Abstract
In recent decades, the major concern of emerging and re-emerging viral diseases has become an increasingly important area of public health concern, and it is of significance to anticipate future pandemic that would inevitably threaten human lives. The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a newly emerged virus that causes mild to severe pneumonia. Coronavirus disease (COVID-19) became a very much concerned issue worldwide after its super-spread across the globe and emerging viral diseases have not got specific and reliable diagnostic and treatments. As the COVID-19 pandemic brings about a massive life-loss across the globe, there is an unmet need to discover a promising and typically effective diagnosis and treatment to prevent super-spreading and mortality from being decreased or even eliminated. This study was carried out to overview nanotechnology-based diagnostic and treatment approaches for emerging and re-emerging viruses with the current treatment of the disease and shed light on nanotechnology's remarkable potential to provide more effective treatment and prevention to a special focus on recently emerged coronavirus.
Keywords: Nanotherapeutics, Coronavirus, Inorganic nanoparticles, Polymeric nanoparticles, Lipid nanoparticles, COVID-19
1. Introduction
Nanotechnology is defined as the application of particles with nanometer range dimension [1], while nanomedicine is the application of nanostructured materials to diagnose, treat, and prevent diseases [2]. Nanoparticles are small size particles (which can facilitate drug delivery into anatomically privileged sites) [1,3] with large surface area to volume ratios (which ensures that large drug payloads can be accommodated) [4] and are tunable surface charge (to facilitate cellular entry across the negatively charged cellular membrane) [5]. Hence, nanoparticles are preferable for viral treatment than macromolecules. Nanotechnology offers some advantages to combat emerging and re-emerging viruses including a) design of innovative drugs with increased activity and decreased toxicity [6,7]; b) design of smart nano-based sensors for early detection of viral infections [8,9]; and c) design of efficient personal protective equipments to avoid viral contamination such as nanoenabled masks [10,11]. Nanomedicines have shown a significant accomplishment in management of viral infections [12]. Nanomedicines can overcome the site-specific delivery of the antiviral drugs. For the case of COVID-19 disease, if the scientists introduce an efficient drug against SARS-CoV-2 virus, then the optimized targeted drug delivery systems based on nanomedicines can be helpful to overcome the site-specific delivery challenge. Nanomedicines are also beneficial for the controlled release and maintenance of the antiviral drugs in the targeted sites which have a significant role in the management of viral infections. Because, a high dose of off-targeted antiviral drugs may result in severe side effects, which can be worse than a viral infection [10].
2. Pathogenesis of COVID-19
In general, coronaviruses are constantly well known in genome change, which basically results from recombination, gene exchange, deletion, and insertion, which in turn could possibly bring about future outbreaks. The first step in viral infection initiation is the interaction of permissive human cells, particularly ciliated epithelial cells with Spike Protein (a protein that facilitates attachment). Genome uncoating occurs after entering the cell cytoplasm and commences the expression of the genes that encode advantageous accessory proteins, which improves the adaptation of coronaviruses to their human host [13].
Based on basic strategies learned from epidemics caused by the previous Coronaviruses, SARS-CoV and MERS-CoV specifically bind to particular cellular receptors angiotensin-converting enzyme 2 (ACE-2) and dipeptidyl peptidase 4 (DPP-4) respectively, and after cell entry, the viral genome is delivered into the cell cytoplasm [14].
After entry, uncoating of viral genetic material begins in the host cell cytoplasm whereby information coded by the viral genome is transcribed and translated. In addition, the viral genome is multiplied and early non-structural, followed by structural proteins, are generated. The viral nucleic acid and proteins are gathered, forming nucleo-capsid in the cell cytoplasm, and then are budded into the lumen of the endoplasmic reticulum. Finally, the virions are then released from the infected host cell through exocytosis. The released virus subsequently infects a variety of host cells, including Kidney cells, Liver cells, intestine, respiratory, and T-lymphocyte, resulting in a wide range of signs and symptoms [15].
3. Diagnosis and treatment options of COVID-19
Samples from cluster pneumonia cases with unknown causes were first analyzed for full-genome analysis in an advanced laboratory setting in China, and finally a novel coronavirus was identified. In accordance with MERS epidemic diagnosis, the most reliable and primary diagnostic technique for COVID-19 is clinical history regarding traveling to where the epidemic is going on, contact with confirmed cases, or contaminated objectives followed by precise laboratory setting [16].
Real-time PCR assay was subsequently developed by using samples from respiratory (particularly lower respiratory tract) and distributed in Wuhan. Also, serological diagnostics are rapidly being developed but are not yet widely used; but much more researches are undergoing in different areas for treatment, vaccine and diagnostic techniques production for COVID-19 [17]. The commonly applied laboratory diagnostic techniques for COVID-19 include Molecular (RT-PCR/Real-time PCR), Serologic tests and viral cell culture, and chest X-ray or Thoracic CT scan. From all the laboratory tests, molecular diagnosis is the most recommendable and reliable way for COVID-19, whereas antibody testing shows low sensitivity, and Viral cell culturing is time-consuming [16]. In short, clinical diagnosis (high body temperature, dry cough, and difficulty of breathing) and history related to a particular geographical location (COVID-19 infected) are the primary forerunners of early diagnosing of the COVID-19, particularly where there is a lack of advanced laboratory setting.
In general, suddenly emerging viral diseases have not found specific and reliable treatments. Besides, most, if not all, viral-caused diseases don't have reliable and very much effective treatment options [18]. Although there are no specific antiviral or immune-modulating agents proven yet for COVID-19, all patients are monitored by regular pulse oximetry. In China, patients were being treated based on National Clinical guidelines recommended by China National Health Commission (NHC); the guidelines include supportive care by clinical category (mild, moderate, severe and critical), as well as the role of investigational treatments such as chloroquine, phosphate, lopinavir/ritonavir, alpha interferon, ribavirin, arbidol [17].
As the COVID-19 brings about a huge life-loss across the worldwide, it is very much necessary to discover a promising and typically effective treatment for decreasing the super-spreading and mortality or even its elimination. Altogether, there is a lot of researches on COVID-19 treatment being undertaken, where some of them reached clinical trials. Recent research revealed inhibitory action of remdesivir and chloroquine on the growth of SARS-CoV-2 in vitro, and an early clinical trial conducted in COVID-19 Chinese patients showed that chloroquine had a significant effect, both in terms of clinical outcome and viral clearance [19,20]. Hydroxychloroquine also presented effective antiviral activity against SARS-CoV-2 with better clinical safety, and it allows a high daily dose [21]. Another study conducted in France recently revealed that Hydroxychloroquine and azithromycin in combination showed very effective antiviral activity against SARS CoV-2 after 100% of patients treated with Hydroxychloroquine and azithromycin combination were Virologically cured comparing with 57.1% in patients treated with Hydroxychloroquine only [22].
4. Emerging nano-based treatment for Coronavirus
Since the outbreak of Coronavirus, researchers have tried to develop nano-based treatments. Nanoparticles like carbon quantum dots are suggested to be of excellent solubility in water. Hence, they can be perfect candidates for winning the battle against Coronavirus because they can easily enter the cell through endocytosis and interact with the virus's protein, thereby preventing viral genome replication and inhibiting protein S receptor interaction [23].
Nanoparticle‐based treatment and vaccine development have been expected to improve efficacy, immunization strategies, and targeted delivery to promote immune responses for coronaviruses [24,25]. Gold nanoparticles (AuNPs) have become the choice for alternative treatments because of their physicochemical properties, which prevent antibody production against viral infections [24]. Nanoparticles exert their antiviral activity by triggering the Production of pro‐inflammatory cytokines and TH1 cytokines [26].
5. Nanotechnology for HIV treatment
HIV treatment is based on the action of drugs that target the life cycle and the multiplication process of the viral antigen. Currently, the antiretroviral therapy (ART) includes nucleotide reverse transcriptase inhibitors [27], non-nucleoside inhibitors [28], protease inhibitors [29], entry/fusion inhibitors [30], CCR5 antagonists [31], and integrase inhibitors [32,33]. To increase the efficacy of treatment and subsequently improve the quality of life, a combination of three or more drugs, called highly active ART (HAART) among HIV infected individuals will be employed [34]. Despite its effectiveness, the treatment is not devoid of unwanted occurrences due to suboptimal adherence, heavy pill burdens, toxicity, treatment side effects, and drug resistance development. Hence, it needs to have novel methods to enhance the inhibition of HIV infection, one of which is nanotechnology.
Modern drug design, which can incorporate ART drug delivery with nano-systems, can decrease the dosage requirements and toxic side effects associated with current heavy pill burdens to improve the treatment's safety and efficacy [35].
Previous studies supported the evidence of effective treatment by nanotechnology. Chiodo et al. conducted a study on the NRTI drugs abacavir (ABC) and lamivudine (3 TC) attaching to glucose nanoparticles (GNPs) in vitro. There was efficient function through the drug's primary hydroxyl groups via an ester bond that can be cleaved off in acidic conditions. After the experiment, the researcher illustrated a new level of multi-functionalization of GNPs as multivalent drug delivery systems for the treatment of HIV [36]. It was previously known that the regulatory T (Treg) cells were a specialized subpopulation of T-cells [37]. These T cells are components of the immune system and susceptible to HIV infection [38]. HIV infection leads to immune hyperactivation, which can subsequently result in erosion, depletion, or exhaustion of T-cells by preventing HIV disease progression [39].
Other researchers demonstrated that carbosilane dendrimers could be used to prevent Treg cell infection with HIV in vitro. The negative phenotypic effects and decreased functionality of these cells due to HIV infection were also decreased with the application of these dendrimers. In addition, high bio-compatibility and a significant reduction in p24 antigen production was observed in cell culture and intracellularly [40].
In a study conducted by Parboosing et al., RNA decoys in the form of a 16-mer oligoribonucleotide originating from the stem loop 3 of the HIV packaging signal were attached to dendrimers to disrupt the packaging process of the HIV life cycle. The results of this study demonstrated efficient delivery into lymphocytes and a modest cytoprotective effect against HIV infection [41]. Jayant et al. [42] demonstrated that an ARV (tenofovir) and an investigational latency-reversing drug (vorinostat) could be co-encapsulated on iron oxide nanoparticles [43].
Similarly, there have been other studies that investigated nanoparticles as novel agents in ARV drug delivery [44,45], and small molecule HIV inhibitors [46,47] for effective treatment of HIV.
6. Nano-based antiviral therapy emerging and re-emerging viruses
The following nanoparticles are the most frequently used treatment options for treating viral infections.
6.1. Inorganic nanoparticles
6.1.1. Silver nanoparticles (AgNPs)
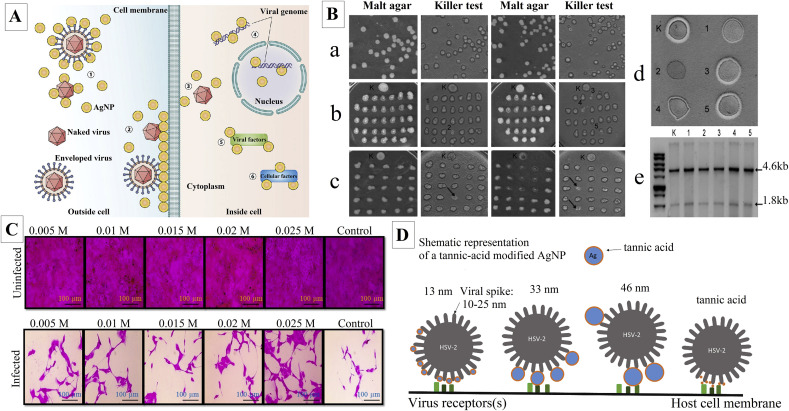
AgNPs are the most effective nano-based inorganic metals against viral infections. These nano-treatments have been checked for effectiveness against other viral infections like Human immunodeficiency virus (HIV)-1, Herpes simplex virus (HSV), Feline Calicivirus (FCV), Poliovirus, Peste des petits ruminants virus, Norovirus, Influenza virus (H7N3), Coxsackievirus, and Saccharomyces cerevisiae dsRNA viruses to date [48]. It was shown that the silver cations released from AgNPs interact directly with phosphorus and sulfur groups of biomolecules such as DNA and RNA. The antiviral mechanism of AgNPs may be due to the interference of AgNPs with viral replication cycle in different stages such as virus binding to the cell membrane and its entry inside the cell, protein synthesis as well as DNA and RNA replication [49]. Fig. 1 A represented a schematic mechanism of antiviral activity of AgNPs on different stages of virus replication [50]. Moreover, it was stated that AgNPs prevented the entry of HIV to susceptible cells by interacting with glycoproteins in HIV envelopes, which avoid their binding and fusion with cell membrane [49]. Rónavári et al. investigated the antiviral activity of two green synthesized AgNPs using coffee (C-AgNPs) and green tea (GT-AgNPs) extracts against five S. cerevisiae dsRNA viruses that are in charge of the killer phenotype of the host strain. The obtained AgNPs were spherical with an average size of 3.2 ± 1.2 and 12.7 ± 5.8 nm for C-AgNPs and GT-AgNPs, respectively. In this study, the effect of AgNPs was observed on viral replication by loss of killer phenotype of the host strain (Fig. 1B). C-AgNPs and GT-AgNPs caused five and three strains with altered phenotypes in the tested strains, respectively. Consequently, the strains treated with C-AgNPs were examined for the stability of the altered phenotype. After around 112 generations, only one strain maintained the nonkiller phenotype (Fig. 1B(d), #2). As shown in Fig. 1B(d), #1, and #4, two strains produced narrow inhibition zones, compared to the control (Fig. 1B(d), [K]). Moreover, in all of the five tested strains, the viral genomes were detected representing that the loss of killer phenotype was not due to viral eradication (Fig. 1B(e)) [51]. Castro-Mayorga et al. reported the antiviral activity of AgNPs with an average size of around 7 ± 3 nm against norovirus surrogates, FCV, and the murine norovirus (MNV). The FCV and MNV were exposed to AgNPs in the concentrations of 2.1, 10.5, and 21 μg/mL for 24 h at 25 °C and then analyzed by cell-culture assays. The results exhibited significant dose-dependent antiviral activity of AgNPs against FCV and MNV even at 2.1 μg/mL [52]. Huy et al. evaluated the antiviral activity of quasi-spherical shaped AgNPs with an average size of 7.1 nm against poliovirus by daily assessment of cytopathic effect (CPE) through inverted light microscopy. CPE refers to viral invasion-induced structural changes in host cells. The host cell in this study was human rhabdomyosarcoma. At the concentration of 3.13 μg/mL of AgNPs, the viral concentration of 1TCID50 (Tissue Culture Infective Dose) and 10TCID50 after 30 and 60 min, respectively, with no CPE and cell viability of up to 98% at 48 h post-infection [53]. Sreekantha et al. stated the antiviral activity of phytosynthesized AgNPs with quasi-spherical morphology ranging from 5 to 15 nm against the influenza A virus (strain A/PR/8) by using the sulforhodamine B (SRB) assay through the normal renal (MDCK) cells infected with or without the influenza A virus as positive and negative controls, respectively. At concentrations of 0.005, 0.01, 0.15, 0.02, and 0.25 M of AgNPs, a dose-dependent inhibitory rates were found to be 5.31%, 4.18%, 5.97%, 7.10%, and 15.12%, respectively (Fig. 1C) [54]. Morris et al. demonstrated the effectiveness of AgNPs in reducing respiratory syncytial virus (RSV) replication as well as a significant drop in production of pro-inflammatory cytokines (i.e., IL-1α, IL-6, TNF-α) and pro-inflammatory chemokines (i.e., CCL2, CCL3, CCL5); both in epithelial cell lines as well as in infected BALB/c mice model. The infected mice that were treated with 4 mg/kg of AgNPs showed the largest reduction in RSV lung titers along with a drop in pro-inflammatory cytokines (i.e., TNF-α, IL-6) [55]. Similarly, tannic acid modified AgNPs in different sizes (i.e., 13, 33 and 46 nm) resulted in reducing HSV-2 infectivity as well as a reduction in inflammation in HSV-2 infected C57BL/6 mice model. The authors believed that tannic acid modified AgNPs-induced antiviral activity was size-related, and required direct interaction and blocked virus attachment. Fig. 1D showed a schematic representation of interaction between tannic acid modified AgNPs or tannic acid and HSV-2 virion [56]. Ochoa-Meza1 et al. reported that AgNPs at the concentration of 12 ng/mL increased 20% survival of white spot syndrome virus infected Penaeus vannamei shrimps by activation of its immunological system, while the same concentration treated healthy shrimps did not show any histological evidence of damage [57].
Fig. 1.
A) Potential antiviral mechanism of AgNPs. (1) AgNPs interact with viral envelope and/or viral surface proteins; (2) AgNPs interact with cell membranes and block viral penetration; (3) AgNPs block cellular pathways of viral entry; (4) AgNPs interact with viral genome; (5) AgNPs interact with viral factors necessary for viral replication; (6) AgNPs interact with cellular factors necessary for productive viral replication [50]; B) Antiviral effect of AgNPs. a) Killer activity of untreated, b) C–AgNP-treated, c) GT-AgNP-treated Saccharomyces cerevisiae SZMC 20733 cells. Numbers (1–5) indicate the colonies of C–AgNP-treated cells where no killer phenotype was observed, whereas GT-AgNP-treated colonies with lost killer activity are indicated with black arrows, d) Killer phenotype of Saccharomyces cerevisiae SZMC 20733 (K) and C–AgNP-treated strains (1–5) after ~112 generations, and e) Viral RNA extracted from SZMC 20733 (K) and from C–AgNP-treated strains (1–5). Arrows indicate the presence of L-A (4.6 kb) and M1 (1.8 kb) virus genomes [51]; C) Antiviral effect of phytosynthesized AgNPs on MDCK cells (A/PR/8 virus-infected) by SRB assay at 24 h [54]; D) Schematic representation of the interaction between tannic acid modified AgNPs or tannic acid and HSV-2 virion [56].
6.1.2. Gold nanoparticles (AuNPs)
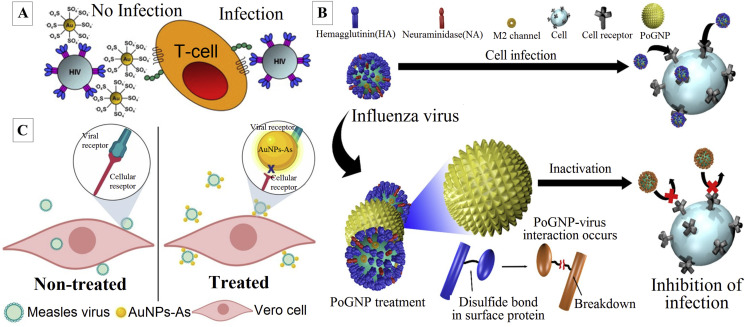
Unlike AgNPs, these nanoparticles have rarely been used for direct antiviral activity. However, they are effective against HIV, H1N1, H3N2, H5N1, dengue virus, bovine viral diarrhea virus, and foot-and-mouth disease virus [58,59]. Their mechanism of action is suggested to be by blocking gp120 attachment with CD4, which results in inhibited viral entry [58], arresting replication at post-entry stage, mostly linked with transcription within the host cell [59,60], in bio-sensing, bio-imaging and catalytic activities [60]. Halder et al. reported the antiviral activity of quasi-spherical AuNPs with an average size of 7.86 nm against HSV-1 and HSV-2 in Vero cells. The AuNPs exhibited antiviral activity in a dose-dependent manner with EC50 of 32.3 and 38.6 μM in HSV-1 and HSV-2, respectively. The authors showed that AuNPs interfered with virus attachment and penetration into the Vero cells [61]. Bawage et al. reported that gold nanorods (GNRs) at 2.5 μg/mL inhibited RSV in HEp-2 cells by 82% and also inhibited RSV in BALB/c mice model by 56% at 20 μg intranasally. The AuNPs caused upregulation of antiviral genes such as Toll-like receptors, RIG-I-like receptor signaling pathways, and NOD-like receptors. The histological and cytokine analysis indicated that GNRs stimulated innate immune response resulting in RSV inhibition [62]. Di Gianvincenzo et al. reported that AuNPs capped with sulfate-ended ligands acted as anti-HIV agents by binding to HIV envelope glycoprotein gp120 with a consequent inhibition of in vitro HIV infection of T-cells at nanomolar concentrations (Fig. 2 A) [63]. Hemagglutinin is a highly conserved surface protein in many influenza viruses that can be considered as an effective target for designing novel antiviral drugs. Notably, hemagglutinin comprises six disulfide bonds. On the other hand, AuNPs have exhibited a high affinity to bind to the disulfide bonds due to gold–thiol interactions. Kim et al. reported the synthesis of porous AuNPs and their strong antiviral activity against influenza viruses, including H1N1, H3N3, and H9N2, by increasing cell viability up to 96.8% through the infected MDCK cells with all tested strains compared to 33.9% cell viability through non-treated infected cells. The authors believed that synthesized AuNPs interact with influenza virus surface proteins such as hemagglutinin and cleave their disulfide bonds resulting in lower viral infectivity to the cells (Fig. 2B) [64]. Meléndez-Villanueva et al. reported effective antiviral effect of phytosynthesized AuNPs against measles virus in Vero cells. The findings revealed that AuNPs significantly inhibited measles virus replication in Vero cells with EC50 of 8.829 μg/mL. In addition, the HAuCl4 exhibited 46.43% antiviral activity at 10 μg/mL indicating that the antiviral activity of AuNPs is better than HAuCl4. Furthermore, the authors proposed that AuNPs inhibited viral infection by direct blocking of viral particles resulting in a potent AuNPs-induced virucidal effect (Fig. 2C) [65].
Fig. 2.
A) AuNPs coated with multiple copies of an amphiphilic sulfate-ended ligand inhibit in vitro the HIV infection of T-cells at nanomolar concentrations [63]; B) Schematic illustration of inactivation of influenza virus treated with porous AuNPs which interact with virus surface proteins and cleave their disulfide bonds [64]; C) Schematic representation of a proposed mode of the virucidal effect of AuNPs on Measles virus infection. In cells treated with AuNPs, the adsorption step is blocked by the binding of AuNPs and the viral envelope; therefore, the infection cannot be initiated [65].
6.1.3. Magnetic nanoparticles (MNPs)
Iron-based nanoparticles have an effective antiviral effect. These particles have direct activity against bacteriophages, zika virus, HCV, and H5N2. They also have applications in hybridized form [66,67]. Kumar et al. reported the antiviral activity of iron oxide nanoparticles (FeNPs) ranging from 10 to 15 nm against H1N1 influenza A virus. The antiviral activity was evaluated by measuring the viral RNA transcripts using RT-PCR. The cells treated with FeNPs exhibited eightfold reductions in viral RNA transcripts within 24 h of virus infection. The authors suggested that the molecular mechanism of FeNPs antiviral activity is due to a reaction of FeNPs with – SH groups of proteins [68]. Choudhary et al. reported the antiviral activity of FeNPs with an average size of 32 nm against the Chikungunya virus in Vero cells. A significant fall in viral titer was found by comparing the FeNPs treated and untreated CHIKVs infected cells at all the tested concentrations of FeNPs (0.05, 0.1, and 0.2 mg/mL) [69]. Park et al. reported effective antiviral activity of magnetic hybrid colloid decorated with AgNPs against bacteriophage φX174, murine norovirus (MNV), and adenovirus serotype 2 (AdV2). The authors suggested the magnetic hybrid colloids as disinfection due to easy recovery of them from the environment resulting from their magnetic properties [70].
6.1.4. Titanium dioxide nanoparticles (TiNPs)
Titanium dioxide (TiO2) a white pigmented molecule which was effectively used as nanoparticle-based against influenza virus and bacteriophages [71,72]. Moreover, researchers cannot rule out their toxicity. Hence, their biomedical application as an antiviral agent is questionable [73]. Syngouna and Chrysikopoulos reported inactivation of MS2 bacteriophage by TiNPs at the concentration of 10 mg/L in the presence of quartz sand with and without ambient light [71]. Mazurkova et al. reported effective inactivation of the influenza virus H3N2 by TiNPs in a dose-dependent manner at the concentrations of 0.2, 2, and 7 mg/mL [72]. Gerrity et al. reported effective photocatalytic inactivation of bacteriophages (MS2, PRD1, phi-X174, and fr) using TiNPs in a dose-dependent manner at the concentrations of 1, 10, 100, and 1000 mg/L at low-pressure UV light [74]. These results were in accordance with the findings of Misstear and Gill [75].
6.1.5. Silica and carbon-based nanocarriers
Currently, the antiviral efficacy of silica nanoparticles (SiNPs) is proven against Papillomavirus, Porcine circovirus, HBV, HSV, and HIV. The most searched mechanism of antiviral activity is by immunization of the host against the virus and inhibiting the viral attachment [76]. Additionally, carbon-based nanoparticles have antiviral activity against Ebola virus (EBOV), Bacteriophage λ, HIV, H3N2, Grass carp reovirus, and Respiratory syncytial virus [77,78]. Lee et al. reported the efficacy of mesoporous silica nanoparticles (MSNs) functionalized with a glycosaminoglycan (GAG) mimetic as viral entry inhibitors of herpes simplex type 1 (HSV-1) and type 2 (HSV-2) viruses in Vero cells. Fig. 3 A depicted that functionalized MSNs bind to HSV and prevented viral attachment to cell membrane ligands [79]. Skrastina et al. reported the influential role of SiNPs with the particle size ranging from 10 to 20 nm as an adjuvant with maximally 40% adsorption of recombinant full-length Hepatitis B virus core and induced strong Th1-biased immune responses in mice. Hence, SiNPs can be considered as an innovative adjuvant for the delivery of virus-like particles [80]. Ye et al. reported significant antiviral activity of graphene oxide (GO) against pseudorabies virus (PRV, a DNA virus) and porcine epidemic diarrhea virus (PEDV, an RNA virus) at a noncytotoxic concentration (6 μg/mL). The authors suggested that the antiviral mechanism of GO may be due to the negative charge of GO. The finding showed that the cationic graphene oxide/poly(diallyl dimethylammonium chloride) composite (GO-PDDA) had no antiviral activity. In contrast, the nonionic graphene oxide/polyvinylpyrrolidone (GO-PVP) showed similar antiviral activity as GO indicating that the negative charge had a role in the antiviral activity of GO. Furthermore, the oxygen-containing group seems not to have a major role in GO's antiviral activity because GO and reduced graphene oxide (rGO) exhibited similar antiviral activity. Fig. 3B represented the possible mechanisms of the antiviral activity of GO [81]. Additionally, carbon-based nanomaterials act as potential nanocarriers for the delivery of antiviral agents. For instance, maximin is naturally isolated from Asian toad Bombina maxima with antiviral activity. Dostalova et al. reported the effective antiviral activity of derivatives of maximin bound to fullerene C60 nanocrystals against bacteriophage k as a model virus (Fig. 3C) [82]. Martinez et al. reported effective antiviral activity of fullerene derivatives on the replication of HIV-1 in human CD4+ T cells in an in vitro assay [83]. Muñoz et al. reported significant antiviral activity of self-assembled glycodendro [60] fullerene mono adducts with uniform size and spherical-shape in an experimental infection assay using EBOV glycoprotein pseudotyped viral particles on Jurkat cells overexpressing DC-SIGN (Dendritic Cell-Specific Intercellular adhesion molecule3-Grabbing Non-integrin) (Fig. 3D) [84]. Du et al. stated the antiviral activity of carbon dots against pseudorabies virus (PRV) and porcine reproductive and respiratory syndrome virus (PRRSV). The results revealed that carbon dots induced expression of interferon-α (IFN-α) and IFN-stimulated genes (ISGs), which led to inhibition of PRV and PRRSV replication [85]. Likewise, Dong et al. reported considerable antiviral activity of carbon dots decorated with different surface passivation molecules, EDA (2,2′-(ethylenedioxy)bis(ethylamine)), and EPA (3-ethoxypropylamine) (Fig. 3E). The results showed that both EDA- and EPA-carbon dots at the concentration of 5 μg/mL had effective antiviral activity against human norovirus virus-like-particles (VLPs), GI.1 and GII.4 VLPs by inhibiting viral attachment to histo-blood group antigens (HBGA) receptors of host cell [86].
Fig. 3.
A) Antiviral activity of functionalized MSNs. a) Schematic illustration and b) TEM image represented that the functionalized MSNs bind to HSV inhibiting viral infection by preventing viral attachment to cell membrane ligands in Vero cells [79]; B) Proposed mechanisms of the antiviral activity of GO. a) Normal viruses are absorbed into cells by interacting with cell receptors to initiate infection. b) Negatively charged GO has more chances to interact with the positively charged viruses, leading to virus damage and the inhibition of infection. c) Infection was blocked by GO conjugated with nonionic PVP but d) not with cationic PDDA [81]; C) Schematic representation of the antiviral activity of derivatives of maximin bound to fullerene C60 nanocrystals against bacteriophage k as a model virus [82]; D) Schematic representation of the antiviral activity of self-assembled glycodendro [60]fullerene monoadducts against Ebola virus glycoprotein pseudotyped viral particles on Jurkat cells overexpressing DC-SIGN [84]; E) Schematic structure of a decorated carbon dot with EDA and EPA [86].
6.1.6. Quantum dots
Quantum dots are semiconductors ranging from 2 to 10 nm with unique physicochemical properties. Quantum dots can be single component materials with uniform internal compositions, such as CdTe and PbS, or consisting of an inorganic semiconductor core (CdSe) and coated shell (e.g., ZnS) to improve optical properties, and can be made to fluorescence when stimulated by light [87,88]. Quantum dots have shown a significant role as nanocarriers for antiviral drug delivery. Blood brain barrier (BBB) has a receptor called transferrin that acts as a receptor-mediated transport mechanism. In an in vitro study, transferrin was conjugated to quantum rods and consequently was loaded with an antiviral drug Saquinavir. The provided nanoformulation exhibited significant transversing ability across an in vitro model of BBB as well as a considerable decrease in HIV-1 viral replication in infected peripheral blood mononuclear cells [89]. Moreover, quantum dots have been utilized as a biosensor for virus detection owing to their specific optical properties. For instance, Norouzi et al. stated the effective identification of human T-lymphotropic virus-1 (HTLV-1) using cadmium–tellurium quantum dots [90]. Similarly, Bentzen et al. successfully utilized quantum dots to identify the presence of RSV and also monitor their progression infection over time [91].
6.2. Organic nanoparticles
When the size of the therapeutic compound is very large, then INPs might not have promising effects. In this case, organic nanoparticles (ONPs) might prove effective treatment [92]. The following organic nanoparticles are investigated to be effective for treating viral infections like NIPAH virus.
6.2.1. Polymeric NPs
These nanoparticles are effective against HIV [93], Influenza A virus, and NiV [94]. HIV and AIDS are retroviruses that cause the demolition of T cells called CD4, among the most serious public health concerns in the world, and inflict a significant cause of morbidity and mortality worldwide. Further, HIV-1 infection causes weakening of the immune system by advanced depletion of CD4+ T lymphocytes [95]. There are types of HIV, namely HIV 1 and HIV 2, with their respective subspecies [96,97]. Worldwide estimation by 2017, 37 million people were infected and mortality 940 000 cases with HIV-1, the infectious agent causing AIDS [95]. This indicates HIV infection as a serious global health concern worth billions of US$ investment. There are different therapeutics available for the treatment and prevention of HIV infections. Treatment of HIV infection directs an early, lifelong treatment and regimens cART (antiretroviral therapy) adherence with combination antiretroviral therapy (cART) [97,98]. Major constraint for the treatment of HIV is dearth of adherence to cART drug regimens, and in terms of formulation the poor availability, high cytotoxicity, and greater metabolism and renal clearance of the drug molecules [98,99]. Further, less effective treatment outcomes result from the nonspecific targeted delivery to the virus. Polymer nanoparticles have been documented as an effective carrier system for drug encapsulation and are now considered as a vehicle for various route of delivery of insoluble therapeutics [100]. Several polymeric nanoparticles have been explored for the therapy to cure different viral diseases, including HIV.
6.2.1.1. Poly(lactic-co-glycolic) acid (PLGA)
PLGA, a degradable polymer that has been used in many FDA approved devices, has long been the standard NP material for intravaginal delivery of therapeutics [101]. HIV uses the brain as a reservoir for infection; hence, it is a likely target to fight this pathology. As most drug therapies (e.g. efavirenz) cannot cross BBB barriers, PLGA based nanoparticles can serve as carriers to the brain [96]. Other protease inhibitors (PIs) have also been extensively used as one of the most efficient methods for the therapy of AIDS. Indinavir, a potent protease inhibitor applied in therapy to HIV. However, its limited aqueous solubility in its unprotonated form is the rate-limiting step of its absorption and onset of action in the gastrointestinal tract fluids. To overcome this issue, Masoumeh Kurd et al. designed and characterized copolymer mPEG-PCL, which is capable of self-assembling into nanoparticles [100]. The nanoparticle system improved oral drug delivery by overcoming the limitation of bioavailability of drugs with poor aqueous solubility.
Further, intravaginal delivery of therapeutics has also emerged as an accomplished means to provide women with local protection, still homing times of these drugs are significantly diminished various factors like protective mucus layer, unstable hormone cycle, and intricate anatomical structure of the reproductive tract. Poly (lactic acid)-hyperbranched polyglycerols (PLA-HPG) NP formulations (surface-modified bioadhesive NPs (BNPs)) with bioadhesive coating after intravaginal administration significantly enhanced and extended delivery of drugs (Fig. 4 A). The results displayed that PLA-HPG BNPs are retained along with the vaginal epithelium longer compared to PLA-HPG NNPs and other PLGA-based mucus-penetrating particles. Further elvitegravir association and retention were extended with epithelial and leukocyte for multiple days compared to free drug groups [101]. KR Jyothi et al. designed a liver-targeted, sustained drug delivery system by conjugating the liver-targeting peptide (LTP) to PEGylated CsA-encapsulated poly (lactic-co-glycolic) acid (PLGA) nanoparticles. One of the problems in treating hepatitis C virus (HCV) infection is drug resistance and adverse side effects. Host associated cyclophilin A (CypA) essential for HCV replication, offers a capable approach for antiviral therapy. Cyclosporine A (CsA) loaded targeted nanoparticles effectively inhibited viral replication in vitro and in an HCV mouse model (Fig. 4B).
Fig. 4.
A) (a) Vaginal delivery of nanoparticles. NNPs and BNPs consist of a hydrophobic interior (PLA and EVG) and hydrophilic exterior (HPG). BNPs have an additional bioadhesive coating on the particle surface produced by the conversion of vicinal diols on NNPs to aldehydes on BNPs (b) Molecular structure of EVG [101]; B). Schematic representation of the preparation of CsANP, CsANP (PEGylated), and CsANP-LTP (PEGylated) formulations. Abbreviations: CsA, cyclosporine A; CsANP, cyclosporine A nanoparticles; CsANP-LTP, CsANP conjugated with LTP; EDC, ethyl (dimethylaminopropyl) carbodiimide; LTP, liver-targeting peptide; NHS, N-hydroxysuccinimide; NP, nanoparticles; PEG, polyethylene glycol; PLGA, poly (lactic-co-glycolic) acid [102]; C) Scanning electron microscopic micrograph of combination antiretroviral nanoparticles (cART NPs) [104]; D) Fluorescent microscopic examination of C6 cell lines and neuro 2a cell lines treated with (a) RhB labelled AZT-GAAD NPs (b) merged image of AZT-GAAD NPS with cell nuclei stained blue with Hoechst 33342 (c) bright-field image of neuro 2a cells and (d) RhB labelled AZT-GAAD NPs [99].
Further decreased immunosuppressive effect and toxic effects were associated with free CsA both in vitro and in vivo models compared to conventional treatment with CsA [102]. Vaccination-based treatment is the most effective strategy to prevent HBV infection and related liver cancer. Mannose-modified poly D,L-lactide-co-glycolic acid (PLGA) was synthesized and nanoparticle (MNP)-loaded hepatitis B surface antigen (HBsAg) protein was prepared to target antigen-presenting cells under the epidermis and induce potent cellular and humoral immune responses upon subcutaneous and parenteral delivery of MNPs. Hence, MNPs can be a promising vehicle for hepatitis B vaccine delivery [103]. Further, a combination of antiviral drugs loaded PLGA based nanoparticle has also been explored to inhibit HIV-1 replication. Annemarie Shibata et al. developed nanoparticles of a biodegradable polymer, poly-(dl-lactide-co-glycolic acid; PLGA) containing efavirenz (EFV) and boosted lopinavir (lopinavir/ritonavir; LPV/r) by a high-pressure homogenization method (Fig. 4C). The designed nanoparticle was localized, released antiretroviral drugs in the nuclear, cytoskeleton, and membrane fractions of cells with no toxicity for a month to IC50 values in concentration at nM level. This study confirmed the efficacy of a novel PLGA NPs formulation for the delivery of cART to inhibit HIV-1 replication [104].
6.2.1.2. Poloxomer
The word 'poloxamer' was conceived by a researcher Irving Schmolka in 1973. Poloxamers are nonionic triblock copolymers composed of a central hydrophobic chain of polyoxypropylene flanked by two hydrophilic chains of polyoxyethylene. Poloxamers are also known by their trade name Pluronics [105]. Dhirender Singh et al. designed abacavir loaded poloxamer P407 (nanoformulated MABC) using high-pressure homogenization. These polymeric nanoformulations enhanced intracellular drug localization and exhibited antiviral activity [97].
6.2.1.3. Alginate
Salts form of alginic acid is one of the most widely considered polymers in drug delivery applications [106]. Because its biocompatibility, nontoxicity, and quick elimination from the body make its application in the development of drug delivery systems [107]. In a separate study, amide functionalized alginate nanoparticles (AZT-GAAD NPs) were encapsulated with zidovudine (AZT) by emulsion solvent evaporation method. The novelty of this study was the absence of chemical cross-linking agents for the preparation of nanoparticles. The results suggested that alginate nanoparticle is a capable delivery vector for effective antiviral drug delivery and particularly for HIV/AIDS therapy. Fig. 4D depicted the fluorescent microscopic examination of C6 cell lines and neuro 2a cell lines treated with (a) RhB labelled AZT-GAAD NPs (b) merged image of AZT-GAAD NPS with cell nuclei stained blue with Hoechst 33342 (c) bright field image of neuro 2a cells (d) RhB labelled AZT-GAAD NPs [99].
6.2.1.4. Chitosan
Chitosan is a well-known polymer with favorable features such as biocompatibility, biodegradability, nontoxic polysaccharide with mucoadhesive properties, and non-immunogenicity, having significance in many fields of study [[108], [109], [110]]. HIV-1 inhibitor peptide derived from GB virus C was loaded in a novel polymeric NPs covered with glycol-chitosan [111]. Further, the nanoparticle was confined to upper-layer epithelial mucosa and released at the site where HIV transmission occurs without any side effects. The results suggested that the fusion inhibitor peptides loaded into the nanoparticles coated with glycol chitosan might be a new way to fight the HIV-1 because this formulation might be able to reach the human epithelial mucosa and therefore release peptide S. In another study, Iranpur Mobarakeh et al. formulated anti-HIV siRNA loaded nanoparticles using different ratio of chitosan, polyethylenimine (PEI) and carboxymethyl dextran (CMD) [109]. These nanoparticles suggestively reduced the RNA and protein expression of HIV-1 that are both stable cells and potentially be used in gene therapy, especially against HIV infection. Further, a protease inhibitor saquinavir loaded in chitosan nanoparticle showed greater encapsulation potential, and cell targeting efficiency resulted in the efficient control of the viral proliferation in target T-cells. Further, the nanoparticles exhibited superior potency compared to free drugs at nanogram levels both in the strains of HIV-NL4-3 and Indie-C1 [98].
An extremely transmissible viral respiratory tract infection caused by the influenza virus potentially has deadly consequences in humans and animals. There is now widespread influenza virus resistance to marketed drugs due to the genetic variation of the virus. Jamali et al. formulated chitosan/siRNA nanoparticles, and the in vitro study showed that they were efficiently transfected into Vero cell lines in vitro and were capable of thwarting influenza virus growth [110]. Further, intranasal administrations of the nanoparticles protected mice against influenza virus. Gupta et al. designed CS coated PCL (CS–PCL) (chitosan-coated poly-(-caprolactone)) nanoparticles produced humoral (both systemic and mucosal) and cellular immune responses upon nasal administration. The study demonstrated high potential of the nanoparticles for their use as a carrier adjuvant for nasally administered influenza antigens [112].
6.2.1.5. Poly(ε-caprolactone) (PCL)
PCL is a synthetic polyester that is partially crystalline, having a low melting point (60 °C) and a glass transition temperature of −60 °C. It is made by ring-opening polymerization of ε-caprolactone. PCL can be readily degraded by lipases and esterases of the microorganism [113]. Amiji and collaborators presented that poly(ethylene oxide)-modified poly(ε-caprolactone) (PEO-PCL) nanoparticles offer a good platform for achieving improved intracellular concentrations of encapsulated drugs in various cell lines by means of non-specific endocytosis [114]. Dapivirine is one of the most promising drug candidates in the microbicides pipeline. Phagocytosis is one of the passive targetings of HIV-target cells. Neves et al. reported the formulation of NNRTI dapivirine containing engineered poly (ε-caprolactone) (PCL) nanoparticulate systems and their ability to promote increased intracellular levels in different cell types relevant to microbicide development [115]. Dapivirine-loaded nanoparticles were readily localized in different cell types, including phagocytic cells. The nanoparticles exhibited similar or improved antiviral activity compared to free drugs.
Various other polymeric material was also utilized for delivering antiviral drugs to targeted organs. For example, Roberta Cavalli et al. synthesized a novel polymer β-cyclodextrin-poly(4-acryloyl morpholine) mono-conjugate (β- CD-PACM), loaded with acyclovir. The nanoparticle was investigated for their cell internalization against two isolates of Herpes simplex virus type 1 (HSV-1) in cell cultures. Coumarin 6-loaded β-CD-PACM nanoparticles were internalized in cells and showed a perinuclear accumulation, providing a sensible elucidation of the improved antiviral activity [116]. Similarly, polyanhydride nanoparticles encapsulating inactivated swine influenza A virus (SwIAV) vaccine was assessed to induce protective immunity against a heterologous IAV challenge in pigs [117]. Poultry industries are also affected by viral disease conditions, for instance Newcastle disease (ND), a serious disease that threatens them in many countries, and no treatments available until date. The vaccine against Newcastle disease virus (NDV) encapsulated in nanoparticles was widely used due to their proven high safety, induced quicker, better mucosal, and humoral immune responses. These studies provide a direction for using the biodegradable materials to research and develop the targeted nano-formulated human vaccines [118].
6.3. Lipid based NPs
Despite a few limitations, lipid-based nanoparticles have antiviral activity against HIV [106,119], Human papillomavirus [120], other viruses like HCV and NiV. Commonly used lipid-based nanoparticles contain liposomes and solid lipid nanoparticles (SLN). Liposomes are spherical colloidal particles prepared using one or more concentric lipid bilayers composed of phospholipids and cholesterol molecules encapsulating an aqueous reservoir [121]. The liposome size varies between nm-μm. There are three types of liposomes [122], liposomes multilamellar, MLV (multilamellar vesicles), relatively stable but polydisperse (100 nm–10 μm); small unilamellar liposomes, SUVs (small unilamellar vesicles) of narrower size distribution (30–50 nm), but less stable and of low capacity; the third type of liposome, the LUVs (large unilamellar vesicles) of average diameter varying between 0.1 and 1 μm, larger capacity. Since the last few decades, these particles have been used as tools for biology and biochemistry as carriers of therapeutic and imaging agents [122,123]. The principl hydrophobic or hydrophilic active agents can be loaded respectively in the bilayers lipid or the aqueous compartment. Their non-toxic and biocompatible character makes these colloids interesting systems for in vivo applications [124]. However, liposomes also have some limitations: they actually showed a low encapsulation capacity (especially for lipophilic molecules trapped in the phospholipid double layer), stability moderate and early release of hydrophilic active agents into the blood [125]. SLNs are structured around a lipid core, generally based on biodegradable triglycerides, bioassimilable and non-toxic [126]. The size of these particles varies between 50 nm and 1 μm. The heart of these particles developed during the 1990s consists of a lipid matrix that is solid at room temperature but also at the temperature of the human body. This matrix more or less crystallized is stabilized by a layer of surfactants. The lipids used are either highly purified triglycerides, or mixtures of glycerides or waxes. These particles have stability and an ability to encapsulate lipophilic molecules greater than those of liposomes. They can also be synthesized in the absence of organic solvent. However, such structures have a set of drawbacks, mainly the increase in their size over time, a tendency to gel, unexpected polymorphic transitions and low encapsulation capacity due to crystal structure of the lipid matrix.
Cationic liposomes in common and, cholesterol-based types in particular are favorable for clinical application because of their low toxicity.
Vitamin E (α-tocopherol) was successfully used for targeted drug delivery systems to deliver the siRNAs from the serum to the liver [127]. Cholesterol-based cationic liposomes was conjugated to vitamin E and used to deliver inhibitory siRNA specifically to the liver in mouse models which led to suppression in both HCV core antigen production and firefly luciferase activity [127] (used as a reporter gene to determine extent of HCV replication) [128]. Carmona et al., designed anti HBV formulations by condensation of siRNA (A component) with cationic liposomes (B component) to form AB core particles (Fig. 5 ) [129]. These core particles are incorporated with an aminoxy cholesterol lipid for convenient surface post coupling of polyethylene glycol to give triggered PEGylated siRNA-nanoparticles sizes of 80–100 nm. Systemic administration of triggered PEGylated siRNA-nanoparticles in HBV transgenic mice results in the suppression of markers of HBV replication by up to 3-fold compared to untreated controls a month period.
Fig. 5.
Chemical components and stability of siRNA nanoparticles. (a) Schematic illustration of structures of lipid components. (b) The aminoxy group of CPA or of CA allows for the convenient addition of PEG2000-(CHO)2 in order to confer biocompatibility and stealth functions. (c) Schematic illustration of a triggered PEGylated siRNA nanoparticle (siRNA-ABC nanoparticle); siRNA (A component) is likely to be condensed as a multilamellar siRNA-lipoplex encapsulated within a lipid bilayer (B component) for cellular uptake and intracellular delivery of siRNA; PEG2000 is added by surface post coupling to act as a stealth/biocompatibility polymer (C component). (d) Change in particle diameter of siRNA-nanoparticle formulations prepared from CDAN/DOPE/CPA (40:50:10, m/m/m) liposomes containing varying amounts of PEG (9 0.1 mol %; 0 0.5 mol %; b 1.0 mol %; O 5.0 mol %), as a function of time in the presence of 80% serum [129].
6.4. Dendrimers and niosomes
Dendrimers are organic compounds which have proven effect against Influenza virus, EBOV, zika virus, HSV, NiV and HIV due to inhibition of viral entry and activation of CD8+ T cells [[130], [131], [132]]. However, their action against NiV is yet to be evaluated. Niosomes are also effective against HSV [133]. Dendrimers are highly branched macromolecules perfectly monodisperse [134]. This strong branching of the polymer molecules gives the dendrimers of different chemical and physical properties of polymer molecules linear, which are of great interest for biomedical and industrial applications. Dendrimers can be synthesized by divergent and convergent methods. In divergent methods [135], the dendrimer grows outwardly from the basic molecule. Monomer containing one reactive group and two dormant groups reacts first with the nucleus forming the first generation dendrimer, and successively, the new rim of the molecule is activated and reacts with several monomers until a large macromolecular structure is formed. This process has the drawbacks that the by-products formed are not easy to purify. In the methods convergent [136], the dendrimer is synthesized in stages from the exterior groups. When branched molecules are large enough, they are attached to a molecule core. The dendrimers generated by these methods are easy to purify and have a structure controlled. Dendrimers have already been used as contrast agents for imaging by magnetic resonance [137], as drug delivery systems [138], as therapeutic agents by boron neutron capture [139], in gene therapy as as vectors of gene transfer across the cell membrane [140], and in medicine regenerative [141].
6.5. Nano micelles and hybrid nanoparticles
Analogously to amphiphilic lipids, the di-block copolymers, with a block hydrophilic and a hydrophobic block, form micelles in some solvents. An excellent example of the use of block copolymer micelles as nanovectors targeted for therapeutic delivery are micelles formed by the block polymer. Polylactic, polymethoxy ethylene glycol, and folate-poly (ethylene glycol) of a diameter of 146 nm developed by Zhu et al. [142]. Polymersomes are artificial vesicles similar in structure to vesicles lipidic, but based on amphiphilic block copolymers [143]. They can be prepared by the same methods as those used for lipid vesicles and liposomes. The preparation methods can be summarized in two groups: techniques without solvent and solvent displacement techniques [144]. Solvent-free techniques consist of the hydration of the block copolymer in the dry state to form vesicles, for example, rehydration of the polymeric film [145] or electroforming [146]. The last method consists of the rehydration of polymers distributed over a pair of electrodes; once the solvent is added, an electric field is applied to facilitate hydration and formation of vesicles. Solvent displacement techniques are methods that require a first step to dissolve the block copolymer in an organic solvent before mixing with some water. After mixing, the organic solvent is removed by various methods. The injection process [147], reverse phase evaporation [148,149] and depletion of detergent [150] are examples of solvent displacement techniques used for polymers. Polymersomes are very attractive due to their structural similarity to biological membranes. Compared to lipid vesicles and liposomes, polymersomes have the advantage of forming flexible structures, high mechanical stability and resistance to an external stimulus [151]. Polymersomes can encapsulate hydrophobic or hydrophilic molecules. They can be used as nanovectors for administration of therapeutic agents.
Nano micelles are effective antiviral particles mostly due to encapsulation, biocompatibility, colloidal stability, and prolonged circulation time [152]. Hybridized nanoparticles are also the most potent antiviral agents. Up to date, these hybrid particles are effective against HIV, Influenza virus, hepatitis virus, Adenovirus, and Ebola [153]. Hepatitis B virus infection is considered a major cause of mortality worldwide due to the risk of cirrhosis, hepatocellular cancer, portal hypertension, and liver failure. Roughly 6% of the global population is chronically infected with HBV, and between 25%, and 40% of carriers of the virus will develop complicating hepatocellular carcinoma (HCC). Major concern in eliminating the hepatitis B virus requires long term treatment and targeted delivery of nanoparticles containing antiviral drugs to the liver infected with hepatitis B [129]. There are some researchers working on hybrid nanoparticles combining the advantages of polymers and lipids [154]. Mohamed Hamdi et al., designed entecavir (E), loaded lipid polymer hybrid LPH nanoparticles (vitamin E coated nanoparticles enriched with lecithin-glyceryl monostearate lipid shell). The hybrid nanoparticle exhibited promising results and enhanced cellular retention in J774 macrophages cells [154].
7. Nanotechnology for Ebola treatment
Like other viral infections, there is a possibility of utilizing nanotechnology for treating the EBOV. Some of the particles include inorganic particles like silica and carbon-based nanocarriers [77,78], and organic particles like dendrimers [130] and hybrid particles [153]. Humans' infection by lethal pathogens such as Ebola and other related viruses has not been properly addressed so far [155]. EBOV is one of the lethal viruses causing infection in humans. Numerous epidemics have been reported, mainly in Central Africa, ever since its initial description in 1976 in Zaire (now the Democratic Republic of the Congo). Zaire EBOV species within the EBOV genus of the family Filoviridae, in which three additional varieties of highly pathogenic agents Sudan, Tai Forest, and Bundibugyo viruses have also been described [156]. EBOV is communicated by direct contact with the body fluids of infected persons and objects contaminated with virus or infected animals [157]. WHO has accredited Ebola as a widespread public health emergency of international concern with severe global economic issues. The recent outbreaks of EBOV in West Africa underscore the urgent need to develop an effective EBOV vaccine [158]. While a variety of therapeutics proved disappointing in tests against EBOV infection, more recently, specific molecules have been prepared. Between 2014 and 16, EBOV outbreak in West Africa has attracted widespread concern [159]. Bazzill et al., report the development of synthetic nanoparticles as a safe and highly immunogenic platform for vaccination against EBOV. The study designed a large recombinant EBOV antigen (rGP) incorporated into lipid-based nanoparticles, termed inter-bilayer-crosslinked multilamellar vesicles (ICMVs) [158]. Illescas et al., presented new approaches directed at the design of efficient entry inhibitors to minimize the development of resistance by viral mutations [155]. In particular, the authors focused on dendrimers as well as fullerene C60 with a unique symmetrical and 3D globular structure as biocompatible carbon platforms for the multivalent presentation of carbohydrates [155].
In terms of diagnosis, quick and sensitive detection methods are essential for diagnosis and Wu et al., proposed a novel method for EBOV detection based on efficient amplification of electroluminescent nanospheres (ENs) coupled with immunomagnetic separation [159]. Using simple ultrasound techniques, uniform ENs are made by embedding abundant amounts of CdSe/ZnS quantum dots into copolymer nanospheres. This method provided consistent reproducibility, specificity, anti-interference ability and is highly promising in clinical diagnosis applications. Further, Tsang et al. proposed a luminescence BaGdF5:Yb/Er upconversion nanoparticles (UCNPs) conjugated with oligonucleotide probe and AuNPs linked with target EBOV oligonucleotide [159]. The author anchored the UCNPs and AuNPs on a nanoporous alumina (NAAO) membrane to form a heterogeneous assay. The approach of combining UCNPs, AuNPs, and NAAO membranes offered low-cost, rapid, and ultrasensitive detection of different diseases [159]. Using Fe3O4 magnetic nanoparticle (MNP) as a nanozyme probe, Duan et al. developed an MNP-based immunochromatographic strip (Nanozyme-strip), detecting the glycoprotein of EBOV as low as 1 ng/mL, which is 100-fold more sensitive than the standard strip method [160]. Nanozyme-strip test can rapidly and sensitively detect EBOV, providing a valuable simple screening tool for diagnosis of infection in Ebola-stricken areas.
8. Challenges and future prospects
Although the nanotechnology-based approaches for the managing of emerging and re-emerging viral infections have attracted significant attantions, there are still several challenges that should be addressed in the future works. Some challenges are the pharmacocynetics and pharmacodynamics of the nano-based drugs as well as the fate of the nanomaterials in the body. Further studies should provide clear information about the safety profile of nano-based drugs. Furthermore, the acute and cronic toxicity of nano-based drugs should be studied. Significantly, to choose a right nanocarrier and a right therapeutic agent to combat a viral infection is critical for the commercial success of a nanotherapeutic system. Another challenge is that the viruses may have numerous reservoirs over the time that make the therapy difficult [7]. In the future, the antiviral diseases such as COVID-19 will be managed by developing of nanotheranostic approaches which not only diagnose the early stage of viral infection, but also will be useful for the treatment of viral infections [6].
9. Conclusion
This review has explored the application of various nanoparticles in the diagnosis and treatment of emerging and re-emerging viruses in general. Different nanotechnological approaches have shown the ability to improve the efficacy against viruses while reducing their toxicity. The development of nanotechnology-based antiviral delivery systems is a potential strategy to improve the efficacy and safety of current therapy. The use of nanoparticles as adjuvants for vaccines has also produced interesting results, which contribute to the development of an efficient and safe anti-viral vaccine design. This comprehensive review offers insight into nanotechnology's remarkable potential in effective diagnosis, prevention, and treatment with a particular focus on the recently emerged virus COVID -19.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X., et al. Coronavirus susceptibility to the antiviral remdesivir (GS5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio. 2018;9 doi: 10.1128/mBio.00221-18. e00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hassan M.Z., Sazzad H.M.S., Luby S.P., Sturm-Ramirez K., Bhuiyan M.U., Rahman M.Z., et al. Nipah virus contamination of hospital surfaces during outbreaks, Bangladesh, 2013-2014. Emerg. Infect. Dis. 2018;24:15–21. doi: 10.3201/eid2401.161758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kulkarni D.D., Tosh C., Venkatesh G., Senthil-Kumar D. Nipah virus infection: current scenario. Indian J. Virol. 2013;24:398–408. doi: 10.1007/s13337-013-0171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valbuena G., Halliday H., Borisevich V., Goez Y., Rockx B. A human lung xenograft mouse model of Nipah virus infection. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeBuysscher B.L., De-Wit E., Munster V.J., Scott D., Feldmann H., Prescott J. Comparison of the pathogenicity of Nipah virus isolates from Bangladesh and Malaysia in the Syrian Hamster. PLoS Neglected Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0002024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varahachalam S.P., Lahooti B., Chamaneh M., Bagchi S., Chhibber T., Morris K., et al. Nanomedicine for the SARS-CoV-2: state-of-the-art and future prospects. Int. J. Nanomed. 2021;16:539–560. doi: 10.2147/IJN.S283686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaushik A. Manipulative magnetic nanomedicine: the future of COVID-19 pandemic/endemic therapy. Expet Opin. Drug Deliv. 2020:1–4. doi: 10.1080/17425247.2021.1860938. [DOI] [PubMed] [Google Scholar]
- 8.Mujawar M.A., Gohel H., Bhardwaj S.K., Srinivasan S., Hickman N., Kaushik A. Nano-enabled biosensing systems for intelligent healthcare: towards COVID-19 management. Materials Today Chemistry. 2020;17:100306. doi: 10.1016/j.mtchem.2020.100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar S., Nehra M., Khurana S., Dilbaghi N., Kumar V., Kaushik A., et al. Aspects of point-of-care diagnostics for personalized health wellness. Int. J. Nanomed. 2021;16:383–402. doi: 10.2147/IJN.S267212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paliwal P., Sargolzaei S., Bhardwaj S.K., Bhardwaj V., Dixit C., Kaushik A. vol. 2. 2020. (Grand Challenges in Bio-Nanotechnology to Manage the COVID-19 Pandemic). [Google Scholar]
- 11.Kaushik A.K., Dhau J.S., Gohel H., Mishra Y.K., Kateb B., Kim N.-Y., et al. Electrochemical SARS-CoV-2 sensing at point-of-care and artificial intelligence for intelligent COVID-19 management. ACS Applied Bio Materials. 2020;3:7306–7325. doi: 10.1021/acsabm.0c01004. [DOI] [PubMed] [Google Scholar]
- 12.Vivekanandhan K., Shanmugam P., Barabadi H., Arumugam V., Daniel Raj Daniel Paul Raj D., Sivasubramanian M., et al. Emerging therapeutic approaches to combat COVID-19: present status and future perspectives. Front. Mol. Biosci. 2021;8:604447. doi: 10.3389/fmolb.2021.604447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahajan S.D., Aalinkeel R., Law W.-C., Reynolds J.L., Nair B.B., Sykes D.E., et al. Anti-HIV-1 nanotherapeutics: promises and challenges for the future. 2012;7:5301. doi: 10.2147/IJN.S25871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiodo F., Marradi M., Calvo J., Yuste E., Penadés SJBjooc Glycosystems in nanotechnology: gold glyconanoparticles as carrier for anti-HIV prodrugs. 2014;10:1339–1346. doi: 10.3762/bjoc.10.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oswald-Richter K., Grill S.M., Shariat N., Leelawong M., Sundrud M.S., Haas D.W., et al. HIV infection of naturally occurring and genetically reprogrammed human regulatory. T-cells. 2004;2:e198. doi: 10.1371/journal.pbio.0020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Méndez-Lagares G., Jaramillo-Ruiz D., Pion M., Leal M., Munoz-Fernandez M., Pacheco Y.M., et al. HIV infection deregulates the balance between regulatory T cells and IL-2–producing CD4 T cells by decreasing the expression of the IL-2 receptor in Treg. 2014;65:278–282. doi: 10.1097/QAI.0000000000000092. [DOI] [PubMed] [Google Scholar]
- 17.Moreno-Fernandez M.E., Zapata W., Blackard J.T., Franchini G., Chougnet C.A. Human regulatory T cells are targets for human immunodeficiency Virus (HIV) infection, and their susceptibility differs depending on the HIV type 1 strain. J. Virol. 2009;83:12925–12933. doi: 10.1128/JVI.01352-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaramillo-Ruiz D., De La Mata F.J., Gómez R., Correa-Rocha R., Muñoz-Fernández MÁJPo. Nanotechnology as a new therapeutic approach to prevent the HIV-infection of Treg cells. 2016;11 doi: 10.1371/journal.pone.0145760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parboosing R., Chonco L., de la Mata F.J., Govender T., Maguire G.E., Kruger H.G. Potential inhibition of HIV-1 encapsidation by oligoribonucleotide–dendrimer nanoparticle complexes. Int. J. Nanomed. 2017;12:317. doi: 10.2147/IJN.S114446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jayant R.D., Atluri V.S., Agudelo M., Sagar V., Kaushik A., Nair MJIjon. Sustained-release nanoART formulation for the treatment of neuroAIDS. 2015;10:1077. doi: 10.2147/IJN.S76517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elliott J.H., Wightman F., Solomon A., Ghneim K., Ahlers J., Cameron M.J., et al. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. 2014;10 doi: 10.1371/journal.ppat.1004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang Y., Cao S., Bright D.K., Bever A.M., Blakney A.K., Suydam I.T., et al. Nanoparticle-based ARV drug combinations for synergistic inhibition of cell-free and cell–cell HIV transmission. 2015;12:4363–4374. doi: 10.1021/acs.molpharmaceut.5b00544. [DOI] [PubMed] [Google Scholar]
- 23.CDC. Functional Carbon Quantum Dots as Medical Countermeasures to Human Coronavirus 2020. [DOI] [PMC free article] [PubMed]
- 24.Ahmad S A.A.Z., Tan H.T., Wong K.K., Lim J., Mohamud R. Targeting dendritic cells through gold nanoparticles: a review on the cellular uptake and subsequent immunological proper ties. Mol. Immunol. 2017;91:123–133. doi: 10.1016/j.molimm.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Pati R., Shevtsov M., Sonawane A. Nanoparticle vaccines against infectious diseases. Front. Immunol. 2018;9:2224. doi: 10.3389/fimmu.2018.02224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dykman L.A., Khlebtsov N.G. Immunological properties of gold nanoparticles. Chem. Sci. 2017;8:1719–1735. doi: 10.1039/c6sc03631g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cihlar T., Ray A.S. Nucleoside and nucleotide HIV reverse transcriptase inhibitors: 25 years after zidovudine. Antivir. Res. 2010;85:39–58. doi: 10.1016/j.antiviral.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 28.De-Béthune M.P. Non-nucleoside reverse transcriptase inhibitors (NNRTIs), their discovery, development, and use in the treatment of HIV-1 infection: a review of the last 20 years; 1989-2009. Antivir. Res. 2010;85:75–90. doi: 10.1016/j.antiviral.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Flexner C. HIV-protease inhibitors N. Engl J Med. 1998;338:1281–1293. doi: 10.1056/NEJM199804303381808. [DOI] [PubMed] [Google Scholar]
- 30.Münch J., Ständker L., Adermann K. Discovery and optimization of a natural HIV-1 entry inhibitor targeting the gp41 fusion peptide. Cell. 2007;129:263–275. doi: 10.1016/j.cell.2007.02.042. [DOI] [PubMed] [Google Scholar]
- 31.Fätkenheuer G., Pozniak A.L., Johnson M.A. Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1. Nat. Med. 2005;11:1170–1172. doi: 10.1038/nm1319. [DOI] [PubMed] [Google Scholar]
- 32.Barbaro G., Scozzafava A., Mastrolorenzo A. Highly active antiretroviral therapy: current state of the art, new agents and their pharmacological interactions useful for improving therapeutic outcome. Curr. Pharmaceut. Des. 2005;11:1805–1843. doi: 10.2174/1381612053764869. [DOI] [PubMed] [Google Scholar]
- 33.Esté J.A., Cihlar T. Current status and challenges of antiretroviral research and therapy. Antivir. Res. 2010;85:25–33. doi: 10.1016/j.antiviral.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Mamo T., Moseman E.A., Kolishetti N. Emerging nanotechnology approaches for HIV/AIDS treatment and prevention. Nanomedicine. 2010;5:269–285. doi: 10.2217/nnm.10.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahajan S.D., Aalinkeel R., C L.-W. Anti-HIV-1 nanotherapeutics: promises and challenges for the future. nt J Nanomedicine. 2012;7:5301–5314. doi: 10.2147/IJN.S25871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiodo F., Marradi M., Calvo J. Glycosystems in nanotechnology: gold glyconanoparticles as carrier for anti-HIV prodrugs. Beilstein J. Org. Chem. 2014;10:1339–1346. doi: 10.3762/bjoc.10.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oswald-Richter K., Grill S.M., Shariat N. HIV infection of naturally occurring and genetically reprogrammed human regulatory T-cells. PLoS Biol. 2004;2:e198. doi: 10.1371/journal.pbio.0020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moreno-Fernandez M.E., Zapata W., Blackard J.T. Human regulatory T cells are targets for human immunodeficiency virus (HIV) infection, and their susceptibility differs depending on the HIV type 1 strain. J. Virol. 2009;83:12925–12933. doi: 10.1128/JVI.01352-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Méndez-Lagares G., Jaramillo-Ruiz D., Pion M. HIV infection deregulates the balance between regulatory T cells and IL-2-producing CD4 T cells by decreasing the expression of the IL-2 receptor in Treg. J. Acquir. Immune Defic. Syndr. 2014;65:278–282. doi: 10.1097/QAI.0000000000000092. [DOI] [PubMed] [Google Scholar]
- 40.Jaramillo-Ruiz D., De-L- Mata F.J., Gómez R. Nanotechnology as a new therapeutic approach to prevent the HIV-infection of Treg cells. PloS One. 2016;11 doi: 10.1371/journal.pone.0145760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parboosing R., Chonco L., De-La-Mata F.J. Potential inhibition of HIV-1 encapsidation by oligoribonucleotide-dendrimer nanoparticle complexes. Int. J. Nanomed. 2017;12:317–325. doi: 10.2147/IJN.S114446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jayant R.D., Atluri V.S.R., Agudelo M., et al. Sustained-release nanoART formulation for the treatment of neuroAIDS. Int. J. Nanomed. 2015;10:1077–1093. doi: 10.2147/IJN.S76517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elliot J.H., Wightman F., Solomon A., et al. Activation of HIV transcription with short course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang Y., Cao S., Bright D.K., et al. Nanoparticle-based ARV drug combinations for synergistic inhibition of cell-free and cell-cell HIV transmission. Mol. Pharm. 2015;12:4363–4374. doi: 10.1021/acs.molpharmaceut.5b00544. [DOI] [PubMed] [Google Scholar]
- 45.Li W., Wang Q., Li Y., et al. A nanoparticle encapsulated non-nucleoside reverse transcriptase inhibitor with enhanced 1anti-HIV-1 activity and prolonged circulation time in plasma. Curr. Pharmaceut. Des. 2015;21:925–935. doi: 10.2174/1381612820666141014125213. [DOI] [PubMed] [Google Scholar]
- 46.Bastian A.R., Nangarlia A., Bailey L.D., et al. Mechanism of multivalent nanoparticle encounter with HIV-1 for potency enhancement of peptide triazole virus inactivation. J. Biol. Chem. 2015;290:529–543. doi: 10.1074/jbc.M114.608315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith K.A., Lin X., Bolshakov O., et al. Activation of HIV-1 with nanoparticle-packaged small molecule protein phosphatase-1-targeting compound. Sci. Pharm. 2015;83:535–548. doi: 10.3797/scipharm.1502-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kerry R.G., Malik S., Redda Y.T., Sahoo S., Patra J.K., Majhi S. Nano-based approach to combat emerging viral (NIPAH virus) infection. Nanomed. Nanotechnol. Biol. Med. 2019;18:196–220. doi: 10.1016/j.nano.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Milovanovic M., Arsenijevic A., Milovanovic J., Kanjevac T., Arsenijevic N. In: Antimicrobial Nanoarchitectonics. Grumezescu A.M., editor. Elsevier; 2017. Chapter 14 - nanoparticles in antiviral therapy; pp. 383–410. [Google Scholar]
- 50.Rai M., Deshmukh S.D., Ingle A.P., Gupta I.R., Galdiero M., Galdiero S. Metal nanoparticles: the protective nanoshield against virus infection. Crit. Rev. Microbiol. 2016;42:46–56. doi: 10.3109/1040841X.2013.879849. [DOI] [PubMed] [Google Scholar]
- 51.Rónavári A., Kovács D., Igaz N., Vágvölgyi C., Boros I.M., Kónya Z., et al. Biological activity of green-synthesized silver nanoparticles depends on the applied natural extracts: a comprehensive study. Int. J. Nanomed. 2017;12:871–883. doi: 10.2147/IJN.S122842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Castro-Mayorga J.L., Randazzo W., Fabra M.J., Lagaron J.M., Aznar R., Sánchez G. Antiviral properties of silver nanoparticles against norovirus surrogates and their efficacy in coated polyhydroxyalkanoates systems. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2017;79:503–510. [Google Scholar]
- 53.Huy T.Q., Hien Thanh N.T., Thuy N.T., Chung P.V., Hung P.N., Le A.-T., et al. Cytotoxicity and antiviral activity of electrochemical – synthesized silver nanoparticles against poliovirus. J. Virol Methods. 2017;241:52–57. doi: 10.1016/j.jviromet.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 54.Sreekanth T.V.M., Nagajyothi P.C., Muthuraman P., Enkhtaivan G., Vattikuti S.V.P., Tettey C.O., et al. Ultra-sonication-assisted silver nanoparticles using Panax ginseng root extract and their anti-cancer and antiviral activities. J. Photochem. Photobiol. B Biol. 2018;188:6–11. doi: 10.1016/j.jphotobiol.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 55.Morris D., Ansar M., Speshock J., Ivanciuc T., Qu Y., Casola A., et al. Antiviral and immunomodulatory activity of silver nanoparticles in experimental RSV infection. Viruses. 2019;11 doi: 10.3390/v11080732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Orlowski P., Tomaszewska E., Gniadek M., Baska P., Nowakowska J., Sokolowska J., et al. Tannic acid modified silver nanoparticles show antiviral activity in herpes simplex virus type 2 infection. PloS One. 2014;9 doi: 10.1371/journal.pone.0104113. e104113-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ochoa-Meza A.R., Álvarez-Sánchez A.R., Romo-Quiñonez C.R., Barraza A., Magallón-Barajas F.J., Chávez-Sánchez A., et al. Silver nanoparticles enhance survival of white spot syndrome virus infected Penaeus vannamei shrimps by activation of its immunological system. Fish Shellfish Immunol. 2019;84:1083–1089. doi: 10.1016/j.fsi.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 58.Vijayakumar S., Ganesan S. Gold nanoparticles as an HIV entry inhibitor. Curr. HIV Res. 2012;10:643–646. doi: 10.2174/157016212803901383. [DOI] [PubMed] [Google Scholar]
- 59.Hafashjani S.R., Rezatofighi S.E., Ardakani M.R., Rastegarzadeh S. Gold nanoparticles impair foot-and-mouth disease virus replication. IEEE Trans. NanoBioscience. 2016;15:34. doi: 10.1109/TNB.2015.2508718. [DOI] [PubMed] [Google Scholar]
- 60.Ahmed S.R., Kim J., Suzuki T., Lee J., Park E.Y. Detection of influenza virus using peroxidase-mimic of gold nanoparticles. Biotechnol. Bioeng. 2016;113:2298–2303. doi: 10.1002/bit.25982. [DOI] [PubMed] [Google Scholar]
- 61.Halder A., Das S., Ojha D., Chattopadhyay D., Mukherjee A. Highly monodispersed gold nanoparticles synthesis and inhibition of herpes simplex virus infections. Mater. Sci. Eng. C. 2018;89:413–421. doi: 10.1016/j.msec.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 62.Bawage S.S., Tiwari P.M., Singh A., Dixit S., Pillai S.R., Dennis V.A., et al. Gold nanorods inhibit respiratory syncytial virus by stimulating the innate immune response. Nanomed. Nanotechnol. Biol. Med. 2016;12:2299–2310. doi: 10.1016/j.nano.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Di Gianvincenzo P., Marradi M., Martínez-Ávila O.M., Bedoya L.M., Alcamí J., Penadés S. Gold nanoparticles capped with sulfate-ended ligands as anti-HIV agents. Bioorg. Med. Chem. Lett. 2010;20:2718–2721. doi: 10.1016/j.bmcl.2010.03.079. [DOI] [PubMed] [Google Scholar]
- 64.Kim J., Yeom M., Lee T., Kim H.-O., Na W., Kang A., et al. Porous gold nanoparticles for attenuating infectivity of influenza A virus. J. Nanobiotechnol. 2020;18:54. doi: 10.1186/s12951-020-00611-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Melendez-Villanueva M.A., Moran-Santibanez K., Martinez-Sanmiguel J.J., Rangel-Lopez R., Garza-Navarro M.A., Rodriguez-Padilla C., et al. Virucidal activity of gold nanoparticles synthesized by green chemistry using garlic extract. Viruses. 2019;11 doi: 10.3390/v11121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Delaviz N., Gill P., Ajamia A., Aarabic M. Aptamer-conjugated magnetic nanoparticles for the efficient removal of HCV particles from human plasma samples. RSC Adv. 2015;5:79433. [Google Scholar]
- 67.Shelby T., Banerjee T., Zegar I., Santra S. Highly sensitive, engineered magnetic nanosensors to investigate the ambiguous activity of Zika virus and binding receptors. Sci. Rep. 2017;7:7377. doi: 10.1038/s41598-017-07620-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kumar R., Nayak M., Sahoo G.C., Pandey K., Sarkar M.C., Ansari Y., et al. Iron oxide nanoparticles based antiviral activity of H1N1 influenza A virus. J. Infect. Chemother. 2019;25:325–329. doi: 10.1016/j.jiac.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 69.Choudhary S., Kumar R., Dalal U., Tomar S., Reddy S.N. Green synthesis of nanometal impregnated biomass – antiviral potential. Mater. Sci. Eng. C. 2020;112:110934. doi: 10.1016/j.msec.2020.110934. [DOI] [PubMed] [Google Scholar]
- 70.Park S., Park H.H., Kim S.Y., Kim S.J., Woo K., Ko G. Antiviral properties of silver nanoparticles on a magnetic hybrid colloid. Appl. Environ. Microbiol. 2014;80:2343–2350. doi: 10.1128/AEM.03427-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Syngouna V.I., Chrysikopoulos C.V. Inactivation of MS2 bacteriophage by titanium dioxide nanoparticles in the presence of quartz sand with and without ambient light. J. Colloid Interface Sci. 2017;497:117–125. doi: 10.1016/j.jcis.2017.02.059. [DOI] [PubMed] [Google Scholar]
- 72.Mazurkova N.A., Spitsyna Y.E., Shikina N.V., Ismagilov Z.R., Zagrebel’nyi S.N., Ryabchikova E.I. Interaction of titanium dioxide nanoparticles with influenza virus. Nanotechnologies in Russia. 2010;5:417–420. [Google Scholar]
- 73.Winkler H.C., Notter T., Meyer U., Naegeli H. Critical review of the safety assessment of titanium dioxide additives in food. J. Nanobiotechnol. 2018;16:51. doi: 10.1186/s12951-018-0376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gerrity D., Ryu H., Crittenden J., Abbaszadegan M. Photocatalytic inactivation of viruses using titanium dioxide nanoparticles and low-pressure UV light. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2008;43:1261–1270. doi: 10.1080/10934520802177813. [DOI] [PubMed] [Google Scholar]
- 75.Misstear D.B., Gill L.W. The inactivation of phages MS2, ΦX174 and PR772 using UV and solar photocatalysis. J. Photochem. Photobiol. B Biol. 2012;107:1–8. doi: 10.1016/j.jphotobiol.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 76.De-Souza E.S.J.M., Hanchuk T.D., Santos M.I., Kobarg J., Bajgelman M.C., Cardoso M.B. Viral inhibition mechanism mediated by surfacemodified silica nanoparticles. ACS Appl. Mater. Interfaces. 2016;8:16564–16572. doi: 10.1021/acsami.6b03342. [DOI] [PubMed] [Google Scholar]
- 77.Dostalova S., Moulick A., Milosavljevic V., Guran R., Kominkova M., Cihalova K., et al. Antiviral activity of fullerene C60 nanocrystals modifiedwith derivatives of anionic antimicrobial peptide maximin H5. Monatsh. Chem. 2016;147:905. [Google Scholar]
- 78.Yang X.X., Li C.M., Li Y.F., Wang J., Huang C.Z. Synergistic antiviral effect of curcumin functionalized graphene oxide against respiratory syncytial virus infection. Nanoscale. 2017;9:16086–16092. doi: 10.1039/c7nr06520e. [DOI] [PubMed] [Google Scholar]
- 79.Lee E.C., Davis-Poynter N., Nguyen C.T.H., Peters A.A., Monteith G.R., Strounina E., et al. GAG mimetic functionalised solid and mesoporous silica nanoparticles as viral entry inhibitors of herpes simplex type 1 and type 2 viruses. Nanoscale. 2016;8:16192–16196. doi: 10.1039/c6nr03878f. [DOI] [PubMed] [Google Scholar]
- 80.Skrastina D., Petrovskis I., Lieknina I., Bogans J., Renhofa R., Ose V., et al. Silica nanoparticles as the adjuvant for the immunisation of mice using hepatitis B core virus-like particles. PloS One. 2014;9 doi: 10.1371/journal.pone.0114006. e114006-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ye S., Shao K., Li Z., Guo N., Zuo Y., Li Q., et al. Antiviral activity of graphene oxide: how sharp edged structure and charge matter. ACS Appl. Mater. Interfaces. 2015;7:21571–21579. doi: 10.1021/acsami.5b06876. [DOI] [PubMed] [Google Scholar]
- 82.Dostalova S., Moulick A., Milosavljevic V., Guran R., Kominkova M., Cihalova K., et al. Antiviral activity of fullerene C60 nanocrystals modified with derivatives of anionic antimicrobial peptide maximin H5. Monatshefte für Chemie - Chemical Monthly. 2016;147:905–918. [Google Scholar]
- 83.Martinez Z.S., Castro E., Seong C.-S., Cerón M.R., Echegoyen L., Llano M. Fullerene derivatives strongly inhibit HIV-1 replication by affecting virus maturation without impairing protease activity. Antimicrob. Agents Chemother. 2016;60:5731–5741. doi: 10.1128/AAC.00341-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Muñoz A., Illescas B.M., Luczkowiak J., Lasala F., Ribeiro-Viana R., Rojo J., et al. Antiviral activity of self-assembled glycodendro[60]fullerene monoadducts. J. Mater. Chem. B. 2017;5:6566–6571. doi: 10.1039/c7tb01379e. [DOI] [PubMed] [Google Scholar]
- 85.Du T., Liang J., Dong N., Liu L., Fang L., Xiao S., et al. Carbon dots as inhibitors of virus by activation of type I interferon response. Carbon. 2016;110:278–285. [Google Scholar]
- 86.Dong X., Moyer M.M., Yang F., Sun Y.P., Yang L. Carbon dots' antiviral functions against Noroviruses. Sci. Rep. 2017;7:519. doi: 10.1038/s41598-017-00675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bera D., Qian L., Tseng T.-K., Holloway P.H. Quantum dots and their multimodal applications: a review. Materials. 2010;3:2260–2345. [Google Scholar]
- 88.Vasudevan D., Gaddam R.R., Trinchi A., Cole I. Core–shell quantum dots: properties and applications. J. Alloys Compd. 2015;636:395–404. [Google Scholar]
- 89.Mahajan S.D., Roy I., Xu G., Yong K.T., Ding H., Aalinkeel R., et al. Enhancing the delivery of anti retroviral drug "Saquinavir" across the blood brain barrier using nanoparticles. Curr. HIV Res. 2010;8:396–404. doi: 10.2174/157016210791330356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Norouzi M., Zarei Ghobadi M., Golmimi M., Mozhgani S.-H., Ghourchian H., Rezaee S.A. Quantum dot-based biosensor for the detection of human T-lymphotropic virus-1. Anal. Lett. 2017;50:2402–2411. [Google Scholar]
- 91.Bentzen E.L., House F., Utley T.J., Crowe J.E., Jr., Wright D.W. Progression of respiratory syncytial virus infection monitored by fluorescent quantum dot probes. Nano Lett. 2005;5:591–595. doi: 10.1021/nl048073u. [DOI] [PubMed] [Google Scholar]
- 92.Mitragotri S., Stayton P. Organic nanoparticles for drug delivery and imaging. MRS Bull. 2014;39:219–223. [Google Scholar]
- 93.Joshy K.S., Snigdha S., Kalarikkal N., Pothen L.A., Thomas S. Gelatin modified lipid nanoparticles for anti-viral drug delivery. Chem. Phys. Lipids. 2017;207:24–37. doi: 10.1016/j.chemphyslip.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 94.Deng L., Mohan T., Chang T.Z., Gonzalez G.X., Wang Y., Kwon Y.M., et al. Double-layered protein nanoparticles induce broad protection against divergent influenza A viruses. Nat. Commun. 2018;9:359. doi: 10.1038/s41467-017-02725-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xu J., Merlier F., Avalle B., Vieillard V., Debré P., Haupt K., et al. Molecularly imprinted polymer nanoparticles as potential synthetic Antibodies for immunoprotection against HIV. ACS Appl. Mater. Interfaces. 2019;11:9824–9831. doi: 10.1021/acsami.8b22732. [DOI] [PubMed] [Google Scholar]
- 96.Govender T., Ojewole E., Naidoo P., Mackraj I. Polymeric nanoparticles for enhancing antiretroviral drug therapy. Drug Deliv. 2008;15:493–501. doi: 10.1080/10717540802321776. [DOI] [PubMed] [Google Scholar]
- 97.Singh D., McMillan J., Hilaire J., Gautam N., Palandri D., Alnouti Y., et al. Development and characterization of a long-acting nanoformulated abacavir prodrug. Nanomedicine (Lond). 2016;11:1913–1927. doi: 10.2217/nnm-2016-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ramana L.N., Sharma S., Sethuraman S., Ranga U., Krishnan U.M. Evaluation of chitosan nanoformulations as potent anti-HIV therapeutic systems. Biochim. Biophys. Acta Gen. Subj. 2014;1840:476–484. doi: 10.1016/j.bbagen.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 99.Joshy K.S., Susan M.A., Snigdha S., Nandakumar K., Laly A.P., Sabu T. Encapsulation of zidovudine in PF-68 coated alginate conjugate nanoparticles for anti-HIV drug delivery. Int. J. Biol. Macromol. 2018;107:929–937. doi: 10.1016/j.ijbiomac.2017.09.078. [DOI] [PubMed] [Google Scholar]
- 100.Kurd M., Sadegh Malvajerd S., Rezaee S., Hamidi M., Derakhshandeh K. Oral delivery of indinavir using mPEG-PCL nanoparticles: preparation, optimization, cellular uptake, transport and pharmacokinetic evaluation. Artif Cells Nanomed Biotechnol. 2019;47:2123–2133. doi: 10.1080/21691401.2019.1616553. [DOI] [PubMed] [Google Scholar]
- 101.Mohideen M., Quijano E., Song E., Deng Y., Panse G., Zhang W., et al. Degradable bioadhesive nanoparticles for prolonged intravaginal delivery and retention of elvitegravir. Biomaterials. 2017;144:144–154. doi: 10.1016/j.biomaterials.2017.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jyothi K.R., Beloor J., Jo A., Nguyen M.N., Choi T.G., Kim J.H., et al. Liver-targeted cyclosporine A-encapsulated poly (lactic-co-glycolic) acid nanoparticles inhibit hepatitis C virus replication. Int. J. Nanomed. 2015;10:903–921. doi: 10.2147/IJN.S74723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhu J., Qin F., Ji Z., Fei W., Tan Z., Hu Y., et al. Mannose-modified PLGA nanoparticles for sustained and targeted delivery in hepatitis B virus immunoprophylaxis. AAPS PharmSciTech. 2019;21:13. doi: 10.1208/s12249-019-1526-5. [DOI] [PubMed] [Google Scholar]
- 104.Shibata A., McMullen E., Pham A., Belshan M., Sanford B., Zhou Y., et al. Polymeric nanoparticles containing combination antiretroviral drugs for HIV type 1 treatment. AIDS Res. Hum. Retrovir. 2013;29:746–754. doi: 10.1089/aid.2012.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mitragotri S., Lahann J. Physical approaches to biomaterial design. Nat. Mater. 2009;8:15–23. doi: 10.1038/nmat2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Prokop A., Davidson J.M. Nanovehicular intracellular delivery systems. J Pharm Sci. 2008;97:3518–3590. doi: 10.1002/jps.21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wee S., Gombotz W.R. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 1998;31:267–285. doi: 10.1016/s0169-409x(97)00124-5. [DOI] [PubMed] [Google Scholar]
- 108.Lai S.K., Wang Y.-Y., Hida K., Cone R., Hanes J. Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses. Proc. Natl. Acad. Sci. Unit. States Am. 2010;107:598–603. doi: 10.1073/pnas.0911748107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Iranpur Mobarakeh V., Modarressi M.H., Rahimi P., Bolhassani A., Arefian E., Atyabi F., et al. Optimization of chitosan nanoparticles as an anti-HIV siRNA delivery vehicle. Int. J. Biol. Macromol. 2019;129:305–315. doi: 10.1016/j.ijbiomac.2019.02.036. [DOI] [PubMed] [Google Scholar]
- 110.Jamali A., Mottaghitalab F., Abdoli A., Dinarvand M., Esmailie A., Kheiri M.T., et al. Inhibiting influenza virus replication and inducing protection against lethal influenza virus challenge through chitosan nanoparticles loaded by siRNA. Drug Deliv Transl Res. 2018;8:12–20. doi: 10.1007/s13346-017-0426-z. [DOI] [PubMed] [Google Scholar]
- 111.Ariza-Sáenz M., Espina M., Bolaños N., Calpena A.C., Gomara M.J., Haro I., et al. Penetration of polymeric nanoparticles loaded with an HIV-1 inhibitor peptide derived from GB virus C in a vaginal mucosa model. Eur. J. Pharm. Biopharm. 2017;120:98–106. doi: 10.1016/j.ejpb.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 112.Gupta N.K., Tomar P., Sharma V., Dixit V.K. Development and characterization of chitosan coated poly-(ϵ-caprolactone) nanoparticulate system for effective immunization against influenza. Vaccine. 2011;29:9026–9037. doi: 10.1016/j.vaccine.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 113.Nair N.R., Sekhar V.C., Nampoothiri K.M., Pandey A. In: Current Developments in Biotechnology and Bioengineering. Pandey A., Negi S., Soccol C.R., editors. Elsevier; 2017. 32 - Biodegradation of Biopolymers; pp. 739–755. [Google Scholar]
- 114.Chawla J.S., Amiji M.M. Biodegradable poly(epsilon -caprolactone) nanoparticles for tumor-targeted delivery of tamoxifen. Int. J. Pharm. 2002;249:127–138. doi: 10.1016/s0378-5173(02)00483-0. [DOI] [PubMed] [Google Scholar]
- 115.das Neves J., Michiels J., Ariën K.K., Vanham G., Amiji M., Bahia M.F., et al. Polymeric nanoparticles affect the intracellular delivery, antiretroviral activity and cytotoxicity of the microbicide drug candidate dapivirine. Pharm. Res. (N. Y.) 2012;29:1468–1484. doi: 10.1007/s11095-011-0622-3. [DOI] [PubMed] [Google Scholar]
- 116.Cavalli R., Donalisio M., Civra A., Ferruti P., Ranucci E., Trotta F., et al. Enhanced antiviral activity of Acyclovir loaded into beta-cyclodextrin-poly(4-acryloylmorpholine) conjugate nanoparticles. J. Contr. Release. 2009;137:116–122. doi: 10.1016/j.jconrel.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 117.Dhakal S., Goodman J., Bondra K., Lakshmanappa Y.S., Hiremath J., Shyu D.-L., et al. Polyanhydride nanovaccine against swine influenza virus in pigs. Vaccine. 2017;35:1124–1131. doi: 10.1016/j.vaccine.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 118.Sun Y., Zhang Y., Shi C., Li W., Chen G., Wang X., et al. Newcastle disease virus vaccine encapsulated in biodegradable nanoparticles for mucosal delivery of a human vaccine. Hum. Vaccines Immunother. 2014;10:2503–2506. doi: 10.4161/hv.29201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Joshy K.S., Snigdha S., Anne G., Nandakumar K., Laly A.P., Sabu T. Poly (vinyl pyrrolidone)-lipid based hybrid nanoparticles for anti viral drug delivery. Chem. Phys. Lipids. 2018;210:82–89. doi: 10.1016/j.chemphyslip.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 120.Gao Y., Han K., Wang Q., Hu Z., Liu Q., Liu L., et al. Development of podophyllotoxin-loaded nanostructured lipid carriers for the treatment of condyloma acuminatum. Mol. Med. Rep. 2018;17:6506–6514. doi: 10.3892/mmr.2018.8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Torchilin V.P. Multifunctional nanocarriers. Adv. Drug Deliv. Rev. 2006;58:1532–1555. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 122.Bajoria R., Sooranna S.R. Liposome as a drug carrier system: prospects for safer prescribing during pregnancy: a review. Placenta. 1998;19:265–287. [Google Scholar]
- 123.Gregoriadis G. The carrier potential of liposomes in biology and medicine (second of two parts) N. Engl. J. Med. 1976;295:765–770. doi: 10.1056/NEJM197609302951406. [DOI] [PubMed] [Google Scholar]
- 124.Gabizon A., Shmeeda H., Barenholz Y. Pharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studies. Clin. Pharmacokinet. 2003;42:419–436. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- 125.Pinto-Alphandary H., Andremont A., Couvreur P. Targeted delivery of antibiotics using liposomes and nanoparticles: research and applications. Int. J. Antimicrob. Agents. 2000;13:155–168. doi: 10.1016/s0924-8579(99)00121-1. [DOI] [PubMed] [Google Scholar]
- 126.Mukherjee S., Ray S., Thakur R.S. Solid lipid nanoparticles: a modern formulation approach in drug delivery system. Indian J. Pharmaceut. Sci. 2009;71:349–358. doi: 10.4103/0250-474X.57282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Duan L., Yan Y., Liu J., Wang B., Li P., Hu Q., et al. Target delivery of small interfering RNAs with vitamin E-coupled nanoparticles for treating hepatitis C. Sci. Rep. 2016;6:24867. doi: 10.1038/srep24867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wakita T., Pietschmann T., Kato T., Date T., Miyamoto M., Zhao Z., et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 2005;11:791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Carmona S., Jorgensen M.R., Kolli S., Crowther C., Salazar F.H., Marion P.L., et al. Controlling HBV replication in vivo by intravenous administration of triggered PEGylated siRNA-nanoparticles. Mol. Pharm. 2009;6:706–717. doi: 10.1021/mp800157x. [DOI] [PubMed] [Google Scholar]
- 130.Chahal J.S., Khan O.F., Cooper C.L., McPartlan J.S., Tsosie J.K., Tilley L.D., et al. Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc. Natl. Acad. Sci. U.S.A. 2016;113:4133–4142. doi: 10.1073/pnas.1600299113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Asgary V., Shoari A., Moayad M.A., Ardestani M.S., Bigdeli R., Ghazizadeh L., et al. Evaluation of G2 citric acid-based dendrimer as an adjuvant in veterinary rabies vaccine. Viral Immunol. 2018;31:47–54. doi: 10.1089/vim.2017.0024. [DOI] [PubMed] [Google Scholar]
- 132.Chahal J.S., Fang T., Woodham A.W., Khan O.F., Ling J., Anderson D.G., et al. An RNA nanoparticle vaccine against Zika virus elicits antibody and CD8+ T cell responses in a mouse model. Sci. Rep. 2017;7:252. doi: 10.1038/s41598-017-00193-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Javad M.P.M., Reza M.S.H., Simin D., Ahmed E.S., Bahram B., Azadeh H. Preparation and evaluation of the antiviral activity of acyclovir loaded nano-niosomes against herpes simplex virus type 1 Pharmaphore. 2014;5:483–493. [Google Scholar]
- 134.Donath E., Sukhorukov G.B., Caruso F., Davis S.A., Möhwald H. Novel hollow polymer shells by colloid-Templated assembly of polyelectrolytes. Angew. Chem. Int. Ed. 1998;37:2201–2205. doi: 10.1002/(SICI)1521-3773(19980904)37:16<2201::AID-ANIE2201>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 135.Caruso F., Caruso R.A., Mohwald H. Nanoengineering of inorganic and hybrid hollow spheres by colloidal templating. Science. 1998;282:1111–1114. doi: 10.1126/science.282.5391.1111. [DOI] [PubMed] [Google Scholar]
- 136.Reibetanz U., Claus C., Typlt E., Hofmann J., Donath E. Defoliation and plasmid delivery with layer-by-layer coated colloids. Macromol. Biosci. 2006;6:153–160. doi: 10.1002/mabi.200500163. [DOI] [PubMed] [Google Scholar]
- 137.Gao C., Moya S., Lichtenfeld H., Casoli A., Fiedler H., Donath E., et al. The decomposition process of melamine formaldehyde cores: the key step in the fabrication of ultrathin polyelectrolyte multilayer capsules. Macromol. Mater. Eng. 2001;286:355–361. [Google Scholar]
- 138.Gao C., Moya S., Donath E., Möhwald H. Melamine formaldehyde core decomposition as the key step controlling capsule integrity: optimizing the polyelectrolyte capsule fabrication. Macromol. Chem. Phys. 2002;203:953–960. [Google Scholar]
- 139.Caruso F., Yang W., Trau D., Renneberg R. Microencapsulation of uncharged low molecular weight organic materials by polyelectrolyte multilayer self-Assembly. Langmuir. 2000;16:8932–8936. [Google Scholar]
- 140.Park M.-K., Xia C., Advincula R.C., Schütz P., Caruso F. Cross-linked, luminescent spherical colloidal and hollow-shell particles. Langmuir. 2001;17:7670–7674. [Google Scholar]
- 141.Yu A., Wang Y., Barlow E., Caruso F. Mesoporous silica particles as Templates for preparing enzyme-loaded biocompatible microcapsules. Adv. Mater. 2005;17:1737–1741. [Google Scholar]
- 142.Singh P. Dendrimers and their applications in immunoassays and clinical diagnostics. Biotechnol. Appl. Biochem. 2007;48:1–9. doi: 10.1042/BA20070019. [DOI] [PubMed] [Google Scholar]
- 143.Kobayashi H., Kawamoto S., Jo S.K., Bryant H.L., Jr., Brechbiel M.W., Star R.A. Macromolecular MRI contrast agents with small dendrimers: pharmacokinetic differences between sizes and cores. Bioconjugate Chem. 2003;14:388–394. doi: 10.1021/bc025633c. [DOI] [PubMed] [Google Scholar]
- 144.Gillies E.R., Fréchet J.M.J. Dendrimers and dendritic polymers in drug delivery. Drug Discov. Today. 2005;10:35–43. doi: 10.1016/S1359-6446(04)03276-3. [DOI] [PubMed] [Google Scholar]
- 145.Wu G., Barth R.F., Yang W., Chatterjee M., Tjarks W., Ciesielski M.J., et al. Site-specific conjugation of boron-containing dendrimers to anti-EGF receptor monoclonal antibody cetuximab (IMC-C225) and its evaluation as a potential delivery agent for neutron capture therapy. Bioconjugate Chem. 2004;15:185–194. doi: 10.1021/bc0341674. [DOI] [PubMed] [Google Scholar]
- 146.Dufès C., Uchegbu I.F., Schätzlein A.G. Dendrimers in gene delivery. Adv. Drug Deliv. Rev. 2005;57:2177–2202. doi: 10.1016/j.addr.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 147.Oliveira J.M., Salgado A.J., Sousa N., Mano J.F., Reis R.L. Dendrimers and derivatives as a potential therapeutic tool in regenerative medicine strategies—a review. Prog. Polym. Sci. 2010;35:1163–1194. [Google Scholar]
- 148.Tanaka K., Inafuku K., Naka K., Chujo Y. Enhancement of entrapping ability of dendrimers by a cubic silsesquioxane core. Org. Biomol. Chem. 2008;6:3899–3901. doi: 10.1039/b812349g. [DOI] [PubMed] [Google Scholar]
- 149.Karakhanov E., Maximov A., Skorkin V., Zolotukhina A., Smerdov A., Tereshchenko A. Nanocatalysts based on dendrimers. Pure and Applied Chemistry - PURE APPL CHEM. 2009;81:2013–2023. [Google Scholar]
- 150.Kim D., Kim E., Lee J., Hong S., Sung W., Lim N., et al. Direct synthesis of polymer Nanocapsules: self-Assembly of polymer hollow spheres through irreversible covalent bond formation. J. Am. Chem. Soc. 2010;132:9908–9919. doi: 10.1021/ja1039242. [DOI] [PubMed] [Google Scholar]
- 151.Pinto Reis C., Neufeld R.J., Ribeiro A.J., Veiga F., Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2006;2:8–21. doi: 10.1016/j.nano.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 152.Talelli M., Barz M., Rijcken C.J., Kiessling F., Hennink W.E., Lammers T. Core-crosslinked polymeric micelles: principles, preparation, biomedical applications and clinical translation. Nano Today. 2015;10:93–117. doi: 10.1016/j.nantod.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Marjomäki V., Lahtinen T., Martikainen M., Koivisto J., Malola S., Salorinne K., et al. Site-specific targeting of enterovirus capsid by functionalized monodisperse gold nanoclusters. Proc. Natl. Acad. Sci. U.S.A. 2014;111:1277–1281. doi: 10.1073/pnas.1310973111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hamdi M., Abdel-Bar H.M., Elmowafy E., Al-Jamal K.T., Awad G.A.S. An integrated vitamin E-coated polymer hybrid nanoplatform: a lucrative option for an enhanced in vitro macrophage retention for an anti-hepatitis B therapeutic prospect. PloS One. 2020;15 doi: 10.1371/journal.pone.0227231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Illescas B.M., Rojo J., Delgado R., Martín N. Multivalent glycosylated Nanostructures to inhibit Ebola virus infection. J. Am. Chem. Soc. 2017;139:6018–6025. doi: 10.1021/jacs.7b01683. [DOI] [PubMed] [Google Scholar]
- 156.Kuhn J.H., Becker S., Ebihara H., Geisbert T.W., Johnson K.M., Kawaoka Y., et al. Proposal for a revised taxonomy of the family Filoviridae: classification, names of taxa and viruses, and virus abbreviations. Arch. Virol. 2010;155:2083–2103. doi: 10.1007/s00705-010-0814-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kaushik A., Tiwari S., Dev Jayant R., Marty A., Nair M. Towards detection and diagnosis of Ebola virus disease at point-of-care. Biosens. Bioelectron. 2016;75:254–272. doi: 10.1016/j.bios.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bazzill J.D., Stronsky S.M., Kalinyak L.C., Ochyl L.J., Steffens J.T., van Tongeren S.A., et al. Vaccine nanoparticles displaying recombinant Ebola virus glycoprotein for induction of potent antibody and polyfunctional T cell responses. Nanomedicine. 2019;18:414–425. doi: 10.1016/j.nano.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wu Z., Hu J., Zeng T., Zhang Z.-L., Chen J., Wong G., et al. Ultrasensitive Ebola virus detection based on electroluminescent nanospheres and immunomagnetic separation. Anal. Chem. 2017;89:2039–2048. doi: 10.1021/acs.analchem.6b04632. [DOI] [PubMed] [Google Scholar]
- 160.Duan D., Fan K., Zhang D., Tan S., Liang M., Liu Y., et al. Nanozyme-strip for rapid local diagnosis of Ebola. Biosens. Bioelectron. 2015;74:134–141. doi: 10.1016/j.bios.2015.05.025. [DOI] [PubMed] [Google Scholar]