Abstract
Objective
Neuroendocrine neoplasms (NENs) are rare and indolent diseases, but the efficacy of treatment without surgical resection is temporary and limited. Targeted immunotherapy is an important treatment strategy in several cancers. However, the tumor and host immune reactions in the NEN microenvironment are poorly understood. Therefore, we investigated the immune checkpoint system and host immune response in pathological NEN specimens.
Methods
The expression of the mismatch repair proteins MSH2, MSH6, PMS2, and MLH1 was immunohistochemically detected in archival tissue samples obtained from 20 patients with gastroenteropancreatic NENs. We additionally assessed the expression of programmed death (PD)-1, PD-L1, and the tumor-infiltrating lymphocyte (TIL) markers CD8 and family of transcription factor P3 (FOXP3).
Results
All 20 NENs expressed the mismatch repair proteins MSH2, MSH6, PMS2, and MLH1. The PD-L1 and/or PD-1 expression in the tumor cells and/or TILs was confirmed in 75% of the cases. PD-1-, CD8-, and FOXP3-positive TILs were more frequently associated with PD-L1-positive tumors than with PD-L1 negative tumors (PD-1: 19.5 vs. 7.3, CD8: 18.1 vs. 7.1, FOXP3: 13.2 vs. 3.2, p=0.438, p=0.419, P=0.603, respectively). The number of cells positive for PD-1 tended to gradually increase in increasing grade of NENs but did not reach significance (Grade 1: 5.8, Grade 2: 10.2, NEC: 18.1, p=0.903).
Conclusion
NENs consistently express mismatch repair proteins but have a high expression of PD-L1 and/or PD-1 in the tumor microenvironment. NEC and PD-L1-positive NENs may be immunologically “hot” tumors, so an immunological approach may be an appropriate treatment strategy for these tumors.
Keywords: GEP-NEN (gastroenteropancreatic neuroendocrine neoplasms), immune checkpoint system, T-cell markers
Introduction
Neuroendocrine neoplasms (NENs) are relatively rare tumors, although the incidence has been increasing in recent years (1,2). Surgical resection is the most effective treatment; however, there are other useful treatment options for unresectable and/or recurrent NENs. Somatostatin analogues, such as octreotide or lanreotide, molecular-targeted agents, such as sunitinib or everolimus, and cytotoxic agents, such as streptozocin or temozolomide, are available treatments for neuroendocrine tumors (NETs), and platinum-based chemotherapy was shown to be effective for treating neuroendocrine carcinoma (NEC); however, its efficacy was temporary and limited (3-8).
The interaction between programmed death 1 (PD1) and its ligand PD-L1 is one of the most important and well-known immunosuppressive mechanisms, functioning by establishing an immune checkpoint in many malignant tumors. Anti-PD-1 or anti-PD-L1 antibody can inhibit the immune checkpoint and activate cytotoxic T cells to attack the cancer (9). Clinically, these immune checkpoint inhibitors (ICIs) are currently used to treat several tumor types, such as melanoma, non-small cell lung cancer, and renal cell carcinoma, showing good efficacy (10-13).
The degree of tumor-infiltrating lymphocytes (TILs) is considered to be a general prognostic factor, and cytotoxic T cells (CTLs) play an important role in the immune response to cancer; however, their activation is blocked by the immune checkpoint system (14), and regulatory T cells (Tregs) can also suppress the tumor immune reaction. Forkhead or winged helix family of transcription factor P3 (FOXP3) is critical for the development and function of Tregs, and an increased tumor FOXP3 expression was shown to be related to a poor prognosis in patients with some types of cancer (15,16). Colon cancer typically shows a good response to ICIs, primarily due to microsatellite instability (MSI) and characterized by abundant lymphocyte infiltration of the tumor (17). MSI is believed to be a useful predictor of the sensitivity to ICIs, and pembrolizumab has been approved for the treatment of any cancer with a high MSI status in the US and Japan.
There have been several clinical trials concerning treatment with ICIs for unresectable NENs. According to the initial clinical trial of the PD1 monoclonal antibody pembrolizumab, the response rate was only 6% in pancreatic and 12% in non-pancreatic PD-L1 positive NETs. A phase II study of previously treated advanced NETs revealed an objective response rate of 3.7%, and all responders had PD-L1-negative tumors (18). In contrast, the Cytotoxic T-Lymphocyte Antigen (CTLA)-4 antibody ipilimumab plus PD1 monoclonal antibody nivolumab demonstrated a 44% objective response rate in patients with non-pancreatic high-grade NETs (19). Therefore, the efficacy of ICIs for NENs is controversial, and more detail examinations are necessary.
At present, a high MSI status is the only available biomarker for solid tumors, including NENs; however, the importance of the PD-L1 and/or PD1 expression in NENs remains controversial, since in most cases, only small series with differences in grade and other major tumor characteristics have been investigated.
The present study explored the pathological characteristics of the tumor immunological microenvironment with a focus on the immune checkpoint system.
Materials and Methods
Study population
We included tumor tissue samples from 20 consecutive patients with gastroenteric-pancreatic (GEP)-NEN from April 2010 to January 2015. All cases had already been pathologically diagnosed with NET or NEC. We reviewed these specimens mainly for their morphological features and reclassified them as NET Grade 1, 2, or 3 or NEC according to the World Health Organization (WHO) 2017. The clinicopathological findings, including the age, gender, primary lesion, tumor size, and stage, were also collected from the medical records of each case.
This study was designed as a historical cohort study and was approved by the Institutional Review Board (IRB B170300001) of Yokohama City University Hospital.
Pathological findings and immunohistochemistry
To confirm the diagnosis of NEN, Hematoxylin and Eosin staining was performed on the tissue sections, along with staining for synaptophysin (Abcam, Tokyo, Japan; clone SY38, dilution 1: 100), chromogranin A (Abcam; dilution 1: 400), and CD56 (Biorbyt, San Francisco, USA; dilution 1: 400). To determine the grade of NEN, the sections were stained for the proliferation marker Ki67, and the Ki67 labeling index was calculated by having 2 pathological specialists count 2,000 tumor cells. Tissue sections (4-μm thick) were deparaffinized in xylene and rehydrated through a graded series of alcohol washes. The endogenous peroxidase activity of the specimens was blocked by incubating the slides in absolute methanol containing 0.3% hydrogen peroxide for 30 minute at room temperature. Antigen retrieval was carried out via autoclave pretreatment (120 °C for 5 minutes) in citrate buffer (pH 6). After washing with phosphate-buffered saline, the specimens were incubated with 10% rabbit serum albumin for 10 minutes and then with the following primary monoclonal antibodies at 37 °C for 1 hour: MLH1 (BioGenex, San Ramon, USA; clone ES05, dilution 1:30), MSH2 (Biocare Medical, Pacheco, USA; clone FE11, dilution 1:100), MSH6 (Leica Microsystems, Tokyo, Japan; clone PU29, dilution 1: 30), PMS2 (Leica, Newcastle, UK; clone M0R4G, dilution 1:50), PD-L1 (Abcam; clone NAT105, dilution 1: 100), PD-1 (Abcam; clone 28-8, dilution 1: 100c), CD8 (Origene Technologies, Rockville, USA; clone 4B11, dilution 1: 50), and FOXP3 (Abcam; clone 42, 1: 100). All eight primary antibodies were treated using PT LINK (Dako, Glostrup, Denmark). Immunostaining was then performed using an automated staining system (BOND-MAX system; Leica Biosystems, Melbourne, Australia).
The evaluation of immunohistochemistry staining
The pathological specimens were evaluated based on a previously reported method (20). When more than 1% of the tumor cells showed PD-L1 expression, the specimen was determined to be positive for that marker and was otherwise considered negative. The first serial section was stained with PD-L1, and the second, third, and fourth sections were sequentially subjected to immunohistochemistry to detect PD-1, CD-8, and FOXP3 antigens. To evaluate the PD-1, CD-8, and FOXP3 expression, lymphocytes were counted in at least 3 different high-power fields (×40 objective and ×10 eyepiece), and the averages were compared. We distinguished and evaluated the lesions of infiltrating lymphocytes, intra-tumoral lesions, and stromal lesions. PD-1-, CD8-, and FOXP3-expressing TILs were manually counted and categorized semi-quantitatively per punch into low (<3 positive lymphocytes) and high (≥3 positive lymphocytes) classes (21). The cell count was performed by two independent researchers (S.H. and N.K.), and the results obtained were compared until an agreement was reached. All mismatch repair (MMR) proteins, such as MSH2, MSH6, PMS2, and MLH1, were assessed in the nuclei of cells. Negative staining was defined as all loss of neoplastic epithelium, i.e. if the nucleus of a neoplastic cell showed any positivity, the cell was considered positive for MMR protein. The nuclear immunoreactions of lymphocytes and stromal cells served as a positive control. An Olympus microscope (Tokyo, Japan; model BX51) was used for all evaluations.
Statistical analyses
Data were analyzed using the SPSS Statistics version 21 (IBM Corporation, Armonk, USA). Data were compared between groups using Pearson’s chi-squared test, Wilcoxon’s rank sum test, and the Kruskal-Wallis test.
Results
Clinicopathological characteristics of NEN patients
The 20 Japanese patients diagnosed with GEP-NET, including 11 men and 9 women, had a median age of 62 (range 28-82) years old (Table 1). The primary sites were the pancreas in 12 patients, small intestine in 4 patients, colon in 2 patients, liver in 1 patient, and unknown in 1 patient. All 20 patients received surgical resection as the first treatment, and specimens evaluated in this study were obtained from the primary lesions for 15 patients and from a metastasis site for 5 patients. Primary unknown cases had adrenal, mediastatic, and bony metastases, and pathological specimens were obtained from metastatic bony tumor. The clinical stages were as IA in 6 patients, IB in 3 patients, II in 3 patients, III in 1 patient. and IV in 7 patients. The median tumor size was 20 (ranging 2-105) mm. Six cases were pathologically graded as NET Grade 1, eight as NET Grade 2, and six as NEC. There were no cases graded as NET Grade 3. Among the six cases of NEC, four were small-cell-type (SCNEC), and two were large-cell-type (LCNEC).
Table 1.
Cilnical Characteristics of This Study Patients.
| Gender, n | |
| Male | 11 |
| Female | 9 |
| Age, years, median (range) | 62 (28-82) |
| Primary lesion (WHO classification G1/G2/G3/NEC), n | |
| Pancreas | 12 (5/6/0/1) |
| Liver | 1 (0/0/0/1) |
| Small intestine | 4 (1/2/0/1) |
| Colon | 2 (0/0/0/1) |
| Unkonwn | 1 (0/0/0/2) |
| Total | 20 (6/8/0/6) |
| Site obtained from | |
| Primary lesion | 15 |
| Metastatic lesion | 5 |
| Clinical Stage (UICC classificasion) | |
| IA/IB/II/III/IV | 6/3/3/1/7 |
| Tumor size, mm, median (range) | 20 (2-105) |
UICC classification, the 8th of the UICC TNM Classification of Malignant Tumors.
MSI status
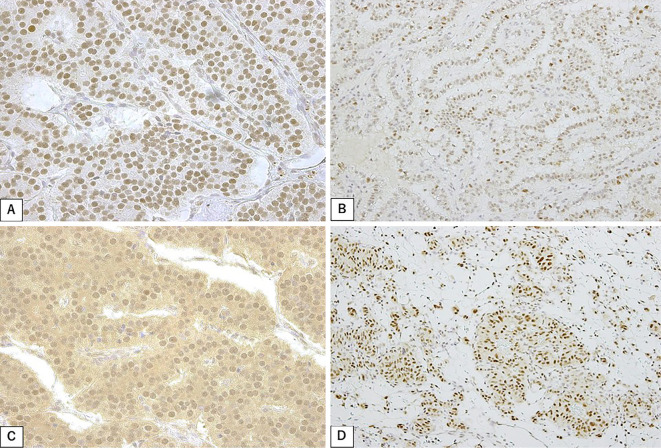
The expression of mismatch repair proteins MSH2, MSH6, PMS2, and MLH1 was positive for all 20 patients (100%). In many cases, these proteins were diffuse and intensively stained in the tumor cell nucleus (Fig. 1).
Figure 1.
Immunohistochemical staining of (A) MSH2, (B) MSH6, (C) PMS2, and (D) MLH1 in neuroendocrine neoplasm cells.
The expression of immune checkpoint proteins and T-cell immune infiltrates
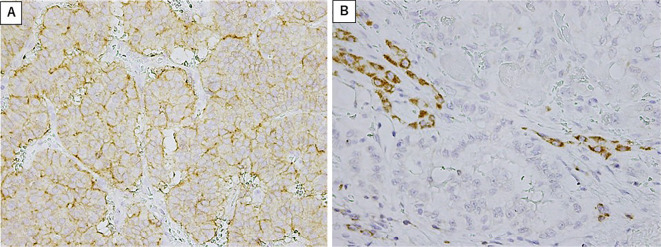
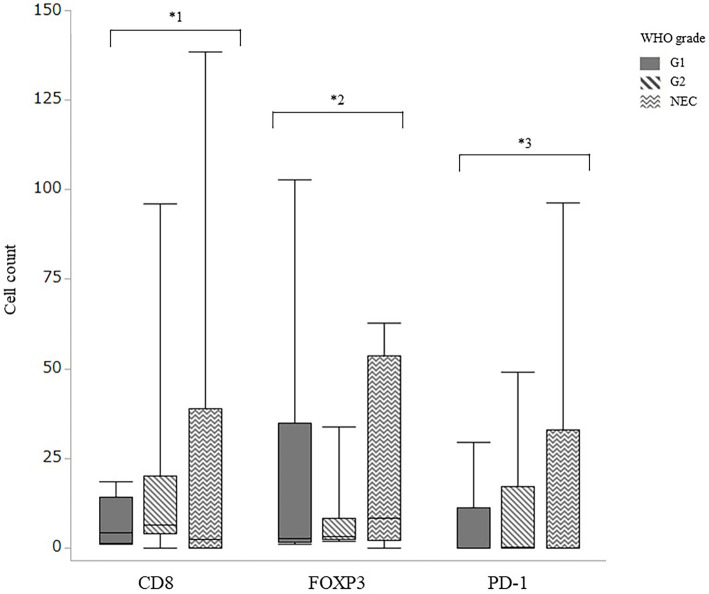
The PD-L1 expression was moderate and diffuse on the tumor cell membrane but lacking or very weak on immune cells (Fig. 2A). In contrast, the PD-1 expression was usually strong and diffuse on the immune cell membrane but lacking or faint on tumor cells (Fig. 2B). A positive PD-L1 expression in tumor cells was found for all six cases of Grade-1 NET, six of the eight cases of Grade-2 NET, and three of the six cases of NEC, with no significant difference in positivity according to grade. With respect to each primary lesion, the PD-L1 expression was positive in 73% (8/11 patients) of pancreatic lesions, 71% (5/7 patients) of gastrointestinal lesions, and 1 sample of unknown origin, with no significant differences. In addition, the number of TILs positive for PD-1, CD8, and FOXP3 expression was comparable among primary lesions from different sites (Table 2). The mean number of PD-1-positive cells was 5.8, 10.2, and 18.1 in WHO Grade 1, Grade 2, and NEC, respectively (Fig. 3). The number of PD-1-positive cells tended to be increased in tumor cells with a higher WHO grade (Grade 1: 5.8, Grade 2: 10.2, NEC: 18.1) but did not reach significance (p=0.903).
Figure 2.
(A) Representative PD-L1 staining on tumor cells. (B) The PD-1 expression on the immune cell membrane.
Table 2.
The Expression Patterns of PD-1, CD8, FOXP3 in NENs.
| N | PD-1 expression | CD8 expression | FOXP3 expression | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tumorhigh | Tumorlow | p value | Tumorhigh | Tumorlow | p value | Tumorhigh | Tumorlow | p value | ||||||||||||
| Total | 20 | 7 | 13 | 14 | 6 | 12 | 8 | |||||||||||||
| WHO grade | 0.9819 | 0.3102 | 0.8262 | |||||||||||||||||
| G1 | 2 | 4 | 4 | 2 | 3 | 3 | ||||||||||||||
| G2 | 3 | 3 | 7 | 1 | 5 | 3 | ||||||||||||||
| NEC | 2 | 4 | 3 | 3 | 4 | 2 | ||||||||||||||
| Primary lesion | 0.8485 | 0.1661 | 0.0404* | |||||||||||||||||
| Pancreas | 4 | 8 | 10 | 2 | 5 | 7 | ||||||||||||||
| Others | 3 | 5 | 4 | 4 | 7 | 1 | ||||||||||||||
| PD-L1 expression | 0.6392 | 0.5492 | 0.6186 | |||||||||||||||||
| Positive | 6 | 10 | 12 | 4 | 9 | 7 | ||||||||||||||
| Negative | 1 | 3 | 2 | 2 | 3 | 1 | ||||||||||||||
PD-1: programed death 1, FOXP3: forkhead or winged helix family of transcription factor P3, NENs: neuroendocrine neoplasms
*stands for the value of p<0.05.
Figure 3.
The number of tumor-infiltrating lymphocytes positive for CD8, FOXP3, and PD-1 for each WHO classification, quantified by the average number of cell counts. Thin bars, SD. *1, p=0.427; *2, p=0.690; *3 p=0.903 (Kruskal-Wallis test).
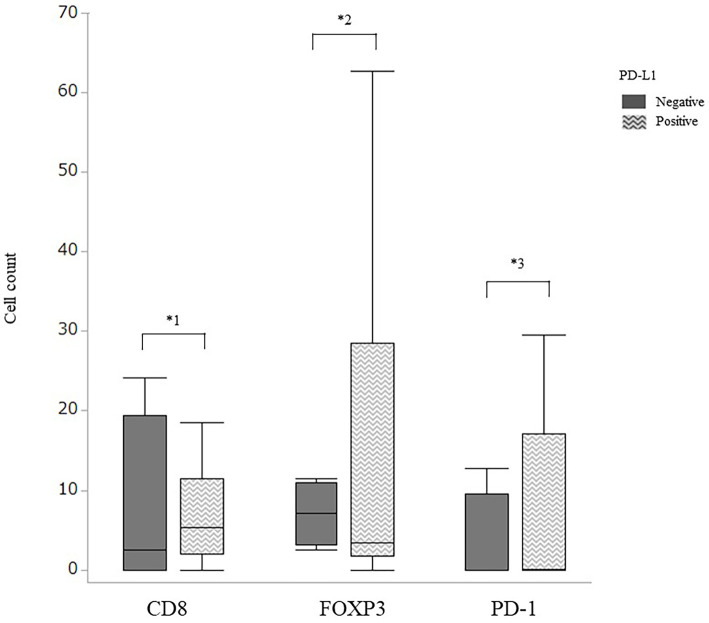
The number of infiltrating lymphocytes for each antibody with and without PD-L1 expression was compared. The number of intra-tumoral lymphocytes expressing PD-1, CD8, and FOXP3 tended to be higher in the presence of PD-L1 expression than in its absence (Fig. 4), but not to a significant degree (PD-1: 19.5 vs. 7.3, CD8: 18.1 vs. 7.1, FOXP3: 13.2 vs. 3.2, p=0.438, p=0.419, p=0.603, respectively)
Figure 4.
The number of tumor-infiltrating lymphocytes positive for CD8, FOXP3, and PD-1 in the presence or absence of the PD-L1 expression, quantified by the average cell count. Thin bars, SD. *1, p=0.438; *2, p=0.603; *3 p=0.419 (Wilcoxon’s rank sum test).
Discussion
We investigated the MSI status and TILs of intra-tumoral and stroma lesions in patients with NEN using an immunohistochemical analysis. All NENs expressed mismatch repair proteins, which is in accordance with previous studies showing that mismatch repair protein deficiency was very rare in NENs (22,23) based on a molecular approach (e.g. polymerase chain reaction) that amplifies DNA at several microsatellite sites from a tumor tissue sample.
We also found a very high frequency of PD-L1 expression on the tumor cell surface. A high PD-L1 expression was previously associated with a high-grade (Grade 2 or 3) WHO classification as determined by immunohistochemistry (20,24). We did not find a clear correlation between the WHO grade and PD-L1 expression in our study, which was attributed to the small number of cases. However, we did find a high frequency of PD1 expression on the surface of TILs. In previously published pathological studies of NENs, a high expression of PD-L1 in tumor cells was associated with a high frequency of PD1-positive lymphocytes and a high number of TILs (21). A high number of TILs, high frequency of PD1-positive lymphocytes, and high expression of PD-L1 in tumor cells were also associated with a poor prognosis (20,21). In the present study, a high expression of PD-L1 in tumor cells was not associated with the overall survival (data not shown in detail). However, a high expression of PD-L1 in tumor cells might be associated with a high frequency of PD1-positive lymphocytes. These results support the hypothesis that the high frequency of PD1-positive lymphocytes infiltrating around the tumor allows tumor cells to upregulate PD-L1 and defend themselves. This immunosuppressive environment established by the tumor and TIL interaction through the PD1/PD-L1 pathway may facilitate tumor cell growth and worsen the host’s prognosis.
A previous study showed that FOXP3-positive TILs were relatively rare in NET (25). However, we found abundant FOXP3-positive TILs in our samples, especially in the tumoral and stromal lesions, and CD8-positive TILs were also detected around the tumor cells. This suggests that the host immune reaction might not be completely absent (or “cold”) in NENs. A previous genomic analysis classified NENs with a low tumor mutation burden and low MSI status as tumors with a cold-type immunoreaction (26). However, we found many lymphocytes infiltrating the tumor cells, including both immunosuppressive TILs (PD1- and FOXP3-positive) and cytotoxic (CD8-positive) TILs. Furthermore, the rate of the positive expression of PD1, CD8, and FOXP3 increased with increasing WHO grade and in cases of PD-L1 positivity. These results suggested that the immunoreaction might be “hot” specifically in high-grade and PD-L1-positive NENs.
A previous study reported the overexpression of PD-L1 in high-grade neuroendocrine carcinoma of the lung. The tumor mutation burden landscape in LCNETs of the lung was also previously investigated, and the median tumor mutation burden was found to be higher than that in small-cell and non-small-cell lung cancer. According to a previous clinical trial of ICIs, combination treatment of ipilimumab and nivolumab demonstrated a 44% objective response rate in patients with non-pancreatic high-grade NEC, with a 0% objective response rate in cases of low/intermediate grade disease (27). These clinical trial data and other investigation findings were consistent with the present results, so PD1/PD-L1 pathway blockade might be a major treatment target for high-grade NENs.
First-line PD-L1 monoclonal antibody atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer resulted in a significantly longer overall survival and progression-free survival than chemotherapy alone (28). The addition of duravalumab to the platinum-etoposide chemotherapy also improved the overall survival in patients with extensive-stage small-cell lung cancer (29). Combination treatment of cytotoxic agents and ICIs may also be effective for treating high-grade NENs, so a clinical trial is expected in the future.
Several limitations associated with the present study warrant mention. First, the sample size was too small to draw general conclusions, and there were no NET Grade 3 cases. High-grade NET was thus equivalent to NEC in this study. Second, we only investigated MMR deficiency by immunostaining and did not evaluate the mRNA levels of the microsatellite domain itself. This method might be insufficient for an accurate MSI analysis. Third, some data were derived from metastatic lesions, which might have different immunological activities from primary lesions. Finally, the PD1 and/or PD-L1 expression is not currently used as a biomarker for predicting the therapeutic effect of PD1 and PD-L1 antibodies on NENs, and the clinical impact may be low with respect to NENs.
In conclusion, all cases of NENs expressed MMR proteins, indicating no MSI-high cases. However, PD-L1 was frequently expressed in NENs, and abundant TILs were identified around the tumor, especially in high-grade and PD-L1-positive NENs. Our findings suggest that a PD1/PD-L1 inhibitor may be an important treatment agent for unresectable high-grade and PD-L1-positive NENs.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the united states. JAMA Oncol 3: 1335-1342, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ito T, Lee L, Hijioka M, et al. The up-to-date review of epidemiological pancreatic neuroendocrine tumors in Japan. J Hepatobiliary Pancreat Sci 22: 574-577, 2015. [DOI] [PubMed] [Google Scholar]
- 3. Rinke A, Müller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 27: 4656-4663, 2009. [DOI] [PubMed] [Google Scholar]
- 4. Caplin ME, Pavel M, Ruszniewski P, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 371: 1556-1557, 2014. [DOI] [PubMed] [Google Scholar]
- 5. Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 364: 514-523, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 364: 501-513, 2011. [DOI] [PubMed] [Google Scholar]
- 7. Yao JC, Fazio N, Singh S, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet 387: 968-977, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moertel CG, Lefkopoulo M, Lipsitz S, et al. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med 326: 519-523, 1992. [DOI] [PubMed] [Google Scholar]
- 9. Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 114: 1537-1544, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515: 563-567, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Badoual C, Hans S, Merillon N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res 73: 128-138, 2013. [DOI] [PubMed] [Google Scholar]
- 12. Ott PA, Elez E, Hiret S, et al. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the phase Ib KEYNOTE-028 study. J Clin Oncol 35: 3823-3829, 2017. [DOI] [PubMed] [Google Scholar]
- 13. Tumeh PC, Hellmann MD, Hamid O, et al. Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res 5: 417-424, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yonesaka K, Haratani K, Takamura S, et al. B7-H3 negatively modulates CTL-mediated cancer immunity. Clin Cancer Res 24: 2653-2664, 2018. [DOI] [PubMed] [Google Scholar]
- 15. Şenbabaoğlu Y, Gejman RS, Winer AG, et al. Tumor immune microenvironment characterization in clear cell renal cell carcinoma identifies prognostic and immunotherapeutically relevant messenger RNA signatures. Genome Biol 17: 231, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kobayashi N, Hiraoka N, Yamagami W, et al. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res 13: 902-911, 2017. [DOI] [PubMed] [Google Scholar]
- 17. Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 18: 1182-1191, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Strosberg JR, Mizuno N, Doi T, et al. Efficacy and safety of pembrolizumab in previously treated advanced neuroendocrine tumors: results from the phase 2 KEYNOTE-158 study. Clin Cancer Res 26: 2124-2130, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patel SP, Othus M, Chae YK, et al. A phase II basket trial of dual anti-CTLA-4 and anti-PD-1 blockade in rare tumors (DART SWOG 1609) in patients with non-pancreatic neuroendocrine tumors. Clin Cancer Res 26: 2290-2296, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim ST, Ha SY, Lee S, et al. The impact of PD-L1 expression in patients with metastatic GEP-NETs. J Cancer 7: 484-489, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bosch F, Bruwer K, Altendorf-Hofmann A, et al. Immune checkpoint markers in gastroenteropancreatic neuroendocrine neoplasia. Endocr Relat Cancer 26: 293-301, 2019. [DOI] [PubMed] [Google Scholar]
- 22. Arnold CN, Sosnowski A, Blum HE. Analysis of molecular pathways in neuroendocrine cancers of the gastroenteropancreatic system. Ann N Y Acad Sci 1014: 218-219, 2004. [DOI] [PubMed] [Google Scholar]
- 23. Ghimenti C, Lonobile A, Campani D, et al. Microsatellite instability and allelic losses in neuroendocrine tumors of the gastro-entero-pancreatic system. Int J Oncol 15: 361-366, 1999. [DOI] [PubMed] [Google Scholar]
- 24. Cavalcanti E, Armentano R, Valentini AM, et al. Role of PD-L1 expression as a biomarker for GEP neuroendocrine neoplasm grading. Cell Death Dis 8: e3004, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. daSilva A, Bowden M, Zhang S, et al. Characterization of the neuroendocrine tumor immune microenvironment. Pancreas 47: 1123-1129, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scarpa A, Chang DK, Nones K, et al. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature 543: 65-71, 2017. [DOI] [PubMed] [Google Scholar]
- 27. Patel SP, Othus M, Chae YK, et al. A Phase II basket trial of dual anti-CTLA-4 and anti-PD-1 blockade in rare tumors (DARTSWOG1609) in patients with nonpancreatic neuroendocrine tumors. Clin Cancer Res. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Horn L, Mansfield AS, Szczęsna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 379: 2220-2229, 2018. [DOI] [PubMed] [Google Scholar]
- 29. Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 394: 1929-1939, 2019. [DOI] [PubMed] [Google Scholar]