Abstract
Objectives
To systematically review the available literature on the efficacy of erythropoietin for wound healing in human patients.
Design
The review was reported following Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines. A descriptive-analytical method was used to analyse and integrate review findings.
Data sources
A primary search of electronic databases was performed using a combination of search terms related to the following areas of interest: ‘efficacy’, ‘erythropoietin’ and ‘wound healing’. A secondary search of the grey literature was conducted in addition to checking the reference list of included studies and review papers.
Results
Seven distinct studies involving 150 patients met the inclusion criteria for the review. The included studies suggest that topical and subcutaneous application of erythropoietin improves the wound healing process via faster re-epithelialization and reducing wound area and depth.
Conclusions
There were a limited number of studies and a great degree of heterogeneity of evidence due to differences in the course of concomitant illness, wound aetiology, and the time and dosing regimens adopted. Further research adopting validated and consistent outcome measures is recommended to determine the efficacy and safety of erythropoietin for wound healing.
Keywords: Efficacy, Erythropoietin, Wound healing, Systematic review
Highlights
-
•
Topical and subcutaneous application of erythropoietin improves the wound healing process in human patients.
-
•
Topical and subcutaneous application of erythropoietin contributes to reducing wound area and depth in human patients.
-
•
Topical and subcutaneous application of erythropoietin has the potential to prevent wounds from becoming chronic.
1. Introduction
Wound healing refers to the replacement, reconstruction, and closure of injured tissue via a cascade of physiological processes and biochemical events [1]. According to Quinn and Wells [2], these processes can be divided into four overlapping stages: a haemostatic stage, an inflammatory stage, a proliferative stage, and the final remodelling stage. Numerous factors such as prolonged infection, perpetual inflammation, and impaired angiogenesis may undermine these processes and lead to unstable hypotrophic/hypertrophic scars or chronic damage. Despite the great progress in wound healing research, the management of chronic and full-thickness wounds remains a significant issue in daily clinical routine.
Human erythropoietin is a glycosylated cytokine secreted by liver and kidney endothelial cells at birth and throughout adulthood [3,4]. More recently, erythropoietin has begun to be perceived as a potentially beneficial therapeutic approach in wound healing due to a number of positive effects at each stage of the wound healing process [1,3]. Firstly, erythropoietin is known to have a haemostatic effect as it can induce coagulation without stimulating thrombosis immediately after skin injury [5]. It has also been reported that erythropoietin activates platelet aggregation and suppresses fibrinolysis by inhibiting the activity of antithrombin, protein C, and protein S [6,7]. Secondly, experimental studies on animals show that erythropoietin can suppress the activity of pro-inflammatory cytokines and inhibit apoptosis, demonstrating anti-inflammatory effects [8,9]. Such effects of erythropoietin may play a crucial role in the treatment of chronic wounds, taking into consideration the fact that the healing of chronic wounds can be complicated by unremitting inflammation and perpetual apoptotic environment [3]. Thirdly, in vitro and in vivo research studies suggest that erythropoietin induces angiogenesis - a crucial process in the proliferative stage of the wound healing process [[10], [11], [12]]. By triggering vascular endothelial growth factors and stimulating the formation of erythroid precursor cells, erythropoietin contributes to diminishing impairment of blood supply and reducing local ischemia [13,14]. Furthermore, it can influence the proliferative stage by increasing the mitosis and migration of mature endothelial cells and endothelial progenitor cells [15]. Finally, erythropoietin can expedite wound closure by triggering the formation of granulation tissue and myofibroblasts [16,17].
Due to the possible far-reaching effects of erythropoietin, interest in its therapeutic application has increased substantially in recent decades. Most studies investigating the effects of erythropoietin on the wound healing process have focused on experimental animals [14,[18], [19], [20], [21], [22]]. A limitation of the current academic literature is that there is very little unifying research on the efficacy of erythropoietin for wound healing in human patients [3]. Such a research gap compromises any attempts to justify the importance of erythropoietin and evaluate its impact on wound healing. Against this background, the present work aims to systematically review the available literature on the efficacy of erythropoietin in wound healing on human patients.
2. Methods
The protocol for this systematic review was registered on PROSPERO (CRD42019139681) in advance. This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [23].
2.1. Search strategy
The following five electronic databases were searched: Scopus, Pubmed, Embase, Web of Science, and The Cochrane Central Register of Controlled Trials on September 8, 2019, and updated on November 12, 2020. Search terms combined three overlapping areas with key words such as ‘efficacy’ AND ‘erythropoietin’ AND ‘wound healing’ (see supplementary files 1 and 2). Publication bias was reduced by searching conference records and unpublished literature using Google Scholar, OpenGrey, EThOS, the British Library Catalogue and Copac theses. Additionally, backward and forward citation tracking was used to include studies and review records.
2.2. Selection criteria
Studies were eligible if they evaluated the efficacy of erythropoietin for wound healing on human patients. Both randomized-controlled trials (RCTs) and non-randomized studies on intervention effects were included expecting a limited number of potentially eligible studies. Studies were excluded if they met one of the following conditions: (1) non-research-based articles, such as conference abstracts, commentaries, opinion pieces, book chapters and editorials; (2) case series with fewer than three cases; (3) were not written using Latin alphabet, Russian or Kazakh; (3) abstract was not available; (4) or full text was not available.
2.3. Review strategy
Titles and abstracts of identified records were exported to the Mendeley reference management software and screened by the first reviewer (MT) to exclude irrelevant records and duplicates. A random subsample of 20% of titles and abstracts were screened by a second reviewer (AS) to ensure accuracy of selection. Full-text articles were inspected again (MT, AS and MD) for relevance according to the inclusion criteria. The level of agreement between MT and AS was 75%, and between MT and MD was 80%. Discrepancies were resolved by involving a fourth reviewer (SK).
2.4. Data extraction and quality assessment
Data from each study, including study details, participant demographics, and key results were extracted into a spreadsheet by the first reviewer (MT). AS and MD ensured the accuracy at this stage by independently extracting data from all included studies. Methodological quality was assessed using the Cochrane Collaboration Risk of Bias Assessment Tool [24] for any randomized-controlled trials, and the Quality Assessment Tool for Case Series Studies [25] developed by the National Heart, Lung, and Blood Institute was adopted for any case series studies.
2.5. Data synthesis
The primary outcome measures were originally intended to be the proportion of healed wounds and the mean wound healing time. However, given the limited numbers of eligible studies and their heterogeneity, a descriptive-analytical method was utilised for the purposes of the current review.
2.6. Patient and public involvement
The results of the analysis were solely based on the previously published literature, as this study did not involve patients or the public.
3. Results
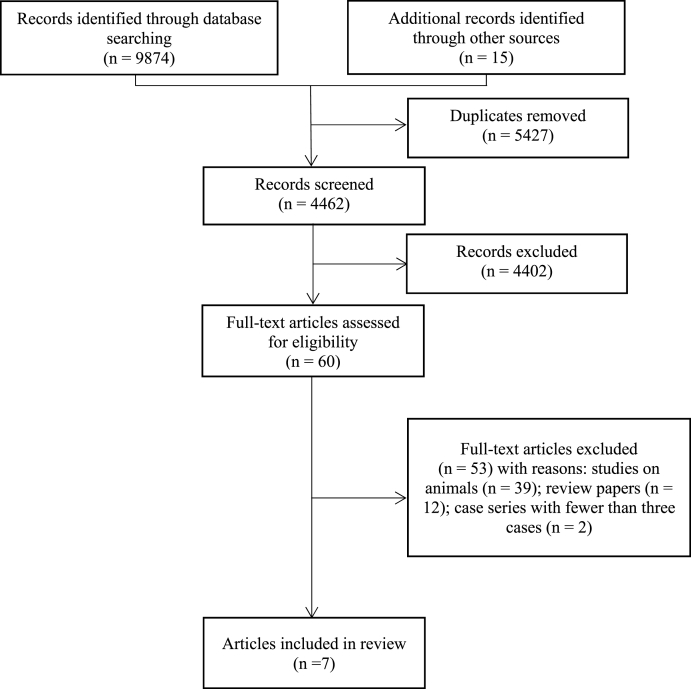
The original search yielded 9874 records through database searching and 15 through other sources. 5427 records were removed as duplicates and 4402 records were excluded for not meeting the inclusion criteria. The full texts of the remaining 60 articles were examined, seven of which were included and represented seven distinct studies. The detailed selection process is presented in the PRISMA flow diagram (Fig. 1) [23].
Fig. 1.
PRISMA flow chart [23].
3.1. Overview of included studies
Included studies were published between 2004 and 2019 and emanated from five different countries, including Canada [26], England [27], Iran [28], Israel [29], and Germany [[30], [31], [32]]. With regard to the study design, three of included studies were case series [26,27,30] and four studies were RCTs [28,29,31,32].
The results of the current review will be presented in two parts. In the first part, case series included in the review will be introduced. In the second part, the findings from the included RCTs will be presented.
3.2. Case series
Case series involved a total of 21 patients with different types of wounds, such as pressure ulcers in refractory anaemia [26], skin ulcers in systematic sclerosis [27], and ulcers in people with diabetes [30]. In two studies, erythropoietin was administered subcutaneously [26,27], whereas one study [30] described its topical application. Treatment period also differed from a minimum of three weeks and a maximum of 25 weeks [30]. Although the course of concomitant illness largely influenced the wound healing process, all studies suggest that erythropoietin can be beneficial in the treatment of chronic wounds. In particular, Keast and Fraser [26] did not report the proportion of wounds with complete healing but noted that ulcers’ depth and size decreased for all patients. Ferri and colleagues [27] observed complete healing of 42.6% of wounds during follow-up after 3–6 months from the beginning of the treatment. Gunter and colleagues [30] reported complete healing for all wounds within an average of 17.3 days. The included case series characteristics are summarised in Table 1.
Table 1.
Characteristics of included case series.
| Study | Keast and Fraser, 2004 [26] | Ferri et al., 2007 [27] | Gunter et al., 2015 [30] |
|---|---|---|---|
| Country | Canada | England | Germany |
| Study design | Case series | Case series | Case series |
| Total number of patients (%) | 4 (100%) | 14 (100%) | 3 (100%) |
| Males | 4 (100%) | 3 (21.4%) | 1 (33.3%) |
| Females | 0 | 11 (78.6%) | 2 (66.7%) |
| Mean age (years) | 59 ± 19 | 52.7 ± 12.1 | 65 ± 4 |
| Wound aetiology | Pressure ulcers in refractory anaemia | Skin ulcers in systematic sclerosis | Diabetic ulcers |
| Type of treatment | subcutaneously | subcutaneously | topical |
| Treatment period | 6 weeks | 8 weeks | 3 weeks (patient 1) 25 weeks (patient 2) 9 weeks (patient 3) |
| Total number of wounds | 12 (100%) | 14 (100%) | 3 (100%) |
| Average wound size (cm2) | Not reported | 27.6 ± 28 | 6 (patient 1) 40 (patient 2) Not reported (patient 3) |
| Number of wounds with complete healing (%) | Not reported (ulcer status was improved for all patients) | 6 (42.85%) | 3 (100%) |
| Mean duration for complete healing (days) | Not reported (data was reported after 6 weeks of treatment) | 135 ± 33.6 (3–6 months) | 17.3 ± 10.1 |
| Follow-up period (months) | Not reported | 6–12 | 9 |
3.3. RCTs
RCTs involved a total of 129 patients with various types of wounds, including diabetic ulcers [29,32], post-traumatic wounds [28], and burn and scalding injuries [31]. Three of the trials were placebo-controlled [28,31,32], and one study [29] used the standard treatment protocol for the control group of patients. Two trials were double-blind, whereas triple- [28] and single-blinding [29] were each adopted in a single trial. All included trials suggest that application of erythrolein enhances the wound healing process. In cases where complete wound healing was not always achieved, reductions in wound area and depth were observed. Chatzikyrkou and colleagues [32] administered erythropoietin subcutaneously over 12 weeks and reported complete closure of 26.7% of diabetic ulcers in the intervention arm compared to 14.3% of diabetic ulcers in the placebo arm. Results presented by Gunter and colleagues [31] were rather inconclusive due to a high withdrawal rate (64 out of 84 patients missed the primary endpoint). Erythropoietin was administered subcutaneously within three weeks and suggested the pro-regenerative effect of erythropoietin in the treatment of burn and scalding injuries [31]. Yaghobee and colleagues [28] described a topical application of erythropoietin administered two times and outlined that the number of wounds with complete epithelisation in the intervention group was significantly higher compared to those in the placebo group on day 21. On day 28, almost all wounds in both groups demonstrated complete closure, suggesting that erythropoietin increases wound healing time. Hamed and colleagues29 described that patients with diabetic ulcers responded positively to a topical application of erythropoietin administered over a 12-week period. On week 12, complete wound closure was observed in 40% of wounds in the intervention arm while none of the wounds healed completely in the control arm. Furthermore, in wounds without complete healing, a significant reduction in the wound areas was observed in the intervention arm compared to the control arm. The overall characteristics of the included RCTs are summarised in Table 2.
Table 2.
Characteristics of included RCTs.
| Study | Chatzikyrkou et al., 2016 [32] | Gunter et al., 2018 [31] | Yaghobee et al., 2018 [28] | Hamed et al., 2019 [29] |
|---|---|---|---|---|
| Country | Germany | Germany | Iran | Israel |
| Study design | RCT (double-blind, placebo controlled) | RCT (double-blind, placebo controlled) | RCT (triple-blind, placebo controlled) | RCT (single blind, standard treatment controlled) |
| Total number of patients (%) | 23 (100%) | 84 (100%) | 12 (100%) | 10 (100%) |
| Males | 22 (95.6%) | 66 (78.6%) | 3 (25%) | 6 (60%) |
| Females | 1 (4.4%) | 18 (21.4%) | 9 (75%) | 4 (40%) |
| Mean age (SD) | 61.91 ± 9.27 | 47.55 ± 15.6 | 44.58 ± 7.5 | 67.8 ± 8.6 |
| Wound aetiology | Diabetic ulcers | Burn and scalding injuries | Post-traumatic wounds (post free gingival grafting) | Diabetic ulcers |
| Type of treatment | subcutaneously | Subcutaneously | topical | topical |
| Treatment period | 12 weeks | 3 weeks | 2 times (after surgery and 2 days after surgery) | 12 weeks |
| Number of patients by arms (%) | ||||
| Intervention arm | 16 (69.6%) | 45 (53.6%) | Not applicable (split mouth design) | 5 (50%) |
| Control arm | 7 (30.4%) | 39 (46.4%) | Not applicable (split mouth design) | 5 (50%) |
| Number of withdrawals (%) | ||||
| Intervention arm | 1 (6.2%) | 36 (80%) | 0 (0%) | 0 (0%) |
| Control arm | 0 | 28 (71,8%) | 0 (0%) | 0 (0%) |
| Number of wounds | ||||
| Intervention arm | 15 | 40 | 12 | 5 |
| Control arm | 17 | 37 | 12 | 5 |
| Mean wound size (cm2) | ||||
| Intervention arm | 3.78 ± 5.07 | Not reported | 1.5 | 5.0 ± 4.9 |
| Control arm | 4.82 ± 5.47 | Not reported | 1.5 | 7.5 ± 5.5 |
| Number of wounds with complete healing (%) | ||||
| Intervention arm | 4 (26.7%) | 9 (23%) on day 16 | 12 (100%) on day 28 | 2 (40%) on week 12 |
| Control arm | 1 (14.3%) | 11 (30%) on day 16 | 11 (91.7%) on day 28 | 0 (0%) on week 12 |
| Mean duration for complete healing (days) | ||||
| Intervention arm | 44 days | Not reported | Not reported | Not reported |
| Control arm | Not reported | Not reported | Not reported | Not reported |
| Follow-up period | 12 weeks | 12 months | Not reported | 12 weeks |
3.4. Risk of bias assessment
On the Quality Assessment Tool for Case Series Studies, one study was rated as weak [26], one as moderate [30], and one as strong [27]. On the Cochrane Collaboration Risk of Bias Assessment Tool for randomized-controlled trials, two studies were rated as demonstrating a high risk of detection [29] and attrition [31] biases each. The risk of bias assessment is presented in supplementary file 3.
4. Discussion
The aim of the current systematic review was to systematically review the available literature on the efficacy of erythropoietin for wound healing in human patients. The results from seven distinct studies suggest that topical and subcutaneous application of erythropoietin improves the wound healing process via faster re-epithelialization and reducing wound area and depth. However, conclusions are tentative due to differences in the course of concomitant illness, wound aetiology, and the time and dosing regimens adopted.
The findings of the current review are consistent with previously described processes associated with erythropoietin-mediated increases in wound healing capacity. Firstly, keratinocytes have been indicated as potential target cells for externally administered erythropoietin [12]. This allows for the increased proliferation and/or migration of keratinocytes, ensuring quicker wound re-epithelialization [3]. Secondly, exogenous erythropoietin has been associated with suppressed production of pro-inflammatory cytokines [33,34]. This may help to reduce the inflammatory response in the wound bed and decrease its depth [33,34]. Thirdly, erythropoietin administration has been associated with increased degranulation and tissue remodelling [33]. A timely resolution to the granulation tissue might facilitate the reduction of wound area and enhance wound repair [33,36]. Finally, erythropoietin has been associated with accelerated angiogenesis [20,22]. The formation of new capillary structures in the wound bed allows for the improvement of its vascularization and ensures a normal wound healing process [[14], [35]].
Based on the findings of this review, erythropoietin can be used for the treatment of both acute [28,31] and chronic [26,27,29,30,32] wounds. In the case of acute wounds, erythropoietin administration has the potential to not only decrease wound healing time but to prevent wounds from becoming chronic [36]. This is particularly crucial for patients suffering from certain chronic conditions because there is a need for wound care after every surgical procedure [3]. In the case of chronic wounds, the healing process often stops during the inflammatory stage, making wounds prone to being resistant to traditional treatment techniques [33]. Therefore, the anti-inflammatory qualities of erythropoietin, in particular, might be beneficial to accelerate healing.
Although the findings of the current review show similar results for subcutaneous and topical administration of erythropoietin, it is important to consider the therapeutic benefits of different approaches to erythropoietin administration. According to Hamed and colleagues [3], topical erythropoietin application is more advantageous than systematic due to the ease of use and the lower risk of adverse events. Systematically administered erythropoietin can potentially activate a prothrombotic state, which can in turn result in life-threatening cardiovascular ischemic events [3,11]. Furthermore, erythropoietin has been associated with tumour growth, and thus prior to systemic administration of erythropoietin patients need to be screened for cancer to determine those at high risk [37].
4.1. Strengths and limitations
To our knowledge, this is the first systematic review of the available literature on the efficacy of erythropoietin for wound healing in human patients. A further strength is that the review employed a comprehensive and reproducible search strategy, without limitations regarding the year of publication, language, or country of origin of the studies. However, this approach presented some limitations. Firstly, due to the limited number of eligible studies and their heterogeneity, it was not possible to conduct a meta-analysis; therefore, the final interpretation was made based on descriptive-analytical procedures. Secondly, the prevalence of studies suggesting that erythropoietin improves the wound healing process could be the result of positive results bias. Thirdly, the comparability of findings across the included studies may be limited due to wide variability in wound aetiology, and time and dosing regimens adopted.
In order to navigate practice implications, future research studies should address a number of research gaps. Firstly, more evidence about the safety and efficacy of erythropoietin for wound healing, particularly among patients with different chronic conditions, is needed. Secondly, there is a need to assess the efficacy of different means of erythropoietin administration, and the timing and dosing regimens used. Thirdly, the potential adverse effects of erythropoietin should be further explored. Future research may also benefit from employing randomized-controlled study designs.
5. Conclusions
This systematic review provides evidence for the effectiveness of erythropoietin in wound healing of human patients. Considering the high heterogeneity and limited quality of included studies, conclusions are tentative. There is a need for large prospective randomized-controlled trials adopting validated and consistent outcome measures to determine the efficacy and safety of erythropoietin for wound healing.
Funding
No funding.
Ethical approval
Not required.
Consent
The results of the analysis were solely based on the previously published literature, as this study did not involve patients or public.
Author contribution
Contributors Medet Toleubayev (MT) and Alina Sabitova (AS) designed the study with input from Saken Kozhakhmetov (SK). MT and AS conducted the systematic searches of the literature, selected the studies, and performed data analysis. AS ensured the consistency of study selection, data extraction and analysis, and drafted the manuscript. Mariya Dmitriyeva (MD) contributed to the analysis and edited the manuscript. All authors approved the final version of the manuscript.
Registration of research studies
-
1.
Name of the registry: PROSPERO.
-
2.
Unique Identifying number or registration ID: CRD42019139681.
-
3.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=139681.
Guarantor
Alina Sabitova
Unit for Social and Community Psychiatry
WHO Collaborating Centre for Mental Health Service Development
Queen Mary University of London
Newham Centre for Mental Health, London E13 8SP
+44 (0)20 7540 4380 x2339
Data sharing statement
All data cited in this manuscript are available from the cited, previously published studies.
Provenance and peer review
Not commissioned, externally peer reviewed.
Declaration of competing interest
None declared.
Acknowledgements
Contributors MT and AS designed the study with input from SK. MT and AS conducted the systematic searches of the literature, selected the studies, and performed data analysis. AS ensured the consistency of study selection, data extraction and analysis, and drafted the manuscript. MD contributed to the analysis and edited the manuscript. All authors approved the final version of the manuscript.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.amsu.2021.102287.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Clark R.A. Cutaneous tissue repair: basic biologic considerations. J. Am. Acad. Dermatol. 1985;5:701–725. doi: 10.1016/s0190-9622(85)70213-7. [DOI] [PubMed] [Google Scholar]
- 2.Quinn J.V., Wells G.A. An assessment of clinical wound evaluation scales. Acad. Emerg. Med. 1998;5:583–586. doi: 10.1111/j.1553-2712.1998.tb02465.x. [DOI] [PubMed] [Google Scholar]
- 3.Hamed S., Bennett C.L., Demiot C., Ullmann Y. Erythropoietin, a novel repurposed drug: an innovative treatment for wound healing in patients with diabetes mellitus. Wound Repair Regen. 2014;22:23–33. doi: 10.1111/wrr.12135. [DOI] [PubMed] [Google Scholar]
- 4.Jelkmann W. Molecular biology of erythropoietin. Intern. Med. 2004;43:649–659. doi: 10.2169/internalmedicine.43.649. [DOI] [PubMed] [Google Scholar]
- 5.Fusté B., Serradell M., Escolar G. Erythropoietin triggers a signaling pathway in endothelial cells and increases the thrombogenicity of their extracellular matrices in vitro. Thromb. Haemostasis. 2002;4:678–685. [PubMed] [Google Scholar]
- 6.Malyszko J., Malyszko J.S., Borawski J. A study of platelet functions, some hemostatic and fibrinolytic parameters in relation to serotonin in hemodialyzed patients under erythropoietin therapy. Thromb. Res. 1995;77:133–143. doi: 10.1016/0049-3848(95)91619-V. [DOI] [PubMed] [Google Scholar]
- 7.Staško J., Drouet L., Soria C., Mazoyer E. Erythropoietin and granulocyte colony-stimulating factor increase plasminogen activator inhibitor-1 release in HUVEC culture. Thromb. Res. 2002;105:161–164. doi: 10.1016/S0049-3848(02)00011-7. [DOI] [PubMed] [Google Scholar]
- 8.Bernaudin M., Marti H.H., Roussel S. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J. Cerebr. Blood Flow Metabol. 1999;19:643–651. doi: 10.1097/00004647-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Strunk T., Härtel C., Temming P., Matzke N. Erythropoietin inhibits cytokine production of neonatal and adult leukocytes. Acta Paediatr. 2008;1:16–20. doi: 10.1111/j.1651-2227.2007.00560.x. [DOI] [PubMed] [Google Scholar]
- 10.Höckel M., Schlenger K., Doctrow S., Kissel T. Therapeutic angiogenesis. Arch. Surg. 1993;128:423–429. doi: 10.1001/archsurg.1993.01420160061009. [DOI] [PubMed] [Google Scholar]
- 11.Brines M., Cerami A. Erythropoietin-mediated tissue protection: reducing collateral damage from the primary injury response. J. Intern. Med. 2008;5:405–432. doi: 10.1111/j.1365-2796.2008.02024.x. [DOI] [PubMed] [Google Scholar]
- 12.Sorg H., Krueger C., Schulz T., Menger M.D. Effects of erythropoietin in skin wound healing are dose related. Faseb. J. 2009;23:3049–3058. doi: 10.1096/fj.08-109991. [DOI] [PubMed] [Google Scholar]
- 13.Stadelmann W.K., Digenis A.G., Tobin G.R. Impediments to wound healing. Am. J. Surg. 1998;2:39–47. doi: 10.1016/s0002-9610(98)00184-6. [DOI] [PubMed] [Google Scholar]
- 14.Buemi M., Galeano M., Sturiale A. Recombinant human erythropoietin stimulates angiogenesis and healing of ischemic skin wounds. Shock. 2004;2:169–173. doi: 10.1097/01.shk.0000133591.47776.bd. [DOI] [PubMed] [Google Scholar]
- 15.Heeschen C., Aicher A., Lehmann R. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood. 2003;4:1340–1346. doi: 10.1182/blood-2003-01-0223. [DOI] [PubMed] [Google Scholar]
- 16.Siebert N., Xu W., Grambow E., Zechner D. Erythropoietin improves skin wound healing and activates the TGF-β signaling pathway. Lab. Invest. 2011;91:1753–1765. doi: 10.1038/labinvest.2011.125. [DOI] [PubMed] [Google Scholar]
- 17.Yang B., Hosgood S.A., Bagul A., Waller H.L., Nicholson M.L. Erythropoietin regulates apoptosis, inflammation and tissue remodelling via caspase-3 and IL-1β in isolated hemoperfused kidneys. Eur. J. Pharmacol. 2011;660(2–3):420–430. doi: 10.1016/j.ejphar.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 18.Fatouros M., Dalekos G.N., Mylonakis E., Vekinis G., Kappas A.M. Alterations in body weight, breaking strength, and wound healing in Wistar rats treated pre- and postoperatively with erythropoietin or granulocyte macrophage-colony stimulating factor: evidence of a previously unknown anabolic effect of erythropoietin? J. Lab. Clin. Med. 1999;3:253–259. doi: 10.1016/s0022-2143(99)90081-1. [DOI] [PubMed] [Google Scholar]
- 19.Buemi M., Vaccaro M., Sturiale A. Recombinant human erythropoietin influences revascularization and healing in a rat model of random ischaemic flaps. Acta Derm. Venereol. 2002;82(6):411–417. doi: 10.1080/000155502762064520. [DOI] [PubMed] [Google Scholar]
- 20.Sayan H., Ozacmak V.H., Guven A., Aktas R.G., Ozacmak I.D. Erythropoietin stimulates wound healing and angiogenesis in mice. J. Invest. Surg. 2006;3:163–173. doi: 10.1080/08941930600674694. [DOI] [PubMed] [Google Scholar]
- 21.Elsherbiny A., Högger D.C., Borozadi M.K. EPO reverses defective wound repair in hypercholesterolaemic mice by increasing functional angiogenesis. J. Plast. Reconstr. Aesthetic Surg. 2012;65(11):1559–1568. doi: 10.1016/j.bjps.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Galeano M., Altavilla D., Bitto A. Recombinant human erythropoietin improves angiogenesis and wound healing in experimental burn wounds. Crit. Care Med. 2006;34(4):1139–1146. doi: 10.1097/01.ccm.0000206468.18653.ec. [DOI] [PubMed] [Google Scholar]
- 23.Stroup D.F., Berlin J.A., Morton S.C. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. J. Am. Med. Assoc. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 24.Higgins J.P.T., Altman D.G., Gøtzsche P.C. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quality assessment tool for case series studies. National Heart, Lung, and blood Institute. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- 26.Keast D.H., Fraser C. Treatment of chronic skin ulcers in individuals with anemia of chronic disease using recombinant human erythropoietin (EPO): a review of four cases. Ostomy/Wound Manag. 2004;50(10):64–70. [PubMed] [Google Scholar]
- 27.Ferri C., Giuggioli D., Sebastiani M., Colaci M. Treatment of severe scleroderma skin ulcers with recombinant human erythropoietin. Clin. Exp. Dermatol. 2007;3:287–290. doi: 10.1111/j.1365-2230.2007.02363.x. [DOI] [PubMed] [Google Scholar]
- 28.Yaghobee S., Rouzmeh N., Aslroosta H., Mahmoodi S., Khorsand A., Kharrazifard M.J. Effect of topical erythropoietin (EPO) on palatal wound healing subsequent to free gingival grafting (FGG) Braz. Oral Res. 2018;32:e55. doi: 10.1590/1807-3107bor-2018.vol32.0055. [DOI] [PubMed] [Google Scholar]
- 29.Hamed S., Belokopytov M., Ullmann Y. Interim results of the remede d'Or study: a multicenter, single-blind, randomized, controlled trial to assess the safety and efficacy of an innovative topical formulation of erythropoietin for treating diabetic foot ulcers. Adv. Wound Care. 2019;8(10):514–521. doi: 10.1089/wound.2018.0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Günter C.I., Kern L., Giri S., Machens H.G., Bader A. First results on three patients treated with topical recombinant human erythropoietin (rhEPO) to improve wound healing in diabetic foot ulcers. J. Transplant. Stem Cell Biol. 2015;2(1):4. [Google Scholar]
- 31.Günter C.I., Machens H.-G., Ilg F.P. A randomized controlled trial: regenerative effects, efficacy and safety of erythropoietin in burn and scalding injuries. Clinical trial. Front. Pharmacol. 2018;9:95. doi: 10.3389/fphar.2018.00951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chatzikyrkou C., Bahlmann F.H., Sushakova N. Low-dose erythropoietin promotes wound-healing of ulcers in diabetics: evidence from a phase-IIa clinical study. Diabetes Metab. 2016;6:466–470. doi: 10.1016/j.diabet.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Eming S.A., Krieg T., Davidson J.M. Inflammation in wound repair: molecular and cellular mechanisms. J. Invest. Dermatol. 2007;3:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 34.Hojman P., Taudorf S., Lundby C., Pedersen B.K. Erythropoietin augments the cytokine response to acute endotoxin-induced inflammation in humans. Cytokine. 2009;45(3):154–157. doi: 10.1016/j.cyto.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Villa P., Bigini P., Mennini T. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J. Exp. Med. 2003;198(6):971–975. doi: 10.1084/jem.20021067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bader A., Lorenz K., Richter A. Interactive role of trauma cytokines and erythropoietin and their therapeutic potential for acute and chronic wounds. Rejuvenation Res. 2011;14(1):57–66. doi: 10.1089/rej.2010.1050. [DOI] [PubMed] [Google Scholar]
- 37.Cao Y. Erythropoietin in cancer: a dilemma in risk therapy. Trends Endocrinol. Metabol. 2013;24(4):190–199. doi: 10.1016/j.tem.2012.10.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.