Abstract
Background
Basic studies suggest that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection can affect chronic rhinosinusitis (CRS), but there is unclear real-world evidence regarding the association of underlying CRS with the risk for SARS-CoV-2 infection and severe coronavirus disease 19 (COVID-19).
Objective
We aimed to determine whether CRS is associated with increased risk for SARS-CoV-2 infection and severe COVID-19.
Methods
Altogether, 219,959 adult patients who tested for SARS-CoV-2 in South Korea from January 1 to May 15, 2020 (excluding self-referral) were identified in this nested case-control study with propensity score matching. Data on SARS-CoV-2 test results and COVID-19 worsened outcomes (ie, the need for oxygen therapy, intensive care, or mechanical ventilation, and death) were obtained from the Health Insurance Review and Assessment Service of Korea.
Results
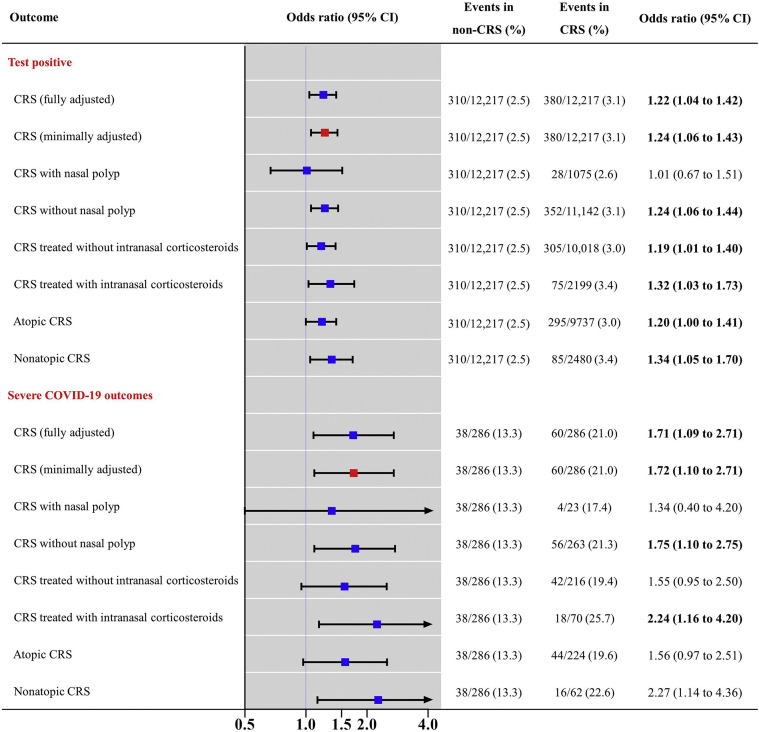
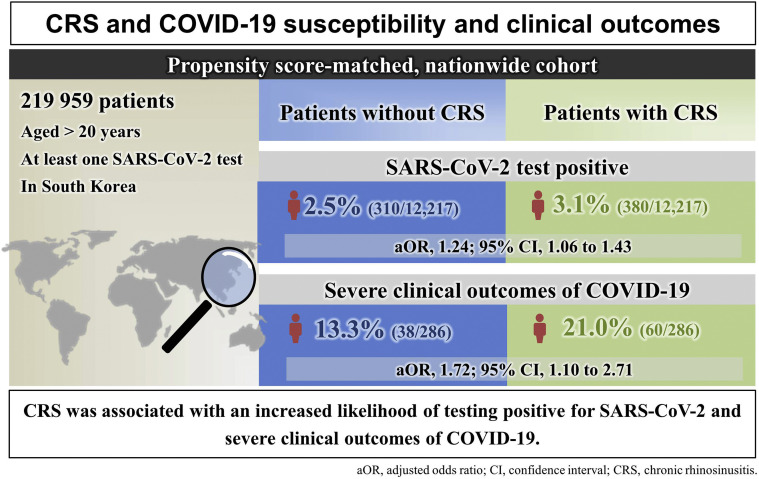
In this matched cohort, 380 of 12,217 patients with CRS (3.1%) tested positive for SARS-CoV-2 infection, compared with 310 patients without CRS (2.5%; adjusted odds ratio = 1.22; 95% confidence interval, 1.04-1.42). Moreover, 60 of 286 COVID-19 patients with CRS (21.0%) had severe COVID-19 outcomes, compared with 38 without CRS (13.3%; adjusted odds ratio = 1.71; 95% confidence interval, 1.09-2.71). Subgroup analysis identified that CRS patients with an absence of nasal polyps, prior intranasal corticosteroid use, or nonatopic type had a greater risk for SARS-CoV-2 infection and severe COVID-19 outcomes.
Conclusions
In patients with CRS, prior intranasal corticosteroid use, the absence of nasal polyps, or nonatopic type was associated with increased risk for SARS-CoV-2 infection and severe COVID-19 in the Korean nationwide cohort. Clinicians should be cautious in determining prognosis and care for patients with CRS amid the COVID-19 pandemic.
Key words: Severe acute respiratory syndrome 2, COVID-19, Chronic rhinosinusitis, Nasal polyp, Intranasal corticosteroids
Abbreviations used: ACE2, Angiotensin converting enzyme II; aOR, Adjusted odds ratio; CI, Confidence interval; COVID-19, Coronavirus disease 2019; CRS, Chronic rhinosinusitis; CRSsNP, Chronic rhinosinusitis without nasal polyp; CRSwNP, Chronic rhinosinusitis with nasal polyp; ICD-10, International Classification of Disease, 10th revision; IFN, Interferon; KCDC, Korea Centers for Disease Control and Prevention; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; SMD, Standardized mean difference; TMPRSS2, Transmembrane serine protease 2
What is already known about this topic? Chronic rhinosinusitis (CRS) is one of the most common upper airway inflammatory diseases and may be associated with epithelial barrier dysfunction, bacterial colonization, exaggerated immune response, and specific antibody deficiency. Basic studies suggest that CRS may increase coronavirus disease susceptibility or severity; however, the potential association of CRS with coronavirus disease 2019 (COVID-19) susceptibility or severity has not been demonstrated.
What does this article add to our knowledge? In our large-scale, population-based nationwide cohort in South Korea, CRS was associated with an increased risk for severe acute respiratory syndrome coronavirus 2 infection and severe COVID-19. In particular, among patients with CRS, prior intranasal corticosteroid use, the absence of nasal polyps, or nonatopic type were associated with a greater risk for severe acute respiratory syndrome coronavirus 2 infection, and severe COVID-19 outcomes.
How does this study impact current management guidelines? Taken together, our findings suggest that clinicians should be cautious in assessing the prognosis and determining care for patients with CRS amid the COVID-19 pandemic.
Introduction
A global pandemic of coronavirus disease 2019 (COVID-19) began in December 2019, when the pathogen of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first identified in Wuhan, China.1, 2, 3 Severe acute respiratory syndrome coronavirus 2 invades host cells by binding its spike proteins to receptor-angiotensin converting enzyme II (ACE2)3 and by their priming and fusion with the cell membrane by host transmembrane serine protease 2 (TMPRSS2).4 In addition to the different expression levels or affinities of viral entry factors, host immunity functions could influence the infectivity or severity of COVID-19.4 Thus, asthma,2 allergic rhinitis,2 chronic obstructive pulmonary disease,5 , 6 and immune-compromised status7 have been reported to be potentially associated with increased susceptibility to or severity of COVID-19.
In addition to the lower airway, COVID-19 has been suggested to have a high impact on the upper airway.8 The viral load of SARS-CoV-2 was found to be higher in nasal swabs than in throat swabs in both symptomatic and asymptomatic patients.9 Furthermore, in healthy human tissues, higher expression levels of viral entry-associated genes ACE2 and TMPRSS2 were observed in nasal epithelial cells than in lower respiratory tract, corneal, and intestinal epithelial cells.10 , 11 In the respiratory tract, a gradient was found in ACE2 expression and SARS-CoV-2 infection levels from the nose to the distal pulmonary epithelium, which suggests that SARS-CoV-2 affinity to the respiratory epithelium combined with inhalation-related viral spreading could explain the pathogenesis of COVID-19.12
Chronic rhinosinusitis (CRS) is one of the most common upper airway inflammatory diseases and could be associated with epithelial barrier dysfunction, bacterial colonization, exaggerated immune response, and specific antibody deficiency.13 , 14 Owing to the upper airway inflammatory condition, epithelial barrier dysfunction, and high expression levels of viral entry genes, CRS may increase COVID-19 susceptibility or severity. Previous preliminary studies were equivocal,11 , 15 and the potential association of CRS with COVID-19 susceptibility or severity has not yet been clarified.
In this nested case-control study in a Korean nationwide cohort, we investigated the potential association of CRS with SARS-CoV-2 infection (SARS-CoV-2 positive test result) and COVID-19 severity (COVID-19 worsened outcomes; including oxygen therapy, intensive care unit admission, use of mechanical ventilation, or death).
Methods
The data were obtained from the Korea Centers for Disease Control and Prevention (KCDC), the Health Insurance Review and Assessment Service of Korea, and the Ministry of Health and Welfare in South Korea. As previously described, the nationwide cohort2 , 3 , 16 , 17 had the following characteristics: (1) the Korean government provided a complimentary medical service for all COVID-19 patients; (2) the data consisted of health insurance claims over the previous 3.5 years (January 1, 2017 to May 15, 2020) of all patients who were tested for SARS-CoV-2 through KCDC or medical referrals (excluding self-referral); and (3) this health insurance claim-based cohort was kept anonymous to guarantee the confidentiality of COVID-19 infection status.
The study protocol was approved by the Institutional Review Board of Sejong University (SJU-HR-E-2020-003). The requirement for written consent was waived by the ethics committee because of urgent medical needs to be met amid the COVID-19 pandemic.
Study population
All adults (aged ≥20 years) who were tested for SARS-CoV-2 in South Korea from January 1, 2020 to May 15, 2020 (total n = 219,959, excluding self-referral) were enrolled. The observation period was from January 1, 2017 to May 15, 2020, and the individual index date was defined as the date of the first SARS-CoV-2 test. The medical history was defined using International Classification of Disease 10th revision (ICD-10) codes and at least two inpatient or outpatient claims within 1 year, as previously described.2 , 3 The Charlson comorbidity index score was calculated, and the region of residence was classified as rural or urban, as previously described.2 , 3 , 18 , 19 The use of medications was defined based on medications taken within 6 months before the individual index date.2 , 3
Exposure
We defined CRS using the ICD-10 code (J32) with at least two claims within 1 year and the use of head and neck computed tomography. We defined CRS with nasal polyp (CRSwNP) using ICD-10 codes J32 plus J33, and CRS without nasal polyp (CRSsNP) using ICD-10 code J32 alone.20 Atopic CRS was defined as CRS with another allergic disease (asthma, atopic dermatitis, or allergic rhinitis), and nonatopic CRS as CRS with no other allergic disease.2 , 21 , 22
Outcomes
The primary outcome was a positive SARS-CoV-2 test result (laboratory-confirmed COVID-19) from real-time reverse transcription-polymerase chain reaction assays of pharyngeal or nasal swabs performed according to the guidelines of the World Health Organization and KCDC.1, 2, 3 Secondary outcomes were severe COVID-19 outcomes, including the requirement of oxygen therapy, intensive care unit admission, use of mechanical ventilation, or death.3
Statistical analysis
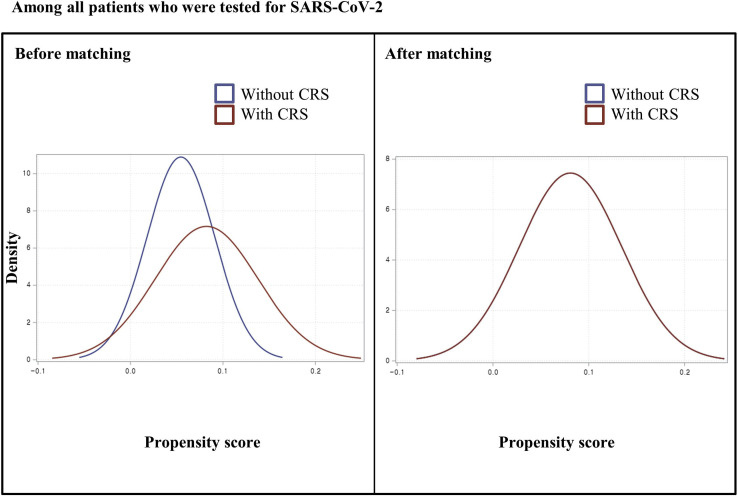
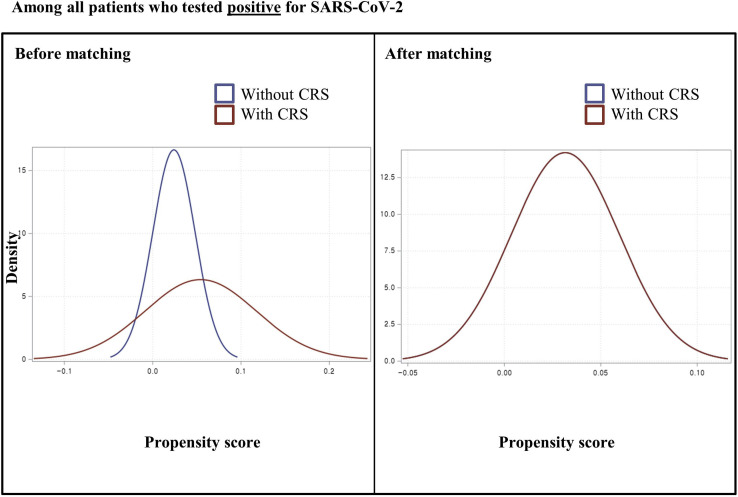
In the nationwide cohort, preexisting CRS was defined as the exposure; a positive SARS-CoV-2 test result or severe COVID-19 outcomes were defined as the outcomes. We performed the exposure propensity score matching method. Therefore, we used a 1:1 ratio greedy nearest-neighbor matching on the logit of the propensity score with the calipers less than 0.001 to balance the covariates of age (continuous); sex; region of residence (urban and rural); a history of diabetes mellitus, cardiovascular disease, cerebrovascular disease, chronic obstructive pulmonary disease, hypertension, or chronic kidney disease; Charlson comorbidity index (0, 1, and ≥2); and use of medication (aspirin, metformin, or statin). We used two rounds of propensity score matching between patients without CRS versus patients with CRS: (1) among all patients who were tested for SARS-CoV-2 (n = 219,959) and (2) among patients with COVID-19 (n = 7340). We confirmed the adequacy of the propensity score matching as assessed by the propensity score densities (see Figures E1 and E2 in this article's Online Repository at www.jaci-inpractice.org) and standardized mean differences (SMDs).2 , 21, 22, 23
Figure E1.
Density of propensity scores before and after matching among patients who received severe acute respiratory syndrome coronavirus 2 testing. CRS, chronic rhinosinusitis.
Figure E2.
Density of propensity scores before and after matching among coronavirus 19 patients. CRS, chronic rhinosinusitis.
Main analysis
Data were analyzed using Firth's bias-reduced logistic regression to reduce the small sample bias and adjusted odds ratios (aORs) with 95% confidence intervals (CIs) in each matched cohort, after adjusting for age; sex; region of residence; a history of diabetes mellitus, cardiovascular disease, cerebrovascular disease, chronic obstructive pulmonary disease, hypertension, chronic kidney disease, or nasal polyp; Charlson comorbidity index; and the use of medication (systemic or intranasal corticosteroids, aspirin, metformin, or statin). We performed several sensitivity analyses, applying stratification by nasal polyp (CRSwNP or CRSsNP), atopic phenotype (atopic CRS or nonatopic CRS), and prior intranasal corticosteroid use (CRS treated with or without intranasal corticosteroids). Statistical analyses were performed using SAS software (version 9.4, SAS Institute Inc, Cary, NC) and R software (version 3.1.1, R Foundation, Vienna, Austria).17 A two-tailed P value less than .05 was considered statistically significant.
Results
Full unmatched cohort
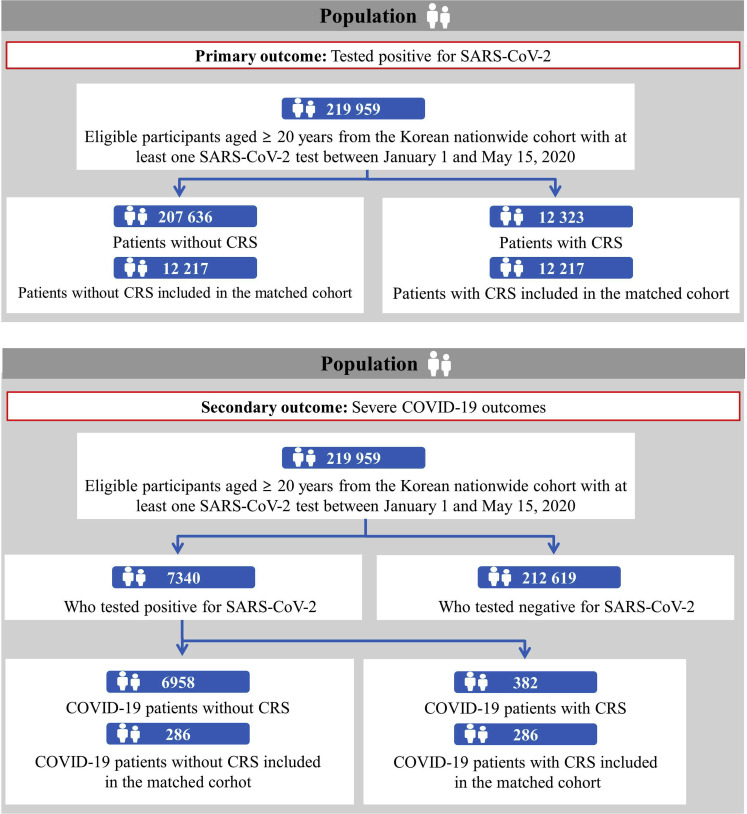
In the entire study population (n = 219,959), we identified 207,636 patients without CRS (94.4%) and 12,323 with CRS (5.6%) (Figure 1 ). Among 7340 patients with laboratory-confirmed COVID-19, there were 6958 without CRS (94.8%) and 382 with CRS (5.2%). The positivity rate of the SARS-CoV-2 test was 3.3% (7340 of 219,959) in the entire study population, 2.3% in patients without CRS (6958 of 207,636), and 3.1% in patients with CRS (382 of 12,323).
Figure 1.
Participant flowchart. COVID-19, coronavirus disease; CRS, chronic rhinosinusitis; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Matched cohort
Table I reports the baseline characteristics of all patients tested for SARS-CoV-2 and all COVID-19 patients stratified by CRS. In the matched cohort (n = 24,434), baseline covariates were balanced between patients with and without CRS (Table II and Figure 2 ) (all SMDs <0.05). Patients with CRS had a 22% greater risk for SARS-CoV-2 infection after fully adjusting for confounding factors compared with patients without CRS (3.1% vs 2.5%; fully aOR = 1.22; 95% CI, 1.04-1.42). The stratification analysis found that patients with CRS had a 61% greater risk for severe COVID-19 compared with those without CRS (see Table E1 in this article's Online Repository at www.jaci-inpractice.org) (0.7% vs 0.4%; fully aOR = 1.61; 95% CI, 1.12-2.29).
Table I.
Baseline characteristics of all patients who underwent severe acute respiratory syndrome coronavirus 2 testing and those with laboratory-confirmed coronavirus disease 2019 in a Korean nationwide cohort
| Characteristic | All patients who underwent severe acute respiratory syndrome coronavirus 2 testing |
Patients with laboratory-confirmed coronavirus disease 2019 |
||||
|---|---|---|---|---|---|---|
| Total | Without CRS | With CRS | Total | Without CRS | With CRS | |
| Total, n (%) | 219,959 (100.0) | 207,636 (94.4) | 12,323 (5.6) | 7340 (100.0) | 6958 (94.8) | 382 (5.2) |
| Age, y (SD) | 49.0 (19.9) | 49.1 (19.9) | 55.0 (19.6) | 47.1 (19.0) | 46.7 (19.0) | 56.1 (17.1) |
| Sex, n (%) | ||||||
| Male | 104,331 (47.4) | 98,444 (47.4) | 5887 (47.8) | 2970 (40.5) | 2818 (40.5) | 152 (39.8) |
| Female | 115,628 (52.6) | 109,192 (52.6) | 6436 (52.2) | 4370 (59.5) | 4140 (59.5) | 230 (60.2) |
| Region of residence, n (%) | ||||||
| Rural | 96,315 (43.8) | 91,252 (44.0) | 5063 (41.1) | 3535 (48.2) | 3388 (48.7) | 147 (38.5) |
| Urban | 123,644 (56.2) | 116,384 (56.1) | 7260 (58.9) | 3805 (51.8) | 3570 (51.3) | 235 (61.5) |
| History of diabetes mellitus, n (%) | 38,396 (17.5) | 34,715 (16.7) | 3681 (29.9) | 951 (13.0) | 845 (12.1) | 106 (27.7) |
| History of cardiovascular disease, n (%) | 32,864 (14.9) | 29,268 (14.1) | 3596 (29.2) | 505 (6.9) | 433 (6.2) | 72 (18.8) |
| History of cerebrovascular disease, n (%) | 22,134 (10.1) | 19,307 (9.3) | 2827 (22.9) | 458 (6.2) | 381 (5.5) | 77 (20.2) |
| History of chronic obstructive pulmonary disease, n (%) | 18,636 (8.5) | 16,157 (7.8) | 2479 (20.1) | 350 (4.8) | 280 (4.0) | 70 (18.3) |
| History of hypertension, n (%) | 66,281 (30.1) | 60,710 (29.2) | 5571 (45.2) | 1638 (22.3) | 1501 (21.6) | 137 (35.9) |
| History of chronic kidney disease, n (%) | 15,360 (7.0) | 13,762 (6.6) | 1598 (13.0) | 254 (3.5) | 223 (3.2) | 31 (8.1) |
| History of nasal polyp, n (%) | 3069 (1.4) | 1985 (1.0) | 1084 (8.8) | 60 (0.8) | 32 (0.5) | 28 (7.3) |
| Charlson comorbidity index, n (%) | ||||||
| 0 | 120,433 (54.8) | 116,627 (56.2) | 3806 (30.9) | 4902 (66.8) | 4758 (68.4) | 144 (37.7) |
| 1 | 25,938 (11.8) | 24,165 (11.6) | 1773 (14.4) | 809 (11.0) | 754 (10.8) | 55 (14.4) |
| ≥2 | 73,588 (33.5) | 66,844 (32.2) | 6744 (54.7) | 1629 (22.2) | 1446 (20.8) | 183 (47.9) |
| Current use of medication, n (%) | ||||||
| Aspirin | 15,706 (7.1) | 14,320 (6.9) | 1386 (11.3) | 310 (4.2) | 272 (3.9) | 38 (9.9) |
| Metformin | 19,252 (8.8) | 17,820 (8.6) | 1432 (11.6) | 550 (7.5) | 496 (7.1) | 54 (14.1) |
| Statin | 41,011 (18.6) | 37,482 (18.1) | 3529 (28.6) | 1078 (14.7) | 949 (13.6) | 129 (33.8) |
| Systemic corticosteroids | 80,943 (36.8) | 74,303 (35.8) | 6640 (53.9) | 2054 (28.0) | 1876 (27.0) | 178 (46.6) |
| Intranasal corticosteroids | 10,384 (4.7) | 8164 (3.9) | 2220 (18.0) | 263 (3.6) | 187 (2.7) | 76 (19.9) |
CRS, chronic rhinosinusitis.
Table II.
One-to-one propensity score–matched baseline characteristics and adjusted ORs (95% confidence intervals) for association of severe acute respiratory syndrome coronavirus 2 test positivity with CRS among all patients who had severe acute respiratory syndrome coronavirus 2 testing
| Characteristic | Without CRS | With CRS | SMD∗ |
|---|---|---|---|
| Total, n (%) | 12,217 | 12,217 | |
| Age, y (SD) | 55.5 (19.8) | 54.9 (19.6) | 0.030 |
| Sex, n (%) | <0.001 | ||
| Male | 5848 (47.9) | 5847 (47.9) | |
| Female | 6369 (52.1) | 6370 (52.1) | |
| Region of residence, n (%) | 0.006 | ||
| Rural | 4996 (40.9) | 5031 (41.2) | |
| Urban | 7221 (59.1) | 7186 (58.8) | |
| History of diabetes mellitus, n (%) | 3636 (29.8) | 3600 (29.5) | 0.007 |
| History of cardiovascular disease, n (%) | 3549 (29.1) | 3504 (28.7) | 0.009 |
| History of cerebrovascular disease, n (%) | 2673 (21.9) | 2730 (22.4) | 0.013 |
| History of chronic obstructive pulmonary disease, n (%) | 2412 (19.7) | 2375 (19.4) | 0.009 |
| History of hypertension, n (%) | 5565 (45.6) | 5484 (44.9) | 0.014 |
| History of chronic kidney disease, n (%) | 1532 (12.5) | 1546 (12.7) | 0.004 |
| Charlson comorbidity index, n (%) | 0.009 | ||
| 0 | 3793 (31.1) | 3806 (31.2) | |
| 1 | 1701 (13.9) | 1772 (14.5) | |
| ≥2 | 6723 (55.0) | 6639 (54.4) | |
| Current use of medication, n (%) | |||
| Aspirin | 1209 (9.9) | 1358 (11.1) | 0.042 |
| Metformin | 1390 (11.4) | 1414 (11.6) | 0.007 |
| Statin | 3461 (28.3) | 3466 (28.4) | <0.001 |
| Coronavirus disease 2019, n (%) | 310 (2.5) | 380 (3.1) | |
| Minimally adjusted OR† (95% CI) | 1.0 (reference) | 1.24 (1.06-1.43) | |
| Fully adjusted OR‡ (95% CI) | 1.0 (reference) | 1.22 (1.04-1.42) |
CRS, chronic rhinosinusitis; SMD, standardized mean difference; OR, odds ratio.
Bolded data indicate significant differences in the regression model (P < .05).
An SMD less than 0.1 indicates no major imbalance. All SMD values were less than 0.05 in the propensity score–matched cohort.
Minimally adjusted for age and sex.
Fully adjusted for age; sex; region of residence; history of diabetes mellitus, cardiovascular disease, cerebrovascular disease, chronic obstructive pulmonary disease, hypertension, chronic kidney disease, or nasal polyp; Charlson comorbidity index; and previous use of medication (systemic or intranasal corticosteroids, aspirin, metformin, or statin).
Figure 2.
Propensity score-matched association of chronic rhinosinusitis (CRS) with coronavirus disease 19 (COVID-19) susceptibility and severity. Firth's bias-reduced logistic regression analysis ORs with 95% CIs are presented. Severe COVID-19 outcomes were the need for oxygen therapy, admission to the intensive care unit, invasive ventilation, or death. The x-axis indicates a log-scale; red dots indicate minimal adjustment and blue dots indicate full adjustment. CI, confidence interval; COPD, chronic obstructive pulmonary disease; OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Next, patients with confirmed COVID-19 were matched for all baseline covariates between those with and without CRS (Table III and Figure 2) (all SMDs <0.1), except for a history of chronic obstructive pulmonary disease (SMD = 0.100). Patients with CRS had a 71% greater risk for severe COVID-19 outcomes after fully adjusting for confounding factors compared with patients without CRS (21.0% vs 13.3%; fully aOR = 1.09; 95% CI, 1.09-2.71).
Table III.
One-to-one propensity score–matched baseline characteristics and adjusted ORs (95% confidence intervals) for association of severe coronavirus disease 2019 with CRS among patients with laboratory-confirmed coronavirus disease 2019
| Characteristic | Without CRS | With CRS | SMD∗ |
|---|---|---|---|
| Total, n (%) | 286 | 286 | |
| Age, y (SD) | 54.7 (16.5) | 55.7 (16.4) | 0.061 |
| Sex, n (%) | 0.058 | ||
| Male | 92 (32.2) | 100 (35.0) | |
| Female | 194 (67.8) | 186 (65.0) | |
| Region of residence, n (%) | 0.014 | ||
| Rural | 122 (42.7) | 124 (43.4) | |
| Urban | 164 (57.3) | 162 (56.6) | |
| History of diabetes mellitus, n (%) | 63 (22.0) | 63 (22.0) | <0.001 |
| History of cardiovascular disease, n (%) | 26 (9.1) | 23 (8.0) | 0.039 |
| History of cerebrovascular disease, n (%) | 25 (8.7) | 23 (8.0) | 0.025 |
| History of COPD, n (%) | 14 (4.9) | 21 (7.3) | 0.100 |
| History of hypertension, n (%) | 89 (31.1) | 89 (31.1) | <0.001 |
| History of chronic kidney disease, n (%) | 13 (4.5) | 17 (5.9) | 0.063 |
| Charlson comorbidity index, n (%) | 0.038 | ||
| 0 | 138 (48.3) | 133 (46.5) | |
| 1 | 44 (15.4) | 47 (16.4) | |
| ≥2 | 104 (36.4) | 106 (37.1) | |
| Current use of medication, n (%) | |||
| Aspirin | 22 (7.7) | 18 (6.3) | 0.049 |
| Metformin | 25 (8.7) | 29 (10.1) | 0.048 |
| Statin | 64 (22.4) | 66 (23.1) | 0.017 |
| Severe outcomes of coronavirus disease 2019†, n (%) | 38 (13.3) | 60 (21.0) | |
| Minimally adjusted OR‡ (95% CI) | 1.0 (Reference) | 1.72 (1.10-2.71) | |
| Fully adjusted OR§ (95% CI) | 1.0 (Reference) | 1.71 (1.09-2.71) |
COPD, chronic obstructive pulmonary disease; CRS, chronic rhinosinusitis; OR, odds ratio; SMD, standardized mean difference.
Bolded data indicate significant differences in the regression model (P < .05).
An SMD less than 0.1 indicates no major imbalance. All SMD values were less than 0.1 in the propensity score–matched cohort, except a history of COPD.
Severe outcomes of coronavirus disease 2019 were the need for oxygen therapy, admission to the intensive care unit, invasive ventilation, or death.
Minimally adjusted for age and sex.
Fully adjusted for age; sex; region of residence; history of diabetes mellitus, cardiovascular disease, cerebrovascular disease, COPD, hypertension, chronic kidney disease, or nasal polyp; Charlson comorbidity index; and previous use of medication (systemic or intranasal corticosteroids, aspirin, metformin, or statin).
Propensity score-matched subgroup analysis
Table IV reports the subgroup analysis of the effect of nasal polyps, intranasal corticosteroid use, and atopic status. Patients with CRSwNP did not experience a greater risk for SARS-CoV-2 infection (fully aOR = 1.01; 95% CI, 0.67-1.51) and severe COVID-19 outcomes (fully aOR = 1.34; 95% CI, 0.40-4.20) compared with patients without CRS. Patients with CRSsNP had a greater risk for SARS-CoV-2 infection (fully aOR = 1.24; 95% CI, 1.06-1.44) and severe COVID-19 outcomes (fully aOR = 1.75; 95% CI, 1.10-2.75) compared with patients without CRS.
Table IV.
Propensity score–matched subgroup analyses for association of risk for positive SARS-CoV-2 test result with CRS by nasal polyp, prior intranasal corticosteroid use, and atopic phenotype among all patients who underwent SARS-CoV-2 testing, and for association of risk for COVID-19 worse outcomes with CRS by nasal polyp, prior intranasal corticosteroid use, and atopic phenotype among patients with laboratory-confirmed COVID-19
| Exposure | Patients who underwent SARS-CoV-2 testing (propensity score–matched n=24,434) |
Patients with laboratory-confirmed SARS-CoV-2 infection (propensity score–matched n=572) |
||
|---|---|---|---|---|
| COVID-19 |
Severe outcomes of COVID-19∗ |
|||
| Event number/total n (%) | Fully adjusted OR† (95% confidence interval) | Event number/total n (%) | Fully adjusted OR† (95% confidence interval) | |
| By nasal polyps | ||||
| None | 310/12,217 (2.5) | 1.0 (reference) | 38/286 (13.3) | 1.0 (reference) |
| CRS with nasal polyp | 28/1075 (2.6) | 1.01 (0.67-1.51) | 4/23 (17.4) | 1.34 (0.40-4.20) |
| CRS without nasal polyp | 352/11,142 (3.1) | 1.24 (1.06-1.44) | 56/263 (21.3) | 1.75 (1.10-2.75) |
| By use of intranasal corticosteroids | ||||
| None | 310/12,217 (2.5) | 1.0 (reference) | 38/286 (13.3) | 1.0 (reference) |
| CRS treated without intranasal corticosteroids | 305/10,018 (3.0) | 1.19 (1.01-1.40) | 42/216 (19.4) | 1.55 (0.95-2.50) |
| CRS treated with intranasal corticosteroids | 75/2199 (3.4) | 1.32 (1.03-1.73) | 18/70 (25.7) | 2.24 (1.16-4.20) |
| By atopic status | ||||
| None | 310/12,217 (2.5) | 1.0 (reference) | 38/286 (13.3) | 1.0 (reference) |
| Atopic CRS | 295/9737 (3.0) | 1.20 (1.00-1.41) | 44/224 (19.6) | 1.56 (0.97-2.51) |
| Nonatopic CRS | 85/2480 (3.4) | 1.34 (1.05-1.70) | 16/62 (22.6) | 2.27 (1.14-4.36) |
COVID-19, coronavirus disease 2019; CRS, chronic rhinosinusitis; OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Bolded data indicate significant differences in the regression model (P < .05).
Severe outcomes of COVID-19 were the need for oxygen therapy, admission to the intensive care unit, invasive ventilation, or death.
Fully adjusted for age; sex; region of residence; history of diabetes mellitus, cardiovascular disease, cerebrovascular disease, chronic obstructive pulmonary disease, hypertension, or chronic kidney disease or nasal polyp; Charlson comorbidity index; and previous use of medication (systemic or intranasal corticosteroids, aspirin, metformin, or statin).
Chronic rhinosinusitis patients treated with intranasal corticosteroids had a greater risk for SARS-CoV2 infection (aOR = 1.32; 95% CI 1.03-1.73) and severe COVID-19 outcomes (fully aOR = 2.24; 95% CI, 1.16-4.20) compared with CRS patients treated without intranasal corticosteroids (SARS-CoV-2 infection fully aOR = 1.19; 95% CI, 1.01-1.40; severe COVID-19 outcomes fully aOR = 1.55; 95% CI, 0.95-2.50). Patients with nonatopic CRS had a greater risk for SARS-CoV2 infection (aOR = 1.34; 95% CI 1.05-1.70) and severe COVID-19 outcomes (fully aOR = 2.27; 95% CI, 1.14-4.36) compared with patients with atopic CRS (SARS-CoV-2 infection fully aOR = 1.20; 95% CI, 1.00-1.41; P < .05; severe COVID-19 outcomes fully aOR = 1.56; 95% CI, 0.97-2.51).
Discussion
We investigated the risk for COVID-19 susceptibility and severity in patients with CRS in a large-scale, population-based nationwide cohort in South Korea (n = 219,959). Patients with CRS had an increased risk for COVID-19 susceptibility and severity compared with people without CRS (Figure 3 ). In particular, CRSsNP, CRS treated with intranasal corticosteroids, and nonatopic CRS conferred a greater risk for SARS-CoV-2 infection and COVID-19 severity, although the event numbers were small.
Figure 3.
Graphical abstract. aOR, adjusted odds ratio; CI, confidence interval; COVID-19, coronavirus disease 19; CRS, chronic rhinosinusitis; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Possible explanations of main results
To the authors’ knowledge, this is the first real-world study to investigate potential associations between a diagnosis of CRS and the risks for SARS-CoV-2 infection and severe COVID-19. Previously, CRS was reported to increase the risks for pneumonia, sepsis, and mortality.24 , 25 Those studies suggested that CRS could provide bacterial or viral reservoirs and reduce nitric oxide production in the sinus, implying impaired local host defense and increased susceptibility to secondary infection.24 , 25 It is known that CRS is associated with immune dysfunction, such as epithelial barrier dysfunction13 , 26 and specific antibody deficiency,14 which may contribute to increased COVID-19 infectivity and severity.
The increased infectivity to SARS-CoV-2 and severity of COVID-19 observed in patients with CRS might be explained by several plausible mechanisms. First, the impairment of sinonasal epithelial barrier functions could increase the exposure of patients with CRS to viral pathogens and their invasion. The mucociliary clearance of viral pathogens might be impeded in CRS patients. Sinonasal epithelial cells in CRS patients have been shown to exhibit morphologic and functional changes in basal cell proliferation, goblet cell hyperplasia, and the loss of ciliated cells.27 Second, patients whose CRS is caused by a viral infection may be more susceptible to viral coinfection, in particular with SARS-CoV-2. In a previous study,28 CRS patients showed a 2.9 times higher rate of viral infection in nasal lavage fluids and nasal mucosa compared with patients without CRS, and approximately 21.6% of patients with CRS showed coronavirus infection.
Third, the high expression levels of ACE2 and TMPRSS2 in the sinonasal cavity might have a role in the viral entry and transmission of SARS-CoV-2.10 , 12 , 29 The sinonasal cavity and the nasal epithelium are two regions with the highest ACE2 protein expression within the human respiratory tract.10 , 12 , 29 Therefore, viral retention in the sinonasal cavity caused by CRS could accelerate the intracellular entry of SARS-CoV-2 by binding the viral receptor to ACE2 and its cofactor to TMPRSS2. Fourth, local inflammatory responses in patients with CRS could modulate susceptibility to COVID-19. Impaired antiviral innate immune responses in sinonasal epithelial cells occur in CRS patients during inflammation.30 Sinonasal inflammatory responses can increase the expression of proinflammatory cytokines such as interferon (IFN) in CRS patients,31 which in turn can upregulate the expression of ACE2.32 Thus, local inflammation with increased IFN in CRS patients could induce ACE2-mediated SARS-CoV2 infection.32 Consistent with local inflammation in CRS that possibly contributes to the development of COVID-19, an inhibitor of interleukin-4 and interleukin-13 (dupilumab) has been reported potentially to alleviate the severity of COVID-19.33 , 34 Altogether, the combined effects of impaired barrier functions, viral coinfection, increased expression of viral entry-associated factors, and local inflammatory response might contribute to the increased susceptibility to SARS-CoV-2 infection and severity of COVID-19 observed in CRS patients.
In our subgroup analysis, patients with CRSsNP or nonatopic CRS showed a higher rate of infection with SARS-CoV-2 and increased severity of COVID-19 compared with patients with CRSwNP or atopic CRS. Patients with CRSwNP or nonatopic CRS may prominently exhibit type 2 immune responses with downregulation of IFNs, which in turn might downregulate ACE2 expression.8 An in vivo study of asthmatic rats with type 2 immune responses, which were given an ACE2 activator, demonstrated decreased expression of ACE2 together with the alleviation of altered airway responsiveness, eosinophilia, and inflammatory changes.35 , 36 Consistent with this observation, our previous real-world study2 suggested a greater risk for a positive SARS-CoV-2 test and severe COVID-19 in patients with nonallergic asthma compared with patients with allergic asthma. Furthermore, a recent study37 found that asthmatic patients with preexisting eosinophilia had a protective association from COVID-19–associated admission and mortality. Altogether, these studies suggest that a prominent type 2 immune response caused by nasal polyps may contribute to lower SARS-CoV-2 infectivity and COVID-19 severity compared with CRSsNP.
Interestingly, we found that prior use of nasal corticosteroids increased the risk for SARS-CoV-2 infection and the severity of COVID-19. Recent in vitro and in vivo studies38 suggest that inhaled corticosteroids may have beneficial or detrimental contributions to the altered susceptibility or severity of COVID-19. Because it is difficult to distinguish between the direct effect of using intranasal corticosteroids and more severe CRS requiring intranasal corticosteroids, the clinical significance of the current intranasal corticosteroid results is not clear.
Limitations and strengths
Several limitations should be considered when interpreting these results, mainly owing to a lack of sufficient information about the study population. In particular, CRS endotypes could not be differentiated because the current study used health insurance claims-based data with ICD-10 codes and lacked laboratory findings and biomarkers. Furthermore, the history of endoscopic sinus surgery was not considered. Because of the heterogeneous types, varying severity, and an inaccurate claims-based definition of CRS in this cohort, this study might have underestimated the relation between CRS and the infectivity of SARS-CoV-2 or severity of COVID-19. The classification of CRS patients according to the atopic status was based on the ICD-10 codes; thus, misdiagnosed cases or untreated cases may have been excluded in this study. Furthermore, ethnic differences in atopic CRS could explain the conflicting results regarding the association of atopic CRS with COVID-19.39 The relatively small study population of atopic patients and that of patients with nasal polyps limited the statistical power in this study. Furthermore, although we adjusted for comorbidities, including chronic obstructive pulmonary disease, other confounding factors such as smoking could exist.40 Second, CRS-related confounders might affect our main results. Although we used a large-scale nationwide cohort and a sophisticated matching technique to reduce bias, anosmia associated with CRS can cause more frequent SARS-CoV-2 testing, which may increase the selection bias.41 Also, CRS can cause sinonasal symptoms, which may make wearing masks less tolerable during the COVID-19 pandemic. Finally, the study population was limited to Koreans, and the association of CRS with the infectivity of SARS-CoV-2 or the severity of COVID-19 could reveal differences in other ethnic groups.42 In particular, type 2/atopic/eosinophilic nasal polyps are less common in Asians.39 The infectivity or severity of COVID-19 in CRS may not show a significant difference in Western countries owing to this geographic and ethnic difference in CRS endotypes.
Despite these limitations, this large-scale, population-based, nationwide cohort study evaluated the potential relation between CRS and SARS-CoV-2 infection and COVID-19 severity. We used a large sample and representative population (n = 219,959), sophisticated statistical techniques to identify accurate predictors of an outcome (propensity score matching to reduce potential confounders and selection bias and Firth bias correction to decrease the small sample bias), and a strict CRS definition (ICD code claims supported by head and neck computed tomography). During the current COVID-19 pandemic, this study provides evidence that CRS is potentially associated with SARS-CoV-2 infectivity and COVID-19 severity.
Conclusions
Chronic rhinosinusitis was associated with an increased risk for SARS-CoV-2 infection and severe COVID-19 in this large-scale, population-based nationwide cohort in South Korea. In particular, CRSsNP, CRS treated with intranasal corticosteroids, or nonatopic CRS was associated with a greater risk for SARS-CoV-2 infection and severe COVID-19 outcomes. Taken together, our findings suggest that clinicians should be cautious in assessing the prognosis and determining care for patients with CRS amid the COVID-19 pandemic.
Acknowledgments
The authors appreciate health care professionals dedicated to treating COVID-19 patients in Korea. The Ministry of Health and Welfare and the Health Insurance Review and Assessment Service of Korea is also acknowledged for sharing invaluable National Health Insurance claims data in a prompt manner.
Footnotes
This work was supported by a National Research Foundation of Korea grant funded by the Korean government (NRF2019R1G1A109977912). The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Conflicts of interest: The authors declare that they have no relevant conflicts of interest.
Online Repository.
Table E1.
Propensity score–matched subgroup analyses for association of risk for positive severe acute respiratory syndrome coronavirus 2 test results with chronic rhinosinusitis by COVID-19 outcome among all patients who underwent severe acute respiratory syndrome coronavirus 2 test (propensity score–matched n = 24,434)
| Event | Variable | None | CRS |
|---|---|---|---|
| COVID-19 | Event n/total n (%) | 310/12,217 (2.5) | 380/12,217 (3.1) |
| Fully adjusted OR‡ (95% CI) | 1.0 (reference) | 1.22 (1.04-1.42) | |
| Severe outcomes of COVID-19∗ | Event n/total n (%) | 50/12,217 (0.4) | 82/12,217 (0.7) |
| Fully adjusted OR‡ (95% CI) | 1.0 (reference) | 1.61 (1.12-2.29) | |
| Nonsevere outcomes of COVID-19† | Event n/total n (%) | 260/12,217 (2.1) | 298/12,217 (2.4) |
| Fully adjusted OR‡ (95% CI) | 1.0 (reference) | 1.13 (0.95-1.32) |
CI, confidence interval; COVID-19, coronavirus disease 2019; OR, odds ratio.
Bolded data indicate significant differences in the regression model (P < .05).
Severe outcomes of COVID-19 were the need for oxygen therapy, admission to the intensive care unit, invasive ventilation, or death.
Nonsevere outcomes of COVID-19 included COVID-19 patients, excluding severe COVID-19 patients.
Fully adjusted for age; sex; region of residence; history of diabetes mellitus, cardiovascular disease, cerebrovascular disease, chronic obstructive pulmonary disease, hypertension, chronic kidney disease, or nasal polyp; Charlson comorbidity index; and previous use of medication (aspirin, metformin, or statin, systemic corticosteroids, or intranasal corticosteroids).
References
- 1.Bae S.H., Shin H., Koo H.Y., Lee S.W., Yang J.M., Yon D.K. Asymptomatic transmission of SARS-CoV-2 on evacuation flight. Emerg Infect Dis. 2020;26:2705–2708. doi: 10.3201/eid2611.203353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang J.M., Koh H.Y., Moon S.Y., Yoo I.K., Ha E.K., You S., et al. Allergic disorders and susceptibility to and severity of COVID-19: a nationwide cohort study. J Allergy Clin Immunol. 2020;146:790–798. doi: 10.1016/j.jaci.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee S.W., Ha E.K., Yeniova A., Moon S.Y., Kim S.Y., Koh H.Y., et al. Severe clinical outcomes of COVID-19 associated with proton pump inhibitors: a nationwide cohort study with propensity score matching. Gut. 2021;70:76–84. doi: 10.1136/gutjnl-2020-322248. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan W.-J., Liang W.-H., Zhao Y., Liang H.-R., Chen Z.-S., Li Y.-M., et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: a Nationwide Analysis. Eur Respir J. 2020;55:2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan W.-J., Liang W.-H., Shi Y., Gan L.-X., Wang H.-B., He J.-X., et al. Chronic respiratory diseases and the outcomes of COVID-19: a nationwide retrospective cohort study of 39,420 cases [published online ahead of print March 6, 2021] https://doi.org/10.1016/j.jaip.2021.02.041 J Allergy Clin Immunol Pract. [DOI] [PMC free article] [PubMed]
- 7.Akalin E., Azzi Y., Bartash R., Seethamraju H., Parides M., Hemmige V., et al. Covid-19 and kidney transplantation. N Engl J Med. 2020;382:2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jian L., Yi W., Zhang N., Wen W., Krysko O., Song W.-J., et al. Perspective: COVID-19, implications of nasal diseases and consequences for their management. J Allergy Clin Immunol. 2020;146:67–69. doi: 10.1016/j.jaci.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pawelka E., Karolyi M., Daller S., Kaczmarek C., Laferl H., Niculescu I., et al. Influenza virus infection: an approach to identify predictors for in-hospital and 90-day mortality from patients in Vienna during the season 2017/18. Infection. 2020;48:51–56. doi: 10.1007/s15010-019-01335-0. [DOI] [PubMed] [Google Scholar]
- 10.Sungnak W., Huang N., Bécavin C., Berg M., Queen R., Litvinukova M., et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saheb Sharif-Askari F., Saheb Sharif-Askari N., Goel S., Fakhri S., Al-Muhsen S., Hamid Q., et al. Are patients with chronic rhinosinusitis with nasal polyps at a decreased risk of COVID-19 infection? Int Forum Allergy Rhinol. 2020;10:1182–1185. doi: 10.1002/alr.22672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou Y.J., Okuda K., Edwards C.E., Martinez D.R., Asakura T., Dinnon K.H., III, et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;182:429–446.e14. doi: 10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho S.H., Hamilos D.L., Han D.H., Laidlaw T.M. Phenotypes of chronic rhinosinusitis. J Allergy Clin Immunol Pract. 2020;8:1505–1511. doi: 10.1016/j.jaip.2019.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keswani A., Dunn N.M., Manzur A., Kashani S., Bossuyt X., Grammer L.C., et al. The clinical significance of specific antibody deficiency (SAD) severity in chronic rhinosinusitis (CRS) J Allergy Clin Immunol Pract. 2017;5:1105–1111. doi: 10.1016/j.jaip.2016.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H., Song J., Pan L., Yao Y., Deng Y.K., Wang Z.C., et al. The characterization of chronic rhinosinusitis in hospitalized patients with COVID-19. J Allergy Clin Immunol Pract. 2020;8:3597–3599.e2. doi: 10.1016/j.jaip.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee S.W., Yang J.M., Moon S.Y., Yoo I.K., Ha E.K., Kim S.Y., et al. Association between mental illness and COVID-19 susceptibility and clinical outcomes in South Korea: a nationwide cohort study. Lancet Psychiatry. 2020;7:1025–1031. doi: 10.1016/S2215-0366(20)30421-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee S.W., Yang J.M., Yoo I.K., Moon S.Y., Ha E.K., Yeniova A., et al. Proton pump inhibitors and the risk of severe COVID-19: a post-hoc analysis from the Korean nationwide cohort [published online ahead of print December 10, 2020] https://doi.org/10.1136/gutjnl-2020-323672 Gut. [DOI] [PubMed]
- 18.Ha J., Lee S.W., Yon D.K. Ten-year trends and prevalence of asthma, allergic rhinitis, and atopic dermatitis among the Korean population, 2008-2017. Clin Exp Pediatr. 2020;63:278–283. doi: 10.3345/cep.2019.01291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koh H.Y., Kim T.H., Sheen Y.H., Lee S.W., An J., Kim M.A., et al. Serum heavy metal levels are associated with asthma, allergic rhinitis, atopic dermatitis, allergic multimorbidity, and airflow obstruction. J Allergy Clin Immunol Pract. 2019;7:2912–2915.e2. doi: 10.1016/j.jaip.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Ryu G., Min C., Park B., Choi H.G., Mo J.H. Bidirectional association between asthma and chronic rhinosinusitis: two longitudinal follow-up studies using a national sample cohort. Sci Rep. 2020;10:9589. doi: 10.1038/s41598-020-66479-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim H.J., Ahn H.S., Kang T., Bachert C., Song W.-J. Nasal polyps and future risk of head and neck cancer: a nationwide population-based cohort study. J Allergy Clin Immunol. 2019;144:1004–1010.e4. doi: 10.1016/j.jaci.2019.06.024. [DOI] [PubMed] [Google Scholar]
- 22.Woo A., Lee S.W., Koh H.Y., Kim M.A., Han M.Y., Yon D.K. Incidence of cancer after asthma development: 2 independent population-based cohort studies. J Allergy Clin Immunol. 2021;147:135–143. doi: 10.1016/j.jaci.2020.04.041. [DOI] [PubMed] [Google Scholar]
- 23.Lee S.W., Yang J.M., Moon S.Y., Ki N., Ahn Y.M., Kim J.-M., et al. Association between mental illness and COVID-19 in South Korea: a post-hoc analysis. Lancet Psychiatry. 2021;8:271–272. doi: 10.1016/S2215-0366(21)00043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huyett P., Rowan N.R., Ferguson B.J., Lee S., Wang E.W. The relationship of paranasal sinus opacification to hospital-acquired pneumonia in the neurologic intensive care unit patient. J Intensive Care Med. 2019;34:844–850. doi: 10.1177/0885066617718458. [DOI] [PubMed] [Google Scholar]
- 25.Deja M., Busch T., Bachmann S., Riskowski K., Campean V., Wiedmann B., et al. Reduced nitric oxide in sinus epithelium of patients with radiologic maxillary sinusitis and sepsis. Am J Respir Crit Care Med. 2003;168:281–286. doi: 10.1164/rccm.200207-640OC. [DOI] [PubMed] [Google Scholar]
- 26.Roland L.T., Pinto J.M., Naclerio R.M. The treatment paradigm of chronic rhinosinusitis with nasal polyps in the COVD-19 era. J Allergy Clin Immunol Pract. 2020;8:2492–2494. doi: 10.1016/j.jaip.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wynne M., Atkinson C., Schlosser R.J., Mulligan J.K. Contribution of epithelial cell dysfunction to the pathogenesis of chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2019;33:782–790. doi: 10.1177/1945892419868588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho G.S., Moon B.J., Lee B.J., Gong C.H., Kim N.H., Kim Y.S., et al. High rates of detection of respiratory viruses in the nasal washes and mucosae of patients with chronic rhinosinusitis. J Clin Microbiol. 2013;51:979–984. doi: 10.1128/JCM.02806-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimura H., Francisco D., Conway M., Martinez F.D., Vercelli D., Polverino F., et al. Type 2 inflammation modulates ACE2 and TMPRSS2 in airway epithelial cells. J Allergy Clin Immunol. 2020;146:80–88.e8. doi: 10.1016/j.jaci.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hwang J.W., Lee K.J., Choi I.H., Han H.M., Kim T.H., Lee S.H. Decreased expression of type I (IFN-β) and type III (IFN-λ) interferons and interferon-stimulated genes in patients with chronic rhinosinusitis with and without nasal polyps. J Allergy Clin Immunol. 2019;144:1551–1565.e2. doi: 10.1016/j.jaci.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morse J.C., Li P., Ely K.A., Shilts M.H., Wannemuehler T.J., Huang L.-C., et al. Chronic rhinosinusitis in elderly patients is associated with an exaggerated neutrophilic proinflammatory response to pathogenic bacteria. J Allergy Clin Immunol. 2019;143:990–1002.e6. doi: 10.1016/j.jaci.2018.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ziegler C.G., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181:1016–1035.e19. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Förster-Ruhrmann U., Szczepek A.J., Bachert C., Olze H. COVID-19 in a patient with severe chronic rhinosinusitis with nasal polyps during therapy with dupilumab. J Allergy Clin Immunol. 2020;146:218–220.e2. doi: 10.1016/j.jaci.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bachert C., Desrosiers M.Y., Hellings P.W., Laidlaw T.M. The role of biologics in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol Pract. 2021;9:1099–1106. doi: 10.1016/j.jaip.2020.11.017. [DOI] [PubMed] [Google Scholar]
- 35.Dhawale V.S., Amara V.R., Karpe P.A., Malek V., Patel D., Tikoo K. Activation of angiotensin-converting enzyme 2 (ACE2) attenuates allergic airway inflammation in rat asthma model. Toxicol Appl Pharmacol. 2016;306:17–26. doi: 10.1016/j.taap.2016.06.026. [DOI] [PubMed] [Google Scholar]
- 36.Laidlaw T.M., Mullol J., Woessner K.M., Amin N., Mannent L.P. Chronic rhinosinusitis with nasal polyps and asthma. J Allergy Clin Immunol Pract. 2021;9:1133–1141. doi: 10.1016/j.jaip.2020.09.063. [DOI] [PubMed] [Google Scholar]
- 37.Ferastraoaru D., Hudes G., Jerschow E., Jariwala S., Karagic M., de Vos G., et al. Eosinophilia in asthma patients is protective against severe COVID-19 illness. J Allergy Clin Immunol Pract. 2021;9:1152–1162.e3. doi: 10.1016/j.jaip.2020.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finney L.J., Glanville N., Farne H., Aniscenko J., Fenwick P., Kemp S.V., et al. Inhaled corticosteroids downregulate the SARS-CoV-2 receptor ACE2 in COPD through suppression of type I interferon. J Allergy Clin Immunol. 2021;147:510–519.e5. doi: 10.1016/j.jaci.2020.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y., Gevaert E., Lou H., Wang X., Zhang L., Bachert C., et al. Chronic rhinosinusitis in Asia. J Allergy Clin Immunol. 2017;140:1230–1239. doi: 10.1016/j.jaci.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 40.Polverino F. Cigarette smoking and COVID-19: a complex interaction. Am J Respir Crit Care Med. 2020;202:471–472. doi: 10.1164/rccm.202005-1646LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Desai M., Oppenheimer J. The importance of considering olfactory dysfunction during the COVID-19 pandemic and in clinical practice. J Allergy Clin Immunol Pract. 2021;9:7–12. doi: 10.1016/j.jaip.2020.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abuelgasim E., Saw L.J., Shirke M., Zeinah M., Harky A. COVID-19: Unique public health issues facing Black, Asian and minority ethnic communities. Curr Probl Cardiol. 2020;45:100621. doi: 10.1016/j.cpcardiol.2020.100621. [DOI] [PMC free article] [PubMed] [Google Scholar]