of follow-up after the first antibody-positive test and with no sign of waning of protective immunity. These findings suggest that induced SARS-CoV-2 immunity, whether induced through natural infection or vaccination, is very efficacious against infection (>90%) and may persist for at least 7 months. The findings also suggest that prioritizing vaccination for those who are antibody-negative, as long as doses of the vaccine remain in short supply, could enhance the health, societal, and economic gains achieved by vaccination.
Alt-text: Unlabelled box
1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has caused extensive disease and death, with social and economic losses [1], [2], [3], [4]. In addition to the risk of first infection, reinfection during this prolonged pandemic has raised additional public health concerns [5], [6], [7], [8], [9].
We previously assessed the cumulative risk and incidence rate of documented reinfection in a cohort of 130,266 SARS-CoV-2 polymerase chain reaction (PCR)-confirmed infected persons in Qatar [5], a country of 2.8 million people [10,11] that experienced a large SARS-CoV-2 epidemic [12], [13], [14], [15], [16]. Benefiting from a centralized data-capture system for nationwide SARS-CoV-2 PCR testing and using viral genome sequencing, we quantified the cumulative risk of reinfection at ~2 reinfections per 10,000 infected persons [5]. Incidence rate of reinfection was estimated at 0.36 (95% CI: 0.28–0.47) per 10,000 person-weeks [5].
Serological testing for SARS-CoV-2 infection has been expanding in Qatar [14,16,17]. The first objective of the present study was to quantify the cumulative risk and incidence rate of documented reinfection in a cohort of 43,044 persons who had a laboratory-confirmed, anti-SARS-CoV-2 positive result, regardless of whether these persons had ever had a diagnosed PCR-confirmed infection. Persons with a PCR-confirmed infection could, in principle, be biologically different from persons with an antibody-confirmed infection, as the former population is more likely to have experienced a symptomatic or even serious primary infection, while the latter population is more likely to have experienced an asymptomatic or mild primary infection that may never have been diagnosed. Moreover, some of those with PCR-confirmed infection may not have developed detectable antibodies [5,7]. In an earlier study in Qatar, we found that 9% of those who were PCR positive >3 weeks before the serology test were antibody negative [12]. The second objective was to estimate the efficacy of natural infection against reinfection by comparing the incidence rate of reinfection to the incidence rate of infection in the complement cohort of 149,923 persons who had a laboratory-confirmed, anti-SARS-CoV-2 negative result.
The present study thus provides an independent assessment of the risk of reinfection in a biologically different population from that of PCR-confirmed infected persons. A major strength of the present study is the long follow-up time of each antibody-positive person in this cohort, which had a median of 16.3 weeks for a total cohort follow-up time of 610,832.6 person-weeks, comparable to or greater than the follow-up time in COVID-19 vaccine trials [18], [19], [20]. An added strength is the comparison to the incidence rate of infection in a large cohort of antibody-negative persons with a similar follow-up time. The study therefore allows assessment of reinfection for more than seven months after primary infection, and provides empirical evidence for possible effects of any waning of immunity.
2. Methods
2.1. Sources of data
We analyzed the centralized, integrated, and standardized national anti-SARS-CoV-2 serological testing database compiled at Hamad Medical Corporation (HMC), the main public healthcare provider and the nationally designated provider for Coronavirus Disease 2019 (COVID-19) healthcare needs. The database covers essentially all serological testing for SARS-CoV-2 conducted in Qatar, including both testing done on residual blood specimens collected for routine clinical care from attendees at HMC [17] and during a series of population-based serological surveys [14,16]. Most serological testing was done on the residual clinical care specimens and tested individuals were not aware of the testing result, nor was the serological result used for case management. The tested population is broadly representative of the urban population of Qatar [17], but less so of the craft and manual workers population who typically receive their primary healthcare at Qatar Red Crescent Society centers [14]. Qatar launched its vaccination campaign on December 21, 2020 [21], around the time this study was concluded (December 31, 2020), so very few individuals had been vaccinated at time of this study.
The antibody database was linked to the HMC national SARS-CoV-2 PCR testing and COVID-19 hospitalization and severity database [22]. The latter includes records for all SARS-CoV-2 PCR testing conducted in Qatar since the start of the epidemic. The database also includes all COVID-19 hospitalizations and their infection severity classifications, assessed through individual chart reviews by trained medical personnel following World Health Organization (WHO) guidelines [23]. Antibody data were also linked to the centralized COVID-19 death registry, which includes all COVID-19 deaths assessed per WHO guidelines [24]. The STROBE statement checklist can be found in Supplementary Table S1.
2.2. Laboratory methods
Antibodies against SARS-CoV-2 in serological samples were detected using the Roche Elecsys® Anti-SARS-CoV-2 assay (Roche, Switzerland), an electrochemiluminescence immunoassay that uses a recombinant protein representing the nucleocapsid (N) antigen for antibody binding. Results were interpreted according to the manufacturer's instructions (reactive: optical density (proxy for antibody titer [25]) cutoff index ≥1.0 vs. non-reactive: optical density cutoff index <1.0).
Nasopharyngeal and/or oropharyngeal swabs (Huachenyang Technology, China) were collected for PCR testing and placed in Universal Transport Medium (UTM). Aliquots of UTM were: extracted on the QIAsymphony platform (QIAGEN, USA) and tested with real-time reverse-transcription PCR (RT-qPCR) using TaqPath™ COVID-19 Combo Kits (Thermo Fisher Scientific, USA) on an ABI 7500 FAST (Thermo Fisher, USA). Samples were extracted using a custom protocol [26] on a Hamilton Microlab STAR (Hamilton, USA) and tested using AccuPower SARS-CoV-2 Real-Time RT-PCR Kits (Bioneer, Korea) on an ABI 7500 FAST, or loaded directly into a Roche cobas® 6800 system and assayed with a cobas® SARS-CoV-2 Test (Roche, Switzerland). The first assay targets the viral S, N, and ORF1ab regions. The second targets the virus’ RdRp and E-gene regions, and the third targets the ORF1ab and E-gene regions. All testing was conducted at HMC Central Laboratory or at Sidra Medicine Laboratory, following standardized protocols.
2.3. Suspected reinfection case eligibility and classification
Reinfection was defined as a PCR positive result in an individual who had a prior infection that had cleared. All SARS-CoV-2 antibody-positive persons in Qatar with at least one PCR-positive swab that occurred ≥14 days after the first-positive antibody test were considered suspected cases of reinfection. These were classified as showing either good evidence, some evidence, or weak (or no) evidence for reinfection based on criteria applied to each case (Table 1). We defined the reinfection swab as the first-positive PCR swab that was identified ≥14 days after the first-positive antibody test. The 14-day cutoff was incorporated to exclude cases in which antibody testing and PCR testing were done around the same time, as part of clinical care of COVID-19 patients. A PCR-positive swab within a few days of an antibody-positive test is likely to reflect active primary infection under clinical consideration rather than a reinfection.
Table 1.
Classification of suspected cases of SARS-CoV-2 reinfection based on the strength of supporting epidemiological evidence.
| Cases of SARS-CoV-2 reinfection | Definition |
|---|---|
| Suspected cases of SARS-CoV-2 reinfection | All antibody-positive persons with at least one PCR-positive swab that occurred ≥14 days after the first-positive antibody test |
| Good evidence for reinfection | Individuals who had a PCR-positive swab with a Ct value ≤30 at least 14 days after the first-positive antibody test and who had not had a PCR-positive swab within the 45 days preceding the reinfection swab |
| Some evidence for reinfection | Individuals who had a PCR-positive swab with a Ct value >30 at least 14 days after the first-positive antibody test and who had not had a PCR-positive swab within the 45 days preceding the reinfection swab |
| Weak evidence for reinfection | Individuals who had a PCR-positive swab at least 14 days after the first-positive antibody test, but who had one or more PCR-positive swabs within the 45 days preceding the reinfection swab |
Ct, cycle threshold; PCR, polymerase chain reaction.
Suspected reinfection cases with a PCR cycle threshold (Ct) value ≤30 for the reinfection swab (suggestive of a recent active infection) [27], [28], [29] and who had not had a PCR-positive swab for 45 days preceding the reinfection swab (to rule out persistent PCR positivity due to non-viable virus fragments) [5,27,[30], [31], [32]], were considered as showing good evidence for reinfection. The decision to use a 45-day duration, instead of a longer duration, was a conservative choice. Had we set this duration for 60 or 90 days, for example, we would have missed some identified reinfections. Suspected reinfection cases who had not had a PCR-positive swab for 45 days preceding the reinfection swab, but whose Ct value for the reinfection swab was >30, were considered as showing some evidence for reinfection. Suspected reinfection cases who had a PCR-positive swab within 45 days preceding the reinfection swab were considered as showing weak (or no) evidence for reinfection, as they were likely to reflect prolonged PCR positivity of the primary infection rather than a reinfection [5,27,[30], [31], [32]].
2.4. Viral genome sequencing and analysis
For a subset of investigated reinfection cases with good or some evidence for reinfection, there were records indicating prior diagnosis of the primary infection. Viral genome sequencing was thus conducted to confirm reinfection in this subset of cases whenever it was possible to retrieve both the first-infection PCR-positive swab and the reinfection swab. Details of viral genome sequencing methods are provided in Supplementary Text S1.
2.5. Reinfection risk and rate
The Kaplan–Meier curve was used to estimate the cumulative risk of documented reinfection, that is, the proportion of cases with good or some evidence for reinfection among all eligible individuals with an antibody-positive test. Incidence rate of documented reinfection was calculated by dividing the number of cases with good or some evidence for reinfection by the number of person-weeks contributed by all anti-SARS-CoV-2 positive cases. The latter was estimated using a Poisson log-likelihood regression model with the STATA 16.1 [33] stptime command. The follow-up person-time was calculated starting 14 days after the first-positive antibody test until the reinfection swab, all-cause death, or end-of-study censoring (on December 31, 2020). The temporal trend in incidence rate was assessed with the Mantel-Haenszel method using the STATA 16.1 [33] stmh command. Adjusted estimates for the cumulative risk of reinfection and the incidence rate of reinfection were derived by applying the confirmation rate obtained from the viral genome sequencing analysis. Sensitivity analyses were conducted.
2.6. Comparator antibody-negative group and efficacy of natural infection against reinfection
SARS-CoV-2 incidence was also assessed in the complement cohort, including all those who tested SARS-CoV-2 antibody-negative in Qatar, to provide an antibody-negative comparator and to assess the efficacy of natural infection against reinfection.
Both cumulative risk of documented infection and incidence rate of documented infection in this antibody-negative cohort were assessed as described above for the antibody-positive cohort, but with the event defined here as the first PCR-positive swab that is ≥14 days after the first antibody-negative test.
The efficacy of natural infection against reinfection was estimated by comparing the incidence rate of reinfection in the antibody-positive cohort to the incidence rate of infection in the comparator antibody-negative cohort:
2.7. Ethical approval
This study was approved by the HMC and Weill Cornell Medicine-Qatar Institutional Review Boards.
2.8. Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the article.
3. Results
3.1. Epidemiological analysis
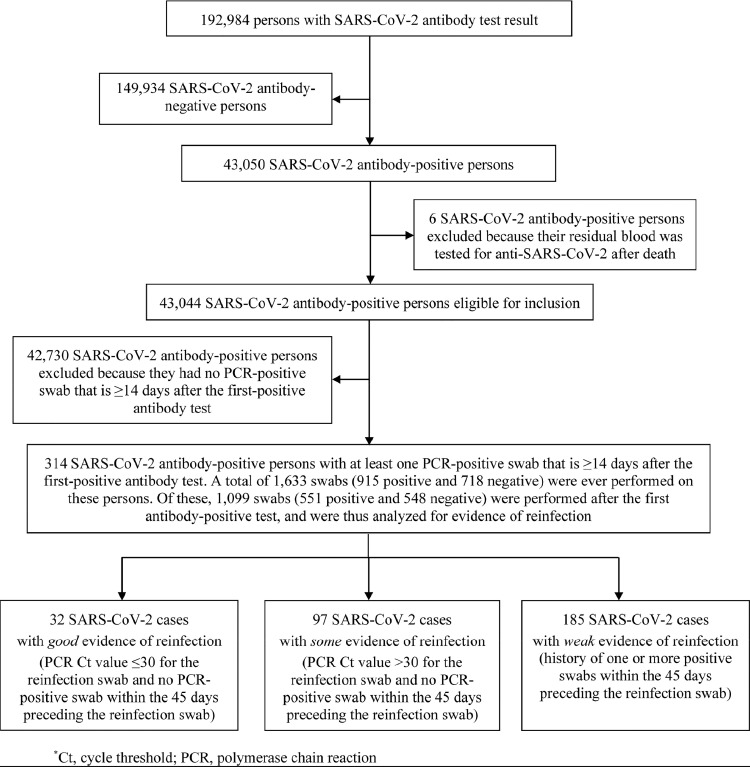
The process for selecting suspected cases of SARS-CoV-2 reinfection is shown in Fig. 1, which summarizes results of their reinfection status evaluation. Of 192,984 persons tested for anti-SARS-CoV-2 using blood specimens collected between April 16, 2020 and December 31, 2020, 149,934 had negative test results, and were excluded. Six of the remaining 43,050 antibody-positive persons were also excluded because their residual blood was tested for SARS-CoV-2 antibodies after death. This yielded a retrospective cohort of 43,044 antibody-positive persons for whom possible reinfection was assessed.
Fig. 1.
Flowchart describing the selection process of suspected cases of SARS-CoV-2 reinfection and summarizing the results of their reinfection status evaluation.
The cohort included 8953 (20.8%) women and 34,091 men (79.2%) of 158 nationalities. Median age was 35 years for women (interquartile range (IQR): 28–45 years) and 38 years for men (IQR: 31–47 years). Of this cohort, 80.7% had received a PCR test with an overall testing frequency of 1.9 tests per person, and of 0.5 tests per person after the first antibody-positive test. Only 19,976 (46.4%) of these persons had ever had a PCR-positive swab preceding their first-positive antibody test. Individual time of follow-up ranged between 0 days and 34.6 weeks, with a median of 16.3 weeks.
Only 314 persons had a PCR-positive swab ≥14 days after the first-positive antibody test, and thus qualified for inclusion in the analysis. There were 1633 swabs (915 positive and 718 negative) collected from these 314 persons, and of these, 1099 (551 positive and 548 negative) were collected after the first-positive antibody test.
Investigation of these 314 suspected cases of reinfection yielded 32 cases with good evidence for reinfection (Ct ≤30 for reinfection swab), 97 cases with some evidence (Ct >30 for reinfection swab), while evidence was weak for the remaining 185 cases.
Characteristics of the 129 cases with good or some evidence for reinfection are shown in Table 2. These individuals had a median age of 37 years (range: <1–72 years) and included 92 men (71.3%). The median time between the first-positive antibody test and the reinfection swab was 52 days (range: 15–212 days). The median Ct value of the reinfection swab was 32.9 (range: 13.9–38.3). Slightly over a third of cases were diagnosed based on clinical suspicion (n = 34; 26.4%) or individual request (n = 9; 7.0%), while the rest (n = 86) were identified incidentally either through random PCR-testing campaigns/surveys (n = 47; 36.4%), through healthcare routine testing (n = 18; 14.0%), through contact tracing (n = 15; 11.6%), or at a port of entry (n = 6; 4.7%).
Table 2.
Characteristics of individuals classified as showing good or some evidence of reinfection.
| Demography |
Ab testing |
PCR testing |
||||||
|---|---|---|---|---|---|---|---|---|
| ID# | Sex | Age group | First-positive Ab test date | Ab test optical density (Ab titers) | Reinfection swab date | Average Ct value* | Reason for swab | Presence of symptoms |
| Good evidence for reinfection | ||||||||
| 1 | Female | 10–14 | 28 Jul | 1.1 | 29 Sep | 21.7 | Clinical suspicion§ | Yes |
| 2 | Female | 20–24 | 02 Jul | 1.2 | 01 Oct | 16.6 | Contact tracing | Yes |
| 3 | Male | 50–54 | 24 Aug | 1.2 | 12 Oct | 22.0 | Clinical suspicion§ | Yes |
| 4 | Female | 25–29 | 21 July | 1.4 | 24 Aug | 30.0 | Individual request† | No |
| 5 | Female | 40–44 | 07 Jul | 2.0 | 20 Sep | 22.2 | Individual request† | Yes |
| 6 | Male | 30–34 | 21 Jul | 2.1 | 30 Sep | 29.5 | Clinical suspicion§ | No†† |
| 7 | Female | 30–34 | 09 Aug | 2.3 | 12 Oct | 21.8 | Clinical suspicion§ | Yes |
| 8 | Female | 40–44 | 03 July | 2.4 | 08 Aug | 20.5 | Port of entry‡ | No |
| 9 | Female | 20–24 | 09 Aug | 2.7 | 06 Nov | 20.5 | Contact tracing | No |
| 10 | Male | 30–34 | 16 Aug | 3.0 | 22 Sep | 28.1 | Clinical suspicion§ | No†† |
| 11 | Female | 30–34 | 02 Aug | 3.0 | 27 Dec | 28.0 | Clinical suspicion§ | Yes |
| 12 | Male | 40–44 | 13 Jul | 4.8 | 07 Aug | 22.6 | Survey⁎⁎ | No |
| 13 | Male | 35–39 | 21 Jun | 5.6 | 14 Sep | 23.3 | Survey⁎⁎ | Not indicated |
| 14 | Male | 30–34 | 16 Jul | 7.6 | 17 Sep | 29.5 | Clinical suspicion§ | No†† |
| 15 | Female | 40–44 | 03 Jul | 7.7 | 16 Sep | 23.4 | Contact tracing | No |
| 16 | Female | 30–34 | 23 Aug | 8.7 | 23 Dec | 13.9 | Survey⁎⁎ | Not indicated |
| 17 | Male | 55–59 | 28 Jun | 8.8 | 12 Aug | 26.4 | Clinical suspicion§ | No†† |
| 18 | Male | 50–54 | 13 Jul | 9.2 | 16 Nov | 29.8 | Individual request† | No |
| 19 | Male | 40–44 | 04 Jul | 11.3 | 12 Oct | 28.1 | Clinical suspicion§ | Yes |
| 20 | Male | 35–39 | 09 Jul | 11.3 | 15 Sep | 28.1 | Contact tracing | Not indicated |
| 21 | Male | 35–39 | 03 Nov | 14.9 | 26 Dec | 28.8 | Survey⁎⁎ | No |
| 22 | Female | 0–9 | 05 Jul | 16.7 | 17 Sep | 29.5 | Clinical suspicion§ | Yes |
| 23 | Male | 40–44 | 20 Aug | 22.2 | 07 Dec | 24.4 | Port of entry‡ | No |
| 24 | Female | 25–29 | 27 Aug | 24.2 | 06 Oct | 29.5 | Clinical suspicion§ | No†† |
| 25 | Female | 20–24 | 25 Aug | 25.9 | 30 Sep | 29.1 | Survey⁎⁎ | No |
| 26 | Male | 65–69 | 01 Jun | 28.3 | 22 Jun | 27.7 | Clinical suspicion§ | Not indicated |
| 27 | Male | 50–54 | 26 Jun | 32.0 | 23 Sep | 29.2 | Healthcare routine testing | No |
| 28 | Male | 65–69 | 30 Oct | 55.9 | 27 Dec | 29.4 | Healthcare routine testing | No |
| 29 | Male | 35–39 | 13 Jul | 75.4 | 18 Aug | 37.6 | Survey⁎⁎ | No |
| 30 | Male | 55–59 | 23 Aug | 85.6 | 12 Dec | 27.8 | Survey⁎⁎ | No |
| 31 | Female | 30–34 | 02 Aug | 60.1 | 06 Oct | 29.1 | Individual request† | Yes |
| 32 | Male | 20–24 | 11 Aug | 140.0 | 28 Aug | 30.0 | Clinical suspicion§ | No†† |
| Some evidence of reinfection | ||||||||
| 33 | Female | 40–44 | 23 Jun | 1.1 | 26 Jul | 36.2 | Survey⁎⁎ | No |
| 34 | Male | 20–24 | 12 Aug | 1.1 | 11 Sep | NR | Contact tracing | No |
| 35 | Male | 30–34 | 16 Jul | 1.2 | 18 Nov | NR | Clinical suspicion§ | Yes |
| 36 | Male | 25–29 | 21 Oct | 1.6 | 17 Nov | NR | Survey⁎⁎ | No |
| 37 | Male | 30–34 | 07 Jul | 1.7 | 01 Sep | NR | Clinical suspicion§ | No†† |
| 38 | Female | 45–49 | 05 Jul | 2.0 | 28 Aug | NR | Healthcare routine testing | No |
| 39 | Female | 65–69 | 06 Jul | 2.0 | 24 Aug | NR | Survey⁎⁎ | No |
| 40 | Male | 60–64 | 12 Jul | 2.5 | 08 Oct | NR | Healthcare routine testing | No |
| 41 | Female | 40–44 | 20 Jun | 3.4 | 24 Aug | NR | Survey⁎⁎ | Not indicated |
| 42 | Male | 35–39 | 18 Aug | 3.7 | 08 Nov | NR | Clinical suspicion§ | No†† |
| 43 | Male | 45–49 | 19 Jul | 3.9 | 24 Aug | 30.5 | Clinical suspicion§ | No†† |
| 44 | Female | 20–24 | 24 Aug | 4.1 | 12 Sep | 35.9 | Survey⁎⁎ | No |
| 45 | Female | 45–49 | 22 Oct | 4.5 | 24 Dec | 31.0 | Clinical suspicion§ | Yes |
| 46 | Male | 60–64 | 19 Jun | 5.2 | 23 Aug | NR | Clinical suspicion§ | No†† |
| 47 | Male | 50–54 | 28 Jun | 5.7 | 16 Sep | 31.3 | Port of entry‡ | No |
| 48 | Female | 40–44 | 26 Aug | 6.2 | 11 Sep | 33.9 | Port of entry‡ | Yes |
| 49 | Male | 35–39 | 09 Jun | 6.3 | 05 Oct | 31.5 | Survey⁎⁎ | No |
| 50 | Male | 25–29 | 12 Jul | 6.9 | 08 Oct | 32.8 | Clinical suspicion§ | No†† |
| 51 | Male | 50–54 | 22 Jul | 7.6 | 19 Aug | 36.4 | Survey⁎⁎ | No |
| 52 | Male | 50–54 | 30 Jun | 7.7 | 11 Oct | NR | Contact tracing | No |
| 53 | Male | 35–39 | 11 Aug | 7.9 | 14 Dec | NR | Survey⁎⁎ | No |
| 54 | Male | 40–44 | 24 Jun | 8.0 | 05 Sep | 34.1 | Survey⁎⁎ | No |
| 55 | Female | 25–29 | 11 Aug | 9.0 | 26 Aug | 34.2 | Healthcare routine testing | Not indicated |
| 56 | Male | 40–44 | 28 Jun | 9.9 | 10 Aug | 32.9 | Survey⁎⁎ | No |
| 57 | Female | 30–34 | 15 Aug | 10.8 | 30 Oct | 30.2 | Clinical suspicion§ | No†† |
| 58 | Male | 25–29 | 21 Jul | 11.0 | 25 Aug | 37.4 | Survey⁎⁎ | Not indicated |
| 59 | Female | 45–49 | 02 Jul | 11.0 | 21 Sep | NR | Contact tracing | No |
| 60 | Male | 50–54 | 01 Jul | 13.1 | 29 Sep | NR | Contact tracing | No |
| 61 | Male | 35–39 | 21 Aug | 13.2 | 07 Sep | 36.8 | Healthcare routine testing | No |
| 62 | Male | 40–44 | 28 May | 13.5 | 26 Dec | NR | Survey⁎⁎ | No |
| 63 | Female | 50–54 | 18 Jul | 14.5 | 25 Aug | 32.3 | Survey⁎⁎ | No |
| 64 | Female | 35–39 | 04 Jul | 14.8 | 30 Aug | NR | Individual request† | No |
| 65 | Female | 35–39 | 18 Jul | 15.8 | 04 Aug | NR | Contact tracing | No |
| 66 | Male | 45–49 | 08 Jul | 16.0 | 15 Oct | NR | Healthcare routine testing | No |
| 67 | Female | 30–34 | 11 Jul | 16.6 | 29 Jul | 36.2 | Survey⁎⁎ | No |
| 68 | Male | 60–64 | 16 Aug | 17.3 | 05 Oct | 31.0 | Healthcare routine testing | No |
| 69 | Male | 35–39 | 25 Aug | 17.4 | 19 Sep | 33.7 | Survey⁎⁎ | Not indicated |
| 70 | Male | 25–29 | 02 Aug | 17.8 | 01 Sep | NR | Clinical suspicion§ | No†† |
| 71 | Male | 35–39 | 24 Aug | 18.0 | 11 Oct | NR | Survey⁎⁎ | Not indicated |
| 72 | Male | 35–39 | 01 Jun | 19.7 | 23 Aug | NR | Survey⁎⁎ | No |
| 73 | Male | 15–19 | 08 Aug | 20.0 | 12 Sep | 34.5 | Healthcare routine testing | No |
| 74 | Male | 50–54 | 08 Jul | 20.1 | 10 Sep | NR | Clinical suspicion§ | No†† |
| 75 | Male | 40–44 | 09 Jul | 20.5 | 30 Aug | NR | Clinical suspicion§ | No†† |
| 76 | Female | 35–39 | 13 Jul | 20.9 | 27 Aug | NR | Survey⁎⁎ | No |
| 77 | Male | 20–24 | 22 Aug | 20.9 | 28 Nov | 34.9 | Survey⁎⁎ | No |
| 78 | Male | 45–49 | 25 Aug | 22.9 | 25 Sep | 34.7 | Survey⁎⁎ | No |
| 79 | Male | 50–54 | 05 Oct | 26.9 | 05 Nov | 35.3 | Survey⁎⁎ | No |
| 80 | Male | 20–24 | 10 Aug | 28.5 | 05 Oct | 33.0 | Survey⁎⁎ | No |
| 81 | Male | 30–34 | 07 Jul | 28.5 | 21 Aug | 34.9 | Clinical suspicion§ | Yes |
| 82 | Male | 30–34 | 26 Aug | 30.4 | 13 Sep | 35.3 | Survey⁎⁎ | Not indicated |
| 83 | Male | 40–44 | 28 Jun | 31.9 | 05 Oct | NR | Individual request† | No |
| 84 | Male | 0–9 | 01 Jul | 32.8 | 01 Aug | NR | Clinical suspicion§ | Yes |
| 85 | Male | 70–74 | 21 Jul | 33.2 | 08 Sep | NR | Healthcare routine testing | No |
| 86 | Male | 40–44 | 17 Jul | 35.8 | 11 Sep | NR | Survey⁎⁎ | No |
| 87 | Male | 30–34 | 21 Jul | 36.8 | 12 Sep | NR | Survey⁎⁎ | No |
| 88 | Male | 30–34 | 01 Jun | 37.9 | 01 Aug | NR | Clinical suspicion§ | Yes |
| 89 | Female | 25–29 | 06 Jun | 38.3 | 23 Jul | 36.0 | Survey⁎⁎ | No |
| 90 | Male | 30–34 | 08 Jul | 39.6 | 23 Jul | 34.2 | Contact tracing | No |
| 91 | Male | 30–34 | 24 Jul | 41.9 | 08 Aug | 34.4 | Survey⁎⁎ | No |
| 92 | Female | 35–39 | 09 Nov | 43.2 | 29 Dec | NR | Healthcare routine testing | No |
| 93 | Male | 25–29 | 05 Jul | 46.0 | 15 Aug | 31.6 | Contact tracing | Not indicated |
| 94 | Male | 20–24 | 27 Jul | 46.2 | 15 Oct | 33.0 | Healthcare routine testing | No |
| 95 | Male | 60–64 | 28 Sep | 47.0 | 22 Oct | 31.3 | Survey⁎⁎ | No |
| 96 | Male | 25–29 | 13 Jul | 47.8 | 28 Jul | NR | Survey⁎⁎ | No |
| 97 | Male | 40–44 | 13 Jul | 48.3 | 30 Aug | NR | Survey⁎⁎ | No |
| 98 | Male | 35–39 | 25 Aug | 49.4 | 26 Sep | 33.6 | Survey⁎⁎ | Not indicated |
| 99 | Male | 25–29 | 23 Aug | 51.7 | 17 Oct | 33.6 | Clinical suspicion§ | No†† |
| 100 | Female | 10–14 | 13 Jul | 52.4 | 29 Sep | 42.4 | Individual request† | Not indicated |
| 101 | Male | 30–34 | 13 Jul | 54.4 | 28 Jul | 35.9 | Survey⁎⁎ | No |
| 102 | Male | 35–39 | 22 Jul | 55.1 | 21 Oct | 37.5 | Clinical suspicion§ | Yes |
| 103 | Male | 35–39 | 05 Jul | 56.1 | 15 Aug | 36.2 | Survey⁎⁎ | No |
| 104 | Male | 40–44 | 12 Aug | 57.2 | 21 Oct | 36.7 | Clinical suspicion§ | Yes |
| 105 | Male | 50–54 | 27 Aug | 57.4 | 03 Dec | 37.3 | Healthcare routine testing | No |
| 106 | Female | 15–19 | 20 Aug | 63.8 | 24 Oct | NR | Individual request† | No |
| 107 | Female | 30–34 | 30 Jul | 65.0 | 29 Sep | 36•4 | Port of entry‡ | No |
| 108 | Male | 25–29 | 20 Jul | 65.3 | 22 Aug | NR | Contact tracing | No |
| 109 | Male | 45–49 | 22 Jun | 66.8 | 12 Jul | 31.3 | Contact tracing | No |
| 110 | Male | 40–44 | 01 Nov | 68.6 | 26 Dec | NR | Survey⁎⁎ | No |
| 111 | Female | 30–34 | 18 Jul | 73.9 | 05 Oct | NR | Survey⁎⁎ | No |
| 112 | Male | 60–64 | 06 Jul | 76.5 | 03 Sep | NR | Healthcare routine testing | No |
| 113 | Female | 30–34 | 14 Jul | 77.3 | 15 Aug | 37.1 | Contact tracing | No |
| 114 | Male | 45–49 | 12 Jul | 81.5 | 20 Aug | 34.6 | Healthcare routine testing | No |
| 115 | Male | 65–69 | 18 Aug | 85.6 | 27 Oct | 35.7 | Port of entry‡ | No |
| 116 | Male | 30–34 | 26 Jul | 92.2 | 12 Dec | NR | Healthcare routine testing | No |
| 117 | Male | 40–44 | 09 Jul | 94.1 | 27 Jul | 38.3 | Survey⁎⁎ | No |
| 118 | Male | 30–34 | 01 Sep | 97.1 | 17 Sep | 35.8 | Healthcare routine testing | No |
| 119 | Male | 40–44 | 24 Aug | 101.0 | 28 Nov | 33.1 | Clinical suspicion§ | Yes |
| 120 | Male | 40–44 | 21 Jul | 101.9 | 29 Aug | 35.0 | Survey⁎⁎ | No |
| 121 | Male | 55–59 | 01 Jul | 105.3 | 17 Aug | NR | Clinical suspicion§ | No†† |
| 122 | Male | 35–39 | 04 Aug | 109.2 | 03 Dec | NR | Survey⁎⁎ | No |
| 123 | Male | 30–34 | 28 Jul | 121.9 | 20 Aug | 35.8 | Contact tracing | No |
| 124 | Male | 35–39 | 09 Aug | 124.4 | 29 Aug | NR | Individual request† | No |
| 125 | Male | 40–44 | 01 Sep | 125.3 | 15 Oct | 35.8 | Clinical suspicion§ | No†† |
| 126 | Female | 60–64 | 29 Jul | 128.0 | 19 Aug | 34.2 | Survey⁎⁎ | No |
| 127 | Male | 35–39 | 11 Aug | 141.0 | 26 Aug | NR | Survey⁎⁎ | No |
| 128 | Male | 35–39 | 31 Aug | 146.0 | 05 Oct | NR | Clinical suspicion§ | Yes |
| 129 | Male | 30–34 | 02 Sep | 150.0 | 27 Sep | 34.1 | Healthcare routine testing | No |
Ab, antibody; Asymp, asymptomatic; Ct, cycle threshold; NR, not reported; PCR, polymerase chain reaction.
The table is sorted by antibody test optical density value (antibody titer).
Persons with ID numbers 5, 64, 72, 88, and 127 are reinfection cases that were confirmed by viral genome sequencing.
Average PCR Ct value over different targets for SARS-CoV-2 genes and/or proteins.
The category “individual request” refers to testing conducted at a healthcare facility based on the individual's request, often because of some requirement for testing, such as for travel.
The category “port of entry” refers to testing conducted at the border or airport upon return from travel.
The category “clinical suspicion” refers to testing conducted at a healthcare facility based on presence of signs or symptoms, or reported history of exposure.
The category “survey” refers to surveillance random PCR testing campaigns conducted in workplaces and residential areas.
The reason for the swab in the hospital record was “clinical suspicion”, but no further details were provided and the person was reported to have no COVID-19 symptoms.
At the time of the reinfection swab, eight cases had records in the severity database. One of these was classified as “severe” and two as “moderate” per WHO classification [23], while the other five were classified as “asymptomatic.” At time of primary infection, 14 cases had records in the severity database, one of whom was classified as “critical”, three as “severe”, five as “moderate”, two as “mild”, and three as “asymptomatic.” For the rest of the reinfection cases, no severity classification was conducted because of minimal or no symptoms to warrant a clinical assessment. For the eight asymptomatic cases above that had a severity assessment, the assessment was conducted because of non-COVID-related hospitalization. No deaths were recorded for any of these reinfection cases.
3.2. Confirmation of reinfection through viral genome sequencing
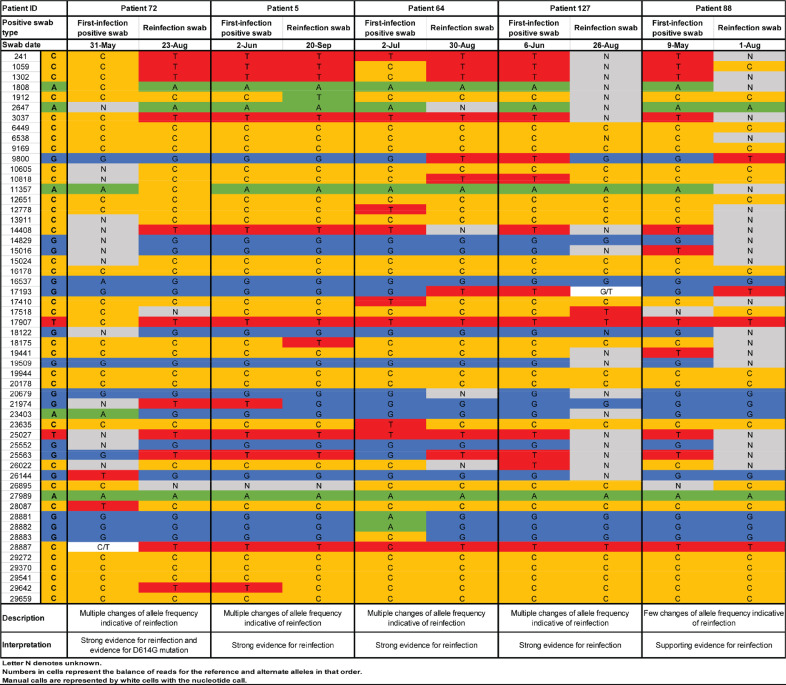
Among the 129 cases with good or some evidence for reinfection, 62 had records indicating prior diagnosis of a primary infection. Paired specimens of the first-infection PCR-positive swab and the reinfection swab were retrieved in 23 cases. Viral genome sequencing results are summarized in Table 3. Detailed analysis for each genome pair is shown in Fig. 2 and Supplementary Figures S1-S2. Genome sequencing results have been also placed in the public domain [34].
Table 3.
Results of reinfection confirmation using viral genome sequencing. Viral genome sequencing was conducted only for a subset of cases with good or some evidence of reinfection, that is, whenever paired samples of the first-infection PCR-positive swab and the reinfection PCR-positive swab were available.
| Viral genome sequencing evidence for reinfection | Indication upon comparing each genome pair | N |
|---|---|---|
| Insufficient evidence to warrant interpretation | One or two genomes of low quality | 7 |
| No evidence for reinfection | One change of allele frequency | 1 |
| Shifting balance of quasi-species with no evidence for reinfection | Few changes of allele frequency but not sufficiently indicative of reinfection | 6 |
| Strong evidence for no reinfection | Both genomes of high quality yet no significant differences found | 4* |
| Supporting evidence for reinfection | Few changes of allele frequency indicative of reinfection | 1 |
| Strong evidence for reinfection | Multiple changes of allele frequency indicative of reinfection | 4 |
| Total | 23 |
PCR, polymerase chain reaction.
Viral genome sequencing for two patients was performed as part of an earlier study assessing the risk of SARS-CoV-2 reinfection in the cohort of PCR-confirmed infected persons in Qatar [5].
Fig. 2.
Viral genome sequencing analysis of paired viral specimens of the primary-infection PCR-positive swab and the reinfection PCR-positive swab for five cases with strong or supporting evidence of reinfection. These genomes have been deposited in the public domain [34].
There was insufficient evidence to warrant interpretation for seven sample pairs because of low genome quality. For seven additional pairs, there were one to several changes of allele frequency indicative, at best, of a shifting balance of quasi-species, thus no evidence for reinfection. For four pairs, there was strong evidence for no reinfection as both genomes were of high quality, yet no differences were found. Three of these cases had a Ct <30 for the reinfection swab, indicating persistent active infection (Table 2). Two of these cases were reported earlier as part of a case report documenting the existence of prolonged infections [35].
Meanwhile, for one pair, there were few changes of allele frequency offering supporting evidence for reinfection. For four other pairs, there were multiple clear changes of allele frequency indicating strong evidence for reinfection. One of the latter pairs also documented the presence of the D614G mutation (23,403 bp A>G) at the reinfection swab—a variant that has progressively replaced the original D614 form [36,37].
In summary, for the 16 cases in which viral genome sequencing evidence was available, five cases were confirmed as reinfections, a confirmation rate of 31.3%. This confirmation rate was similar to that found in our earlier study of reinfection among those with a PCR-confirmed infection at 33.3% [5].
3.3. Assessment of risk and incidence rate of reinfection
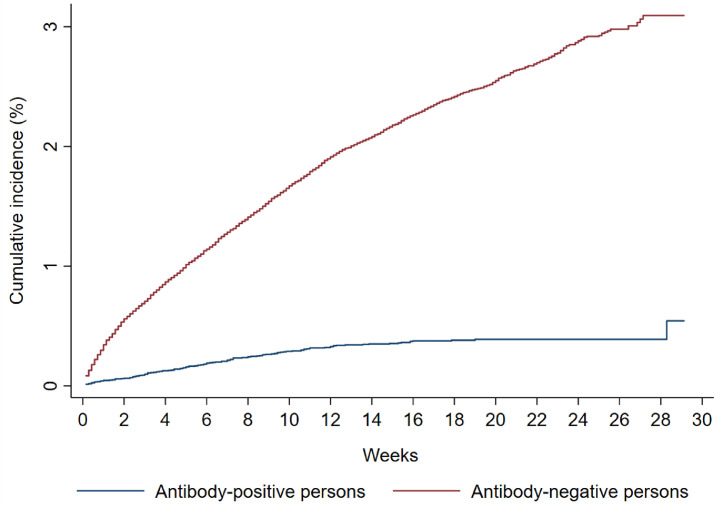
The Kaplan–Meier curve for the risk (incidence) of documented reinfection is shown in Fig. 3. Applying the 31.3% confirmation rate obtained through viral genome sequencing, this yielded a cumulative risk of documented reinfection of 0.17% (95% confidence interval (CI): 0.10–0.30%) after 34.6 weeks of follow-up.
Fig. 3.
Kaplan-Meier curves showing the cumulative risk (incidence) of documented reinfection and of documented infection with SARS-CoV-2 in the antibody-positive and antibody-negative cohorts, respectively.
The incidence rate of documented reinfection was estimated at 0.66 per 10,000 person-weeks (95% CI: 0.56–0.78). That is 31.3% of 129 reinfection events in a follow-up person-time of 610,832.5 person-weeks.
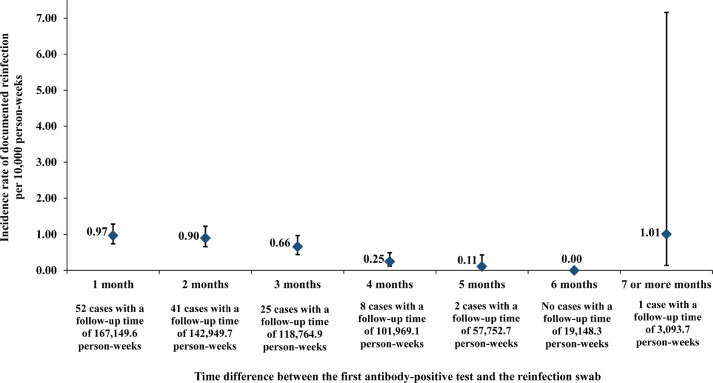
Fig. 4 shows the incidence rate of documented reinfection versus month of follow-up in this cohort of antibody-positive persons. There was evidence for a decreasing trend in the incidence rate of reinfection with each additional month of follow-up (Mantel-Haenszel trend analysis p-value: <0.001).
Fig. 4.
Incidence rate of documented SARS-CoV-2 reinfection versus month of follow-up in the cohort of 43,044 antibody-positive persons. Error bars indicate 95% confidence interval.
3.4. Comparator antibody-negative group and efficacy of natural infection against reinfection
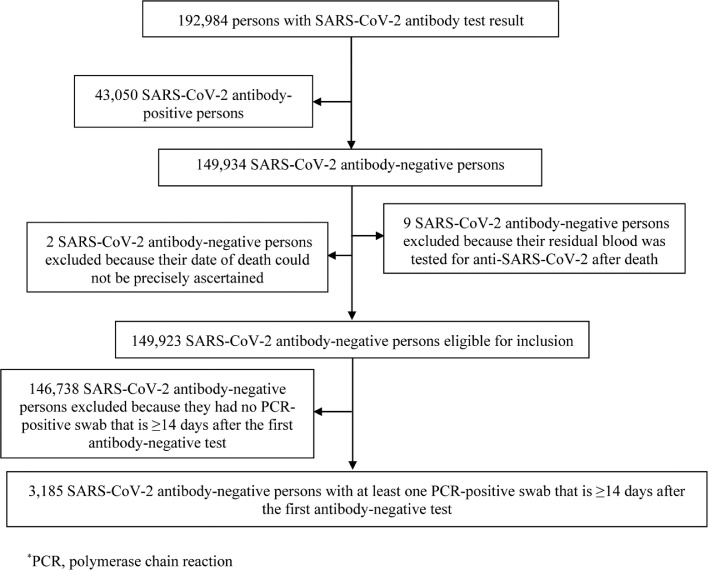
The complement cohort of all those who tested SARS-CoV-2 antibody-negative included 149,934 individuals. Of those, nine were excluded because their residual blood was tested for SARS-CoV-2 antibodies after death. Two other individuals were excluded because their date of death could not be precisely ascertained. This yielded a retrospective cohort of 149,923 antibody-negative persons to be assessed for SARS-CoV-2 infection incidence (Fig. 5).
Fig. 5.
Flowchart describing the process for identifying SARS-CoV-2 incident infections in the complement cohort of antibody-negative individuals.
This cohort included 75,904 (50.6%) women and 74,019 men (49.4%) of 167 nationalities. Median age was 35 years for women (interquartile range (IQR): 28–47 years) and 39 years for men (IQR: 30–50 years). Individual time of follow-up ranged between 0 days and 45.6 weeks, with a median of 17.0 weeks. These characteristics are similar to those of the antibody-positive cohort apart from the higher proportion of women. The higher proportion of women is a consequence of the fact that men were several-fold more affected than women by the SARS-CoV-2 epidemic and much more likely to be seropositive [12,14,16,17]. The men craft and manual worker population, that comprises 60% of the total population [38], was the most affected segment of the population with a seroprevalence that is much higher than the rest of the population [12,14,16,17].
Of this cohort, 69.2% had received a PCR test with an overall testing frequency of 1.7 tests per person, and of 0.9 tests per person after the first antibody-negative test. Of 149,923 antibody-negative individuals, 3185 individuals had at least one PCR-positive swab ≥14 days after the first antibody-negative test. The Kaplan–Meier curve for the risk (incidence) of documented infection is shown in Fig. 3. The cumulative risk of documented infection was estimated at 3.09% (95% CI: 2.93–3.27%) after 45.6 weeks of follow-up.
The incidence rate of documented infection was estimated at 13.69 per 10,000 person-weeks (95% CI: 13.22–14.14), that is 3185 infections in a follow-up person-time of 2326,572.0 person-weeks.
The efficacy of natural infection against reinfection was estimated by comparing the incidence rate of reinfection in the antibody-positive cohort to the incidence rate of infection in the comparator antibody-negative cohort:
yielding an efficacy estimate of 95.2% (95% CI: 94.1–96.0%).
There were no statistically significant differences in efficacy between women and men, or for those <50 years of age versus those ≥50 years of age. The PCR testing frequency (after the antibody test) among those antibody-positive was lower than that among those antibody-negative, probably reflecting the lower incidence of infection among them. Adjusting the efficacy estimate for differences in testing frequency reduced the estimate to 91.3% (95% CI: 89.4–92.9%), not materially different from the original estimate.
As a sensitivity analysis, the Mantel-Haenszel approach was used to provide another estimate for the efficacy by factoring all PCR testing (after the antibody test) on the combined cohorts stratified by calendar week, that is adjusting for the phase of the epidemic. After applying the viral genome sequencing confirmation rate and excluding PCR-positive cases with weak evidence for reinfection, the efficacy against reinfection was estimated at 92.7% (95% CI: 91.3–93.9%), also not materially different from the original estimate.
4. Discussion
The results provide concrete evidence for the presence of reinfection in some individuals with detectable antibodies for SARS-CoV-2 infection, even in some with high antibody titers (Table 2). However, the cumulative risk of documented reinfection was rare, at ~2 per 1000 infected persons, at least for a few months after the first antibody-positive test in the young and international population of Qatar where <9% of the population are ≥50 years of age [11].
There was also no evidence that antibody-positive persons experienced any waning of protective immunity over time, as the incidence rate of reinfection versus month of follow-up did not show an increasing trend over seven months following the first antibody-positive test (Fig. 4). To the contrary, there was a trend of decreasing incidence rate, possibly explained by the (very) slowly declining incidence rate in the wider population of Qatar during this study [15,39], or possibly by strengthening of protective immunity due to repeated exposures that did not lead to established infection. Notably, a recent study from Qatar indicated an association between higher antibody titers and repeated exposures to the virus [17]. Further follow-up of this cohort of antibody-positive persons over time may allow a more long-term assessment of the persistence of protection against reinfection.
Remarkably, the incidence rate of reinfection found here for those with antibody-confirmed infection at ~1 per 10,000 person-weeks is very similar to that found for those with PCR-confirmed infection, as reported in our earlier reinfection study [5]. This suggests that these two populations are functionally similar. Evidence of exposure to SARS-CoV-2, regardless of the biomarker used to assess infection, appears sufficient to indicate protection against reinfection.
These findings are striking, as the epidemic in Qatar has been intense, with half of the population estimated to have acquired this infection at some point since its introduction into Qatar early in 2020 [[14], [15], [16], [17],39]. It is highly probable that a proportion of the population has been repeatedly exposed to SARS-CoV-2, but such re-exposures did not lead to more than a limited number of documentable reinfections. Other lines of evidence also support a low frequency of reinfection. The epidemic in Qatar grew rapidly and declined rapidly [15,39], consistent with a susceptible-infected-recovered “SIR” epidemic dynamic in which infection elicits strong immunity against reinfection. No second wave materialized in 2020 following the epidemic peak in May 2020, despite easing of public health restrictions [15,39]. Other studies of reinfection also indicated lower incidence of infection in those antibody-positive or with a prior PCR-confirmed infection [5,[40], [41], [42], [43], [44], [45], [46]], and a study of immunological memory in a cohort of COVID-19 patients indicated durability of the immune response for at least 6–8 months [47].
The study estimated the efficacy of natural infection against reinfection at 95.2% by comparing SARS-CoV-2 incidence in those antibody-positive to those antibody-negative. The efficacy can also be estimated by comparing the incidence rate of documented reinfection to the incidence rate of documented infection throughout the epidemic in 2020 that was estimated at ~15 per 10,000 person-weeks [15]. This yielded an efficacy of 95.6%, confirming the above estimate. Remarkably, this efficacy estimate is similar to the efficacy reported for the two mRNA COVID-19 vaccines [18,19], our earlier reinfection study [5], and a study of reinfection in Switzerland [45], but is higher than that reported recently in other studies of reinfection, ranging between 80 and 90% [[40], [41], [42], [43], [44],46].
While one reinfection was severe, none were critical or fatal and a large proportion of reinfections were minimally symptomatic (if not asymptomatic) to the extent that they were discovered only incidentally, such as through contact tracing or random testing campaigns/surveys (Table 2). The severity of reinfection was also less than that of primary infection. These findings suggest that reinfections (when they rarely occur) appear well tolerated and no more symptomatic than primary infections.
This study has some limitations. By study design, primary infection was indirectly ascertained through serological testing, thereby including only a subset with documented PCR-confirmed primary infections. Having said so, serological testing was based on a high-quality, validated platform, the Roche platform, one of the best available and most extensively used and investigated commercial platforms, with a specificity of at least 99.8% [48,49]. Thus, it is unlikely that misclassified antibody-positives could have biased our findings. We assessed risk of only documented reinfections, but other reinfections may have occurred, but went undocumented, perhaps because of minimal/mild or no symptoms. Recent studies from Denmark and the USA report strong, but still lower protection against reinfection at about 80% [40,46], possibly because of higher diagnosis of asymptomatic and minimally mild reinfections [50]. The follow-up times varied among individuals and a proportion of individuals were followed for a short duration. The antibody-negative cohort had a higher proportion of women than the antibody-positive cohort, due to the differential spread of the infection among women versus men in Qatar [12,14,16,17]. Antigen testing has had limited use in Qatar, and positive results had to be confirmed by PCR testing, therefore it is not likely that reinfections diagnosed through antigen testing could have been missed. Travel history of members of the two cohorts was not available to assess whether there is any loss of follow-up due to travel out of the country.
Viral genome sequencing analysis was possible for only a subset of reinfections, either because primary infection was only identified through antibody testing with no record of earlier PCR testing, or because the reinfection swab could not be retrieved. Reinfections were confirmed by noting differences in the viral genome between the primary infection and the reinfection. While not likely, it is theoretically possible that these differences may have occurred due to within-host evolution of the virus, as in the context of a prolonged infection [35,51].
Unlike in blinded, randomized clinical trials, the two observational cohorts of those antibody-positive and antibody-negative were not randomized. However, most of those antibody-positive or antibody-negative were not aware of their antibody status; thus, it is not likely that awareness of antibody status could have biased the results. In a small proportion of those antibody-negative, there was a record of a prior PCR-positive result. Nonetheless, excluding those with a prior PCR-positive result did not affect the estimated protection against reinfection, which was estimated once more at 95.0% (95% CI: 93.9–95.9). During the course of follow-up, some of those antibody-negative may have developed antibodies, but the infection was not documented by PCR, and so misclassified as controls. It is also possible that those antibody-positive may have been at higher risk of reinfection because of a higher number of contacts, or may have been at lower risk of reinfection because their peers within their social network were also previously infected and thereby less likely to be infected and to transmit the infection to them. The overall potential effect of these departures from the hypothetical ideal of a randomized clinical trial is perhaps an overestimation, rather than underestimation of the incidence of reinfection, thereby affirming the conclusion of the rarity of reinfections.
In conclusion, SARS-CoV-2 reinfection was investigated in a large cohort of antibody-positive individuals who were followed for as long as 35 weeks. While the study documented some reinfections, they constitute a rare phenomenon, with natural infection eliciting protection against reinfection with an efficacy of ~95%. This points to development of robust immunity following primary infection, which lasts for at least seven months. These findings suggest that prioritizing vaccination for those who are antibody-negative, as long as doses of the vaccine remain in short supply, could enhance the health, societal, and economic gains attained by vaccination.
Funding
The authors are grateful for support from the Biomedical Research Program, the Biostatistics, Epidemiology, and Biomathematics Research Core, and the Genomics Core, all at Weill Cornell Medicine-Qatar, as well as for support provided by the Ministry of Public Health and Hamad Medical Corporation. The authors are also grateful for support from the Qatar Genome Programme for supporting the viral genome sequencing. The statements made herein are solely the responsibility of the authors.
Author contributions
LJA conceived and designed the study, led the statistical analyses, and co-wrote the first draft of the article. HC contributed to study design, performed the data analyses, and co-wrote the first draft of the article. HC and LJA had access to raw data. JAM led the viral genome sequencing analyses and AAA, YAM, and SY conducted these analyses. All authors contributed to data collection and acquisition, database development, discussion and interpretation of the results, and to the writing of the manuscript. All authors have read and approved the final manuscript.
Data sharing
All relevant data are available within the manuscript and its supplementary materials.
Declaration of Competing Interest
We declare no competing interests.
Acknowledgements
We acknowledge the many dedicated individuals at Hamad Medical Corporation, the Ministry of Public Health, the Primary Health Care Corporation, and the Qatar Biobank for their diligent efforts and contributions to make this study possible.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.100861.
Appendix. Supplementary materials
References
- 1.World Health Organization (WHO). WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11-march-2020. Accessed on March 14, 2020.
- 2.De Walque D, Friedman J, Gatti RV, Mattoo A. How two tests can help contain COVID-19 and revive the economy. Available from: http://documents.worldbank.org/curated/en/766471586360658318/pdf/How-Two-Tests-Can-Help-Contain-COVID-19-and-Revive-the-Economy.pdf. Accessed on April 16, 2020. Research & Policy Briefs, World Bank Malaysia Hub 2020.
- 3.Kaplan J, Frias L, McFall-Johnsen M. A third of the global population is on coronavirus lockdown. Available from: https://www.businessinsider.com.au/countries-on-lockdown-coronavirus-italy-2020-3. Accessd on: April 25, 2020. Business Insider Australia 2020.
- 4.Nicola M., Alsafi Z., Sohrabi C. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg. 2020;78:185–193. doi: 10.1016/j.ijsu.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abu-Raddad L.J., Chemaitelly H., Malek J.A. Assessment of the risk of SARS-CoV-2 reinfection in an intense re-exposure setting. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1846. ciaa1846. doi: 10.1093/cid/ciaa1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tillett R.L., Sevinsky J.R., Hartley P.D. Genomic evidence for reinfection with SARS-CoV-2: a case study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.To K.K., Hung I.F., Ip J.D. COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Elslande J., Vermeersch P., Vandervoort K. Symptomatic SARS-CoV-2 reinfection by a phylogenetically distinct strain. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prado-Vivar B., Becerra-Wong M., Guadalupe J.J. COVID-19 re-infection by a phylogenetically distinct SARS-CoV-2 variant, first confirmed event in South America. SSRN. 2020 (published online Sept 8.) (preprint) https://doi.org/10.2139/ssrn.3686174. [Google Scholar]
- 10.Planning and Statistics Authority- State of Qatar. Qatar Monthly Statistics. Available from: https://www.psa.gov.qa/en/pages/default.aspx. Accessed on: may 26,2020. 2020.
- 11.Planning and Statistics Authority-State of Qatar. The Simplified Census of Population, Housing & Establishments. Available from: https://www.psa.gov.qa/en/statistics/Statistical%20Releases/Population/Population/2018/Population_social_1_2018_AE.pdf. Accessed on: April 2, 2020. 2019.
- 12.Abu-Raddad L.J., Chemaitelly H., Ayoub H.H. Characterizing the Qatar advanced-phase SARS-CoV-2 epidemic. Sci Rep. 2021;11(1):6233. doi: 10.1038/s41598-021-85428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al Kuwari H.M., Abdul Rahim H.F., Abu-Raddad L.J. Epidemiological investigation of the first 5685 cases of SARS-CoV-2 infection in Qatar, 28 February–18 April 2020. BMJ Open. 2020;10(10) doi: 10.1136/bmjopen-2020-040428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Thani M.H., Farag E., Bertollini R. Seroprevalence of SARS-CoV-2 infection in the craft and manual worker population of Qatar. medRxiv. 2020 2020.11.24.20237719 (non-peer-reviewed preprint) [Google Scholar]
- 15.Ayoub H.H., Chemaitelly H., Seedat S. Mathematical modeling of the SARS-CoV-2 epidemic in Qatar and its impact on the national response to COVID-19. J Glob Health. 2021;11:05005. doi: 10.7189/jogh.11.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeremijenko A., Chemaitelly H., Ayoub H.H. Evidence for and level of herd immunity against SARS-CoV-2 infection: the ten-community study. Emerging Infectious Diseases (in press) 2021 [Google Scholar]
- 17.Coyle P.V., Chemaitelly H., Kacem M.A.B.H. SARS-CoV-2 seroprevalence in the urban population of Qatar: an analysis of antibody testing on a sample of 112,941 individuals. medRxiv. 2021 doi: 10.1016/j.isci.2021.102646. 2021.01.05.21249247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polack F.P., Thomas S.J., Kitchin N. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020 doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson L.A., Anderson E.J., Rouphael N.G. An mRNA vaccine against SARS-CoV-2 - preliminary report. N Engl J Med. 2020;383(20):1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voysey M., Clemens S.A.C., Madhi S.A. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2020 doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayoub HH, Chemaitelly H, Makhoul M, et al. Epidemiological impact of prioritizing SARS-CoV-2 vaccination by antibody status: mathematical modeling analyses. BMJ Innov; Epub ahead of print: [31 March 2021]. doi: 10.1136/bmjinnov-2021-000677. [DOI] [PubMed]
- 22.Hamad Medical Corporation. National SARS-CoV-2 PCR testing, infection severity, and hospitalization database. 2020.
- 23.World Health Organization. Clinical management of COVID-19. Available from: https://www.who.int/publications-detail/clinical-management-of-covid-19. Accessed on: May 31st 2020.
- 24.World Health Organization. International guidelines for certification and classification (coding) of COVID-19 as cause of death. Available from: https://www.who.int/classifications/icd/Guidelines_Cause_of_Death_COVID-19-20200420-EN.pdf?ua=1. Document Number: WHO/HQ/DDI/DNA/CAT. Accessed on June 1, 2020.
- 25.Oved K., Olmer L., Shemer-Avni Y. Multi-center nationwide comparison of seven serology assays reveals a SARS-CoV-2 non-responding seronegative subpopulation. EClinicalMedicine. 2020;29 doi: 10.1016/j.eclinm.2020.100651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalikiri M.K.R., Hasan M.R., Mirza F., Xaba T., Tang P., Lorenz S. High-throughput extraction of SARS-CoV-2 RNA from nasopharyngeal swabs using solid-phase reverse immobilization beads. medRxiv. 2020 2020.04.08.20055731. [Google Scholar]
- 27.Sethuraman N., Jeremiah S.S., Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020;323(22):2249–2251. doi: 10.1001/jama.2020.8259. [DOI] [PubMed] [Google Scholar]
- 28.Singanayagam A., Patel M., Charlett A. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill. 2020;25(32) doi: 10.2807/1560-7917.ES.2020.25.32.2001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drew R.J., O'Donnell S., LeBlanc D., McMahon M., Natin D. The importance of cycle threshold values in interpreting molecular tests for SARS-CoV-2. Diagn Microbiol Infect Dis. 2020;98(3) doi: 10.1016/j.diagmicrobio.2020.115130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wajnberg A., Mansour M., Leven E. Humoral response and PCR positivity in patients with COVID-19 in the New York City region, USA: an observational study. Lancet Microbe. 2020;1(7):e283–e2e9. doi: 10.1016/S2666-5247(20)30120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cha S, Smith J. Explainer: South Korean findings suggest 'reinfected' coronavirus cases are false positives. Reuters Available from: https://wwwreuterscom/article/us-health-coronavirus-southkorea-explain-idUSKBN22J0HR. Accessed on: November 24, 2020.
- 32.Bo-gyung K. Tests in recovered patients found false positives, not reinfections, experts say. The Korea Herald Available from: http://wwwkoreaheraldcom/viewphp?ud=20200429000724. Accessed on November 24, 2020.
- 33.StataCorp . College station. Stata Corporation; TX: 2019. Statistical Software: release 16.1. [Google Scholar]
- 34.Weill Cornell Medicine in Qatar. SARS-CoV-2 reinfection in a cohort of 43,000 antibody-positive individuals followed for up to 35 weeks. https://www.ncbi.nlm.nih.gov/bioproject/PRJNA698478/. https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA699251/. 2021.
- 35.Abu-Raddad L.J., Chemaitelly H., Malek J.A. Two prolonged viremic SARS-CoV-2 infections with conserved viral genome for two months. Infect Genet Evol. 2020;88 doi: 10.1016/j.meegid.2020.104684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korber B., Fischer W.M., Gnanakaran S. Tracking changes in SARS-CoV-2 Spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182(4) doi: 10.1016/j.cell.2020.06.043. 812-27 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grubaugh N.D., Hanage W.P., Rasmussen A.L. Making sense of mutation: what D614G means for the COVID-19 pandemic remains unclear. Cell. 2020;182(4):794–795. doi: 10.1016/j.cell.2020.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Planning and Statistics Authority- State of Qatar. Labor force sample survey. Available from: https://www.psa.gov.qa/en/statistics/Statistical%20Releases/Social/LaborForce/2017/statistical_analysis_labor_force_2017_En.pdf. Accessed on: May 01, 2020. 2017.
- 39.Seedat S., Chemaitelly H., Ayoub H. SARS-CoV-2 infection hospitalization, severity, criticality, and fatality rates. medRxiv. 2020 doi: 10.1038/s41598-021-97606-8. 2020112920240416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansen C.H., Michlmayr D., Gubbels S.M., Molbak K., Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet. 2021 doi: 10.1016/S0140-6736(21)00575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lumley S.F., O'Donnell D., Stoesser N.E. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2020 doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harvey R.A., Rassen J.A., Kabelac C.A. Association of SARS-CoV-2 seropositive antibody test with risk of future infection. JAMA Intern Med. 2021 doi: 10.1001/jamainternmed.2021.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall V., Foulkes S., Charlett A. Do antibody positive healthcare workers have lower SARS-CoV-2 infection rates than antibody negative healthcare workers? Large multi-centre prospective cohort study (the SIREN study), England: june to November 2020. medRxiv. 2021 2021.01.13.21249642. [Google Scholar]
- 44.Pilz S., Chakeri A., Ioannidis J.P. SARS-CoV-2 re-infection risk in Austria. Eur J Clin Invest. 2021;51(4):e13520. doi: 10.1111/eci.13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leidi A., Koegler F., Dumont R. Risk of reinfection after seroconversion to SARS-CoV-2: a population-based propensity-score matched cohort study. medRxiv. 2021 doi: 10.1093/cid/ciab495. 2021.03.19.21253889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheehan M.M., Reddy A.J., Rothberg M.B. Reinfection rates among patients who previously tested positive for COVID-19: a retrospective cohort study. medRxiv. 2021 doi: 10.1093/cid/ciab234. 2021.02.14.21251715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dan J.M., Mateus J., Kato Y. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021 doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.The Roche Group. Roche's COVID-19 antibody test receives FDA Emergency Use Authorization and is available in markets accepting the CE mark. Available from: https://www.roche.com/media/releases/med-cor-2020-05-03.htm. Accessed on: June 5, 2020.
- 49.Public Health England. Evaluation of Roche Elecsys AntiSARS-CoV-2 serology assay for the detection of anti-SARS-CoV-2 antibodies. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/891598/Evaluation_of_Roche_Elecsys_anti_SARS_CoV_2_PHE_200610_v8.1_FINAL.pdf. Accessed on June 5, 2020.
- 50.Boyton R.J., Altmann D.M. Risk of SARS-CoV-2 reinfection after natural infection. Lancet. 2021 doi: 10.1016/S0140-6736(21)00662-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.European Centre for Disease Prevention and Control. Rapid increase of a SARS-CoV-2 variant with multiple spike protein mutations observed in the United Kingdom –20 December 2020. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/SARS-CoV-2-variant-multiple-spike-protein-mutations-United-Kingdom.pdf. Accessed on: January 7, 2021. ECDC: Stockholm, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.