Abstract
Laboratory-derived temperature dependencies of life-history traits are increasingly being used to make mechanistic predictions for how climatic warming will affect vector-borne disease dynamics, partially by affecting abundance dynamics of the vector population. These temperature–trait relationships are typically estimated from juvenile populations reared on optimal resource supply, even though natural populations of vectors are expected to experience variation in resource supply, including intermittent resource limitation. Using laboratory experiments on the mosquito Aedes aegypti, a principal arbovirus vector, combined with stage-structured population modelling, we show that low-resource supply in the juvenile life stages significantly depresses the vector's maximal population growth rate across the entire temperature range (22–32°C) and causes it to peak at a lower temperature than at high-resource supply. This effect is primarily driven by an increase in juvenile mortality and development time, combined with a decrease in adult size with temperature at low-resource supply. Our study suggests that most projections of temperature-dependent vector abundance and disease transmission are likely to be biased because they are based on traits measured under optimal resource supply. Our results provide compelling evidence for future studies to consider resource supply when predicting the effects of climate and habitat change on vector-borne disease transmission, disease vectors and other arthropods.
Keywords: climatic warming, abundance, temperature, population fitness, resource limitation, vector-borne disease
1. Introduction
The global burden of human, animal and plant vector-borne diseases has increased substantially in recent decades [1,2]. The transmission patterns of these diseases are strongly linked to the spatiotemporal distributions and abundances of their vectors [3,4]. Therefore, there is growing concern that climate and land-use change coupled with rapid globalization may shift the distributions and abundances of vector species, and thus, the diseases they transmit [5,6]. However, we currently lack a mechanistic understanding of how changes in multiple environmental drivers interact to affect the abundance of disease vectors [7–9].
Because most disease vectors are small ectotherms, environmental temperature in particular can have large effects on their population fitness [5,6,10–15]. Many biological rates (including metabolic, development and fecundity rate [7]) of ectotherms increase approximately exponentially with temperature up to some optimum before declining to a baseline [16]. A number of recent studies have used laboratory data on the thermal responses of functional traits of vectors to predict how temperature will affect vector abundance and disease transmission in the field, leading to new insights, including a much lower optimal temperature for malaria transmission than previously thought [17]. However, these laboratory-derived temperature–trait relationships are generally measured in populations reared on optimal resource supply, whereas natural vector populations are expected to experience variation in resource supply, including intermittent resource limitation.
Indeed, along with temperature, resource supply is another ubiquitous environmental driver that is expected to limit the fitness of vector populations in nature [18–20]. Moreover, temperature and resource supply are expected to act interactively [9,21]. The primary reason for this prediction is that while the energy cost of somatic maintenance, growth and ontogenetic development of individuals generally increases with temperature [22,23], the ability to meet this increasing demand depends on resource supply. If the resources available to an individual do not keep pace with increasing energy requirements, its growth, development and survival would be compromised. This resource limitation should negatively affect fitness, with the severity of these effects increasing with temperature. While the importance of resource supply in mediating the effect of temperature on population abundance may seem obvious, this problem remains largely unresolved theoretically and empirically, not just in vector-borne disease research, but in thermal ecology in general. For example, ecological metabolic theories (including the metabolic theory of ecology (MTE) and dynamic energy budget (DEB) theory), which seek to link organismal metabolic rates to ontogenetic and population growth, generally assume that external resource supply is not a limiting factor [22–25].
Resource supply can also interact with temperature to affect the population fitness of ectotherms by determining size at maturity. Generally, size at maturity decreases with rising temperature (the size–temperature rule [26], which also applies to disease vectors, such as mosquitoes [27]). The size–temperature rule also remains largely untested under resource limitation in disease vectors and other ectotherms [26,28]. For vectors specifically, female size is demographically and epidemiologically important because it is associated with longevity, fecundity and biting behaviour [29,30].
In general, the question of whether and how temperature and resource supply interact to modulate disease transmission together through effects on underlying traits remains open. Here, we seek to address this gap in knowledge by investigating the effect of realistic variation in resource supply on the temperature dependence of population-level fitness in Aedes aegypti, a principal mosquito vector of human arboviruses (e.g. dengue, yellow fever and Zika; [31]). Because resource competition between larvae is expected to be a major regulator of adult mosquito abundance, many studies have examined how resource supply and larval density interact to affect fitness [32–35], while others have investigated the effect of resource supply and larval density separately [36,37]. However, none of these studies considered environmental temperature. On the other hand, studies that have considered temperature have not examined how the effects of temperature and resource supply on underlying fitness traits can propagate through the system to affect population fitness [38,39]. By taking a trait-based approach, we seek to gain general, mechanistic insights into how resource availability and temperature may together affect the abundance of disease vectors and other arthropods in the field.
2. Methods
To investigate the effects of temperature and resource supply on mosquito life history, we employed a 3 × 2 factorial design comprised of three temperatures (22, 26 and 32°C) and two resource supply levels: 0.1 (low-resource supply) and 1 mg larva−1 day−1 (high-resource supply). These experimental temperatures span the range of average annual temperatures [40] that this strain of Ae. aegypti is likely to experience in the wild (F16–19 originating from Fort Meyer, FL; [41]). Our low-resource supply level was chosen because previous work has found that it is the highest resource limitation that can be applied to this species without resulting in complete juvenile mortality [18,19]; a level of limitation that might be expected in wild populations. We also determined that the low-resource level was appropriate with a preliminary assay (electronic supplementary material, tables S4 and S5). The high-resource supply level corresponds to the upper mid-range of the high-resource supply levels used in Arrivillaga & Barrera [18] and Barrera et al. [19] and is consistent with the levels of resource supply commonly used in laboratory studies on this species [38,42].
Batches of approximately 300 Ae. aegypti eggs were randomly assigned to one of the three experimental temperatures and immersed in plastic tubs containing 150 ml of dechlorinated tap water. Each tub was provided with a pinch of powdered fish food (Cichlid Gold®, Hikari, Kyrin Food Industries Ltd, Japan) to stimulate overnight hatching. The tubs were then submerged in water baths (Grant Instruments: JAB Academy) set at either 22, 26 or 32°C. Water baths were situated in a 20°C climate-controlled insectary with a 12 L : 12 D photoperiod and 30 min of gradual transition of light levels to simulate sunrise and sunset. On the following day, first instar larvae were separated into cohorts of 30 and held in tubs containing 150 ml of water. We created three replicate tubs per treatment (90 individuals/treatment). We conducted a preliminary assay to determine the adequacy of this replication level to detect statistically significant effect sizes (electronic supplementary material, tables S4 and S5). Low-resource supply treatments were provided 3 mg of food and high-resource supply treatments received 30 mg. Thereafter, resource levels were adjusted daily according to the number of living individuals in each tub prior to feeding each day such that resource levels were maintained at an approximately constant level during the juvenile stages. Rearing tubs were cleaned and refilled with fresh tap water daily. Water volumes were also adjusted daily in accordance with mortality to maintain larval density (0.2 larvae ml–1). Electronic supplementary material, figure S1 is a schematic of the experimental design and the traits measured.
(a). Fitness calculation
To calculate population-level fitness, we used our data to parameterize stage-structured matrix projection models [43], which describe a change in a population over time:
| 2.1 |
where Nt is a vector of abundances in the stage classes at time t and M is the population projection matrix. The first row of M is populated by daily fecundity (the number of female offspring produced per female at age i). The sub-diagonal of M is populated with the proportions of survival from age i to age i+1. Multiplying the transition matrix (M; equation (2.1)) and stage-structured population size vector (Nt) sequentially across time intervals yields the stage-structured population dynamics. Once the stable stage distribution of the abundance vector is reached, the dominant eigenvalue of the system is the finite population rate of increase (λ) [43]. Then, the intrinsic rate of population growth is
This is a population's inherent capacity to reproduce and therefore a measure of population-level fitness [24,44,45]. Negative rmax values indicate decline and positive ones, growth. The projection matrices were built and analysed using the popbio R package [46,47].
(b). Parameterization
(i). Immature development time and immature and adult survival proportions
Matrix survival elements (the sub-diagonal of the matrix M; equation (2.1)) were populated with continuous survival proportions estimated using the Kaplan–Meier survival function in the survival R package [46,48]. We assumed life stage duration (i.e. larva-to-pupa-to-adult) was the mean duration of transitioning into and out of that stage and a fixed age of adult emergence at the mean age of emergence. Adult survival elements were populated with the Kaplan–Meier proportions. Hatching-to-adult development times were calculated by recording the day and time that egg eclosion, pupation and adult emergence occurred for each individual. Upon pupation, mosquitoes were held in individual falcon tubes containing 5 ml of tap water. This enabled pupa-to-adult development durations and the lifespans of individual starved adults to be recorded. Starvation forces adults to metabolize nutritional reserves accumulated during larval development, so starved lifespan should increase with body size. Therefore, starved adult lifespan is a useful indicator of the carry over effects of temperature and resource availability in the larval habitat [49,50].
(ii). Daily fecundity rate
The use of scaling relationships between fecundity and size is common in predictions of population growth in Aedes mosquitoes [51,52]. A detailed description of our method for estimating fecundity is provided in the electronic supplementary material (electronic supplementary material, figure S2). Briefly, we measured wing length as a proxy for body size, and estimated lifetime fecundity using previously published datasets on the temperature- and resource supply-dependent scaling between wing length and lifetime fecundity [42,50]. Daily fecundity rate is required for the first row of M (equation (2.1)), so lifetime fecundity was divided by lifespan and multiplied by 0.5 (assuming a 1 : 1 male-to-female offspring ratio) to give temperature-specific individual daily fecundity.
(c). Parameter sensitivity
We used the delta method to approximate 95% confidence intervals (CIs) for our fitness calculations [43,53] to account for how uncertainty in survival and fecundity estimates is propagated through to the rmax estimate. This method requires the standard errors of the survival and fecundity element estimates. For survival, we used the standard errors estimated by the Kaplan–Meier survival function in the survival R package. For fecundity, we calculated the standard errors of the mean daily fecundity rates (electronic supplementary material, table S2) for each treatment using the Rmisc R package [54]. As an additional sensitivity analysis, we recalculated fitness using the upper and lower 95% CIs of the exponents for the scaling of wing length and lifetime fecundity (figure 3; electronic supplementary material, figure S2).
Figure 3.
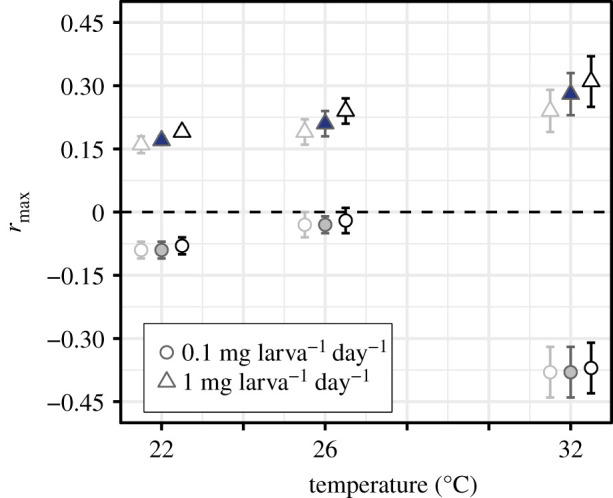
Population-level Ae. aegypti fitness (rmax) by resource supply (0.1 (low) or 1 mg larva−1 day−1 (high)) across temperatures. Fitness estimates for each treatment, with 95% confidence intervals (CIs). The three data points for each treatment represent rmax estimated using the 95% CI bounds of the exponents for the scaling of lifetime fecundity with wing length (electronic supplementary material, equation S1, figure S2). The lightest greyscale hue estimates derive from the lower 95% CIs, the mid-range hue estimates with closed symbols derive from the slopes, and the darkest hue derives from the upper 95% CIs.
(d). Elasticity analysis
Elasticities were used to quantify the proportional contributions of individual life-history traits to rmax. Elasticity, eij, measures the proportional effect on λ of an infinitesimal change in an element of M (equation (2.1)) with all other elements held constant (the partial derivative) [55,56]. This partial derivative of λ, with respect to each element of M, is sij = ∂λ/∂aij = viwj with the dot product 〈w, v〉 = 1. Here, w is the dominant right eigenvector (the stage distribution vector of M), v is the dominant left eigenvector (the reproductive value vector of M) and aij is the i × jth element of M. Elasticities can then be calculated using the relationship: eij = aij/λ × sij. Multiplying an elasticity by λ gives the absolute contribution of its corresponding aij to λ [55,56]. Absolute contributions for juvenile and adult elements were summed and changed proportionally to quantify the sensitivity of rmax to these traits.
(e). Statistical analyses
All statistical analyses were conducted using R [46]. We used full factorial generalized linear models (GLM) with gamma distributions and identity link functions (predictor effects were considered additive) to determine the significance of each predictor on the thermal responses of development time, lifespan and wing length. Replicate was included in these GLMs as a fixed effect.
We investigated the effect of temperature and resource supply on juvenile mortality rate by fitting a set of candidate distributions (exponential, log-logistic, Gompertz and Weibull) to the survival data with R package flexsurv [57]. The Gompertz survival function was the best fit to these data according to the Akaike information criterion (AIC) (electronic supplementary material, table S3). The final mortality model was obtained by dropping terms from the full model (consisting of temperature × resource supply + replicate as fixed effect predictors). If removing a term worsened model fit (ΔAIC > −2), then it was retained. Otherwise, it was removed (electronic supplementary material, table S3). For each treatment, maximum-likelihood methods executed in flexsurv estimated the mortality parameters (and their 95% CIs) of the Gompertz model, μx = aebx, where a is the baseline morality rate, and b is the change in mortality rate with time. These parameter estimates were then used to determine the significance of the effects of temperature and resource supply on juvenile mortality.
3. Results
All trait responses varied significantly with temperature and resource supply, with a significant interaction between the two environmental variables (figures 1 and 2; electronic supplementary material, tables S1 and S2). Thus, the realized effect of temperature on trait responses was consistently and significantly mediated by resource supply.
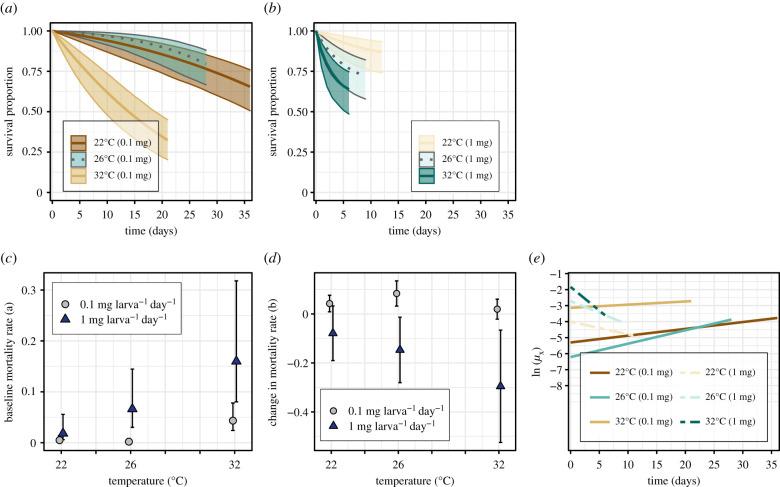
Figure 1.
The effect of resource supply on the temperature dependence of juvenile survival. Survival curves at (a) low- and (b) high-resource supply by temperature with 95% confidence bounds. Predicted survival for each treatment was 22°C at low-resource supply = 65%, at high-resource supply = 87%; 26°C at low-resource supply = 80%, at high-resource supply = 83%; 32°C at low-resource supply = 32%, at high-resource supply = 0.64%. (c) Baseline mortality rates (a) by resource supply level across temperatures with 95% CIs. Mortality rates were significantly lower at low-resource supply than at high-resource supply as temperatures increased from 22 to 32°C (95% CIs at 26 and 32°C do not overlap). (d) Change in mortality rate trajectories (b) by resource supply level across temperatures with 95% CIs. Rate trajectories were significantly lower at high-resource supply than at low-resource supply as temperatures increased from 22°C (95% CIs at 26 and 32°C do not overlap). (e) Logged daily mortality rates (ln(μx) = ln(a) + bx) show how mortality rates at low-resource supply started low and increased with time; at high-resource supply, they started high and decreased with time.
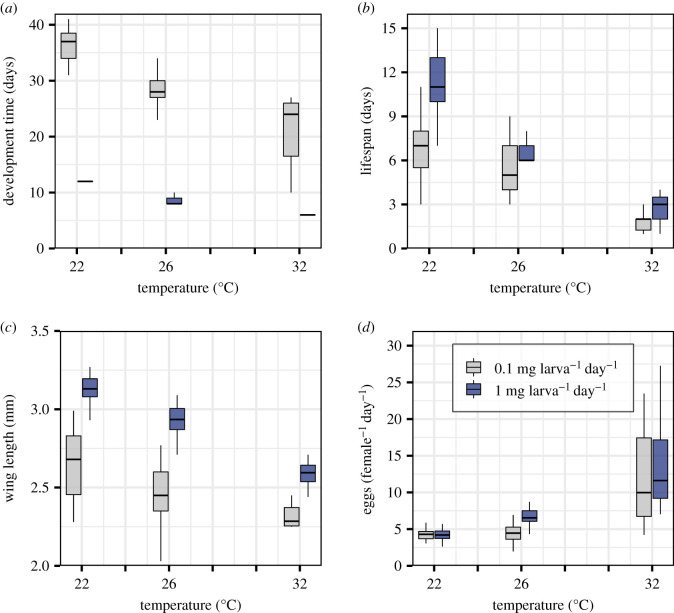
Figure 2.
The combined effect of temperature and resource supply level on Ae. aegypti life-history traits. (a–c) Resource supply significantly modulated the effect of temperature on all directly measured traits, except adult lifespan at 26°C (b). The resulting ANOVAs of the GLMs for each trait are presented in the electronic supplementary material, table S1. The GLM-estimated trait means with 95% CIs calculated from the standard errors are shown in the electronic supplementary material, table S2. (d) Predicted fecundity increased significantly with the temperature at both resource levels (non-overlapping 95% CIs). The extent to which fecundity increased with temperature and resource level was only significant at 26°C. The numbers of females that survived to adulthood (n) in each treatment were 22°C at low-resource supply n = 23, at high-resource supply n = 37; 26°C at low-resource supply n = 29, at high-resource supply n = 30; 32°C at low-resource supply n = 10, at high-resource supply n = 27. Boxplot horizontal lines represent medians. Lower and upper hinges are the 25th and 75th percentiles. Upper whiskers extend from the hinge to the largest value no further than 1.5 × inter-quartile range (IQR) from the hinge. The lower whisker extends from the hinge to the smallest value at most 1.5 × IQR of the hinge.
At a low-resource supply, daily juvenile mortality rates, μx, increased with time at all temperatures, whereas at high-resource supply, they decreased with time (figure 1e). Baseline juvenile mortality rates, a, were significantly lower at low-resource supply than at high-resource supply as temperatures increased from 22 to 32°C (non-overlapping 95% CIs at 26 and 32°C; figure 1c). Mortality rate trajectories, b, were significantly lower at high-resource supply than at low-resource supply as temperatures increased from 22 to 32°C (non-overlapping 95% CIs at 26 and 32°C; figure 1d).
Development time varied significantly with the interaction between temperature and resource supply (ANOVA; F2,0.75 = 24.11, p < 0.001; electronic supplementary material, table S1). Whereas development time decreased both at warmer temperatures and at high-resource supply, the decrease with temperature was greater at low-resource supply than at high-resource supply. At low-resource supply, development time decreased by 15.45 days as temperatures increased from 22 to 32°C, whereas at high-resource supply, it decreased by 6.38 days across this range (figure 2a; electronic supplementary material, table S2).
Adult lifespan varied significantly with the interaction between temperature and resource supply (ANOVA; F2, 2.41 = 14.95, p < 0.001; electronic supplementary material, table S1). Although lifespan decreased both at warmer temperatures and at low-resource supply, the decrease with temperature was greater at high-resource supply than at low-resource supply. High-resource supply lifespan decreased by 8.89 days, whereas low-resource supply lifespan decreased by 4.71 days as temperatures increased from 22 to 32°C (figure 2b; electronic supplementary material, table S2).
The interaction between temperature and resource supply resulted in significant variation in size at maturity (wing length) between resource levels (ANOVA; F2,0.03 = 4.36, p = 0.01; electronic supplementary material, table S1). Adult size decreased both at warmer temperatures and at low-resource supply, though the decrease with temperature was greater at high-resource supply than at low-resource supply. As temperatures increased from 22 to 32°C, size decreased by 0.54 mm at high-resource supply, whereas size at low-resource supply decreased by 0.37 mm (figure 2c; electronic supplementary material, table S2).
(a). Population fitness (rmax)
Resource limitation depressed rmax to negative values at all temperatures, with a unimodal relationship of rmax with temperature (figure 3; electronic supplementary material, table S2). Low-resource supply rmax increased from −0.09 at 22°C to −0.03 at 26°C and then decreased acutely to −0.38 at 32°C. By contrast, at high-resource supply, rmax was always positive and increased with temperature from 0.17 at 22°C to maximal growth (0.28) at 32°C.
(b). Elasticity analysis
Juvenile development and survival were the most important contributors to rmax (electronic supplementary material, figure S3). For example, at low-resource supply at 32°C, a 0.5 proportional increase in juvenile traits would almost halve the rate of decline from −0.380 to −0.202 (electronic supplementary material, figure S3a). By contrast, for the same treatment, a proportional increase of the same magnitude for adult survival would increase rmax from −0.380 to −0.376 (electronic supplementary material, figure S3c), and fecundity would increase rmax from −0.380 to −0.372 (electronic supplementary material, figure S3d). This underlines how the temperature dependence of rmax derives mainly from how resource supply level impacts juvenile mortality and development, which determine the number of reproducing individuals and the timing of reproduction, respectively. Fecundity and adult survival, on the other hand, have relatively negligible effects on rmax, which suggests that the carry over effect of reduced size at maturity on rmax is relatively weak.
4. Discussion
Our results show that juvenile resource regimes can have far-reaching effects on the temperature dependence of population-level fitness, rmax. Differences between the thermal response of traits at low- versus high-resource supply resulted in a marked divergence of the temperature dependence of rmax between the two resource levels (figure 3). At low-resource supply, fitness was negative for all three temperatures tested and was much lower at 32°C than 26°C. This indicates that population fitness becomes increasingly and nonlinearly constrained by resource limitation as temperatures increase. By contrast, fitness at high-resource supply was positive and increased moderately from 26°C to 32°C. While recent studies show that interactions between temperature and resource availability can mediate population growth in single-celled plankton [21,58,59], studies of how such interactions can affect eukaryotes are rare (but see [87]). Our study shows that the effects of temperature × resource availability interactions need to be considered to accurately predict and understand how natural disease vector populations and other arthropods will respond to environmental change.
The elasticity analysis (electronic supplementary material, figure S3) shows that the primary mechanism underlying the divergent temperature dependence of rmax across resource levels is decreased juvenile survival and increased juvenile development time at low resources. Population-level reproductive output decreased at low resources because decreased juvenile survival (figure 1) reduced the number of reproducing individuals, and increased juvenile development time (figure 2a) delayed the onset of reproduction. At low-resource supply, the daily mortality rate started low and then increased over time, while at high-resource supply, it started high and then decreased to very low levels (figure 1e). Resource limitation substantially increased development time at all temperatures (figure 2a).
Fecundity and adult lifespan had comparatively negligible effects on rmax, which suggests that the carry over effect of reduced size at maturity on rmax is relatively weak. For example, at high-resource supply, adult lifespan and body size were greater at 26°C than at 32°C, yet fitness at 32°C was predicted to be 25% higher (figures 2c and 3). This pattern occurs because high-resource supply and increased temperature minimized juvenile mortality and optimized development rate. These effects allowed faster recruitment at 32°C, leading to increased fitness as greater numbers of individuals could contribute to population growth through reproductive output sooner than for other treatments. This result is consistent with empirical and theoretical studies of ectotherm fitness [60–63], including mosquitoes [32]. This finding is key, as it implies that predictions about the effect of warming on vector abundance and disease transmission based on laboratory-derived trait data (which are generally from populations under high- or optimal resource supply) likely underestimate the effect of temperature on development time and juvenile survival, and overestimate effects of temperature on lifespan and fecundity.
Indeed, the trait-level responses of our high-resource supply treatments correspond with studies that have synthesized laboratory-derived high-resource supply trait responses to temperature to estimate vector fitness and R0. In these studies, juvenile survival is expected to be approximately 80% at 22 and 26°C and approximately 70% at 32°C [8]. In the present study, juvenile survival at high-resource supply was predicted to follow a similar pattern (approx. 80% at 22 and 26°C, and 64% at 32°C; figure 1). By contrast, juvenile survival at low-resource supply in the present study was predicted to be 65% at 22°C, 80% at 26°C and 32% at 32°C (figure 1).
Further, the juvenile development rate of most mosquito vectors is expected to increase from approximately 0.07 day−1 at 22°C to approximately 0.14 day−1 at 32°C [8], which is congruent with the present study's development rate (1/development time, figure 2a) at high-resource supply (approx. 0.08 to approx. 0.17 day−1) across the same temperature range. By contrast, at low-resource supply, we found juvenile development rate was approximately 0.05 day−1 at 32°C (figure 2a), which is consistent with other studies on the effects of temperature and low-resource supply on juvenile development rate [38]. Such differences in juvenile trait responses are likely to substantially alter predictions about the temperature dependence of R0. This underlines the importance of considering resource supply when predicting the temperature dependence of R0 for vector-borne diseases.
Juvenile survival decreased significantly with temperature and was overall significantly lower at low-resource supply (figure 1). This reduction in survival is likely because somatic maintenance costs increase with metabolic rate [22], which cannot be met below a threshold resource supply level. Such metabolic costs could explain why the highest level of mortality occurred at 32°C at low-resource supply, where the energy supply-demand deficit was expected to be the largest.
The Gompertz-shaped juvenile survival curves observed at 22 and 26°C at low-resource supply (figure 1a) may well arise from the amount of resource being sufficient for somatic maintenance, but not for development. This hypothesis could be a key line of future investigation because it points to the importance of understanding how resource availability combines with temperature and other environmental factors to affect natural mosquito populations and other arthropods. For example, the negative effects of resource limitation on population growth through increased juvenile development time and mortality may be exacerbated, as individuals remain in the vulnerable juvenile stages for longer, which may increase predation threat [64] and/or the risk of exposure to breeding habitat evaporation [65]. If resource availability increases with climatic warming, the negative effects of predation and breeding habitat evaporation on population growth could be offset by increased development and recruitment rates [66]. Alternatively, population growth could be dampened, if climate change reduces the quantity of food available to ectotherms [67,68]. This effect could simultaneously decrease the burden of vector-borne diseases and agricultural pests, but increase the extinction risk of vulnerable species [69,70].
We did not measure the effect of temperature and resource supply on fecundity directly, but used the size-scaling of this trait to estimate this effect. This approach is appropriate because most of the effect of resource limitation on juveniles is expected to affect adult mosquitoes indirectly by reducing size at emergence and lifespan [30,50]. Predicted fecundity increased with temperature, with a larger increase from 26°C to 32°C than between 22°C and 26°C (figure 2d; electronic supplementary material, table S2). Across both resource levels, these fecundity estimates are similar to datasets that are used to parameterize mosquito-borne disease transmission models (e.g. [71]). However, even substantial under- or overestimation of fecundity by our size-scaling predictions and the use of starved adult lifespans would not affect our main conclusions because predicted fitness was relatively insensitive to these traits (figure 3; electronic supplementary material, figure S3).
While the increased negative carry over effects of temperature at resource limitation on adult traits had a relatively weak impact on fitness compared to juvenile traits, temperature × resource supply interactions may have important effects on other components of vector-borne disease transmission [72]. For example, larger individuals may have greater transmission potential because they are more likely to outlive a pathogen's extrinsic incubation period [73]. However, the interactive effects of temperature and resource availability can alter the relationships between body size, longevity and vector competence [74,75]. Indeed, as we have shown here, resource limitation can exaggerate the negative relationship between size and temperature [26]. This effect could increase transmission probability as smaller Ae. aegypti may compensate for poor larval nutrition by biting more frequently [76]. Also, larval nutrition [37] and temperature [77] can independently influence within-vector parasite development, but future studies could consider how the combined effects of temperature and resource supply affect this, and other important transmission traits.
Another important implication of this study's findings for vector-borne disease research is that it underlines the need to develop realistic and tractable methods of measuring density-dependent effects on population fitness in the field. Without such datasets, it will not be possible to link temperature- and resource-dependent fitness to vector abundance dynamics and VBD dynamics. Semi-field systems offer a way to track the entire mosquito life cycle under ambient environmental conditions [78]. Such systems are generally being used to test the effectiveness of novel biocontrol strategies, such as transgenic fungi [79], but they also could allow for the effects of temperature × resource interactions on fitness and abundance to be explored under conditions that more closely resemble natural environments. State of the art insect traps and geospatial mapping of microclimates and vegetation indices could also be used to study the effects of variation in temperature and resource availability on vector populations in the field [80,81].
In this study, we did not consider the temperature dependence of resource supply itself (supply was held constant across temperatures in our experiments). In nature, the availability of resources may in fact be temperature dependent. This relationship occurs because microbial growth rates increase with temperature to some optimum, which may increase the concentration of food in the environment [9,82,83]. For example, Anopheles [84] and Aedes [85] mosquitoes can be reared exclusively on cultures of Asaia bacteria. We also did not address larval competition for resources by manipulating the number of larvae for a given resource supply level. Variation in larval density may introduce additional fitness constraints through interference and exploitative competition. It could also interact with temperature-dependent resource supply because a higher larval density will increase accumulation of waste products. These are interesting and potentially important avenues for future investigation.
We note that experimenting with more resource levels would not change our qualitative results, which we have shown to be robust using thorough sensitivity analyses. Indeed, the two resource levels we have chosen represent extremes, and it is reasonable to conclude that mosquito population fitness in the field fluctuates with resource availability between the radically different temperature responses as we have found here (figure 3). One avenue for future work is to find more accurate methods to estimate effective temperature-dependent fitness values in the field, accounting for resource fluctuations.
Organisms experience significant resource limitation over space and time in nature. This is particularly true for insects such as mosquitoes, which have juvenile stages restricted to small, ephemeral aquatic habitats that are susceptible to resource fluctuations [18–20,86]. Our study underlines the importance of the effects of resource supply on the temperature dependence of population-level fitness of an important disease vector. In doing so, our findings suggest that current projections of how climatic warming affects vector-borne disease transmission may prove inaccurate because they generally fail to consider resource limitations. Our findings also underline the need for a future research effort to be directed at better understanding how temperature and resource supply interact in the field, and how this, and interactions between other environmental factors, may influence other components of vector-borne disease systems. While recent studies have shown that interactions between temperature and resource availability can have important effects on population fitness in single-celled organisms [21,58,59], our results show that such interactions also need to be considered when predicting how eukaryotes will respond to environmental change.
Supplementary Material
Data accessibility
All data for analyses are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.h9w0vt4gg [88]. A preprint version of this manuscript is available from the bioRxiv preprint server: https://doi.org/10.1101/2020.05.29.123315 [89].
Authors' contributions
All authors contributed to the conception of the study and designed the experiments, L.J.C. provided the mosquitoes; P.J.H. and S.P. performed the modelling; P.J.H. collected the data and analysed it. P.J.H. wrote the first draft of the manuscript, and all authors contributed substantially to revisions.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by Natural Environment Research Council (NE/L002515/1). We also acknowledge joint Centre funding from the UK Medical Research Council and Department for International Development (MR/R0156600/1).
References
- 1.Stanaway JD, et al. 2016. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect. Dis. 16, 712-723. ( 10.1016/S1473-3099(16)00026-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. 2014. A global brief on vector-borne diseases. World Heal. Organ. WHO/DCO/WHD/2014. 1, 1–56.
- 3.Li R, Xu L, Bjørnstad ON, Liu K, Song T, Chen A, Xu B, Liu Q, Stenseth NC. 2019. Climate-driven variation in mosquito density predicts the spatiotemporal dynamics of dengue. Proc. Natl Acad. Sci. 116, 3624-3629. ( 10.1073/pnas.1806094116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng K-C, Chaves L, Tsai K-H, Chuang T-W. 2018. Increased adult Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae) abundance in a dengue transmission hotspot, compared to a coldspot, within Kaohsiung City, Taiwan. Insects 9, 98. ( 10.3390/insects9030098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mordecai EA, Ryan SJ, Caldwell JM, Shah MM, LaBeaud AD. 2020. Climate change could shift disease burden from malaria to arboviruses in Africa. Lancet Planet. Health 4, e416-e423. ( 10.1016/S2542-5196(20)30178-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan SJ, Carlson CJ, Tesla B, Bonds MH, Ngonghala CN, Mordecai EA, Johnson LR, Murdock CC. 2021. Warming temperatures could expose more than 1.3 billion new people to Zika virus risk by 2050. Glob. Chang. Biol. 27, 84-93. ( 10.1111/gcb.15384) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cator LJ, et al. 2020. The role of vector trait variation in vector-borne disease dynamics. Front. Ecol. Evol. 8, 1-25. ( 10.3389/fevo.2020.00189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mordecai EA, et al. 2019. Thermal biology of mosquito-borne disease. Ecol. Lett. 22, 1690-1708. ( 10.1111/ele.13335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cross WF, Hood JM, Benstead JP, Huryn AD, Nelson D. 2015. Interactions between temperature and nutrients across levels of ecological organization. Glob. Chang. Biol. 21, 1025-1040. ( 10.1111/gcb.12809) [DOI] [PubMed] [Google Scholar]
- 10.Iwamura T, Guzman-Holst A, Murray KA. 2020. Accelerating invasion potential of disease vector Aedes aegypti under climate change. Nat. Commun. 11, 2130. ( 10.1038/s41467-020-16010-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parham PE, Michael E. 2010. Modeling the effects of weather and climate change on malaria transmission. Environ. Health Perspect. 118, 620-626. ( 10.1289/ehp.0901256) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu-Helmersson J, Stenlund H, Wilder-Smith A, Rocklö J. 2014. Vectorial capacity of Aedes aegypti: effects of temperature and implications for global dengue epidemic potential. PLoS ONE 9, e89783. ( 10.1371/journal.pone.0089783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miazgowicz KL, et al. 2020. Age influences the thermal suitability of Plasmodium falciparum transmission in the Asian malaria vector Anopheles stephensi. Proc. R. Soc. B 287, 20201093. ( 10.1098/rspb.2020.1093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shocket MS, Verwillow AB, Numazu MG, Slamani H, Cohen JM, El Moustaid F, Rohr J, Johnson LR, Mordecai EA. 2020. Transmission of West Nile and five other temperate mosquito-borne viruses peaks at temperatures between 23°C and 26°C. Elife 9, 1-67. ( 10.7554/eLife.58511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamlet A, Jean K, Perea W, Yactayo S, Biey J, Van Kerkhove M, Ferguson N, Garske T. 2018. The seasonal influence of climate and environment on yellow fever transmission across Africa. PLoS Negl. Trop. Dis. 12, e0006284. ( 10.1371/journal.pntd.0006284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dell AI, Pawar S, Savage VM. 2011. Systematic variation in the temperature dependence of physiological and ecological traits. Proc. Natl Acad. Sci. 108, 10 591-10 596. ( 10.1073/pnas.1015178108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mordecai EA, et al. 2013. Optimal temperature for malaria transmission is dramatically lower than previously predicted. Ecol. Lett. 16, 22-30. ( 10.1111/ele.12015) [DOI] [PubMed] [Google Scholar]
- 18.Arrivillaga J, Barrera R. 2004. Food as a limiting factor for Aedes aegypti in water-storage containers. J. Vector Ecol. 29, 11-20. [PubMed] [Google Scholar]
- 19.Barrera R, Amador M, Clark GG. 2006. Ecological factors influencing Aedes aegypti (Diptera: Culicidae) productivity in artificial containers in Salinas, Puerto Rico. J. Med. Entomol. 43, 484-492. ( 10.1093/jmedent/43.3.484) [DOI] [PubMed] [Google Scholar]
- 20.Walsh RK, Facchinelli L, Ramsey JM, Bond JG, Gould F. 2011. Assessing the impact of density dependence in field populations of Aedes aegypti. J. Vector Ecol. 36, 300-307. ( 10.1111/j.1948-7134.2011.00170.x) [DOI] [PubMed] [Google Scholar]
- 21.Thomas MK, Aranguren-Gassis M, Kremer CT, Gould MR, Anderson K, Klausmeier CA, Litchman E. 2017. Temperature-nutrient interactions exacerbate sensitivity to warming in phytoplankton. Glob. Chang. Biol. March. 23, 3269-3280. ( 10.1111/gcb.13641) [DOI] [PubMed] [Google Scholar]
- 22.Kooijman SALM. 2000. Dynamic energy and mass budgets in biological systems. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 23.West GB, Brown JH, Enquist BJ. 2001. A general model for ontogenetic growth. Nature 413, 628-631. ( 10.1038/35098076) [DOI] [PubMed] [Google Scholar]
- 24.Savage VM, Gillooly JF, Brown JH, West GB, Charnov EL. 2004. Effects of body size and temperature on population growth. Am. Nat. 163, 429-441. ( 10.1086/381872) [DOI] [PubMed] [Google Scholar]
- 25.Amarasekare P, Savage V. 2012. A framework for elucidating the temperature dependence of fitness. Am. Nat. 179, 178-191. ( 10.1086/663677) [DOI] [PubMed] [Google Scholar]
- 26.Atkinson D. 1994. Temperature and organism size—a biological law for ectotherms? Adv. Ecol. Res. 25, 1-58. ( 10.1016/S0065-2504(08)60212-3) [DOI] [Google Scholar]
- 27.Rueda LM, Patel KJ, Axtell RC, Stinner RE. 1990. Temperature-dependent development and survival rates of Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 27, 892-898. ( 10.1093/jmedent/27.5.892) [DOI] [PubMed] [Google Scholar]
- 28.Forster J, Hirst AG, Atkinson D. 2012. Warming-induced reductions in body size are greater in aquatic than terrestrial species. Proc. Natl Acad. Sci. USA 109, 19 310-19 314. ( 10.1073/pnas.1210460109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nasci RS. 1991. Influence of larval and adult nutrition on biting persistence in Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 28, 522-526. ( 10.1093/jmedent/28.4.522) [DOI] [PubMed] [Google Scholar]
- 30.Steinwascher K. 1982. Relationship between pupal mass and adult survivorship and fecundity for Aedes aegypti. Environ. Entomol. 11, 150-153. ( 10.1093/ee/11.1.150) [DOI] [Google Scholar]
- 31.Brady OJ, Hay SI. 2020. The global expansion of dengue: how Aedes aegypti mosquitoes enabled the first pandemic arbovirus. Annu. Rev. Entomol. 65, 191-208. ( 10.1146/annurev-ento-011019-024918) [DOI] [PubMed] [Google Scholar]
- 32.Dye C. 1984. Models for the population dynamics of the yellow fever mosquito, Aedes aegypti. J. Anim. Ecol. 53, 247. ( 10.2307/4355) [DOI] [Google Scholar]
- 33.Gilpin ME, McClelland GA. 1979. Systems analysis of the yellow fever mosquito Aedes aegypti. Fortschr. Zool. 25, 355-388. [PubMed] [Google Scholar]
- 34.Walsh RK, Aguilar CL, Facchinelli L, Valerio L, Ramsey JM, Scott TW, Lloyd AL, Gould F. 2013. Regulation of Aedes aegypti population dynamics in field systems: quantifying direct and delayed density dependence. Am. J. Trop. Med. Hyg. 89, 68-77. ( 10.4269/ajtmh.12-0378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gimnig JE, Ombok M, Otieno S, Kaufman MG, Vulule JM, Walker ED. 2002. Density-dependent development of Anopheles gambiae (Diptera: Culicidae) larvae in artificial habitats. J. Med. Entomol. 39, 162-172. ( 10.1603/0022-2585-39.1.162) [DOI] [PubMed] [Google Scholar]
- 36.Romeo Aznar V, Alem I, De Majo MS, Byttebier B, Solari HG, Fischer S. 2018. Effects of scarcity and excess of larval food on life history traits of Aedes aegypti (Diptera: Culicidae). J. Vector Ecol. 43, 117-124. ( 10.1111/jvec.12291) [DOI] [PubMed] [Google Scholar]
- 37.Shapiro LLM, Murdock CC, Jacobs GR, Thomas RJ, Thomas MB. 2016. Larval food quantity affects the capacity of adult mosquitoes to transmit human malaria. Proc. R. Soc. B 283, 20160298. ( 10.1098/rspb.2016.0298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Couret J, Dotson E, Benedict MQ. 2014. Temperature, larval diet, and density effects on development rate and survival of Aedes aegypti (Diptera: Culicidae). PLoS ONE 9, 1-9. ( 10.1371/journal.pone.0087468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Padmanabha H, Correa F, Legros M, Nijhout HF, Lord C, Lounibos LP. 2012. An eco-physiological model of the impact of temperature on Aedes aegypti life history traits. J. Insect Physiol. 58, 1597-1608. ( 10.1016/j.jinsphys.2012.09.015) [DOI] [PubMed] [Google Scholar]
- 40.Arguez A, Durre I, Applequist S, Vose RS, Squires MF, Yin X, Heim RR, Owen TW. 2012. NOAA's 1981–2010U.S. Climate normals: an overview. Bull. Am. Meteorol. Soc. 93, 1687-1697. ( 10.1175/BAMS-D-11-00197.1) [DOI] [Google Scholar]
- 41.Bargielowski IE, Lounibos LP, Carrasquilla MC. 2013. Evolution of resistance to satyrization through reproductive character displacement in populations of invasive dengue vectors. Proc. Natl Acad. Sci. USA 110, 2888-2892. ( 10.1073/pnas.1219599110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farjana T, Tuno N. 2012. Effect of body size on multiple blood feeding and egg retention of Aedes aegypti (L.) and Aedes albopictus (Skuse) (Diptera: Culicidae). Med. Entomol. Zool. 63, 123-131. ( 10.7601/mez.63.123) [DOI] [Google Scholar]
- 43.Caswell H. 1989. Matrix population models construction, analysis, and interpretation. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 44.Birch LC. 1948. The intrinsic rate of natural increase of an insect population. J. Anim. Ecol. 17, 15. ( 10.2307/1605) [DOI] [Google Scholar]
- 45.Cole LC. 1954. The population consequences of life history phenomena. Q. Rev. Biol. 29, 103-137. ( 10.1086/400074) [DOI] [PubMed] [Google Scholar]
- 46.R Core Team. 2018. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See http://www.R-project.org/.
- 47.Stubben C, Milligan B. 2007. Estimating and analyzing demographic models using the popbio package in R. J. Stat. Softw. 22, 1-23. ( 10.18637/jss.v022.i11) [DOI] [Google Scholar]
- 48.Therneau T. 2021. A package for survival analysis in R. R package version 3.2-10. See https://CRAN.R-project.org/package=survival.
- 49.Agnew P, Hide M, Sidobre C, Michalakis Y. 2002. A minimalist approach to the effects of density-dependent competition on insect life-history traits. Ecol. Entomol. 27, 396-402. ( 10.1046/j.1365-2311.2002.00430.x) [DOI] [Google Scholar]
- 50.Briegel H. 1990. Metabolic relationship between female body size, reserves, and fecundity of Aedes aegypti. J. Insect Physiol. 36, 165-172. ( 10.1016/0022-1910(90)90118-Y) [DOI] [Google Scholar]
- 51.Focks DA, Haile D, Daniels E, Mount GA. 1993. Dynamic life table model for Aedes aegypti (Diptera: Culicidae): simulation results and validation. J. Med. Entomol. 30, 1018-1028. ( 10.1093/jmedent/30.6.1018) [DOI] [PubMed] [Google Scholar]
- 52.Juliano SA. 1998. Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition? Ecology 79, 255. ( 10.2307/176880) [DOI] [Google Scholar]
- 53.Skalski JR, Millspaugh JJ, Dillingham P, Buchanan RA. 2007. Calculating the variance of the finite rate of population change from a matrix model in Mathematica. Environ. Model. Softw. 22, 359-364. ( 10.1016/j.envsoft.2005.12.003) [DOI] [Google Scholar]
- 54.Hope RM. 2013. Rmisc: Rmisc: Ryan Miscellaneous. See https://CRAN.R-project.org/package=Rmisc.
- 55.Caswell H, Naiman RJ, Morin R. 1984. Evaluating the consequences of reproduction in complex salmonid life cycles. Aquaculture 43, 123-134. ( 10.1016/0044-8486(84)90016-4) [DOI] [Google Scholar]
- 56.de Kroon H, Plaisier A, van Groenendael J, Caswell H. 1986. Elasticity: the relative contribution of demographic parameters to population growth rate. Ecology 67, 1427-1431. ( 10.2307/1938700) [DOI] [Google Scholar]
- 57.Jackson C. 2016. flexsurv: a platform for parametric survival modeling in R. J. Stat. Softw. 70, 1-33. ( 10.18637/jss.v070.i08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bestion E, Schaum C-E, Yvon-Durocher G. 2018. Nutrient limitation constrains thermal tolerance in freshwater phytoplankton. Limnol. Oceanogr. Lett. 3, 436-443. ( 10.1002/lol2.10096) [DOI] [Google Scholar]
- 59.Hall E, Cotner J. 2007. Interactive effect of temperature and resources on carbon cycling by freshwater bacterioplankton communities. Aquat. Microb. Ecol. 49, 35-45. ( 10.3354/ame01124) [DOI] [Google Scholar]
- 60.Huey RB, Berrigan D. 2001. Temperature, demography, and ectotherm fitness. Am. Nat. 158, 204-210. ( 10.1086/321314) [DOI] [PubMed] [Google Scholar]
- 61.Caswell H. 1978. A general formula for the sensitivity of population growth rate to changes in life history parameters. Theor. Popul. Biol. 14, 215-230. ( 10.1016/0040-5809(78)90025-4) [DOI] [PubMed] [Google Scholar]
- 62.Kammenga JE, Busschers M, Van Straalen NM, Jepson PC, Bakker J. 1996. Stress induced fitness reduction is not determined by the most sensitive life-cycle trait. Funct. Ecol. 10, 106. ( 10.2307/2390268) [DOI] [Google Scholar]
- 63.Forbes VE, Olsen M, Palmqvist A, Calow P. 2010. Environmentally sensitive life-cycle traits have low elasticity: implications for theory and practice. Ecol. Appl. 20, 1449-1455. ( 10.1890/09-1063.1) [DOI] [PubMed] [Google Scholar]
- 64.Benrey B, Denno RF. 1997. The slow-growth–high-mortality hypothesis: a test using the cabbage butterfly. Ecology 78, 987. ( 10.2307/2265852) [DOI] [Google Scholar]
- 65.Fillinger U, Sonye G, Killeen GF, Knols BGJ, Becker N. 2004. The practical importance of permanent and semipermanent habitats for controlling aquatic stages of Anopheles gambiae sensu lato mosquitoes: operational observations from a rural town in western Kenya. Trop. Med. Int. Heal. 9, 1274-1289. ( 10.1111/j.1365-3156.2004.01335.x) [DOI] [PubMed] [Google Scholar]
- 66.Culler LE, Ayres MP, Virginia RA. 2015. In a warmer Arctic, mosquitoes avoid increased mortality from predators by growing faster. Proc. R. Soc. B 282, 20151549. ( 10.1098/rspb.2015.1549) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lister BC, Garcia A. 2018. Climate-driven declines in arthropod abundance restructure a rainforest food web. Proc. Natl Acad. Sci. 115, E10397-E10406. ( 10.1073/pnas.1722477115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huey RB, Kingsolver JG. 2019. Climate warming, resource availability, and the metabolic meltdown of ectotherms. Am. Nat. 194, E140-E150. ( 10.1086/705679) [DOI] [PubMed] [Google Scholar]
- 69.Wagner DL, Grames EM, Forister ML, Berenbaum MR, Stopak D. 2021. Insect decline in the Anthropocene: death by a thousand cuts. Proc. Natl Acad. Sci. 118, e2023989118. ( 10.1073/pnas.2023989118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lehmann P, et al. 2020. Complex responses of global insect pests to climate warming. Front. Ecol. Environ. 18, 141-150. ( 10.1002/fee.2160) [DOI] [Google Scholar]
- 71.Yang HM, Macoris MLG, Galvani KC, Andrighetti MTM, Wanderley DMV. 2009. Assessing the effects of temperature on the population of Aedes aegypti, the vector of dengue. Epidemiol. Infect. 137, 1188-1202. ( 10.1017/S0950268809002040) [DOI] [PubMed] [Google Scholar]
- 72.Parham PE, et al. 2015. Climate, environmental and socio-economic change: weighing up the balance in vector-borne disease transmission. Phil. Trans. R. Soc. B 370, 20130551. ( 10.1098/rstb.2013.0551) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bellan SE. 2010. The importance of age dependent mortality and the extrinsic incubation period in models of mosquito-borne disease transmission and control. PLoS ONE 5, e10165. ( 10.1371/journal.pone.0010165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barreaux AMG, Stone CM, Barreaux P, Koella JC. 2018. The relationship between size and longevity of the malaria vector Anopheles gambiae (s.s.) depends on the larval environment. Parasit. Vectors 11, 485. ( 10.1186/s13071-018-3058-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barreaux AMG, Barreaux P, Thievent K, Koella JC. 2016. Larval environment influences vector competence of the malaria mosquito Anopheles gambiae. MalariaWorld J. 7, 1-6. ( 10.1186/s13071-018-3058-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scott TW, Morrison AC, Lorenz LH, Clark GG, Strickman D, Kittayapong P, Zhou H, Edman JD. 2000. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: population dynamics. J. Med. Entomol. 37, 77-88. ( 10.1603/0022-2585-37.1.77) [DOI] [PubMed] [Google Scholar]
- 77.Shapiro LLM, Whitehead SA, Thomas MB. 2017. Quantifying the effects of temperature on mosquito and parasite traits that determine the transmission potential of human malaria. PLoS Biol. 15, e2003489. ( 10.1371/journal.pbio.2003489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jones RT, Ant TH, Cameron MM, Logan JG. 2021. Novel control strategies for mosquito-borne diseases. Phil. Trans. R. Soc. B 376, 20190802. ( 10.1098/rstb.2019.0802) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lovett B, Bilgo E, Millogo SA, Ouattarra AK, Sare I, Gnambani EJ, Dabire RK, Diabate A, St. Leger RJ. 2019. Transgenic Metarhizium rapidly kills mosquitoes in a malaria-endemic region of Burkina Faso. Science 364, 894-897. ( 10.1126/science.aaw8737) [DOI] [PubMed] [Google Scholar]
- 80.Wimberly MC, Davis JK, Evans MV, Hess A, Newberry PM, Solano-Asamoah N, Murdock CC. 2020. Land cover affects microclimate and temperature suitability for arbovirus transmission in an urban landscape. PLoS Negl. Trop. Dis. 14, e0008614. ( 10.1371/journal.pntd.0008614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Høye TT, et al. 2021. Deep learning and computer vision will transform entomology. Proc. Natl Acad. Sci. 118, e2002545117. ( 10.1073/pnas.2002545117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Craine JM, Fierer N, McLauchlan KK. 2010. Widespread coupling between the rate and temperature sensitivity of organic matter decay. Nat. Geosci. 3, 854-857. ( 10.1038/ngeo1009) [DOI] [Google Scholar]
- 83.Smith TP, Thomas TJH, García-Carreras B, Sal S, Yvon-Durocher G, Bell T, Pawar S. 2019. Community-level respiration of prokaryotic microbes may rise with global warming. Nat. Commun. 10, 5124. ( 10.1038/s41467-019-13109-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chouaia B, et al. 2012. Delayed larval development in Anopheles mosquitoes deprived of Asaia bacterial symbionts. BMC Microbiol. 12, S2. ( 10.1186/1471-2180-12-S1-S2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Souza RS, Virginio F, Riback TIS, Suesdek L, Barufi JB, Genta FA. 2019. Microorganism-based larval diets affect mosquito development, size and nutritional reserves in the yellow fever mosquito Aedes aegypti (Diptera: Culicidae). Front. Physiol. 10, 1-24. ( 10.3389/fphys.2019.00152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Subra R, Mouchet J. 1984. The regulation of preimaginal populations of Aedes aegypti (L.) (Diptera: Culicidae) on the Kenya coast. Ann. Trop. Med. Parasitol. 78, 63-70. ( 10.1080/00034983.1984.11811774) [DOI] [PubMed] [Google Scholar]
- 87.Orcutt JD, Porter KG. 1984. The synergistic effects of temperature and food concentration of life history parameters of Daphnia. Oecologia 63, 300-306. ( 10.1007/BF00390657) [DOI] [PubMed] [Google Scholar]
- 88.Huxley PJ, Murray KA, Pawar S, Cator LJ. 2021. Data from: The effect of resource limitation on the temperature dependence of mosquito population fitness. Dryad Digital Repository. ( 10.5061/dryad.h9w0vt4gg) [DOI] [PMC free article] [PubMed]
- 89.Huxley PJ, Murray KA, Pawar S, Cator LJ. 2020. The effect of resource limitation on the temperature-dependence of mosquito population fitness. bioRxiv 1–15. ( 10.1101/2020.05.29.123315) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Huxley PJ, Murray KA, Pawar S, Cator LJ. 2021. Data from: The effect of resource limitation on the temperature dependence of mosquito population fitness. Dryad Digital Repository. ( 10.5061/dryad.h9w0vt4gg) [DOI] [PMC free article] [PubMed]
- Huxley PJ, Murray KA, Pawar S, Cator LJ. 2020. The effect of resource limitation on the temperature-dependence of mosquito population fitness. bioRxiv 1–15. ( 10.1101/2020.05.29.123315) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
All data for analyses are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.h9w0vt4gg [88]. A preprint version of this manuscript is available from the bioRxiv preprint server: https://doi.org/10.1101/2020.05.29.123315 [89].