Abstract
The efficacy of epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) in lung squamous cell carcinoma is said to be low. Thus far, only four cases of osimertinib in lung squamous cell carcinoma have been published. We experienced a case of EGFR mutant lung squamous cell carcinoma in which fifth-line treatment with osimertinib was effective after T790M EGFR mutation turned positive. Osimertinib was resumed after sixth-line chemotherapy was ineffective, showing efficacy again. Osimertinib may be a promising treatment option for EGFR mutant lung squamous cell carcinoma. This is the first report to show its effect in a case of rechallenge after intervening chemotherapy. It may therefore be important to evaluate EGFR in never-smoker lung squamous cell carcinoma patients.
Keywords: epidermal growth factor receptor, EGFR, tyrosine kinase inhibitor, TKI, adenosquamous, rechallenge
Introduction
Epidermal growth factor receptor (EGFR) mutations can sometimes be found in lung squamous cell carcinoma. The frequency is reported to be less than 5% (1). This is markedly lower than in lung adenocarcinoma, in which EGFR mutations are found in 19.2% of Caucasians and 47.9% of Asians (2). The efficacy of EGFR-tyrosine kinase inhibitors (TKIs) in EGFR mutant lung squamous cell carcinoma is also reported to be lower than in adenocarcinoma. Collective reports on the effectiveness of EGFR-TKIs in squamous cell carcinoma are limited (3,4), and there are none concerning osimertinib. Thus far, only four cases concerning the use of osimertinib in lung squamous cell carcinoma have been described (5-7), and there are none describing the efficacy of rechallenge with osimertinib in lung squamous cell carcinoma.
We experienced a case of EGFR mutant lung squamous cell carcinoma with a secondary T790M EGFR mutation that showed good response to fifth-line treatment with osimertinib as well as subsequent rechallenge as seventh-line therapy. We present this valuable case and a literature review of the efficacy of osimertinib in lung squamous cell carcinoma.
Case Report
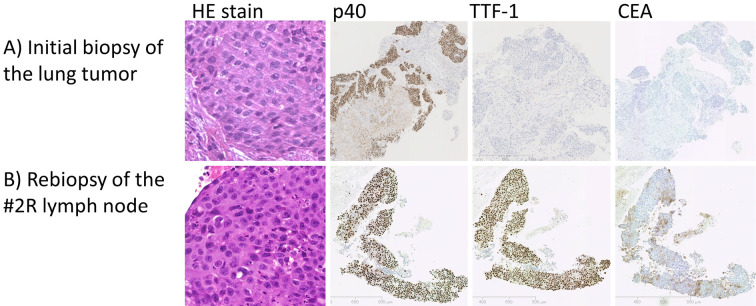
A 60-year-old woman with no medical history was referred for persistant cough and right lower lung opacity on chest X-ray. There was no family history of cancer. She was a never-smoker. Computed tomography (CT) of the chest revealed a large mass and atelectasis in the right lower lobe, and right pleural effusion. 18F-fluorodeoxyglucose (FDG) positron emission tomography showed a high FDG uptake in the lung tumor, bilateral mediastinal lymph nodes, and right pleural lesions. Serum cytokeratin 19 fragment (CYFRA) was elevated (14.9 ng/mL), and carcinoembryonic antigen (CEA) was within the normal limits (3.9 ng/mL). Bronchoscopy revealed a visible tumor in the right lower bronchus, and a biopsy showed lung squamous cell carcinoma. Immunostaining was consistent with squamous cell carcinoma; p40 and Cytokeratin 14 (CK14) were positive, while adenocarcinoma markers thyroid transcription factor 1 (TTF-1), carcinoembryonic antigen (CEA), and Napsin A were negative (Fig. 1A). This confirmed the diagnosis of lung squamous cell carcinoma T4N3M1a clinical stage IVA. EGFR exon 21 L858R was identified on a molecular analysis.
Figure 1.
Biopsy specimens. A) The initial biopsy of the lung tumor. Hematoxylin and Eosin (H&E) staining shows squamous cell carcinoma morphology. Immunostaining with p40 and Cytokeratin 14 (CK14) were positive, while adenocarcinoma markers thyroid transcription factor 1 (TTF-1), carcinoembryonic antigen (CEA), and Napsin A were negative. B) The rebiopsy of the #2R lymph node. H&E staining shows definite squamous cell carcinoma morphology, identical to that of the primary lung tumor. Immunostaining shows features of squamous cell carcinoma (positive p40 and CK 14) along with weak positivity in TTF-1, Napsin A, and CEA.
First-line treatment with afatinib was initiated, showing a partial response (PR) according to RECIST 1.1 criteria. The serum CYFRA level decreased to a normal level. Eight months later, while the primary tumor was stable, a newly enlarged #2R lymph node proved progressive disease (PD). Endobronchial ultrasound-guided transbronchial needle aspiration of the #2R lymph node was performed. Hematoxylin and eosin (HE) staining showed squamous cell carcinoma morphology, identical to that of the primary lung tumor. Immunostaining showed features of squamous cell carcinoma (positive p40 and CK 14) along with weak positivity for TTF-1, Napsin A, and CEA, suggesting squamous cell carcinoma with some adenocarcinoma features (Fig. 1B). T790M was not found in either the rebiopsy specimen or the liquid biopsy. Second- to fourth-line treatments with cytotoxic chemotherapy and immune checkpoint inhibitors were performed.
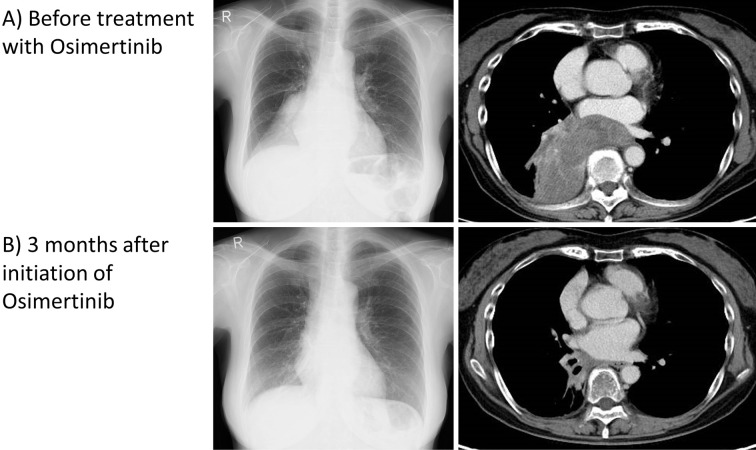
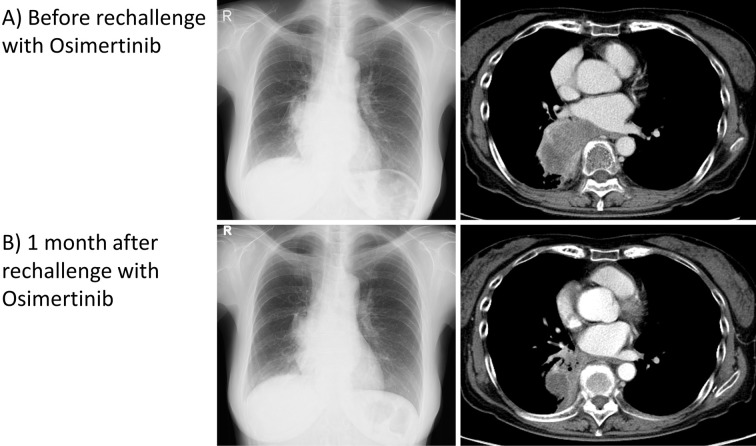
After fourth-line treatment failed and the tumor grew (Fig. 2A), the serum CYFRA level rose to 10.5 ng/mL, while the CEA level stayed within the normal limits. A liquid biopsy revealed a positive T790M EGFR mutation. Therefore, osimertinib was selected as the fifth-line treatment. Osimertinib was effective, and in three months, the tumor size shrank considerably (Fig. 2B). Osimertinib maintained a PR for 7 months, after which the serum CEA level rose to 20.1 ng/mL, while the CYFRA level was within the normal limits. The primary tumor had grown, indicating PD, and the patient also developed Trousseau's syndrome. Sixth-line treatment with chemotherapy showed no effect, and the serum CEA level remained high. As cytotoxic chemotherapy showed hematologic toxicities, osimertinib was resumed as seventh-line treatment, and this rechallenge was also effective for another two months, with the lung tumor decreasing in size (Fig. 3). Eighth-line treatment with cytotoxic chemotherapy was ineffective, and the patient died three years after the initial diagnosis.
Figure 2.
Chest X-ray and chest CT with contrast. A) Before treatment with osimertinib, a large tumor in the right lower lobe had caused atelectasis. B) Three months after initiation of osimertinib, the tumor size had shrunk greatly, and the atelectasis was improved.
Figure 3.
Chest X-ray and chest CT with contrast. A) Before rechallenge treatment with osimertinib, a large tumor in the right lower lobe could be seen. B) One month after restarting treatment with osimertinib, the tumor size had decreased significantly.
Discussion
A case of EGFR mutant squamous cell carcinoma with a secondary T790M EGFR mutation showed a good response to fifth-line treatment with osimertinib for seven months in addition to two months as a rechallenge. Osimertinib may be a reasonable choice of treatment in patients with EGFR mutant lung squamous cell carcinoma.
The efficacy of EGFR-TKIs in EGFR mutant lung squamous cell carcinoma is reported to be lower than in adenocarcinoma. A previous study showed that 33 (13.3%) of 249 patients with squamous cell carcinoma had EGFR mutations (3). Twenty of these cases received EGFR-TKI treatment (either erlotinib or gefitinib), and the response rate was 25.0% [95% confidence interval (CI), 8.7-49.1%]. The progression-free survival (PFS) was 1.4 months, and the overall survival (OS) was 14.6 months. A pooled analysis of EGFR mutant non-adenocarcinoma non-small-cell lung carcinoma (NSCLC) patients treated with gefitinib reported a 27% response rate, 67-70% disease control rate, and 3.0-month median PFS (4). The overall effect was significantly poorer than in EGFR mutant adenocarcinoma patients but better than in EGFR wild-type squamous cell carcinoma. Therefore, the Japanese guidelines state the recommendation level regarding the use of EGFR-TKIs in advanced EGFR mutant lung squamous cell carcinoma as “2D,” in contrast to “1A” for adenocarcinoma (8). A recent study including 1709 adenocarcinoma and 77 non-adenocarcinoma patients with an EGFR mutation-positive status investigated the efficacy of erlotinib and gefitinib on these patients. The median OS for patients receiving EGFR-TKI treatment was significantly longer than that of patients who did not receive this treatment. There were no significant differences in the clinical characteristics between those who responded to EGFR-TKIs and those who did not (9).
However, the efficacy of the new EGFR-TKI osimertinib remains unclear at present. In the FLAURA and AURA trials, which studied the effectiveness of osimertinib in lung cancer, squamous cell carcinoma accounted for less than 1% of cases (10,11). Therefore, there are no data available on the clinical response in such cases. To our knowledge, publications concerning the use of osimertinib in primary lung squamous cell carcinoma are limited to case reports (5-7). In two of these cases as well as our present case, osimertinib was effective once T790M turned positive after other EGFR-TKI failures (5,6). Recently, osimertinib has been approved as the first-line therapy for EGFR mutant NSCLC. The effect of osimertinib as a first-line treatment for EGFR mutant lung squamous cell carcinoma has also been reported in only two cases (7). Collective data are awaited regarding the effects of osimertinib on lung squamous cell carcinoma.
Few reports have documented the efficacy of osimertinib for lung adenocarcinoma with secondary T790M mutation-positive squamous cell carcinoma transformation after failure of other EGFR-TKIs (12,13). Osimertinib was selected in four cases, and all showed clinical efficacy. EGFR-TKI therapy can alter the tumor histology from adenocarcinoma to either a small cell or squamous cell phenotype. Our case had squamous cell morphology on HE staining of both the primary lung tumor as well as the lymph node, which was biopsied later in the course. However, the immunostaining status changed to include some features of adenocarcinoma in addition to the original squamous cell carcinoma features. This may have been due to the squamous cell carcinoma acquiring adenocarcinoma features during the treatment course. Alternatively, the tumor may have originally been adenosquamous carcinoma, and the biopsied specimen (both at the initial diagnosis and rebiopsy) were simply taken from the squamous cell morphology proportion. Hata et al. pointed out the possibility of poorly differentiated adenocarcinoma morphologically mimicking squamous cell carcinoma (3). They stated that, regardless of the histology, the sensitivity of EGFR-TKIs in patients with non-adenocarcinoma harboring EGFR mutations might depend on the proportion of EGFR mutated adenocarcinoma components within the whole tumor. Changes in the tumor characteristics over the treatment course may influence the effects of EGFR-TKIs in certain squamous cell carcinoma cases. In addition, the positive T790M EGFR mutation status may have been responsible for the substantial effect of osimertinib in our case.
As a bronchoscopy biopsy only allows for a small specimen, it is difficult to diagnose squamous cell carcinoma. In non-surgical specimens, even when the pathology reports suggest squamous cell carcinoma, there is a possibility that it may be adenocarcinoma or adenosquamous carcinoma. As EGFR mutations have been found more frequently in never-smokers than in ever-smokers (14), it may therefore be important to test for EGFR in never-smoker NSCLC patients. Current ESMO guidelines also recommend testing for EGFR mutations in current non-smokers or light smokers (15).
There are no known factors that contribute to patients with EGFR mutant lung squamous cell carcinoma showing a good response to EGFR-TKIs. However, some patients in the previous study in whom gefitinib or erlotinib was effective were never smokers, had high CEA levels, or had positive immunostaining findings for TTF-1 (3). A non-smoking history is a factor that suggests the effectiveness of EGFR-TKIs in adenocarcinoma, so this is understandable. High CEA levels and positive TTF-1 staining indicate adenocarcinoma features. EGFR mutant patients with these features may benefit from osimertinib, even if the morphology is squamous cell carcinoma. Therefore, osimertinib may be a promising treatment option for these cases.
Rechallenge with an EGFR-TKI after intervening chemotherapy is a treatment option that has been reported to be effective in some cases. It is said that the intervening chemotherapy eradicates the clones of cancer cells that are responsible for clinical resistance to an EGFR-TKI, allowing EGFR-TKI-sensitive cells to regrow and thereby resensitizing the tumor to the EGFR-TKI (16). Rechallenge with the same EGFR-TKI has shown efficacy, such as a 52.5% clinical benefit with gefitinib after its initial failure (17). In addition, rechallenge with the second-generation EGFR-TKI afatinib after failure with cytotoxic chemotherapy and either elrotinib or gefitinib was also reported to be effective, with a response rate of 11.6% and a median PFS of 3.9 months (18). However, publications on the rechallenge of osimertinib are still limited to case reports (16).
Our case was sensitive to rechallenge of osimertinib after intervening chemotherapy, although efficacy was only noted for two months (compared to the initial effect of seven months). In patients with EGFR mutant lung cancer, osimertinib may also be a promising treatment choice, especially in late lines where there are few options left. Data regarding its rechallenge are also awaited.
Conclusion
It may be advisable to consider EGFR testing in patients with lung squamous cell carcinoma who have little or no smoking history. Osimertinib may be a reasonable treatment choice in patients with EGFR mutant lung squamous cell carcinoma. In cases showing efficacy with osimertinib, rechallenge after intervening chemotherapy may also be a viable treatment choice.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Perez-Moreno P, Brambilla E, Thomas R, Soria JC. Squamous cell carcinoma of the lung: Molecular subtypes and therapeutic opportunities. Clin Cancer Res 18: 2443-2451, 2012. [DOI] [PubMed] [Google Scholar]
- 2. Dearden S, Stevens J, Wu YL, Blowers D. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Ann Oncol 24: 2371-2376, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hata A, Katakami N, Yoshioka H, et al. How sensitive are epidermal growth factor receptor-tyrosine kinase inhibitors for squamous cell carcinoma of the lung harboring EGFR gene-sensitive mutations? J Thorac Oncol 8: 89-95, 2013. [DOI] [PubMed] [Google Scholar]
- 4. Shukuya T, Takahashi T, Kaira R, et al. Efficacy of gefitinib for non-adenocarcinoma non-small-cell lung cancer patients harboring epidermal growth factor receptor mutations: a pooled analysis of published reports. Cancer Sci 102: 1032-1037, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yamasaki M, Funaishi K, Daido W, Hattori N. Acquired T790M-positive squamous cell lung carcinoma that responded to osimertinib. Arch Bronconeumol 55: 602-603, 2019. [DOI] [PubMed] [Google Scholar]
- 6. Cortiula F, De Maglio G, Cangi MG, et al. Third-generation tyrosine kinase inhibitor in the treatment of epidermal growth factor receptor mutated squamous cell lung cancer: a tailored therapy approach. Ann Transl Med 7: 14, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shoji S, Watanabe S, Takamura K, Umezu H, Kikuchi T. First-line osimertinib treatment in patients with lung squamous cell carcinoma harboring active epidermal growth factor receptor mutations. Lung Cancer 140: 113-115, 2020. [DOI] [PubMed] [Google Scholar]
- 8. Akamatsu H, Ninomiya K, Kenmotsu H, et al. The Japanese Lung Cancer Society Guideline for non-small cell lung cancer, stage IV. Int J Clin Oncol 24: 731-770, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kobayashi K, Soejima K, Fukunaga K, et al. Key prognostic factors for EGFR-mutated non-adenocarcinoma lung cancer patients in the Japanese Joint Committee of Lung Cancer Registry Database. Lung Cancer 146: 236-243, 2020. [DOI] [PubMed] [Google Scholar]
- 10. Vansteenkiste J, Wauters E. Tyrosine kinase inhibition of EGFR: a successful history of targeted therapy for NSCLC since 20 years. Ann Oncol 29 (Suppl): i1-i2, 2018. [DOI] [PubMed] [Google Scholar]
- 11. Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 376: 629-640, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamaguchi F, Kato E, Wakabayashi A, Shikama Y. Effect of osimertinib treatment on lung adenocarcinoma with squamous cell transformation harboring the T790M mutation: a case report and literature review. Mol Clin Oncol 11: 127-131, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roca E, Pozzari M, Vermi W, et al. Outcome of EGFR-mutated adenocarcinoma NSCLC patients with changed phenotype to squamous cell carcinoma after tyrosine kinase inhibitors: a pooled analysis with an additional case. Lung Cancer 127: 12-18, 2019. [DOI] [PubMed] [Google Scholar]
- 14. Huang Y, Wang R, Pan Y, et al. Clinical and genetic features of lung squamous cell cancer in never-smokers. Oncotarget 7: 35979-35988, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 27 (Suppl): v1-v27, 2016. [DOI] [PubMed] [Google Scholar]
- 16. Metro G, Baglivo S, Siggillino A, et al. Successful response to osimertinib rechallenge after intervening chemotherapy in an EGFR T790M-positive lung cancer patient. Clin Drug Investig 38: 983-987, 2018. [DOI] [PubMed] [Google Scholar]
- 17. Cappuzzo F, Morabito A, Normanno N, et al. Efficacy and safety of rechallenge treatment with gefitinib in patients with advanced non-small cell lung cancer. Lung Cancer 99: 31-37, 2016. [DOI] [PubMed] [Google Scholar]
- 18. Landi L, Tiseo M, Chiari R, et al. Activity of the EGFR-HER2 dual inhibitor afatinib in EGFR-mutant lung cancer patients with acquired resistance to reversible EGFR tyrosine kinase inhibitors. Clin Lung Cancer 15: 411-417.e4, 2014. [DOI] [PubMed] [Google Scholar]