Introduction
Immune checkpoint inhibitors (ICI) such as anti-programmed cell death protein 1 (PD-1) or anti-programmed death-ligand 1 (PDL-1) antibodies represent a breakthrough in the treatment of advanced neoplasms such as melanoma, lung, or renal cancer. The therapeutic use of these agents is rapidly growing in both active as well as adjuvant settings. Anti-PD-1 agents such as pembrolizumab or nivolumab target PD-1 present on the surface of T cells and other immune cells, enhancing the antitumoral response. Although they offer a better safety profile than anti-CTLA-4 antibodies such as ipilimumab, immune-related adverse events (irAE) still have an important impact on morbidity during treatment. Gastrointestinal and skin irAE are the most frequent adverse events associated with their use and tend to occur early in treatment. Maculopapular rash and lichenoid dermatitis are among the most common skin irAE, affecting approximately 3%-20% of the patients. Most of these reactions are mild and are easily managed with topical corticosteroids, rendering immunotherapy withdrawal unnecessary.
Psoriasis might also be exacerbated or present as de novo, both with nivolumab or pembrolizumab, although the exact incidence rate has not been established. This immune-related psoriasis has also been observed during ipilimumab therapy, but its severity has not been defined according to clinical scales such as the psoriasis area severity index (PASI), body surface area (BSA), or physician's global assessment (PGA). Most published series report mild cases with a good outcome after treatment with topical steroids, ultraviolet B phototherapy, or acitretin. Here, we describe 3 patients with moderate to severe psoriasis related to nivolumab treatment, with a favorable response to apremilast and no impairment of antitumoral efficacy.
Case series
Table I summarizes the main characteristics of these 3 patients and their response to treatment with apremilast, based on clinical scales and imaging performed at day 1 and month 2.
Table I.
Main features of the 3 patients reported with moderate to severe immune induced/aggravated psoriasis
| Sex/age | Cancer/anti-PD-1 | Psoriasis type | Latency period | Past psoriatic treatment | Apremilast treatment period | Body sites affected | Initial psoriasis severity | Final psoriasis severity | Malignancy progression/ time to progression with apremilast | Estimated progression free survival mean time of anti-PD1 treatment/reference | Apremilast adverse effects |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male/50 years | Stage IV Uveal melanoma/nivolumab | New onset plaque psoriasis with psoriatic arthritis | 5 months | –∗ | 12 months | Trunk, soles, upper and lower extremities Asymmetric oligoarthritic involvement |
PASI 12 BSA 15 % PGA 3 |
PASI 3.40 BSA 8% PGA 1 |
None/–∗ | 2.8 months12 | Auto limited diarrhea |
| Male/70 years | Stage IV laryngeal carcinoma/nivolumab | Exacerbated Plaque psoriasis with intertriginous psoriasis | 2 weeks | Acitretin 25 mg/day | 10 months | Trunk, upper and lower extremities, axillar and sub-mammary folds | PASI 13.80 BSA 15% PGA 3 |
PASI 3 BSA 4% PGA 1 |
Yes/10 months | 2 months13 | Mild headache |
| Male/60 years | Stage IV squamous lung cell carcinoma/nivolumab | Exacerbated plaque psoriasis with intertriginous psoriasis | 1 week | Mometasone furoate ointment | 10 months | Lower extremities, inguinal and genital folds | PASI 6.40 BSA 4% PGA 3 |
PASI 4 BSA 3% PGA 1 |
Yes/10 months | 3.5 months14 | None |
BSA, Body surface area; PASI, psoriasis area severity index; PD1, programmed cell death protein 1; PGA, physician's global assessment.
– = not applicable.
Case 1
We report the case of a 50-year-old man with stage IV uveal melanoma who developed erythematous psoriatic desquamative plaques on the chest, abdomen, back, legs and both hands after 5 months while taking nivolumab every 2 weeks (Fig 1, A). He had neither a personal nor a family history of psoriasis. He also suffered from inflammatory pain located on certain distal interphalangeal joints on both hands and the left knee, which showed no response to intraarticular corticosteroid injections or a low oral dose of prednisone (5 mg per day). He was referred to the rheumatology clinic, and articular exploration proved compatible with psoriatic arthritis (pSA). He also developed vitiligo lesions on his trunk and both arms. His psoriasis did not respond optimally to topical mometasone furoate, which achieved a PASI score of 12 and affected 15% of his BSA. His PGA score was 3. We initiated apremilast 30 mg twice daily, and the response was good for both articular and skin conditions. At a 1-year follow-up, his clinical scores were as follows: PASI 3.40, BSA 8%, and PGA 1, achieving a PASI 75 response (Fig 1, B). The patient reported rapid joint pain relief within the first month of treatment. After over 2 years of follow-up, the metastatic melanoma disease has remained stable; thus, nivolumab has not been discontinued to date. PASI 75 and pSA responses remain to date.
Fig 1.
Patient 1. A, Psoriatic plaques covering the right palm. B, Response to apremilast 30 mg twice daily.
Case 2
This case involves a 70-year-old man with stage IV laryngeal carcinoma, who experienced a moderate worsening of his previous psoriasis after 2 weeks of initial nivolumab treatment. He developed severe intertriginous psoriatic plaques, located on his back, chest, and extremities (PASI 13.80, BSA 15%, and PGA 3) (Fig 2, A). In the past, his psoriasis had responded well to acitretin 25 mg/daily, so it was reintroduced along with topical mometasone. After 6 weeks, no improvement was observed. We then administered apremilast 30 mg twice daily, which resulted in a good clinical response, with a PASI of 3, a BSA of 4%, and a PGA of 1 (Fig 2, B). Given the fact that tumor progression occurred after 10 months of nivolumab and apremilast treatment, both were discontinued. The patient died 3 months later after a lack of response to standard chemotherapy treatment and progression of his disease.
Fig 2.
Patient 2. A, Severe psoriatic plaques covering the patient's axillary fold. B, Response to apremilast 30 mg twice daily.
Case 3
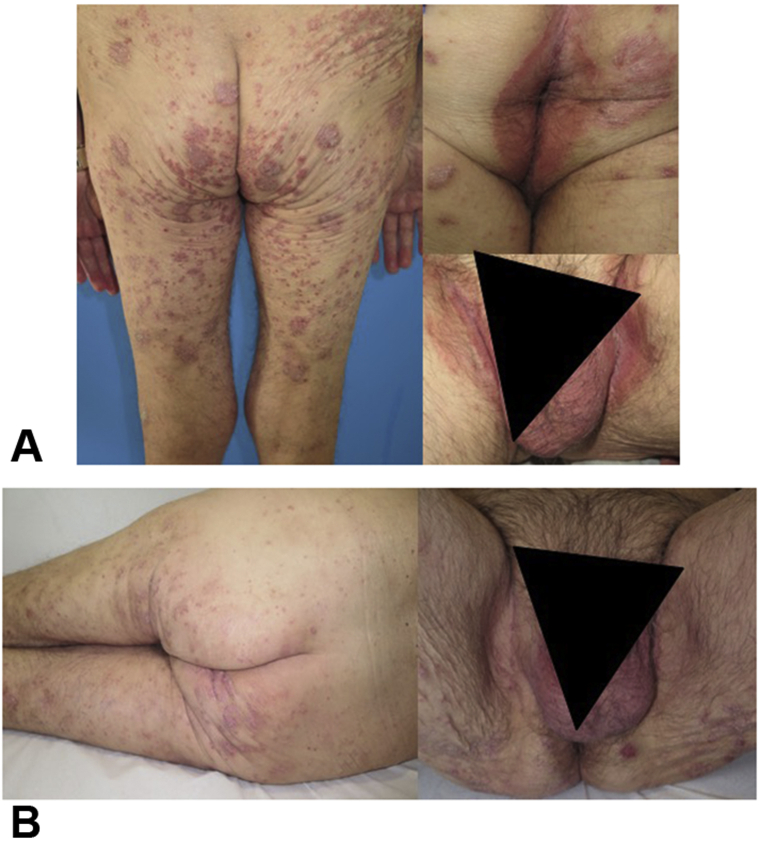
A 60-year-old man with stage IV squamous lung cell carcinoma experienced a significant worsening of previous plaque psoriasis, with painful intertriginous involvement of the genitocrural area, only 1 week subsequent to treatment with nivolumab (Fig 3, A). The patient developed moderate psoriasis (PASI 6.40, BSA 4%, and PGA 3) involving the lower extremities, buttocks, and inguinal folds, despite topical steroid therapy. We initiated apremilast 30 mg twice daily and achieved a positive clinical response (PASI 4, BSA 3%, and PGA 1), especially regarding inverse psoriasis (Fig 3, B). After 10 months of follow-up, tumor progression occurred, leading to the death of the patient.
Fig 3.
Patient 3. A, Severe inverse psoriasis. B, Response to apremilast 30 mg twice daily.
Discussion
ICI are novel agents approved to treat advanced malignancies.1 Skin toxicities are one of the most common irAE. These include maculopapular rashes, lichenoid dermatitis, bullous pemphigus, vitiligo, and pruritus as the most frequent conditions appearing during anti-PD-1/PDL-1 blockade.2 Psoriasis has also been reported as a rare irAE,3 although specific rates have yet to be reported. Exacerbation of preexisting psoriasis and new onset psoriasis during immunotherapy have both been previously described. The largest series of cases to date involves 21 patients with plaque, and less commonly, guttate psoriasis.4 Clinical severity was not reported; however, most cases improved with topical therapy, some with additional oral acitretin. Phototherapy responses have also been reported, although phototherapy might be contraindicated in patients with melanoma.5 Cyclosporine or biological therapies might be discouraged, given such treatments are contraindicated for patients with active cancer. Clinical improvement has not been described in most of the reports, due to the limited application of clinical scales such as PASI, BSA, or PGA, nor have long-term follow-up and impact on tumor progression free survival (PFS) time. As such, patients receiving ICI have not yet shown an improved outcome with regard to immune-induced psoriasis, as has occurred among patients with other skin irAE, such as vitiligo or lichenoid dermatitis.2 Few cases of pSA have been published related to the use of ICI.6 An increase in TH17 activity and IL-17 production is thought to be enhanced by ICI, which could explain how psoriasis is triggered, although this hypothesis warrants further research.3
Apremilast is an orally available phosphodiesterase 4 (PDE4) inhibitor approved for treatment of adults with moderate to severe psoriasis and active psoriatic arthritis.7,8 PDE4 inhibition leads to the accumulation of intracellular cyclic adenosine monophosphate, which gives rise to both an increase in anti-inflammatory cytokines from macrophages, such as IL-10, as well as a decrease in pro-inflammatory cytokines, such as IL-17, IL-22, and IL-13.9 Apremilast has shown clinical efficacy in both skin and arthritic psoriasis.10,11 Generally, the safety of apremilast at a dosage of 30 mg bid appears to be sound, given the most frequent adverse events include light-to-mild nausea and diarrhea during the first weeks of treatment. Apremilast's safety profile is further emphasized by the absence of tuberculosis reactivation, serious infections, and malignancies.9 For this reason, it is regarded more as an immunomodulating agent rather than an immunosuppressant drug.
We have described 3 cases with moderate to severe immune-enhanced psoriasis during anti-PD-1 treatment for advanced malignancies. All 3 patients presented with plaque psoriasis. Two had severe intertriginous affection, and 1 also presented concomitant pSA, diagnosed based on rheumatologic evaluation. All were resistant to topical treatment. Apremilast was given at a standard dosage, with favorable clinical improvement on PASI, BSA, and PGA reduction scales. Although some flare-ups were observed near new nivolumab infusions, a PASI 75 response was achieved for all 3 patients. Patient 1 also showed a good response to articular inflammation. In all 3 cases, PFS was not significantly lower than mean PFS rates reported for anti-PD-1 therapy due to their malignancy (Table I).12, 13, 14 Patients 2 and 3 showed malignant progression of their disease after 10 months of anti-PD-1 and apremilast therapy. The mean PFS for nivolumab in advanced head and neck cancer is only up to 2 months, whereas it is up to 3 months for squamous cell lung cancer.13,14 Thus, it appears highly unlikely that apremilast affected anti-pd1 antitumor activity. The first patient still shows stable malignant disease on anti-PD-1 blockade and presents a favorable clinical psoriasis response to apremilast. Tolerance has been good in all 3 patients, and no significant adverse events have been observed. Fattore et al15 recently reported a case of apremilast efficacy and safety for treating immune-related psoriasis in a patient receiving ICI for advanced cancer. The follow-up period was only 6 weeks.
Conclusions
Psoriasis can develop or worsen as an irAE during ICI therapy against cancer. Precise incidence rates and clinical objective characterization have yet to be defined. Despite the fact that most cases reported to date have shown a good clinical response to topical therapy, acitretin, or phototherapy, more treatment options are needed because the current options are not always effective or suitable. Moreover, moderate to severe psoriasis cases could arise as anti-PD-1/PDL-1 drugs become more widely used for patients with cancer. Safe, long-term therapies should soon be tested and developed in this clinical setting.
We report good clinical responses and safety with apremilast for 3 patients with immune-triggered psoriasis during ICI treatment. Anti-PD-1 blockade was not affected in our patients by apremilast if we compare the PFS with the expected mean PFS in clinical trials. Apremilast could be a more suitable option for immune-related psoriasis and pSA than other therapies due to its safety profile. More reliable data on the safety of apremilast and other therapies are needed for advanced cancer patients receiving immunotherapy. Apremilast for immune-related psoriasis and pSA should be tested in clinical trials involving the use of anti-PD-1/PDL-1 in a diverse cancer setting.
Conflicts of interest
None declared.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Topalian S.L., Hodi F.S., Brahmer J.R. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sibaud V., Meyer N., Lamant L., Vigarios E., Mazieres J., Delord J.P. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. 2016;28(4):254–263. doi: 10.1097/CCO.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 3.Matsumura N., Ohtsuka M., Kikuchi N., Yamamoto T. Exacerbation of psoriasis during nivolumab therapy for metastatic melanoma. Acta Derm Venereol. 2016;96(2):259–260. doi: 10.2340/00015555-2212. [DOI] [PubMed] [Google Scholar]
- 4.Bonigen J., Raynaud-Donzel C., Hureaux J. Anti-PD1-induced psoriasis: a study of 21 patients. J Eur Acad Dermatol Venereol. 2017;31(5):e254–e257. doi: 10.1111/jdv.14011. [DOI] [PubMed] [Google Scholar]
- 5.Voudouri D., Nikolaou V., Laschos K. Anti-PD1/PDL1 induced psoriasis. Curr Probl Cancer. 2017;41(6):407–412. doi: 10.1016/j.currproblcancer.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Ruiz-Bañobre J., Pérez-Pampín E., García-González J. Development of psoriatic arthritis during nivolumab therapy for metastatic non-small cell lung cancer, clinical outcome analysis and review of the literature. Lung Cancer. 2017;108:217–221. doi: 10.1016/j.lungcan.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Torres T., Puig L. Apremilast: a novel oral treatment for psoriasis and psoriatic arthritis. Am J Clin Dermatol. 2018;19(1):23–32. doi: 10.1007/s40257-017-0302-0. [DOI] [PubMed] [Google Scholar]
- 8.Schafer P.H., Parton A., Capone L. Apremilast is a selective PDE4 inhibitor with regulatory effects on innate immunity. Cell Signal. 2014;26(9):2016–2029. doi: 10.1016/j.cellsig.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83(12):1583–1590. doi: 10.1016/j.bcp.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Reich K., Gooderham M., Green L. The efficacy and safety of apremilast, etanercept and placebo in patients with moderate-to-severe plaque psoriasis: 52-week results from a phase IIIb, randomized, placebo-controlled trial (LIBERATE) J Eur Acad Dermatol Venereol. 2017;31(3):507–517. doi: 10.1111/jdv.14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wells A., Adebajo A.O., Aelion J.A. Apremilast, an oral phosphodiesterase 4 inhibitor, is associated with long-term (52-week) improvement in the signs and symptoms of psoriatic arthritis in DMARD-naive patients: results from a phase 3, randomized, controlled trial [abstract no. 1543] Arthritis Rheumatol. 2014;66(Suppl 10):S680. [Google Scholar]
- 12.Algazi A.P., Tsai K.K., Shoushtari A.N. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer. 2016;122(21):3344–3353. doi: 10.1002/cncr.30258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferris R.L., Blumenschein G., Jr., Fayette J. Nivolumab vs investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018;81:45–51. doi: 10.1016/j.oraloncology.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horn L., Spigel D.R., Vokes E.E. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase iii trials (CheckMate 017 and CheckMate 057) J Clin Oncol. 2017;35(35):3924–3933. doi: 10.1200/JCO.2017.74.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fattore D., Annunziata M.C., Panariello L., Marasca C., Fabbrocini G. Successful treatment of psoriasis induced by immune checkpoint inhibitors with apremilast. Eur J Cancer. 2019;110:107–109. doi: 10.1016/j.ejca.2019.01.010. [DOI] [PubMed] [Google Scholar]