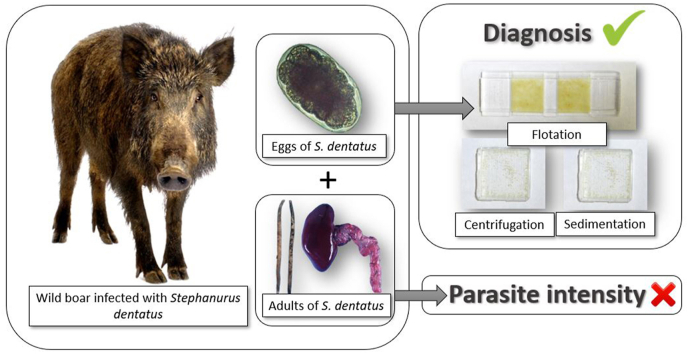
Abstract
Stephanurus dentatus is a nematode that parasitizes the urinary tract of domestic and wild Suidae, especially in tropical areas. However, there is a lack of information about stephanurosis in wild boar (Sus scrofa), thus making it necessary to develop sensitive techniques with which to diagnose this pathogen in order to carry out further research. In Spain, the high prevalence of this nematode has been evidenced in Doñana National Park (DNP). The objective of the present work is twofold. The first is to compare the efficacy of three parasitological techniques to detect S. dentatus eggs in the urine of infected wild boar: (i) gravity sedimentation, (ii) sedimentation by centrifugation, and (iii) flotation techniques, while the second is to determine whether the quantification of eggs can serve as an indicative value of the host's parasite intensity. In order to accomplish these purposes, 27 wild boars from DNP were necropsied, and the urinary system of each animal was examined in order to determine parasite intensity. While all the aforementioned techniques can be used to detect eggs in urine, the most effective in terms of egg quantification are sedimentation by gravity and by centrifugation, as they allow a greater number of S. dentatus eggs to be detected. However, none of the results obtained with these techniques significantly correlated with the number of adult nematodes parasitizing the host, signifying that counts in urine can provide guidance on only the parasite intensity of wild boar.
Keywords: Diagnosis, Parasite eggs, Stephanurus dentatus, Sus scrofa, Urine
Graphical abstract
Highlights
-
•
All the studied parasitological techniques are useful to detect S. dentatus eggs in urine.
-
•
The most effective parasitological technique was sedimentation by centrifugation.
-
•
None of techniques can be reliably applied to infer the parasite intensity.
-
•
Counts of eggs in urine can provide guidance on the parasite intensity of wild boar.
1. Introduction
Stephanurosis is a parasitic disease of great significance that is widely distributed in domestic and wild Suidae (Stewart et al., 1964) and is caused by Stephanurus dentatus (Diesing, 1839), a nematode known as the pig's kidney worm. Adult nematodes are located in cysts and nodular granulomatous lesions in the perirenal fat, ureters and kidney (Morosco et al., 2017). The clinical signs are rare, but it has been shown that pigs with high parasite intensities can be harmed owing to the destruction of the functional tissue of the kidney (Hale and Marti, 1983). This can lead to the retention of metabolic waste, which results in a poor appetite and, therefore, a loss of weight and weakness in the infected animal (Islam et al., 2015). This nematode has principally been reported in tropical and subtropical countries, and is particularly common in pigs reared in traditional free-range production systems (Batte et al., 1960; Waddall, 1969). Although S. dentatus is mainly present in tropical areas, it has also occasionally been detected in different areas of the Iberian Peninsula (Cádiz, Granada, Madrid and Portugal) (Cordero del Campillo et al., 1994). However, there is a lack of information about S. dentatus in wild boar (Sus scrofa), and its distribution and prevalence in wildlife, therefore, remain unknown. In a recent study conducted in several populations of wild boar in South-central Spain, infection was evidenced only in wild boar from Doñana National Park (DNP), with a remarkably high prevalence (76.5%), suggesting a clustered distribution of this parasite (Moratal et al., 2018). However, since this is the single epidemiological study of stephanurosis conducted in wild boar in Europe, additional research is needed to clarify the role of wild boar as potential reservoir of S. dentatus for domestic pigs. In fact, there are large extensions of Iberian pig farms near DNP that are extensive systems. Although S. dentatus has never been identified as a problem for the pig industry in the Iberian Peninsula, the wild boar is a reservoir of many diseases that are potentially transmissible to the domestic pig, since there is a flow of pathogens between these two sympatric species (Gortázar et al., 2007). Stephanurosis might, therefore, imply an important health problem that could be highly relevant in specific areas.
Precise diagnosis is a fundamental element when studying the various epidemiological and health aspects of different parasitosis in order to implement surveillance, control and preventive programs. Endo-macroparasite infections in wildlife can be investigated by employing post-mortem examination when the sampling of dead animals is feasible. However, the diagnosis of nematode infections in living hosts relies mostly on non-invasive methods to detect the presence of eggs or larvae in host excreta, such as feces or urine. Individual intensity measures through the use of non-invasive methods can provide important information on nematode distribution throughout the host population, such as transmission rates or seasonal host-parasite interactions in wildlife, thus increasing our understanding of parasite epidemiology (Arneberg et al., 1998; Cattadori et al., 2005).
Considering that stephanurosis has barely been studied in the Iberian Peninsula, and taking into account the hyperendemic focus recently detected in DNP (Moratal et al., 2018), it is necessary to assess sensitive diagnosis techniques to design further studies. In this context, the aim of our study was: (i) to compare the efficacy of three parasitological techniques (gravity sedimentation, sedimentation by centrifugation, and flotation) in order to detect S. dentatus eggs in the urine of infected wild boar, and (ii) to determine whether the quantification of eggs in the urine can serve as a proxy for the adult parasite intensity in this host species.
2. Material and methods
2.1. Sampling, necropsy, and parasite intensity
The study was carried out on twenty-seven wild boar (two females and twenty-five males; seven sub-adults and twenty adults), which were shot by park rangers as part of the DNP health-monitoring and population control program, approved by the park's Research Commission in accordance with the management rules established by the Autonomous Government of Andalusia. The sex-ratio in this study is skewed in favor of males, since urine samples are more difficult to obtain in females in which the bladder is normally emptied after the death. The necropsy of these animals was performed in the field, and the urinary system, including the perirenal fat, kidneys and ureters, was collected as described by Moratal et al. (2018) from those animals whose urinary tract was in a good condition in order to determine the intensity of parasites (n = 23). Furthermore, urine samples were collected from those animals in which there was bladder content (n = 27). The bladder was specifically removed from the animal by making a cut in the lower part of the urethra to prevent the contents from leaking out. The bladder was subsequently shaken, and its content transferred to sterile jars. The urine was preserved by the immediate addition of 10% formalin of the total sample volume as a preservation method.
2.2. Laboratory procedures
The urinary tract of each wild boar was dissected to detect S. dentatus specimens. Adult nematodes were collected in 70% ethanol and later counted and morphologically identified in accordance with the method described by Skryabin (1991). The sex ratio was calculated by means of the microscopic examination (Motic, B1 Series) of adult nematodes at 40x magnification, as described by Skryabin (1991), and were found to be 1 female per 1.1 male and relatively constant among the infected wild boar (±5.1%). In order to compare the performances of the techniques employed to quantify parasite eggs, each urine sample was analyzed using three different techniques, based on the Manual of Veterinary Parasitological Laboratory Techniques (MAFF, 1986), with some variations, as detailed below. Before carrying out the different techniques, the urine samples were shaken in order to homogenize them. In the case of the gravity sedimentation technique with a Favatti counting chamber (hereafter referred to as “sedimentation”), 4 ml were taken from each sample and placed in a 15 ml falcon tube where they were left to settle at room temperature for 1 h, after which 4.5 ml of the supernatant was removed. A dilution of 1:10 of the sediment was made in distilled water in order to facilitate the count of eggs. 0.5 ml were taken from this dilution, and this volume was deposited in a Favatti chamber of 1 × 1 cm. The egg count was performed under a microscope (Motic, B1 Series) with a magnification of 40x. In the case of the sedimentation by centrifugation technique (hereafter referred to as “centrifugation”) and the subsequent counting in the Favatti chamber, the protocol followed was the same as that described above, but in this case the sample was sedimented by means of centrifugation at 800×g for 5 min. Finally, the flotation technique (hereafter denominated as “flotation”) was performed by taking 4 ml of each sample and centrifuging it at 800×g for 5 min, after which 4.5 ml of the supernatant was removed. In this case, zinc sulfate was used (specific gravity: 1.200) to perform the 1:10 dilution of the sediment and as a floating solution. The quantification of the eggs was performed by waiting for 5 min after the sample had been deposited and using a microscope with a magnification of 40x in a McMaster chamber. These techniques were compared on the basis of the number of S. dentatus eggs in wild boar urine that each parasitological method detected, since there is no gold standard technique in scientific literature or previous studies on the diagnosis of this parasite. This assumption is based on the fact that parasitic diagnostic techniques that detect a greater number of eggs have a greater precision (Godber et al., 2015). Therefore, we assumed that the highest values obtained by the different methods were the closest to the real value. The S. dentatus eggs were identified on the basis of the descriptions provided by Skryabin (1991).
2.3. Statistical analysis
The association between the number of eggs detected (continuous response variable) and the technique used (explicatory factor) was studied by performing a mixed generalized linear model, in which the random variable was the host. We also included the number of parasites present in the urinary tractas (continuous explanatory factor), and the interaction between the technique and the number of parasites. A negative binomial error and a loglink were used. The P-value was set at 0.05. Analyses were conducted using IBM SPSS V21 (StatSoft Inc.).
3. Results and discussion
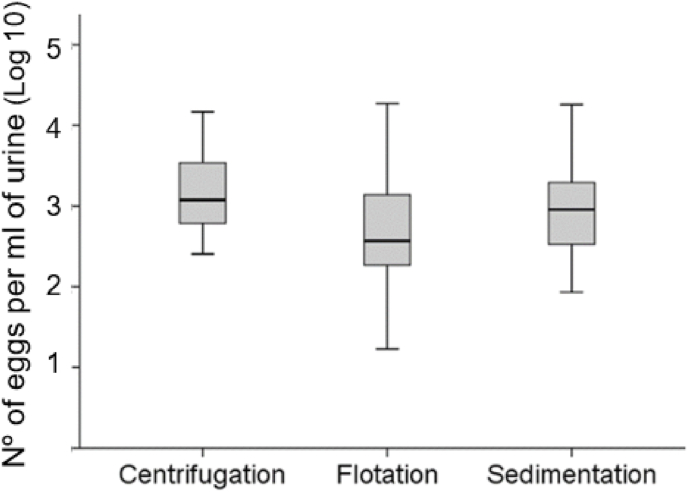
Stephanurus dentatus eggs were detected in all infected animals, showing that the sensitivity of all three techniques is 100% (Table 1). These results indicate that any of the three techniques can be used as an accurate method to diagnose the presence of this nematode species in the urine of Suidae hosts. However, there are significant differences between the three techniques in terms of the number of eggs counted in the urine (F = 4.40, 2, 66 d.f., p = 0.015; Fig. 1), with the sedimentation by centrifugation and by gravity being more efficient than the flotation technique. In general, flotation methods are usually employed for the laboratory diagnosis of nematode infections using the McMaster chamber (MAFF, 1986; Kaminsky, 2014), since it is a rapid method and has a high diagnostic sensitivity. However, in our study, the flotation technique detected the lowest number of eggs (Fig. 1).
Table 1.
Mean (±SD), maximum and minimum number of Stephanurus dentatus adults found in infected wild boar (n = 23) and number of eggs counted in urine according to each parasitological technique (n = 27).
| Technique | Minimum | Maximum | Mean | ±SD | N | |
|---|---|---|---|---|---|---|
| Nº of eggs per ml | Gravity sedimentation | 85 | 18180 | 2923.37 | 4637.91 | 27 |
| Sedimentation by centrifugation | 254 | 62415 | 7116.5 | 14953.5 | 27 | |
| Flotation | 1 | 18724 | 2220.11 | 4730.72 | 27 | |
| Nº of adult nematodes per animal | Necropsy | 3 | 239 | 61.43 | 63.6 | 23 |
Fig. 1.
Average number of Stephanurus dentatus eggs (Log10± S-E.) detected by the three techniques.
With regard to the possible negative effect of the sample preservation method on the estimates of the number of eggs recovered and the technique performance, Maurelli et al. (2014) carried out several diagnostic methods to detect the eggs of Capillaria plica, a nematode that parasitizes the urinary tract of dogs, using fresh urine and urine conserved in formalin; after using different flotation solutions, the aforementioned authors concluded that the flotation technique with saturated saline solution and fresh urine provided the best results. In our study, the samples were taken in the field and could not be processed immediately, so they were preserved in 10% formalin. Therefore, we could not compare our method with other preservation methods, which is a factor to be considered in future studies. Interestingly, in our study we have observed the formation of aggregates in the urine, possibly as a result of the addition of formalin, as described elsewhere (Boon and Kok, 2008); this flocculation process may have influenced the detection of S. dentatus eggs, especially when using the flotation technique. In particular, aggregates of coagulated proteins may have prevented the eggs from floating freely, and they might, therefore, have been outside the upper detection area of the McMaster chamber. This could explain why the flotation technique was the least effective diagnostic method in our study.
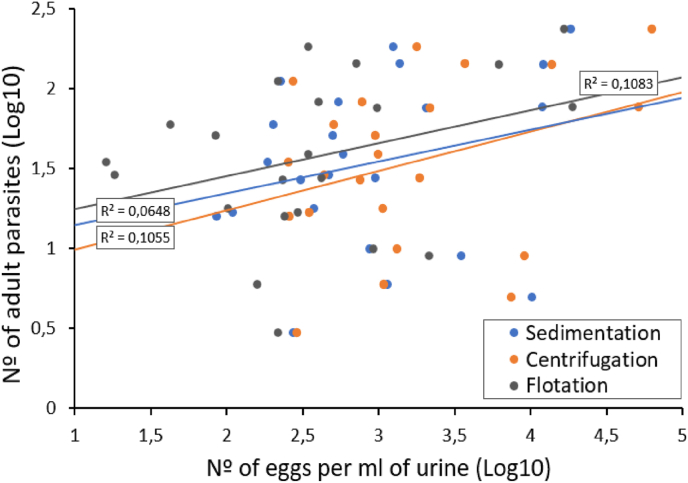
Although there was a positive trend, none of the egg counts obtained with any of the techniques can be reliably applied in order to infer the number of adult nematodes infecting the host (F = 3.05, 2, 66 d.f., p = 0.054; Fig. 2), and counts in urine can, therefore, provide only guidance on the parasite intensity in wild boar. Numerous studies have attempted to develop non-invasive methods with which to determine parasite intensity by associating the number of excreted eggs or larvae with the adult parasites found in the host (Budischak et al., 2015; Seivwright et al., 2004). However, previous studies have shown that the estimation of parasite intensity through the use of the non-invasive method of counting parasite stages excreted by the infected host is not always reliable (Gillespie, 2006; Romeo et al., 2014). There are several factors related not only to the parasite, the host and the environment, but also to the diagnostic methods, that determine the number of eggs and larvae excreted and detected. In particular, it has been shown that the elimination of parasitic forms is usually intermittent over time, as the excretion of eggs and larvae of some nematodes that infect wild ungulates have daily or seasonal fluctuations (Vicente et al., 2005). One relevant determinant of parasite eggs is the number of adult reproductive females present in the host, along with the presence of mature males (Stear et al., 1997). This factor is not relevant for our study since, as mentioned above, the sex ratio of mature nematodes remained relatively constant in our study population. Several host dependent factors (e.g., age, sex, nutritional status and mating or breeding season) that potentially influencing the host immune response may condition the number of reproductive females that are eventually installed and the number of eggs that they successfully produce and excrete (Vicente et al., 2007).
Fig. 2.
Association between the number of eggs counted in urine and the parasite intensity of each infected wild boar, based on the results obtained using the three techniques. Values in log10.
4. Conclusion
This is the first study to investigate and compare different diagnostic methods by which to detect the presence of S. dentatus in infected Suidae. Moreover, if we assume that the technique is equally effective in the domestic pig, which is a sympatric species of wild boar, the diagnostic techniques described here would be useful to detect and monitor the presence of S. dentatus in the pig farming sector, especially in endemic tropical and subtropical areas in which it may lead to economic losses. Further studies are required in order to develop a precise and effective diagnostic method, and to propose essential tools for the study of the epidemiology of this parasitic disease that has rarely been studied in Europe. There is also a need to evaluate other potential factors that may affect the S. dentatus egg count in urine, such as the sample preservation method.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. We would like to thank the staff of Doñana National Park and to the IREC (Animal Health group) for their collaboration in taking field samples.
References
- Arneberg P., Skorping A., Grenfell B., Read A.F. Host densities as determinants of abundance in parasite communities. Proc. R. Soc. B Biol. Sci. 1998 doi: 10.1098/rspb.1998.0431. [DOI] [Google Scholar]
- Batte E.G., Harkema R., Osborne J.C. Observations on the life cycle and pathogenicity of the swine kidney worm (Stephanurus dentatus) J. Am. Vet. Med. Assoc. 1960;136:622–625. [PubMed] [Google Scholar]
- Boon M.E., Kok L.P. Theory and practice of combining coagulant fixation and microwave histoprocessing. Biotech. Histochem. 2008 doi: 10.1080/10520290802553476. [DOI] [PubMed] [Google Scholar]
- Budischak S.A., Hoberg E.P., Abrams A., Jolles A.E., Ezenwa V.O. A combined parasitological molecular approach for noninvasive characterization of parasitic nematode communities in wild hosts. Mol. Ecol. Resour. 2015 doi: 10.1111/1755-0998.12382. [DOI] [PubMed] [Google Scholar]
- Cattadori I.M., Boag B., Bjørnstad O.N., Cornell S.J., Hudson P.J. Peak shift and epidemiology in a seasonal host-nematode system. Proc. R. Soc. B Biol. Sci. 2005 doi: 10.1098/rspb.2004.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero del Campillo M., Castanon Ordonez L., Reguera Feo A. Index-catalogue of Iberian Parasites of Animals. second ed. Universidad de León: Secretariado de Publicaciones; Leon: 1994. [Google Scholar]
- Gillespie T.R. Noninvasive assessment of gastrointestinal parasite infections in free-ranging primates. Int. J. Primatol. 2006 doi: 10.1007/s10764-006-9064-x. [DOI] [Google Scholar]
- Godber O.F., Phythian C.J., Bosco A., Ianniello D., Coles G., Rinaldi L., Cringoli G. A comparison of the FECPAK and Mini-FLOTAC faecal egg counting techniques. Vet. Parasitol. 2015 doi: 10.1016/j.vetpar.2014.12.029. [DOI] [PubMed] [Google Scholar]
- Gortázar C., Ferroglio E., Höfle U., Frölich K., Vicente J. Diseases shared between wildlife and livestock: a European perspective. Eur. J. Wildl. Res. 2007 doi: 10.1007/s10344-007-0098-y. [DOI] [Google Scholar]
- Hale O.M., Marti O.G. Influence of an experimental infection of swine kidneyworm (Stephanurus dentatus) on performance of pigs. J. Anim. Sci. 1983 doi: 10.2527/jas1983.563616x. [DOI] [PubMed] [Google Scholar]
- Islam A., Anisuzzaman J., Roy M., Yasin G., Labony S.S., Hossain M., Alim M.A. Swine kidney worm in Bangladesh: an abattoir survey. Eurasian J. Vet. Sci. 2015 doi: 10.15312/eurasianjvetsci.2015413527. [DOI] [Google Scholar]
- Kaminsky R.G. third ed. Universidad Nacional Autónoma de Honduras. Facultad de Ciencias Médicas; Honduras: 2014. Manual de Parasitología. Tecnicas para Laboratorio de Atencion Primaria de Salud y para el Diagnóstico de las Enfermedades Infecciosas Desatendidas. [Google Scholar]
- Maff. Ministry of Agriculture Fisheries and Food . Manual of Veterinary Parasitological Laboratory Techniques. third ed. H.M.S.O; London: 1986. [Google Scholar]
- Maurelli M., Rinaldi L., Rubino G., Lia R., Musella V., Cringoli G. FLOTAC and Mini-FLOTAC for uro-microscopic diagnosis of Capillaria plica (syn. Pearsonema plica) in dogs. BMC Res. Notes. 2014 doi: 10.1186/1756-0500-7-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moratal S., Ruíz de Ybáñez R., Barroso P., Granados J.E., Höfle U., Martínez-Carrasco C., Acevedo P., Vicente J. High prevalence and intensity of Stephanurus dentatus in a population of wild boar (Sus scrofa) in south western Spain. Vet. J. 2018 doi: 10.1016/j.tvjl.2018.09.004. [DOI] [PubMed] [Google Scholar]
- Morosco D., Illanes O., Fuentealba C. Renal stephanuriasis: gross and microscopic findings in naturally-infected pigs from St. Kitts, West Indies. Austral J. Vet. Sci. 2017 doi: 10.4067/S0719-81322017000100053. [DOI] [Google Scholar]
- Romeo C., Wauters L.A., Cauchie S., Martinoli A., Matthysen E., Saino N., Ferrari N. Faecal egg counts from field experiment reveal density dependence in helminth fecundity: Strongyloides robustus infecting grey squirrels (Sciurus carolinensis) Parasitol. Res. 2014 doi: 10.1007/s00436-014-4005-7. [DOI] [PubMed] [Google Scholar]
- Seivwright L.J., Redpath S.M., Mougeot F., Watt L., Hudson P.J. Faecal egg counts provide a reliable measure of Trichostrongylus tenuis intensities in free-living red grouse Lagopus lagopus scoticus. J. Helminthol. 2004 doi: 10.1079/joh2003220. [DOI] [PubMed] [Google Scholar]
- Skryabin K.I. Key to Parasitic Nematodes. third ed. vol. 3. Strongylata; Leiden: 1991. [Google Scholar]
- Stear M.J., Bairden K., Duncan J.L., Holmes P.H., McKellar Q.A., Park M., Strain S., Murray M. How hosts control worms [7] Nature. 1997 doi: 10.1038/37895. [DOI] [PubMed] [Google Scholar]
- Stewart T.B., Hale O.M., Andrews J.S. Eradication of the swine kidney worm, Stephanurus dentatus, from experimental pastures by herd management. Am. J. Vet. Res. 1964;25:1141–1150. [PubMed] [Google Scholar]
- Vicente J., Fierro Y., Gortazar C. Seasonal dynamics of the fecal excretion of Elaphostrongylus cervi (Nematoda, Metastrongyloidea) first-stage larvae in Iberian red deer (Cervus elaphus hispanicus) from southern Spain. Parasitol. Res. 2005 doi: 10.1007/s00436-004-1255-9. [DOI] [PubMed] [Google Scholar]
- Vicente J., Pérez-Rodríguez L., Gortazar C. Sex, age, spleen size, and kidney fat of red deer relative to infection intensities of the lungworm Elaphostrongylus cervi. Naturwissenschaften. 2007 doi: 10.1007/s00114-007-0231-5. [DOI] [PubMed] [Google Scholar]
- Waddall A.H. The parasitic life-cycle of the swine kidney worm Stephanurus dentatus Diesing. Aust. J. Zool. 1969 doi: 10.1071/ZO9690607. [DOI] [Google Scholar]