Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease in the developed countries. The aim of this study was to evaluate the NAFLD prevalence in European adults and children/adolescents of the general population and specific subgroups.
Method
Search for all articles published between 01/1990-06/2019 reporting NAFLD prevalence from European countries.
Results
Nineteen studies with adults and 9 with children/adolescents were included. Pooled NAFLD prevalence in adults was 26.9%, being higher in studies using ultrasonography (27.2%) or fatty liver index (FLI) (30.1%) than liver biochemical tests (19.1%) and without differences between Mediterranean and non-Mediterranean countries or publication periods. Pooled NAFLD prevalence was higher in men than women (32.8% vs. 19.6%) and in patients with than those without metabolic syndrome (75.3% vs. 17.9%) or any of its components (always P<0.01). Ultrasound and FLI performed equally in estimating NAFLD prevalence in most subgroups. A higher prevalence was reported using FLI in obese and in diabetic patients, whereas a higher prevalence was observed with ultrasound in non-obese patients and in individuals without metabolic syndrome. NAFLD prevalence was 2.7% in unselected and 31.6% in obese/overweight children/adolescents.
Conclusions
NAFLD prevalence exceeds 25% in European adults, being higher in those with metabolic syndrome component(s)-related comorbidities. It remains low in unselected NAFLD population, but increased in overweight/obese European children/adolescents, particularly from Mediterranean countries.
Keywords: Fatty liver, Europe, prevalence, metabolic syndrome, adults
Introduction
Nonalcoholic fatty liver disease (NAFLD) is characterized by fat accumulation (steatosis) in >5% of hepatocytes, in the absence of other causes including alcohol over-consumption [1]. NAFLD spectrum ranges from benign hepatic steatosis, to nonalcoholic steatohepatitis (NASH), characterized by steatosis, inflammation and hepatocyte ballooning, and liver cirrhosis and/or hepatocellular carcinoma (HCC) [2,3].
Today, NAFLD represents the most common cause of chronic liver disease in developed countries with a global prevalence of 25% among adults [4] being higher in patients with metabolic syndrome (MetS) or its components [5]. Moreover, liver transplantation (LT) performed in Europe for NASH-related decompensated cirrhosis and HCC increased from 0.9% to 5.0% and from 0.2% to 1.2% from 2014 to 2017, respectively [6], while in the USA NASH became the second leading cause for LT in 2015 [7], and is expected to be the first one soon [8].
Additionally, NAFLD is associated with an increased incidence of cardiovascular diseases (CVD) [1,2], dyslipidemia, insulin resistance, type II diabetes, and/or arterial hypertension, which represent components of MetS [1,2]. A recent meta-analysis [9] demonstrated that NAFLD and particularly NASH carry significant clinical and economic burden [9]. However, NAFLD epidemiology in European countries alone has not been systematically investigated, despite the need for detailed description of the current situation that could guide precise patient management in this distinct region.
This systematic review and meta-analysis aimed to assess the NAFLD prevalence in European-only adults and children/adolescents overall as well as different subgroups.
Materials and methods
Data sources and searches
PubMed/Medline from 1990 to June 2019 was searched according to the Preferred Reporting Items for Systemic Reviews and Meta-Analyses (PRISMA) guidelines [10] for a meta-analysis of observational studies to identify all relevant medical literature included under the following search text terms: “non-alcoholic fatty liver” OR “NAFLD” AND “prevalence” OR “epidemiology”. Also, a full manual search of all relevant review articles and the original studies retrieved was performed. PRISMA checklist is provided in the Supplementary Appendix A (450.2KB, pdf) .
Study selection
All studies published in English as full papers were included if they fulfilled all of the following criteria: 1) they were observational (case-control or cohort) studies; 2) they included random samples of the general population or specific subpopulations with well-defined inclusion criteria from European countries; 3) they included adults (≥18 years old) or children/adolescents (<18 years old) with NAFLD diagnosis; 4) they excluded other common causes of liver diseases, such as hepatitis B and C, as well as excess alcohol consumption in cases with NAFLD diagnosis; 5) they provided data on the prevalence of NAFLD based on any diagnostic method, i.e., biopsy, imaging [ultrasonography, magnetic resonance imaging (MRI) or other scans] or liver biochemical enzymes. Multicenter studies with participants from both European and non-European countries that did not provide the European data separately were excluded. If 2 studies of the same cohort were published, only the largest study was considered.
Data extraction and quality assessment
Data extraction from selected papers was carried out based on a predefined form by 2 authors (MP, GM) for adults and for children-adolescents (IP, EC) according to the PRISMA guidelines [10]. The Newcastle-Ottawa scale [11] was used to assess the quality of the included studies.
Any queries regarding data extraction were arbitrated by a discussion with another author (GP). Data extracted from selected studies included country and center(s), date of publication, type of study (case-control or cohort), primary study question, sample size, age and gender in total population and among NAFLD cases, method of NAFLD diagnosis, and number of patients with NAFLD in the total population as well as in specific subgroups according to sex, smoking habits, presence of MetS and its parameters, presence of comorbidities such as obesity (based on the body mass index [BMI]), CVD, and chronic kidney disease. A pilot data extraction form was tested and revised.
Risk of bias was assessed by the Newcastle-Ottawa scale. This scale assigns a maximum score of 5 for selection, 2 for comparability, and 2 for outcome. Studies with a score 7-9, 4-6 and 1-3 were considered of high quality (low risk of bias), fair quality (moderate risk of bias), and low quality (high risk of bias), respectively.
Statistical analysis
The outcome of interest involved NAFLD prevalence in the total population and specific subgroups defined by gender, smoking habits, MetS and its parameters and comorbidities (obesity, CVD, chronic kidney disease) according to the diagnostic method, geographical region (Mediterranean or non-Mediterranean countries) and publication period, whenever data were available. In addition, pooled mean values were evaluated in participants with and without NAFLD, whenever data were available.
Meta-analysis was performed using a generalized linear mixed model [12] and Clopper-Pearson confidence intervals (exact binomial interval) for individual studies [13]. Between studies, variance was estimated using the maximum likelihood estimator. Heterogeneity was examined visually in the forest plots and its extent was described using the I2 measure, as proposed by Higgins et al [14]. We used a test statistic based on a weighted linear regression of the treatment effect on the inverse of the total sample size using the variance of the average event rate as weights, as described by Peters [15]. The pooled prevalence rates (95% confidence intervals [CI]) are reported. A prediction interval (PI) for the treatment effect of a new study was also calculated as proposed by Higgins [16,17]. Random-effects meta-analysis was chosen in advance as the analysis method to incorporate the assumption that the true effect varies across studies. In cases of zero responders, zero was replaced by 0.5, and the number of participants was corrected accordingly. Analysis was performed in R v3.6.0 [18] using the meta [19] and the metaphor [20] packages.
Results
In total, 28/3580 observational studies were included in the meta-analysis; 19 studies in adults published with a total of 85,486 subjects [21-39] and 9 studies in children/adolescents with a total of 19,891 subjects [40-48] (Supplementary Fig. 1 (450.2KB, pdf) ). Study and subject characteristics for adults and children/adolescents are presented in Supplementary Tables 1 (450.2KB, pdf) and 2 (450.2KB, pdf) , respectively.
Adults
Nineteen studies were included for the analysis of NAFLD prevalence in adults. Two of them evaluated patients with MetS and were used only for the relevant subgroup analysis [21,29]. The remaining 17 studies [22-28,30-39] were from 8 European countries (Germany: 6, Italy: 2, Netherlands: 2, Spain: 2, Finland: 2, Portugal: 1, United Kingdom (UK): 1, Greece: 1). The mean Newcastle-Ottawa Scale quality assessment score was 7 (range 5-9), whereas 12 studies were of high and 7 of fair quality.
The 17 adult studies evaluated 85,203 participants (NAFLD patients, n=19,922). Five studies were published between 2004-2010 [22-26], 6 between 2011-2015 [27,28,30-33], and 6 between 2016-2019 [34-39]. NAFLD was diagnosed by ultrasonography in 11 (n=14,393), liver biochemical tests (aminotransferases ± g-glutamyl transpeptidase [GGT]) in 2 (n=5,829) and combination of biochemical markers and clinical parameters, e.g., fatty liver index (FLI) in 4 studies (n=64,981).
Prevalence of NAFLD in the general population
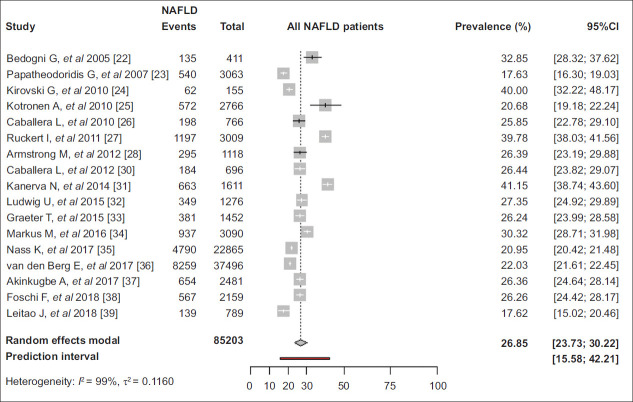
The overall pooled NAFLD prevalence was 26.9% (95%CI 23.7-30.2, 95%PI 15.6-42.2, primary-study range 17.6-41.2%) (Fig. 1) [22-28,30-39]. The prevalence of NAFLD was 23.9% (95%CI 19.9-28.5) and 28.5% (95%CI 24.5-32.9) in studies from Mediterranean [22,23,26,30,38,39] and non-Mediterranean countries [24,25,27,28,31-37] (P=0.14), as well as 27.2% (95%CI 24.6-29.9), 19.1% (95%CI 17.1-21.3) and 30.1% (95%CI 21.6-40.2) in studies using ultrasonography [22,24,26,28,30,32-34,37-39], only liver biochemical tests [23,25] and FLI [27,31,35,36] for NAFLD diagnosis, respectively (P<0.01) (Fig. 2).
Figure 1.
Pooled prevalence of nonalcoholic fatty liver disease (NAFLD) in adult general population in Europe
CI, confidence interval
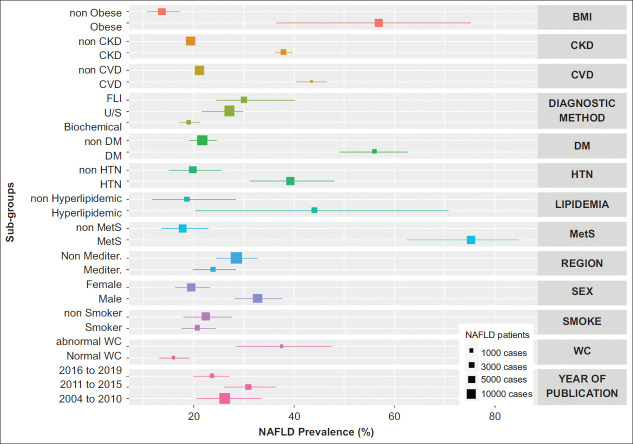
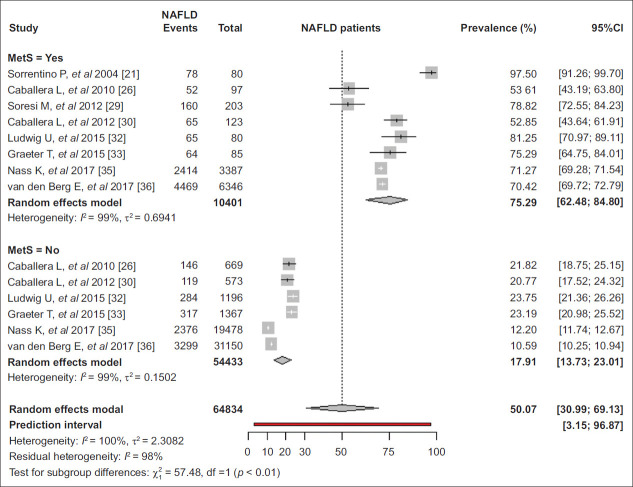
Figure 2.
Composite forest plot of prevalence of nonalcoholic fatty liver disease (NAFLD) in major subgroups of adults in Europe
CKD, chronic kidney disease; CVD, cardiovascular disease; FLI, fatty liver index; US, ultrasonography; DM, diabetes mellitus; HTN, hypertension; MetS, metabolic syndrome; Mediter, Mediterranean; WC, waist circumference; BMI, body mass index
The prevalence of NAFLD varied according to the period of publication being 26.2% (95%CI 20.0-33.6), 31.0% (95%CI 26.1-36.3) and 23.8% (95%CI 20.7-27.2) in studies published between 2004-2010 [22-26], 2011-2015 [27,28,30-33] and 2016-2019 [34-39], respectively (P=0.06) (Fig. 2).
When only the studies using ultrasonography for NAFLD diagnosis were taken into consideration, the prevalence of NAFLD was 25.4% (95%CI 21.4-30.0) and 28.2% (95%CI 26.0-30.4) in studies from Mediterranean and non-Mediterranean countries (P=0.27) and 31.9% (95%CI 25.9-38.6), 26.6% (95%CI 25.4-27.9) and 25.0% (95%CI 20.6-29.9) in studies published between 2004-2010, 2011-2015 and 2016-2019, respectively (P=0.19).
Prevalence of NAFLD in subpopulations
Sex
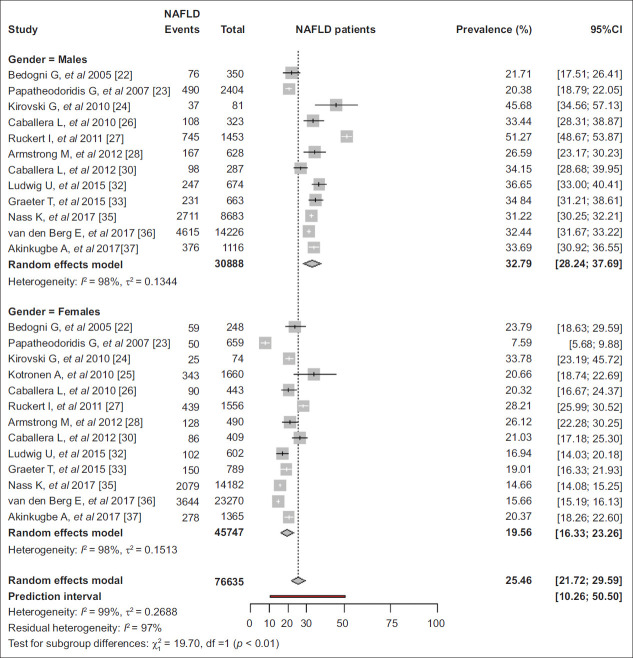
The pooled prevalence of NAFLD was higher in men than women (32.8% vs. 19.6%, P<0.01) [22-28,30,32,33,35-37] (Fig. 3), regardless of publication period, diagnostic method or geographical area (Table 1).
Figure 3.
Pooled prevalence of nonalcoholic fatty liver disease (NAFLD) in adults in Europe by sex
CI, confidence interval
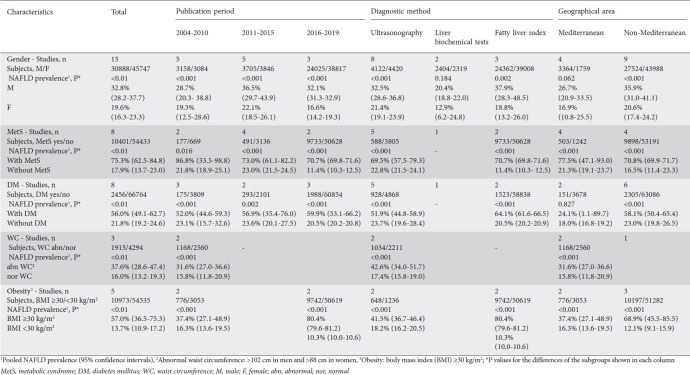
Table 1.
Prevalence of nonalcoholic fatty liver disease (NAFLD) in adult subpopulations. Pooled data coming from at least 2 studies for each subpopulation
MetS
The pooled prevalence of NAFLD was higher in those with than those without MetS (75.3% vs. 17.9%, P<0.01) [21,26,29,30,32,33,35,36] (Fig. 4). In patients with MetS, the prevalence of NAFLD was 69.5% in studies using ultrasonography [26,29,30,32,33] and 70.7% in studies using FLI [35,36] (P=0.82), as well as 97.5% in one study based on liver biopsy [21]. Moreover, NAFLD prevalence was 86.8%, 73.0% and 70.7% in studies published between 2004-2010 [21,26], 2011-2015 [29,30,32,33] and 2016-2019 [35,36], respectively (P=0.69) in the same setting. In participants without MetS, the prevalence of NAFLD was 22.8% and 11.4% in studies in which NAFLD was diagnosed by ultrasonography [26,30,32,33] and FLI [35,36], respectively (P<0.01) and 21.8% 23.0% and 11.4% in studies published between 2004-2010 [26], 2011-2015 [30,32,33] and 2016-2019 [35,36], respectively (P<0.01) (Table 1).
Figure 4.
Pooled prevalence of nonalcoholic fatty liver disease (NAFLD) in adults in Europe by metabolic syndrome
CI, confidence interval
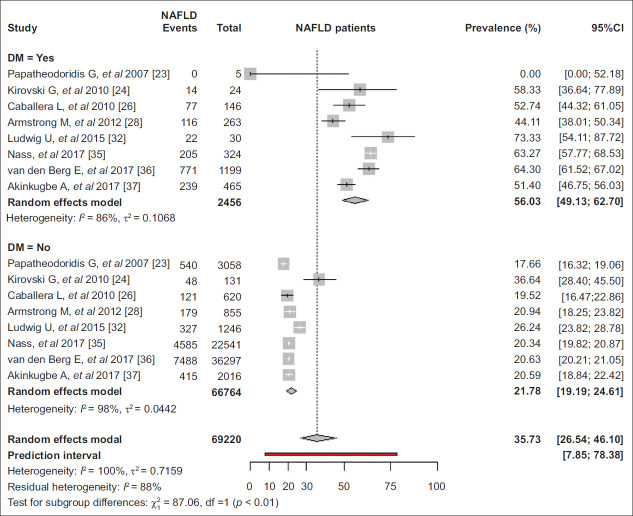
MetS parameters
The pooled prevalence of NAFLD was 56.0% (95%CI 49.1-62.7) and 21.8% (95%CI 19.2-24.6) in participants with and without diabetes (P<0.01) [23,24,26,28,32,35-37] (Fig. 5) and 39.3% (95%CI 31.3-48.0) and 19.9% (95%CI 15.2-25.6) in participants with and without arterial hypertension, respectively (P<0.01) [24,26,28,32,35,36]. Among patients with diabetes, the prevalence of NAFLD was higher in studies using FLI [35,36] than ultrasonography [24,26,28,32,37] (64.1% vs. 51.9%, P<0.01), but did not differ in relation to the publication period. In contrast, in individuals without diabetes, the prevalence of NAFLD was similar in studies using ultrasonography [24,26,28,32,36] or FLI [35,36] as well as in studies published between 2004-2010 [23,24,26], 2011-2015 [28,32] or 2016-2019 [35-37] (Table 1). Based on available data from 2 studies, the pooled prevalence of NAFLD was 44.0% (95%CI 20.4-70.8) and 18.7% (95%CI 11.7-28.4) in individuals with and without hyperlipidemia, respectively (P=0.053) [26,35].
Figure 5.
Pooled prevalence of nonalcoholic fatty liver disease (NAFLD) in adults in Europe by diabetes mellitus
CI, confidence interval
Finally, the pooled prevalence of NAFLD in patients with abnormal waist circumference (defined as >102 cm in men and >88 cm in women) was significantly higher, compared to those with normal waist circumference (37.6% vs. 16.0%, P<0.01) [23,26,37]. Among individuals with abnormal waist circumference, the prevalence of NAFLD was 42.6% in studies using ultrasonography [26,37] and 28.9% in the only study using liver biochemical tests [23] for NAFLD diagnosis (P<0.01) as well as 31.6% in studies published between 2004-2010 [23,26] and 48.7% in the only study [37] published between 2016-2019 (P<0.01). Similarly, in individuals with normal waist circumference, the prevalence of NAFLD was 17.4% [26,37] and 13.0% [23] in studies where NAFLD was diagnosed by ultrasonography and liver biochemical tests, respectively (P<0.01) as well as 15.8% and 16.7% in studies published between 2004-2010 [23,26] and 2016-2019 [37], respectively (P=0.71). There was no data for the NAFLD prevalence in relation to the waist circumference from studies published between 2011-2015 (Table 1).
Other comorbidities
As expected, the pooled NAFLD prevalence was higher in obese patients (BMI ≥30 kg/m2) than non-obese (BMI <30 kg/m2) individuals (57.0% vs. 13.7%, P<0.01) [23,26,28,35,36] (Supplementary Fig. 2 (450.2KB, pdf) ). In obese individuals, the prevalence of NAFLD was higher in studies using FLI [35,36] than ultrasonography [26,28] (80.4% vs. 41.5%, P<0.01) as well as in the more recently published studies (2004-2010 [23,26]: 37.4%, 2011-2015 [28]: 39.3%, 2016-2019 [35,36]: 80.4%, P<0.01). In non-obese subjects, the prevalence of NAFLD was lower in studies using FLI [35,36] than ultrasonography [26,28] for NAFLD diagnosis (10.3% vs. 18.2%, P<0.01) as well as in the 2 more recent studies published between 2016-2019, both of which used FLI for NAFLD diagnosis (2004-2010 [23,26]: 16.3%, 2011-2015 [28]: 17.5%, 2016-2019 [35,36]: 10.3%, P<0.01) (Table 1).
NAFLD prevalence in adults in relation to smoking
There were 3 studies [23,36,37] with evaluable data regarding smoking: 9,373 participants were current smokers, and 33,658 participants were ex or never smokers. Based on the available data from these three studies, the pooled prevalence of NAFLD was similar between current smokers and ex/never smokers [20.8% (95%CI 17.6-24.4) vs. 22.5% (95%CI 18.0-27.7), P=0.58].
NAFLD prevalence in adults in relation to CVD or chronic kidney disease
Based on the available data derived from 2 studies, the pooled prevalence of NAFLD was 43.5% (95%CI 40.5-46.6) and 21.2% (95%CI 20.7-21.7) in individuals with and without CVD (P<0.001) [35,36]. In addition, NAFLD was more frequent in those with than in those without chronic kidney disease [pooled prevalence 37.9% (95%CI 36.2-39.7) vs. 19.5% (95%CI 18.9-20.1), P<0.001] [35,36].
Children and adolescents
Eight of the 9 studies including children and/or adolescents were from single European countries (3 from Germany, 2 from Italy, 1 from UK, 1 from Poland and 1 from Greece) and 1 was multicenter having subjects from Germany, Austria and Switzerland [40-48]. Six studies included 17,590 only obese/overweight children/adolescents [40-44,46,48] and 3 studies included 2,352 children/adolescents from the general population [41,45,47] (in 1 [41] of these 3 studies, the prevalence of NAFLD in obese/overweight children/adolescents was also provided). Based on the Newcastle-Ottawa scale, the mean quality assessment score was 7 (range 6-9), whereas 5 studies were of high and 4 studies of fair quality.
Four of the 9 studies were published between 2004-2011 and 5 studies between 2012-2019. The method used for NAFLD diagnosis was ultrasonography in 6 studies (including 3,192 participants), liver biochemical tests (aminotransferases and/or GGT) in 1 study (including 16,390 participants), MRI in 1 study (including only 44 participants), and autopsy/postmortem liver biopsy in 1 study (including 265 participants).
General population
All studies assessing the prevalence of NAFLD in unselected children/adolescents were from non-Mediterranean countries (Germany, Poland and UK) [41,45,47]. The overall NAFLD prevalence was 2.7% (95%CI 2.1-3.4; primary-study range: 2.4-4.1%). The prevalence of NAFLD was: 2.8% (95%CI 1.9-4.2) in boys and 2.2% (95%CI 1.5-3.3) in girls [41,47], as well as 2.4% (95%CI 1.3-4.5) and 2.7% (95%CI 2.1-3.6) in studies published between 2004-2011 and 2012-2019, respectively [41,45,47]. NAFLD prevalence was 2.5% (95%CI 1.9-3.3) and 4.2% (95%CI 2.3-7.3) in studies in which NAFLD was diagnosed by ultrasonography and autopsy/postmortem liver biopsy, respectively [41,45,47] (always P>0.05).
Obese/overweight children/adolescents
The overall prevalence of NAFLD in obese/overweight children/adolescents was 31.6% (95%CI 17.6-49.9; primary-study range: 11.58-81.82) [40-44,46,48]. The prevalence of NAFLD was highest in studies using ultrasonography [37.7% (95%CI 19.4-60.2)] [41,42,44,46,48], intermediate in the study using MRI [31.8% (95%CI 19.8-46.8)] (40) and lowest in the study using liver biochemical tests for NAFLD diagnosis [11.6% (95%CI 11.1-12.1)] [43] (P<0.001). NAFLD prevalence was higher in studies from Mediterranean [40,44,48] than non-Mediterranean countries [41-43,46] [53.1% (95%CI 27.5-77.3) vs. 19.8% (95%CI 13.1-28.7), P=0.01; or 63.8% (95%CI 33.1-86.3) vs. 27.0% (95%CI 24.4-29.8), P=0.016, when only studies using ultrasonography for NAFLD diagnosis were considered]. Finally, NAFLD prevalence was higher in studies published between 2012-2019 [44,46,48] than 2004-2011 [40-43] [50.7% (95%CI 24.0-77.0) vs. 20.0% (95%CI 12.7-30.0), P=0.03; or 50.7% (95%CI 24.0-77.0) vs. 26.9% (95%CI 23.5-30.7), P=0.09, when only studies using ultrasonography for NAFLD diagnosis were considered].
Sex
Based on the available data, NAFLD was more frequent in obese/overweight boys than girls [32.5% (95%CI 22.7-44.0) vs. 15.5% (95%CI 7.6-29.0); P=0.04] [40-44,46]. The higher prevalence of NAFLD in obese/overweight boys than girls was maintained in both publication periods [2004-2011: 28.0% (95%CI 17.0-42.5) vs. 11.1% (95%CI 4.1-26.6); 2012-2019: 40.4% (95%CI 34.4-46.7) vs. 25.6% (95%CI 13.0-44.3)], in studies with NAFLD diagnosis by ultrasonography [40.6% (95%CI 36.4-44.9) vs. 15.1% (95%CI 5.2-36.6)] as well as in non-Mediterranean countries [30.6% (95%CI 19.0-45.2) vs. 10.8% (95%CI 6.0-18.8)] (always P<0.05), but not in studies from Mediterranean countries [39.7% (95%CI 28.8-51.7) vs. 37.9% (95%CI 26.5-51.0); P=0.84].
Discussion
Our meta-analysis, which is the first one exclusively focusing on NAFLD epidemiology in European adults and children/adolescents, confirms that the overall prevalence of NAFLD in European adults is high, exceeding 25%, without difference between Mediterranean and non-Mediterranean countries and publication period. NAFLD prevalence in adults is higher in men than women and patients with than without MetS or any of its components or with any related comorbidity such as obesity, CVD or chronic kidney disease (approximately 37-75% depending on the patient subgroup). The overall NAFLD prevalence remains low (<3%) in unselected children/adolescents, but it appears to exceed 30% in obese/overweight children/adolescents from European countries and to increase in the recent years.
Our findings regarding the overall pooled NAFLD prevalence in European adults (27%) is in accordance with estimations for US or global population [3,4], while rates range widely from 14% in non-obese participants to 75% in those with MetS. Moreover, the lower overall NAFLD prevalence in adult studies using liver biochemical tests (19%) compared to ultrasonography or FLI (27% and 30%, respectively) indicates that aminotransferases are unreliable for NAFLD diagnosis leading to underestimation of the true prevalence and that ultrasonography or FLI should be used for NAFLD screening, as suggested [1]. In our meta-analysis, ultrasonography was the most commonly used screening test (n=11 studies), followed by FLI (n=4) and liver function tests (aminotransferases/GGT, n=2), while, in only 1 study including adults with MetS, histological diagnosis was reported.
The absence of statistically significant differences in NAFLD rates between Mediterranean and non-Mediterranean countries (24% vs. 29%, P=0.14) might be explained by the widespread westernized lifestyle across Europe today, irrespective of the geographical region. Also, it is quite surprising that NAFLD prevalence in Europe did not increase significantly among different 5-year periods, (24% vs. 31% vs. 26%, in 2016-2019 vs. 2011-2015 vs. 2004-2010, P-value not significant), despite seemingly rising rates worldwide, perhaps because urbanization and lifestyle changes had already been established in Europe during the last 15 years, when all the included studies were published.
As expected, adults with MetS presented the highest prevalence of NAFLD among all subgroups (75%), irrespective of diagnostic modality and time period. In patients without MetS [26,30,32,33,35,36], the pooled prevalence of NAFLD was 18% and seemed alarmingly lower in studies using FLI compared to ultrasonography for NAFLD diagnosis (11% vs. 23%, P<0.01). Thus, ultrasonography rather than FLI may be safer to be used in patients without MetS, while the diagnostic method (ultrasound vs. FLI) seems not to be important in patients with MetS.
Comorbidities with MetS components or related diseases were associated with a higher prevalence of NAFLD indicating their role as risk factors and underlying the complex clinical management needed in these patients. In most subgroups, a higher NAFLD prevalence was reported with the use of ultrasonography and FLI compared to liver function tests, but ultrasonography compared to FLI seemed to underestimate the NAFLD prevalence in patients with diabetes [35,36].
Our meta-analysis also showed that more than half of the obese European adults have NAFLD (57%), while the relatively low rates among non-obese adults (14%) remain clinically relevant, particularly since studies have suggested that NAFLD in non-obese may hide more severe histological lesions and progress more rapidly to end-stage liver disease than obese patients [49]. Similar to the subgroup of diabetics, the prevalence of NAFLD in obese adults was lower in studies using ultrasonography than FLI (42% vs. 80%, P<0.01) suggesting that ultrasonography may underestimate NAFLD prevalence given the technical difficulties leading to inaccurate evaluation in obese subjects. In the same subgroup, the increased disease burden in the recent years (80% vs. 37-39% in 2016-2019 vs. 2004-2015, P<0.01) is also a finding that should not go unnoticed.
Last but not least, we evaluated, at the same depth as above, NAFLD prevalence among children/adolescents from Europe and unveiled some intriguing observations for the pediatric population where relative available epidemiological data were scarce. First, we showed that overall pooled prevalence of pediatric NAFLD is quite low (3%) without differences between boys and girls, among diagnostic methods or publication periods. However, the overall prevalence of NAFLD in obese/overweight children/adolescents seems worryingly high (32%) and highlights an essential clinical problem, with concealed risks for the future adults. Notably and contrary to the data from adults, there was a significantly higher NAFLD prevalence in obese children from the Mediterranean than non-Mediterranean countries (53% vs. 20%, P=0.01) and in obese boys than girls (33% vs. 16%, P=0.04), implicating additional perils for the populations of the specific regions and possible further deviation from the traditional protective Mediterranean diet and lifestyle. More severe obesity, behavioral and dietary differences as well as genetic differences may be responsible for the differences in NAFLD prevalence in children from Mediterranean and non-Mediterranean countries. Moreover, the higher rates in obese children/adolescents from studies using ultrasound or MRI than aminotransferases (38% or 32% vs. 12%, P<0.01) might indicate again that imaging techniques should be preferred for NAFLD diagnosis, similarly to adults. Finally, NAFLD rates in obese children/adolescents appear increased in more recent studies (2012-2019 than 2005-2011: 51% vs. 20%, P=0.03) and therefore, increased vigilance is considered of vital importance to control the NAFLD burden in Europe in the next decades.
Our systematic review has several limitations including data shortage from several European countries, a relatively small sample size of some studies, inclusion of participants only from urban areas in most studies, and use of different methods for NAFLD diagnosis. In addition, the sensitivity of ultrasonography, the most common diagnostic method, may vary among operators or time periods. However, these limitations do not reflect on the quality and/or importance of the meta-analysis rather than on the inherent weaknesses of the currently available epidemiological studies in this setting.
In short, our meta-analysis confirmed that NAFLD prevalence in Europe is similar to the global rates (>25%), and higher in patients with obesity and/or MetS. Specifically for these high-risk subgroups, ultrasonography seems to underestimate the true prevalence and therefore, FLI might be considered preferential for screening. As there are no epidemiological data from Europe based on newer non-invasive NAFLD diagnostic methods, future studies employing those modalities are needed to shed more light in the true prevalence rates. Finally, further research is warranted, especially in the Mediterranean region, to confirm the increasing NAFLD rates in obese/overweight children/adolescents.
Summary Box.
What is already known:
Nonalcoholic fatty liver disease (NAFLD) represents the most common cause of chronic liver disease in the developed countries
It is estimated that the global prevalence of NAFLD is 25% among adults being higher in patients with metabolic syndrome (MetS) or its components
What the new findings are:
In this first systematic review including exclusively European populations, the overall prevalence of NAFLD in adults was high, exceeding 25%, without difference between Mediterranean and non-Mediterranean countries and publication period
NAFLD prevalence in European adults is higher in men than women and patients with than without MetS or any of its components or with any related comorbidity such as obesity, cardiovascular disease or chronic kidney disease (ranging between 37% and 75%)
Ultrasound and fatty liver index (FLI) performed equally in estimating NAFLD prevalence in most subgroups; a higher prevalence was reported using FLI in obese and in diabetic patients, whereas a higher prevalence was observed with ultrasound in non-obese patients and in individuals without MetS
Biography
Medical School of National and Kapodistrian University, General Hospital of Athens “Laiko”, Athens; National and Kapodistrian University of Athens, P. & A. Kyriakou Children’s Hospital, Athens; Social-Preventive Medicine and Medical Statistics and Epidemiology, Medical School of Aristotle University of Thessaloniki, Thessaloniki, Greece
References
- 1.European Association for the Study of the Liver (EASL);European Association for the Study of Diabetes (EASD);European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease:Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 3.Vernon G, Baranova A, Younossi ZM. Systematic review:the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 4.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 5.Sayiner M, Koenig A, Henry L, Younossi ZM. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in the United States and the rest of the world. Clin Liver Dis. 2016;20:205–214. doi: 10.1016/j.cld.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Belli LS, Perricone G, Adam R, et al. Impact of DAAs on liver transplantation:Major effects on the evolution of indications and results. An ELITA study based on the ELTR registry. J Hepatol. 2018;69:810–817. doi: 10.1016/j.jhep.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg D, Ditah IC, Saeian K, et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology. 2017;152:1090–1099. doi: 10.1053/j.gastro.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noureddin M, Vipani A, Bresee C, et al. NASH leading cause of liver transplant in women:updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol. 2018;113:1649–1659. doi: 10.1038/s41395-018-0088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes:A systematic review and meta-analysis. J Hepatol. 2019;71:793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 10.PRISMA transparent reporting of systematic reviews and meta-analyses. [Accessed 17 January 2021]. Available from: http://www.prisma-statement.org/
- 11.Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in metaanalyses. 2004. [Accessed 25 January 2021]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp .
- 12.Stijnen T, Hamza TH, Ozdemir P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med. 2010;29:3046–3067. doi: 10.1002/sim.4040. [DOI] [PubMed] [Google Scholar]
- 13.Julious SA. Two-sided confidence intervals for the single proportion:comparison of seven methods by Robert G. Newcombe Statistics in Medicine 1998; 17:857-872. Stat Med. 2005;24:3383–3384. doi: 10.1002/sim.2164. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters JL, Sutton AJ, Jones DR, et al. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295:676–680. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172:137–159. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.IntHout J, Ioannidis JP, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6:e010247. doi: 10.1136/bmjopen-2015-010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R Core Team. R:A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. 2020. [Accessed 25 January 2021]. Available from: http://www.r-project.org/index.html .
- 19.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R:a practical tutorial. Evid Based Ment Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 21.Sorrentino P, Tarantino G, Conca P, et al. Silent non-alcoholic fatty liver disease-a clinical-histological study. J Hepatol. 2004;41:751–757. doi: 10.1016/j.jhep.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Bedogni G, Miglioli L, Masutti F, et al. Prevalence of and risk factors for nonalcoholic fatty liver disease:the Dionysos nutrition and liver study. Hepatology. 2005;42:44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 23.Papatheodoridis GV, Goulis J, Christodoulou D, et al. High prevalence of elevated liver enzymes in blood donors:associations with male gender and central adiposity. Eur J Gastroenterol Hepatol. 2007;19:281–287. doi: 10.1097/MEG.0b013e328011438b. [DOI] [PubMed] [Google Scholar]
- 24.Kirovski G, Schacherer D, Wobser H, et al. Prevalence of ultrasound-diagnosed non-alcoholic fatty liver disease in a hospital cohort and its association with anthropometric, biochemical and sonographic characteristics. Int J Clin Exp Med. 2010;3:202–210. [PMC free article] [PubMed] [Google Scholar]
- 25.Kotronen A, Yki-Järvinen H, Männistö S, et al. Non-alcoholic and alcoholic fatty liver disease - two diseases of affluence associated with the metabolic syndrome and type 2 diabetes:the FIN-D2D survey. BMC Public Health. 2010;10:237. doi: 10.1186/1471-2458-10-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caballería L, Pera G, Auladell MA, et al. Prevalence and factors associated with the presence of nonalcoholic fatty liver disease in an adult population in Spain. Eur J Gastroenterol Hepatol. 2010;22:24–32. doi: 10.1097/MEG.0b013e32832fcdf0. [DOI] [PubMed] [Google Scholar]
- 27.Rückert IM, Heier M, Rathmann W, et al. Association between markers of fatty liver disease and impaired glucose regulation in men and women from the general population:the KORA-F4-study. PLoS One. 2011;6:e22932. doi: 10.1371/journal.pone.0022932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armstrong MJ, Houlihan DD, Bentham L, et al. Presence and severity of non-alcoholic fatty liver disease in a large prospective primary care cohort. J Hepatol. 2012;56:234–240. doi: 10.1016/j.jhep.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 29.Soresi M, Noto D, Cefalù AB, et al. Nonalcoholic fatty liver and metabolic syndrome in Italy:results from a multicentric study of the Italian Arteriosclerosis society. Acta Diabetol. 2013;50:241–249. doi: 10.1007/s00592-012-0406-1. [DOI] [PubMed] [Google Scholar]
- 30.Caballería L, Pera G, Rodríguez L, et al. Metabolic syndrome and nonalcoholic fatty liver disease in a Spanish population:influence of the diagnostic criteria used. Eur J Gastroenterol Hepatol. 2012;2:1007–1011. doi: 10.1097/MEG.0b013e328355b87f. [DOI] [PubMed] [Google Scholar]
- 31.Kanerva N, Sandboge S, Kaartinen NE, et al. Higher fructose intake is inversely associated with risk of nonalcoholic fatty liver disease in older Finnish adults. Am J Clin Nutr. 2014;100:1133–1138. doi: 10.3945/ajcn.114.086074. [DOI] [PubMed] [Google Scholar]
- 32.Ludwig U, Holzner D, Denzer C, et al. Subclinical and clinical hypothyroidism and non-alcoholic fatty liver disease:a cross-sectional study of a random population sample aged 18 to 65 years. BMC Endocr Disord. 2015;15:41. doi: 10.1186/s12902-015-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graeter T, Niedermayer PC, Mason RA, et al. Coffee consumption and NAFLD:a community based study on 1223 subjects. BMC Res Notes. 2015;8:640. doi: 10.1186/s13104-015-1645-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Markus MR, Meffert PJ, Baumeister SE, et al. Association between hepatic steatosis and serum liver enzyme levels with atrial fibrillation in the general population:The Study of Health in Pomerania (SHIP) Atherosclerosis. 2016;245:123–131. doi: 10.1016/j.atherosclerosis.2015.12.023. [DOI] [PubMed] [Google Scholar]
- 35.Nass KJ, van den Berg EH, Faber KN, et al. High prevalence of apolipoprotein B dyslipoproteinemias in non-alcoholic fatty liver disease:The lifelines cohort study. Metabolism. 2017;72:37–46. doi: 10.1016/j.metabol.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 36.van den Berg EH, Amini M, Schreuder TC, et al. Prevalence and determinants of non-alcoholic fatty liver disease in lifelines:A large Dutch population cohort. PLoS One. 2017;12:e0171502. doi: 10.1371/journal.pone.0171502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akinkugbe AA, Avery CL, Barritt AS, et al. Do Genetic markers of inflammation modify the relationship between periodontitis and nonalcoholic fatty liver disease?Findings from the SHIP Study. J Dent Res. 2017;96:1392–1399. doi: 10.1177/0022034517720924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foschi FG, Bedogni G, Domenicali M, et al. Prevalence of and risk factors for fatty liver in the general population of Northern Italy:the Bagnacavallo Study. BMC Gastroenterol. 2018;18:177. doi: 10.1186/s12876-018-0906-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leitão J, Carvalhana S, Silva AP, et al. No evidence for lower levels of serum Vitamin D in the presence of hepatic steatosis. A study on the portuguese general population. Int J Med Sci. 2018;15:1778–1786. doi: 10.7150/ijms.26586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Radetti G, Kleon W, Stuefer J, Pittschieler K. Non-alcoholic fatty liver disease in obese children evaluated by magnetic resonance imaging. Acta Paediatr. 2006;95:833–837. doi: 10.1080/08035250500449890. [DOI] [PubMed] [Google Scholar]
- 41.Imhof A, Kratzer W, Boehm B, et al. Prevalence of non-alcoholic fatty liver and characteristics in overweight adolescents in the general population. Eur J Epidemiol. 2007;22:889–897. doi: 10.1007/s10654-007-9181-7. [DOI] [PubMed] [Google Scholar]
- 42.Denzer C, Thiere D, Muche R, et al. Gender-specific prevalences of fatty liver in obese children and adolescents:roles of body fat distribution, sex steroids, and insulin resistance. J Clin Endocrinol Metab. 2009;94:3872–3881. doi: 10.1210/jc.2009-1125. [DOI] [PubMed] [Google Scholar]
- 43.Wiegand S, Keller KM, Röbl M, et al. Obese boys at increased risk for nonalcoholic liver disease:evaluation of 16,390 overweight or obese children and adolescents. Int J Obes (Lond) 2010;34:1468–1474. doi: 10.1038/ijo.2010.106. [DOI] [PubMed] [Google Scholar]
- 44.Papandreou D, Karabouta Z, Pantoleon A, Rousso I. Investigation of anthropometric biochemical and dietary parameters of obese children with and without non-alcoholic fatty liver disease. Appetite. 2012;59:939–944. doi: 10.1016/j.appet.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 45.Rorat M, Jurek T, Kuchar E, et al. Liver steatosis in Polish children assessed by medicolegal autopsies. World J Pediatr. 2013;9:68–72. doi: 10.1007/s12519-012-0387-8. [DOI] [PubMed] [Google Scholar]
- 46.Schlieske C, Denzer C, Wabitsch M, et al. Sonographically measured suprailiac adipose tissue is a useful predictor of non-alcoholic fatty liver disease in obese children and adolescents. Pediatr Obes. 2015;10:260–266. doi: 10.1111/ijpo.265. [DOI] [PubMed] [Google Scholar]
- 47.Lawlor DA, Callaway M, Macdonald-Wallis C, et al. Nonalcoholic fatty liver disease, liver fibrosis, and cardiometabolic risk factors in adolescence:a cross-sectional study of 1874 general population adolescents. J Clin Endocrinol Metab. 2014;99:E410–E417. doi: 10.1210/jc.2013-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valentini D, Alisi A, di Camillo C, et al. Nonalcoholic fatty liver disease in Italian children with Down syndrome:prevalence and correlation with obesity-related features. J Pediatr. 2017;189:92–97. doi: 10.1016/j.jpeds.2017.05.077. [DOI] [PubMed] [Google Scholar]
- 49.Denkmayr L, Feldman A, Stechemesser L, et al. Lean patients with non-alcoholic fatty liver disease have a severe histological phenotype similar to obese patients. J Clin Med. 2018;7:E562. doi: 10.3390/jcm7120562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.