Abstract
Selective serotonin reuptake inhibitors (SSRIs) are one of the most commonly prescribed antidepressants worldwide and recent data show significant impairment of fracture healing after treatment with the SSRI fluoxetine in mice. Here, we provide evidence that the negative effects of SSRIs can be overcome by administration of the beta-blocker propranolol at the time of fracture. First, in vitro experiments established that propranolol does not affect osteogenic differentiation. We then used a murine model of intramembranous ossification to study the potential rescue effect of propranolol on SSRI-induced impaired fracture healing. Micro-CT analysis revealed that fluoxetine treatment resulted in a smaller bony regenerate and that this decrease in bone formation can be overcome by co-treatment with propranolol. We then tested this in a clinically relevant model of endochondral ossification. Fluoxetine-treated mice with a femur fracture were treated with propranolol initiated at the time of fracture, and a battery of analyses demonstrated a reversal of the detrimental effect of fluoxetine on fracture healing in response to propranolol treatment. These experiments show for the first time to our knowledge that the negative effects of SSRIs on fracture healing can be overcome by co-treatment with a beta-blocker. © 2020 American Society for Bone and Mineral Research.
Keywords: SSRI, PROPRANOLOL, FRACTURE HEALING, FLUOXETINE, ANTIDEPRESSANTS, ENDOCHONDRAL OSSIFICATION
Introduction
Selective serotonin reuptake inhibitors (SSRIs) are one of the most commonly prescribed classes of medication for chronic depression. A recent study from the Center of Disease Control and Prevention (CDC) reported that 1 in every 10 Americans reports using an antidepressant, and this figure is estimated to significantly increase.(1) SSRIs inhibit the serotonin transporter and, in doing so, increase the extracellular, intrasynaptic serotonin concentration. Because of its molecular specificity, the side effect profile is minimal, further leading to an increased use. SSRIs have recently been linked to alterations in bone remodeling in both animal and in vitro studies; however, the exact mechanism has yet to be fully elucidated.(2,3) Chronic SSRI usage has been linked to an increased risk of osteoporosis and an associated increased relative fracture risk of 70% compared with non-SSRI users.(1,4,5) SSRIs exert their peak catabolic effect at 8 months after treatment initiation and at this point exert their greatest fracture risk.(6) Just as chronic SSRI usage affects bone deposition during bone homeostasis, it could theoretically impair bone deposition in the setting of fracture healing. A recent animal study demonstrated that fracture calluses in mice treated with fluoxetine (flx), the most commonly prescribed SSRI, were smaller in size and biomechanically weaker compared with the fracture calluses of control animals.(7) Interestingly, cessation of the medication did not lead to improved bone healing but rather led to the development of nonunions, indicating a prolonged half-life in bone tissue with detrimental effects on the program of bone repair.(7) Ortuño and colleagues(8) recently reported that propranolol, a widely used beta-blocker medication, can rescue the negative effect of fluoxetine on bone homeostasis. Here we show that, similar to the protective effect of propranolol during bone homeostasis, the beta-blocker also mitigates the negative effects of the SSRI on fracture healing.
Materials and Methods
Experimental animals
All procedures were approved by the New York University Committee on Animal Research. Studies were conducted on 12-week-old C57BL/6 male mice (Mus musculus) purchased from Jackson Laboratory (Bar Harbor, ME, USA). Mice were maintained on a 12-hour light/dark cycle with food and water provided ad libitum.
Fluoxetine and propranolol administration
Fluoxetine (Teva Pharmaceuticals, Sellersville, PA, USA) was delivered in the drinking water. Mice were randomly selected to receive either treatment or control fluid. A 240 mg/L fluoxetine solution was prepared so that the mice received 10 mg/kg/d.(9) To simulate chronic fluoxetine use, mice received oral treatment for 3 weeks before the surgical procedures(9) and their treatment was continued until euthanasia. Propranolol (Sigma, St. Louis, MO, USA) was administered via drinking water, 166 mg/L (0.5 mg/d).
Surgical procedures
We utilized a femoral shaft fracture model to study the effect of fluoxetine and propranolol on endochondral bone formation.(10) Briefly, after induction of anesthesia with isoflurane inhalation (1% to 5%), an incision was made over the anterolateral femur. A 27-gauge syringe needle was inserted into the distal femur through a small incision medial to the patella tendon. The needle was partially withdrawn and the mid-diaphysis of the femur was transected with small surgical scissors. The needle was reinserted into the femur to stabilize the fracture. The free edge of the muscle flap was placed over the fracture site with a single suture, and the skin was closed. Mice were euthanized by CO2 asphyxiation, followed by cervical dislocation on postoperative day (POD) 14 or 21.
Intramembranous ossification was studied using a monocortical tibial defect model, as previously described.(11,12) After adequate anesthesia and analgesia, the surgical site was clipped and prepped with betadine. A 3 mm incision was performed over the anteromedial tibia, and the pes anserine insertion was identified and sharply elevated off of the tibia. A 1 mm monocortical defect was drilled with a dental drill (NSK Ultimate XL, Kanuma, Japan) in the location of the pes insertion (approximately 3 mm distal from joint line), which allows consistent placement of the injury site. Next, the anterior muscle compartment, which was previously elevated, was then laid over the defect and sutured in place with a 7–0 Vicryl, followed by skin closure. Mice were allowed to ambulate freely postoperatively. Mice were euthanized as described above on postoperative day 14.
Micro-CT analyses
Femurs and tibias from all experimental groups were analyzed with micro-computed tomography (micro-CT) on postoperative days 14 and 21. After euthanasia, the skeletal elements were carefully dissected and fixed in 4% paraformaldehyde overnight at 4°C. Implants were removed from the femurs before scanning. Specimens were scanned using a high-resolution SkyScan micro-CT system (SkyScan 1172, Kontich, Belgium). CT images were acquired at 10 μm isotropic resolution using a 10 MP digital detector, 10 W energy (100 kV and 100 A) and a 0.5 mm aluminum filter with a 9.7 μm image voxel size. A fixed global thresh-old method was used based on the manufacturer’s recommendations and preliminary studies, which showed that mineral variation between groups was not high enough to warrant adaptive thresholding. The following parameters were analyzed: total bone volume (BV), total tissue volume (TV), respective mineralized volume fraction (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), and trabecular spacing (Tb.Sp) following the guidelines described by Bouxsein and colleagues.(13) Cortical bone of proximal femurs was analyzed for cross-sectional thickness, bone perimeter, cross-sectional bone area, and closed porosity. The amount of mineralized bone in callus (callus mineral density) was assessed by micro-CT after calibration using two phantoms with defined hydroxyapatite (HA) contents (0.25 and 0.75 g/cm3).
Histology and histomorphometry
Femurs and tibias were dissected and fixed overnight in 4% paraformaldehyde at 4°C. The samples were decalcified in 19% ethylenediaminetetraacetic acid (EDTA) for 3 weeks at 4°C. Decalcified samples were dehydrated in a graded ethanol series, embedded into paraffin, and cut into 10-μm-thick sections. Pentachrome and aniline blue staining were used to detect osseous tissues as previously described.(11)
In vivo calcein/alizarin labeling and calculation of mineral apposition rate (MAR)
Calcein/alizarin labeling and MAR calculation were performed in control (n = 4), fluoxetine-treated (n = 4), and combination of fluoxetine and propranolol-treated mice (n = 3) (treatment from 3 weeks before fracture to euthanasia). Calcein and alizarin were intraperitoneally injected with the following dosage: 30 mg/kg of alizarin (Sigma-Aldrich) in a 2% sodium bicarbonate solution at POD 15 and 20 mg/kg of calcein (Sigma-Aldrich) in a 2% sodium bicarbonate solution at POD 20 after femur fracture. On POD 21, the femurs were fixed in 4% paraformaldehyde for 24 hours at 4°C, embedded as described,(14) then cryosectioned at 50 μm thickness. The distance between the midpoints of the two labels was measured with ImageJ software (US National Institutes of Health, Bethesda, MD, USA), and values obtained were divided by the time between the alizarin and calcein injections to obtain the MAR (μm/d). Bone formation rate per bone surface (BFR/BS; μm2/μm3/d) was analyzed by Bioquant Osteo software (BIOQUANT Image Analysis Corporation, Nashville, TN, USA).
Immunostaining
Paraffin-embedded femur and tibia samples were sectioned at 10 μm and subjected to immunostaining for PCNA. Antigen retrieval was performed on the sections with IHC-Tek Epitope Retrieval solution (IHC World, Woodstock, MD, USA) at 70°C for 20 minutes. Sections were blocked in 1% Donkey IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) with 0.2% ovalbumin (Sigma-Aldrich). Rabbit anti-PCNA antibody (1:400; Cell Signaling Technology, Danvers, MA, USA) was applied over-night at 4°C, followed by biotinylated anti-Rabbit IgG (Abcam, Cambridge, UK) and peroxidase-conjugated streptavidin (Jackson ImmunoResearch Laboratories) incubation, and visualized by diaminobenzidine substrate (DAB; Life Technologies, Carlsbad, CA, USA). PCNA-positive cells were counted using ImageJ software (US National Institutes of Health, Bethesda, MD, USA).
Bone marrow harvest for in vitro experiments
For the in vitro experiments, tibial and femoral bone marrow was extracted by centrifugation from untreated 12-week-old mice.(15) Cells were resuspended in growth media (GM), which consisted of DMEM (Thermo Fisher Scientific, Waltham, MA, USA) containing 10% FBS (Thermo Fisher Scientific) and 1% penicillin/streptomycin (Thermo Fisher Scientific), followed by plating in T75 tissue culture flasks. Media was replenished every 3 days. Approximately 1 week from harvest, the cells were trypsinized and passaged for the following assays.
Osteogenic differentiation
Cells were plated in 24-well plates with a density of 10,000 cells/well. After overnight attachment in GM, cells were treated with osteogenic differentiation induction (OI) media containing DMEM, 10% FBS, 100 μg/mL ascorbic acid, 10 mM ß-glycerophosphate, and 1% penicillin/streptomycin. Cells were cultured in OI media alone, containing fluoxetine, propranolol, or a combination of both. Media was replenished every 3 days. The cells were fixed and stained for alkaline phosphatase activity and alizarin red at days 7 and 14, respectively. After 7 days, the cells were harvested and alkaline phosphatase activity was analyzed with an Alkaline Phosphatase Assay kit (Abcam) following the manufacturer’s instructions. After 14 days, RNA was isolated as described below. Data were collected with Soft Max Pro (Molecular Devices, San Jose, CA, USA) software. Means and standard of the mean were calculated in GraphPad Prism 7 software (GraphPad, La Jolla, CA, USA).
RNA isolation and quantitative real-time PCR
Cells were plated in 24-well plates at a density of 10,000 cells/well. The cells were cultured in OI media alone or containing 1 or 10 μM propranolol with or without 10 μM fluoxetine. Media was replenished every 3 days. After 7 days, RNA was isolated (RNeasy Kit, Qiagen, Hilden, Germany), genomic DNA was removed (RNase-free DNase set, Qiagen), and the RNA reverse-transcribed (High Capacity cDNA RT Kit, Applied Biosystems, Carlsbad, CA, USA). Quantitative real-time PCR was carried out using the Applied Biosystems Step One Plus detection system (Thermo Fisher Scientific) and RT2 SYBR Green ROX PCR Master Mix (Qiagen). Specific primers were designed based on PrimerBank (http://pga.mgh.harvard.edu/primerbank/) sequence (Table 1). Results are presented as 2−ΔΔCt values normalized to the expression of 18S samples. All reactions were performed in duplicates; means and standard of the mean were calculated in GraphPad Prism 7 software.
Table 1.
qPCR Primers
| Primer name | Sequence (5′ - 3′) |
|---|---|
| 18S forward | ACGAGACTCTGGCATGCTAACTAGT |
| 18S reverse | CGCCACTTGTCCCTCTAAGAA |
| Sp7 (Osterix) forward | CTGCTTGAGGAAGAAGCTC |
| Sp7 (Osterix) reverse | CTTCTTTGTGCCTCCTTTCC |
Results
Fluoxetine treatment impairs osteogenesis in vitro
Our previous research has convincingly shown that the SSRI fluoxetine exerts a direct, inhibitory effect on osteoblast differentiation and mineralization, shown in two disparate murine models of bone repair.(7) Here, we are evaluating a potential remedy for the negative effect of fluoxetine on bone formation. As shown by Ortuño and colleagues, the beta-blocker propranolol is able to overcome the osteocatabolic effect of fluoxetine during bone homeostasis.(8) To identify whether this mechanism is regulated centrally rather than on a direct cellular level, we studied the effect of propranolol on bone marrow stromal cells in an in vitro environment, which is devoid of central stimuli. We isolated bone marrow stromal cells from adult, male 12-week-old C57B/L6 mice and subjected them to fluoxetine, propranolol, and fluoxetine/propranolol in presence or absence of osteogenic differentiation induction (OI) media. We then assessed osteogenic differentiation using alkaline phosphatase activity (ALP), alizarin red staining, and expression levels of Osterix (osx), a key transcription factor required for osteogenic differentiation (Fig. 1A–E). ALP activity after 7 days in cells treated with fluoxetine alone demonstrated significantly less activity compared with the control cells (osteogenic differentiation media alone) (Fig. 1A, C). Cells treated with fluoxetine and either 1 μM and 10 μM propranolol exhibited similar ALP activity, indicating no direct effect of propranolol on ALP activity in bone marrow stromal cells (Fig. 1A, C). The alizarin red assay, which is staining for mineralized matrix in vitro, revealed similar findings. Propranolol treatment did not affect mineral deposition or rescue the fluoxetine phenotype (Fig. 1B, E). After 14 days, we performed qRT-PCR to evaluate osx expression and detected a similar trend as observed in the ALP and alizarin red assay (Fig. 1D). From these experiments, we conclude that fluoxetine exerts a direct effect on cells and cannot be overcome by the addition of propranolol, which is in line with other studies demonstrating a central, pro-osteogenic effect of the beta-blocker rather than a direct effect on the bone marrow stromal cell.(8)
Fig. 1.
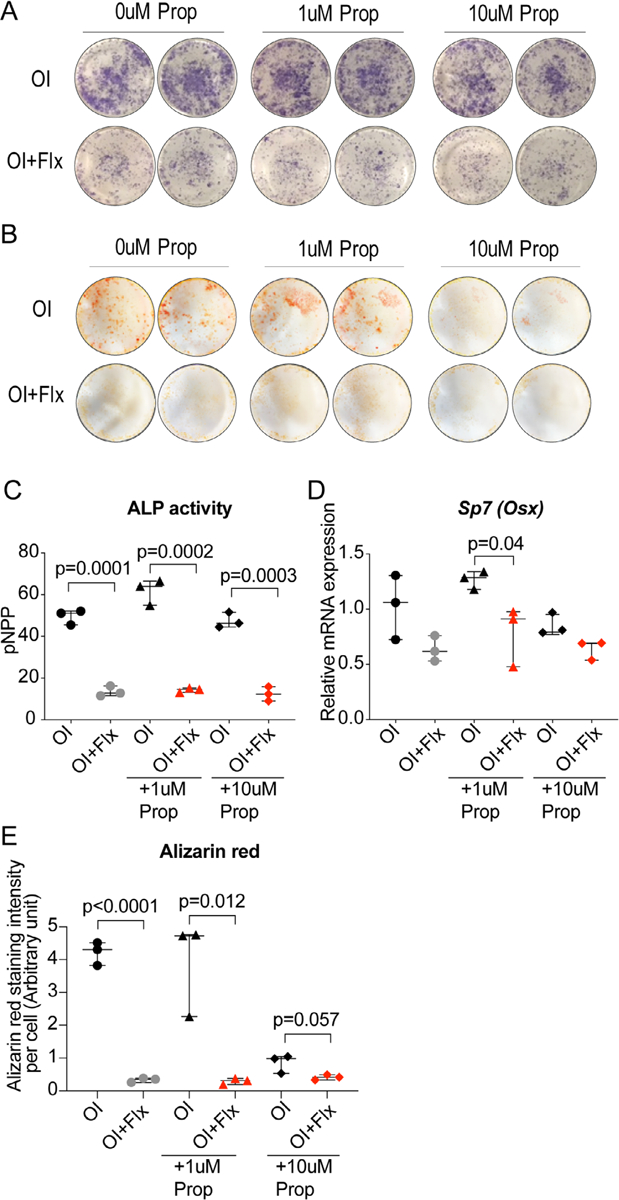
Fluoxetine effect on osteogenic differentiation is not mitigated by propranolol co-treatment in vitro. (A) Alkaline phosphatase (ALP) activity and (B) alizarin red staining of bone marrow stromal cells in osteogenic differentiation media treated with fluoxetine and varying concentrations of propranolol and fluoxetine. (C) Quantification of ALP activity. (D) Sp7 (osterix) gene expression. (E) Quantification of alizarin red staining. Statistical analysis: t test. Flx = fluoxetine; prop = propranolol; ALP = alkaline phosphatase; OI = osteogenic differentiation induction media.
Propranolol co-treatment prevents negative effect of fluoxetine on intramembranous bone formation
Having shown that the beta-blocker propranolol cannot overcome the negative effects of fluoxetine in vitro, we now set out to test its effect in an in vivo model of intramembranous bone formation. A mono-cortical defect was performed in tibias of 12-week-old male mice treated with either control drinking water, fluoxetine drinking water, or a combination of fluoxetine and propranolol. Mice were euthanized after 2 weeks and the tibias were processed for histology and micro-CT analyses (Fig. 2A). Aniline blue histology confirmed our previous finding that fluoxetine treatment results in decreased bone matrix deposition in an intramembranous ossification model (Fig. 2B).(7) However, when mice were treated with fluoxetine and propranolol, we observed a complete restoration of callus volume comparable to the untreated control animal using histomorphometry (Fig. 2C). We examined osteoblast number and proliferation and detected a decrease in osteoblast number with fluoxetine treatment. A significant increase in proliferation resulted in a normalization of the osteoblast number in fluoxetine + propranolol–treated animals (Fig. 2D, E). We then analyzed callus volume (TV) using micro-CT at 2 weeks postoperation. Callus volume included the entire callus including soft and hard tissue. As expected, fluoxetine treatment resulted in a smaller callus (Fig. 2F). In contrast, callus volume in mice treated with both fluoxetine and propranolol was significantly larger than the callus volume in mice treated with fluoxetine alone (Fig. 2F). Similarly, total bone volume within the callus showed a decrease in SSRI-treated animals and an increase back to normal in animals treated with the combination of fluoxetine and propranolol (Fig. 2G). Ratio of bone volume over tissue volume (BV/TV) did not change at this time point (Fig. 2H). Trabecular number, thickness, and spacing were not affected (Fig. 2I–K).
Fig. 2.
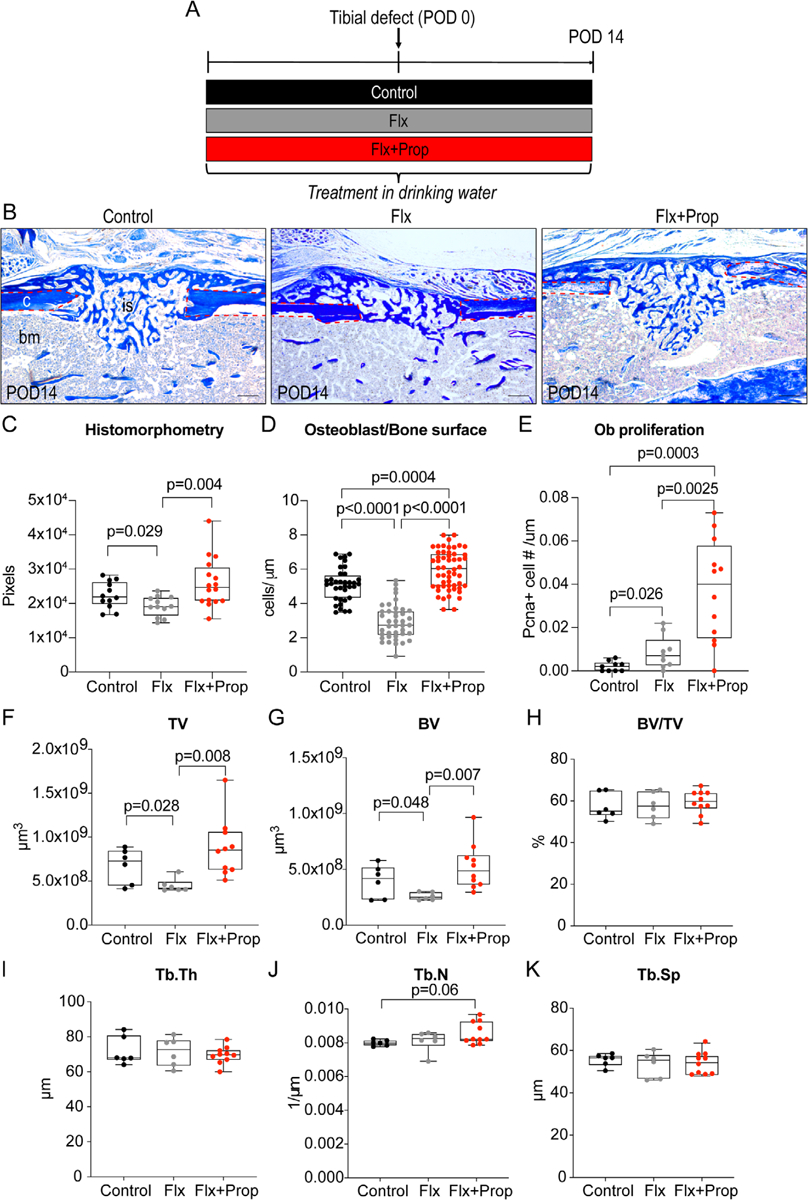
Propranolol co-treatment reverses fluoxetine-induced decline in osteogenic differentiation during intramembranous bone formation. (A) Experimental schematic outlining drug treatment started 3 weeks before injury and maintained until euthanasia 2 weeks after injury. (B) Aniline blue histology staining of tibial defects 14 days after injury in mice treated with control, fluoxetine, and fluoxetine + propranolol treatment. Red dotted line outlines cortical bone. Scale bar = 200 μm. (C) Graph depicting histomorphometric quantification of bone volume within the callus, (D) osteoblasts per μm bone surface measurement, and (E) osteoblast proliferation within the defect. (F–K) Micro-CT analysis of tibial defects 2 weeks after injury. Statistical analysis: t test. bm = bone marrow; c = cortical bone; is = injury site, ob = osteoblast; PCNA = proliferating cell nuclear antigen; TV = total volume; BV = bone volume; Tb. Th. = trabecular thickness; Tb.N. = trabecular number; Tb.Sp. = trabecular spacing.
These in vivo data suggest that beta-blocker treatment can overcome the negative effect of SSRI treatment on osteogenesis during intramembranous ossification. However, to be tter understand the clinical utility of this approach, we devised an experiment that better modeled the clinical reality.
Propranolol treatment overcomes negative effect of fluoxetine on endochondral ossification
Using an endochondral ossification model, we next assessed if the combination of fluoxetine and propranolol can prevent the detrimental effects of the SSRI on osteogenic differentiation (Fig. 3A). During this mode of bone healing, chondrogenic differentiation makes up the first phase of repair, followed by osteogenic differentiation once the soft callus has formed. We analyzed the callus using histology, histomorphometry, and dynamic histomorphometry. Histological staining of the fracture callus at 21 days after fracture revealed a stark difference in callus size between the control water-treated mice and the mice treated with fluoxetine. The callus of the fluoxetine-treated mice appeared smaller (Fig. 3B, C). In contrast, the callus from mice treated with both fluoxetine and propranolol demonstrated a similar size as the control callus (Fig. 3B, D), suggesting a rescue of the fluoxetine effect on fracture healing. We next set out to objectively measure the callus bone volume using histomorphometry of the osseous callus stained with aniline blue (Fig. 3E–G). Histomorphometry confirmed the observation (Fig. 3H). Although fluoxetine treatment resulted in a significant reduction in callus size and callus bone volume, fluoxetine and propranolol co-treatment resulted in a callus size and callus bone volume comparable to that of control animals (Fig. 3H). Next, we analyzed the MAR and BFR using calcein and alizarin labels. This technique allows assessment of the functional osteoblast capacity in response to a treatment. Analysis of the matrix that had formed between the two fluorescent labels demonstrated that fluoxetine treatment resulted in a decreased mineral apposition rate and bone formation rate, while combination treatment resulted in an increased MAR and BFR/BS (Fig. 3I–M).
Fig. 3.
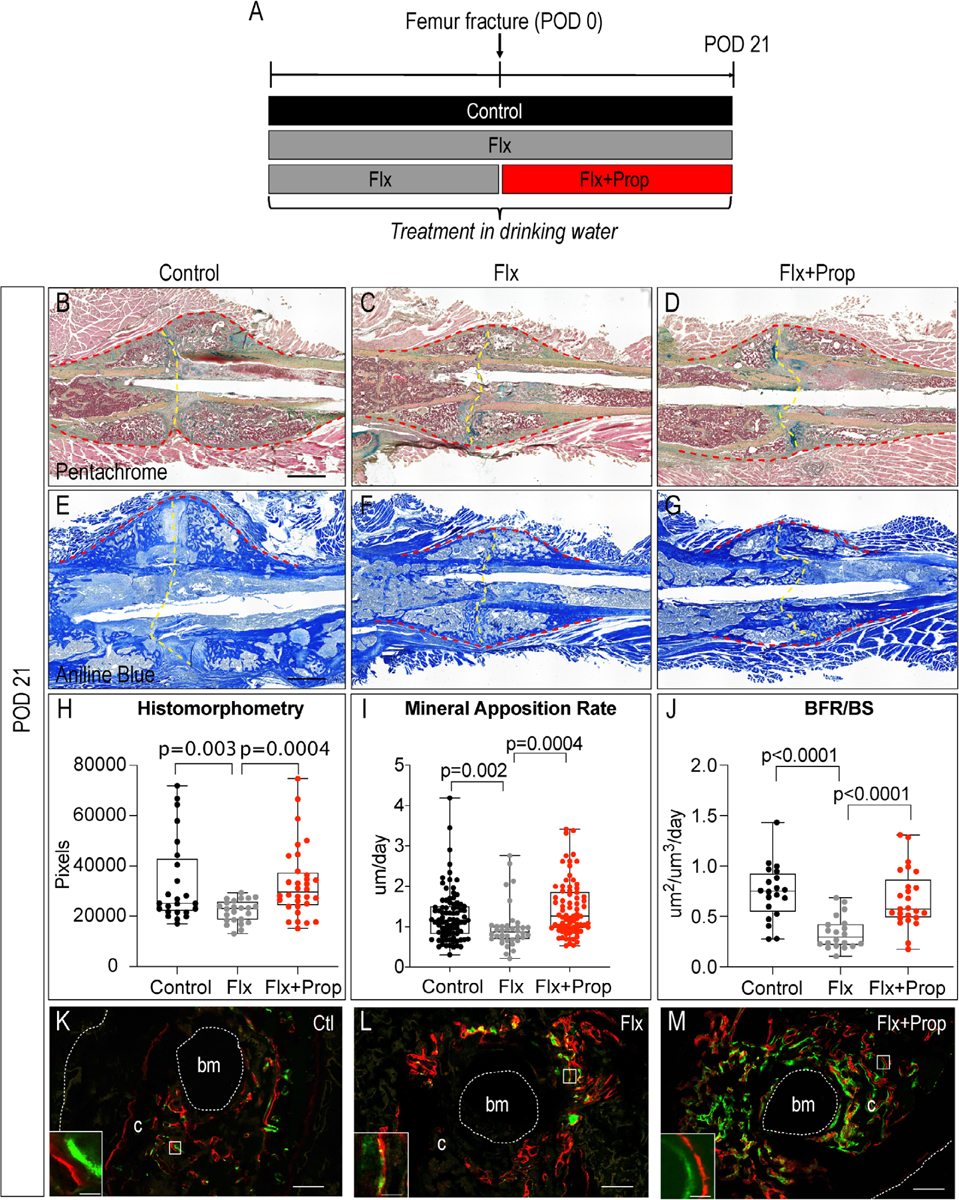
Combination treatment of fluoxetine and propranolol prevents SSRI-induced impaired bone healing in clinically relevant femur fracture model. (A) Experimental schematic. (B–D) Pentachrome staining and (E–G) aniline blue staining of femur fractures at postoperative day 21 in mice treated with control water (B, E), fluoxetine (C, F), and fluoxetine + propranolol (D, G). Red dotted line outlines callus and yellow dotted line indicates fracture site. Scale bar = 1 mm. (H) Histomorphometric quantification of osseous callus volume. (I) Quantification of mineral apposition rate and (J) bone formation rate within the callus and (K–M) representative images. Scale bar = 200 μm; scale bar in insert = 20 μm. Statistical analysis: t test. BFR = bone formation rate; bm = bone marrow; BS = bone surface; c = callus.
To verify the histomorphometric analysis and the MAR measurements, we employed micro-CT analysis. Plain visual analyses of the longitudinal CT reconstructions at 21 days show a decreased callus size in the fluoxetine group and a larger callus, similar to that found in wild-type mice, in the combination group (fluoxetine and propranolol) (Fig. 4A). Analysis of the callus volume confirmed that BV/TV decreased in the fluoxetine alone group, whereas there was no difference between the controls and the combination treatment, confirming our hypothesis that the combination treatment can revert the negative SSRI effect (Fig. 4B). In addition, combination treatment resulted in an increased bone mineral density compared with the fluoxetine treatment alone (Fig. 4B). Together these data provide evidence that both in intramembranous and endochondral ossification, propranolol can overcome the detrimental effects of fluoxetine.
Fig. 4.
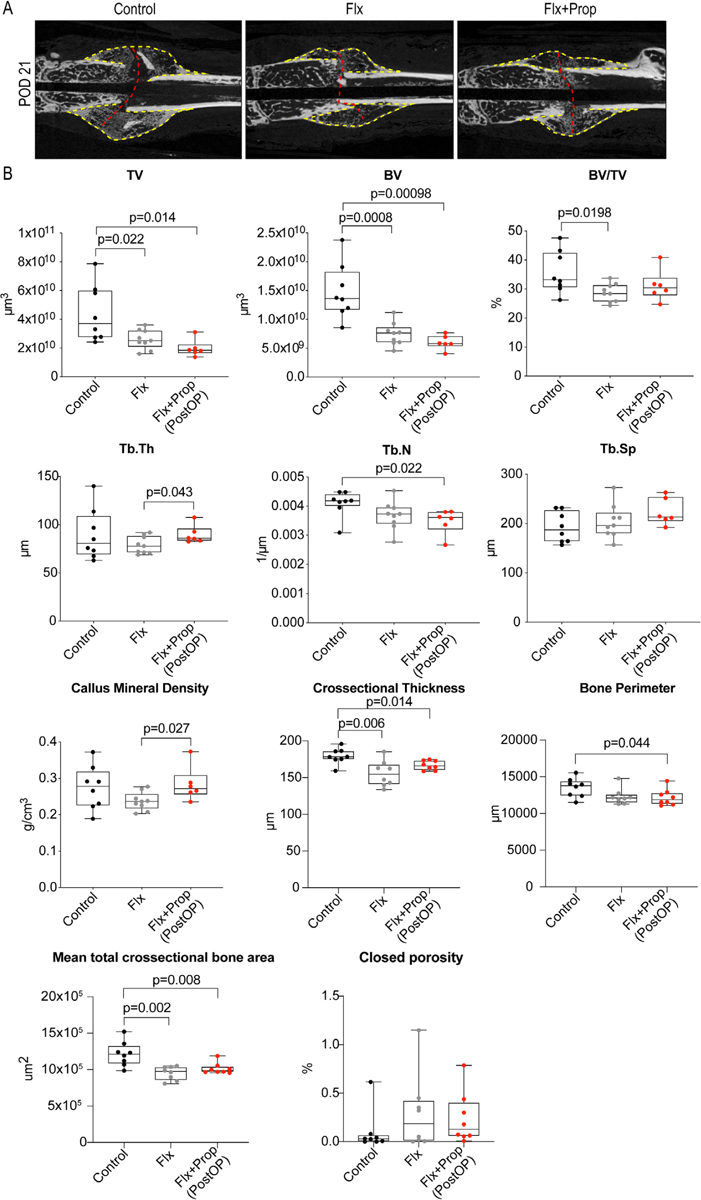
Combination treatment of fluoxetine and propranolol rescues fluoxetine-induced bone mineral density loss in fracture callus. (A) Longitudinal micro-CT reconstructions through the fracture callus of the three experimental groups. Yellow dotted line indicates callus and red dotted line indicates fracture site. (B) Micro-CT analysis of the fracture callus at POD 21. Statistical analysis: t test. TV = total volume; BV = bone volume; Tb.Th. = trabecular thickness; Tb.N. = trabecular number; Tb.Sp. = trabecular spacing.
Propranolol rescue in femur fractures may be a consequence of larger cartilaginous template
To better understand how the combination of fluoxetine and propranolol accomplished a partial rescue of the fluoxetine phenotype during endochondral ossification, we analyzed the fracture callus at an earlier time point during the healing process. At postoperative day 14, the cartilaginous template is the largest in size, thus we chose this time point to assess whether our treatment regimens had an effect on chondrogenesis. Histology revealed a gross difference in soft callus volume between the fluoxetine and combination group (fluoxetine and propranolol) (Fig. 5A). Further histomorphometric analyses confirmed this observation. The combination group showed a significantly larger cartilaginous callus (Fig. 5B). In addition, we performed PCNA staining to assess proliferation at 14 days after fracture and observed a significant decrease in proliferation in response to fluoxetine treatment. This decrease did not occur in mice that were treated with propranolol (Fig. 5C).
Fig. 5.
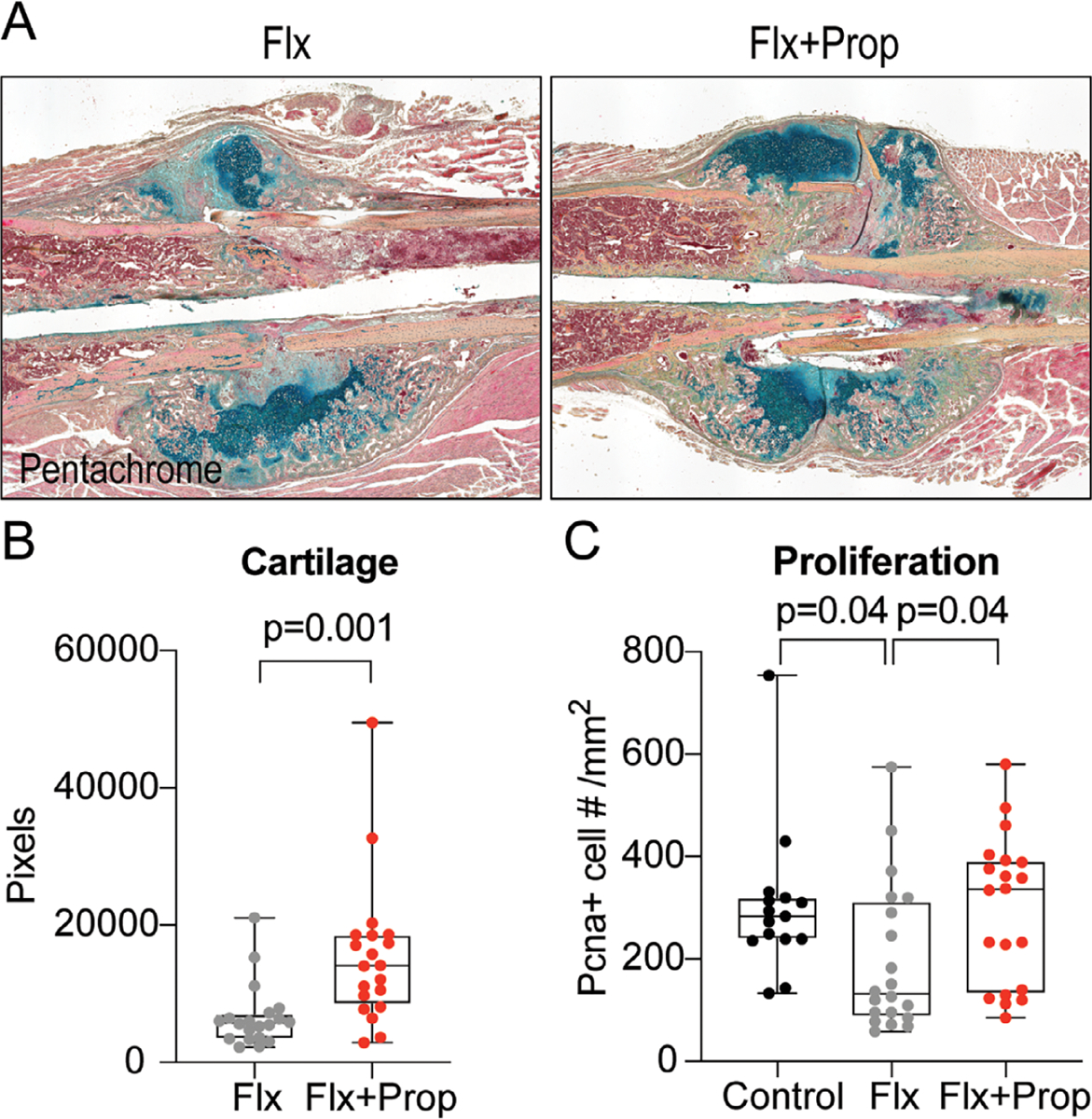
Cartilaginous callus volume is increased at POD14 after combination treatment with fluoxetine and propranolol. (A) Pentachrome staining of longitudinal section through the fracture callus at 2 weeks postfracture revealing difference in cartilage volume (dark green). (B) Histomorphometric quantification of cartilage volume at 2 weeks postfracture. (C) Proliferation within the fracture callus at 14 days postfracture. Statistical analysis: t test.
In summary, our data suggest that co-treatment with the beta-blocker propranolol mitigates the negative effects of fluoxetine observed during fracture healing. In particular, the co-treatment resulted in a larger cartilaginous soft callus, an increased bone mineral deposition, and increased osteoblast number and proliferation.
Discussion
Animal studies and human clinical data have shown that long-term SSRI use can lead to osteoporosis.(16–19) More recently, both in vivo and in vitro studies have shown that along with altering bone homeostasis, SSRIs can also negatively affect bone regeneration.(7) Therefore, we utilized an animal model to examine a potential pharmacological rescue of the negative effects of fluoxetine on fracture healing by co-treating the animals with a beta-blocker, propranolol. Ortuño and colleagues recently published convincing data showing that treatment with propranolol reverses the negative effects of the SSRI during bone homeostasis.(8) The process of bone homeostasis and remodeling is characterized by osteoclastic resorption followed by bone matrix deposition from osteoblasts. This is a continuous, slow process that takes place at many sites within the skeleton. Bone regeneration in response to injury is comparable to the program of remodeling; however, the individual steps occur at an accelerated pace. During bone regeneration, skeletal stem and progenitor cells home to the fracture, then divide, followed by chondrogenic and osteogenic differentiation. These complex steps are all accomplished within the first 21 days during murine fracture healing. Because of the similarities between homeostasis and acute fracture healing, we postulated that propranolol could have a similar effect on fluoxetine-treated bones as shown by Ortuño and colleagues(8) We utilized both an intramembranous and an endochondral bone healing model to study this potential rescue using propranolol. First, we observed the previously published decrease in bone formation in response to fluoxetine treatment.(7) We then treated femur fractures at the time of injury with propranolol and observed a healing response similar to the one found in the control animals. From these data, we concluded that propranolol can rescue the deleterious effect of fluoxetine on fracture healing. To gain mechanistic insights into the process, we utilized an intramembranous bone repair model and in doing so revealed that propranolol treatment restored osteogenic differentiation and thus callus volume in fluoxetine-treated animals to levels comparable to the control animals. in vitro experiments provided further insights in the mechanism. When bone marrow stromal cells were treated with fluoxetine alone, we observed decreased osteogenic differentiation. Addition of propranolol did not result in a rescue of this decline in matrix deposition, suggesting that propranolol does not exert its effect directly on bone marrow stromal cells or osteoblasts. SSRIs lead to an increase in systemic epinephrine and norepinephrine, which has been shown to have a bone catabolic effect.(20) Ortuño and colleagues suggested that using beta-blockers to reduce the fluoxetine-dependent increase in cate-cholamines would inhibit this catabolic effect. This central effect of propranolol explains why the in vitro data presented herein do not show a direct effect of propranolol on bone marrow stromal cells.
Although these animal data are showing a promising result, we still need further clinical evidence to be in a position to recommend this treatment for patients taking fluoxetine for the treatment of depression. We will continue to investigate the underlying mechanisms to better understand the cellular and molecular steps that govern the side effects of fluoxetine. We are confident that eventually, we will be able to abrogate the deleterious side effects of fluoxetine.
Acknowledgments
The authors thank Gina Yildirim (New York University College of Dentistry) for assistance with the micro-CT imaging (supported by NIH S10 OD010751-01A1). This work was supported by an Orthopaedic Trauma Association (OTA) research grant to PL. PL is supported by K08AR069099 from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin and R01AG056169 from the National Institutes of Health/National Institute of Aging.
Footnotes
Disclosures
All authors state that they have no conflicts of interest.
References
- 1.Feuer AJ et al. Use of selective serotonin reuptake inhibitors and bone mass in adolescents: an NHANES study. Bone. 2015;78:28–33. [DOI] [PubMed] [Google Scholar]
- 2.Ducy P, Karsenty G. The two faces of serotonin in bone biology. J Cell Biol. 2010;191(1):7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karsenty G, Yadav VK. Regulation of bone mass by serotonin: molecular biology and therapeutic implications. Annu Rev Med. 2011;62: 323–31. [DOI] [PubMed] [Google Scholar]
- 4.Richter T, Paluch Z, Alusik S. The non-antidepressant effects of citalopram: a clinician’s perspective. Neuro Endocrinol Lett. 2014;35 (1):7–12. [PubMed] [Google Scholar]
- 5.Rauma PH, Honkanen RJ, Williams LJ, et al. Effects of antidepressants on postmenopausal bone loss — a 5-year longitudinal study from the OSTPRE cohort. Bone. 2016;89:25–31. [DOI] [PubMed] [Google Scholar]
- 6.Rizzoli R, Cooper C, Reginster JY, et al. Antidepressant medications and osteoporosis. Bone. 2012;51(3):606–13. [DOI] [PubMed] [Google Scholar]
- 7.Bradaschia-Correa V, Josephson AM, Mehta D, et al. The selective serotonin reuptake inhibitor fluoxetine directly inhibits osteoblast differentiation and mineralization during fracture healing in mice. J Bone Miner Res. 2017;32(4):821–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ortuño MJ, Robinson ST, Subramanyam P, et al. Serotonin-reuptake inhibitors act centrally to cause bone loss in mice by counteracting a local anti-resorptive effect. Nat Med. 2016;2(10): 1170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychophar-macology. 2004;29(7):1321–30. [DOI] [PubMed] [Google Scholar]
- 10.Bonnarens F, Einhorn TA. Production of a standard closed fracture in laboratory animal bone. J Orthop Res. 1984;2(1):97–101. [DOI] [PubMed] [Google Scholar]
- 11.Leucht P, Kim JB, Wazen R, et al. Effect of mechanical stimuli on skeletal regeneration around implants. Bone. 2007;40(4):919–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minear S, Leucht P, Jiang J, et al. Wnt proteins promote bone regeneration. Sci Transl Med. 2010;2(29):29ra30. [DOI] [PubMed] [Google Scholar]
- 13.Bouxsein ML, Boyd SK, Christiansen BA, et al. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25(7): 1468–86. [DOI] [PubMed] [Google Scholar]
- 14.Kusumbe AP, Ramasamy SK, Starsichova A, Adams RH. Sample preparation for high-resolution 3D confocal imaging of mouse skeletal tissue. Nat Protoc. 2015;10(12):1904–14. [DOI] [PubMed] [Google Scholar]
- 15.Kelly NH, Schimenti JC, Patrick Ross F, van der Meulen MC. A method for isolating high quality RNA from mouse cortical and cancellous bone. Bone. 2014;68:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warden SJ, Hassett SM, Bond JL, et al. Psychotropic drugs have contrasting skeletal effects that are independent of their effects on physical activity levels. Bone. 2010;46(4):985–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warden SJ, Nelson IR, Fuchs RK, Bliziotes MM, Turner CH. Serotonin (5-hydroxytryptamine) transporter inhibition causes bone loss in adult mice independently of estrogen deficiency. Menopause. 2008;15(6):1176–83. [DOI] [PubMed] [Google Scholar]
- 18.Warden SJ, Robling AG, Sanders MS, Bliziotes MM, Turner CH. Inhibition of the serotonin (5-hydroxytryptamine) transporter reduces bone accrual during growth. Endocrinology. 2005;146(2):685–93. [DOI] [PubMed] [Google Scholar]
- 19.Fernandes BS, Hodge JM, Pasco JA, Berk M, Williams LJ. Effects of depression and serotonergic antidepressants on bone: mechanisms and implications for the treatment of depression. Drugs Aging. 2016;33(1):21–5. [DOI] [PubMed] [Google Scholar]
- 20.Blardi P, de Lalla A, Auteri A, et al. Plasma catecholamine levels after fluoxetine treatment in depressive patients. Neuropsychobiology. 2005;51(2):72–6. [DOI] [PubMed] [Google Scholar]


